Recent advancement in the synthesis of quinoline derivatives via multicomponent reactions†
Arnab
Mandal
 and
Abu Taleb
Khan
and
Abu Taleb
Khan
 *
*
Department of Chemistry, Indian Institute of Technology Guwahati, Guwahati-781039, Assam, India. E-mail: atk@iitg.ac.in
First published on 27th February 2024
Abstract
The synthesis of quinoline derivatives through multicomponent reactions (MCRs) has emerged as an efficient and versatile strategy in organic synthesis. MCRs offer the advantage of constructing complex molecular architectures in a single step, utilising multiple starting materials in a convergent manner. This review provides an overview of recent advancements in the field of quinoline synthesis via MCRs. Various MCRs, such as the Povarov reaction, the Gewald reaction, and the Ugi reaction have been successfully employed for the synthesis of diverse quinoline scaffolds. These methodologies not only showcase high atom economy but also allow the incorporation of structural diversity into the final products. The versatility of MCRs enables the introduction of functional groups and substitution patterns tailored to specific applications. This review highlights the significance of quinoline derivatives in medicinal chemistry, materials science, and other interdisciplinary areas. The continuous innovation and development of novel MCR-based approaches for quinoline synthesis hold great promise for the rapid and efficient generation of valuable compounds with a wide range of biological and physicochemical properties.
1. Introduction
The realm of organic chemistry never ceases to captivate scientists and researchers with its boundless complexity and diversity. Among its vast collection of chemical compounds, quinolines have long been recognized for their intriguing structural motifs and versatile applications.1–4 They are some of the most important naturally occurring compounds and have various applications in pharmaceutical and other industries. It is also a widely studied molecule in synthetic organic chemistry, and there are numerous papers in the literature reporting a wide variety of synthesis procedures for the creation of this scaffold for its importance in biological and medicinal chemistry.Quinoline was first obtained from coal tar by the German scientist Runge5 in 1834, and later it was extracted from cinchonine by French chemist Gerhardt6 in 1842. It took some years before pure quinoline was available for synthesis and other studies. The main sources of quinoline include petroleum, coal processing, wood preservation and shale oil. However, numerous synthetic processes have been developed for various quinoline derivatives, such as transition metal catalysts, Brønsted acid/base catalysts and even catalyst-free synthesis. Some of the well-known synthetic techniques are Friedländer, Pfitzinger, Povarov, Skraup, Doebner and Conrad–Limpach–Knorr synthesis. The nitrogen source will depend on the specific reaction conditions and the desired quinoline derivative. The most commonly used starting material is aniline; however, ammonia, hydrazine, nitriles, and nitroso compounds are also utilized in numerous syntheses. Nevertheless, the majority of these approaches entail severe reaction conditions, elevated temperatures, acid catalysts, harmful solvents and limited selectivity in terms of the desired reaction outcome. Therefore, more recently, several alterations to these reactions involving photocatalytic, electrocatalytic, microwave and ultrasound-mediated synthesis using organo and transition metal-catalysed synthesis have been reported.
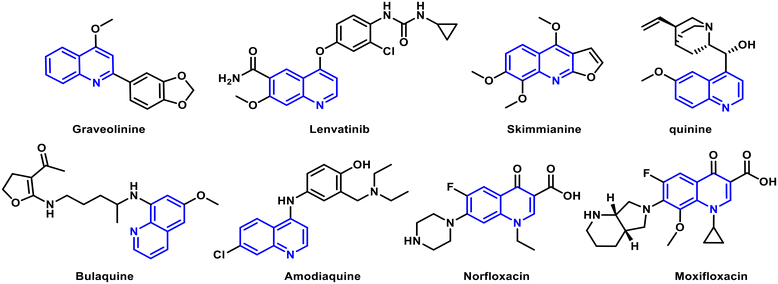
Basic types of quinoline derivatives have various applications in the production of dyes, paints, insecticides and antibacterial compounds. However, the quinoline structure is also a crucial building block for many natural products, serving as a fundamental framework in medicinal chemistry.7,8 A quinoline ring is typically found in numerous synthetic antimalarial, antibacterial, antifungal and anti-inflammatory drugs, and natural substances with potent properties in inhibiting the growth of parasites9–13 such as quinine, chloroquine and amodiaquine have been employed in the treatment of malaria. Certain quinoline derivatives have demonstrated antibacterial properties, particularly against Mycobacterium tuberculosis, the causative agent of tuberculosis. Quinolines have also shown antifungal activity against various fungal pathogens.14 Styrylquinoline derivatives for instance, have been identified as effective agents against Candida albicans, a common fungal pathogen responsible for various infections.
Not only this, quinoline derivatives have typically been explored for their potential as anticancer agents as well.15 Compounds like lenvatinib act as kinase inhibitors, interfering with cell signaling pathways involved in cancer growth and progression (Fig. 1).16,17 Many of these compounds showed PDE4 inhibitory properties in vitro. Quinoline-based drugs, like pitavastatin, are employed to reduce cholesterol levels, aiding in the management of cardiovascular diseases.18 Quinoline derivatives have been investigated for their potential in treating neurodegenerative disorders like Alzheimer's and Parkinson's diseases due to their ability to interact with certain neurotransmitter systems and receptors.19 Some quinoline derivatives possess anti-inflammatory properties and modulate immune responses, suggesting potential applications in autoimmune and inflammatory conditions. The diverse pharmacological activities of quinoline compounds make them valuable candidates for drug development across a wide spectrum of therapeutic areas, from infectious diseases to cancer and neurological disorders.
Multicomponent reactions (MCRs) are synthetic processes that yield a single product from three or more reactants in a single reaction, achieved through a series of simple reactions. They are vital in organic chemistry for their efficiency, enabling the synthesis of complex molecules in one step from multiple starting materials. They enhance diversity, atom economy, and synthetic versatility, accelerating drug discovery and materials science while uncovering new reactivity. MCRs facilitate the rapid generation of compound libraries for screening purposes in drug discovery. This acceleration of lead optimization can significantly shorten the time required to identify potential drug candidates. Beyond drug discovery, MCRs have applications in materials science for the synthesis of polymers, dendrimers, and other complex materials with tailored properties. They differ from traditional sequential two-component synthesis methods by utilising as many as seven initial components and yielding higher quantities of products compared with classical chemical processes. In an optimal scenario, all reaction equilibria within the intricate MCR mixture are reversible, with the final step of product formation being irreversible. This irreversible step acts as the driving force, pushing all intermediates towards the formation of a single product.20 The simplicity and adaptability of the experimental methods that provide access to a wide range of goods through the myriad reagent combination options are the driving forces behind MCRs’ success. Multicomponent reactions are a recently developed strategy for eco-friendly synthesis that enables the rapid production of intricate products from basic reagents, without requiring additional purification steps. An excellent illustration of this is the synthesis of 2,3-disubstituted quinolines using aniline, aldehydes, and acetylene derivatives, which can be catalysed using organic or metal catalysts.
In view of potential applications, there are several established methods available for creating the quinoline ring, which can be easily adapted to produce a variety of quinolines with different substitutions. MCR is one of the most promising techniques for synthesising the complicated framework of quinoline in a single step. So far, a huge number of review articles have covered the synthesis of quinoline. However, we focus specifically on the recent multicomponent approach towards the synthesis of quinoline analogues, which has not been covered in the previous reports. This review includes the literature from 2012 to date and focusses on the synthesis of quinolines.
2. Metal-catalysed reactions
Over the last few decades, transition-metal-catalysed methods have played a dominant role in the synthesis of complex quinoline-based heterocycles, particularly in organic and medicinal chemistry. This is because transition metals and their complexes possess excellent catalytic abilities, allowing for the generation of lead drug candidates. This has provided an advantage over traditional synthetic methods, as it allows for the construction of complex compound libraries from readily available starting materials. Furthermore, these metals and their complexes can serve as a cost-effective and less hazardous alternative to other catalysts for the preparation of therapeutic molecules based on quinoline from easily accessible materials.2.1. Iron(III) catalyst
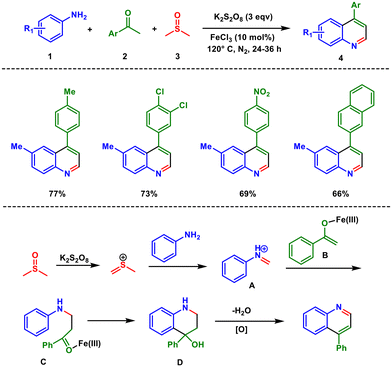
Iron catalysis plays a pivotal role in modern chemical synthesis, offering a sustainable and cost-effective alternative to more expensive and less abundant transition metals. Iron catalysis has revolutionized the synthesis of quinoline, a vital structural motif in pharmaceuticals and materials. This field has gained significant attention due to iron's ability to facilitate a wide range of reactions, ranging from cross-coupling reactions to asymmetric transformations and even more complex processes like cycloisomerizations and C–H functionalizations.21,22 Iron-catalysed reactions have profound implications for green chemistry as iron is abundant, inexpensive, and non-toxic compared with other transition metals like palladium or rhodium. As a result, these catalytic processes contribute to minimizing the environmental impact and cost of chemical synthesis, aligning with the principles of sustainability.In 2017, Singh and coworkers reported a FeCl3-catalysed oxidative annulation reaction toward the synthesis of 4-aryl quinoline involving anilines 1, aryl ketone 2 and dimethyl sulfoxide (DMSO) 3 promoted by K2S2O8 (Scheme 1).23 The reaction readily accommodated a wide range of substituents, both electron-donating and electron-withdrawing, as well as halides. These substituents were easily converted into their corresponding quinolines with consistently high yields. Here, DMSO serves as a methine source that reacts with K2S2O8 in the presence of aniline to produce the intermediate A. This iminium intermediate reacts with enolate B to generate C, which undergoes cyclization by C–N bond formation. Dehydration followed by oxidation affords the desired quinoline 4.
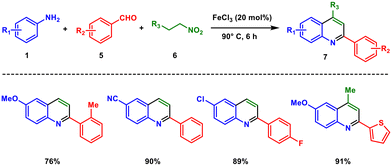
Likewise, Majee and coworkers in the year 2019 developed a one-pot methodology for the synthesis of 2-arylquinolines catalysed by FeCl3 under ambient conditions.24 This protocol involves three-component coupling of simple anilines 1, aldehydes 5, and nitroalkanes 6 at 90 °C for 6 hours (Scheme 2). After optimization of the different reaction parameters, a library of compounds was synthesized. This synthetic procedure possesses a broad scope, and more than 55 derivatives were synthesized along with gram-scale synthesis in good yield.
The reaction most likely occurs via imine formation followed by a sequential aza-Henry cyclization. By rearrangement of the nitro group, the aza-Henry intermediate produces a dihydroquinoline carbene, which on rearrangement and subsequent oxidation produces the desired 2,4-substituted quinoline 7. This method has several advantages, including the use of easily accessible chemicals as starting materials, a low-cost metal catalyst, the ability to carry out the reaction in the presence of oxygen, and the ability to tolerate a wide range of functional groups.
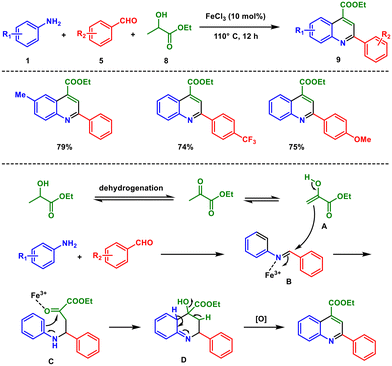
Shortly afterwards, Yang and Wan reported a three-component reaction catalysed by FeCl3 that shows high efficiency allowing access to 2,4-disubstituted quinoline.25 The reaction involves anilines 1, aryl aldehyde 5 and ethyl lactate 8via simple iron(III) chloride catalysis under neat reaction conditions. This approach is very sustainable and environmentally friendly because it utilises biomass, alcohol dehydrogenation, and diversity-oriented synthesis to produce quinoline derivatives 9 (Scheme 3). Firstly, ethyl pyruvate A is formed from ethyl lactate through dehydrogenation. On the other hand, an imine B is generated from aniline and the aldehyde. Then the ethyl pyruvate attacks the imine facilitated by the ferric ion. The adduct C subsequently undergoes an intramolecular addition where the nucleophilic aryl C–H bond reacts with the ketone carbonyl, leading to the formation of intermediate D. Dehydration-induced elimination of this intermediate, followed by aromatization, results in the formation of the desired product.
The iron-catalysed synthesis of quinolines offers a versatile and efficient method for generating these important heterocyclic compounds. This approach leverages the unique reactivity of iron catalysts to facilitate the formation of quinolines from readily available starting materials. Overall, this method stands as a promising strategy in the synthesis of quinolines, showcasing the potential for further developments and applications in organic synthesis.
2.2. Copper catalysts
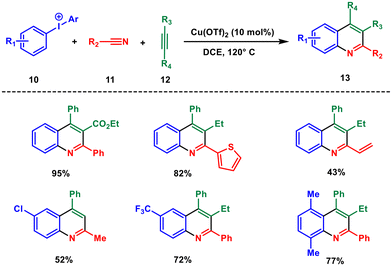
The synthesis of quinolines through copper catalysis offers a powerful tool for chemists to access a wide range of structurally diverse quinoline derivatives. Here, we delve into various multicomponent approaches of copper-catalysed quinoline synthesis, reaction conditions, catalytic systems, and key steps involved in this synthetic process catalysed by copper.Anilines are the most used starting materials for quinoline synthesis, but nitriles and azides are also introduced as a source of the nitrogen atom. Nitriles can form anilide through an iminium cation which acts as an electron acceptor. In this regard, the Chen group in the year 2013 proposed a new route to forge a wide range of poly-substituted quinolines via diaryliodonium salts 10, nitriles 11 and alkynes 12, as environmentally benign reagents with low toxicity.26 This three-component regioselective cyclisation is catalysed by Cu(OTf)2 and the aryl group of the diaryliodonium acts as a C2 building block (Scheme 4). This approach represents a notable departure from the established methods relying on condensation chemistry and enables wide variation in the substitution patterns on the quinolines 13. The cascade annulation involved a series of cationic intermediates, thus ensuring an efficient process and high regioselectivity.
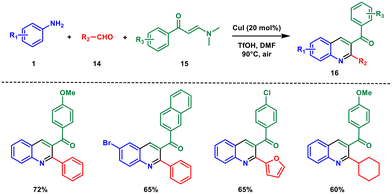
In 2017, Wan and colleagues developed a copper(I)-catalysed three-component regioselective synthesis of functionalised quinolines using anilines 1, aldehydes 14, and enaminones 15.27 In contrast to many alternative approaches, Povarov-type reactions stand out due to their benefits, such as the convenient accessibility of initial compounds and the swift creation of a wide range of products through multi-component processes (Scheme 5). In light of this, the development of Povarov-type three-component reactions that allow for the specific production of quinolines displaying varied substitution arrangements going beyond the traditional Povarov reaction, holds great importance.
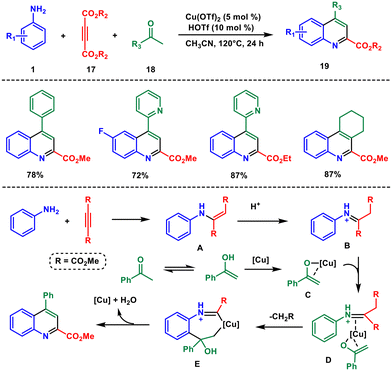
Yi and co-workers in 2018 reported the synthesis of 2,4-disubstituted quinolines in a single step by using a copper triflate catalyst28 to combine simple anilines 1, alkyne esters 17 and (hetero)aryl/cycloalkyl ketone 18 in acetonitrile. The reaction has excellent tolerance for various functional groups and produces the desired quinolines 19 with exclusive regioselectivity (Scheme 6). Initially, an enamine A is generated rapidly from in situ nucleophilic addition of aniline to the alkyne ester. Subsequently, the enol form B coordinates with the Cu(II) catalyst, leading to the formation of intermediate C. This is succeeded by additional coordination with B, giving rise to intermediate D. The regioselective migratory insertion of the enol form results in the formation of intermediate E, involving a comparable C–C bond cleavage. Protonolysis of E produces the final product and restores the active Cu(II) catalyst, accompanied by the expulsion of a water molecule.
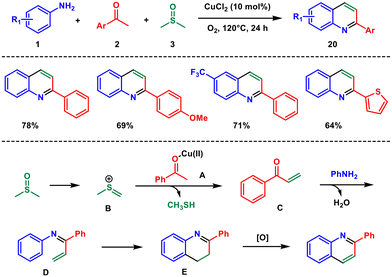
Shortly afterwards, Guo and co-workers reported a method for synthesising 2-aryl quinolines using a copper catalyst, O2 as an oxidant, and DMSO as a carbon source.29 This method involves a cyclization reaction of simple anilines 1 and aryl ketones 2 under aerobic conditions in a DMSO 3 medium. A variety of electron-donating and electron-withdrawing groups in aryl ketone and aniline participated in the reaction and converted to the respected quinolines 20 with moderate to good yields (Scheme 7). The protocol is atom-efficient and straightforward, as a variety of ketones and anilines can be used to directly generate 2-aryl quinolines. Initially, DMSO gets activated to form B. This intermediate B can react with the enolate A and undergo demethylthioation to produce species C. Aniline then condenses with C to produce imine D, which further undergoes annulation followed by aromatization to yield the final product, 20. It can be noted that the starting materials are the same as for Scheme 1, but the product is 4-substituted quinolines for the iron(III)-catalysed reaction whereas it is 2-substituted for the copper(II)-catalysed reaction. This is because in the presence of K2S2O8 and Fe(III) DMSO reacts with aniline and produces an imine which attacks the enolate. But here in the presence of Cu(II), DMSO reacts with the aryl ketone to produce a vinyl ketone which reacts with aniline to produce 2-arylquinoline.
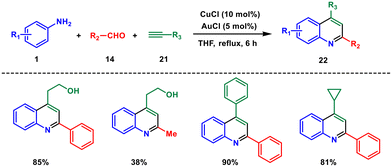
In the same year, Lin et al. proposed a new A3 coupling reaction which involves an amine, aldehyde and alkyne to construct a 4-hydroxyalkyl quinoline derivative via Cu(I) and Au(I) sequential catalysis.30 The reaction involves cyclization of anilines 1, aldehydes 14 and aliphatic/aromatic alkynes 21, respectively (Scheme 8). This protocol provides easy access to various 2,4-substituted quinolines that contain an aliphatic chain substituent at the 2,4-position in high yields. The reaction entails a sequence of events starting with domino imine formation, followed by imine addition to propargyl amine, cyclization, oxidation, and ultimately, the alkynyl–Cu(I) complex undergoes nucleophilic decomposition of the metal complex, resulting in the formation of the final product 22. The authors provided DFT calculations to investigate the proposed mechanism by transition state searches by computation.
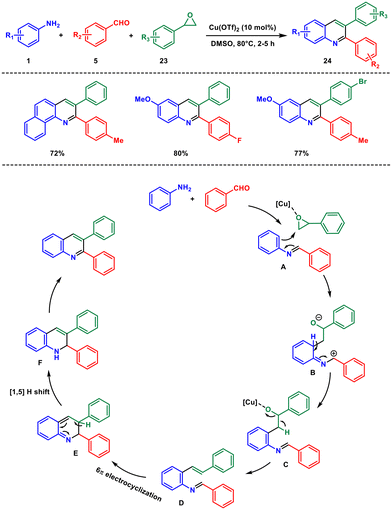
In 2021, our group devised an efficient method to synthesize 2,3-diarylquinoline derivatives using easily accessible aryl amines 1, aryl aldehydes 5, and styrene oxides 23.31 This process relies on 10 mol% copper(II) triflate in a three-component reaction (Scheme 9). By combining the in situ-formed imine with styrene oxide, we successfully generated the desired products. Our approach offers multiple benefits, including high atom efficiency, precise regioselectivity, sequential creation of one C–N and two C–C bonds, shorter reaction times, a wider range of applicable starting materials and good yields.
Mechanistically the reaction between the amine and aldehyde forms a Schiff's base intermediate A. Subsequently, intermediate A reacts with styrene oxide to yield intermediate B, which, upon aromatization, produces C. Then, elimination of water from intermediate C results in the formation of D, which undergoes 6π-electrocyclic ring closure to generate intermediate E. Intermediate E experiences a [1,5] hydrogen shift,32 leading to dihydroquinoline F, followed by aerial oxidation to furnish the desired quinoline.
The copper-catalysed synthesis of quinolines represents a valuable and robust approach in organic synthesis. This method harnesses the catalytic power of copper to facilitate the construction of quinoline structures from diverse starting materials. It offers notable advantages such as mild reaction conditions, high efficiency and excellent functional group tolerance. The versatility and reliability of copper catalysis in quinoline synthesis underscores its significance in accessing these important heterocyclic compounds.
2.3. Metal photocatalyst
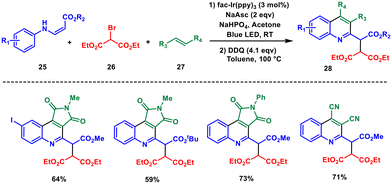
Metal photocatalysts are a promising class of materials with unique properties that enable them to harness light energy for various catalytic processes, typically in the presence of oxygen or another oxidizing agent. Metal-based coordination complexes which strongly absorb in the visible light region such as fac-Ir(ppy)3 and [Ru(bpy)3]2+ are the most used photocatalysts in recent years, as they provide novel routes to access various diverse organic scaffolds.33,34 In this context, the synthesis of quinoline compounds using photocatalysis has gained significant attention in recent years as a green and sustainable method.35For instance, Choi and Park in 2018 constructed highly substituted quinolines based on a three-component radical cascade reaction of β-aminoacrylate 25 with diethyl bromomalonate 26 and an alkene (mostly N-methyl maleimide) 27 in acetonitrile. The cyclization was carried out using 3 mol% Ir(ppy)2(dtbbpy)PF6 and base under 12 W blue LED lamp at room temperature (Scheme 10).36 Under optimised conditions, a tandem coupling reaction exhibits excellent chemoselectivity due to the distinct electronic characteristics of the reacting components. When electron-rich β-aminoacrylates are combined with electron-deficient halides and alkenes, quinolines are produced in favourable yields. The final step involves the in situ oxidation of tetrahydroquinolines using DDQ at 100 °C, resulting in the formation of quinolines.
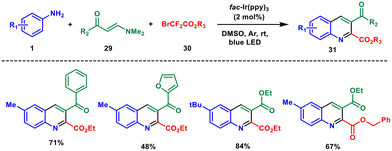
Recently in 2022, Wang and coworkers developed an iridium-catalysed photoinduced multicomponent reaction. A mixture of aryl amine 1, enaminone 29, ethyl bromodifluoroacetate 30 and fac-Ir(ppy)3 (2 mol%) was irradiated under 6 W blue LED irradiation under an argon atmosphere for 5 h.37 The authors synthesized a library of compounds of alkyl, aryl, electron-donating and electron-withdrawing substituents in moderate yields (Scheme 11). The crucial steps involving the sequential cleavage of C–Br and C–F bonds were the decisive steps in this reaction, followed by intermolecular [3 + 3] cyclization between in situ-generated 1,3-vinylimine ions and arylamines. This strategy features broad functional group tolerance and wide substrate scope that enables further synthetic applications of the obtained products.
2.4. Other metal catalysts
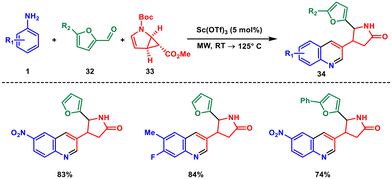
Various other transition and post-transition metals such as scandium, cobalt, zinc or silver can serve as catalysts, enabling the formation of quinolines through different catalytic cycles and enabling the creation of diverse quinoline derivatives.Back in 2012, the Reiser group reported a scandium triflate-mediated multicomponent stereoselective synthesis of 3-pyrrolidinone-substituted quinolines under microwave irradiation using aryl amine 1, furancarbaldehyde 32 and pyrrole derivatives 33 (Scheme 12). This is a Povarov-type reaction which combines donor–acceptor-induced cyclopropane ring opening, 1,4-furan ring migration and formation of the quinoline ring.38 A variety of modified cis-pyrrolidinones were successfully synthesised with a high degree of stereoselectivity, resulting in excellent yields. These compounds hold significance as fundamental building blocks in biologically significant compounds with pharmaceutical relevance. The reaction proceeds through an intermediate having endo and exo epimers with respect to the stereochemistry of the furan substituent which can be separable on silica. Interestingly the endo isomer is arranged in such a way that it gives the desired product 34 while the exo epimer yields a ring-opened polycyclic imine.
Polyoxometalates (POMs), known for their versatile molecular and electronic structural variations, play a crucial role in a wide range of academic fields due to their unique structure. After Chester's research was published in 1970,39 CoIIIW125− and similar POM anions have been employed as precise agents for outer-sphere electron transfers. Conversely, the potential efficacy of various POM anions as electron-transfer agents has been harnessed in the selective catalytic oxidation of both inorganic and organic substrates.
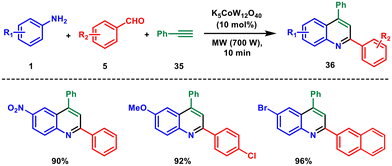
In this context, in 2012 Mohammadpoor-Baltork et al. reported a microwave-assisted one-pot three-component reaction using aromatic amines 1, aromatic aldehydes 5, and phenylacetylene 35 catalysed by POM.40 They used a small amount of catalytic potassium dodecatungstocobaltate trihydrate to produce 2,4-substituted quinolines 36 in high yields. This method is notable for its ability to selectively convert aromatic aldehydes over aliphatic ones, making it valuable for synthesising quinoline derivatives (Scheme 13). The reaction proceeds through a Co(II) radical cation generated from an electron transfer from the phenyl acetylene moiety. Additionally, the catalyst could be reused multiple times without significant loss of activity.
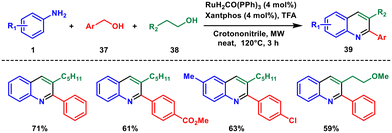
In 2015, Porcheddu et al. presented an innovative method for synthesising diverse substituted quinolines through a ruthenium-catalysed dehydrogenative cross-coupling of primary alcohols and imines.41 This transformative process utilised trifluoroacetic acid (TFA) and a ruthenium catalyst to facilitate the in situ generation of imines from various anilines 1 and benzyl alcohols 37, employing a hydrogen-transfer procedure (Scheme 14). Notably, the same catalyst was employed throughout the process, allowing for the introduction of various primary alcohols 38via a telescopic approach, resulting in the formation of quinolines 39 in satisfactory to commendable yields. This methodology offers several advantages, such as the utilization of alcohols as starting materials, the absence of potent oxidizing agents, and the wide array of potential precursor compounds. In comparison with conventional quinoline synthesis methods, this contemporary adaptation of the Skraup reaction stands out due to its enhanced efficiency and versatility.
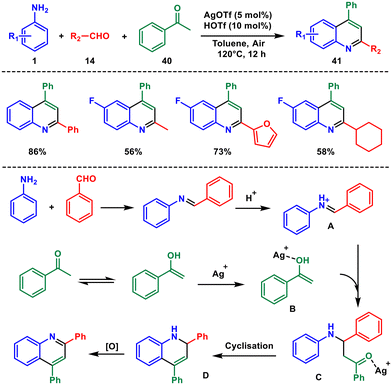
Shortly after that, Zhang and co-worker reported a new method for synthesising a range of polysubstituted quinolines using silver triflate as a catalyst.42 It emerged as an important catalyst for the construction of many architecturally intriguing molecules, including quinoline. This method involves combining arylamines 1, aldehydes 14, and acetophenone 40 (Scheme 15). The technique works well with a broad range of substrates, allowing for greater flexibility in the creation of the heterocyclic framework using a single catalytic system to facilitate multiple chemical transformations. It is an important step toward developing more efficient and environmentally friendly synthesis strategies for complex molecules from simple starting materials.
Mechanistically, the equilibrium between acetophenone and its enol form is highly inclined toward the enol B in the presence of the silver catalyst. The generated enol immediately reacts with imine A, forming intermediate C which via intramolecular cyclization produces D. Oxygen in the air serves as a potent oxidizing agent for the aromatization of D to the final quinoline 41 product.
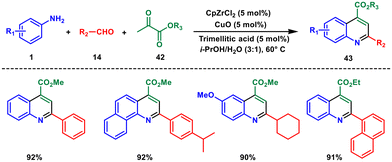
Later in 2017, Gao and coworkers introduced a new system for catalysis that involves three types of cooperation and relays. The system is used to trigger the Mannich addition, followed by C–C construction and oxydehydrogenation.43 This triple cooperative and relay catalytic system used aryl amines 1, aldehydes 14 and ketones 42 for the synthesis of polysubstituted quinoline 43 under mild conditions The catalytic system is composed of zirconocene dichloride and trimellitic acid that forms a new zirconocene species Cp2Zr(OOC)2PhCOOH which promotes the Mannich addition and C–C bond construction reactions (Scheme 16). Additionally, the system features copper oxide (CuO) that allows for relay catalysis for oxydehydrogenation. The use of this system was shown to be highly effective in synthesising substituted quinolines from commercially available anilines, aldehydes, and ketones. The substrate and the product of this reaction are similar to those in Scheme 3. However, the yield of this reaction is much higher, despite using much milder reaction conditions.
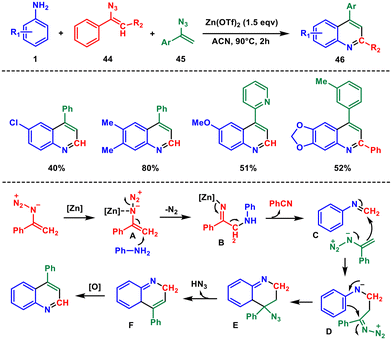
In the same year, Jiang and co-worker found an unprecedented Zn(OTf)2-promoted selective cleavage of vinyl azides 44 and 45 with aryl amine 1 for the synthesis of 4-substituted quinolines 46,44 where vinyl azides operate as a dual synthon by undergoing simultaneous cleavage of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–N bonds, leading to the formation of two C
C and C–N bonds, leading to the formation of two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds and one C
C bonds and one C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond in a single-step process (Scheme 17). However, various anilines with electron-withdrawing substituents did not effectively participate in the reaction. Control experiments revealed that the methylene end of the vinyl azide is important because the reaction does not take place with methyl-substituted vinyl azide. Mechanistically, intermediate A is formed through the coordination of zinc with the nitrogen in the azide group, which enhances the electrophilic nature of the olefin. Following that, aniline initiates a nucleophilic attack, resulting in intermediate B, accompanied by the removal of nitrogen. Subsequently, intermediate B transforms into imine intermediate C by breaking a C–C bond,45 resulting in the production of benzonitrile as a by-product. Following this, intermediate C undergoes an intramolecular cyclization, specifically a [4 + 2]-annulation with compound 45, leading to the formation of intermediate E. This, in turn, gives rise to intermediate F with the elimination of HN3. Finally, F undergoes aromatization when exposed to O2 in the air, yielding the desired quinoline 46.
N bond in a single-step process (Scheme 17). However, various anilines with electron-withdrawing substituents did not effectively participate in the reaction. Control experiments revealed that the methylene end of the vinyl azide is important because the reaction does not take place with methyl-substituted vinyl azide. Mechanistically, intermediate A is formed through the coordination of zinc with the nitrogen in the azide group, which enhances the electrophilic nature of the olefin. Following that, aniline initiates a nucleophilic attack, resulting in intermediate B, accompanied by the removal of nitrogen. Subsequently, intermediate B transforms into imine intermediate C by breaking a C–C bond,45 resulting in the production of benzonitrile as a by-product. Following this, intermediate C undergoes an intramolecular cyclization, specifically a [4 + 2]-annulation with compound 45, leading to the formation of intermediate E. This, in turn, gives rise to intermediate F with the elimination of HN3. Finally, F undergoes aromatization when exposed to O2 in the air, yielding the desired quinoline 46.
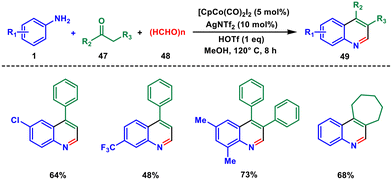
In 2017, the Yi group developed a new method for the synthesis of diverse quinolines using a Co(III) catalyst for the first time. This method involves the one-pot synthesis of 3,4-substituted quinolines 49 from simple aryl amines 1 and ketones 47 with paraformaldehyde 48.46 The yield of the reaction is good to excellent, and there is excellent tolerance for different functional groups (Scheme 18). The only by-products of the reaction are water and hydrogen gas. The method also shows exclusive site- or/and region-selectivity when unsymmetrical ketones and meta-substituted anilines are used. This method has significant potential for the synthesis of biologically important quinoline frameworks in an environmentally friendly and atom-economical manner with exclusive site selectivity.
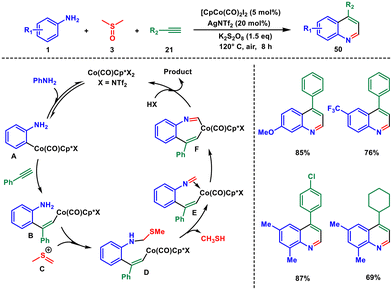
The same group in 2018 reported C–H activation of anilines with alkynes for synthesis of quinolines with exclusive regioselectivity.47 The same Co(III)-catalyst is used in this DMSO-involved cyclization of simple anilines 1 with alkynes 21 for direct and highly efficient synthesis of privileged quinolines 50 with broad substrate tolerance and in good to excellent yields (Scheme 19). Here DMSO served the dual role of solvent and the C1 building block for the synthesis of quinoline products.
In terms of the mechanism, the process begins with the formation of the active cationic Co(III) catalyst. This catalyst then coordinates with aniline, leading to the EAS pathway and subsequent ortho-metalation, resulting in the formation of intermediate A. The increased polarization of the carbon–cobalt bond in intermediate A promotes the 1,2-regioselective insertion of an alkyne,48 yielding intermediate B. In the presence of K2S2O8, DMSO is activated, producing compound C, which combines with B to generate intermediate D. Subsequently, D undergoes oxidation, leading to the formation of an imine species E and the elimination of CH3SH. Subsequently, Co–C migratory insertion occurs, yielding intermediate F. Ultimately, protonolysis of F yields the final product 50, concurrently releasing the active Co(III) catalyst.
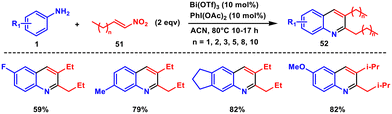
In 2020, we investigated the reaction behaviour of aryl amines 1 with nitroalkenes 51 in the presence of bismuth(III) triflate and diacetoxyiodobenzene.49 This approach offers advantages including consecutive formation of a C–N bond and two C–C bonds, high regioselectivity, broad substrate scope, and favourable yields. Instead of the anticipated 3-alkylindole derivatives, we obtained 2,3-dialkylquinoline derivatives 52. This reaction provides a new route to synthesize 2,3-dialkylquinoline derivatives under more gentle conditions (Scheme 20). Mechanistic understanding determined through theoretical calculations revealed a preference for the conventional aza-Michael reaction over the Michael addition. The aza-Michael adduct formed leads to an imine by water elimination, which may tautomerize to the corresponding amine. Subsequent reactions between the resulting imine and enamine intermediates led to the desired quinoline derivatives.
Metal–organic frameworks (MOFs) represent a novel category of porous materials formed through the combination of metal ions and organic building blocks. MOFs have a unique porous structure with organic and inorganic active sites, and their structural flexibility makes them excellent heterogeneous catalysts. Compared with homogeneous transition-metal catalysts, MOF-based catalysts offer advantages such as a large internal surface area, microporosity, and well-organized porous structures. The choice of MOF, its structure, and the specific metal ions or ligands used can greatly influence its catalytic activity and selectivity. Researchers continue to explore and design MOFs with tailored properties to address various challenges in organic synthesis and catalysis.
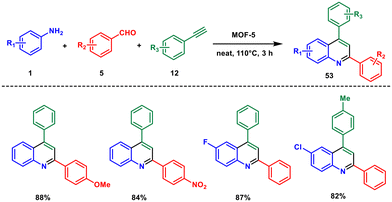
In view of developing another MOF-catalysed reaction, Zhang and coworkers in 2020 developed a new zinc-based MOF-catalysed three-component coupling reaction for the synthesis of 2,4-disubstituted quinoline derivatives. They used aromatic amines 1, aldehydes 5 and alkynes 12 reacted neat at 110 °C to produce quinoline 53 in excellent yields.50 The advantages of the method are easy recovery and reusability of the catalyst, broad substrate scope, short reaction time, and solvent-free conditions (Scheme 21). The reaction proceeds via generation of an imine which is coordinated by the MOF-5 and enhances its electrophilicity. Then the addition of alkyne to imine forms a propargylamine intermediate, which then undergoes an intramolecular hydroarylation, followed by oxidative aromatization with oxygen to afford the desired product. This reaction bears significant similarities to Scheme 13, including similar starting materials, products, and being a solvent-free reaction. However, the yields with the POM catalyst are slightly higher than with the MOF catalyst.
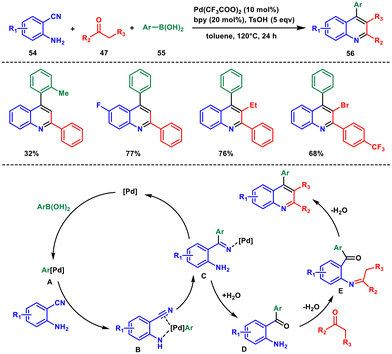
Recently, Chen and co-workers proposed a new approach for the synthesis of poly-substituted quinolines through a three-component tandem reaction of 2-aminobenzonitriles 54, arylboronic acids 55 and ketones 47.51 The method utilises easily accessible materials, employing straightforward palladium-catalysed conditions and a wide range of functional groups (Scheme 22) giving good yield. The authors synthesized a library of alkyl, aryl and halide-substituted quinolines in moderate to good yields. Moreover, a variety of aryl groups placed at the C-4 position, alkyl and aryl groups at the C-2 position and halide groups at the C-3 position were found to be compatible under the given conditions.
To begin, the Pd(II) catalyst engages in transmetalation with an arylboronic acid, forming arylpalladium intermediate A. This species can potentially bind with 2-aminobenzonitrile, creating an N-bound intermediate labeled as B. Following this, the intramolecular carbopalladation of the cyano group leads to the generation of an imine palladium complex C. Subsequently, in the presence of water, complex C undergoes hydrolysis to yield intermediate D. From there, intermediate D takes part in a series of reactions involving condensation and cyclization, ultimately resulting in the formation of poly-substituted quinolines 56.
3. Non-metal-catalysed reactions
Non-metal catalysis is considered an important development in green chemistry due to the mild reaction conditions and eco-friendly catalysts that can be used in organic synthesis. Green chemistry principles have spurred the innovation of using organocatalysts instead of metal catalysts for synthesizing quinoline derivatives, aligning with sustainability goals.52 They have several benefits, including their insensitivity to moisture and oxygen, low cost, easy accessibility, and low toxicity. These advantages make them particularly useful in the production of pharmaceutical compounds when compared with metal catalysts.3.1. Iodine-catalysed MCR of quinolines
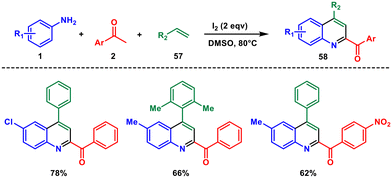
Iodine can act as a catalyst in various chemical reactions. It is used extensively in a variety of chemical reactions and is a green alternative to transition metals in organic chemistry.53 The most common method for quinoline synthesis involving iodine as a catalyst is the Friedländer synthesis. The specific mechanism and conditions under which iodine serves as a catalyst can vary depending on the reaction. The choice of iodine as a catalyst is often influenced by factors such as reaction selectivity and efficiency.In 2014, Wu and co-workers introduced an efficient method for synthesising 2,4-disubstituted quinolines 58 directly from aryl amines 1, aryl ketones 2 and styrenes 57.54 This new approach utilised molecular iodine as a catalyst and involved a unique variant of the Povarov reaction (Scheme 23). Instead of the usual carbonyl carbon, the methyl group of the methyl ketone was found to participate in the reaction. The authors proposed a mechanistic pathway involving a series of sequential reactions, namely iodination, Kornblum oxidation, Povarov reaction, and aromatization. This self-sequenced cascade reaction, driven by molecular iodine, allowed for the sequential occurrence of three distinct reactions within a single reactor. Furthermore, this catalytic process was straightforward to carry out and offered significant advantages.
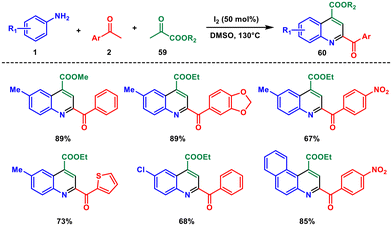
In the next year, the same research group published a significant finding on the use of iodine as a catalyst in a Povarov-type reaction involving the same arylamines 1 and aryl ketones 2 along with α-ketoesters 59.55 This approach demonstrated high efficiency by employing a catalytic amount of hydrogen iodide (HI), which was generated in situ through iodination and Kornblum oxidation steps (Scheme 24). The generated HI acted as a promoter for the subsequent Povarov step, eliminating the need for additional additives. The outcome was the synthesis of substituted quinolines 60, showcasing a simple and intriguing procedure with excellent compatibility for various functional groups. This reaction introduces a novel reactivity pathway for the Povarov reaction, offering promising possibilities in terms of functional group tolerance.
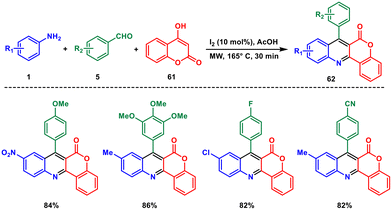
In the same year, Sashidhara and coworkers introduced a straightforward and efficient method for selectively synthesising chromeno[4,3-b]quinolin-6-one 62 derivatives.56 This process, conducted under microwave irradiation, utilised molecular iodine as the sole catalyst. The reaction involved a three-component tandem annulation of aromatic amines 1, aromatic aldehydes 5 and 4-hydroxycoumarin 61 resembling a Povarov-type reaction (Scheme 25). The reaction proceeds through formation of a Knoevenagel intermediate between the aldehyde and coumarin followed by rearrangement and 1,3-H shift to afford the product. The authors supported the mechanism by control experiments. The method offered high yields and proved to be a valuable approach for generating diverse libraries of hybrid drug-like scaffolds. By employing this strategy, a complex central structure could be constructed in a single step using readily available starting materials and environmentally friendly oxidants, contributing to a greener and more sustainable synthetic approach.
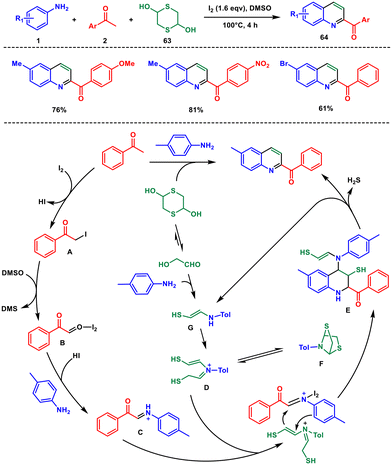
In a 2017 study, again Wu et al. introduced a highly efficient method for synthesising 2-acylquinolines through a [4 + 2] cycloaddition reaction.57 They utilised I2 as a catalyst and employed 1,4-dithiane-2,5-diol 63 as a substitute for ethylene. It is the first example where an arylamine 1 substrate played a crucial role in activating 1,4-dithiane-2,5-diol for participation in the Povarov reaction (Scheme 26). In this process, 1,4-dithiane-2,5-diol acted as an ethylene surrogate. Furthermore, this method presented a novel approach for utilizing 1,4-dithiane-2,5-diol as a C2 building block in the synthesis of nitrogen-containing heterocycles, instead of its typical use in constructing sulfur-containing heterocycles through desulfurization.
In the initial step, acetophenone is converted into phenylglyoxal B through a process involving the release of HI and DMS, achieved through an iodination/Kornblum oxidation sequence. Intermediate B then undergoes a dehydration reaction with p-toluidine to form the iminium ion C. Meanwhile, mercapto acetaldehyde, produced from 1,4-dithiane-2,5-diol under equilibrium conditions, gets initiated by p-toluidine to create enamine intermediate D, which subsequently reacts with C to generate intermediate E. It's worth noting that intermediate D can also undergo intramolecular nucleophilic attack independently to produce F when intermediate C is absent. The transformation between intermediate D and compound F is reversible, with compound F readily converting back to intermediate D, which then promptly combines with intermediate C to yield the desired product 64. Intermediate E then goes through an oxidative aromatization process, resulting in the final product 64via desulfurization and deamination steps.58 Enamine intermediate G continues to participate in the reaction throughout this mechanistic sequence.
In the same year, they also developed a novel approach involving iodine–amine synergistic promotion for a formal [4 + 2] cycloaddition reaction. This reaction involved the use of arylamines 1, methyl ketones 2 and aldehydes 65. The authors used the aryl(alkyl)aldehydes as alkene surrogates in a DMSO solvent.59 Unlike previous methods that mainly yielded 2-substituted quinolines, this protocol enabled the modular synthesis of diverse 2-acyl-3-aryl(alkyl)quinolines (Scheme 27). Consequently, it expanded the scope of Povarov-type reactions. The arylamines played dual pivotal roles in this process. They not only acted as reactants but also served as essential catalysts for promoting enamine formation. Furthermore, the mechanistic investigation suggested that the reaction proceeded through an iodination/Kornblum oxidation/Povarov/aromatization sequence.
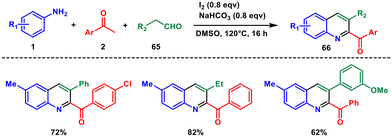
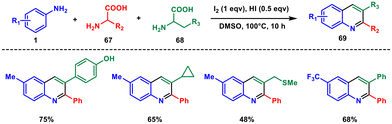
Later that year, they developed a novel method for generating a wide range of 2,3-disubstituted quinolines. This time they used aryl amine 1 and two distinct amino acids 67 and 68 with high efficiency and diversity that facilitates the creations of pharmaceutical derivatives, photochemical active compounds, and challenging scaffolds (Scheme 28).60 Using this method, challenging fused rings and biquinolines which have never been prepared before can be readily reached. They introduced the concept of using two amino acids as heterocyclic precursors for the first time. Utilising renewable resources like amino acids and operating under metal-free conditions render this reaction environmentally friendly, potentially valuable in pharmaceutical exploration, photochemical applications, and industrial manufacturing. Mechanistically, an I2-facilitated decarboxylation and deamination process activate two separate amino acids in situ. Subsequently, I2 facilitates the formation of new C–N and C–C bonds as a terminal oxidant.
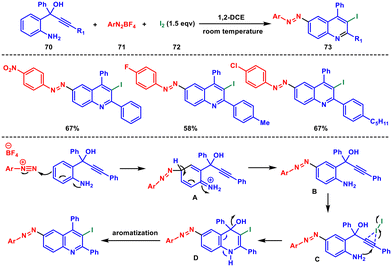
In 2018, Yaragorla and coworkers developed a comprehensive and diverse approach to synthesize quinoline derivatives. They employed a sequential process involving azo-coupling and electrophilic cyclization reactions to prepare 6-(aryldiazenyl)-3-iodoquinolines 73.61 This one-pot, three-component method utilised 2-aminoaryl propargyl alcohols 70, aryldiazonium salts 71 and molecular iodine 72. The reaction proceeded through a series of steps including azo-coupling, regioselective iodocyclization, and aromatization, resulting in the formation of the desired quinoline derivatives 73 (Scheme 29). The reaction begins with the azo-coupling reaction of aniline and diazonium salts to produce B through the intermediate A. Then the triple bond of the amino propargyl alcohol attaches with the iodine to generate intermediate C. This species then undergoes iodocyclization followed by aromatization to produces the desired quinoline product.
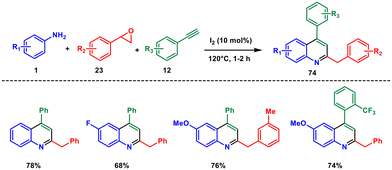
In 2021, our group reported a metal- and solvent-free synthesis of 2-benzyl-4-arylquinoline derivatives from aryl amines 1, styrene oxides 23 and aryl acetylenes 12 in the presence of 10 mol% molecular iodine (Scheme 30).62 This reaction occurs at 120 °C, which sidesteps the need for metal catalysts, reducing metal waste. Unlike the [4 + 2] imino-Diels–Alder route, our process involves an alternative pathway where an activated imine is captured by a terminal alkyne, forming a benzylic vinylic cation. This cation then undergoes intramolecular cyclization, followed by a [1,3] H shift and oxidation, resulting in the desired quinoline 74. Key aspects of this methodology include employing uncomplicated starting materials, achieving high regioselectivity, shorter reaction times, maximizing atom economy, generating one C–N and two C–C bonds in a single step, and accommodating a broad spectrum of functional groups.
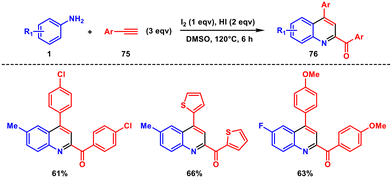
In the same year, Wu and coworkers introduced a new approach to the Povarov reaction using iodine and HI as a mediator.63 This method enables the synthesis of 2,4-substituted quinolines 76 by utilizing anilines 1 and arylacetylenes 75. Notably, arylacetylenes serve a dual role as both diene precursors and dienophiles. The C(sp)–H bond in arylacetylenes undergoes oxidation to form an aldehyde by the I2/DMSO system (Scheme 31). Subsequently, the aldehyde reacts with aniline in situ, generating a diene C–acylimine. This innovation in the Povarov reaction expands the range of applicable diene precursors and broadens the substrate scope to include compounds beyond carbonyl compounds.
The iodine-catalysed synthesis of quinolines stands as a valuable and practical method in organic chemistry. Leveraging iodine as a catalyst enables the efficient construction of quinoline frameworks from diverse starting materials. This approach offers distinct advantages, including mild reaction conditions, operational simplicity, and broad substrate compatibility. The effectiveness of iodine catalysis in quinoline synthesis highlights its significance as a versatile and environmentally friendly strategy. Continued exploration and optimization of iodine-catalysed methods holds promise for further enhancing the scope, efficiency, and applicability of quinoline synthesis in various synthetic endeavours.
3.2. Acid-catalysed reactions
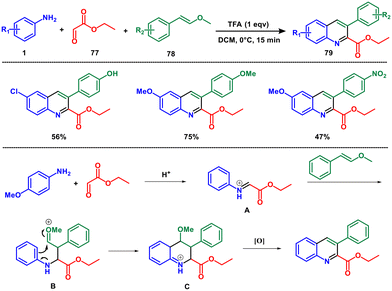
Acid catalysts play an important role in the chemical and pharmaceutical industries, catalysing various process such as esterification, hydrolysis, dehydration, polymerization, and many organic synthesis reactions. They serve as a proton source thereby lowering the activation energy required for the reaction to occur.For instance, in 2016, McNulty et al. introduced a novel three-component coupling reaction for the preparation of 2-carboxyl-3-aryl quinoline derivatives from anilines 1, ethyl glyoxalate 77 and enol ethers 78 as phenylacetaldehyde surrogates. The reaction takes place quickly and in dichloromethane, catalysed by trifluoroacetic acid (TFA) under mild conditions and delivers quinolines with good to high yields.64 This method provides a more straightforward route to obtain 3-aryl quinolines, avoiding complications encountered with phenylacetaldehyde derivatives (Scheme 32). When aniline reacts with ethyl glyoxylate, it forms an imine, which upon protonation yields intermediate A. The reaction progresses through a stepwise Mannich-aldol-type process progressing through intermediate B which upon rearrangement leading to intermediate C. Loss of a proton to restore aromaticity followed by elimination of methanol and spontaneous oxidation results in the desired product 79. By using aryl enol ethers, it is also possible to obtain products with reversed regioselectivity compared with standard Povarov products. This chemistry has been successfully employed to synthesize a wide range of 3-aryl quinolines, as well as a more limited selection of vaulted diaryl ether analogues.
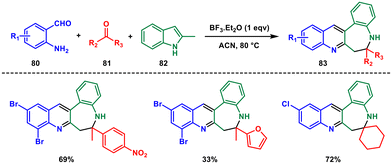
In 2016, Gu and coworkers developed a boron trifluoride-catalysed three-component Mannich-type reaction involving o-amino benzaldehyde 80, ketone 81 and 2-methylindole 82.65 This reaction provided a straightforward route to diverse quinoline-fused 1-benzazepine derivatives 83 using a Lewis acid in acetonitrile solvent under mild conditions (Scheme 33). The key intermediate in this process was a previously unreported C,N-1,6-bisnucleophile, formed through an indole-to-quinoline transformation between o-aminobenzaldehyde and 2-methylindole. A variety of keto- or aldocarbonyl-containing compounds can serve as electrophiles to engage with this bisnucleophile, leading to a considerable enhancement in product diversity. Nevertheless, the reaction yields obtained were generally moderate in the majority of cases.
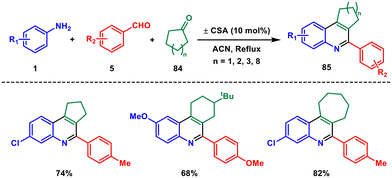
In 2017, our research group published a study presenting a straightforward and efficient method for synthesising various fused quinoline, benzoquinoline, and naphthoquinoline derivatives.66 This method involved a one-pot three-component reaction using aryl amines 1, aromatic aldehydes 5 and cyclic ketones 84, with camphorsulfonic acid serving as the catalyst (Scheme 34). The protocol offered numerous advantages, including the use of a readily available and inexpensive catalyst, short reaction times, mild reaction conditions, easy isolation procedures, a wide range of applicable substrates, and an environmentally friendly approach with high atom efficiency. First, the cyclic ketone reacts with CSA to form an active intermediate which reacts with the imine formed from the aldehyde and ketone followed by an intramolecular Michael addition, and subsequent oxidation produces the desired product 85. We also explored the application of pregnenolone acetate and terephthalaldehyde in the synthesis of steroid-substituted benzo[f]quinoline and bis-benzo[f]quinoline, respectively. These novel quinoline and benzoquinoline derivatives represented the first instances of their synthesis using camphorsulfonic acid as a catalyst.
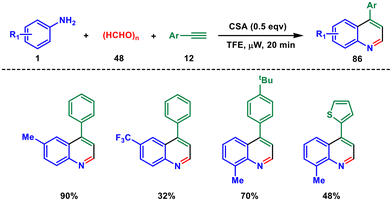
In 2019 Sharma and coworkers developed a rapid microwave-assisted Povarov-type multicomponent synthesis of 4-aryl quinolines.67 The reaction involves anilines 1, alkynes 12 and paraformaldehyde 48 and proceeds through [4 + 2] cycloaddition of imine and alkynes in the presence of camphor sulphonic acid (CSA), without any metal catalyst. The Povarov reaction, which involves a [4 + 2] cycloaddition of imines and olefins, has traditionally been a highly anticipated method for producing substituted N-heterocycles (Scheme 35).68 However, when it comes to the synthesis of 4-substituted quinolines, terminal alkynes and paraformaldehyde are seldom employed in Povarov-type reactions. Instead, inorganic Lewis acids are typically used to catalyze this reaction, while the potential of organic acids in this context remains largely unexplored.69,70 The reaction proceeds through the formation of in situ-generated imine from condensation of aniline and paraformaldehyde. This imine then undergoes Povarov-type cyclisation with the alkyne, which on spontaneous oxidation yields the final product.
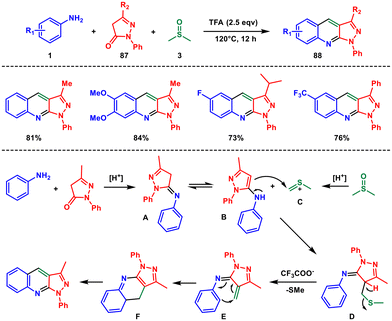
In 2021, Tiwari and colleagues presented a novel method for synthesising 3-substituted-1-aryl-1H-pyrazolo-[3,4-b]quinoline compounds using an acid-mediated and DMSO-assisted one-pot tandem synthesis.71 This approach allows for the selective formation of these valuable heterocycles without the need for transition metals or oxidants, making it an environmentally friendly process. The reaction involves the use of various substituted aryl amines 1 and pyrazolones 87, which undergo a cascade mechanism to produce a series of pyrazolo[4,3-c] quinolines 88. This is the first example of DMSO activation in pyrazolone (Scheme 36). Here DMSO not only acts as a solvent but also serves as the source of methine to the quinoline moiety.
Initially the process begins with the condensation of aniline and pyrazolone in the presence of TFA, leading to the formation of an imine A that undergoes isomerization to a more stable pyrazole B. However, in the presence of acid, DMSO is activated and generates an electrophilic thionium ion C that reacts with pyrazole B to form an intermediate D. Through the elimination of CH3SH, the intermediate D generates an azadiene intermediate E. This azadiene intermediate then undergoes annulation to produce an intermediate F, which ultimately undergoes aromatization to yield the desired product.
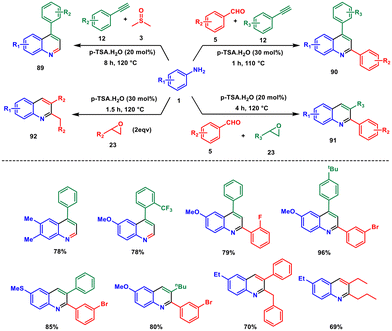
Recently our group has developed several three-component metal-free methods for the synthesis of differently substituted quinolines using p-TSA as catalyst. Firstly, we adopted a green and non-metallic approach to creating 4-arylquinoline 89 utilizing easily accessible arylamine 1, arylacetylene 12 and DMSO 3 with 20 mol% p-TSA.72 In this method, DMSO serves both as a reactant, contributing the C2 carbon atom to the quinoline structure, and as a solvent. Importantly, this process demonstrates high effectiveness and efficiency, all without requiring metal catalysts, ligands, co-catalysts as additives, or inert atmospheric conditions. Then, the synthesis of 2,4-diarylquinolines 90 was reported via a three-component reaction of arylamine 1, aryl aldehyde 5 and aryl acetylene 12 under solvent-free conditions at a temperature of 110 °C, employing 30 mol% p-toluenesulfonic acid as catalyst.73 Next, we developed a new eco-friendly method for producing 2,3-diarylquinolines 91 in a one-step process that combines arylamines 1, benzaldehyde 5 and styrene oxide 23.74 The reaction takes place at 120 °C in the presence of 20 mol% p-TSA, eliminating the need for additional metal catalysts, additives, or oxidizing agents. Detailed investigations into the mechanism of the reaction established the vital function of p-TSA and demonstrated that air serves as the exclusive oxidizing agent. Then, we documented a highly efficient method for creating 2-benzyl-3-arylquinoline 92 compounds. This synthesis involves using aryl amines 1 and styrene oxides 23 with the assistance of 20 mol% p-toluenesulfonic acid, all without the need for metals or solvents.75 This process is achieved through a pseudo-three-component reaction. This is the first metal-free synthesis of 2-benzyl-3-phenylquinoline using aniline and styrene oxide derivative (Scheme 37).
4. Catalyst-free reactions
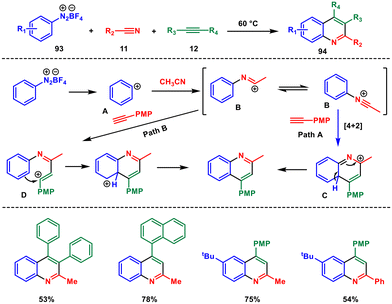
In traditional chemical reactions, catalysts are often used to speed up reactions or facilitate specific chemical transformations. However, catalyst-free organic transformations have gained significance as they are more environmentally friendly and cost efficient, and eliminate the need for potentially toxic or expensive catalysts, reducing waste and minimizing environmental impact. However, there are very few reactions that can occur without a catalyst. These reactions often rely on appropriate reaction conditions, such as temperature, concentration, and choice of solvent, to drive the desired chemical changes.For instance, in 2017, Shen and Yu et al. published a study on a fast and effective method for synthesising multiple substituted quinolines.76 This technique utilises a three-component cascade annulation process involving commonly available aryl diazonium salts 93, nitriles 11, and alkynes 12. The reaction does not require any catalysts or additives (Scheme 38). A wide range of aryl diazonium salts, nitriles, and alkynes can be used in this process, resulting in yields of up to 83%. This environmentally friendly and cost-effective procedure holds great promise for the advancement of potential industrial applications.
Mechanistically, upon heating the aryl diazonium salt undergoes decomposition, yielding the aryl cation A with concominant release of N2 gas. Acetonitrile functions as a nucleophile, engaging with the aryl cation A to produce the nitrilium cation B. There are two potential routes for product formation. In pathway A, the nitrilium cation reacts with an alkyne through a concerted Diels–Alder reaction, leading to the formation of intermediate C. Subsequent deprotonation of the intermediate results in the corresponding product. Conversely, the same product can be formed through a stepwise process in pathway B. In this case, intermediate B is attacked by the alkyne, generating the vinyl cation D, followed by a Friedel–Crafts-type cyclization that ultimately yields the desired quinoline 94.
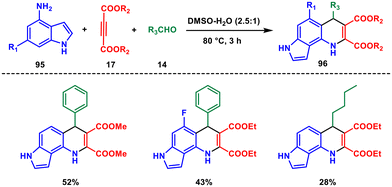
In 2020, Zhu and Liao introduced an effective and gentle catalyst-free three-component reaction for synthesising two novel series of dihydropyrrolo[2,3-h]quinolines with moderate to good yields.77 The reaction employed readily available 4-aminoindoles 95, but-2-ynedioates 17 and aldehydes 14 as starting materials, with water–DMSO mixtures serving as the solvent (Scheme 39). They showed that the ratio of water–DMSO mixtures played a crucial role and observed a synergistic effect of water and DMSO, as they formed hydrogen bonds and acted as proton donors and acceptors, respectively. This synergistic behaviour facilitated the smooth progress of the reaction, leading to fewer byproducts. This study represents the first investigation into the catalyst-free one-pot synthesis of pyrrolo[2,3-h]quinolines 96. Furthermore, the dihydropyrrolo[2,3-h]quinoline ring could be nearly completely oxidized to the pyrrolo[2,3-h]quinoline ring at room temperature using Cu(NO3)2 as an oxidizing agent. Most organic reactions are conducted using different solvents, the intriguing synergistic promotion effect of water–DMSO observed in this study encourages the exploration of new solvent mixtures with similar effects, which attracts further theoretical research on solvent mixture synergistic promotion.
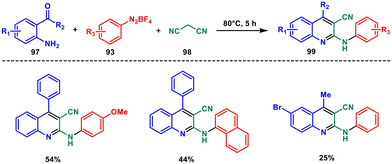
In 2020 Liu and coworkers developed a new method for the preparation of 2-amino-3-cyanoquinolines from readily available 2-aminoarylketones 97, aryldiazonium salts 93 and malononitrile 98via a cascade reaction.78 The one-pot method described involves the formation of an N-arylnitrilium intermediate through the direct reaction of aryldiazonium salts and malononitrile (Scheme 40). This intermediate undergoes intermolecular amination, Knoevenagel condensation, and subsequent aromatization, resulting in the formation of the desired compound in moderate to good yields. This approach enables the rapid synthesis of quinolines with functional groups at positions C2 and C3.
5. Miscellaneous reactions
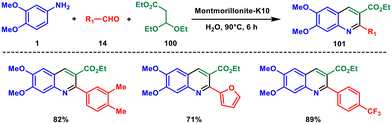
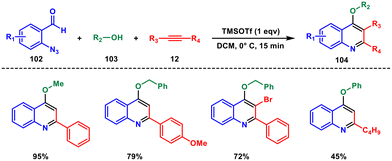
In 2012, Pal et al. achieved a direct one-pot synthesis of 2-substituted quinolines employing a 3-component reaction of aniline 1, aldehydes 14 and ethyl 3,3-diethoxypropionate 100 in aqueous medium with oxygen from air.79 Montmorillonite K-10 was identified as a green and reusable catalyst (Scheme 41). The reaction likely involves a Mannich reaction between an imine and 3-hydroxy acrylate, followed by intramolecular cyclization to produce a 1,2-dihydroquinoline intermediate. Subsequent oxidation using atmospheric oxygen yielded the desired product. This green approach holds promise for creating a diverse collection of 2-substituted quinoline-related molecules in an eco-friendly pathway.In 2017, Gharpure and coworkers developed a metal-free, Lewis acid-mediated multisegment cascade coupling approach for the synthesis of diversely substituted quinolines using 2-azido benzaldehyde 102, alcohol 103 and alkyne 12.80 The reaction proceeds via an oxonium ion-triggered alkyne carboamination sequence involving C–C and C–N bond formations to produce densely substituted 4-alkoxy quinolines 104 (Scheme 42). The versatility and practicality of this methodology were showcased through the post-functionalization of the obtained products, as well as its application in the synthesis of potent drug molecules. Furthermore, this high-yielding and straightforward technique exhibited excellent selectivity towards different functional groups, allowing for the synthesis of cyclic ether-fused quinolines and sugar-fused quinoline derivatives. They have also shown that the protocol gave a rapid access to biologically active natural products and drug molecules. Overall, this approach not only demonstrated efficient performance within a short timeframe but also allowed for sequential execution of multiple cascade reactions.
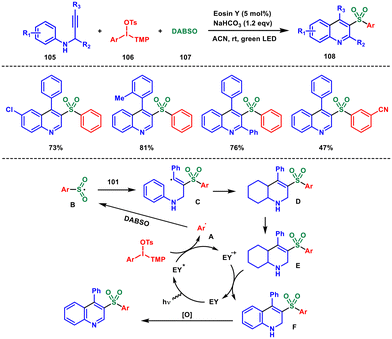
Considering the benefits of organophotocatalysed reactions compared with metal-based photocatalysts, Zhang and coworkers in 2018 reported a novel method for synthesising 3-arylsulfonylquinoline derivatives 108 using visible light with Eosin Y acting as the photocatalyst (Scheme 43). They used N-propargyl aromatic amines 105, diaryliodonium salts 106 and sulfur dioxide 107 in this three-component synthesis allowing the formation of C–S bonds and quinolines in a single step.81 The approach not only demonstrates high efficiency but also boasts excellent substrate scope and tolerance towards different functional groups. This protocol is particularly appealing for the utilization of easily manageable diaryliodonium salts, sulfur dioxide sources, and the cost-effective photocatalyst Eosin Y.
To begin, the photocatalyst Eosin Y was activated by visible light, leading to the creation of Eosin Y*. Then it underwent oxidative quenching in conjunction with diaryliodonium salts, resulting in the formation of aryl radical A and Eosin Y+. Following this, radical A engaged in a reaction with DABSO to produce another radical B which reacted with the N-propergyl amine, forming an alkenyl radical C. Subsequently, the alkenyl radical underwent an intramolecular cyclization process, giving rise to an aryl radical D. The deprotonation of radical D resulted in the formation of a radical anion E. This radical anion was then oxidized by Eosin Y+, resulting in the production of the sulfonated compound 1,2-dihydroquinoline, labelled as F. Finally, compound F underwent a dehydro-aromatization reaction to yield the corresponding final product.
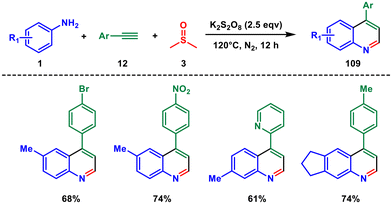
In 2018, Tiwari and coworkers introduced a transition-metal-free three-component cascade reaction using anilines 1 and alkynes 12, along with potassium persulfate (K2S2O8) in dimethyl sulfoxide (DMSO) for an environmentally friendly approach to synthesize 4-arylquinolines 109.82 They demonstrated that a wide range of alkynes and anilines with various substitution patterns could be successfully employed in this one-pot cascade process (Scheme 44). The use of DMSO as a carbon source in this methodology significantly improved atom efficiency and minimized environmental impact. Moreover, the authors expanded the synthetic applicability of this approach to the preparation of biologically significant compounds such as 4-aryl-2-morpholinoquinoline and 4-aryl-2-tosylquinoline.
In the future, the development of novel multicomponent reactions for quinoline synthesis could lead to more efficient and diverse routes to access quinoline derivatives. This could enable the rapid generation of structurally diverse compound libraries for drug discovery and materials science applications. Further optimization of MCRs for quinoline synthesis with an emphasis on sustainability and atom economy can contribute to the principles of green chemistry. Designing MCRs with minimal waste generation and environmentally benign reagents would be beneficial for large-scale production. Computational modelling and virtual screening approaches can help identify promising reaction pathways, predict reactivity, and optimize reaction conditions, thereby accelerating the development of efficient synthetic routes. Continued research efforts in these areas are essential to unlock the full potential of MCRs for the synthesis of quinoline derivatives with diverse properties and applications.
6. Conclusion
This review article describes various strategies for quinoline synthesis via multicomponent reaction. Different synthetic techniques based on catalyst, viz. (i) iron-catalysed, (ii) copper-catalysed, (iii) other metal-catalysed, (iv) iodine-catalysed, (v) acid-catalysed, and (vi) catalyst-free syntheses, are illustrated. The broad scope of multicomponent reactions allows for the incorporation of various starting materials, making them adaptable to the synthesis of diverse quinoline derivatives. As research in this field continues to advance, we can anticipate the development of new, more efficient, and selective multicomponent reactions for quinoline synthesis, further expanding the possibilities for their incorporation into complex molecule construction. Despite the promise offered by existing techniques, there exists an opportunity to explore new pathways in the field. This includes the pursuit of diversity-oriented synthesis and the creation of enriched quinoline structures using affordable starting materials and catalysts. Such endeavours lay the groundwork for the development of even more captivating methodologies within this field.Conflicts of interest
The authors have no conflict of interest to declare.Acknowledgements
AM thanks IIT Guwahati for providing an Institute Postdoctoral Fellowship through R&D (Project No. IITG/R&D/IPDF/2022-23/20220922P138). ATK is thankful to the Department of Science and Technology, New Delhi, for financial support (Grant No.: CRG/2022/002751/OC). We also thank Prof. S. S. Ghosh for his valuable suggestions. We are thankful to the editor as well as all the referees for their valuable comments and suggestions on our manuscript. We also thank all the researchers who have contributed to the research related to quinolines.References
- G. Lewinska, J. Sanetra and K. W. Marszalek, J. Mater. Sci.: Mater. Electron., 2021, 32, 18451–18465 CrossRef CAS.
- C. Dos Santos Chagas, F. L. A. Fonseca and I. A. Bagatin, Mater. Sci. Eng., C, 2019, 98, 1043–1052 CrossRef CAS PubMed.
- O. O. Ajani, K. T. Iyaye and O. T. Ademosun, RSC Adv., 2022, 12, 18594–18614 RSC.
- S. Rajendran, K. Sivalingam, R. P. Karnam Jayarampillai, W. Wang and C. O. Salas, Chem. Biol. Drug Des., 2022, 100, 1042–1085 CrossRef CAS PubMed.
- F. F. Runge, Ann. Phys., 1834, 107, 65–78 CrossRef.
- G. Collin and H. Höke, in Ullmann's Encyclopedia of Industrial Chemistry, John Wiley & Sons, Ltd, 2000 Search PubMed.
- Y.-Q. Zhao, X. Li, H.-Y. Guo, Q.-K. Shen, Z.-S. Quan and T. Luan, Molecules, 2023, 28, 6478 CrossRef CAS.
- I. Balderas-Renteria, P. Gonzalez-Barranco, A. Garcia, B. K. Banik and G. Rivera, Curr. Med. Chem., 2012, 19, 4377–4398 CrossRef CAS PubMed.
- O. Afzal, S. Kumar, M. R. Haider, M. R. Ali, R. Kumar, M. Jaggi and S. Bawa, Eur. J. Med. Chem., 2015, 97, 871–910 CrossRef CAS PubMed.
- S. Vandekerckhove and M. D′hooghe, Bioorg. Med. Chem., 2015, 23, 5098–5119 CrossRef CAS PubMed.
- H.-G. Fu, Z.-W. Li, X.-X. Hu, S.-Y. Si, X.-F. You, S. Tang, Y.-X. Wang and D.-Q. Song, Molecules, 2019, 24, 548 CrossRef PubMed.
- S. Mukherjee and M. Pal, Drug Discovery Today, 2013, 18, 389–398 CrossRef CAS PubMed.
- P. Sridhar, M. Alagumuthu, S. Arumugam and S. R. Reddy, RSC Adv., 2016, 6, 64460–64468 RSC.
- A. Dorababu, Arch. Pharm., 2021, 354, 2000232 CrossRef CAS.
- C. S. Manohar, A. Manikandan, P. Sridhar, A. Sivakumar, B. Siva Kumar and S. R. Reddy, J. Mol. Struct., 2018, 1154, 437–444 CrossRef CAS.
- J. L. Roberts, A. Poklepovic, L. Booth and P. Dent, Cell. Signalling, 2020, 70, 109573 CrossRef CAS.
- K. S. Kumar, S. Kiran Kumar, B. Yogi Sreenivas, D. R. Gorja, R. Kapavarapu, D. Rambabu, G. Rama Krishna, C. M. Reddy, M. V. Basaveswara Rao, K. V. L. Parsa and M. Pal, Bioorg. Med. Chem., 2012, 20, 2199–2207 CrossRef CAS PubMed.
- B. S. Matada, R. Pattanashettar and N. G. Yernale, Bioorg. Med. Chem., 2021, 32, 115973 CrossRef CAS PubMed.
- M. Sc. S. Chauhan, T. Umar and M. K. Aulakh, ChemistrySelect, 2023, 8, e202204960 CrossRef CAS.
- L. Weber, Drug Discovery Today, 2002, 7, 143–147 CrossRef CAS.
- B. D. Sherry and A. Fürstner, Acc. Chem. Res., 2008, 41, 1500–1511 CrossRef CAS.
- C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Rev., 2011, 111, 1293–1314 CrossRef CAS.
- S. D. Jadhav and A. Singh, Org. Lett., 2017, 19, 5673–5676 CrossRef CAS PubMed.
- S. Mahato, A. Mukherjee, S. Santra, G. V. Zyryanov and A. Majee, Org. Biomol. Chem., 2019, 17, 7907–7917 RSC.
- L. Yang and J.-P. Wan, Green Chem., 2020, 22, 3074–3078 RSC.
- Y. Wang, C. Chen, J. Peng and M. Li, Angew. Chem., Int. Ed., 2013, 52, 5323–5327 CrossRef CAS PubMed.
- Y. Li, X. Cao, Y. Liu and J.-P. Wan, Org. Biomol. Chem., 2017, 15, 9585–9589 RSC.
- W. Wu, Y. Guo, X. Xu, Z. Zhou, X. Zhang, B. Wu and W. Yi, Org. Chem. Front., 2018, 5, 1713–1718 RSC.
- Y. Liu, Y. Hu, Z. Cao, X. Zhan, W. Luo, Q. Liu and C. Guo, Adv. Synth. Catal., 2018, 360, 2691–2695 CrossRef CAS.
- K.-M. Jiang, J.-A. Kang, Y. Jin and J. Lin, Org. Chem. Front., 2018, 5, 434–441 RSC.
- S. Ali and A. T. Khan, Org. Biomol. Chem., 2021, 19, 3255–3262 RSC.
- L. G. Qiang and N. H. Baine, J. Org. Chem., 1988, 53, 4218–4222 CrossRef CAS.
- W.-J. Yoo and S. Kobayashi, Green Chem., 2014, 16, 2438–2442 RSC.
- I. Ghosh, R. S. Shaikh and B. König, Angew. Chem., Int. Ed., 2017, 56, 8544–8549 CrossRef CAS PubMed.
- A. K. Dhiya, A. Monga and A. Sharma, Org. Chem. Front., 2021, 8, 1657–1676 RSC.
- J.-H. Choi and C.-M. Park, Adv. Synth. Catal., 2018, 360, 3553–3562 CrossRef CAS.
- J. Huo, X. Geng, W. Li, P. Zhang and L. Wang, Adv. Synth. Catal., 2022, 364, 3539–3543 CrossRef.
- S. Roy and O. Reiser, Angew. Chem., Int. Ed., 2012, 51, 4722–4725 CrossRef CAS PubMed.
- A. W. Chester, J. Org. Chem., 1970, 35, 1797–1800 CrossRef CAS.
- S. Anvar, I. Mohammadpoor-Baltork, S. Tangestaninejad, M. Moghadam, V. Mirkhani, A. R. Khosropour and R. Kia, RSC Adv., 2012, 2, 8713 RSC.
- M. G. Mura, S. Rajamäki, L. De Luca, E. Cini and A. Porcheddu, Adv. Synth. Catal., 2015, 357, 576–582 CrossRef CAS.
- X. Xu, W. Liu, Z. Wang, Y. Feng, Y. Yan and X. Zhang, Tetrahedron Lett., 2016, 57, 226–229 CrossRef CAS.
- Y. Luo, H. Sun, W. Zhang, X. Wang, S. Xu, G. Zhang, Y. Jian and Z. Gao, RSC Adv., 2017, 7, 28616–28625 RSC.
- J. Cen, J. Li, Y. Zhang, Z. Zhu, S. Yang and H. Jiang, Org. Lett., 2018, 20, 4434–4438 CrossRef CAS PubMed.
- S. Chiba, L. Zhang, G. Y. Ang and B. W.-Q. Hui, Org. Lett., 2010, 12, 2052–2055 CrossRef CAS PubMed.
- X. Xu, Y. Yang, X. Chen, X. Zhang and W. Yi, Org. Biomol. Chem., 2017, 15, 9061–9065 RSC.
- X. Xu, Y. Yang, X. Zhang and W. Yi, Org. Lett., 2018, 20, 566–569 CrossRef CAS PubMed.
- X. Zhou, Y. Pan and X. Li, Angew. Chem., Int. Ed., 2017, 56, 8163–8167 CrossRef CAS PubMed.
- S. Ali, R. Gattu, V. Singh, S. Mondal, A. T. Khan, G. Dubey and P. V. Bharatam, Org. Biomol. Chem., 2020, 18, 1785–1793 RSC.
- S.-T. Xiao, C.-T. Ma, J.-Q. Di and Z.-H. Zhang, New J. Chem., 2020, 44, 8614–8620 RSC.
- Y. Zhang, L. Chen, Y. Shao, F. Zhang, Z. Chen, N. Lv, J. Chen and R. Li, Org. Chem. Front., 2021, 8, 254–259 RSC.
- V. F. Batista, D. C. G. A. Pinto and A. M. S. Silva, ACS Sustainable Chem. Eng., 2016, 4, 4064–4078 CrossRef CAS.
- M. S. Yusubov and V. V. Zhdankin, Resour.-Effic. Technol., 2015, 1, 49–67 Search PubMed.
- Q. Gao, S. Liu, X. Wu and A. Wu, Org. Lett., 2014, 16, 4582–4585 CrossRef CAS PubMed.
- Q. Gao, S. Liu, X. Wu, J. Zhang and A. Wu, J. Org. Chem., 2015, 80, 5984–5991 CrossRef CAS PubMed.
- K. V. Sashidhara, G. R. Palnati, L. R. Singh, A. Upadhyay, S. R. Avula, A. Kumar and R. Kant, Green Chem., 2015, 17, 3766–3770 RSC.
- X. Wu, X. Geng, P. Zhao, J. Zhang, X. Gong, Y. Wu and A. Wu, Org. Lett., 2017, 19, 1550–1553 CrossRef CAS PubMed.
- R. Yan, X. Liu, C. Pan, X. Zhou, X. Li, X. Kang and G. Huang, Org. Lett., 2013, 15, 4876–4879 CrossRef CAS PubMed.
- X. Geng, X. Wu, P. Zhao, J. Zhang, Y.-D. Wu and A.-X. Wu, Org. Lett., 2017, 19, 4179–4182 CrossRef CAS PubMed.
- J.-C. Xiang, Z.-X. Wang, Y. Cheng, S.-Q. Xia, M. Wang, B.-C. Tang, Y.-D. Wu and A.-X. Wu, J. Org. Chem., 2017, 82, 9210–9216 CrossRef CAS PubMed.
- S. Yaragorla and A. Pareek, Eur. J. Org. Chem., 2018, 1863–1871 CrossRef CAS.
- S. Ali and A. T. Khan, Org. Biomol. Chem., 2021, 19, 8772–8782 RSC.
- X.-X. Yu, P. Zhao, Y. Zhou, C. Huang, L.-S. Wang, Y.-D. Wu and A.-X. Wu, J. Org. Chem., 2021, 86, 8381–8388 CrossRef CAS PubMed.
- C. E. Brown, J. McNulty, C. Bordón, R. Yolken and L. Jones-Brando, Org. Biomol. Chem., 2016, 14, 5951–5955 RSC.
- L. Min, B. Pan and Y. Gu, Org. Lett., 2016, 18, 364–367 CrossRef CAS.
- R. Gattu, P. R. Bagdi, R. S. Basha and A. T. Khan, J. Org. Chem., 2017, 82, 12416–12429 CrossRef CAS.
- D. Chandra, A. K. Dhiman, R. Kumar and U. Sharma, Eur. J. Org. Chem., 2019, 2753–2758 CrossRef CAS.
- X. Jiang and R. Wang, Chem. Rev., 2013, 113, 5515–5546 CrossRef CAS.
- O. Ghashghaei, C. Masdeu, C. Alonso, F. Palacios and R. Lavilla, Drug Discovery Today: Technol., 2018, 29, 71–79 CrossRef.
- F. Shi, G.-J. Xing, Z.-L. Tao, S.-W. Luo, S.-J. Tu and L.-Z. Gong, J. Org. Chem., 2012, 77, 6970–6979 CrossRef CAS PubMed.
- P. Yadav, A. Awasthi, S. Gokulnath and D. K. Tiwari, J. Org. Chem., 2021, 86, 2658–2666 CrossRef CAS PubMed.
- S. Faraz and A. T. Khan, Org. Biomol. Chem., 2023, 21, 7553–7560 RSC.
- S. Faraz, S. Yashmin, M. Dilip Marathe and A. Taleb Khan, Tetrahedron Lett., 2023, 120, 154433 CrossRef CAS.
- S. Faraz and A. T. Khan, Synthesis, 2023, 55, 3195–3203 CrossRef CAS.
- S. Faraz, M. Kumar, A. Taleb Khan, S. Ponneganti and P. Radhakrishnanand, Tetrahedron Lett., 2023, 115, 154283 CrossRef CAS.
- H. Wang, Q. Xu, S. Shen and S. Yu, J. Org. Chem., 2017, 82, 770–775 CrossRef CAS.
- H. Liao and Q. Zhu, Org. Biomol. Chem., 2020, 18, 215–219 RSC.
- M. Ramanathan, J. Wan, Y.-H. Liu, S.-M. Peng and S.-T. Liu, Org. Biomol. Chem., 2020, 18, 975–982 RSC.
- T. R. Reddy, L. S. Reddy, G. R. Reddy, K. Yarbagi, Y. Lingappa, D. Rambabu, G. R. Krishna, C. M. Reddy, K. S. Kumar and M. Pal, Green Chem., 2012, 14, 1870 RSC.
- S. J. Gharpure, S. K. Nanda, P. A. Adate and Y. G. Shelke, J. Org. Chem., 2017, 82, 2067–2080 CrossRef CAS PubMed.
- D. Sun, K. Yin and R. Zhang, Chem. Commun., 2018, 54, 1335–1338 RSC.
- M. Phanindrudu, S. B. Wakade, D. K. Tiwari, P. R. Likhar and D. K. Tiwari, J. Org. Chem., 2018, 83, 9137–9143 CrossRef CAS PubMed.
Footnote |
| † Celebrating the 100th birthday of Prof Sukh Dev. |
| This journal is © The Royal Society of Chemistry 2024 |