Cu(OTf)2/HFIP catalyzed regioselective cycloisomerization of indole-C3-functionalized alkynols to carbazoles†
Srinivasarao
Yaragorla
 *,
Tabassum
Khan
and
Sayonika
Chakroborty
*,
Tabassum
Khan
and
Sayonika
Chakroborty
School of Chemistry, University of Hyderabad, 500046, Telangana, India. E-mail: Srinivas.yaragorla@uohyd.ac.in; ysruoh@gmail.com
First published on 15th April 2024
Abstract
We report here a simple and atom economic cycloisomerization reaction of indole-tethered alkynols for constructing diverse carbazoles using Cu(OTf)2/HFIP as the excellent promoter system. The reaction proceeds through a one-pot, domino process of spiro cyclization and 1,2-migration followed by aromatization to deliver carbazoles.
Introduction
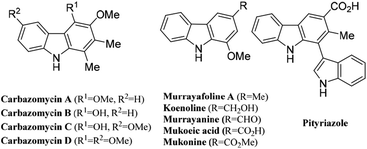
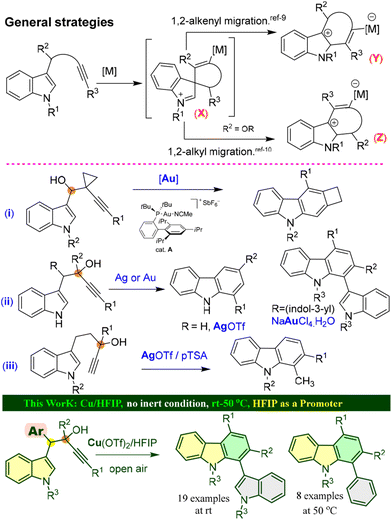
Carbazole structural frameworks have received overwhelming attention from the scientific community due to their rich natural abundance and broad range of biological activities such as anticancer, antipsychotic, antibiotic, anti-inflammatory, antioxidative, and antimicrobial activities (Fig. 1).1 Moreover, carbazole templates serve as useful platforms for the design of new organic materials because of their efficiency in hole-transporting and their light-emitting and photoconductive properties.2 Therefore, the development of efficient and economical synthetic methods for carbazoles continues to be a desirable task in organic synthesis.3 Despite several classical and elegant synthetic methods, the benzannulation of a C-2 or a C-3 alkyne tethered indole moiety is considered as one of the most accessible means for synthesizing carbazoles due to its efficient conversion and atom economy;4–7 most of these methods are catalyzed by platinum,4 gold,5 or silver complexes.6 In particular, the annulation of C3-alkynol tethered indoles becomes more interesting for the construction of carbazoles via spiro-cyclization. Based upon the nature of substitutions, electronic and steric effects, and also the carbon chain length, they undergo either an endo- or exo-cyclization to form intermediate X, as shown in Fig. 2.8 Subsequently, the spirane intermediate can undergo a 1,2-alkenyl migration9 to form carbazole Y or carbazole Zvia 1,2-alkyl migration.10 For example, the Min Shi group developed an intramolecular cyclization of 1-(indol-3-yl)-3-alkyn-1-ols (alcohol being on the a-carbon) in the presence of a cationic gold(I) complex to form a substituted carbazole through a 1,2-alkyl migration and aromatization (Fig. 2, eqn (i)).11 The Unsworth group developed an Ag(OTf) mediated carbazole synthesis from indole-C3-tethered propargyl alcohols (alcohol being on the b-carbon, Fig. 2, eqn (ii)).12 Next, a detailed study on a 1,2-migration of a gold(III)-catalyzed cycloisomerization of α-bis(indol-3-yl)methyl alkynols to afford 1-(indol-3-yl)carbazoles was reported by the R. Sanz group.13 Baire's group reported a silver triflate-mediated cycloisomerization of (indol-3-yl)pentyn-3-ols to produce tetrahydrocarbazoles, which underwent a dehydrative aromatization to form carbazoles in the presence of pTSA.14 In continuation of our research aimed at developing nitrogen heterocycles15 from propargyl alcohols, recently, we developed an iodo-cycloisomerization of indole-tethered propargylic alcohols to furnish 3-iodocarbazoles via selective 1,2-alkyl migration8b and 2-iodocarbazoles via 1,2-alkenyl migration.8c Though elegant approaches are available, there is still a need to develop a sustainable method that avoids excess solvents, inert conditions and expensive catalysts.Results and discussion
In continuation of our pursuit to study and understand aryl migrations during 1,2-shifts of indole-tethered propargyl alcohols to furnish carbazoles,8b,c we chose compound 1a as the model substrate. Therefore, we commenced our study by treating 1a with 10 mol% pTSA in 1,2-dichloromethane (DCE) at room temperature; after 24 h, we isolated the desired product 2a in 10% yield (entry 1, Table 1). Encouraged by this observation, we then screened some more Brønsted acids, such as triflic acid (entry 2), methanesulfonic acid (entry 3) and triflimide (entry 4) in DCE at rt. All these reactions led to the formation of a complex TLC along with a trace of 2a. Next, we switched to Lewis acid catalysts, like BF3·Et2O (entry 5), Ca(NTf2)2 (entry 6) and Mg(OTf)2 (entry 7) and found only Ca(NTf2)2 catalyzed the reaction for the formation of 2a in 12% yield. Then, we performed the reaction with Earth-abundant transition metal catalysts; FeCl3 did not catalyze the reaction, but Cu(OTf)2 furnished 2a in 43% yield. With this, we identified Cu(OTf)2 as the right catalyst and started screening various solvents. 45% 2a was formed in toluene, but no product was observed in DMSO, and acetonitrile gave only 25% product yield. In recent years, HFIP showed excellent results in catalysis; therefore,16 we used HFIP as the solvent (2 mL) in our reaction, and to our surprise, HFIP/Cu(OTf)2 promoted the formation of 2a in 82% yield at rt (entry 13). When we used 2 equiv. of HFIP (twice the total weight of 1a + catalyst), i.e. 0.2 mL, a quantitative yield of 2a (96%) was observed. Looking at the sharp rise in the reaction yield due to HFIP, we were curious to know if the catalyst is really required for this transformation. Therefore, we stirred 1a with only HFIP and found that no reaction was initiated, which confirms the role of HFIP. Further attempts with protic solvents (methanol, ethanol, water) and mixed solvents (MeOH/DCE, MeOH/CH3CN) were not encouraging (entries 16–20). Copper acetate was catalysed to furnish a moderate yield of 2a (entry 21), but no reaction occurred with copper iodide (entry 21). Finally, we concluded that 10 mol% Cu(OTf)2 and HFIP (0.2 mL) at rt were the best reaction conditions for the cycloisomerization (entry 14, Table 1).| Entry | Reaction conditionsa | Yieldb (%) |
|---|---|---|
| a Unless mentioned, all the reactions were carried out with 1a (100 mg) in 2 mL solvent at rt under specified conditions. b Isolated yields. c Complex TLC. d Optimum conditions. nr = no reaction. | ||
| 1 | pTSA (10), DCE, 25 °C, 24 h | 10 |
| 2 | TfOH (10), DCE, 25 °C, 4 h | —c |
| 3 | MeSO3H (10), DCE, 25 °C, 4 h | —c |
| 4 | HNTf2 (10), DCE, 25 °C, 4 h | —c |
| 5 | BF3·Et2O (10), DCE, 25 °C, 4 h | —c |
| 6 | Ca(NTf2)2/Bu4NPF6 (10), DCE, 25 °C, 8 h | 12 |
| 7 | Mg(OTf)2 (10), DCE, 25 °C, 24 h | nr |
| 8 | FeCl3 (10), DCE, 25 °C, 24 h | nr |
| 9 | Cu(OTf)2 (10), DCE, 25 °C, 4 h | 43 |
| 10 | Cu(OTf)2 (10), toluene, 25 °C, 1 h | 45 |
| 11 | Cu(OTf)2 (10), DMSO, 25 °C, 24 h | nr |
| 12 | Cu(OTf)2 (10), CH3CN, 25 °C, 2 h | 25 |
| 13 | Cu(OTf)2 (10), HFIP, 25 °C, 15 min | 82 |
| 14 | Cu(OTf) 2 (10), HFIP (0.2 mL), 25 °C, 15 min | 96 |
| 15 | HFIP, 25 °C, 24 h | nr |
| 16 | Cu(OTf)2 (10), MeOH, 25 °C, 6 h | 43 |
| 17 | Cu(OTf)2 (10), EtOH, 25 °C, 6 h | 48 |
| 18 | Cu(OTf)2 (10), H2O, 25 °C, 24 h | nr |
| 19 | Cu(OTf)2 (10), MeOH/DCE, 25 °C, 4 h | 54 |
| 20 | Cu(OTf)2 (10), MeOH/CH3CN, 25 °C, 8 h | 50 |
| 21 | Cu(OAc)2 (10), HFIP, 25 °C, 4 h | 60 |
| 22 | CuI (10), HFIP, 25 °C, 24 h | nr |
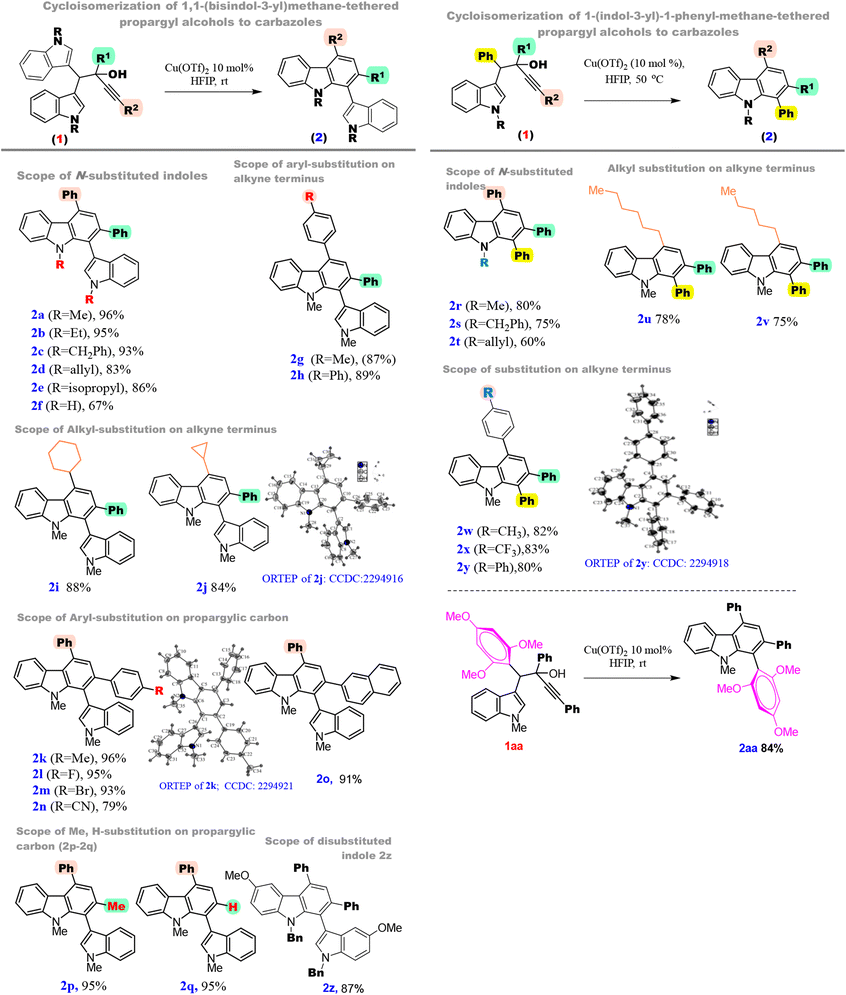
With the optimum reaction conditions in hand, we were keen to check the generality of the protocol (Table 2). Initially, we studied the scope of N-substituted indoles with methyl, ethyl, benzyl, allyl, and isopropyl groups; all reacted smoothly to yield corresponding carbazoles 2a–2e in excellent yields. However, it was observed that N–H indoles gave moderate yields when compared to N-alkyl indoles. Next, we noticed that p-tolyl and p-biphenyl substitutions on the alkyne terminus also furnished carbazoles 2g and 2h in excellent yields.
| a All the reactions were performed with 100 mg of propargyl alcohol (1) in 0.2 mL of HFIP. |
|---|
|
Moreover, substrates bearing cyclohexyl and cyclopropyl groups on the alkyne terminus reacted smoothly to furnish 2i and 2j. Single crystal-X-ray data of 2j were obtained to understand the regioselectivity of this protocol. Next, we focussed on understanding the electronic effects on the phenyl ring at the propargylic carbon. The benzene ring bearing, methyl, fluoro, and bromo substitutions at the para position showed quantitative yields of carbazoles 2k–2m. However, the yield in the case of p-cyanobenzene was slightly lower due to the strong-I effect (2n). We have also obtained the single crystal X-ray data of 2k to understand the regioselectivity of the protocol. Besides, we were quite successful in demonstrating the reactivity of substrates having 2-naphthyl, methyl and hydrogen (a secondary propargylic system) substitutions at the propargylic carbon to synthesize carbazoles 2o–2q in excellent yields. Motivated by this outcome, with bis(indolyl) systems, we intended to study the indole-phenyl tethered propargylic alcohols. Accordingly, we subjected compound 1r to the standard reaction conditions, and gratifyingly, the reaction gave the desired product 2r but in a lower yield. Yet, we circumvented this issue by performing the reaction at 50 °C and obtained 2r in 80% yield. Furthermore, we found that N-Me, N-benzyl and N-allyl derivatives of indoles also reacted smoothly at 50 °C to furnish the desired carbazoles 2r–2t in good yields. N-Hexyl and n-pentyl substituted alkynols (1u and 1v) also showed excellent reactivity to furnish 2u and 2v. Similarly, p-phenyl substituted benzene on an alkyne terminus was also shown to produce 2w–2y in good yields. Single-crystal-X-ray data of 2y were obtained. Carbazole 2z was synthesized in 87% yield from the 5-methoxyindole derivative 1z. Interestingly, 1,3,5-trimethoxy benzene substitution was also well tolerated under the standard conditions to furnish the desired carbazole 2aa in 84% yield.
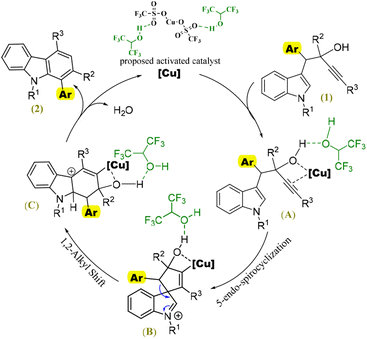
From the reaction screening (Table 1), it was clear that HFIP alone could not initiate the reaction, and Cu(OTf)2 without HFIP furnished a poor yield of 2a. Based on these observations and previous reports,16 we believe that HFIP activates both the catalyst and substrate through hydrogen bonding, as depicted in Fig. 3. The Cu/HFIP activates the substrate via the classical formation of a p-Lewis acid complex (A), which undergoes a 5-endo-dig spirocyclization to form the spirane B. A subsequent 1,2-alkyl migration of the spirane forms intermediate C, and the subsequent protodemetallation and aromatization furnishes the desired carbazole 2.
Conclusions
In conclusion, we have developed a simple, operationally friendly protocol for the annulation of indole-C3-attached propargyl alcohols to construct functionally diverse carbazoles. The reaction was performed using catalytic Cu(OTf)2 and HFIP at rt for bisindolyl (2a–2q and 2z) and indolyl-trimethoxybenzene (2aa) systems in open air. The indole-phenyl system required moderate heating (2r–2y). Furthermore, this protocol does not require inert conditions and solvent media. Broad substrate scope, high yields, and open-air reactions using a minimum amount of HFIP are the key highlights of our method.Experimental
General information
Unless otherwise noted, all reagents were used as received from commercial suppliers, and indenols were synthesized by following the reported procedures. Reactions were performed in flame-dried or oven-dried glassware with magnetic stirring and were monitored using thin-layer chromatography (TLC) with aluminum sheets of silica gel 60 F254 from Merck. TLC plates were visualized with UV light (254 nm), iodine treatment, or using p-anisaldehyde or vanillin stain. Column chromatography was carried out using silica gel 60–120 and 100–200 mesh size as the stationary phase. NMR spectra were recorded at 500 MHz and 400 MHz (1H) and 125 MHz and 100 MHz (13C{1H}), respectively, on a Bruker Avance spectrometer. NMR spectra were solved by using the Bruker Topspin software. Chemical shifts (δ) are reported in ppm, using the residual solvent peak in CDCl3 (1H: δ = 7.26 and 13C{1H}: δ = 77.16 ppm) as the internal standard, and coupling constants (J) are given in Hz. HRMS were recorded using ESI-TOF techniques. Melting points were measured with LABINDIA's melting point apparatus (MEPA).9-Methyl-1-(1-methyl-1H-indol-3-yl)-2,4-diphenyl-9H-carbazole (2a). Isolated as a pale yellow solid; yield: 96% (0.092 g); mp: 224–225 °C; 1H NMR (400 MHz, CDCl3): δ 7.71 (d, J = 7.0 Hz, 2H), 7.53 (t, J = 7.0 Hz, 2H), 7.49–7.46 (m, 2H), 7.38–7.35 (m, 2H), 7.33 (d, J = 8.0 Hz, 1H), 7.26–7.22 (m, 2H), 7.19 (s, 1H), 7.15–7.13 (m, 2H), 7.08–7.05 (m, 4H), 6.97 (t, J = 7.5 Hz, 1H), 6.70 (s, 1H), 3.72 (s, 3H), 3.25 (s, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 142.9, 141.8, 141.5, 140.9, 136.5, 136.3, 131.2, 129.7, 129.5, 129.4, 128.5, 127.6, 127.3, 125.9, 125.5, 123.6, 122.3, 121.8, 120.5, 120.4, 119.8, 118.7, 115.2, 112.0, 109.2, 108.8, 32.9, 31.4 ppm; HRMS (ESI-TOF): m/z calculated for C34H27N2 [M + H]+ 463.2174, found 463.2177.
9-Ethyl-1-(1-ethyl-1H-indol-3-yl)-2,4-diphenyl-9H-carbazole (2b). Isolated as a pale yellow solid; yield: 95% (0.091 g); mp: 181–182 °C; 1H NMR (400 MHz, CDCl3): δ 7.72 (d, J = 7.0 Hz, 2H), 7.53 (t, J = 7.5 Hz, 2H), 7.48–7.46 (m, 2H), 7.37–7.37 (m, 3H), 7.29 (d, J = 7.0 Hz, 1H), 7.23 (t, J = 3.5 Hz, 1H), 7.21–7.19 (m, 1H), 7.11–7.10 (m, 2H), 7.06–7.02 (m, 4H), 6.96 (t, J = 7.5 Hz, 1H), 6.79 (s, 1H), 4.14–4.03 (m, 2H), 3.93–3.85 (m, 1H), 3.84–3.77 (m, 1H), 1.26 (t, J = 7.0 Hz, 3H), 0.86 (t, J = 6.5 Hz, 3H) ppm; 13C{1H} (125 MHz, CDCl3) δ 143.0, 142.1, 141.8, 141.5, 139.8, 136.5, 135.1, 131.2, 129.6, 129.5, 128.5, 127.5, 127.4, 127.1, 125.8, 125.4, 123.4, 122.8, 122.5, 121.7, 120.8, 120.5, 119.6, 118.7, 115.2, 112.0, 109.4, 109.1, 40.9, 38.4, 15.8, 14.3 ppm; HRMS (ESI-TOF): m/z calculated for C36H31N2 [M + H]+ 491.2487, found 491.2487.
9-Benzyl-1-(1-benzyl-1H-indol-3-yl)-2,4-diphenyl-9H-carbazole (2c). Isolated as a white solid; yield: 93% (0.090 g); mp: 174–176 °C; 1H NMR (500 MHz, CDCl3): δ 7.76 (d, J = 7.0 Hz, 2H), 7.56 (t, J = 7.0 Hz, 2H), 7.53–7.48 (m, 2H), 7.35 (d, J = 8.0 Hz, 1H), 7.32–7.28 (m, 1H), 7.24–7.22 (m,1H), 7.21 (d, J = 7.5 Hz, 1H), 7.17–7.15 (m, 3H), 7.14–7.13 (m, 3H), 7.12–7.09 (m, 3H), 7.06–6.97 (m, 6H), 6.61–6.59 (m, 2H), 6.51–6.49 (m, 2H), 6.23 (s, 1H), 5.12–5.02 (m, 3H), 4.69–4.65 (m, 1H) ppm; 13C{1H}(100 MHz, CDCl3) δ 176.1, 164.3, 161.8, 151.4, 149.2, 130.9, 129.2, 127.9, 127.8, 127.4, 127.0, 126.6, 124.9, 121.9, 120.0, 116.3, 116.1, 112.6, 49.5, 31.2, 19.4 ppm; HRMS (ESI-TOF): m/z calculated for C46H35N2 [M + H]+ 615.2800, found 615.2790.
9-Allyl-1-(1-allyl-1H-indol-3-yl)-2,4-diphenyl-9H-carbazole (2d). Isolated as a white solid; yield: 83% (0.080 g); mp: 162–163 °C; 1H NMR (400 MHz, CDCl3): δ 7.73–7.72 (m, 2H), 7.54 (t, J = 7.0 Hz, 2H), 7.48 (t, J = 8.0 Hz, 2H), 7.35–7.32 (m, 2H), 7.27 (d, J = 8.0 Hz, 1H), 7.25–7.21 (m, 1H), 7.20–7.19 (m, 2H), 7.16–7.14 (m, 2H), 7.05–7.02 (m, 4H), 6.97 (t, J = 7.5 Hz, 1H), 6.97 (d, J = 1.0 Hz, 1H), 5.86–5.79 (m, 1H), 5.50–5.42 (m, 1H), 5.05 (d, J = 10.5 Hz, 1H), 4.85 (d, J = 10.0 Hz, 1H), 4.72–4.66 (m, 2H), 4.63–4.57 (m, 2H), 4.45–4.35 (m, 2H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 142.8, 142.2, 141.4, 140.1, 136.5, 135.5, 133.7, 133.2, 130.7, 129.6, 129.5, 128.5, 128.4, 127.6, 127.2, 125.9, 125.4, 123.6, 122.6, 122.3, 121.9, 120.6, 119.8, 119.0, 116.6, 115.5, 115.1, 111.8, 48.4, 46.3 ppm; HRMS (ESI-TOF): m/z calculated for C38H31N2 [M + H]+ 515.2487, found 515.2487.
9-Isopropyl-1-(1-isopropyl-1H-indol-3-yl)-2,4-diphenyl-9H-carbazole (2e). Isolated as a yellowish solid; yield: 86% (0.083 g); mp: 199–200 °C; 1H NMR (400 MHz, CDCl3): δ 7.70 (d, J = 7.0 Hz, 2H), 7.55 (d, J = 8.5 Hz, 1H), 7.52 (t, J = 8.0 Hz, 2H), 7.49–7.45 (m, 1H), 7.43 (d, J = 7.5 Hz, 1H), 7.37–7.33 (m, 2H), 7.30–7.27 (m, 1H), 7.21–7.18 (m, 1H), 7.16 (s, 1H), 7.08–7.06 (m, 2H), 7.03–6.99 (m, 4H), 6.94–6.91 (m, 1H), 6.84 (s, 1H), 4.96–4.90 (m, 1H), 4.64–4.59 (m, 1H), 1.46 (d, J = 6.5 Hz, 3H), 1.24–1.20 (m, 6H), 1.14 (d, J = 7.0 Hz, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 143.2, 142.3, 141.6, 140.4, 140.3, 136.2, 135.3, 130.0, 129.5, 128.5, 127.5, 127.0, 125.7, 124.6, 123.8, 123.3, 122.6, 121.6, 120.9, 120.6, 119.4, 118.3, 115.6, 112.7, 112.6, 109.3, 46.9, 46.5, 22.9, 22.8, 21.0, 20.0 ppm; HRMS (ESI-TOF): m/z calculated for C38H31N2 [M + H]+ 519.2800, found 519.2805.
1-(1H-Indol-3-yl)-2,4-diphenyl-9H-carbazole (2f). Isolated as a brown viscous compound; yield: 67% (0.064 g); 1H NMR (500 MHz, CDCl3): δ 8.23 (s, 1H), 8.13 (s, 1H), 7.77 (d, J = 7.0 Hz, 2H), 7.60 (d, J = 8.0 Hz, 1H), 7.55 (t, J = 7.0 Hz, 2H), 7.50–7.46 (m, 3H), 7.32–7.27 (m, 6H), 7.17–7.12 (m, 4H), 7.00–6.97 (m, 1H), 6.88 (d, J = 2.0 Hz, 1H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 142.2, 141.2, 140.2, 140.0, 139.3, 136.5, 136.2, 130.0, 129.4, 128.5, 127.7, 127.6, 127.1, 126.3, 125.6, 124.8, 123.8, 123.2, 122.5, 120.4, 120.2, 119.6, 119.1, 114.9, 112.2, 111.6, 110.6 ppm; HRMS (ESI-TOF): m/z calculated for C32H23N2 [M + H]+ 435.1861, found 435.1867.
9-Methyl-1-(1-methyl-1H-indol-3-yl)-2-phenyl-4-(p-tolyl)-9H-carbazole (2g). Isolated as a white solid; yield: 87% (0.084 g); mp: 223–224 °C; 1H NMR (500 MHz, CDCl3): δ 7.71 (d, J = 7.0 Hz, 2H), 7.53 (t, J = 7.5 Hz, 2H), 7.49–7.46 (m, 2H), 7.37–7.34 (m, 3H), 7.27 (s, 1H), 7.24 (s, 1H), 7.17 (s, 1H), 7.09–7.06 (m, 1H), 7.04 (d, J = 7.5 Hz, 2H), 6.98–6.95 (m, 1H), 6.87 (d, J = 8.0 Hz, 2H), 6.73 (s, 1H), 3.75 (s, 3H), 3.23 (s, 3H), 2.22 (s, 3H) ppm; 13C{1H}(125 MHz, CDCl3) δ 176.1, 151.4, 150.4, 132.2, 131.2, 130.1, 129.2 (2), 129.1, 129.0, 125.8 (2), 125.4, 123.6, 120.3, 118.5, 116.7, 113.7, 49.4, 31.2, 19.4 ppm; HRMS (ESI-TOF): m/z calculated for C35H29N2 [M + H]+ 477.2331, found 477.2330.
4-([1,1′-Biphenyl]-4-yl)-9-methyl-1-(1-methyl-1H-indol-3-yl)-2-phenyl-9H-carbazole (2h). Isolated as a white solid; yield: 89% (0.086 g); mp: 225–226 °C; 1H NMR (500 MHz, CDCl3): δ 7.82–7.77 (m, 4H), 7.75 (d, J = 7.5 Hz, 2H), 7.63 (d, J = 8.0 Hz, 1H), 7.49 (t, J = 7.5 Hz, 2H), 7.40–7.36 (m, 3H), 7.33 (d, J = 8.5 Hz, 1H), 7.27–7.23 (m, 3H), 7.16–7.15 (m, 2H), 7.08–7.06 (m, 4H), 7.01–6.98 (m, 1H), 6.71 (s, 1H), 3.72 (s, 3H), 3.27 (s, 3H) ppm; 13C{1H}(100 MHz, CDCl3) δ 142.9, 141.9, 141.0, 140.5, 140.3, 136.3, 136.0, 131.2, 129.9, 129.7, 129.4, 128.9, 127.4, 127.3, 127.2 (2), 126.0, 125.5, 123.6, 122.4, 122.3, 121.8, 120.5, 120.3, 119.8, 118.8, 115.3, 112.0, 109.2, 108.8, 32.9, 31.5 ppm; HRMS (ESI-TOF): m/z calculated for C40H31N2 [M + H]+ 539.2487, found 539.2481.
4-Cyclohexyl-9-methyl-1-(1-methyl-1H-indol-3-yl)-2-phenyl-9H-carbazole (2i). Isolated as a white solid; yield: 88% (0.085 g); mp: 220–221 °C; 1H NMR (500 MHz, CDCl3): δ 8.19 (d, J = 7.5 Hz, 1H), 7.45–7.42 (m, 1), 7.31 (d, J = 3.5 Hz, 1H), 7.29–7.27 (m, 3H), 7.23–7.20 (m, 1H), 7.19 (s, 1H), 7.13–7.11 (m, 2H), 7.08–7.06 (m, 3H), 7.04–7.01 (m, 1H), 6.64 (s, 1H), 3.70 (s, 3H), 3.66–3.56 (m, 1H), 3.22 (s, 3H), 2.31–2.28 (m, 2H), 1.99 (s, 2H), 1.89 (d, J = 7.5 Hz, 1H), 1.72–1.63 (m, 4H), 1.43–1.36 (m, 1H) ppm; 13C{1H}(100 MHz, CDCl3) δ 143.6, 142.8, 142.3, 142.1, 140.8, 136.3, 131.2, 129.7, 129.4, 127.2, 125.8, 124.9, 122.7, 122.4, 121.6, 120.6, 120.4, 119.6, 119.1, 118.5, 113.6, 112.3, 109.1, 108.8, 41.0, 33.3, 33.2, 32.9, 31.4, 27.5, 27.4, 26.7 ppm; HRMS (ESI-TOF): m/z calculated for C34H33N2 [M + H]+ 469.2644, found 469.2648.
4-Cyclopropyl-9-methyl-1-(1-methyl-1H-indol-3-yl)-2-phenyl-9H-carbazole (2j). Isolated as a yellowish solid; yield: 84% (0.081 g); mp: 246–247 °C; 1H NMR (500 MHz, CDCl3): 8.56 (d, J = 7.5 Hz, 1H), 7.48–7.44 (m, 1H), 7.32–7.29 (m, 3H), 7.28–7.26 (m, 1H), 7.23–7.20 (m, 1H), 7.11–7.09 (m, 2H), 7.08–7.05 (m, 4H), 7.04–7.01 (m, 1H), 6.64 (s, 1H), 3.70 (s, 3H), 3.23 (s, 3H), 2.67–2.62 (m, 1H), 1.20–1.17 (m, 2H), 1.00–0.96 (m, 2H) ppm; 13C{1H}(125 MHz, CDCl3) δ (ppm) 143.3, 142.7, 141.8, 140.6, 136.6, 136.3, 131.2, 129.6, 129.4, 127.2, 125.8, 125.1, 123.1, 123.0, 122.5, 121.7, 120.5, 120.3, 119.7, 119.0, 114.1, 112.1, 109.1, 108.6, 32.9, 31.3, 15.0, 6.9 (2) ppm; HRMS (ESI-TOF): m/z calculated for C31H27N2 [M + H]+ 427.2174, found 427.2175.
9-Methyl-1-(1-methyl-1H-indol-3-yl)-4-phenyl-2-(p-tolyl)-9H-carbazole (2k). Isolated as a white solid; yield: 96% (0.093 g); mp: 208–209 °C; 1H NMR (400 MHz, CDCl3): δ 7.71 (d, J = 7.5 Hz, 2H), 7.52 (t, J = 7.5 Hz, 2H), 7.47 (d, J = 7.5 Hz, 2H), 7.37–7.32 (m, 3H), 7.25–7.22 (m, 2H), 7.17 (s, 1H), 7.08–7.03 (m, 3H), 6.96 (t, J = 7.5 Hz, 1H), 6.86 (d, J = 7.5 Hz, 2H), 6.71 (s, 1H), 3.72 (s, 3H), 3.22 (s, 3H), 2.21 (s, 3H) ppm; 13C{1H}(100 MHz, CDCl3) δ 142.9, 141.8, 141.5, 141.0, 139.9, 136.4, 136.3, 135.4, 131.3, 129.5, 129.3, 128.5, 128.0, 127.5, 125.4, 123.7, 122.3, 121.7, 120.5, 120.2, 119.8, 118.7, 115.1, 112.1, 109.2, 108.7, 32.9, 31.4, 21.1 ppm; HRMS (ESI-TOF): m/z calculated for C35H29N2 [M + H]+ 477.2331, found 477.2329.
2-(4-Fluorophenyl)-9-methyl-1-(1-methyl-1H-indol-3-yl)-4-phenyl-9H-carbazole (2l). Isolated as a pale yellow solid; yield: 95% (0.092 g); mp: 234–235 °C; 1H NMR (400 MHz, CDCl3): δ 7.72–7.70 (m, 2H), 7.55–7.52 (m, 2H), 7.50–7.45 (m, 2H), 7.38–7.33 (m, 3H), 7.26–7.23 (m, 3H), 7.14 (s, 1H), 7.11–7.05 (m, 3H), 6.99–6.95 (m, 1H), 6.76–6.72 (m, 2H), 6.71 (s, 1H), 3.75 (s, 3H), 3.25 (s, 3H) ppm; 13C{1H}(100 MHz, CDCl3) δ 162.7, 160.2, 142.9, 141.3, 140.9, 140.7, 138.8, 136.5, 136.3, 131.1, 131.0, 129.4, 129.3, 128.5, 127.6, 125.5, 123.4, 122.3, 122.2, 121.9, 120.5, 120.4, 119.9, 118.8, 115.2, 114.2, 114.0, 111.9, 109.3, 108.8, 33.0, 31.4 ppm; HRMS (ESI-TOF): m/z calculated for C34H26FN2 [M + H]+ 481.2080, found 481.2076.
2-(4-Bromophenyl)-9-methyl-1-(1-methyl-1H-indol-3-yl)-4-phenyl-9H-carbazole (2m). Isolated as a white solid; yield: 93% (0.090 g); mp: 200–201 °C; 1H NMR (500 MHz, CDCl3): δ 7.70–7.68 (m, 2H), 7.55–7.52 (m, 2H), 7.50–7.48 (m, 1H), 7.46 (d, J = 8.0 Hz, 1H), 3.84 (s, 3H), 7.38–7.32 (m, 3H), 7.25 (t, J = 9.0 Hz, 2H), 7.18–7.16 (m, 2H), 7.12 (s, 1H), 7.09–7.05 (m, 1H), 7.03–7.00 (m, 2H), 6.98–6.95 (m, 1H), 6.70 (s, 1H), 3.75 (s, 3H), 3.23 (s, 3H) ppm; 13C{1H}(125 MHz, CDCl3) δ 142.9, 141.9, 141.3, 140.9, 140.5, 136.7, 136.3, 131.3, 131.1, 130.4, 129.4, 129.3, 128.5, 127.7, 125.6, 123.2, 122.4, 122.2, 122.0, 120.6, 120.4, 120.2, 120.0, 118.8, 115.1, 111.8, 109.4, 108.8, 33.0, 31.4 ppm; HRMS (ESI-TOF): m/z calculated for C34H26BrN2 [M + H]+ 541.1279, found 541.1274.
4-(9-Methyl-1-(1-methyl-1H-indol-3-yl)-4-phenyl-9H-carbazol-2-yl)benzonitrile (2n). Isolated as a yellowish solid; yield: 79% (0.076 g); mp: 300–301 °C; 1H NMR (CDCl3, 500 MHz): δ 7.70–7.68 (m, 2H), 7.56–7.53 (m, 2H), 7.51–7.48 (m, 1H), 7.46 (d, J = 8.0 Hz, 1H), 7.41–7.39 (m, 1H), 7.37 (d, J = 8.5 Hz, 1H), 7.34–7.32 (m, 3H), 7.29–7.26 (m, 2H), 7.25–7.24 (m, 2H), 7.12 (s, 1H), 7.08 (t, J = 7.5 Hz, 1H), 6.99 (t, J = 7.5 Hz, 1H), 6.70 (s, 1H), 3.76 (s, 3H), 3.27 (s, 3H) ppm; 13C{1H} NMR (CDCl3, 125 MHz): 148.0, 142.9, 141.0, 140.7, 139.7, 136.8, 136.2, 131.1, 130.8, 130.3, 129.3, 128.6, 127.8, 125.9, 122.7, 122.4, 122.2, 122.0, 121.1, 120.2, 120.1, 119.3, 119.0, 115.0, 111.3, 109.5, 108.8, 33.0, 31.4 ppm; HRMS (ESI-TOF): m/z calculated for C35H26N3 [M + H]+ 488.2127, found 488.2125.
9-Methyl-1-(1-methyl-1H-indol-3-yl)-2-(naphthalen-2-yl)-4-phenyl-9H-carbazole (2o). Isolated as a yellow solid; yield: 91% (0.088 g); mp: 291–292 °C; 1H NMR (CDCl3, 400 MHz): δ 7.77 (s, 1H), 7.75 (d, J = 6.5 Hz, 2H), 7.70–7.64 (m, 2H), 7.55 (t, J = 7.0 Hz, 2H), 7.51–7.47 (m, 2H), 7.46–7.43 (m, 2H), 7.41–7.39 (m, 1H), 7.40–7.35 (m, 3H), 7.33 (d, J = 8.0 Hz, 1H), 7.29 (s, 1H), 7.28–7.27 (m, 1H), 7.14–7.09 (m, 2H), 7.00–6.97 (m, 1H), 6.68 (s, 1H), 3.65 (s, 3H), 3.28 (s, 3H) ppm; 13C{1H} NMR (CDCl3, 100 MHz): 143.0, 141.6, 141.5, 141.0, 140.8, 136.6, 136.3, 133.2, 131.9, 131.3, 131.0, 129.6, 129.5, 128.5, 128.4, 128.2, 128.0, 127.6, 127.5, 126.3, 125.6, 125.5, 125.4, 123.9, 122.3, 121.9, 120.5, 119.9, 118.8, 115.4, 112.0, 109.3, 108.8, 32.9, 31.4 ppm; HRMS (ESI-TOF): m/z calculated for C38H29N2 [M + H]+ 513.2331, found 513.2330.
2,9-Dimethyl-1-(1-methyl-1H-indol-3-yl)-4-phenyl-9H-carbazole (2p). Isolated as a white solid; yield: 95% (0.092 g); mp: 88–89 °C; 1H NMR (500 MHz, CDCl3): δ 7.67 (d, J = 7.0 Hz, 2H), 7.54 (t, J = 8.0 Hz, 2H), 7.49–7.46 (m, 1H), 7.44 (d, J = 9.0 Hz, 1H), 7.39 (d, J = 8.0 Hz, 1H), 7.33–7.28 (m, 3H), 7.21 (d, J = 8.0 Hz, 1H), 7.11–7.10 (m, 1H), 7.08 (s, 1H), 7.06 (m, 1H), 6.94–6.91 (m, 1H), 3.94 (s, 3H), 3.21 (s, 3H), 2.26 (s, 3H) ppm; 13C{1H}(100 MHz, CDCl3) δ 142.3, 141.7, 141.2, 137.0, 136.8, 136.4, 129.7, 129.4, 128.4, 127.4, 125.0, 123.1, 122.5, 122.1, 122.0, 120.5, 119.7, 119.2, 118.5, 115.8, 112.5, 109.4, 108.5, 33.1, 31.4, 20.8 ppm; HRMS (ESI-TOF): m/z calculated for C29H25N2 [M + H]+ 401.2018, found 401.2013.
9-Methyl-1-(1-methyl-1H-indol-3-yl)-4-phenyl-9H-carbazole (2q). Isolated as a white solid; yield: 95% (0.092 g); mp: 97–98 °C; 1H NMR (500 MHz, CDCl3): δ 7.67 (d, J = 2 Hz, 1H), 7.54 (d, J = 6.5 Hz, 2H), 7.50–7.48 (m, 1H), 7.47 (d, J = 8.0 Hz, 1H), 7.43–7.41 (m, 2H), 7.39 (s, 1H), 7.38–7.36 (m, 1H), 7.31–7.28 (m, 2H), 7.22 (s, 1H), 7.13–7.09 (m, 2H), 6.98–6.95 (m, 1H), 3.93 (s, 3H), 3.45 (s, 3H) ppm; 13C{1H}(125 MHz, CDCl3) δ 142.5, 141.6, 140.2, 136.7, 136.6, 129.9, 129.6, 129.4, 128.5, 127.9, 127.5, 125.5, 122.7, 122.4, 122.1, 121.4, 120.7, 120.5, 119.9, 118.7, 117.2, 114.9, 109.4, 108.8, 33.1, 31.8 ppm; HRMS (ESI-TOF): m/z calculated for C28H23N2 [M + H]+ calculated 387.1861, found 387.1863.
9-Methyl-1,2,4-triphenyl-9H-carbazole (2r). Isolated as a yellow solid; yield: 80% (0.077 g); mp: 179–180 °C; 1H NMR (400 MHz, CDCl3): δ 7.54 (d, J = 8.0 Hz, 1H), 7.50–7.48 (m, 4H), 7.41 (t, J = 7.0 Hz, 2H), 7.36–7.33 (m, 2H), 7.32–7.30 (m, 5H), 7.28–7.26 (m, 2H), 7.23 (d, J = 8.5 Hz, 1H), 7.14 (t, J = 8.5 Hz, 1H), 7.11 (s, 1H), 7.04 (t, J = 7.5 Hz, 1H), 3.10 (s, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 143.0, 140.5, 138.3, 137.2, 136.2, 135.9, 135.7, 133.8, 132.6, 131.8, 131.2, 130.5, 130.3, 129.9, 129.7, 128.5, 128.4, 128.1, 128.0, 127.8, 127.6, 127.2, 126.6, 125.5, 122.2, 121.8, 120.2, 120.1, 110.5, 34.3 ppm; HRMS (ESI-TOF): m/z calculated for C31H24N [M + H]+ 410.1909, found 410.1903.
9-Benzyl-1,2,4-triphenyl-9H-carbazole (2s). Isolated as a yellow solid; yield: 75% (0.072 g); mp: 179–180 °C; 1H NMR (400 MHz, CDCl3): δ 7.74–7.72 (m, 2H), 7.56–7.53 (m, 2H), 7.51–7.48 (m, 2H), 7.31–7.27 (m, 1H), 7.17 (s, 1H), 7.15 (d, J = 8.0 Hz, 1H), 7.12–7.09 (m, 6H), 7.08–7.06 (m, 3H), 7.04–7.02 (m, 2H), 7.00–6.96 (m, 3H), 6.61–6.59 (m, 2H), 5.01 (s, 2H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 142.6, 142.1, 141.2, 140.8, 139.7, 138.1, 137.5, 136.8, 136.1, 135.9, 135.7, 135.2, 134.4, 134.2, 133.2, 131.9, 131.2, 130.5, 129.9, 129.7, 129.5, 129.1, 128.5, 128.4, 128.1, 128.0, 127.8, 127.3, 127.2, 127.0, 126.9, 126.7, 126.6, 126.3, 126.1, 125.4, 122.7, 122.5, 122.0, 121.8, 121.6, 120.5, 120.4, 120.1, 119.8, 111.8, 110.0, 49.9 ppm; HRMS (ESI-TOF): m/z calculated for C37H28N [M + H]+ 486.2222, found 486.2223.
9-Allyl-1,2,4-triphenyl-9H-carbazole (2t). Isolated as a yellow solid; yield: 60% (0.057 g); mp: 179–180 °C; 1H NMR (400 MHz, CDCl3): δ 7.71–7.69 (m, 2H), 7.53 (t, J = 7.5 Hz, 2H), 7.49–7.46 (m, 1H), 7.44 (d, J = 8.0 Hz, 1H),7.36–7.33 (m, 1H), 7.30–7.28 (m, 2H), 7.27 (s, 1H), 7.25–7.22 (m, 3H), 7.16–7.10 (m, 6H), 6.97 (t, J = 7.0 Hz, 1H), 5.57–5.49 (m, 1H), 4.96 (dd, J1 = 1.0 Hz, J2 = 10.5 Hz, 1H), 4.65 (dd, J1 = 1.0 Hz, J2 = 16.0 Hz, 1H), 4.32–4.31 (m, 2H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 142.3, 141.2, 139.9, 138.5, 138.1, 136.6, 133.3, 131.7, 130.3, 129.4, 128.5, 127.6, 127.3, 126.0, 125.6, 123.6, 123.2, 122.4, 119.1, 115.8, 109.7, 46.7 ppm; HRMS (ESI-TOF): m/z calculated for C33H26N [M + H]+ 436.2065, found 436.2063.
4-Hexyl-9-methyl-1,2-diphenyl-9H-carbazole (2u). Isolated as a yellow solid; yield: 78% (0.075 g); mp: 179–180 °C; 1H NMR (400 MHz, CDCl3): δ 8.10 (d, J = 7.5 Hz, 1H), 7.40–7.37 (m, 1H), 7.26 (d, J = 8.0 Hz, 1H), 7.21–7.19 (m, 1H), 7.18 (s, 1H), 7.17 (s, 4H), 7.08–7.04 (m, 5H), 7.00 (s, 1H), 3.21 (t, J = 7.5 Hz, 2H), 3.13 (s, 3H), 1.87–1.81 (m, 2H), 1.54–1.47 (m, 2H), 1.34–1.28 (m, 4H), 0.84 (t, J = 7.0 Hz, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 142.7, 142.4, 139.7, 139.3, 138.7, 132.1, 130.4, 127.5, 127.4, 126.9, 125.9, 125.2, 122.6, 122.4, 121.9, 120.9, 108.8, 34.6, 32.8, 31.9, 29.8, 29.7, 22.8, 14.2 ppm; HRMS (ESI-TOF): m/z calculated for C31H32N [M + H]+ 418.2535, found 418.2537.
9-Methyl-4-pentyl-1,2-diphenyl-9H-carbazole (2v). Isolated as a yellow solid; yield: 75% (0.072 g); mp: 179–180 °C; 1H NMR (400 MHz, CDCl3): δ 8.17 (d, J = 7.5 Hz, 1H), 7.48–7.44 (m, 1H), 7.33 (d, J = 8.0 Hz, 1H), 7.29–7.27 (m, 1H), 7.26–7.25 (m, 1H), 7.24–7.23 (m, 4H), 7.17–7.10 (m, 5H), 7.07 (s, 1H), 3.28 (t, J = 8.0 Hz, 2H), 3.20 (s, 3H), 1.95–1.89 (m, 2H), 1.61–1.53 (m, 2H), 1.49–1.40 (m, 2H), 0.94 (t, J = 7.5 Hz, 1H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 142.7, 142.4, 139.7, 139.3, 138.6, 137.1, 132.1, 130.3, 127.5, 127.4, 126.9, 125.9, 125.2, 122.6, 122.5, 122.4, 121.9, 120.9, 119.3, 108.8, 34.5, 32.8, 32.3, 29.5, 22.8, 14.3 ppm; HRMS (ESI-TOF): m/z calculated for C30H30N [M + H]+ 404.2378, found 404.2377.
9-Methyl-1,2-diphenyl-4-(p-tolyl)-9H-carbazole (2w). Isolated as a yellow solid; yield: 82% (0.079 g); mp: 179–180 °C; 1H NMR (400 MHz, CDCl3): δ 7.58 (d, J = 8.0 Hz, 2H), 7.53 (d, J = 8.0 Hz, 1H), 7.40–7.36 (m, 2H), 7.34–7.33 (m, 2H), 7.32–7.29 (m, 3H), 7.28–7.26 (m, 2H), 7.24 (s, 1H), 7.16–7.15 (m, 2H), 7.14 (s, 2H), 7.13–7.10 (m, 1H), 7.00–6.97 (m, 1H), 3.23 (s, 3H), 2.49 (s, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 142.9, 142.1, 139.6, 139.3, 138.5, 138.2, 137.3, 136.6, 132.0, 130.4, 129.3, 129.2, 127.6, 127.4, 127.1, 126.0, 125.6, 123.5, 123.0, 122.4, 122.2, 120.5, 118.8, 108.7, 32.8, 21.5 ppm; HRMS (ESI-TOF): m/z calculated for C32H26N [M + H]+ 424.2065, found 424.2066.
9-Methyl-1,2-diphenyl-4-(4-(trifluoromethyl)phenyl)-9H-carbazole (2x). Isolated as a yellow solid; yield: 83% (0.080 g); mp: 179–180 °C; 1H NMR (400 MHz, CDCl3): δ 7.76–7.71 (m, 4H), 7.36–7.32 (m, 2H), 7.26–7.20 (m, 6H), 7.18 (s, 1H), 7.9–7.08 (m, 4H), 7.06 (d, J = 3.5 Hz, 1H), 7.05 (s, 1H), 6.95–6.92 (m, 1H), 3.18 (s, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 145.0, 143.0, 141.7, 139.8, 139.4, 138.1, 134.9, 131.9, 130.3, 129.9, 127.7, 127.5, 127.3, 126.2, 126.0, 125.5, 123.9, 123.3, 122.1, 121.7, 120.2, 119.1, 109.0, 32.8 ppm; HRMS (ESI-TOF): m/z calculated for C32H23F3N [M + H]+ 478.1783, found 478.1783.
4-([1,1′-Biphenyl]-4-yl)-9-methyl-1,2-diphenyl-9H-carbazole (2y). Isolated as a yellow solid; yield: 80% (0.077 g); mp: 179–180 °C; 1H NMR (400 MHz, CDCl3): δ 7.78 (s, 3H), 7.76–7.74 (m, 2H), 7.60 (d, J = 8.0 Hz, 1H), 7.50 (t, J = 7.5 Hz, 2H), 7.41–7.37 (m, 3H), 7.33–7.31 (m, 3H), 7.29–7.27 (m, 3H), 7.20 (s, 1H), 7.17–7.16 (m, 2H), 7.15–7.11 (m, 3H), 7.01–6.98 (m, 1H), 3.25 (s, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 142.9, 142.0, 141.0, 140.4, 140.2, 139.7, 139.4, 138.4, 136.1, 132.0, 130.6, 130.4, 129.9, 129.0, 127.6, 127.4, 127.2, 126.1, 125.7, 126.1, 125.7, 123.5, 123.2, 122.4, 122.1, 120.5, 118.9, 108.4, 32.9 ppm; HRMS (ESI-TOF): m/z calculated for C37H28N [M + H]+ 486.2222, found 486.2221.
9-Benzyl-1-(1-benzyl-5-methoxy-1H-indol-3-yl)-6-methoxy-2,4-diphenyl-9H-carbazole (2z). Isolated as a yellowish solid; yield: 87% (0.084 g); mp: 185–186 °C; 1H NMR (500 MHz, CDCl3): δ 7.77 (d, J = 7.5 Hz, 2H), 7.57 (t, J = 7.0 Hz, 2H), 7.52–7.48 (m, 1H), 7.21 (s, 1H), 7.18–7.12 (m, 6H), 7.11–7.09 (m, 4H), 7.08–7.03 (m, 4H), 7.01–6.99 (m, 1H), 6.98–6.96 (m, 2H), 6.78–6.72 (m, 2H), 6.61 (d, J = 7.0 Hz, 2H), 6.51–6.5 (m, 2H), 6.24 (s, 1H), 5.07 (s, 3H), 4.73–4.60 (m, 1H), 3.69 (s, 3H), 3.61 (s, 3H) ppm; 13C{1H}(125 MHz, CDCl3) δ 154.6, 153.2, 142.8, 142.5, 141.2, 140.8, 139.0, 137.5, 136.6, 131.5, 130.6, 129.7, 129.6, 128.6, 128.4, 128.1, 127.7, 127.3, 127.2, 126.4, 126.2, 125.9, 125.2, 123.1, 122.7, 120.4, 115.3, 114.9, 112.4, 111.0, 110.6, 109.9, 105.3, 101.4, 55.8, 55.6, 49.7, 47.4 ppm; HRMS (ESI-TOF): m/z calculated for C28H23N2O2 [M + H]+ calculated 675.8520, found 675.8524.
9-Methyl-2,4-diphenyl-1-(2,4,6-trimethoxyphenyl)-9H-carbazole (2aa). Isolated as a yellow solid; yield: 84% (0.081 g); mp: 186–187 °C; 1H NMR (400 MHz, CDCl3): δ 7.72 (d, J = 7.0 Hz, 2H), 7.52–7.50 (m, 2H), 7.49–7.43 (m, 2H), 7.36–7.33 (m, 1H), 7.28 (d, J = 8.5 Hz, 1H), 7.19–7.17 (m, 2H), 7.15–7.08(m, 4H), 6.94–6.91 (m, 1H), 6.05 (s, 2H), 3.81 (s, 3H), 3.54 (s, 3H), 3.42 (s, 3H) ppm; 13C{1H} NMR (125 MHz, CDCl3): δ 161.5, 159.4, 143.0, 142.4, 141.7, 141.1, 140.1, 136.2, 129.6, 129.2, 128.3, 127.3, 127.0, 125.9, 125.1, 123.2, 122.5, 122.2, 120.2, 118.3, 114.6, 108.7, 108.3, 90.1, 55.6, 55.3, 30.6 ppm; HRMS (ESI-TOF): m/z calculated for C34H30NO3 [M + H]+ 500.2226, found 500.2220.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from the IOE research grant (no-IOE/RC1/20/008).References
- (a) R. Hegden, P. B. D. Emmanuel, J. Beevi and S. S. Dharan, J. Pharm. Sci. Res., 2020, 12, 1271–1277 Search PubMed; (b) A. Caruso, J. Ceramella, D. Iacopetta, C. Saturnino, M. V. Mauro, R. Bruno, S. Aquaro and M. S. Sinicropi, Molecules, 2019, 24, 1912–1936 CrossRef CAS PubMed; (c) S. Sellamuthu, G. Gutti, D. Kumar and S. K. Singh, Mini-Rev. Org. Chem., 2018, 15, 498–507 CrossRef CAS.
- (a) J. Yin, Y. Ma, G. Li, M. Peng and W. Lin, Coord. Chem. Rev., 2020, 412, 213257–213279 CrossRef CAS; (b) I. Gupta and P. E. Kesavan, Front. Chem., 2019, 7, 841–872 CrossRef CAS PubMed; (c) K. Karon and M. Lapkowski, J. Solid State Electrochem., 2015, 19, 2601–2610 CrossRef CAS; (d) H. Jiang, J. Sun and J. Zhang, Curr. Org. Chem., 2012, 16, 2014–2025 CrossRef CAS; (e) J. Li and A. C. Grimsdale, Chem. Soc. Rev., 2010, 39, 2399–2410 RSC.
- (a) T. Mandal and J. Dash, Org. Biomol. Chem., 2021, 19, 9797–9808 RSC; (b) T. Aggarwal, Sushmita and A. K. Verma, Org. Biomol. Chem., 2019, 17, 8330–8342 RSC; (c) S. N. Georgiades and P. G. Nicolau, Adv. Heterocycl. Chem., 2019, 129, 1–88 CrossRef CAS; (d) N. Yoshikai and Y. Wei, Asian J. Org. Chem., 2013, 2, 466–478 CrossRef CAS; (e) A. W. Schmidt, K. R. Reddy and H.-J. Knölker, Chem. Rev., 2012, 112, 3193–3328 CrossRef CAS PubMed; (f) X. Fang, L. Fang and S. Gou, Chin. J. Org. Chem., 2012, 32, 1217–1231 CrossRef CAS; (g) H.-J. Knölker and K. R. Reddy, Chem. Rev., 2002, 102, 4303–4428 CrossRef PubMed.
- (a) J. Zhou, Y. Qiu, J. Li, C. Fu, X. Zhang and S. Ma, Chem. Commun., 2017, 53, 4722–4725 RSC; (b) W. Kong, C. Fu and S. Ma, Org. Biomol. Chem., 2012, 10, 2164–2173 RSC; (c) W. Kong, C. Fu and S. Ma, Chem. Commun., 2009, 4572–4574 RSC.
- (a) B. Alcaide, P. Almendros, J. M. Alonso, E. Busto, I. Fernández, M. P. Ruiz and G. Xiaokaiti, ACS Catal., 2015, 5, 3417–3421 CrossRef CAS; (b) Y. Qiu, J. Zhou, C. Fu and S. Ma, Chem. – Eur. J., 2014, 20, 14589–14593 CrossRef CAS PubMed; (c) Y. Qiu, W. Kong, C. Fu and S. Ma, Org. Lett., 2012, 14, 6198–6201 CrossRef CAS PubMed; (d) L. Wang, G. Li and Y. Liu, Org. Lett., 2011, 13, 3786–3789 CrossRef CAS PubMed; (e) C. Praveen and P. T. Perumal, Synlett, 2011, 521–524 CAS.
- (a) N. Inprung, M. J. James, R. J. Taylor and W. P. Unsworth, Org. Lett., 2021, 23, 2063–2068 CrossRef CAS PubMed; (b) A. K. Clarke, H. E. Ho, J. A. Rossi-Ashton, R. J. Taylor and W. P. Unsworth, Chem. – Asian J., 2019, 11, 1900–1911 CrossRef PubMed.
- A. Banerjee, S. Kundu, A. Bhattacharyya, S. Sahu and M. S. Maji, Org. Chem. Front., 2021, 8, 2710–2771 RSC.
- For examples of exo-cyclizations, see: (a) J. Zhu, J. Li, L. Zhang, S. Sun, Z. Wang, X. Li, L. Yang, M. Cheng, B. Lin and Y. Liu, J. Org. Chem., 2023, 88, 5483–5496 CrossRef CAS PubMed; (b) S. Yaragorla, R. Dada and D. Bag, Eur. J. Org. Chem., 2019, 6983–6988 CrossRef CAS; (c) S. Yaragorla, D. Bag, R. Dada and K. J. Jose, ACS Omega, 2018, 3, 15024–15034 CrossRef CAS PubMed; (d) R. Sanz, D. Miguel, M. Mohain, P. García-García, M. A. Fernández-Rodríguez, A. González-Pérez, O. Nieto-Faza, A. R. de Lera and F. Rodríguez, Chem. – Eur. J., 2010, 16, 9818–9828 CrossRef CAS PubMed; (e) R. Sanz, D. Miguel and F. Rodríguez, Angew. Chem., Int. Ed., 2008, 47, 7354–7357 CrossRef CAS; (f) C. Ferrer, C. H. M. Amijs and A. M. Echavarren, Chem. – Eur. J., 2007, 13, 1358–1373 CrossRef CAS.
- (a) S. J. Heffernan, J. P. Tellam, M. E. Queru, A. C. Silvanus, D. Benito, M. F. Mahon, A. J. Hennessy, B. I. Andrews and D. R. Carbery, Adv. Synth. Catal., 2013, 355, 1149–1159 CrossRef CAS; (b) C. J. Loh, J. Badorrek, G. Raabe and D. Enders, Chem. – Eur. J., 2011, 17, 13409–13414 CrossRef CAS PubMed; (c) X. Xie, X. Du, Y. Chen and Y. Liu, J. Org. Chem., 2011, 76, 9175–9181 CrossRef CAS PubMed; (d) Y. Lu, X. Du, X. Jia and Y. Liu, Adv. Synth. Catal., 2009, 351, 1517–1522 CrossRef CAS.
- (a) A. S. K. Hashmi, W. Yang and F. Rominger, Chem. – Eur. J., 2012, 18, 6576–6580 CrossRef CAS PubMed; (b) G. Li and Y. Liu, J. Org. Chem., 2010, 75, 3526–3528 CrossRef CAS PubMed.
- Z. Zhang, X. Tang, Q. Xu and M. Shi, Chem. – Eur. J., 2013, 19, 10625–10631 CrossRef CAS PubMed.
- M. J. James, R. E. Clubley, K. Y. Palate, T. J. Procter, A. C. Wyton, P. ÓBrien, R. J. K. Taylor and W. P. Unsworth, Org. Lett., 2015, 17, 4372–4375 CrossRef CAS PubMed.
- (a) A. Suárez, S. Suárez-Pantiga, O. Nieto-Faza and R. Sanz, Org. Lett., 2017, 19, 5074–5077 CrossRef PubMed; (b) M. Solas, M. A. Muñoz-Torres, F. Martínez-Lara, L. Renedo, S. Suárez-Pantiga and R. Sanz, ChemPlusChem, 2023, 88, e2023003, DOI:10.1002/cplu.202300382.
- P. Tharra and B. Baire, Org. Lett., 2018, 20, 1118–1121 CrossRef CAS PubMed.
- (a) L. Ali, D. S. Latha, A. Kumar and S. Yaragorla, J. Org. Chem., 2023, 88, 5457–5472 CrossRef CAS PubMed; (b) S. Yaragorla and D. Arun, J. Org. Chem., 2022, 87, 14250–14263 CrossRef CAS PubMed; (c) S. Yaragorla and D. S. Latha, ACS Omega, 2022, 7, 34693–34706 CrossRef CAS PubMed; (d) J. Vannada, M. Sulthan, D. Arun, R. Dada and S. Yaragorla, J. Org. Chem., 2020, 85, 6697–6708 CrossRef CAS PubMed; (e) R. Dada, M. Sulthan and S. Yaragorla, Org. Lett., 2020, 22, 279–283 CrossRef CAS PubMed; (f) S. Yaragorla and A. Pareek, Eur. J. Org. Chem., 2018, 1863–1871 CrossRef CAS.
- (a) H. F. Motiwala, A. M. Armaly, J. G. Cacioppo, T. C. Coombs, K. R. K. Koehn, V. M. Norwood IV and J. Aubé, Chem. Rev., 2022, 122, 12544–12747 CrossRef CAS PubMed; (b) V. Pozhydaiev, M. Power, V. Gandon, J. Moran and D. Lebœuf, Chem. Commun., 2020, 56, 11548–11564 RSC , and references cited therein.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2294916, 2294918 and 2294921. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ob00421c |
| This journal is © The Royal Society of Chemistry 2024 |