Material design of biodegradable primary batteries: boosting operating voltage by substituting the hydrogen evolution reaction at the cathode
Shunsuke
Yamada
 * and
Takashi
Honda
* and
Takashi
Honda
Department of Electrical and Electronic Engineering, Kyushu Institute of Technology, 1-1 Sensuicho, Tobataku, Kitakyushu, Fukuoka 804-8550, Japan. E-mail: yamada@ele.kyutech.ac.jp
First published on 26th September 2024
Abstract
Transient primary batteries (TPBs) degrade after use without leaving harmful toxic substances, providing power sources for developing low-invasive and environmentally benign sensing platforms. Magnesium and zinc, both abundant on Earth, possess low anodic potentials and good biodegradability, making them useful as anode materials. However, molybdenum, a biodegradable metal, causes the hydrogen evolution reaction (HER) at the cathode, reducing the operating voltage of cells because of its low cathodic potential. In this review, we examine recent material designs to increase the operating voltage by introducing alternative electrochemical reactions at the cathode, including the oxygen reduction reaction, metal-ion intercalation into transition metal oxides, and halogen ionization, all of which have higher cathodic potentials than the HER. After discussing the characteristics, constituents, and demonstration of TPBs, we conclude by exploring their potential as power sources for implants, wearables, and environmental sensing applications.
1. Introduction
Emission of greenhouse gases, including carbon dioxide (CO2), leads to increasing global average temperature, resulting in worldwide climate change,1–3 loss of species,4–6 and natural disasters.7–9 To protect society, the environment, and wildlife, humans must address global climate change. Lifestyle changes, such as adopting renewable energy sources to generate electricity, can reduce overall carbon footprints; energy storage devices play critical roles in storing this electricity. Conventional research on batteries has been focused on large capacity to power electronic devices for an extended period with a single full charge, and is helping in the design of electrolytes, electrodes, and active materials.10–12 However, most battery materials are toxic, causing environmental pollution during outdoor usage as well as severe harm to human bodies when used as implants. If devices degrade after use without producing harmful substances,13 their application expands from indoor to outdoor or even inside human bodies. Transient electronics comprising bioderived materials satisfy this demand because of their intrinsic nature of biocompatibility, biodegradability, and environmental benignity. Early demonstrations of applying transient electronics have been as transistors,14–17 sensors,18–21 and optical devices22–24 using metals25–27 that include magnesium (Mg), zinc (Zn) molybdenum (Mo), tungsten (W) and iron (Fe), semiconductors (silicon (Si) and zinc oxide (ZnO)28), and polymers (silk and poly(lactic-co-glycolic acid) (PLGA)) for implants that monitor mouse temperature, acceleration, and brain waves. Si and these metals can be dissolved in water by hydrolysis, forming hydroxide or oxide ions (e.g., Si(OH)4, Mg(OH)2, Zn(OH)2,WO4−, MoO4− or Fe(OH)4−) via the following reactions,16,29–31 where oxides can sometimes be formed as intermediates:32Si:
| Si + 4H2O → Si(OH)4 + 2H2 | (1) |
Mg:
| Mg + 2H2O → Mg(OH)2 + H2 | (2) |
Zn:
| Zn + 2H2O → Zn(OH)2 + H2 | (3) |
W:
| W + 4H2O → WO42− + 8H+ + 6e− | (4) |
Mo:
| Mo + 4H2O → MoO42− + 8H+ + 6e− | (5) |
and Fe:
| 4Fe + 3O2 + 10H2O → 4Fe(OH)4− + 4H+ | (6) |
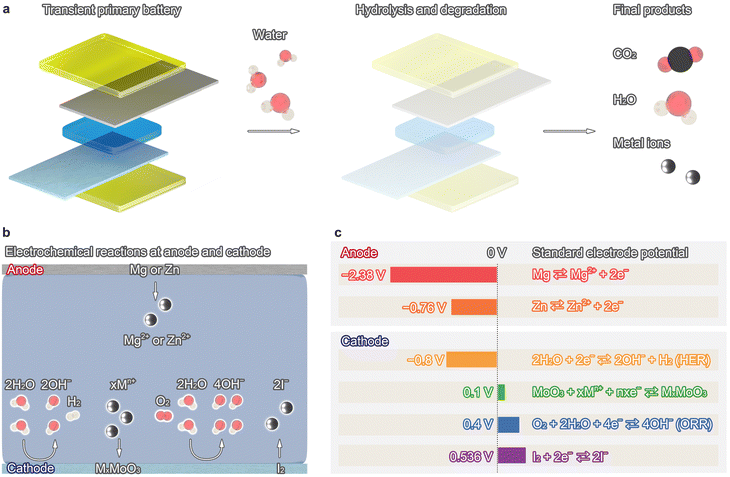
Similarly, silk is water-soluble and biodegradable via enzymatic degradation;16 PLGA degrades into lactic acid and glycolic acid through hydrolysis.33 This research area has gradually shifted to ionic devices, such as tactile devices,34–37 temperature sensors,38,39 and energy storage devices,40 adopting ions as carriers for device operation. Whereas transient supercapacitors41,42 and pseudo-capacitors31,43,44 show high power density and high cycling stability, transient primary batteries (TPBs) have minimalistic structures, are easy to fabricate, and are power sources for transient devices. TPBs are invaluable for enabling transient gadgets to operate inside human bodies and in outdoor environments where retrieval after use is not possible, addressing the issue of electronic waste through biodegradation (Fig. 1a).45 The output voltage is determined by the difference in the standard electrode potentials between the cathode and anode; however, TPBs are restricted by their low operating voltages due to the high potential of the hydrogen evolution reaction (HER) at the cathode. The output voltage is ∼0.8 V or less, and such low voltage requires multiple TPBs connected in series to operate light-emitting diodes (LEDs) and RF circuits.
In this review, we examine recent progress in TPBs and focus on their materials, reactions at the anode and cathode, and electrochemical characteristics. Transient electronics is an emerging research field that is undergoing intense study, and recent literature and reviews have discussed materials and their degradation behavior. This perspective focuses on the electrochemical reactions of anodes and cathodes to improve their electrochemical characteristics. We first highlight the fundamental reactions of TPBs and their performance. The next section reviews cathode materials and reactions to increase output voltage, power, and capacity (Fig. 1b and c). Finally, this perspective concludes with an outlook for expanding the use of TPBs for practical applications in implants and environmental sensing.
2. Suppression of the HER
2.1. Mg, Zn–metal batteries
Highlighted in recent literature, Mg is a biodegradable metal with low standard electrode potential E° vs. standard hydrogen electrode (SHE), exhibiting the following half-reaction:29| Mg → Mg2+ + 2e− (E° = −2.38 V vs. SHE) | (7) |
Such a low E° is beneficial for an anode material to achieve high operating voltage. Furthermore, Fe, Mo, and W exhibit high E° values of −0.44, −0.15, and 0.20 V vs. SHE, respectively. They are relatively stable in electrolytes and are adopted as cathode materials. The first report on TPBs by Yin et al. employed Mg and metal X (X = Fe, Mo, or W) as the anode and cathode, respectively, in which the electrolytes and encasements were phosphate-buffered saline (PBS) and polyanhydride, respectively (Fig. 2a).29 In Mg–X batteries, the dominant electrochemical reactions at the anode and cathode are Mg corrosion reactions and the HER, respectively, as follows:
Mg corrosion:
| Mg → Mg2+ + 2e− (E° = −2.38 V vs. SHE) | (8) |
The HER:
| 2H2O + 2e− → H2 + 2OH− (−1.05 V vs. Ag/AgCl) | (9) |
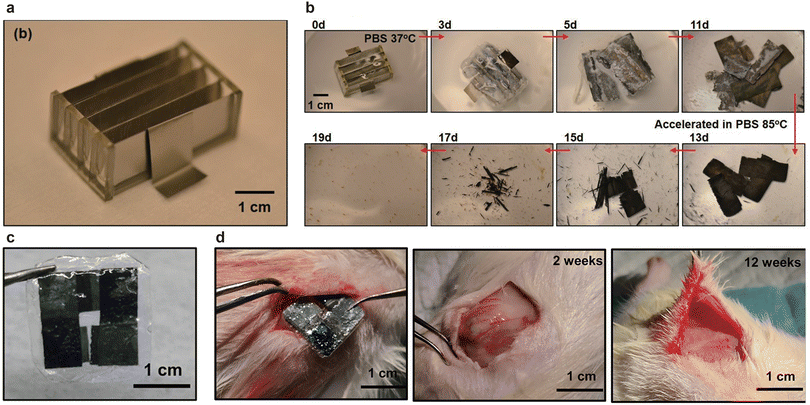 | ||
| Fig. 2 Transient primary batteries incorporating the HER at the cathode. Photographs of Mg–Mo batteries (a) after assembly and (b) during testing of their transient behavior in PBS at 37 °C and 80 °C. Reprinted with permission from ref. 29, Copyright 2014 Wiley–VCH. (c) Photographs of Zn–Mo batteries and (d) their transient behavior in vivo, implanted in SD rats. Reprinted with permission from ref. 46, Copyright 2023 American Chemical Society. | ||
Simultaneously, the following oxygen reduction reaction (ORR) occurs at the cathode:
| O2 + 2H2O + 4e− → 4OH− (0.179 V vs. Ag/AgCl) | (10) |
If the overall reaction at the cathode is the ORR, as shown in eqn (3), the operating voltage (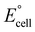 ) is ∼2.8 V, which is calculated as the difference in cathodic (
) is ∼2.8 V, which is calculated as the difference in cathodic (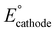 ) and anodic (
) and anodic (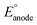 ) potentials as follows:
) potentials as follows:
 | (11) |
The actual output voltages for the Mg–Fe, Mg–W, and Mg–Mo batteries were ∼0.75, ∼0.65, and ∼0.45 V, respectively, because the HER and ORR occur simultaneously. The cathodic potential ranged from −1.05 to 0.179 V vs. Ag/AgCl with a low discharge current density of <0.1 mA cm−2, indicating that the HER and ORR occurred simultaneously. As the discharge current density increased, the HER became dominant at the cathode, resulting in a cathodic potential of −(1.2–1.3 V) vs. Ag/AgCl and an output voltage of <0.3 V at 1.5 mA cm−2. The transient behavior of the battery confirmed that the polyanhydride encasement disappeared first, leaving partially dissolved Mg and Mo foils after 11 d in PBS at 37 °C. Accelerated aging in PBS at 85 °C fully dissolved the Mo foils after another 8 d (Fig. 2b).
Huang et al. developed Zn–Mo batteries with long and stable operation without extra hydrogen production (Fig. 2c).46 The reactions at the anode and cathode were similar to those of Mg–Mo batteries, substituting Mg with Zn as follows:
Zn anode:
| Zn → Zn2+ + 2e− (E0 = −0.76 V vs. SHE) | (12) |
Mo cathode:
| 2H2O + 2e− → H2 + 2OH− (−1.05 V vs. Ag/AgCl) | (13) |
or
| O2 + 2H2O + 4e− → 4OH− (0.179 V vs. Ag/AgCl) | (14) |
Despite the Zn anodic potential being considerably larger than the Mg anodic potential, leading to a low operating voltage, Zn porous electrodes demonstrated a remarkable operating voltage of ∼0.6 V at 10 μA cm−2 in near-neutral environments. This value surpassed that of Zn foil electrodes. Moreover, when multiple Zn–Mo batteries were connected in series, they produced a sufficiently high operating voltage of >2.0 V for four and six cells. Four cells of Zn–Mo delivered nitric oxide (NO) on demand and modulated cell behavior. The battery fully degraded without substantial inflammatory response 12 weeks after implantation into the subcutaneous area of the posterior back of SD rats, underscoring the desirable biocompatibility and biodegradation of the batteries (Fig. 2d).
2.2. Mg air batteries
Mg–Mo or Zn–Mo batteries exhibit excellent biodegradability and biocompatibility as power sources for implants, yet the low operating voltage and power require multiple batteries connected in series to satisfy the power consumption of electronic devices. Conversely, Mg and Zn anodes have a sufficiently low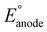 to increase
to increase 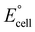 , and the low potential of the HER at the cathode leads to a reduction of
, and the low potential of the HER at the cathode leads to a reduction of 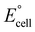 . Jia et al. adopted gold (Au) for the cathode to suppress the HER and make the ORR dominant in a Mg–Au battery,47 accelerating the ORR through the catalytic behavior of the Au foil (Fig. 3a). The main reactions are written as follows:
. Jia et al. adopted gold (Au) for the cathode to suppress the HER and make the ORR dominant in a Mg–Au battery,47 accelerating the ORR through the catalytic behavior of the Au foil (Fig. 3a). The main reactions are written as follows:
Anode:
| 2Mg + 4OH−→ 2Mg(OH)2 + 4e− (−2.69 V vs. SHE) | (15) |
Cathode:
| O2 + 2H2O + 4e− → 4OH− (0.40 V vs. SHE) | (16) |
Overall reaction:
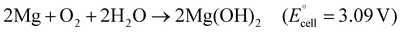 | (17) |
 | ||
| Fig. 3 Transient primary batteries with cathodes suppressing or substituting the HER. (a) Discharge profile for Mg–Au air batteries using anodes with different thicknesses. The open-circuit voltage of the encapsulated batteries under PBS or ambient air environments. Reprinted with permission from ref. 47, Copyright 2017 American Chemical Society. Photographs of (b) the Mo cathode with the Mo2C MXene coating and (c) the Mg–Mo2C air battery. Reprinted with permission from ref. 54, Copyright the author under the CC BY 4.0 license https://creativecommons.org/licenses/by/4.0/. (d) Demonstration of Mg–MoO3 illuminating a red LED in PBS solution for 16 h. Reprinted with permission from ref. 58, Copyright 2018 Wiley–VCH. (e) Mg–MoO3 with kirigami-patterned electrodes and its mechanical deformation with (f) stretching and twisting. Reprinted with permission from ref. 59, Copyright 2022 Wiley–VCH. | ||
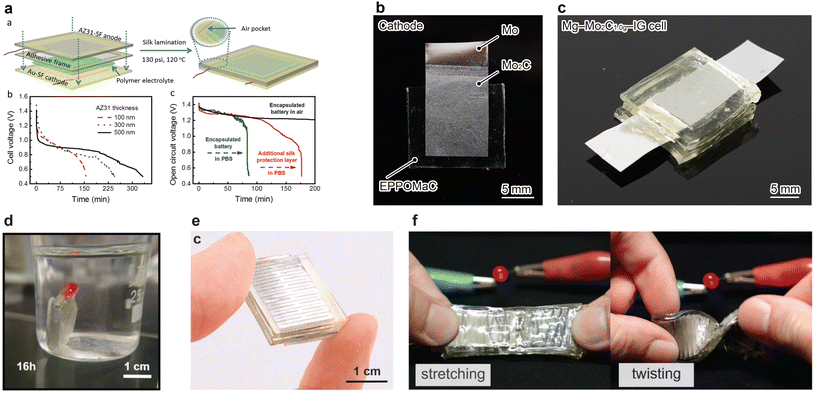
The open-circuit voltage of the Mg–Au battery was 1.58–1.45 V just after assembly of the cell, which was larger than that of Mg–Mo or Zn–Mo batteries by 0.50–1.0 V. The cell capacity was 2.2 mA h cm−2 with an operating voltage of ∼1.03 V and a discharge current density of 5.0 μA cm−2. Soaked in a buffered protease solution at 37 °C, the encapsulated battery (170 μm thickness) nearly disappeared after 45 d. The degradation of the silk substrate caused the Au foil to fragment in the solution. Although this result indicates that Au is beneficial for suppressing the HER and increasing the operating voltage, Au is a precious metal unsuitable for mass production applications. Mg air batteries need an abundant, biodegradable alternative cathode that suppresses the HER, highlighting the need for a more practical and sustainable solution in the field of Mg air batteries.
MXenes, comprising transition metals and carbon or nitrogen, have attracted attention as a new class of two-dimensional (2D) materials due to their large surface area, high conductivity, and various atomic compositions.48–51 In addition to the aforementioned characteristics, molybdenum carbide (Mo2C)–MXene shows biocompatibility and biodegradability and is used for biomedical applications.52 Bulk Mo2C has a unique energy diagram with energy barriers that make the HER kinetics slow in an alkaline environment.53 Similarly, Mo2C–MXene exhibits this property, making the ORR dominant in boosting the operating voltage. We reported Mg–Mo2C MXene air batteries using Mo2C, Mg, and ionic gel as the cathode, anode, and electrolyte, respectively.54 Unless otherwise noted, Mo2C represents the MXene form henceforth. The bioderived ionic liquid and poly(vinyl) alcohol (PVA) are classified as readily biodegradable, forming a biodegradable ionic gel.55,56 As for Mg–Mo cells, the Mo cathode exhibited a potential of −1.2 V vs. Ag/AgCl with 40 wt% [Ch][Lac], and 60 wt% water during operation, the value of which was comparable or less than those of previous works. Furthermore, the Mg–Mo2C cells (Mo cathode coated with Mo2C) (Fig. 3b) showed a cathodic potential of −0.3 V vs. Ag/AgCl just after discharge. This value was close to that of the ORR potential of 0.179 V vs. Ag/AgCl, indicating that Mo2C hinders the HER in the cell. The liquid electrolyte penetrated the Mo2C layer and made contact with the Mo foil to decrease the cathodic potential. We solidified the liquid electrolyte in poly(vinyl) alcohol to create ionic gels, preventing soaking of the electrolyte. All solid-state Mg–Mo2C–IG 1 cells yielded an open-circuit potential and operating voltage of 1.6 and 1.4 V at 0.1 mA cm−2 (Fig. 3c). The operating voltage was 3.1 and 2.3 times higher than that of Mg–Mo and Zn–Mo cells, respectively. Furthermore, the Mg–Mo2C–IG cell afforded a maximum power of 0.92 mW cm−2, which was >100 times higher than those of Zn–Mo cells. The Mg–Mo2C–IG 2 cells successfully operated a wireless sensor module, indicating a power source for environmental sensing. In its transient behavior, the Mg–Mo2C–IG cell dissolved in 100 mL of 10 mM PBS solution at 37 °C after 123 d of soaking, leaving only small fragments of Mo and polymer substrate.
3. Adoption of alternative reactions
3.1. Mg–MoO3 battery
As discussed above, suppression of the HER is beneficial in boosting the operating voltage, and another approach is to adopt alternative electrochemical reactions with high cathodic potentials. MoO3 has been comprehensively investigated as a material for lithium-ion batteries, involving Mo(VI) reduction and Mg intercalation reactions. Furthermore, Mo is recognized as a safe material for human bodies, with a daily Mo intake of 300 μg57 and solubility in aqueous solution of ∼1 g L−1.58 MoO3 is, therefore, a promising choice for biodegradable batteries. Huang et al. developed Mg–MoO3 batteries,58 substituting the HER at the cathode with metal-ion (M) intercalation reactions as follows:| MoO3 + Mxn+ + nxe− → MxMoO3 | (18) |
The MoO3 crystal has a layered crystalline structure, forming a tiny gap (van der Waals gap, vdW gap) at the interlayers.31 When MoO3 is immersed in electrolytes containing small ions, Li+, Na+, Mg2+, and Ca2+, the ions absorb and desorb onto the surface of the oxide, and the ions are additionally intercalated and deintercalated into the vdW gap. They reported that the Mg–MoO3 cell showed an operating voltage of >1.6 V, achieving an increase in voltage by 1.0 V compared with the Mg–Mo cell. The maximum power and capacity were 0.27 mW cm−2 and 1.72 mA h cm−2, respectively, with a discharge current of 45 μA cm−2. The Mg–MoO3 cell operated a red-light LED with a threshold voltage of ∼1.5 V for 16 h and a calculator with a liquid crystal display (Fig. 3d). Mg, sodium alginate hydrogel, and the MoO3−PLGA layer completely dissolved in PBS (pH 7.4) at 37 °C within 9 d, and Mo disappeared at elevated temperature (85 °C) in another 10 d. An in vivo study of Mg–MoO3 biodegradability on Sprague–Dawley rats revealed that the battery fully degraded in 4 weeks. Karami–Mosammam et al. employed Kirigami-patterned electrodes to make the Mg–MoO3 cell stretchable by 50%, and the cell could illuminate a red LED, even when stretched and twisted (Fig. 3e and f).59 Mg, MoO3, calcium alginate electrolyte and the poly(glycerol sebacate) package dissolved in PBS (pH 7.4) at 37 °C after 10 weeks, and an additional degradation test completely degraded the Mg–MoO3 battery at elevated temperature (85 °C) in another 3 weeks.
3.2. The Mg–I2 battery
Similarly, Huang et al. adopted iodine(I), which shows a higher cathodic potential than that of the HER as follows:60| I2 + 2e− ⇄ 2I− (E° = 0.536 V vs. SHE) | (19) |
The key design of the developed Mg–I2 battery was a dual-electrolyte system comprising a choline chloride/urea-based ionic liquid and an aqueous medium as the catholyte and anolyte, respectively. The conventional design allowed the H2 produced at the anode to attack the I2 cathode (H2 poisoning), quickly dissolving I2. In the proposed design, the catholyte and a polyanhydride encapsulation protected I2 from dissolution and retained I2 in the cathode. The Mg–I2 battery achieved a high operating voltage (∼1.8 V) with an areal capacity of ∼9.8 mA h cm−2, areal energy density of ∼17.7 mW h cm−2, areal power density of ∼0.7 mW cm−2, volumetric energy density of ∼93.0 mW h cm−3, and volumetric power density of ∼3.8 mW cm−3. The degradation behavior in PBS solution at 37 °C began with the hydrolysis of Mg (0.05–0.5 mm h−1 under physiological conditions) and polyanhydride (10−2 μg d−1 in physiological conditions), followed by the dissolution of I2 into ions. Finally, the Mg–I2 battery fully dissolved under slow hydrolysis of Mo (10−4–10−3 μm h−1 at room temperature).
4. Conclusions and outlook
Recent progress in TPBs enables the operation of transient devices in living organisms and the environment that can be degraded after use without producing harmful substances. In the early stages of their development, TPBs were restricted by low operating voltage due to the HER, and the introduction of alternative electrochemical reactions at the cathode became an essential feature of substituting or suppressing the HER to advance battery performance (Table 1). The latest TPBs operate wireless sensors for temperature and humidity monitoring using conventional nondegradable electronic devices,54 and the integration of transient devices, including energy storage, sensors, and transistors, is crucial for practical applications of transient electronics. Biodegradability is an attractive characteristic for environmental sensing and implants; nonetheless, ensuring stability during device operation is an important issue. TPBs have a natural tendency to degrade because of the biodegradability of the materials. Encapsulation materials that are stable yet biodegradable are essential for TPBs. The advancement of development routines is essential for the mass production of TPBs. One potential application of TPBs is as a power source for transient electronics, which requires flexibility and stretchability to adhere to the human body without discomfort. Consequently, TPBs must be developed on flexible and stretchable substrates to ensure mechanical softness. Printing methods can deposit hydrogels61,62 and ionic gels63–65 on such substrates; however, conventional chemical or physical vapor deposition cannot be applied to create metal electrodes on the stretchable substrates due to the difference in metal and substrate thermal expansion. Therefore, transfer printing is advantageous for laying highly conductive materials on soft substrates, using self-assembly monolayers (SAM, Fig. 4a and b),66 sacrificial metals,67–71 and polymer layers.72 The surface area of electrodes determines the capacity and power of energy storage devices; therefore, thick, porous, and highly conductive electrodes are required. Electrochemical sintering is a promising method to yield electrodes such as those of Zn. In humid air, the surfaces of Zn particles are passivated with ZnO and Zn(OH)2, which slowly convert to zinc hydroxy carbonates, Zn4CO3(OH)·6H2O and Zn5(CO3)2·(OH)6.73 When Zn particles are soaked in acetic acid, the solution strips the passivation layer and promotes self-exchange between Zn and Zn2+ at the Zn/H2O interfaces between the particles (Fig. 4c and d). This sintering yields porous Zn electrodes with a conductivity of over 105 S m−1 and a BET surface area of 8.3 m2 g−1.74 Electrochemical sintering is compatible with conventional screen printing and this process can fabricate functional Zn cathodes for TPBs on a large scale.75 Polymer–metal particle composites offer a combination of high conductivity, stretchability, and flexibility; printing these biodegradable composites promises transient electrodes for TPBs. Adding metal particles to polymers results in bio/eco-resorbable, conductive, and printable inks. These inks include Mo microparticles and polyanhydride (Fig. 4e);76 Mo microparticles and polybutylene adipate terephthalate (PBAT),77 W powder, beeswax,78 and glycofurol, and W powder and natural wax (candelilla, myrtle, and soy wax, Fig. 4f and g).79 The addition of materials that suppress or substitute the HER enables the production of printable inks for TPB cathodes, allowing for large-scale manufacturing on soft substrates. Primary batteries are not rechargeable because of the irreversible electrochemical reactions that govern their operation;80–83 however, rechargeable secondary batteries may be promising candidates for long-term operation. Integrating batteries, electrical circuits, and energy harvesting will promise power sources that continuously operate electronic devices until they disappear via biodegradation.84–86 The biodegradability and benignity of TPBs are great advantages, compared with those of conventional batteries, and their applications range from biomedical applications to smart agriculture and livestock industry uses. Wireless power transfer operates various transient devices implanted in rat bodies.87–89 While wireless power transfer eliminates the need for a battery, it requires large receiver and transmitter coils, and the output voltage decreases with increasing distance. Wireless power transfer can face challenges in supplying power to transient devices implanted deep within human bodies. In contrast, TPBs are self-sustaining power sources that can supply energy to implanted transient devices, even in locations inaccessible by wireless power transfer. TPBs provide opportunities for monitoring the real-time healing of tissues or injuries, which can support personalized care systems and rehabilitation protocols.90 In smart agriculture and the livestock industry, the growing global population makes it essential to increase the yield of agricultural products and livestock to address food shortages worldwide.91 Smart agriculture systems comprise sensor networks, information systems, advanced machinery, and informed management practices, monitoring soil conditions, livestock health, and crop development through sensors and wearables for plants and livestock, collecting critical data for decision-making and management.92,93 TPBs can be a promising power source for these sensing systems without contaminating food or soil due to their biodegradability and environmental friendliness. The rapid advancement of transient electronics will enable the development of biomedical, wearable, and environmentally friendly devices that decompose after use.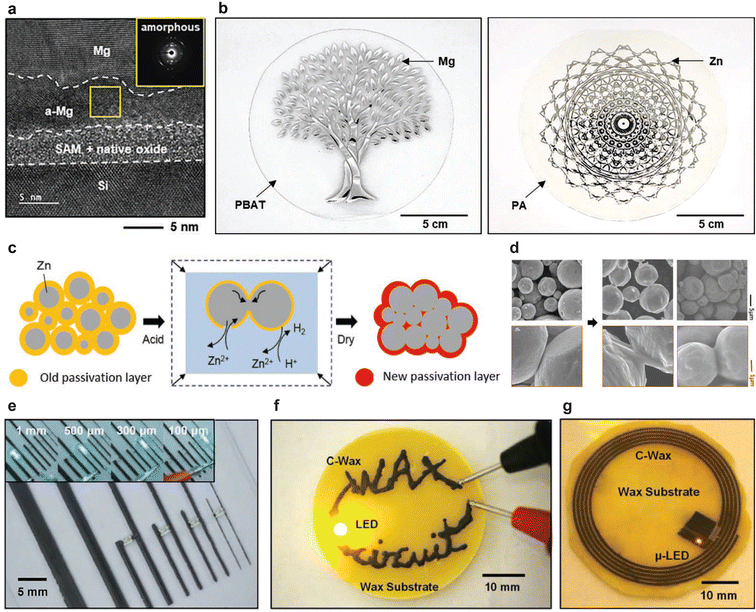 | ||
| Fig. 4 Printing methods and inks for fabricating electrodes on biodegradable substrates. (a) Cross-sectional transmission electron microscopy image of Mg and SAM layers. (b) Photographs of transferred Zn and Mg patterns on biodegradable substrates. Reprinted with permission from ref. 66, Copyright 2024 Wiley–VCH. (c) Mechanism of Zn sintering and (d) image of sintered Zn particles. Reprinted with permission from ref. 73, Copyright 2017 Wiley–VCH. (e) Biodegradable inks composed of Mo microparticles and polyanhydride. Reprinted with permission from ref. 76, Copyright 2018 Elsevier Ltd. (f) Conductive wax made from candelilla and W powder, and (g) its application in wireless power transfer. Reprinted with permission from ref. 79, Copyright 2018 Wiley–VCH. | ||
| No. | Cell | Electrolyte | Operating voltage | Maximum power | Capacity | Lifetime | Year | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Mg–Mo, W, or Fe | Phosphate buffered saline (PBS) solution | 0.45 V | — | 276 mA h g−1 at 0.1 mA cm−2 (Mg–Mo) | 11 d in PBS at 37 °C and another 8 d in PBS at 85 °C | 2014 | 29 |
| 2 | Zn–Mo | 0.9 wt% NaCl saline or hydrogel | 0.6 V | 6 μW cm−2 | 1596 μW h at 5 μA cm−2, 1728 μW h at 10 μA cm−2 | 12 weeks in the subdermal region of SD rats | 2023 | 46 |
| 3 | Mg–Au | Hydrogel (silk fibroin aqueous solution 7.5 wt%) | 1.0 V | 8.7 μW cm−2 | 0.06 mA h cm−2 at 10 μA cm−2 (unsealed 1.43 mA h cm−2) | 45 d in buffered protease solution at 37 °C. | 2017 | 47 |
| 4 | Mg–Mo2C | Ionic gel [Ch][Lac]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DIW DIW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PVA = 34 PVA = 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
1.4 V | 0.92 mW cm−2 | 0.57 mA h g−1 at 0.1 mA cm−2 | 123 d in PBS at 37 °C leaving EPPOMaC fragments | 2024 | 54 |
| 5 | Mg–MoO3 | Hydrogel (sodium alginate), PBS solution | 1.6 V | 0.27 mW cm−2 | 6.5 mA h cm−2 | 9 d in PBS at 37 °C and another 10 d at 85 °C, 4 weeks in SD rats | 2018 | 58 |
| 6 | Mg–MoO3 | Hydrogel (sodium alginate), PBS solution | 1.5 V | 0.196 mW cm−2 | 1.72 mA h cm−2 at 45 μA cm−2 | 10 weeks in PBS at 35 °C + 3 weeks in PBS at 85 °C | 2022 | 59 |
| 7 | Mg–I2 | PBS/ChCl urea-based ILs (anolyte/catholyte) | 1.8 V | ∼0.7 mW cm−2 | 3.9 mA h cm−2 at 0.4 mA cm−2 | 7 d in PBS at 37 °C, and another 21 d PBS at 85 °C | 2022 | 60 |
Author contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript. Credits: Shunsuke Yamada, conceptualization, writing – original draft, writing – review and editing, supervision, and funding acquisition; Takashi Honda, writing – review and editing.Data availability
No new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP24K01313, Iwatani Naoji Foundation, ENEOS Tonen General Sekiyu Research/Development Encouragement and Scholarship Foundation, and The Ebara Hatakeyama Memorial Foundation.References
- L. V. Gatti, L. S. Basso, J. B. Miller, M. Gloor, L. Gatti Domingues, H. L. G. Cassol, G. Tejada, L. E. O. C. Aragão, C. Nobre, W. Peters, L. Marani, E. Arai, A. H. Sanches, S. M. Corrêa, L. Anderson, C. Von Randow, C. S. C. Correia, S. P. Crispim and R. A. L. Neves, Nature, 2021, 595, 388–393 CrossRef CAS PubMed
.
- T. M. Lenton, C. Xu, J. F. Abrams, A. Ghadiali, S. Loriani, B. Sakschewski, C. Zimm, K. L. Ebi, R. R. Dunn, J.-C. Svenning and M. Scheffer, Nat. Sustain., 2023, 6, 1237–1247 CrossRef
.
- A. K. Magnan, H.-O. Pörtner, V. K. E. Duvat, M. Garschagen, V. A. Guinder, Z. Zommers, O. Hoegh-Guldberg and J.-P. Gattuso, Nat. Clim. Change, 2021, 11, 879–885 CrossRef
.
- V. Barbarossa, J. Bosmans, N. Wanders, H. King, M. F. P. Bierkens, M. A. J. Huijbregts and A. M. Schipper, Nat. Commun., 2021, 12, 1701 CrossRef CAS PubMed
.
- F. Benedetti, M. Vogt, U. H. Elizondo, D. Righetti, N. E. Zimmermann and N. Gruber, Nat. Commun., 2021, 12, 5226 CrossRef CAS PubMed
.
- C. Chaudhary, A. J. Richardson, D. S. Schoeman and M. J. Costello, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2015094118 CrossRef CAS
.
- A. Dai, Nat. Clim. Change, 2013, 3, 52–58 CrossRef
.
- T. Geiger, J. Gütschow, D. N. Bresch, K. Emanuel and K. Frieler, Nat. Clim. Change, 2021, 11, 861–866 CrossRef
.
- R. Newman and I. Noy, Nat. Commun., 2023, 14, 6103 CrossRef CAS
.
- S. Deng, H. Zhu, G. Wang, M. Luo, S. Shen, C. Ai, L. Yang, S. Lin, Q. Zhang, L. Gu, B. Liu, Y. Zhang, Q. Liu, G. Pan, Q. Xiong, X. Wang, X. Xia and J. Tu, Nat. Commun., 2020, 11, 132 CrossRef CAS
.
- L. Sun, Y. Liu, J. Wu, R. Shao, R. Jiang, Z. Tie and Z. Jin, Small, 2022, 18, 2102894 CrossRef CAS PubMed
.
- F. Wang, B. Wang, J. Li, B. Wang, Y. Zhou, D. Wang, H. Liu and S. Dou, ACS Nano, 2021, 15, 2197–2218 CrossRef CAS PubMed
.
- A. Nedjalkov, J. Meyer, M. Köhring, A. Doering, M. Angelmahr, S. Dahle, A. Sander, A. Fischer and W. Schade, Batteries, 2016, 2, 5 CrossRef
.
- J. K. Chang, H. Fang, C. A. Bower, E. M. Song, X. G. Yu and J. A. Rogers, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E5522–E5529 CAS
.
- S. W. Hwang, C. H. Lee, H. Y. Cheng, J. W. Jeong, S. K. Kang, J. H. Kim, J. Shin, J. Yang, Z. J. Liu, G. A. Ameer, Y. G. Huang and J. A. Rogers, Nano Lett., 2015, 15, 2801–2808 CrossRef CAS PubMed
.
- S. W. Hwang, H. Tao, D. H. Kim, H. Y. Cheng, J. K. Song, E. Rill, M. A. Brenckle, B. Panilaitis, S. M. Won, Y. S. Kim, Y. M. Song, K. J. Yu, A. Ameen, R. Li, Y. W. Su, M. M. Yang, D. L. Kaplan, M. R. Zakin, M. J. Slepian, Y. G. Huang, F. G. Omenetto and J. A. Rogers, Science, 2012, 337, 1640–1644 CrossRef CAS
.
- D.-H. Kim, Y.-S. Kim, J. Amsden, B. Panilaitis, D. L. Kaplan, F. G. Omenetto, M. R. Zakin and J. A. Rogers, Appl. Phys. Lett., 2009, 95, 133701 CrossRef PubMed
.
- M. Baumgartner, F. Hartmann, M. Drack, D. Preninger, D. Wirthl, R. Gerstmayr, L. Lehner, G. Mao, R. Pruckner and S. Demchyshyn, Nat. Mater., 2020, 19, 1102–1109 CrossRef CAS
.
- C. M. Boutry, Y. Kaizawa, B. C. Schroeder, A. Chortos, A. Legrand, Z. Wang, J. Chang, P. Fox and Z. N. Bao, Nat. Electron., 2018, 1, 314–321 CrossRef
.
- G. A. Salvatore, J. Sülzle, F. Dalla Valle, G. Cantarella, F. Robotti, P. Jokic, S. Knobelspies, A. Daus, L. Büthe and L. Petti, Adv. Funct. Mater., 2017, 27, 1702390 CrossRef
.
- S.-K. Kang, R. K. Murphy, S.-W. Hwang, S. M. Lee, D. V. Harburg, N. A. Krueger, J. Shin, P. Gamble, H. Cheng and S. Yu, Nature, 2016, 530, 71–76 CrossRef CAS PubMed
.
- A. Espinha, C. Dore, C. Matricardi, M. I. Alonso, A. R. Goñi and A. Mihi, Nat. Photonics, 2018, 12, 343–348 CrossRef CAS
.
- G. H. Lee, H. Moon, H. Kim, G. H. Lee, W. Kwon, S. Yoo, D. Myung, S. H. Yun, Z. Bao and S. K. Hahn, Nat. Rev. Mater., 2020, 5, 149–165 CrossRef
.
- W. B. Han, J. H. Lee, J. W. Shin and S. W. Hwang, Adv. Mater., 2020, 32, 2002211 CrossRef CAS PubMed
.
- S. Lee, J. Koo, S. K. Kang, G. Park, Y. J. Lee, Y. Y. Chen, S. A. Lim, K. M. Lee and J. A. Rogers, Mater. Today, 2018, 21, 207–215 CrossRef CAS
.
- Y. Liu, Y. Zheng, X. H. Chen, J. A. Yang, H. Pan, D. Chen, L. Wang, J. Zhang, D. Zhu and S. Wu, Adv. Funct. Mater., 2019, 29, 1805402 CrossRef
.
- L. Yin, H. Y. Cheng, S. M. Mao, R. Haasch, Y. H. Liu, X. Xie, S. W. Hwang, H. Jain, S. K. Kang, Y. W. Su, R. Li, Y. G. Huang and J. A. Rogers, Adv. Funct. Mater., 2014, 24, 645–658 CrossRef CAS
.
- C. Dagdeviren, S. W. Hwang, Y. Su, S. Kim, H. Cheng, O. Gur, R. Haney, F. G. Omenetto, Y. Huang and J. A. Rogers, Small, 2013, 9, 3398–3404 CrossRef CAS PubMed
.
- L. Yin, X. Huang, H. X. Xu, Y. F. Zhang, J. Lam, J. J. Cheng and J. A. Rogers, Adv. Mater., 2014, 26, 3879–3884 CrossRef CAS PubMed
.
- S. Yamada, ACS Appl. Nano Mater., 2023, 6, 7229–7233 CrossRef CAS
.
- H. Sheng, J. Zhou, B. Li, Y. He, X. Zhang, J. Liang, J. Zhou, Q. Su, E. Xie and W. Lan, Sci. Adv., 2021, 7, eabe3097 CrossRef CAS PubMed
.
- J.-S. Shim, J. A. Rogers and S.-K. Kang, Mater. Sci. Eng., R, 2021, 145, 100624 CrossRef
.
- J. Lee, H. R. Cho, G. D. Cha, H. Seo, S. Lee, C. K. Park, J. W. Kim, S. Qiao, L. Wang, D. Kang, T. Kang, T. Ichikawa, J. Kim, H. Lee, W. Lee, S. Kim, S. T. Lee, N. Lu, T. Hyeon, S. H. Choi and D. H. Kim, Nat. Commun., 2019, 10, 5205 CrossRef
.
- Y. Dobashi, D. Yao, Y. Petel, T. N. Nguyen, M. S. Sarwar, Y. Thabet, C. L. Ng, E. Scabeni Glitz, G. T. M. Nguyen and C. Plesse, Science, 2022, 376, 502–507 CrossRef CAS
.
- T. Li, Y. Wang, S. Li, X. Liu and J. Sun, Adv. Mater., 2020, 32, 2002706 CrossRef
.
- S. Yamada, T. Sato and H. Toshiyoshi, Appl. Phys. Lett., 2017, 110, 253501 CrossRef
.
- S. Yamada and H. Toshiyoshi, Sens. Actuators, A, 2023, 361, 114574 CrossRef CAS
.
- I. You, D. G. Mackanic, N. Matsuhisa, J. Kang, J. Kwon, L. Beker, J. Mun, W. Suh, T. Y. Kim and J. B.-H. Tok, Science, 2020, 370, 961–965 CrossRef CAS
.
- S. Yamada and H. Toshiyoshi, ACS Appl. Mater. Interfaces, 2020, 12, 36449–36457 CrossRef CAS
.
- S. Yamada, ACS Appl. Mater. Interfaces, 2022, 14, 26595–26603 CrossRef CAS
.
- G. Lee, S. K. Kang, S. M. Won, P. Gutruf, Y. R. Jeong, J. Koo, S. S. Lee, J. A. Rogers and J. S. Ha, Adv. Energy Mater., 2017, 7, 1700157 CrossRef
.
- L. Migliorini, C. Piazzoni, K. Pohako-Esko, M. Di Girolamo, A. Vitaloni, F. Borghi, T. Santaniello, A. Aabloo and P. Milani, Adv. Funct. Mater., 2021, 31, 2102180 CrossRef CAS
.
- H. Lee, G. Lee, J. Yun, K. Keum, S. Y. Hong, C. Song, J. W. Kim, J. H. Lee, S. Y. Oh, D. S. Kim, M. S. Kim and J. S. Ha, Chem. Eng. J., 2019, 366, 62–71 CrossRef CAS
.
- S. Yamada, Small, 2023, 19, 2205598 CrossRef CAS
.
- S. Yamada, Adv. Energy Sustainability Res., 2023, 4, 2300083 CrossRef
.
- X. Y. Huang, H. Q. Hou, B. B. Yu, J. Bai, Y. J. Guan, L. Wang, K. T. Chen, X. B. Wang, P. C. Sun, Y. P. Deng, S. B. Liu, X. Cai, Y. Wang, J. Peng, X. Sheng, W. Xiong and L. Yin, ACS Nano, 2023, 17, 5727–5739 CrossRef CAS
.
- X. T. Jia, C. Y. Wang, V. Ranganathan, B. Napier, C. C. Yu, Y. F. Chao, M. Forsyth, F. G. Omenetto, D. R. MacFarlane and G. G. Wallace, ACS Energy Lett., 2017, 2, 831–836 CrossRef CAS
.
- M. Ghidiu, M. R. Lukatskaya, M.-Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78–81 CrossRef CAS
.
- M. R. Lukatskaya, O. Mashtalir, C. E. Ren, Y. Dall'Agnese, P. Rozier, P. L. Taberna, M. Naguib, P. Simon, M. W. Barsoum and Y. Gogotsi, Science, 2013, 341, 1502–1505 CrossRef CAS
.
- Q. Z. Zhu, J. P. Li, P. Simon and B. Xu, Energy Storage Mater., 2021, 35, 630–660 CrossRef
.
- W. Liu, X. Wang, F. Wang, K. Du, Z. Zhang, Y. Guo, H. Yin and D. Wang, Nat. Commun., 2021, 12, 6776 CrossRef CAS
.
- W. Feng, R. Wang, Y. Zhou, L. Ding, X. Gao, B. Zhou, P. Hu and Y. Chen, Adv. Funct. Mater., 2019, 29, 1901942 CrossRef
.
- W. Liu, X. Wang, F. Wang, K. Du, Z. Zhang, Y. Guo, H. Yin and D. Wang, Nat. Commun., 2021, 12, 6776 CrossRef CAS
.
- S. Yamada, ACS Appl. Mater. Interfaces, 2024, 16, 14759–14769 CrossRef CAS
.
- S. Yamada, Small, 2023, 19, 2302385 CrossRef CAS
.
- S. Yamada and H. Toshiyoshi, Small, 2018, 14, 1800937 CrossRef
.
- K. V. Rajagopalan, Annu. Rev. Nutr., 1988, 8, 401–427 CrossRef CAS
.
- X. Huang, D. Wang, Z. Yuan, W. Xie, Y. Wu, R. Li, Y. Zhao, D. Luo, L. Cen and B. Chen, Small, 2018, 14, 1800994 CrossRef
.
- M. Karami-Mosammam, D. Danninger, D. Schiller and M. Kaltenbrunner, Adv. Mater., 2022, 34, 2204457 CrossRef CAS PubMed
.
- I. Huang, Y. M. Zhang, H. M. Arafa, S. P. Li, A. Vazquez-Guardado, W. Ouyang, F. Liu, S. Madhvapathy, J. W. Song, A. Tzavelis, J. Trueb, Y. Choi, W. J. Jeang, V. Forsberg, E. Higbee-Dempsey, N. Ghoreishi-Haack, I. Stepien, K. Bailey, S. L. Han, Z. J. Zhang, C. Good, Y. G. Huang, A. J. Bandodkar and J. A. Rogers, Energy Environ. Sci., 2022, 15, 4095–4108 RSC
.
- Y. Hui, Y. Yao, Q. Qian, J. Luo, H. Chen, Z. Qiao, Y. Yu, L. Tao and N. Zhou, Nat. Electron., 2022, 5, 893–903 CrossRef CAS
.
- K. Li, J. Zhao, A. Zhussupbekova, C. E. Shuck, L. Hughes, Y. Dong, S. Barwich, S. Vaesen, I. V. Shvets and M. Möbius, Nat. Commun., 2022, 13, 6884 CrossRef CAS PubMed
.
- C. Jin, W. Liu, Y. Huang, Y. Xu, Y. Nie, G. Zhang, P. He, J. Sun and J. Yang, Appl. Phys. Lett., 2022, 120, 233701 CrossRef CAS
.
- C. Jin, W. Liu, Y. Xu, Y. Huang, Y. Nie, X. Shi, G. Zhang, P. He, J. Zhang and H. Cao, Nano Lett., 2022, 22, 3372–3379 CrossRef CAS PubMed
.
- Y. Wang, Y. Yuan, H. Geng, W. Yang and X. Chen, Adv. Funct. Mater., 2024, 2400887 CrossRef CAS
.
- S. M. Lee, W. J. Lee, J. Y. Bae, J. W. Gu, S. Lee, K. B. Yeo, J. Lee, J. W. Kim, J. Y. Lee and J. Kim, Adv. Funct. Mater., 2024, 34, 2310612 CrossRef CAS
.
- J. K. Chang, H. P. Chang, Q. Guo, J. Koo, C. I. Wu and J. A. Rogers, Adv. Mater., 2018, 30, 1704955 CrossRef
.
- S. H. Jin, S.-K. Kang, I.-T. Cho, S. Y. Han, H. U. Chung, D. J. Lee, J. Shin, G. W. Baek, T.-I. Kim and J.-H. Lee, ACS Appl. Mater. Interfaces, 2015, 7, 8268–8274 CrossRef CAS
.
- J.-H. Kim, A. Nizami, Y. Hwangbo, B. Jang, H.-J. Lee, C.-S. Woo, S. Hyun and T.-S. Kim, Nat. Commun., 2013, 4, 2520 CrossRef PubMed
.
- C. H. Lee, D. R. Kim and X. Zheng, Nano Lett., 2011, 11, 3435–3439 CrossRef CAS
.
- D. S. Wie, Y. Zhang, M. K. Kim, B. Kim, S. Park, Y.-J. Kim, P. P. Irazoqui, X. Zheng, B. Xu and C. H. Lee, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, E7236–E7244 CrossRef CAS PubMed
.
- T. Makita, A. Yamamura, J. Tsurumi, S. Kumagai, T. Kurosawa, T. Okamoto, M. Sasaki, S. Watanabe and J. Takeya, Sci. Rep., 2020, 10, 4702 CrossRef CAS
.
- Y. K. Lee, J. Kim, Y. Kim, J. W. Kwak, Y. Yoon and J. A. Rogers, Adv. Mater., 2017, 29, 1702665 CrossRef
.
- W. Tian, Y. Li, J. Zhou, T. Wang, R. Zhang, J. Cao, M. Luo, N. Li, N. Zhang and H. Gong, ACS Appl. Mater. Interfaces, 2021, 13, 8285–8293 CrossRef CAS PubMed
.
- N. Fumeaux and D. Briand, npj Flexible Electron., 2023, 7, 14 CrossRef CAS
.
- S. Lee, J. Koo, S.-K. Kang, G. Park, Y. J. Lee, Y.-Y. Chen, S. A. Lim, K.-M. Lee and J. A. Rogers, Mater. Today, 2018, 21, 207–215 CrossRef CAS
.
- K.-S. Kim, J. Yoo, J.-S. Shim, Y.-I. Ryu, S. Choi, J.-Y. Lee, H. M. Lee, J. Koo and S.-K. Kang, Adv. Mater. Technol., 2022, 7, 2001297 CrossRef CAS
.
- K. S. Kim, W.-Y. Maeng, S. Kim, G. Lee, M. Hong, G.-B. Kim, J. Kim, S. Kim, S. Han and J. Yoo, Mater. Today Bio, 2023, 18, 100541 CrossRef CAS
.
- S. M. Won, J. Koo, K. E. Crawford, A. D. Mickle, Y. Xue, S. Min, L. A. McIlvried, Y. Yan, S. B. Kim, S. M. Lee, B. H. Kim, H. Jang, M. R. MacEwan, Y. Huang, R. W. Gereau IV and J. A. Rogers, Adv. Funct. Mater., 2018, 28, 1801819 CrossRef
.
- K. Fu, Z. Wang, C. Yan, Z. Liu, Y. Yao, J. Dai, E. Hitz, Y. Wang, W. Luo and Y. Chen, Adv. Energy Mater., 2016, 6, 1502496 CrossRef
.
- M. H. Lee, J. Lee, S. K. Jung, D. Kang, M. S. Park, G. D. Cha, K. W. Cho, J. H. Song, S. Moon and Y. S. Yun, Adv. Mater., 2021, 33, 2004902 CrossRef CAS
.
- A. Lahiri, L. Yang, O. Hofft and F. Endres, Mater. Adv., 2021, 2, 2676–2683 RSC
.
- N. Mittal, A. Ojanguren, D. Kundu, E. Lizundia and M. Niederberger, Small, 2023, 19, 2206249 CrossRef CAS
.
- S. Yamada and H. Toshiyoshi, Sens. Actuators, A, 2023, 354, 114306 CrossRef CAS
.
- S. Yamada and H. Toshiyoshi, IEEE Electron Device Lett., 2017, 38, 1477–1480 CAS
.
- S. Yamada and H. Toshiyoshi, Appl. Phys. Lett., 2023, 123, 264101 CrossRef CAS
.
- J. Koo, M. R. MacEwan, S.-K. Kang, S. M. Won, M. Stephen, P. Gamble, Z. Xie, Y. Yan, Y.-Y. Chen and J. Shin, Nat. Med., 2018, 24, 1830–1836 CrossRef CAS
.
- H. Tao, S.-W. Hwang, B. Marelli, B. An, J. E. Moreau, M. Yang, M. A. Brenckle, S. Kim, D. L. Kaplan and J. A. Rogers, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 17385–17389 CrossRef CAS
.
- J. Koo, S. B. Kim, Y. S. Choi, Z. Xie, A. J. Bandodkar, J. Khalifeh, Y. Yan, H. Kim, M. K. Pezhouh and K. Doty, Sci. Adv., 2020, 6, eabb1093 CrossRef CAS PubMed
.
- C. M. Boutry, Y. Kaizawa, B. C. Schroeder, A. Chortos, A. Legrand, Z. Wang, J. Chang, P. Fox and Z. Bao, Nat. Electron., 2018, 1, 314–321 CrossRef
.
- S. Neethirajan and B. Kemp, Sens. Bio-Sens. Res., 2021, 32, 100408 CrossRef
.
- H. Yin, Y. Cao, B. Marelli, X. Zeng, A. J. Mason and C. Cao, Adv. Mater., 2021, 33, 2007764 CrossRef CAS
.
- K. Muthumalai, N. Gokila, Y. Haldorai and R. T. Rajendra Kumar, Adv. Sens. Res., 2024, 3, 2300107 CrossRef
.
| This journal is © The Royal Society of Chemistry 2024 |