Artificial enzyme innovations in electrochemical devices: advancing wearable and portable sensing technologies
Long
Zheng†
a,
Mengzhu
Cao†
a,
Yan
Du
 b,
Quanyi
Liu
b,
Mohammed Y.
Emran
c,
Ahmed
Kotb
c,
Mimi
Sun
*a,
Chong-Bo
Ma
b,
Quanyi
Liu
b,
Mohammed Y.
Emran
c,
Ahmed
Kotb
c,
Mimi
Sun
*a,
Chong-Bo
Ma
 *a and
Ming
Zhou
*a and
Ming
Zhou
 *a
*a
aKey Laboratory of Polyoxometalate and Reticular Material Chemistry of Ministry of Education, Key Laboratory of Nanobiosensing and Nanobioanalysis at Universities of Jilin Province, Analysis and Testing Center, Department of Chemistry, Northeast Normal University, Changchun, Jilin Province 130024, China. E-mail: sunmm467@nenu.edu.cn; macb806@nenu.edu.cn; zhoum739@nenu.edu.cn
bState Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130000, China
cChemistry Department, Faculty of Science, Al-Azhar University, Assiut 71524, Egypt
First published on 30th November 2023
Abstract
With the rapid evolution of sensing technologies, the integration of nanoscale catalysts, particularly those mimicking enzymatic functions, into electrochemical devices has surfaced as a pivotal advancement. These catalysts, dubbed artificial enzymes, embody a blend of heightened sensitivity, selectivity, and durability, laying the groundwork for innovative applications in real-time health monitoring and environmental detection. This minireview penetrates into the fundamental principles of electrochemical sensing, elucidating the unique attributes that establish artificial enzymes as foundational elements in this field. We spotlight a range of innovations where these catalysts have been proficiently incorporated into wearable and portable platforms. Navigating the pathway of amalgamating these nanoscale wonders into consumer-appealing devices presents a multitude of challenges; nevertheless, the progress made thus far signals a promising trajectory. As the intersection of materials science, biochemistry, and electronics progressively intensifies, a flourishing future seems imminent for artificial enzyme-infused electrochemical devices, with the potential to redefine the landscapes of wearable health diagnostics and portable sensing solutions.
1. Introduction
Natural enzymes, revered as nature's exquisite catalysts, are indispensable in orchestrating a myriad of biological processes, driving reactions with unparalleled specificity and efficiency. These biological marvels accelerate reactions significantly, making them invaluable in various applications ranging from industrial biocatalysis to medical diagnostics.1–4 However, despite their specificity, natural enzymes possess inherent limitations. They are often sensitive to environmental conditions like pH and temperature, losing their activity outside optimal conditions. Additionally, the processes of extraction, purification, and stabilization of these enzymes are not only intricate but also economically burdensome.5Addressing these challenges, there has been burgeoning interest in developing artificial enzyme materials. These meticulously designed synthetic catalysts, which emulate the catalytic functions of their natural counterparts, offer a slew of advantages.6–11 Engineered for enhanced stability, artificial enzymes can operate efficiently across a broader spectrum of conditions. Their production, scalable and economically viable, is devoid of the complexities tethered to the extraction from natural sources. Moreover, the properties of artificial enzymes can be meticulously tailored to cater to specific applications, offering a versatility that is elusive with natural enzymes.
In the specialized domain of electrochemical sensing, the transition from natural to artificial enzymes has been markedly evident.12,13 Electrochemical sensors, adept at transducing chemical signals into electrical readouts, have reaped substantial benefits from the robustness and adaptability proffered by artificial enzyme materials. These innovative materials bolster the sensors’ catalytic efficiency, facilitating faster response times and enhanced sensitivity. The design flexibility inherent to artificial enzymes also paves the way for sensors capable of detecting a diverse array of analytes with pinpoint accuracy. With the burgeoning demand for real-time, reliable sensing in critical areas such as medical diagnostics and environmental monitoring, the pivot towards artificial enzyme materials marks a significant trend in electrochemical sensing.14
Over the past decade, there has been a discernible shift in the technological landscape towards miniaturization and personalization, with portable and wearable electrochemical sensors at the forefront of this evolution.15–19 These sophisticated devices represent a synergy of miniaturized electronics, avant-garde materials science, and groundbreaking biotechnological innovations, aiming to democratize health monitoring and environmental analysis.
Portable electrochemical sensors are renowned for their exceptional convenience and adaptability.20–29 These compact and user-friendly devices may not always be designed for continuous monitoring, but they excel in facilitating intermittent checks and field tests. Their design makes them particularly useful in scenarios where traditional laboratory setups are impractical or unavailable, thereby offering a versatile tool for various sensing and diagnostic applications.
Following this, wearable sensors, which are ingeniously integrated into the fabric of daily life, have emerged as powerful tools offering continuous, non-invasive monitoring.30–41 These innovative devices are swiftly becoming indispensable, providing invaluable insights into various physiological parameters, ranging from monitoring glucose levels in diabetics to tracking lactate thresholds in athletes. The continuous data streams generated by these sensors furnish a comprehensive view of an individual's health and environmental exposure, thereby facilitating timely interventions and personalized health feedback.
The integration of artificial enzyme materials into both portable and wearable sensors has been revolutionary. These advanced materials enhance the sensors’ capabilities, enabling ultra-sensitive detection limits, a broader spectrum of detectable analytes, and robust stability under diverse conditions. The versatility of artificial enzymes allows for the customization of sensors for myriad applications, ranging from monitoring water quality to detecting vital biomarkers in bodily fluids.
In a time where prompt, data-driven decisions are crucial for both individual health management and environmental stewardship, these sensors transcend their role as mere devices; they are enablers of a healthier and more informed society. With ongoing research continually expanding the possibilities of artificial enzymes, the future of portable and wearable electrochemical sensors appears limitless, signalling the dawn of a new era in decentralized and empowered health and environmental monitoring.
2. Artificial enzymes: an overview
2.1. Definition and classification of artificial enzymes
Artificial enzymes, also known as enzyme mimics or synthetic enzymes, are designed and synthesized catalysts that emulate the catalytic functions of natural enzymes.42–45 These constructs aim to combine the specificity and efficiency of biological enzymes with the robustness and versatility of synthetic materials, providing adaptable catalytic solutions for various conditions and applications.Artificial enzymes can be classified into several categories, each contributing uniquely to the advancement of portable and wearable electrochemical sensing technologies (Fig. 1).
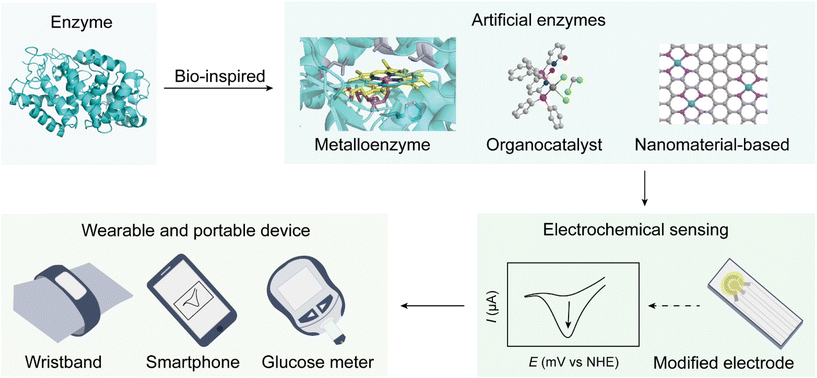 | ||
| Fig. 1 Schematic illustration of advancements in artificial enzyme technology for enhanced electrochemical sensing technologies in wearable and portable devices. | ||
2.2. Mechanism of action and unique properties of artificial enzymes
Each class of artificial enzymes operates through distinct mechanisms shaped by their structural composition:Versatility. They can be tailored for specific reactions, allowing for extensive modification of their catalytic properties.
Stability. Many artificial enzymes demonstrate enhanced resilience, functioning across a wider range of conditions than their natural counterparts.
Reusability. Some, especially those of a solid nature, can be easily separated from reaction mixtures and reused, offering sustainable and cost-effective solutions.
Multi-functionality. Certain artificial enzymes are designed to handle multiple reactions, either simultaneously or in sequence.
3. Electrochemical sensing: principles and techniques
3.1. Basic principles of electrochemical sensing
Electrochemical sensing is a technique grounded in the interaction between electrical and chemical processes. When a potential is applied to an electrode within an electrolyte, it initiates redox reactions, facilitating electron transfer between the electrode and the analyte. The resulting current, proportional to the analyte concentration, is crucial for quantification in electrochemical sensors.68,69 The electrochemical technique selected, whether voltammetry, amperometry, or impedance spectroscopy, provides varied insights into the analyte behavior and reaction mechanisms.70 Voltammetry involves varying the electrode potential to analyze the resulting current response, identifying changes in the oxidation or reduction state of the analyte. Amperometry measures the current at a fixed potential, which is directly proportional to the concentration of the analyte. Impedance spectroscopy assesses the change in electrical impedance of the solution or surface, providing insights into reaction kinetics and surface properties. Electrochemical sensors are valued for their rapid response, high sensitivity, and miniaturization potential. They are versatile, used in various applications from glucose detection in blood to environmental pollutant monitoring, facilitating real-time, on-site analysis without the need for extensive laboratory equipment.713.2. Common techniques and methods in electrochemical sensing
Following the foundational principles outlined in Section 3.1, we now focus on specific techniques employed in electrochemical sensing. Cyclic voltammetry (CV) is utilized for a comprehensive analysis of redox processes, offering insights into electrochemical behaviors. Differential pulse voltammetry (DPV) is chosen for its enhanced sensitivity and resolution, which is particularly effective for detecting analytes at low concentrations. Amperometry is used for continuous analysis, measuring current at a constant potential, ideal for real-time monitoring. The selection of these techniques is determined by the unique requirements of each sensing application, considering factors like the type of analyte, required sensitivity, and the speed of detection.4. Artificial enzymes in portable electrochemical sensors
The relentless pursuit of miniaturization and portability in sensing technology has significantly marked its evolution, with a pronounced emphasis in the sphere of electrochemical sensors. The integration of avant-garde materials, notably artificial enzymes, has pioneered unprecedented pathways for compact, real-time monitoring devices indispensable in diverse domains, from environmental scrutiny to point-of-care diagnostics.4.1. Design and fabrication of portable sensors with artificial enzyme components
Crafting portable electrochemical sensors necessitates a meticulous amalgamation of components, with the sensing element-increasingly represented by artificial enzymes-at its core, serving as a transformative catalyst. These synthetic entities, meticulously engineered to mirror the catalytic finesse of their natural counterparts, bring to the table unparalleled advantages in sensitivity, selectivity, and stability.The incorporation process demands an in-depth comprehension of the catalytic mechanisms of these enzyme mimics and the governing electrochemical principles. Decisions regarding substrate selection, electrode material, and the method employed for enzyme immobilization on the electrode are crucial, significantly influencing the sensor's efficacy and efficiency.
Moreover, the fabrication protocol must ensure the preservation of the artificial enzyme's activity and stability. Techniques like electrodeposition, layer-by-layer assembly, and covalent bonding have been adeptly utilized to secure these mimics onto sensor platforms, guaranteeing steadfast performance even in daunting conditions.
4.2. Benefits of artificial enzymes in improving portability, stability, and real-time sensing capabilities
Artificial enzymes’ integration into portable electrochemical sensors heralds an epoch of augmented performance and functionality. These tailor-engineered catalysts offer a myriad of advantages essential for portable sensing applications:4.3. Examples of innovative portable electrochemical sensors based on artificial enzymes
The domain of portable electrochemical sensors has undergone noteworthy advancements, especially through the incorporation of artificial enzymes. These mimetics of enzymes not only amplify the performance metrics of sensors but also instigate innovative designs and functionalities. The noteworthy progress in this niche area has been the spotlight of several comprehensive reviews, as evidenced by ref. 28 and 72–78. Nonetheless, our narrative takes a distinct turn, illuminating particular instances where artificial enzymes play a crucial role. Delving deeper into the specifics, we spotlight a series of pioneering studies and sensor designs that leverage the prowess of artificial enzymes, thereby delineating their wide-ranging applications in practical settings. Each selected study eloquently testifies to the transformative potential harbored by these synthetic catalysts, which are instrumental in redefining the contours of portable electrochemical sensing technology.Focusing on metal–organic frameworks (MOFs) and covalent organic frameworks (COFs), it is evident that their unique structural and functional attributes have garnered significant attention in the realm of electrochemical sensing. These frameworks, endowed with high surface areas, tunable porosity, and the ability to accommodate diverse functional groups, naturally excel as substrates for artificial enzymes, optimizing sensor performance, sensitivity, and selectivity. The subsequent discussions will concentrate on pivotal studies that skillfully utilize MOFs and COFs as foundational platforms, spotlighting their integral role in the advancement of electrochemical sensing technologies through the innovative integration of artificial enzymes. These explorations elucidate the multifaceted contributions of these frameworks in optimizing and innovating sensor technology, offering profound insights into their practical utility and future potentials.
In the rich tapestry of innovation within electrochemical sensors, one study distinguishes itself by introducing a highly sensitive tool crafted from a nano-composite material. This material, a fusion of iron(II) phthalocyanine (PcFe) and zinc-based MOF (ZIF-8), was tailor-made for the precise detection of trichloroacetic acid (TCAA). Harnessing ZIF-8's impressive surface area and structure, it adeptly adsorbs TCAA. Within this dynamic, PcFe emerges as the star player, undergoing a key redox process in the presence of TCAA that allows for its quantification. This sensor excels in both sensitivity and specificity but has not yet made its mark in portable detection.79
Transitioning from this, another intriguing work introduces a dual-mode mobile sensor, fine-tuned for tracking hydrogen peroxide (H2O2) and hydrogen sulfide (H2S) emitted by cells. This device leans heavily on the capabilities of the MOF-818 nanozyme, which facilitates both colorimetric and electrochemical detections with impressive precision (Fig. 2A). Its promising applications span clinical diagnostics to cell biology, hinting at a bright future.80
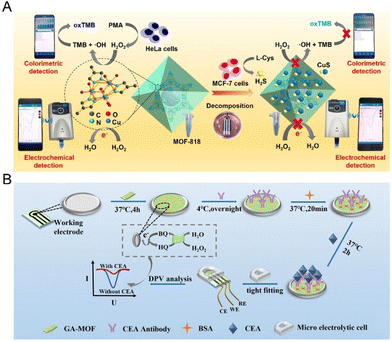 | ||
| Fig. 2 The portable electrochemical sensors based on artificial enzymes. (A) Schematic of a smartphone-based dual-mode sensing platform utilizing MOF-818 nanozyme for real-time detection of H2O2 and H2S released by living cells. Reproduced from ref. 80 with permission from Elsevier, copyright 2023. (B) Schematic of the construction procedure for the Cu–Ni MOF-based electrochemical immunosensor. Reproduced from ref. 81 with permission from Royal Society of Chemistry, copyright 2023. | ||
Taking a slightly different route, Hong and his team melded 3D printing tech with electrochemical prowess. They devised a microfluidic sensor, boasting a flexible screen-printed electrode (SPE) fortified with porous Mn2O3 extracted from a manganese-based MOF. This gadget's standout feature is its ability to detect heavy metal ions, surpassing WHO standards for sensitivity.82
Transitioning to a different stride, another innovative feat is witnessed in the creation of a hybrid button sensor. Employing cobalt-based MOF (Co–MOF), this sensor ingeniously enhances non-enzymatic glucose detection. Its design promotes usability, cost-effectiveness, and adaptability, illustrating significant promise in point-of-care applications for disease prevention and personalized diagnostics.83
In a subsequent scholarly contribution focusing on Co MOF, researchers developed a novel electrochemical sensor for detecting the breast cancer biomarker, carcinoembryonic antigen (CEA). This sensor utilizes a nanocomposite of Co MOF and ionic liquid (IL) as the foundational matrix for the electrode, with the Co MOF serving as the conductive scaffold. The work not only contributes a new approach to rapid breast cancer diagnosis but also showcases the sensor's broad detection range and satisfactory selectivity, reproducibility, and recovery rates.84
Liu's study further enriches the field with the introduction of an intelligent device for monitoring circulating tumor cells (CTCs), specifically designed for diagnosing diseases like pheochromocytoma (PCC). The research employs COF@Pt as a peroxidase mimic, enhancing the electrochemical response and inducing a color change for tumor cell detection. This work stands as a testament to the potential of portable, sensitive, and visual diagnostic platforms for various diseases, meeting the demands for rapid and precise point-of-care diagnostics.85
Navigating further into the realm of MOFs, we uncover the sophistication of bimetallic MOFs. In this advanced domain, complexity merges with functionality, creating a dynamic interplay of properties. These dual-metal systems harness the robust attributes of their monometallic counterparts while revealing unprecedented synergistic effects due to their multi-metallic cores. Such enhancements lead to improved catalytic activity, selectivity, and stability, vital characteristics that underpin the superior performance of electrochemical sensors.
Within this context, Shu's research emerges as a noteworthy example, illuminating the path with an integrated electrochemical immunosensor adept at sensitive and efficient detection of CEA. As shown in Fig. 2B, this sensor is skillfully built upon carbon printed electrodes (CPE) paired with a bimetallic copper–nickel MOF (Cu–Ni MOF). Created via solvothermal methods and seamlessly integrated onto the CPE, this MOF exhibits remarkable peroxidase-like activity. Acting as an efficient catalyst, the Cu–Ni MOF boosts the reaction between hydroquinone and H2O2, intensifying the crucial electrochemical signal for accurate CEA detection. The introduction of immune complexes further refines its catalytic prowess, enabling precise CEA quantification. In comparison to their single-metal counterparts, the bimetallic Cu–Ni MOF truly shines, elevating the sensor's sensitivity. With a wide linear range and impressive detection limits, this sensor hints at significant clinical potential, poised to revolutionize early cancer diagnosis and monitoring.81
Venturing beyond the confines of MOFs and COFs, our exploration leads us to the frontier of other groundbreaking materials in the realm of portable sensors. Here, the spotlight shines brightly on artificial enzymes and catalysts, encompassing the likes of nanozymes and enzyme-mimicking materials. Whether they stand alone in their prowess or join forces with natural enzymes, these materials unfurl a tapestry of unique properties and functionalities. These attributes, in turn, elevate the performance metrics of portable sensing devices to unprecedented heights. In the passages that follow, we aim to unravel the profound impact of these artificial enzymes and catalysts on the evolution of portable electrochemical sensors, emphasizing their invaluable contributions to crafting devices that are not only sensitive and selective but also steadfastly reliable across diverse applications.
