 Open Access Article
Open Access ArticleConsecutive one-pot alkyne semihydrogenation/alkene dioxygenation reactions by Pt(II)/Cu(II) single-chain nanoparticles in green solvent†
Jokin
Pinacho-Olaciregui
ab,
Ester
Verde-Sesto
ac,
Daniel
Taton
 b and
José A.
Pomposo
b and
José A.
Pomposo
 *acd
*acd
aCentro de Física de Materiales (CSIC – UPV/EHU) – Materials Physics Center MPC, P° Manuel Lardizabal 5, E-20018 Donostia, Spain. E-mail: josetxo.pomposo@ehu.eus
bLaboratoire de Chimie des Polymères Organiques (LCPO), Université de Bordeaux INP-ENSCBP, 16 av. Pey Berland, 33607 Pessac cedex, France
cIKERBASQUE – Basque Foundation for Science, Plaza Euskadi 5, E-48009 Bilbao, Spain
dDepartamento de Polímeros y Materiales Avanzados: Física, Química y Tecnología. University of the Basque Country (UPV/EHU), P° Manuel Lardizabal 3, E-20800 Donostia, Spain
First published on 29th April 2024
Abstract
Heterobimetallic Pt(II)/Cu(II) single-chain polymer nanoparticles (SCNPs) were sequentially synthesized from a polymeric precursor featuring both α-diazo-β-ketoester and naked β-ketoester functional groups. Photoactivated carbene generation at λexc = 365 nm from α-diazo-β-ketoester moieities was triggered for bonding Pt(II) ions from dichloro(1,5-cyclooctadiene)Pt(II) to the polymeric precursor, whereas Cu(II) ions were subsequently incorporated via Cu(II)-(β-ketoester)2 complex formation using Cu(II) acetate. Both intrachain Pt(II) bonding and Cu(II) complexation were found to contribute to the folding of the polymeric precursor generating Pt(II)/Cu(II)-SCNPs as evidenced by infrared spectroscopy, size exclusion chromatography and dynamic light scattering. These heterobimetallic SCNPs proved highly efficient as soft nanocatalysts for the consecutive one-pot alkyne semihydrogenation/alkene dioxygenation reactions at room temperature in N-butylpyrrolidone, as a non-toxic alternative solvent to N,N-dimethylformamide.
Metal–ligand coordination allows intramolecular folding of ligand-decorated discrete synthetic polymer chains to metallo-folded single-chain polymer nanoparticles (SCNPs).1 SCNPs are intra-chain cross-linked single polymer chains with manifold promising applications, mainly as catalysts, nanosensors and drug nanocarriers.2,3 In general, intramolecular folding of the isolated synthetic chains generates locally compact zones within the SCNPs for efficient immobilization of catalytic active species, luminophores or drugs.4 To some extent, the intrachain folding of discrete synthetic polymer chains to SCNPs resembles the folding of certain proteins to their precise functional conformation (i.e., native state).5 In particular, metallo-folded SCNPs leverage the dual role played by the metal: as a folding element via intra-chain metal–ligand coordination, and as an immobilized functional center for subsequent catalysis.6 The number and catalytic applications of metallo-folded SCNPs as enzyme-mimetic nanoentities have grown significantly in recent years.7,8 In a seminal work, Terashima et al. reported Ru-containing amphiphilic SCNPs to catalyse the reduction of cyclohexanone to cyclohexanol in water.9 Pomposo et al. pioneered the introduction of metallo-folded Cu(II)-containing SCNPs showing catalytic selectivity in alkyne homocoupling reactions,6 and single-chain globules mimicking the morphology and polymerase activity of metalloenzymes.10 He et al. prepared metallo-folded SCNPs containing Ni-thiolate complexes showing excellent thermal stability under aerobic conditions and excellent activity and selectivity during the photocatalytic reduction of CO2 to CO.11 Zimmerman et al. developed “clickase” SCNPs displaying enzyme-like “click” catalysis in vivo and enabling efficient cell surface glycan editing.12 Taton et al. reported SCNPs containing Ag(I)-N-heterocyclic carbene (NHC) linkages as NHC pre-catalysts for the benzoin condensation reaction.13 Yang et al. synthesized metal-containing SCNPs in concentrated solutions at room temperature (r.t.) by introducing electrostatic repulsion and intra-chain crosslinking by coordination with Cu(II) or Fe(II) or Fe(III) ions.14 Tan et al. synthesized enzyme-mimetic SCNPs with chiral Fe(II)-oxazoline complexes for efficient asymmetric sulfa-Michael addition of thiols to α,β-unsaturated ketones in water at r.t.15 More recently, Pomposo et al. developed a method to upcycling poly(vinyl chloride) (PVC) waste to efficient catalytic Cu(II)-containing SCNPs.16 Current advances in catalysis utilizing SCNPs have been recently reviewed by the Barner-Kowollik team.17
However, introduction of at least two distinct metal species in SCNPs although highly desirable is still synthetically challenging, since it requires designing a polymer chain able to complex both metal species while maintaining their orthogonality. In a pioneering work, Lemcoff et al. synthesized Rh(I)/Ir(I) SCNPs although their catalytic properties were not evaluated.18 Subsequently, Barner-Kowollik et al. synthesized heterobimetallic Eu(III)/Pt(II)-SCNPs19 and Au(I)/Y(III)-SCNPs20 in which only one of the two metal ions, Pt(II) or Au(I), was used for catalysis, while the other was employed for sensing or intrachain folding (Eu(III) or Y(III), respectively). More recently, the same group decorated SCNPs folded through ferrocene units with Pd(II) atoms, which proved to be an active catalyst for the intramolecular hydroamination of an aminoalkyne.21
Advanced heterobimetallic nanocatalysts for carrying out multistep chemical processes in a single reaction vessel are currently of great interest in academia and industry.22 Often, however, major costs of many consumer products synthesized in multistep processes are incurred in the purification and isolation of intermediates.23 Additionally, replacement of toxic organic solvents by green solvents has attracted significant interest.24 To the best of our knowledge, bimetallic SCNPs that would allow consecutive one-pot reactions to be performed in a green solvent have not been reported. To fill this gap, we report herein the proof of concept of heterobimetallic SCNPs through the synthesis of Pt(II)/Cu(II)-SCNPs, since these SCNPs could show synergistic effects for catalysis and other applications. We show that these can serve as advanced soft nanocatalysts to perform consecutive one-pot alkyne semihydrogenation/alkene dioxygenation reactions in N-butylpyrrolidone (NBP) as a green solvent.
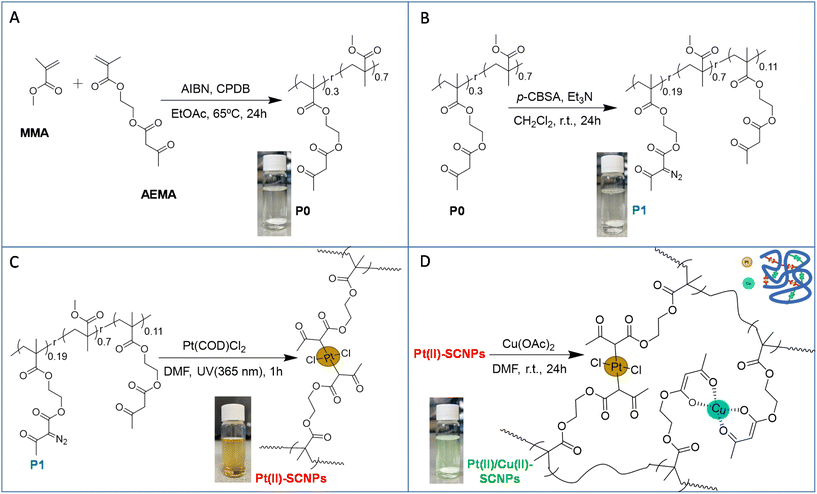
The synthetic procedure followed in this work to produce Pt(II)/Cu(II)-SCNPs is depicted schematically in Fig. 1. Initially, the monomers (2-acetoacetoxy)ethyl methacrylate (AEMA) and methyl methacrylate (MMA) were copolymerized via reversible addition fragmentation chain-transfer (RAFT) polymerization6 yielding the random copolymer P0 (Fig. 1A). Subsequently, P0 with a content of β-ketoester functional groups of 30 mol%, a weight-average molecular weight of Mw = 73.6 kDa and a narrow dispersity of Đ = 1.17 was post-functionalized with 19 mol% of α-diazo-β-ketoester functional motifs-using p-carboxybenzenesulfonazide (p-CBSA) as the diazo transfer reagent-25 leaving 11 mol% β-ketoester functional groups unreacted (see Fig. 1B and ESI†). Upon the diazo transfer reaction, the resulting polymeric precursor P1 showed Mw = 75.3 kDa and Đ = 1.15, in very good agreement with the expected Mw (75.4 kDa) based on its chemical composition. Infrared (IR) spectroscopy measurements confirmed the appearance of the characteristic infrared vibration band of the diazo moieties centred at ν ≈ 2200 cm−1 (Fig. 2A). Heterobimetallic Pt(II)/Cu(II)-SCNPs were sequentially synthesized from P1 containing both α-diazo-β-ketoester and unreacted β-ketoester functional groups. Thus, P1 was first irradiated with UV light at λexc = 365 nm at high dilution in the presence of dichloro(1,5-cyclooctadiene)Pt(II) (Pt(COD)Cl2) to generate the highly reactive carbene species from the α-diazo-β-ketoester functional groups and to promote intrachain cross-linking via Pt(II) bonding (see Fig. 1C).
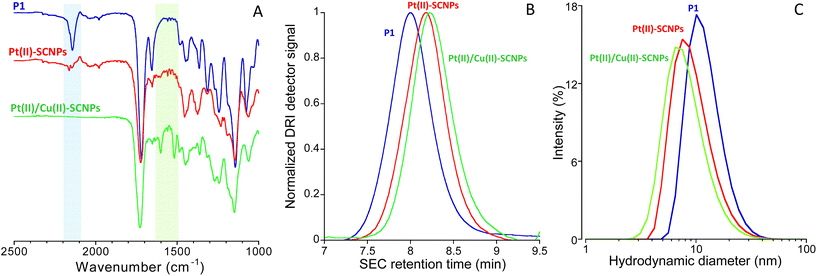 | ||
| Fig. 2 (A) Infrared (IR) spectra of polymeric precursor P1, Pt(II)-SCNPs and Pt(II)/Cu(II)-SCNPs. (B) Size exclusion chromatography (SEC) traces (differential refractive index (DRI) detector, DMF, 1 mL min−1) of P1, Pt(II)-SCNPs and Pt(II)/Cu(II)-SCNPs. (C) Dynamic light scattering (DLS) size distributions of P1, Pt(II)-SCNPs and Pt(II)/Cu(II)-SCNPs (see ESI† for details). | ||
Successful folding of P1 to Pt(II)-SCNPs was confirmed through size exclusion chromatography (SEC) and dynamic light scattering (DLS) measurements, whereas the IR spectrum of Pt(II)-SCNPs showed the disappearance of the characteristic diazo IR vibration band after 1 h of UV irradiation (see Fig. 2A). SEC results showed an increase in retention time, hence, a reduction in hydrodynamic size for the Pt(II)-SCNPs when compared to P1 (Fig. 2B). The average hydrodynamic diameter of P1, Dh = 12.5 nm as determined by DLS, was found to decrease to Dh = 9.9 nm upon formation of the Pt(II)-SCNPs (Fig. 2C). In a final step, Cu(II) ions were incorporated using Cu(II) acetate6 to form – in an orthogonal manner – Cu(II)-(β-ketoester)2 complexes from free β-ketoester functional groups of the Pt(II)-SCNPs (11 mol%) affording the heterobimetallic Pt(II)/Cu(II)-SCNPs (see Fig. 1D). Successful (additional) folding of Pt(II)-SCNPs into Pt(II)/Cu(II)-SCNPs was revealed by SEC (Fig. 2B) and DLS (Fig. 2C) analyses. The average hydrodynamic diameter of Pt(II)/Cu(II)-SCNPs was found to be Dh = 8.6 nm. The less pronounced reduction in hydrodynamic size upon the second folding event can be attributed to the reduced degrees of freedom available after the first Pt(II)-induced compaction.26 Complexation of Cu(II) ions by the residual β-ketoester groups was confirmed from the IR spectrum of the Pt(II)/Cu(II)-SCNPs. Fig. 2A indeed shows the characteristic vibration bands located at ca. 1600 cm−1 (stretching C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration, enol tautomer bonded to Cu) and ca. 1515 cm−1 (stretching C
O vibration, enol tautomer bonded to Cu) and ca. 1515 cm−1 (stretching C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibration, enol tautomer bonded to Cu).6 Inductively coupled plasma-mass spectrometry (ICP-MS) measurements showed a content of Pt(II) and Cu(II) ions in the Pt(II)/Cu(II)-SCNPs of 9.7 μg mg−1 and 9.5 μg mg−1, respectively (see ESI†).
C vibration, enol tautomer bonded to Cu).6 Inductively coupled plasma-mass spectrometry (ICP-MS) measurements showed a content of Pt(II) and Cu(II) ions in the Pt(II)/Cu(II)-SCNPs of 9.7 μg mg−1 and 9.5 μg mg−1, respectively (see ESI†).
With the Pt(II)/Cu(II)-SCNPs in hand, we envisioned to use them as advanced soft nanocatalysts for consecutive one-pot (incompatible) reactions. To this end, we targered the preparation of β-keto-N-alkoxyphthalimides, known intermediates of great utility for the pharmaceutical and agricultural industries,27 by using phenylacetylene substrates.
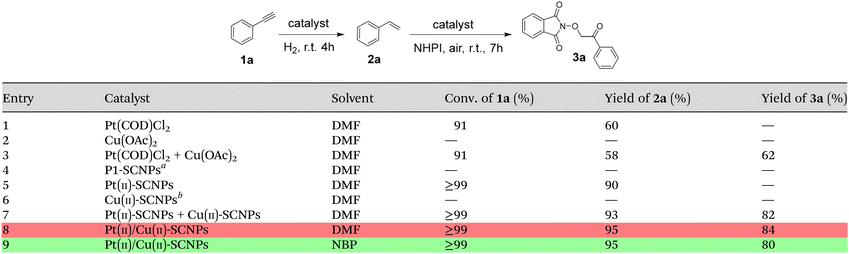
Table 1 shows a comparison of Pt(II)/Cu(II)-SCNPs with different control catalysts for the consecutive one-pot phenylacetylene (1a) semihydrogenation/styrene (2a) dioxygenation with air and N-hydroxyphthalimide (NHPI) at r.t. to give the β-keto-N-alkoxyphthalimide 3a as the target product (see ESI†). With Pt(COD)Cl2 (1 mol% Pt(II)) 1a was transformed into 2a in DMF with 60% yield, along with a large portion of 1a completely hydrogenated to ethylbenzene (Table 1, entry 1). As expected, Cu(OAc)2 (1 mol% Cu(II)) proved inefficient for the semihydrogenation of 1a (Table 1, entry 2). A combination of Pt(COD)Cl2 for the semihydrogenation reaction, and subsequently adding Cu(OAc)2 for the dioxygenation reaction gives a yield of 2a and 3a of 58% and 62%, respectively (Table 1, entry 3). Pt(II)-SCNPs (i.e., 0.13 mol% Pt(II)) provided 2a in 90% yield (Table 1, entry 5) whereas both P1-SCNPs synthesized–as a control–from P1 without Pt(COD)Cl2 and Cu(II)-SCNPs synthesized also as a control from P1-SCNPs, were totally inefficient (Table 1, entries 4 and 6). A combination of Pt(II)-SCNPs for the semihydrogenation reaction, and subsequently addition of Cu(II)-SCNPs (0.41 mol% Cu(II)) for the dioxygenation reaction provided 2a and 3a in 93% and 82% yield, respectively (Table 1, entry 7). Remarkably, the heterobimetallic Pt(II)/Cu(II)-SCNPs (0.14 mol% Pt(II) and 0.43 mol% Cu(II)) allowed the consecutive one-pot semihydrogenation/dioxygenation reaction to be carried out in DMF with exceptional selectivity and yield (Table 1, entry 8). Moreover, a similar efficiency and selectivity was found for the heterobimetallic Pt(II)/Cu(II)-SCNPs when the consecutive one-pot semihydrogenation of 1a/dioxygenation of 2a with air and NHPI was carried out in NBP as a green solvent to replace toxic DMF.
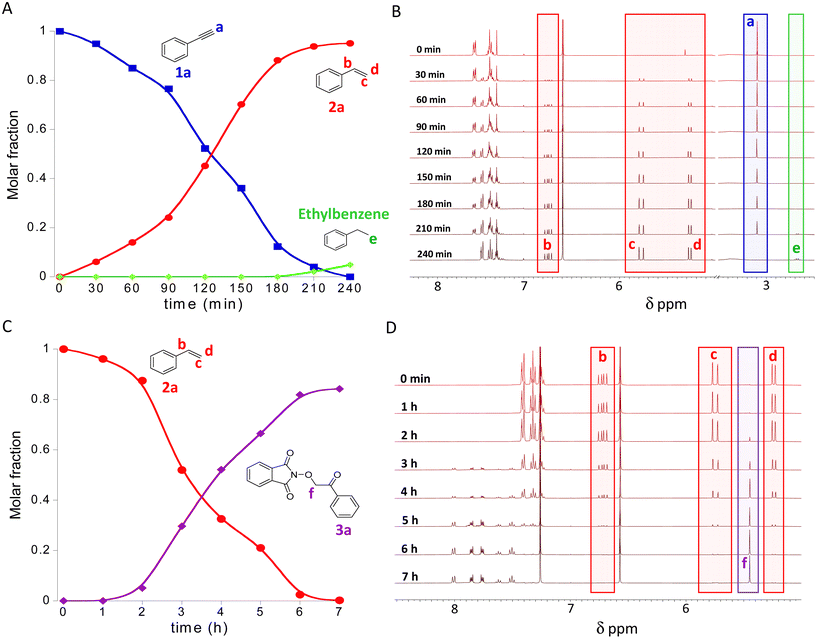
Fig. 3A shows the transformation of 1a into 2a over time during the semihydrogenation reaction in NBP, as monitored by 1H NMR spectroscopy. The kinetics of this reaction was followed through the disappearance of the alkyne proton of 1a, denoted as a in Fig. 3B and the concomitant appearance of the vinylic protons, denoted as b, c and d of 2a (Fig. 3B). In the late stages, i.e., after 210 min of reaction time, hydrogenation of 2a to ethylbenzene (secondary product) takes place (see Fig. 3B). After 4 h of reaction time, the conversion of 1a reached completion with a final yield in 2a of 95%. The turnover number (moles of product per mole of catalyst, TON) of the Pt(II)/Cu(II)-SCNPs in the semihydrogenation reaction was TON = 679 (a value 10 times higher than that of Pt(COD)Cl2 at 1 mol% Pt(II), see ESI, Table S3†). Without isolation of 2a, the crude product of the semi-hydrogenation reaction containing the bimetallic Pt(II)/Cu(II)-SCNPs was used for the dioxygenation of 2a with air and NHPI at r.t. in the same reaction vessel. Fig. 3C illustrates the consumption of 2a and the generation of the β-keto-N-alkoxyphthalimide 3a, as the target product, over reaction time. Also in this case, the kinetics of the dioxygenation reaction in NBP was directly monitored by 1H NMR spectroscopy through the disappearance of the vinylic protons, denoted as b, c and d of 2a (Fig. 3D) and the simultaneous appearance of the methylene protons denoted as f in Fig. 3D of 3a. After 7 h of reaction, the conversion of 2a was nearly complete and the yield of 3a was 80%. The turnover number of the Pt(II)/Cu(II)-SCNPs in the dioxygenation reaction was TON = 186. This value is 30-fold higher than that corresponding to the classical procedure27 involving Cu(OAc)2 as catalysts (see Table S3†).
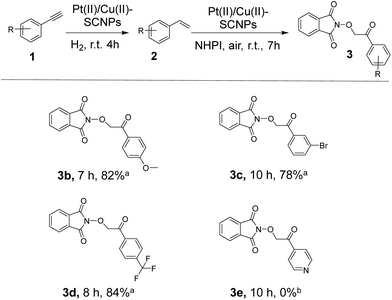
Finally, we investigated the substrate scope of the consecutive one-pot alkyne semihydrogenation/alkene dioxygenation reactions catalysed by Pt(II)/Cu(II)-SCNPs in NBP (see Scheme 1). Substituted phenylacetylene at the para-position with a methoxy group gave the β-keto-N-alkoxyphthalimide 3b in 82% isolated yield. The reaction of phenylacetylene bearing a bromine atom at meta-position produced the corresponding β-keto-N-alkoxyphthalimide 3c in 78% isolated yield. The introduction of a trifluoromethyl subtituent in phenylacetylene at the para-position afforded the β-keto-N-alkoxyphthalimide 3d in 84% isolated yield. In contrast, 4-ethynylpyridine failed to react under our reaction conditions during the dioxygenation reaction, which we attribute to the deactivation of the Cu(II) catalytic sites induced by the presence of the nitrogen atom in the aromatic ring. Further work–which is outside the scope of this communication–is guaranteed to gain insight into the complete reaction mechanism (a tentative mechanism is provided in Table S4†).
Conclusions
We report bimetallic SCNPs as highly efficient soft nanocatalysts allowing consecutive one-pot (incompatible) reactions to be performed at r.t. in a green solvent. For this purpouse, a polymeric precursor, P1, containing both α-diazo-β-ketoester and naked β-ketoester functional motifs was rationally designed. Pt(II) and Cu(II) ions were sequentially added not only to trigger intra-chain folding of P1 chains at high dilution, but also to install two distinct type of metallic species for consecutive catalytic reactions. Efficient compaction was revealed by combining IR, SEC and DLS measurements, yielding catalytically active Pt(II)/Cu(II)-SCNPs. This unique heterobimetallic soft nanocatalyst was shown to be highly efficient for consecutive one-pot alkyne semihydrogenation/alkene dioxygenation reactions at r.t. in NBP, as a non-toxic alternative solvent to DMF, affording β-keto-N-alkoxyphthalimides as intermediates of great utility for the pharmaceutical and agricultural industries. Critically, this work paves the way to multistep chemical reactions in a single reaction vessel with complex multi-metallic SCNPs as advanced nanocatalysts.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge Grant PID2021-123438NB-I00 funded by MCIN/AEI/10.13039/501100011033 and “ERDF A way of making Europe”, Grant TED2021-130107A-I00 funded by MCIN/AEI/10.13039/501100011033 and Unión Europea “NextGenerationEU/PRTR” and Grant IT-1566-22 from Eusko Jaurlaritza (Basque Government). E.V.-S. acknowledges financial support from RyC program (RYC2022-037590-I). The authors sincerely thank Davide Arena for technical support.References
- S. Mavila, C. E. Diesendruck, S. Linde, L. Amir, R. Shikler and N. G. Lemcoff, Angew. Chem., Int. Ed., 2013, 52, 5767 CrossRef CAS PubMed.
- E. Verde-Sesto, A. Arbe, A. J. Moreno, D. Cangialosi, A. Alegría, J. Colmenero and J. A. Pomposo, Mater. Horiz., 2020, 7, 2292 RSC.
- A. Nitti, R. Carfora, G. Assanelli, M. Notari and D. Pasini, ACS Appl. Nano Mater., 2022, 5, 13985 CrossRef CAS.
- J. A. Pomposo, Single-Chain Polymer Nanoparticles: Synthesis; Characterization, Simulations, and Applications, John Wiley & Sons, 2017 Search PubMed.
- J. A. Pomposo, Polym. Int., 2014, 63, 589 CrossRef CAS.
- A. Sanchez-Sanchez, A. Arbe, J. Colmenero and J. A. Pomposo, ACS Macro Lett., 2014, 3, 439 CrossRef CAS PubMed.
- H. Rothfuss, N. D. Knöfel, P. W. Roesky and C. Barner-Kowollik, J. Am. Chem. Soc., 2018, 140, 5875 CrossRef CAS PubMed.
- J. Rubio-Cervilla, E. González and J. A. Pomposo, Nanomaterials, 2017, 7, 341 CrossRef PubMed.
- T. Terashima, T. Mes, T. F. A. De Greef, M. A. J. Gillissen, P. Besenius, A. R. A. Palmans and E. W. Meijer, J. Am. Chem. Soc., 2011, 133, 4742 CrossRef CAS PubMed.
- A. Sanchez-Sanchez, A. Arbe, J. Kohlbrecher, J. Colmenero and J. A. Pomposo, Macromol. Rapid Commun., 2015, 36, 1592 CrossRef CAS PubMed.
- S. Thanneeru, J. Nganga, A. S. Amin, B. Liu, L. Jin, A. Angeles-Boza and J. He, ChemCatChem, 2017, 9, 1157 CrossRef CAS.
- J. Chen, J. Wang, K. Li, Y. Wang, M. Gruebele, A. L. Ferguson and S. C. Zimmerman, J. Am. Chem. Soc., 2019, 141, 9693 CrossRef CAS PubMed.
- S. Garmendia, S. B. Lawrenson, M. C. Arno, R. K. O'Reilly, D. Taton and A. P. Dove, Macromol. Rapid Commun., 2019, 40, e1900071 CrossRef PubMed.
- W. Xu, D. Xiang, J. Xu, Y. Ye, D. Qiu and Z. Yang, Polym. Chem., 2021, 12, 172 RSC.
- W. Wang, J. Wang, S. Li, C. Li, R. Tan and D. Yin, Green Chem., 2020, 22, 4645 RSC.
- A. Blazquez-Martin, E. Verde-Sesto, A. Arbe and J. A. Pomposo, Angew. Chem., Int. Ed., 2023, 62, e202313502 CrossRef CAS PubMed.
- K. Mundsinger, A. Izuagbe, B. T. Tuten, P. W. Roesky and C. Barner-Kowollik, Angew. Chem., Int. Ed., 2024, 63, e202311734 CrossRef CAS PubMed.
- S. Mavila, I. Rozenberg and N. G. Lemcoff, Chem. Sci., 2014, 5, 4196 RSC.
- N. D. Knöfel, H. Rothfuss, P. Tzvetkova, B. Kulendran, C. Barner-Kowollik and P. W. Roesky, Chem. Sci., 2020, 11, 10331 RSC.
- J. L. Bohlen, B. Kulendran, H. Rothfuss, C. Barner-Kowollik and P. W. Roesky, Polym. Chem., 2021, 12, 4016 RSC.
- S. Gillhuber, J. O. Holloway, H. Frisch, F. Feist, F. Weigend, C. Barner-Kowollik and P. W. Roesky, Chem. Commun., 2023, 59, 4672 RSC.
- T. Dang-Bao, D. Pla, I. Favier and M. Gómez, Catalysts, 2017, 7, 207 CrossRef.
- S. Kar, H. Sanderson, K. Roy, E. Benfenati and J. Leszczynski, Chem. Rev., 2022, 122, 3637 CrossRef CAS PubMed.
- J. Sherwood, F. Albericio and B. G. de la Torre, ChemSusChem, 2024, e202301639 CrossRef CAS PubMed.
- J. De-La-Cuesta and J. A. Pomposo, ACS Omega, 2018, 3, 15193 CrossRef CAS PubMed.
- T. K. Claus, J. Zhang, L. Martin, M. Hartlieb, H. Mutlu, S. Perrier, G. Delaittre and C. Barner-Kowollik, Macromol. Rapid Commun., 2017, 38, 1700264 CrossRef PubMed.
- R. Bag, D. Sar and T. Punniyamurthy, Org. Lett., 2015, 17, 2010 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr01261e |
| This journal is © The Royal Society of Chemistry 2024 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Isolated yield.
Isolated yield.