Applications of nanotheranostics in the second near-infrared window in bioimaging and cancer treatment
Huimin
Li
a,
Pengju
Li
b,
Jiarui
Zhang
a,
Ziyi
Lin
a,
Lintao
Bai
a and
Heyun
Shen
 *a
*a
aBeijing Key Laboratory of Bioprocess, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: shenhy@mail.buct.edu.cn
bDepartment of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Fudan University, 220 Handan Road, Shanghai 200433, P. R. China
First published on 30th September 2024
Abstract
Achieving accurate and efficient tumor imaging is crucial in the field of tumor treatment, as it facilitates early detection and precise localization of tumor tissues, thereby informing therapeutic strategies and surgical interventions. The optical imaging technology within the second near-infrared (NIR-II) window has garnered significant interest for its remarkable benefits, such as enhanced tissue penetration depth, superior signal-to-background ratio (SBR), minimal tissue autofluorescence, reduced photon attenuation, and lower tissue scattering. This review explained the design and optimization strategies of nano-agents responsive to the NIR-II window, such as single-walled carbon nanotubes, quantum dots, lanthanum-based nanomaterials, and noble metal nanomaterials. These nano-agents enable non-invasive, deep-tissue imaging with high spatial resolution in the NIR-II window, and their superior optical properties significantly improve the accuracy, efficiency, and versatility of imaging-guided tumor treatments. And we discussed the characteristics and advantages of fluorescence imaging (FL)/photoacoustic imaging (PA) in NIR-II window, providing a comprehensive overview of the latest research progress of different nano-agents in FL/PA imaging-guided tumor therapy. Furthermore, we exhaustively reviewed the latest applications of multifunctional nano-phototherapy technologies carried out by NIR-II light including photothermal therapy (PTT), photodynamic therapy (PDT), and combined modalities like photothermal-chemodynamic therapy (PTT-CDT), photothermal-chemotherapy (PTT-CT), and photothermal- immunotherapy (PTT-IO). These imaging-guided integrated tumor therapy approaches within the NIR-II window have gradually matured over the past decade and are expected to become a safe and effective non-invasive tumor treatment. Finally, we outlined the prospects and challenges of development and innovation of the NIR-II integrated diagnosis and therapy nanoplatform. This review aims to provide insightful perspectives for future advancements in NIR-II optical tumor diagnosis and integrated treatment platforms.
1. Introduction
Advancing “tumor visualization” and “tumor treatment” technology is essential for breakthroughs in tumor theranostics. A variety of effective clinical diagnostic imaging techniques have been developed, such as X-ray computed tomography (X-CT), magnetic resonance imaging (MRI), positron emission tomography (PET), ultrasound, fluorescence (FL), and photoacoustic (PA) imaging.1,2 These technologies are instrumental in precisely determining the location and size of tumors and offer real-time imaging capabilities. Remarkably, FL and PA imaging technologies stand out due to their ability to identify tumor margins with high sensitivity and spatiotemporal resolution within seconds, all while minimizing the risks associated with ionizing radiation throughout the diagnostic, treatment, and monitoring phases.3–5 In addition, the biological imaging window has been extended from the visible window (400–700 nm) to the first near-infrared window (NIR-I, 700–900 nm) and the second near-infrared window (NIR-II, 1000–1700 nm).6 In the field of biomedical imaging, the NIR II window imaging system surpasses the visible light window and NIR I window due to its distinct advantages.7 The longer wavelengths of NIR-II light penetrate deeper into tissues, significantly enhancing the detection of tumors and lesions.8 This imaging range also encounters lower autofluorescence interference from tissues, which improves the signal-to-background ratio and results in clearer images. Additionally, NIR-II light undergoes less scattering and attenuation, which optimizes light delivery and enhances image quality. Furthermore, the longer wavelengths of NIR-II induce minimal thermal damage to tissues owing to lower absorption rates.9,10 The improved resolution and sensitivity of NIR-II imaging allow for more precise delineation of tumor margins and increased detection sensitivity, making it a superior choice for advanced medical diagnostics.Moreover, NIR-II phototherapy technology is often combined with NIR-II imaging technology to achieve effective tumor treatment or resection under the guidance of accurate tumor imaging, so as to monitor the whole tumor treatment process.11 NIR-II phototherapy has the characteristics of deep tissue penetration, non-invasion, high selectivity, and accurate targeting, leading to potential in minimizing the damage to normal tissues, reducing side effects and promoting the rehabilitation of patients, making it a promising medical treatment method.12 The applications of NIR-II phototherapy include: photothermal therapy (PTT),13 photodynamic therapy (PDT),14 and combination therapies such as photothermal-chemodynamic therapy (PTT-CDT),15 photothermal-chemotherapy (PTT-CT),16,17 and photothermal-immunotherapy (PTT-IO).18,19 Each of these therapies capitalizes on the unique properties of NIR-II phototherapy to achieve accurate targeted and effective treatment outcomes, indicating the adaptability and potential of NIR-II phototherapy in addressing complex and challenging tumor cases.
By leveraging the distinct advantages of NIR-II optical imaging and NIR-II phototherapy, multi-modal diagnosis and treatment integrated nano-platforms have been constructed. A variety of NIR-II photoresponsive nano-agents with good photostability, bleaching resistance, long fluorescence lifetime, targeting, and high drug loading efficiency can further enrich and enhance the optical diagnosis and treatment capabilities of tumors in the NIR-II window.20,21 And this integrative treatment effect enables precise targeted treatments, minimizes damage to surrounding healthy tissues, provides real-time monitoring, and adjustment of treatment parameters.
Currently, there is a lack of comprehensive reviews on nano-agents that combine NIR-II FL/PA imaging and phototherapy for tumor diagnosis and treatment. Therefore, in this review, we summarized in detail the latest progress of NIR-II tumor diagnosis and treatment integrated nano-platform (Scheme 1). Firstly, we mainly introduced the design and optimization of nano-agents with optical properties in NIR-II window, and summarized the characteristics of NIR-II FL/PA imaging technology and their application in imaging-guided tumor therapy. Secondly, we analyzed and discussed the advanced NIR-II nano-agents using different photoresponse properties to achieve effective tumor treatment. Finally, the future development prospects and obstacles of NIR-II nano-agents in realm of optical diagnosis and treatment of tumors were discussed. This review is expected to provide illuminating ideas and effective suggestions for subsequent researchers in the NIR-II diagnosis and treatment nano-platform.
 | ||
| Scheme 1 The recent progress in NIR-II biomedical imaging detection and application in cancer treatment. | ||
2. Design and optimization of NIR-II nano-agents
With the continuous development of the research field of NIR-II imaging-guided therapy, various emerging optical nano-agents have been studied, such as single-walled carbon nanotubes, quantum dots, lanthanum-based nanomaterials, noble metal nanomaterials. Under the excitation of NIR-II light with excellent penetration depth and imaging contrast, different materials have exhibited their unique charm and advantages. This greatly broadens the range of materials selection and design for researchers in fundamental studies and clinical transformation application. In this section, we will focus on the properties of various nano-agents as well as their design and optimization to realize bioimaging and cancer treatment in the NIR-II window.2.1 Single-walled carbon nanotubes
Single-walled carbon nanotubes (SWNTs) are cylindrical nanostructures with diameters ranging from 1 to 2 nm and lengths spanning from 50 nm to 1 cm. SWNTs exhibit a large surface area with all atoms on the surface, capable of chemically or physically connecting many molecules.22,23 Due to their distinctive physical structures, shapes, surface chemical and optical properties, SWNTs have garnered significant attentions. Actually, the fluorescence emission of SWNTs is the band gap photoluminescence. The band gap between each semiconducting SWNTs is ∼1 eV, which causes electrons to van Hove transitions across the band gap between different energy levels by absorbing certain energy, thus emitting fluorescence.24 By modifying the diameter of carbon nanotubes, incorporating dopants such as nitrogen or boron, or attaching various chemical groups or molecules to influence their electronic structures and energy band gap, it is possible to effectively tune the fluorescence emission wavelength to the NIR-II window, which can reduce excitation scattering and suppress autofluorescence, thus leading to an enhancement of the imaging sensitivity and tissue penetration depth (Fig. 1).25 In view of the excellent properties mentioned above, SWNTs have been extensively studied in bioimaging.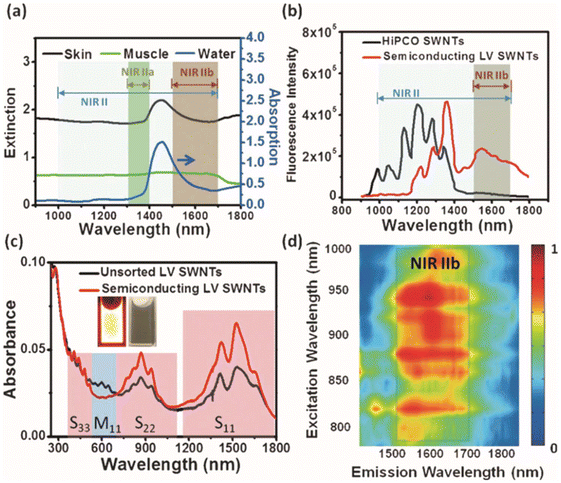 | ||
| Fig. 1 (a) Absorption spectrum of water alongside extinction spectra for mouse skin and muscle. (b) NIR fluorescence spectra of HiPCO and semiconducting LV SWNTs. (c) Absorption spectra of pristine and separated semiconducting LV SWNTs. (d) The photoluminescence versus excitation map of semiconducting LV SWNTs.25 Reproduced with permission from ref. 25. Copyright 2015 John Wiley and Sons. | ||
Enhancing the water solubility and biocompatibility of SWNTs is crucial for improving their suitability in tissue imaging and guidance during surgical treatments in vivo.26,27 Modification of SWNTs with amphiphilic polymers, specifically coating them with phospholipid-polyethylene glycol (PL-PEG) after initial treatment with sodium cholate, has proven effective for enhancing solubility and biocompatibility. Welsher et al. used this approach and pointed out that PL-PEG coated SWNTs had low cytotoxicity, which made them suitable for guiding brain tissue imaging compared to other encapsulation methods.28 In addition, protein coating also can diminish the cytotoxicity of SWNTs, such as bovine serum albumin (BSA),29,30 fibrinogen31 and so on.
It was widely recognized that SWNTs own significant excitonic binding and emit light across a wide wavelength range in the NIR-II window. However, the mobility of excitons decreases the photoluminescence quantum yield (PLQY) due to nonradiative processes.32 Interestingly, researchers found that by doping sp3 defects on the surface of SWNTs, these quantum defects produce local potential wells, which could effectively capture moving excitons and enable them to recombine radiatively, resulting in a significant enhancement of the PLQY.33,34 These defects could be achieved by arylation,34–37 alkylation38,39 (Fig. 2). Berger et al. reported a phase-transfer technique using diazonium salts dissolved in organic non-halogenated solvents to selectively react with polymer-wrapped SWNTs, resulting in the formation of aryl defects. After optimization, the PLQY of the SWNTs reached as high as 4% in the NIR-II window.36 All in all, the PLQY of SWNTs was significantly improved by functional group modification.
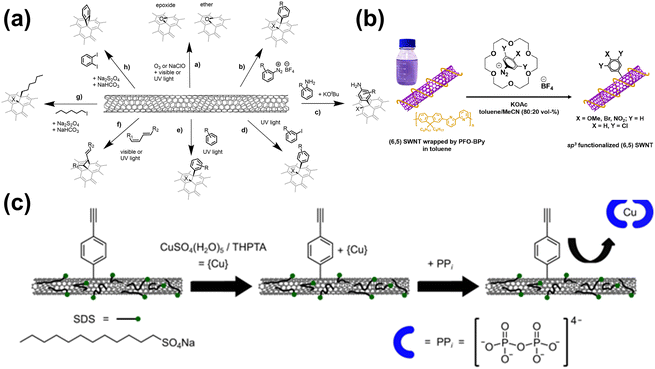 | ||
| Fig. 2 Different sp3 modifications on the surface of SWNTs. (a) A range of chemical reactions were chosen to induce luminescent defects in SWNTs.34 Reproduced with permission from ref. 34. Copyright 2021 John Wiley and Sons. (b) The functionalization process for PFO-BPy-wrapped (6, 5) SWNTs using aryldiazonium salts in a toluene/acetonitrile solution with 18-crown-6 serving as a phase-transfer catalyst.36 Reproduced with permission from ref. 36. Copyright 2019 American Chemical Society. (c) Design strategy for the detection of PPi with sp3-functionalized (6, 5) SWNTs.37 Reproduced with permission from ref. 37. Copyright 2024 Nature. | ||
Besides, in order to apply ultra-low doses of SWNTs to in vivo imaging, the chirality classification of SWNTs has become a breakthrough. Since SWNTs have various chirality,40 which correspond to different excitation and emission wavelengths.41 In mixed chiral SWNTs, non-ideal chiral structures increase energy transfer and non-radiative recombination, thereby reducing the PLQY. Therefore, in pure chiral SWNTs, identical optical and electronic properties minimize these effects, allowing for stronger emission and higher PLQY under limited light excitation.42 Chirality purification methods have made progress to date, including aqueous two-phase extraction (ATPE),43 gel filtration,44–46 DNA wrapping chromatography,47 and density gradient centrifugation (DGU).48,49 Langenbacher et al. obtained single-chirality SWNTs by ATPE, then SWNTs were wrapped with polycarbodiimide polymers.50 The results demonstrated that functionalized single-chirality SWNTs could target to specific subcellular compartments, resulting in multiplexed cellular imaging in the NIR-II window.
In a word, through covalent/non-covalent functionalization and chiral purification, the biocompatibility, long circulation in vivo, PLQY, and tumor tissue accumulation of SWNTs have been further optimized. And the application prospect of SWNTs in NIR-II imaging will be broader in future, owing to their extensive surface area and strong fluorescence intensity.
2.2 Quantum dots
Quantum dots (QDs) have gained widespread interest in the fields of bio-optical detection and imaging tracing. The luminescence of QDs is the result of the recombination of an electron and a hole.51,52 When sufficient energy, at least equal to the band gap (the energy difference between the highest occupied valence band and the lowest unoccupied conduction band), is absorbed by an electron in the valence band, then electron transitions to the conduction band. This movement of the electron leaves behind a hole in the valence band, effectively creating an electron–hole pair by Coulomb force, namely an exciton, which plays a crucial role in the properties of the material.53 When an electron returns to the valence band, it can annihilate an exciton and emit energy as photons (Fig. 3).54 In addition, QDs are capable of emitting light in various colors determined by their composition and size. By modifying their structural characteristics, the fluorescence emission can be regulated to the NIR-II window.55,56 NIR-II QDs exhibit outstanding properties, including excitation across a broad NIR-II wavelength range, emission of narrow and symmetrical spectra, high photostability, stronger photobleaching resistance, and easy decoration of surface properties for targeted biological recognition.57–60 Crucially, QDs in the NIR-II window have advantages in real-time imaging with higher resolution and non-invasive imaging of deep tissues compared to those in the visible and the NIR-I window.61 Wang et al. reported a core–shell lead sulfide/cadmium sulfide QDs, which can realize non-invasive cell resolution imaging of mouse head and lymph nodes under the excitation of 1880 nm.62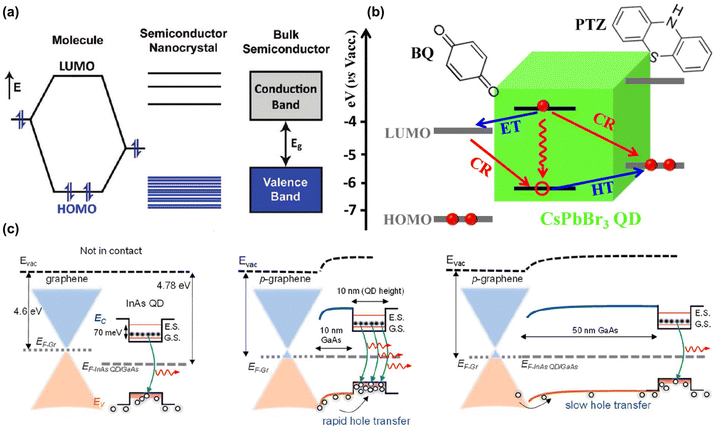 | ||
| Fig. 3 (a) Transfer process of semiconductor electronic energy state.51 Reproduced with permission from ref. 51. Copyright 2009 American Chemical Society. (b) Possible pathways of charge separation and recombination of CsPbBr3 QDs–benzoquinone and QDs–phenothiazine complexes.52 Reproduced with permission from ref. 52. Copyright 2015 American Chemical Society. (c) The alignment of energy bands in a graphene/quantum dot heterostructure.54 Reproduced with permission from ref. 54. Copyright 2023 American Chemical Society. | ||
However, QDs as crystals may have lattice defects, which will destroy the periodicity of crystal atoms and produce defect energy levels.63 Therefore, this will lead to an increase in nonradiative relaxation, a decrease in band-edge recombination of electrons and holes, and a decrease in PLQY of QDs. Up to now, a lot of efforts have been made to improve the PLQY of QDs. Zhou et al. synthesized Zn-doped Ag2Te QDs modified by polyethylene glycol, which PLQY can reach 4.03% (200-fold higher than commercial IR-26 dye) in the NIR-II window.64 The principle was that Zn dopant inhibited the crystal defects of QDs, thus reducing non-radiative transitions and then improving the PLQY.65 Shu et al. synthesized Pb-doped Ag2S QDs by “one-pot” synthesis method.66 Pb2+ made up for the lattice defects that arose from the high mobility of Ag+ within Ag2S nanocrystals. When the ratio of Pb2+/Ag+ was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the PLQY reached the maximum value of 4.14%. The fluorescence intensity of Pb
1, the PLQY reached the maximum value of 4.14%. The fluorescence intensity of Pb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag2S was 3.76 times as much as undoped Ag2S QDs. In addition, some QDs contained heavy metal elements such as cadmium, lead, or tellurium. It was of great significance to develop high biological safety QDs suitable for imaging deep tissues in vivo.67,68 Zebibula et al. applied a dual-layer coating of silica and amphiphilic polymer (Pluronic F-127) to PbS@CdS QDs, which significantly improved them biological safety and could achieve long circulation in blood vessels for up to 16 h.69 There were no obvious signs of infection, allergy and toxic reaction in blood routine and liver and kidney function tests. Awasthi et al. synthesized Ag2S QDs encapsulated by PEGylated polythiourea dendrimer (PATU) for NIR-II imaging by one-pot method.70 The QDs had excellent water dispersibility and biocompatibility. Li et al. reported on BSA-coated Ag2Te QDs.71 The BSA coating notably decreased their biotoxicity, enabling their effective to be used in CT/NIR-II imaging of the gastrointestinal tract in mice.
Ag2S was 3.76 times as much as undoped Ag2S QDs. In addition, some QDs contained heavy metal elements such as cadmium, lead, or tellurium. It was of great significance to develop high biological safety QDs suitable for imaging deep tissues in vivo.67,68 Zebibula et al. applied a dual-layer coating of silica and amphiphilic polymer (Pluronic F-127) to PbS@CdS QDs, which significantly improved them biological safety and could achieve long circulation in blood vessels for up to 16 h.69 There were no obvious signs of infection, allergy and toxic reaction in blood routine and liver and kidney function tests. Awasthi et al. synthesized Ag2S QDs encapsulated by PEGylated polythiourea dendrimer (PATU) for NIR-II imaging by one-pot method.70 The QDs had excellent water dispersibility and biocompatibility. Li et al. reported on BSA-coated Ag2Te QDs.71 The BSA coating notably decreased their biotoxicity, enabling their effective to be used in CT/NIR-II imaging of the gastrointestinal tract in mice.
Furthermore, it is worth mentioning that the modified QDs can achieve targeted tumor cell imaging.72,73 Zhang et al. synthesized Ag2Te QDs modified by poly(lactic-co-glycolic acid), and QDs were coated with membrane-derived vesicles (CVs).74 This NIR-II fluorescence nano-biological probe not only had good biological safety, the ability to effectively escape the phagocytosis of macrophages, and long blood circulation time, but also had obvious homotypic tumor targeting effect (Fig. 4a). Lu et al. enhanced Ag2S QDs by attaching polyethylene glycol, significantly boosting their blood circulation and tumor targeting capabilities (Fig. 4b).75 Tian et al. modified PbS@CdS QDs by attaching an anti-CD3 antibody, increasing their selectivity for cancer-related sentinel lymph nodes (LNs) (Fig. 4c).76 Combined with IR-FD (a donor–acceptor–donor dye prepared by fluorene and dioctyl chains substituted 3,4-propylenedioxy thiophene), these QDs served as a dual NIR-II probe for imaging LNs and tumors, assisting in biopsy surgeries, enhancing the understanding of tumor metastasis.
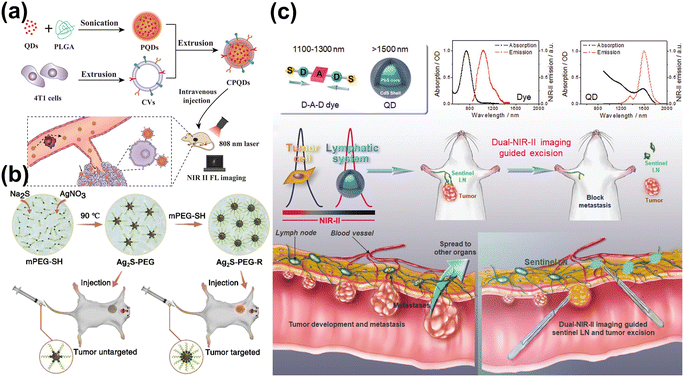 | ||
| Fig. 4 (a) The preparation and application of Ag2Te QDs.74 Reproduced with permission from ref. 74. Copyright 2019 John Wiley and Sons. (b) The method for preparing PEGylated Ag2S QDs for in vivo tumor imaging.75 Reproduced with permission from ref. 75. Copyright 2020 Elsevier. (c) Sentinel lymph node surgery guided by dual-NIR-II imaging.76 Reproduced with permission from ref. 76. Copyright 2020 John Wiley and Sons. | ||
In the future, enhancing the PLQY of QDs while ensuring their biological safety will be crucial. As a traditional and effective fluorescent material, QDs are not only used in basic FL imaging and drug tracing, but also how to detect the target substance more innovatively and intelligently and stably label the whole process of tumor treatment is a direction worthy of researchers’ consideration.
2.3 Lanthanum-based nanomaterials
Lanthanum-based nanoparticles are mainly composed of inorganic matrix and rare-earth-doped ions.77 Inorganic matrix generally employed fluorides, oxides, vanadate, oxyfluorides, and other materials with excellent optical transparency, high light damage threshold, and chemical stability. The most commonly used matrices include NaGdF4,78,79 LaF3,80 and Na3ZrF7.81 Rare-earth-doped ions mainly include two parts: luminescent center (activator) and sensitizer.82 The activators require uniform, discrete energy levels, and long metastable lifetimes, for example, Er3+ (ref. 83 and 84) and Tm3+ (ref. 85 and 86) are considered excellent activators. Sensitizers are particularly effective at absorbing excitation light and transferring energy to activators, such as Yb3+ (ref. 83, 85 and 86) and Nd3+.79,85,87,88 Therefore, by changing the composition of inorganic matrix and adjusting the composition and proportion of rare-earth-doped ions, the fluorescence emission wavelength can be easily adjusted to the NIR-II window, so as to obtain more forceful penetration depth and imaging sensitivity.89 There are five basic mechanisms for lanthanum-based nanoparticles to emit FL by up-conversion type: excited-state absorption (ESA), energy transfer upconversion (ETU), cooperative sensitization upconversion (CSU), cross relaxation (CR), and photon avalanche (PA) (Fig. 5a).90 The 4f electron orbitals of lanthanide elements are not fully filled and are typically located within the more completely filled 5s and 5p orbitals. When 4f electrons absorb energy, they produce a sharp f-f transition similar to that of atoms. When they jump to a lower energy level again, they release energy and emit fluorescence (Fig. 5b and c).91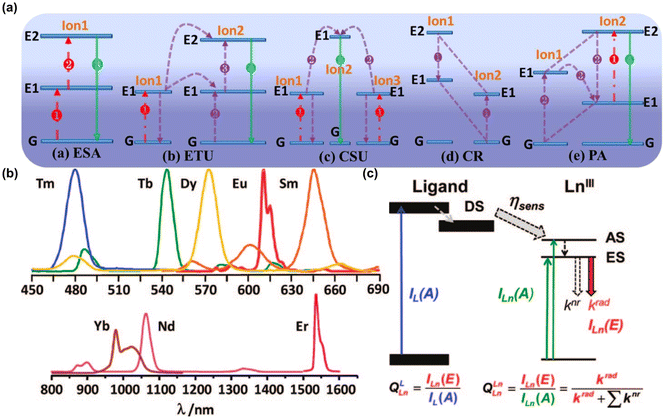 | ||
| Fig. 5 Mechanism of fluorescence emission of lanthanum-based NPs. (a) Main upconversion processes in lanthanide-doped UCNPs.90 Reproduced with permission from ref. 90. Copyright 2014 American Chemical Society. (b) Luminescence spectra of various lanthanide tris(β-diketonates). (c) Simplified diagram showing the definitions of QLLn and QLnLn.91 Reproduced with permission from ref. 91. Copyright 2010 American Chemical Society. | ||
It is urgent to develop Ln3+ doped nanoparticles with efficient emission of in the NIR-II window, to satisfy the demands of sensitive biological imaging.92 The brightness of lanthanum-based nanoparticles can be effectively improved by inhibiting the surface-related luminescence quenching effects,93 minimizing the energy migration-induced losses,94 and sensitizing by organic dyes.95 It has been demonstrated that the inert shell, epitaxially grown on the core nanoparticles, can separate the excited states of the lanthanide ions within the core from the surface quenching centers. This separation helps conserve the NIR-II excitation energy, leading to more efficacious luminescence.96 Huang et al. achieved excellent multicolor emission in core–shell lanthanide nanostructures by modifying the atomic vacancies in the inert shell.97 Besides, adjusting the process of energy transfer by doping lanthanides is also a good way to enhance the fluorescence emission intensity.98 Nd3+ was often used to regulate the process of energy transfer, this is because Nd3+ is excited to the excited state level after absorbing pump light with different wavelengths, then transits to the metastable 4F3/2 level in the form of non-radiative transition, finally returns to the ground state through the 4F3/2–4I11/2 channel, releasing laser with the wavelength of 1064 nm. Wang et al. synthesized LuPO4:Nd3+ nanoparticles using hydrothermal method, which emitted stable and bright fluorescence at 1064 nm after absorbing laser at 808 nm.99 In addition, Yu et al. prepared water-soluble CaF2:Y3+,Nd3+ NPs NIR-II luminescent nanoparticles.100 By doping Nd3+, these nano-agents had more excellent fluorescence emission intensity in the NIR-II window.
Traditional fluorescence technology will be influenced and interfered by different absorption and emission spectra in deep tissue structures (including muscle, bone, and fat) during imaging.101 However, lanthanum-based nanoparticles typically exhibit long emission wavelengths and distinct fluorescence lifetimes. And photons generated by lanthanide nanoparticles with different fluorescence lifetimes at the same emission wavelength have different exponential decay rates, which can be used as signals to avoid chromatic aberration that is caused by tissue heterogeneity. This provides a sophisticated method for multi-channel imaging by leveraging the distinct lifetime signals of different fluorophores in the time domain. This technique enables the separation and analysis of multiple signals simultaneously, enhancing the clarity and detail of biological imaging studies.102 Li et al. developed a lifetime cascade system with temporal domain that span the nanosecond to millisecond (Fig. 6a–c).103 The intermediate meso-tetra(4-carboxyphenyl)porphine (TCPP) served as an intermediate energy relay to bridge the energy transfer of long lifetime ZGO:Mn,Eu to the NIR-II lanthanide Yb3+ for the generation of a lifetime cascade with a controllable lifetime range across three time domains. Besides, Guo et al. developed a series of NIR-II-emitting β-NaErF4: 2% Ce@NaYbF4@NaYF4 core–shell nanoparticles.104 By adjusting the thickness of the NaYF4 inert layer, they could regulate the fluorescence lifetime at 1525 nm from 4.8 ms to 6.5 ms, enabling high signal-to-noise ratio whole-body vascular fluorescence lifetime imaging in mice (Fig. 6d–h).
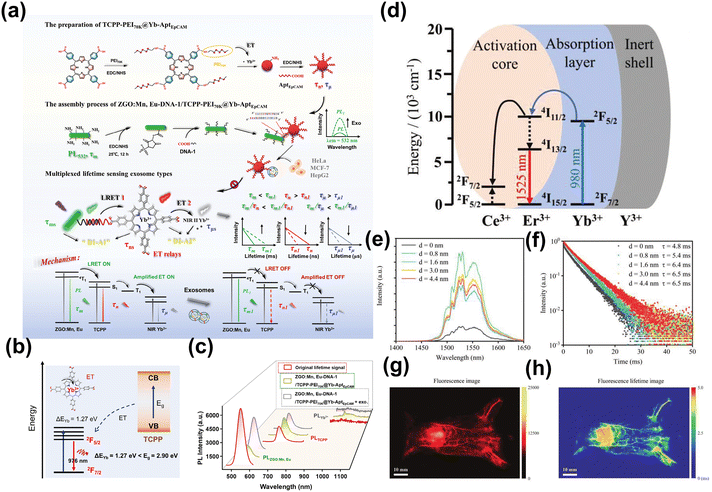 | ||
| Fig. 6 (a) The scheme of a lifetime cascade system ranging from nanoseconds to milliseconds in the temporal domain. (b) Proposed mechanism of energy transfer from TCPP to Yb3+. (c) Photoluminescence spectra of the energy transfer process of ZGO: Mn, Eu-DNA-1/TCPP-PEI70K@Yb-AptEpCAM.103 Reproduced with permission from ref. 103. Copyright 2024 John Wiley and Sons. (d) An energy level diagram depicting the fluorescence emission process of the core–shell–shell nanoparticles. (e) Spectra of fluorescence emission of core–shell nanoparticles. (f) Fluorescence lifetime decay curves recorded at 1525 nm. (g) Fluorescence image of whole-body vascular in mice. (h) Fluorescence lifetime imaging for mapping the entire vascular network in mice.104 Reproduced with permission from ref. 104. Copyright 2023 John Wiley and Sons. | ||
In summary, NIR-II lanthanum-based nanoparticles, characterized by their extended fluorescence lifetime and high PLQY, represent a promising new approach for the future development of convenient and effective fluorescence labeling tools. By leveraging multiplexing imaging technology, these nanoparticles could significantly enhance the capability to simultaneously image multiple targets, thereby improving the efficiency and accuracy of biomedical imaging applications.105
2.4 Noble metal nanomaterials
Noble metals typically encompass eight metallic elements, including gold, silver, ruthenium, rhodium, palladium, osmium, iridium, and platinum. By varying the composition of metal elements, reducing agents, templates, and other conditions, it is possible to coordinate the morphology, particle size, and optical properties of various noble metal nanomaterials.106 According to the size of the particles, the noble metal nanomaterials can be divided into two categories: nanoclusters (NCs) (<2 nm)107 and nanoparticles (NPs) (>2 nm).108 Because of their different size, noble metal NCs and NPs show completely different properties. Next, we will summarize the application of noble metal nanomaterials in the NIR-II window biological imaging and therapy based on these two distinctions.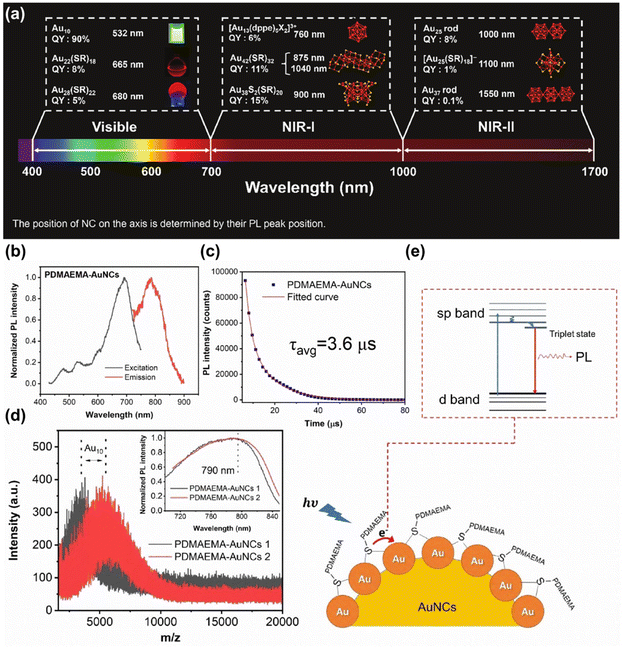 | ||
| Fig. 7 (a) A range of luminescent MNCs showcasing their QY and peak emission wavelengths.115 Reproduced with permission from ref. 115. Copyright 2023 John Wiley and Sons. (b) Normalized excitation and emission spectra (c) Time-resolved PL spectrum (d) matrix-assisted laser desorption/ionization-time of flight mass spectra and (e) schematic representation of the PL origins of PDMAEMA-AuNCs.116 Reproduced with permission from ref. 116. Copyright 2023 John Wiley and Sons. | ||
MNCs synthesized from gold and emitting in the NIR-II spectrum exhibit exceptional fluorescence properties, making them ideally suited for photoactivation and in vivo detection. Bright AuNCs possess deep penetration in biological tissues, and have been successfully used in gastric acid imaging,118 cerebrovascular imaging,119 brain tissue organelle imaging,120 marking tumor metastasis, and guiding bone tissue imaging.121 Additionally, ultra-small AuNCs have the advantage of being safely metabolized and excreted by the body, posing no risk of acute or long-term toxic effects. Although MNCs have been widely developed, compared with QDs and organic dyes, there is still a defect of lower PLQY.122 Over recent years, a range of strategies have been progressively devised to enhance the fluorescence efficiency of MNCs, aiming to optimize their optical properties and broaden their applicability in bioimaging, sensing, and optoelectronics, such as alloy NCs (AuAgNCs, AuAgCuNCs),123,124 surface modification,125 and adjustment of metal atom motifs.126 Additionally, adjusting ligands is also an effective method to enhance the fluorescence efficiency of MNCs.127 The results demonstrated that the luminescence of MNCs could be significantly enhanced by adjusting the structure or interaction between surface ligands molecules.128–130 Through host–guest interactions, the supramolecular host–guest assembly formed between ligand molecules capped on the metal core effectively inhibits the intramolecular vibration nuclear rotation of the ligand, which greatly prohibits the energy loss process on the surface of nanoclusters, resulting in high PLQY.
Besides being used for FL imaging, MNC was also widely used in tumor treatment because of its excellent long emission wavelength and the ability to produce singlet oxygen (1O2) (Fig. 8a).131 Chen et al. co-modified alkyl thio-substituted NCs with human serum albumin (HSA) and catalase (CAT) to obtain multifunctional nano-agents integrating optics and therapy.132 Under the excitation of a 1064 nm laser, these AuNCs produced 1O2. Concurrently, CAT catalyzed the decomposition of endogenous H2O2 in the tumor microenvironment, releasing O2 and significantly enhancing the therapeutic efficacy. Dan et al. used AuNCs (BSA@Au) with NIR-II fluorescence and catalase-like activity as photosensitizers.133 AuNCs visualized the tumor location with high signal-to-background ratio (∼7.3) and biodistribution, and realized high-performance imaging-guided photodynamic therapy (PDT) (Fig. 8b). Apart from AuNCs, AgNCs was also extensively used in PDT.134,135 However, there are few reports about AgNCs used in NIR-II PDT, because the emission wavelength of AgNCs is usually located in the visible window, and AgNCs are prone to oxidation and have poor stability compared with AuNCs, which limits their use in biomedical applications.
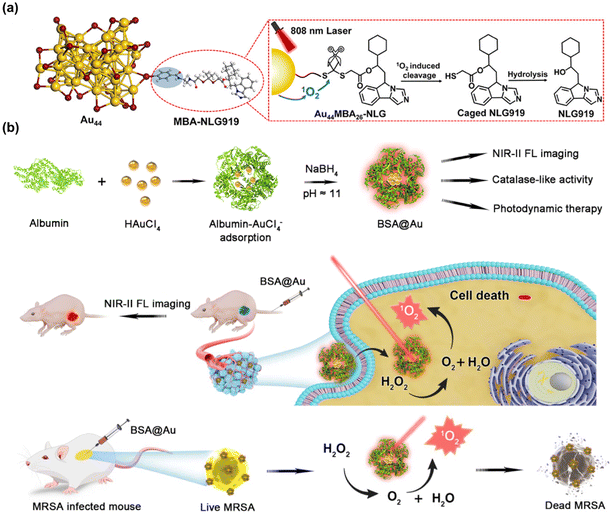 | ||
| Fig. 8 (a) Working principle of the NIR photoactivation of Au44MBA26-NLG.131 Reproduced with permission from ref. 131. Copyright 2023 American Chemical Society. (b) Scheme of NIR-II BSA@Au enhancing PDT.133 Reproduced with permission from ref. 133. Copyright 2022 Multidisciplinary Digital Publishing Institute. | ||
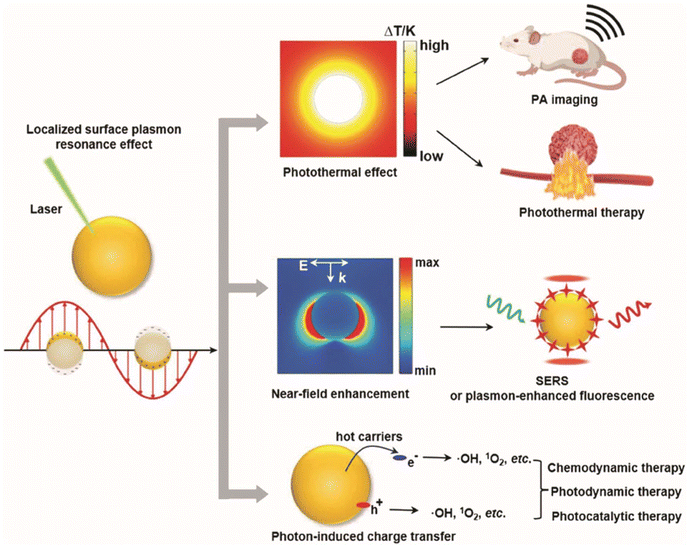 | ||
| Fig. 9 Schematic illustration of the LSPR excitation in MNPs facilitates photothermal effects, near-field enhancement, and charge transfer, enabling applications in imaging, therapy, and catalysis in biomedicine.139 Reproduced with permission from ref. 139. Copyright 2024 John Wiley and Sons. | ||
Because of LSPR effect, MNPs also have the effect of enhancing fluorescence.140 When the fluorophore is positioned in close proximity the MNPs, the free surface electrons of the noble metal resonate when irradiated by light with appropriate wavelength, which can couple with the fluorophore electrons to change the fluorescence emission intensity. By adjusting the size and morphology of MNPs to change the location and intensity of LSPR and local “hot spots”, or by adjusting the distance between MNPs and fluorophore or interlayer materials, the fluorescence properties of some fluorescent nano-agents/fluorescent organic molecules with poor photostability or low PLQY in the NIR-II window can be improved as shown in Fig. 10a–d.141 Xu et al. reported that the Er3+ induced fluorescence emission at 1527 nm of NaGdF4:Yb,Er, Ce@NaGdF4:Yb,Nd@NaGdF4 core–shell–shell NPs was enhanced sixfold by coupling with a Au hole-cap nanoarray (Fig. 10e–g).142
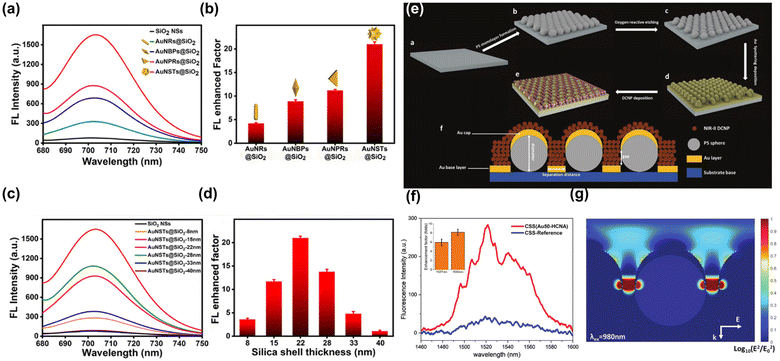 | ||
| Fig. 10 (a) Fluorescence intensity of AuNPs with different morphology in the presence of miRNA-21. (b) The distance between miRNA-21-labeled fluorophores and AuNPs corresponding to (a). (c) Fluorescence intensity of AuNPs with different SiO2 shell thickness in the presence of miRNA-21. (d) The distance between miRNA-21-labeled fluorophores and AuNPs corresponding to (c).141 Reproduced with permission from ref. 141. Copyright 2021 American Chemical Society. (e) The scheme of MEF on Au-HCNA films. (f) Photoluminescence spectra of CSS-DCNPs on glass and on Au50-HCNA. (g) Local E-field enhancement calculated by 3D-FDTD modelling for Au-50 HCNA.142 Reproduced with permission from ref. 142. Copyright 2023 John Wiley and Sons. | ||
Besides AuNPs, AgNPs also have good electrical conductivity and high density of free electrons, thus them also been extensively utilized in NIR-II PA imaging. Cui et al. fabricated AgBr@PLGA nanocrystals with ultra-high sensitivity and tumor-specific PA properties.143 After the GSH activation, AgBr was reduced to AgNPs, and tumor-specific photoacoustography with deep imaging depth could be achieved under NIR-II laser irradiation. Furthermore, the in-band and inter-band electronic transitions of MNPs represent key pathways for non-radiative decay in LSPR. Enhancing these electronic transitions and reducing resistance losses in plasma metals can further refine the electronic structure of the metals, thereby improving its optical properties.144 Alloying is one of the effective strategies, and different optical properties can be selectively obtained by adjusting the types and the atomic ratio of alloys. Shan et al. reported the NIR-II plasma Au@Au–Ag dot-in-cubic nano-frames, which showed tunable plasmonic properties extending beyond 1400 nm.145 This was achieved through the selective etching of Ag atoms and the simultaneous deposition of Au and Ag atoms at the edges and corners of the Au@Ag structures. Additionally, when these probes were injected to the depth of about 2 mm under the mouse skin, the PA signal intensity linearly increased with the probe concentration (Fig. 11a and b). Zhang et al. synthesized 2D PtAg nanoplates capable of absorbing broadband light from the NIR-I to NIR-II window (Fig. 11c and d).146 This alloy nanoplate demonstrated enhanced photothermal and PA effects compared to previously reported Ag nanoplates and Pt nanoparticles.
 | ||
| Fig. 11 (a) The synthesis process of Au@Au–Ag dot-in-cubic nanoframes. (b) Adjustable plasmonic properties across the NIR-I to the NIR-II windows of nanoframes.145 Reproduced with permission from ref. 145. Copyright 2021 John Wiley and Sons. (c) Ultrathin PtAg nanosheets were used for targeted PTT guided by PA imaging. (d) PA imaging showcased PtAg nanosheet suspensions in the axial and cross-sections of photoacoustic tubes under 1064 nm lasers.146 Reproduced with permission from ref. 146. Copyright 2021 John Wiley and Sons. | ||
Due to their unique properties, MNPs have given many surprises beyond fluorescence, such as NIR-II PA imaging, metal-enhanced fluorescence, NIR-II PTT effects, and the generation of ROS for NIR-II PDT. At present, among precious MNPs, Au is the most widely accepted and well-known, followed by Ag and Pt. In recent years, Cu has also been recognized as a low-cost material. In the future, it is worthy of attention to develop new MNPs that can replace noble metals, further reduce the experimental and clinical conversion costs, and improve their imaging and therapeutic performance.
3. NIR-II nano-agents for biological imaging
Optical imaging technology has emerged as a crucial research tool in biomedicine due to its non-invasive property and high safety profile. Particularly significant is the biological imaging technology at NIR-II window, which longer emission wavelengths substantially decrease photon scattering in biological tissues while enhancing their light absorption capacity. This permits deeper tissue penetration, enabling imaging with superior temporal and spatial resolution as depicted in Fig. 12a.147,148 Currently, NIR-II fluorescence imaging and NIR-II photoacoustic imaging are the primary techniques extensively employed for biological imaging diagnosis and treatment of tumors, playing a pivotal role in advancing medical diagnostics and therapeutic strategies.149–151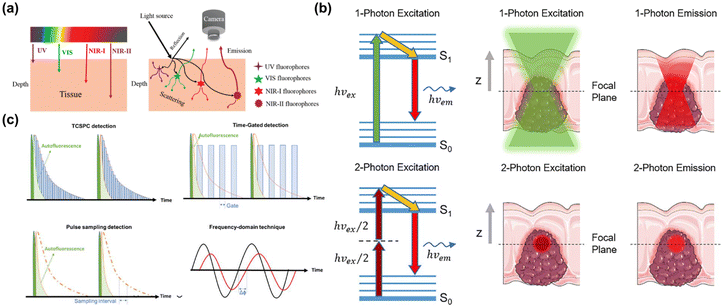 | ||
| Fig. 12 (a) Diagram illustrating the depth of light penetration in tissue at different wavelengths, and interactions like reflection, absorption, and FL.147 Reproduced with permission from ref. 147. Copyright 2018 Royal Society of Chemistry. (b) Comparative schematic of single-photon and two-photon imaging.154 Reproduced with permission from ref. 154. Copyright 2022 Frontiers. (c) Illustration of time-resolved lifetime imaging techniques including TCSPC, time-gated, pulse sampling, and frequency-domain techniques.155 Reproduced with permission from ref. 155. Copyright 2022 John Wiley and Sons. | ||
3.1 NIR-II fluorescence imaging
In the NIR-II window single-photon FL imaging system, the PLQY is a pivotal parameter for evaluating the luminescence efficiency of FL materials. This parameter crucially measures it by comparing the ratio of photons emitted by molecules in their excited state to those absorbed by molecules in their ground state.156 It is fundamental for optimizing the performance of imaging systems in this spectral range, as it directly influences the efficacy of light conversion from absorbed to emitted photons. By improving the degree of π electron conjugation and molecules rigidity, the PLQY can be improved and the FL emission wavelength can be red shifted to the NIR-II window.157,158 Moreover, the performance of imaging system is also very important for obtaining effective FL image. Building upon the inherent deep penetration depth of the NIR-II window, a high-sensitivity imaging system can more effectively capture the emission signals of FL substances and provide more accurate anatomical structure, functional, and molecular images. At present, researchers primarily focus on adjusting material structures to obtain higher PLQY to develop noble NIR-II FL nano-agents.
Two-photon FL imaging technology is increasingly recognized as an effective method for significantly enhancing penetration depth.159 It allows for the absorption of two photons with the same or different frequencies almost simultaneously, and then emits a FL photon through radiation transition. This technique reduces fluorescence background, Rayleigh scattering, and tissue damage while enabling deep tissue imaging to avoid the photobleaching and allowing fluorophores to be excited in a long wavelength window. In addition, it has been made clear that the optimal excitation spectral window of two-photon located in the NIR-II window, which make the research of NIR-II two-photon FL imaging in biological imaging is favored.160 At present, NIR-II absorption and luminescence materials used in two-photon biological imaging mainly include organic FL groups,161 aggregation-induced emission dyes,162 carbon dots,163 QDs,164 noble metal nanomaterials and so on.
NIR-II FL lifetime imaging is an imaging technology that uses the intrinsic characteristics of FL materials–FL lifetime, which refers to the time required for the energy loss of excited FL groups to decrease exponentially to 1/e through FL and other non-radiation processes.165 And the FL lifetime is independent of external factors, such as excitation wavelength, excitation intensity, whether excitation is single photon or multi-photon and photobleaching. The combination of FL lifetime imaging and NIR-II excitation can achieve deep penetration depth and avoid FL scattering of biological tissues, thus achieving high-fidelity multiple detection of multiple analyte biosensors with higher spatial resolution.166 The technical methods of NIR-II FL lifetime imaging include time gate imaging (TGL)167,168 and multi-channel optical imaging.169 At present, the most prevalently used FL materials for NIR-II FL lifetime imaging are nano-agents doped with rare earth elements,170 and the precise adjustment of FL lifetime can be realized by adjusting the ion concentration and energy conversion process of rare earth elements.
Currently, researchers are increasingly focusing on the development of intelligent “off–on” NIR-II FL nano-agents that can be activated by the tumor microenvironment (TME). For example, GSH175,176 and pH-responsive177–181 diagnostic FL nano-agents have been designed for tumor imaging to effectively overcome the interference of background signals in normal tissues and enhance the signal-to-noise ratio. Additionally, the activation of FL signal can be realized by coupling the substrate of enzyme in tumor tissue and the specific recognition polypeptide of antibody over-expressed in tumor tissue on the surface of FL nano-agents.182 Dai et al. prepared a GSH-activated NIR-II diagnostic nanoplatform mediating by iron ion-mediated self-assembly of Ag2S QD and NIR-II semiconductor polymers (Fig. 13a).183 The over-expressed GSH in tumor tissue led to its decomposition via the destruction of metal coordination, thereby turning off the Fluorescence Resonance Energy Transfer (FRET) effect between Ag2S QD and NIR-II DBZ polymer-based nanoparticles (Pdots) and restoring the FL of Ag2S QD under the excitation of 808 nm (Fig. 13b and c). Liu et al. proposed Ag2S vesicles (Ag2S Ve) assembled by self-assembly of Ag2S QDs, and then coated with pH-sensitive copolymer thiolated polystyrene-co-poly(4-vinyl pyridine) (Fig. 13d).184 Under low pH conditions characteristic of tumor tissue, these vesicles triggered the release of Ag2S QDs and enabled the recovery of NIR-II FL (Fig. 13e). This controllable disassembly method demonstrated specific illumination towards diseased tissue, which provided a powerful strategy for accurate cancer treatment.
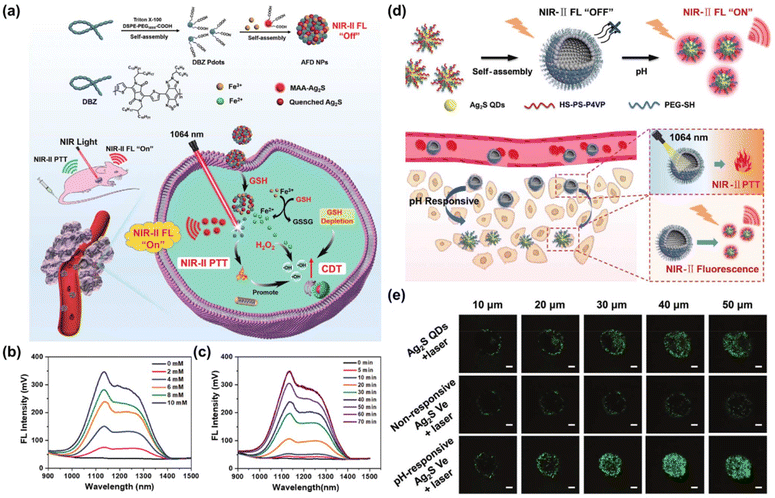 | ||
| Fig. 13 (a) The synthesis of TME activated AFD NPs for tumor specific phototheranostic applications. (b) NIR-II FL recovery of AFD NPs triggered by varying concentrations of GSH. (c) NIR-II FL recovery under different incubation time.183 Reproduced with permission from ref. 183. Copyright 2023 John Wiley and Sons. (d) The synthesis of Ag2S Ve and it's pH-activated FL-guided NIR-II PTT applications. (e) The images of MCF-7 multicellular spheroids incubated with different types of FITC-labeled Ag2S Ve for 6 h and exposed to 1064 nm laser irradiations (1 W cm−2) for 5 min.184 Reproduced with permission from ref. 184. Copyright 2021 John Wiley and Sons. | ||
Additionally, tumor surgery guided by NIR-II FL imaging represents a cutting-edge technology that precisely delineates tumor edges through targeted imaging of the tumor. And it is anticipated to boost survival rates by facilitating the comprehensive removal of tumor tissues.180,181 Dang et al. reported a biodegradable, hollow, virus-like neodymium oxide (Nd2O3) Nd-nanoprobe, which were modified with cyclic arginine-glycine-aspartic acid (cRGD) pentapeptide (Fig. 14a).185
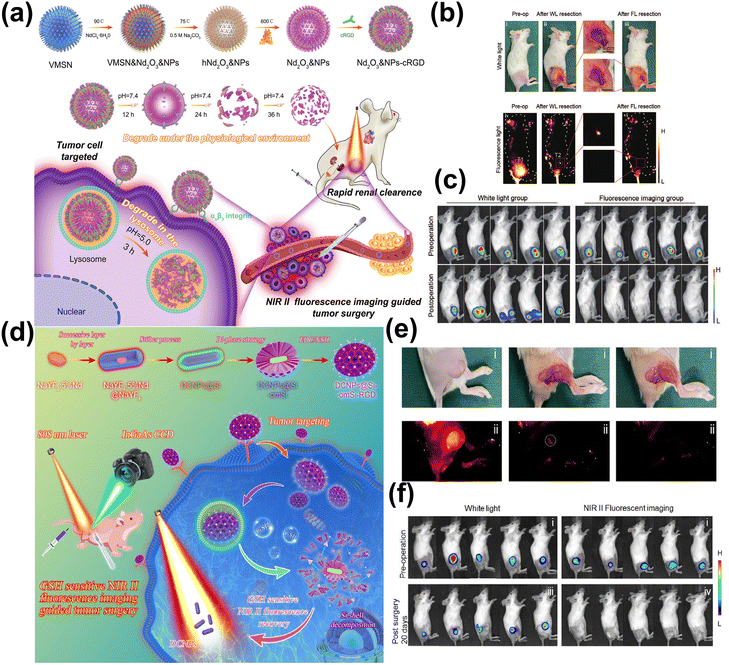 | ||
| Fig. 14 (a) The synthetic method of Nd2O3 &NPs-cRGD for NIR-II image-guided breast cancer surgery. (b) The different stages of NIR-II FL-guided tumor surgery in the subcutaneous mouse model. (c) The images of mice from pre-operation (up) and 14 days post-operation (down) after tumor movement under NIR-II FL and white light guidance.185 Reproduced with permission from ref. 185. Copyright 2024 John Wiley and Sons. (d) The fabrication of DCNPs@Si-omSi-RGD for liver tumor precise resection. (e) The quantitative analysis of fluorescent intensity related to liver tumor surgery. (f) The images of mice from pre-operation (up) and 20 days post-operation (down) after tumor movement under NIR-II FL and white light guidance.186 Reproduced with permission from ref. 186. Copyright 2022 Elsevier. | ||
Fourteen days post-operation, the group treated with NIR-II imaging-guided surgery using Nd2O3&NPs-cRGD showed no signs of local recurrence or metastasis. In contrast, all mice in the control group undergoing surgery guided only by white light, experienced in situ tumor recurrence (Fig. 14b and c). Li et al. designed mesoporous silica shell doped with GSH-sensitive tetrasulfide bond, encapsulated Nd-doped down-conversion nanocrystals, and modified RGD peptide on the surface to target liver tumor tissue (Fig. 14d).186 At high GSH concentration in tumor tissue, the tetrasulfide bond could be destroyed, which led to the recovery of NIR-II FL explosion. Guided by the NIR-II FL, the solid liver tumor in mice was successfully excised, and without any recurrence within 20 days post-surgery (Fig. 14e and f).
The NIR-II window for FL imaging is increasingly recognized as an exceptional candidate for biological applications due to its deeper tissue penetration and clearer imaging capabilities. Furthermore, the innovation of intelligent “off–on” FL probes elevates NIR-II imaging technology to unprecedented levels. Rendering it is pivotal not only in fundamental research but also in clinical settings where it provides highly accurate image-guided treatments. Consequently, the utilization of NIR-II FL nano-agents holds immense potential for advancing tumor diagnosis and therapy, offering invaluable assistance in both research laboratories and clinical environments.
3.2 NIR-II photoacoustic imaging
The scattering effect of biological tissues is pronounced at shorter wavelengths (200–700 nm), leading to an exponential decrease in light intensity and PA amplitude with depth increasing.190 In contrast, the NIR-I and NIR-II window exhibits lower blood absorption and improved light penetration, making it extensively used for deep tissue imaging.191 Recent advancements have focused on PA imaging technology in the NIR-II window, which has garnered attention due to its low sound scattering properties.192 This characteristic allows it to overcome the diffusion limit of light, achieving a penetration depth of up to 5 cm,193 thereby facilitating multi-scale, high-resolution imaging of biological structures ranging from organelles to entire organs.194,195 Additionally, imaging contrast is significantly enhanced in the NIR-II window due to the absence of background interference from non-absorbing tissue components. Most importantly, the Maximum Permissible Exposure (MPE) for humans is higher in the NIR-II range, permitting the utilization of higher intensity pulsed lasers and resulting in a stronger PA signal.196–198 Therefore, NIR-II PA imaging offers substantial improvements over NIR-I PA imaging, in both basic research and practical applications. Overall, NIR-II PA imaging, with its 3D stereoscopic depiction and deeper penetration capabilities, merges the benefits of optical and acoustic imaging. This integration resolves the limitations of NIR-I PA imaging and FL imaging in tumor diagnostics, presenting a more effective and detailed approach.
In addition to choosing the appropriate wavelength for PA imaging, it is crucial to develop a variety of NIR-II PA contrast agent with high NIR-II absorption rates and efficient PA signal conversion capabilities in order to improve the PA imaging performance.199,200 Recent advancements have seen a surge in the creation of exogenous NIR-II PA contrast agents, which boast high molar extinction coefficients (MEC), low PLQY, and excellent photostability, making them highly effective for PA imaging purposes. Various categories of materials are being explored for this purpose, including metal nanomaterials,201,202 organic semiconductor conjugated polymers,203–206 QDs,207 small molecular organic dyes208,209 and so on. He et al. adjusted the LSPR peak of GNR into the NIR-II window by coating SiO2 on gold nanorods, which enhanced the PA imaging capabilities and improved its photothermal stability.210 Zheng et al. made inorganic nanoparticles have strong NIR-II absorption by adjusting the silicon–carbon ratio in zeolite–carbon-based nanozymes, resulting in excellent PA imaging performance.211 Jiang et al. used the donor–receptor–donor structure and active intramolecular motion to design an organic small molecular structure, the NIR absorption peak is red-shifted to obtain excellent NIR-II PA imaging performance.212 These innovative designs have significantly advanced research on NIR-II PA nano-agents, which is anticipated to unlock new possibilities in non-invasive imaging technologies and clinical applications.
Furthermore, NIR-II PA imaging significantly enhances capabilities in the realm of deep tumor imaging. In vitro experiments demonstrated an impressive imaging depth of up to 5 cm in chicken breast tissue, while in vivo studies on mice show a imaging depth of approximately 2 cm. Wu et al. synthesized a semiconducting polymer based on thienoisoindigo that exhibited strong absorbance in NIR-II window.219 This polymer could be effectively imaged through chicken breast tissue samples as thick as 5.3 cm using 1064 nm laser excitation. Chitgupi et al. reported a micelle formed from NIR-II absorbing cyanoalkyl phosphate (CYFAP) salt dye, which could be imaged by chicken breast tissue of 11.6 cm in vitro.220 The NIR-II PA imaging technique was successfully applied to imaging human breast cancer by utilizing it on three female volunteers through compressed breast tissue, achieving imaging depths ranging from 2.6 to 5.1 cm (Fig. 15a–e). Additionally, developing contrast agents with high permeability represents a promising strategy to enhance the contrast of NIR-II PA imaging in deep tumor tissues. Li et al. proposed that hyaluronidase and Ag2S nanodots were encapsulated by acid-labile metal–organic framework, which could be passively transported to the tumor and catalyze the breakdown of hyaluronic acid around the tumor tissue. Thus, achieving deep penetration, and Ag2S nanodots exhibited significant NIR-II PA signal under the excitation of 1064 nm (Fig. 15f–g).221 Li et al. proposed a Janus AuNR-Pt nano-motor (JAuNR@Pt) driven by H2O2. In mice treated with it, the NIR-II PA signal intensity at depths of 1.8 mm and 3.6 mm was increased by approximately 20% and 76%, respectively, compared to those treated with AuNR@Pt (Fig. 15h–k).222 Moreover, because the acoustic waves in PA imaging are the signal obtained after the biological tissues absorb photons and produce thermoelastic expansion, the temperature usually rise in the process of PA imaging. Therefore, innovative designs incorporating temperature sensitivity have significantly broadened the applications of NIR-II PA imaging. Li et al. described a reversible temperature-responsive tungsten-doped vanadium dioxide nanoparticle (W-VO2@PEG) coated with polyethylene glycol (Fig. 16a).223 This NPs transitioned from a metal phase to an insulator phase when the temperature rose from 35 °C to 45 °C. During this phase changed, W-VO2@PEG NPs exhibited a significant increase in NIR-II light absorption, the PA signal amplitude at 1064 nm escalated by as much as 260% (Fig. 16b and c). Sun et al. also used a similar W-VO2@PEG NPs material to achieve the quantitative 3D temperature rendering of deep tumors (Fig. 16d).224 When the temperature increased from 35 °C to 45 °C, the PA signal amplitude at 1064 nm increased by 2 times (Fig. 16e).
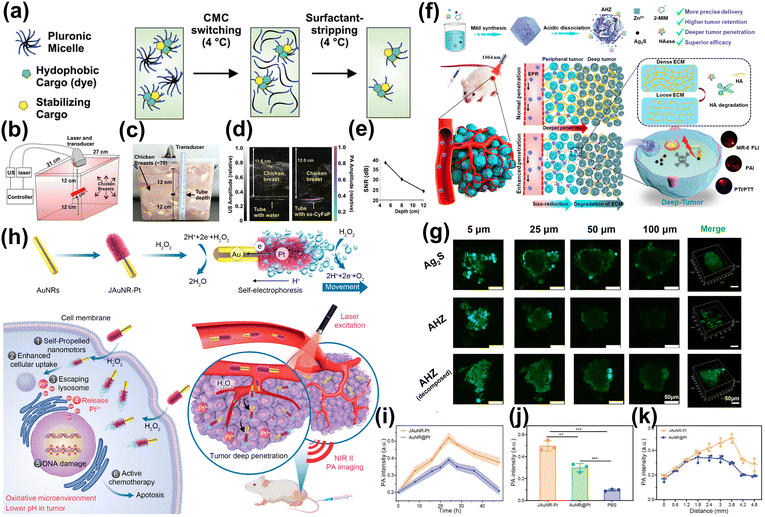 | ||
| Fig. 15 (a) Surfactant-stripped CyFaP (ss-CyFaP). (b) The imaging setup. (c) A container filled with chicken breast was used for in vitro penetration depth test. (d) Overlaid with US images in grayscale, showing differences between tubes filled with water and ss-CyFaP micelles. (e) SNR of PA signal as a function of tissue thickness on top of the tube.220 Reproduced with permission from ref. 220. Copyright 2019 John Wiley and Sons. (f) The Preparation and Tumor Penetration of AHZ NPs. (g) The images of Ag2S or AHZ in 4T1 tumor cell spheroids penetrated.221 Reproduced with permission from ref. 221. Copyright 2022 American Chemical Society. (h) The preparation of JAuNR-Pt nanomotor and its application. (i) Comparison of PA signal intensities at various time points post-injection of JAuNR-Pt nanomotors versus AuNR@Pt. (j) Corresponding PA signal intensity of tumor in vivo at 24 h. (k) Corresponding PA signal intensity in the different depths at 24 h.222 Reproduced with permission from ref. 222. Copyright 2022 American Chemical Society. | ||
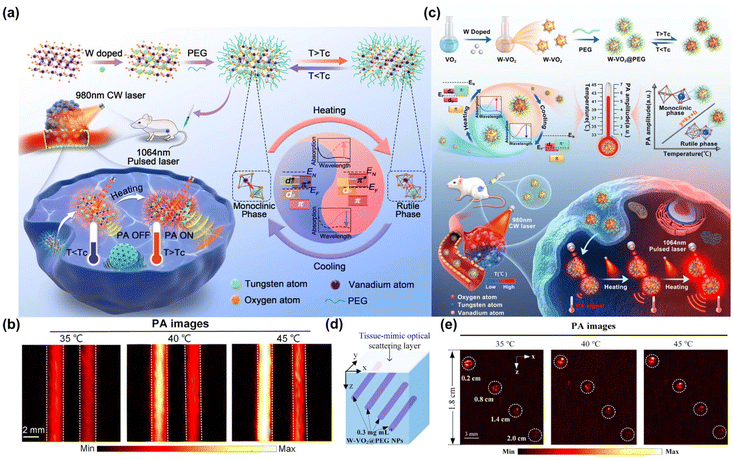 | ||
| Fig. 16 (a) The dynamic-enhanced NIR PA imaging using W-VO2@PEG NPs triggered by near-human-body temperature. (b) PA imaging of W-VO2@PEG NPs and graphene samples at different temperatures.223 Reproduced with permission from ref. 223. Copyright 2022 American Chemical Society. (c) The synthesize of W-VO2@PEG NThems and its application of quantitative 3D temperature rendering of deep tumors. (d) The depth PA imaging of the W-VO2@PEG NThems in an agar phantom sample. (e) X–Z tomographic imaging at different temperatures of (d).224 Reproduced with permission from ref. 224. Copyright 2023 American Chemical Society. | ||
In the future, the NIR-II window severs as an ideal response window of PA imaging, will significantly improving the precision of tumor tissue localization and facilitating tumor treatment guidance by PA imaging.
4. NIR-II imaging guided NIR-II phototherapy
In recent years, phototherapy has emerged as a highly effective method for treating tumors. The NIR-II window broadens the depth of tumor treatment and provides a powerful means for tumor detection and treatment. Nano-agents with NIR-II FL imaging or PA imaging performance can effectively kill tumor cells through PTT, PDT, PTT-CDT, PTT-CT, and PTT-IO. These technologies not only allow for precise visualization of tumors but also facilitate targeted treatment strategies, thereby realizing a dual breakthrough in cancer management.4.1 NIR-II imaging guided NIR-II PTT
The therapeutic and diagnostic nano-agents combining NIR-II optical biological imaging with high sensitivity and high penetration depth with PTT have been considered as an advanced technology to improve detection and treatment. The semiconductor polymer TRPV1 prepared by Wu et al. can even be used to realize brain imaging and treatment without implantation.225 PTT represents a prominent photon-triggered therapeutic modality that eradicates tumor cells through localized heating, which is generated by photothermal agents activated by visible or NIR light.226,227 Notably, compared with visible light and NIR-I window PTT, NIR-II window PTT has more advantages. This is because there is lower light absorption and scattered light in the NIR-II window, which allows deeper tissue penetration. Additionally, the maximum permissible exposure (MPE) intensity in the NIR-II window is higher-1 W cm−2 for 1064 nm, 0.72 W cm−2 for 980 nm and 0.33 W cm−2 for 808 nm-enhancing both the safety and efficacy of the treatment. These attributes make NIR-II PTT a more effective option for targeting deeper-seated tumors with minimal damage to surrounding tissues.228,229At present, PTT guided by NIR-II PA imaging offers considerable advantages, because the heat energy generated during PA imaging can be utilized directly to treat tumors. This method eliminates the need for adding extra photothermal agents or fluorescent dyes, streamlining the treatment process and potentially reducing side effects associated with additional compounds.230–234 Li et al. described the synthesis of a semiconductor polymer nanoparticles (SPNs) prepared by the NIR-absorbing SPNs (PFTDPP) and SnO-based NO Donors (S-nitro so-n-acetyl cellulose, Snap), which could generate heat through vibration relaxation and provide NIR-II FL/PA imaging to guide PTT.235 Shao et al. reported the titanium nitride QDs (Ti2NQDs) prepared from nitride-based MXene, which showed excellent photothermal performance and strong photothermal stability under laser irradiation in both NIR-I (808 nm) and NIR-II (1064 nm) biological windows, and the experimental group showed complete tumor ablation under the guidance of PA imaging.236
Photothermal conversion efficiency (PCE, η) is a critical parameter of photothermal agents, directly influencing the required intensity of irradiation light.237,238 The use of high-intensity light to activate photothermal agents can inadvertently harm healthy skin and tissues. Consequently, it is very important to develop photothermal agents with high PCE to reduce the radiation intensity and improve the safety of PTT for living tissues. Some nano-agents with excellent PCE are shown in Table 1. For inorganic NIR-II photothermal agents, enhancing NIR-II absorption is commonly employed to augment the photothermal effect.239 AuNPs are extensively utilized in the field of PTT due to their ability to generate efficient local heating through the excitation of LSPR (as discussed in 2.4.2 above).240–244 Ding et al. constructed Au@Dopamine, and its PCE was 75.7% (1064 nm, 1.0 W cm−2).245 Even with an 8 mm thickness of in vitro pork tissue, the temperature of the NPs solution increased to above 40 °C. Chen et al. prepared AuNPs coated UiO-66-NH2 (NMOF).246 By adjusting the growth of Au nanoshells, with the peak value exceeding 1300 nm and the PCE as high as 74.0%. In addition, ultra-thin two-dimensional (2D) nanomaterials have interesting physical and optical properties, which provide new possibilities for realizing efficient photothermal agents with high extinction coefficient and high PCE.247–249 Organic semiconductor polymer nano-agents have the characteristics of optically adjustable NIR-II window. By designing donor–acceptor (D–A) structure, they can show strong absorption extinction and high PCE. Adding weak electron donor units to the semiconductor skeleton to inhibit the non-radiation decay pathway can effectively improve NIR-II absorption.250–252
| Classification of nano-materials | NIR-II photothermal agent | λ ex/λem [nm] | PCE % | Ref. |
|---|---|---|---|---|
| AuNPs | TA–Si–Au | 1064 | 24.1 | 240 |
| Au/Ag core/shell NRs | 1064 | 28.8 | 241 | |
| AuNCs@SiO2 nanochains | 1064 | 82.2 | 242 | |
| Janus Au-PbS NPs | 808/1300 | 45.16 | 243 | |
| 808/1500 | 63.27 | |||
| AuPd-GOx-HA (APGH) nanocatalysts | 1064 | 50.7 | 244 | |
| Au@PDA/PEG-PI (T-NPs) | 808 | 86.6 | 245 | |
| 1064 | 75.7 | |||
| Gold nanoshells on UiO-66-NH2 (UGs) | 1064/1300 | 74.0 | 246 | |
| 2D nanomaterials | TeO2/(NH4)xWO3 nanoribbons (TONWNRs) | 1064 | 43.6 | 247 |
| 2D SnTe@MnO2-SP NSs | 808 | 38.2 | 248 | |
| 1064 | 43.9 | |||
| PNS/PEG-Ag2S QDs | 808/1050 | 52.46 | 249 | |
| Organic semiconductor polymer nano-agents | AuGSNO | 1064 | 80.0 | 250 |
| SP4 NPs | 1064 | 46.5 | 251 | |
| Semiconducting polymer P2 based NPs (P2NPs) | 1064 | 38.8 | 252 |
Additionally, the single bond linkage between the donor and acceptor in multiple-acceptor D–A type polymers potentially bestows them with a wealth of flexible intramolecular rotors along the polymer backbone. These rotors are considered highly advantageous for promoting active intramolecular motion, which in turn induce an effective photothermal effect. Theoretically, this arrangement can significantly enhance the PCE in aggregates, potentially leading to the improvement of performance in photothermal applications.253
4.2 NIR-II imaging guided NIR-II PDT
NIR-II PDT involves the activation of photosensitizers by NIR-II laser irradiation, the photosensitizers absorb energy and transfer it to molecular oxygen, generating reactive oxygen species (ROS), such as hydroxyl radical (˙OH), singlet oxygen (1O2), and superoxide (O2˙−), which leads to oxidative stress and triggers apoptosis in tumor cells.254,255 Two primary forms of ROS production exist: photosensitizer molecules are excited from the ground state to the singlet excited state upon photoexcitation. They are then excited to the long-lived triplet excited state via cross-process between systems, which can undergo electron transfer reaction with biological substrates/chromophores to generate free radical anions, and the free radical anions further interact with oxygen to generate ˙OH and O2˙− (the type I reaction).256–260 Alternatively, the excited state energy of triplet photosensitizer molecules can be transferred to oxygen to generate 1O2 (the type II reaction).261,262Currently, the field of NIR-II imaging-guided NIR-II PDT is experiencing a surge in groundbreaking research, exploring innovative techniques that enhance the efficacy and precision of this therapeutic approach. It has been demonstrated that certain NIR-II FL dyes, such as cyanine dyes,263,264 porphyrins and their derivatives265,266 can produce ROS under NIR-II laser irradiation. Furthermore, photosensitizers with low PLQY, such as phthalocyanine and naphthalene phthalocyanine267,268 are effective as PA contrast agents due to their robust absorption in the NIR-II window. Consequently, these small molecular photosensitizers enhance the therapeutic efficacy of NIR-II PDT by serving as dual roles: as therapeutic agents and as NIR-II contrast agents, thereby optimizing treatment outcomes.269,270 In addition, the biocompatibility and photostability of photosensitizers can be further improved by loading/encapsulating them in inorganic NPs or forming semiconductor polymers though self-assembly, which also improves the targeted delivery to tumor cells and boosts the production of ROS, effectively enhancing the capability to eradicate tumor cells.271–275
Even more interesting is that among many inorganic nano-agents, up-conversion NPs can be used not only as carriers and NIR-II FL emitting materials, but also as energy converters to activate the attached photosensitizers via FRET, enabling deep-tissue PDT.276–278 Er3+-doped UCNPs can be used to excite IR808279/Ce6280 photosensitizer via energy transfer to enhance the NIR-I-excited PDT, and be used to excite ZnPc photosensitizer to enhance the NIR-II-excited PDT.281 In addition, some nano-agents can directly generate ROS for tumor treatment in the absence of organic photosensitizers,282 which has following advantages: (1) higher photostability; (2) resistance to enzymatic degradation; (3) superior molar extinction coefficient, at least 3–7 orders of magnitude higher than that of most organic photosensitizers; (4) tunable light absorbs to a longer wavelength, resulting in deeper tissue penetration. Plasma MNPs have the characteristic of LSPR, which can generate two types of high-energy charge carriers under laser irradiation, namely electrons and holes. The generated high-energy charges can promote the generation of ROS through the energy conversion process, which endows plasma MNPs with photodynamic characteristics.283,284 Meng et al. synthesized plasmonic Bi2S3−x–Au@HA heterostructure nanocomposites (Fig. 17a).285 The modified AuNPs can accept e− on the Bi2S3−x semiconductor conduction band and transfer it to O2 for generating O2˙−, which can be further oxidized by h+ to 1O2 under 1064 nm laser irradiation (Fig. 17b and c). In addition, bimetallic QDs, carbon dots QDs and graphene QDs can induce 1O2 to generate ROS by transferring energy from excited QDs state to ground state triplet oxygen, which is called triplet energy transfer (TET). Chen et al. reported an effective photosensitizer Cu2−xSe QDs, which could efficiently generate dreadful ROS (˙OH and 1O2) by NIR-II (1064 nm) laser to trigger PDT (Fig. 17d).286 Wang et al. reported UCNPs@SiO2-GQD, upon 980 nm laser irradiation, allowed NaYF4:Yb3+,Er3+ UCNPs to emit green and NIR FL.287 The green FL aligned with the excitation band of GQDs, facilitating energy transfer from UCNPs to GQDs. This interaction resulted in the generation of 1O2 and produced additional NIR FL (Fig. 17e).
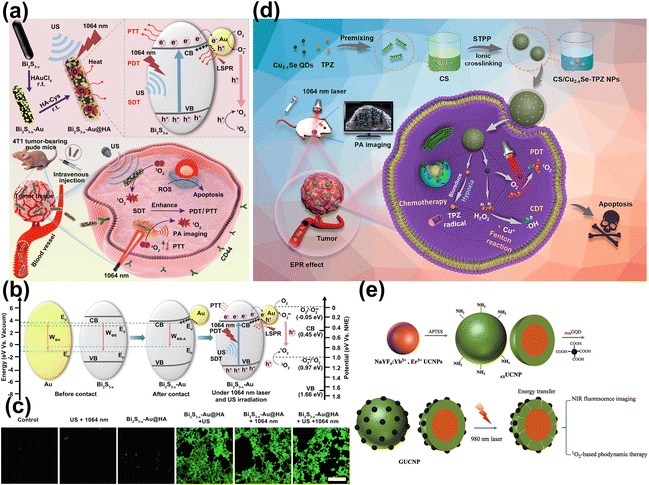 | ||
| Fig. 17 (a) The main synthesis procedure, ROS generation and antitumor mechanism of Bi2S3−x–Au@HA. (b) The proposed charge separation/transfer in Bi2S3−x–Au@HA and ROS generation mechanism. (c) The images of 4T1 cells stained with DCFH-DA to detect ROS generation following different treatments.285 Reproduced with permission from ref. 285. Copyright 2023 Elsevier. (d) The Construction of CS/Cu2−x Se-TPZ Nanoplatform.286 Reproduced with permission from ref. 286. Copyright 2023 American Chemical Society. (e) Scheme illustrating the design of GUCNPs.287 Reproduced with permission from ref. 287. Copyright 2021 Royal Society of Chemistry. | ||
4.3 NIR-II imaging guided NIR-II combinational phototherapy
Although NIR-II-guided single PTT and/or PDT exhibits considerable promise in the treatment of cancer, there is a bottleneck in its single method that prevents the therapeutic effect from being further enhanced. Adopting combinational therapies, which combine NIR-II PTT with CDT, CT and IO, are the promising strategy to overcome this bottleneck. Under the guidance of NIR-II imaging, these multi-elements combined therapies offer a promising approach to achieve a synergistic “1 + 1 > 2” anticancer effect, which can further overcome the defects of insufficient photothermal killing ability, multidrug resistance, and poor metastasis prevention ability.| Fe2+ + H2O2 → Fe3+ + (OH)− + ˙OH | (1) |
| H2O2 + 2Fe3+ → 2Fe2+ + O2 + 2H+ | (2) |
| O2 + Fe2+ → Fe3+ + O2− | (3) |
At present, a variety of nano-agents with good photothermal properties have been developed, which can effectively promote CDT to kill tumor cells through photothermal stimulation under the NIR-II. Such as Bi nanodots,294 Fe/Mn bimetal-doped ZIF-8,295 Fe-doped polyoxometalate (Fe-POM),296,297 carbon-encapsulated magnetite nanodoughnuts,298 Pt@V2C MXene,299 Cu2O-supportedmolyb-denum disulfide (MoS2) nanoflowers,300 Cu2−xTe nanosheets,301 ultrathin 2D transition metal chalco-genides (TMCs) nanosheets (NSs),302 nickel-based nanomaterials,303 CuS NPs,304,305 Ag2S–Ag Janus probes.306 In recent years, Cu+-mediated CDT has also been widely studied. Li et al. reported a dual-plasmonic Au@Cu2−xSe core–shell nanocrystals (dpGCS NCs) (Fig. 18a), facilitating tumor-specific CDT through the production of ˙OH triggered by TME-activated dpGCS NCs-catalyzed in situ Fenton-like reactions, which were further enhanced by NIR-II laser exposure.307 Besides, the robust NIR-II LSPR absorption of dpGCS NCs significantly enhanced their photothermal performance for NIR-II PTT, as depicted in Fig. 18b and c. Zhang et al. designed and synthesized an oxidized molybdenum polyoxometalate-Cu nanocomposite (Ox-POM@Cu) (Fig. 18d).308 The inclusion of Cu in Ox-POM@Cu created oxygen vacancies, increasing carrier concentration, accelerating electron transfer, and enhancing redox activity, thus providing efficient catalysis (Fig. 18e and f). In addition, this composite also served as an NIR-II PA imaging probe and a cancer therapy agent. In a similar vein, Li et al. developed an elesclomol (ES) loaded Cu2O nanocomposite (PEG@Cu2O-ES) (Fig. 18g).309 The NIR-II induced PTT effect of Cu2O speed up the release of Cu+, generating a high level of ROS that attacked the ATP-Cu pump on cancer cell membranes, thereby aggravating Cu overload and effectively killing tumor cells (Fig. 18h and i).
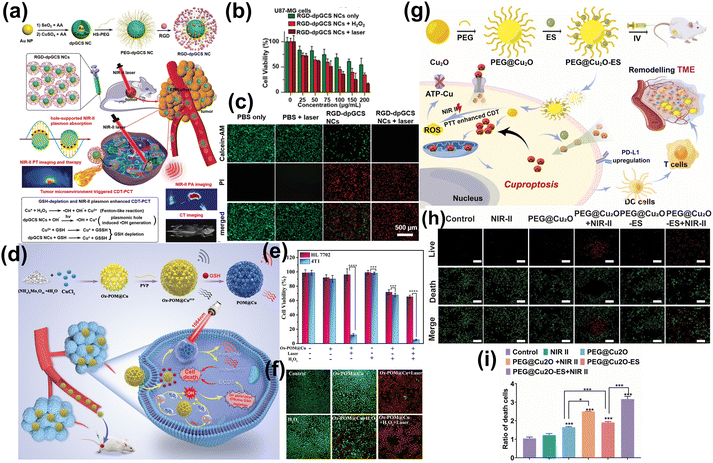 | ||
| Fig. 18 (a) The synthesis of RGD-dpGCS NCs and its therapeutic mechanism for NIR-II phototheranostics. (b) The image of U87-MG cancer cells after various treatments. (c) The images of calcein-AM/PI co-stained U87-MG cells after various treatments.307 Reproduced with permission from ref. 307. Copyright 2021 John Wiley and Sons. (d) The preparation, PAI, and therapeutic mechanism of Ox-POM@Cu nanoenzyme. (e) Evaluation of drug efficacy of Ox-POM@Cu. (f) The images of live and dead 4T1 cells with calcein-AM/PI double staining and different treatments.308 Reproduced with permission from ref. 308. Copyright 2021 John Wiley and Sons. (g) The cuprous oxide nanocomposites for inducing Cu proptosis in breast cancer. (h and i) The live & dead images and fluorescence statistics of 4T1 cells after treatment with NIR-II.309 Reproduced with permission from ref. 309. Copyright 2024 Elsevier. | ||
Loading enzymes that can catalyze the CDT reaction onto a carrier with PTT capability to achieve synergistic treatment of both therapeutic approaches is also a promising approach.310 Li et al. reported a plasmonic trienzyme-integrated metal–organic framework (plasEnMOFs) nanoplatform, which incorporated NIR-II plasmonic Au nanorods and natural enzymes—catalase (CAT), glucose oxidase (GOx), and horseradish peroxidase (HRP)—within zeolite imidazolate framework 8 (ZIF-8) MOFs.311 Thus, it effectively reduced intratumor glucose levels and produced toxic ROS for simultaneous starvation therapy and CDT, complemented by plasma thermotherapy. Additionally, the NIR-II PTT effect further boosts glucose depletion and ROS generation. Yang et al. reported that by conjugating GOx with strontium copper tetrasilicate (SrCuSi4O10) nanosheets (NSs).312 The photothermal effect of it could amplify the catalytic activity of GOx under NIR-II exposure, markedly increasing the depletion of intertumoral glucose and the production of H2O2, thereby enhancing the effectiveness of the therapy.
Nano-agents possessing enzyme-like properties offer an innovative approach to cancer treatment by generating a substantial quantity of ROS, thus reducing reliance on the sole use of NIR-II PTT. Additionally, NIR-II PTT can significantly enhance the ionization process in the Fenton-like reaction, boosting ROS production. This therapy also increases blood flow and oxygen supply at the tumor site, which strengthens the β-lapachone cyclic reaction, leading to copious H2O2 generation. Overall, the synergistic integration of NIR-II PTT with CDT harnesses their complementary strengths, optimizing the therapeutic impact and broadening the scope for effectively targeting and eliminating tumor cells.
At present, researchers have developed a variety of nano-agents with NIR-II PTT properties. By encapsulating CT drugs, targeted transportation or in situ injection into tumor sites, the drugs are accurately released under the conditions of NIR-II laser319–325 or low pH,238,326 which greatly improved the safety and efficiency of CT. Lan et al. developed an “all-in-one” nanoplatform that integrated at the NIR-II window dye IR1061 and anticancer drug paclitaxel (PTX) into an apoferritin (AFN) nanocage (IR-AFN@PTX) (Fig. 19a and b).327 Remarkably, 78.6% of PTX was released under acidic conditions (pH 5.0) with NIR-II laser irradiation, significantly more than the 3.2% released at physiological pH 7.4 (Fig. 19c). Deng et al. constructed Au nanostar@ZIF-8 (Au@MOF) nanoparticles, which encapsulated the DOX (Fig. 19d).137 Upon exposured to the 1064 nm laser irradiation, the heat generated led to the dissociation of the Zn–O coordination within ZIF-8, doubling the release rate of DOX compared to conditions without irradiation (Fig. 19e). Shen et al. crafted thermosensitive liposomes (DG@TLs) activated by NIR-II (1064 nm) light, which encapsulated gambogic acid (GA).328 These liposomes leveraged a phase transition in the phase change materials (PCMs) from solid to liquid under hyperthermia induced by NIR-II PTT, facilitating controlled release of GA (Fig. 19f and g). The released GA enhanced the efficacy of NIR-II PTT by inhibiting HSP90 activity, reducing tumor thermoresistance and displaying potent chemotherapeutic effects, thus achieving a synergistic anti-tumor impact.
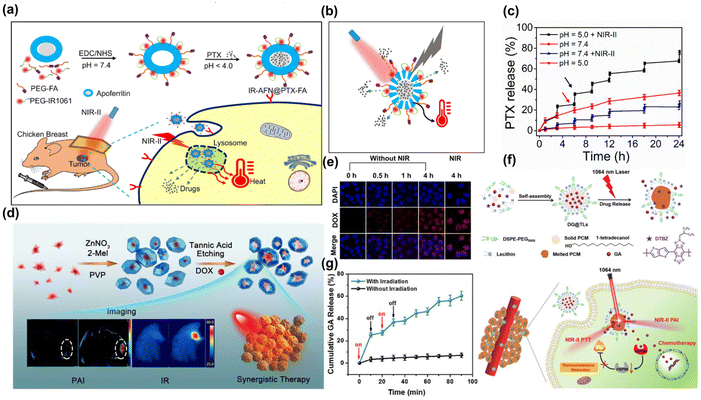 | ||
| Fig. 19 (a) The preparation and application of IR-AFN@PTX-FA. (b) The scheme of pH/NIR-II responded drug release and heat generation. (c) Release kinetics of PTX from IR-AFN@PTX-FA.327 Reproduced with permission from ref. 327. Copyright 2021 Dove Medical Press. (d) The fabrication process and application of Au@MOF-DOX. (e) The viability rate of L929 cells incubated with Au@MOF at various Au concentrations.137 Reproduced with permission from ref. 137. Copyright 2019 American Chemical Society. (f) Synthesis of DG@TLs and its anti-tumor therapy. (g) Cumulative GA release from DG@TLs under 1064 nm laser.328 Reproduced with permission from ref. 328. Copyright 2021 Royal Society of Chemistry. | ||
Currently, encasing or loading CT prodrugs on nano-agents is a more advanced technological approach, which can be then transformed into CT drugs under NIR-II laser/TME. In this way, CT drugs can be transformed into effective molecular structures or drug monomers only at tumor site, which is more intelligent and avoids the toxicity of long-term circulation in the body. Cisplatin is the most commonly used CT drug in clinic and experiment, but its severe dose-limiting toxicity and drug resistance have greatly hindered its wider application. Tetravalent cisplatin (cispt(IV)) prodrug can be reduced by over-expressed GSH in TME and activated into more toxic cispt(II).329 Hyperthermia promotes the metabolism of cancer cells, thus further improving the intake of CT drugs. At present, numerous NIR-II PTT nanocarriers are employed for delivering cisplatin prodrugs for tumor treatment, such as ultrasmall CuS-modified Fe(III)-metal–organic frameworks (MIL-88),330 Pt(IV)-initiated photo-polymerized methacrylate gelatin (GelMA)-based hydrogel microparticles,331 NIR-II light excitable photothermal lipid NPs (Fig. 20a).332 Additionally, disulfiram (DSF) combined with Cu2+ is used in antitumor therapies. In the physiological environment, DSF is quickly metabolized into diethyldithiocarbamate (DTC), which forms a complex with Cu2+-bis (N,N-diethyldithiocarbamate) (Cu(DTC)2). This complex has been utilized in photonic hyperthermia to enhance DSF-initiated cancer CT (Fig. 20b).333 In addition, there are some strategies to realize tumor treatment by cutting off covalent bonds and releasing CT drugs under NIR-II laser. Lu et al. reported that co-encapsulating of the redox-responsive prodrug camptothecin-combretastatin A4 (CPT-CA4) with the DPP-BT-TPA dye.334 These NPs were specifically designed to respond to the high levels of GSH in the TME, facilitating the release of the CT drug (CPT) and the angiogenesis inhibitor CA4 (Fig. 20c). This release process could be expedited by elevated temperatures, utilizing laser-induced hyperthermia to control drug release and boost therapeutic outcomes. Zhu et al. developed a versatile nanoplatform that enhanced CT with phototherapy, encapsulating a poly-prodrug (PEG–TPZ) inside the semiconducting polymer TDPP (Fig. 20d).335 The platform released tirapazamine (TPZ) by hydrolyzing the acrylamide bonds within the TME. This release triggered damage to blood vessels, which was then intensified by the hyperthermia produced during treatment.
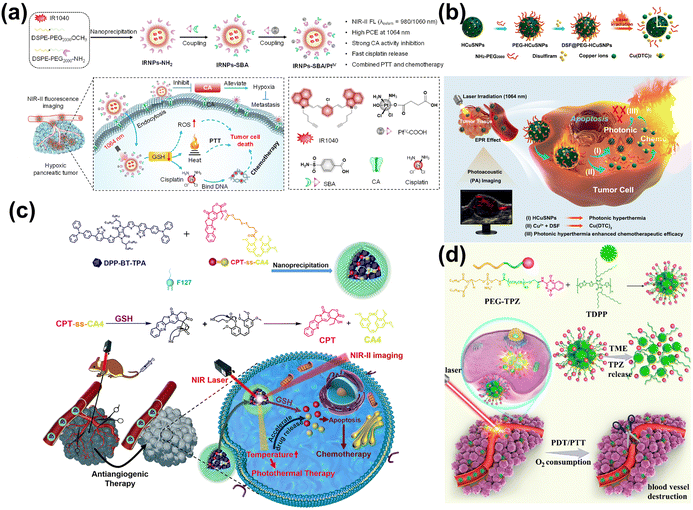 | ||
| Fig. 20 (a) The preparation of IRNPs-SBA/Pt IV and its mechanism for NIR-II FL-guided combined PTT and CT.332 Reproduced with permission from ref. 332. Copyright 2023 Elsevier. (b) The fabrication of DSF@PEG-HCuSNPs and the corresponding synergy of PTT and CT.333 Reproduced with permission from ref. 333. Copyright 2021 American Chemical Society. (c) The fabrication process and drug release mechanism of DCssC NPs.334 Reproduced with permission from ref. 334. Copyright 2022 Elsevier. (d) The formulation of TDPP@PEG–TPZ and its applications.335 Reproduced with permission from ref. 335. Copyright 2023 Royal Society of Chemistry. | ||
At present, a variety of CT drugs have transitioned from experimental research to clinical application, driven by the primary goal of benefiting cancer patients. The challenge of enhancing the effectiveness of these drugs while minimizing harm to normal cells remains a critical area of scientific inquiry. The integration of NIR-II PTT with CT drugs presents a promising solution. This combination therapy leverages the relative safety of mild PTT, additionally, it facilitates the controlled release of CT drugs or prodrugs, potentially improving treatment outcomes and minimizing side effects.
Heat shock protein (HSP) are a diverse group of proteins found extensively in bacteria and mammals, synthesized in response to high temperatures to protect the organism.336,337 Thus, the over-expression of HSP induced cell heat resistance will reduce the therapeutic effect of NIR-II PTT.338,339 In IO, activated T cells can secrete TNF-α, a cytokine that reduces the expression of HSPs in tumor cells, thereby increasing their sensitivity to photothermal stimulation.340,341 Yang et al. reported a NIR-II photothermal agent (BPBBT dots) coated with artificial dendritic cell (DC) membrane, this configuration preserved the antigen-presenting capability and enabled binding with endogenous T cells (Fig. 21a).342 Consequently, it promoted TNF-α secretion by T cells and diminished HSP expression, significantly enhancing the efficacy of NIR-II PTT. Du et al. reasonably designed the nano-epigenetic therapeutic platform (GNR-siYTH1), which is consisted of a nuclear localization signal peptide-conjugated PEI-PLA polymer-coated gold nanorod and small interfering RNA targeting YTHDF1 (Fig. 21b).343 This platform decreased the translation of HSP70, which decreased the thermo-resistance of tumor cells and enhanced photothermal performance. Additionally, reducing ATP production in tumor cells disrupted the energy supply needed for HSP production, thereby diminishing the thermal resistance of tumors to NIR-II PTT (Fig. 21c).344 Wei et al. constructed a facile biomimetic nano-immunoactivator (CuS/Z@M4T1), by incorporating NIR-II photothermal agent CuS nanodots and 4T1 tumor cell membrane into a ZIF-8 structure (Fig. 21d).345 The overloaded Zn2+ lead to NAD depletion and systematic energy exhaustion by disrupting the metabolic processes, thus causing a reduction in intratumoral ATP levels, inhibiting HSP synthesis and enhancing the therapeutic efficacy of NIR-II PTT.
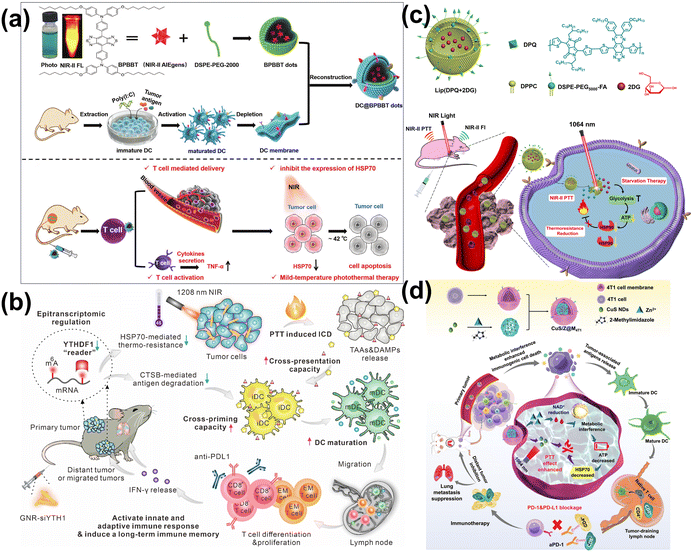 | ||
| Fig. 21 (a) The preparation and assembly of DC@BPBBT dots and its imaging-guided PTT.342 Reproduced with permission from ref. 342. Copyright 2022 John Wiley and Sons. (b) The preparation of N6-methyladenosine reader YTHDF1 siRNA loaded Au nanorods.343 Reproduced with permission from ref. 343. Copyright 2022 Elsevier. (c) Synthesis of Lip(DPQ + 2DG) NPs and its applications.344 Reproduced with permission from ref. 344. Copyright 2021 Elsevier. (d) The fabrication procedures and applications of the CuS/Z@M 4T1.345 Reproduced with permission from ref. 345. Copyright 2023 John Wiley and Sons. | ||
The combination of NIR-II PTT and IO is a two-way promotion effect. In addition to reducing the expression of HSP and weakening the heat insensitivity of tumor cells, the heat generated by NIR-II PTT can improve the immunogenicity of tumors and create a favorable niche for tumor-infiltrating lymphocytes (TIL) recruitment, thus promoting the transformation of “cold” tumors (non-immunogenic tumors) into “hot” tumors (immunogens with increased T cell infiltration) in immune checkpoint blockade (ICB) therapy.346–348 Wang et al. reported a AuPtAg-GOx nanozyme, which could produce mild PTT under 1064 nm laser and recruitment of TILs to reprogram the “cold” TME.349 Besides, the α-PD-L1 on the AuPtAg-GOx could effectively inhibit both primary and distant tumors, thus enhancing the potent tumor treatment capabilities of NIR-II PTT when combined with IO. Furthermore, NIR-II PTT can induce dying tumor cells to release tumor-associated antigens (TAAs), leading to immunogenic cell death (ICD) and thereby activating the immune response. At present, a range of nano-agents have been developed specifically for treating photothermal-induced immunogenic death, such as semiconducting polymer (SP) grafted with imiquimod R837 and indoximod IND (SPRIN-micelle),350 tumor cell membrane coated redox nanozyme (CMO-R@4T1),351 semiconducting polymer nano engager (SPNE),352 Ag@CuS-TPP@HA NPs,353 GdOF@PDA-HA-R837-hydrogel complex,354 theragnostic thermosensitive liposome (PLDD) which contained a multifunctional photothermal agent (DTTB) and an immune potentiator STING pathway agonist (DMXAA),355 NIR-II light-activated semiconducting polymer nano mediators (SPNaiB).356
This approach effectively facilitates the release of cellular contents and inflammatory cytokines, and enhances the anti-tumor immune response by synergizing with cell death induced by NIR-II PTT. The human immune system can leverage these effects to trigger a cascade of immune reactions, effectively eradicating tumor cells. This combination therapy offers benefits such as low side effects, lack of drug resistance, and broad-spectrum anti-cancer properties. Looking ahead, the development of various vaccine adjuvants that harness the NIR-II photothermal effect to prevent or treat tumor diseases represents a promising, well-tolerated, and effective treatment strategy for patients.
5. Challenges and future prospects
Although NIR-II optical imaging and phototherapy hold great potential, they face several challenges. The complexity of biological tissues—affected by factors such as absorption, scattering, and blood content—may hinder the effectiveness of NIR-II imaging and treatment. Furthermore, the stability and biocompatibility of nano-agents, which are crucial for NIR-II diagnostics and therapy, need enhancement to avoid immune reactions or tissue damage. Additionally, accurately targeting nano-agents to lesions and finely tuning phototherapy performance to ensure safety and efficacy remain challenging.In future development, researchers can improve the effectiveness of NIR-II optical diagnosis and treatment integration nanoplatforms in three main aspects: (1) design and optimization of nano-agents: by controlling their morphology, surface functionalization, and structural stability. Nano-agents can be tailored for targeted delivery and tracking, serving not only as precise diagnostic and therapeutic tools but also as drug carriers with controlled release capabilities. (2) By integrating NIR-II optical imaging with other modalities like MRI or CT and employing techniques such as biomarkers and immunohistochemical labeling, imaging accuracy and detail can be significantly improved, thus better guiding treatment planning and adjustment. (3) Precise control of NIR-II phototherapy temporal-spatial light response mechanism: developing optical-controlled drug release systems within the NIR-II window could enable precise drug delivery and phototherapy dose regulation, maximizing therapeutic outcomes while minimizing side effects.
Moreover, as NIR-II optical diagnosis and treatment integration nanoplatforms progress towards clinical application, creating personalized treatment plans represents a vital direction for future clinical transformation. By leveraging the deep tissue penetration of NIR-II light combined with the precision of localized phototherapy, personalized treatment plans can significantly enhance treatment efficacy and patient quality of life.
6. Conclusion
In summary, the construction of NIR-II optical diagnosis and treatment integration nanoplatforms using a variety of nano-agents with outstanding optical properties—such as high FL intensity, strong PA imaging signals, efficient PCE, significant ROS production, and targeted CT drug release—facilitates multiple phototherapy mechanisms guided by NIR-II FL and PA imaging. This approach substantially addresses the limitations of poor tissue penetration, high tissue absorption and scattering, and low spatial and temporal resolution associated with the visible and NIR-I windows. Consequently, this emerging platform will open new vistas of cancer diagnosis and treatment in the future.Author contributions
Conceptualization, H.M. L., P.J. L., and H.Y. S.; visualization, H.M. L. and J.R. Z.; investigation, Z.Y. L., L.T. B.; methodology, H.M. L. and H.Y. S.; writing—original draft, H.M. L., P.J. L. and J.R. Z.; writing—review & editing, H.M. L. and H.Y. S. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Major Research Plan of the National Natural Science Foundation of China (92359202), the Beijing Natural Science Foundation (L212001), the National Natural Science Foundation of China (51972343).References
- M. Zhang, Y. Zhang, L. Hang, T. Zhang, C. Luo, W. Li, Y. Sun, H. Wen, Y. Chen, G. Jiang and X. Ma, Nanoscale, 2024, 16, 6095–6108 RSC.
- K. Du, S. Zhao, J. Feng, X. Gao, K. Liu, X. Wang, M. Zhang, Y. Li, Y. Lu and H. Zhang, J. Mater. Chem. B, 2021, 9, 7216–7228 RSC.
- Y. Luo, S. Wang, J. Zhao, F. Ye, S. Zhao, S. Hu and L. Zhang, ACS Appl. Mater. Interfaces, 2024, 16, 25879–25891 CrossRef CAS PubMed.
- C. Dong, Z. Zhang, H. Wu, X. Liang, S. Pang, K. Wu, J. Sun, X. Dong, L. Sun, X. Gu and C. Zhao, Chem. Sci., 2024, 15, 10969–10979 RSC.
- W. Pan, M. Rafiq, W. Haider, Y. Guo, H. Wang, M. Xu, B. Yu, H. Cong and Y. Shen, Coord. Chem. Rev., 2024, 514, 215907 CrossRef CAS.
- Z. Hu, C. Fang, B. Li, Z. Zhang, C. Cao, M. Cai, S. Su, X. Sun, X. Shi, C. Li, T. Zhou, Y. Zhang, C. Chi, P. He, X. Xia, Y. Chen, S. S. Gambhir, Z. Cheng and J. Tian, Nat. Biomed. Eng., 2019, 4, 259–271 CrossRef PubMed.
- A. M. Smith, M. C. Mancini and S. Nie, Nat. Nanotechnol., 2009, 4, 710–711 CrossRef CAS PubMed.
- M. Zhang, J. Yue, R. Cui, Z. Ma, H. Wan, F. Wang, S. Zhu, Y. Zhou, Y. Kuang, Y. Zhong, D.-W. Pang and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 6590–6595 CrossRef CAS PubMed.
- Q. Jiang, J. Li, Z. Du, M. Li, L. Chen, X. Zhang, X. Tang, Y. Shen, D. Ma, W. Li, L. Li, N. Alifu, Q. Hu and J. Liu, Adv. Healthcare Mater., 2024, 2400962 CrossRef CAS PubMed.
- Y. Chu, X.-Q. Xu and Y. Wang, J. Phys. Chem. Lett., 2022, 13, 9564–9572 CrossRef CAS PubMed.
- B. Huang, T. Tang, F. Liu, S.-H. Chen, Z.-L. Zhang, M. Zhang and R. Cui, Chin. Chem. Lett., 2024, 109694 CrossRef CAS.
- Y. Wu, D. Hu, D. Gao, C. Liu, H. Zheng and Z. Sheng, Adv. Healthcare Mater., 2022, 11, 2202379 CrossRef CAS PubMed.
- X. Sun, Y. Li, X. Liu, D. Cui, Y. Shi and G. Huang, J. Nanobiotechnol., 2024, 22, 326 CrossRef CAS PubMed.
- W. Zhou, D. D. He, K. Zhang, N. Liu, Y. Li, W. Han, W. Zhou, M. Li, S. Zhang, H. Huang and C. Yu, Biosens. Bioelectron., 2024, 259, 116424 CrossRef CAS PubMed.
- Z. Liu, C. Hong, C. Pan, Y. Sun, Y. Lv, Y. Wei, X. Wang, W. Zang, Q. Mao, X. Deng, P. Wang, W. Zhu, T. Chen, M. Wu, J. Li and A. Wu, ACS Mater. Lett., 2024, 6, 1593–1605 CrossRef CAS.
- P. Sun, D. Hu, P. Chen, X. Wang, Q. Shen, S. Chen, D. Li and Q. Fan, Adv. Sci., 2024, 2309446 CrossRef CAS PubMed.
- H. Zhao, Z. Wang, X. He, J. Li, K. Chen, Y. Pan, W. Hu, Q. Fan and Q. Shen, ACS Appl. Nano Mater., 2024, 7, 12053–12063 CrossRef CAS.
- X. Zhang, D. Li, W. Wang, X. Zheng, C. Zhang, Y. Jin, S. Meng, J. Li, R. Dai, W. Kang, H. Wu, Z. Zheng and R. Zhang, Mater. Today Bio, 2024, 26, 101052 CrossRef CAS PubMed.
- Z. Huang, J. Song, S. Huang, S. Wang, C. Shen, S. Song, J. Lian, Y. Ding, Y. Gong, Y. Zhang, A. Yuan, Y. Hu, C. Tan, Z. Luo and L. Wang, Nano Lett., 2024, 24, 7764–7773 CrossRef CAS PubMed.
- M. Zhao and X. Chen, Acc Mater. Res., 2024, 5, 600–613 CrossRef CAS.
- S. H. Chen, H. Liu, B. Huang, J. Zheng, Z. L. Zhang, D. W. Pang, P. Huang and R. Cui, Small, 2024, 20, 2310795 CrossRef CAS PubMed.
- C. Ma, J. M. Mohr, G. Lauer, J. T. Metternich, K. Neutsch, T. Ziebarth, A. Reiner and S. Kruss, Nano Lett., 2024, 24, 2400–2407 CrossRef CAS PubMed.
- L. Chio, R. L. Pinals, A. Murali, N. S. Goh and M. P. Landry, Adv. Funct. Mater., 2020, 30, 1910556 CrossRef CAS.
- M. J. O'Connell, S. M. Bachilo, C. B. Huffman, V. C. Moore, M. S. Strano, E. H. Haroz, K. L. Rialon, P. J. Boul, W. H. Noon, C. Kittrell, J. Ma, R. H. Hauge, R. B. Weisman and R. E. Smalley, Science, 2002, 297, 593–596 CrossRef PubMed.
- S. Diao, J. L. Blackburn, G. Hong, A. L. Antaris, J. Chang, J. Z. Wu, B. Zhang, K. Cheng, C. J. Kuo and H. Dai, Angew. Chem., Int. Ed., 2015, 54, 14758–14762 CrossRef CAS PubMed.
- Y. Nagai, R. Hamano, K. Nakamura, I. A. Widjaja, N. Tanaka, M. Zhang, T. Tanaka, H. Kataura, M. Yudasaka and T. Fujigaya, Carbon, 2024, 218, 118728 CrossRef CAS.
- A. G. Godin, J. A. Varela, Z. Gao, N. Danné, J. P. Dupuis, B. Lounis, L. Groc and L. Cognet, Nat. Nanotechnol., 2016, 12, 238–243 CrossRef PubMed.
- K. Welsher, Z. Liu, S. P. Sherlock, J. T. Robinson, Z. Chen, D. Daranciang and H. Dai, Nat. Nanotechnol., 2009, 4, 773–780 CrossRef CAS PubMed.
- L. Muzi, F. Tardani, C. La Mesa, A. Bonincontro, A. Bianco and G. Risuleo, Nanotechnology, 2016, 27, 155704 CrossRef PubMed.
- B. D. Holt, K. N. Dahl and M. F. Islam, Small, 2011, 7, 2348–2355 CrossRef CAS PubMed.
- N. Lu, Y. Sui, Y. Ding, R. Tian and Y.-Y. Peng, J. Mater. Sci.: Mater. Med., 2018, 29, 115 CrossRef PubMed.
- T. Shiraki, Y. Miyauchi, K. Matsuda and N. Nakashima, Acc. Chem. Res., 2020, 53, 1846–1859 CrossRef CAS PubMed.
- C. Ma, C. A. Schrage, J. Gretz, A. Akhtar, L. Sistemich, L. Schnitzler, H. Li, K. Tschulik, B. S. Flavel and S. Kruss, ACS Nano, 2023, 17, 15989–15998 CrossRef CAS PubMed.
- J. Zaumseil, Adv. Opt. Mater., 2021, 10, 2101576 CrossRef.
- Y. Piao, B. Meany, L. R. Powell, N. Valley, H. Kwon, G. C. Schatz and Y. Wang, Nat. Chem., 2013, 5, 840–845 CrossRef CAS PubMed.
- F. J. Berger, J. Lüttgens, T. Nowack, T. Kutsch, S. Lindenthal, L. Kistner, C. C. Müller, L. M. Bongartz, V. A. Lumsargis, Y. Zakharko and J. Zaumseil, ACS Nano, 2019, 13, 9259–9269 CrossRef CAS PubMed.
- S. Settele, C. A. Schrage, S. Jung, E. Michel, H. Li, B. S. Flavel, A. S. K. Hashmi, S. Kruss and J. Zaumseil, Nat. Commun., 2024, 15, 706 CrossRef CAS PubMed.
- A. Borel, F. Rapisarda, S. K. Doorn, C. Voisin and Y. Chassagneux, Nano Lett., 2024, 24, 3456–3461 CrossRef CAS PubMed.
- H. Kwon, A. o. Furmanchuk, M. Kim, B. Meany, Y. Guo, G. C. Schatz and Y. Wang, J. Am. Chem. Soc., 2016, 138, 6878–6885 CrossRef CAS PubMed.
- S. Han, N. T. Hung, Y. Xie, R. Saito, J. Zhang and L. Tong, Nano Lett., 2023, 23, 8454–8459 CrossRef CAS PubMed.
- R. Nißler, L. Kurth, H. Li, A. Spreinat, I. Kuhlemann, B. S. Flavel and S. Kruss, Anal. Chem., 2021, 93, 6446–6455 CrossRef PubMed.
- F. Yang, M. Wang, D. Zhang, J. Yang, M. Zheng and Y. Li, Chem. Rev., 2020, 120, 2693–2758 CrossRef CAS PubMed.
- H. Li, C. M. Sims, R. Kang, F. Biedermann, J. A. Fagan and B. S. Flavel, Carbon, 2023, 204, 475–483 CrossRef CAS.
- Y. Yomogida, T. Tanaka, M. Zhang, M. Yudasaka, X. Wei and H. Kataura, Nat. Commun., 2016, 7, 12056 CrossRef CAS PubMed.
- B. S. Flavel, K. E. Moore, M. Pfohl, M. M. Kappes and F. Hennrich, ACS Nano, 2014, 8, 1817–1826 CrossRef CAS PubMed.
- X. Wei, T. Tanaka, T. Hirakawa, M. Tsuzuki, G. Wang, Y. Yomogida, A. Hirano and H. Kataura, Carbon, 2018, 132, 1–7 CrossRef CAS.
- X. Tu, S. Manohar, A. Jagota and M. Zheng, Nature, 2009, 460, 250–253 CrossRef CAS PubMed.
- P. Zhao, E. Einarsson, G. Lagoudas, J. Shiomi, S. Chiashi and S. Maruyama, Nano Res., 2011, 4, 623–634 CrossRef CAS.
- Y. Zhang, M. Wang, D. Li, H. Zhao and Y. Li, ACS Appl. Nano Mater., 2019, 2, 5440–5449 CrossRef CAS.
- R. Langenbacher, J. Budhathoki-Uprety, P. V. Jena, D. Roxbury, J. Streit, M. Zheng and D. A. Heller, Nano Lett., 2021, 21, 6441–6448 CrossRef CAS PubMed.
- A. M. Smith and S. Nie, Acc. Chem. Res., 2010, 43, 190–200 CrossRef CAS PubMed.
- K. Wu, G. Liang, Q. Shang, Y. Ren, D. Kong and T. Lian, J. Am. Chem. Soc., 2015, 137, 12792–12795 CrossRef CAS PubMed.
- I. Swart, Z. Sun, D. Vanmaekelbergh and P. Liljeroth, Nano Lett., 2010, 10, 1931–1935 CrossRef CAS PubMed.
- Q. N. D. Lung, R. J. Chu, Y. Kim, T. Laryn, M. A. Madarang, O. Kovalchuk, Y.-W. Song, I.-H. Lee, C. Choi, W. J. Choi and D. Jung, Nano Lett., 2023, 23, 3344–3351 CrossRef CAS PubMed.
- H. Van Avermaet, P. Schiettecatte, S. Hinz, L. Giordano, F. Ferrari, C. Nayral, F. Delpech, J. Maultzsch, H. Lange and Z. Hens, ACS Nano, 2022, 16, 9701–9712 CrossRef CAS PubMed.
- K. Wang, K.-H. Deng, Y.-S. Tian, M.-Y. Sun, Z.-l. Yu, Z.-Q. Tian and Z.-L. Zhang, ACS Appl. Nano Mater., 2023, 6, 14289–14299 CrossRef CAS.
- H. Zhang, C. Sun, L. Sun, W. Xu, W. Wu, J. Chen, B. Wang, J. Yu, P. Cui, F. Zhang and Y. Tang, Angew. Chem., Int. Ed., 2022, 61, e202203851 CrossRef CAS PubMed.
- H. Yang, R. Li, Y. Zhang, M. Yu, Z. Wang, X. Liu, W. You, D. Tu, Z. Sun, R. Zhang, X. Chen and Q. Wang, J. Am. Chem. Soc., 2021, 143, 2601–2607 CrossRef CAS PubMed.
- Y. Zhang, Y. Zhang, Y. Li, Y. Fu, Y. Zhao, W. Zhao, R. Li, Y. Xian, K. Tu, F. Wu, C. Li, Y. Hou and M. Zhang, Chem. Eng. J., 2023, 474, 145685 CrossRef CAS.
- P. Zhang, Y. Wang, X. Liu, L. Yuan, J. Liu, R. Guo and Y. Tian, ACS Appl. Mater. Interfaces, 2024, 16, 8509–8517 CrossRef CAS PubMed.
- H. Li, Z. Du, L. Zhu, C. Zhang, J. Xiong, B. Zhou, B. Dong, X. Zhang and N. Alifu, ACS Appl. Mater. Interfaces, 2024, 16, 32045–32057 CrossRef CAS PubMed.
- F. Wang, F. Ren, Z. Ma, L. Qu, R. Gourgues, C. Xu, A. Baghdasaryan, J. Li, I. E. Zadeh, J. W. N. Los, A. Fognini, J. Qin-Dregely and H. Dai, Nat. Nanotechnol., 2022, 17, 653–660 CrossRef CAS PubMed.
- M. Yu, X. Yang, Y. Zhang, H. Yang, H. Huang, Z. Wang, J. Dong, R. Zhang, Z. Sun, C. Li and Q. Wang, Small, 2021, 17, 2006111 CrossRef CAS PubMed.
- Y. Zhou, B. Huang, S.-H. Chen, S.-L. Liu, M. Zhang and R. Cui, Nano Res., 2022, 16, 2719–2727 CrossRef.
- X. Shi, S. Chen, M.-Y. Luo, B. Huang, G. Zhang, R. Cui and M. Zhang, Nano Res., 2020, 13, 2239–2245 CrossRef CAS.
- Y. Shu, J. Yan, Q. Lu, Z. Ji, D. Jin, Q. Xu and X. Hu, Sens. Actuators, B, 2020, 307, 127593 CrossRef.
- Y. Yao, T. Zhang and M. Tang, Environ. Pollut., 2023, 317, 120676 CrossRef CAS PubMed.
- T. Wei, T. Zhang and M. Tang, Environ. Pollut., 2022, 311, 119865 CrossRef CAS PubMed.
- A. Zebibula, N. Alifu, L. Xia, C. Sun, X. Yu, D. Xue, L. Liu, G. Li and J. Qian, Adv. Funct. Mater., 2017, 28, 1703451 CrossRef.
- P. Awasthi, X. An, J. Xiang, N. Kalva, Y. Shen and C. Li, Nanoscale, 2020, 12, 5678–5684 RSC.
- B. Li, G. Wang, Y. Tong, Y. Zhang, S.-K. Sun and C. Yu, ACS Biomater. Sci. Eng., 2022, 9, 449–457 CrossRef PubMed.
- L. Yang, M. Yuan, P. a. Ma, X. Chen, Z. Cheng and J. Lin, Small Methods, 2023, 7, 2201706 CrossRef CAS PubMed.
- H. Xiong, S. Shao, Y. Yang, W. Wang, H. Xiong, Y. Han, Z. Wang, X. Hu, L. Zeng, Z. Yang and T. Su, Biomed. Pharmacother., 2024, 170, 116011 CrossRef CAS PubMed.
- J. J. Zhang, Y. Lin, H. Zhou, H. He, J. J. Ma, M. Y. Luo, Z. L. Zhang and D. W. Pang, Adv. Healthcare Mater., 2019, 8, 1900341 CrossRef PubMed.
- F. Lu, W. Ju, N. Zhao, T. Zhao, C. Zhan, Q. Wang, Q. Fan and W. Huang, Biochem. Biophys. Res. Commun., 2020, 529, 930–935 CrossRef CAS PubMed.
- R. Tian, H. Ma, S. Zhu, J. Lau, R. Ma, Y. Liu, L. Lin, S. Chandra, S. Wang, X. Zhu, H. Deng, G. Niu, M. Zhang, A. L. Antaris, K. S. Hettie, B. Yang, Y. Liang and X. Chen, Adv. Mater., 2020, 32, 1907365 CrossRef CAS PubMed.
- T. Jia and G. Chen, Coord. Chem. Rev., 2022, 471, 214724 CrossRef CAS.
- A. Puccini, N. Liu and E. Hemmer, ACS Mater. Lett., 2024, 6, 1327–1337 CrossRef CAS.
- M. Zhao, R. Wang, B. Li, Y. Fan, Y. Wu, X. Zhu and F. Zhang, Angew. Chem., Int. Ed., 2019, 58, 2050–2054 CrossRef CAS PubMed.
- S. Cheng, L. Liu, Q. Yang, Y. Li and S. Zeng, J. Rare Earths, 2019, 37, 931–936 CrossRef CAS.
- Q. Wang, H. Shangguan, H. Yu, X. Rong, B. Zhou, Z. Tang, C. Li, S. Liu, Y. Lu and J. Xu, Nano Lett., 2024, 24, 2876–2884 CrossRef CAS PubMed.
- H. Li, X. Wang, T. Y. Ohulchanskyy and G. Chen, Adv. Mater., 2020, 33, 2000678 CrossRef PubMed.
- X. Zheng, Y. Du, Z. Dang, K. Lan, G. Liu, J. Ren, X. Wang and S. Zhu, Adv. Funct. Mater., 2024, 34, 2313278 CrossRef CAS.
- Y. Xu, H. Liang, Q. Zeng, F. He, C. Liu, S. Gai, H. Ding and P. Yang, J. Colloid Interface Sci., 2024, 659, 149–159 CrossRef CAS PubMed.
- Y. Zhan, P. Yu, X. Wang, Y. Xie, H. Zhang and F. Zhang, Adv. Funct. Mater., 2023, 33, 2301683 CrossRef CAS.
- H. Zhang, Y. Fan, P. Pei, C. Sun, L. Lu and F. Zhang, Angew. Chem., Int. Ed., 2019, 58, 10153–10157 CrossRef CAS PubMed.
- M. Zhao, B. Li, Y. Wu, H. He, X. Zhu, H. Zhang, C. Dou, L. Feng, Y. Fan and F. Zhang, Adv. Mater., 2020, 32, 2001172 CrossRef CAS PubMed.
- M. Zhang, W. Zheng, Y. Liu, P. Huang, Z. Gong, J. Wei, Y. Gao, S. Zhou, X. Li and X. Chen, Angew. Chem., Int. Ed., 2019, 58, 9556–9560 CrossRef CAS PubMed.
- H. Zhu, X. Ding, C. Wang, M. Cao, B. Yu, H. Cong and Y. Shen, J. Mater. Chem. B, 2024, 12, 1947–1972 RSC.
- G. Chen, H. Qiu, P. N. Prasad and X. Chen, Chem. Rev., 2014, 114, 5161–5214 CrossRef CAS PubMed.
- J.-C. G. Bünzli, Chem. Rev., 2010, 110, 2729–2755 CrossRef PubMed.
- K. Malhotra, D. Hrovat, B. Kumar, G. Qu, J. V. Houten, R. Ahmed, P. A. E. Piunno, P. T. Gunning and U. J. Krull, ACS Appl. Mater. Interfaces, 2023, 15, 2499–2528 CrossRef CAS PubMed.
- X. Zhu, H. Zhang and F. Zhang, ACS Mater. Lett., 2022, 4, 1815–1830 CrossRef CAS.
- Z. Zhuo, Y. Liu, D. Liu, P. Huang, F. Jiang, X. Chen and M. Hong, Chem. Sci., 2017, 8, 5050–5056 RSC.
- X. Wang, R. R. Valiev, T. Y. Ohulchanskyy, H. Ågren, C. Yang and G. Chen, Chem. Soc. Rev., 2017, 46, 4150–4167 RSC.
- Y. Fan, L. Liu and F. Zhang, Nano Today, 2019, 25, 68–84 CrossRef CAS.
- J. Huang, J. Li, X. Zhang, W. Zhang, Z. Yu, B. Ling, X. Yang and Y. Zhang, Nano Lett., 2020, 20, 5236–5242 CrossRef CAS PubMed.
- Y. Fan and F. Zhang, Adv. Opt. Mater., 2019, 7, 1801417 CrossRef.
- X. Wang, J. Shi, P. Li, S. Zheng, X. Sun and H. Zhang, J. Lumin., 2019, 209, 420–426 CrossRef CAS.
- Z.-f. Yu, J.-p. Shi, J.-l. Li, P.-h. Li and H.-w. Zhang, J. Mater. Chem. B, 2018, 6, 1238–1243 RSC.
- Y. Fan, P. Wang, Y. Lu, R. Wang, L. Zhou, X. Zheng, X. Li, J. A. Piper and F. Zhang, Nat. Nanotechnol., 2018, 13, 941–946 CrossRef CAS PubMed.
- X. Zhu, H. Zhang and F. Zhang, Acc Mater. Res., 2023, 4, 536–547 CrossRef CAS.
- H. Li, J. Bai, Y. Chen, C. Du, M. Chen and J. Wang, Small, 2024, 2309955 CrossRef CAS PubMed.
- Y. Guo, J. Hu, P. Wang, H. Yang, S. Liang, D. Chen, K. Xu, Y. Huang, Q. Wang, X. Liu and H. Zhu, Small, 2023, 19, 2300392 CrossRef CAS PubMed.
- Z. H. Chen, X. Wang, M. Yang, J. Ming, B. Yun, L. Zhang, X. Wang, P. Yu, J. Xu, H. Zhang and F. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202311883 CrossRef CAS PubMed.
- Y. Y. Cai, Y. C. Choi and C. R. Kagan, Adv. Mater., 2022, 35, 2108104 CrossRef PubMed.
- E. L. Albright, T. I. Levchenko, V. K. Kulkarni, A. I. Sullivan, J. F. DeJesus, S. Malola, S. Takano, M. Nambo, K. Stamplecoskie, H. Häkkinen, T. Tsukuda and C. M. Crudden, J. Am. Chem. Soc., 2024, 146, 5759–5780 CrossRef CAS PubMed.
- G. Habibullah, J. Viktorova and T. Ruml, Nanoscale Res. Lett., 2021, 16, 47 CrossRef CAS PubMed.
- Y. Xiao, Z. Wu, Q. Yao and J. Xie, Aggregate, 2021, 2, 114–132 CrossRef CAS.
- Q. Yao, T. Chen, X. Yuan and J. Xie, Acc. Chem. Res., 2018, 51, 1338–1348 CrossRef CAS PubMed.
- Q. Yao, Z. Wu, Z. Liu, Y. Lin, X. Yuan and J. Xie, Chem. Sci., 2021, 12, 99–127 RSC.
- F. Xu, T. Qing and Z. Qing, Nano Today, 2021, 36, 101021 CrossRef CAS.
- Á. Turcsányi, D. Ungor, M. Wojnicki and E. Csapó, J. Mol. Liq., 2023, 370, 121002 CrossRef.
- T. Jia, Z.-J. Guan, C. Zhang, X.-Z. Zhu, Y.-X. Chen, Q. Zhang, Y. Yang and D. Sun, J. Am. Chem. Soc., 2023, 145, 10355–10363 CrossRef CAS PubMed.
- Z. Liu, L. Luo and R. Jin, Adv. Mater., 2024, 36, 2309073 CrossRef CAS PubMed.
- S. Zhou, L. Gustavsson, G. Beaune, S. Chandra, J. Niskanen, J. Ruokolainen, J. V. I. Timonen, O. Ikkala, B. Peng and R. H. A. Ras, Angew. Chem., Int. Ed., 2023, 62, e202312679 CrossRef CAS PubMed.
- S. Yi, Q. Hu, Y. Chi, H. Qu and Y. Xiao, ACS Mater. Lett., 2023, 5, 2164–2173 CrossRef CAS.
- M. Liang, Q. Hu, S. Yi, Y. Chi and Y. Xiao, Biosens. Bioelectron., 2023, 224, 115062 CrossRef CAS PubMed.
- H. Liu, G. Hong, Z. Luo, J. Chen, J. Chang, M. Gong, H. He, J. Yang, X. Yuan, L. Li, X. Mu, J. Wang, W. Mi, J. Luo, J. Xie and X. D. Zhang, Adv. Mater., 2019, 31, 1901015 CrossRef CAS PubMed.
- K. Qiu, A. Yadav, Z. Tian, Z. Guo, D. Shi, C. K. Nandi and J. Diao, ACS Mater. Lett., 2022, 4, 1565–1573 CrossRef CAS.
- D. Li, Q. Liu, Q. Qi, H. Shi, E. C. Hsu, W. Chen, W. Yuan, Y. Wu, S. Lin, Y. Zeng, Z. Xiao, L. Xu, Y. Zhang, T. Stoyanova, W. Jia and Z. Cheng, Small, 2020, 16, 2003851 CrossRef CAS PubMed.
- D. Li, Z. Chen and X. Mei, Adv. Colloid Interface Sci., 2017, 250, 25–39 CrossRef CAS PubMed.
- L. Fu, X. Gao, S. Dong, H.-Y. Hsu and G. Zou, Anal. Chem., 2021, 93, 4909–4915 CrossRef CAS PubMed.
- X.-H. Ma, J.-T. Jia, P. Luo, Z.-Y. Wang, S.-Q. Zang and T. C. W. Mak, Nano Res., 2022, 15, 5569–5574 CrossRef CAS.
- W. Wang, Y. Kong, J. Jiang, Q. Xie, Y. Huang, G. Li, D. Wu, H. Zheng, M. Gao, S. Xu, Y. Pan, W. Li, R. Ma, M. X. Wu, X. Li, H. Zuilhof, X. Cai and R. Li, Angew. Chem., Int. Ed., 2020, 59, 22431–22435 CrossRef CAS PubMed.
- A. Chen, B. Yin, B. Huang, Y. Liu, S. Chen, Y. Pei and M. Zhu, J. Phys. Chem. Lett., 2022, 13, 4139–4144 CrossRef CAS PubMed.
- M. Guo, G. Zhang, R. Zhao, H. Ma, Y. Yan, S. Yang, J. Meng, Y. Huang, X.-D. Zhang, H. Wang and R. Zhang, ACS Appl. Nano Mater., 2023, 6, 15945–15958 CrossRef CAS.
- Y. Zhong, J. Zhang, T. Li, W. Xu, Q. Yao, M. Lu, X. Bai, Z. Wu, J. Xie and Y. Zhang, Nat. Commun., 2023, 14, 658 CrossRef CAS PubMed.
- H.-H. Deng, X.-Q. Shi, F.-F. Wang, H.-P. Peng, A.-L. Liu, X.-H. Xia and W. Chen, Chem. Mater., 2017, 29, 1362–1369 CrossRef CAS.
- Y. Peng, L. Gao, G. Pidamaimaiti, D. Zhao, L. Zhang, G. Yin and F. Wang, Nanoscale, 2022, 14, 8342–8348 RSC.
- G. Yang, X. Pan, W. Feng, Q. Yao, F. Jiang, F. Du, X. Zhou, J. Xie and X. Yuan, ACS Nano, 2023, 17, 15605–15614 CrossRef CAS PubMed.
- Q. Chen, J. Chen, Z. Yang, L. Zhang, Z. Dong and Z. Liu, Nano Res., 2018, 11, 5657–5669 CrossRef CAS.
- Q. Dan, Z. Yuan, S. Zheng, H. Ma, W. Luo, L. Zhang, N. Su, D. Hu, Z. Sheng and Y. Li, Pharmaceutics, 2022, 14, 1645 CrossRef CAS PubMed.
- Y. Yao, N. Li, X. Zhang, J. Ong'achwa Machuki, D. Yang, Y. Yu, J. Li, D. Tang, J. Tian and F. Gao, ACS Appl. Mater. Interfaces, 2019, 11, 13991–14003 CrossRef CAS PubMed.
- Y. Yu, J. Geng, E. Y. X. Ong, V. Chellappan and Y. N. Tan, Adv. Healthcare Mater., 2016, 5, 2528–2535 CrossRef CAS PubMed.
- M. Wang, X. Zhang, Q. Chang, H. Zhang, Z. Zhang, K. Li, H. Liu, D. Liu, L. An and Q. Tian, Acta Biomater., 2023, 168, 606–616 CrossRef PubMed.
- X. Deng, S. Liang, X. Cai, S. Huang, Z. Cheng, Y. Shi, M. Pang, P. a. Ma and J. Lin, Nano Lett., 2019, 19, 6772–6780 CrossRef CAS PubMed.
- Y. Zhang, T. Song, T. Feng, Y. Wan, N. T. Blum, C. Liu, C. Zheng, Z. Zhao, T. Jiang, J. Wang, Q. Li, J. Lin, L. Tang and P. Huang, Nano Today, 2020, 35, 100987 CrossRef CAS.
- Y. Lu and M. Li, Adv. Funct. Mater., 2024, 34, 2312753 CrossRef CAS.
- S. M. Fothergill, C. Joyce and F. Xie, Nanoscale, 2018, 10, 20914–20929 RSC.
- Y. Gao, J. Wang, W. Wang, T. Zhao, Y. Cui, P. Liu, S. Xu and X. Luo, Anal. Chem., 2021, 93, 2480–2489 CrossRef CAS PubMed.
- J. Xu, M. Fu, C. Ji, A. Centeno, D. K. Kim, K. Evers, S. E. M. Heutz, R. Oulton, M. P. Ryan and F. Xie, Adv. Opt. Mater., 2023, 11, 2300477 CrossRef CAS.
- D. Cui, Y. Shi, D. Xing and S. Yang, Nano Lett., 2021, 21, 6914–6922 CrossRef CAS PubMed.
- C.-c. Jian, J. Zhang and X. Ma, RSC Adv., 2020, 10, 13277–13285 RSC.
- B. Shan, L. Li, Y. Zhao, H. Wang and M. Li, Adv. Funct. Mater., 2021, 31, 2103186 CrossRef CAS.
- Y. Zhang, Q. Shen, Q. Li, P. He, J. Li, F. Huang, J. Wang, Y. Duan, C. Shen, F. Saleem, Z. Luo and L. Wang, Adv. Sci., 2021, 8, 2100386 CrossRef CAS PubMed.
- S. He, J. Song, J. Qu and Z. Cheng, Chem. Soc. Rev., 2018, 47, 4258–4278 RSC.
- N. Li, M. Wang, J. Zhou, Z. Wang, L. Cao, J. Ye and G. Sun, Eur. J. Med. Chem., 2024, 267, 116173 CrossRef CAS PubMed.
- S. Wang, X. Zhang, Y. Zhang, K. Zhu, X. Liu, J. Zhang, G. Wang, D. Liu, K. Zhao, X. Wang, J. Chen and J. Song, Adv. Opt. Mater., 2023, 12, 2302796 CrossRef.
- X. Ge, Q. Fu, L. Su, Z. Li, W. Zhang, T. Chen, H. Yang and J. Song, Theranostics, 2020, 10, 4809–4821 CrossRef CAS PubMed.
- T. Chen, L. Su, X. Ge, W. Zhang, Q. Li, X. Zhang, J. Ye, L. Lin, J. Song and H. Yang, Nano Res., 2020, 13, 3268–3277 CrossRef CAS.
- Y. Chen, S. Wang and F. Zhang, Nat. Rev. Bioeng., 2023, 1, 60–78 CrossRef.
- J. Zhao, D. Zhong and S. Zhou, J. Mater. Chem. B, 2018, 6, 349–365 RSC.
- P. A. Shaw, E. Forsyth, F. Haseeb, S. Yang, M. Bradley and M. Klausen, Front. Chem., 2022, 10, 921354 CrossRef CAS PubMed.
- R. Feng, G. Li, C.-N. Ko, Z. Zhang, J.-B. Wan and Q.-W. Zhang, Small Struct., 2022, 4, 2200131 CrossRef.
- K. Nawara and J. Waluk, Anal. Chem., 2017, 89, 8650–8655 CrossRef CAS PubMed.
- L. Fang, R. Ai, W. Wang, L. Tan, C. Li, D. Wang, R. Jiang, F. Qiu, L. Qi, J. Yang, W. Zhou, T. Zhu, W. Tan, Y. Jiang and X. Fang, Nano Lett., 2023, 23, 8734–8742 CrossRef CAS PubMed.
- M. Li, Z. Lu, J. Zhang, L. Chen, X. Tang, Q. Jiang, Q. Hu, L. Li, J. Liu and W. Huang, Adv. Mater., 2023, 35, 2209647 CrossRef CAS PubMed.
- S. Wang, J. Liu, G. Feng, L. G. Ng and B. Liu, Adv. Funct. Mater., 2019, 29, 1808365 CrossRef.
- Q. Yang, Z. Ma, H. Wang, B. Zhou, S. Zhu, Y. Zhong, J. Wang, H. Wan, A. Antaris, R. Ma, X. Zhang, J. Yang, X. Zhang, H. Sun, W. Liu, Y. Liang and H. Dai, Adv. Mater., 2017, 29, 1605497 CrossRef PubMed.
- H. Matsuura, R. Kawakami, M. Isoe, M. Hoshihara, Y. Minami, K. Yatsuzuka, T. Tsuda, M. Murakami, Y. Suzuki, J. Kawamata, T. Imamura, S. Hadano, S. Watanabe and Y. Niko, ACS Appl. Mater. Interfaces, 2022, 14, 40481–40490 CrossRef CAS PubMed.
- S. Wang, J. Liu, C. C. Goh, L. G. Ng and B. Liu, Adv. Mater., 2019, 31, 1904447 CrossRef CAS PubMed.
- D. Li, P. Jing, L. Sun, Y. An, X. Shan, X. Lu, D. Zhou, D. Han, D. Shen, Y. Zhai, S. Qu, R. Zbořil and A. L. Rogach, Adv. Mater., 2018, 30, 1705913 CrossRef PubMed.
- H. Ni, Y. Wang, T. Tang, W. Yu, D. Li, M. He, R. Chen, M. Zhang and J. Qian, Photonics Res., 2021, 10, 189–196 CrossRef.
- B. del Rosal and A. Benayas, Small Methods, 2018, 2, 1800075 CrossRef.
- P. Pei, Y. Chen, C. Sun, Y. Fan, Y. Yang, X. Liu, L. Lu, M. Zhao, H. Zhang, D. Zhao, X. Liu and F. Zhang, Nat. Nanotechnol., 2021, 16, 1011–1018 CrossRef CAS PubMed.
- B. del Rosal, D. H. Ortgies, N. Fernández, F. Sanz-Rodríguez, D. Jaque and E. M. Rodríguez, Adv. Mater., 2016, 28, 10188–10193 CrossRef CAS PubMed.
- M. Sakiyama, H. Sugimoto and M. Fujii, Nanoscale, 2018, 10, 13902–13907 RSC.
- J. Liao, L. Yang, S. Wu, Z. Yang, J. Zhou, D. Jin and M. Guan, J. Lumin., 2022, 242, 118597 CrossRef CAS.
- X. Zhu, X. Wang, H. Zhang and F. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202209378 CrossRef CAS PubMed.
- S. Roy, N. Bag, S. Bardhan, I. Hasan and B. Guo, Adv. Drug Delivery Rev., 2023, 197, 114821 CrossRef CAS PubMed.
- E. L. Schmidt, Z. Ou, E. Ximendes, H. Cui, C. H. C. Keck, D. Jaque and G. Hong, Nat. Rev. Methods Primers, 2024, 4, 23 CrossRef CAS.
- Y. Dai, X. Li, Y. Xue, K. Chen, G. Jiao, L. Zhu, M. Li, Q. Fan, Y. Dai, Q. Zhao and Q. Shen, Acta Biomater., 2023, 166, 496–511 CrossRef CAS PubMed.
- Y. Li, J. Gao, S. Wang, S. Li, X. Hou, Y. Pan, J. Gao, X. Qiao, Z. Tian, D. Chen, H. Deng, Z. Deng, X. Hong and Y. Xiao, J. Controlled Release, 2022, 342, 157–169 CrossRef CAS PubMed.
- Y. Huang, F. Tian, L. Sun, C. Ji, C. A. Grimes and Q. Cai, Sens. Actuators, B, 2023, 381, 133457 CrossRef CAS.
- S. Bi, X. Wen, G. Sun and S. Zeng, Nano Today, 2023, 53, 102027 CrossRef CAS.
- P. Wang, J. Li, M. Wei, R. Yang, K. Lou, Y. Dang, W. Sun, F. Xue and X. Liu, Biomaterials, 2022, 287, 121636 CrossRef CAS.
- X. Liao, Y. Zheng, Z. Lin, Y. Shen, H. Lin, X. Liu, D. Zhang and B. Li, Chem. Eng. J., 2020, 400, 125882 CrossRef CAS.
- L. Zhu, D. Gao, L. Xie, Y. Dai and Q. Zhao, Mol. Pharmaceutics, 2020, 17, 3720–3729 CrossRef CAS.
- K. Dou, C. Fan, W. Feng, Y. Kong, Y. Xiang, Z. Wang and Z. Liu, CCS Chem., 2022, 4, 3609–3626 CrossRef CAS.
- J. Li, K. Lou, Y. Dang, H. Tian, Q. Luo, C. Huang, R. Liu, X. Gong, S. Wang, H. Liu, P. Wang and X. Liu, Mater. Des., 2023, 227, 111703 CrossRef CAS.
- C. Chen, R. Tian, Y. Zeng, C. Chu and G. Liu, Bioconjugate Chem., 2020, 31, 276–292 CrossRef CAS PubMed.
- Y. Dai, F. Zhang, K. Chen, Z. Sun, Z. Wang, Y. Xue, M. Li, Q. Fan, Q. Shen and Q. Zhao, Small, 2023, 19, 2206053 CrossRef CAS PubMed.
- T. Liu, X. Zhang, D. Liu, B. Chen, X. Ge, S. Gao and J. Song, Adv. Opt. Mater., 2021, 9, 2100233 CrossRef CAS.
- Y. Dang, J. Bai, K. Lou, R. Yang, Y. Gao, H. Tian, J. Li, L. Lin, R. Lv, P. Wang and G. Zhang, Adv. Funct. Mater., 2024, 34, 2311673 CrossRef CAS.
- J. Li, F. Zhu, K. Lou, H. Tian, Q. Luo, Y. Dang, X. Liu, P. Wang and L. Wu, Mater. Today Bio, 2022, 16, 100397 CrossRef CAS PubMed.
- Q. Fu, R. Zhu, J. Song, H. Yang and X. Chen, Adv. Mater., 2018, 31, 1805875 CrossRef PubMed.
- K. Zhu, X. Zhang, Y. Wu and J. Song, Acc. Chem. Res., 2023, 56, 3223–3234 CrossRef CAS PubMed.
- W.-W. Liu and P.-C. Li, J. Biomed. Sci., 2020, 27, 3 CrossRef PubMed.
- A. Sun, H. Guo, Q. Gan, L. Yang, Q. Liu and L. Xi, Opt. Express, 2020, 28, 389714 CAS.
- G. Hong, A. L. Antaris and H. Dai, Nat. Biomed. Eng., 2017, 1, 0010 CrossRef CAS.
- Kenry, Y. Duan and B. Liu, Adv. Mater., 2018, 30, 1802394 CrossRef PubMed.
- Y. Liu, H. Liu, H. Yan, Y. Liu, J. Zhang, W. Shan, P. Lai, H. Li, L. Ren, Z. Li and L. Nie, Adv. Sci., 2019, 6, 1801615 CrossRef PubMed.
- X. L. Deán-Ben, S. Gottschalk, B. Mc Larney, S. Shoham and D. Razansky, Chem. Soc. Rev., 2017, 46, 2158–2198 RSC.
- L. V. Wang and S. Hu, Science, 2012, 335, 1458–1462 CrossRef CAS PubMed.
- A. Sharma, Srishti, V. Periyasamy and M. Pramanik, J. Biomed. Opt., 2019, 24, 121904 CAS.
- J. Zhang, L. Ning, Z. Zeng and K. Pu, Trends Chem., 2021, 3, 305–317 CrossRef CAS.
- C. Du, L. Zhou, J. Qian, M. He, C.-M. Dong, J.-D. Xia, Z.-G. Zhang and R. Zhang, J. Mater. Chem. B, 2021, 9, 5484–5491 RSC.
- S. Jiang, J. Lin and P. Huang, Adv. Healthcare Mater., 2022, 12, 2202208 CrossRef PubMed.
- Z. Li, C. Zhang, X. Zhang, J. Sui, L. Jin, L. Lin, Q. Fu, H. Lin and J. Song, Bioconjugate Chem., 2022, 33, 67–86 CrossRef CAS PubMed.
- J. Zheng, H. Liu, S.-H. Chen, B. Huang, T. Tang, P. Huang and R. Cui, Anal. Chem., 2024, 96, 5315–5322 CrossRef CAS PubMed.
- T. Chen, L. Su, L. Lin, X. Ge, F. Bai, M. Niu, C. Wang, J. Song, S. Guo and H. Yang, Nano Res., 2022, 15, 4154–4163 CrossRef CAS.
- P. Xiao, Y. Sun, M. Liang, S. Yang, J. Li, L. e. Zhang, X. Jiang and W. Wu, Mater. Today Nano, 2023, 24, 100404 CrossRef CAS.
- Y. Jiang, P. K. Upputuri, C. Xie, Z. Zeng, A. Sharma, X. Zhen, J. Li, J. Huang, M. Pramanik and K. Pu, Adv. Mater., 2019, 31, 1808166 CrossRef PubMed.
- J. Chen, R. Chen, C. V. Chau, A. C. Sedgwick, Q. Xue, T. Chen, S. Zeng, N. Chen, K. K. Y. Wong, L. Song, Y. Ren, J. Yang, J. L. Sessler and C. Liu, J. Am. Chem. Soc., 2024, 146, 4620–4631 CrossRef CAS PubMed.
- X. Zhen, K. Pu and X. Jiang, Small, 2021, 17, 2004723 CrossRef CAS PubMed.
- Y. Li, P. Zhang, W. Tang, K. J. McHugh, S. V. Kershaw, M. Jiao, X. Huang, S. Kalytchuk, C. F. Perkinson, S. Yue, Y. Qiao, L. Zhu, L. Jing, M. Gao and B. Han, ACS Nano, 2022, 16, 8076–8094 CrossRef CAS PubMed.
- J. Li, J. Wang, L. Xu, H. Chi, X. Liang, J. Yoon and W. Lin, Angew. Chem., Int. Ed., 2023, 63, e202312632 CrossRef PubMed.
- Z. Wang, Y. Liu, C. He, X. Zhang, X. Li, Y. Li, Y. Tang, X. Lu and Q. Fan, Small, 2023, 20, 2307829 CrossRef PubMed.
- T. He, C. Jiang, J. He, Y. Zhang, G. He, J. Wu, J. Lin, X. Zhou and P. Huang, Adv. Mater., 2021, 33, 2008540 CrossRef CAS PubMed.
- Z. Zheng, Z. Jia, Y. Qin, R. Dai, X. Chen, Y. Ma, X. Xie and R. Zhang, Small, 2021, 17, 2103252 CrossRef CAS PubMed.
- Z. Jiang, C. Zhang, X. Wang, M. Yan, Z. Ling, Y. Chen and Z. Liu, Angew. Chem., Int. Ed., 2021, 60, 22376–22384 CrossRef CAS PubMed.
- S. Zheng, D. Gao, Y. Wu, D. Hu, Z. Li, Y. Wang, H. Zheng, Y. Li and Z. Sheng, Adv. Sci., 2023, 10, 2206979 CrossRef CAS PubMed.
- Z. Li, Q. Fu, J. Ye, X. Ge, J. Wang, J. Song and H. Yang, Angew. Chem., Int. Ed., 2020, 59, 22202–22209 CrossRef CAS PubMed.
- Q. Fu, Z. Li, J. Ye, Z. Li, F. Fu, S.-L. Lin, C. A. Chang, H. Yang and J. Song, Theranostics, 2020, 10, 4997–5010 CrossRef CAS PubMed.
- B. Park, K. M. Lee, S. Park, M. Yun, H.-J. Choi, J. Kim, C. Lee, H. Kim and C. Kim, Theranostics, 2020, 10, 2509–2521 CrossRef CAS PubMed.
- B. Guo, Z. Sheng, D. Hu, C. Liu, H. Zheng and B. Liu, Adv. Mater., 2018, 30, 1802591 CrossRef PubMed.
- Y. Yang, J. Chen, Y. Yang, Z. Xie, L. Song, P. Zhang, C. Liu and J. Liu, Nanoscale, 2019, 11, 7754–7760 RSC.
- J. Wu, L. You, L. Lan, H. J. Lee, S. T. Chaudhry, R. Li, J. X. Cheng and J. Mei, Adv. Mater., 2017, 29, 1703403 CrossRef PubMed.
- U. Chitgupi, N. Nyayapathi, J. Kim, D. Wang, B. Sun, C. Li, K. Carter, W. C. Huang, C. Kim, J. Xia and J. F. Lovell, Adv. Mater., 2019, 31, 1902279 CrossRef CAS PubMed.
- S. Li, Z. Ma, K. Zhang, W. Zhang, Z. Song, W. Wang, X. Yu and H. Han, ACS Appl. Mater. Interfaces, 2022, 14, 41684–41694 CrossRef CAS PubMed.
- Q. Li, L. Liu, H. Huo, L. Su, Y. Wu, H. Lin, X. Ge, J. Mu, X. Zhang, L. Zheng and J. Song, ACS Nano, 2022, 16, 7947–7960 CrossRef CAS PubMed.
- L. Li, H. Chen, Y. Shi and D. Xing, ACS Nano, 2022, 16, 2066–2076 CrossRef CAS PubMed.
- T. Sun, Z. Zhang, D. Cui, G. Mu, X. Sun, X. Su and Y. Shi, ACS Nano, 2023, 17, 14604–14618 CrossRef CAS PubMed.
- X. Wu, Y. Jiang, N. J. Rommelfanger, F. Yang, Q. Zhou, R. Yin, J. Liu, S. Cai, W. Ren, A. Shin, K. S. Ong, K. Pu and G. Hong, Nat. Biomed. Eng., 2022, 6, 754–770 CrossRef CAS PubMed.
- M. Zhao, Y. Li, Y. Liu, L. Bai, J. Ma, M. Ren, J. Liu and H. Shen, Bioconjugate Chem., 2022, 33, 1934–1943 CrossRef CAS PubMed.
- J. Ma, P. Li, W. Wang, S. Wang, X. Pan, F. Zhang, S. Li, S. Liu, H. Wang, G. Gao, B. Xu, Q. Yuan, H. Shen and H. Liu, ACS Nano, 2018, 12, 9022–9032 CrossRef CAS PubMed.
- G. Liu, S. Wang, S. Wang, R. Wu, H. Li, M. Zha, J. Song, Y. Yin, K. Li, J. Mu and Y. Shi, J. Nanobiotechnol., 2023, 21, 151 CrossRef CAS PubMed.
- C. Zhang, W. Sun, Y. Wang, F. Xu, J. Qu, J. Xia, M. Shen and X. Shi, ACS Appl. Mater. Interfaces, 2020, 12, 9107–9117 CrossRef CAS PubMed.
- Y. Yao, Z. Zhao, J. He, B. Ali, M. Wang, F. Liao, J. Zhuang, Y. Zheng, W. Guo and D.-Y. Zhang, Acta Biomater., 2023, 172, 369–381 CrossRef CAS PubMed.
- F. Hong, X. Geng, G. Min, X. Sun, B. Zhang, Y. Yao, R. Li, J. Wang, H. Zhao, P. Guo, Z. Yuan, X. Wen, L. Nie, G. Liu, X. Chen and Q. Zhao, Chem. Eng. J., 2022, 449, 137846 CrossRef CAS.
- Y. Zhang, Y. Li, J. Li, F. Mu, J. Wang, C. Shen, H. Wang, F. Huang, B. Chen, Z. Luo and L. Wang, Adv. Healthcare Mater., 2023, 12, 2300267 CrossRef CAS PubMed.
- L. Liu, Y. Pan, L. Ye, T. Zhang, Y. Chen, C. Liang, D. Chen, X. Mou, X. Dong and Y. Cai, Adv. Healthcare Mater., 2023, 12, 2301116 CrossRef CAS PubMed.
- G. Yang, X. Mu, X. Pan, Y. Tang, Q. Yao, Y. Wang, F. Jiang, F. Du, J. Xie, X. Zhou and X. Yuan, Chem. Sci., 2023, 14, 4308–4318 RSC.
- J. Li, R. Jiang, Q. Wang, X. Li, X. Hu, Y. Yuan, X. Lu, W. Wang, W. Huang and Q. Fan, Biomaterials, 2019, 217, 119304 CrossRef CAS PubMed.
- J. Shao, J. Zhang, C. Jiang, J. Lin and P. Huang, Chem. Eng. J., 2020, 400, 126009 CrossRef CAS.
- K. Li, X. Ma, S. He, L. Wang, X. Yang, G. Zhang, S. Guan, X. Qu, S. Zhou and B. Xu, ACS Biomater. Sci. Eng., 2022, 8, 540–550 CrossRef CAS PubMed.
- H. Shen, B. Wu, Q. Zhang, J. Ni, M. Liang, Y. Liu, X.-F. Zang, S. Wang, Y.-Y. Quan, X. Ye and Z.-S. Huang, Chem. Eng. J., 2023, 468, 143726 CrossRef CAS.
- L. Zhang, H. Forgham, X. Huang, A. Shen, T. P. Davis, R. Qiao and B. Guo, Mater. Today Adv., 2022, 14, 100226 CrossRef CAS.
- L. Sun, Y. Chen, F. Gong, Q. Dang, G. Xiang, L. Cheng, F. Liao and M. Shao, J. Mater. Chem. B, 2019, 7, 4393–4401 RSC.
- Z. Mei, D. Gao, D. Hu, H. Zhou, T. Ma, L. Huang, X. Liu, R. Zheng, H. Zheng, P. Zhao, J. Zhou and Z. Sheng, Biomaterials, 2020, 251, 120092 CrossRef CAS PubMed.
- C. Zhou, L. Zhang, T. Sun, Y. Zhang, Y. Liu, M. Gong, Z. Xu, M. Du, Y. Liu, G. Liu and D. Zhang, Adv. Mater., 2020, 33, 2006532 CrossRef PubMed.
- X.-H. Shi, L. Tao, L. Wang, X. Liu, S.-L. Liu and Z.-G. Wang, Chem. Mater., 2024, 36, 2776–2789 CrossRef CAS.
- Z. Tang, Y. Hou, S. Huang, N. S. Hosmane, M. Cui, X. Li, M. Suhail, H. Zhang, J. Ge, M. Z. Iqbal and X. Kong, Acta Biomater., 2024, 177, 431–443 CrossRef CAS PubMed.
- X. Ding, S. Bai, F. Liu, N. Michał, S. Roman, N. Peng and Y. Liu, Acta Biomater., 2023, 157, 487–499 CrossRef CAS PubMed.
- M. Chen, X.-T. Chen, L.-Y. Zhang, W. Meng, Y.-J. Chen, Y.-S. Zhang, Z.-C. Chen, H.-M. Wang, C.-M. Luo, X.-D. Shi, W.-H. Zhang, M.-S. Wang and J.-X. Chen, J. Nanobiotechnol., 2023, 21, 138 CrossRef CAS PubMed.
- Y. Cheng, F. Yang, G. Xiang, K. Zhang, Y. Cao, D. Wang, H. Dong and X. Zhang, Nano Lett., 2019, 19, 1179–1189 CrossRef CAS PubMed.
- H. Zhang, W. Zeng, C. Pan, L. Feng, M. Ou, X. Zeng, X. Liang, M. Wu, X. Ji and L. Mei, Adv. Funct. Mater., 2019, 29, 1903791 CrossRef.
- X. Luan, H. Hu, Z. Sun, P. He, D. Zhu, Y. Xu, B. Liu and G. Wei, J. Colloid Interface Sci., 2024, 663, 111–122 CrossRef CAS PubMed.
- J. Cui, F. Zhang, D. Yan, T. Han, L. Wang, D. Wang and B. Z. Tang, Adv. Mater., 2023, 35, 2302639 CrossRef CAS PubMed.
- J. Liu, J. Li, Y. Shi, A. Zhu, M. Ding, N. Yu, Y. Liu, P. Wang, J. Li and Y. Liu, Small Struct., 2024, 5, 2300508 CrossRef CAS.
- J. Li, M. Kang, Z. Zhang, X. Li, W. Xu, D. Wang, X. Gao and B. Z. Tang, Angew. Chem., Int. Ed., 2023, 62, e202301617 CrossRef CAS PubMed.
- D. Li, C. Zhang, X. Tai, D. Xu, J. Xu, P. Sun, Q. Fan, Z. Cheng and Y. Zhang, Chem. Eng. J., 2022, 445, 136836 CrossRef CAS.
- B. Yang, Y. Chen and J. Shi, Chem. Rev., 2019, 119, 4881–4985 CrossRef CAS PubMed.
- J. Lin, D. Li, C. Li, Z. Zhuang, C. Chu, K. Ostrikov, E. W. Thompson, G. Liu and P. Wang, Nanoscale, 2023, 15, 11813–11833 RSC.
- J. Zhuang, B. Wang, H. Chen, K. Zhang, N. Li, N. Zhao and B. Z. Tang, ACS Nano, 2023, 17, 9110–9125 CrossRef CAS PubMed.
- K. Chen, B. Yin, Q. Luo, Y. Liu, Y. Wang, Y. Liao, Y. Li, X. Chen, B. Sun, N. Zhou, H. Liu, C. Peng, S. Liu, W. Cheng and G. Song, Theranostics, 2023, 13, 4469–4481 CrossRef CAS PubMed.
- H. Xiao, Y. Wang, J. Chen, S. Xi, Z. Duan, Q. Zhan, Y. Tian, L. Wang, J. Qu and R. Liu, Adv. Healthcare Mater., 2024, 2303183 CrossRef CAS PubMed.
- L. Li, C. Shao, T. Liu, Z. Chao, H. Chen, F. Xiao, H. He, Z. Wei, Y. Zhu, H. Wang, X. Zhang, Y. Wen, B. Yang, F. He and L. Tian, Adv. Mater., 2020, 32, 2003471 CrossRef CAS PubMed.
- K. Wen, H. Tan, Q. Peng, H. Chen, H. Ma, L. Wang, A. Peng, Q. Shi, X. Cai and H. Huang, Adv. Mater., 2022, 34, 2108146 CrossRef CAS PubMed.
- X. Zhang, Y. Dou, S. Liu, P. Chen, Y. Wen, J. Li, Y. Sun and R. Zhang, Adv. Healthcare Mater., 2024, 2303842 CrossRef CAS PubMed.
- X. Wu, Y. Fan, K. Wang, Y. Miao, Y. Chang, J. Ming, X. Wang, S. Lu, R. Liu, F. Zhang, Y. Zhang, H. Qin and J. Shi, Sci. Bull., 2024, 69, 1263–1274 CrossRef CAS PubMed.
- H. Bian, D. Ma, X. Zhang, K. Xin, Y. Yang, X. Peng and Y. Xiao, Small, 2021, 17, 2100398 CrossRef CAS.
- H. Zhao, K. Chen, M. Liu, Z. Wang, L. Li, M. Li, P. Sun, H. Zhou, Q. Fan and Q. Shen, J. Med. Chem., 2023, 67, 467–478 CrossRef PubMed.
- J. Chen, A. C. Sedgwick, S. Sen, Y. Ren, Q. Sun, C. Chau, J. F. Arambula, T. Sarma, L. Song, J. L. Sessler and C. Liu, Chem. Sci., 2021, 12, 9916–9921 RSC.
- W.-L. Chan, C. Xie, W.-S. Lo, J.-C. G. Bünzli, W.-K. Wong and K.-L. Wong, Chem. Soc. Rev., 2021, 50, 12189–12257 RSC.
- Y. Y. Zhao, X. Zhang, Z. Chen, Y. Xu, H. Kim, H. Jeong, Y. R. Lee, J. Lee, X. Li and J. Yoon, Aggregate, 2024, 5, e514 CrossRef CAS.
- P.-C. Lo, M. S. Rodríguez-Morgade, R. K. Pandey, D. K. P. Ng, T. Torres and F. Dumoulin, Chem. Soc. Rev., 2020, 49, 1041–1056 RSC.
- H. Luo and S. Gao, J. Controlled Release, 2023, 362, 425–445 CrossRef CAS PubMed.
- Y. Liu, Y. Liang, P. Lei, Z. Zhang and Y. Chen, Adv. Sci., 2022, 10, 2203669 CrossRef PubMed.
- A. Grebinyk, O. Chepurna, M. Frohme, J. Qu, R. Patil, L. O. Vretik and T. Y. Ohulchanskyy, J. Photochem. Photobiol., C, 2024, 58, 100652 CrossRef CAS.
- X. Gu, J. Shen, Z. Xu, W. Wang, Y. Wu, W. Zhou, C. Xie and Q. Fan, Nano Res., 2024, 17, 5399–5408 CrossRef CAS.
- Y. Gu, H. Lai, Z. Y. Chen, Y. Zhu, Z. Sun, X. Lai, H. Wang, Z. Wei, L. Chen, L. Huang, Y. Zhang, F. He and L. Tian, Angew. Chem., Int. Ed., 2023, 62, e202303476 CrossRef CAS PubMed.
- Y. Tian, D. Zhao, X. Huang, X. Guan, F. Wang and X. Wei, ACS Appl. Mater. Interfaces, 2022, 14, 7626–7635 CrossRef CAS PubMed.
- X. He, Y. Luo, Y. Li, Y. Pan, R. T. K. Kwok, L. He, X. Duan, P. Zhang, A. Wu, B. Z. Tang and J. Li, Aggregate, 2023, e396 Search PubMed.
- C. Liu, B. Liu, J. Zhao, Z. Di, D. Chen, Z. Gu, L. Li and Y. Zhao, Angew. Chem., Int. Ed., 2020, 59, 2634–2638 CrossRef CAS PubMed.
- Y. Wang, M. Feng, B. Lin, X. Peng, Z. Wang and R. Lv, Nanoscale, 2021, 13, 18125–18133 RSC.
- Q.-X. Wang, Y.-F. Yang, X.-F. Yang, Y. Pan, L.-D. Sun, W.-Y. Zhang, Y. Shao, J. Shen, J. Lin, L. Li and C.-H. Yan, Nano Today, 2022, 43, 101439 CrossRef CAS.
- C. Tan, X. Li, Z. Li, S. Lu, F. Wang, Y. Liu, F. Wen, R. Li, D. Tu, Z. Chen and X. Chen, Nano Today, 2024, 54, 102089 CrossRef CAS.
- X. Han, L. Zhou, H. Zhuang, P. Wei, F. Li, L. Jiang and T. Yi, ACS Appl. Mater. Interfaces, 2022, 14, 18031–18042 CrossRef CAS PubMed.
- S. Bi, Z. Deng, J. Huang, X. Wen and S. Zeng, Adv. Mater., 2022, 35, 2207038 CrossRef PubMed.
- R. Vankayala and K. C. Hwang, Adv. Mater., 2018, 30, 1706320 CrossRef PubMed.
- S. Linic, U. Aslam, C. Boerigter and M. Morabito, Nat. Mater., 2015, 14, 567–576 CrossRef CAS PubMed.
- Y. Yang, T. Hu, Y. Bian, F. Meng, S. Yu, H. Li, Q. Zhang, L. Gu, X. Weng, C. Tan and R. Liang, Adv. Mater., 2023, 35, 2211205 CrossRef PubMed.
- N. Meng, P. Xu, C. Wen, H. Liu, C. Gao, X.-C. Shen and H. Liang, J. Colloid Interface Sci., 2023, 644, 437–453 CrossRef CAS PubMed.
- B.-Q. Chen, Y. Zhao, Y.-J. Pan, D.-G. Zhang, H. Liu, Y.-H. Shi, C.-Y. Li, R. K. Kankala, Y. Zhang, S.-B. Wang, G. Liu and A.-Z. Chen, ACS Mater. Lett., 2023, 6, 321–334 CrossRef.
- Y. Wang, X. Sun, Y. Chang and H. Zhang, Biomater. Sci., 2021, 9, 4662–4670 RSC.
- Z. Tang, P. Zhao, H. Wang, Y. Liu and W. Bu, Chem. Rev., 2021, 121, 1981–2019 CrossRef CAS PubMed.
- C. Zhang, J. Huang, X. Guo, X. Da, Z. Dai, M. Hassan, Y. Yu, X. Wang and Q. Zhou, Nano Today, 2023, 50, 101824 CrossRef CAS.
- Z. Zheng, X. Chen, Y. Ma, R. Dai, S. Wu, T. Wang, J. Xing, J. Gao and R. Zhang, Small, 2022, 18, 2203531 CrossRef CAS PubMed.
- G. l. Wu, X. Tan and Q. Yang, Adv. Healthcare Mater., 2023, 13, 2303451 CrossRef PubMed.
- Y. Jiang, X. Zhao, J. Huang, J. Li, P. K. Upputuri, H. Sun, X. Han, M. Pramanik, Y. Miao, H. Duan, K. Pu and R. Zhang, Nat. Commun., 2020, 11, 1857 CrossRef CAS PubMed.
- Y. Luo, L. Zhang, S. Wang, Y. Wang, J. Hua, C. Wen, S. Zhao and H. Liang, ACS Appl. Mater. Interfaces, 2023, 15, 38309–38322 CrossRef CAS PubMed.
- L. Yang, P. Jia, S. Song, Y. Dong, R. Shen, F. He and S. Gai, ACS Appl. Mater. Interfaces, 2022, 14, 21787–21799 CrossRef CAS PubMed.
- C. Li, J. Ye, X. Yang, S. Liu, Z. Zhang, J. Wang, K. Zhang, J. Xu, Y. Fu and P. Yang, ACS Nano, 2022, 16, 18143–18156 CrossRef CAS PubMed.
- G. Liu, J. Zhu, H. Guo, A. Sun, P. Chen, L. Xi, W. Huang, X. Song and X. Dong, Angew. Chem., Int. Ed., 2019, 58, 18641–18646 CrossRef CAS PubMed.
- Y. Shi, J. Zhang, H. Huang, C. Cao, J. Yin, W. Xu, W. Wang, X. Song, Y. Zhang and X. Dong, Adv. Healthcare Mater., 2020, 9, 2000005 CrossRef CAS PubMed.
- X. Meng, Z. Zhang, Y. Qian, X. Wang, Y. Lin, X. Shi, W. Lin, M. Zhang and H. Wang, Adv. Healthcare Mater., 2023, 12, 2301926 CrossRef CAS PubMed.
- X. He, Y. Lv, Y. Lin, H. Yu, Y. Zhang, Y. Tong and C. Zhang, Adv. Mater., 2024, 36, 2400366 CrossRef CAS PubMed.
- J. Huang, G. Deng, S. Wang, T. Zhao, Q. Chen, Y. Yang, Y. Yang, J. Zhang, Y. Nan, Z. Liu, K. Cao, Q. Huang and K. Ai, Adv. Sci., 2023, 10, 2302208 CrossRef CAS PubMed.
- G. Zhou and M. Li, Chem. Eng. J., 2022, 450, 138348 CrossRef CAS.
- S. Wang, T. Hu, G. Wang, Z. Wang, D. Yan, R. Liang and C. Tan, Chem. Eng. J., 2021, 419, 129458 CrossRef CAS.
- Y. Qian, J. Zhang, J. Zou, X. Wang, X. Meng, H. Liu, Y. Lin, Q. Chen, L. Sun, W. Lin and H. Wang, Theranostics, 2022, 12, 3690–3702 CrossRef CAS PubMed.
- T. Xu, S. Liu, Z. Wei, W. Zhang, G.-Y. Zhang, T. Liu, L. Dai, P. Yin and Y. Song, ACS Appl. Mater. Interfaces, 2024, 16, 14489–14502 CrossRef CAS PubMed.
- Q. Zou, H. Pan, X. Zhang and C. Zhang, J. Mater. Chem. B, 2023, 11, 4740–4751 RSC.
- J. Bao, R. Liu, Z. Yu, Z. Cheng and B. Chang, Adv. Funct. Mater., 2024, 2316646 CrossRef CAS.
- B. Shan, H. Liu, L. Li, Y. Lu and M. Li, Small, 2021, 18, 2105638 CrossRef PubMed.
- S. Wang, J. Zhao, L. Zhang, C. Zhang, Z. Qiu, S. Zhao, Y. Huang and H. Liang, Adv. Healthcare Mater., 2021, 11, 2102073 CrossRef PubMed.
- W. Li, Y. Xiao, G. Guo, J. Peng, N. Zhu, Z. Chen, B. Peng, Z. Jiang, B. Li, G. Yu, Z. Guo, M. Liang and W. Guo, Nano Today, 2024, 56, 102223 CrossRef CAS.
- Y. Liu, R. Lu, M. Li, D. Cheng, F. Wang, X. Ouyang, Y. Zhang, Q. Zhang, J. Li and S. Peng, Mater. Horiz., 2024, 11, 2406–2419 RSC.
- G. Zhou and M. Li, Adv. Mater., 2022, 34, 2200871 CrossRef CAS PubMed.
- C. Yang, M. R. Younis, J. Zhang, J. Qu, J. Lin and P. Huang, Small, 2020, 16, 2001518 CrossRef CAS PubMed.
- J. Costoya, B. Surnar, A. A. Kalathil, N. Kolishetti and S. Dhar, Mol. Aspects Med., 2022, 83, 101043 CrossRef CAS PubMed.
- G. Szakács, J. K. Paterson, J. A. Ludwig, C. Booth-Genthe and M. M. Gottesman, Nat. Rev. Drug Discovery, 2006, 5, 219–234 CrossRef PubMed.
- P. Gao, K. Wang, R. Wei, X. Shen, W. Pan, N. Li and B. Tang, Biomater. Sci., 2023, 11, 7616–7622 RSC.
- H. Yu, Y. Wang, Y. Chen, M. Cui, F. Yang, P. Wang and M. Ji, Nano Convergence, 2023, 10, 3 CrossRef CAS PubMed.
- X. Lin, Z. Li, S. Du, Q. Wang, Y. Guan, G. Cheng, H. Hong, J. Li, X. Chen and T. Chen, Chem. Eng. J., 2023, 464, 142732 CrossRef CAS.
- R. Hu, C. Dai, C. Wang, J. Lin, H. Hu, Z. Li, H. Lin, L. Ding, Y. Chen and B. Zhang, Adv. Funct. Mater., 2021, 31, 2101660 CrossRef CAS.
- Q. Cai, C. Wang, S. Gai and P. Yang, ACS Appl. Mater. Interfaces, 2022, 14, 3809–3824 CrossRef CAS PubMed.
- X. Zhang, H. Wei, C. Dong, J. Wang, T. Zhang, L. Huang, D. Ni and Y. Luo, Chem. Eng. J., 2023, 461, 141855 CrossRef CAS.
- Y.-J. Pan, Y. Zhang, B.-Q. Chen, Y. Zhao, J.-Y. Wang, C.-Y. Li, D.-G. Zhang, R. K. Kankala, S.-B. Wang, G. Liu and A.-Z. Chen, Bioact. Mater., 2024, 33, 311–323 CAS.
- B. Yang, Y. Zhang, L. Sun, J. Wang, Z. Zhao, Z. Huang, W. Mao, R. Xue, R. Chen, J. Luo, T. Wang, J. Jiang and Y. Qin, Adv. Funct. Mater., 2022, 33, 2211251 CrossRef.
- J. Sun, J. Wang, W. Hu, Y. Wang, Q. Zhang, X. Hu, T. Chou, B. Zhang, C. Gallaro, M. Halloran, L. Liang, L. Ren and H. Wang, ACS Nano, 2022, 16, 10711–10728 CrossRef CAS PubMed.
- P. Sun, W. Yang, J. He, L. He, P. Chen, W. Xu, Q. Shen, D. Li and Q. Fan, Adv. Healthcare Mater., 2023, 12, 2302099 CrossRef CAS PubMed.
- L. Wu, M. Jiang, C. Chu, T. Luo, Y. Hui, W. Zhou, S. Geng and X. F. Yu, Adv. Sci., 2023, 11, 2305762 CrossRef PubMed.
- D. Yu, Y. Wang, J. Chen, S. Liu, S. Deng, C. Liu, I. McCulloch, W. Yue and D. Cheng, Acta Biomater., 2022, 137, 238–251 CrossRef CAS PubMed.
- L. He, F. Qing, M. Li and D. Lan, Int. J. Nanomed., 2020, 15, 2337–2349 CrossRef CAS PubMed.
- Y. Dai, W. Du, D. Gao, H. Zhu, F. Zhang, K. Chen, H. Ni, M. Li, Q. Fan and Q. Shen, Biomater. Sci., 2022, 10, 435–443 RSC.
- J. Zhang, J. Hu, W. Zhu, Y. Liu, S. Li, H. Chen, F. Liao, Q. Zhang, X. Sun, X. Li, Y. Xiao and J. Chen, Chem. Eng. J., 2024, 479, 147692 CrossRef CAS.
- S. Qiang, X. Hu, R. Li, W. Wu, K. Fang, H. Li, Y. Sun, S. Liang, W. Zhao, M. Wang, Y. Lin, S. Shi and C. Dong, ACS Appl. Mater. Interfaces, 2022, 14, 36503–36514 CrossRef CAS PubMed.
- Q. Zhang, X. Wang, G. Kuang and Y. Zhao, Bioact. Mater., 2023, 24, 185–196 CAS.
- Y. Yang, Y. Liu, J. Weng, X. Wen, Y. Liu and D. Ye, Biomaterials, 2024, 305, 122454 CrossRef CAS PubMed.
- W. Liu, H. Xiang, M. Tan, Q. Chen, Q. Jiang, L. Yang, Y. Cao, Z. Wang, H. Ran and Y. Chen, ACS Nano, 2021, 15, 6457–6470 CrossRef CAS PubMed.
- F. Lu, R. Sang, Y. Tang, H. Xia, J. Liu, W. Huang, Q. Fan and Q. Wang, Acta Biomater., 2022, 151, 528–536 CrossRef CAS PubMed.
- J. Zhu, Y. Zhang, Z. Li, X. Bao, Y. Zhou, B. Ma, Y. Xie, P. Yan, Z. Wu, Q. Zhang, J. Zou and X. Chen, Mater. Horiz., 2023, 10, 3014–3023 RSC.
- C. Caruso Bavisotto, A. Marino Gammazza, C. Campanella, F. Bucchieri and F. Cappello, Semin. Cancer Biol., 2022, 86, 36–45 CrossRef CAS PubMed.
- A. Jiang, Y. Liu, L. Ma, F. Mao, L. Liu, X. Zhai and J. Zhou, ACS Appl. Mater. Interfaces, 2019, 11, 6820–6828 CrossRef CAS PubMed.
- S. Qiao, F. Xin, M. Wu, Y. Zheng, B. Zhao, C. Zhang, X. Liu, Z. Wei and J. Liu, J. Mater. Chem. B, 2021, 9, 5083–5091 RSC.
- T. Sun, L. Zhang, S. Xiao, Q. Xie, M. Wang, Y. Zhao, C. Zhou, M. Gong and D. Zhang, ACS Mater. Lett., 2024, 6, 985–998 CrossRef CAS.
- M. Niwa, K. Hotta, A. Hara, K. Hirade, H. Ito, K. Kato and O. Kozawa, Life Sci., 2006, 80, 181–186 CrossRef CAS PubMed.
- Z. Sun, G. Deng, X. Peng, X. Xu, L. Liu, J. Peng, Y. Ma, P. Zhang, A. Wen, Y. Wang, Z. Yang, P. Gong, W. Jiang and L. Cai, Biomaterials, 2021, 279, 121228 CrossRef CAS PubMed.
- X. Yang, T. Yang, Q. Liu, X. Zhang, X. Yu, R. T. K. Kwok, L. Hai, P. Zhang, B. Z. Tang, L. Cai and P. Gong, Adv. Funct. Mater., 2022, 32, 2206346 CrossRef CAS.
- Y. Du, X. Dai, M. Han, Z. Wang, Y. Wang, Z. Shi, F. Yan and S. Feng, Chem. Eng. J., 2023, 453, 139635 CrossRef CAS.
- Y. Dai, Z. Sun, H. Zhao, D. Qi, X. Li, D. Gao, M. Li, Q. Fan, Q. Shen and W. Huang, Biomaterials, 2021, 275, 120935 CrossRef CAS PubMed.
- X. Wei, H. Huang, J. Guo, N. Li, Q. Li, T. Zhao, G. Yang, L. Cai, H. Yang, C. Wu and Y. Liu, Small, 2023, 19, 2304370 CrossRef CAS PubMed.
- J. Galon and D. Bruni, Nat. Rev. Drug Discovery, 2019, 18, 197–218 CrossRef CAS PubMed.
- N. A. Ullman, P. R. Burchard, R. F. Dunne and D. C. Linehan, J. Clin. Oncol., 2022, 40, 2789–2805 CrossRef CAS PubMed.
- S. Guo, D. Tang, M. Zhang, H. Yang, T. Zhang, B. Hu, C. Xu, Y. Weng, K. Shang and Y. Huang, Adv. Mater., 2024, 36, 2400228 CrossRef CAS PubMed.
- M. Wang, M. Chang, P. Zheng, Q. Sun, G. Wang, J. Lin and C. Li, Adv. Sci., 2022, 9, 2202332 CrossRef CAS PubMed.
- G. Liu, J. Li, X. Wang, H. Ren and Y. Zhang, Adv. Healthcare Mater., 2024, 13, 2303305 CrossRef CAS PubMed.
- D. Jana, B. He, Y. Chen, J. Liu and Y. Zhao, Adv. Mater., 2022, 36, 2206401 CrossRef PubMed.
- C. Xu, Y. Jiang, Y. Han, K. Pu and R. Zhang, Adv. Mater., 2021, 33, 2008061 CrossRef CAS PubMed.
- Y. Bai, J. Hua, J. Zhao, S. Wang, M. Huang, Y. Wang, Y. Luo, S. Zhao and H. Liang, Adv. Sci., 2023, 11, 2306375 CrossRef PubMed.
- Y. Wang, W. Li, B. Lin, Y. Yuan, P. Ning, X. Tao and R. Lv, Biomater. Sci., 2023, 11, 5177–5185 RSC.
- Q. Long, Y. Yang, F. Liao, H. Chen, D. He, S. Li, P. Li, W. Guo and Y. Xiao, J. Mater. Chem. B, 2023, 11, 8528–8540 RSC.
- M. Li, M. Zhao, Y. Zhang, M. Ding, N. Yu, S. Peng, X. Shi and J. Li, Nano Today, 2023, 50, 101833 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |
