A seed-like structured Mo@ZrS2 catalyst on graphene nanosheets for boosting the performance of rechargeable Zn–air batteries†
Ramasamy
Santhosh Kumar
a,
Dilmurod
Sayfiddinov
a,
S.
Tamilarasi
a and
Dong Jin
Yoo
 *ab
*ab
aDepartment of Energy Storage/Conversion Engineering of Graduate School (BK21 FOUR), Hydrogen and Fuel Cell Research Center, Jeonbuk National University, Jeonju, Jeollabuk-do, 54896 Republic of Korea. E-mail: djyoo@jbnu.ac.kr; Fax: +82-(0) 63-270-3909; Tel: +82-(0) 63-270-3608
bDepartment of Life Science, Jeonbuk National University, Jeonju-si, Jeollabuk-do, 54896 Republic of Korea
First published on 8th July 2024
Abstract
Novel composite materials are being studied by researchers for energy storage and renewable energy applications. Here, a seed-like Mo-doped ZrS2 catalyst was developed on a reduced graphene oxide (rGO) surface by an annealing and hydrothermal method. Using photoelectron spectroscopy, scanning microscopy, and X-ray diffraction analyses, the structure of Mo@ZrS2/rGO and the impact of heteroatoms are demonstrated, providing insight into the catalyst. Furthermore, it is demonstrated that Mo@ZrS2/rGO has been utilized as an efficient energy storage electrocatalyst by offering a very low half-wave potential of 0.80 V for the oxygen reduction reaction in an alkaline solution. Furthermore, Zn–air batteries with a high-power density of 128.6 mW cm−2 and exceptional cycling stability are demonstrated by the developed array electrocatalyst. Ultimately, the research findings suggest novel perspectives on the structure of ZrS2 nanoseeds created by Mo surface doping, promote the usage of Zn–air batteries in practical scenarios, and offer a fascinating idea for creating a redox electrocatalyst.
1. Introduction
Zinc–air batteries (ZABs) are a promising alternative in modern battery technology due to their excellent theoretical energy density (around 1084 W h kg−1), low production cost, and environmental friendliness.1–4 However, the high polarization and short lifespan of the air electrodes restrict the practical deployment of ZABs. These benefits come naturally from oxygen-based electrochemistry in aqueous systems.5,6 Consequently, it is critical to create ZAB electrodes that can withstand the slow kinetics of the oxygen evolution reaction (OER) and the oxygen reduction reaction (ORR) during an extended cycling time. Pt and RuO2/IrO2 are two of the precious noble metals that are necessary for the synthesis of modern ORR and OER catalysts.7–10 More obstacles to the use of scaling are the low abundance, high cost, and inadequate stability of the noble metals.11–13 Therefore, to advance the use of ZABs, it is essential to use low-cost bifunctional electrocatalysts with adequate activity and long-term stability. To date, it has been difficult to find single or bi-metal ZAB catalysts with long cycle lives that can simultaneously offer effective OER and ORR functionalities.14,15A number of noble-metal-free catalysts have demonstrated excellent efficacy in catalyzing these important processes, including transition-metal oxides,16 chalcogenides,17 oxynitrides,18 and their complexes. The development of hybrid catalysts like metal sulfide composites and graphitic carbon frameworks has greatly advanced their potential applications.19,20 Curiously, when early-transition-metal sulfide nanoparticles were supported by heteroatom-doped carbon or carbon nanotubes, the combined impact of the heteroatom-doped conductive carbon and nanoparticles could produce exceptional ORR activity.21–23 Similarly, nanoscale metal sulfides show excellent activity and durability for the OER.24–26 Moreover, doping Zr-based materials with an extra transition metal implies that oxygen from atoms can be readily adsorbed on their surface to speed up ORR kinetics.19,27 Zr-based catalysts for the ORR, such as carbides, nitrides, and oxides of zirconium, have thus been the subject of extensive investigation.27,28 For the ORR involving four-electron transfer, Zr-based oxides are thought to be electrocatalytically ineffective due to their innately low electrical conductivity.29 However, there has not been much research on catalysts based on zirconium sulfide.25 To increase ORR performances, Zr-based sulfide electrocatalysts exhibiting improved electrical conductivity must be carefully designed.
In this work, on the basis of these outstanding findings, we report a process of doping Mo on the ZrS2 seed surface on rGO (reduced graphene oxide) nanosheets (Mo@ZrS2/rGO) to produce a sulfur-based ORR catalyst with good performance. Mo was doped on ZrS2/rGO in a later step after a one-pot process employing Zr and S precursors to produce a ZrS2/rGO composite with varying atomic ratios. Furthermore, we observed that ZrS2 nanoseeds doped with Mo exhibit cutting-edge ORR and ZAB capabilities, demonstrating that Mo is able to both strengthen the contact with ZrS2 and modify its electrical properties. Microscopy, diffraction, and photoelectron spectroscopy probes were used to describe the structural and chemical compositions of the produced Mo@ZrS2/rGO and ZrS2/rGO catalysts. According to the electrochemical data, the ZrS2/rGO catalyst with the most active ORR and ZAB performances was synthesised with a lower atomic ratio of Mo. With a half-wave potential of just 0.80 V in a 0.1 M KOH solution, the Mo@ZrS2/rGO electrocatalyst outperformed ORR electrocatalysts in terms of performance. The rechargeable Zn–air battery assembly for the ORR electrocatalyst demonstrated a remarkable power density and specific capacity of 128.6 mW cm−2 and 807.9 mA h g−1, respectively. This work offers novel findings on the structure of Mo@ZrS2 nanoseeds on rGO nanosheets, which is promising for a variety of energy storage applications.
2. Experimental
Materials
Zirconium(IV) oxynitrate dihydrate (Sigma Aldrich, 99%), thiourea (Alfa Aesar, 99%), sodium molybdate dihydrate (Sigma Aldrich, 99.0%), and ammonium fluoride (Sigma Aldrich, 99.99%) were used. Alfa Aesar supplied urea (99.3%), graphite powder (99.99%), and Nafion in 5 weight percentage and 20 weight percent of commercial Pt–C. Samchun Pure Chemicals Co. in South Korea provided both ethanol and potassium hydroxide (KOH).Synthesis of ZrS2/rGO and Mo@ZrS2/rGO
Using a modified Hummers process, graphite flakes were converted into graphene oxide (GO).30–32 Typically, 2 mmol ZrO(NO3)2·2H2O, 4 mmol thiourea, and 10 mg of GO were dissolved in 40 ml of deionized (DI) water. While the mixture was being stirred, 2 mmol ammonium fluoride was introduced into the solution. After stirring for 30 min at room temperature, the solution was transferred to a Teflon-lined hydrothermal autoclave at 180 °C for 4 h. Finally, the obtained material was washed with DI water and ethanol, and then dried at 60 °C for 24 h to produce ZrS2/rGO.The synthesis method of ZrS2/rGO is similar to that of Mo@ZrS2/rGO. 20 mg of ZrS2/rGO was dispersed in 40 ml of DI water and sonicated for 10 min. 1 mmol Na2MoO4·2H2O was added dropwise into pre-dispersed ZrS2/rGO solution with continuous stirring at room temperature for 1 h. Then, the mixture was introduced into an autoclave for synthesis by the hydrothermal method at 180 °C for 4 h. Following this, the as-obtained product was washed with DI water and ethanol, and then dried at 60 °C for 24 h. Lastly, the final product was calcined at 400 °C for 2 h to obtain Mo@ZrS2/rGO. Additionally, the Zn–air battery test, materials, and electrochemical characterization are all described in the ESI† clearly.
3. Results and discussion
Synthesis and characterization of ZrS2/rGO and Mo@ZrS2/rGO nanocatalysts
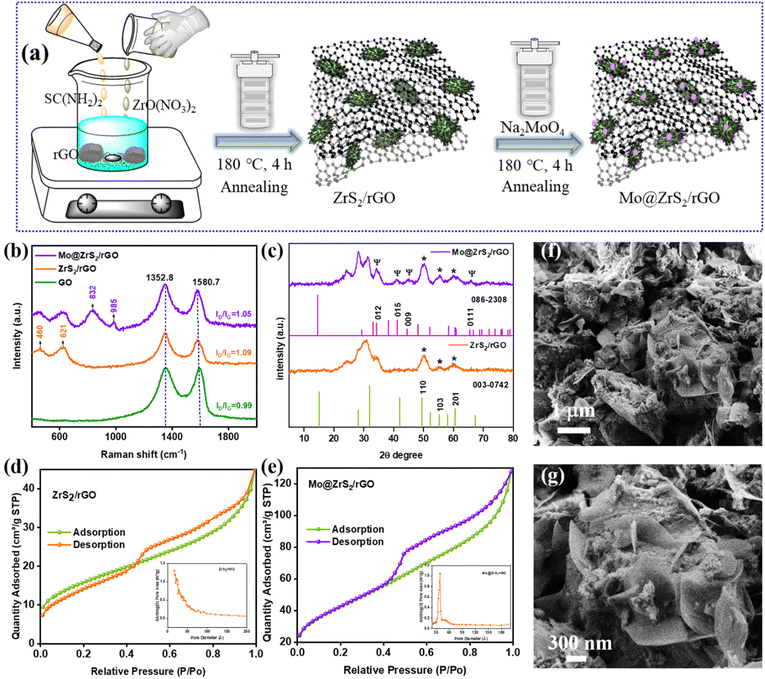
As shown in Fig. 1a, a hydrothermal and annealing approach were used to synthesise ZrS2 on the rGO (ZrS2/rGO) utilizing metal-precursors namely zirconium oxynitrate and thiourea in a sample of GO solution. Strong contacts between the GO sheet and the metal precursors are produced by the functional groups present in the GO.34 The absorbed metal precursors consistently formed a seed-like structured ZrS2 on rGO during the hydrothermal reaction, and at the same time, GO was converted into rGO since thiourea was present. In the present study, the structure-tuning agent employed to regulate the production of the ZrS2 nanocatalyst with a seed-like structure was ammonium fluoride. Consequently, as seen in Fig. S1a–d,† the FE-SEM (field emission scanning electron microscopy) images of ZrS2/rGO reveal the seed-like structure of ZrS2 growing on the rGO sheet. The extra atom needs to be doped in order to enhance the physical interaction of ZrS2/rGO nanocatalysts that show enhanced electrical conductivity. As a result, we have doped the Mo atom on the ZrS2/rGO nanocatalyst surface here using Na2MoO4 as the precursor Mo atom dopant. Because, as shown in Fig. S1e and f,† the addition of a Mo atom to the ZrS2/rGO nanocatalyst did not harm the seed-like structure, the addition caused the nanoparticle to form on the seed-like structure (Mo@ZrS2/rGO). Moreover, the elemental analysis of energy dispersive X-ray (EDS) spectroscopy verified the presence of every species in the final catalyst and showed that Mo had been successfully deposited (Fig. S1g†). We firmly expect that the doped atom will enhance the catalytic activity and form robust chemical bonds with Zr–S atoms, and rGO functional groups since the contact between metals (Mo–Zr) facilitates catalytic reactions.Moreover, as shown in Fig. 1b, Raman spectroscopy was used to illustrate the graphitic and defective surroundings of GO, ZrS2/rGO, and Mo@ZrS2/rGO composites. Two prominent peaks in the Raman spectra of graphitic oxide (GO) may be seen at 1352.8 and 1580.7 cm−1, respectively. These peaks correspond to the D and G bands, which denote the defect structure of carbon graphitic and the sp2 character of carbon atoms. The fact that the ID/IG values of GO, at roughly 0.99, were lower compared to those of ZrS2/rGO and Mo@ZrS2/rGO (ID/IG = 1.09 and 1.05, respectively) indicates that the synthesis of both catalysts was successful.35 The further peaks at 832 and 985 cm−1 were due to Mo doping in the final catalyst,36 while the development of peaks at 460 and 621 cm−1 suggests that ZrS2 was successfully deposited onto rGO.37 ZrS2 and Mo@ZrS2 have successfully formed on a rGO nanocomposite, according to these findings.
Moreover, the crystal structure characteristics of ZrS2/rGO and Mo@ZrS2/rGO were examined using X-ray diffraction (XRD). The ZrS2/rGO and Mo@ZrS2/rGO samples showed a large peak (002) at 30.3°, which is typically connected to the rGO plane. This observation confirms that GO has been effectively reduced to rGO (Fig. 1c). The XRD pattern shows small peaks of ZrS2 at 50.0, 55.2, and 59.7° appropriate to the (1 1 0), (1 0 3), and (2 0 1) planes, respectively. JCPDS-003-0742 shows that the diffraction pattern of these peaks is a regular, characteristic ZrS2/rGO (hexagonal structure with the P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1 space group).38 However, after Mo was added to produce the final catalyst, each peak of ZrS2 and rGO grew more distinct. Moreover, a low-intensity peak corresponding to Mo appeared at 34.1, 41.1, 44.4, and 65.5° indicating the (0 1 2), (0 1 5), (0 0 9), and (0 1 11) planes, respectively.39 The diffraction pattern of these peaks corresponds to the regular, distinctive Mo@ZrS2/rGO (rhombohedral structure with the R3m space group), as demonstrated by JCPDS-086-2308. The crystal planes showed plenty of heteroatom production on the rGO nanosheet and were confirmed by HR-TEM (high resolution-transmission electron microscopy) images. Furthermore, Brunauer–Emmett–Teller (BET) analysis was used to examine the large surface area of the seed-like structured ZrS2/rGO (56.0 cm2 g−1) and Mo@ZrS2/rGO (150.8 cm2 g−1) nanocatalyst (Fig. 1d and e). Furthermore, the resulting seed-like structured Mo@ZrS2/rGO nanocatalyst exhibits a pore diameter in the range of 52.3 and 49.0 Å, according to the BJH pore size distribution (Fig. 1d and e insert image), which is very advantageous for the charge-transfer phase of electrocatalytic activity. Consequently, the Mo@ZrS2/rGO nanocatalyst with a seed-like structure exhibits a large surface area for Mo atoms with a spherical shape that are dispersed on ZrS2 seeds (Fig. 1f and g).
m1 space group).38 However, after Mo was added to produce the final catalyst, each peak of ZrS2 and rGO grew more distinct. Moreover, a low-intensity peak corresponding to Mo appeared at 34.1, 41.1, 44.4, and 65.5° indicating the (0 1 2), (0 1 5), (0 0 9), and (0 1 11) planes, respectively.39 The diffraction pattern of these peaks corresponds to the regular, distinctive Mo@ZrS2/rGO (rhombohedral structure with the R3m space group), as demonstrated by JCPDS-086-2308. The crystal planes showed plenty of heteroatom production on the rGO nanosheet and were confirmed by HR-TEM (high resolution-transmission electron microscopy) images. Furthermore, Brunauer–Emmett–Teller (BET) analysis was used to examine the large surface area of the seed-like structured ZrS2/rGO (56.0 cm2 g−1) and Mo@ZrS2/rGO (150.8 cm2 g−1) nanocatalyst (Fig. 1d and e). Furthermore, the resulting seed-like structured Mo@ZrS2/rGO nanocatalyst exhibits a pore diameter in the range of 52.3 and 49.0 Å, according to the BJH pore size distribution (Fig. 1d and e insert image), which is very advantageous for the charge-transfer phase of electrocatalytic activity. Consequently, the Mo@ZrS2/rGO nanocatalyst with a seed-like structure exhibits a large surface area for Mo atoms with a spherical shape that are dispersed on ZrS2 seeds (Fig. 1f and g).
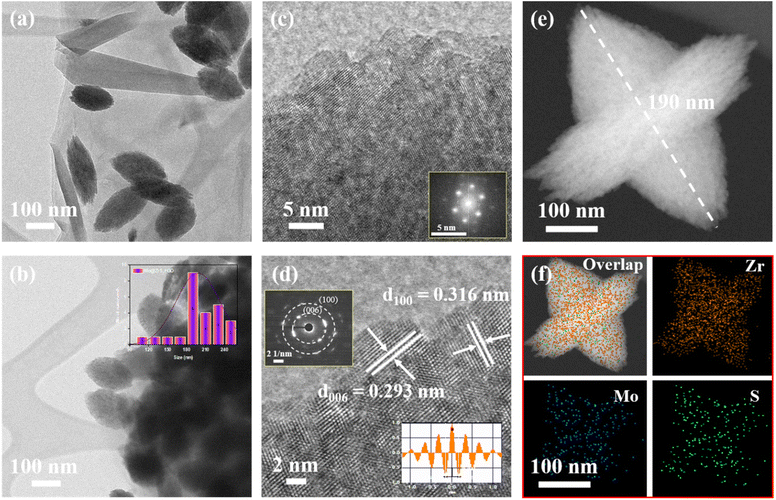
In addition to the perfect seed-like structured Mo@ZrS2 nanocatalyst on the developing rGO surface, the morphology was studied using images from transmission electron microscopy (TEM). The production of seed-like structures that were evenly distributed across the outermost layer of rGO nanosheets is evident in Fig. S3a and b.† Moreover, the size of the seed-like structured Mo@ZrS2 nanocatalyst was determined utilizing the results of individual histograms. On the outermost layer of rGO containing active sites, this revealed a mean length spacing of nanoseeds with an average particle diameter size of around 190 nm (Fig. 2a and b). The HR-TEM analysis of the seed-like structured Mo@ZrS2 nanocatalyst is presented in Fig. 2c and d. These images demonstrate the presence of distinct lattice fringes, measuring approximately 0.316 and 0.293 nm in equivalent crystalline size. The associated crystalline peaks are assigned to the (100) and (006) planes, respectively. Fig. S2a–c† illustrates the interparticle link between neighboring seed-like organized Mo@ZrS2 nanocatalysts. Through electrocatalysis, interparticle structures can enhance electron transfer across the seed-like structures, sustain mechanical strength, and confirm heteroatom formation.17
Moreover, the fast Fourier transform (FFT) and selected area electron diffraction (SAED) patterns verified the creation of a seed-like ordered Mo@ZrS2 nanocatalyst through polycrystalline structures (insert image; Fig. 2c and d). As seen in the Fig. 2d inset image, distinct lattice edges were found to have a d-spacing of 0.317 nm correlating with the (100) layer of Mo@ZrS2. TEM-EDS-elemental mapping in Fig. 2e and f, and S3c† demonstrated the successful doping of the Mo atom inside the novel structure of the seed-like ZrS2/rGO nanocatalyst. By using high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) imaging, the uniform distribution of Mo, Zr, S, O, and C elements was also determined. This demonstrated that the elements of Mo@ZrS2 nanocatalysts were similarly distributed across the rGO surface. Furthermore, Mo and Zr ratios in the product were found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 by energy-dispersive X-ray (EDX) spectroscopy, as is seen in Fig. S3d.†
3 by energy-dispersive X-ray (EDX) spectroscopy, as is seen in Fig. S3d.†
X-ray photoelectron spectroscopy (XPS) was used to confirm the chemical composition and binding states of Mo@ZrS2/rGO and ZrS2/rGO composites. The XPS spectra show that distinct peaks were dispersed throughout the rGO sheet for each element in the ZrS2/rGO and Mo@ZrS2/rGO composites. In addition, as illustrated in Fig. 3a, the survey spectrum determines the elemental compositions of the ZrS2/rGO and Mo@ZrS2/rGO samples, which comprise C, O, Zr, S, and extra Mo in the following sample. Specifically, ZrS2/rGO contains 44.4% carbon, 42.94% oxygen, 10.9% zirconium, and 1.72% sulfur, whereas Mo@ZrS2/rGO contains 48.14% carbon, 41.07% oxygen, 8.24% zirconium, 1.06% sulfur, and 1.49% molybdenum (inset image; Fig. 3a).
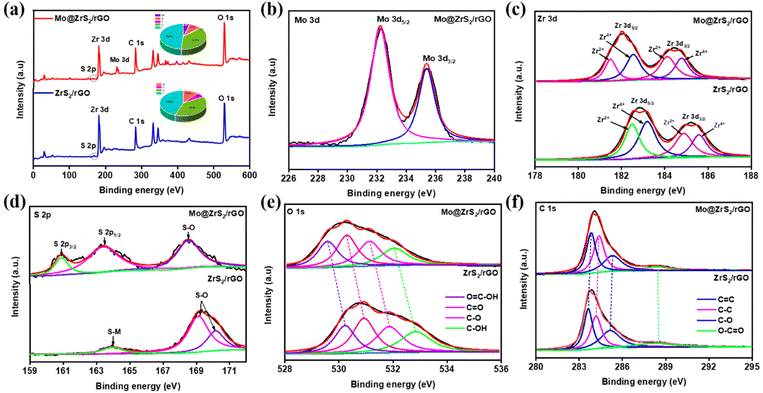 | ||
| Fig. 3 XPS analysis of ZrS2/rGO and Mo@ZrS2/rGO nanocomposites: (a) survey spectra and high-resolution XPS spectra of (b) Mo 3d, (c) Zr 3d, (d) S 2p, (e) O 1s, and (f) C 1s. | ||
The final synthesized catalyst was found to contain Mo 3d doublets (3d5/2 and 3d3/2) with high-resolution binding energies of 232.3 and 235.4 eV, respectively (Fig. 3b).40–42 The presence of ZrS2 and ZrS in ZrS2/rGO is confirmed by the peaks seen at 182.8 and 185.2 eV, which can be attributed to Zr 3d5/2 and Zr 3d3/2, respectively.43,44 In line with predictions, these peaks moved to 182.1 and 184.5 eV when Mo was added, signifying the creation of MoZrS2/rGO, which are shown in Fig. 3c. The addition of Mo to Mo@ZrS2/rGO causes an increase in the sulfur–metal bonding peaks, which appear as S 2p3/2 and S 2p1/2 at 160.9 and 163.4 eV, respectively.41,45 The high-resolution S 2p spectrum shows a low ratio of sulfur–metal bonding in ZrS2/rGO (Fig. 3d). However, as can be seen in Fig. 3e, the inclusion of Mo dramatically changes the binding energy of the O 1s spectrum: for O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH, C
C–OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O, and C–OH, respectively, it shifts from 529.6, 530.3, 531.1, and 532.1 eV to 530.2, 530.9, 531.9, and 532.1 eV.9 According to the high-resolution C 1s spectra study, ZrS2/rGO bonds with C
O, C–O, and C–OH, respectively, it shifts from 529.6, 530.3, 531.1, and 532.1 eV to 530.2, 530.9, 531.9, and 532.1 eV.9 According to the high-resolution C 1s spectra study, ZrS2/rGO bonds with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–C, C–O, and O–C
C, C–C, C–O, and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 283.6, 284.2, 285.2, and 288.5 eV, in that order (Fig. 3f).9,30 Furthermore, the C 1s spectrum is barely altered by the addition of Mo to ZrS2/rGO. Ultimately, our investigations verified that the rGO nanosheets were successfully formed from a seed-like structure. This suggests that nanocatalysts can enhance the electrochemical activity for applications using ORR and ZABs.
O at 283.6, 284.2, 285.2, and 288.5 eV, in that order (Fig. 3f).9,30 Furthermore, the C 1s spectrum is barely altered by the addition of Mo to ZrS2/rGO. Ultimately, our investigations verified that the rGO nanosheets were successfully formed from a seed-like structure. This suggests that nanocatalysts can enhance the electrochemical activity for applications using ORR and ZABs.
Evaluate the ORR performance of ZrS2/rGO and Mo@ZrS2/rGO nanocatalysts
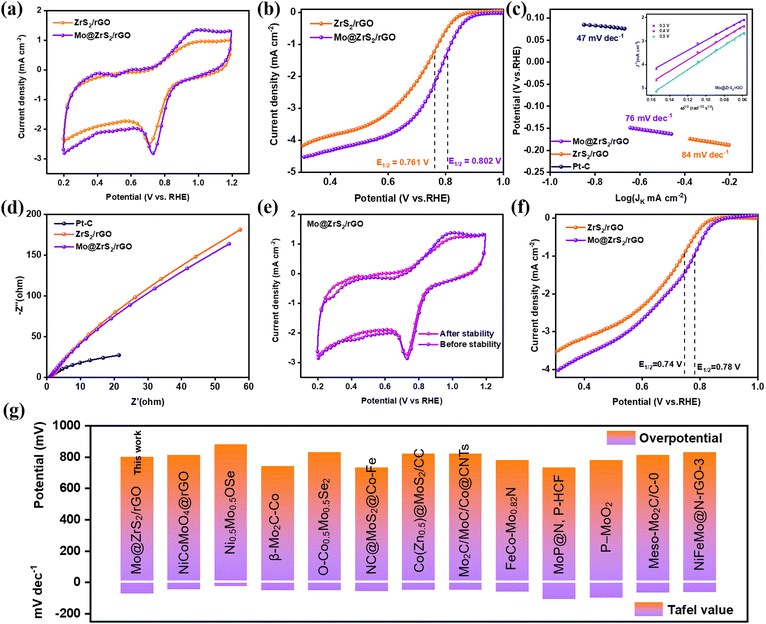
The ORR performance of ZrS2/rGO, Mo@ZrS2/rGO, and commercial Pt–C electrodes was investigated to explore the electrocatalytic activity in addition to having excellent cyclic durability. The electrocatalytic efficiency of the catalysts that were synthesized for the ORR was investigated using cyclic voltammograms (CVs) using 0.1 M KOH that were saturated with N2 and O2. However, when the electrolyte was saturated with O2, distinct cathodic reduction peaks emerged (Fig. 4a and S4a†), where the ORR electrocatalytic efficiency is represented by the strength and location of the reduction peaks. For the catalysts that were in the N2 saturated solution, there was no reduction peak. Mo@ZrS2/rGO demonstrated superior ORR performance, with a bigger higher peak potential at ≈0.80 V compared to the remaining catalysts (ZrS2/rGO; 0.76 V), apart from Pt–C (≈0.85 V). The various catalysts were subjected to linear sweep voltammograms (LSVs) utilizing an oscillating disk electrode (RDE) in a 0.1 M O2-saturated solution of KOH at different rotation speeds (400 to 2800 rpm) as shown in Fig. S4b–d.† Unless otherwise indicated, the blank response that was recorded under N2 was used to adjust the current density. The onset potential (Eonset = 0.86 V) and half-wave potential (E1/2 = 0.76 V) of ZrS2/rGO are comparatively lower than those of the other materials, as shown in Fig. 4b at 1600 rpm. This difference in ORR activity may be due to the presence of the stable state (Zr4+) in the active region and the significant aggregation of ZrS2/rGO throughout electrochemical testing. However, the highly conductive GO significantly increased the Eonset and E1/2 of ZrS2/rGO and also prevented the aggregation of ZrS2.Additionally, compared to ZrS2/rGO, Mo@ZrS2/rGO exhibits somewhat better Eonset (0.88 V) and E1/2 (0.80 V). This is largely because Mo-doped ZrS2 forms a large number of active locations (Mo4+ state) for an effective ORR. When compared to the remaining synthesis materials, Mo@ZrS2/rGO showed superior values, which are very similar to commercial Pt–C values (0.94 V and 0.84 V) (see in Fig. 4g and Table S1† for a comparison study). Furthermore, the limiting current (JL) at Mo@ZrS2/rGO displayed quicker ORR kinetics than Pt–C (9.6 mA cm−2) and was greater than that at ZrS2/rGO (4.1 mA cm−2) and Mo@ZrS2/rGO (4.6 mA cm−2).33 The Tafel slope for a prepared material was then computed, which is crucial for assessing the rate kinetics and figuring out the electrochemical processes of the reaction mechanistic pathway.46 According to Fig. 4c, Mo@ZrS2/rGO had a Tafel slope of 76 mV dec−1, which was considerably lower than those of ZrS2/rGO (84 mV dec−1) and commercial Pt–C (47 mV dec−1), indicating that reaction rates at Mo@ZrS2/rGO are faster than those at ZrS2/rGO.
LSV tests with different rotation speeds and the corresponding computations were carried out to study the mechanism of electron transfer through the ORR (Fig. S4b–d†). The Mo@ZrS2/rGO modified RDE was subjected to the Koutecky–Levich (K–L) relationship throughout a potential range of 0.3–0.5 V, as shown in the insert image; Fig. 4c, which shows how the current density increased as rotation speed increased. Remarkable linear relationships with almost identical gradients that were equivalent to commercial Pt–C were seen in all of the K–L plots, indicating first-order kinetics behavior for the ORR.47,48 The K–L equation was used to compute the amount of electrons transferred for the ORR at Mo@ZrS2/rGO, and the result was 2.12. This indicates that the catalyst has a 2e− ORR mechanism (O2 + H2O + 2e− → HO2− + OH− (2e)).47 Finally, among the produced catalysts, ZrS2/rGO, Mo@ZrS2/rGO, Pt–C, and electrolytic ions at the interface all showed a comparatively quick energy transfer rate, and Mo@ZrS2/rGO also showed a low resistance to charge transfer (Rct) (Fig. 4d).9 In addition, the cycling stability of the ZrS2/rGO and Mo@ZrS2/rGO was assessed using the CV approach. Fig. 4e and f demonstrates that the current density at Mo@ZrS2/rGO only decreased by 20 mV of its starting value after 1000 cycles at a scan rate of 500 mV. Fig. S5a–d† show the individual CV and LSV curves of Pt–C, ZrS2/rGO, and Mo@ZrS2/rGO electrocatalysts for assessing before and after cycling stability. Additionally, the Mo@ZrS2/rGO catalyst's post-morphology was examined, which is depicted in Fig. S7,† for cycling stability following the ORR. This picture demonstrates how the seed-like structure is still intact and how each seed is distributed equally across the rGO nanosheet surface (Fig. S7a–f†). Additionally, the as-produced seed-like structured Mo@ZrS2/rGO catalysts exhibit stable crystal planes, as shown in the HR-TEM image (Fig. S7g–i†), making them more stable and suitable for future energy storage applications.
Zn–air battery performance of ZrS2/rGO and Mo@ZrS2/rGO nanocatalysts
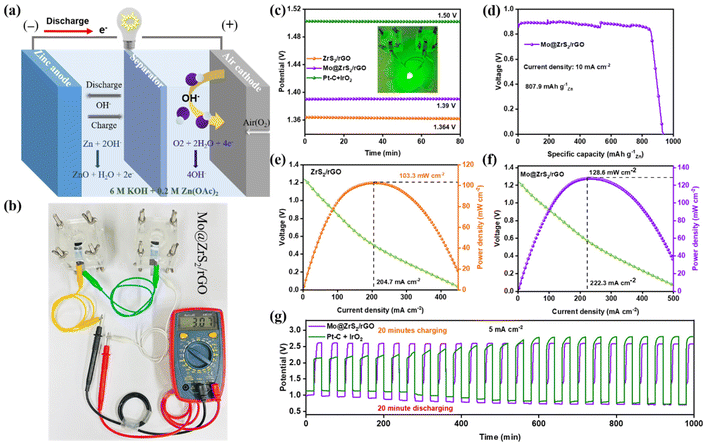
Because ZrS2/rGO, Mo@ZrS2/rGO, and Pt–C have excellent ORR characteristics, rechargeable ZABs were constructed by employing them as air electrodes. As seen in Fig. 5a, the Mo@ZrS2/rGO electrocatalyst is used as the air cathode in a ZAB, where a Zn plate functions as the anode and zinc acetate (0.2 M) is present in the 6 M KOH electrolyte.1,9 Using the same setup and the commercial Pt–C + IrO2 catalyst, comparative testing was also carried out. As seen in Fig. 5b,c, the open-circuit voltage (OCV) of the liquid ZAB was significantly different from that of Mo@ZrS2/rGO (1.39 V) and marginally lower than that of Pt–C + IrO2, indicating that Mo enhanced the electrocatalytic activity of the seed structure catalyst. Additionally, as illustrated in Fig. 5c, a green light-emitting device (LED) could be illuminated by joining two liquid ZABs with compared OCV.The Mo@ZrS2/rGO-based ZAB demonstrated a higher voltage platform than Pt–C + IrO2 when both were exposed to the same current densities. As the current densities increase, the discharge voltage differential becomes increasingly noticeable. Furthermore, the Mo@ZrS2/rGO battery exhibits a specific capacity and an associated energy density (measured in relation to the mass of Zn consumed) of 807.9 mA h g−1 (Fig. 5d), which is considerably greater than those of ZrS2/rGO (559 mA h g−1) and Pt–C + IrO2 (762 mA h g−1). This means that a variety of electronic gadgets can make use of the created Zn–air batteries. Because Mo@ZrS2/rGO electrocatalysts exhibit stronger ORR catalytic activity (Table S1†), charging and discharging polarization curves (Fig. S6c and d†) demonstrate a smaller voltage difference in comparison with the Mo@ZrS2/rGO and Pt–C + IrO2 catalyst. The discharge polarization curves and the corresponding power densities of Zn–air batteries are also shown in Fig. 5e and f and S6a and b.† It have been seen that the Mo@ZrS2/rGO electrocatalyst outperformed the ZrS2/rGO and comparable Pt–C + IrO2 electrodes, with the highest power density of 128.6 mW cm−2, compared to 103.3 and 223 mW cm−2.
Next, one of the most important characteristics of zinc–air batteries is their ability to withstand prolonged charge–discharge cycles, which is essential for assessing the rechargeability of air electrodes.33,49 Because of the cathodic Mo@ZrS2/rGO electrocatalyst, the ZAB charge–discharge potential curve exhibits remarkable stability for 25 cycles up to 16 h. As illustrated in Fig. 5g, the charge–discharge cycling experiments were conducted at a steady current density of 5 mA cm−2, with the discharge lasting 20 minutes and the charge lasting an extra 20 minutes. The Pt–C + IrO2 cathode, on the other hand, has a stability period of over 16 hours. Furthermore, the Mo@ZrS2/rGO electrocatalyst performed better than the Pt–C + IrO2 electrocatalyst at an average current density of 5 mA cm−2, with an electrode voltage gap between charge and discharge cycles of 1.6 V compared to 1.01 V for the Pt–C + IrO2 cathode (Fig. S6c and d†). This further implies that Mo@ZrS2/rGO is a bifunctional catalyst for cyclability and ORR activities that is highly effective. It is noteworthy that in the presence of Mo, ZrS2/rGO exhibits a constant charge voltage.
Lastly, the redox reactions that take place in alkaline solutions across the cathode and anode can be used to characterize the complete electrochemical system (eqn (1)–(3)).
Cathodic reaction:
| O2 + 2H2O + 4e− ⇌ 4OH− (E° = 0.4 V vs. SHE) | (1) |
Anodic reaction:
| Zn + 4OH− − 2e− ⇌ Zn(OH)42− (E° = −1.25 V vs. SHE) | (2) |
Overall reaction:
| Zn + ½ O2 ⇌ ZnO (E° = +1.65 V) | (3) |
4. Conclusion
A unique seed-like structure of ZrS2 on rGO was generated and structural deformation was restricted by the doped Mo atom on the ZrS2 surface. This was achieved by a simple hydrothermal-based annealing process. XRD, XPS, and TEM characterization methods were used to examine the produced catalysts. The resulting seed-like structure was used in ZABs and showed remarkable catalytic capabilities for ORRs. Mo@ZrS2/rGO has the longest cycling stability (at a scan rate of 500 mV) and the lowest half-wave potential (0.80 V) for the ORR in a 0.1 M KOH solution. In addition, Mo@ZrS2/rGO showed remarkable performance as an air cathode in zinc–air batteries due to its longer cycling stability, higher specific capacity (807.9 mA h g−1Zn), higher power density (128.6 mW cm−2), and higher OCV (1.39 V). Lastly, this is the first time that Mo-doped zirconium sulfide catalyst has been developed with a seed-like structure, and the findings offer a variety of prospects for further research and development to increase catalytic activity and incorporate novel features for energy storage and conversion applications.Author contributions
Ramasamy Santhosh Kumar: conceptualization, experimental, investigation, formal analysis, and writing – review of the original draft. Dilmurod Sayfiddinov and S. Tamilarasi: characterization and data visualization. Dong Jin Yoo: data visualization, review of the original draft, funding acquisition, and research supervision of the work.Data availability
The data supporting the findings of this study are available within the article and its ESI.† Source data are provided in this paper.Request can be made to the corresponding author for accessing the data.
Conflicts of interest
The authors declare no competing interests.Acknowledgements
This research was supported by the “Regional Innovation Strategy (RIS)” through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) (2024RIS-008). This research was supported by "Regional Integrated Hydrogen Industry Human Resource Development (jeonbuk National University) Program" grant funded by the Ministry of Trade, Industry and Energy (H2 Korea).References
- J. Balamurugan, P. M. Austeria, J. B. Kim, E.-S. Jeong, H.-H. Huang, D. H. Kim, N. Koratkar and S. O. Kim, Electrocatalysts for Zinc–Air Batteries Featuring Single Molybdenum Atoms in a Nitrogen-Doped Carbon Framework, Adv. Mater., 2023, 35, 2302625 CrossRef CAS
.
- J. Zhang, Z. Zhao, Z. Xia and L. Dai, A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions, Nat. Nanotechnol., 2015, 10, 444–452 CrossRef CAS
.
- Z. P. Cano, D. Banham, S. Ye, A. Hintennach, J. Lu, M. Fowler and Z. Chen, Batteries and fuel cells for emerging electric vehicle markets, Nat. Energy, 2018, 3, 279–289 CrossRef
.
- H. Zhang, G. Meng, Q. Liu, Y. Luo, M. Niederberger, L. Feng, J. Luo and X. Liu, Metal Phosphorous Chalcogenide: A Promising Material for Advanced Energy Storage Systems, Small, 2023, 19, 2303165 CrossRef CAS PubMed
.
- W. Sun, F. Wang, B. Zhang, M. Zhang, V. Küpers, X. Ji, C. Theile, P. Bieker, K. Xu, C. Wang and M. Winter, A rechargeable zinc-air battery based on zinc peroxide chemistry, Science, 2021, 371, 46–51 CrossRef CAS
.
- Y. Ma, S. Tang, H. Wang, Y. Liang, D. Zhang, X. Xu, Q. Wang and W. Li, Bimetallic ZIFs-derived electrospun carbon nanofiber membrane as bifunctional oxygen electrocatalyst for rechargeable zinc-air battery, J. Energy Chem., 2023, 83, 138–149 CrossRef CAS
.
- M. Luo and M. T. M. Koper, A kinetic descriptor for the electrolyte effect on the oxygen reduction kinetics on Pt(111), Nat. Catal., 2022, 5, 615–623 CrossRef CAS
.
- A. Grimaud, A. Demortière, M. Saubanère, W. Dachraoui, M. Duchamp, M.-L. Doublet and J.-M. Tarascon, Erratum: Activation of surface oxygen sites on an iridium-based model catalyst for the oxygen evolution reaction, Nat. Energy, 2017, 2, 17002 CrossRef
.
- R. S. Kumar, P. Mannu, S. Prabhakaran, T. T. T. Nga, Y. Kim, D. H. Kim, J.-L. Chen, C.-L. Dong and D. J. Yoo, Trimetallic Oxide Electrocatalyst for Enhanced Redox Activity in Zinc–Air Batteries Evaluated by In Situ Analysis, Adv. Sci., 2023, 10, 2303525 CrossRef CAS
.
- F. Zhang, Y. Zhu, Y. Zhong, J. Zou, Y. Chen, L. Zu, Z. Wang, J. J. Hinsch, Y. Wang, L. Zhang, Z. Shao and H. Wang, Tuning the charge distribution and crystal field of iron single atoms via iron oxide integration for enhanced oxygen reduction reaction in zinc-air batteries, J. Energy Chem., 2023, 85, 154–163 CrossRef CAS
.
- J. Ban, H. Xu, G. Cao, Y. Fan, W. K. Pang, G. Shao and J. Hu, Synergistic Effects of Phase Transition and Electron-Spin Regulation on the Electrocatalysis Performance of Ternary Nitride, Adv. Funct. Mater., 2023, 33, 2300623 CrossRef CAS
.
- S. Yi, R. Xin, X. Li, Y. Sun, M. Yang, B. Liu, H. Chen, H. Li and Y. Liu, “Setaria viridis”-like cobalt complex derived Co/N-doped carbon nanotubes as efficient ORR/OER electrocatalysts for long-life rechargeable Zn–air batteries, Nanoscale, 2023, 15, 16612–16618 RSC
.
- A. Singh, R. Sharma and A. Halder, Flexible solid-state Zn–air battery based on polymer-oxygen-functionalized g-C3N4 composite membrane, Nanoscale, 2024, 16, 4157–4169 RSC
.
- Y.-P. Deng, Y. Jiang, R. Liang, S.-J. Zhang, D. Luo, Y. Hu, X. Wang, J.-T. Li, A. Yu and Z. Chen, Dynamic electrocatalyst with current-driven oxyhydroxide shell for rechargeable zinc-air battery, Nat. Commun., 2020, 11, 1952 CrossRef CAS
.
- L. Liu, X. Zhang, F. Yan, B. Geng, C. Zhu and Y. Chen, Self-supported N-doped CNT arrays for flexible Zn–air batteries, J. Mater. Chem. A, 2020, 8, 18162–18172 RSC
.
- R. J. Toh, A. Y. S. Eng, Z. Sofer, D. Sedmidubsky and M. Pumera, Ternary Transition Metal Oxide Nanoparticles with Spinel Structure for the Oxygen Reduction Reaction, ChemElectroChem, 2015, 2, 982–987 CrossRef CAS
.
- R. Santhosh Kumar, P. Muthu Austeria, C. Sagaya Selvam Neethinathan, S. Ramakrishnan, K. Sekar, A. R. Kim, D. H. Kim, P. J. Yoo and D. J. Yoo, Highly mixed high-energy d-orbital states enhance oxygen evolution reactions in spinel catalysts, Appl. Surf. Sci., 2023, 641, 158469 CrossRef CAS
.
- M. Chisaka, A. Ishihara, K.-I. Ota and H. Muramoto, Synthesis of carbon-supported titanium oxynitride nanoparticles as cathode catalyst for polymer electrolyte fuel cells, Electrochim. Acta, 2013, 113, 735–740 CrossRef CAS
.
- V. Kashyap and S. Kurungot, Zirconium-Substituted Cobalt Ferrite Nanoparticle Supported N-doped Reduced Graphene Oxide as an Efficient Bifunctional Electrocatalyst for Rechargeable Zn–Air Battery, ACS Catal., 2018, 8, 3715–3726 CrossRef CAS
.
- N. Logeshwaran, I. R. Panneerselvam, S. Ramakrishnan, R. S. Kumar, A. R. Kim, Y. Wang and D. J. Yoo, Quasihexagonal Platinum Nanodendrites Decorated over CoS2-N-Doped Reduced Graphene Oxide for Electro-Oxidation of C1-, C2-, and C3-Type Alcohols, Adv. Sci., 2022, 9, 2105344 CrossRef CAS
.
- H. Yang, B. Wang, H. Li, B. Ni, K. Wang, Q. Zhang and X. Wang, Trimetallic Sulfide Mesoporous Nanospheres as Superior Electrocatalysts for Rechargeable Zn–Air Batteries, Adv. Energy Mater., 2018, 8, 1801839 CrossRef
.
- J. Zhang, X. Bai, T. Wang, W. Xiao, P. Xi, J. Wang, D. Gao and J. Wang, Bimetallic Nickel Cobalt Sulfide as Efficient Electrocatalyst for Zn–Air Battery and Water Splitting, Nano-Micro Lett., 2019, 11, 2 CrossRef CAS PubMed
.
- Z. Wang, C. Li, Y. Liu, Y. Wu, S. Zhang and C. Deng, Atomically dispersed Fe-Ni dual sites in heteroatom doped carbon tyres for efficient oxygen electrocatalysis in rechargeable Zn-Air battery, J. Energy Chem., 2023, 83, 264–274 CrossRef CAS
.
- M. Kumar and T. C. Nagaiah, A multifunctional cobalt iron sulfide electrocatalyst for high performance Zn–air batteries and overall water splitting, J. Mater. Chem. A, 2022, 10, 4720–4730 RSC
.
- M. Wang, L. Zhang, Y. He and H. Zhu, Recent advances in transition-metal-sulfide-based bifunctional electrocatalysts for overall water splitting, J. Mater. Chem. A, 2021, 9, 5320–5363 RSC
.
- H. Chen, Z. Yu, Y. Hou, R. Jiang, J. Huang, W. Tang, Z. Cao, B. Yang, C. Liu and H. Song, Double MOF gradually activated S bond induced S defect rich MILN-based Co(z)-NiMoS for efficient electrocatalytic overall water splitting, Nanoscale, 2021, 13, 20670–20682 RSC
.
- X. Cao, S. Zheng, T. Wang, F. Lin, J. Li and L. Jiao, N-doped ZrO2 nanoparticles embedded in a N-doped carbon matrix as a highly active and durable electrocatalyst for oxygen reduction, Fundam. Res., 2022, 2, 604–610 CrossRef CAS PubMed
.
- X. Zhao, J. Wang, J. Wang, M. Yang, C. Yan, G. Zou, J. S. Tse, C. Fernandez and Q. Peng, Atomically dispersed quintuple nitrogen and oxygen co-coordinated zirconium on graphene-type substrate for highly efficient oxygen reduction reaction, Cell Rep. Phys. Sci., 2022, 3, 100773 CrossRef CAS
.
- H. Sudrajat and S. Babel, Comparison and mechanism of photocatalytic activities of N-ZnO and N-ZrO2 for the degradation of rhodamine 6G, Environ. Sci. Pollut. Res., 2016, 23, 10177–10188 CrossRef CAS
.
- S. Tamilarasi, R. S. Kumar, K.-B. Cho, C.-J. Kim and D. J. Yoo, High-performance electrochemical detection of glucose in human blood serum using a hierarchical NiO2 nanostructure supported on phosphorus doped graphene, Mater. Today Chem., 2023, 34, 101765 CrossRef CAS
.
- R. Santhosh Kumar, K. Govindan, S. Ramakrishnan, A. R. Kim, J.-S. Kim and D. J. Yoo, Fe3O4 nanorods decorated on polypyrrole/reduced graphene oxide for electrochemical detection of dopamine and photocatalytic degradation of acetaminophen, Appl. Surf. Sci., 2021, 556, 149765 CrossRef CAS
.
- Y. Liang, Y. Li, H. Wang, J. Zhou, J. Wang, T. Regier and H. Dai, Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction, Nat. Mater., 2011, 10, 780–786 CrossRef CAS PubMed
.
- R. S. Kumar, S. Prabhakaran, S. Ramakrishnan, S. C. Karthikeyan, A. R. Kim, D. H. Kim and D. J. Yoo, Developing Outstanding Bifunctional Electrocatalysts for Rechargeable Zn-Air Batteries Using High-Purity Spinel-Type ZnCo2Se4 Nanoparticles, Small, 2023, 19, 2207096 CrossRef CAS PubMed
.
- R. Santhosh Kumar, S. Ramakrishnan, S. Prabhakaran, A. R. Kim, D. R. Kumar, D. H. Kim and D. J. Yoo, Structural, electronic, and electrocatalytic evaluation of spinel transition metal sulfide supported reduced graphene oxide, J. Mater. Chem. A, 2022, 10, 1999–2011 RSC
.
- R. Santhosh Kumar, S. C. Karthikeyan, S. Ramakrishnan, S. Vijayapradeep, A. R. Kim, J.-S. Kim and D. J. Yoo, Anion dependency of spinel type cobalt catalysts for efficient overall water splitting in an acid medium, Chem. Eng. J., 2023, 451, 138471 CrossRef CAS
.
- S. B. Patil, M. S. Raghu, B. Kishore and G. Nagaraju, Enhanced electrochemical performance of few-layered MoS2–rGO nanocomposite for lithium storage application, J. Mater. Sci.: Mater. Electron., 2019, 30, 316–322 CrossRef CAS
.
- A. Smirnov, N. W. Solís Pinargote, N. Peretyagin, Y. Pristinskiy, P. Peretyagin and J. F. Bartolomé, Zirconia Reduced Graphene Oxide Nano-Hybrid Structure Fabricated by the Hydrothermal Reaction Method, Materials, 2020, 13, 687 CrossRef CAS PubMed
.
- P. Fadojutimi, C. Masemola, M. Maubane-Nkadimeng, E. Linganiso, Z. Tetana, J. Moma, N. Moloto and S. Gqoba, Room temperature sensing of primary alcohols via polyaniline/zirconium disulphide, Heliyon, 2023, 9, e16216 CrossRef CAS
.
- J. Chen, Y. Xia, J. Yang and B. Chen, Fabrication of monolayer MoS2/rGO hybrids with excellent tribological performances through a surfactant-assisted hydrothermal route, Appl. Phys. A, 2018, 124, 430 CrossRef
.
- G. Tai, T. Zeng, J. Yu, J. Zhou, Y. You, X. Wang, H. Wu, X. Sun, T. Hu and W. Guo, Fast and large-area growth of uniform MoS2 monolayers on molybdenum foils, Nanoscale, 2016, 8, 2234–2241 RSC
.
- H. Yu, C. Ma, B. Ge, Y. Chen, Z. Xu, C. Zhu, C. Li, Q. Ouyang, P. Gao, J. Li, C. Sun, L. Qi, Y. Wang and F. Li, Three-Dimensional Hierarchical Architectures Constructed by Graphene/MoS2 Nanoflake Arrays and Their Rapid Charging/Discharging Properties as Lithium-Ion Battery Anodes, Chem. – Eur. J., 2013, 19, 5818–5823 CrossRef CAS PubMed
.
- A. Wang, S. Gao, J. Yan, C. Zhao, M. Yu and W. Wang, Vacancy-modified bimetallic FeMoSx/CoNiPx heterostructure array for efficient seawater splitting and Zn-air battery, J. Energy Chem., 2023, 81, 533–542 CrossRef CAS
.
- J. Zhou, X. Zha, F. Y. Chen, Q. Ye, P. Eklund, S. Du and Q. Huang, A Two-Dimensional Zirconium Carbide by Selective Etching of Al3C3 from Nanolaminated Zr3Al3C5, Angew. Chem., Int. Ed., 2016, 55, 5008–5013 CrossRef CAS PubMed
.
- A. P. Rizzato, C. V. Santilli, S. H. Pulcinelli, Y. Messaddeq and P. Hammer, XPS Study of the Corrosion Protection of Fluorozirconate Glasses Dip-Coated with SnO2 Transparent Thin Films, J. Sol-Gel Sci. Technol., 2004, 32, 155–160 CrossRef CAS
.
- M. Otomo, M. Hamada, R. Ono, I. Muneta, K. Kakushima, K. Tsutsui and H. Wakabayashi, Chemical states of PVD-ZrS2 film underneath scaled high-k film with self-oxidized ZrO2 film as interfacial layer, Jpn. J. Appl. Phys., 2023, 62, SC1015 CrossRef
.
- T. Shinagawa, A. T. Garcia-Esparza and K. Takanabe, Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion, Sci. Rep., 2015, 5, 13801 CrossRef PubMed
.
- R. Zhou, Y. Zheng, M. Jaroniec and S.-Z. Qiao, Determination of the Electron Transfer Number for the Oxygen Reduction Reaction: From Theory to Experiment, ACS Catal., 2016, 6, 4720–4728 CrossRef CAS
.
- T. Wang, Q. Zhang, K. Lian, G. Qi, Q. Liu, L. Feng, G. Hu, J. Luo and X. Liu, Fe nanoparticles confined by multiple-heteroatom-doped carbon frameworks for aqueous Zn-air battery driving CO2 electrolysis, J. Colloid Interface Sci., 2024, 655, 176–186 CrossRef CAS PubMed
.
- S. Gao, X. Wang, X. Liu, C. Guo, Q. Liu and G. Hu, Recent progress in iron-series-element-based electrocatalysts for Zn–air batteries, Mater. Chem. Front., 2024, 8, 485–506 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr01191k |
| This journal is © The Royal Society of Chemistry 2024 |