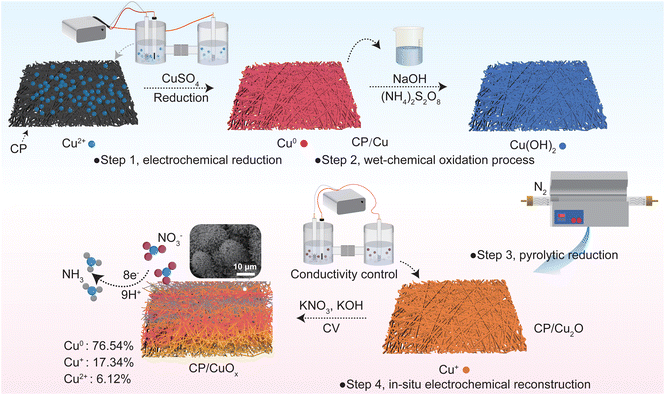
Conductivity-mediated in situ electrochemical reconstruction of CuOx for nitrate reduction to ammonia†
Hao
Liang‡
a,
Yinqiao
Zhang‡
a,
Xiaona
Zhang
a,
Erzhuo
Zhao
c,
Wendan
Xue
 d,
Enguang
Nie
e,
Jianqiu
Chen
*a,
Sijin
Zuo
d,
Enguang
Nie
e,
Jianqiu
Chen
*a,
Sijin
Zuo
 *a and
Minghua
Zhou
b
*a and
Minghua
Zhou
b
aState of Key Laboratory of Natural Medicines, School of Engineering, China Pharmaceutical University, Nanjing 210009, PR China. E-mail: sjzuo@cpu.edu.cn; cjqer@163.com
bKey Laboratory of Pollution Process and Environmental Criteria, Ministry of Education, College of Environmental Science and Engineering, Nankai University, Tianjin 300350, PR China
cSchool of Environment, Tsinghua University, Beijing 100084, PR China
dDepartment of Chemistry, Tsinghua University, Beijing 100084, PR China
eCollege of Bioscience and Biotechnology, Yangzhou University, Yangzhou, 225009, PR China
First published on 21st June 2024
Abstract
The electrocatalytic nitrate reduction reaction (NO3RR) is an ideal NH3 synthesis route with ease of operation, high energy efficiency, and low environmental detriment. Electrocatalytic cathodes play a dominant role in the NO3RR. Herein, we constructed a carbon fiber paper-supported CuOx nanoarray catalyst (CP/CuOx) by an in situ electrochemical reconstruction method for NO3−-to-NH3 conversion. A series of characterization techniques, such as X-ray diffraction (XRD) and in situ Raman spectroscopy, unveil that CP/CuOx is a polycrystalline-faceted composite copper nanocatalyst with a valence composition containing Cu0, Cu+ and Cu2+. CP/CuOx shows more efficient NO3−-to-NH3 conversion than CP/Cu and CP/Cu2O, which indicates that the coexistence of various Cu valence states could play a dominant role. CP/CuOx with a suitable Cu2+ content obtained by adjusting the conductivity during the in situ electrochemical reconstruction process exhibited more than 90% faradaic efficiencies for the NO3RR in a broad range of −0.3 to −1.0 V vs. RHE, 28.65 mg cm−2 h−1 peak ammonia yield, and stable NO3RR efficiencies for ten cycles. These findings suggest that CP/CuOx with suitable copper valence states obtained by fine-tuning the conductivity of the electrochemical reconstruction may provide a competitive cathode catalyst for achieving excellent activity and selectivity of NO3−-to-NH3 conversion.
1. Introduction
Ammonia (NH3), as the essential chemical feedstock of pharmaceutical manufacturing, fertilizer production, energy supply, etc., plays an important role in global energy and agricultural production.1,2 At present, industrial-scale NH3 synthesis is dominated by the energy-intensive Haber–Bosch (H–B) process. However, the H–B process requires high temperature (400–500 °C) and high pressure (150–300 atm), leading to the consumption of 2% of the world's annual energy output and 400 Mt of carbon dioxide emission per year.3 Therefore, finding a new method for NH3 synthesis that is environmentally friendly, efficient, and can utilize renewable energy sources has important research value and application prospects.Electrocatalytic technologies are beginning to show their potential in NH3 synthesis due to their ease of operation, high energy efficiency, and low environmental detriment.4–6 The electrocatalytic nitrate reduction reaction (NO3RR) is an ideal NH3 synthesis route, which not only complements the traditional energy-intensive and costly NH3 production process, but also helps to alleviate the environmental problems caused by nitrate pollution (such as eutrophication).7–10 Nitrate reduced to ammonia would undergo a multi-step electroreduction process at the cathode that involves the transfer of nine protons and eight electrons to ultimately produce NH3 and H2O in acidic and neutral electrolytes.11,12 Throughout the electrochemical reaction at the cathode, the rate-determining step (*NH3 to NH3) and the competing hydrogen evolution reaction (HER) dominantly impede the kinetics and faradaic efficiency of the NO3RR.13–16 As a result, it is crucial to rationally design cathode catalysts to achieve excellent activity and selectivity of the NO3RR.
Various catalysts, including noble and transition metals, alloys, and non-metallic electrodes, have been explored to enhance the selectivity and efficiency of the NO3RR.17,18 For example, Liu et al. developed atomically precise silver (Ag) nanocluster catalysts for an efficient NO3RR to synthesize NH3, achieving high stability in neutral media.19 A transition metal-based Ni/Ni(OH)2 catalyst was reported to enable ammonia production at ampere-level current densities. Metal-free amorphous graphene was also found to benefit the direct electroreduction of nitrates to ammonia.20 However, common issues with these catalysts include their limited availability, high cost, and potential deactivation over long-term use. Copper (Cu), on the other hand, shows promise in addressing these issues due to its abundance, lower cost, and superior NO3− adsorption and reduction of *NO3 to *NO2, which is attributed to the highly occupied d-orbitals and the energy level of Cu matching the lowest unoccupied *π orbital of NO3-N.21 Nevertheless, pure Cu would rapidly deactivate owing to the NO3RR intermediates (e.g., NO2−) accumulating on the Cu surface.22 To overcome this limitation, researchers have proved that optimizing the valence state of Cu is a preferable way, as Cuδ+ species (e.g., CuOx) with a relatively low energy barrier for the NO3RR are more active in adsorbing, activating, and even desorbing the intermediates.23–25 For instance, Wu et al. reported a strategy for controlling the oxidation state of copper using aryl diazonium salts for covalently binding aryl groups onto the copper surface.26
In this study, a carbon fiber paper-supported CuOx nanoarray catalyst (CP/CuOx) obtained by an in situ electrochemical reconstruction method was used for NO3−-to-NH3 conversion. The crystalline state, morphology, and electronic state of Cu were investigated by various spectroscopy characterization techniques. The performance of the CP/CuOx cathode was assessed in the electrocatalytic reduction of NO3− to NH3 including NH3 yield, faradaic efficiency and stability. The electrochemical measurements, density functional theory (DFT) calculations, and spectroscopy characterization studies of CP/Cu, CP/Cu2O and CP/CuOx cathodes were compared to evaluate the role of CuOx in NO3−-to-NH3 conversion. Finally, the effects of different physicochemical morphologies of CP/CuOx constructed by conductivity modulation on NH3 yield and faradaic efficiency were investigated.
2. Materials and methods
2.1 Working electrode preparation
The CP/Cu precursor was first synthesized using a chronoamperometry (i–t) method. As shown in Scheme 1, a carbon fiber paper sheet was used as the cathode (1 cm × 2 cm), a platinum sheet was used as the anode, and a saturated calomel electrode (SCE) was used as the reference electrode, respectively. 25 mL of electrolyte containing 70 mM CuSO4 and 0.50 M Na2SO4 solution was added to a single-cell reactor. Then copper ions can be electrochemically reduced to metallic copper and deposited onto the carbon fiber paper at a constant stirring rate of 600 rpm and a deposition potential of 0.3 V vs. RHE for a duration of 800 seconds.The acquired CP/Cu precursor was immersed in an alkaline solution which consisted of 2.5 M NaOH and 0.125 M (NH4)2S2O8 for 15 min to convert Cu to Cu(OH)2. After washing 3–5 times with deionized water, the Cu(OH)2 was dried. The Cu(OH)2 was then pyrolyzed to obtain Cu2O-loaded CP (CP/Cu2O) at 550 °C for 2 hours in a tube furnace (inert gas atmosphere).
CP/Cu2O was treated by cyclic voltammetry (CV) for 50 electrolysis cycles (−0.5 to 0.2 V vs. RHE) in the alkaline electrolyte (1 M KOH) with varying nitrate concentrations, as displayed in Scheme 1. The nitrate concentrations of the alkaline electrolyte used were 0.1, 0.5, 0.8, and 1 M, and the corresponding conductivities of the electrolytes were 112, 128, 138, and 143 μS cm−1, respectively.
2.2 Ammonia electrosynthesis
Electrochemical measurements were performed in an H-cell configured with the anodic and cathodic chambers (100 mL each) being separated by an anion exchange membrane (Nafion 115). 60 mL of 1 M KOH containing 0.1 M KNO3 was added to each chamber. CP/CuOx was used as the working electrode. Electrochemical methods including CV, linear scanning voltammetry (LSV), and electrochemical impedance spectroscopy (EIS) were used for characterization of the NO3RR performance of the CP/CuOx catalysts. LSV tests were performed in the range of −1 to 1 V vs. RHE at 5 mV s−1. CV curves were recorded in the range of −0.52 to −0.62 V vs. RHE at 20, 40, 60, 80, 100, and 120 mV s−1 in 1 M KOH. EIS was conducted at 0.15 V vs. RHE and frequencies from 0.1 to 100 kHz. The measurement methods of NO2−, NO3−, NH4+, N2H4, N2 and H2 are depicted in the ESI,† and their standard curves are shown in ESI Fig. S1–S5.†3. Results and discussion
3.1 Preparation and characterization of electrodes
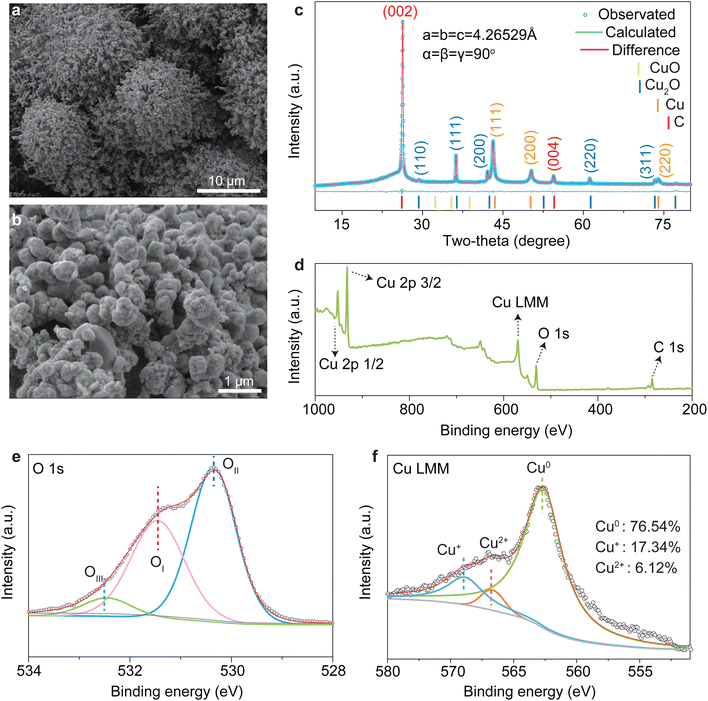
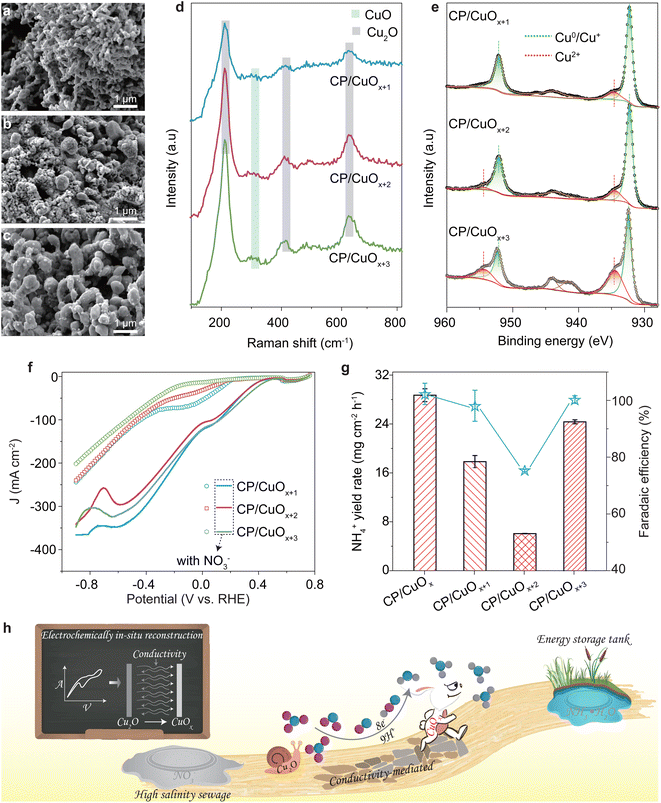
The preparation process of the CP/CuOx electrode is shown in Scheme 1. First, the CP was pre-treated to remove the impurities on the fiber surface and to improve the attachment point of the copper grains. CP loaded with copper grains (CP/Cu) was obtained by chronoamperometry, and the optimum copper deposition state was obtained by tuning the deposition potential and reaction time (ESI Fig. S6 and S7†). The copper grains on CP/Cu were converted to Cu(OH)2 by wet chemistry. They were then annealed to obtain CP/Cu2O by the thermal decomposition method. Finally, CP/Cu2O was reduced to a composite Cu nanocatalyst (CP/CuOx) with better electrochemical stability in solutions containing nitrates. During the in situ electrochemical reconstruction process, the CV curves of the first 40 cycles varied significantly and with little overlap, indicating that Cu2O was reduced on the surface of the carbon fibers (ESI Fig. S8a and b†). In subsequent cycles, the CV curves show good overlap, demonstrating that the catalyst has formed a stable chemical state (ESI Fig. S8c and d†).As shown in Fig. 1a and b, after the in situ electrochemical reconstruction process, CP/CuOx formed a cluster-like structure consisting of many spherical nanograins on its surface at a conductivity of 112 μS cm−1. The characteristic diffraction peaks of C, Cu and Cu2O are observed in the XRD spectra of the CP/CuOx electrocatalyst, as shown in Fig. 1c and ESI Table S1.† The diffraction peaks of C are from the graphitic carbon structure of the CP, with 26.5° being attributed to the (002) facet and 54.5° being attributed to the (004) plane of the carbon substrate.27 The weaker diffraction peaks observed at 43.3° and 50.5° are attributed to the Cu (100) facet and the Cu (111) plane, respectively. It is important to note that the (200) diffraction peak is a secondary diffraction originating from the (100) plane.28,29 The CP/CuOx electrocatalyst showed weakly (100)- and (111)-oriented CuO crystalline phases and also strongly (100)-, (111)-, and (220)-oriented Cu2O crystalline phases. The elemental composition and valence states of CP/CuOx were further investigated by XPS. One can see in Fig. 1d that CP/CuOx exhibits the characteristic peaks of C 1s, O 1s, Cu LMM, Cu 2p3/2 and Cu 2p1/2.30,31 As displayed in Fig. 1e, there are three different types of oxygens present in CP/CuOx, namely OI (oxygen on the complex structure), OII (oxygen on the Cu/Cu2O structure) and OIII (oxygen-deficient species).32–35 This also indicates that CP/CuOx is a mixed-valence copper nanocatalyst. Fig. 1f shows the high-resolution Cu LMM XPS spectrum of CP/CuOx. The three peaks observed at 567.76, 573.94 and 571.80 eV correspond to Cu0 (76.54%), Cu+ (17.34%) and Cu2+ (6.12%), respectively.36–38 In addition, our Cu 2p XPS spectrum (ESI Fig. S9†) also showed a similar phenomenon of mixed Cu2+ and Cu+/Cu0. These results collectively demonstrated that CP/CuOx is a polycrystalline-faceted composite copper nanocatalyst.
3.2 Evaluation of ammonia electrosynthesis
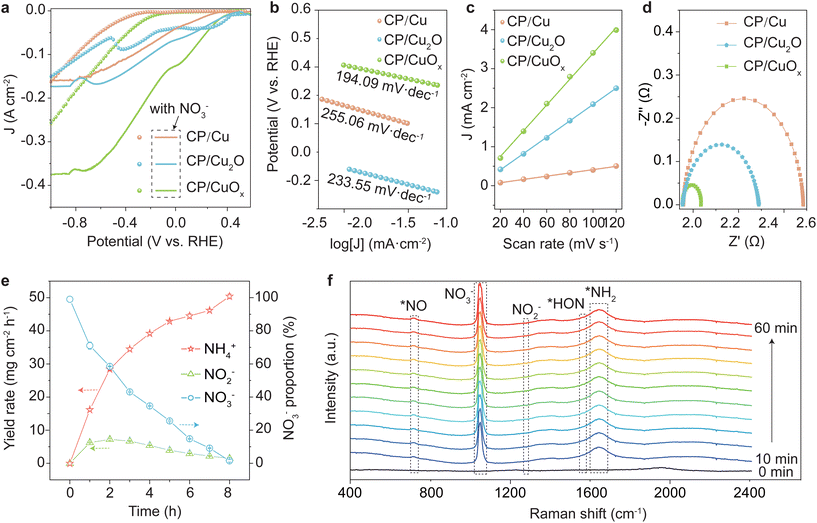
The catalytic activity of CP/Cu, CP/Cu2O and CP/CuOx for the electroreduction of nitrate to ammonia was investigated in H-cells. Before investigating the NO3RR performance of the catalysts, their LSV curves were tested until the polarization curves reached a steady state. Fig. 2a shows the LSV curves of the three catalysts. In general, the presence of NO3− leads to an increase of current density for the three catalysts. The phenomenon indicates that the NO3− reduction process occurred on the three catalysts.39,40 Moreover, the current density of CP/CuOx with NO3− shows the most significant increase compared to that of virgin CP, CP/Cu and CP/Cu2O (Fig. 2a and ESI Fig. S10†). In particular, at −0.4 V vs. RHE, the current density of CP/CuOx increased to −311 mA cm−2, which is approximately 2.78 times higher than that of CP/Cu. The results suggest that more NO3− reduction occurred on the CP/CuOx cathode.In addition, we further investigated the kinetics of the NO3RR by analyzing the Tafel curves of the three catalysts (Fig. 2b). CP/CuOx exhibited the smallest Tafel slope (194.09 mV dec−1) compared to CP/Cu (255.06 mV dec−1) and CP/Cu2O (233.55 mV dec−1), suggesting that the composite state Cu interface of CP/CuOx is beneficial for improving the reaction kinetics compared to pure Cu. The higher electrochemically active surface area (ECSA) and the electrochemical double-layer capacitance (Cdl) values of CP/CuOx than those of CP/Cu and CP/Cu2O (Fig. 2c and ESI Fig. S11†) indicate more catalytically active sites on CP/CuOx. Furthermore, EIS measurement was also used to further confirm the excellent electrocatalytic performance of CP/CuOx. The Nyquist plots of EIS show that CP/CuOx exhibited a lower charge transfer impedance than the control groups, implying a more efficient electron transfer on the surface of CP/CuOx (Fig. 2d).
In the electrolysis experiments after 8 h, the CP/CuOx electrocatalyst was able to promote the conversion of NO3− to NH4+ consistently and efficiently, with the intermediate product NO2− almost not accumulating but being rapidly converted (Fig. 2e). In order to gain insight into the reduction of NO3− to NH3 over CP/CuOx in a mixed solution of 0.1 M KNO3 and 1 M KOH, electrochemical in situ Raman spectroscopy measurements were performed at −0.6 V vs. RHE for a duration of 1 h. As shown in Fig. 2f, CP/CuOx shows peaks stretching at 730 and 1047 cm−1, which were attributed to the vibrational modes of N–O (NO3−) and free NO3− in the liquid environment.41,42 The stretching intensity of these peaks increased gradually as the reaction time extended from 0 to 60 min, indicating the aggregation of NO3− on the catalyst due probably to the strong adsorption of NO3− on the CP/CuOx surface. The vibrational peaks at 1280 and 1548 cm−1 are attributed to the reaction intermediates of *NO2 and *HON, and the Raman signals of these intermediates are weak during the reaction (ESI Fig. S12†).43,44 Nevertheless, the Raman peak at 1664 cm−1 attributed to NH2 is clearly stronger.45 These phenomena indicated that the NO3RR could rapidly convert to ammonia accompanied by some detectable intermediates through a stepwise deoxygenated hydrogenation pathway on the CP/CuOx cathode.
The efficient catalytic performance of CP/CuOx in ammonia production from nitrate reduction can be attributed to the suitable valence of Cu. With respect to the detailed role of Cu in each specific valence state, we tentatively considered them from the viewpoint of Gibbs free energy of reaction intermediates (ESI Fig. S13†) by DFT calculations. The calculated results indicate that Cu0 has a positive contribution to the conversion of NO3− to *NH3 but a thermodynamically unfavorable process is the desorption of *NH3. Cu+ displays a favorable NO3− adsorption (*NO3) compared to Cu0 and Cu2+ because of a higher downhill energy barrier of −4.02 eV, nevertheless only −1.88 and −1.84 eV on Cu0 and Cu2+ respectively. Unfortunately, Cu+ has the very difficult step in the conversion of “*NO to *N” owing to an uphill energy barrier of 1.74 eV. Regarding Cu2+, it almost displays all beneficial steps in coordinate numbers of 1–8 from the conversion of “NO3− to *NH3” aside from a slight uphill of 1.24 eV (versus 1.74 eV on Cu+) in the foregoing step of “*NO to *N”. Importantly, Cu2+ displays the smallest uphill energy barrier for the final desorption of the *NH3 intermediate which serves as the rate-determining step for Cu0, Cu+ and Cu2+. Therefore, the valence composition of Cu plays a key role in our NO3RR performance.
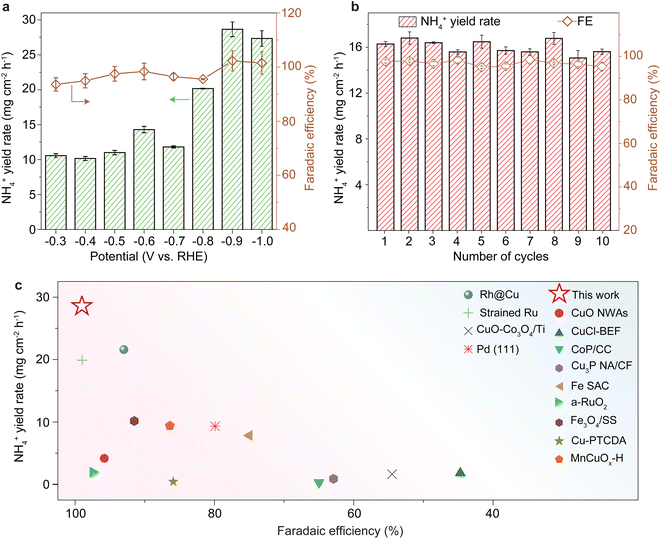
The performance of CP/CuOx in ammonia synthesis at different potentials (−0.1 to −1.0 V vs. RHE) was deeply investigated. To verify that ammonia production originated from the electrochemical reduction of NO3− entirely, CP/CuOx was placed in electrolyte with and without NO3− for electrocatalytic tests (please see 2.2 Ammonia electrosynthesis). As shown in ESI Fig. S14,† almost no ammonia generation was detected in the solution without NO3−, further confirming that the ammonia originated totally from the reduction of NO3−. The complementary 15N isotopic labeling experiment showed a typical double peak with chemical shifts at 6.85 and 6.97 ppm in the 1H NMR spectrum (ESI Fig. S15†), indicating the above similar conclusion. As shown in Fig. 3a and ESI Fig. S16a,† CP/CuOx exhibited faradaic efficiencies of more than 90% in the range of −0.3 to −1.0 V vs. RHE and realized a peak ammonia yield of 28.65 mg cm−2 h−1 at a potential of −0.9 V vs. RHE. Such high selectivity to NH3 was probably attributed to the suitable adsorption energy and a favorable thermodynamic process for some key intermediates over our electrocatalyst. Other by-products such as H2 or N2H4 were not detectable in our system (ESI Fig. S4, S5 and S17†). However, the faradaic efficiency still not being equal to 100% was probably attributed to the detectable reaction-incomplete intermediate NO2− (Fig. 2e). CP/CuOx performed well in terms of ammonia yield and faradaic efficiency, well in agreement with the results of their LSV, ECSA, and EIS tests. A suitable potential of −0.6 V vs. RHE was chosen to test the long-term stability of CP/CuOx, as can be seen in Fig. 3b. The results show that there was almost no performance attenuation in the continuous ammonia electrosynthesis with the CP/CuOx electrocatalyst during 10 cycles. In addition, no significant changes were still observed in the CV curves (ESI Fig. S18†), Cu LMM XPS spectra (ESI Fig. S19a†) and Raman spectra (ESI Fig. S20†) of the catalysts before and after the reaction, and even the neglected Cu leaching (ESI Fig. S19b and c†) in each repeated run, together indicating that the catalyst composition remained robust even after ten cycle tests. Compared to the reported copper catalysts and other advanced catalysts, the CP/CuOx electrocatalyst in this study showed superior faradaic efficiency and NH4+ yield for the NO3RR (Fig. 3c and ESI Table S2†).
3.3 Unveiling of the role of conductivity in the reconstruction of CuOx
To investigate the surface compositions and valence states of Cu for the NO3RR, the CP/CuOx, CP/CuOx+1, CP/CuOx+2 and CP/CuOx+3 catalysts were prepared in 112, 128, 138 and 143 μS cm−1 electrolyte, respectively, through an in situ electrochemical reconstruction process (please see 2.1 Working electrode preparation). Note that the increased oxygen element indicates the increased valence of copper. With increasing the conductivity of electrolyte from 128 to 143 μS cm−1, the morphologies of CP/CuO& ranged from a spherical-like cluster consisting of numerous rod-shaped copper grains (128 μS cm−1, Fig. 4a) to irregularly distributed copper grains (138 μS cm−1, Fig. 4b) and a more ordered distribution of large rod-like copper grains (143 μS cm−1, Fig. 4c). Changes in the chemical compositions of these CP/CuO& electrocatalysts were first detected by Raman spectroscopy (Fig. 4d). The characteristic Raman peaks of Cu+ and Cu2+ could be clearly observed, such as the stretching peak at about 300 cm−1 pointed to the CuO (Cu2+) phase.46 This result is consistent with the valence changes revealed by XPS, as shown in Fig. 4e. In the XPS spectra of CP/CuOx+1, CP/CuOx+2 and CP/CuOx+3, the signals of the Cu2+ species become more prominent with increasing conductivity. The results indicate that the four catalysts consist of Cu0, Cu+ and Cu2+ and that the amount of Cu2+ increases from 6.94% to 25.91% with increasing conductivity (ESI Table S3†). In addition, the XRD results in ESI Fig. S21† support a similar conclusion, with the intensity of the Cu (100), (111) and Cu (220) facets decreasing progressively as the conductivity of electrolyte increases, while the intensities of the diffraction peaks representing the CuO phase increase gradually.The in situ electrochemical reconstruction by CV was found to be two stages of dissolution (oxidation process) and redeposition (reduction process) of copper ions.47 The Cu2+ ions of the catalyst would leach into the electrolyte during the oxidation process, and then the Cu+ or Cu0 would redeposit onto the catalyst during the reduction process. A decrease in the conductivity of the electrolyte leads to an increase in electrical resistance, which slows down the ion migration rate in turn.48 Consequently, in a low conductivity microenvironment, slow ion migration causes a higher accumulation of Cu2+ on the catalyst surface. This would contribute to the formation of a clustered deposit with some lower valence copper during the reduction process (ESI Fig. S22†). In contrast, at higher conductivity, Cu2+ ions can migrate and diffuse faster, resulting in a decrease in the local Cu2+ concentration in the electrolyte which would cause the slight reduction of Cu2+ on the catalyst (Cu2+liquid → Cu0/Cu+solid). Thus, we could obtain more Cu2+ on the catalyst in a higher conductivity microenvironment (Fig. 4e and ESI Table S3†).
LSV analysis shows the current responses of CP/CuOx+1, CP/CuOx+2 and CP/CuOx+3 to NO3−, and the current densities vary, in the sequence of CP/CuOx+3 > CP/CuOx+1 > CP/CuOx+2 (Fig. 4f). This order is accordingly consistent with their ammonia yields and faradaic efficiency (Fig. 4g and ESI Fig. S16†). In terms of NO3RR performance, CP/CuOx produced with the lowest conductivity performs best, followed by CP/CuOx+3 with the highest conductivity. The Raman spectrum of CP/CuOx+3 was similar to that of CP/CuOx after 1 or 10 electrolysis experiments (ESI Fig. S20c†). The phenomenon indicates that CP/CuOx+3 is unstable during electrolysis, and this would lead to a shift towards a similar chemical composition to CP/CuOx, even for the initial CP/CuOx+3 electrocatalyst with the highest Cu2+ content. As a result, the NO3RR performance of CP/CuOx+3 was close to that of CP/CuOx (Fig. 4g).
These results clearly indicate that conductivity-mediated in situ electrochemical reconstruction of CuOx can not only tune the physicochemical characteristics of catalysts, such as the physical morphology (Fig. 4a–c), but also, importantly, effectively control the valence state of copper which plays a key role in the NO3RR performance. The comparison of the NO3RR performance of CP/CuOx with that of CP/Cu2O and CP/Cu in Fig. 2a shows that the catalysts with all three valence states (Cu0, Cu+ and Cu2+) exhibit desirable nitrate reduction. Furthermore, the results in Fig. 3 and 4f, g proved the significance of a suitable content of Cu2+ in the CuOx electrocatalyst. The phenomena could be attributed to the easy formation of some key reaction intermediates during the NO3RR process and effective suppression of the competitive cathodic HER (see ESI Fig. S13 and S17†) on the CuOx interface.49,50 Therefore, for achieving a high NH3 yield during the NO3RR process, an appropriate conductivity microenvironment is needed to optimize the ratio of Cu valence states.
4. Conclusions
This study introduces a carbon fiber paper-supported CuOx nanoarray catalyst (CP/CuOx) obtained by an in situ electrochemical reconstruction method for NO3−-to-NH3 conversion. The CP/CuOx catalyst with various valence states of Cu shows an obvious improvement in NO3−-to-NH3 conversion in comparison with CP/Cu and CP/Cu2O. CP/CuOx with a suitable Cu2+ content exhibited good stability, high faradaic efficiencies and high ammonia yields in a wide potential range. The results suggest that CP/CuOx obtained by the conductivity-mediated in situ electrochemical reconstruction route may provide a commercially competitive electrocatalyst for achieving excellent activity and selectivity of NO3−-to-NH3 conversion (Fig. 4h).Author contributions
Hao Liang: methodology, data curation, formal analysis, and writing – original draft. Yinqiao Zhang: visualization and writing – review and editing. Xiaona Zhang: methodology and visualization. Erzhuo Zhao: validation and formal analysis. Wendan Xue: supervision and formal analysis. Enguang Nie: formal analysis and writing – review and editing. Minghua Zhou: writing – review and editing. Jianqiu Chen: methodology. Sijin Zuo: conceptualization, supervision, project administration, and funding acquisition.Conflicts of interest
There is no conflict of interest to declare.Acknowledgements
We are thankful for the financial support of the National Natural Science Foundation of China (No. 22306202 and 22306201) and the Natural Science Foundation of Jiangsu Province (No. BK20231026).References
- K. Zhang, A. Cao, L. H. Wandall, J. Vernieres, J. Kibsgaard, J. K. Norskov and I. Chorkendorff, Spin-mediated promotion of Co catalysts for ammonia synthesis, Science, 2024, 383, 1357–1363 CrossRef CAS PubMed.
- X. Zheng, J. C. Hao, Z. C. Zhuang, Q. Kang, X. F. Wang, S. L. Lu, F. Duan, M. L. Du and H. Zhu, Emerging electrospinning platform toward nanoparticle to single atom transformation for steering selectivity in ammonia synthesis, Nanoscale, 2024, 16, 4047–4055 RSC.
- S. T. Wang, Y. Liu, K. Zhang and S. Y. Gao, Self-powered electrocatalytic nitrate to ammonia driven by lightweight triboelectric nanogenerators for wind energy harvesting, Nano Energy, 2023, 112, 108434 CrossRef CAS.
- S. Han, H. Li, T. Li, F. Chen, R. Yang, Y. Yu and B. Zhang, Ultralow overpotential nitrate reduction to ammonia via a three-step relay mechanism, Nat. Catal., 2023, 6, 402–414 CrossRef CAS.
- J. Chen, M. Q. Gu, Y. F. Zhou, D. J. Wan, Q. C. He, Y. H. Shi and Y. D. Liu, Efficient nitrate and perchlorate removal from aqueous solution via a novel electro-dialysis ion-exchange membrane bioreactor, Chem. Eng. J., 2022, 430, 132952 CrossRef CAS.
- J. Z. Qin, K. Wu, L. Z. Chen, X. T. Wang, Q. L. Zhao, B. J. Liu and Z. F. Ye, Achieving high selectivity for nitrate electrochemical reduction to ammonia over MOF-supported RuxOy clusters, J. Mater. Chem. A, 2022, 10, 3963–3969 RSC.
- L. Jia, H. Xue, F. Xian, Y. Sugahara, N. Sakai, J. Nan, Y. Yamauchi, T. Sasaki and R. Z. Ma, Porous and partially dehydrogenated Fe2+-containing iron oxyhydroxide nanosheets for efficient electrochemical nitrogen reduction reaction (ENRR), Small, 2023, 19, 2303221 CrossRef CAS.
- Z. C. Zheng, D. Wu, L. Chen, S. Chen, H. Wan, G. Chen, N. Zhang, X. Liu and R. Z. Ma, Collaborative optimization of thermodynamic and kinetic for Ni-based hydroxides in electrocatalytic urea oxidation reaction, Appl. Catal., B, 2024, 340, 123214 CrossRef CAS.
- F. B. Yao, M. C. Jia, Q. Yang, F. Chen, Y. Zhong, S. J. Chen, L. He, Z. J. Pi, K. J. Hou, D. B. Wang and X. M. Li, Highly selective electrochemical nitrate reduction using copper phosphide self-supported copper foam electrode: Performance, mechanism, and application, Water Res., 2021, 193, 116881 CrossRef CAS PubMed.
- S. J. Zuo, W. D. Xue, Y. Q. Zhang, J. Q. Chen and Z. Q. Lin, Atomically dispersed Fe motif-based electrocatalysts for hydrogen peroxide synthesis, ChemNanoMat, 2024, 10, e202300476 CrossRef CAS.
- B. Wang, X. Zhu, X. D. Pei, W. G. Liu, Y. C. Leng, X. W. Yu, C. Wang, L. H. Hu, Q. M. Su, C. P. Wu, Y. F. Yao, Z. Q. Lin and Z. G. Zou, Room-Temperature laser planting of high-loading single-atom catalysts for high-efficiency electrocatalytic hydrogen evolution, J. Am. Chem. Soc., 2023, 145, 13788–13795 CrossRef CAS PubMed.
- L. Fang, S. Wang, C. Song, X. H. Yang, Y. K. Li and H. Liu, Enhanced nitrate reduction reaction via efficient intermediate nitrite conversion on tunable CuxNiy/NC electrocatalysts, J. Hazard. Mater., 2022, 421, 126628 CrossRef CAS.
- S. J. Zuo, R. X. Guo, W. D. Xue, J. G. Shang, J. Q. Chen and Y. Q. Zhang, Decipher the key role of ketone toward singlet oxygen evolution in Fenton-like process for water decontamination, Appl. Catal., B, 2023, 339, 123100 CrossRef CAS.
- R. D. Zhao, Q. Y. Yan, L. H. Yu, T. Yan, X. Y. Zhu, Z. Y. Zhao, L. Liu and J. Y. Xi, A Bi–Co corridor construction effectively improving the selectivity of electrocatalytic nitrate reduction toward ammonia by nearly 100%, Adv. Mater., 2023, 35, 2306633 CrossRef CAS PubMed.
- J. Martínez, A. Ortiz and I. Ortiz, State-of-the-art and perspectives of the catalytic and electrocatalytic reduction of aqueous nitrates, Appl. Catal., B, 2017, 207, 42–59 CrossRef.
- J. N. Gao, B. Jiang, C. C. Ni, Y. F. Qi, Y. Q. Zhang, N. Oturan and M. A. Oturan, Non-precious Co3O4-TiO2/Ti cathode based electrocatalytic nitrate reduction: Preparation, performance and mechanism, Appl. Catal., B, 2019, 254, 391–402 CrossRef CAS.
- S. Liang, M. Y. Zhang, G. M. Biesold, W. Choi, Y. J. He, Z. L. Li, D. F. Shen and Z. Q. Lin, Recent advances in synthesis, properties, and applications of metal halide perovskite nanocrystals/polymer nanocomposites, Adv. Mater., 2021, 33, 2005888 CrossRef CAS.
- S. Xu, A. Dong, Y. Hu, Z. Yang, S. Huang and J. Qian, Multidimensional MOF-derived carbon nanomaterials for multifunctional applications, J. Mater. Chem. A, 2023, 11, 9721–9747 RSC.
- L. Liu, S. Zheng, H. Chen, J. Cai and S. Zang, Tandem nitrate-to-ammonia conversion on atomically precise silver nanocluster/MXene electrocatalyst, Angew. Chem., Int. Ed., 2024, 63, e202316910 CrossRef CAS.
- L. Cheng, Ti. Ma, B. Zhang, L. Huang, W. Guo, F. Hu, H. Zhu, Z. Wang, T. Zheng, D. Yang, C. Siu, Q. Liu, Y. Ren, C. Xia, B. Z. Tang and R. Ye, Steering the topological defects in amorphous laser-induced graphene for direct nitrate-to-ammonia electroreduction, ACS Catal., 2022, 12, 11639–11650 CrossRef CAS.
- R. Hao, Y. Song, L. Yang, Y. Guo, X. Wu, Z. Ma, Z. Qian, F. Liu, Z. Wu and L. Wang, Electrochemical reduction of flue gas denitrification wastewater to ammonia using a dual-defective Cu2O@Cu heterojunction electrode, Environ. Sci. Technol., 2024, 58, 5557–5566 CrossRef CAS PubMed.
- E. Murphy, Y. Liu, I. Matanovic, M. Rüscher, Y. Huang, A. Ly, S. Guo, W. Zang, X. Yan, A. Martini, J. Timoshenko, B. R. Cuenya, I. V. Zenyuk, X. Pan, E. D. Spoerke and P. Atanassov, Elucidating electrochemical nitrate and nitrite reduction over atomically-dispersed transition metal sites, Nat. Commun., 2023, 14, 4554 CrossRef CAS PubMed.
- Z. Liu, F. Shen, L. Shi, Q. Tong, M. Tang, Y. Li, M. Peng, Z. Jiao, Y. Jiang, L. Ao, W. Fu, X. Lv, G. Jiang and L. Hou, Electronic structure optimization and proton-transfer enhancement on titanium oxide-supported copper nanoparticles for enhanced nitrogen recycling from nitrate-contaminated water, Environ. Sci. Technol., 2023, 57, 10117–10126 CrossRef CAS PubMed.
- Y. Li, J. X. Ma, Z. C. Wu and Z. W. Wang, Direct electron transfer coordinated by oxygen vacancies boosts selective nitrate reduction to N2 on a Co-CuO electroactive filter, Environ. Sci. Technol., 2022, 56, 8673–8681 CrossRef CAS PubMed.
- A. Baghdasaryan and T. Bürgi, Copper nanoclusters: designed synthesis, structural diversity, and multiplatform applications, Nanoscale, 2021, 13, 6283–6340 RSC.
- H. Wu, L. Huang, J. Timoshenko, K. Qi, W. Wang, J. Liu, Y. Zhang, S. Yang, E. Petit, V. Flaud, J. Li, C. Salameh, P. Miele, L. Lajaunie, B. Cuenya, D. Rao and D. Voiry, Selective and energy-efficient electrosynthesis of ethylene from CO2 by tuning the valence of Cu catalysts through aryl diazonium functionalization, Nat. Energy, 2024, 9, 422–433 CrossRef CAS.
- Y. L. Zhao, Y. Liu, Z. J. Zhang, Z. K. Mo, C. Y. Wang and S. Y. Gao, Flower-like open-structured polycrystalline copper with synergistic multi-crystal plane for efficient electrocatalytic reduction of nitrate to ammonia, Nano Energy, 2022, 97, 107124 CrossRef CAS.
- G. A. Cerrón-Calle, A. S. Fajardo, C. M. Sánchez-Sánchez and S. Garcia-Segura, Highly reactive Cu-Pt bimetallic 3D-electrocatalyst for selective nitrate reduction to ammonia, Appl. Catal., B, 2022, 302, 120844 CrossRef.
- Y. T. Wang, W. Zhou, R. R. Jia, Y. F. Yu and B. Zhang, Unveiling the activity origin of a copper-based electrocatalyst for selective nitrate reduction to ammonia, Angew. Chem., Int. Ed., 2020, 59, 5350–5354 CrossRef CAS PubMed.
- Y. H. Wang, A. Xu, Z. Y. Wang, L. S. Huang, J. Li, F. W. Li, J. Wicks, M. C. Luo, D. H. Nam, C. S. Tan, Y. Ding, J. W. Wu, Y. W. Lum, C. T. Dinh, D. Sinton, G. F. Zheng and E. H. Sargent, Enhanced nitrate-to-ammonia activity on copper-nickel alloys via tuning of intermediate adsorption, J. Am. Chem. Soc., 2020, 142, 5702–5708 CrossRef CAS PubMed.
- J. F. Su, I. Ruzybayev, I. Shah and C. P. Huang, The electrochemical reduction of nitrate over micro-architectured metal electrodes with stainless steel scaffold, Appl. Catal., B, 2016, 180, 199–209 CrossRef CAS.
- Q. G. Zhu, X. F. Sun, D. X. Yang, J. Ma, X. C. Kang, L. R. Zheng, J. Zhang, Z. H. Wu and B. X. Han, Carbon dioxide electroreduction to C2 products over copper-cuprous oxide derived from electrosynthesized copper complex, Nat. Commun., 2019, 10, 3851 CrossRef PubMed.
- R. L. Hao, Y. C. Song, L. L. Yang, Y. X. Guo, X. H. Wu, Z. Ma, Z. Qian, F. Liu and L. D. Wang, Electrochemical reduction of flue gas denitrification wastewater to ammonia using a dual-defective Cu2O@Cu heterojunction electrode, Environ. Sci. Technol., 2024, 58, 5557–5566 CrossRef CAS PubMed.
- A. R. Puigdollers, P. Schlexer, S. Tosoni and G. Pacchioni, Increasing oxide reducibility: The role of metal/oxide interfaces in the formation of oxygen vacancies, ACS Catal., 2017, 7, 6493–6513 CrossRef.
- F. Cavalca, R. Ferragut, S. Aghion, A. Eilert, O. Diaz-Morales, C. Liu, A. L. Koh, T. W. Hansen, L. G. M. Pettersson and A. Nilsson, Nature and distribution of stable subsurface oxygen in copper electrodes during electrochemical CO2 reduction, J. Phys. Chem. C, 2017, 121, 25003–25009 CrossRef CAS.
- J. Zhao, Z. Shen, J. Yu, Y. Guo, M. A. Mushtaq, Y. B. Ding, Z. Q. Song, W. Zhang, X. Huang, Y. Li, D. Q. Liu and X. K. Cai, Constructing Cu-CuO heterostructured skin on Cu cubes to promote electrocatalytic ammonia production from nitrate wastewater, J. Hazard. Mater., 2022, 439, 129653 CrossRef CAS.
- Z. J. Zhang, Y. Liu, X. Z. Su, Z. W. Zhao, Z. K. Mo, C. Y. Wang, Y. L. Zhao, Y. Chen and S. Y. Gao, Electro-triggered joule heating method to synthesize single-phase CuNi nano-alloy catalyst for efficient electrocatalytic nitrate reduction toward ammonia, Nano Res., 2023, 10, 6632–6641 CrossRef.
- J. F. Zhang, Y. Wang, Z. Y. Li, S. Xia, R. Cai, L. Ma, T. Y. Zhang, O. Ackley, S. Z. Yang, Y. C. Wu and J. J. Wu, Grain boundary-derived Cu+/Cu0 interfaces in CuO nanosheets for low overpotential carbon dioxide electroreduction to ethylene, Adv. Sci., 2022, 9, 2200454 CrossRef CAS.
- S. Ingavale, P. Marbaniang, M. Palabathuni, V. N. Kale and N. Mishra, Decoration of boron nanoparticles on a graphene sheet for ammonia production from nitrate, Nanoscale, 2023, 15, 11497–11505 RSC.
- X. D. Wen and J. Q. Guan, Recent advancement in the electrocatalytic synthesis of ammonia, Nanoscale, 2020, 12, 8065–8094 RSC.
- Y. Liu, J. W. Ma, S. L. Huang, S. Y. Niu and S. Y. Gao, Highly dispersed copper-iron nanoalloy enhanced electrocatalytic reduction coupled with plasma oxidation for ammonia synthesis from ubiquitous air and water, Nano Energy, 2023, 117, 108840 CrossRef CAS.
- X. Y. Li, P. Deng, M. Q. Xu, Z. B. Peng, Y. H. Zhou, G. Jia, W. Wang, P. Gao and W. Wang, Multi-layer core-shell metal oxide/nitride/carbon and its high-rate electroreduction of nitrate to ammonia, Nanoscale, 2023, 15, 14439–14447 RSC.
- Y. T. Wang, C. H. Wang, M. Y. Li, Y. F. Yu and B. Zhang, Nitrate electroreduction: Mechanism insight, in situ characterization, performance evaluation, and challenges, Chem. Soc. Rev., 2021, 50, 6720–6733 RSC.
- F. C. Lei, K. Li, M. Yang, J. Yu, M. M. Xu, Y. M. Zhang, J. F. Xie, P. Hao, G. W. Cui and B. Tang, Electrochemical reduction of nitrate on silver surface and an in situ Raman spectroscopy study, Inorg. Chem. Front., 2022, 9, 2734–2740 RSC.
- Y. H. Wang, S. S. Zheng, W. M. Yang, R. Y. Zhou, Q. F. He, P. Radjenovic, J. C. Dong, S. N. Li, J. X. Zheng, Z. L. Yang, G. Attard, F. Pan, Z. Q. Tian and J. F. Li, In situ Raman spectroscopy reveals the structure and dissociation of interfacial water, Nature, 2021, 600, 81–85 CrossRef CAS.
- J. J. Zhou, F. Pan, Q. F. Yao, Y. Q. Zhu, H. R. Ma, J. F. Niu and J. P. Xie, Achieving efficient and stable electrochemical nitrate removal by in situ reconstruction of Cu2O/Cu electroactive nanocatalysts on Cu foam, Appl. Catal., B, 2022, 317, 121811 CrossRef CAS.
- G. C. Halford and M. L. Personick, Bridging colloidal and electrochemical nanoparticle growth with in situ electrochemical measurements, Acc. Chem. Res., 2023, 56, 1228–1238 CrossRef CAS PubMed.
- H. X. Sun and Z. F. Hu, Electrochemical behavior of Al-1.5Mg-0.05Sn-0.01Ga alloy in KOH ethanol-water solutions for Al-Air battery, J. Electrochem. Soc., 2019, 166, A2477–A2484 CrossRef CAS.
- Y. Xu, Y. S. Wen, T. L. Ren, H. J. Yu, K. Deng, Z. Q. Wang, X. N. Li, L. Wang and H. J. Wang, Engineering the surface chemical microenvironment over CuO nanowire arrays by polyaniline modification for efficient ammonia electrosynthesis from nitrate, Appl. Catal., B, 2023, 320, 121981 CrossRef CAS.
- Y. Zhang, M. Q. Xu, X. D. Xu, X. Y. Li, G. P. Zhu, G. Jia, B. C. Yang, R. L. Yin, P. Gao and W. Ye, Efficient interlayer confined nitrate reduction reaction and oxygen generation enabled by interlayer expansion, Nanoscale, 2022, 15, 204–214 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr01625d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |