Self-assembly of water-filled molecular saddles to generate diverse morphologies and high proton conductivity†
Nyaya Prakash
Pradhan
 a,
Sweety
Gupta‡
a,
Swapnendu Narayan
Ghosh‡
a,
Sweety
Gupta‡
a,
Swapnendu Narayan
Ghosh‡
 b,
Amit
Paul
b,
Amit
Paul
 *a,
Santanu
Talukder
*a,
Santanu
Talukder
 *b and
Aasheesh
Srivastava
*b and
Aasheesh
Srivastava
 *a
*a
aDepartment of Chemistry, Indian Institute of Science Education and Research Bhopal (IISER Bhopal), Bhauri, Bhopal Bypass Road, Bhopal, 462 066, Madhya Pradesh, India. E-mail: asrivastava@iiserb.ac.in
bDepartment of Electrical Engineering and Computer Science, Indian Institute of Science Education and Research Bhopal (IISER Bhopal), Bhauri, Bhopal Bypass Road, Bhopal, 462 066, Madhya Pradesh, India
First published on 27th March 2024
Abstract
The design of single-component organic compounds acting as efficient solid-state proton conduction (SSPC) materials has been gaining significant traction in recent times. Molecular design and controlled self-assembly are critical components in achieving highly efficient SSPC. In this work, we report the design, synthesis, and self-assembly of an organic macrocyclic aza-crown-type compound, P2Mac, which complements synthetic ease with efficient SSPC. P2Mac is derived from the pyridine-2,6-dicarboxamide (PDC) framework and contains polar amide and amine residues in its inner region, while aromatic residues occupy the periphery of the macrocycle. The crystal structure analysis revealed that P2Mac adopts a saddle-shaped geometry. Each P2Mac molecule interacts with one water molecule that is present in its central polar cavity, stabilized by a network of five hydrogen bonds. We could self-assemble P2Mac in a variety of unique, aesthetically pleasing morphologies such as micron-sized octahedra, hexapods, as well as hollow nanoparticles, and microrods. The water-filled polar channels formed through the stacking of P2Mac allow attaining a high proton conductivity value of 21.1 mS cm−1 at 27 °C under a relative humidity (RH) of 95% in the single crystals of P2Mac, while the as-prepared P2Mac pellet sample exhibited about three-orders of magnitude lower conduction under these conditions. The low activation energy of 0.39 eV, calculated from the Arrhenius plot, indicates the presence of the Grotthus proton hopping mechanism in the transport process. This report highlights the pivotal role of molecular design and self-assembly in creating high-performance SSPC organic materials.
Introduction
The development of organic solid-state proton conduction (SSPC) materials holds great significance across a wide range of technical domains ranging from energy storage and conversion devices such as Proton Exchange Membrane (PEM) fuel cells, hydrogen separation and batteries to sensors and biomedical devices.1–5 SSPC refers to the ability of materials to facilitate the transport of protons through them, allowing for the conversion of chemical energy into electrical energy.6 Designing efficient SSPC materials is crucial for advancing clean and renewable energy technologies and overcoming certain challenges related to conventional proton-conducting systems.7,8 The current SSPC materials based on metal–organic frameworks (MOFs),9–12 carbon–organic frameworks (COFs),13–16 polymer electrolytes,17–19 perovskite oxide,20–24etc. offer limited solution processability and the use of metal ions can lead to adverse environmental consequences and loss of activity upon leaching of metal ions. In this context, there is a need for developing highly efficient single-component molecular SSPC materials that can offer advantageous features such as light weight, improved processability, and diversification through molecular design.25The single-component hydrogen-bonded organic framework (HOF) has also gained prominence as a potential SSPC material since the first report by Müllen et al., who prepared a phosphonic acid-based HOF with a self-assembled columnar structure, displaying a consistently high proton conductivity of 3.2 mS cm−1 in the temperature range of 120 to 180 °C under 1 bar H2O atmosphere.26 The conductivity was ascribed to the intramolecular H-bonded network formed by phosphonic acid groups within the framework. Later, in 2016, Chen et al. introduced a metal-free porphyrin-based single-component HOF, exhibiting a moderately high proton conductivity of 3.4 × 10−3 mS cm−1 at room temperature and 97% RH.27 In a recent study, Zhang and co-workers showcased the achievement of superprotonic conductivity (σ = 22.1 mS cm−1) in a HOF material by inserting DMSO as guest molecules under anhydrous conditions at 100 °C.28 Such organic SSPC materials can benefit from exploiting supramolecular interactions such as hydrogen bonding, π–π stacking, and CH–π interactions, as well as charge transfer (CT) interactions to direct molecular self-assembly in order to create proton conducting conduits that ease the flow of H+ through them.29–31 These interactions play a crucial role in stabilizing the functional supramolecular self-assemblies.32–34 Thus, understanding the principles underlying the self-assembly process and its impact on the resulting structural arrangement is essential for enhancing the proton conducting ability. It is evident from the literature reports that both molecular design and spatial organization of molecules play critical roles in creating efficient SSPC organic motifs. Previously, Wang et al. demonstrated that highly ordered nanostructures obtained through self-assembly through H-bonding and other interactions facilitate superior SSPC.35 In this study, melamine and trimesic acid were utilized to create crystalline nanowires, where the trimesic acid acts as a proton source in the system. However, the design of single-component SSPC organic molecular materials is rather rare and holds great relevance in the realm of designing pure organic electronics.
Our group has been designing pyridine-2,6-dicarboxamide (PDC) based molecular scaffolds demonstrating diverse capabilities such as anion sensing,36 host–guest extraction,37 charge-transfer interactions (CTI),38 and photoisomerization.39 Recently, we have reported a PDC-based amphiphilic molecular clip that can efficiently co-transport both H+ and Cl− ions across synthetic lipid vesicles.40 Furthermore, we have developed a helical scaffold based on the PDC framework that can exist in two distinct forms (yellow and orange) in polar and nonpolar solvents and also shows high SSPC (up to 1.2 × 10−1 mS cm−1 at 95 °C and 95% RH).41 Taking these explorations further, we aimed to create a novel compact macrocyclic system for efficient SSPC. The macrocycle named P2Mac (Fig. 1) is strategically designed to facilitate a more ordered and symmetrical arrangement of molecules. It contains a hydrophilic inner core comprising amide and amine –NH residues, forming a polar aza-crown-like structure that can establish a strong H-bonding network during self-assembly. Meanwhile, the outer region incorporates nonpolar aromatic residues for efficient stacking of the macrocycles to create an extended proton-transporting channel.
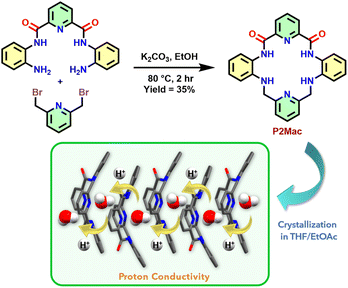 | ||
| Fig. 1 Molecular design and synthetic scheme for preparing the aza-crown-like macrocycle P2Mac and exploring its solid-state proton conductivity. | ||
Results and discussion
Synthesis and crystallographic studies
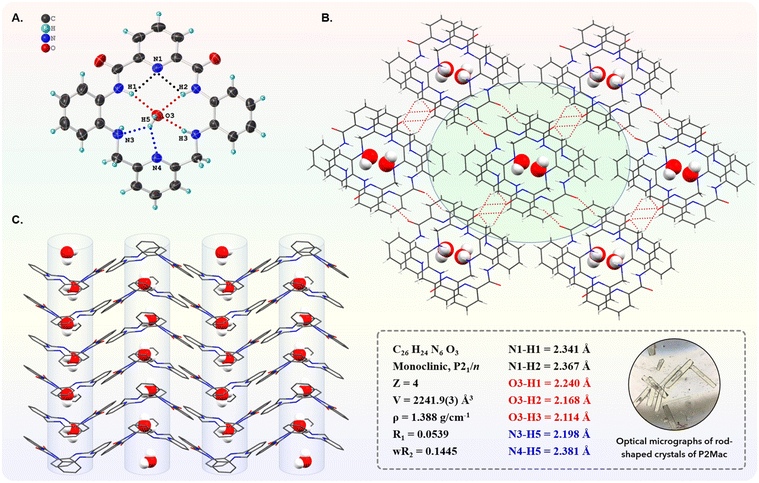
The macrocycle P2Mac was readily synthesized in 35% yield by clipping the previously reported PDC-based precursor P240 with 2,6-bis(bromomethyl)pyridine (Fig. 1). The macrocycle was well characterized through various techniques such as mass, NMR, and single-crystal X-ray diffraction (ESI† section). In order to get structural insights into the macrocycle, single crystals of P2Mac were obtained by slow infusion of ethyl acetate vapors into its THF solution over the course of two to three days at 4 °C. The SC-XRD data revealed that the molecule crystallizes in the centrosymmetric monoclinic space group P21/n, with four P2Mac molecules per unit cell along with four water molecules. It is likely that these water molecules are either present in the solvent or are taken up by P2Mac molecules from the atmosphere. From the crystal structure, it was observed that the molecule adopts a saddle-shaped geometry in order to minimize the steric repulsion between the rings (Fig. 2A). The two phenyl rings present in the molecule are oriented on one side, while the two pyridine rings are oriented on the other side with a dihedral angle of 128.6° and 115.3°, respectively (Fig. S2A and S2B†). Additionally, a water molecule resides within the central cavity of each molecule, stabilized by a strong H-bonded network. We found that the presence of water molecules is crucial for stacking the macrocycles and establishing an organized and symmetrical arrangement within the crystal structure. Each water molecule formed five H-bonds with the macrocycle (O3⋯H1, 2.240 Å; O3⋯H2, 2.168 Å; O3⋯H3, 2.114 Å; N3⋯H5, 2.198 Å and N4⋯H5, 2.381 Å) and one with the amine –NH residue of the adjacent macrocycle (H6⋯N5, 2.008 Å), forming a strong H-bonded stacked water channel (Fig. 2 and S3A, S3B†). Additionally, the PDC nitrogen is engaged in intramolecular bifurcated H-bonding (H1⋯N1⋯H2) with the neighboring amide –NH groups at a distance of 2.341 Å and 2.367 Å, respectively. As a result of these interactions, the P2Mac molecules are stacked in a head-to-tail fashion, where PDC residues are oriented oppositely in adjacent molecules along the b-axis, albeit with a slight slip-stacked configuration, rather than being precisely aligned on top of each other (Fig. S3B†). In addition, short contacts between the amide –CO and the adjacent phenyl and pyridyl –CH were also observed, ranging from 2.4 to 2.6 Å (Fig. 2B and S3C†). These short intermolecular interactions play a crucial role in forming a more symmetrical arrangement of the molecules in all three directions. Furthermore, the addition of 2% water to the THF solution of P2Mac led to the formation of elongated rod-shaped crystals (Fig. S4†), which were subsequently investigated for their proton conductivity properties. A time-dependent crystal growth experiment in this solvent medium demonstrated the slow growth of the rod-shaped crystals to form elongated rods of millimeter length (Fig. S5†). The water molecules present during the crystal formation play a crucial role in the directional stacking of P2Mac molecules through interlinking adjacent P2Mac molecules via hydrogen bonds, thus setting them up for crystallization through oriented assembly. Thus, the addition of water aids in stacking the P2Mac molecules and promotes the directional growth of the crystals to form such elongated rods. Nonetheless, the crystals obtained in the presence of water had crystal parameters and arrangement of molecules similar to those observed for crystals obtained without any added water in the medium.DFT calculations and EPR studies
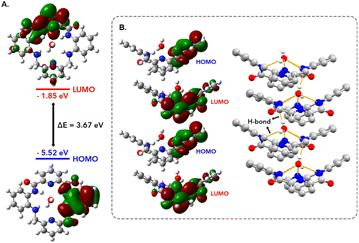
To gain further insights into the molecular packing, DFT calculations were performed using Gaussian 09 software, utilizing the coordinates obtained from the crystal structure. We performed the energy optimization of P2Mac using the B3LYP/6-311G++(d,p) basis set to simulate the spatial distribution of frontier molecular orbitals (FMOs) in the molecule. The HOMO is localized on one of the phenyl residues of the molecule, whereas the LUMO lies on the PDC backbone (Fig. 3A), with a HOMO–LUMO gap of ∼3.67 eV. As a result, while packing along the b-axis, rather than stacking directly on top of one another, P2Mac molecules are slip-stacked in a head-to-tail fashion with opposite orientations in order to attain spatial proximity for the HOMO and LUMO in the stacks (Fig. 3B). We performed an electron paramagnetic resonance (EPR) experiment at low temperature to verify the HOMO/LUMO charge-transfer interactions (CTI) in the molecular packing of the macrocycle.42 The solid-state EPR experiment revealed a strong peak in the spectrum with a g-factor of 2.007 at 140 K (Fig. S6†). The intensity of the EPR signal was further enhanced when P2Mac was exposed to UV light, suggesting the formation of active radicals during the charge transfer process.Self-assembling P2Mac into diverse morphologies
We wished to investigate how H-bonding solvent can alter the morphology of P2Mac self-assemblies. We varied the water/THF ratio as well as the rate of addition of THF solution of P2Mac (0.1 mg mL−1) in the water–THF mixture to control the nucleation and growth kinetics of the self-assemblies. Here, THF would act as a good solvent for P2Mac while water would act as a poor solvent. By maintaining a final water/THF ratio of 50% v/v and adding the THF solution of P2Mac at an addition rate of 100 μL per 60 s, followed by keeping the solution undisturbed for 1 hour, we obtained uniform hollow nanoparticles with an average diameter of approximately 400 ± 25 nm (Fig. 4A and B). The hollowness of the nanoparticles is clearly evident in the SEM and TEM images (Fig. 4B and S7†). Conversely, a rapid addition at 100 μL s−1 of the P2Mac THF solution results in the formation of microrods at a final water/THF ratio of 30% v/v (Fig. 4C and D). However, at an intermediate addition rate of 100 μL per 30 s, we managed to generate two distinct micron-sized morphologies, namely octahedron (at a final water/THF ratio of 30% v/v) and hexapod (at a 90% v/v water/THF ratio). The schematic representation of the formation of various morphologies under different water/THF ratios and addition rates is shown in Fig. 5, and a detailed procedure for creating these morphologies is provided in the ESI.† An intricate interplay of solvent–solute interaction generated these diverse morphologies through the controlled self-assembly of P2Mac molecules. A detailed investigation into the generation of these faceted morphologies, such as the octahedron and the hexapod, is pending and will be the subject of future studies.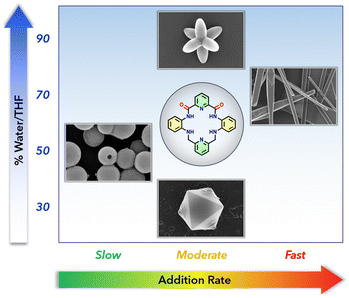 | ||
| Fig. 5 Schematic illustration of the formation of diverse morphologies of P2Mac under different conditions. | ||
Conductivity studies of P2Mac
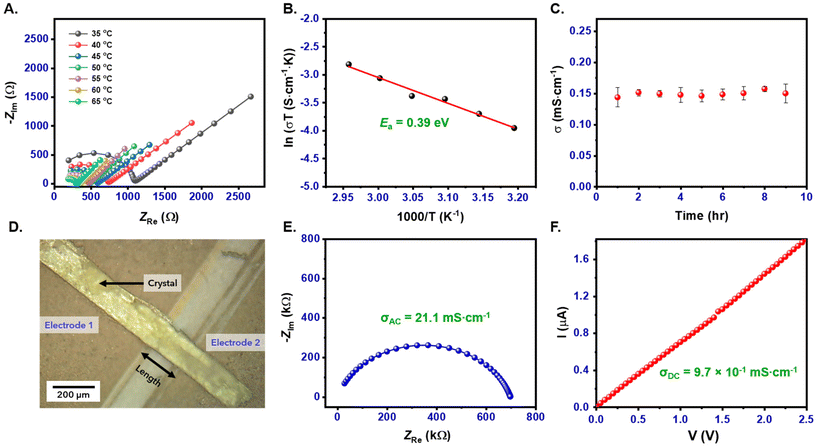
The formation of water channels in the stacks of P2Mac seen in the crystal structure encouraged us to explore its solid-state proton conduction (SSPC) ability in both pellet and crystal forms. The AC proton conductivity of P2Mac was investigated through electrochemical impedance spectroscopy (EIS) using compressed pellets. These pellets were initially exposed to HCl vapors for 4 hours and subsequently placed inside a temperature and humidity-controlled chamber. The conductivity experiment was conducted in the frequency range of 5 MHz to 1 Hz. The semicircle obtained from the Nyquist plot was fitted according to the equivalent circuit shown in Fig. S8,† where the first semicircle that appears in the high-frequency region shows the proton conductivity value of the sample. The experiments were conducted at varying temperatures, ranging from 27 °C to 65 °C, under a relative humidity (RH) of 95%. The proton conductivity of P2Mac pellets, measured at 27 °C and 95% RH, was 2.17 × 10−2 mS cm−1, exhibiting a notable increase to 1.79 × 10−1 mS cm−1 at 65 °C as illustrated in Fig. 6A. These values are remarkably high for single-component SSPC materials, which is attributed to the existence of polar self-assembled water-filled channels in P2Mac stacks that facilitate the migration of H+ through them. However, since P2Mac lacks a source of protons to transmit, we kept the P2Mac pellets as well as the rod-shaped crystals in an environment of aqueous HCl (ca. 5.5 M) for 4 h to enhance the conductivity of the material. Nevertheless, no changes in the crystal structure or molecular arrangement were observed in the crystals upon such exposure, except for a color change in the pellet from light to dark yellow (Fig. S9†). To gain further insight into the mechanism of the proton transfer pathway in the pelletized P2Mac sample, activation