An eco-friendly polycaprolactone/graphite composite as a robust freestanding electrode platform for supercapacitive energy storage†
Rajeev
Gupta
 *a and
Monika
Singhal
b
*a and
Monika
Singhal
b
aDepartment of Applied Chemistry, School of Sciences, ITM(SLS) Baroda University, Vadodara, Gujarat 391510, India. E-mail: rajeevguptachem@gmail.com
bShree PM Patel Institute of PG Studies and Research in Science (Affiliated to Sardar Patel University VV Nagar), Anand, Gujarat 388001, India
First published on 2nd October 2024
Abstract
We present the successful development and characterization of a novel eco-friendly polycaprolactone–graphite (PCLGr) composite as a freestanding platform, serving as a bulk conducting chip electrode for supercapacitor applications. Notably, this is the first report of using this biodegradable polymer for making such a self-standing conductive platform. Traditional polymer and carbon-based electrodes often rely on insulating supports or non-eco-friendly materials, which we have addressed in our work. Direct deposition of the redox material, polyaniline (PANI), onto the electrode via the galvanostatic method has been achieved. The specific capacitance of PANI demonstrates comparability to previous studies utilizing conventional current collectors. Notably, the electrode exhibits exceptional stability in highly acidic environments. Comprehensive characterization utilizing bulk conductivity measurements, XRD, TGA, DSC, SEM, and stress–strain analyses shows advanced properties of the electrode. It complements the evaluation of PANI's supercapacitive performance through cyclic voltammetry, charge–discharge measurements, and impedance spectroscopy. We achieved a specific capacitance of ≈162 F g−1 at 0.5 A g−1. This innovative electrode presents a promising alternative to conventional counterparts across various electrochemical applications.
1 Introduction
Energy storage attracts attention from electrochemists and the scientific community due to its fascinating potential and multifaceted implications.1,2 The urgent need for sustainable energy solutions has fueled significant interest in enhancing the efficiency of energy storage devices, driving intensive research and development efforts in this field.3–10 Electrochemical capacitors (ECs) or pseudocapacitors have been extensively researched for their potential as sustainable energy storage solutions, addressing concerns surrounding sustainability in various systems and industries.11–15 Numerous conducting polymers such as polythiophene, polypyrrole, PEDOT, and polyaniline have been employed in energy storage applications due to their versatile electrochemical properties, high conductivity, and compatibility with various electrode materials, offering promising avenues for advancing energy storage technologies.16–20 Polyaniline stands out as a promising candidate among these polymers due to its facile electrochemical synthesis directly onto electrode surfaces. This unique feature simplifies fabrication processes, enhances device integration, and enables tailored electrode designs, contributing to its attractiveness for energy storage applications.17 Polyaniline21 can be readily synthesized using various electrochemical techniques such as galvanostatic, potentiostatic, and potentiodynamic methods. However, its strong adhesion to current collectors poses challenges, necessitating careful selection of collectors crucial for supercapacitance or any other application to ensure optimal performance and stability of the electrodes.22Current collectors are pivotal in supercapacitor devices, acting as substrates for the electrodeposition of high surface area conductive materials. Various collectors are reported in the literature, including carbon fabrics,23,24 polymer films with conductive coatings,25 metal foils like aluminum and nickel,26,27 nickel foams28 and stainless-steel fabrics.29 These collectors offer diverse properties such as high conductivity, mechanical strength, and surface area, crucial for optimizing the device performance and stability. Carbon fabrics are known for their flexibility and mechanical robustness, but they often come with a high cost. Polymer films with conductive coatings of materials like carbon nanotubes (CNTs) or graphene offer a viable alternative, yet their coatings may delaminate over time, exposing non-conductive surfaces and compromising the device performance. Metal foils such as aluminum and nickel, while offering rigidity or flexibility, face challenges with stability in acidic or primary media. Oxidation of these metals in liquid electrolytes leads to corrosion, making them less environmentally friendly. Nickel foam shares similar properties with nickel metal and is limited in its application range due to its inability to withstand anodic potentials, restricting electrochemical measurements primarily to cathodic potentials. Stainless steel fabrics stand out for their superior conductivity, mechanical strength, and neutrality towards acids or bases. However, they still face limitations at high anodic potentials in liquid systems and are not entirely eco-friendly. Many researchers resort to protecting stainless steel from corrosion by coating it with conducting polymers. While each current collector option offers advantages and limitations, the choice depends on specific application requirements, balancing factors such as the cost, stability, environmental impact, and electrochemical performance.
An easily synthesizable, flexible, cost-effective, and environmentally friendly current collector holds significant promise for direct deposition of conducting polymers without the need for binders, offering considerable potential for supercapacitor applications. Materials such as high-surface-area carbon nanomaterials, graphene, or reduced graphene oxide (rGO) can be affixed to these collectors using adhesives, providing versatility in design. Graphite plates and rods are commonly utilized as electrode surfaces for electrochemical reactions, including polyaniline electrosynthesis.17 However, these graphite electrodes are inherently inflexible, heavy, and at times, cost-prohibitive, limiting their utility for portable devices. Despite this, graphite boasts stability across a broad potential window, making it an ideal substrate for various electrochemical processes. To address the issues of rigidity and weight, different aliphatic polyesters can be selected to tailor the current collector's flexibility. The development of composite electrodes presents significant advantages in electroanalysis. These electrodes can be fabricated with remarkable flexibility in terms of size and shape, allowing for easy adaptation to various electrode configurations. The preparation of a composite varies depending on the nature and quantities of each phase involved, and the targeted application.30 The main methods of constructing composite electrodes described in the literature include dissolution/drying, mechanical compression, melting/cooling, and in situ polymerization.31–35 Through solution casting, some polyesters form rigid films, while others produce flexible ones, depending on the polymer's nature. The choice of a biodegradable polymer like PLA adds an extra benefit, enhancing the environmental friendliness of the current collector.36 For example, polylactic acid (PLA) yields rigid films and has been successfully combined with graphite in previous work to create volume conductive electrodes.31 Choosing a biodegradable polymer adds extra benefit to this type of current collector, which makes it environment-friendly. Another aliphatic polyester, polycaprolactone (PCL), shares similar properties with PLA but offers increased flexibility. PCL has garnered attention for its enhanced flexibility, further augmenting the adaptability and applicability of such current collectors for supercapacitor devices. PCL biodegrades depending on the amount of crystallinity, polymer molecular mass, and other degradation parameters, such as environment, temperature, pH, and salinity.37 Overall, these developments signify a promising step towards sustainable and versatile energy storage solutions.38
In a report, an electrochemical sensor based on graphite and PCL was prepared for the determination of antihypertensive drugs. This is an example of a bulk conducting electrode but it uses a syringe as a support and copper wire provides a conductive channel in it.39 It has been shown that the graphite and PCL composite prepared by the injection molding technique shows good mechanical properties.40 Due to its non-toxic and flexible nature, PCL is a thermoplastic commonly used in materials such as wound sutures and in blends with other polymers for medicinal purposes.41 Indeed, the unique properties of PCL have prompted researchers to consider it as a binding material in composite electrodes. Its versatility and lack of prior use in this application make it an intriguing candidate for electrode fabrication. Moreover, its light weight nature and ease of molding offer significant advantages, facilitating straightforward and rapid electrode preparation processes.
Polyaniline deposition on conductive substrates under anodic potentials in highly acidic media presents an attractive avenue for energy storage applications. While conventional methods involve chemical synthesis followed by binder-based coating onto current collectors, electrochemically grown polyaniline offers a more direct and environmentally friendly approach. However, research in this area is limited, particularly concerning its integration with low-cost, bulk-conducting, eco-friendly, and flexible chip-like current collectors. Current testing methods often rely on high-cost inert electrodes e.g. glassy carbon and inter-metals, underscoring the demand for carbon-based, flexible, and low-cost electrodes that are easily fabricated and stable for electroanalytical and supercapacitor applications. Developing such electrodes could significantly advance the field by providing accessible and sustainable solutions for energy storage needs.
In this report, we describe in detail the fabrication and characterization of PCL/graphite chip electrodes, followed by the electro-synthesis of polyaniline onto them, and their subsequent supercapacitor applications. PCL serves as the polymeric matrix, while graphite acts as the conductive filler, yielding self-standing, bulk-conductive, mechanically robust, and flexible electrodes. PCL's biodegradability and graphite's favorable surface properties make these electrodes environmentally friendly. Enhanced wettability of graphite with the polymer matrix and its electrochemical inertness contribute to superior adhesion and acid resistance. These attributes position the eco-friendly plastic chip electrodes as ideal candidates for aqueous systems, offering a promising solution for sustainable energy storage applications.
2 Experimental
2.1 Chemicals and materials
For the fabrication of PCLGr, graphite powder (particle size: 50 μm) from CDH Pvt. Ltd served as the conductive filler. PCL, consisting of shining white pellets, was sourced from Sigma Aldrich. This polymer exhibits complete solubility in chlorine-containing organic solvents such as chloroform but remains insoluble in water. AR Grade chloroform from S.D. Fine Chem was employed to dissolve PCL. Aniline, procured from Sigma-Aldrich and subjected to double distillation, was stored in a refrigerator. H2SO4 from SRL Chemicals was used to prepare the electrolyte solution for electrochemical analysis. All other chemicals used were of AR grade.2.2 Fabrication of pristine PCL films and PCL–graphite (PCLGr) composites
For the preparation of PCL films, all materials were used as received. The solution casting method, previously described in detail in our work,31 was employed. PCL was dissolved in chloroform to create a 10% w/v solution, resulting in a more viscous solution. This solution (40.5 mL) was then cast in a 7.2 cm × 7.5 cm glass mold, constructed using four glass strips adhered to a glass plate to form a square-shaped mold, without the use of a polyester sheet. The decision to omit the polyester sheet was due to the strong adhesion between the PCL film and the sheet, making removal challenging after drying. The solution was carefully poured into the mold to prevent spillage, and the assembly was covered with a thin paper to facilitate slow chloroform evaporation. After 24 hours of drying, intact, white, and comparably flexible PCL films were easily separated from the glass sheet.To fabricate PCLGr composite chips, the solution casting method was employed, with preparative steps outlined in Fig. 1. Nine different compositions of PCLGr were prepared by varying the ratio of the components from 10% to 90%. A 10% w/v PCL solution in chloroform was used, totaling 200 mL of solution. Nine square-shaped glass molds, each measuring 7.2 cm × 7.5 cm (54 cm2), were employed for solution casting. Care was taken during assembling to prevent solution spillage from the molds.
To achieve a homogeneous suspension of graphite in the PCL solution, ultrasonication and stirring with a glass rod for 10 minutes were performed. The viscous slurries were then poured directly onto glass sheet bases in the molds and covered with a thin paper to ensure a slow evaporation rate of the organic solvent, promoting the formation of high-quality chips. The assemblies were left at room temperature for approximately 24 hours to allow for solvent evaporation and chip formation. Once dried, the PCLGr composite sheets easily detached from the glass sheet bases and were peeled off from the molds (photographs of various composites are shown in Fig. S1; ESI†).
The material composition of the PCLGr composites is provided in Table S1 (ESI).†
Following drying, the chips were stored in airtight plastic pouches. These PCLGr composites were then cut into specific dimensions for use as current collectors in electrochemical experiments. Only one composition, PCL4Gr6 (polymer 40% and graphite 60%), was selected for application as a current collector in the supercapacitor study. The rationale for choosing this particular composition is discussed in the Results and Discussion section, particularly in relation to bulk conductivity considerations.
2.3 Spectroscopic and morphological characterization
The conductivity of various compositions of PCLGr was measured by sandwiching square-sized chips (1 cm × 1 cm) between two platinum electrodes in a spring-loaded sample holder. A voltage window of ±5 V was applied, and the current was measured using a Keithley Model 2635A source meter unit, which integrates multiple functions into one compact instrument. Powder X-ray diffraction (XRD) measurements of PCL pellets, the PCL film (1.5 cm × 1.5 cm), the PCL4Gr6 electrode, and graphite powder samples were conducted using a PANalytical powder X-ray diffractometer with Cu-Kα (λ = 0.15406 nm) radiation. To check the thermal stability of the PCL film and the PCL4Gr6 electrode, thermogravimetric analyses (TGA) were performed using a NETZSCH TG209F1Libra instrument at a heating rate of 10 °C min−1 under nitrogen flow, by heating the samples from room temperature to 800 °C in alumina crucibles. Differential scanning calorimetry (DSC) analysis was carried out using a NETZSCH DSC 204 F1 Phoenix instrument, by heating the samples from −75 °C to 200 °C at a rate of 10 °C min−1 in aluminum pans with an empty pan being used as a reference. The morphologies of PCL films, PCL4Gr6, and PANI-coated PCL4Gr6 were evaluated using a field emission scanning electron microscope (FE-SEM) (model JEOL JSM 7100F) at an acceleration voltage of 15 kV. Samples were prepared by sticking small sections onto carbon tape on conductive stubs and dried for 2 hours at 50 °C before imaging. Tensile strength analyses of the PCL film and PCL4Gr6 electrodes were carried out using a universal testing machine (ZwickRoell, type X force P, S/N 756324) at room temp. The samples were cut into small strips with the same width of 1 cm. The PCL film thickness was 0.40 mm (cross sectional area of 4 mm2) and for the PCL4Gr6 electrode it was 0.45 mm (cross sectional area of 4.5 mm2). A preload of 0.5 N was applied at the rate of 10 mm min−1 in the beginning. The test speed was 50 mm min−1 during the experiment. The experiment was repeated up to four times to assess the reproducibility, with consistent results being observed across repetitions.2.4 Electrochemical deposition of PANI on PCL4Gr6
The schematic procedure for galvanostatic deposition of polyaniline over PCL4Gr6 is illustrated in Fig. S2a (ESI).† The galvanostatic coating of PANI was conducted following established protocols. A suitable current density of 0.5 mA cm−2 was applied, using a 0.1 M aniline monomer prepared in 1 M H2SO4 solution for PANI deposition on the electrode surface. In the three-electrode assembly, the working electrode's active area was set to 1 cm2, with Ag/AgCl (sat. KCl) and platinum foil serving as the reference and counter electrodes, respectively. The polymerization process occurred in a beaker containing 10 mL of the monomer solution, with the galvanostatic current density being maintained at 0.5 mA cm−2 for 15 minutes, as depicted in the galvanostatic polymerization curve in Fig. S2b (ESI).†Following polymerization, the coated working electrodes were rinsed with deionized water and dried in an oven at 55 °C for 12 hours. The resulting electrode, referred to as PCL4Gr6/PANI, had the deposited PANI mass recorded using an analytical balance with a precision of four decimal places, by subtracting the weight of the working electrode from that of the PANI-coated electrode. Approximately 1 mg of PANI was deposited on each electrode within the 15 minute duration, and multiple samples were prepared for subsequent studies. The deposited mass value is provided in Table S2 (ESI),† which was utilized for specific capacitance calculations.
2.5 Electrochemical characterization of supercapacitive electrodes
Electrochemical measurements of the PANI-coated electrode were conducted using a three-electrode system in a 1 M H2SO4 electrolyte. Platinum foil and an Ag/AgCl (sat. KCl) electrode were employed as the counter and reference electrodes, respectively. For electrochemical testing, cyclic voltammetry (CV) and galvanostatic charge–discharge (GCD) were recorded on a potentiostat/galvanostat (Autolab PGSTAT 204), while electrochemical impedance spectroscopy (EIS) was performed on a PARSTAT Model 2273 with a built-in frequency response analyzer. These measurements were conducted at room temperature under ambient conditions, covering a frequency range of 20 kHz to 100 mHz with a sinusoidal voltage amplitude of 10 mV superimposed on 0 V DC (vs. open circuit). Cyclic voltammetry measurements were conducted within a potential range of −0.2 to 0.8 V vs. Ag/AgCl (sat. KCl) for PANI-coated electrodes, with the scan rate being varied from 5 mV s−1 to 100 mV s−1. Galvanostatic charge–discharge measurements were carried out with a constant current ranging from 0.5 A g−1 to 10 A g−1 within a potential window of 0.0 to 0.8 V.3 Results and discussion
3.1 Bulk conductivity of different compositions of PCL–graphite composites (electrical characterization)
By analyzing the results presented in Table 1 and Fig. 2, it was observed that the bulk conductivity increased with the higher graphite content, with the percolation threshold being noted at 50 wt% of the filler content (Fig. 2b). Subsequent minor increase in the conductivity was observed with a further increase in the graphite content. Unlike our previous study on polylactic acid-based composites,31 where conductivity exhibited a dramatic change, here, the increase was more gradual, possibly due to better wettability of the filler induced by the PCL matrix. The I–V plots (Fig. 2a) of all samples exhibited linear behavior within the voltage window of ±5 V, indicating the ohmic nature of the composites. Beyond 50 wt% of the graphite filler, additional conductivity increments were minimal. Such substantial variations in conductivity values occurring within the graphite content range of 40 to 50 wt% suggest the onset of percolation at 50 wt% of graphite. | ||
| Fig. 2 (a) I–V plots of polycaprolactone–graphite composites with nine different compositions. (b) Effect of the graphite content on the bulk conductivity of PCL–graphite composite chips. | ||
| Sr. no. | Sample code | Composition of the PCLGr chip (w/w%) | Bulk conductivity (mS cm−1) |
|---|---|---|---|
| 1 | PCL1Gr9 | PCL (10%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) graphite (90%) graphite (90%) |
82.9 mS cm−1 |
| 2 | PCL2Gr8 | PCL (20%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) graphite (80%) graphite (80%) |
80.2 mS cm−1 |
| 3 | PCL3Gr7 | PCL (30%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) graphite (70%) graphite (70%) |
77.9 mS cm−1 |
| 4 | PCL4Gr6 |
PCL (40%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) graphite (60%) graphite (60%) |
65.6 mS cm −1 |
| 5 | PCL5Gr5 | PCL (50%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) graphite (50%) graphite (50%) |
65.1 mS cm −1 |
| 6 | PCL6Gr4 | PCL (60%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) graphite (40%) graphite (40%) |
25.9 mS cm−1 |
| 7 | PCL7Gr3 | PCL (70%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) graphite (30%) graphite (30%) |
2.4 mS cm−1 |
| 8 | PCL8Gr2 | PCL (80%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) graphite (20%) graphite (20%) |
2.6 × 10−1 mS cm−1 |
| 9 | PCL9Gr1 | PCL (90%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) graphite (10%) graphite (10%) |
1.4 × 10−5 mS cm−1 |
Therefore, composite electrodes containing 50%–60% graphite can be suitable for electrochemical experiments. According to the percolation theory, a graphite content of 60 wt% is sufficient to demonstrate percolation and achieve adequate conductivity. Additionally, XRD, thermal analysis, scanning electron microscopy, and stress–strain analysis were conducted on PCL4Gr6. While samples with a higher graphite content, like PCL3Gr7, exhibited slightly better conductivity, their increased fragility and brittle nature rendered them less practical for application.
3.2 XRD pattern of the PCL pellets, film and PCL–graphite chip electrode (PCL4Gr6)
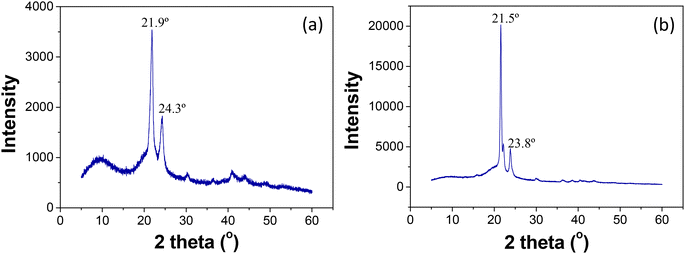
The crystalline phases present in the samples were identified using XRD. PCL pellets were analyzed in their original form. Fig. 3a and b display the XRD patterns of both PCL pellets and the PCL film. In the XRD pattern of PCL pellets, strong and sharp crystalline peaks were observed at 2θ = 21.9° (with a high intensity peak; corresponding to a d-spacing of 4.05 Å) and at 2θ = 24.3° (with a relatively lower intensity peak; corresponding to a d-spacing of 3.65 Å). These peaks correspond to the [110] and [200] crystallographic planes of PCL crystals, respectively, and are characteristic of PCL in XRD patterns.42 PCL being a semi-crystalline material, its sharp and distinct peaks in XRD suggest a high degree of crystallinity within the polymer. In contrast, the XRD pattern of the PCL film revealed a higher intensity peak at 2θ = 21.5° (corresponding to a d-spacing of 4.12 Å) and a lower intensity peak at 2θ = 23.8° (corresponding to a d-spacing of 3.73 Å). The increased intensity of peaks in the PCL film suggests an increase in the crystallinity following solution casting and film drying. Additionally, both peaks in the PCL film were shifted to lower values, indicating a reduction in the compactness of the crystalline state compared to PCL pellets.The XRD pattern of PCL4Gr6 is depicted in Fig. 4. Notably, the characteristic peak of graphite appears highly intense and sharp at 2θ = 26.7°, corresponding to a d-spacing of 3.33 Å. Peaks corresponding to the PCL matrix are observed at lower 2θ values compared to graphite. Differences in the pattern are evident within the range of 2θ = 5° to 2θ = 25° for both materials. For comparison, the curves for PCL4Gr6 and neat graphite powder are displayed in the inset of Fig. 4. All peaks present in the composite are also observed in the graphite XRD pattern. However, two distinct peaks at 2θ = 21.6° and 2θ = 24.1°, corresponding to d spacings of 4.11 Å and 3.68 Å, respectively, are unique to the PCL component of the composite.
The shifting of peak positions to higher angles, specifically at 21.6° and 24.1° for PCL in the composite, compared to the pristine PCL film peaks at 21.5° and 23.8°, suggests a more compact crystalline state of PCL in PCL4Gr6. Furthermore, the peak intensities of PCL in PCL4Gr6 are reduced, and peak broadening is observed, indicating a smaller crystallite size and lower crystallinity of PCL in the composite compared to the pristine PCL film. This phenomenon can be attributed to the faster nucleation of PCL during the drying process induced by the presence of graphite powder particles, which act as nucleating agents. This results in the formation of disordered lamellae, and greater interaction induces less crystallinity in PCL during electrode formation.
3.3 Thermal analysis of the polycaprolactone film and PCL–graphite chip electrode (PCL4Gr6)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) are close to the pure PCL polymer as received. Two phase transitions are observed from the curve. The first one is Tg is due to the conversion into a more amorphous phase of PCL and the second one is Tm due to the melting of the crystalline phase. Here no major difference in Tg and Tm values of PCL in the composite from neat PCL illustrates that the addition of graphite does not affect these values and the composition of these two components makes the composite.
000) are close to the pure PCL polymer as received. Two phase transitions are observed from the curve. The first one is Tg is due to the conversion into a more amorphous phase of PCL and the second one is Tm due to the melting of the crystalline phase. Here no major difference in Tg and Tm values of PCL in the composite from neat PCL illustrates that the addition of graphite does not affect these values and the composition of these two components makes the composite.
3.4 Scanning electron microscopy of the polycaprolactone film and polycaprolactone–graphite chip electrode (PCL4Gr6)
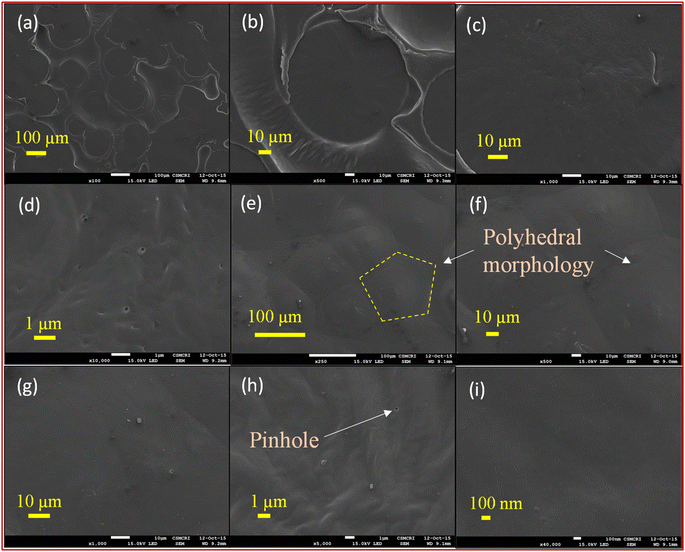
The SEM images of the lower surface and top surface of the PCL film at different magnifications are presented in Fig. 7. The lower surface of the film (Fig. 7a–d) exhibits relatively fewer features, with the presence of large bubble-like structures and small pinholes. These characteristics are typical of solution-cast polymer films and are attributed to the evaporation of chloroform during the drying process.In contrast, the top surface of the film (Fig. 7e–i) displays a distinct morphology. Polyhedral structures are prominently visible and distributed uniformly across the entire surface (as marked in Fig. 7e). These polyhedral morphologies result from the growth of crystalline domains of PCL from heterogeneous nucleation centers followed by impingement. The presence of pinholes further supports this observation, indicating the presence of crystalline domains covered with some amorphous regions formed due to solvent evaporation.
The tightly packed polyhedral structures resemble spherulites and are approximately 60–70 μm in size. These structures are formed by the branching of crystalline networks of PCL outward in a radial direction from the nucleation centers, as described above.45,46
SEM images at different magnifications of the lower surface of PCL4Gr6 are presented in Fig. 8a–f. In a broad view, the lower surface appears relatively smooth and shiny compared to the top surface. This is attributed to the presence of graphite particles with rough and undefined shapes, randomly distributed at the bottom during the solution casting process, with the PCL matrix filling the empty spaces between the particles. Small pores are formed due to solvent evaporation. In contrast, the top surface of PCL4Gr6 exhibits a significantly different morphology, as depicted in Fig. 8g–l. Here, graphite particles are more prominent, resembling floating islands, and are arranged in a closely packed manner, resulting in a rougher surface compared to the lower surface. The presence of a large amount of graphite particles in the matrix reduces the possibility of polyhedral formation, as observed in neat PCL films. Additionally, the graphite particles prevent the polymer from forming spherulite-like structures. At lower magnifications, only the graphite part is prominently visible, while at higher magnifications, the polymer matrix can be clearly observed binding the graphite particles, ensuring their intactness on the surface. The porous areas depicted in Fig. 8k and l are composed of the polymer matrix, with the pores being formed as a result of solvent evaporation, while such pores are absent on the graphite part of the surface.
3.5 Mechanical properties of the polycaprolactone film and polycaprolactone–graphite chip electrode (PCL4Gr6) (stress–strain analysis)
Young's modulus (Emod), tensile strength at break (σb) and elongation at break (εb) were evaluated from the stress–strain curves of the PCL film and PCL4Gr6. Many samples were analyzed to observe the reproducibility of stress–strain properties. Young's modulus was calculated from the regression slope in the elastic region of the stress–strain curve. The tensile strength at break is the value of stress where it is the maximum and after that the film breaks. Elongation at break is the numerical value of strain where the film breaks.The stress–strain curves for the PCL film and PCL4Gr6 samples are depicted in Fig. 9. The obtained results align well with those reported in the literature.40 The influence of graphite in PCL4Gr6 is evident from the curves, which exhibit distinct differences. Both materials were completely dry, with the PCL film demonstrating its typical flexible nature. However, the insertion of graphite in PCL rendered the films brittle and hard, although they remained flexible and robust for the electrodes.
Young's modulus (Emod) for the PCL film was measured at 0.21 GPa, which is significantly lower than 1.11 GPa observed for PCL4Gr6 (with the R2 value being close to one (first-order polynomial) for the regression slope). This substantial increase in Young's modulus for PCL4Gr6, nearly five times higher than that of the PCL film, is attributed to the presence of graphite particles. The graphite fraction in PCL4Gr6 enhances its Young's modulus by impeding the displacement of polymer macromolecules with respect to one another. This effect arises from the presence of graphite particles, which act as reinforcing agents, reinforcing the polymer matrix and increasing its stiffness. On the other hand, the tensile strength at break (σb) for the PCL film was 18.06 MPa, which is notably higher than 10.49 MPa observed for PCL4Gr6. Similarly, the elongation at break (εb) for the PCL film was much greater at 28.27% compared to 2.76% for PCL4Gr6. These differences can be attributed to the presence of graphite particles, which reduce the mutual slip of PCL macromolecules and hinder the ability of PCL chains to straighten under tensile force in PCL4Gr6.
The addition of graphite powder to the PCL matrix results in an increase in both the tensile strength and Young's modulus. However, this enhancement comes at the expense of a decrease in both the tensile strength at break and elongation at break.
3.6 Scanning electron microscopy of the PANI coated PCL4Gr6 chip electrode
The SEM micrograph of the PCL4Gr6/PANI electrode is shown in Fig. 10b. The micrograph suggests that the coating of the conducting polymer is smooth and homogeneous. Some larger pores (gaps) are observed which originated during the fabrication of PCL4Gr6 during the drying process. Some granular dots are also visible in the micrograph with radial lines of the polymer around them. The dots are the nuclei formed during the initiation of polymerization which can be observed in the chronopotentiometry plot during polymerization of PANI (Fig. S2b) (ESI).† On the onset of the polymerization the potential was high, around 0.81 V, but after some time it decreased to around 0.75 V.47 The initial potential surge is attributed to the nucleation process of PANI which dropped down during the propagation stage. A photograph of the coated electrode is also shown in Fig. 10a. | ||
| Fig. 10 PCL4Gr6/PANI electrode: (a) photograph of the coated electrode and (b) SEM image of polyaniline coated on the PCL4Gr6 electrode. | ||
3.7 Electrochemical studies of the supercapacitor electrode (PCL4Gr6/PANI)
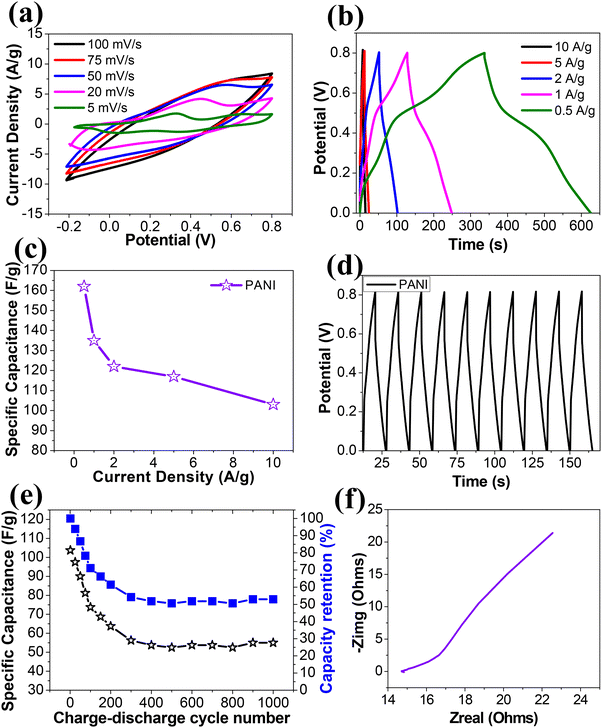
The results obtained for PCL4Gr6/PANI electrodes in supercapacitive testing were consistent with previous reports.31 Potentiodynamic cycling was performed with PCL4Gr6/PANI electrodes in 1 M H2SO4 with a three electrode configuration at various scan rates to test the charge–discharge properties. The corresponding cyclic voltammograms are depicted in Fig. 11a, revealing large rectangular loops with two oxidation and two reduction peaks. These peaks were clearly visible at low scan rates but became less distinct at higher scan rates The charge–discharge characteristics were also evaluated using the Galvanostatic mode, as illustrated in Fig. 11b, which shows charging–discharging curves recorded in the cut-off voltage range from 0.0 V to 0.8 V (versus Ag/AgCl) at five different applied current densities. Galvanostatic charge–discharge curves exhibited non-linear potential characteristics during the measurements due to the redox behavior of PANI. The specific capacitances were calculated from the charge–discharge curves. Specific capacitances calculated at current densities of 0.5 A g−1, 1 A g−1, 2 A g−1, 5 A g−1 and 10 A g−1 were 162 F g−1, 135 F g−1, 122 F g−1, 117 F g−1 and 103 F g−1, respectively as shown in Fig. 11c. To assess the electrode's lifespan, continuous charge–discharge was performed for 1000 cycles. Fig. 11d illustrates the initial 10 cycles of charge–discharge of the electrode within a cut-off potential window of 0.0 to 0.8 V at a constant current density of 10 A g−1. The triangular profiles observed during the charge–discharge process indicate good charge–discharge reversibility. Fig. 11e presents the measured specific capacitances of the electrode over 1000 cycles, showing an initial decrease until the 200th cycle followed by stabilization. A 50% capacitance loss occurred during the initial 200 cycles, after which no further loss was observed until the 1000th cycle. The Nyquist impedance plot of the electrode recorded in 1 M H2SO4 is shown in Fig. 11f, revealing a semicircle in the high-frequency region and an inclined line with a 45° angle in the low-frequency region. The semicircle represents the parallel combination of resistance and capacitance, while the inclined line corresponds to the Warburg impedance. The phase angle between the current and voltage at selected frequencies is provided in Table 2. Additionally, an intercept on the real axis of the impedance plot, measuring approximately 15 ohms, corresponds to the pure resistive component, indicating solution resistance and electrical connections in the circuit.| Sr. no. | Frequency (Hz) | Phase angle (°) |
|---|---|---|
| 1 | 0.01 | 78.01 |
| 2 | 0.13 | 43.49 |
| 3 | 1.02 | 08.64 |
| 4 | 13.39 | 02.92 |
| 5 | 104.78 | 01.02 |
| 6 | 1371.01 | 00.29 |
The specific capacitances were calculated from the charge–discharge curves using the following eqn (1):
| C = IΔt/mΔV | (1) |
After discussing the supercapacitive behavior of the system, it is imperative to examine the electrochemical behavior of the current collector in its pristine form (without PANI coating). The Nyquist impedance plot of the PCL4Gr6 electrode in 1 M H2SO4 solution with the same frequency range is presented in Fig. S3 (ESI).† The electrode exhibits only a very large semicircle in the plot, with the absence of Warburg resistance, indicating both resistive and capacitive behavior at the electrode–electrolyte interface within the applied frequency range. Thus, it is evident from the curve that the electrode solely serves as a current collector and does not contribute to demonstrating supercapacitance. The observed supercapacitive properties are attributed to the coated electroactive material.
4 Conclusion
In this study, PCLGr composite chips of various compositions were successfully fabricated, with particular focus on PCL4Gr6 for comprehensive characterization and electrochemical applications. The PCL4Gr6 current collector exhibited promising properties, demonstrating a high bulk conductivity of 65.6 mS cm−1 and good thermal stability. Morphological studies provided detailed insights into the surface morphology of the electrode. The comparison of crystallinity between the composite chip, neat graphite, and neat PCL provided valuable insights into the material properties, demonstrating an intrinsic understanding of their behavior. Moreover, stress–strain analysis revealed a fivefold increase in Young's modulus compared to that of the pristine PCL film, emphasizing the reinforcing effect of graphite in the chip, and highlighting its robustness and flexibility.The development of a supercapacitor electrode, PCL4Gr6/PANI, via electrodeposition demonstrated excellent adhesion and homogeneity, leading to comparable specific capacitance values in aqueous media. Notably, both electric double-layer capacitance (EDLC) and pseudocapacitive behavior were observed in PCL4Gr6/PANI electrodes, indicating their potential for versatile energy storage applications. The electrodes exhibited satisfactory stability in acidic solutions during electrochemical experiments. Impedance measurements further validated the supercapacitive behavior of both coated and uncoated current collectors. Overall, this study presents a technically efficient and economically viable approach utilizing green, robust, flexible, and chip-like electrodes for diverse electrochemical applications.
Author contributions
Rajeev Gupta: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, and writing – original draft. Monika Singhal: data curation and formal analysis.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors have no conflicts of interest to declare.Acknowledgements
We are thankful to CSIR India for providing instrumental facility. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.References
- J. Vatamanu and D. Bedrov, J. Phys. Chem. Lett., 2015, 6, 3594–3609 CrossRef CAS PubMed.
- P. Simon, P. Knauth, J. L. Tirado, J. Plocharski, W. Wieczorek, A. Barnabe, J.-M. Tarascon and C. Masquelier, J. Chem. Educ., 2011, 88, 1203–1206 CrossRef CAS.
- I. Shown, A. Ganguly, L.-C. Chen and K.-H. Chen, Energy Sci. Eng., 2015, 3, 2–26 CrossRef CAS.
- D. Li, Y. Li, Y. Feng, W. Hu and W. Feng, J. Mater. Chem. A, 2015, 3, 2135–2143 RSC.
- Z. Yang, J. Ren, Z. Zhang, X. Chen, G. Guan, L. Qiu, Y. Zhang and H. Peng, Chem. Rev., 2015, 115, 5159–5223 CrossRef CAS PubMed.
- B. Liu, Y. Ye, M. Yang, Y. Liu, H. Chen, H. Li, W. Fang and J. Qiu, Adv. Funct. Mater., 2024, 34, 2310534 CrossRef CAS.
- W. Zhang, B. Liu, M. Yang, Y. Liu, H. Li and P. Liu, J. Mater. Sci. Technol., 2021, 95, 105–113 CrossRef CAS.
- A. Mahto, R. Gupta, K. K. Ghara, D. N. Srivastava, P. Maiti, K. D, P.-Z. Rivera, R. Meena and S. K. Nataraj, J. Hazard. Mater., 2017, 340, 189–201 CrossRef CAS.
- R. Gupta, N. Vadodariya, A. Mahto, J. P. Chaudhary, D. B. Parmar, D. N. Srivastava, S. K. Nataraj and R. Meena, J. Appl. Electrochem., 2018, 48, 37–48 CrossRef CAS.
- J. P. Chaudhary, R. Gupta, A. Mahto, N. Vadodariya, K. Dharmalingm, N. Sanna Kotrappanavar and R. Meena, ACS Sustainable Chem. Eng., 2019, 7, 174–186 CrossRef CAS.
- S. Ghosh, T. Maiyalagan and R. N. Basu, Nanoscale, 2016, 8, 6921–6947 RSC.
- Q. Xie, R. Bao, A. Zheng, Y. Zhang, S. Wu, C. Xie and P. Zhao, ACS Sustainable Chem. Eng., 2016, 4, 1422–1430 CrossRef CAS.
- X. Zhang, X. Cui, C.-H. Lu, H. Li, Q. Zhang, C. He and Y. Yang, Chem. Eng. J., 2020, 401, 126031 CrossRef CAS.
- L. Li, D. Du, C. He, K. Han, W. Xu, L. Xia, G. Cai, X. Cui, Y. Chen, L. Yu and L. Kong, Polymer, 2024, 306, 127200 CrossRef CAS.
- L. Li, Y. Jiang, C. Guo, K. Han, X. Cui, C. He, Y. Chen and Y. Yang, Appl. Surf. Sci., 2024, 653, 159388 CrossRef CAS.
- R. Holze and Y. P. Wu, Electrochim. Acta, 2014, 122, 93–107 CrossRef CAS.
- B. Jugovic, M. Gvozdenovic, J. Stevanovic, T. Trisovic and B. Grgur, Mater. Chem. Phys., 2009, 114, 939–942 CrossRef CAS.
- M. Caglar, A. Arslan, R. Kilic and E. Hur, Synth. Met., 2015, 206, 8–14 CrossRef CAS.
- R. Gupta, A. Paul, J. C. Chaudhari and D. N. Srivastava, Synth. Met., 2016, 220, 95–101 CrossRef CAS.
- R. Gupta, M. Singhal and J. P. Chaudhary, Colloids Surf., A, 2022, 632, 127806 CrossRef CAS.
- Sowmya, Y. N. Sudhakar and M. Selvakumar, Ionics, 2016, 22, 1729–1739 CrossRef CAS.
- M. Bazzaoui, J. I. Martins, E. A. Bazzaoui, A. Albourine and L. Martins, Mater. Corros., 2014, 65, 67–75 CrossRef CAS.
- K. Jost, C. R. Perez, J. K. McDonough, V. Presser, M. Heon, G. Dion and Y. Gogotsi, Energy Environ. Sci., 2011, 4, 5060–5067 RSC.
- J. Xu, Q. Wang, X. Wang, Q. Xiang, B. Liang, D. Chen and G. Shen, ACS Nano, 2013, 7, 5453–5462 CrossRef CAS.
- L. Nyholm, G. Nystroem, A. Mihranyan and M. Stromme, Adv. Mater., 2011, 23, 3751–3769 CrossRef CAS PubMed.
- N. W. Duffy, W. Baldsing and A. G. Pandolfo, Electrochim. Acta, 2008, 54, 535–539 CrossRef CAS.
- R.-S. Kuehnel, J. Reiter, S. Jeong, S. Passerini and A. Balducci, Electrochem. Commun., 2014, 38, 117–119 CrossRef CAS.
- X.-L. Wu, T. Wen, H.-L. Guo, S. Yang, X. Wang and A.-W. Xu, ACS Nano, 2013, 7, 3589–3597 CrossRef CAS PubMed.
- J. Yu, J. Wu, H. Wang, A. Zhou, C. Huang, H. Bai and L. Li, ACS Appl. Mater. Interfaces, 2016, 8, 4724–4729 CrossRef CAS.
- A. L. Silva, M. M. Corrêa, G. C. de Oliveira, R. C. Michel, F. S. Semaan and E. A. Ponzio, New J. Chem., 2018, 42, 19537–19547 RSC.
- Kirti, R. Gupta and D. N. Srivastava, Electrochem., 2022, 3, 379–396 CrossRef CAS.
- M. Mascini, F. Pallozzi and A. Liberti, Anal. Chim. Acta, 1973, 64, 126–131 CrossRef CAS.
- L. N. Klatt, D. R. Connell, R. E. Adams, I. L. Honigberg and J. C. Price, Anal. Chem., 1975, 47, 2470–2472 CrossRef CAS.
- K. Štulík, V. Pacáková and B. Stárková, J. Chromatogr., A, 1981, 213, 41–46 CrossRef.
- H. S. Swofford and R. L. Carman, Anal. Chem., 1966, 38, 966–969 CrossRef CAS.
- C. Zhang, T. Zhai, L.-S. Turng and Y. Dan, Ind. Eng. Chem. Res., 2015, 54, 9505–9511 CrossRef CAS.
- M. Thakur, I. Majid, S. Hussain and V. Nanda, Packag. Technol. Sci., 2021, 34, 449–461 CrossRef CAS.
- S. Naseri, M. Diba, S. Golkar, A. R. Boccaccini and R. N. Klupp Taylor, Mater. Lett., 2014, 130, 164–167 CrossRef CAS.
- E. M. da Silva, G. C. de Oliveira, A. B. de Siqueira, A. J. Terezo and M. Castilho, Microchem. J., 2020, 158, 105228 CrossRef CAS.
- M. Zenkiewicz and J. Richter, Polimery, 2013, 58, 224–227 CrossRef CAS.
- M. A. Woodruff and D. W. Hutmacher, Prog. Polym. Sci., 2010, 35, 1217–1256 CrossRef CAS.
- K. K. Gupta, A. Kundan, P. K. Mishra, P. Srivastava, S. Mohanty, N. K. Singh, A. Mishra and P. Maiti, Phys. Chem. Chem. Phys., 2012, 14, 12844–12853 RSC.
- J. Y. Liu, L. Reni, Q. Wei, J. L. Wu, S. Liu, Y. J. Wang and G. Y. Li, eXPRESS Polym. Lett., 2011, 5, 742–752 CrossRef CAS.
- O. Persenaire, M. Alexandre, P. Degée and P. Dubois, Biomacromolecules, 2001, 2, 288–294 CrossRef CAS PubMed.
- C. P. Teng, K. Y. Mya, K. Y. Win, C. C. Yeo, M. Low, C. He and M.-Y. Han, NPG Asia Mater., 2014, 6, e142 CrossRef.
- S. M. Martins-Franchetti, A. Campos, T. A. Egerton and J. R. White, J. Mater. Sci., 2008, 43, 1063–1069 CrossRef CAS.
- M. A. Shishov, V. A. Moshnikov and I. Y. Sapurina, Chem. Pap., 2013, 67, 909–918 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr03113j |
| This journal is © The Royal Society of Chemistry 2024 |