Open Access Article
Open Access ArticleN-Heterocyclic carbene/palladium synergistic catalysis in organic synthesis†
Chhanda
Debnath
,
Saswat Ranjan
Bhoi
and
Shikha
Gandhi
 *
*
Department of Chemical Sciences, Indian Institute of Science Education and Research Berhampur, 760010, India. E-mail: sgandhi@iiserbpr.ac.in
First published on 23rd May 2024
Abstract
The cooperation of two distinct catalytic cycles to activate different reactive centers leading to a chemical transformation has been classified as synergistic catalysis. The synergistic combination of NHC with palladium catalysis has emerged as a powerful strategy in the last few years. Merging the ability of NHCs to inverse the polarity of a functional group with the unique reactivity of palladium enables transformations that cannot be accomplished by either of these catalysts alone. Despite the associated challenges, such as quenching of catalysts, reactivity mismatch etc., significant development has been achieved in the field of NHC/Pd synergistic catalysis. The recent incorporation of photoredox catalysis with NHC/Pd synergistic catalysis has further advanced this area. This review highlights the developments made in the area of NHC/Pd synergistic catalysis.
1. Introduction
Development of novel catalytic processes for the synthesis of useful organic molecules is the leading area of research in chemistry. One of the most recent developments in this area is the use of several catalysts in a single reaction to facilitate desired chemical changes.1 Among these approaches, synergistic catalysis (cooperative catalysis), the process by which at least two distinct catalysts collaborate through their independent catalytic cycles, resulting in activated complexes that then participate in a chemical reaction, has gained much attention.2 Along with offering several advantages such as enabling challenging transformations, and enhancements in yields, reactivity and stereoselectivity, cooperative catalysis has also faced some challenges like reactivity mismatch, quenching of catalysts, etc. Thus, a strategic blend of planning and practical methods is essential to effectively combine catalysts and achieve desired transformations.Organocatalysts have been extensively studied in the past few decades because of their potent catalytic activity.3 N-Heterocyclic carbenes (NHCs), in particular, are highly effective organocatalysts, due to their ability to reverse the polarity of a functional group (umpolung) and their wide applicability in asymmetric synthesis.4 In order to enhance the utilization of NHC catalysis, multiple research teams have overcome the constraints of NHC single catalysis through an array of cooperative systems that employ an NHC catalyst with another catalyst.5 Over the years, the merging of NHC organocatalysis with transition-metal catalysis in a cooperative manner has gained popularity.1c,5 Due to the coordinating ability of NHCs with transition metals, which might render both the catalysts inactive, this approach offered a significant challenge. Nonetheless, several groups have successfully combined NHC catalysis synergistically with different transition metals like Pd, Ru, Cu, Ni, Ir, Co, and Au.6–11 Among these, Pd has emerged as the most employed transition metal in synergistic catalysis with NHCs. This combination has been successfully utilized for the synthesis of diverse molecules. To date, no comprehensive review focusing exclusively on NHC/palladium synergistic catalysis has been published. This review covers the progress made in this area. The review is structured into two segments. The first segment covers the applications of NHC/palladium synergistic catalysis using aldehydes as substrates and the second one covers the developments using α,β-unsaturated carbonyl systems. When an NHC reacts with an aldehyde, it leads to the formation of a Breslow intermediate,12 whereas, in the case of α,β-unsaturated carbonyls, an extended Breslow intermediate13 is formed.
2. Aldehydes as substrates
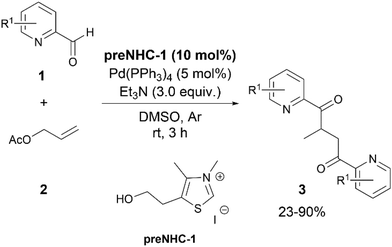
In 2014, Liu and his group reported an application of NHC/palladium synergistic catalysis for the synthesis of 1,4-diones employing pyridine-2-carbaldehydes 1 and allyl acetate 2 as substrates (Scheme 1).14 In their initial attempts utilizing Pd(PPh3)4, preNHC-1, and triethylamine, the product 3 was isolated in 53% yield as a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 coupling product. The addition of 1 (as a 1 M DMSO solution) using a syringe pump within 2 hours increased the yield of 3 to 90%. Pyridine-3-carbaldehydes and pyridine-4-carbaldehydes did not form the desired product under the same reaction conditions, indicating an important role of the ortho-N in the reaction. The authors observed that replacement of 1 by benzaldehyde led to the formation of benzoin as the sole product under the same reaction conditions.
1 coupling product. The addition of 1 (as a 1 M DMSO solution) using a syringe pump within 2 hours increased the yield of 3 to 90%. Pyridine-3-carbaldehydes and pyridine-4-carbaldehydes did not form the desired product under the same reaction conditions, indicating an important role of the ortho-N in the reaction. The authors observed that replacement of 1 by benzaldehyde led to the formation of benzoin as the sole product under the same reaction conditions.
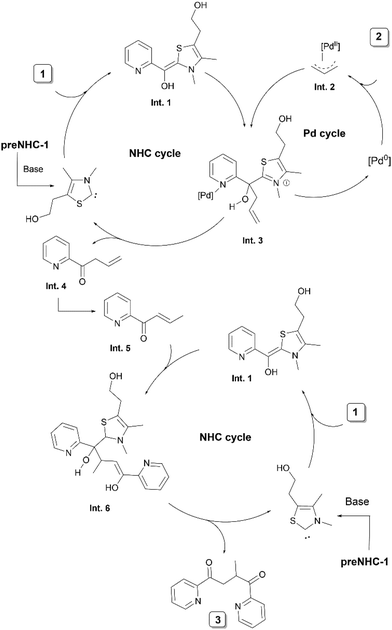
The authors proposed a mechanism involving a synergistic action of the NHC and palladium catalysts (Scheme 2). The initial addition of the NHC to pyridine-2-carbaldehydes 1 forms a nucleophilic Breslow intermediate Int. 1. In parallel, a π-allyl Pd complex Int. 2 is generated from 2. The co-ordination of the N-atom of the pyridine ring with the palladium of the π-allyl complex facilitates the convergence of the intermediates. Subsequent nucleophilic addition of the Breslow intermediate to the π-allyl Pd complex yields the intermediate Int. 3. Catalyst removal leads to a β,γ-unsaturated ketone Int. 4, which upon subsequent proton transfer in the basic medium forms an α,β-unsaturated ketone Int. 5. This is followed by the Michael addition of Int. 5 with another Breslow intermediate Int. 1, ultimately forming the compound 3viaInt. 6.
 | ||
| Scheme 2 Proposed mechanism of NHC/Pd synergistically catalysed coupling of pyridine-2-carbaldehydes and allylic acetate. | ||
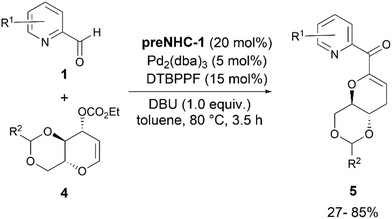
The same group extended the applications of NHC/Pd synergistic catalysis to couple glycals 4 with 1 (Scheme 3).15 A preliminary study with Pd(PPh3)4, DPPB, preNHC-1, and triethylamine in DCM at 50 °C gave the desired C-glycoside product 5 in 20% yield along with a 22% yield of the O-glycoside, formed by the attack of ethanol, generated during decarboxylation of the electrophile. A further screening of Pd catalysts, ligands, and bases improved the yield of the desired product to 85%. The use of a Pd(II) catalyst as the palladium source did not give the desired product.
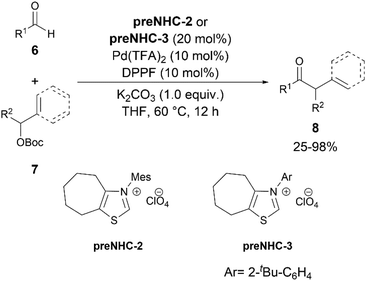
The first development of a C(sp2)–H benzylation and allylation of general aldehydes was achieved by Ohmiya and team in 2018 (Scheme 4).16 They reported an NHC/palladium catalysed synergistic coupling of aldehydes 6 and benzyl tert-butyl carbonates or allylic carbonates 7. This development provided a new synthetic pathway to α-arylated ketones and β,γ-unsaturated ketones 8. A combination of thiazolium preNHCs 2 or 3 and Pd(TFA)2-DPPF catalysts was found to be optimal for the reaction. Based on the observation that DPPF was found to be the most suitable ligand for the reaction, the authors implied that the bulkiness of the bidentate ligand might prevent the unwanted coordination of the NHC to the Pd.
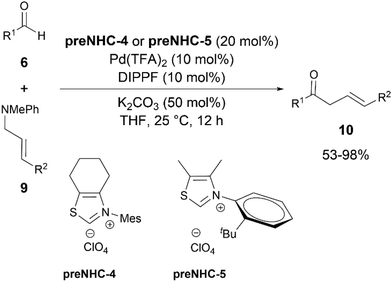
The same group, in the subsequent year, reported a synergistic NHC/palladium catalytic system for the acylation of aliphatic aldehydes 6 with allylic amines 9via C–N bond cleavage (Scheme 5).17 Pd(TFA)2 was used as the Pd catalyst and DIPPF in place of DPPF was found to be the optimal ligand to synthesise β,γ-unsaturated ketones 10. Thiazolium NHCs were found to be compatible catalysts for this transformation whereas imidazolium and triazolium NHCs were not suitable.
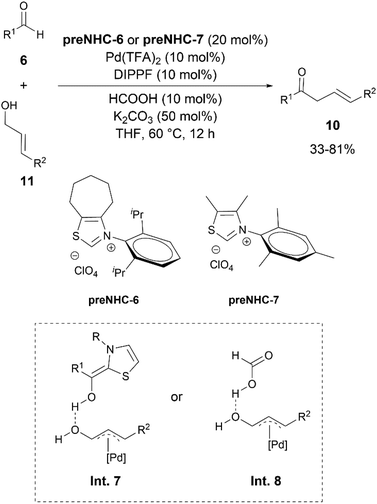
A dehydrative allylation between widely available aldehydes 6 and allylic alcohols 11 was also developed by the group of Ohmiya in 2019 to afford β,γ-unsaturated ketones 10 (Scheme 6).18 The addition of a catalytic amount of formic acid was found to be beneficial for the reaction. Furan- and pyridine-based allylic alcohols also reacted to produce the corresponding ketones 10. Aromatic aldehydes did not react under the reaction conditions. However, the authors did not provide an explanation for this. Based on the DFT calculations, a reaction pathway involving intermediacy of intermediates Int. 7 or 8, formed via the assistance of the Breslow intermediate (as a Brønsted acid) or formic acid in the oxidative addition step, was proposed.
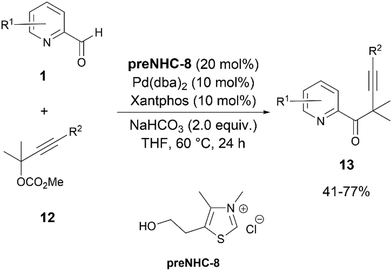
A further advancement in the field of NHC/palladium synergistic catalysis was brought by Wang and co-workers through the development of the reaction of the Breslow intermediate of 1 with the π-allyl complexes derived from propargylic carbonates 12 (Scheme 7).19 The thiazolium preNHC-8 and Pd(dba)2 appeared to be the most effective catalysts. A bidentate ligand, Xantphos, was found to be the most suitable, while the monodentate ligand triphenylphosphine failed to give the desired product 13. This reaction could be applied to propargylic carbonates with naphthyl, thienyl, or n-butyl groups along with differently substituted aryl groups.
 | ||
| Scheme 7 Coupling of pyridine-2-carbaldehydes and propargylic carbonates via NHC/Pd synergistic catalysis. | ||
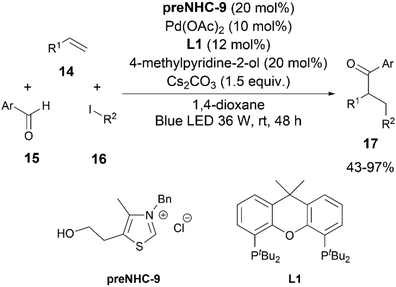
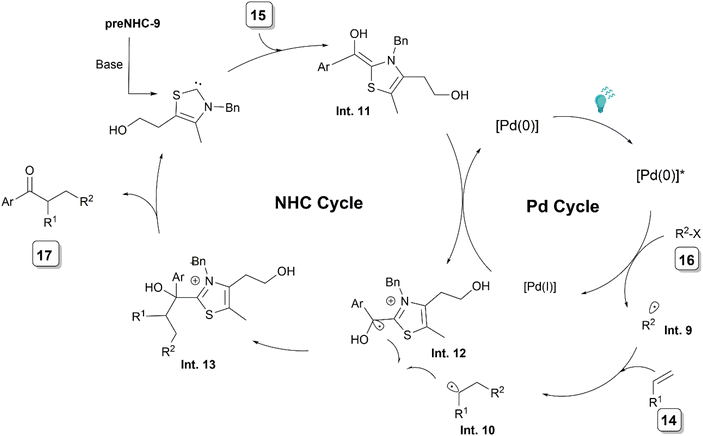
In 2022, Ye and his team opened a new vista in the field of NHC/palladium cooperative catalysis through the incorporation of photoredox catalysis (Scheme 8).20 They reported a photoredox21 cooperative NHC/Pd-catalyzed alkylacylation of simple alkenes 14 with aldehydes 15 and unactivated alkyl halides 16. The reaction was efficiently catalysed by using catalytic amounts of Pd(OAc)2, a bidentate phosphine, and preNHC-9 along with a base and 4-methylpyridine-2-ol as an additive under blue LED irradiation. Although the clear role of the additive was not elucidated, it could be acting as a competitive ligand. Based on UV-visible absorption spectroscopy studies of a combination of substrates and catalysts, a palladium species was proposed to be the photocatalyst for the reaction.
 | ||
| Scheme 8 Proposed mechanism of photoredox NHC/Pd synergistically catalysed alkylacylation of alkenes. | ||
The plausible catalytic cycle involved the generation of an alkyl radical species Int. 9 and a Pd(I) species from the photoexcited Pd(0) complex and an alkyl halide (Scheme 9). The addition of Int. 9 to the alkene 14 generates the alkyl radical Int. 10. Concurrently, Breslow intermediate Int. 11, formed from the NHC and aldehyde 15, undergoes SET with Pd(I), generating ketyl radical Int. 12 and Pd(0). The coupling of radicals Int. 10 and Int. 12 leads to Int. 13, which releases the product 17 along with the regeneration of the NHC.
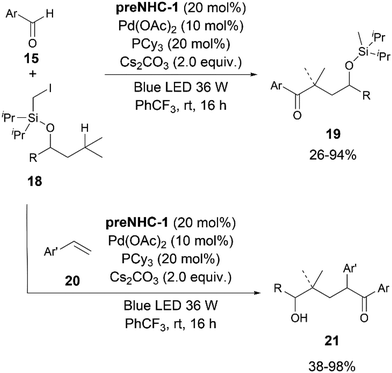
In the subsequent year, the Ye group published another report on photoredox cooperative NHC/Pd-catalyzed ketone synthesis via activation of C(sp3)–H bonds (Scheme 10).22 A variety of aromatic aldehydes 15 could be coupled to different iodomethylsilyl ethers 18. The reaction proceeded via a 1,n-HAT from the C(sp3)–H by the initially generated silyl methyl radicals under photoredox conditions. The methodology was also extended to a three-component reaction by the addition of styrenes. ε-Hydroxylketones could be synthesized in good yields after a desilylation step.
A probable catalytic cycle was proposed by the authors (Scheme 11). The Pd(0) species upon excitation with the blue light, after a SET to iodomethylsilyl ether 18, produces a Pd(I) complex and radical Int. 14. A subsequent intramolecular 1,n-hydrogen atom transfer (HAT) leads to radical Int. 15. A single electron oxidation of the Breslow intermediate Int. 11, generated from the NHC and aldehyde, by Pd(I), leads to the radical species Int. 12. A subsequent combination of the radicals Int. 12 and Int. 15 forms Int. 16, which then releases the product and eliminates the NHC. In the presence of styrene, the radical Int. 15 attacks the styrene to form the benzylic radical Int. 17, which couples with the ketyl radical Int. 12 to give the product 21.
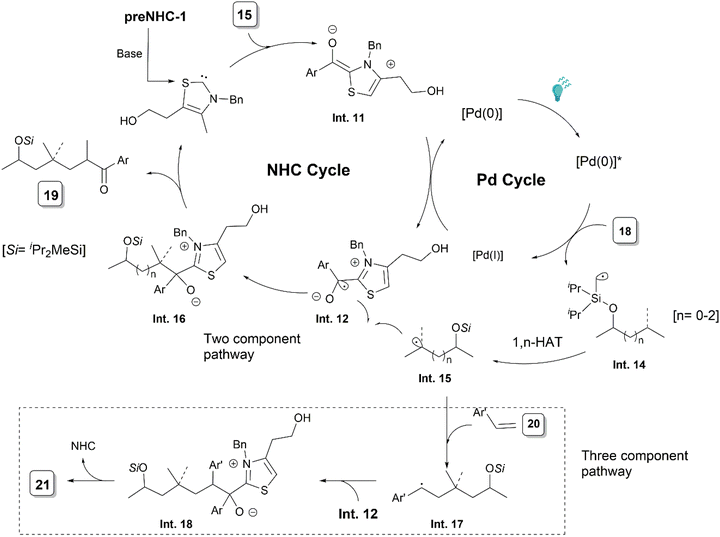 | ||
| Scheme 11 Proposed mechanism of photoredox NHC/Pd synergistically catalysed alkyl C(sp3)–H functionalisation. | ||
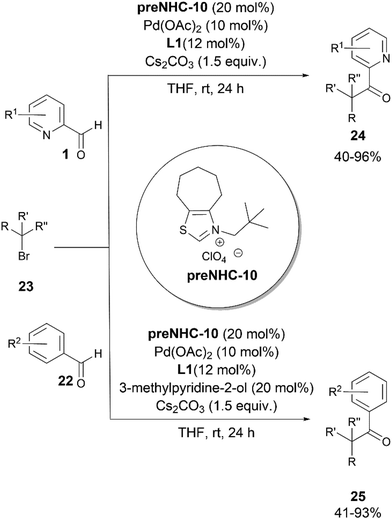
Recently, the same group reported the direct C–H alkylation of aldehydes 1 and 22 with alkyl bromides 23 using an NHC/Pd synergistic system (Scheme 12).23 The method provided a direct access to ketones 24 and 25. The reactions with aldehydes 22 required the addition of 3-methyl-pyridin-2-ol as an additive to provide the desired ketones. The feasibility of the reaction at rt has been ascribed to the coordination of the pyridine nitrogen to Pd(0), facilitating the SET from the Pd(0) complex to the alkyl bromide. Secondary, tertiary and benzylic alkyl bromides could be employed as coupling partners under the reaction conditions. The proposed reaction mechanism proceeding via the coupling of alkyl radical Int. 19 and ketyl radical Int. 21, generated from Breslow Int. 20, was supported by the mechanistic studies performed by the authors (Scheme 13).
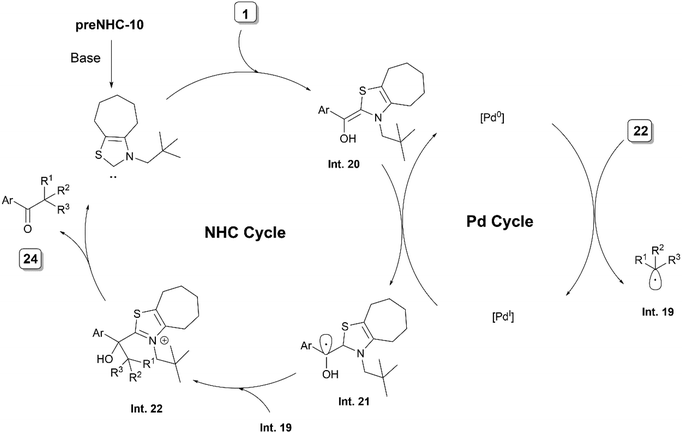 | ||
| Scheme 13 Proposed mechanism of NHC/Pd synergistic catalysis for coupling of aliphatic aldehydes with alkyl bromides. | ||
3. For α,β-unsaturated carbonyls
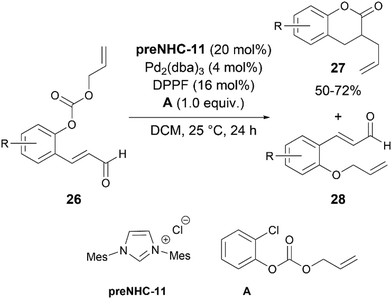
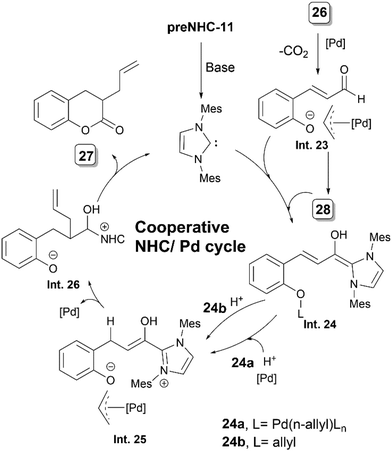
Scheidt's group applied NHC–Pd synergistic catalysis for the synthesis of 3-allyl dihydrocoumarin derivatives 27via the allylation and acylation of O-alloc α,β-unsaturated aldehydes 26 (Scheme 14).24preNHC-11 was found to be the most suitable catalyst for this transformation. They also postulated that the inclusion of a bidentate ligand could inhibit irreversible Pd–NHC binding, hence preventing catalytic quenching. During the course of the reaction, formation of 28 was observed, indicating this to be an intermediate in the reaction.The authors proposed a mechanism where O-alloc aldehyde 26 is rapidly consumed, and palladium insertion precedes NHC addition (Scheme 15). Intermediate Int. 23 or aldehyde 28 enters the catalytic cycle, adding on to the NHC to form extended Breslow intermediates Int. 24a or 24b. β-Protonation of Int. 24b, or β-protonation and palladium insertion of 24a, leads to the intermediate Int. 25. An ionic interaction between the phenoxide anion and Pd[π-allyl]Ln species facilitates a pseudo-intramolecular allylation, leading to Int. 26. Intramolecular acylation of allylated acyl azolium Int. 26 completes the catalytic cycle, yielding the desired product 27 and regenerating the NHC catalyst.
In 2016, the Glorius group reported a synergistic NHC/palladium catalyzed asymmetric synthesis of benzazepines 31 (Scheme 16).25 The reaction proceeded via the nucleophilic addition of NHC generated homoenolates from enals 29 to the π-allyl Pd-species generated from 30, followed by cyclisation. This also constituted the first example of asymmetric NHC-Pd synergistic catalysis for an intermolecular reaction. Under the optimized reaction conditions employing NHC precatalyst preNHC-12 (15 mol%), Pd(PPh3)4 (5 mol%) and Cs2CO3 (1 equiv.) in THF, the products could be obtained in high yields (up to 85%) and enantioselectivities (up to 99% ee).
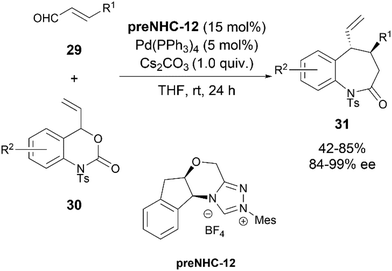 | ||
| Scheme 16 Enantioselective umpolung [2 + 5] annulation of enals and vinyl oxazinanones via NHC/Pd synergistic catalysis. | ||
In the following year, the group published the details of the mechanistic investigations on their above mentioned cooperative asymmetric NHC/palladium catalyzed transformation.26 These suggested a dual role of the NHC catalyst, both as an organocatalyst and as a chiral ligand for palladium. ESI-MS analysis supported the presence of a mixed ligand palladium complex ([Pd(π-allyl)(NHC)(PPh3)]). The absence of a phosphine ligand resulted in no product formation, indicating its crucial role. The effect of varying the phosphine ligand on the enantioselectivity indicated its role in the enantio-determining step.
As per the proposed mechanism, the coordination of vinyl benzoxazinanone 30 to the NHC–Pd–phosphine complex initiates the formation of an electrophilic allyl–palladium complex Int. 28viaInt. 27 (Scheme 17). Simultaneously, NHC addition to enal 29 generates a homoenolate intermediate Int. 30. Conjugate addition of Int. 30 to Int. 28 leads to the intermediate Int. 31. The subsequent step involves N-acylation cyclisation to yield the final product 31, regenerating the NHC catalyst.
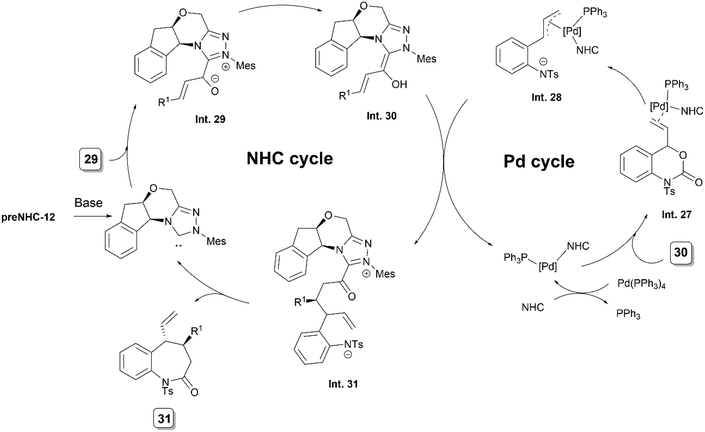 | ||
| Scheme 17 Proposed mechanism of NHC/Pd synergistically catalysed umpolung annulation of enals and vinyl oxazinanones. | ||
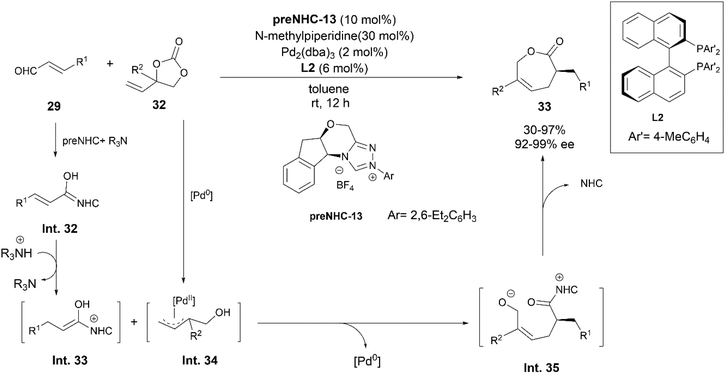
In 2018, Glorius and his team took up the challenge to form ε-caprolactones enantioselectively using NHC–Pd synergistic catalysis (Scheme 18).27 [5 + 2] annulation of vinylethylene carbonates 32 and α,β-unsaturated aldehydes 29 using a chiral NHC as well as a chiral phosphine ligand led to the desired products in high yields and enantioselectivities. Their studies indicated that the use of a bidentate ligand inhibited the coordination of the NHC to Pd, leaving the role of the NHC as that of an organocatalyst only.
 | ||
| Scheme 18 NHC/Pd synergistic catalysis for enantioselective [2 + 5] annulation of enals and vinylethylene carbonates. | ||
Based on their mechanistic studies, they postulated that the reaction is initiated by the formation of the extended Breslow intermediate (Int. 32) of 29 which upon protonation forms a Z-enol intermediate Int. 33 (Scheme 18). The attack of Int. 33 at the terminal position of the Pd–π-allyl complex Int. 34, followed by a cyclization step leads to formation of 33viaInt. 35 with regeneration of the free NHC and Pd catalysts.