energy (Ea) was calculated from the Arrhenius plot (Fig. 6B and Table S2†). The calculated Ea was found to be 0.39 eV, indicating that the proton transfer proceeds through the Grotthus mechanism.43 Furthermore, a time-dependent stability experiment was also performed for P2Mac pellets at 50 °C and 95% RH, which showed that they were stable for 9 hours (Fig. 6C and Table S3†). The NMR spectra before and after conductivity measurement also demonstrated the stability of P2Mac (Fig. S10†).The rod-shaped crystals of P2Mac, with sub-mm length, inspired us to investigate both the AC and DC conductivity of the material in its crystalline state as well (Fig. S11†). For that purpose, we fabricated a single crystal device of P2Mac (Fig. 6D), and the detailed fabrication procedure is outlined in section 6.4.1 of the ESI.† The AC proton conductivity of the crystal sample was measured using the EIS technique in the frequency range of 1 MHz to 1 kHz. At 27 °C and 95% RH, the conductivity was calculated to be 21.1 mS cm−1 from the first semicircle obtained from the Nyquist plot, as depicted in Fig. 6E. Compared to the pelletized sample, the crystal of P2Mac exhibited a remarkable three-order enhancement in proton conductivity under similar conditions. The DC current–voltage (I–V) characteristics of the P2Mac crystal showed a moderate conductivity value of 9.1 × 10−4 mS cm−1 at 27 °C and 50% RH. However, under high humidity conditions (95% RH), the same crystal showed a three-order increase in conductivity up to 9.7 × 10−1 mS cm−1 (Fig. 6F). Both the AC and DC conductivity values of the P2Mac crystal are comparable to those of some of the current high-performance single-component organic SSPC materials (Table S4†). The exceptionally high AC and DC conductivities observed in the crystal sample can be attributed to the highly ordered crystal packing, which reduces grain boundaries and eliminates extrinsic proton transfer across particle gaps commonly present in powder samples.
Conclusions
In conclusion, this study presents a suitably designed organic macrocycle P2Mac that acts as a competent single-component organic material with enhanced solid-state proton conduction (SSPC) ability. We also demonstrate how the self-assembly of P2Mac can be modulated in a controlled manner to produce diverse morphologies, including hollow nanoparticles, microrods, and micron-sized octahedra as well as hexapods through the use of different water/THF ratios and addition rates. By leveraging the molecular self-assembly of P2Mac in the single crystal and the spontaneous formation of a water channel in it, high SSPC values up to 21.1 mS cm−1 were achievable at 27 °C and 95% relative humidity. This exploration presents a useful amalgamation of molecular design and self-assembly to augment the prospects of designing efficient single-component organic proton conductors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the SERB India for funding this work through project no. CRG/2021/007029. We acknowledge the Central Instrumentation Facility (CIF) IISER Bhopal for the spectroscopic characterization of the samples. We also thank the FIST-funded TEM facility of the Department of Chemistry, IISER Bhopal. N. P. P. thanks the CSIR for a Senior Research Fellowship, and S. G. and S. N. G. thank the IISER Bhopal for fellowships.This article is dedicated to Prof. Santanu Bhattacharya on his 65th birthday.
References
- K.-D. Kreuer, Chem. Mater., 1996, 8, 610–641 CrossRef CAS.
- M. M. Tellez-Cruz, J. Escorihuela, O. Solorza-Feria and V. Compañ, Polymers, 2021, 13, 3064 CrossRef CAS PubMed.
- H. Iwahara, Solid State Ionics, 1996, 86–88, 9–15 CrossRef CAS.
- S. Yu, M. Yang, Y. Liu and M. Liu, Mater. Chem. Front., 2023, 7, 3560–3575 RSC.
- P. Colomban, Solid State Ionics, 2019, 334, 125–144 CrossRef CAS.
- C. E. Thomas, Int. J. Hydrogen Energy, 2009, 34, 6005–6020 CrossRef CAS.
- D. Vignesh and E. Rout, Energy Fuels, 2023, 37, 3428–3469 CrossRef CAS.
- D. A. Medvedev, Curr. Opin. Green Sustainable Chem., 2021, 32, 100549 CrossRef CAS.
- S. Zhang, Y. Xie, R. J. Somerville, F. F. Tirani, R. Scopelliti, Z. Fei, D. Zhu and P. J. Dyson, Small, 2023, 19, 2206999 CrossRef CAS PubMed.
- C. Xiao, Z. Chu, X.-M. Ren, T.-Y. Chen and W. Jin, Chem. Commun., 2015, 51, 7947–7949 RSC.
- O. Basu, A. Das, T. Jana and S. K. Das, ACS Appl. Energy Mater., 2023, 6, 9092–9107 CrossRef CAS.
- S. S. Nagarkar, S. M. Unni, A. Sharma, S. Kurungot and S. K. Ghosh, Angew. Chem., Int. Ed., 2014, 53, 2638–2642 CrossRef CAS PubMed.
- S. Tao, L. Zhai, A. D. Dinga Wonanke, M. A. Addicoat, Q. Jiang and D. Jiang, Nat. Commun., 2020, 11, 1981 CrossRef CAS PubMed.
- L. Zhu, P. Ye, L. Zhang, Y. Ren, J. Liu, J. Lei and L. Wang, Small, 2024, 20, 2304575 CrossRef CAS PubMed.
- L. Zhu, L. Zhang, Y. Ren, J. Lei, L. Wang and J. Liu, Adv. Funct. Mater., 2023, 2313844 CrossRef.
- W. Zou, G. Jiang, W. Zhang, L. Zhang, Z. Cui, H. Song, Z. Liang and L. Du, Adv. Funct. Mater., 2023, 33, 2213642 CrossRef CAS.
- M. N. Chai and M. I. N. Isa, Sci. Rep., 2016, 6, 27328 CrossRef CAS PubMed.
- T. Regu, C. Ambika, K. Karuppasamy, J.-H. Jeon, Y.-T. Jeong, D. Vikraman, T. A. B. Raj and H.-S. Kim, Ionics, 2019, 25, 5117–5129 CrossRef CAS.
- R.-Y. Wang, S. Jeong, H. Ham, J. Kim, H. Lee, C. Y. Son and M. J. Park, Adv. Mater., 2023, 35, 2203413 CrossRef CAS PubMed.
- H. Iwahara, H. Uchida and S. Tanaka, Solid State Ionics, 1983, 9–10, 1021–1025 CrossRef CAS.
- D. S. Saini, A. Ghosh, S. Tripathy, A. Kumar, S. K. Sharma, N. Kumar, S. Majumdar and D. Bhattacharya, Sci. Rep., 2020, 10, 3461 CrossRef CAS PubMed.
- C. A. Fuller, Q. Berrod, B. Frick, M. R. Johnson, M. Avdeev, J. S. O. Evans and I. R. Evans, Chem. Mater., 2020, 32, 4347–4357 CrossRef CAS.
- C. A. Fuller, D. A. Blom, T. Vogt, I. R. Evans and J. S. O. Evans, J. Am. Chem. Soc., 2022, 144, 615–624 CrossRef CAS PubMed.
- S. Fop, J. A. Dawson, D. N. Tawse, M. G. Skellern, J. M. S. Skakle and A. C. Mclaughlin, Chem. Mater., 2022, 34, 8190–8197 CrossRef CAS PubMed.
- Y.-R. Liu, Y.-Y. Chen, H.-Y. Zhao and G. Li, Coord. Chem. Rev., 2024, 499, 215516 CrossRef CAS.
- L. Jiménez-García, A. Kaltbeitzel, W. Pisula, J. S. Gutmann, M. Klapper and K. Müllen, Angew. Chem., Int. Ed., 2009, 48, 9951–9953 CrossRef PubMed.
- W. Yang, F. Yang, T.-L. Hu, S. C. King, H. Wang, H. Wu, W. Zhou, J.-R. Li, H. D. Arman and B. Chen, Cryst. Growth Des., 2016, 16, 5831–5835 CrossRef CAS.
- X.-L. Wang, K.-Y. Niu, F.-F. Yang, J.-H. Wang, L. Liang and X.-M. Zhang, Cryst. Growth Des., 2023, 23, 6221–6227 CrossRef CAS.
- Z. Yang, N. Zhang, L. Lei, C. Yu, J. Ding, P. Li, J. Chen, M. Li, S. Ling, X. Zhuang and S. Zhang, JACS Au, 2022, 2, 819–826 CrossRef CAS PubMed.
- S. Louie, Y. Zhong, S. T. Bao, C. Schaack, A. Montoya, Z. Jin, N. M. Orchanian, Y. Liu, W. Lei, K. Harrison, J. Hone, A. Angerhofer, A. M. Evans and C. P. Nuckolls, J. Am. Chem. Soc., 2023, 145, 4940–4945 CrossRef CAS PubMed.
- D. Shao, L. Shi, G. Liu, J. Yue, S. Ming, X. Yang, J. Zhu and Z. Ruan, Cryst. Growth Des., 2023, 23, 5035–5042 CrossRef CAS.
- J.-H. Deng, J. Luo, Y.-L. Mao, S. Lai, Y.-N. Gong, D.-C. Zhong and T.-B. Lu, Sci. Adv., 2020, 6, eaax9976 CrossRef CAS PubMed.
- X.-Z. Luo, X.-J. Jia, J.-H. Deng, J.-L. Zhong, H.-J. Liu, K.-J. Wang and D.-C. Zhong, J. Am. Chem. Soc., 2013, 135, 11684–11687 CrossRef CAS PubMed.
- H.-H. Huang, J.-H. Zhang, M. Dai, L. Liu, Z. Ye, J. Liu, D.-C. Zhong, J.-W. Wang, C. Zhao and Z. Ke, Proc. Natl. Acad. Sci., 2022, 119, e2119267119 CrossRef CAS PubMed.
- H. Wang, X. Xu, N. M. Johnson, N. K. R. Dandala and H.-F. Ji, Angew. Chem., Int. Ed., 2011, 50, 12538–12541 CrossRef CAS PubMed.
- R. Kumar and A. Srivastava, Chem. – Eur. J., 2016, 22, 3224–3229 CrossRef CAS PubMed.
- R. Kumar, H. Aggarwal, R. Bhowal, D. Chopra and A. Srivastava, Chem. – Eur. J., 2019, 25, 10756–10762 CrossRef CAS PubMed.
- R. Kumar, S. Semwal, J. Choudhury and A. Srivastava, Chem. – Eur. J., 2017, 23, 15012–15016 CrossRef CAS PubMed.
- D. Pathak and A. Srivastava, Chem. Commun., 2022, 58, 12653–12656 RSC.
- N. P. Pradhan, K. R. Namdev and A. Srivastava, Org. Biomol. Chem., 2024, 22, 74–79 RSC.
- H. Aggarwal, P. A. Gaikwad, A. Dahat, S. Narayan Ghosh, P. Mehra, A. Paul, S. Talukder and A. Srivastava, Chem. – Eur. J., 2023, 29, e202300019 CrossRef CAS PubMed.
- L. Sun, W. Zhu, W. Wang, F. Yang, C. Zhang, S. Wang, X. Zhang, R. Li, H. Dong and W. Hu, Angew. Chem., Int. Ed., 2017, 56, 7831–7835 CrossRef CAS PubMed.
- T. Miyake and M. Rolandi, J. Phys.: Condens. Matter, 2015, 28, 023001 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2326935. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4nr00456f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |