Open Access Article
Open Access ArticleEnhanced photocatalytic performance of SnS2 under visible light irradiation: strategies and future perspectives
Ardiansyah
Taufik
 *a,
Rosari
Saleh
*a,
Rosari
Saleh
 bc and
Gimyeong
Seong
bc and
Gimyeong
Seong
 *d
*d
aWPI – Advanced Institute for Materials Research (WPI-AIMR), Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai, Miyagi 980-8577, Japan. E-mail: ardiansyah.taufik.d2@tohoku.ac.jp
bDepartement Fisika, FMIPA Universitas Indonesia, Kampus UI Depok, Depok 16424, Indonesia
cIntegrated Laboratory of Energy and Environment FMIPA Universitas Indonesia, Kampus UI Depok, Depok 16424, Indonesia
dDepartment of Environmental and Energy Engineering, The University of Suwon, 17, Wauan-gil, Bongdam-eup, Hwaseong-si, Gyeonggi-do 18323, Republic of Korea
First published on 17th April 2024
Abstract
Tin(II) sulfide (SnS2) has emerged as a promising candidate for visible light photocatalytic materials. As a member of the transition metal dichalcogenides (TMDs) family, SnS2 features a band gap of approximately 2.20 eV and a layered structure, rendering it suitable for visible light activation with a high specific surface area. However, the application of SnS2 as a visible light photocatalyst still requires improvement, particularly in addressing the high recombination of electrons and holes, as well as the poor selectivity inherent in its perfect crystal structure. Therefore, ongoing research focuses on strategies to enhance the photocatalytic performance of SnS2. In this comprehensive review, we analyze recent advances and promising strategies for improving the photocatalytic performance of SnS2. Various successful approaches have been reported, including controlling the reactive facets of SnS2, inducing defects in the crystal structure, manipulating morphologies, depositing noble metals, and forming heterostructures. We provide a detailed understanding of these phenomena and the preparation techniques involved, as well as future considerations for exploring new science in SnS2 photocatalysis and optimizing performance.
1 Introduction
The family of transition metal dichalcogenides (TMDs) has garnered significant attention in photocatalytic applications owing to its visible light band gap and layered structure, which provides a high specific surface area.1,2 However, the traditional utilization of primary TMD materials such as MoS2, MoSe2 and WS2 has predominantly been as supporting photocatalysts alongside other materials like ZnO,3–6 TiO2,7–10 C3N4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and others, due to their properties akin to graphene.12,13 In contrast, unlike MoS2, MoSe2 and WS2, SnS2 has emerged as a promising photocatalyst under visible light irradiation, attributable to its suitable band gap energy for visible light.14,15
11 and others, due to their properties akin to graphene.12,13 In contrast, unlike MoS2, MoSe2 and WS2, SnS2 has emerged as a promising photocatalyst under visible light irradiation, attributable to its suitable band gap energy for visible light.14,15
SnS2 belongs to the family of layered structure transition metal dichalcogenides (TMDs), characterized as an n-type semiconductor with a band gap ranging from 2.18 to 2.44 eV.16 In practical applications, SnS2 has found widespread use across various fields such as batteries,17,18 sensors,19,20 and photocatalysis due to its low cost, low toxicity, excellent stability, and abundant natural reserves.21 SnS2 can be synthesized using various techniques, including hydrothermal,22,23 solvothermal,24,25 CVD,26,27 and pulse laser deposition,28–30 among others. Additionally, SnS2 can exhibit diverse morphologies, such as flower-like structures, plate-like forms, nanoparticles, and quantum dots, rendering it particularly intriguing for both study and application purposes.
Various reports have shown that SnS2 can be utilized in numerous photocatalytic applications, such as hydrogen production,31–34 wastewater treatment,35–39 antibacterial and antifungal activity,40–43 and air purification.44 For example, Yu et al.45 first reported the photocatalytic hydrogen production of SnS2 nanosheets under solar light simulation with H2 evolution activity of 1.06 mmol h−1 g−1. In the case of waste water remediation, there are numerous reports for different kinds of waste water such as heavy metals,46–48 organic dyes,49,50 and pharmaceutical waste.51–53 For antibacterial activity, SnS2 has been applied to inactivate S. aureus and E. coli as reported by Fakhri et al.,40,41 and for air purification SnS2 has been reported to be able to remove various VOC gasses such as trichloroethylene54 and formaldehyde55 as well as NOx gas.44 Other applications have also been reported by researchers such as CO2 reduction and nitrogen fixation which make SnS2 a promising photocatalyst candidate. (For detailed summaries see sub-section 2.2.)
As a photocatalyst, some characteristics of SnS2 have become key for an excellent performance. These include low recombination and hole rates,56–59 a high specific surface area,60,61 high-reactivity facets,62,63 and good stability.64,65 Despite the confirmed photocatalytic performance of SnS2, there are still limitations that hinder its efficiency, such as a high recombination of electrons and holes,66,67 and low specific surface area. Consequently, many researchers have focused on modifying SnS2 to meet the requirements.
Several strategies have been widely adopted to enhance photocatalytic performance, including morphological modification,68,69 structural engineering,70,71 and heterostructure formation.72–74 Each of these strategies has unique advantages. For instance, morphological modification can alter the surface structure of SnS2, influencing parameters such as surface area and facet arrangement, which are beneficial for improving photocatalytic performance. Structural engineering involves disturbing the crystal structure of SnS2 through various treatments, including vacancy generation, doping addition, and strain engineering. These treatments effectively decrease electron and hole recombination, thereby enhancing photocatalytic performance. Noble metal doping, such as Au, Pt, and Ag, is a well-known method for improving photocatalytic performance due to its ability to act as an electron acceptor, slowing down the recombination of electrons and holes.75 Meanwhile, heterostructure formation can generate a synergistic effect between one sample and another, effectively improving the photocatalytic performance.76
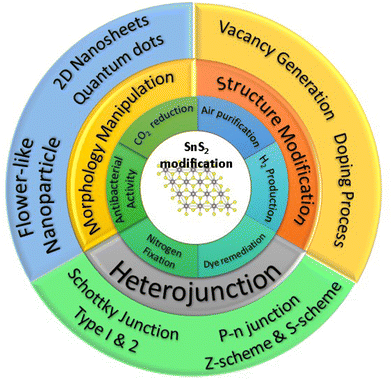
Presently, there are several reports that have reviewed the photocatalytic performance of SnS2. Sharma et al.77 reviewed the photocatalytic performance of SnS2 related to energy conversion and a heterostructure perspective approach, while Guo et al.78 reviewed the advancement of the photocatalytic performance of SnS2 by comparing pure SnS2 and SnS2-based composite. While these two reviews have addressed some important aspects of improvement strategies, the detailed explanation and fundamental mechanism of the photocatalytic enhancement of SnS2 have not been thoroughly explored. In this study, we systematically investigate the current advancement strategies for enhancing photocatalytic performance, which can be classified into three categories: structural modification, morphology engineering, and heterojunction formation. We will elucidate the factors from each strategy that have the most significant impact on the photocatalysis of SnS2. Furthermore, we will propose future perspectives for the development of photocatalytic studies on SnS2 based on the existing literature. Fig. 1 illustrates three distinct strategies for enhancing photocatalytic performance across various applications, discussed in this review. The first strategy involves structural modification, encompassing vacancy generation and doping processes. The second strategy focuses on morphological manipulation, featuring a range of morphologies such as nanoflowers, nanoparticles, nanosheets, and quantum dots. The final strategy concerns heterojunction formation, encompassing Schottky junctions, heterojunctions types I and II, p–n junctions, as well as Z-scheme and S-scheme photocatalysts. Additionally, we address current challenges and offer insights into future developments.
2 SnS2 properties and photocatalytic applications
2.1 Physical properties of SnS2
Tin disulfide (SnS2) belongs to the IV–VI chalcogenide materials and exhibits a golden-yellowish color, resembling the configuration of transition metal dichalcogenide (TMD) materials.77 The atomic arrangement of SnS2 consists of a Sn atom sandwiched between two hexagonally close-packed sulfur atoms. In layered SnS2, planar threefold layers (TLs) are formed, where strong covalent bonding exists in a plane, while weak van der Waals interactions dominate out of plane, as shown in Fig. 2(a).79 The SnS2 structure belongs to the covalent bonding group of P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1 (164) with lattice parameters a = b = 0.3643 nm and c = 1.7683 nm.79 Like the other members of the TMD family, SnS2 has a layered structure with several interesting properties such as a high optical absorption coefficient, tunable bandgap, low toxicity, and natural abundance, which makes it very promising for 2D material applications such as photocatalysis, photoanodes,80 and gas sensors.20 SnS2 corresponds to an n-type semiconductor that possesses a narrow energy band gap of 2.0–2.4 eV. The band positions of SnS2 were determined by linear extrapolation of the band edges and secondary electron cut-off, to the baseline, and yielded an ionization potential (Evac − EVBM) of 6.44 ± 0.07 eV and an electron affinity (Evac − ECBM) of 4.16 ± 0.17 eV. A bandgap (ECBM − EVBM) of 2.28 ± 0.15 eV is thus found that corresponds to the indirect band gap as shown in Fig. 2(b). SnS2 also has higher electron mobility (∼230 cm2 V−1 s−1), higher capacitance (645 mA h g−1), and lower environmental noxiousness.81 The photocatalytic reaction process occurs when the band positions of the photocatalyst align with the redox potentials required for specific chemical reactions. For instance, the conversion of H+ to H2 can occur at 0 V vs. NHE energy, where electrons in the conduction band with an energy lower than 0 V can reduce H+ to H2. Conversely, the transformation of OH− to OH˙ requires 1.93 V vs. NHE, necessitating holes with an energy higher than 1.93 vs. NHE to oxidize OH− to OH˙. In this context, the band positions of SnS2 fulfill both criteria, with the conduction band at −1.5 V vs. NHE and the valence band at 2.13 V vs. NHE,82 as illustrated in Fig. 2(c). Various chemical reactions may yield different potential energy requirements, thus influencing the applicability of SnS2 in photocatalysis.
m1 (164) with lattice parameters a = b = 0.3643 nm and c = 1.7683 nm.79 Like the other members of the TMD family, SnS2 has a layered structure with several interesting properties such as a high optical absorption coefficient, tunable bandgap, low toxicity, and natural abundance, which makes it very promising for 2D material applications such as photocatalysis, photoanodes,80 and gas sensors.20 SnS2 corresponds to an n-type semiconductor that possesses a narrow energy band gap of 2.0–2.4 eV. The band positions of SnS2 were determined by linear extrapolation of the band edges and secondary electron cut-off, to the baseline, and yielded an ionization potential (Evac − EVBM) of 6.44 ± 0.07 eV and an electron affinity (Evac − ECBM) of 4.16 ± 0.17 eV. A bandgap (ECBM − EVBM) of 2.28 ± 0.15 eV is thus found that corresponds to the indirect band gap as shown in Fig. 2(b). SnS2 also has higher electron mobility (∼230 cm2 V−1 s−1), higher capacitance (645 mA h g−1), and lower environmental noxiousness.81 The photocatalytic reaction process occurs when the band positions of the photocatalyst align with the redox potentials required for specific chemical reactions. For instance, the conversion of H+ to H2 can occur at 0 V vs. NHE energy, where electrons in the conduction band with an energy lower than 0 V can reduce H+ to H2. Conversely, the transformation of OH− to OH˙ requires 1.93 V vs. NHE, necessitating holes with an energy higher than 1.93 vs. NHE to oxidize OH− to OH˙. In this context, the band positions of SnS2 fulfill both criteria, with the conduction band at −1.5 V vs. NHE and the valence band at 2.13 V vs. NHE,82 as illustrated in Fig. 2(c). Various chemical reactions may yield different potential energy requirements, thus influencing the applicability of SnS2 in photocatalysis.
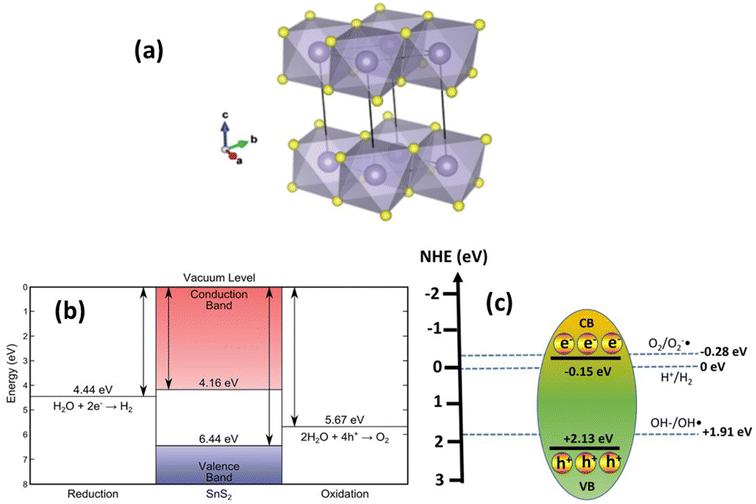 | ||
| Fig. 2 (a) Crystal structure and (b) band position of SnS2. (Reproduced from ref. 79 with permission from the Royal Society of Chemistry, copyright 2016.)79 (c) Band position of SnS2 for photocatalytic reactions. | ||
2.2 Photocatalytic applications of SnS2
The utilization of SnS2 as a photocatalyst was initially documented in 2007 by He et al.83 who explored the formation of a heterostructure between SnS2 and TiO2 for methyl orange degradation. However, after this initial investigation, research on SnS2 photocatalysis remained limited until 2017, when the number of publications on the subject notably increased, as illustrated in Fig. 3(a). Presently, the total number of publications on SnS2 as a photocatalyst remains at less than 500, significantly lower compared with conventional photocatalysts such as TiO2 or ZnO, and even other transition metal dichalcogenide (TMD) materials like MoS2 and WS2 (based on Scopus data). As a 2D photocatalyst material, SnS2 has found applications across various domains, including air purification,44,55 wastewater treatment,47,84–87 hydrogen production,88–97 and antibacterial purposes,41 CO2 reduction, and89,91,98–100 nitrogen fixation.92,94,101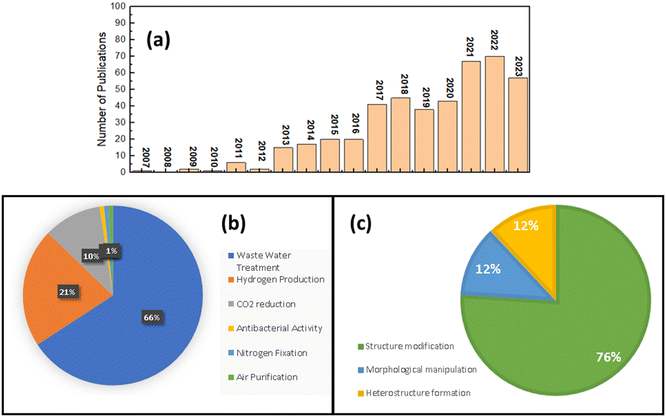 | ||
| Fig. 3 (a) Number of SnS2 photocatalytic publications; (b) summary of practical applications of SnS2 photocatalytic. (c) Graphical illustration for photocatalytic improvement strategies for SnS2. | ||
According to Scopus data, illustrated in Fig. 3(b), the predominant focus of photocatalytic applications involving SnS2 is wastewater treatment, constituting 66% of the total research publications to date. Following closely, hydrogen production and CO2 reduction make up 21% and 10% of the total publications on SnS2 as a photocatalyst. The least explored applications include nitrogen fixation, antibacterial activity, and air purification. Wastewater treatment represents the most extensively studied application when investigating SnS2 photocatalytic performance. Given the high demand for clean water in our daily lives, the removal of wastewater from water resources is of paramount importance. Various types of wastewater, containing pollutants such as organic dyes, heavy metals, pharmaceutical waste, and even uranium(VI), pose significant threats to our health. Until now (2024 January 10th), based on Scopus data there are 337 publications that have reported the use of SnS2 photocatalyst for wastewater treatment under visible light irradiation and 192 of them are about organic dye removal such as methylene blue,102–104 methyl orange,105 and rhodamine B (RhB).49,106 For heavy metals, hexavalent chromium (Cr(VI)) is the most studied waste water model for photocatalytic investigations of SnS2, with 99 publications, which makes SnS2 one of the most promising materials for Cr(VI) reduction under visible light irradiation. In the pharmaceutical waste field, tetracycline is the most studied wastewater, with 11 reports showing a good performance for tetracycline removal by a SnS2 photocatalyst. Table 1 shows the summary of various types of application of SnS2 photocatalysts. It can be seen that the SnS2 photocatalyst exhibits great potential for various types of photocatalytic application.
| Sample | Sample | Light source | Photocatalytic result | Additional information | Ref. |
|---|---|---|---|---|---|
| CO2 reduction | Cu-doped SnS2 | 420 nm LED | CH3OH yield 0.99 mmol g−1 | 97 | |
| S-vacancy SnS2 | 400 W metal halide lamp | CO yield = 2.44 μmol g−1 h−1. 1.48 times that of pure SnS2 | 106 | ||
| SnS2 flower-like | 300 W xenon light | CO production rate 8.91 μmol g−1 h−1 | 135 | ||
| Z-scheme SnO2/SnS2/Cu2SnS3 | 300 W xenon lamp | Ethanol yield 28.44 mmol g−1 h−1 3 times higher than SnS2 | 110 | ||
| SnS2/SnO2 nanoflower | 300 W xenon lamp | CO yield 60.85 μmol g−1 h−1 | 136 | ||
| Partly oxidized SnS2 | 300 W xenon lamp | CO yield 12.28 μmol g−1 h−1 | 67 | ||
| Carbon-doped SnS2 | 300 W halogen lamp | CH3CHO yield 125.66 μmol per 100 g per 3 h | 67 | ||
| Carbon-doped SnS2 | 150 W halogen lamp | CH4 yield 0.64 μmol g−1 h−1 | 67 | ||
| SnS2 QDs/g-C3N4 | 300 W xenon lamp (420 nm cut-off) | CH3OH yield 2.3 μmol g−1 h−1 | 137 | ||
| Hydrogen production | SnS2-nanosheets | 400 W mercury lamp | H2 production rate: 0.566 mmol g−1 h−1 | 31 | |
| 2.5 mol% Ni–SnS2 | 400 W mercury lamp | 1429 μmol g−1 h−1 | 138 | ||
| In3+-doped SnS2 | 300 W xenon lamp | H2 production rate: 470 mmol g−1 for 12 h | 139 | ||
| S-scheme SnS2/CdS | Simulated solar light (100 mW cm−2) | 360.75 μL h−1 | 116 | ||
| SnS2/g-C3N4 | 300 W xenon lamp (λ ≥ 420 nm) | 1818.8 μM g−1 h−1 | 124 | ||
| SnS2/CdS/TiO2 | 300 W xenon lamp (λ ≥ 420 nm) | 97.14 μmol h−1 cm−2 | 140 | ||
| SnS2/BiVO4 | 100 mW cm−2 xenon lamp, 0.8 V | 900 μmol cm−2 | 117 | ||
| SnS2/Cd0.5Zn0.5S | 300 W xenon lamp (λ ≥ 420 nm) | 3.19 mmol g−1 h−1 | 141 | ||
| Air purification | Bi4O5Br2–SnS2 | Visible light | 40% NOx removal | NOx abatement | 44 |
| Ni-doped SnS2/SnO2 | 8 W, 256 nm light | 85% | Trichloroethylene | 54 | |
| SnS2/TiO2 | 40 W fluorescence lamp | 55% removal | Formaldehyde | 55 | |
| Nitrogen fixation | SnS2 nanosheets | 330 W xenon lamp | 27.5 μmol g−1 h−1 | 101 | |
| SnS2/MoO3 | 300 W xenon lamp | 30.04 μg h−1 mg−1 | 92 | ||
| Bi/Bi2S3/SnS2 | 300 W xenon lamp | 96.4 μmol g−1 h−1 | 94 | ||
| Antibacterial activity | SnS2 nanoparticles | 500 W xenon lamp | 75.28 ± 2.3% | S. aureus (ATCC 25923) | 41 |
| SnS2 nanoparticles | 500 W xenon lamp | 78.95 ± 2.0% | E. coli (ATCC 13534) | 41 | |
| SnS2 nanoparticles | 500 W xenon lamp | 81.22 ± 1.1% | E. coli (ATCC 25922) | 41 | |
| SnS2 | Visible light | 50.39 | E. coli | 43 | |
| Pr-doped SnS2 | Visible light | 71.03 | E. coli | 43 | |
| Waste water treatment | SnS2 nanoparticle | 250 W xenon lamp (λ ≥ 420 nm) | k MO: 0.0594 min−1 | Methyl orange | 105 |
| MoS2/SnS2 | Visible light | k MB: 0.0594 min−1 | Methylene blue | 122 | |
| SnS2 decorated biochar | Natural sunlight | k MB: 0.013 min−1 | Methylene blue | 142 | |
| S–Sn–S vacancy SnS2 | 300 W Xe lamp | k RhB = 0.136 min−1 | Rhodamine B | 143 | |
| CdZnS/SnS2/SnO2 | 500 W xenon lamp | k RhB = 0.0267 min−1 | Rhodamine B | 49 | |
| Zr-doped SnS2 | Larger than 420 nm | k Cr(VI) = 0.059 min−1 | Cr(VI) | 144 | |
| SnS2 nanoparticle | 300 W xenon lamp | k Cr(VI) = 0.00401 min−1 | Cr(VI) | 129 | |
| SnS2@SnO2 | 300 W xenon lamp | k Cr(VI) = 0.0907 min−1 | Cr(VI) | 129 | |
| SnS2/SnS | Direct sunlight | k Cr(VI) = 0.086 min−1 | Cr(VI) | 145 | |
| SnS2/CNTs | 300 W xenon lamp (λ ≥ 420 nm) | k Cr(VI) = 0.07750 min−1 | Cr(VI) | 146 | |
| SnS2 nanoplates | Solar irradiance was assumed to be 221 W m−2 | k TC = 0.001 min−1, kCP = 0.0005 min−1 | Tetracycline and ciprofloxacin | 134 | |
| SnS2/Bi2WO6 | Solar irradiance was assumed to be 221 W m−2 | k TC = 0.027 min−1, kCP = 0.024 min−1 | Tetracycline and ciprofloxacin | 134 | |
| SnS2 nanoplates | Natural sunlight | k CP = 0.0024 min−1 | Ciprofloxacin | 132 | |
| SnS2/BiVO4 | Natural sunlight | k CP = 0.018424 min−1 | Ciprofloxacin | 132 | |
| SnS2 nanoplates | LED light 500 W m−2 | k GM = 0.1492 min−1 | Gemifloxacin | 37 | |
| Platinum/polypyrrole–carbon black/SnS2 | LED light 500 W m−2 | k GM = 0.0429 min−1 | Gemifloxacin | 37 | |
| SnS2 nanoparticle | Visible light (λ ≥ 420 nm) | k TC = 0.00129 min−1 | Tetracycline | 38 | |
| SnS2/Bi2MoO6−x | Visible light (λ ≥ 420 nm) | k TC = 0.01245 min−1 | Tetracycline | 38 | |
| SnS2 nanoplates | Solar simulator | k NP = 0.014 min−1 | Norfloxacin | 147 |
2.2.6.1 Organic dyes. Organic dyes are prevalent pollutants in wastewater within our immediate environment, often originating from direct discharge by the textile industry, presenting significant environmental concern. Utilizing photocatalytic wastewater treatment emerges as a promising solution to address this issue, offering notable advantages such as cost-effectiveness, simplicity, and the capability to convert the chemical composition of organic dyes into harmless byproducts such as CO2.120,121 In the realm of employing SnS2 as a photocatalyst for organic dye degradation, extensive research has been conducted, targeting various dye types including methylene blue,122 methyl orange,123 rhodamine B,124–126 crystal violet,127 and others. Table 1 depicts the efficacy of SnS2 photocatalysts in removing organic dyes. Notably, the data demonstrate promising outcomes across all the tested dyes. The underlying mechanism of organic dye degradation can be elucidated as follows: upon irradiation, electron–hole pairs are generated, which subsequently react with O2 and OH− to yield highly reactive O2˙− and ˙OH radicals. These radicals possess strong oxidative capabilities, facilitating the decomposition of diverse synthetic dyes into CO2 and water.
2.2.6.2 Heavy metals. In addition to organic dyes, the reduction of heavy metals represents a significant area of study in the photocatalytic application of SnS2. Among various heavy metals, hexavalent chromium (Cr(VI)) has been extensively researched. Several strategies have been developed to enhance Cr(VI) removal, including vacancy generation,48 doping formation,128 and heterostructure formation.129 The basic mechanism of Cr(VI) reduction involves the generation of electrons and holes upon irradiation, where the photoexcited electrons can directly decompose Cr(VI) ions into Cr(III). The photocatalytic ability of SnS2 depends on the extent to which electrons react with Cr(VI) molecules, which may be attributed to increasing active sites or improving charge carrier separation efficiency. Table 1 presents several studies confirming the degradation of Cr(VI) using SnS2 photocatalysts with varying capabilities. A detailed analysis will be provided in the subsequent section.
2.2.6.3 Antibiotics. Pharmaceutical waste, including compounds like tetracycline and others, presents a significant challenge in wastewater management, particularly with many pharmaceutical industries directly discharging their waste into water bodies. Consequently, effective treatment methods are imperative to mitigate the presence of these hazardous chemicals in water. Photocatalysis has emerged as a promising technique for efficiently removing pharmaceutical waste from water. However, the utilization of SnS2 photocatalysts for antibiotic removal remains limited. Only a small number of research papers, fewer than 30, have explored the use of SnS2 photocatalysts for this purpose, with most focusing on the formation of heterojunctions with SnS2, such as Z-scheme and S-scheme configurations.42,104,130,131 For instance, Kumar et al.132 demonstrated the effectiveness of Z-scheme SnS2/BiVO4 in removing the antibiotic ciprofloxacin from aqueous solutions. They found that the optimized batch with 0.15SnS2/BiVO4 exhibited a significantly higher apparent rate constant (k = 0.0184 min−1) compared with BiVO4 (k = 0.0049 min−1) and SnS2 (k = 0.0024 min−1) alone. This improved performance was attributed to enhanced charge separation efficiency, enabling electrons from SnS2 to directly react with O2 to form O2˙−, ultimately degrading ciprofloxacin into CO2 and water.133 Other antibiotics have also been shown to be degradable by SnS2 photocatalysts. For example, Kumar et al.134 reported the use of SnS2/Bi2WO6 for the removal of ciprofloxacin and tetracycline. While SnS2 alone exhibited minimal removal capabilities for these antibiotics, the combination with Bi2WO6 significantly enhanced the degradation rates. These findings suggest that SnS2 photocatalysts alone are insufficient for effectively decomposing various types of pharmaceutical waste. One of the key limitations is the rapid recombination of electron–hole pairs, which impedes the reaction of electrons with O2 to generate reactive oxygen species (ROS). Therefore, the development of hybrid photocatalytic systems, combining SnS2 with other materials, holds promise for improving the efficiency of pharmaceutical waste removal from wastewater. Continued research in this area is crucial for advancing sustainable water treatment technologies.
Although there are many publications about photocatalytic performance with different applications, we can categorize their strategies into three different categories.
(1) Structural modification: This strategy involves altering the structure of SnS2 to enhance its photocatalytic properties. This could include changes in the composition, arrangement of atoms, or other structural aspects.
(2) Morphological manipulation: Morphological manipulation focuses on changing the physical shape or form of SnS2 to improve its photocatalytic performance. This might involve controlling the particle size, shape, or surface structure.
(3) Heterostructure formation: This approach involves creating composite materials by combining SnS2 with other materials, possibly forming interfaces or layered structures. The high percentage suggests that researchers find this strategy particularly effective or promising for enhancing photocatalytic performance.
Based on these three classifications, the quantitative analysis has been made and plotted in Fig. 3(c) based on the Scopus data until now. The structural modification and morphological manipulation SnS2 strategies are just 12% each of the total of publications of SnS2 photocatalytic. On the other hands, the heterostructure formation exceeded more than 75%, which indicates, at the current stage, that the heterostructure formation of SnS2 is the most popular strategy for improving strategies, which probably attributed to the unique characteristics of composite structures which imply to the better performance.
3 Photocatalytic improvement strategies
3.1 Structural modification
Structural modification is a valuable strategy for enhancing photocatalytic performance. By adjusting the structural properties of the photocatalyst, it is possible to improve its electronic and surface characteristics for a range of photocatalytic applications, including optimizing light utilization, enhancing charge transfer kinetics, and creating additional active sites.148,149 Several established techniques can be employed to modify the structure of the photocatalyst, including vacancy generation and doping. These methods can increase the photocatalyst's charge separation efficiency and surface reactivity by raising the adsorption energy.The synthesis of sulfur vacancy-generating SnS2 was reported by Qiang et al.,48 prepared via hydrothermal reaction for Cr(VI) reduction under visible light irradiation. They observed that adjusting the molar ratio between the Sn and S precursors during synthesis is crucial for controlling the defect concentration on SnS2 nanoparticles. A higher Sn to S ratio indicates a higher defect content, confirmed by EPR analysis (Fig. 4(a)). An intense resonance signal from EPR analysis for the sample with a Sn to S ratio of five was observed, indicating a higher defect concentration compared with the sample with a lower Sn to S ratio due to sulfur vacancy. Photocatalytic Cr(VI) reduction also demonstrated that the sample with a high defect content exhibits the best photocatalytic ability (Fig. 4(b)). This phenomenon was attributed to the prolonged charge carrier lifetime of the sulfur vacancy sample, as demonstrated in the photocurrent analysis of all the prepared samples (Fig. 4(c)). SnS2 nanosheets with an optimal amount of sulfur vacancies exhibited a superior photoreduction rate of Cr(VI) (100% in 20 min), roughly 18.09 times higher than that of pure SnS2 (Table 2), indicating vast prospects for photocatalytic wastewater treatment. The presence of sulfur vacancies in the crystal structure of SnS2 serves to create an electron trap between the valence band and conduction band, effectively slowing down the recombination of electrons and holes. In that case the trapped electrons are still able to generate chemical reactions with Cr(VI) ions to change into less hazardous ions (Cr(III)).
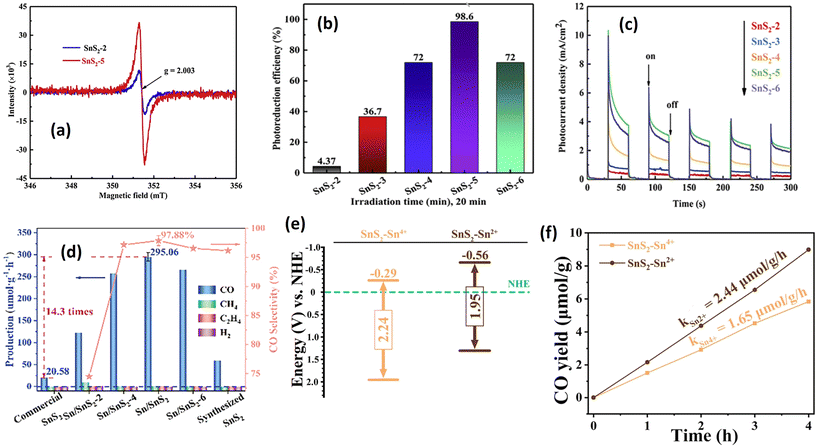 | ||
| Fig. 4 (a) Room-temperature EPR spectra of SnS2-2 and SnS2-5. (b) Comparison of Cr(VI) photoreduction over SnS2−X (X = 2, 3, 4, 5, 6) under visible light irradiation. (c) Photocurrent response of SnS2−X (X = 2, 3, 4, 5, 6).48 (Reproduced from ref. 48 with permission from Elsevier, copyright 2020.) (d) Photocatalytic CO2 reduction performance of vacancy generated SnS2 prepared at different L-cystine concentrations (−1, −2, −4, −6 mol).150 (Reproduced from ref. 150 with permission from Wiley, copyright 2023.) (e) Energy band of SnS2–Sn4+ and SnS2–Sn2+. (f) CO2 reduction of SnS2–Sn4+ and SnS2–Sn2+.106 (Reproduced from ref. 106 with permission from Elsevier, copyright 2023.) | ||
| Vacancy type | Light source | Photocatalytic testing | Photocatalytic performance | Ref. |
|---|---|---|---|---|
| S-vacancy SnS2 | 500 W Xe lamp | 50 mg L−1 Cr(VI) reduction | Degradation efficiency (100% in 20 min), roughly 18.09 times that of pure SnS2 | 48 |
| S-vacancy SnS2 | 400 W metal halide lamp | (1 × 10−5 M) rhodamine B (RhB) degradation | k RhB = 0.0102 min−1 for S-vacancy SnS2, ∼2.1 times that of SnS2 | 106 |
| S-vacancy SnS2 | 400 W metal halide lamp | CO2 reduction (1 atm) | CO yield = 2.44 μmol g−1 h−1. 1.48 times that of pure SnS2 | 106 |
| S–Sn-vacancy SnS2 | 300 W Xe lamp | RhB, and methylene blue (MB) degradation | k RhB = 0.054 min−1 (3.17 times that of pure SnS2), kMB = 0.043 min−1 (3.07 times that of pure SnS2) | 143 |
| S–Sn–S-vacancy SnS2 | 300 W Xe lamp | RhB and MB degradation | k RhB = 0.136 min−1 (8.00 times that of pure SnS2), kMB = 0.116 min−1 (8.28 times that of pure SnS2) | 143 |
| C-doped SnS2 | Visible light | CO2 reduction (acetaldehyde formation) | Acetaldehyde formation: 1256.6 μmol g−1 (228.47 times that of SnS2) | 67 |
| Cu-doped SnS2 | 420 nm LED | CO2 reduction (CH3OH production) | Cu-doped SnS2: 0.99 mmol g−1 h−1 (2.06 times that of SnS2) | 97 |
| Cu-doped SnS2 | 300 W xenon lamp | Hydrogen evolution | Cu-doped SnS2: 1.37 mmol g−1 h−1 far exceeding 6 times that of pure SnS2 | 153 |
| Ce-doped SnS2 | Solar irradiation | 10 and 20 ppm of methyl orange (MO) | k MO = 0.096 and kMO = 0.080 for 10 ppm and 20 ppm. SnS2: kMO = 0.045 and kMO = 0.035 for 10 and 20 ppm | 87 |
| Zr-doped SnS2 | Larger than 420 nm | 40 mg L−1 Cr(VI) reduction | k Cr(VI) = 0.059 min−1 nearly 2.2 times that of SnS2 | 144 |
| In3+-doped SnS2 | White LED 48 W | 20 ppm Cr(VI) reduction | k Cr(VI) = 0.123 min−1. 39.4 times that of SnS2 | 128 |
| In3+-doped SnS2 | 300 W xenon lamp | Hydrogen evolution | H2 production rate: 470 mmol g−1 for 12 h. 1.95 times higher than SnS2 | 139 |
| Sr-doped SnS2 | 250 W xenon lamp | 10 ppm RhB degradation | k RhB = 0.00721 min−1 (1.55 times that of SnS2) | 154 |
Sulfur vacancy generation is not only suitable for Cr(VI) reduction but also for other applications, including CO2 reduction and phenol degradation. In a recent publication, Zhang et al.150 employed a synthesis method using L-cystine as the sulfur precursor to generate S-vacancies in SnS2 for CO2 reduction (see Fig. 4(d)). Their investigation demonstrated a significant enhancement in the CO2 reduction efficacy of SnS2 with S-vacancies, showcasing a 14.3-fold improvement compared with commercially available SnS2 products. Their objective was to elucidate the underlying mechanism of CO2 reduction and discern the role of Sn0 atoms in CO2 adsorption. Through DFT analysis, it was observed that the CO2 molecule approached the surface of Sn/SnS2, where the Sx2− ions in proximity transferred photogenerated electrons to the oxygen atom of the CO2, thereby activating the CO2 molecule and facilitating adsorption. Sn0 particles functioned as electron transport bridges, enabling the further transfer of photogenerated electrons to the Sx2− ions, consequently reducing the adsorption energy barrier and accelerating the reaction rate. In a parallel investigation, Li et al.106 explored variations in the Sn precursor's valence state (Sn2+ and Sn4+) to induce S-vacancies in the crystal structure. Based on their investigation, the Sn2+ precursor yielded a higher S-vacancy content than the Sn4+ precursor, with a determined vacancy ratio (via EPR analysis) of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for Sn2+
1 for Sn2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn4+. This increase in S-vacancy quantity resulted in a band position shift, as illustrated in Fig. 4(e), leading to a more potent CO2 reduction capability. Photocatalytic CO2 reduction revealed a 1.47 times enhancement with the Sn2+ precursor compared with Sn4+, attributed to the heightened S-vacancy concentration (see Fig. 4(f)). The enhanced photocatalytic performance following an increase in S-vacancy concentration can be attributed to several factors, including improved light absorption and increased efficiency in the separation of photogenerated charge carriers. Beyond S-vacancies, other vacancy types, such as Sn–S vacancies and S–Sn–S vacancies, may exist, imparting distinct effects on the physical properties and photocatalytic performance of SnS2. Guo et al.143 addressed these defects by introducing a specific amount of 2,2′-bipyridine during the hydrothermal reaction. Their research has confirmed several phenomena directly correlated with the improvements in photocatalysis. The presence of S-vacancies and S–Sn–S vacancies could decrease the band gap energy, resulting in an upward shift of the valence and conduction bands. This shift facilitates a stronger reduction ability, enabling more efficient reactions with O2 molecules. Furthermore, the S–Sn–S vacancy demonstrates the highest adsorption energy, followed by Sn–S and S vacancies, respectively. This trend is also reflected in the electron transfer ability, with the S–Sn–S vacancy exhibiting an electron transfer ability 4.7 times higher than that of S vacancy. These findings suggest that the presence of S–Sn–S vacancies is more favorable for improving photocatalytic performance, a conclusion also supported by Guo et al.143 for phenol decomposition (Table 2).
Sn4+. This increase in S-vacancy quantity resulted in a band position shift, as illustrated in Fig. 4(e), leading to a more potent CO2 reduction capability. Photocatalytic CO2 reduction revealed a 1.47 times enhancement with the Sn2+ precursor compared with Sn4+, attributed to the heightened S-vacancy concentration (see Fig. 4(f)). The enhanced photocatalytic performance following an increase in S-vacancy concentration can be attributed to several factors, including improved light absorption and increased efficiency in the separation of photogenerated charge carriers. Beyond S-vacancies, other vacancy types, such as Sn–S vacancies and S–Sn–S vacancies, may exist, imparting distinct effects on the physical properties and photocatalytic performance of SnS2. Guo et al.143 addressed these defects by introducing a specific amount of 2,2′-bipyridine during the hydrothermal reaction. Their research has confirmed several phenomena directly correlated with the improvements in photocatalysis. The presence of S-vacancies and S–Sn–S vacancies could decrease the band gap energy, resulting in an upward shift of the valence and conduction bands. This shift facilitates a stronger reduction ability, enabling more efficient reactions with O2 molecules. Furthermore, the S–Sn–S vacancy demonstrates the highest adsorption energy, followed by Sn–S and S vacancies, respectively. This trend is also reflected in the electron transfer ability, with the S–Sn–S vacancy exhibiting an electron transfer ability 4.7 times higher than that of S vacancy. These findings suggest that the presence of S–Sn–S vacancies is more favorable for improving photocatalytic performance, a conclusion also supported by Guo et al.143 for phenol decomposition (Table 2).
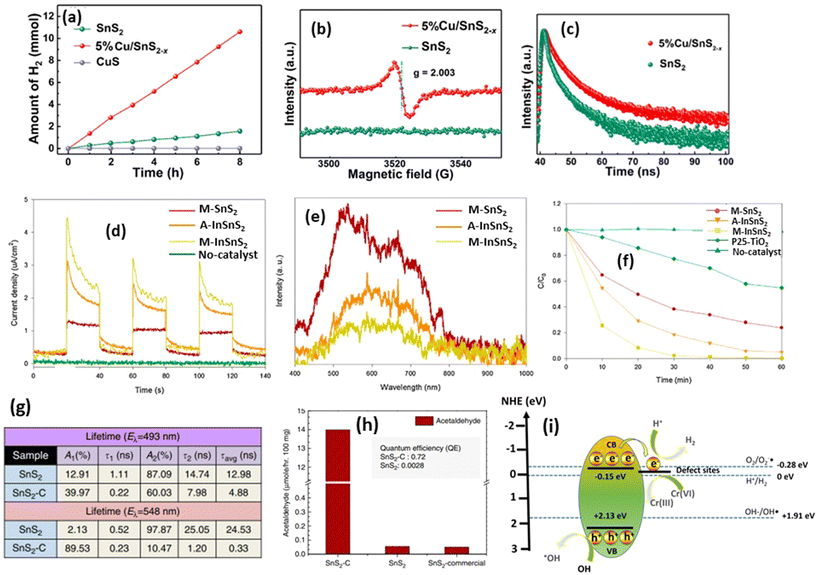 | ||
| Fig. 5 (a) Photocatalytic water reduction capability of SnS2, CuS, and Cu-doped SnS2. (b) EPR spectra of SnS2 and Cu-SnS2, and (c) time-resolved photoluminescence spectra of SnS2 and Cu-doped SnS2.97 (Reproduced from ref. 97 with permission from the Royal Society of Chemistry, copyright 2023.) (d) Photocurrent measurements of In3+-doped SnS2. (e) Photoluminescence measurement of In3+-doped SnS2. (f) Cr(VI) photodegradation by using In3+-doped SnS2.128 (Reproduced from ref. 128 with permission from Elsevier, copyright 2017.) (g) Charge life-time of C-doped SnS2. (h) Photocatalytic CO2 reduction into acetaldehyde by using carbon-doped SnS2.67 (Reproduced from ref. 67 with permission from Springer Nature, copyright 2018.) (i) Photocatalytic reaction mechanism of S-vacancy on the SnS2 structure. | ||
Doping in SnS2 structures, not only within the transition metal family but also through carbon infusion, has been demonstrated to markedly enhance photocatalytic efficacy. In a study by Shown et al.,67 carbon-doped SnS2 was investigated for its capacity to catalyze CO2 reduction to acetaldehyde gas under 300 W halogen irradiation. The carbon doping regimen involved the use of L-cystine as both a sulfur and carbon source. The results revealed that carbon doping induced macrostrain within the SnS2 lattice, leading to distinct photophysical properties. Moreover, carbon doping facilitated the adsorption of CO2 molecules on the surface, with a relatively low dissociation barrier observed. In Fig. 5(g), the observed average lifetimes for SnS2–C are 4.88 and 0.33 ns, markedly shorter than the 12.98 and 24.53 ns for SnS2 at 493 and 548 nm, respectively. This decrease in lifetime in SnS2–C implies the presence of a nonradiative pathway, characterized by the delocalization of electrons from SnS2 to C, thereby facilitating effective carrier separation. Additionally, the resulting carbon-doped SnS2–C exhibited smaller nanosheets composed of only a few atomic layers, thereby reducing the charge diffusion times compared with pure SnS2. These findings collectively underscore the exceptional quantum efficiency of carbon-doped SnS2, which demonstrated an improvement of over 257 times compared with pristine SnS2. This enhanced quantum efficiency manifested as a remarkable increase in acetaldehyde production on carbon-doped SnS2, outperforming pure SnS2 by 228.47 times (Fig. 5(h)).
Based on the results, structural modification can enhance photocatalytic performance by several factors including S-vacancy generation, strain engineering, and ion trapping which successfully impeded the recombination of electrons and holes through an electron trap. However, since the majority of the publications state that the S-vacancy was the most responsible species for improving the photocatalytic reaction, therefore the photocatalytic mechanism of SnS2's structural modification can be generally explained as follows (Fig. 5(i)): upon exposure to light, electrons from the valence band become excited to the conduction band of SnS2. Without a vacancy state, the electron would easily recombine with holes in the valence band. However, due to the presence of a vacancy state below the conduction band of SnS2, electrons tend to be trapped, providing sufficient time to initiate photocatalytic reactions. These reactions have potential applications in various fields, including hydrogen production, wastewater treatment, and many others.
All these structural modification strategies exhibit varying degrees of improvement in photocatalytic applications, as summarized in Table 2. Among these strategies, the carbon doping of SnS2 emerges as one of the most promising approaches, showing an enhancement ability over 228.47 times greater than pristine SnS2 in the reduction of CO to acetaldehyde.67 Additionally, In3+ doping in the SnS2 structure demonstrates a superior photocatalytic performance for wastewater treatment, exhibiting an improvement degree approximately 39.4 times higher than pristine SnS2.128 This suggests that In3+ doping enhances the charge separation efficiency more effectively than other cation doping methods and direct S-vacancy formation. However, the underlying mechanisms of this phenomenon remain unclear, as each doping process generates S-vacancies in the system, and the role of the dopant itself is not fully understood. Therefore, one plausible explanation for this phenomenon may lie in the variation in vacancy content and surface properties of the SnS2 after doping, including an increased surface area and other factors.
3.2 Morphological manipulation
Beyond the structural factor, the photocatalytic activity of SnS2 is also influenced by its surface properties. These properties are intricately linked to changes in morphology, impacting the surface area, the active facets, and the reaction process for photocatalytic reactions. Researchers have explored various SnS2 morphologies, each demonstrating distinct photocatalytic performances. These morphologies encompass flower-like structures, nanosheets, nanoparticles, quantum dots, and more.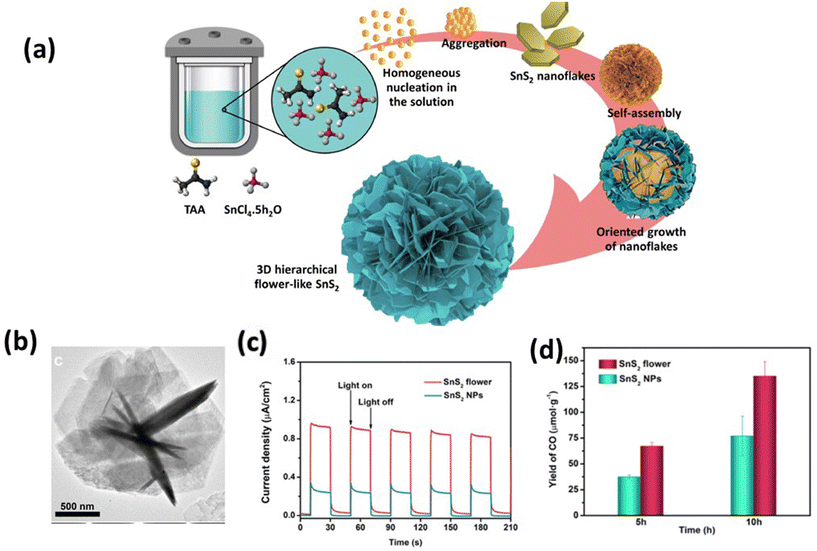 | ||
| Fig. 6 (a) Formation of SnS2 rose flower-like morphology.155 (Reproduced from ref. 155 with permission from Elsevier, copyright 2021.) (b) TEM images of an individual SnS2 flower. (c) Transient photocurrent and (d) comparative CO formation yield of the SnS2 flower and SnS2 NPs within the reaction times of 5 and 10 h, respectively.135 (Reproduced from ref. 135 with permission from the American Chemical Society, copyright 2021.) | ||
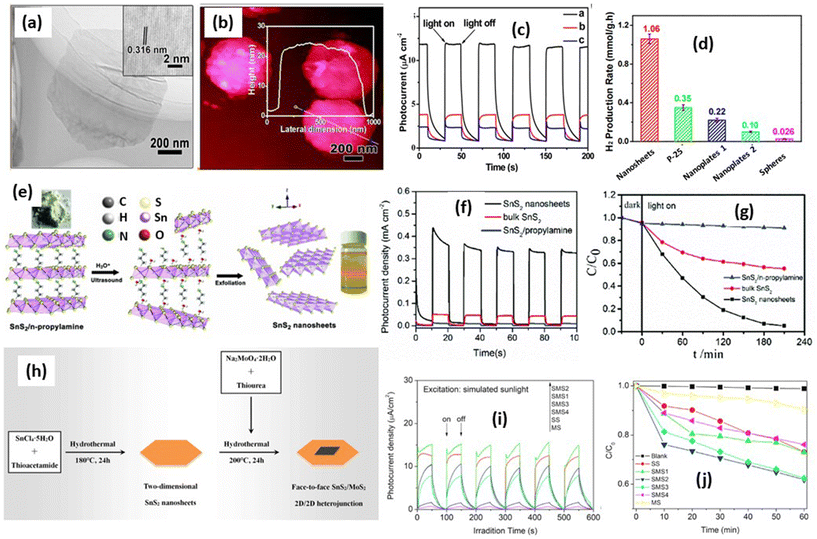 | ||
Fig. 7 (a) TEM image of an individual nanosheet. Inset is an HRTEM image. (b) High-magnification AFM image and corresponding height profile.45 (c) The photoelectrochemical response of the samples at 0.8 V versus SCE electrode under 300 W Xe lamp illumination (λ > 420 nm). (Reproduced from ref. 45 with permission from the American Chemical Society, copyright 2014.) (d) Comparison of the photocatalytic hydrogen production rate of the samples. (e) Schematic illustration of the exfoliation of bulk SnS2/n-propylamine into few-layer SnS2 nanosheets. (f) Chronoamperometry at 0.6 V vs. Hg/Hg2SO4. (g) Photocatalytic reduction of 100 mg L−1 Cr(VI) with (1) the bulk SnS2/n-propylamine hybrid, (2) bulk SnS2, and (3) SnS2 nanosheets.163 (Reproduced from ref. 163 with permission from the Royal Society Chemistry, copyright 2019.) (h) A schematic drawing of the synthetic process of SnS2/MoS2. (i) Photocurrent and (j) photocatalytic activities of SS (SnS2), SMS1 (SnS2/MoS2 (Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn = 1%)), SMS2 (SnS2/MoS2 (Mo Sn = 1%)), SMS2 (SnS2/MoS2 (Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn = 2.5%)), SMS3 (SnS2/MoS2 (Mo Sn = 2.5%)), SMS3 (SnS2/MoS2 (Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn = 5%)), SMS4 (SnS2/MoS2 (Mo Sn = 5%)), SMS4 (SnS2/MoS2 (Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn = 75%)) and MS (MoS2) for methylene blue decomposition under UV-vis irradiation.162 (Reproduced from ref. 162 with permission from Elsevier, copyright 2019.) Sn = 75%)) and MS (MoS2) for methylene blue decomposition under UV-vis irradiation.162 (Reproduced from ref. 162 with permission from Elsevier, copyright 2019.) | ||
Another report from Liu et al.163 reported the formation of 2D nanosheets by the sonication of SnS2/n-propylamine in HNO3 solution to exfoliate the bulk SnS2 into SnS2 nanosheets, as shown in Fig. 7(e). The exfoliation of inorganic (SnS2)–organic hybrids that are composed of few- or single-layered inorganic targeting slabs sandwiched by a single-layer organic molecule via coordinated bonds. The inserting organic molecule can be removed by the ion exchange method. In this research, they also confirmed the improvement of the charge carrier lifetime investigated by photocurrent measurement compared with bulk materials as shown in Fig. 7(f). Moreover, the charge carrier lifetime was observed to be around 0.78 ns which is almost 10 times higher than bulk SnS2. The Cr(VI) removal under visible light irradiation shows a significant improvement compared with bulk SnS2 (Fig. 7(g)) with a degradation rate about 0.01436 min−1 which is 6 times higher than SnS2 bulk materials. It indicates that the improvement of charge carrier separation from SnS2 nanosheets has a direct correlation with the photocatalytic performance. Moreover, since SnS2 nanosheets are a layered structure, the heterostructure of SnS2 with other 2D materials was also interesting and has been explored by many researchers. For example, Zhang et al.162 have prepared a 2D heterojunction SnS2/MoS2 photocatalyst for methylene blue degradation. In this work, they prepared two-step hydrothermal reactions, as shown in Fig. 7(h). At the initial stage the nanolayer of SnS2 was prepared, and the MoS2 layers grew on the SnS2 surface using a hydrothermal reaction. The formation of a 2D heterostructure of SnS2/MoS2 nanosheets can increase the charge separation efficiency, which leads to an increase in the photocurrent signal, as shown in Fig. 7(i). The photocurrent density of SnS2/MoS2 was 15.20 mA cm−2 in the stable second cycle, which exhibited an 8.8 times enhancement compared with that of SnS2 (∼1.55 mA cm−2) and a 23.1 times improvement compared with that of MoS2 (∼0.63 mA cm−2). This phenomenon occurred due to the improvement of the charge separation efficiency due to the charge transfer between MoS2 and SnS2, which can prolong the charge carrier lifetimes. The photocatalytic activity is also directly correlated with the photocurrent result as shown in Fig. 7(j). The best sample SnS2/MoS2 demonstrated an enhanced photocatalytic rate of 81% compared with SnS2.
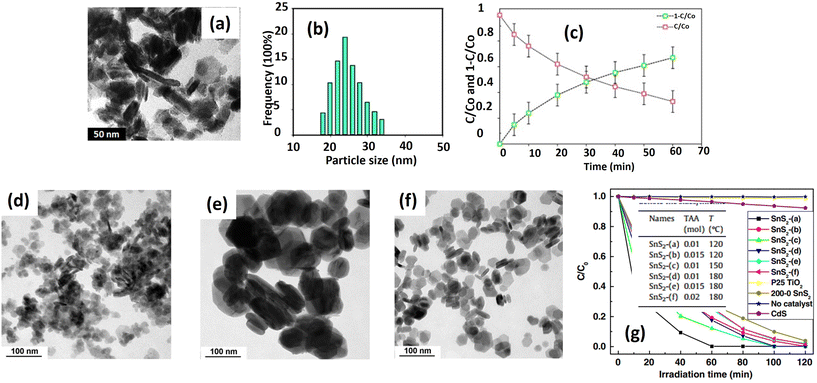 | ||
| Fig. 8 (a) TEM image and (b) particle size distribution of SnS2 NPs; (c) the photodegradation of ENRO by SnS2 NPs under visible light.41 (Reproduced from ref. 41 with permission from Elsevier, copyright 2015.) TEM images of SnS2 prepared with (d) 0.01 mol of TAA at 120 °C; (e) 0.01 mol of TAA at 180 °C; and (f) 0.02 mol of TAA at 180 °C; (g) photocatalytic activities of SnS2, flower-like CdS and P25 TiO2 in degrading MO in distilled water under visible light (420 nm) irradiation.105 (Reproduced from ref. 105 with permission from Elsevier, copyright 2011.) | ||
Zhang et al.105 conducted a more detailed investigation into SnS2 nanoparticle formation and its photocatalytic ability. They controlled the particle diameter and specific surface area of the SnS2 particle by adjusting parameters such as sulfur source concentration and reaction temperature during the hydrothermal process. Increasing the reaction temperature resulted in larger particle sizes, as depicted in Fig. 8(d) and (e). For instance, particles prepared at 180 °C had an average size of around 142 nm, while those from the 120 °C hydrothermal reaction were about 23 nm in size. Conversely, higher sulfur source concentrations led to smaller particle sizes, illustrated in Fig. 8(e) and (f). For instance, the particle size decreased to 46 nm when the sulfur source concentration was increased from 0.01 mol to 0.02 mol. Higher reaction temperatures promote lower viscosity and enhance the diffusion of constituent Sn4+ and S2− ions. Moreover, they accelerate Ostwald ripening rates, resulting in larger particle sizes.105
On the other hand, increasing the initial amount of thioacetamide from 0.01 to 0.02 mol releases more S2− ions, saturating the reaction solution further. Consequently, a greater number of SnS2 crystal nuclei form in the initial stages, reducing the available Sn4+ for subsequent crystal growth during the hydrothermal reaction and resulting in smaller particle sizes. Photocatalytic degradation of methyl orange reveals that SnS2 synthesized at 120 °C exhibits superior performance compared with the sample prepared at 180 °C (Fig. 8(g)). The variation in particle size accounts for the observed differences, significantly influencing the available surface area and creating additional active sites to facilitate photocatalytic reactions. Furthermore, smaller particle sizes commonly lead to defect formation, serving as electron traps that prolong the lifetime of electrons and holes, thus promoting the photocatalytic degradation of methyl orange.
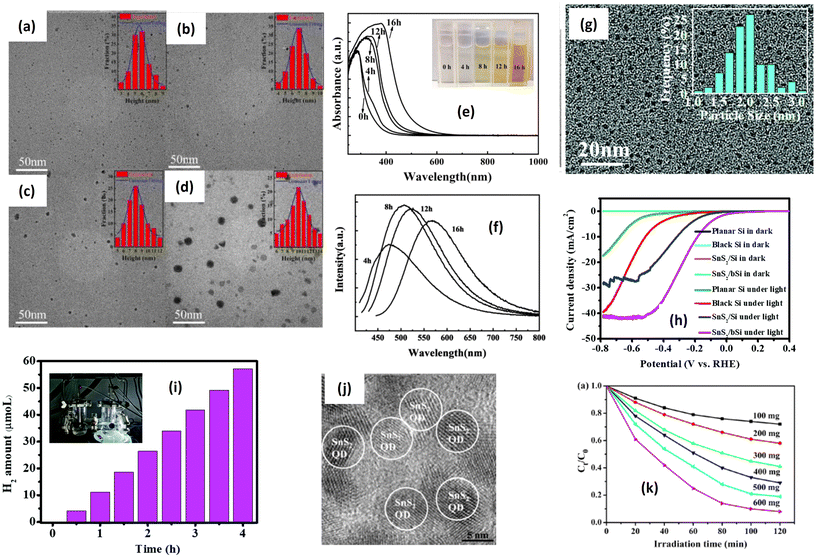 | ||
| Fig. 9 TEM (inset: size distribution) images of SnS2 QDs after heating for (a) 4 h; (b) 8 h; (c) 12 h; and (d) 16 h. (e) Absorption spectra of SnS2 QDs with different heating times. (f) Luminescence analysis of SnS2 QDs with different heating times.169 (Reproduced from ref. 169 with permission from Elsevier, copyright 2023.) (g) TEM images of as-synthesized SnS2 QDs. (h) Lateral size distribution of SnS2 QDs;173 (reproduced from ref. 173 with permission from the Royal Society of Chemistry, copyright 2019). (i) The calculated photocurrent density under various wavelengths; (j) HR-TEM images of SnS2 QDs; (k) effect of the dosage of the SnS2 QD photocatalyst on the photocatalytic reduction of 100 mg L−1 Cr(VI) under visible-light irradiation (λ = 420 nm).47 (Reproduced from ref. 47 with permission from Elsevier, copyright 2016.) | ||
Interestingly, the UV-Vis absorbance of SnS2 quantum dots largely shifted to the larger wavelength, indicating the narrower band gap value after increasing the particle size (Fig. 9(e)). This behavior is a typical characteristic of quantum dot particles where the band gap energy is greatly affected by size. Quantum-sized particles have a higher surface area-to-volume ratio, which increases the probability of photon absorption; moreover, quantum-sized particles often exhibit higher charge carrier mobility due to their unique electronic structure and reduced dimensionality. Enhanced mobility facilitates the efficient separation and migration of photogenerated electron–hole pairs, which are essential for driving redox reactions in photocatalysis. The luminescence analysis (Fig. 9(f)) has also confirmed the emission shift because of electron excitation. The significant shift in the band gap with a slight increase in size is one of the unique features of quantum dots due to the quantum confinement effect, which is beneficial for luminescence applications. On the other hand, Wang et al.173 reported the fabrication of a Si-photoanode incorporating SnS2 quantum dots for the hydrogen evolution reaction. In Fig. 9(g), the TEM image displays the particle distribution of SnS2 quantum dots, with an average size of approximately 2 nm. Fig. 9(h) illustrates the polarization curves of planar Si, SnS2/pSi, black Si, and SnS2/bSi samples. All the Si electrodes exhibit noticeable current responses under illumination, contrasting with dark conditions. Additionally, the SnS2/bSi photocathode demonstrates a maximum photocurrent of ∼41 mA cm−2 at approximately −0.51 V, attributed to the light-harvesting nanostructure of black Si and the active sites of the SnS2 catalyst. The hydrogen evolution reaction (Fig. 9(i)) indicates a hydrogen amount of about 55 μmol L−1 after irradiation. The photocatalytic performance of SnS2 quantum dots has been also reported by Tu et al.47 for Cr(VI) reduction under visible light irradiation. The quantum size SnS2 has been successfully prepared for hydrothermal reaction with L-cystine as a particle-controlling agent. SnS2 quantum dots with a size of 6.32 nm have been obtained, as shown in Fig. 9(j). The photocatalytic performance of SnS2 quantum dots also shows good potential for wastewater treatment (Fig. 9(k)). Comparing various morphologies, it is anticipated that the most suitable morphology for photocatalytic applications can be identified. Different morphologies can be achieved by adjusting several parameters during the synthesis process. As shown in Table 3, diverse morphologies, including flower-like structures, nanolayers, nanoparticles, and quantum dots, have been synthesized using different methods. Upon evaluating the apparent rate constants derived from the catalytic activities, it becomes evident that the flower-like structure exhibits the most effective photocatalytic reaction, with a degradation rate of approximately 0.0692 min−1, followed by the nanoparticle morphology with the highest observed degradation rate of about 0.0594 min−1. The superior photocatalytic performance of the flower-like morphology is attributed to its large specific surface area, excellent interfacial contact, and rapid electron transfer.157 The enhanced photocatalytic properties of the flower-like structure can be elucidated as follows: the abundant petal-like structures provide more surface area for photochemical reactions to occur upon irradiation and interaction with chemicals, while the arrangement of flower-like structures traps light, thereby increasing electron–hole generation.174 Furthermore, the open and porous nature of flower-like structures facilitates a better mass transfer of reactants and products,175 enhancing accessibility to active sites and promoting more effective contact between the photocatalyst and target pollutants in the surrounding environment.
| Morphology type | Light source | Synthesis method | Photocatalytic testing | Photocatalytic result | Ref. |
|---|---|---|---|---|---|
| SnS2-flower-like | 300 W xenon lamp | Hydrothermal (at 180 °C, 9 h) 80 mL 0.01 M SnCl4·5H2O and L-cysteine | 100 mg L−1 rhodamine B degradation | Degradation rate: 0.0251 min−1 | 50 |
| SnS2 flower-like | 300 W xenon lamp | Solvothermal in ethanol, (180 °C, 12 h) 2 mmol SnCl4·5H2O and 5 mmol CH3CSNH2 (thioacetamide) | 10 ppm rhodamine B degradation | Degradation rate: 0.0692 min−1 | 36 |
| SnS2 flower-like | 400 W metal halide lamp | Hydrothermal (200 °C, 24 h) 10 mmol SnCl4·5H2O, and 3.04 g (40 mmol) thiourea | 10 ppm methylene blue degradation | Degradation rate: 0.0179 min−1 | 176 |
| SnS2 flower-like | 300 W xenon lamp | Hydrothermal (180 °C, 3 days) 100.0 mg SnO2 hollow multi-shelled microspheres and 1.0 g thioacetamide | CO2 reduction | CO production rate 8.91 μmol g−1 h−1 | 135 |
| SnS2-nanosheets | 300 W xenon lamp | Solvothermal in ethanol with acetic acid (180 °C, 12 h) 0.25 mmol SnCl4·5H2O, 0.625 mmol of thioacetamide | Nitrogen fixation | NH4+ yield: 27.5 μmol g−1 h−1 | 101 |
| SnS2-nanosheets | 300 W xenon lamp | Sonication in an ice-water bath for 1 h | 50 mg L−1 Cr(VI) reduction | k Cr(VI) = 0.01436 min−1 | 163 |
| SnS2-nanosheets | 300 W xenon lamp 320 nm filter | Solvothermal in triethylene glycol (220 °C, 12 h). 1 mmol SnCl2·2H2O, 2 mmol TAA and 0.5 g PVP | Hydrogen generation | H2 production rate: 1.06 mmol g−1 h−1 | 45 |
| SnS2-nanosheets | 400 W mercury lamp | Hydrothermal with ethylenediamine (200 °C, 24 h). 3.5 g (10 mmol) of stannic(IV) chloride and 3.04 g (40 mmol) of thiourea | Hydrogen generation | H2 production rate: 0.566 mmol g−1 h−1 | 31 |
| SnS2-nanosheets | 400 W mercury lamp | Hydrothermal with ethylenediamine (200 °C, 24 h). 3.5 g (10 mmol) of stannic(IV) chloride and 3.04 g (40 mmol) of thiourea | Methylene blue degradation | k MB: 0.0178 min−1 | 31 |
| SnS2-nanoparticle | 250 W xenon lamp 420 nm filter | Hydrothermal (130–170 °C). 5 mmol SnCl4·5H2O, 10 mmol thioacetamide | 50 mg L−1 Cr(VI) reduction | k Cr(VI): 0.0394 min−1 | 164 |
| SnS2-nanoparticle | 250 W xenon lamp 420 nm filter | Hydrothermal (140–180 °C). 5 mmol SnCl4·5H2O, 10 mmol thioacetamide | 20 mg L−1 methyl orange degradation | k MO: 0.0594 min−1 | 105 |
| SnS2-nanoparticle | 500 W xenon lamp | Hydrothermal + acetic acid (130–170 °C, 12 h). 0.005 mol SnCl4·5H2O and 0.01–0.02 mol thioacetamide | 10 mL of log phase culture's antibacterial reaction | The bacteria killing ability of 0.11 g mL−1 NPs: 75.28 ± 2.3% for S. aureus (ATCC 25923), 78.95 ± 2.0% for E. coli (ATCC 13534), 81.22 ± 1.1% for E. coli (ATCC 25922) | 41 |
| SnS2 quantum dots | 300 W Xe lamp equipped with a cut-off filter (λ > 420 nm) | Hydrothermal at (160 °C, 16 h) Sn source: SnCl4·5H2O S source: L-cystine modifier | 100 mg L−1 of Cr(VI) reduction | Degradation ability: 92% for 2 h | 47 |
3.3 Heterostructure formation
Heterostructure photocatalysts have garnered widespread acceptance as a highly promising technique for the development of high-performance photocatalysts. The heterostructures can be defined as the combination of photocatalysts with other materials (other photocatalysts or co-catalysts). The key advantage of heterostructure formation lies in the synergistic effects between the different materials, wherein each component mutually reinforces the other, thereby enhancing the overall photocatalytic performance.177,178 As illustrated in Fig. 3(b), the current literature demonstrates that approximately 75% of published works on SnS2-based photocatalysts involve heterostructure formation. Diverse materials, including semiconductors, metals, and 2D materials such as graphene and its derivatives, have been combined with SnS2 and exhibit a significant impact on the photocatalytic properties, such as the improvement of specific surface area, heightened light sensitivity, and enhanced reactivity. Furthermore, a crucial aspect is its ability to mitigate the recombination of electrons and holes through the formation of heterojunctions. A heterojunction is an electrical transfer between two or more materials resulting from differing electronic states and band positions. This phenomenon extends the lifetimes of electrons and holes, providing them with more time to promote redox reactions for photocatalytic reactions. To date, various types of heterojunction formation exhibit distinct characteristics and effects on photocatalytic reactions, including type I, type II, p–n junction, Schottky junction, and Z-scheme, as illustrated in Fig. 10.179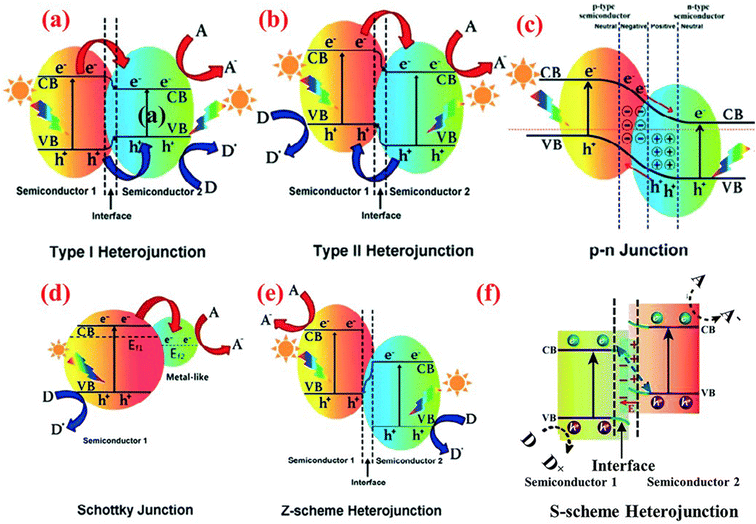 | ||
| Fig. 10 Band structure of various types of heterojunction in a photocatalytic hybrid nanocomposite: (a) type I heterojunction; (b) type II heterojunction; (c) p–n junction; (d) Schottky junction; (e) Z-scheme heterojunction; and (f) S-scheme heterojunction.179 (Reproduced from ref. 179 with permission from the Royal Society of Chemistry, copyright 2021.) | ||
The exploration of Schottky junction processes in SnS2 photocatalysts remains limited in the existing literature, with only a few reports delving into this phenomenon with different types of noble metal including Pt,37 Au,181 and Ag.182 Among other noble metals, gold (Au) has emerged as one of the extensively studied supports for SnS2 photocatalysts. Feng et al.183 reported the deposition of Au on SnS2 nanoflowers prepared via hydrothermal synthesis for methylene blue degradation under visible light irradiation. The transmission electron microscopy (TEM) images in Fig. 11(a and b) reveal a 20 nm size of Au deposition on 3 μm size SnS2 nanoflowers, indicating intimate contact between the Au and SnS2, which enhances the charge transfer efficiency. Fig. 11(c) demonstrates the methylene blue degradation ability of Au-deposited SnS2 with varying Au concentrations, showcasing a significant enhancement in degradation after Au deposition. Specifically, the degradation rate of Au–SnS2 was approximately 3.91 times higher than that of SnS2 nanoflowers alone (Table 4). The pronounced improvement in methylene blue degradation post-Au deposition can be attributed to the enhanced charge carrier efficiency facilitated by the Schottky junction formation between Au and SnS2 (Fig. 11(d)). As the Fermi level of Au is lower than that of SnS2, electron transfer from SnS2 to Au occurs, effectively preventing electron–hole recombination. This allows the generation of radicals necessary for methylene blue molecule dissociation. Charge separation efficiency, typically reflected in the number of mobile electrons on the surface, was assessed through photocurrent measurement (Fig. 11(e)). Mondal et al.184 endeavored to decompose benzylamine using Au–SnS2 nanosheets under solar irradiation. Synthesized via a hydrothermal reaction followed by gold photoreduction using a 400 W xenon lamp, Au-deposited SnS2 nanosheets achieved a conversion rate of approximately 98% under solar light (Fig. 11(f)), surpassing SnS2 and standard P-25 conversion rates of about 20% and 35%, respectively (Table 4).
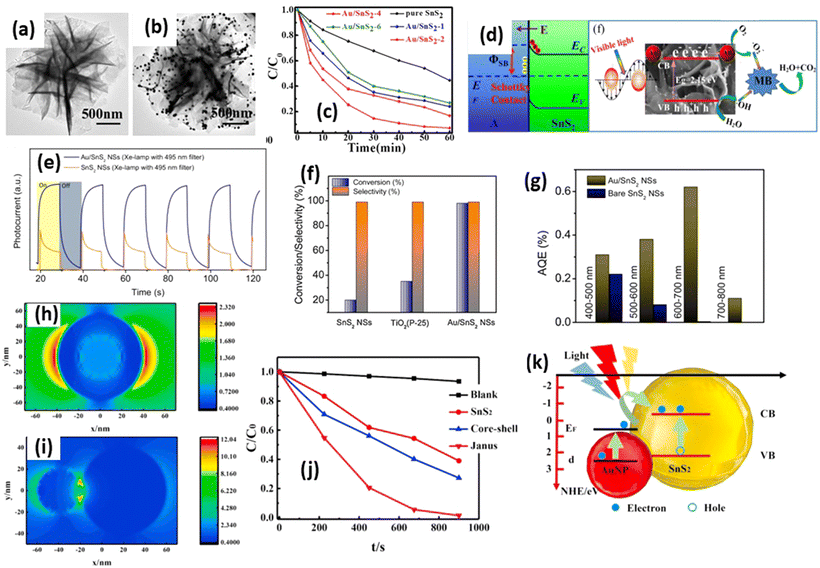 | ||
| Fig. 11 TEM images of (a) SnS2 and (b) Au/SnS2. (c) Methylene blue degradation by Au/SnS2. (d) Illustration of the band alignment formed at the interface between the SnS2 and Au NPs.183 (Reproduced from ref. 183 with permission from Elsevier, copyright 2019.) (e) Transient photocurrent recorded on bare SnS2 and Au/SnS2 NSs using a 400 W Xe lamp (with an attached 495 nm cut-off filter). (f) Comparison plots of benzylamine oxidation efficiency with different catalysts under natural sunlight and ambient conditions for 2 h in open air. (g) Plot of AQE at different wavelength ranges under LED illumination in air at room temperature for bare SnS2 and Au/SnS2.184 (Reproduced from ref. 184 with permission from Elsevier, copyright 2021.) (h) The near-field distribution of the core–shell structure and the (i) Janus structure with Au nanoparticle s, respectively. (j) Degradation curves of methyl orange under visible light. (k) The schematic diagram of the LSPR-enhanced visible light catalysis of SnS2 and the heterostructures with Au nanoparticles.186 (Reproduced from ref. 186 with permission from Elsevier, copyright 2021.) | ||
| Materials | Heterojunction type | Light source | Photocatalytic testing | Photocatalytic performance | Ref. |
|---|---|---|---|---|---|
| Au–SnS2 | Schottky junction | 330 W xenon lamp | 100 mg L−1 rhodamine B degradation | k RhB: 0.0514 min−1. 3.91 times that of SnS2 | 183 |
| Au–SnS2 | Schottky junction | 400 W xenon lamp | Benzylamine oxidation | Conversion: 98%, 4.9 times that of SnS2 | 184 |
| Au–SnS2 | Schottky junction | 250 W xenon lamp | 50 mg L−1 Cr(VI) reduction | k Cr(VI): 0.0234 min−1. 2.07 times that of SnS2 | 73 |
| BiFeO3/SnS2 | p–n junction | 250 W mercury lamp | 15 mg L−1 methylene blue degradation | k MB: 0.0428 min−1. 7.50 times that of SnS2 | 188 |
| BiOI/SnS2 | p–n junction | UV-vis (385–740 nm) | 10 mg L−1 rhodamine B degradation | k RhB 100% within 30 min 3 times that of SnS2 | 187 |
| TiO2/SnS2 | Type I heterojunction | Xe lamp, 100 mW cm−2 | Hydrogen evolution reaction (HER) | H2 yield: 652.4 μmol g−1 h−1 7.51 times that of SnS2 | 190 |
| ZnIn2S4/SnS2 | Type II heterojunction | 300 W xenon lamp | 50 mg L−1 Cr(VI) reduction | k Cr(VI): 0.01273 min−1 2.2 times that of SnS2 | 196 |
| ZnWO4/SnS2 | Type II heterojunction | Solar hotspot 5 kW h m−2 day−1 | 50 mg L−1 Cr(VI) reduction | k Cr(VI) = 0.061 min−1 3.58 times that of SnS2 | 104 |
| ZnWO4/SnS2 | Type II heterojunction | Solar hotspot 5 kW h m−2 day−1 | 20 mg L−1 tetracycline | k TC = degradation rate: 0.023 min−1 3.28 times that of SnS2 | 104 |
| In2S3/SnS2 | Type II heterojunction | 300 W xenon lamp | 10 mg L−1 rhodamine B degradation | k RhB: 0.11716 min−1. 15.3 times that of SnS2 | 192 |
| Ag3PO4/SnS2 | Z-scheme | A 500 W xenon-arc lamp | 10 mg L−1 methylene blue | k MB = 0.063 min−1 7.08 times that of SnS2 | 201 |
| Bi2WO4/SnS2 | Z-scheme | Sunlight | 20 mg L−1 tetracycline degradation | k TC = 0.027 min−1 27 times that of SnS2 | 134 |
| BiOBr/SnS2 | Z-scheme | 400 W xenon lamp | 10 mg L−1 rhodamine B degradation | k RhB = 0.1203 min−1 75 times that of SnS2 | 197 |
| SnO2/SnS2 | S-scheme | 500 W xenon lamp | 10 mg L−1 methyl orange degradation | k MO: 0.01182 min−1. 2.19 times that of SnS2 | 199 |
| ZnIn2S4/SnS2 | S-scheme | 300 W xenon lamp | H2 production | H2 yield: 1.113 mmol g−1 h−1 16.14 times that of ZnIn2S4 | 200 |
| CdS/SnS2 | S-scheme | 300 W xenon lamp | H2 production | H2 yield: 5.18 mmol g−1 h−1 5.95 times that of CdS | 198 |
To investigate the photocatalytic enhancement in Au–SnS2 nanosheets, the apparent quantum efficiency (AQE) was examined at different wavelengths (400–500 nm, 500–600 nm, 600–700 nm, 700–800 nm) (Fig. 11(g)). Compared with SnS2 nanosheets, Au–SnS2 exhibited a 1.2 to 3 times higher AQE at 400–500 nm and 500–600 nm wavelengths, attributed to improved electron–hole separation mediated by Au nanoparticles (Fig. 11(g)). Notably, at 600–700 nm, where SnS2 excitation is minimal, Au-deposited SnS2 exhibited a strong AQE, indicating an efficient ‘hot-electron’ injection mechanism from Au nanoparticles to SnS2 nanosheets.185 Furthermore, the formation of Au–SnS2 is influenced by Au positioning relative to the SnS2 photocatalyst. Fu et al.186 investigated different composite structures of Au–SnS2 for methyl orange degradation under visible light. Two structures were examined: core–shell and Janus structures. Near-field distribution analysis (Fig. 11(h and i)) revealed a low light enhancement effect in the core–shell structures, leading to diminished photocatalytic ability. Conversely, Janus structures exhibited a significant near-field enhancement, resulting in superior methyl orange degradation (Fig. 11(j)). Among the images, the Janus structures exhibit the highest reaction rate (4.0 × 10−3 s−1), followed by the core–shell structures (1.2 × 10−3 s−1). A significant increase in photocatalytic activity, approximately four times greater than that of sole SnS2 nanoparticles (1.0 × 10−3 s−1), is observed in the Janus structures. This enhancement is attributed to the improved extinction effect. Photoluminescence lifetime analysis revealed longer electron lifetimes in nanoparticle structures (1.74 ns) than Janus structures (1.53 ns) compared with core–shell structures (1.16 ns), indicating an enhanced photocatalytic efficiency. Fig. 11(k) illustrates the proposed mechanism of surface resonance analysis of Janus Au–SnS2; due to the near-field enhancement, electron excitation becomes more pronounced in SnS2 nanoparticles, significantly enhancing photocatalysis. Besides the enhanced optical fields, the localized surface plasmon resonance (LSPR) induces electron injection from AuNPs to the conduction band edge of SnS2, further contributing to the photocatalytic process. Among these mechanisms, the excited electrons in the conduction band edge of the SnS2 nanoparticles facilitate the generation of reactive oxygen radicals, including superoxide, hydroxyl, and singlet oxygen, through a series of oxidation–reduction processes in the solution. These oxygen radicals exhibit high activity and play a crucial role in degrading methyl orange molecules.186
Wang et al.187 confirmed the p–n junction formation between BiOI and SnS2 for rhodamine B degradation under visible light irradiation, as illustrated in Fig. 12(a). Notably, SnS2 has a layered morphology, while BiOI comprises the nanoparticles that disperse on the top of the SnS2 layer. The conduction band of SnS2 is situated at −0.96 V, with a valence band at 1.5 V. Conversely, BiOI exhibits a valence band at +0.53 V and a conduction band at −1.29 V. Upon electrical contact, band alignment ensues, facilitating electron and hole transfer between the BiOI and SnS2 until equilibrium is attained, with the Fermi level equalized. Upon irradiation, electron–hole pairs form in the conduction and valence bands of SnS2 and BiOI. Due to the p–n junction, electrons readily transfer from BiOI to SnS2, while holes undergo the reverse process. This reaction process enhances electron mobility and correlates directly with photocatalytic efficiency. Photocurrent analysis (Fig. 12(b)) demonstrates increased electron densities of BiOI/SnS2 heterostructures compared with BiOI and SnS2. The active electrons in the conduction band react with O2 to form O2˙−, while the holes react with OH− to produce OH˙, facilitating rhodamine B degradation. Rhodamine B degradation analysis (Fig. 12(c)) indicates a significant improvement after p–n junction formation, three times higher than that observed with SnS2 alone (Table 4), a finding consistent with the photocurrent analysis.
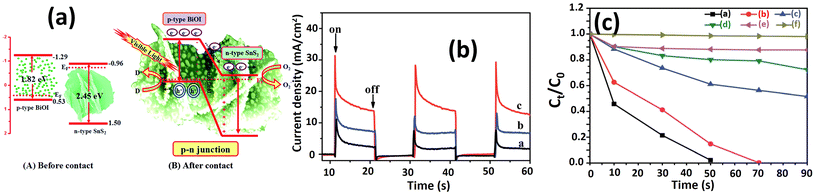 | ||
| Fig. 12 (a) Schematic diagrams illustrating the energy bands of p-BiOI and n-SnS2 before contact, the formation of a p–n junction, and the equilibrium energy band diagram. Additionally, the transfer of photoinduced electrons from p-BiOI to n-SnS2 under visible-light irradiation is depicted; (b) comparison of the transient photocurrent response of SnS2 (black), BiOI (blue), and BiOI/SnS2 (red) in 0.1 M Na2SO4 aqueous solution under visible-light irradiation; (c) visible-light photocatalytic activities: (black) BiOI/SnS2, (red) BiOI, (blue) SnS2, (green) P25, (violet) adsorption in the dark, and (yellow) degradation of 10 mg rhodamine B without photocatalyst under visible light (λ > 420 nm) irradiation.187 (Reproduced from ref. 187 with permission from the Royal Society of Chemistry, copyright 2015.) | ||
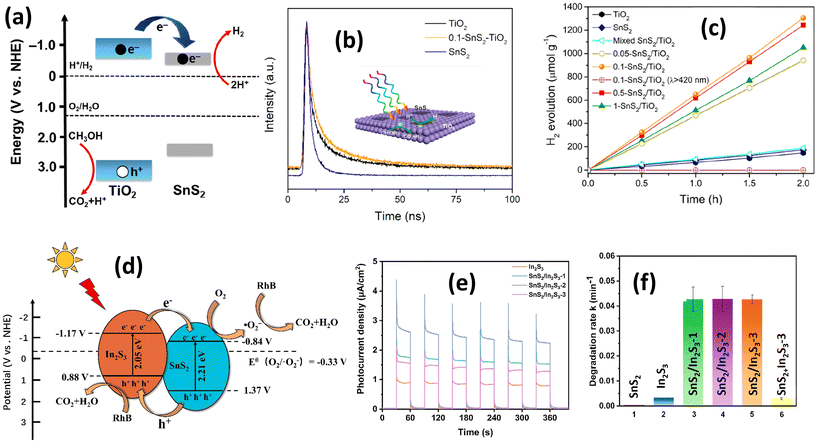 | ||
| Fig. 13 (a) Corresponding band energy diagrams and charge transfer path; (b) time-resolved photoluminescence decay spectra of TiO2, SnS2, and 0.1-SnS2/TiO2; (c) photocatalytic H2 evolution curves with different catalysts.190 (Reproduced from ref. 190 with permission from Elsevier, copyright 2019.) (d) Schematic diagram of the possible photocatalytic degradation mechanism of RhB by the SnS2/In2S3 heterostructure under visible light irradiation; (e) transient photocurrent responses of In2S3 and SnS2/In2S3 heterostructures; (f) the apparent rate constants of SnS2/In2S3.192 (Reproduced from ref. 192 with permission from Elsevier, copyright 2022.) | ||
Differing from type I heterojunctions, type II heterojunctions involve semiconductor photocatalysts where two types of semiconductor exhibit distinct band structure levels. One semiconductor possesses a more positive valence band, while the other semiconductor has a more negative conduction band. In this arrangement, electrons flow from the semiconductor with the more negative conduction band position, while holes flow from the semiconductor with the more positive valence band. This electrical transfer scheme helps prevent electron and hole recombination. An example of a type II heterojunction formation of SnS2 was reported by Zhang et al.192 investigating the formation of SnS2 with In2S3. Fig. 13(d) illustrates the catalytic reaction mechanism of the SnS2/In2S3 heterostructure for rhodamine B degradation under visible light irradiation. Upon irradiation, electron transfer occurs from the conduction band of In2S3 to the conduction band of SnS2 due to the more negative level of the conduction band position of In2S3, while hole transfer operates oppositely due to the greater positivity of the valence band level of SnS2. Similar to a heterojunction type I, this charge transfer process between In2S3 and SnS2 significantly enhances charge mobility efficiency, as demonstrated in Fig. 13(e); the photocurrent of the In2S3/SnS2 heterostructure is 3–4 times higher than In2S3 only, which shows a marked improvement in current density after heterojunction formation. RhB degradation testing indicates a significant enhancement in RhB degradation by conducting In2S3/SnS2 heterojunction formation (Fig. 13(f)), where the degradation rate was 99 times and 15 times higher than with SnS2 and In2S3 alone, respectively. The theoretical explanation of the In2S3/SnS2 heterojunction as type II heterojunction formation was confirmed by Wang et al.193 through EPR analysis for detecting superoxide radicals. The lower intensity of the EPR signal of In2S3/SnS2 compared with In2S3 indicates electron transfer from In2S3 to SnS2, confirming type II heterojunction formation. Other materials have also formed type II heterojunctions combined with SnS2, such as g-C3N4,194 ZnWO4,104 SnO2,195 and ZnIn2S4.196
Several studies have confirmed the formation of direct Z-scheme photocatalysts. For instance, Luo et al.15 initiated Z-scheme formation by integrating SnS2 with Ag3PO4 for methyl orange degradation under visible light irradiation. The schematic representation in Fig. 14(a) illustrates the Z-scheme formation of the SnS2/Ag3PO4 heterostructure. The realization of a direct Z-scheme photocatalyst depends on the band positions of each constituent material. In the case of the SnS2/Ag3O4 heterostructure, the Z-scheme configuration is inferred from the disparate band positions of the SnS2 and Ag3PO4.
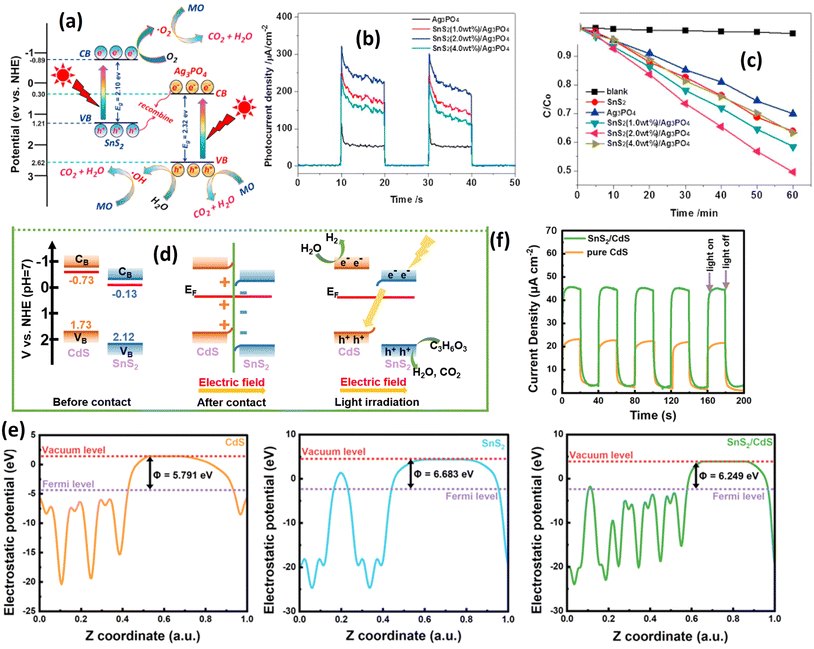 | ||
| Fig. 14 (a) Schematic diagram of the possible photoinduced electron–hole pair separation process and direct Z-scheme photodegradation mechanism of methyl orange over the SnS2/Ag3PO4 heterojunction photocatalyst under visible light irradiation. (b) Photocurrent response over the as-prepared samples under visible light irradiation. (c) Photocatalytic degradation curves for the photodegradation of MO.15 (Reproduced from ref. 15 with permission from Elsevier Ltd.) (d) S-scheme illustration of the possible charge transfer processes in the SnS2/CdS heterostructures; (e) work function analysis of CdS, SnS2 and SnS2/CdS. (f) Photocurrent analysis of CdS and SnS2/CdS.198 (Reproduced from ref. 198 with permission from the Royal Society of Chemistry.) | ||
The formation of a direct Z-scheme photocatalyst relies on the band positions of each material. In the case of the SnS2/Ag3PO4 heterostructure, the existence of the Z-scheme can be inferred from the disparate band positions of each material. The valence band (VB) maximum and conduction band (CB) minimum of SnS2 are about 1.21 eV and −0.89 eV, respectively, while the VB and CB potentials of Ag3PO4 are about 2.62 eV and 0.30 eV, respectively. The CB position of Ag3PO4 is more positive than the standard reduction potential of O2/O2˙− (0.13 eV). The electron from Ag3PO4 is unable to reduce O2 into O2˙− and instead reacts with the hole of the SnS2. Consequently, recombination is prevented, leading to a more efficient charge separation process, as demonstrated by photocurrent analysis. This correlation directly corresponds to the observed improvements in photocatalytic activity (see Fig. 14(b and c)). As mentioned earlier, the Z-scheme can be generated if one of the conduction bands (CBs) of the sample position is more positive than the reduction potential of O2/O2˙− (0.13 eV), thereby preventing the reduction reaction. Other reports also confirm the same understanding about the Z-scheme formation, such as Qiu et al.,197 Li et al.,49 and Kumar et al.134
Slightly different from the Z-scheme, the S-scheme can be generated through the inner electric field phenomenon. This electric field emerges when a charge moves between semiconductors, creating a field within each semiconductor due to differing Fermi energies. Fig. 14(d) illustrates an instance of S-scheme formation between SnS2 and CdS nanoparticles.198 Before contact, SnS2 and CdS exhibit different Fermi energy levels; CdS registers at 5.791 eV (Fig. 14(e)), while SnS2 is at 6.683 eV. Upon interaction, Fermi energy alignment occurs until an equilibrium is established. In this state, the electron density at the SnS2 interface surpasses that of CdS, generating an inner electric field. Consequently, the valence band of CdS bends, and the conduction band of SnS2 shows observable curvature. Upon light irradiation, both SnS2 and CdS generate electron–hole pairs, and the inner electric field facilitates electron transfer from SnS2 to CdS, enhancing the charge separation efficiency. This improvement is supported by photocurrent analysis (Fig. 14(f)). The photocatalytic activity of the S-scheme SnS2/CdS photocatalyst for H2 production, summarized in Table 4, yields 5.18 mmol g−1 h−1 of hydrogen, 5.95 times higher than that of CdS alone. Similar S-scheme SnS2-based photocatalysts have been reported for other materials, including BiOBr,197 SnO2,199 ZnIn2S4.200
Table 4 presents various reports on the photocatalytic performance of SnS2 heterojunction formations. Different strategies yield varying degrees of improvement compared with SnS2 alone. According to current data, Au/SnS2 demonstrates a 3.91-fold enhancement over SnS2. Among the p–n junction formations, BiFeO3/SnS2 exhibits the most significant improvement, with a degradation rate approximately 7.50 times higher than SnS2. Similarly, heterojunctions of type I and type II show comparable enhancement, boasting 7.51 times improvements over SnS2. Notably, Z-scheme configurations outperform other heterojunction types, with BiOBr/SnS2 achieving the highest improvement, nearly 75 times higher than SnS2 alone. This superior enhancement in Z-scheme heterojunctions is attributed to enhanced charge separation efficiency and stronger reduction and oxidation capabilities, resulting in accelerated degradation rates, and increased photochemical reaction yields.
4 Future perspectives
The current strategies for modifying SnS2 have been thoroughly examined, encompassing structural modification, morphological manipulation, and heterojunction formation. Despite the comprehensive elucidation of each photocatalytic advancement mechanism and synthesis technique in this review, challenges persist, hindering the further advancement of SnS2 photocatalysts.In terms of structural modification, it is established that S-vacancies play a pivotal role in enhancing the photocatalytic performance, primarily by improving electron–hole separation efficiency. While the authors attribute this enhancement to electron traps between the valence and conduction bands, empirical evidence remains elusive. Future research endeavors should focus on elucidating the mechanism of the S-vacancy effect on mid-gap formation to substantiate this claim. Additionally, the role of dopants in photocatalytic reactions remains unclear due to their concurrent generation with S-vacancy formation during doping processes. To advance research in this area, techniques minimizing the impact of S-vacancies in doping must be developed to gain a comprehensive understanding of doping phenomena for photocatalytic enhancement.
In morphology engineering, the flower-like morphology has emerged as the most suitable structure for various photocatalytic applications, including wastewater treatment and hydrogen production. Enhanced mass transfer and light harvesting capabilities are key attributes contributing to the photocatalytic performance of flower-like structures. However, challenges in material preparation persist, particularly in hydrothermal synthesis methods, which remain dominant in SnS2 flower-like morphology production. Although hydrothermal synthesis is known for producing flower-like morphologies, it is important to note that other morphologies can also be generated using the same method and precursor. This variability may hinder further development, as precise control over flower-like morphology parameters such as size, petal-like dimensions, and facet arrangements are essential for further research development of the morphological effect on the photocatalytic performance of SnS2. Therefore, for future development, it is necessary to find the key factors which control the growth of morphology through hydrothermal reactions encompassing the precursor, solvent, temperature control, and other factors.
While the proliferation of heterojunction formation is widespread, it does not guarantee a comprehensive understanding of photocatalytic mechanisms. Heterostructure formation, considered one of the most extensively studied systems for SnS2 photocatalysts, relies on the synergistic effects of its constituent materials. Various types of heterostructure have been reported, each with unique characteristics. Among these, the Z-scheme exhibits significant promise for enhancing photocatalytic performance due to its capacity to augment oxidation and reduction potentials for photocatalytic reactions. However, several considerations must be addressed for the optimal utilization of Z-scheme heterojunction photocatalysts. Beyond the reliance on electronic properties for photocatalytic enhancements, factors such as particle interaction and surface optimization are also pivotal. Particle interaction hinges on the specific facets of each particle, as different facets exhibit distinct electronic and adsorption behaviors. Theoretical investigations, particularly those utilizing density functional theory (DFT) calculations, are essential to fully comprehend these interactions. Additionally, optimizing the surface properties of Z-scheme particles for specific applications, including increasing the surface area and modifying the charge, is imperative for optimizing the adsorption process and enhancing active sites for photocatalytic reactions. To address these issues, for future research directions, it is interesting to employ the comprehensive investigation of photocatalyst design while considering the structural design alongside surface optimization and Z-scheme heterojunction formation.
We have thoroughly discussed the current results and outlined future perspectives for three distinct strategies. Building on this understanding, future research endeavors will focus on integrating these strategies into a unified photocatalyst system. The S-vacancy SnS2, characterized by a prolonged charge carrier lifetime, can be elaborately engineered into a flower-like morphology with enhanced mass transfer, light harvesting capabilities, and a large surface area. Concurrently, nanoparticles with suitable band gaps, such as CdS and Ag3VO4, can be selectively deposited atop the flower-like SnS2 system for generating a Z-scheme photocatalyst, as illustrated in Fig. 14. The successful amalgamation of these components is anticipated to yield a photocatalyst system of exceptional performance, aligning with industrial standards.
Furthermore, it is imperative to address a critical aspect pertaining to the chemical stability of SnS2 itself, given its susceptibility to sulfur atom replacement by oxygen upon exposure to air. Factors influencing stability, including the synthesis process and the photocatalyst environment, necessitate meticulous examination. Such analysis assumes paramount importance for practical applications, wherein long-term stability is imperative for industrial viability.
5 Summary
For summarizing this review, Fig. 15 was used for understanding the current findings and future directions. In this review, we have summarized recent strategies for improving the photocatalytic performance of SnS2, focusing on three fundamental approaches: crystal structure engineering, morphology engineering, and heterojunction formation. SnS2-based photocatalysts have been utilized in various applications, including CO2 reduction, H2 production, nitrogen fixation, antibacterial activity, air purification, and wastewater treatment technologies.At the current stage, the structural engineering of SnS2 has been carried out through two methods: vacancy generation and doping processes. These approaches have successfully increased the photocatalytic performance of SnS2. The fundamental phenomenon observed in S-vacancy generation and doping processes is the ability to decrease the recombination of electrons and holes, thereby facilitating redox reactions. Additionally, altering crystal structures results in a decrease in the crystallite size of SnS2, which is beneficial for increasing the surface area and the number of active sites for chemical reactions.
Various morphologies of SnS2, such as flower-like structures, nanoparticles, nanosheets, and quantum dots, exhibit distinct effects on the photocatalytic performance. Flower-like structures, with their high specific surface area and unique morphology, demonstrate superior light harvesting ability and increased active sites compared with nanosheet and nanoparticle morphologies. Although nanosheets have a smaller surface area than flower-like structures, they facilitate the efficient migration of photogenerated electrons and holes to reaction sites, thereby enhancing the photocatalytic efficiency. Conversely, nanoparticle morphology is influenced by the size of the nanoparticles; smaller nanoparticles also contribute to an improved specific surface area, thereby enhancing photocatalytic activity.
Heterojunction formation is by far the most effective strategy for improving photocatalytic reactions, as it enables the generation of synergistic effects between SnS2 and other materials, significantly enhancing the photocatalytic efficiency. Among various types of heterojunction, Z-scheme photocatalytic reactions are the most promising strategy for improving the photocatalytic performance of SnS2. This is due to their stronger oxidation and reduction capabilities, coupled with highly efficient charge carrier separation efficiency.
For further development, several aspects are still limited in current literature. Firstly, there is a lack of research on the application of SnS2 for antibacterial activity, N2 fixation, and air purification. Therefore, future research should focus more on investigating these types of photocatalytic application. Secondly, there is uncertainty regarding the effect of dopants on vacancy generation. Since the doping process directly leads to vacancy formation, the individual impact of each dopant is not well understood. To address this issue, both synthesis processes and theoretical approaches should be conducted simultaneously to comprehensively understand the doping effect on improving charge carrier separation in SnS2. Moreover, the growth of morphology is not well understood. Therefore, for future development, it is necessary to find the key factor which controls the growth of morphology through hydrothermal reactions encompassing precursor, solvent, temperature control, and other factors.
However, one of the most crucial areas of research is the design of the ideal photocatalyst based on the aforementioned findings. Developing a Z-scheme SnS2-based photocatalyst with a high specific surface area and efficient charge carrier separation is essential. One promising technique is to combine SnS2 flower-like structures with very small nanoparticles, allowing the particles to attach to the flower-like structures, thereby improving the light harvesting process and charge carrier separation efficiency. Lastly, the stability of SnS2 is a critical consideration. Since SnS2 is prone to oxidation, stability studies must be conducted to meet industrial standards.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the research grant of The University of Suwon in 2023.References
- R. Yang, Y. Fan, Y. Zhang, L. Mei, R. Zhu, J. Qin, J. Hu, Z. Chen, Y. Hau Ng, D. Voiry, S. Li, Q. Lu, Q. Wang, J. C. Yu and Z. Zeng, Angew. Chem., Int. Ed., 2023, 62, 1–29 Search PubMed.
- W. Peng, Y. Li, F. Zhang, G. Zhang and X. Fan, Ind. Eng. Chem. Res., 2017, 56, 4611–4626 CrossRef CAS.
- V. Dhiman and N. Kondal, Phys. B, 2022, 628, 413569 CrossRef CAS.
- W. Yu, X. Chen, W. Mei, C. Chen and Y. Tsang, Appl. Surf. Sci., 2017, 400, 129–138 CrossRef CAS.
- R. A. Geioushy, I. M. Hegazy, S. M. El-Sheikh and O. A. Fouad, J. Environ. Chem. Eng., 2022, 10, 107337 CrossRef CAS.
- Y. J. Huang, L. M. Lyu, C. Y. Lin, G. C. Lee, K. Y. Hsiao and M. Y. Lu, ACS Omega, 2022, 7, 2217–2223 CrossRef CAS PubMed.
- B. Chen, Y. Meng, J. Sha, C. Zhong, W. Hu and N. Zhao, Nanoscale, 2018, 10, 34–68 RSC.
- Y. Lin, P. Ren and C. Wei, CrystEngComm, 2019, 21, 3439–3450 RSC.
- E. C. Cho, C. W. Chang-Jian, J. H. Zheng, J. H. Huang, K. C. Lee, B. C. Ho and Y. S. Hsiao, J. Taiwan Inst. Chem. Eng., 2018, 91, 489–498 CrossRef CAS.
- W. Ho, J. C. Yu, J. Lin, J. Yu and P. Li, Langmuir, 2004, 20, 5865–5869 CrossRef CAS PubMed.
- X. Zhang, K. Zhu, C. Xie and P. Yang, Carbon, 2024, 220, 118884 CrossRef CAS.
- Y. Chen, H. Sun and W. Peng, Nanomaterials, 2017, 7, 1–11 Search PubMed.
- R. Tong, K. W. Ng, X. Wang, S. Wang, X. Wang and H. Pan, J. Mater. Chem. A, 2020, 8, 23202–23230 RSC.
- L. Han, Y. L. Zhong, K. Lei, D. Mao, Y. Z. Dong, G. Hong, Y. T. Zhou and D. Fang, J. Phys. Chem. C, 2019, 123, 2398–2409 CrossRef CAS.
- J. Luo, X. Zhou, L. Ma, L. Xu, X. Xu, Z. Du and J. Zhang, Mater. Res. Bull., 2016, 81, 16–26 CrossRef CAS.
- X. Hu, G. Song, W. Li, Y. Peng, L. Jiang, Y. Xue, Q. Liu, Z. Chen and J. Hu, Mater. Res. Bull., 2013, 48, 2325–2332 CrossRef CAS.
- Y. Shan, Y. Li and H. Pang, Adv. Funct. Mater., 2020, 30, 200198 CrossRef.
- H. Li, Q. Su, J. Kang, H. Feng, P. Huang, M. Feng, M. Huang and G. Du, Mater. Res. Bull., 2018, 108, 106–112 CrossRef CAS.
- W. J. Yan, D. Y. Chen, H. R. Fuh, Y. L. Li, D. Zhang, H. Liu, G. Wu, L. Zhang, X. Ren, J. Cho, M. Choi, B. S. Chun, C. Coileáin, H. J. Xu, Z. Wang, Z. Jiang, C. R. Chang and H. C. Wu, RSC Adv., 2019, 9, 626–635 RSC.
- J. Sun, W. Xiong, J. Zhang, Y. Zhang and B. Xie, Mater. Lett., 2021, 308, 131214 CrossRef.
- C. S. Diko, M. Abitonze, Y. Liu, Y. Zhu and Y. Yang, Nanomaterials, 2022, 12, 4497 CrossRef CAS PubMed.
- D. Ma, W. Zhang, Q. Tang, R. Zhang, W. Yu and Y. Qian, J. Nanosci. Nanotechnol., 2005, 5, 806–809 CrossRef CAS PubMed.
- H. Zhu, X. Ji and D. Yang, J. Mater. Sci., 2006, 41, 3489–3492 CrossRef CAS.
- R. K. Mishra, G. W. Baek, K. Kim, H. I. Kwon and S. H. Jin, Appl. Surf. Sci., 2017, 425, 923–931 CrossRef CAS.
- B. Hai, K. Tang, C. Wang, C. An, Q. Yang, G. Shen and Y. Qian, J. Cryst. Growth, 2001, 225, 92–95 CrossRef CAS.
- A. Sánchez-Juárez, A. Tiburcio-Silver and A. Ortiz, Thin Solid Films, 2005, 480–481, 452–456 CrossRef.
- G. Su, V. G. Hadjiev, P. E. Loya, J. Zhang, S. Lei, S. Maharjan, P. Dong, P. M. Ajayan, J. Lou and H. Peng, Nano Lett., 2015, 15, 506–513 CrossRef CAS PubMed.
- J. Johny, S. S. Guzman, B. Krishnan, J. A. A. Martinez, D. Avellaneda Avellaneda and S. Shaji, Appl. Surf. Sci., 2019, 470, 276–288 CrossRef CAS.
- J. Johny, S. Sepulveda-Guzman, B. Krishnan, D. Avellaneda and S. Shaji, Appl. Surf. Sci., 2018, 435, 1285–1295 CrossRef CAS.
- W. Wang, T. Zhang, A. Seliverstov, H. Zhang, Y. Wang, F. Wang, X. Peng, Q. Lu, C. Qin, X. Pan, Y. J. Zeng, C. Van Haesendonck and Z. Ye, Adv. Electron. Mater., 2019, 6, 1901020 CrossRef.
- S. R. Damkale, S. S. Arbuj, G. G. Umarji, R. P. Panmand, S. K. Khore, R. S. Sonawane, S. B. Rane and B. B. Kale, Sustainable Energy Fuels, 2019, 3, 3406–3414 RSC.
- N. Jawale, S. Arbuj, G. Umarji, M. Shinde, B. Kale and S. Rane, RSC Adv., 2023, 13, 2418–2426 RSC.
- A. C. Ok and C. Sarıoğlu, Int. J. Hydrogen Energy, 2024, 52, 561–568 CrossRef CAS.
- B. Balan, M. M. Xavier and S. Mathew, ACS Omega, 2023, 8, 25649–25673 CrossRef CAS PubMed.
- T. Qiang, L. Chen, Y. Xia and X. Qin, J. Cleaner Prod., 2021, 291, 125213 CrossRef CAS.
- X. Bai, Y. Du, W. Xue, X. Hu, J. Fan, J. Li and E. Liu, Nanoscale Adv., 2020, 2, 4220–4228 RSC.
- M. Faisal, J. Ahmed, J. S. Algethami, A. S. Alkorbi and F. A. Harraz, J. Saudi Chem. Soc., 2024, 28, 101806 CrossRef CAS.
- X. Zou, B. Sun, L. Wang, H. Bai, X. Meng, C. Li and Z. Li, Chem. Eng. J., 2024, 482, 148818 CrossRef CAS.
- P. Huang, F. Chen, Y. Tang, W. Sun, Y. Song and Y. Sun, Mater. Sci. Semicond. Process., 2024, 173, 108182 CrossRef CAS.
- A. Fakhri, S. Behrouz and M. Pourmand, J. Photochem. Photobiol., B, 2015, 149, 45–50 CrossRef CAS PubMed.
- A. Fakhri and S. Behrouz, Sol. Energy, 2015, 117, 187–191 CrossRef CAS.
- V. Gadore, S. R. Mishra and M. Ahmaruzzaman, J. Hazard. Mater., 2023, 461, 132458 CrossRef PubMed.
- A. N. Ech-Chergui, F. Bennabi, M. Isik, Y. Khane, F. J. G. García, A. S. Kadari, M. Guezzoul, A. Rahman, M. M. Khan, A. Mehdi, K. Driss-Khodja and B. Amrani, Colloids Surf., A, 2024, 686, 133362 CrossRef CAS.
- F. Chang, Z. Wei, Z. Zhao, Y. Qi and D. Liu, J. Ind. Eng. Chem., 2023, 117, 265–272 CrossRef CAS.
- J. Yu, C. Y. Xu, F. X. Ma, S. P. Hu, Y. W. Zhang and L. Zhen, ACS Appl. Mater. Interfaces, 2014, 6, 22370–22377 CrossRef CAS PubMed.
- H. P. Nogueira, S. H. Toma, A. T. Silveira, A. A. C. Carvalho, A. M. Fioroto and K. Araki, Microchem. J., 2019, 149, 104025 CrossRef CAS.
- J. R. Tu, X. F. Shi, H. W. Lu, N. X. Yang and Y. J. Yuan, Mater. Lett., 2016, 185, 303–306 CrossRef CAS.
- T. Qiang and Y. Xia, J. Alloys Compd., 2020, 845, 156155 CrossRef CAS.
- L. Li, Y. Du, H. Sun, H. Zhang and Z. Zhong, ChemCatChem, 2022, 15, 1436 Search PubMed.
- J. Zai, X. Wei, M. Sun, H. Tian, X. Liu, R. Qi and X. Qian, J. Photochem. Photobiol., A, 2021, 415, 113320 CrossRef CAS.
- B. Xia, F. Deng, S. Zhang, L. Hua, X. Luo and M. Ao, J. Hazard. Mater., 2020, 392, 122345 CrossRef CAS PubMed.
- S. Singla, S. Basu and P. Devi, J. Ind. Eng. Chem., 2023, 118, 119–131 CrossRef CAS.
- M. Kovacic, J. Papac, H. Kusic, P. Karamanis and A. Loncaric Bozic, Chem. Eng. J., 2020, 381, 1222826 Search PubMed.
- N. Afzali, M. Torka Beydokhti, A. A. Khodadadi and Y. Mortazavi, J. Environ. Chem. Eng., 2022, 10, 107793 CrossRef CAS.
- G. Lu, X. Xie, X. Wang, G. Shi, Q. Zeng, D. Segets and J. Sun, J. Photochem. Photobiol., A, 2018, 364, 725–731 CrossRef CAS.
- A. K. Ganguli, G. B. Kunde, W. Raza, S. Kumar and P. Yadav, Molecules, 2022, 27(22), 7778 CrossRef CAS PubMed.
- Y. Liu, D. A. Cullen and T. Lian, J. Am. Chem. Soc., 2021, 143, 20264–20273 CrossRef CAS PubMed.
- W. Gao, J. Lu, S. Zhang, X. Zhang, Z. Wang, W. Qin, J. Wang, W. Zhou, H. Liu and Y. Sang, Adv. Sci., 2019, 6, 1244 Search PubMed.
- B. Ohtani, Catalysts, 2013, 3, 942–953 CrossRef CAS.
- F. Amano, K. Nogami, M. Tanaka and B. Ohtani, Langmuir, 2010, 26, 7174–7180 CrossRef CAS PubMed.
- H. Cheng, J. Wang, Y. Zhao and X. Han, RSC Adv., 2014, 4, 47031–47038 RSC.
- N. Roy, Y. Park, Y. Sohn, K. T. Leung and D. Pradhan, ACS Appl. Mater. Interfaces, 2014, 6, 16498–16507 CrossRef CAS PubMed.
- Z. Zheng, Z. Wang, L. Xie, Z. Fang, W. Feng, M. Huang and P. Liu, Appl. Surf. Sci., 2015, 353, 714–722 CrossRef CAS.
- X. Zhang, C. Bo, S. Cao, Z. Cheng, Z. Xiao, X. Liu, T. Tan and L. Piao, J. Mater. Chem. A, 2022, 10, 24381–24387 RSC.
- R. A. Carcel, L. Andronic and A. Duta, Mater. Charact., 2012, 70, 68–73 CrossRef CAS.
- L. Jiang, Z. Y. Jiang, Y. M. Lin and J. M. Zheng, Phys. Status Solidi RRL, 2021, 15, 340 Search PubMed.
- I. Shown, S. Samireddi, Y. C. Chang, R. Putikam, P. H. Chang, A. Sabbah, F. Y. Fu, W. F. Chen, C. I. Wu, T. Y. Yu, P. W. Chung, M. C. Lin, L. C. Chen and K. H. Chen, Nat. Commun., 2018, 9(1), 169 CrossRef PubMed.
- X. Shuai, Y. Wang, J. Zhang, R. Zhao, T. Guo, J. Du and J. Li, ChemistrySelect, 2022, 7(33), 1068 CrossRef.
- J. H. Liu, G. F. Huang, W. Q. Huang, H. Miao and B. X. Zhou, Mater. Lett., 2015, 161, 480–483 CrossRef CAS.
- Y. Zhao, D. Sun, K. Hu, W. Zhao and F. Huang, Inorg. Chem. Commun., 2020, 114, 107849 CrossRef CAS.
- S. Mondal, S. Das and U. K. Gautam, J. Colloid Interface Sci., 2021, 603, 110–119 CrossRef CAS PubMed.
- S. Paul, D. Barman, C. Chowdhury, P. K. Giri and S. K. De, CrystEngComm, 2021, 23, 2276–2288 RSC.
- F. Zhang, L. Shen, J. Li, Y. Zhang, G. Wang and A. Zhu, Powder Technol., 2021, 383, 371–380 CrossRef CAS.
- Y. Liu, D. Pan, M. Xiong, Y. Tao, X. Chen, D. Zhang, Y. Huang and G. Li, Chin. J. Catal., 2020, 41, 1554–1563 CrossRef CAS.
- A. Bumajdad and M. Madkour, Phys. Chem. Chem. Phys., 2014, 16, 7146–7158 RSC.
- M. Lin, H. Chen, Z. Zhang and X. Wang, Phys. Chem. Chem. Phys., 2023, 25, 4388–4407 RSC.
- K. Sharma, S. Patial, P. Singh, A. A. P. Khan, V. Saini, A. K. Nadda, C. M. Hussain, V. H. Nguyen, C. C. Nguyen, T. B. Hac Nguyen, S. Y. Kim, Q. Van Le and P. Raizada, Sol. Energy, 2022, 231, 546–565 CrossRef CAS.
- X. Guo, F. Zhang, Y. Zhang and J. Hu, J. Mater. Chem. A, 2023, 11, 7331–7343 RSC.
- L. A. Burton, T. J. Whittles, D. Hesp, W. M. Linhart, J. M. Skelton, B. Hou, R. F. Webster, G. O'Dowd, C. Reece, D. Cherns, D. J. Fermin, T. D. Veal, V. R. Dhanak and A. Walsh, J. Mater. Chem. A, 2016, 4, 1312–1318 RSC.
- S. Deng, Y. Chen, Q. Li, J. Sun, Z. Lei, P. Hu, Z. H. Liu, X. He and R. Ma, Nanoscale, 2022, 14, 14097–14105 RSC.
- P. Shinde and C. S. Rout, Mater. Chem. Front., 2021, 5, 516–556 RSC.
- W. Zhao, Z. Wei, L. Ma, J. Liang and X. Zhang, Materials, 2019, 12(4), 582 CrossRef CAS PubMed.
- H. Y. He, J. Lu, L. Y. Cao and M. Li, Res. Chem. Intermed., 2012, 38, 537–547 CrossRef CAS.
- L. Dashairya, M. Sharma, S. Basu and P. Saha, J. Alloys Compd., 2019, 774, 625–636 CrossRef CAS.
- R. R. Srivastava, P. Kumar Vishwakarma, U. Yadav, S. Rai, S. Umrao, R. Giri, P. S. Saxena and A. Srivastava, Front. Nanotechnol., 2021, 3, 711368 CrossRef.
- Z. Li, R. Fan, Z. Hu, W. Li, H. Zhou, S. Kang, Y. Zhang, H. Zhang and G. Wang, J. Hazard. Mater., 2020, 394, 122525 CrossRef CAS PubMed.
- V. Govindan, H. Imran, V. Dharuman and K. Sankaranarayanan, J. Mater. Sci.: Mater. Electron., 2018, 29, 17670–17680 CrossRef CAS.
- Z. Liu, C. Liu, S. Mao and X. Huang, ACS Appl. Mater. Interfaces, 2023, 15, 7529–7537 CrossRef CAS PubMed.
- Y. Kawabe, Y. Ito, Y. Hori, S. Kukunuri, F. Shiokawa, T. Nishiuchi, S. Jeong, K. Katagiri, Z. Xi, Z. Li, Y. Shigeta and Y. Takahashi, ACS Nano, 2023, 17, 11318–11326 CrossRef CAS PubMed.
- H. Wang, Z. Liu, L. Wang, Q. Shou, M. Gao, H. Wang, A. Nazir and P. Huo, J. Mater. Sci.: Mater. Electron., 2023, 34, 350 CrossRef CAS.
- C. Jin, W. Feng and X. Zhao, IOP Conference Series: Earth and Environmental Science, IOP Publishing Ltd, 2021, vol. 766 Search PubMed.
- Y. Nie, J. Liu, N. Li, Y. Wang, Q. Cheng, S. He, Q. Guo, R. Zhao and F. Pan, Fuel Process. Technol., 2023, 250, 107871 CrossRef CAS.
- L. Xiang, S. Liu, L. Zhao, S. Yuan, X. Li and N. Li, J. Alloys Compd., 2023, 945, 169201 CrossRef CAS.
- M. Lan, X. Dong, N. Zheng, X. Zhang, Y. Wang and X. Zhang, J. Mater. Sci. Technol., 2023, 167, 237–247 CrossRef CAS.
- S. R. Kadam, S. Ghosh, R. Bar-Ziv and M. Bar-Sadan, Chem. – Eur. J., 2020, 26, 6679–6685 CrossRef CAS PubMed.
- J. W. Shi, Y. Zou, D. Ma, Z. Fan, L. Cheng, D. Sun, Z. Wang, C. Niu and L. Wang, Nanoscale, 2018, 10, 9292–9303 RSC.
- T. Di, T. Cao, H. Liu, S. Wang and J. Zhang, Phys. Chem. Chem. Phys., 2023, 25, 5196–5202 RSC.
- A. R. Woldu, P. Talebi, A. G. Yohannes, J. Xu, X. D. Wu, S. Siahrostami, L. Hu and X. C. Huang, Angew. Chem., Int. Ed., 2023, 62, e2023016 CrossRef PubMed.
- T. Billo, I. Shown, A. Anbalagan, T. A. Effendi, A. Sabbah, F. Y. Fu, C. M. Chu, W. Y. Woon, R. S. Chen, C. H. Lee, K. H. Chen and L. C. Chen, Nano Energy, 2020, 72, 104717 CrossRef CAS.
- X. Jiao, X. Li, X. Jin, Y. Sun, J. Xu, L. Liang, H. Ju, J. Zhu, Y. Pan, W. Yan, Y. Lin and Y. Xie, J. Am. Chem. Soc., 2017, 139, 18044–18051 CrossRef CAS PubMed.
- G. Li, W. Liu, J. Wang, B. Li, S. Yang, W. Wang, W. Sun and Y. Sun, Chem. Phys. Lett., 2022, 807, 140063 CrossRef CAS.
- G. Ma, Z. Pan, Y. Liu, Y. Lu and Y. Tao, Materials, 2023, 16, 4436 CrossRef CAS PubMed.
- G. Kumar, J. Kumar, M. Bag and R. Kumar Dutta, Sep. Purif. Technol., 2022, 292, 121040 CrossRef CAS.
- G. Kumar and R. K. Dutta, Environ. Sci. Pollut. Res., 2022, 29, 57758–57772 CrossRef CAS PubMed.
- Y. C. Zhang, Z. N. Du, K. W. Li and M. Zhang, Sep. Purif. Technol., 2011, 81, 101–107 CrossRef CAS.
- L. Li, Z. Chai, W. Jin, H. Sun, J. He, G. Wu and W. Xia, J. Alloys Compd., 2023, 932, 167658 CrossRef CAS.
- X. Zhang and P. Yang, Carbon, 2024, 216, 118584 CrossRef CAS.
- Y. Sun, G. Li, J. Xu and Z. Sun, Mater. Lett., 2016, 174, 238–241 CrossRef CAS.
- G. Li, Y. Sun, S. Sun, W. Chen, J. Zheng, F. Chen, Z. Sun and W. Sun, Adv. Powder Technol., 2020, 31, 2505–2512 CrossRef CAS.
- F. Wang, S. Zhang, W. Jing, H. Qiu, Y. Liu and L. Guo, J. Mater. Sci. Technol., 2024, 189, 146–154 CrossRef.
- T. Song, X. Zhang, C. Xie and P. Yang, Carbon, 2023, 210, 118052 CrossRef CAS.
- K. Perović, M. Kovačić, M. Kraljić Roković, H. Kušić, B. Genorio, U. Lavrenčić Štangar and A. Lončarić Božić, Mater. Res. Bull., 2023, 167, 112418 CrossRef.
- J. Wang, J. Xuan, X. Wei, Y. Zhang, J. Fan, L. Ni, Y. Yang, J. Liu, Y. Tian and L. Duan, Int. J. Hydrogen Energy, 2024, 54, 979–989 CrossRef CAS.
- Y. Li, Z. Liu, S. Wu, M. Zhu and Y. Zhang, Chem. Phys. Lett., 2022, 812, 140248 CrossRef.
- M. Awais, S. Aslam, M. N. Ashiq, M. Mirza and M. Safdar, New J. Chem., 2024, 48, 3247–3257 RSC.
- P. A. K. Reddy, H. Han, K. C. Kim and S. Bae, ACS Sustainable Chem. Eng., 2024, 12, 4979–4992 CrossRef CAS.
- N. Ma, C. Lu, Y. Liu, T. Han, W. Dong, D. Wu and X. Xu, Small, 2024, 20, 2304839 CrossRef CAS PubMed.
- A. Fakhri, S. Behrouz and M. Pourmand, J. Photochem. Photobiol., B, 2015, 149, 45–50 CrossRef CAS PubMed.
- X. Zhang, P. Yang, H. S. Chen and S. P. Jiang, Chem. Eng. J., 2023, 479, 147609 CrossRef.
- D. Wang, P. Zhao, J. Yang, G. Xu, H. Yang, Z. Shi, Q. Hu, B. Dong and Z. Guo, Colloids Surf., A, 2020, 603, 125147 CrossRef CAS.
- S. Thirumalairajan, K. Girija, V. R. Mastelaro and N. Ponpandian, New J. Chem., 2014, 38, 5480–5490 RSC.
- K. Tamilarasu, R. Ranjith, P. Maadeswaran, R. Ramesh, R. Thammasak, G. Periyasami, P. Karthikeyan and C. Umarani, J. Mater. Sci.: Mater. Electron., 2024, 35, 607 CrossRef CAS.
- Y. Ben Smida, O. Oyewo, S. Ramaila, L. Mavuru, R. Marzouki, D. C. Onwudiwe and A. H. Hamzaoui, J. Inorg. Organomet. Polym. Mater., 2022, 32, 4679–4693 CrossRef CAS.
- Y. Cheng, J. He and P. Yang, Colloids Surf., A, 2023, 680, 132678 CrossRef.
- K. M. Alnahdi, Inorg. Chem. Commun., 2023, 160, 111870 CrossRef.
- X. Zheng, M. Xu, C. Cai, Y. Yuan, F. Lin, W. Chen and F. Yang, J. Alloys Compd., 2024, 980, 173630 CrossRef CAS.
- V. Gadore, S. R. Mishra and M. Ahmaruzzaman, J. Hazard. Mater., 2023, 444, 130301 CrossRef CAS PubMed.
- S. Park, R. Selvaraj, M. A. Meetani and Y. Kim, J. Ind. Eng. Chem., 2017, 45, 206–214 CrossRef CAS.
- X. Wu, H. Hu, L. Cheng, Y. Zhang, Q. Jiang, P. Wang, L. Xu, P. Lin and C. Cui, J. Mater. Sci.: Mater. Electron., 2024, 35, 545 CrossRef CAS.
- P. Cao, Y. Zhang, D. Gao, H. Chen, M. Zhou, Y. He, P. Song and R. Wang, J. Alloys Compd., 2022, 904, 164061 CrossRef CAS.
- T. T. Salunkhe, V. Kumar, A. N. Kadam, M. Mali and M. Misra, Ceram. Int., 2024, 50, 1826–1835 CrossRef CAS.
- G. Kumar, J. Inorg. Organomet. Polym. Mater., 2023, 33, 2710–2720 CrossRef CAS.
- M. Rajamani, A. Rajan and B. Neppolian, J. Environ. Chem. Eng., 2022, 11, 109129 CrossRef.
- G. Kumar and R. K. Dutta, J. Phys. Chem. Solids, 2022, 164, 110639 CrossRef CAS.
- F. You, X. Hou, P. Wei and J. Qi, Inorg. Chem., 2021, 60(24), 18598–18602 CrossRef CAS PubMed.
- F. You, Y. Zhou, D. Li, H. Zhang, D. Gao, X. Ma, R. Hao and J. Liu, J. Colloid Interface Sci., 2023, 629, 871–877 CrossRef CAS PubMed.
- T. Di, B. Zhu, B. Cheng, J. Yu and J. Xu, J. Catal., 2017, 352, 532–541 CrossRef CAS.
- N. Jawale, S. Arbuj, G. Umarji, M. Shinde, B. Kale and S. Rane, RSC Adv., 2023, 13, 2418–2426 RSC.
- G. Li, Y. Sun, X. Hong, W. Lu, W. Chen, Y. Deng, Z. Sun and W. Sun, J. Mater. Sci., 2021, 56, 10847–10858 CrossRef CAS.
- S. Zhu, G. Wu, Z. Liu, S. Zhao, D. Cao, C. Li and G. Liu, Ceram. Int., 2023, 49, 5893–5904 CrossRef CAS.
- H. Zhao, B. Zhao, H. Liu and X. Li, Dalton Trans., 2023, 53, 591–600 RSC.
- F. Ganaie, Z. Haq, A. Bashir, A. Qureashi, I. Nazir, K. Fatima, A. H. Pandith and M. A. Bhat, New J. Chem., 2024, 48, 7111–7124 RSC.
- S. Q. Guo, B. Yang, Z. Hu, M. Zhen, B. Gu and B. Shen, Nano Res., 2023, 16, 2102–2110 CrossRef CAS.
- S. Liu, Mater. Lett., 2023, 330, 133256 CrossRef CAS.
- X. He, T. He, R. Xia, Y. Qi, Y. Zhou, B. Song, F. Liao, Z. Kang and L. Jiang, Sep. Purif. Technol., 2023, 324, 124515 CrossRef CAS.
- Z. Wan, Q. Mao, J. Xiang, D. Ma and H. Tang, J. Mater. Sci. Technol., 2023, 161, 233–244 CrossRef CAS.
- S. Kar, T. Pal and S. Ghosh, ChemistrySelect, 2023, 8, e202300878 CrossRef CAS.
- S. Bai, N. Zhang, C. Gao and Y. Xiong, Nano Energy, 2018, 53, 296–336 CrossRef CAS.
- J. Liu, Z. Wei and W. Shangguan, ChemCatChem, 2019, 11, 6177–6189 CrossRef CAS.
- H. Zhang, L. Mao, J. Wang, Y. Nie, Z. Geng, D. Zhong, X. Tan, J. Ye and T. Yu, Small, 2023, 20, 5727 Search PubMed.
- M. Chen, S. Wan, L. Zhong, D. Liu, H. Yang, C. Li, Z. Huang, C. Liu, J. Chen, H. Pan, D. S. Li, S. Li, Q. Yan and B. Liu, Angew. Chem., Int. Ed., 2021, 60, 26233–26237 CrossRef CAS PubMed.
- G. Kiruthigaa, C. Manoharan, M. Bououdina, S. Ramalingam and C. Raju, Solid State Sci., 2015, 44, 32–38 CrossRef CAS.
- Y. Liu, Y. Zhou, X. Zhou, X. Jin, B. Li, J. Liu and G. Chen, Chem. Eng. J., 2021, 407, 127180 CrossRef CAS.
- A. Sharma, A. Makhija, S. Dahiya, A. Ohlan, R. Punia and A. S. Maan, Mater. Res. Bull., 2023, 168, 112464 CrossRef CAS.
- M. Setayeshmehr, M. Haghighi and K. Mirabbaszadeh, Electrochim. Acta, 2021, 376, 137987 CrossRef CAS.
- P. Bharathi, S. Harish, M. Shimomura, M. Krishna Mohan, J. Archana and M. Navaneethan, Mater. Lett., 2023, 335, 133691 CrossRef CAS.
- P. Sengodu, C. H. Li, C. F. Wei, R. Bendi and C. C. Chen, ChemistrySelect, 2016, 1, 3328–3334 CrossRef CAS.
- C. Mondal, M. Ganguly, J. Pal, A. Roy, J. Jana and T. Pal, Langmuir, 2014, 30, 4157–4164 CrossRef CAS PubMed.
- X. Zhang, A. Chen, L. Chen and Z. Zhou, Adv. Energy Mater., 2022, 12, 2003841 CrossRef CAS.
- Y. Hu, X. Chen, X. Ren, Z. Huang, X. Qi and J. Zhong, J. Mater. Sci.: Mater. Electron., 2018, 29, 19614–19619 CrossRef CAS.
- B. Luo, G. Liu and L. Wang, Nanoscale, 2016, 8, 6904–6920 RSC.
- J. Zhang, G. Huang, J. Zeng, X. Jiang, Y. Shi, S. Lin, X. Chen, H. Wang, Z. Kong, J. Xi and Z. Ji, J. Alloys Compd., 2019, 775, 726–735 CrossRef CAS.
- Y. Liu, X. Mi, J. Wang, M. Li, D. Fan, H. Lu and X. Chen, Inorg. Chem. Front., 2019, 6, 948–954 RSC.
- Y. C. Zhang, J. Li, M. Zhang and D. D. Dionysiou, Environ. Sci. Technol., 2011, 45, 9324–9331 CrossRef CAS PubMed.
- J. Gajendiran and V. Rajendran, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2021, 2(1), 15001 Search PubMed.
- S. H. Chaki, M. P. Deshpande, D. P. Trivedi, J. P. Tailor, M. D. Chaudhary and K. Mahato, Appl. Nanosci., 2013, 3, 189–195 CrossRef CAS.
- X. L. Gou, J. Chen and P. W. Shen, Mater. Chem. Phys., 2005, 93, 557–566 CrossRef CAS.
- Y. Ren, H. An, W. Zhang, S. Wei, C. Xing and Z. Peng, Nanophotonics, 2022, 11, 4781–4792 CrossRef CAS.
- H. Zhao, X. Li, Y. Chen, Z. Zhao, M. Zhang, H. Su, H. Liang, W. Zhang, K. Tao and L. Li, J. Lumin., 2023, 235, 118068 CrossRef.
- Y. Huang, W. Jiao, Z. Chu, X. Nie, R. Wang and X. He, ACS Appl. Mater. Interfaces, 2020, 12, 25178–25188 CrossRef CAS PubMed.
- Y. J. Yuan, D. Q. Chen, X. F. Shi, J. R. Tu, B. Hu, L. X. Yang, Z. T. Yu and Z. G. Zou, Chem. Eng. J., 2017, 313, 1438–1446 CrossRef CAS.
- T. Ali, X. Wang, K. Tang, Q. Li, S. Sajjad, S. Khan, S. A. Farooqi and C. Yan, Electrochim. Acta, 2019, 300, 45–52 CrossRef CAS.
- B. Wang, M. Chen, J. Lv, G. Xu, X. Shu and Y. C. Wu, Dalton Trans., 2021, 50, 13329–13336 RSC.
- D. Ursu, M. Vajda and M. Miclau, Micro Nano Lett., 2019, 14, 872–876 CrossRef CAS.
- Y. Liang, N. Guo, L. Li, R. Li, G. Ji and S. Gan, Appl. Surf. Sci., 2015, 332, 32–39 CrossRef CAS.
- J. M. Mali, S. S. Arbuj, J. D. Ambekar, S. B. Rane, U. P. Mulik and D. P. Amalnerkar, Sci. Adv. Mater., 2013, 5, 1994–1998 CrossRef CAS.
- Z. Liu, K. Xu, H. Yu and Z. Sun, Int. J. Energy Res., 2021, 45, 6850–6862 CrossRef CAS.
- L. L. Zulfa, R. Ediati, A. R. P. Hidayat, R. Subagyo, N. Faaizatunnisa, Y. Kusumawati, D. Hartanto, N. Widiastuti, W. P. Utomo and M. Santoso, RSC Adv., 2023, 13, 3818–3834 RSC.
- Y. Zhong, C. Peng, Z. He, D. Chen, H. Jia, J. Zhang, H. Ding and X. Wu, Catal. Sci. Technol., 2021, 11, 27–42 RSC.
- G. Li and K. A. Gray, Chem. Phys., 2007, 339, 173–187 CrossRef CAS.
- A. Mohanty and K. Kamali, ACS Appl. Nano Mater., 2024, 7, 3326–3338 CrossRef CAS.
- N. R. Barveen, J. L. Xu and Y. W. Cheng, J. Environ. Chem. Eng., 2024, 12, 112200 CrossRef CAS.
- J. Feng, Y. Sun, J. Mu, L. Chen, T. Han, H. Miao, E. Liu, J. Fan and X. Hu, Mater. Lett., 2019, 236, 534–537 CrossRef CAS.
- S. Mondal, L. Sahoo, C. P. Vinod and U. K. Gautam, Appl. Catal., B, 2021, 2, 119927 CrossRef.
- S. I. Naya, K. Kimura and H. Tada, ACS Catal., 2013, 3, 10–13 CrossRef CAS.
- R. Fu, L. Li, X. Li, B. Li, C. Shao, Z. Liu and A. Shen, Mater. Chem. Phys., 2021, 267, 124702 CrossRef CAS.
- T. Wang, H. Meng, X. Yu, Y. Liu, H. Chen, Y. Zhu, J. Tang, Y. Tong and Y. Zhang, RSC Adv., 2015, 5, 15469–15478 RSC.
- M. Bagherzadeh and R. Kaveh, J. Photochem. Photobiol., A, 2018, 359, 11–22 CrossRef CAS.
- H. Derikvandi and A. Nezamzadeh-Ejhieh, J. Colloid Interface Sci., 2017, 490, 628–641 CrossRef CAS PubMed.
- L. Sun, Z. Zhao, S. Li, Y. Su, L. Huang, N. Shao, F. Liu, Y. Bu, H. Zhang and Z. Zhang, ACS Appl. Nano Mater., 2019, 2, 2144–2151 CrossRef CAS.
- Q. Liu, S. Liu, A. Wu, H. Huang and L. Zhou, J. Alloys Compd., 2020, 834, 155174 CrossRef CAS.
- L. Zhang, X. Dong, Y. Wang, N. Zheng, H. Ma and X. Zhang, Appl. Surf. Sci., 2022, 579, 152088 CrossRef CAS.
- L. Wang, S. K. Karuturi and L. Zan, Appl. Surf. Sci., 2023, 537, 148063 CrossRef.
- Y. Liu, C. Lv, J. Sun, X. Zhou, Y. Zhou and G. Chen, Adv. Mater. Interfaces, 2022, 9, 2200153 CrossRef CAS.
- Sunaina, K. K. Yadav, Ankush, S. K. Guchhait, K. Sood, S. K. Mehta, A. K. Ganguli and M. Jha, Sep. Purif. Technol., 2020, 242, 116835 CrossRef CAS.
- J. Pan, Z. Guan, J. Yang and Q. Li, Chin. J. Catal., 2020, 41, 200–208 CrossRef CAS.
- F. Qiu, W. Li, F. Wang, H. Li, X. Liu and J. Sun, J. Colloid Interface Sci., 2017, 493, 1–9 CrossRef CAS PubMed.
- X. Chen, Z. Han, Z. Lu, T. Qu, C. Liang, Y. Wang, B. Zhang, X. Han and P. Xu, Sustainable Energy Fuels, 2023, 7, 1311–1321 RSC.
- W. Ren, J. Yang, W. Chen, J. Zhang, Y. Sun, Y. Zheng, H. Zhao and B. Liang, Mater. Res. Bull., 2022, 153, 111884 CrossRef CAS.
- C. Zhang, J. Ma, H. Zhu, H. Ding, H. Wu, K. Zhang, X. L. Zhao, X. Wang and C. Cheng, J. Alloys Compd., 2023, 960, 170932 CrossRef CAS.
- Q. Li, S. He, L. Wang, J. Song, J. Wang, C. Shao, Z. Tian and Y. Liu, CrystEngComm, 2023, 25, 2882–2891 RSC.
| This journal is © The Royal Society of Chemistry 2024 |