Casting our gaze back to 2013, we encounter a seminal piece of research that laid the foundation for a flexible electrochemical biosensor. This avant-garde sensor is meticulously crafted from free-standing graphene paper, interwoven with a binary nanocomposite of PtAu alloy and MnO2. Such an intricate design bestows upon the sensor an unparalleled prowess in non-enzymatic amperometric glucose detection. It boasts a vast linear range, amplified sensitivity, a minimal detection threshold, unwavering selectivity and stability, and a commendable resilience to mechanical stress. While the lexicon of the study might not explicitly echo the terms “nanozymes” or “artificial enzymes”, the materials in play resonate with the essence of artificial enzyme materials. This trailblazing work not only underscores the potential of co-synthesizing metals and metal oxides on autonomous carbon bases but also charts a visionary course for the next generation of high-caliber flexible electrochemical sensors.86
Building on our exploration of the transformative potential of nanozymes, Zhu and his team charted new territory by unveiling a groundbreaking biomolecular detection platform. This ingenious design seamlessly marries nanozymes with lateral flow sensors, paving the way for the adept detection of non-glucose biomarkers. The crux of this platform lies in its ability to transmute analytes, such as 8-hydroxy-2′-deoxyguanosine (8-OHdG) and prostate-specific antigen (PSA), into glucose. This resultant glucose, when quantified using a glucose meter, mirrors the concentration of the original biomarkers in the sample. The detection cascade commences with the meticulous binding of biomolecules to their respective antibodies or antigens on the test strip. Introducing a sucrose solution, which acts as the nanozyme's substrate, triggers the hydrolysis of sucrose, culminating in the production of glucose. The glucose levels then serve as a beacon, reflecting the biomarker concentration (Fig. 3). Zhu's approach stands out not just for its simplicity and cost-effectiveness, but also as a beacon of hope for broadening the horizons of medical diagnostics.87
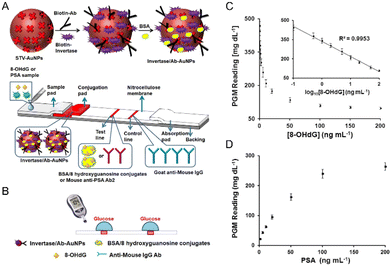 | ||
| Fig. 3 (A) The development of test strips for the quantitative measurement of 8-OHdG and (B) the detection mechanism using a PGM. (C) The performance of 8-OHdG and (D) PSA in buffer based on the PGM. Reproduced from ref. 87 with permission from Elsevier, copyright 2019. | ||
Diverging slightly, another intriguing piece of research unfurled a novel strategy tailored for the ultra-sensitive detection of α-naphthaleneacetic acid (NAA) residues, often found lurking in agricultural environments and products. The linchpin of this strategy is a flexible electrode, a brainchild of nanozymes birthed from 2D phosphorene (BP) and graphene-like titanium carbide MXene (Ti3C2–MXene). What sets this approach apart is its embrace of machine learning for the astute identification of NAA residues. The detection dance, orchestrated via linear sweep voltammetry (LSV) on a nimble portable workstation, boasts an impressive detection range and an enviable low detection threshold for NAA. This groundbreaking endeavor marks the emergence of a wireless, intelligent nanozyme-based flexible sensing platform-an efficient, swift, and cost-effective solution-for the vigilant detection of environmental hazards.88
Venturing further into the realm of biosensing, Yu et al. introduced a trailblazing technology, one that harnesses the power of multi-signal output mechanisms, spanning optical visualization to electrochemical signaling. Their primary objective was the nuanced detection and prognosis of coronary heart disease (CHD). The team's magnum opus is a cascade colorimetric-photothermal biosensor model, which synergizes hollow Prussian blue nanoparticles (PBNPs) with a signal visualization platform. This union, adaptable to an enhanced co-immunoassay, facilitates the meticulous detection of the cTnI protein. But the true genius of this work lies in its theoretical foundation for machine learning-assisted multimodal biosensors, potentially heralding a new era for ultra-sensitive non-enzymatic biosensors. At its core, this avant-garde biosensor, indispensable for CHD patient monitoring, employs machine learning as its guiding star, amplifying both its sensitivity and precision. This marks a significant stride in the ever-evolving landscape of non-enzymatic biosensors.89
Building upon the theme of innovative materials and their applications, a study by Sun et al. unveiled a groundbreaking method for crafting flexible multifunctional nano-hybrid paper electrodes. This was achieved by ultrasonically electrodepositing PtPd alloy nanoparticles onto free-standing, ion liquid (IL)-functionalized graphene paper, broadening its potential in electrochemical catalysis and sensing systems. These electrodes, characterized by their high flexibility and superior mechanical strength, stand out as exemplary free-standing flexible electrodes for electrochemical devices. The integration of IL-functionalized graphene paper not only amplifies the electroactive surface area but also bolsters the adhesion and dispersion of metal nanoparticles. Such enhancements, coupled with the ultrasonic electrodeposition technique, ensure optimal distribution, size, and morphology of PtPd alloy nanoparticles. As a result, this nano-hybrid paper electrode showcases remarkable catalytic activity and stability, especially in the electro-oxidation of fuel molecules like methanol and ethanol. Furthermore, when tested for non-enzymatic electrochemical detection of biomarkers such as glucose and hydrogen peroxide, the electrode's performance was commendable in terms of selectivity, sensitivity, and biocompatibility. This paves the way for its potential use in versatile applications, from bendable chip-type batteries to wearable biosensors.90
Building on the theme of Pd and Pt composite materials, another intriguing study delved into the use of a distinctive nanomaterial marker, the mesoporous core–shell palladium–platinum nanoparticles (Pd@Pt NPs). These nanoparticles displayed peroxidase-like activity. This property was ingeniously leveraged for the quantitative analysis of herbicide residues. The researchers employed 3D printing technology to craft a multi-channel immunosensor, seamlessly integrating lateral flow immunoassay with electrochemical detection techniques. This innovative approach promises swift, accurate, and cost-effective detection of herbicides, such as atrazine and acetochlor, on portable devices. The study further accentuates the transformative potential of Pd and Pt-based materials in advancing the frontiers of chemical analysis and point-of-care diagnostics.91
Expanding on the innovative use of materials, a study ventured into the development of a unique electrochemical bioassay method, emphasizing the sensitive and selective analysis of alkaline phosphatase (ALP). This approach ingeniously diagnoses ALP activity by gauging the ratio of two distinct electrochemical signals. While one signal is intrinsically tied to ALP, the other emerges as a response to the generation of PO43−, catalyzed by the CoOOH nanozyme. The beauty of this method lies in its simplicity: it is label-free and requires no modification. Such a streamlined bioassay method paves the way for more straightforward, user-friendly electroanalytical tools in biosensor design.92
Venturing further into the synthesis of unique materials, a subsequent investigation crafted a material that seamlessly blends CdS nanoparticles with MOFs, positioning it as a potent artificial enzyme catalyst. This blend showcased remarkable catalytic prowess, especially in the oxidation of 4-chloro-1-naphthol (4-CN). The resulting photoelectrochemical immunosensor, infused with this synthesized concoction, exhibited an amplified photocurrent response even under subdued light conditions. Augmented by an Au@CuO/Cu2O heterostructure, the sensor boasts of dual-mode detection capabilities, capturing both fluorescence and photocurrent signals. Such a multifaceted integration heralds the onset of a portable device marked by its heightened sensitivity and dual detection prowess, holding promise for diverse applications ranging from biomedical diagnostics to food safety evaluations.93
Building upon the theme of innovative nano-catalysts, a research endeavor unveiled a groundbreaking nano-catalyst that emulates nitroreductase. This catalyst, ingeniously crafted from 2H–MoS2/Co3O4 nano-hybrids, is tailored for the pinpoint detection of elusive nitroaromatic compounds. What is striking about this nano-catalyst is its pronounced affinity and catalytic prowess towards nitroaromatic compounds. Delving deeper, the researchers fashioned an astoundingly sensitive electrochemical microsensor for 2,4,6-trinitrotoluene (TNT) detection, achieving a staggering detection threshold of 1 pM. The underlying catalytic mechanism, enriched with abundant oxygen vacancies, showcases a Michaelis–Menten constant that is notably lower than its natural nitroreductase counterpart, signifying its superior catalytic affinity. This venture not only introduces a novel nano-enzyme paradigm but also propels the development of a portable TNT detection microsensor.94
Transitioning to the work spearheaded by Li and co-workers, a compelling composite material emerged, blending cobalt oxide-functionalized MoS2 with reduced graphene oxide (rGO). Synthesized via a streamlined hydrothermal approach, this composite material displayed remarkable catalytic zeal towards glucose, aiding its conversion to gluconolactone. Harnessing this composite's potential, a highly sensitive non-enzymatic glucose sensor was birthed, with the working electrode of an SPE sensor adorned with this material (Fig. 4A). Probing its electrocatalytic mettle through cyclic voltammetry and amperometry, the findings underscored its stellar catalytic activity for glucose oxidation, coupled with an impressively low detection threshold of 30 nM. A standout feature was the sensor's unparalleled selectivity amidst potential disruptors like uric acid and ascorbic acid. This enzyme-mimetic nanomaterial, amenable to screen-printing technology, sets the stage for real-time, non-enzymatic electrochemical glucose detection in portable devices.95
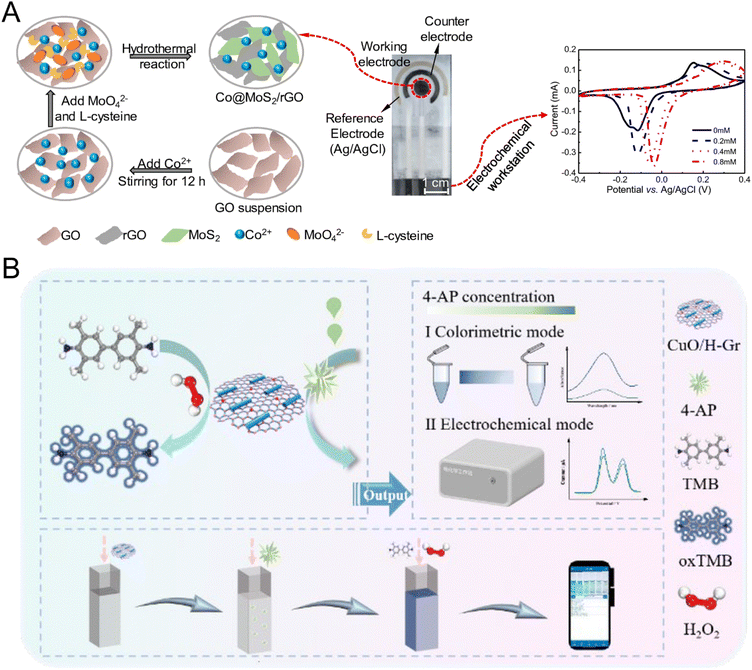 | ||
| Fig. 4 The portable electrochemical sensors based on artificial enzymes. (A) Schematic of the production and electrochemical sensing of the screen-printed biosensor using a working electrode modified with ternary non-enzymatic Co@MoS2/rGO. Reproduced from ref. 95 with permission from Springer Nature, copyright 2020. (B) Schematic of CuO/H–Gr serving as a peroxidase mimic for the quantification of 4-AP. Reproduced from ref. 96 with permission from Royal Society of Chemistry, copyright 2023. | ||
Building upon the theme of innovative nanocomposite materials, a subsequent investigation reveals the synthesis of copper oxide nanorods modified hematin-functionalized graphene (CuO/H–Gr). Tailored with precision, this material is a beacon for the detection of 4-aminophenol (4-AP). Its inherent catalytic prowess facilitates dual modalities: colorimetric and electrochemical detection. The resulting dual-mode sensor, birthed from this nanocomposite, stands as a testament to concurrent colorimetric and electrochemical detection capabilities. With its robust detection metrics, this sensor not only validates its efficacy in real water samples but also champions a smartphone-based detection paradigm. Such a leap in sensor technology underscores the pivotal role of rapid and efficient 4-AP detection in environmental and health monitoring (Fig. 4B).96
The recent work of Li et al. introduced an innovative electrochemiluminescence (ECL) platform featuring a FeMoOv nanoenzyme-bipolar electrode (NM-BPE), representing a significant advancement in this field. This platform sets a new gold standard in the nuanced detection of H2O2 and prostate-specific antigen (PSA). By harnessing the modifiable facets of the bipolar electrode (BPE), the research team integrates a dynamic duo: an anode with the ECL reagent Ru(bpy)32+ and a cathode amalgamated with iron-doped molybdenum oxide/gold nanoparticles (FeMoOv/AuNPs). This combination, exhibiting stellar enzyme-mimicking activities, paves the way for a sandwich immunosensor. This sensor, leveraging FeMoOv/AuNPs as recognition probes, culminates in a heightened ECL response for PSA detection. Further enhancing this innovation, a novel mobile phone ECL imaging system emerges, adept at discerning PSA across varied concentrations. This seminal work, a pioneering effort, marries nanoenzymes with bipolar electrodes for ECL diagnostics and imaging. It not only broadens the horizons for nanoenzyme applications but also blazes a trail in the confluence of bipolar electrodes and ECL imaging, hinting at a future replete with multiplexed detection avenues.97
Researchers have unveiled a sophisticated sensing platform, melding the robustness of violet phosphorus (VP) material with the versatility of phosphorus-doped multistage porous carbon microspheres (PCM). This platform is meticulously tailored for the intelligent analysis of methylphenolic acid (MPA) in corn and wheat silage, with machine learning acting as the linchpin for data interpretation. The VP-PCM/SPCE (SPCE refers to screen printed carbon electrode) sensor, a brainchild of this synergy, showcases remarkable sensitivity, discerning MPA across a spectrum of concentrations. What is particularly commendable is the ML model's predictive prowess, streamlining the quantitative analysis of MPA residues. This venture into bio-mimetic sensing, particularly with VP-PCM, is a beacon for the evolution of MPA analytical methodologies, especially in the context of agricultural safety. Delving deeper, the study offers insights into the resilience of violet phosphorus vis-à-vis black phosphorus, the potential applications of VP-PCM in electrochemical sensing, and the transformative role of machine learning in refining data processing. Moreover, the narrative underscores the perils associated with MPA, the imperative of its detection, and the edge that electrochemical sensors bring to the table. The meticulous detailing of the VP-PCM material's preparation, characterization, and its performance metrics in MPA detection further enriches the discourse.98
Shifting gears to a pressing healthcare challenge, diabetes, a subsequent exploration introduces a trailblazing non-enzymatic glucose sensor. This sensor, with its laser-sharp focus on urine glucose detection, aspires to empower diabetes patients in their quest for optimal blood sugar management. At the core of this innovation lie the laser-induced graphene (LIG) electrodes, which are seamlessly integrated with Au@CuO nano-catalysts. These catalysts, in their essence, emulate the functionality of glucose oxidase. This ingenious marriage of Au@CuO and LIG electrodes not only charts a novel trajectory in non-enzymatic glucose sensing but also culminates in a sensor that stands out for its unparalleled sensitivity, swift responsiveness, and razor-sharp detection precision. Given its affordability and disposability, this sensor is not just a clinical asset but a beacon of hope for countless diabetes patients. This pioneering endeavor heralds a new era in glucose sensing, potentially revolutionizing diabetes monitoring and management.99 Cai et al. have brought to light the potential of a unique material, Mnx(PO4)y artificial enzyme. This material, intriguingly, mirrors the activity of superoxide dismutase (SOD), paving the way for the electrochemical detection of superoxide ions. Central to this innovation is the crafting of a portable electrochemical sensor, laser-focused on discerning superoxide anions (O2˙−) emanating from cells. Driving the functionality of this sensor is the advanced portable potentiostat, xenSTAT, which serves as a clear demonstration of the sensor's exceptional sensitivity, selectivity, and reliability. What sets this device apart is its agility in response, cost-effectiveness, and intuitive design, positioning it as a frontrunner for real-time data acquisition, especially when paired with contemporary digital devices like smartphones or computers. This digital integration not only streamlines data reading but also empowers users to tailor parameters and electrochemical methodologies to their specific needs. The device's prowess in pinpointing O2˙− cannot be understated, as it heralds a new era, potentially catalyzing the commercial trajectory of portable O2˙− sensors and democratizing the use of bespoke potentiostats.100
This section has presented a comprehensive look at the evolution of portable electrochemical sensors enhanced by the use of artificial enzymes. These developments represent a significant advancement in creating compact, efficient monitoring devices applicable in a variety of fields, from environmental analysis to point-of-care diagnostics. The integration of artificial enzymes into these sensors has led to improvements in portability, stability, and real-time sensing capabilities. The case studies and examples provided clearly demonstrate the innovative use of these enzymes in sensor design, offering insights into their wide-ranging practical applications. This exploration not only highlights the remarkable capabilities of artificial enzymes in portable sensing technologies but also sets a foundation for future breakthroughs in this dynamic area of research.
5. Artificial enzymes in wearable electrochemical sensors
Wearable electrochemical sensors signify a transformative evolution in portable diagnostic technologies, representing a specialized iteration of portable sensors. Their innovation is heightened by incorporating artificial enzymes, an audacious leap propelling advancements in wearable diagnostics, environmental surveillance, and individualized health monitoring.5.1. Integration of artificial enzymes into wearable sensing platforms
The incorporation of artificial enzymes has profoundly marked the ascent of wearable electrochemical sensors. These remarkable synthetic catalysts are masterfully engineered to replicate the catalytic finesse of natural enzymes, infusing wearable sensors with extraordinary sensitivity and selectivity, thus navigating the nuanced terrains of human body dynamics with refined precision.Crafting wearable sensors interlaced with artificial enzymes presents a formidable challenge, demanding components resilient to the body's fluctuating environments, such as divergent pH levels, temperatures, and the myriad of other present substances. Artificial enzymes, with their adaptable attributes, emerge as promising heralds of enhanced functionality, able to maintain steadfast activity across a more extensive spectrum of conditions. Their design flexibility facilitates the forging of bespoke binding domains and active sites, honing specificity for the targeted analytes and mitigating the risks of erroneous results, which is paramount in wearable applications where accuracy is non-negotiable.
A hallmark of these synthetic enzymes lies in their amenability to miniaturization, fostering the emergence of sleek, lightweight sensors, uncompromising in potency and precision. This attribute shines particularly in wearable contexts, where the fusion of compact design with robust performance heralds a new epoch in the realm of wearable electrochemical sensors, imbuing them with a sophisticated confluence of functionality and convenience.
5.2. Advantages of artificial enzymes in enhancing sensor performance, sensitivity, and selectivity
The integration of artificial enzymes into wearable electrochemical sensors is not merely a trend but a strategic move to harness their unique properties for enhanced sensor performance:5.3. Case studies of notable wearable electrochemical sensors utilizing artificial enzymes
Exploring the dynamic landscape of wearable electrochemical sensors uncovers a wealth of innovation, primarily driven by the integration of artificial enzymes and nanozymes. These meticulously designed catalysts enhance sensor functionalities, giving rise to transformative designs that adeptly manage the complexities of biological interactions and mechanical resilience.While extensive research has been conducted in the field of portable sensors, it is worth noting that the landscape of wearable sensors is still relatively young but rapidly evolving. In recent years, there have been notable comprehensive reviews that synthesize the advancements in wearable sensor technologies, encompassing various aspects of materials, fabrication methods, and applications.104–117
The integration of artificial enzymes into wearable sensors represents a pivotal area within this broader context, showcasing the potential to revolutionize health monitoring, environmental sensing, and beyond. As we delve deeper into this transformative research area, it is essential to leverage the insights gleaned from these comprehensive reviews to build upon existing knowledge and chart a path forward toward even more innovative and impactful wearable sensor technologies.
In this realm where challenges and innovations intertwine, instances of successful artificial enzyme integration stand as pioneering landmarks, painting the canvas of possibility with strokes of enhanced sensitivity, specificity, and unprecedented functionalities. Our journey forward will navigate through carefully curated case studies, each illuminating the transformative potential of artificial enzymes in the wearable sensor landscape, unfolding chapters of practical ingenuity, and future horizons.
In the development of wearable electrochemical sensors, we encounter a mix of challenges and achievements. Significant advancements have been made, notably the introduction of materials such as MOFs, which have played a crucial role in guiding progress due to their unique structures and functional versatility.
These materials, characterized by their extensive surface areas, adjustable porosity, and compatibility with numerous functional groups, herald a new phase in wearable diagnostics. They resonate with enhanced sensitivity, specificity, and non-invasive capabilities. As we delve into detailed case studies, each will highlight the innovative integration of MOFs with artificial enzymes, suggesting their collective potential to shape the future of wearable electrochemical sensors.
Zhu and his team pioneered a groundbreaking wearable, nonenzymatic glucose sensor employing palladium (Pd) nanoparticles ensconced within ZIF-67 (Pd@ZIF-67). Remarkably, this state-of-the-art sensor operates with precision at physiological pH levels, circumventing the need for extraneous reagents. When ingeniously integrated into a sweatband, it offers real-time glucose surveillance in sweat, with a seamless data transmission to smartphones (Fig. 5A). Notably, the system boasts robustness, maintaining its sensitivity and stability over a commendable two-month span under standard conditions, adeptly discerning glucose concentrations between 10 × 10−6 and 1000 × 10−6 M (Fig. 5B).118
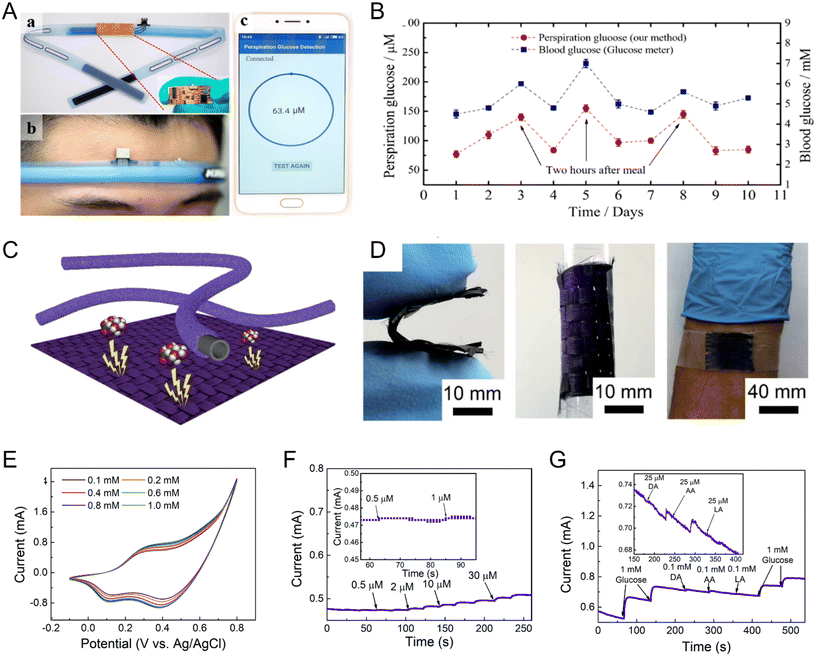 | ||
| Fig. 5 The wearable electrochemical devices based on artificial enzymes. (A) The (a) design of Pd@ZIF-67-based glucose sensor system integrated into a sweatband. The digital picture of (b) sweatband wearing by a volunteer and (c) the smartphone equipped with a corresponding app for perspiration analysis. (B). Monitoring of glucose concentration in both perspiration and blood of a human subject over a span of 10 days. Reproduced from ref. 118 with permission from American Chemical Society, copyright 2019. (C) The schematic of the functional smart fiber and its associated functional textile and (D) digital picture under bending and rolling conditions, as well as when it is attached to human skin. (E) The CV curves of Co–PSF under different glucose concentrations. (F) I–t curve of Co–PSF with the successive addition of glucose or (G) other interfering substance. Reproduced from ref. 119 with permission from Royal Society of Chemistry, copyright 2020. | ||
Wei and associates unveiled a cutting-edge, flexible platform by juxtaposing a conductive, leaf-inspired Co–MOF onto pliable carbon cloth (CC). Devised for the electrocatalytic oxidation of glucose in alkaline milieu, this sensor exhibits a linear detection expanse from 0.004 × 10−3 to 4.428 × 10−3 M and an impressive sensitivity cresting at 1113 μA mM−1 cm−2. Its detection threshold (LOD) stands commendably at 1.2 × 10−6 M. Of importance, this avant-garde sensor finds its niche in glucose detection in human blood serum.120 In another compelling research, Xuan and collaborators engineered a flexible carbon fiber electrode, fortified with a Ni–MOF, tailor-made for non-enzymatic electrochemical glucose sensing in sweat. The innovative configuration of the Ni–MOF promotes longitudinal expansion, augmenting the active sites of Ni ions, thereby amplifying glucose detection sensitivity. Married with a PVA/NaOH solid-state electrolyte, this Ni–MOF-augmented flexible sensor discerns glucose concentrations in sweat from 0 to 1600 × 10−6 M, achieving a remarkable sensitivity of 470.40 μA mM−1 cm−2.121
The scientific community has also witnessed an array of trailblazing endeavors targeting the creation of flexible, non-enzymatic electrochemical glucose sensors, capitalizing on diverse MOF composites. This inventive tapestry encompasses materials such as CuO/NiO/carbon nanocomposites on cello tape,122 Cu–MOF on sandpaper,123 Co–MOF/CC/paper,124 ZIF-67 on carbon fibers (Fig. 5C–G),119 Cu–CAT MOF fractals on carbon paper,125 and the bimetallic 2D Cu–Co–ZIF derivatives on CC.126 A standout iteration in this series is the bimetallic 2D Cu–Co–ZIF derivative on a flexible CC electrode. Its superlative sensitivity, eclipsing other MOFs and MOF-derived materials, is credited to its distinct 2D blueprint and the synergetic redox dynamism of Cu and Co ions in alkaline surroundings. It is pivotal to note that a majority of these groundbreaking MOF-centric sensors have undergone rigorous testing across varied body fluids, notably blood serum, urine, and saliva.126
In a recent study, Wang et al. heralded a significant leap in sweat sensing methodologies. Given the pocket composed by tryptophan (Trp)/histidine (His) and copper sites, natural ascorbate oxidase can selectively captured and identified ascorbate (Fig. 6A). Through an astute chemical refinement, they amplified the selectivity of Cu–MOFs while orchestrating reactions. The meticulous amalgamation of Cu–MOFs with Trp and His yielded a highly selective and dynamic material (Fig. 6B). This innovative material, mirroring the functionality of ascorbic acid oxidase, flaunts unparalleled sensitivity, establishing itself as a beacon for future-sensitive sweat sensors (Fig. 6C). This material exhibits stellar sensitivity in both acidic and alkaline sweat environs, with sensitivity coefficients of 0.18 mA mM−1 cm−2 and 0.48 mA mM−1 cm−2, respectively. And the sensing platform demonstrates remarkable sensitivity to the continuous flow of sweat and delivers a rapid current response to sweat ascorbate. Notably, it maintains a low background current of 0.01 mA cm−2 within just 5 seconds (Fig. 6D). This paradigm-shifting research not only provides a highly specific detection modality but also propels sweat sensing technology into a new era. Its unparalleled potential in personalized health monitoring is evident, positioning this innovation as a luminary in the domain. Its adeptness in replicating natural enzymatic processes, synergized with enhanced selectivity via chemical enhancements, paves the path for highly specific and bespoke health monitoring applications.127
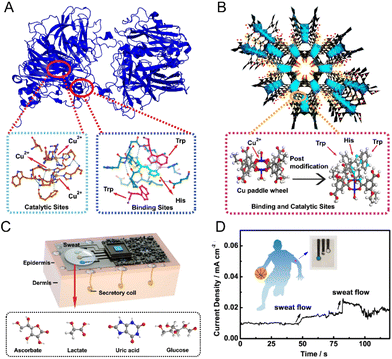 | ||
| Fig. 6 (A) A natural ascorbate oxidase with Cu catalytic and specific binding sites formed by histidine (His) and tryptophan (Trp) for selective catalysis of ascorbate. (B) The concept of an ascorbate oxidase-mimicking MOF. (C) The sweat sensors design for noninvasive health monitoring using Cu–MOFs artificial enzymes and (D) corresponding sensing performance toward ascorbate. Reproduced from ref. 127 with permission from Springer Nature, copyright 2023. | ||
The innovative use of MOFs in sensor technology, as highlighted in the preceding examples, primarily centers around their function in catalyzing reactions and enhancing sensor sensitivity. However, the versatility of MOFs extends beyond these traditional roles. They are increasingly recognized for their ability to act as carriers for natural enzymes, providing crucial support that enhances enzyme stability and activity. This emerging application of MOFs represents a significant shift from their conventional usage, utilizing their unique structural and functional properties to improve the performance of natural enzymes in sensor technologies.
This new paradigm is aptly demonstrated in a study which introduced a pioneering wearable electrochemical sensor for uric acid detection in sweat. In this sensor, the hydrophilic MAF-7 MOF is employed not as a direct catalyst but as a stabilizing carrier for uricase. MAF-7, known for its hydrophilicity and stability, encapsulates uricase, preventing enzyme aggregation and ensuring high enzymatic activity. Such strategic use of MAF-7 allows the sensor to maintain high sensitivity in detecting uric acid, with a detection range of 2 μM-70 μM and a limit as low as 0.34 μM. Coupled with a flexible microfluidic chip, this sensor exemplifies the innovative integration of MOFs in wearable technology, opening new possibilities for non-invasive health monitoring and disease-related metabolite detection.128
Similarly, in Xiao's research, they introduced an innovative electrochemical sensor for levodopa quantification in sweat, vital for treating Parkinson's disease. This sensor employs a unique MOF/graphene oxide (ZIF-8/GO) composite, where ZIF-8 is instrumental in constructing the composite and immobilizing tyrosinase. ZIF-8 enhances enzyme stability and catalytic efficiency by co-precipitating with the enzyme on the graphene oxide surface. It protects the enzyme, maintaining its activity in biocatalysis with large substrates. The ZIF-8/GO composite, by increasing the accessible surface area of the exposed enzyme and enhancing its stability, improves the cost-effectiveness of biocatalysis. This non-invasive, portable device offers significant potential in real-time health monitoring and medication management for Parkinson's patients, with its high sensitivity, low detection limit, excellent selectivity, and robust stability, demonstrating its effectiveness for continuous, non-invasive drug monitoring.129
Also, another noninvasive wearable immunosensor was fabricated by combining ZIF-8 and cortisol monoclonal antibodies for cortisol detection in sweat. This ZIF-8/antibody composite enhances antibody stability and antigen-binding capacity, improving the sensor's selectivity and sensitivity. The sensor demonstrates a robust linear detection range from 1 pg mL−1 to 1 μg mL−1, with a low detection limit of 0.26 pg mL−1. Additionally, it maintains good persistence, showing only a 4.1% decay after 9 days of storage. This innovative approach in antibody assembly and integration with MOFs marks a significant step towards long-term, continuous biomarker detection in sweat, highlighting its potential for practical, real-time health monitoring and stress management applications.130
Moving beyond the scope of MOFs, we are on the threshold of broader advancements in wearable electrochemical sensors. There are other innovative materials poised to further revolutionize the field. As we shift our focus from MOFs, three pivotal studies emerge, each demonstrating unique strengths in sensitivity, selectivity, and stability. This exploration highlights the diverse advancements in the field, setting the stage for an in-depth analysis of these unique yet transformative research efforts.
Foremost among these is the Berlin green carbon ink, a crafted marvel acting as a metalloenzyme mimic. It adorns electrode arrays with an extraordinary palette of qualities such as remarkable sensitivity, stability, and reproducibility—essential attributes for the precise detection of hydrogen peroxide and glucose. Its adaptability, biocompatibility, and ease of mass production herald its prowess, setting a benchmark in the development of formidable wearable smart sensors.131
In the theatre of technological marvels, a captivating performance unveils itself through a flexible tri-electrode system. It presents the TIOWE (Two-in-One Working Electrode) as its star, an innovative concoction brewed with a Ni2P-based composite electrode ink. The TIOWE, with its intrinsic glucose oxidase-like activity, meticulously tuned, sets the stage for tracking glucose levels in human sweat with finesse. This magnum opus symbolizes a milestone, enriching the tapestry of wearable biosensing technologies with its ingenuity for real-time, non-invasive monitoring.132
Completing this symphony of innovations, the canvas is illuminated by the ingenuity of Xu et al. Their masterpiece, an electrochemical biosensor, is an orchestrated ensemble of PEDOT:PSS conductive hydrogel and Prussian blue nanoparticles (PBNPs). Conducting a catalytic symphony with hydrogen peroxide (H2O2), this fusion emerges as a guardian of continuous and non-invasive glucose monitoring on human skin, echoing virtues of sensitivity, specificity, and robust operational stability in the realms of sensor technology.133
This section highlights the significant role of artificial enzymes in the development of wearable electrochemical sensors. These sensors represent a major step forward in diagnostic technology, combining high sensitivity with user-friendly designs. The discussion has shown how the unique properties of artificial enzymes contribute to the creation of wearable sensors that are both sensitive and versatile, capable of functioning in varied environments. The case studies featured in this section provide a clear picture of the current state of wearable sensor technology, emphasizing their growing importance in health monitoring and environmental detection. These examples not only demonstrate the current capabilities of wearable sensors but also point towards future possibilities in this rapidly advancing field, offering a vision of enhanced personal health monitoring and environmental awareness.
6. Challenges and opportunities
6.1. Technical challenges in integrating artificial enzymes into wearable and portable sensors
The integration of artificial enzymes into portable and wearable electrochemical sensors presents both a promising avenue and a technical puzzle. While artificial enzymes have shown immense potential, they currently do not match the selectivity and catalytic activity of their natural counterparts. However, this gap does not signify an insurmountable barrier but rather a challenge to be addressed.Visualize a technological advancement where tailored modifications elevate the performance of artificial enzymes, enabling them to rival or even surpass their natural equivalents. Such a breakthrough, rooted in rigorous scientific research and pragmatic applications, would have profound implications not only for the scientific community but for broader societal applications as well. Achieving this would be akin to refining raw materials into unparalleled tools of precision.
This section examines the tangible hurdles faced when enhancing the properties of artificial enzymes. The goal is not merely academic but lies in striking a harmony between theoretical advances and practical solutions. As scientists work to enhance these enzymes, they are striving for more than just scientific excellence; they are working towards a vision that reshapes the landscape of wearable sensors. Amidst these challenges, we also see a horizon rich with opportunities, suggesting a future where artificial enzymes not only compete with but may excel beyond the capabilities of their natural peers.
6.2. Opportunities for future research and development in this interdisciplinary field
The future in the interdisciplinary arena of artificial enzymes stretches out, brimming with unexplored pathways and immense possibilities for research and development. The essence lies not just in theoretical exploration, but in practical, transformative advancements that are both tangible and consequential.One immediate opportunity lies in advancing nanoengineering techniques. Fine-tuning the manipulation of artificial enzyme nanoparticles’ size, shape, and composition stands out as a pivotal area. There is an imperative to move beyond theoretical potential to real-world applicability, ensuring that our advancements in nanoengineering translate directly into enhanced catalytic efficiency and functionality of artificial enzymes.
Emphasis should also be placed on the seamless integration of artificial enzymes with various substrates, moving towards flexibility and biocompatibility. Envision synthetic enzymes that not only coalesce effortlessly with organic tissues and wearable devices but also power real-time, non-invasive health monitoring systems. We should focus on turning this vision into a reality, cultivating technologies that resonate with the practical necessities of personalized healthcare and continuous disease monitoring.
The intersection of artificial enzymes with artificial intelligence also holds profound potential. Instead of merely dwelling in possibilities, there is a need to cultivate concrete strategies where machine learning algorithms actively participate in deciphering and optimizing enzymatic patterns, catalyzing a revolution in the design and efficiency of synthetic enzymes.
Sustainability is another crucial frontier. Prioritize strategies that genuinely align artificial enzymes with green chemistry principles, fostering innovations that are not just theoretically profound but also resonate with urgent calls for environmentally sustainable industrial applications. Focusing on practical pathways to realize the blend of synthetic biology with enzymatic catalysis, guiding the field towards solutions that champion sustainable and efficient production methods, is essential.
Lastly, the collaborative spirit in this field must be nurtured and harnessed effectively. The integration of diverse expertise, from biochemists and material scientists to engineers and data analysts, is not merely a desirable aspect but a crucial ingredient in unlocking the full potential of artificial enzymes. Collaborations should be aimed at fostering a culture of tangible innovations and solutions, ensuring that our combined efforts and shared visions drive the field towards practical brilliance, enhancing the real-world applicability and impact of artificial enzymes.
6.3 Recap the transformative potential of artificial enzymes in advancing wearable and portable electrochemical sensing technologies
In retrospect, the transformative potential of artificial enzymes in advancing wearable and portable electrochemical sensing technologies is nothing short of revolutionary. These synthetic marvels, meticulously crafted to mimic the intricacies of natural enzymes, have ushered in a new era of possibilities in the realm of biosensors.One of the most remarkable aspects of artificial enzymes lies in their adaptability. These engineered biomimetics can be tailored to target specific molecules with unprecedented precision. This level of customization has paved the way for highly sensitive and selective sensors, enabling the detection of even the slightest biochemical signals in various bodily fluids and environmental samples.
Moreover, the portability and miniaturization facilitated by artificial enzyme-based sensors have redefined point-of-care diagnostics. These sensors, integrated into wearable devices or handheld gadgets, empower individuals to monitor their health parameters in real-time, transforming how diseases are managed and diagnosed. The convenience and immediacy offered by these sensors have the potential to revolutionize healthcare delivery, especially in remote or resource-limited areas.
Additionally, artificial enzyme-based sensors have opened new avenues in environmental monitoring. From detecting pollutants in water sources to monitoring air quality, these sensors have become indispensable tools in safeguarding our ecosystem. Their rapid response, coupled with their ability to detect a wide range of analytes, positions them as vital instruments in environmental conservation efforts.
Furthermore, the fusion of artificial enzymes with emerging technologies such as Internet of Things (IoT) and big data analytics has magnified their impact. Real-time data transmission and analysis enable timely interventions and informed decision-making. This amalgamation of biotechnology and digital innovation holds immense promise in enhancing the efficiency of healthcare systems and environmental management strategies.
The transformative journey of artificial enzymes in the realm of wearable and portable electrochemical sensing technologies has transcended scientific boundaries. From personalized healthcare to environmental stewardship, these synthetic biocatalysts have demonstrated their potential to shape a healthier, more sustainable future. As research continues to unravel their complexities and refine their functionalities, the horizon of possibilities broadens, promising even more astounding breakthroughs in the fascinating intersection of biology, materials science, and technology.
6.4. Emphasize the interdisciplinary nature of this research area and its implications for healthcare, environmental monitoring, and beyond
The interdisciplinary nature of research in the field of artificial enzymes for wearable and portable electrochemical sensing technologies underscores a profound paradigm shift in scientific exploration. This convergence of diverse disciplines, including biochemistry, materials science, electronics, and data analytics, has not only expanded the horizons of scientific understanding but also translated into tangible advancements with far-reaching implications.In the realm of healthcare, this interdisciplinary synergy has given rise to innovative diagnostic tools that transcend the limitations of traditional methods. Artificial enzyme-based sensors, integrated into wearable devices, provide continuous, real-time monitoring of vital biomarkers. This continuous stream of data offers unprecedented insights into individual health profiles, enabling early disease detection, personalized treatment strategies, and improved patient outcomes. The marriage of biotechnology, electronics, and data analysis in this context has the potential to revolutionize healthcare delivery, making it more proactive, precise, and patient-centric. In the healthcare sector, a notable example is the collaboration between biotechnologists, medical researchers, and electronic engineers, which has culminated in the creation of wearable sensors that provide real-time health monitoring. These devices, capable of tracking vital biomarkers, are revolutionizing patient care by offering personalized and proactive health management solutions. In the arena of environmental monitoring, the interdisciplinary approach has led to the development of sensors capable of detecting minute quantities of pollutants in air, water, and soil. By leveraging the unique catalytic properties of artificial enzymes, these sensors offer high sensitivity and selectivity, making them invaluable tools in environmental conservation efforts. Real-time monitoring facilitated by these sensors aids in timely interventions, contributing significantly to the preservation of ecosystems and the well-being of communities. For instance, the development of advanced artificial enzyme-based sensors for environmental monitoring has been a result of collaborative efforts between chemists, environmental scientists, and engineers. This synergy has led to sensors capable of detecting trace levels of pollutants with greater accuracy and speed, significantly advancing our ability to monitor and protect our natural ecosystems. Beyond healthcare and environmental applications, the interdisciplinary research in artificial enzymes holds promise in diverse fields. In agriculture, these sensors could revolutionize crop monitoring and pest management, leading to increased agricultural productivity and sustainability. In industrial settings, they could enhance the efficiency of manufacturing processes by enabling precise monitoring of chemical reactions and product quality. Moreover, the integration of artificial enzyme-based sensors with smart infrastructure could create intelligent cities where resources are utilized optimally, promoting sustainability and resilience.
The collaborative efforts of experts from various disciplines have not only propelled the development of cutting-edge technologies but also fostered a culture of innovation and discovery. This collaborative spirit has the potential to unlock novel solutions to some of the most pressing challenges facing humanity, ranging from healthcare disparities to environmental degradation. As researchers continue to explore the intersections of different fields, the interdisciplinary nature of artificial enzyme research will undoubtedly catalyze transformative changes, shaping a future where scientific innovation transcends traditional boundaries for the betterment of society as a whole.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the support from the National Key Research and Development Program of China (no. 2022YFE0121100), Science and technology research project from Education Department of Jilin Province [grant number JJKH20231296KJ], the Natural Science Foundation of Science and Technology Department of Jilin Province (Joint Fund Project) [grant number YDZJ202201ZYTS340], National Natural Science Foundation of China (no. 22274018, 22004014, 22322410, 22174137), China Postdoctoral Science Foundation (no. 2022M720698), Fundamental Research Funds for the Central Universities (China, no. 2412023QD010, 2412022ZD013), “111” Project (China, no. B18012), Jilin Provincial Department of Education (China), the Key Laboratory of Nanobiosensing and Nanobioanalysis at Universities of Jilin Province (China), and Analysis and Testing Center of Northeast Normal University (China). This paper is based upon the work supported by Science, Technology & Innovation Funding Authority (STDF) under the grant number 44279.References
- K. Zajki-Zechmeister, G. S. Kaira, M. Eibinger, K. Seelich and B. Nidetzky, ACS Catal., 2021, 11, 13530–13542 CrossRef CAS.
- T. J. Lawton and A. C. Rosenzweig, J. Am. Chem. Soc., 2016, 138, 9327–9340 CrossRef CAS PubMed.
- W.-L. Li and T. Head-Gordon, ACS Cent. Sci., 2021, 7, 72–80 CrossRef CAS.
- G. Xu, M. Lai, R. Wilson, A. Glidle, J. Reboud and J. M. Cooper, Microsyst. Nanoeng., 2019, 5, 37 CrossRef PubMed.
- C. Garcia-Galan, Á. Berenguer-Murcia, R. Fernandez-Lafuente and R. C. Rodrigues, Adv. Synth. Catal., 2011, 353, 2885–2904 CrossRef CAS.
- Q. Liu, S. Zhao, Y. Zhang, Q. Fang, W. Liu, R. Wu, G. Wei, H. Wei and Y. Du, Adv. Mater., 2023, 2305555 CrossRef CAS PubMed.
- A. Shamsabadi, T. Haghighi, S. Carvalho, L. C. Frenette and M. M. Stevens, Adv. Mater., 2023, 2300184 CrossRef.
- C.-B. Ma, Y. Xu, L. Wu, Q. Wang, J.-J. Zheng, G. Ren, X. Wang, X. Gao, M. Zhou, M. Wang and H. Wei, Angew. Chem., Int. Ed., 2022, 61, e202116170 CrossRef CAS PubMed.
- Y. Zhang, L. Zhang, W. Wang, Q. Deng, M. Liu, Z. Zhu, H. Liu, J. Ren and X. Qu, Angew. Chem., Int. Ed., 2023, 62, e202306395 CrossRef CAS.
- P. Zhang, D. Sun, A. Cho, S. Weon, S. Lee, J. Lee, J. W. Han, D.-P. Kim and W. Choi, Nat. Commun., 2019, 10, 940 CrossRef PubMed.
- L. Zhao, J. Cai, Y. Li, J. Wei and C. Duan, Nat. Commun., 2020, 11, 2903 CrossRef CAS.
- J. Hovancová, I. Šišoláková, R. Oriňaková and A. Oriňak, J. Solid State Electrochem., 2017, 21, 2147–2166 CrossRef.
- R. G. Mahmudunnabi, F. Z. Farhana, N. Kashaninejad, S. H. Firoz, Y.-B. Shim and M. J. A. Shiddiky, Analyst, 2020, 145, 4398–4420 RSC.
- Kenry, J. C. Yeo and C. T. Lim, Microsyst. Nanoeng., 2016, 2, 16043 CrossRef CAS.
- H. Teymourian, M. Parrilla, J. R. Sempionatto, N. F. Montiel, A. Barfidokht, R. Van Echelpoel, K. De Wael and J. Wang, ACS Sens., 2020, 5, 2679–2700 CrossRef CAS PubMed.
- M. Parrilla and K. De Wael, Adv. Funct. Mater., 2021, 31, 2107042 CrossRef CAS.
- M. Mathew, S. Radhakrishnan, A. Vaidyanathan, B. Chakraborty and C. S. Rout, Anal. Bioanal. Chem., 2021, 413, 727–762 CrossRef CAS.
- L. Su, Int. J. Electrochem. Sci., 2022, 17, 220841 CrossRef CAS.
- J. R. Sempionatto, J. A. Lasalde-Ramírez, K. Mahato, J. Wang and W. Gao, Nat. Rev. Chem., 2022, 6, 899–915 CrossRef PubMed.
- N. Akhtar, S. A. El-Safty, M. E. Abdelsalam and H. Kawarada, Adv. Healthcare Mater., 2015, 4, 2110–2119 CrossRef CAS.
- J. Choi, M. Jeun, S.-S. Yuk, S. Park, J. Choi, D. Lee, H. Shin, H. Kim, I.-J. Cho, S. K. Kim, S. Lee, C. S. Song and K. H. Lee, ACS Nano, 2019, 13, 812–820 CrossRef CAS.
- H. Eynaki, M. A. Kiani and H. Golmohammadi, Nanoscale, 2020, 12, 18409–18417 RSC.
- J. Kampeera, P. Pasakon, C. Karuwan, N. Arunrut, A. Sappat, S. Sirithammajak, N. Dechokiattawan, T. Sumranwanich, P. Chaivisuthangkura, P. Ounjai, S. Chankhamhaengdecha, A. Wisitsoraat, A. Tuantranont and W. Kiatpathomchai, Biosens. Bioelectron., 2019, 132, 271–278 CrossRef CAS PubMed.
- V. Kumar, K. Vaid, S. A. Bansal and K.-H. Kim, Biosens. Bioelectron., 2020, 165, 112382 CrossRef CAS PubMed.
- M. K. Rayappa, P. A. Viswanathan, G. Rattu and P. M. Krishna, J. Agric. Food Chem., 2021, 69, 4578–4603 CrossRef CAS PubMed.
- M. Sun, X. Pei, T. Xin, J. Liu, C. Ma, M. Cao and M. Zhou, Anal. Chem., 2022, 94, 1890–1900 CrossRef CAS.
- M. Sun, T. Xin, Z. Ran, X. Pei, C. Ma, J. Liu, M. Cao, J. Bai and M. Zhou, ACS Sens., 2021, 6, 275–284 CrossRef CAS PubMed.
- D. Zhang and Q. Liu, Biosens. Bioelectron., 2016, 75, 273–284 CrossRef CAS.
- H. Zhou, G. Ran, J.-F. Masson, C. Wang, Y. Zhao and Q. Song, Biosens. Bioelectron., 2018, 105, 226–235 CrossRef.
- Y. Bi, M. Sun, J. Wang, Z. Zhu, J. Bai, M. Y. Emran, A. Kotb, X. Bo and M. Zhou, Anal. Chem., 2023, 95, 6690–6699 CrossRef CAS PubMed.
- G. Cappon, G. Acciaroli, M. Vettoretti, A. Facchinetti and G. Sparacino, Electronics, 2017, 6, 65 CrossRef.
- D. Castaneda, A. Esparza, M. Ghamari, C. Soltanpur and H. Nazeran, Int. J. Biosens. Bioelectron., 2018, 4, 195–202 Search PubMed.
- W. Gao, S. Emaminejad, H. Y. Y. Nyein, S. Challa, K. Chen, A. Peck, H. M. Fahad, H. Ota, H. Shiraki, D. Kiriya, D.-H. Lien, G. A. Brooks, R. W. Davis and A. Javey, Nature, 2016, 529, 509–514 CrossRef CAS PubMed.
- H. Kim, Y.-S. Kim, M. Mahmood, S. Kwon, N. Zavanelli, H. S. Kim, Y. S. Rim, F. Epps and W.-H. Yeo, Adv. Sci., 2020, 7, 2000810 CrossRef CAS.
- H. Lee, E. Kim, Y. Lee, H. Kim, J. Lee, M. Kim, H.-J. Yoo and S. Yoo, Sci. Adv., 2018, 4, eaas9530 CrossRef CAS PubMed.
- S. P. Lee, G. Ha, D. E. Wright, Y. Ma, E. Sen-Gupta, N. R. Haubrich, P. C. Branche, W. Li, G. L. Huppert, M. Johnson, H. B. Mutlu, K. Li, N. Sheth, J. A. Wright, Y. Huang, M. Mansour, J. A. Rogers and R. Ghaffari, npj Digital Med., 2018, 1, 2 CrossRef.
- A. Monteriù, M. R. Prist, E. Frontoni, S. Longhi, F. Pietroni, S. Casaccia, L. Scalise, A. Cenci, L. Romeo, R. Berta, L. Pescosolido, G. Orlandi and G. M. Revel, Sensors, 2018, 18, 2310 CrossRef.
- X. Pei, M. Sun, J. Wang, J. Bai, X. Bo and M. Zhou, Small, 2022, 18, 2205061 CrossRef CAS.
- J. R. Sempionatto, M. Lin, L. Yin, E. De la paz, K. Pei, T. Sonsa-ard, A. N. de Loyola Silva, A. A. Khorshed, F. Zhang, N. Tostado, S. Xu and J. Wang, Nat. Biomed. Eng., 2021, 5, 737–748 CrossRef CAS.
- M. Sun, Y. Gu, X. Pei, J. Wang, J. Liu, C. Ma, J. Bai and M. Zhou, Nano Energy, 2021, 86, 106061 CrossRef CAS.
- J. Wang, M. Sun, X. Pei, L. Zheng, C. Ma, J. Liu, M. Cao, J. Bai and M. Zhou, Adv. Funct. Mater., 2022, 32, 2209697 CrossRef CAS.
- J. Czulak, A. Jakubiak-Marcinkowska and A. Trochimczuk, Adv. Mater. Sci. Eng., 2013, 2013, 464265 Search PubMed.
- A. R. Cardoso, M. F. Frasco, V. Serrano, E. Fortunato and M. G. F. Sales, Biosensors, 2021, 11, 152 CrossRef CAS.
- Z. Wang, Z. Wang, W. W. Lu, W. Zhen, D. Yang and S. Peng, NPG Asia Mater., 2017, 9, e435–e435 CrossRef CAS.
- Z. Yang, C. Wang and X. Lu, Sci. China Mater., 2018, 61, 653–670 CrossRef CAS.
- J. Cho, R. Sarangi and W. Nam, Acc. Chem. Res., 2012, 45, 1321–1330 CrossRef CAS.
- S. A. Cook and A. S. Borovik, Acc. Chem. Res., 2015, 48, 2407–2414 CrossRef CAS.
- C. Zhou, Y. Zeng, Z. Song, Q. Liu, Y. Zhang, M. Wang and Y. Du, Anal. Chem., 2022, 94, 13261–13268 CrossRef CAS PubMed.
- A. T. Fiedler and A. A. Fischer, JBIC, J. Biol. Inorg. Chem., 2017, 22, 407–424 CrossRef CAS PubMed.
- L. Que and W. B. Tolman, Nature, 2008, 455, 333–340 CrossRef CAS PubMed.
- C. Shen, X. Cheng, L. Wei, R.-Q. Wang and C.-J. Wang, Chem. Sci., 2021, 12, 15882–15891 RSC.
- M. Bortolami, I. I. Bogles, C. Bombelli, F. Pandolfi, M. Feroci and F. Vetica, Molecules, 2022, 27, 5150 CrossRef CAS.
- J. Czescik, S. Zamolo, T. Darbre, R. Rigo, C. Sissi, A. Pecina, L. Riccardi, M. De Vivo, F. Mancin and P. Scrimin, Angew. Chem., Int. Ed., 2021, 60, 1423–1432 CrossRef CAS PubMed.
- C. Tan, J. Zhao, P. Sun, W. Zheng and G. Cui, New J. Chem., 2020, 44, 4916–4926 RSC.
- L. Yao, M.-M. Zhao, Q.-W. Luo, Y.-C. Zhang, T.-T. Liu, Z. Yang, M. Liao, P. Tu and K.-W. Zeng, ACS Nano, 2022, 16, 9228–9239 CrossRef CAS PubMed.
- L. Zhao, Z. Wu, G. Liu, H. Lu, Y. Gao, F. Liu, C. Wang, J. Cui and G. Lu, J. Mater. Chem. B, 2019, 7, 7042–7051 RSC.
- S. He, J. Huang, Q. Zhang, W. Zhao, Z. Xu and W. Zhang, Adv. Funct. Mater., 2021, 31, 2105198 CrossRef CAS.
- H. Wang, P. Li, D. Yu, Y. Zhang, Z. Wang, C. Liu, H. Qiu, Z. Liu, J. Ren and X. Qu, Nano Lett., 2018, 18, 3344–3351 CrossRef CAS.
- X. Ruan, D. Liu, X. Niu, Y. Wang, C. D. Simpson, N. Cheng, D. Du and Y. Lin, Anal. Chem., 2019, 91, 13847–13854 CrossRef CAS PubMed.
- D. Wang, X. Song, P. Li, X. J. Gao and X. Gao, J. Mater. Chem. B, 2020, 8, 9028–9034 RSC.
- E. L. Almeida, A. F. Carrillo Rincón, S. A. Jackson and A. D. W. Dobson, Front. Microbiol., 2019, 10, 2187 CrossRef.
- N. Zhang, K. Mei, P. Guan, X. Hu and Y. Zhao, Small, 2020, 16, 1907256 CrossRef CAS.
- C.-B. Ma and M. Zhou, Chin. J. Anal. Chem., 2022, 50, 535–544 CAS.
- S. Bang, Y.-M. Lee, S. Hong, K.-B. Cho, Y. Nishida, M. S. Seo, R. Sarangi, S. Fukuzumi and W. Nam, Nat. Chem., 2014, 6, 934–940 CrossRef CAS PubMed.
- X. Chen, D. J. Aschaffenburg and T. Cuk, Nat. Catal., 2019, 2, 820–827 CrossRef CAS.
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu, L. Qin and H. Wei, Chem. Soc. Rev., 2019, 48, 1004–1076 RSC.
- T. Sergeyeva, O. Piletska and S. Piletsky, BBA Adv., 2023, 3, 100070 CrossRef CAS PubMed.
- H. J. Im and Y. J. Park, Sci. Rep., 2022, 12, 527 CrossRef CAS PubMed.
- O. V. Yarmolenko, G. R. Baymuratova, K. G. Khatmullina, G. Z. Tulibayeva, A. V. Yudina, T. A. Savinykh, I. K. Yakushchenko, P. A. Troshin and A. F. Shestakov, Inorganics, 2022, 10, 176 CrossRef CAS.
- B. A. Elsayed, E. A. Gomaa, M. M. Awad and A. A. Abdallah, Al-Azhar Bull. Sci., 2021, 32, 37–45 CrossRef.
- K. Halicka and J. Cabaj, Int. J. Mol. Sci., 2021, 22, 6357 CrossRef CAS PubMed.
- P. V. V. Romanholo, C. A. Razzino, P. A. Raymundo-Pereira, T. M. Prado, S. A. S. Machado and L. F. Sgobbi, Biosens. Bioelectron., 2021, 185, 113242 CrossRef CAS.
- M. Peng, Y. Zhao, D. Chen and Y. Tan, ChemCatChem, 2019, 11, 4222–4237 CrossRef CAS.
- J. Zhang, J. Lei, Z. Liu, Z. Chu and W. Jin, Environ. Res., 2022, 214, 113858 CrossRef CAS.
- Q. He, B. Wang, J. Liang, J. Liu, B. Liang, G. Li, Y. Long, G. Zhang and H. Liu, Mater. Today Adv., 2023, 17(100340) Search PubMed.
- Y. Zhang, Z.-M. Song and Y. Du, Chin. J. Anal. Chem., 2023, 51, 800–810 CAS.
- K. Bialas, D. Moschou, F. Marken and P. Estrela, Microchim. Acta, 2022, 189, 172 CrossRef CAS.
- F. Zhao, W. Wu, M. Zhao, S. Ding, Y. Lin, Q. Hu and L. Yu, TrAC, Trends Anal. Chem., 2023, 158, 116833 CrossRef CAS.
- Z. Zeng, X. Fang, W. Miao, Y. Liu, T. Maiyalagan and S. Mao, ACS Sens., 2019, 4, 1934–1941 CrossRef CAS PubMed.
- K. Yu, M. Li, H. Chai, Q. Liu, X. Hai, M. Tian, L. Qu, T. Xu, G. Zhang and X. Zhang, Chem. Eng. J., 2023, 451, 138321 CrossRef CAS.
- Y. Shu, L. Yan, M. Ye, L. Chen, Q. Xu and X. Hu, Analyst, 2023, 148, 4721–4729 RSC.
- Y. Hong, M. Wu, G. Chen, Z. Dai, Y. Zhang, G. Chen and X. Dong, ACS Appl. Mater. Interfaces, 2016, 8, 32940–32947 CrossRef CAS PubMed.
- X. Wei, J. Guo, H. Lian, X. Sun and B. Liu, Sens. Actuators, B, 2021, 329, 129205 CrossRef CAS.
- A. Luo, Y. Cai, M. Liu, S. Tang, Z. Zhu, R. Haotian, B. Xie, Y. Yi, Z. Hao and A. Liang, J. Electrochem. Soc., 2022, 169, 117504 CrossRef CAS.
- X. Liu, F. Wang, Y. Meng, L. Zhao, W. Shi, X. Wang, Z. He, J. Chao and C. Li, Biosens. Bioelectron., 2022, 207, 114208 CrossRef CAS PubMed.
- F. Xiao, Y. Li, H. Gao, S. Ge and H. Duan, Biosens. Bioelectron., 2013, 41, 417–423 CrossRef CAS.
- X. Zhu, M. Sarwar, J.-J. Zhu, C. Zhang, A. Kaushik and C.-Z. Li, Biosens. Bioelectron., 2019, 126, 690–696 CrossRef CAS.
- X. Zhu, L. Lin, R. Wu, Y. Zhu, Y. Sheng, P. Nie, P. Liu, L. Xu and Y. Wen, Biosens. Bioelectron., 2021, 179, 113062 CrossRef CAS PubMed.
- Z. Yu, H. Gong, M. Li and D. Tang, Biosens. Bioelectron., 2022, 218, 114751 CrossRef CAS PubMed.
- Y. Sun, H. Zheng, C. Wang, M. Yang, A. Zhou and H. Duan, Nanoscale, 2016, 8, 1523–1534 RSC.
- X. Ruan, Y. Wang, E. Y. Kwon, L. Wang, N. Cheng, X. Niu, S. Ding, B. J. Van Wie, Y. Lin and D. Du, Biosens. Bioelectron., 2021, 184, 113238 CrossRef CAS PubMed.
- H. Rao, J. Li, M. Luo, K. Zhang, H. Gou, H. Liu and Z. Xue, Anal. Bioanal. Chem., 2022, 414, 2991–3003 CrossRef CAS PubMed.
- A. Qileng, W. Liu, Z. Sun, W. Zhou, Y. Fang, H. Lei, Y. Liu and S. Zhang, Adv. Funct. Mater., 2022, 32, 2203244 CrossRef CAS.
- Y. Ma, M. Deng, X. Wang, X. Gao, H. Song, Y. Zhu, L. Feng and Y. Zhang, Anal. Chim. Acta, 2022, 1221, 340078 CrossRef CAS.
- X. Li, M. Zhang, Y. Hu, J. Xu, D. Sun, T. Hu and Z. Ni, Biomed. Microdevices, 2020, 22, 17 CrossRef CAS PubMed.
- M. Li, X. Peng, Z. Liu, Y. Dai, Y. Han, L. Fan and Y. Guo, Analyst, 2023, 148, 2709–2716 RSC.
- H. Li, Q. Cai, J. Wang and G. Jie, Biosens. Bioelectron., 2023, 232, 115315 CrossRef CAS PubMed.
- Y. Ge, P. Liu, Q. Chen, M. Qu, L. Xu, H. Liang, X. Zhang, Z. Huang, Y. Wen and L. Wang, Biosens. Bioelectron., 2023, 237, 115454 CrossRef CAS PubMed.
- F. Cui, H. Sun, X. Yang, H. Zhou, Y. Wu, J. Li, H. Li, J. Liu, C. Zeng, B. Qu, J. Zhang and Q. Zhou, Chem. Eng. J., 2023, 457, 141303 CrossRef CAS.
- X. Cai, Q. Gao, S. Zuo, H. Zhao and M. Lan, Electroanalysis, 2020, 32, 598–605 CrossRef CAS.
- W. G. X. Wang, Y. Hu, J. Wu and H. Wei, Nanozymes: Next Wave of Artificial Enzymes, Springer, Berlin, Heidelberg, 2016 Search PubMed.
- H. Cheng, X. Wang and H. Wei, in Encyclopedia of Physical Organic Chemistry, 2017, pp. 1–64, DOI:10.1002/9781118468586.epoc5016.
- G. Brunetti, F. Padovani, A. De Pastina, C. Rotella, A. Monahan, S. L. Hoffman, S. A. Jongo, S. Abdulla, G. Corradin, G. Pluschke, C. Daubenberger and M. Hegner, Nanoscale, 2021, 13, 2338–2349 RSC.
- J. Wu, M. Li, W.-Q. Chen, D.-H. Kim, Y.-S. Kim, Y.-G. Huang, K.-C. Hwang, Z. Kang and J. A. Rogers, Acta Mech. Sin., 2010, 26, 881–888 CrossRef.
- S. Choi, H. Lee, R. Ghaffari, T. Hyeon and D.-H. Kim, Adv. Mater., 2016, 28, 4203–4218 CrossRef CAS PubMed.
- H.-R. Lim, H. S. Kim, R. Qazi, Y.-T. Kwon, J.-W. Jeong and W.-H. Yeo, Adv. Mater., 2020, 32, 1901924 CrossRef CAS PubMed.
- C. Wang, K. Xia, H. Wang, X. Liang, Z. Yin and Y. Zhang, Adv. Mater., 2019, 31, 1801072 CrossRef.
- A. Levin, S. Gong and W. Cheng, Biosensors, 2023, 13, 462 CrossRef CAS PubMed.
- X. Jin, C. Liu, T. Xu, L. Su and X. Zhang, Biosens. Bioelectron., 2020, 165, 112412 CrossRef CAS PubMed.
- T. Q. Trung and N. E. Lee, J. Mater. Chem. C, 2017, 5, 2202–2222 RSC.
- Y. Huang, T. Xu, W. Wang, Y. Wen, K. Li, L. Qian, X. Zhang and G. Liu, Microchim. Acta, 2020, 187, 70 CrossRef CAS.
- M. Bariya, H. Y. Y. Nyein and A. Javey, Nat. Electron., 2018, 1, 160–171 CrossRef.
- J. Yoo, S. Tang and W. Gao, Nat. Rev. Bioeng., 2023, 1, 308–310 CrossRef.
- Y. Chen, Y. Zhang, Z. Liang, Y. Cao, Z. Han and X. Feng, npj Flexible Electron., 2020, 4, 2 CrossRef.
- H. Niu, H. Zhang, W. Yue, S. Gao, H. Kan, C. Zhang, C. Zhang, J. Pang, Z. Lou, L. Wang, Y. Li, H. Liu and G. Shen, Small, 2021, 17, 2100804 CrossRef CAS.
- O. Parlak, V. F. Curto, E. Ojeda, L. Basabe-Desmonts, F. Benito-Lopez and A. Salleo, in Wearable Bioelectronics, ed. O. Parlak, A. Salleo and A. Turner, Elsevier, 2020, pp. 65–88, DOI:10.1016/B978-0-08-102407-2.00004-7.
- B. Wang, A. Wilhelm, A. Wilhelm, S. Pilehvar, S. Moshfeghi, P. Stout, K. Salahi and S. Emaminejad, in Wearable Bioelectronics, ed. O. Parlak, A. Salleo and A. Turner, Elsevier, 2020, pp. 49–63, DOI:10.1016/B978-0-08-102407-2.00003-5.
- X. Zhu, S. Yuan, Y. Ju, J. Yang, C. Zhao and H. Liu, Anal. Chem., 2019, 91, 10764–10771 CrossRef CAS.
- Z. Zhao, Y. Kong, X. Lin, C. Liu, J. Liu, Y. He, L. Yang, G. Huang and Y. Mei, J. Mater. Chem. A, 2020, 8, 26119–26129 RSC.
- Z. Wei, W. Zhu, Y. Li, Y. Ma, J. Wang, N. Hu, Y. Suo and J. Wang, Inorg. Chem., 2018, 57, 8422–8428 CrossRef CAS.
- X. Xuan, M. Qian, L. Pan, T. Lu, L. Han, H. Yu, L. Wan, Y. Niu and S. Gong, J. Mater. Chem. B, 2020, 8, 9094–9109 RSC.
- V. Archana, Y. Xia, R. Fang and G. Gnana kumar, ACS Sustainable Chem. Eng., 2019, 7, 6707–6719 CrossRef CAS.
- L. Hou, H. Zhao, S. Bi, Y. Xu and Y. Lu, Electrochim. Acta, 2017, 248, 281–291 CrossRef CAS.
- X. Wei, J. Guo, H. Lian, X. Sun and B. Liu, Sens. Actuators, B, 2021, 329, 129205 CrossRef CAS.
- Z. Wang, T. Liu, L. Jiang, M. Asif, X. Qiu, Y. Yu, F. Xiao and H. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 32310–32319 CrossRef CAS.
- C. Chen, Y. Zhong, S. Cheng, Y. Huanga, T. Li, T. Shi, G. Liao and Z. Tang, J. Electrochem. Soc., 2020, 167, 027531 CrossRef CAS.
- Z. Wang, Y. Huang, K. Xu, Y. Zhong, C. He, L. Jiang, J. Sun, Z. Rao, J. Zhu, J. Huang, F. Xiao, H. Liu and B. Y. Xia, Nat. Commun., 2023, 14, 69 CrossRef CAS.
- J. Xiao, Y. Luo, L. Su, J. Lu, W. Han, T. Xu and X. Zhang, Anal. Chim. Acta, 2022, 1208, 339843 CrossRef CAS.
- J. Xiao, C. Fan, T. Xu, L. Su and X. Zhang, Sens. Actuators, B, 2022, 359, 131586 CrossRef CAS.
- G. Tian, Z. Zhou, M. Li, X. Li, T. Xu and X. Zhang, Anal. Chem., 2023, 95, 13250–13257 CrossRef CAS PubMed.
- J. Ma, Y. Jiang, L. Shen, H. Ma, T. Sun, F. Lv, A. Kiran and N. Zhu, Biosens. Bioelectron., 2019, 144, 111637 CrossRef CAS.
- Y. Wang, Y. Liu, X. Wang, X. Cao, J. Xia and Z. Wang, Anal. Chim. Acta, 2023, 1278, 341688 CrossRef CAS PubMed.
- C. Xu, D. Jiang, Y. Ge, L. Huang, Y. Xiao, X. Ren, X. Liu, Q. Zhang and Y. Wang, Chem. Eng. J., 2022, 431, 134109 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |