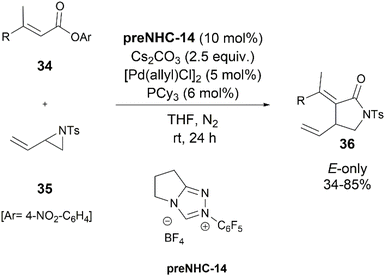
Later, in 2020, Du and coworkers reported the first example of synergistic NHC/palladium catalyzed reactions of vinyl enolates 34 with vinylaziridine 35 for the simple (3 + 2) synthesis of the (E)-3-ethylidene-4-vinylpyrrolidin-2-ones 36 (Scheme 19).28 They also discovered that having an excess of 35 was crucial for this change due to dimerization of 35 in the reaction.
 | ||
| Scheme 19 NHC/Pd synergistic catalysis for [3 + 2] annulation of vinyl enolates and vinyl aziridines. | ||
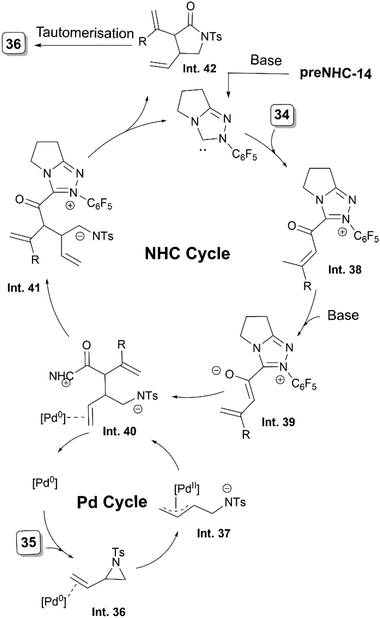
As proposed by the authors, an initial coordination of the vinyl aziridine with Pd (Int. 36) facilitates its ring opening and leads to an (η3-allyl)palladium complex Int. 37 (Scheme 20). A simultaneous activation of vinyl enolate 34 by the NHC, forms the intermediate Int. 38, which undergoes a γ-H deprotonation to form the nucleophilic NHC-bound vinyl enolate Int. 39. The attack of Int. 39 on the π-allyl complex Int. 37 present in the reaction medium results in the C–C bond formation. Removal of Pd and the subsequent N-acylation forms the annulated intermediate Int. 42viaInt. 41 and regenerates the NHC. Subsequently, the more stable product 36 is formed from Int. 42 by tautomerisation.
 | ||
| Scheme 20 Proposed mechanism of [3 + 2] annulation of vinyl enolates and vinyl aziridines via NHC/Pd synergistic catalysis. | ||
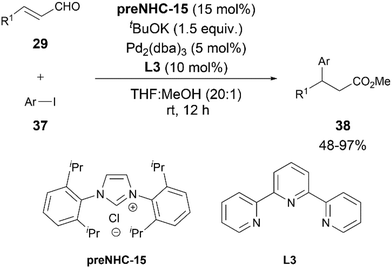
To enhance the utility of NHC-bound nucleophilic intermediates in C–C bond-forming processes, in 2020, Mao and Walsh reported the first 1,4-addition of aryl iodides 37 to enals 29 (Scheme 21).29 A combination of preNHC-15, tBuOK as the base with Pd2(dba)3 and terpyridine ligand L3 was found to be the most suitable combination for the desired arylation. The addition of MeOH to the reaction led to the regeneration of the NHC and formation of β,β-diarylpropanoates.
 | ||
| Scheme 21 Umpolung 1,4 addition of aryl iodides to α,β-unsaturated aldehydes using NHC/Pd synergistic catalysis. | ||
In the organocatalytic cycle, the NHC leads to the formation of NHC bound homoenolate intermediate Int. 43 whereas oxidative addition of palladium into aryl iodide 37 generates the intermediate Int. 44 (Scheme 22). The homoenolate Int. 43 then attacks the palladium, displacing the iodide and leading to the formation of a Pd–C bond (Int. 45). Upon reductive elimination, Int. 45 leads to Int. 46, which is followed by liberation of the NHC by MeOH and formation of the desired product 38.
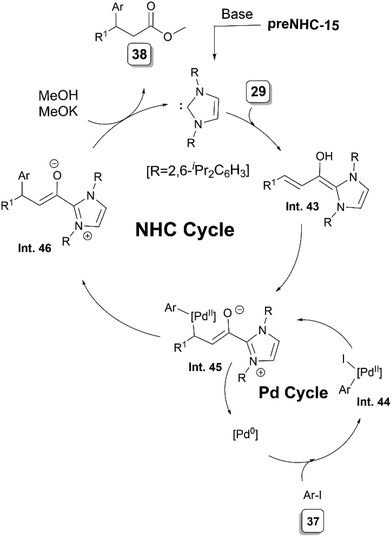 | ||
| Scheme 22 Proposed mechanism of NHC/Pd synergistically catalysed umpolung 1,4-addition of aryl iodides to enals. | ||
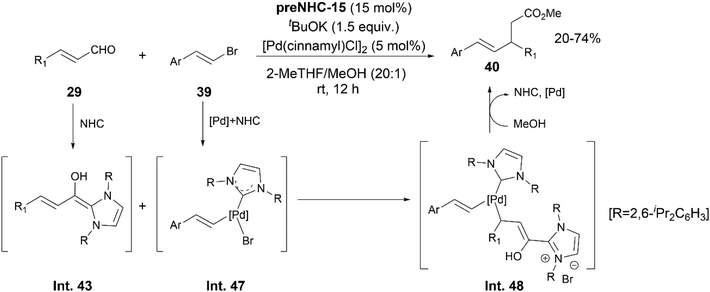
Mao extended the same strategy for 1,4 addition of vinyl bromides 39 to cinnamaldehyde 29, which led to the formation of γ,δ-unsaturated carbonyl derivatives 40 (Scheme 23).30 Based on some experiments, the authors postulated the NHC to be the catalyst as well as ligand in the reaction. Intermediates Int. 43 and Int. 47 were proposed to be generated via the activation of substrates 29 and 39 by the NHC and Pd catalysts, respectively. A combination of these two intermediates, followed by a reductive elimination and NHC regeneration aided by MeOH, led to the desired product 40viaInt. 48.
4. Conclusion
The field of NHC/palladium synergistic catalysis has witnessed significant developments over the last decade, showcasing its potential in several synthetic transformations. The reactions of Breslow as well as extended Breslow intermediates, generated from the activation of aldehydes and α,β-unsaturated carbonyls by NHCs, with a diverse range of substrates activated by palladium complexes have enabled several transformations that could not be accomplished by either of these catalysts alone. Innovative extensions, such as the incorporation of photoredox catalysis, have further expanded the scope of NHC/palladium synergistic catalysis. The development of new synthetic pathways, such as alkylacylation of alkenes and activation of C(sp3)–H bonds, highlights the adaptability of this collaborative strategy to address challenges posed by unreactive substrates. Despite the progress made, challenges such as reactivity mismatch and catalyst quenching persist. Ongoing efforts are required to refine reaction conditions, and catalyst and ligand selection for enhanced efficiency and a broader substrate scope. The development of enantioselective processes using NHC/palladium synergistic catalysis has not gained much momentum, in spite of the significant advancement in the enantioselective transformations catalysed by NHCs and palladium catalysts separately.The cooperative catalysis with NHCs and palladium represents a vibrant and promising pathway in synthetic chemistry. As research in this field progresses, NHC/palladium synergistic catalysis is poised to make substantial contributions to the development of innovative synthetic methodologies.
Conflicts of interest
The authors have no conflict of interest to declare.Acknowledgements
The authors are thankful to IISER Berhampur for funding. C. D. acknowledges the Ministry of Education, Government of India for PMRF.References
-
(a) Q. Wang, Y. Meng, L. Wu and E.-Q. Li, Chin. Chem. Lett., 2023, 108544 CrossRef CAS
; (b) C. C. Malakar, L. Dell'Amico and W. Zhang, Eur. J. Org. Chem., 2023, e202201114 CrossRef CAS
; (c) N. Chakraborty, B. Das, K. K. Rajbongshi and B. K. Patel, Eur. J. Org. Chem., 2022, e202200273 CrossRef CAS
; (d) D.-F. Chen and L.-Z. Gong, J. Am. Chem. Soc., 2022, 144, 2415–2437 CrossRef CAS PubMed
; (e) L.-Z. Gong, Asymmetric Organo-Metal Catalysis, Wiley-VCH, Weinheim, 2022 CrossRef
; (f) B. Zhang, G. Yang, D. Guob and J. Wang, Org. Chem. Front., 2022, 9, 5016–5040 RSC
; (g) S. Martínez, L. Veth, B. Lainer and P. Dydio, ACS Catal., 2021, 11, 3891–3915 CrossRef
; (h) S. P. Sancheti, Urvashi, M. P. Shah and N. T. Patil, ACS Catal., 2020, 10, 3462–3489 CrossRef CAS
; (i) H. Pellissier, Adv. Synth. Catal., 2020, 362, 2289–2325 CrossRef CAS
; (j) A. Galván, F. J. Fañanás and F. Rodríguez, Eur. J. Inorg. Chem., 2016, 2016, 1306–1313 CrossRef
; (k) D.-F. Chen, Z.-Y. Han, X.-L. Zhou and L.-Z. Gong, Acc. Chem. Res., 2014, 47, 2365–2377 CrossRef CAS PubMed
; (l) Z. Du and Z. Shao, Chem. Soc. Rev., 2013, 42, 1337–1378 RSC
; (m) Z. Shao and H. Zhang, Chem. Soc. Rev., 2009, 38, 2745–2755 RSC
.
-
(a) L. Wei, C. Fu, Z.-F. Wang, H.-Y. Tao and C.-J. Wang, ACS Catal., 2024, 14, 3812–3844 CrossRef CAS
; (b) C. D.-T. Nielsen, J. D. Linfoot, A. F. Williams and A. C. Spivey, Org. Biomol. Chem., 2022, 20, 2764–2778 RSC
; (c) U. B. Kim, D. J. Jung, H. J. Jeon, K. Rathwell and S.-G. Lee, Chem. Rev., 2020, 120, 13382–13433 CrossRef CAS PubMed
; (d) D.-S. Kim, W.-J. Park and C.-H. Jun, Chem. Rev., 2017, 117, 8977–9015 CrossRef CAS PubMed
; (e) S. Afewerki and A. Córdova, Chem. Rev., 2016, 116, 13512–13570 CrossRef CAS PubMed
; (f) Y. Deng, S. Kumar and H. Wang, Chem. Commun., 2014, 50, 4272–4284 RSC
; (g) A. E. Allen and D. MacMillan, Chem. Sci., 2012, 3, 633 RSC
.
-
(a) B. Panda, Curr. Organocatal., 2023, 10, 134–146 CrossRef CAS
; (b) B. Han, X.-H. He, Y.-Q. Liu, G. He, C. Peng and J.-L. Li, Chem. Soc. Rev., 2021, 50, 1522–1586 RSC
; (c) H. Pellesier, Organocatalysis in Domino Processes, in Domino Reactions, ed. L. F. Tietze, Wiley-VCH, Weinheim, 2014, ch. 10, pp. 325–405 Search PubMed
; (d) D. MacMillan, Nature, 2008, 455, 304–308 CrossRef CAS PubMed
; (e) B. List, Chem. Rev., 2007, 107, 5413–5415 CrossRef CAS
.
-
(a) G. Zhen, K. Jiang and B. Yin, ChemCatChem, 2022, 14, e202200099 CrossRef CAS
; (b) R. Song, Y. Xie, Z. Jin and Y. R. Chi, Angew. Chem., Int. Ed., 2021, 60, 26026–26037 CrossRef CAS PubMed
; (c) Y. Que and H. He, Eur. J. Org. Chem., 2020, 5917–5925 CrossRef CAS
; (d) H. Ohmiya, ACS Catal., 2020, 10, 6862–6869 CrossRef CAS
; (e) A. T. Biju, N-Heterocyclic Carbenes in Organocatalysis, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2018 CrossRef
; (f) D. M. Flanigan, F. Romanov-Michailidis, N. A. White and T. Rovis, Chem. Rev., 2015, 115, 9307–9387 CrossRef CAS PubMed
; (g) M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485–496 CrossRef CAS PubMed
.
-
(a) Q. Jia, Y. Li, Y. Lin and Q. Ren, Catalysts, 2019, 9, 863 CrossRef CAS
; (b) K. Nagao and H. Ohmiya, Top. Curr. Chem., 2019, 377, 35 CrossRef PubMed
; (c) M. H. Wang and K. A. Scheidt, Angew. Chem., Int. Ed., 2016, 55, 14912–14922 CrossRef CAS PubMed
.
- J. Zhao, C. Mück-Lichtenfeld and A. Studer, Adv. Synth. Catal., 2013, 355, 1098–1106 CrossRef CAS
.
- Z. Zhang, L. Zhang, R. Geng, J. Song, X. Chen and L. Gong, Angew. Chem., Int. Ed., 2019, 58, 12190–12194 CrossRef CAS PubMed
.
- T. Fan, J. Song and L. Gong, Angew. Chem., 2022, 134, e202201678 CrossRef
.
- S. Singha, E. Serrano, S. Mondal, C. G. Daniliuc and F. Glorius, Nat. Catal., 2019, 3, 48–54 CrossRef
.
- A. M. Siddiqui, A. Khalid, A. Khan, C. S. Azad, M. Samim and I. A. Khan, ChemCatChem, 2020, 12, 4281–4287 CrossRef CAS
.
- L. Zhou, X. Wu, X. Yang, C. Mou, R. Song, S. Yu, H. Chai, L. Pan, Z. Jin and Y. R. Chi, Angew. Chem., Int. Ed., 2019, 59, 1557–1561 CrossRef PubMed
.
-
(a) A. Wessels, M. Klussmann, M. Breugst, N. E. Schlörer and A. Berkessel, Angew. Chem., Int. Ed., 2022, 61, e202117682 CrossRef CAS PubMed
; (b) M. Pareek, Y. Reddi and R. B. Sunoj, Chem. Sci., 2021, 12, 7973–7992 RSC
.
-
(a) X. Chen, H. Wang, Z. Jin and Y. R. Chi, Chin. J. Chem., 2020, 38, 1167–1202 CrossRef CAS
; (b) L. R. Mills and S. A. L. Rousseaux, Eur. J. Org. Chem., 2019, 8–26 CrossRef CAS
; (c) R. S. Menon, A. T. Biju and V. Nair, Chem. Soc. Rev., 2015, 44, 5040–5052 RSC
; (d) V. Nair, R. S. Menon, A. T. Biju, C. R. Sinu, R. R. Paul, A. Jose and V. Sreekumar, Chem. Soc. Rev., 2011, 40, 5336 RSC
.
- Y. Bai, S. Xiang, M. L. Leow and X. W. Liu, Chem. Commun., 2014, 50, 6168–6170 RSC
.
- Y. Bai, W. L. Leng, Y. Li and X. W. Liu, Chem. Commun., 2014, 50, 13391–13393 RSC
.
- S. Yasuda, T. Ishii, S. Takemoto, H. Haruki and H. Ohmiya, Angew. Chem., Int. Ed., 2018, 57, 2938–2942 CrossRef CAS PubMed
.
- N. Ohnishi, S. Yasuda, K. Nagao and H. Ohmiya, Asian J. Org. Chem., 2019, 8, 1133–1135 CrossRef CAS
.
- H. Haruki, S. Yasuda, K. Nagao and H. Ohmiya, Chem. – Eur. J., 2019, 25, 724–727 CrossRef CAS PubMed
.
- W. Bi, Y. Yang, S. Ye and C. Wang, Chem. Commun., 2021, 57, 4452–4455 RSC
.
- Y.-F. Han, Y. Huang, H. Liu, Z.-H. Gao, C.-L. Zhang and S. Ye, Nat. Commun., 2022, 13, 5754 CrossRef CAS PubMed
.
-
(a) N. Holmberg-Douglas and D. A. Nicewicz, Chem. Rev., 2021, 122, 1925–2016 CrossRef PubMed
; (b) J. Xie, H. Jin and A. S. K. Hashmi, Chem. Soc. Rev., 2017, 4, 5193 RSC
; (c) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed
; (d) J. Xuan and W.-J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828–6838 CrossRef CAS PubMed
; (e) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102–113 RSC
.
- H.-Y. Wang, X.-H. Wang, B.-A. Zhou, C.-L. Zhang and S. Ye, Nat. Commun., 2023, 14, 4044 CrossRef CAS PubMed
.
- Y. Huang, Y.-F. Han, C.-L. Zhang and S. Ye, ACS Catal., 2023, 13, 11033–11040 CrossRef CAS
.
- K. Liu, M. T. Hovey and K. A. Scheidt, Chem. Sci., 2014, 5, 4026–4031 RSC
.
- C. Guo, M. Fleige, D. Janssen-Müller, C. G. Daniliuc and F. Glorius, J. Am. Chem. Soc., 2016, 138, 7840–7843 CrossRef CAS PubMed
.
- C. Guo, D. Janssen-Müller, M. Fleige, A. Lerchen, C. G. Daniliuc and F. Glorius, J. Am. Chem. Soc., 2017, 139, 4443–4451 CrossRef CAS PubMed
.
- S. Singha, T. Patra, C. G. Daniliuc and F. Glorius, J. Am. Chem. Soc., 2018, 140, 3551–3554 CrossRef CAS PubMed
.
- J. Gao, J. Zhang, S. Fang, J. Feng, T. Lu and D. Du, Org. Lett., 2020, 22, 7725–7729 CrossRef CAS PubMed
.
- W. Yang, B. Ling, B. Hu, H. Yin, J. Mao and P. J. Walsh, Angew. Chem., Int. Ed., 2019, 59, 161–166 CrossRef PubMed
.
- B. Ling, W. Yang, Y.-E. Wang and J. Mao, Org. Lett., 2020, 22, 9603–9608 CrossRef CAS PubMed
.
Footnote |
| † Celebrating the 100th birthday of Prof. Sukh Dev. |
| This journal is © The Royal Society of Chemistry 2024 |