Recent advances in synthesis and properties of silver nanoclusters
Xiaolin
Liu†
ab,
Taeyoung
Ki†
ab,
Guocheng
Deng
ab,
Seungwoo
Yoo
ab,
Kangjae
Lee
ab,
Byoung-Hoon
Lee
c,
Taeghwan
Hyeon
 *ab and
Megalamane S.
Bootharaju
*ab
*ab and
Megalamane S.
Bootharaju
*ab
aCenter for Nanoparticle Research, Institute for Basic Science (IBS), Seoul 08826, Republic of Korea. E-mail: thyeon@snu.ac.kr; msbootharaju@snu.ac.kr
bSchool of Chemical and Biological Engineering, and Institute of Chemical Processes, Seoul National University, Seoul 08826, Republic of Korea
cKU-KIST Graduate School of Converging Science and Technology, Korea University, Seoul, Republic of Korea
First published on 10th June 2024
Abstract
Achieving atomic precision in nanostructured materials is essential for comprehending formation mechanisms and elucidating structure–property relationships. Within the realm of nanoscience and technology, atomically precise ligand-protected noble metal nanoclusters (NCs) have emerged as a rapidly expanding area of interest. These clusters manifest quantum confinement-induced optoelectronic, photophysical, and chemical properties, along with remarkable catalytic capabilities. Among coinage metals, silver distinguishes itself for the fabrication of stable nanoclusters, primarily due to its cost-effectiveness compared to gold. This minireview provides an overview of recent advancements since 2020 in synthetic methodologies and ligand selections toward attaining NCs boasting a minimum of two free valence electrons. Additionally, it explores strategies for fine-tuning optical properties. The discussion extends to surface reactivity, elucidating how exposure to ligands, heat, and light induces transformations in size and structure. Of paramount significance are the applications of silver NCs in catalytic reactions for energy and chemical conversion, supplemented by in-depth mechanistic insights. Furthermore, the review delineates challenges and outlines future directions in the NC field, with an eye toward the design of new functional materials and prospective applications in diverse technologies, including optoelectronics, energy conversion, and fine chemical synthesis.
1. Introduction
The use of silver traces back to ancient civilizations like Rome and China, valued for its aesthetic allure and believed medicinal properties.1 Craftsmen combined silver with other metals for decorative brilliance and employed silver vessels to safeguard liquids from contamination. Over the decades, silver has undergone significant advancements, particularly following Faraday's preparation of colloidal gold and its catalytic application by Haruta, which redirected focus towards silver.2,3 Turkevich's synthesis of analogous silver colloids and Brust's pioneering use of thiolate ligands for surface functionalization and size control of gold nanoparticles further contributed to this evolution.1 Subsequently, researchers developed synthetic methods for producing atomically monodispersed gold and silver nanoparticles, commonly referred to as atomically precise nanoparticles or nanoclusters (NCs), typically with diameters below 3 nm.4,5 These NCs, bridging the gap between plasmonic nanoparticles and molecular precursors, offer insights into the evolution of material properties at discrete atom levels.6–10 Due to quantum confinement effects, NCs exhibit molecule-like optical, photophysical, and chiroptical properties.11–18 Their high surface-to-volume ratio and defined metal–ligand interface make NCs ideal model catalysts for unravelling molecular-level insights into the origins of catalysis through combined experimental and computational approaches.19–24Among coinage metals, gold has been extensively utilized to investigate the structure–property relationship of gold NCs due to their enhanced stability, facilitating structural characterization via single-crystal X-ray crystallography.1,2 Despite challenges in fabricating stable silver NCs, their lower cost renders them economically competitive, prompting the development of new synthesis strategies.3 It is noteworthy that silver forms stable Ag(I) clusters with few or no free electrons. Serving as models for metallic nanoparticles, silver NCs with free valence electrons are invaluable for understanding various properties, including identifying the size regime of non-metallic to metallic transitions and emerging plasmonic nanocatalysis.25–27 Hence, this minireview focuses on the recent advances in ligand-protected Ag NCs possessing free valence electrons since 2020, covering synthetic methodologies, modulation of optical properties, surface reactivity, and promising catalytic applications (Scheme 1). Additionally, we discuss the challenges and future directions in the field of metal NCs.
2. Synthetic strategies
2.1. Ligand-controlled synthesis
The synthesis of ligand-protected metal NCs is typically carried out through the chemical reduction of metal–ligand precursors in appropriate solvents at low or room temperature. Sodium borohydride is commonly employed as a reducing agent.28–30 Additionally, arylsilane, cyanoborohydride, and borane alkylamine complex are used to control the rate of reduction of metal–ligand complexes and thereby the size and structure of the resultant NCs.31–35 Understanding the molecular-level mechanism of the formation of NCs is challenging since the reaction solutions involve different phases, disabling the analysis of reaction intermediates through mass spectrometry. Nevertheless, ligands play a critical role in directing the formation of suitable intermediates, which lead to the formation of NCs of a specific size.Zhu and co-workers36 developed a promising synthetic strategy called “dual-level kinetic control”, in which the added secondary ligand (phosphine), in addition to the primary thiolate ligand, and the low reaction temperature are found to help achieve first- and second-level kinetic control, respectively. In the absence of a secondary ligand, polydisperse thiolated Ag nanoparticles were obtained. By employing phosphine and diphosphine ligands of different molecular structures, the “dual-level kinetic control” method offers a family of Ag NCs, including [Ag25(SR)18]−, [Ag34(SR)18(DPPP)3Cl4]2+, [Ag36(SR)26S4]2+, [Ag37(SR)25Cl1]+, and [Ag52(SR)28Cl4]2+ (Fig. 1) (DPPP: 1,3-bis(diphenylphosphino)propane; SR: 2-adamantanethiolate). In the first-level kinetic control (Fig. 1A), the phosphine ligands retarded the reduction rate, resulting in different Ag NCs correlating with the phosphine ligand type. In the second-level kinetic control (Fig. 1B), lowering the reaction temperature using an ice bath further slowed down the reduction rate, which led to advanced kinetic control and improved synthetic yields of Ag NCs.
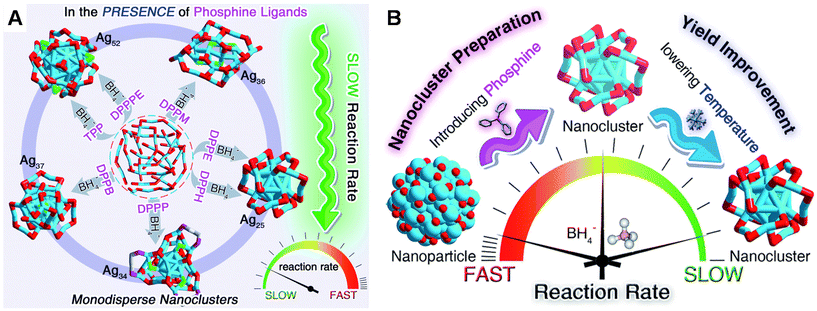 | ||
| Fig. 1 Schematic illustration of (A) first-level and (B) dual-level kinetic control via introducing phosphine ligands and low reaction temperature, leading to synthesis of a series of Ag NCs with improved yields. Reproduced with permission from ref. 36. Copyright (2022). | ||
By employing various combinations of thiol and diphosphine, a rare collection of super-atomic Ag NCs with sizes Ag19, Ag22, Ag26, and Ag30, possessing 2, 4, 6, and 8 free valence electrons respectively, has been reported.37 Interestingly, the cores of these clusters consist of one decahedral Ag7, perpendicular bidecahedra, three-dimensional penta-decahedra, and hexadecahedra, respectively. The strong structural correlation demonstrates the motif-to-core evolution of the surface Ag (on AgS2) to construct additional decahedral blocks, offering opportunities to investigate the impact of decahedral evolution patterns on the properties of NCs. In the presence and absence of phosphine ligands, a dithiol is shown to form Ag NCs of different sizes and structures. Specifically, 1,2-benzenedithiolate (1,2-BDT) forms Ag14(1,2-BDT)6(PPh3)8 in the presence of PPh3,38 while [Ag9(1,2-BDT)6]3− forms in the absence of the PPh3 ligand.39 The Ag14 cluster possesses an inner core of Ag6 arranged in an octahedral shape, surrounded by an outer cubic shell of Ag8. Conversely, the Ag atoms of the Ag9 cluster are arranged in an elongated body-centered cubic format. These examples demonstrate the crucial role of phosphine in the size-selected synthesis of NCs. In contrast, when an isomeric dithiolate, 1,3-benzenedithiolate (1,3-BDT), is used, Ag29 NCs are synthesized both in the presence and absence of the PPh3 ligand.40 The formation of the Ag29 cluster is attributed to its geometrical and electronic stability. However, the presence of PPh3 facilitates single-crystal growth and increases the yield of Ag29 clusters.40 Notably, the replacement of PPh3 with diphosphines also yields Ag29 clusters.41
The Zang group synthesized novel Ag(0)-containing Ag NCs by controlling the molecular structures of auxiliary phosphine ligands while keeping the thiolate ligand unchanged.42 Specifically, they synthesized [Ag17(L)12(PPh3)6]3+ (L = quinoline-2-thiol (deprotonated)-4-carboxyl) and [Ag32(L)18(P(Ph-pCH3)3)6Cl2]4+ (where P(Ph-pCH3)3 = tri-p-tolylphosphine). The Ag17 cluster features an Ag5 trigonal bipyramid core, while the Ag32 cluster has an Ag20 inner core formed by the fusion of two Ag13 icosahedra. However, both clusters possess surface structures based on Ag4L4P2 units and exhibit photoluminescence in the near-infrared region. The rigidity of the surface structure of Ag17 results in a higher photoluminescence quantum yield (PLQY: 3.09%) compared to that (less than 0.1%) of Ag32.
2.2. Counterion- and solvent-controlled synthesis
Counterions (cation or anion) are provided to balance the core charge of the NCs, resulting in electrostatic stabilization. Additionally, the bulkiness of the counterion can direct the formation of NCs with specific size and structure. Zang and co-workers43 observed the unusual cocrystallization of two anionic Ag NCs, [Ag62(MNT)24(TPP)6]8− and [Ag22(MNT)12(TPP)4]4− (Fig. 2) (MNT2−: dimercaptomaleonitrile; TPP: triphenylphosphine). The inter- and intra-cluster noncovalent interactions drive the cocrystallization of NCs. Interestingly, when the synthesis is carried out in the presence of positively charged counterions with varied bulkiness, a specific sized NC is obtained. Tetrabutylammonium and tetraphenylphosphonium direct the formation of the truncated-tetrahedral Ag22 and the octahedral Ag62 NCs, respectively.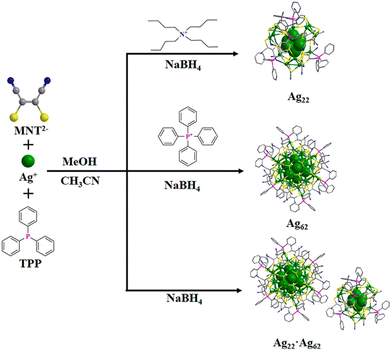 | ||
| Fig. 2 Synthetic route for counter cation-directed synthesis of [Ag62(MNT)24(TPP)6]8− and [Ag22(MNT)12(TPP)4]4− NCs. Color legend: green, Ag; pink, P; yellow, S; blue, N; gray, C. Reproduced with permission from ref. 43. Copyright (2023). | ||
The Kang group obtained three medium-sized Ag NCs, namely Ag52, Ag54, and Ag58, co-protected by thiolate, halide, and hydride ligands.44 The Ag52 and Ag54 NCs are synthesized in the presence of tetraphenylphosphonium, whereas the Ag58 NC is produced in the presence of tetraoctylammonium counterion. The Ag52 and Ag54 NCs are structurally homologous with respect to the kernel structures and the overall metal-atom configurations, while the Ag58 NC has a completely different geometric structure. The formation of different cluster compounds is attributed to the following: (i) the flexibility of the hydrocarbon chain of tetraoctylammonium and the rigidity of the phenyl rings of tetraphenylphosphonium. The different degrees of steric hindrance of the counterions would have resulted in structural rearrangement at distinct levels during the formation of NCs; (ii) different and/or specific interactions among cluster intermediate species and counterions would have remarkably affected the synthesis of NCs of a specific size.
The solvent medium, in which metal–ligand precursors form nuclei and further grow to yield NCs, also plays a crucial role in controlling the size and structure of metal NCs. Organic solvents with varying polarities, such as alcohols (methanol and ethanol), halocarbons (dichloromethane and chloroform), acetonitrile, and dimethylformamide (DMF), are commonly used for the synthesis of Ag NCs since both the precursors and the NC products dissolve well in these solvents. Although the exact reasons for the size selection of NCs are unknown, changing solvents can lead to NCs with distinct structures.
The [Ag30(TC4A)4(TBPMT)8] NCs are synthesized in a methanol-chloroform mixture (where TC4A4− = p-tert-butylthiacalix[4]arene; TBPMT = 4-tert-butylbenzenemethanethiolate).45 Interestingly, by replacing methanol in the solvent mixture with acetone, different-sized NCs [Ag34Na4Cl4(TC4A)4(TBPMT)11(solvents)]3+ are obtained. The 6-electron-Ag148+ kernel of Ag30 is formed by the central Ag6 octahedron sandwiched between two orthogonally oriented Ag5 trigonal bipyramids through sharing vertices. On the other hand, the 4-electron-Ag106+ core of Ag34 is formed by two edge-shared octahedrons. These two clusters exhibit distinct optical absorption profiles due to the differences in their geometric and electronic structures. Similarly, n-butanol, a rarely used alcohol for the synthesis of NCs, is found to be critical in the synthesis of a large Ag NC [Ag155(CyS)40(TC4A)5Cl2] (where CyS = cyclohexanethiolate).46 The use of other common alcohols is unable to produce this NC. This cluster consists of a metallic core of four concentric shells, Ag13@Ag42@Ag30@Ag70. In some instances, special precursors are required to obtain particular clusters. For example, [Ag(pz)]n (where Hpz = pyrazole) is necessary to obtain the [Ag78(iPrPhS)30(dppm)10Cl10]4+ cluster.47 Here, the pz-ligand is not incorporated into the cluster, but its strong coordination effect serves to make [Ag(pz)]n a controlled-releasing silver ion source. Such ligand-assisted synthesis is applicable to other NCs as well.48,49 The presence of the halide ligand originates from the in situ decomposition of the halocarbon solvent or other sources, a phenomenon also observed in other cluster systems.35,47,50,51
2.3. Photochemical and metal-mediated approach
Light serves as a promising energy source for the reduction of noble metal precursors to their metallic state. Inspired by the photochemical synthesis of plasmonic nanoparticles,52 Zhang and co-workers attempted to synthesize ligand-protected Ag NCs.53 By irradiating a solution of suitable metal and ligand precursors along with an organic base, they successfully synthesized atomically monodisperse [Ag25(4-MePhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)20(Dpppe)3]3+ NCs (as depicted in Fig. 3A) (where Dpppe = 1,5-bis(diphenylphosphino)pentane; 4-MePhC
C)20(Dpppe)3]3+ NCs (as depicted in Fig. 3A) (where Dpppe = 1,5-bis(diphenylphosphino)pentane; 4-MePhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH = 4-methylphenylacetylene). The photochemical formation mechanism of the NCs involves a photoinduced electron-transfer (PET) process. Upon photoexcitation of amines with light wavelength <455 nm, electrons from the amine are transferred to Ag+ ions, resulting in the oxidation of the amine and the formation of amine N-oxide.
CH = 4-methylphenylacetylene). The photochemical formation mechanism of the NCs involves a photoinduced electron-transfer (PET) process. Upon photoexcitation of amines with light wavelength <455 nm, electrons from the amine are transferred to Ag+ ions, resulting in the oxidation of the amine and the formation of amine N-oxide.
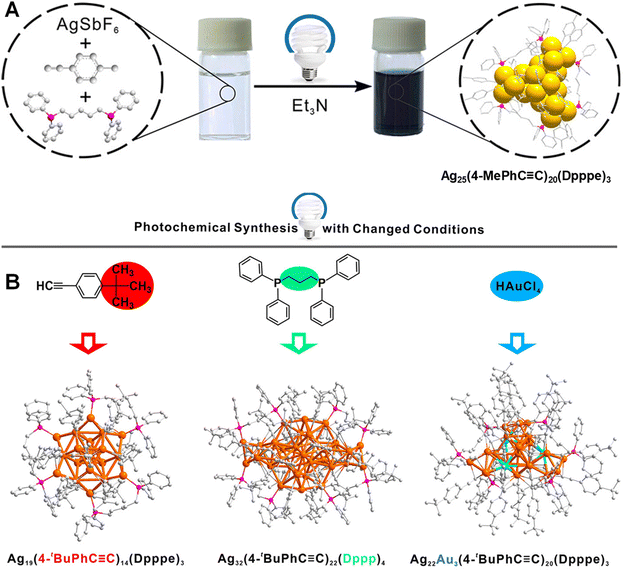 | ||
| Fig. 3 (A) Schematic illustration of the photochemical synthesis of Ag25 NCs by irradiating the precursor solution by <455 nm light for 24 h. (B) Schematic of the photochemical synthesis of Ag NCs of different size, structure and composition. Reproduced with permission from ref. 53. Copyright (2023). | ||
The PET process is supported by experimental and density functional theory results. The absence of light and amine leads to no formation of NCs. A minimum photon energy of 2.7 eV is required to initiate the synthesis of NCs. The structure of Ag25 consists of an Ag13 core in the form of an anticuboctahedron, a type of hexagonally close-packed (hcp) lattice. This core differs from the Ag13 icosahedral core of thiolated Ag25 clusters.54 This difference likely originates from variations in the coordination modes of thiolate and alkynyl ligands with silver. The remaining 12 Ag atoms form three distorted tetrahedra, which are trigonally distributed around the Ag13 core. Subsequently, the metal atoms of the cluster are co-protected by diphosphine and alkynyl ligands. The photochemical method has been found to be applicable to the synthesis of Ag NCs of various sizes and structures by using alkynyl and phosphine ligands with different molecular structures and functionalities (as shown in Fig. 3B). Interestingly, by substituting 4-tert-butylphenylacetylene (4-tBuPhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH) for 4-MePhC
CH) for 4-MePhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH, [Ag19(4-tBuPhC
CH, [Ag19(4-tBuPhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)14(Dpppe)3]3+ is synthesized with an overall structure similar to that of Ag25. However, the Ag19 cluster possesses an Ag13 cuboctahedron core unlike the anticuboctahedron Ag13 core of the Ag25 cluster. Additionally, by introducing 1,3-bis(diphenylphosphino)propane (Dppp), the synthesized product contains [Ag32(4-tBuPhC
C)14(Dpppe)3]3+ is synthesized with an overall structure similar to that of Ag25. However, the Ag19 cluster possesses an Ag13 cuboctahedron core unlike the anticuboctahedron Ag13 core of the Ag25 cluster. Additionally, by introducing 1,3-bis(diphenylphosphino)propane (Dppp), the synthesized product contains [Ag32(4-tBuPhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)22(Dppp)4]3+. The structure of Ag32 comprises an Ag24 kernel and two distorted Ag4 tetrahedra. Interestingly, it exhibits electron paramagnetic resonance (EPR) activity due to its 7 free valence electrons. Furthermore, this photochemical route can be used to synthesize alloy NCs, such as [Ag22Au3(4-tBuPhC
C)22(Dppp)4]3+. The structure of Ag32 comprises an Ag24 kernel and two distorted Ag4 tetrahedra. Interestingly, it exhibits electron paramagnetic resonance (EPR) activity due to its 7 free valence electrons. Furthermore, this photochemical route can be used to synthesize alloy NCs, such as [Ag22Au3(4-tBuPhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)20(Dpppe)3]3+, by introducing corresponding metal precursors.
C)20(Dpppe)3]3+, by introducing corresponding metal precursors.
Attempts to synthesize heteroatom-doped Ag NCs sometimes lead to the formation of undoped Ag NCs, which are only possible in the presence of that particular heterometal atom. For instance, Pd is found to mediate the synthesis of Ag33(SCH2CH2Ph)24(PPh3)4 NC.55 In its absence or in the presence of Au or Pt, a different sized NC, Ag23(PPh3)8(SC2H4Ph)18, is obtained. Control experiments indicated that Pd(PPh3)4 is formed first by borohydride reduction, which further catalyzes the reduction of Ag+ and the growth of the Ag33 cluster. Similarly, Cu is found to be essential in synthesizing Ag44(EBT)26(TPP)4 NC (EBT: 2-ethylbenzenethiolate),56 whose size and core structure are analogous to an all-thiolated Ag44(SR)30 cluster. The formation of AgCu alloy NCs is believed to mediate the synthesis of electronically and geometrically stable Ag44 clusters. On the other hand, by replacing Cu with Au, AgAu alloy clusters are formed, indicating the unique role of specific metals in the synthesis of certain Ag NCs.
2.4. Other ligands for synthesis of NCs
Coinage metal NCs are typically synthesized using soft-base ligands such as thiolates, phosphines, selenolates, and alkynylides. The use of hard-base ligands with O and N donors is rare because the combination with soft bases is not stable. Recently, the [Ag8(pfga)6]6− cluster (pfga: perfluoroglutarate) was synthesized with an unprecedented rhombohedral Ag86+ core.57 This two-electron cluster exhibits visible light absorption and emits bright green-yellow light. Following this work, 9-anthracene carboxylate, a bulky monocarboxylate, and different phosphines were used to synthesize two-electron [Ag16(L)8(9-AnCO2)12]2+ clusters.58 Its structure features an [Ag8@Ag8]14+ core–shell structure, with an [Ag8]6+ superatomic inner core adopting an unprecedented structure showing a distorted hexagonal bipyramidal structure. The outer Ag8 shell is composed of the unique “Ag(PR3)–AnCOO–Ag(PR3)” staple motifs.Using N donor ligands, Wang and co-workers synthesized [Ag61(dpa)27]4+ (Hdpa = dipyridylamine), the longest silver supercluster based on icosahedral Ag13 building blocks with vertex sharing.59 In this large cluster of clusters, the icosahedron units are bridged by dpa ligands. The quantum size effects contribute significantly to the enhanced metal-related absorptions and the red-shift in the near-infrared region as the length of the cluster increases from 8-electron [Ag21(dpa)12]+ NC to 30-electron [Ag61(dpa)27]4+ NC (Fig. 4). Furthermore, electron coupling is developed between constituent Ag13 building units. Notably, a disc-like largest structurally characterized Ag cluster of clusters,31 [Ag93(PPh3)6(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)50]3+ (R = 4-CH3OC6H4) is observed for a phosphine and alkynyl combination.
CR)50]3+ (R = 4-CH3OC6H4) is observed for a phosphine and alkynyl combination.
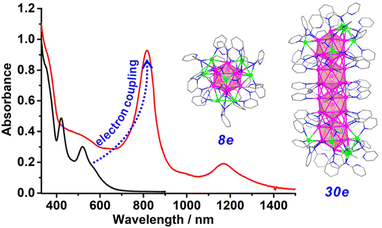 | ||
| Fig. 4 Optical absorption spectra of [Ag21(dpa)12]+ and [Ag61(dpa)27]4+ NCs and corresponding atomic-level structures. Reproduced with permission from ref. 59. Copyright (2021). | ||
Continuing the use of N donor ligands for Ag NC synthesis, Wang and co-workers60 designed tetradentate formamidinates and successfully obtained two fcc clusters: [Ag52(5-F-dpf)16Cl4]2+ (5-F-Hdpf = N,N′-di(5-fluoro-2-pyridinyl)formamidine) and [Ag53(5-Me-dpf)18]5+ (5-Me-Hdpf = N,N′-di(5-methyl-2-pyridinyl)formamidine). All silver atoms are engaged in fcc packing with [111] facets, and no staple motifs are present due to the linear arrangement of the four N donors of the dpf ligands. The Ag53 cluster is the largest well-defined [111] facet with 14 fcc Ag atoms. In combination with organic ligands, inorganic ligands such as oxometalates are used to stabilize and tune the cluster size.61,62 For example, 20-electron Ag NCs such as Ag28(dppb)6(MoO4)4, Ag28(dppb)6(WO4)4 and Ag32(dppb)12(MoO4)4(NO3)4 (dppb: 1,4-bis(diphenylphosphino)butane) are synthesized through co-protection. Combination of novel multiple ligands can lead to new assembled clusters. For instance, alkynyl, trifluoroacetate, and diphenylphosphinate set forms luminescent one-dimensional high-nuclearity silver polyclusters,63 such as [Ag16(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)6(tfa)6(Ph2PO2)4(CH3OH)]n, and [Ag21(C
CtBu)6(tfa)6(Ph2PO2)4(CH3OH)]n, and [Ag21(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CtBu)8(tfa)6(Ph2PO2)7]n.
CtBu)8(tfa)6(Ph2PO2)7]n.
2.5. Stability, yield, and functionalization
Metal NCs are typically metastable, which limits their potential applications in optoelectronics and catalysis. For example, the weak Ag–P bonding in the 58-electron Ag64(PnBu3)16Cl6 cluster makes the NC unstable.64 However, the stability of NCs has been significantly improved by employing a combination of ligands. Zhu and co-workers65 designed a strategy termed “surface environment complication”, in which the use of a bidentate PPh2py ligand with dual (P and N) coordination sites helps stabilize the extremely unstable Ag29(S-Adm)18(PPh3)4 cluster by forming a new Ag29(S-Adm)15(NO3)3(PPh2py)4 cluster. The Cl intercalation in plasmonic Ag307Cl62(SPhtBu)110 cluster leads to its superstability (stable for a week at 55 °C).66 Pradeep and co-workers67 used carboranethiol for the preparation of propeller-shaped [Ag21(MCT)12(PPh3)2]+ (MCT: m-carborane-9-thiolate). This cluster is found to be remarkably thermally stable up to 100 °C. The use of macrocyclic ligands in combination with thiolate made the 155-atom Ag NC ultrastable.46 It exhibits an outstanding photothermal effect. This cluster can attain a temperature of 59.1 °C within 300 s. The heating ability of the cluster remains nearly intact through 6 cycles of heating and cooling processes.High yields of NCs are crucial for exploring their properties. Since controlling the monodispersity of clusters in large-scale synthesis is challenging, more efforts are indeed required to achieve satisfactory yields. Fast and high-yield (83%) synthesis of Ag44(SR)30 clusters is achieved through the development of the CTAB (cetyltrimethylammonium bromide) reverse micelle method.68 Forming reverse micelles using CTAB to construct a sealed chemical environment is found to be critical in achieving fast and high-yield synthesis. Functionalization of the cluster surface can be useful in improving stability and other properties. Tang and co-workers69 used a thiophenol ligand with silane substituents, which not only enabled the synthesis of [Ag44(SPhSi(OEt)3)30]4− clusters but also improved their stability up to 60 °C in air for 13 hours. This silica-coated NCs may be useful in photothermal and photoacoustic imaging applications.
3. Tuning optical properties
The PL of Ag NCs is an attractive characteristic with potential applications in sensing and imaging. It is influenced by several factors, including the metal core, ligand, dopant, solvent, intercluster distance, ion-pairing, and supramolecular interactions, which have been covered in the previous reviews.70–75 The hydrophilic Ag NCs typically exhibit weak PL due to quenching. To investigate the underlying mechanism of PL quenching in hydrophobic Ag NCs in an aqueous phase, Zhu and colleagues developed a surface functionalization strategy (Fig. 5).76 Specifically, they synthesized a functionalized [Ag29(BDTA)12(DPBA)4]3− NC (Ag29-AC; AC denotes all-around-carboxyl functionalization; BDTA: 3,5-dithiolbenzoic acid; DPBA: 4-(diphenylphosphino)benzoic acid) by replacing the unfunctionalized carbon-tail-containing dithiolate and phosphine ligands of [Ag29(BDT)12(PPh3)4]3− NC (Ag29-BDT; BDT = 1,3-benzenedithiolate). The water solubility of Ag29-AC, while retaining its size and structure, allows it to serve as a model NC compound to elucidate the effect of functionalization on PL. The quenching of PL (PLQY: 0.39%; lifetime: 0.21 μs) in Ag29-AC(H2O) clusters is triggered by phase transfer, leading to molecular decoupling. This increased mobility of Ag29-AC(H2O) clusters in water weakens the radiative transition. However, the emission recovery of the quenched NCs is achieved (PLQY: 4.7%; lifetime: 2.85 μs) through a supramolecular recoupling route induced by glutathione addition, which promotes the aggregation of NCs. This aggregation restricts intracluster motion and intercluster rotation, thereby strengthening the radiative transition of the clusters.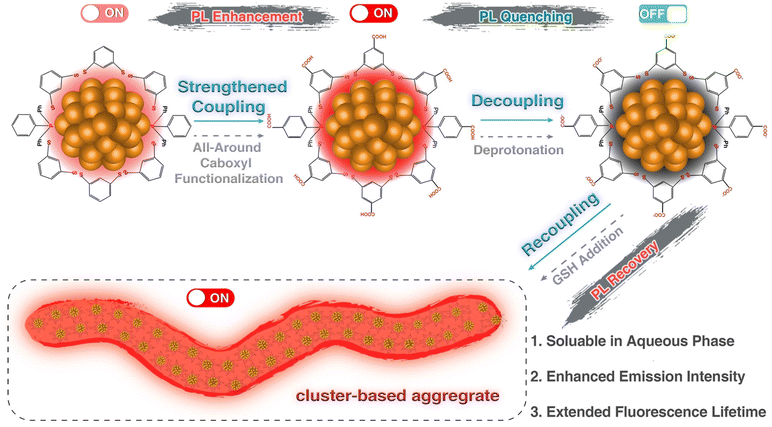 | ||
| Fig. 5 Schematic of the emission enhancement of the Ag29-AC NC through the strengthened coupling, the emission quenching of Ag29-AC in the aqueous phase caused by molecular decoupling, and the emission recovery of the Ag29-AC NC by its supramolecular recoupling in the presence of reduced glutathione (GSH). Reproduced with permission from ref. 76. Copyright (2024). | ||
The selective peripheral modification of [Ag29(BDT)12(TPP)4]3− NC with Ag(I) complexes, resulting in Ag29 + Ag NCs, causes the PL peak to shift from 680 to 770 nm, accompanied by an increase in PLQY from 1.4 to 39%.77 Despite minor changes in the ground-state geometric and electronic structure, the excited-state properties undergo significant alterations. In addition to the inherent ultrafast intersystem crossing from the S1 to T1 state, the Ag29 + Ag NC exhibits an additional excited-state relaxation to another triplet state (T′1) with a time constant of 287 ps. This additional relaxation may be associated with internal motions within the Ag29 + Ag NC, such as rearrangement of the ion-pair structure. Such modifications hold promise for applications in photocatalysis.
Ligands and their functional groups modulate the structure and optical absorption features of NCs. The Ag14 cluster structure is found to be robust, as it can be formed by a range of thiolate ligands, including monothiol and dithiol.38 The dithiolated Ag14 cluster exhibits unique absorption peaks at 425, 600, and 860 nm, extending into the near-infrared (NIR) region, primarily due to transitions derived from ligands. It also shows dual visible/NIR emission at approximately 680 and 997 nm. Conversely, the monothiolated Ag14 cluster has visible absorption peaks at 368 and 530 nm, with yellow PL.78 Due to the rigidity of the dithiolate, the resulting cluster is thermally robust. Furthermore, the electronic effects of halogen substituents on the thiolate ligand influence the optical and electrochemical bandgap values, governed by intracluster and intercluster π–π interactions.79 Additionally, the electronegativity of ligand substituents affects ultrafast relaxation dynamics by tuning the electron–phonon interaction.80 Intracluster interactions among thiolate and diphosphine ligands of Ag21 clusters have been shown to enhance the PLQY up to 7%.81
In addition to ligands, electron-vibration coupling plays a crucial role in the PL of metal NCs. Weaker and stronger electron-vibration coupling leads to higher and lower NIR PLQY in Ag25(SR)18 (3.5%) and Au25(SR)18 (1%) clusters, respectively.82 Doping a single Au atom into Ag25 further suppresses the nonradiative decay rate, enhancing PLQY up to 35%.83 Pradeep and co-workers84 improved the PL of Ag14 NCs co-protected with naphthalenethiol and 1,6-bis(diphenylphosphino)hexane ligands by three-fold through secondary ligand-induced orthogonal self-assembly. The resulting supracolloidal structures, including helical fibers, spheres, and nanosheets, possess restricted intramolecular motion through noncovalent and supramolecular interactions, directly influencing radiative excited-state relaxation. Chiral NCs with light emission exhibit circularly polarized luminescence (CPL). The Zang group synthesized enantiomeric superatomic Ag clusters,85 [Ag17(R/S-NYA)12]3+, stabilized by chiral alkynyl ligands (R/S-NYA: N-((R/S)-1-(naphthalen-4-yl)ethyl)prop-2-yn-1-amine). They exhibit NIR PLQY of 8% as well as CPL due to the chirality of the excited states.
4. Transformation and surface reactivity
The molecular nature and intermediate stability of NCs render them reactive when subjected to external stimuli such as light, ligands, metal ions, temperature, and others. This reactivity often leads to their conversion into NCs of different sizes, including reduction or growth in size. Thus formed clusters may have novel electronic and geometric structures with exciting optoelectronic and photophysical properties.4.1. Light-induced transformation
Pradeep and colleagues86 reported that the [Ag42(CBDT)15(TPP)4]2− NC undergoes light-activated downsize conversion to form the Ag14(CBDT)6(TPP)6 cluster (CBDT: ortho-carborane-1,2-dithiolate; TPP: triphenylphosphine). Despite the high thermal stability of Ag42 cluster up to 200 °C in its solid-state, it undergoes conversion in solution under light irradiation (Fig. 6A). The transformation proceeds through two sets of intermediates (Fig. 6B): (i) [Ag37(CBDT)12(TPP)4]3− and [Ag35(CBDT)8(TPP)4]2− formed after 8 hours, and (ii) [Ag30(CBDT)8(TPP)4]2−, [Ag26(CBDT)11(TPP)4]2−, and [Ag26(CBDT)7(TPP)7]2− formed after 16 hours. After 24 hours, these intermediates transform into the final product, the Ag14 cluster. The kernel-centered stable excited states of Ag42 are found to be responsible for light-induced cluster conversion. The Ag42 cluster emits light at 980 nm with a phosphorescence lifetime of >1.5 μs, whereas Ag14 shows red fluorescence emission at 626 nm with a lifetime of 550 ps.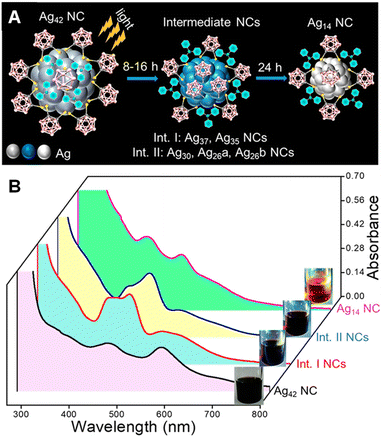 | ||
| Fig. 6 (A) Schematic of the light-activated conversion of Ag42 to Ag14 through intermediate clusters. (B) UV–vis absorption spectra of Ag42 NC before and after light irradiation along with the mixture of intermediate clusters. Insets: photographs of the color change of cluster solutions during the light-irradiation. Reproduced with permission from ref. 86. Copyright (2021). | ||
In a recent study, the same group observed a light-activated size-expansion process, wherein the [Ag31(TRZ)10]2−/1− cluster transformed into the [Ag42(TRZ)13]2+ cluster (TRZ: 6-(dibutylamino)-1,3,5-triazine-2,4-dithiolate).87 The size conversion was demonstrated using absorption spectroscopy of NCs with fingerprint absorption features, as well as a clear color change from violet to greenish. The Ag31 cluster exhibits photoresponsive behavior, and the resulting intermediate species are covered with solvated electrons, contributing to the expansion of the cluster size. Both the Ag31 and Ag42 clusters exhibit NIR and red emission, respectively. Notably, the final product Ag42 cluster exhibits strikingly short excited-state carrier dynamics compared to the Ag31 cluster. In other words, the stable photo-excited-state carriers and unique electronic and geometric structure of the Ag31 cluster likely lead to the light-induced size growth of NCs.
4.2. Ligand-exchange-induced transformation
When introduced to a foreign ligand, NCs undergo transformations depending on the inherent electronic effects of the new ligand and the stability of ligand-exchange intermediates. The Zhu group88 utilized aqueous Agm(SG)n clusters (where SG = L-glutathione) in a biphasic ligand-exchange process with two different thiols (HStBu: tert-butyl mercaptan; CySH: cyclohexanethiol). This process yielded Ag42(StBu)24 and Ag61(SCy)40Cl. Ag42 features a tetrahedral kernel, while Ag61 possesses a face-fused bi-tetrahedral Ag14 kernel. Interestingly, Ag42 can also convert to Ag61 through a ligand etching process with excess HSCy. When Ag25(Capt)18 NC (Capt: captopril) reacts with 2-isopropylbenzenethiol, the cluster size remains intact,89 indicating that ligands determine both the cluster size and metal arrangements (i.e., structure). The [Ag18H16(PPh3)10]2+ clusters react with 2-pyrene imine thiol (2-PIT), forming the [Ag35(2-PIT)7(TPP)7@(H2O)]3+ cluster.90 This cluster emits blue and NIR light under UV excitation due to pyrene-to-metal core charge transfer and charge transfer within the metal core, respectively.Liu and colleagues91 observed the formation of a two-electron silver superatom, Ag6(S2P(OiPr)2)4(dppm)2, by reacting an eight-electron superatom, Ag20(S2P(OiPr)2)12, with a diphosphine (dppm: bis(diphenylphosphino)methane). Here, dppm acts as a chemical scissor, pruning the icosahedron-based Ag20 NC to an octahedral Ag6 NC. The Ag6 cluster exhibits a bright yellow emission (PLQY: 16.3%) under UV light at room temperature. Sometimes, ligands react with clusters to form even smaller clusters or metal–ligand complex precursors.92 When Pt2Ag23Cl7(PPh3)10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 93 NCs react with a diphosphine ligand dppe (1,2-bis(diphenylphosphino)ethane), a self-assembled Ag2Cl2(dppe)2 cluster material is obtained.94 This material exhibits a high PLQY of ∼18% due to metallophilic interactions and crystallization, which restrict intermolecular rotations and minimize nonradiative relaxation paths of the excited-state. Bidentate ligands can assemble the NCs if they have uncoordinated metal sites. The 4,4′-bipyridine (4,4′-bpy) links two two-electron primary building units Ag10(S2P(OiPr)2)8 to make the smallest self-assembled structure of [Ag10(S2P(OiPr)2)8]2(μ-4,4′-bpy)95 through N coordination with open Ag site of the cluster. Upon heat treatment, an Ag(I) hydride cluster Ag7(H)(S2P(OiPr)2)6 undergoes self-redox reaction to produce Ag10(S2P(OiPr)2)8 cluster without addition of any reducing agent.96 The bicapped trigonal bipyramid metal framework of Ag7 cluster is well correlated with that of Ag10, suggesting Ag7 acts as the template in the growth of the cluster.
93 NCs react with a diphosphine ligand dppe (1,2-bis(diphenylphosphino)ethane), a self-assembled Ag2Cl2(dppe)2 cluster material is obtained.94 This material exhibits a high PLQY of ∼18% due to metallophilic interactions and crystallization, which restrict intermolecular rotations and minimize nonradiative relaxation paths of the excited-state. Bidentate ligands can assemble the NCs if they have uncoordinated metal sites. The 4,4′-bipyridine (4,4′-bpy) links two two-electron primary building units Ag10(S2P(OiPr)2)8 to make the smallest self-assembled structure of [Ag10(S2P(OiPr)2)8]2(μ-4,4′-bpy)95 through N coordination with open Ag site of the cluster. Upon heat treatment, an Ag(I) hydride cluster Ag7(H)(S2P(OiPr)2)6 undergoes self-redox reaction to produce Ag10(S2P(OiPr)2)8 cluster without addition of any reducing agent.96 The bicapped trigonal bipyramid metal framework of Ag7 cluster is well correlated with that of Ag10, suggesting Ag7 acts as the template in the growth of the cluster.
4.3. Surface reactivity
When a cluster possesses accessible surfaces or labile ligands with bulkiness, it tends to exhibit distinct reactivity, which can be harnessed for specific applications through materials design. For example, the [Ag29(SSR)12(PPh3)4]3− cluster is hydrophobic, limiting its utility in applications involving aqueous media. However, the accessible surface and high magnitude of negative electronic charge of this robust cluster enable surface interactions with solvent-conjoined Na+ cations (Na1(NMP)5 or Na3(DMF)12) (NMP: N-Methyl-2-pyrrolidone).97 The strong electrostatic attraction between the anionic cluster and these positive ions facilitates the migration of the clusters [Ag29(SSR)12(PPh3)4]3−[Na1(NMP)5]3+ into the aqueous medium, thus enabling biological applications. Such a micellization strategy has been found to be applicable to most anionic clusters.Zheng and colleagues98 synthesized a solvent-coordinated chiral Ag cluster, [Ag14(SPh(CF3)2)12(PPh3)4(DMF)4] (Ag14-DMF), in which surface ligands are highly labile. The high surface reactivity of Ag14-DMF allows DMF to dissociate or be exchanged with new ligands. Utilizing the surface reactivity of the cluster, a fully amine-ligated Ag14 cluster (where all PPh3 and DMF are replaced) could be obtained with fine-tuned optical properties by exposing the cluster to excess amine. Interestingly, by introducing chiral amines (R-1-PPA: R-1-phenylpropylamine and S-1-PPA: S-1-phenylpropylamine), the racemic Ag14-DMF clusters (Fig. 7A) are converted to homochiral Ag14 clusters (Fig. 7B) with a good chiroptical response. By employing bidentate linkers with different chiral configurations,99 such as (1R,2R,N1E,N2E)-N1,N2-bis(pyridin-3-ylmethylene)cyclohexane-1,2-diamine (LR) and its chiral analog LS, chiral cluster-based metal–organic frameworks are synthesized using Ag14-DMF clusters. These cluster-organic frameworks demonstrate assembly-disassembly process.
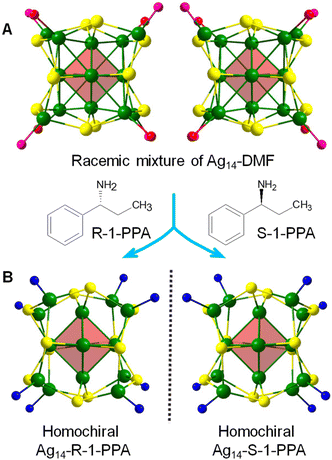 | ||
| Fig. 7 Core structures of (A) Ag14-DMF enantiomer pair and (B) Ag14-R-1-PPA (left) and Ag14-S-1-PPA (right). Reproduced with permission from ref. 98. Copyright (2021). | ||
The surface reactivity of NCs with metal ions can also serve as a useful strategy to obtain novel NCs. Zhu and co-workers100 reported on the role of metal ion quantity in NC transformation. When [Ag25(2,5-DMBT)16(DPPF)3]+ reacts with less than 0.5 equiv. of Cu2+, size-retained doped clusters [Ag25−xCux(2,5-DMBT)16(DPPF)3]+ are formed (2,5-DMBT: 2,5-dimethylbenzenethiolate; DPPF: 1,1′-bis(diphenylphosphino)ferrocene). On the other hand, one equiv. of Cu2+ results in a structural transformation process, yielding [Ag22−xCux(2,5-DMBT)12(DPPF)4Cl4]2+ clusters. In the former case, Cu2+ acts as a dopant, while in the latter case, it acts as an oxidant. Control experiments demonstrate that Cu+ forms doped clusters, whereas Cu2+ and other common oxidizers produce size-transformed products, indicating the unique role of metal ions in NC reactions.
5. Catalytic applications
Metal NCs with well-defined size, structure, and composition can serve as model nanoparticle analogs to understand the molecular-level mechanisms of catalytic properties. In the following, we will cover various reactions catalyzed by Ag NCs.5.1. CO2 conversion
In addressing global energy demands and closing the carbon cycle, CO2 conversion plays a crucial role. Electrocatalysis holds great potential for converting CO2 into useful fuels and chemicals using electrical energy produced from renewable sources. Recently, Ag NCs have begun to be utilized for CO2 reduction. Tang and colleagues101 synthesized a body-centered cubic (bcc) structured homoleptic Ag15(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C-tBu)12+ cluster and employed it as a catalyst for the electrochemical CO2 reduction reaction (eCO2RR). The cluster catalyst exhibits high selectivity for CO, with a highest Faradaic efficiency (FECO) of 95% at −0.6 V and a maximal turnover frequency of 6.37 s−1 at −1.1 V, along with excellent long-term stability. Theoretical calculations indicate that the Ag site created upon stripping a single alkynyl ligand acts as the active site.
C-tBu)12+ cluster and employed it as a catalyst for the electrochemical CO2 reduction reaction (eCO2RR). The cluster catalyst exhibits high selectivity for CO, with a highest Faradaic efficiency (FECO) of 95% at −0.6 V and a maximal turnover frequency of 6.37 s−1 at −1.1 V, along with excellent long-term stability. Theoretical calculations indicate that the Ag site created upon stripping a single alkynyl ligand acts as the active site.
In another study, the same group utilized two Ag NCs of identical size, but one was protected by alkynyl ligands and the other was co-protected by thiolate and diphosphine ligands to understand the effect of ligands on eCO2RR.102 The clusters are Ag32L24 (L = 3,5-bis(trifluoromethylbenzene)acetylide) and [Ag32(dppe)5(SR)24]2− (SR: trifluoromethylbenzenethiolate). The alkynyl-protected Ag32 cluster exhibits a high FECO of 96.4% at −0.8 V, whereas the thiolated cluster shows an FECO of only 56.6% at −1.0 V, suggesting the critical role of ligands in eCO2RR. Theoretical calculations indicate that the energy barrier for the formation of the *COOH intermediate is similar for both clusters. However, the thiolated cluster has the lowest thermodynamic barrier for the formation of H2 gas (0.05 eV) compared to that (0.51 eV) of the alkynylated cluster, explaining the high CO selectivity in the latter case.
The thiacalix[4]arene-capped 6-electron [Ag30(TC4A)4(iPrS)8] NC and the chain-like Ag(I) polymer [H2Ag5(TC4A)(iPrS)3]∞ are compared with their eCO2RR performance.103 The NC exhibits high CO selectivity (93.4% at −0.9 V versus RHE) and long-term stability (24 h). Density functional theory (DFT) calculations show that the stabilization of the *COOH intermediate is better on the cluster than on the polymer. Furthermore, the jCO of Ag30 is −33.29 mA cm−2 at −1.2 V versus RHE, which is 2.5 times higher than that of the Ag(I) polymer (−13.2 mA cm−2).
Recently, we have demonstrated that the local hydrophobicity of the surface of NCs influences eCO2RR by affecting the interfacial water structure.89 To understand the molecular-level mechanism, two Ag NCs, namely Ag25(Capt)18 and Ag25(IPBT)18, were synthesized. They exhibit identical metal–ligand arrangements except for the difference in the ligand structure, wherein the carboxyl group of the Capt ligand makes the NC water-soluble, while the isopropyl on the benzene ring of the IPBT ligand makes the NC hydrophobic. Despite loading the NCs on a carbon support, the local hydrophilicity of Ag25(Capt)18 is confirmed using contact angle measurements. The eCO2RR activity of the Ag25(IPBT)18 cluster is dramatically enhanced by locally induced hydrophobicity due to bulky alkyl functionality near its surface (Fig. 8A). This enhancement is reflected in the FECO (90.3%) and higher jCO observed in an H-cell compared to the Ag25(Capt)18 cluster with confined hydrophilicity (FECO: 66.6%) (Fig. 8B). Notably, the hydrophobic Ag25 cluster exhibits an impressive jCO as high as −240 mA cm−2 with FECO >90% at a cell potential of −3.4 V in a gas-fed membrane electrode assembly device (Fig. 8C). Furthermore, this cluster demonstrates stable eCO2RR activity over a period of 120 hours (Fig. 8D). Operando surface-enhanced infrared absorption spectroscopy and theoretical simulations reveal that the hydrophilic cluster surface accommodates more water molecules due to hydrogen bonding. In contrast, the interfacial water structure on the hydrophobic cluster is similar to bulk water. These differences influence the thermodynamics and kinetics of eCO2RR.
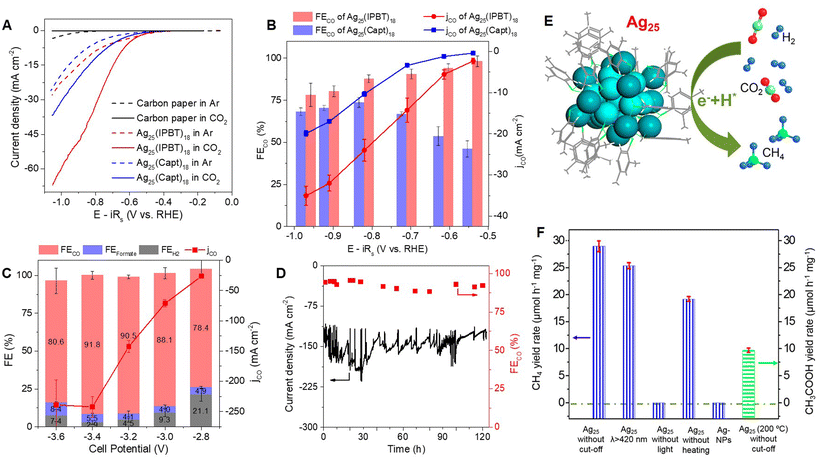 | ||
Fig. 8 (A) Polarization curves and (B) FECO and jCO of Ag25(IPBT)18 and Ag25(Capt)18 catalysts in the H-cell. (C) FECO and jCO of Ag25(IPBT)18 in the cell potential range from −2.8 to −3.6 V in the MEA cell. (D) Long-term operation of Ag25(IPBT)18 at −3.2 V cell potential in the MEA cell. Reproduced with permission from ref. 89. Copyright (2024). (E) Structure and photocatalytic CO2 hydrogenation of Ag25(DMBT)18 cluster. (F) CH4 and CH3COOH yields obtained via CO2 hydrogenation over Ag25 clusters (CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 1 H2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v, 1.5 MPa). Reproduced with permission from ref. 104. Copyright (2021). 3 v/v, 1.5 MPa). Reproduced with permission from ref. 104. Copyright (2021). | ||
Jin and co-workers104 utilized the Ag25(DMBT)18 cluster (DMBT: 2,4-dimethylbenznethiolate) for photocatalytic CO2 hydrogenation (Fig. 8E). The product selectivity is 100% towards CH4 at a relatively mild temperature (100 °C). CO2 adsorption on Ag25 is found to be energetically favorable. Operando infrared spectroscopy reveals CO2 methanation proceeds through a –H-assisted multielectron reaction pathway via the transformation of formyl and formaldehyde species into surface CHx. CO2 photoreduction is promoted by Ag25 clusters, yielding methane at a rate of 28.95 μmol h−1 mg−1 at 100 °C under Xe lamp illumination without a cutoff filter (Fig. 8F). With a 420 nm cutoff filter, Ag25 produces CH4 at a rate of 25.34 μmol h−1 mg−1. No activity is observed without light, and heating promotes CO2 photoreduction rate. Interestingly, the selectivity switches from CH4 to acetic acid with a rise in bed temperature. It is suggested that the CO intermediate might bind with Ag–CH3 intermediate at high temperatures (200 °C) to form acetic acid. The Ag NCs stabilized by lacunary polyoxometalates, [Ag24(Si2W18O66)3] and [Ag27(Si2W18O66)3], form HCOOH with 90% selectivity, along with minor products CO and H2.105 This result indicates that tuning product selectivity may be possible by changing the ligand shell of the NCs.
5.2. Hydrogen evolution
The Pinna group deposited Ag44(SR)30 NCs onto a wide-bandgap semiconductor TiO2.106 It produces hydrogen under simulated solar light from water splitting at a rate of 7.4 mmol h−1 gcatalyst−1, which is three orders of magnitude higher than under visible light irradiation. The former value is five times higher than that of TiO2 modified with plasmonic Ag nanoparticles, indicating the importance of NCs in photocatalysis. Energy band alignment and transient absorption spectroscopy reveal the formation of a type II heterojunction charge-transfer route under UV-vis irradiation, with the cluster acting as a small bandgap semiconductor. Consequently, clusters function as cocatalysts rather than mere photosensitizers.Synthesizing core–shell nanomaterials with atomic-level control poses significant challenges despite their potential for enhancing optical and catalytic properties. We reported the synthesis and crystal structure of [Au12Ag32(SePh)30]4−, the first instance of selenolated Au–Ag core–shell NCs.107 These clusters feature a gold icosahedron core encased within a silver dodecahedron, protected by an Ag12(SePh)30 shell. The presence of the gold core strongly influences the overall electronic structure, leading to synergistic effects that enhance stability and near-infrared-II photoluminescence. Both Au12Ag32 and its homometal analog Ag44 exhibit strong interactions with oxygen vacancies on TiO2, facilitating interfacial charge transfer for photocatalysis. Indeed, Au12Ag32/TiO2 demonstrates remarkable solar H2 production (6810 μmol g−1 h−1), surpassing Ag44/TiO2 and TiO2 by approximately 6.2 and 37.8 times, respectively (Fig. 9A and B). Furthermore, Au12Ag32/TiO2 exhibits good stability and recyclability with minimal catalytic activity loss (Fig. 9C and D). Experimental and computational findings suggest that Au12Ag32 serves as an efficient cocatalyst due to its favorable electronic structure, which aligns well with TiO2 bands, promoting enhanced separation of photoinduced charge carriers, facilitated by the relatively negatively charged Au12 core.
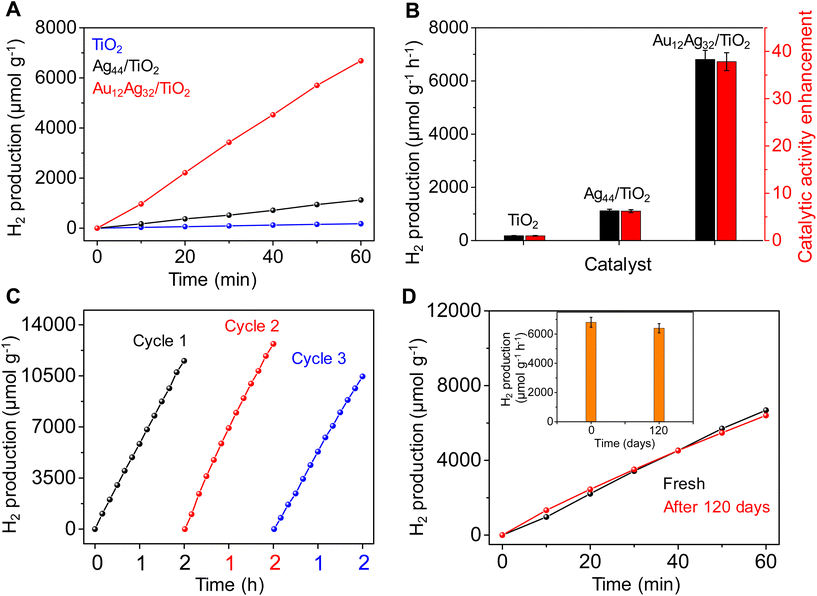 | ||
| Fig. 9 (A and B) Photocatalytic H2 production and improvement with Ag44/TiO2 and Au12Ag32/TiO2 compared to TiO2. (C) H2 evolution activity of recycled Au12Ag32/TiO2. (D) H2 production by fresh and aged Au12Ag32/TiO2. Inset: Bar diagram demonstrating nearly unchanged photocatalytic activity of Au12Ag32/TiO2 clusters after four months of storage. Reproduced with permission from ref. 107. Copyright (2023). | ||
Heteroatom doping may not always have a positive effect on photocatalysis. For instance, when the Ag25 cluster is doped with Au and Pd to form isostructural centrally doped AuAg24 and PdAg24 clusters, respectively, the photocatalytic hydrogen production is lower than that of the parent Ag25 cluster.108 Furthermore, the hydrogen production rate of Ag25/TiO2 is 10 times higher than that of TiO2 alone due to the enhanced charge separation at the interface. Measurements using ultraviolet photoelectron spectroscopy and DFT calculations indicate that the lower activities of doped clusters result from an energy mismatch between the levels of doped NCs and TiO2.
Zhu and co-workers109 systematically designed an electrocatalytic hydrogen evolution catalyst based on an Ag29 cluster by core-alloying with Pt and grafting optimal Mn onto the cluster's surface. In the resulting catalyst, PtAg28-BTT-Mn(10), the Mn site exhibits a ΔGH* value of 0.18 eV, which is suitable for the hydrogen evolution reaction. This suggests that the Mn sites, rather than the Ag3 faces of the Ag29 cluster, serve as the catalytic sites.
5.3. Nitrate reduction
The Zang group110 loaded [Ag9(mba)9](NH4)9 NCs onto Ti3C2 MXene to form Ag9/MXene composite catalyst for highly efficient nitrate reduction to ammonia under ambient conditions with improved stability in neutral medium. The Ag clusters promote the conversion of nitrate to nitrite. The nitrite-enriched MXene promotes the sustained reaction of nitrite conversion to ammonium ion, establishing a tandem catalytic system. Furthermore, compared to solely Ag9 NCs, Ag9/MXene shows enhanced catalytic stability, with no decay in current density after 108 hours of reaction. Tang and colleagues111 synthesized a homoleptic alkynyl-protected two-electron [Ag30Pd4(C6H9)26]2+ cluster and employed it for nitrate to ammonia reduction. The Pd atoms occupy the subcenter of the metal core. This Ag30Pd4 cluster acts as an efficient electrocatalyst for ammonia production with a selectivity of over 90%. In situ infrared spectroscopy results show that nitrate to nitrite formation takes place at the silver site, and Pd sites contribute majorly to the nitrite to ammonia conversion. These experimental observations are consistent with DFT calculations, wherein nitrate preferably binds with silver. Upon binding with a water molecule, nitrite is released. This nitrite is then transferred to the vicinally exposed Pd site to complete the reaction. These results demonstrate tandem catalysis of NCs rather than the usually thought synergistic catalysis.5.4. Other energy related applications
A molecular hybrid of an atomically precise silver NC and polyoxometalates is found to cleave H2 into protons and electrons, which are stored at the polyoxometalate surface and silver, respectively.112 The cluster (Ag27)17+ accommodates electrons by becoming (Ag27)13+. The polyoxometalate stabilizes the unstable Ag cluster without structural transformation, providing a unique interface for H2 dissociation and storing protons on its negatively charged basic surface. This example may open new avenues for fuel cell applications. A stable supercapacitor electrode is fabricated using a two-dimensional high-nucleus Ag NC.113 The high stability of the Ag NC polymer, enabled by its strong coordination with decafluoroazelaic acid (CF2), exhibits a high energy density of 372 F g−1 at 4.5 A g−1, along with excellent cycling stability and capacity. This NC demonstrates both high power density and long cycle life, enduring over 6000 cycles with a retention rate of 95%. Additionally, it can tolerate a wide range of scan rates from 5 mV s−1 to 1 V s−1, suggesting its potential as a sustainable energy source.Ag NCs catalyse the hypergolic activity of inert o-carboranealkynyl ligand when they contact with white fuming nitric acid oxidizer.114 Spontaneous ignition and combustion produce heat, making o-carboranealkynyl-protected Ag clusters as potential components of propellant systems. Similarly, Ag cluster-based organic frameworks also exhibit high-performance hypergolic properties.115 The graphitic carbon nitride (g-C3N4)-loaded Ag33(4-MePhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)22(Dppp)4 NC serves as excellent photocatalyst for degradation of methyl orange.116 Compared to pristine g-C3N4, the cluster-loaded catalyst shows a 16-fold activity enhancement due to the enhanced separation of photogenerated charge carriers. The co-assembled [Ag29(BDT)12(TPP)2]3− clusters and Ru(bpy)32+ photosensitizer (i.e., Ag29Ru coassembly) exhibits a greatly enhanced singlet oxygen production capacity under visible light irradiation as compared to pristine Ag29 cluster.117 Narrowing of the HOMO–LUMO gap of the Ag29Ru coassembly is suggested to be the reason for enhancement in singlet oxygen production.
C)22(Dppp)4 NC serves as excellent photocatalyst for degradation of methyl orange.116 Compared to pristine g-C3N4, the cluster-loaded catalyst shows a 16-fold activity enhancement due to the enhanced separation of photogenerated charge carriers. The co-assembled [Ag29(BDT)12(TPP)2]3− clusters and Ru(bpy)32+ photosensitizer (i.e., Ag29Ru coassembly) exhibits a greatly enhanced singlet oxygen production capacity under visible light irradiation as compared to pristine Ag29 cluster.117 Narrowing of the HOMO–LUMO gap of the Ag29Ru coassembly is suggested to be the reason for enhancement in singlet oxygen production.
5.5. Organic transformation
The Wang group118 synthesized two identically sized Ag NCs with different types of ligands, enabling the study of the effect of ligands on the stability and catalytic activity trade-off in the hydrogenation of nitro compounds. The clusters [Ag21(H2BTCA)3(O2PPh2)6]+ and [Ag21(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H3-3,5-R2)6(O2PPh2)10]+ (Fig. 10A and B, respectively) where H4BTCA represents p-tert-butylthiacalix[4]arene and R represents OMe, possess identical icosahedral kernels. The thiacalix[4]arene-protected Ag21 is found to be an 8-electron superatomic cluster, exhibiting robust stability under high temperatures (90 °C) in solution and oxidative conditions (30% H2O2). On the other hand, the alkynylated Ag21 is a four-electron cluster with an open-shell electronic configuration, demonstrating good ambient stability. However, the alkynylated cluster could catalyse nitrophenol reduction to aminophenol (Fig. 10C) and complete the reaction in 16 minutes (rate constant: 0.228 min−1), while the other cluster could not finish the reaction even after 1.5 hours (rate constant: 0.023 min−1). Notably, the thiacalix[4]arene-protected Ag21 shows significantly higher catalytic activity than the catalyst support. These results highlight the importance of the electronic structure of clusters in catalysis, which can be modulated by an appropriate combination of protecting ligands.
CC6H3-3,5-R2)6(O2PPh2)10]+ (Fig. 10A and B, respectively) where H4BTCA represents p-tert-butylthiacalix[4]arene and R represents OMe, possess identical icosahedral kernels. The thiacalix[4]arene-protected Ag21 is found to be an 8-electron superatomic cluster, exhibiting robust stability under high temperatures (90 °C) in solution and oxidative conditions (30% H2O2). On the other hand, the alkynylated Ag21 is a four-electron cluster with an open-shell electronic configuration, demonstrating good ambient stability. However, the alkynylated cluster could catalyse nitrophenol reduction to aminophenol (Fig. 10C) and complete the reaction in 16 minutes (rate constant: 0.228 min−1), while the other cluster could not finish the reaction even after 1.5 hours (rate constant: 0.023 min−1). Notably, the thiacalix[4]arene-protected Ag21 shows significantly higher catalytic activity than the catalyst support. These results highlight the importance of the electronic structure of clusters in catalysis, which can be modulated by an appropriate combination of protecting ligands.
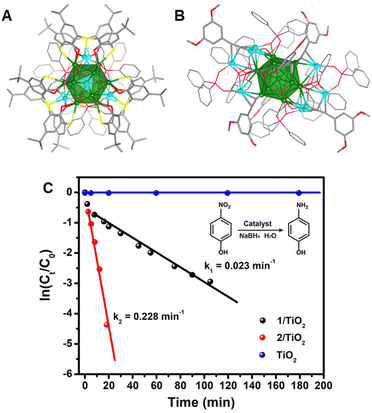 | ||
| Fig. 10 Molecular structures of (A) thiacalixarene- and (B) alkynyl-protected Ag21 NCs. (C) Catalytic activity of Ag21(thiacalixarene)/TiO2 (1/TiO2) and Ag21(alkynyl)/TiO2 (2/TiO2) and TiO2 in nitrophenol reduction. Reproduced with permission from ref. 118. Copyright (2022). | ||
The Chen group119 synthesized three identical Ag33(SR)24(PPh3)4 NCs with three different thiolate ligands: 2-phenylethanethiol, 4-chlorobenzylmercaptan, and 4-methoxybenzylmercaptan. The benzylthiolated Ag33 NCs exhibit superior hydrogenation activity compared to the phenylethanethiolated Ag33 cluster. This ligand-dependent catalysis is attributed to the noncovalent (H–π and π–π) interactions of ligands and substrates through phenyl rings, facilitating facile adsorption on the cluster catalyst. Furthermore, the cluster catalysts can be recycled more than five times without significantly losing activity. The surface-exposed Ag30 clusters are incorporated into molecular metal oxide cavities,120 which demonstrate exceptional hydrogenation activities using H2 gas. The substrate scope is very broad for these cluster catalysts with high yields.
The Ag25(SR)18 NCs serve as templates to fabricate AgPd bimetallic nanocatalysts with controlled Pd content and distribution.121 Initially, the Ag25(SR)18 NCs are activated at low temperature, acting as a template for subsequent Pd deposition. By employing low and high amounts of Pd, the location of Pd is manipulated at the subsurface and surface, respectively. The AgPd nanostructure with Pd at the subsurface (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-Ag
1-Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd/carbon) exhibits controlled alkyne (2-methyl-3-butyn-2-ol) to alkene (2-methyl-3-buten-2-ol) hydrogenation with 96.6% selectivity. On the other hand, the catalyst with a high Pd content (1
Pd/carbon) exhibits controlled alkyne (2-methyl-3-butyn-2-ol) to alkene (2-methyl-3-buten-2-ol) hydrogenation with 96.6% selectivity. On the other hand, the catalyst with a high Pd content (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6-Ag
6-Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd/carbon) shows fully hydrogenated products, suggesting the use of Ag NCs in catalyst design. Upon controlled activation of Ag25 clusters at 250 °C with an unchanged cluster size,122 they function as excellent catalysts for styrene oxidation with high selectivity for styrene oxide. However, above this temperature, the clusters sinter to form nanoparticles, and the catalytic performance decreases dramatically with poor styrene oxide selectivity. The Ag4 NC is synthesized for catalyzing the cyclization of propargylamine with CO2.123 The alkyne-captured cluster catalyst intermediate is confirmed by mass spectrometry. This cluster catalyst exhibits a broad substrate scope with high turnover number values and recyclability for several cycles. Furthermore, gram-scale cyclized products could be achieved using the Ag4 cluster-based catalyst.
Pd/carbon) shows fully hydrogenated products, suggesting the use of Ag NCs in catalyst design. Upon controlled activation of Ag25 clusters at 250 °C with an unchanged cluster size,122 they function as excellent catalysts for styrene oxidation with high selectivity for styrene oxide. However, above this temperature, the clusters sinter to form nanoparticles, and the catalytic performance decreases dramatically with poor styrene oxide selectivity. The Ag4 NC is synthesized for catalyzing the cyclization of propargylamine with CO2.123 The alkyne-captured cluster catalyst intermediate is confirmed by mass spectrometry. This cluster catalyst exhibits a broad substrate scope with high turnover number values and recyclability for several cycles. Furthermore, gram-scale cyclized products could be achieved using the Ag4 cluster-based catalyst.
6. Summary and perspectives
We have conducted a brief overview of the recent advancements in Ag(0)-containing atomically precise Ag nanoclusters, highlighting the innovative strategies employed in their design and synthesis. By leveraging a rare combination of ligands and meticulously crafting atomic-level structures, researchers have achieved significant progress in enhancing stability, functionalization, and tuning optical and photophysical properties of these nanoclusters. Moreover, the transformative potential of these clusters in catalytic applications, particularly in understanding the role of structures and ligands in catalytic activity and selectivity, has been extensively discussed.Despite these strides, several challenges remain to be addressed to propel Ag nanocluster research forward. Chief among these is the need to synthesize a greater variety of nanoclusters with consistent size, atomic arrangement, and ligand environment, enabling a comprehensive understanding of their dependency on optical and photophysical characteristics. This necessitates the development of novel synthetic routes (e.g., solid-state124,125 and biphasic89 reactions) and the exploration of unconventional precursors, solvents, and reducing agents. Additionally, efforts to upscale synthesis for practical applications must be intensified, requiring advancements in design principles and synthetic chemistry to increase yield and scalability.
The intriguing transformation chemistry of Ag nanoclusters, especially under light-induced conditions, presents an exciting avenue for future exploration. By precisely controlling irradiation parameters and capturing intermediate clusters, researchers can unlock new catalytic possibilities. Furthermore, there is a pressing need to synthesize Ag nanoclusters analogous to existing Au counterparts, such as the elusive Ag38(SR)24 analogue, to facilitate comparative studies and expand our understanding of chiroptical and catalytic properties.
While the applications of Ag nanoclusters have been explored to a limited extent, their lower stability compared to gold counterparts presents both challenges and opportunities. The metastability of Ag clusters can be leveraged to create novel catalysts by probing interactions with support materials and elucidating photo-excited-state dynamics. Additionally, the utilization of Ag clusters in CO2 electrolyzers, owing to their high atom utilization efficiency, holds promise for practical applications. Exploring interactions with other clusters, metal ions, ligands, surfaces, and interfaces will undoubtedly unlock new avenues and possibilities in cluster chemistry. The use of heterometal atoms during the synthesis of silver nanoclusters may lead to either doped silver clusters or novel Ag clusters different from those obtained in the absence of heterometal precursors. Although predicting the choice of heterometal precursors is challenging, considering their ability to form complexes with ligands or transient alloy species may be helpful in assisting the controlled nucleation and growth stages. The heteroatom-doped silver clusters are anticipated to exhibit enhanced catalytic performance and stability due to synergistic and cooperative effects.
In conclusion, the future of cluster chemistry is exceptionally bright, with Ag nanoclusters poised to play a pivotal role in advancing catalysis, energy conversion, and materials science. Continued interdisciplinary efforts and innovative approaches will be essential in harnessing the full potential of these atomically precise nanomaterials.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Institute for Basic Science (IBS) with Project Codes IBS-R006-D1 and IBS-R006-Y2.References
- I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208–8271 CrossRef CAS PubMed.
- Y. Du, H. Sheng, D. Astruc and M. Zhu, Chem. Rev., 2020, 120, 526–622 CrossRef CAS PubMed.
- R. Jin, G. Li, S. Sharma, Y. Li and X. Du, Chem. Rev., 2021, 121, 567–648 CrossRef CAS PubMed.
- M. F. Matus and H. Häkkinen, Nat. Rev. Mater., 2023, 8, 372–389 CrossRef CAS.
- Y. Negishi, K. Nobusada and T. Tsukuda, J. Am. Chem. Soc., 2005, 127, 5261–5270 CrossRef CAS PubMed.
- Z. Wang, M.-D. Li, J.-Y. Shi, H.-F. Su, J.-W. Liu, L. Feng, Z.-Y. Gao, Q.-W. Xue, C.-H. Tung, D. Sun and L.-S. Zheng, CCS Chem., 2022, 4, 1788–1795 CrossRef CAS.
- Y.-M. Su, B.-Q. Ji, Z. Wang, S.-S. Zhang, L. Feng, Z.-Y. Gao, Y.-W. Li, C.-H. Tung, D. Sun and L.-S. Zheng, Sci. China: Chem., 2021, 64, 1482–1486 CrossRef CAS.
- S.-S. Zhang, R.-C. Liu, X.-C. Zhang, L. Feng, Q.-W. Xue, Z.-Y. Gao, C.-H. Tung and D. Sun, Sci. China: Chem., 2021, 64, 2118–2124 CrossRef CAS.
- Z. Wang, Q.-P. Qu, H.-F. Su, P. Huang, R. K. Gupta, Q.-Y. Liu, C.-H. Tung, D. Sun and L.-S. Zheng, Sci. China: Chem., 2020, 63, 16–20 CrossRef CAS.
- Z. Wang, H.-F. Su, G.-L. Zhuang, M. Kurmoo, C.-H. Tung, D. Sun and L.-S. Zheng, CCS Chem., 2020, 2, 663–672 CrossRef CAS.
- S. Takano and T. Tsukuda, J. Am. Chem. Soc., 2021, 143, 1683–1698 CrossRef CAS PubMed.
- K. Zheng, X. Yuan and J. Xie, Chem. Commun., 2017, 53, 9697–9700 RSC.
- H. Lin, X. Song, O. J. H. Chai, Q. Yao, H. Yang and J. Xie, Adv. Mater., 2024 DOI:10.1002/adma.202401002.
- X. Liu, J. Chen, J. Yuan, Y. Li, J. Li, S. Zhou, C. Yao, L. Liao, S. Zhuang, Y. Zhao, H. Deng, J. Yang and Z. Wu, Angew. Chem., Int. Ed., 2018, 57, 11273–11277 CrossRef CAS PubMed.
- N. Xia, J. Yang and Z. Wu, Nanoscale, 2015, 7, 10013–10020 RSC.
- S. Biswas, A. K. Das and S. Mandal, Acc. Chem. Res., 2023, 56, 1838–1849 CrossRef CAS PubMed.
- G. Deng, K. Lee, H. Deng, M. S. Bootharaju, N. Zheng and T. Hyeon, J. Phys. Chem. C, 2022, 126, 20577–20583 CrossRef CAS.
- K. Lee, G. Deng, M. S. Bootharaju and T. Hyeon, Acc. Chem. Res., 2023, 56, 1118–1127 CrossRef CAS PubMed.
- W. Jing, H. Shen, R. Qin, Q. Wu, K. Liu and N. Zheng, Chem. Rev., 2023, 123, 5948–6002 CrossRef CAS PubMed.
- T. Kawawaki, Y. Kataoka, M. Hirata, Y. Akinaga, R. Takahata, K. Wakamatsu, Y. Fujiki, M. Kataoka, S. Kikkawa, A. S. Alotabi, S. Hossain, D. J. Osborn, T. Teranishi, G. G. Andersson, G. F. Metha, S. Yamazoe and Y. Negishi, Angew. Chem., Int. Ed., 2021, 60, 21340–21350 CrossRef CAS PubMed.
- T. Kawawaki, T. Okada, D. Hirayama and Y. Negishi, Green Chem., 2024, 26, 122–163 RSC.
- A. K. Das, S. Biswas, A. Pal, S. S. Manna, A. Sardar, P. K. Mondal, B. Sahoo, B. Pathak and S. Mandal, Nanoscale, 2024, 16, 3583–3590 RSC.
- Y. Yun, H. Sheng, K. Bao, L. Xu, Y. Zhang, D. Astruc and M. Zhu, J. Am. Chem. Soc., 2020, 142, 4126–4130 CrossRef CAS PubMed.
- S. Wang, S. Jin, S. Yang, S. Chen, Y. Song, J. Zhang and M. Zhu, Sci. Adv., 2015, 1, e1500441 CrossRef PubMed.
- A. Desireddy, B. E. Conn, J. Guo, B. Yoon, R. N. Barnett, B. M. Monahan, K. Kirschbaum, W. P. Griffith, R. L. Whetten, U. Landman and T. P. Bigioni, Nature, 2013, 501, 399–402 CrossRef CAS PubMed.
- H. Yang, Y. Wang, H. Huang, L. Gell, L. Lehtovaara, S. Malola, H. Häkkinen and N. Zheng, Nat. Commun., 2013, 4, 2422 CrossRef PubMed.
- H. Chang, M. S. Bootharaju, S. Lee, J. H. Kim, B. H. Kim and T. Hyeon, Bull. Korean Chem. Soc., 2021, 42, 1386–1399 CrossRef CAS.
- X.-H. Ma, Y. Si, L.-L. Luo, Z.-Y. Wang, S.-Q. Zang and T. C. W. Mak, ACS Nano, 2022, 16, 5507–5514 CrossRef CAS PubMed.
- X. Ma, Y. Bai, Y. Song, Q. Li, Y. Lv, H. Zhang, H. Yu and M. Zhu, Angew. Chem., Int. Ed., 2020, 59, 17234–17238 CrossRef CAS PubMed.
- G. Deng, T. Ki, S. Yoo, X. Liu, K. Lee, M. S. Bootharaju and T. Hyeon, Nanoscale, 2024 10.1039/D4NR01471E.
- F. Hu, R.-L. He, Z.-J. Guan, C.-Y. Liu and Q.-M. Wang, Angew. Chem., Int. Ed., 2023, 62, e202304134 CrossRef CAS PubMed.
- G. Deng, H. Yun, M. S. Bootharaju, F. Sun, K. Lee, X. Liu, S. Yoo, Q. Tang, Y. J. Hwang and T. Hyeon, J. Am. Chem. Soc., 2023, 145, 27407–27414 CrossRef CAS PubMed.
- G. Deng, J. Kim, M. S. Bootharaju, F. Sun, K. Lee, Q. Tang, Y. J. Hwang and T. Hyeon, J. Am. Chem. Soc., 2023, 145, 3401–3407 CrossRef CAS PubMed.
- G. Deng, T. Ki, X. Liu, Y. Chen, K. Lee, S. Yoo, Q. Tang, M. S. Bootharaju and T. Hyeon, Chem. Commun., 2024, 60, 1289–1292 RSC.
- F. Hu, J.-J. Li, Z.-J. Guan, S.-F. Yuan and Q.-M. Wang, Angew. Chem., Int. Ed., 2020, 59, 5312–5315 CrossRef CAS PubMed.
- X. Wei, C. Xu, H. Li, X. Kang and M. Zhu, Chem. Sci., 2022, 13, 5531–5538 RSC.
- X. Ma, S. He, Q. Li, Q. Li, J. Chai, W. Ma, G. Li, H. Yu and M. Zhu, Inorg. Chem., 2023, 62, 15680–15687 CrossRef CAS PubMed.
- M. Bodiuzzaman, E. Khatun, K. S. Sugi, G. Paramasivam, W. A. Dar, S. Antharjanam and T. Pradeep, J. Phys. Chem. C, 2020, 124, 23426–23432 CrossRef CAS.
- B. J. Alamer, M. S. Bootharaju, S. M. Kozlov, Z. Cao, A. Shkurenko, S. Nematulloev, P. Maity, O. F. Mohammed, M. Eddaoudi, L. Cavallo, J.-M. Basset and O. M. Bakr, Inorg. Chem., 2021, 60, 4306–4312 CrossRef CAS PubMed.
- L. G. AbdulHalim, M. S. Bootharaju, Q. Tang, S. Del Gobbo, R. G. AbdulHalim, M. Eddaoudi, D.-e. Jiang and O. M. Bakr, J. Am. Chem. Soc., 2015, 137, 11970–11975 CrossRef CAS PubMed.
- E. Khatun, A. Ghosh, P. Chakraborty, P. Singh, M. Bodiuzzaman, P. Ganesan, G. Nataranjan, J. Ghosh, S. K. Pal and T. Pradeep, Nanoscale, 2018, 10, 20033–20042 RSC.
- Q.-Q. Ma, X.-J. Zhai, J.-H. Huang, Y. Si, X.-Y. Dong, S.-Q. Zang and T. C. W. Mak, Nanoscale, 2024, 16, 9361–9366 RSC.
- Y. Li, X.-M. Luo, P. Luo, Q.-X. Zang, Z.-Y. Wang and S.-Q. Zang, ACS Nano, 2023, 17, 5834–5841 CrossRef CAS PubMed.
- H. Li, X. Wei, X. Kang and M. Zhu, Nanoscale, 2024, 16, 1254–1259 RSC.
- Z. Wang, H. Zhao, Y.-Z. Li, C. Zhang, R. K. Gupta, C.-H. Tung and D. Sun, Nano Lett., 2024, 24, 458–465 CrossRef CAS PubMed.
- Z. Wang, F. Alkan, C. M. Aikens, M. Kurmoo, Z.-Y. Zhang, K.-P. Song, C.-H. Tung and D. Sun, Angew. Chem., Int. Ed., 2022, 61, e202206742 CrossRef CAS PubMed.
- W.-J. Zhang, Z. Liu, K.-P. Song, C. M. Aikens, S.-S. Zhang, Z. Wang, C.-H. Tung and D. Sun, Angew. Chem., Int. Ed., 2021, 60, 4231–4237 CrossRef CAS PubMed.
- M. S. Bootharaju, H. Chang, G. Deng, S. Malola, W. Baek, H. Häkkinen, N. Zheng and T. Hyeon, J. Am. Chem. Soc., 2019, 141, 8422–8425 CrossRef CAS PubMed.
- M. Qu, H. Li, L.-H. Xie, S.-T. Yan, J.-R. Li, J.-H. Wang, C.-Y. Wei, Y.-W. Wu and X.-M. Zhang, J. Am. Chem. Soc., 2017, 139, 12346–12349 CrossRef CAS PubMed.
- M. S. Bootharaju, W. Baek, G. Deng, K. Singh, O. Voznyy, N. Zheng and T. Hyeon, Chem, 2022, 8, 2978–2989 CAS.
- A. Gonzàlez-Rosell, S. Malola, R. Guha, N. R. Arevalos, M. F. Matus, M. E. Goulet, E. Haapaniemi, B. B. Katz, T. Vosch, J. Kondo, H. Häkkinen and S. M. Copp, J. Am. Chem. Soc., 2023, 145, 10721–10729 CrossRef PubMed.
- L. Maretti, P. S. Billone, Y. Liu and J. C. Scaiano, J. Am. Chem. Soc., 2009, 131, 13972–13980 CrossRef CAS PubMed.
- Y.-X. Wang, J. Zhang, H.-F. Su, X. Cui, C.-Y. Wei, H. Li and X.-M. Zhang, ACS Nano, 2023, 17, 11607–11615 CrossRef CAS PubMed.
- C. P. Joshi, M. S. Bootharaju, M. J. Alhilaly and O. M. Bakr, J. Am. Chem. Soc., 2015, 137, 11578–11581 CrossRef CAS PubMed.
- F. Tian and R. Chen, J. Am. Chem. Soc., 2019, 141, 7107–7114 CrossRef CAS PubMed.
- M. S. Bootharaju, S. Lee, G. Deng, S. Malola, W. Baek, H. Häkkinen, N. Zheng and T. Hyeon, Angew. Chem., Int. Ed., 2021, 60, 9038–9044 CrossRef CAS PubMed.
- K.-G. Liu, X.-M. Gao, T. Liu, M.-L. Hu and D.-e. Jiang, J. Am. Chem. Soc., 2020, 142, 16905–16909 CrossRef CAS PubMed.
- H.-H. Wang, J. Wei, F. Bigdeli, F. Rouhani, H.-F. Su, L.-X. Wang, S. Kahlal, J.-F. Halet, J.-Y. Saillard, A. Morsali and K.-G. Liu, Nanoscale, 2023, 15, 8245–8254 RSC.
- S.-F. Yuan, C.-Q. Xu, W.-D. Liu, J.-X. Zhang, J. Li and Q.-M. Wang, J. Am. Chem. Soc., 2021, 143, 12261–12267 CrossRef CAS PubMed.
- F. Hu, H.-W. Luyang, R.-L. He, Z.-J. Guan, S.-F. Yuan and Q.-M. Wang, J. Am. Chem. Soc., 2022, 144, 19365–19371 CrossRef CAS PubMed.
- G.-X. Duan, J. Han, B.-Z. Yang, Y.-P. Xie and X. Lu, Nanoscale, 2020, 12, 1617–1622 RSC.
- S.-S. Zhang, F. Alkan, H.-F. Su, C. M. Aikens, C.-H. Tung and D. Sun, J. Am. Chem. Soc., 2019, 141, 4460–4467 CrossRef CAS PubMed.
- J. Wei, F. Bigdeli, L.-X. Wang, L.-L. Hou, A. Panjehpour, Y. Ma, K.-Z. Wang, A. Morsali and K.-G. Liu, Inorg. Chem., 2023, 62, 10185–10192 CrossRef CAS PubMed.
- M. Diecke, C. Schrenk and A. Schnepf, Angew. Chem., Int. Ed., 2020, 59, 14418–14422 CrossRef CAS PubMed.
- C. Xu, Q. Yuan, X. Wei, H. Li, H. Shen, X. Kang and M. Zhu, Chem. Sci., 2022, 13, 1382–1389 RSC.
- M.-X. Ma, X.-L. Ma, G.-M. Liang, X.-T. Shen, Q.-L. Ni, L.-C. Gui, X.-J. Wang, S.-Y. Huang and S.-M. Li, J. Am. Chem. Soc., 2021, 143, 13731–13737 CrossRef CAS PubMed.
- A. Jana, P. M. Unnikrishnan, A. K. Poonia, J. Roy, M. Jash, G. Paramasivam, J. Machacek, K. N. V. D. Adarsh, T. Base and T. Pradeep, Inorg. Chem., 2022, 61, 8593–8603 CrossRef CAS PubMed.
- L. Qin, F. Zhang, X. Ma, Y. Tang, G. Ma and Z. Tang, Dalton Trans., 2021, 50, 562–567 RSC.
- J. Yang, S. Xie, H. Zhang, W. Xu, A. Dong and Y. Tang, Chem. Commun., 2022, 58, 6849–6852 RSC.
- J. Yang and R. Jin, J. Phys. Chem. C, 2021, 125, 2619–2625 CrossRef CAS.
- X. Kang and M. Zhu, Chem. Soc. Rev., 2019, 48, 2422–2457 RSC.
- S. Wang, X. Meng, A. Das, T. Li, Y. Song, T. Cao, X. Zhu, M. Zhu and R. Jin, Angew. Chem., Int. Ed., 2014, 53, 2376–2380 CrossRef CAS PubMed.
- X. Wang, B. Yin, L. Jiang, C. Yang, Y. Liu, G. Zou, S. Chen and M. Zhu, Science, 2023, 381, 784–790 CrossRef CAS PubMed.
- S. Chen, W. Du, C. Qin, D. Liu, L. Tang, Y. Liu, S. Wang and M. Zhu, Angew. Chem., Int. Ed., 2020, 59, 7542–7547 CrossRef CAS PubMed.
- M. S. Bootharaju, S. M. Kozlov, Z. Cao, A. Shkurenko, A. M. El-Zohry, O. F. Mohammed, M. Eddaoudi, O. M. Bakr, L. Cavallo and J.-M. Basset, Chem. Mater., 2018, 30, 2719–2725 CrossRef CAS.
- H. Shen, J. Xu, Z. Fu, X. Wei, X. Kang, W. Shi and M. Zhu, Angew. Chem., Int. Ed., 2024, 63, e202317995 CrossRef CAS PubMed.
- W. Ishii, Y. Okayasu, Y. Kobayashi, R. Tanaka, S. Katao, Y. Nishikawa, T. Kawai and T. Nakashima, J. Am. Chem. Soc., 2023, 145, 11236–11244 CrossRef CAS PubMed.
- H. Yang, J. Lei, B. Wu, Y. Wang, M. Zhou, A. Xia, L. Zheng and N. Zheng, Chem. Commun., 2013, 49, 300–302 RSC.
- A. K. Das, R. Mekkat, S. Maity, A. S. Nair, S. Bhandary, R. Bhowal, A. Patra, B. Pathak, D. Chopra and S. Mandal, Inorg. Chem., 2021, 60, 19270–19277 CrossRef CAS PubMed.
- S. Kolay, S. Maity, S. Chakraborty, S. Ghosh and A. Patra, J. Phys. Chem. C, 2023, 127, 3769–3777 CrossRef CAS.
- S. Kolay, S. Chakraborty, S. Pramanik and A. Patra, J. Phys. Chem. C, 2024, 128, 7643–7651 CrossRef CAS.
- Z. Liu, M. Zhou, L. Luo, Y. Wang, E. Kahng and R. Jin, J. Am. Chem. Soc., 2023, 145, 19969–19981 CrossRef CAS PubMed.
- M. S. Bootharaju, C. P. Joshi, M. R. Parida, O. F. Mohammed and O. M. Bakr, Angew. Chem., Int. Ed., 2016, 55, 922–926 CrossRef CAS PubMed.
- K. S. Sugi, A. P. Sandra, Nonappa, D. Ghosh, J. S. Mohanty, M. Paulthangam Kannan, B. S. Sooraj, P. Srikrishnarka, J. Roy, W. A. Dar and T. Pradeep, Nanoscale, 2023, 15, 11927–11934 RSC.
- M.-M. Zhang, X.-Y. Dong, Z.-Y. Wang, X.-M. Luo, J.-H. Huang, S.-Q. Zang and T. C. W. Mak, J. Am. Chem. Soc., 2021, 143, 6048–6053 CrossRef CAS PubMed.
- A. Jana, M. Jash, A. K. Poonia, G. Paramasivam, M. R. Islam, P. Chakraborty, S. Antharjanam, J. Machacek, S. Ghosh, K. N. V. D. Adarsh, T. Base and T. Pradeep, ACS Nano, 2021, 15, 15781–15793 CrossRef CAS PubMed.
- A. Jana, W. A. Dar, S. K. Jana, A. K. Poonia, V. Yadav, J. Roy, S. Chandra, K. N. V. D. Adarsh, R. H. A. Ras and T. Pradeep, Chem. Mater., 2023, 35, 7020–7031 CrossRef CAS.
- T. Chen, S. Yang, Y. Song, J. Chai, Q. Li, X. Ma, G. Li, H. Yu and M. Zhu, Chem. Commun., 2020, 56, 7605–7608 RSC.
- S. Yoo, S. Yoo, G. Deng, F. Sun, K. Lee, H. Jang, C. W. Lee, X. Liu, J. Jang, Q. Tang, Y. J. Hwang, T. Hyeon and M. S. Bootharaju, Adv. Mater., 2024, 36, 2313032 CrossRef CAS PubMed.
- A. Jana, P. Chakraborty, W. A. Dar, S. Chandra, E. Khatun, M. P. Kannan, R. H. A. Ras and T. Pradeep, Chem. Commun., 2020, 56, 12550–12553 RSC.
- Y.-M. Tseng, J.-H. Liao, T.-H. Chiu, H. Liang, S. Kahlal, J.-Y. Saillard and C. W. Liu, Inorg. Chem., 2023, 62, 3866–3874 CrossRef CAS PubMed.
- A. Baksi, M. S. Bootharaju, P. K. Chhotaray, P. Chakraborty, B. Mondal, S. Bhat, R. Gardas and T. Pradeep, J. Phys. Chem. C, 2017, 121, 26483–26492 CrossRef CAS.
- M. S. Bootharaju, S. M. Kozlov, Z. Cao, M. Harb, N. Maity, A. Shkurenko, M. R. Parida, M. N. Hedhili, M. Eddaoudi, O. F. Mohammed, O. M. Bakr, L. Cavallo and J.-M. Basset, J. Am. Chem. Soc., 2017, 139, 1053–1056 CrossRef CAS PubMed.
- M. S. Bootharaju, S. Lee, G. Deng, H. Chang, W. Baek and T. Hyeon, J. Chem. Phys., 2021, 155, 014307 CrossRef CAS PubMed.
- W.-J. Yen, J.-H. Liao, T.-H. Chiu, Y.-S. Wen and C. W. Liu, Inorg. Chem., 2024, 63, 5320–5324 CrossRef CAS PubMed.
- Y.-J. Zhong, J.-H. Liao, T.-H. Chiu, S. Kahlal, C.-J. Lin, J.-Y. Saillard and C. W. Liu, Angew. Chem., Int. Ed., 2021, 60, 12712–12716 CrossRef CAS PubMed.
- X. Kang, X. Wei, P. Xiang, X. Tian, Z. Zuo, F. Song, S. Wang and M. Zhu, Chem. Sci., 2020, 11, 4808–4816 RSC.
- G. Deng, S. Malola, P. Yuan, X. Liu, B. K. Teo, H. Häkkinen and N. Zheng, Angew. Chem., Int. Ed., 2021, 60, 12897–12903 CrossRef CAS PubMed.
- G. Deng, B. K. Teo and N. Zheng, J. Am. Chem. Soc., 2021, 143, 10214–10220 CrossRef CAS PubMed.
- S. Wang, Y. Tan, T. Li, Q. Zhou, P. Li, S. Yang, H. Yu and M. Zhu, Inorg. Chem., 2022, 61, 18450–18457 CrossRef CAS PubMed.
- L. Qin, F. Sun, X. Ma, G. Ma, Y. Tang, L. Wang, Q. Tang, R. Jin and Z. Tang, Angew. Chem., Int. Ed., 2021, 60, 26136–26141 CrossRef CAS PubMed.
- L. Chen, F. Sun, Q. Shen, L. Qin, Y. Liu, L. Qiao, Q. Tang, L. Wang and Z. Tang, Nano Res., 2022, 15, 8908–8913 CrossRef CAS.
- L.-J. Li, Y.-T. Luo, Y.-Q. Tian, P. Wang, X.-Y. Yi, J. Yan, Y. Pei and C. Liu, Inorg. Chem., 2023, 62, 14377–14384 CrossRef CAS PubMed.
- Y. Xiong, H. Chen, Y. Hu, S. Yang, X. Xue, L. He, X. Liu, J. Ma and Z. Jin, Nano Lett., 2021, 21, 8693–8700 CrossRef CAS PubMed.
- Y. Feng, F. Fu, L. Zeng, M. Zhao, X. Xin, J. Liang, M. Zhou, X. Fang, H. Lv and G.-Y. Yang, Angew. Chem., Int. Ed., 2024, 63, e202317341 CrossRef CAS PubMed.
- Y. Wang, X.-H. Liu, Q. Wang, M. Quick, S. A. Kovalenko, Q.-Y. Chen, N. Koch and N. Pinna, Angew. Chem., Int. Ed., 2020, 59, 7748–7754 CrossRef CAS PubMed.
- M. S. Bootharaju, C. W. Lee, G. Deng, H. Kim, K. Lee, S. Lee, H. Chang, S. Lee, Y.-E. Sung, J. S. Yoo, N. Zheng and T. Hyeon, Adv. Mater., 2023, 35, 2207765 CrossRef CAS PubMed.
- Y. Liu, D. Long, A. Springer, R. Wang, N. Koch, M. Schwalbe, N. Pinna and Y. Wang, Sol. RRL, 2023, 7, 2201057 CrossRef CAS.
- H. Shen, Q. Zhu, J. Xu, K. Ni, X. Wei, Y. Du, S. Gao, X. Kang and M. Zhu, Nanoscale, 2023, 15, 14941–14948 RSC.
- L. Liu, S.-J. Zheng, H. Chen, J. Cai and S.-Q. Zang, Angew. Chem., Int. Ed., 2024, 63, e202316910 CrossRef CAS PubMed.
- L. Qin, F. Sun, Z. Gong, G. Ma, Y. Chen, Q. Tang, L. Qiao, R. Wang, Z.-Q. Liu and Z. Tang, ACS Nano, 2023, 17, 12747–12758 CrossRef CAS PubMed.
- K. Yonesato, S. Yamazoe, D. Yokogawa, K. Yamaguchi and K. Suzuki, Angew. Chem., Int. Ed., 2021, 60, 16994–16998 CrossRef CAS PubMed.
- J. Zhuge, F. Rouhani, F. Bigdeli, X.-M. Gao, H. Kaviani, H.-J. Li, W. Wang, M.-L. Hu, K.-G. Liu and A. Morsali, Dalton Trans., 2021, 50, 2606–2615 RSC.
- Q.-Y. Wang, J. Wang, S. Wang, Z.-Y. Wang, M. Cao, C.-L. He, J.-Q. Yang, S.-Q. Zang and T. C. W. Mak, J. Am. Chem. Soc., 2020, 142, 12010–12014 CrossRef CAS PubMed.
- C. Wang, Y.-J. Wang, C.-L. He, Q.-Y. Wang and S.-Q. Zang, JACS Au, 2021, 1, 2202–2207 CrossRef CAS PubMed.
- H. Li, W. Duan, X. Cui, S. Liu, Y. Bai, Y.-X. Wang, S. Feng, Y. Wang, M. Qu and X.-M. Zhang, ACS Appl. Nano Mater., 2022, 5, 14251–14255 CrossRef CAS.
- J.-Y. Wang, Y.-K. Li, X. Jing, P. Luo, X.-Y. Dong and S.-Q. Zang, ACS Mater. Lett., 2022, 4, 960–966 CrossRef CAS.
- Z.-J. Guan, R.-L. He, S.-F. Yuan, J.-J. Li, F. Hu, C.-Y. Liu and Q.-M. Wang, Angew. Chem., Int. Ed., 2022, 61, e202116965 CrossRef CAS PubMed.
- J. Yuan, X. Huang, W. Zhang, M. Zhou, G. Li, F. Tian and R. Chen, Inorg. Chem., 2023, 62, 17668–17677 CrossRef CAS PubMed.
- K. Yonesato, D. Yanai, S. Yamazoe, D. Yokogawa, T. Kikuchi, K. Yamaguchi and K. Suzuki, Nat. Chem., 2023, 15, 940–947 CrossRef CAS PubMed.
- K. O. Sulaiman, A. Bueckert, A. Abdellah, S. K. Veeranmaril, D. C. Higgins and R. W. J. Scott, J. Phys. Chem. C, 2022, 126, 16117–16126 CrossRef CAS.
- K. O. Sulaiman, V. Sudheeshkumar and R. W. J. Scott, RSC Adv., 2019, 9, 28019–28027 RSC.
- L. Li, Y. Lv, H. Sheng, Y. Du, H. Li, Y. Yun, Z. Zhang, H. Yu and M. Zhu, Nat. Commun., 2023, 14, 6989 CrossRef CAS PubMed.
- T. U. B. Rao, B. Nataraju and T. Pradeep, J. Am. Chem. Soc., 2010, 132, 16304–16307 CrossRef CAS PubMed.
- T. Udayabhaskararao, M. S. Bootharaju and T. Pradeep, Nanoscale, 2013, 5, 9404–9411 RSC.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |