Open Access Article
Open Access ArticleStereodivergent synthesis of 2-oxo-oligopyrrolidines by an iterative coupling strategy†
Yasuki
Soda
a,
Kumpei
Tatsumi
a,
Matteo
Forner
 ab,
Shunsei
Sato
a,
Kana
Shibuya
a,
Tomoe
Matagawa
a,
Siro
Simizu
a,
Noritaka
Chida
ab,
Shunsei
Sato
a,
Kana
Shibuya
a,
Tomoe
Matagawa
a,
Siro
Simizu
a,
Noritaka
Chida
 a,
Toshitaka
Okamura
a,
Toshitaka
Okamura
 *a and
Takaaki
Sato
*a and
Takaaki
Sato
 *a
*a
aDepartment of Applied Chemistry, Faculty of Science and Technology, Keio University, 3-14-1, Hiyoshi, Kohoku-ku, Yokohama, 223-8522, Japan. E-mail: t-okamura@applc.keio.ac.jp; takaakis@applc.keio.ac.jp
bDepartment of Pharmaceutical and Pharmacological Sciences, University of Padova, Via Marzolo, 5, 35131 Padova, PD, Italy
First published on 28th March 2024
Abstract
Natural linear polyamines play diverse roles in physiological processes by interacting with receptors at the cellular level. Herein, we describe the stereodivergent synthesis of oligopyrrolidines, which are conformationally constrained polyamines. We synthesized dimeric and trimeric 2-oxo-oligopyrrolidines using an iterative coupling strategy. The key to our success is an iridium-catalyzed trans/cis-selective nucleophilic addition and subsequent threo/erythro-stereoselective reduction. The synthesized pyrrolidines show varying cytotoxicities against a human cancer cell line depending on the number of rings and their stereochemistry.
Introduction
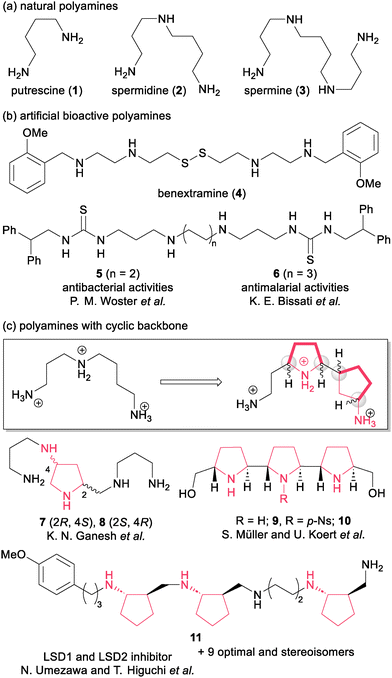
Polyamines are organic cations containing a repeating structure of acyclic alkyl amines. Natural polyamines such as putrescine (1), spermidine (2) and spermine (3) exhibit a broad range of physiological functions such as cell replication and the modulation of gene expression (Scheme 1a).1 Moreover, polyamines have attracted attention as “privileged” templates to induce versatile biological activities (Scheme 1b).2 In 1988, the Melchiorre group documented tetraamine disulfide benextramine (4) as an irreversible α-adrenoreceptor antagonist.2a Subsequently, 4 was also found to bind to other receptors such as nicotinic receptors and muscarinic receptors. These studies indicated that synthetic polyamines are attractive candidates in drug discovery. Woster and co-workers reported antibacterial polyamines targeting bacterial membranes.3 Compound 5 exhibits bactericidal activity against various Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA) due to electrostatic interaction between the positively charged polyamines and the negatively charged bacterial membrane. Bissati and co-workers reported thiourea-based polyamine 6 as being potent against the malaria parasite.4Cyclic polyamines with stereogenic centers are promising structural motifs for controlling the three-dimensional arrangements of protonated amine groups, which is crucial for recognizing the target polyanion structures, as seen in DNA and mRNA (Scheme 1c).5 In 1997, Ganesh's group reported the first example using spermine analogues 7 and 8, which have a pyrrolidine backbone. Their synthetic analogues exhibited stronger binding to the DNA triplex than natural spermine.6 Müller, Koert and co-workers synthesized the trans-threo-trans oligopyrrolidines 9 and 10, which have helical conformations due to the rigid structure of pyrrolidines.7 The rate of ssRNA cleavage by oligopyrrolidine 10 was faster than that of natural spermine. Higuchi and colleagues reported pentamine-based inhibitors of lysine-specific demethylase 1 (LSD1) and LSD2 such as 11, which are promising potent anticancer agents.8 These pentamines exhibit better LSD1- and 2-inhibitory activities than simple linear polyamines, probably through conformational restriction with the three trans-cyclopentane units contained in the six stereogenic centers. They also found that the LSD-inhibitory activities depended on the stereochemistry of each artificial polyamine.
Results and discussion
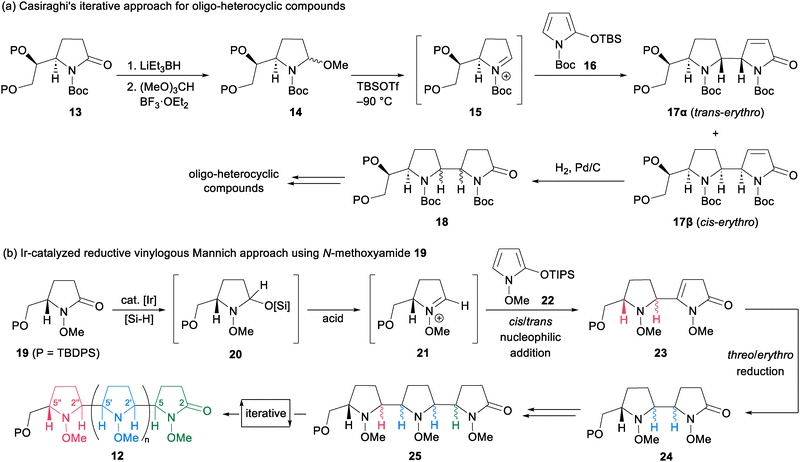
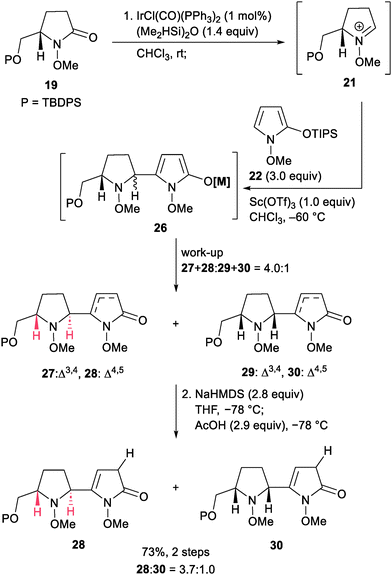
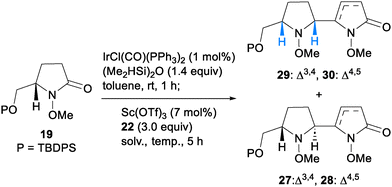
To obtain three-dimensionally constrained polyamines, we took interest in 2-oxo-oligopyrrolidines 12 possessing diverse stereochemistry at C2′–C5 on the cyclic amines (Scheme 2). Casiraghi and coworkers documented pioneering reports on a building-block-based synthesis of 2-oxo-oligopyrrolidines by a coupling reaction using N-Boc-lactam 13 and 2-silyloxypyrrole 16 (Scheme 2a).9 The method began with LiEt3BH reduction of N-Boc-lactam 13, followed by acid-mediated formation of N,O-acetal 14. Addition of TBSOTf to 14 at −90 °C formed the N-acyliminium ion 15, which underwent the vinylogous Mannich reaction10,11 with 16 to give trans-erythro and cis-erythro 2-oxo-bispyrrolidines 17α and 17β. Pd/C-catalyzed hydrogenation of the resulting bispyrrolidines 17 provided 18, which could be converted to oligo-heterocyclic compounds by the same four-step sequence. Their pioneering work successfully demonstrated the utility of the iterative approach. However, some synthetic issues remained unsolved. First, reduction of amide carbonyls required a stoichiometric amount of a strong reductant. Second, transformation of the resulting hemiaminal to N,O-acetal 14 prior to the vinylogous Mannich reaction was essential to form the unstable N-acyliminium ion 15 under acidic conditions. In addition, only two out of the four possible stereoisomers were obtained by their method. To resolve these issues, we envisioned an iterative approach12 based on N-methoxylactams.13,14 Our method begins with an iridium-catalyzed reductive vinylogous Mannich reaction between N-methoxylactam 19 and 2-silyloxypyrrole 22.15,16 Iridium-catalyzed hydrosilylation of 19 would give N,O-acetal 20. Subsequent addition of an acid to 20 forms the N-methoxyiminium ion 21, which could undergo a cis/trans-selective vinylogous Mannich reaction with 22 to provide enamide 23. We employed N-methoxylactams instead of N-Boc-lactams due to the following reasons. N-Methoxylactams undergo Ir-catalyzed hydrosilylation under mild conditions, while N-Boc-lactams do not.17 Formation of the N-methoxyiminium ion 21 is feasible by direct addition of an acid without isolation of N,O-acetal 20. Thus, a reductive vinylogous Mannich reaction would give enamide 23 in a one-pot process. In addition, N-methoxyiminium ions are known to be more electrophilic than N-alkyliminium ions such as the N-benzyliminium ion, leading to a high yielding vinylogous Mannich reaction.18 The resulting enamide 23 could be converted to threo- or erythro-2-oxo-bispyrrolidines 24 by stereoselective reduction. These sequences would enable the stereodivergent synthesis of all four possible diastereomers of 24. The key to success is the development of appropriate reaction conditions to control the cis/trans configurations on the pyrrolidine ring, and the threo/erythro configurations on the rotational bond. Ultimately, we found that the stereocontrol of trans- or cis-selectivity could be achieved using kinetic or thermodynamic conditions, respectively. Subsequent threo/erythro-selectivity was controlled by the choice of reducing agent. Iterative application of this process enabled the construction of 2-oxo-oligopyrrolidines 12. In this paper, we demonstrate the synthesis of all four possible diastereomers of 2-oxo-bispyrrolidines 24. The method is applicable to the synthesis of 2-oxo-trispyrrolidines 25en route to 2-oxo-oligopyrrolidines 12.Our stereodivergent nucleophilic addition was evaluated using N-methoxylactam 19 and 2-silyloxypyrrole 22 (Scheme 3). After extensive screening, trans-selective nucleophilic addition under kinetic control was realized as follows. Treatment of a solution of 13 in CHCl3 with (Me2HSi)2O and a catalytic amount of IrCl(CO)(PPh3)2 initiated hydrosilylation to generate the N-methoxyiminium ion 21. Subsequently, the addition of Sc(OTf)3 and 2-silyloxypyrrole 22 at −60 °C generated the N-methoxyiminium ion 26. Aqueous work-up afforded a mixture of four lactams and two enamides 27–30.19 Successful trans-selectivity is dependent on the nature of the solvent used, and CHCl3 was found to be the best solvent [trans-(27 + 28)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis-(29 + 30) = 4
cis-(29 + 30) = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1]. Although the vinylogous Mannich reaction produced a mixture of six diastereomers, these diastereomers converged to two enamides 28 and 30 through deprotonation with NaHMDS, and α-selective protonation with AcOH at −78 °C. Thus, the reductive vinylogous Mannich reaction and subsequent α-selective protonation gave enamides 28 and 30 in a trans-selective fashion (73% for 2 steps, 28
1]. Although the vinylogous Mannich reaction produced a mixture of six diastereomers, these diastereomers converged to two enamides 28 and 30 through deprotonation with NaHMDS, and α-selective protonation with AcOH at −78 °C. Thus, the reductive vinylogous Mannich reaction and subsequent α-selective protonation gave enamides 28 and 30 in a trans-selective fashion (73% for 2 steps, 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 = 3.7
30 = 3.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).20
1).20
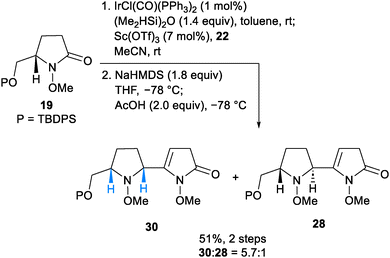
With the optimized conditions for the trans-selective nucleophilic addition reaction in hand, we examined the cis-selective reaction as shown in Scheme 4. After iridium-catalyzed hydrosilylation of 19, the vinylogous Mannich reaction using MeCN as a co-solvent at room temperature was found to show cis-selectivity. The resulting mixture converged to two enamides 30 and 28 by a regioselective deprotonation/protonation sequence (51%, 2 steps, cis/trans = 5.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).20
1).20
To elucidate the mechanism leading to the cis-selectivity, we investigated the effect of reaction temperature (Table 1). After iridium-catalyzed hydrosilylation of 19 in toluene at room temperature, addition of 22 in the presence of 7 mol% of Sc(OTf)3 at −78 °C promoted the vinylogous Mannich reaction. Interestingly, a mixture of four lactams and two enamides 27–30 was obtained with slight trans-selectivity [99%, cis-(29 + 30)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans-(27 + 28) = 1
trans-(27 + 28) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7] (entry 1). The vinylogous Mannich reaction at room temperature was found to be cis-selective [31%, cis-(29 + 30)
1.7] (entry 1). The vinylogous Mannich reaction at room temperature was found to be cis-selective [31%, cis-(29 + 30)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans-(27 + 28) = 3.1
trans-(27 + 28) = 3.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1], although some decomposition was observed (entry 2). To increase both yield and selectivity, the addition of MeCN was proved to be effective [62%, cis-(29 + 30)
1], although some decomposition was observed (entry 2). To increase both yield and selectivity, the addition of MeCN was proved to be effective [62%, cis-(29 + 30)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans-(27 + 28) = 5.5
trans-(27 + 28) = 5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1]. These results indicated that the cis-selectivity was achieved under thermodynamic conditions.
1]. These results indicated that the cis-selectivity was achieved under thermodynamic conditions.
| Entry | Temp. | Solv. |
cis-(29 + 30)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans-(27 + 28) trans-(27 + 28) |
Combined yield (%) |
|---|---|---|---|---|
| a Optimal reaction conditions: 19 (1 equiv.), IrCl(CO)(PPh3)2 (1 mol%), (Me2HSi)2O (1.4 equiv.), toluene, rt, 1 h; Sc(OTf)3 (7 mol%), 22 (3.0 equiv.), co-solvent, 5 h. | ||||
| 1 | −78 °C | None | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 1.7 |
99 |
| 2 | rt | None | 3.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
31 |
| 3 | rt | MeCN | 5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
62 |
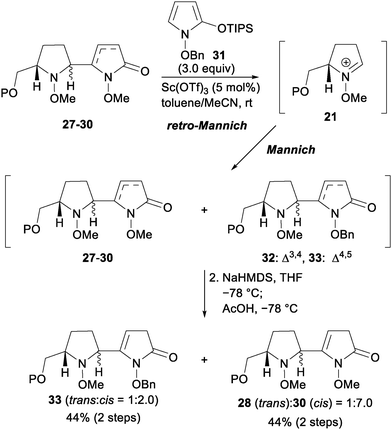
To clarify the thermodynamic nature, the reversibility of the vinylogous Mannich reaction was further confirmed by a crossover experiment (Scheme 5). A solution of 27–30 in toluene/MeCN was treated with 1-benzyloxy-2-silyloxypyrrole 31 in the presence of a catalytic amount of Sc(OTf)3. Subsequent isomerization to enamides through the deprotonation/protonation protocol gave enamide 33 [44% (2 steps), trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis = 1
cis = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0], along with a mixture of 28 and 30 [44% (2 steps), 28
2.0], along with a mixture of 28 and 30 [44% (2 steps), 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 = 1
30 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.0].These results clearly indicated that enamide 33 was formed by a retro-Mannich/Mannich reaction under equilibrium conditions.
7.0].These results clearly indicated that enamide 33 was formed by a retro-Mannich/Mannich reaction under equilibrium conditions.
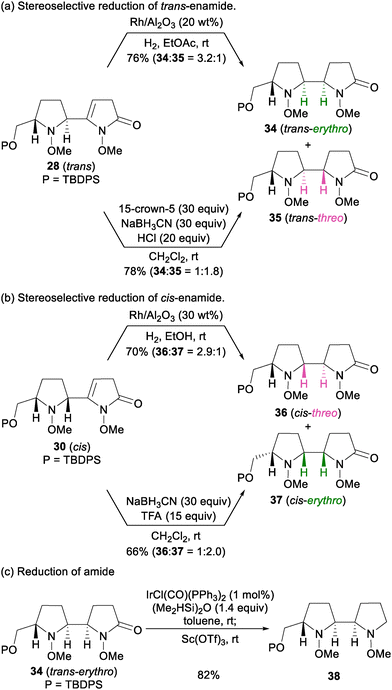
Having established the cis/trans-selective vinylogous Mannich reaction, we investigated the stereoselective reduction of trans-enamide 28 and cis-enamide 30 (Scheme 6). Hydrogenation of trans-enamide 28 with Rh/Al2O3 formed the 2-oxo-bispyrrolidines, favoring erythro lactam 34 over threo lactam 35 (Scheme 6a, 76%, 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 = 3.2
35 = 3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).20 In contrast, hydride reduction under acidic conditions reversed this stereoselectivity. Thus, treatment of trans-enamide 28 with NaBH3CN and HCl in the presence of 15-crown-5 gave a mixture of 2-oxo-bispyrrolidines 34 and 35 with threo selectivity (78%, 34
1).20 In contrast, hydride reduction under acidic conditions reversed this stereoselectivity. Thus, treatment of trans-enamide 28 with NaBH3CN and HCl in the presence of 15-crown-5 gave a mixture of 2-oxo-bispyrrolidines 34 and 35 with threo selectivity (78%, 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 = 1
35 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8).20,21cis-Enamide 30 showed similar stereoselectivity (Scheme 6b). In contrast, Rh/Al2O3-catalyzed hydrogenation of cis-enamide 30 predominantly provided threo-lactam 36 (70%, 36
1.8).20,21cis-Enamide 30 showed similar stereoselectivity (Scheme 6b). In contrast, Rh/Al2O3-catalyzed hydrogenation of cis-enamide 30 predominantly provided threo-lactam 36 (70%, 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 = 2.9
37 = 2.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1),20 and hydride reduction with NaBH3CN and TFA produced erythro lactam 37 as a major product (66%, 36
1),20 and hydride reduction with NaBH3CN and TFA produced erythro lactam 37 as a major product (66%, 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 = 1
37 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0). Reduction of trans-erythro lactam 34 was achieved by hydrosilylation with 1 mol% of IrCl(CO)(PPh3)2 and (Me2HSi)2O, followed by the addition of Sc(OTf)3 to give bispyrrolidine 38.
2.0). Reduction of trans-erythro lactam 34 was achieved by hydrosilylation with 1 mol% of IrCl(CO)(PPh3)2 and (Me2HSi)2O, followed by the addition of Sc(OTf)3 to give bispyrrolidine 38.
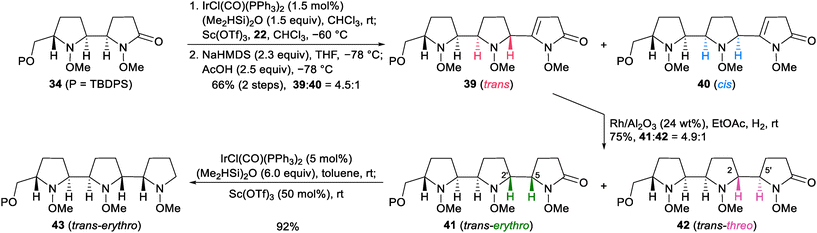
We demonstrated the feasibility of iterative assembly of a pyrrolidine unit by investigating the stereoselective synthesis of 2-oxo-trispyrrolidines 41 and 42 (Scheme 7). Under the optimized kinetic conditions for trans-selective addition, trans-erythro lactam 34 was converted to trans enamide 39, along with the formation of minor cis enamide 40 (66% (2 steps), 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 = 4.5
40 = 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).20 Rh/Al2O3-catalyzed hydrogenation promoted erythro-selective reduction of enamide 39, providing erythro lactam 41 and threo lactam 42 in 75% yield (41
1).20 Rh/Al2O3-catalyzed hydrogenation promoted erythro-selective reduction of enamide 39, providing erythro lactam 41 and threo lactam 42 in 75% yield (41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 = 4.9
42 = 4.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).20 The lactam carbonyl of 2-oxo-trispyrrolidine 41 was successfully reduced by iridium-catalyzed hydrosilylation followed by the addition of Sc(OTf)3 to give trispyrrolidine 43.
1).20 The lactam carbonyl of 2-oxo-trispyrrolidine 41 was successfully reduced by iridium-catalyzed hydrosilylation followed by the addition of Sc(OTf)3 to give trispyrrolidine 43.
The antiproliferative activity of the series of mono-, bis-, and 2-oxo-trispyrrolidines against Jurkat cells (human T-cell leukemia cells) is summarized in Table 2. Comparison of the activity of 2-oxo-pyrrolidine 19, 2-oxo-bispyrrolidines 34–37, and 2-oxo-trispyrrolidines 41 and 42 revealed clear relationships between the number of rings and the compound's antiproliferative activity. 2-Oxo-bispyrrolidines 34–37 were found to be more potent than 2-oxo-pyrrolidine 19, with 2-oxo-trispyrrolidines 42 displaying the strongest activity, approximately 10-fold when compared to monomeric 2-oxo-pyrrolidine 19. Stereochemistry played an important role in the antiproliferative activity of the compounds. Of the 2-oxo-bispyrrolidines 34–37, trans-threo lactam 35 exhibited the highest activity. A similar tendency was observed for the trimeric compounds, although only two diastereomers were tested. Thus, the activity of 2-oxo-trispyrrolidine 42 with a trans-threo configuration at C2–C5′ was 8-fold higher than that of 34 with a trans-erythro configuration. Interestingly, 2-oxo-oligopyrrolidines 34 and 41 exhibited stronger activity than the corresponding 38 and 43, their fully reduced counterparts.
| (a) IC50 values of mono- and 2-oxo-bispyrrolidines in Jurkat cells | (b) IC50 values of 2-oxo-trispyrrolidines in Jurkat cells | ||
|---|---|---|---|
| Compounds | IC50 [μM] | Compounds | IC50 [μM] |
| a The antiproliferative activity against Jurkat cells was evaluated by the standard MTT assay. Cells were treated for 72 h with increasing concentrations of the evaluated compounds. | |||
| 19 | 22.9 | 41 | 15.5 |
| 34 | 14.0 | 42 | 2.00 |
| 35 | 5.50 | 43 | 44.1 |
| 36 | 10.7 | ||
| 37 | 17.0 | ||
| 38 | 27.6 | ||
Conclusion
In conclusion, the results reported here provide an iterative and stereodivergent method to construct 2-oxo-oligopyrrolidines. trans/cis-Selectivity in the nucleophilic addition to amide carbonyls was controlled by choosing either kinetic or thermodynamic conditions. Subsequent erythro/threo-selective reduction of the enamides was realized by hydrogenation with Rh/Al2O3 or hydride reduction with NaBH3CN. The developed conditions enabled the stereodivergent synthesis of all four possible diastereomers of the 2-oxo-bispyrrolidines. Moreover, the established method was successfully applied to the synthesis of the trimeric derivatives. The antiproliferative activity of the synthetic lactams against human cancer cells revealed the role of the number of rings and their stereochemistry in the cytotoxicity of the compounds. Detailed investigations of the antiproliferative activities and RNA binding assays of compounds are underway.Author contributions
Conceptualization: Y.S., N.C., T.O., and T.S.; data curation: Y.S., K.T., M.F., and T.M.; formal analysis: M.F., T.M.; funding acquisition: N.C., T.S.; investigation: Y.S., K.T., M.F., Su.S., K.S., and T.M.; methodology: Y.S., M.F., T.M.; project administration: T.O., T.S.; resource: Si.S., N.C., T.S.; software: N/A; supervision: Si.S., N.C., T.O., T.S.; validation: N/A; visualization: Y.S., T.O., T.S.; writing – original draft: Y.S., T.O.; writing – review and editing: Si.S., N.C., T.O., T.S.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by a Grant-in-Aid for Scientific Research (B) from MEXT (22H02084), the Tobe Maki Foundation, the JGC-S Scholarship Foundation, the Kato Memorial Bioscience Foundation, the Sasakawa Scientific Research Grant from The Japan Science Society (2022-0613), and the Amano Institute of Technology Foundation. The financial support of the Yoshida Scholarship Foundation to Y. Soda is gratefully acknowledged.References
- (a) A. E. Pegg, IUBMB Life, 2009, 61, 880–894 CrossRef CAS PubMed; (b) A. E. Pegg, J. Biol. Chem., 2016, 291, 14904–14912 CrossRef CAS PubMed; (c) E. W. Gerner and F. L. Meyskens, Nat. Rev. Cancer, 2004, 4, 781–792 CrossRef CAS PubMed; (d) B. Li, J. Liang, H. R. Baniasadi, M. A. Phillips and A. J. Michael, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2214165120 CrossRef CAS PubMed; (e) F. Madeo, T. Eisenberg, F. Pietrocola and G. Kroemer, Science, 2018, 359, eaan2788 CrossRef PubMed.
- (a) M. L. Bolognesi, A. Minarini, R. Budriesi, S. Cacciaguerra, A. Chiarini, S. Spampinato, V. Tumiatti and C. Melchiorre, J. Med. Chem., 1988, 41, 4150–4160 CrossRef PubMed; (b) C. Melchiorre, M. L. Bolognesi, A. Minarini, M. Rosini and V. Tumiatti, J. Med. Chem., 2010, 53, 5906–5914 CrossRef CAS PubMed.
- B. Wang, B. Pachaiyappan, J. D. Gruber, M. G. Schmidt, Y.-M. Zhang and P. M. Woster, J. Med. Chem., 2016, 59, 3140–3151 CrossRef CAS PubMed.
- K. El Bissati, H. Redel, L.-M. Ting, J. D. Lykins, M. J. McPhillie, R. Upadhya, P. M. Woster, N. Yarlett, K. Kim and L. M. Weiss, Front. Cell. Infect. Microbiol., 2019, 9, 9 CrossRef CAS PubMed.
- K. Igarashi and K. Kashiwagi, Biochem. Biophys. Res. Commun., 2000, 271, 559–564 CrossRef CAS PubMed.
- (a) K. G. Rajeev, G. J. Sanjayan and K. N. Ganesh, J. Org. Chem., 1997, 62, 5169–5173 CrossRef CAS; (b) D. Nagamani and K. N. Ganesh, Org. Lett., 2001, 3, 103–106 CrossRef CAS PubMed.
- H.-D. Arndt, R. Welz, S. Müller, B. Ziemer and U. Koert, Chem. – Eur. J., 2004, 10, 3945–3962 CrossRef CAS PubMed.
- (a) N. Umezawa, K. Tsuji, S. Sato, M. Kikuchi, H. Watanabe, Y. Horai, M. Yamaguchi, Y. Hisamatsu, T. Umehara and T. Higuchi, RSC Adv., 2018, 8, 36895–36902 RSC; (b) Y. Yoshikawa, N. Umezawa, Y. Imamura, T. Kanbe, N. Kato, K. Yoshikawa, T. Imanaka and T. Higuchi, Angew. Chem., Int. Ed., 2013, 52, 3712–3716 CrossRef CAS PubMed; (c) N. Umezawa, Y. Horai, Y. Imamura, M. Kawakubo, M. Nakahira, N. Kato, A. Muramatsu, Y. Yoshikawa, K. Yoshikawa and T. Higuchi, ChemBioChem, 2015, 16, 1811–1819 CrossRef CAS PubMed.
- (a) F. Zanardi, L. Battistini, G. Rassu, L. Pinna, M. Mor, N. Culeddu and G. Casiraghi, J. Org. Chem., 1998, 63, 1368 CrossRef CAS; (b) F. Zanardi, L. Battistini, G. Rassu, L. Auzzas, L. Pinna, L. Marzocchi, D. Acquotti and G. Casiraghi, J. Org. Chem., 2000, 65, 2048 CrossRef CAS PubMed; (c) C. Curti, B. Ranieri, L. Battistini, G. Rassu, V. Zambrano, G. Pelosi, G. Casiraghi and F. Zanardia, Adv. Synth. Catal., 2010, 352, 2011 CrossRef CAS.
- For selected reviews on the vinylogous Mannich reaction, see: (a) G. Rassu, F. Zanardi, L. Battistini and G. Casiraghi, Chem. Soc. Rev., 2000, 29, 109–118 RSC; (b) S. K. Bur and S. F. Martin, Tetrahedron, 2001, 57, 3221–3242 CrossRef CAS; (c) G. Casiraghi, L. Battistini, C. Curti, G. Rassu and F. Zanardi, Chem. Rev., 2011, 111, 3076–3154 CrossRef CAS PubMed.
- For selected examples of 2-silyloxyfuran or 2-silyloxypyrrole to construct five-membered iminium ions, see: (a) S. F. Martin and J. W. Corbett, Synthesis, 1992, 55–57 CrossRef CAS; (b) ref. 9a; ; (c) S. F. Martin, K. J. Barr, D. W. Smith and S. K. Bur, J. Am. Chem. Soc., 1999, 121, 6990–6997 CrossRef CAS; (d) ref. 9b; ; (e) M. Yoritate, Y. Takahashi, H. Tajima, C. Ogihara, T. Yokoyama, Y. Soda, T. Oishi, T. Sato and N. Chida, J. Am. Chem. Soc., 2017, 139, 18386–18391 CrossRef CAS PubMed.
- For a selected review, see: J. W. Lehmann, D. J. Blair and M. D. Burke, Nat. Rev. Chem., 2018, 2, 0115 CrossRef PubMed.
- For a selected review on pyroglutamic acid as a unique synthon, see: S. K. Panday, J. Prasad and D. K. Dikshit, Tetrahedron: Asymmetry, 2009, 20, 1581–1632 CrossRef CAS.
- For selected examples of enantioselective transformation of amides, see: (a) H. Chen, Z.-Z. Wu, D.-Y. Shao and P.-Q. Huang, Sci. Adv., 2022, 8, eade3431 CrossRef CAS PubMed; (b) F.-F. Xu, J.-Q. Chen, D.-Y. Shao and P.-Q. Huang, Nat. Commun., 2023, 14, 6251 CrossRef CAS PubMed.
- For selected reviews on nucleophilic addition to amides, see: (a) V. Pace, W. Holzer and B. Olofsson, Adv. Synth. Catal., 2014, 356, 3697–3736 CrossRef CAS; (b) T. Sato, M. Yoritate, H. Tajima and N. Chida, Org. Biomol. Chem., 2018, 16, 3864–3875 RSC; (c) D. Kaiser, A. Bauer, M. Lemmerer and N. Maulide, Chem. Soc. Rev., 2018, 47, 7899–7925 RSC; (d) P.-Q. Huang, Acta Chim. Sin., 2018, 76, 357–365 CrossRef CAS; (e) D. Matheau-Raven, P. Gabriel, J. A. Leitch, Y. A. Almehmadi, K. Yamazaki and D. J. Dixon, ACS Catal., 2020, 10, 8880–8897 CrossRef CAS; (f) D. Y. Ong, J.-h. Chen and S. Chiba, Bull. Chem. Soc. Jpn., 2020, 93, 1339–1349 CrossRef CAS.
- For selected pioneering examples of iridium-catalyzed reductive nucleophilic addition to amides, see: (a) P.-Q. Huang, W. Ou and F. Han, Chem. Commun., 2016, 52, 11967–11970 RSC; (b) P. W. Tan, J. Seayad and D. J. Dixon, Angew. Chem., Int. Ed., 2016, 55, 13436–13440 CrossRef CAS PubMed; (c) Á. L. Fuentes de Arriba, E. Lenci, M. Sonawane, O. Formery and D. J. Dixon, Angew. Chem., Int. Ed., 2017, 56, 3655–3659 CrossRef PubMed; (d) L.-G. Xie and D. J. Dixon, Nat. Commun., 2018, 9, 2841 CrossRef PubMed; (e) W. Qu, F. Han, X.-N. Hu, H. Chen and P.-Q. Huang, Angew. Chem., Int. Ed., 2018, 57, 11354–11358 CrossRef PubMed; (f) Z. Li, F. Zhao, W. Ou, P.-Q. Huang and X. Wang, Angew. Chem., Int. Ed., 2021, 60, 26604–26609 CrossRef CAS PubMed; (g) F. Jiang, F. Zhao, Y. He, X. Luo and X. Wang, Cell Rep. Phys. Sci., 2022, 3, 100955 CrossRef CAS.
- Y. Motoyama, M. Aoki, N. Takaoka, R. Aoto and H. Nagashima, Chem. Commun., 2009, 1574–1576 RSC.
- (a) Y. Kurosaki, K. Shirokane, T. Oishi, T. Sato and N. Chida, Org. Lett., 2012, 14, 2098–2101 CrossRef CAS PubMed; (b) M. Nakajima, Y. Oda, T. Wada, R. Minamikawa, K. Shirokane, T. Sato and N. Chida, Chem. – Eur. J., 2014, 20, 17565–17571 CrossRef CAS PubMed.
- α-Protonation of the oxypyrrole moiety in compound 19 provides trans- or cis-enamide (28 and 30), and γ-protonation gave two trans- or cis-α,β-unsaturated compounds each (27α, 29α, 29β and 29β). The details are described in the ESI.†.
- These diastereomers were separated by HPLC, see the ESI for details.†.
- Their stereochemistry was determined by NOESY measurement of the converted cyclic urea (see the ESI).†.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ob00350k |
| This journal is © The Royal Society of Chemistry 2024 |