Bisindole-based small molecules as transmembrane anion transporters and potential anticancer agents†
Swati Bansi
Salunke
 a,
Shreyada N.
Save
a,
Shreyada N.
Save
 b,
Naveen J.
Roy
b,
Naveen J.
Roy
 a,
Ronedy
Naorem
a,
Ronedy
Naorem
 a,
Shilpy
Sharma
a,
Shilpy
Sharma
 b and
Pinaki
Talukdar
b and
Pinaki
Talukdar
 *a
*a
aDepartment of Chemistry, Indian Institute of Science Education and Research (IISER) Pune, Dr. Homi Bhabha Road, Pashan, Pune 411008, Maharashtra, India. E-mail: ptalukdar@iiserpune.ac.in
bDepartment of Biotechnology, Savitribai Phule Pune University (Formerly University of Pune), Pune 411007, Maharashtra, India
First published on 27th May 2024
Abstract
Few synthetic ion transporters have been reported incorporating indole as the core moiety. We have developed a novel bisindole-based transporter capable of efficient transmembrane anion antiport. This system induced cytotoxicity in MCF-7 breast cancer cells via chloride ion homeostasis disruption and the associated ROS generation, mitochondrial membrane depolarization, and lysosomal deacidification.
Introduction
The transport of ions across the cell envelope is facilitated by various membrane-embedded proteins that aid the polar ion in overcoming the hydrophobic barrier of the lipid membrane.1 This tightly regulated transport process, also known as ion homeostasis, is central to vital cellular processes.1–3 It is possible to disrupt this delicate ion balance using small molecules that mimic these protein functions, either as ion carriers or channels, and initiate unregulated transmembrane ion transport. Such systems find application in the killing of diseased and cancerous cells.4 Reports in recent literature show synthetic chloride ion transporters to be capable of inducing cancer cell death through the apoptotic pathway via perturbation of cellular chloride ion concentrations,5–8 making the design of such small molecules to be of interest in supramolecular chemistry.Prodigiosins are a well-known family of naturally occurring tripyrrolic transporters capable of transmembrane chloride transport9,10 that show promising anticancer, antimicrobial, and antifungal properties.11,12 The anticancer activity of prodigiosin is linked to its ion transport ability, where it can destabilize the ion homeostasis and induce apoptotic cell death.13 Pyrrole with its hydrogen bond donor N–H forms the core of this system and has thus inspired the development of many pyrrole-based transporters including tambjamines,14,15 calixpyrroles.16,17 and amidopyrroles.18,19 Like pyrrole, indole also has a single NH H-bond donor capable of anion binding, but surprisingly has been less studied as an anion transporter, even though indole NH is more acidic than pyrrole NH (pKa in DMSO: pyrrole 23.0 and indole 21.0).20 Until 2004, indole anion complexation was only recognized in biological systems as a binding core in the form of tryptophan in the nitrate,21 sulfate,22 and bicarbonate23 binding proteins, and in haloalkane dehalogenase.24 The first indole-based anion receptor was a macrocycle reported by Jeong and co-workers in 2005,25 following which many more were reported by Gale and co-workers that included simple indole/biindole,26 acyclic indole,27 2-amidoindole,28 and 2,7-functionalized indole29 moieties. Thenceforth, ion transporters having an indole core were pioneered by this group, who have reported drug-like properties of indole-substituted urea and thiourea-based anion transporters30 and fused the pyrrole and imine part of prodigiosin with indole to develop perenosins.31 These along with the indole amide system from our lab32 are the only known reports of indole-based transporters, making indole less explored (relative to pyrrole) in the field of artificial ion transport.
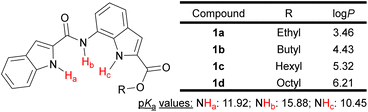
Herein, we have designed and synthesized a series of substituted bisindole-based anionophores (1a–1d) by the coupling of indole-2-carboxylic acid with ester derivatives of 7-aminoindole-2-carboxylic acid. Tuning of the transporter logP value was achieved by variation of the chain length of alkyl groups attached to the ester moiety (Fig. 1). From the predicted N–H group pKa values (MarvinSketch),33 11.92 for (indole) N–Ha, 15.88 for (amide) N–Hb, and 10.45 for (indole) N–Hc (Fig. 1), we hypothesized the bisindole system could effectively complex with an anion through hydrogen bonding with its three N–H groups and transport the anion across the membrane, while the indole rings efficiently shielded the charge from the hydrophobic environment of the lipid bilayer.
Results and discussion
Synthesis
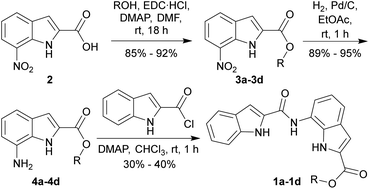
7-Nitroindole-2-carboxylic acid (2) was synthesized from ortho-nitroaniline according to the known literature procedure.34 Ester derivatives 3a–3d were synthesized by the coupling of acid 2 with different aliphatic alcohols in the presence of EDC·HCl and DMAP (Scheme 1). The corresponding amines 4a–4d were obtained by the reduction of the nitro group of 3a–3d using H2, Pd/C. Finally, coupling of amines 4a–4d with indole-2-carboxylic chloride yielded the desired anionophores 1a–1d. Prediction of logP of derivatives with calculator plugins of MarvinSketch yielded values close to 5 (Fig. 1), which ensured good lipid membrane permeability in line with Lipinski's rule, making them suitable for transmembrane ion transport.Amid all synthesized compounds 1a–1d, the crystal for 1d was obtained by slow evaporation of solvent from the saturated solution of 1d in DMSO. The transporter was found to co-crystallize with DMSO, where the amide N–Hb and indole N–Hc interacted with the oxygen atom of the solvent through hydrogen bonds, while indole N–Ha formed H-bond with the amide carbonyl group of the neighboring bisindole molecule, contributing to the 3D crystal packing (Fig. S15†).
Ion transport studies
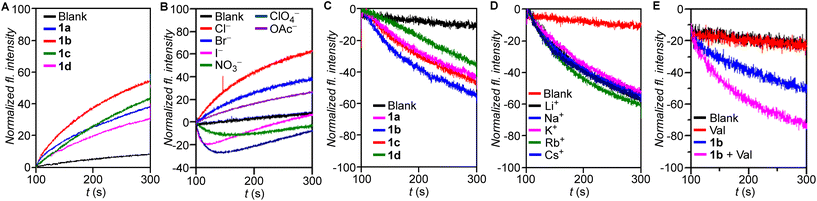
The ion transport properties of compound 1a–1d were investigated across 8-hydroxypyrene-1,3,6-trisulfonate (HPTS) dye entrapped large unilamellar vesicles (LUVs), prepared from egg-yolk phosphatidylcholine (EYPC) (EYPC–LUVs⊃HPTS) (Fig. S1†) suspended in NaCl (100 mM)–HEPES (10 mM) buffer (pH = 7.0).5 A pH gradient (pHin = 7.0, pHout = 7.8) was applied across the membrane by addition of NaOH following which the transporters (1a–1d) were added. The resultant ion transport induced dissipation of the pH gradient by 1a–1d was measured by monitoring the time-based fluorescence intensity of HPTS at 450 nm (λem = 510 nm). Comparison of ion transport activities of 1a–1d offered the activity sequence 1b > 1c ≈ 1a > 1d at 500 nM (0.80 mol%) concentration (Fig. 2A). Dose-dependent ion transport studies of 1a–1c (Fig. S2–S4†) were conducted across EYPC–LUVs⊃HPTS and the EC50 values and Hill coefficients (n) of the transporters were obtained by fitting to the Hill equation (eqn (S2)†). The Hill plot analysis of most active transporter 1b furnished EC50 = 347 nM (0.55 mol%) and the Hill coefficient, n = 1.3 suggesting only one molecule of 1b interacts with an anion for active transporter complex formation. The calculated EC50 values for 1a and 1c were 751 nM (1.20 mol%) and 748 nM (1.19 mol%), and Hill coefficients 1.78 and 1.51, respectively. Hill analysis could not be performed for 1d due to its precipitation at higher concentrations in the buffer.Since transporter 1b furnished the highest ion transport activity, mechanistic studies were conducted using this derivative. Variation of the extravesicular anion (Cl−, Br−, I−, NO3−, ClO4−, and OAc−) in EYPC–LUVs⊃HPTS (Fig. S5†) resulted in significant changes in the transport activity profile of 1b (0.6 μM, 0.96 mol%) (Fig. 2B), indicating the process to be anion dependent. The dual gradient of pH and dissimilar anions created across the vesicular membrane in this assay can act in opposite directions, depending on the nature of the anion. In the case of the extravesicular I−, NO3−, and ClO4−, which are hydrophobic anions, the ease of anion dehydration,35 coupled with the higher hydrophobicity of the resulting transporter-anion complex allows for higher membrane permeability36 and, thus, faster rates of ion transport compared to the corresponding chloride complex. Thus, in the case of I−, NO3−, and ClO4−, the anion gradient dominates the initial phase of the transport process, leading to an influx of A− balanced, at least in part, by OH− efflux following transporter addition. This leads to an initial decrease in HPTS fluorescence as a consequence of the drop in intravesicular pH. The subsequent enhancement of the pH gradient eventually allows it to dominate the transport process, leading to OH− influx balanced by Cl− efflux which results in the increase of intravesicular pH and thus the dye fluorescence. This is not observed for the hydrophilic anions (Cl−, Br−, and OAc−), where the pH gradient solely dictates the direction of anion transport.
As the ion transport process was anion dependent, the transporter induced Cl− influx of 1a–1d were evaluated across LUVs entrapped with lucigenin (EYPC–LUVs⊃lucigenin), across which a Cl− gradient was generated (Fig. S6†). The Cl− transport process was monitored from the change in fluorescence intensity of lucigenin at λem = 535 nm (λex = 455 nm). Comparative studies showed the same order of transport activity as observed in HPTS assay, i.e., 1b > 1c ≈ 1a > 1d (Fig. 2C). Dose-dependent studies of 1a and 1b across EYPC–LUVs⊃lucigenin followed by Hill analysis (Fig. S7 and S8†) yielded n = 2.05 (EC50 = 1.91 μM, 2.04 mol%) for 1a, while n = 1.34 (EC50 = 1.24 μM, 1.32 mol%) was obtained for 1b which agreed with data from the HPTS assay. Hill analysis could not be performed for compounds 1c and 1d due to precipitation at higher concentrations. No change in the rate of transport of 1b was observed on variation of extravesicular cations (Li+, Na+, K+, Rb+, and Cs+) across EYPC–LUVs⊃lucigenin revealing the absence of cation transport (Fig. 2D). This indicated the transport process to be cation independent, suggesting either H+/Cl− symport or Cl−/X− antiport. Hence, the Cl− transport properties of 1b were evaluated in the presence and absence of the K+ selective transporter valinomycin (Fig. S8†).19 A significant increase in the ion transport activity of 1b across EYPC–LUVs⊃lucigenin was observed following valinomycin treatment (Fig. 2E), confirming anion antiport as the mechanism of transport in bisindoles. The dual K+, Cl− gradient generated across the membrane at the start of the assay triggers unidirectional transport/influx of K+ by valinomycin. The resulting charge gradient was neutralized by the Cl− transport (influx) by the bisindole, resulting in a synergistic effect that aids both transport processes. Since net charge neutrality is maintained in this coupled transport process the reverse movement (efflux) of the NO3− by the transporter becomes redundant. Overall, a significant increase in the rate of Cl− influx would be observed, leading to faster quenching of the lucigenin fluorescence if the mechanism of transport is anion antiport. For a symporter, no change in transport activity would be observed as the net transfer of charge is zero and is not influenced by the K+ gradient.
Compounds 1a and 1b were tested for carrier mechanism of transmembrane ion transport through a variable temperature assay across 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) liposomes entrapping HPTS dye (DPPC–LUVs⊃HPTS) (Fig. S11†).37 DPPC undergoes gel to liquid-crystalline phase transition at Tc = 41.3 °C.38 The transport ability of both 1a and 1b (2.5 μM, 3.64 mol%) were inhibited at 25 °C, when lipid was in gel phase, which then recovered above Tc at 45 °C (Fig. S12†). This confirmed the mobile carrier mechanism of ion transport, as carrier mobility is greatly inhibited in the gel phase.37 Transport through ion channels are unaffected by the lipid phase due to their membrane spanning nature and thus this mechanism of ion transport can be ruled out for the bisindole system.
Hill analysis data indicated the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (n ≈ 1, [1b + Cl−]) transmembrane ion transport complex for transporter 1b while 1a formed a 2
1 (n ≈ 1, [1b + Cl−]) transmembrane ion transport complex for transporter 1b while 1a formed a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (n ≈ 2, [(1a)2 + Cl−]) complex, the theoretical prediction of whose structures were attempted. An initial prediction of the most probable geometry of [1b + Cl−] complex using the CONFLEX 8 conformation search software package39,40 using MMFF94S (2010-12-04HG) force field with a search limit of 10 kcal mol−1 yielded 101 conformers. Conformers with a predicted Boltzmann population <5% were discarded, leaving 7 conformers whose Density Functional Theory (DFT) geometry optimization was conducted on the Gaussian 09 program suite41 using the B3LYP exchange–correlation functional42,43 and 6-31G(d,p) basis set.44,45 The resultant outputs were highly similar and the conformer with the lowest Hartree–Fock (HF) energy was chosen, which showed all three N–H groups to be involved in anion binding (Fig. 3A and S20†), as per our initial hypothesis. The binding energy was calculated to be −38.69 kcal mol−1.
1 (n ≈ 2, [(1a)2 + Cl−]) complex, the theoretical prediction of whose structures were attempted. An initial prediction of the most probable geometry of [1b + Cl−] complex using the CONFLEX 8 conformation search software package39,40 using MMFF94S (2010-12-04HG) force field with a search limit of 10 kcal mol−1 yielded 101 conformers. Conformers with a predicted Boltzmann population <5% were discarded, leaving 7 conformers whose Density Functional Theory (DFT) geometry optimization was conducted on the Gaussian 09 program suite41 using the B3LYP exchange–correlation functional42,43 and 6-31G(d,p) basis set.44,45 The resultant outputs were highly similar and the conformer with the lowest Hartree–Fock (HF) energy was chosen, which showed all three N–H groups to be involved in anion binding (Fig. 3A and S20†), as per our initial hypothesis. The binding energy was calculated to be −38.69 kcal mol−1.
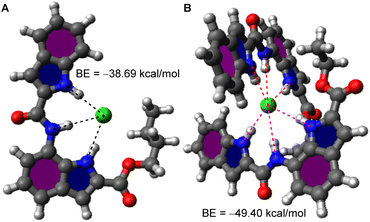 | ||
| Fig. 3 Geometry optimized structures of ion transport complexes (A) [1b + Cl−] and (B) [(1a)2 + Cl−] along with their calculated binding energies. | ||
A similar conformation search for [(1a)2 + Cl−] complex yielded 10 726 predictions of which 6 conformers having Boltzmann population ≥ 5% were chosen and the rest were rejected. DFT geometry optimization (B3LYP/6-31G(d,p)) of the chosen conformers yielded highly similar structures of which the conformer with lowest HF energy was chosen. The resultant [(1a)2 + Cl−] complex showed two molecules of 1a orthogonal to each other binding to Cl− (Fig. 3B, S18†) with all N–H groups participating in binding. The binding energy was calculated to be −49.40 kcal mol−1.
For further verification of this interesting variation in ion complex formation in 1a and 1b, co-crystallization of the transporters with chloride ion (using tetrabutylammonium chloride (TBACl) as the Cl− source) was attempted in various solvent systems but ultimately unsuccessful. Hence, 1H NMR ion titration studies were conducted with 1a and 1b in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 CD3COCD3/CD3CN solvent system using TBACl as the chloride source (Fig. S13 and S14†). Downfield shifts were observed for all three NH peaks ((indole) N–Ha, (amide) N–Hb, and (indole) N–Hc) thereby confirming their participation in anion binding. The data was fitted to host–guest binding models using the BindFit program.46 Data for transporter 1a yielded a 2
9 CD3COCD3/CD3CN solvent system using TBACl as the chloride source (Fig. S13 and S14†). Downfield shifts were observed for all three NH peaks ((indole) N–Ha, (amide) N–Hb, and (indole) N–Hc) thereby confirming their participation in anion binding. The data was fitted to host–guest binding models using the BindFit program.46 Data for transporter 1a yielded a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 transporter-ion binding stoichiometry with Ka1:1 = 137.7 M−1 ± 8.8% and Ka2:1 = 5862.7 M−1 ± 6.7%, while 1b yielded a 1
1 transporter-ion binding stoichiometry with Ka1:1 = 137.7 M−1 ± 8.8% and Ka2:1 = 5862.7 M−1 ± 6.7%, while 1b yielded a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry with Ka1:1 = 2489.5 M−1 ± 3.7%, both of which were in agreement with data obtained from ion transport experiments, thereby validating our theoretical models. The large value of Ka2:1 for transporter 1a indicated a strong preference for the formation of 2
1 stoichiometry with Ka1:1 = 2489.5 M−1 ± 3.7%, both of which were in agreement with data obtained from ion transport experiments, thereby validating our theoretical models. The large value of Ka2:1 for transporter 1a indicated a strong preference for the formation of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 transporter-ion complex over a 1
1 transporter-ion complex over a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex. Fitting of the data to the converse binding stoichiometries were tested, i.e., 1
1 complex. Fitting of the data to the converse binding stoichiometries were tested, i.e., 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for transporter 1a and 2
1 for transporter 1a and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 1b, but these models yielded large error values for the fit and were thus rejected.
1 for 1b, but these models yielded large error values for the fit and were thus rejected.
Biological studies
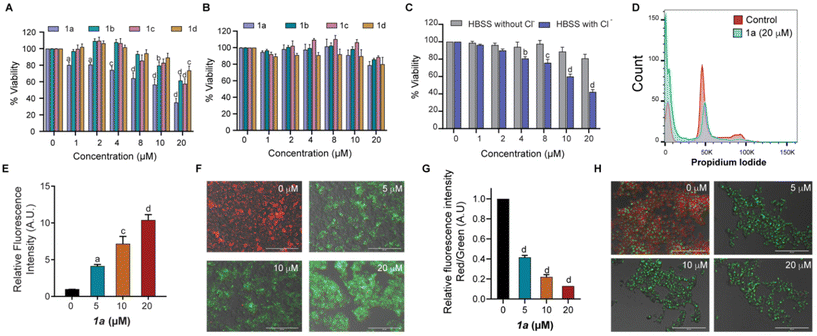
The promising results obtained from ion transport studies conducted for the bisindoles 1a–1d encouraged us to assess their activities in biological systems. Initially, cell viability was assessed in the presence of 1a–1d using MTT assay in non-cancerous mouse embryonic fibroblasts (MEFs) and MCF-7 breast cancer cells. While significant cytotoxicity was observed in MCF-7 cells following incubation with 1a–1d for 24 h (Fig. 4A); negligible cytotoxicity was observed in the non-cancerous MEFs (Fig. 4B). This indicated of some level of selectivity of our bisindole system in the MCF-7 cancerous cells at the concentrations tested. Amongst the different derivatives tested, transporter 1a was found to be most potent in terms of its anti-cancerous activity, although highest ion transport activity was observed for 1b. The discrepancy in the results obtained could likely be attributed to the difference in membrane composition between the mammalian cells and the artificial liposomes used in the study. An IC50 value of 15 μM was obtained for 1a in MCF-7 cells.To further evaluate whether the cytotoxicity mediated by 1a was dependent on Cl− transport, an MTT assay was performed in the presence and absence of Cl− ion in the extracellular media. In line with our previous observations, cytotoxicity was observed in MCF-7 cells grown in extracellular media containing Cl− ions when compared to cells cultured in extracellular media without Cl− ions upon exposure to 1a (Fig. 4C). These results confirmed the importance of extracellular Cl− ions for bisindole-mediated toxicity observed in MCF-7 cells, thereby indicating transporter-mediated chloride transport to be the initiator of cell death.8,47 This was associated with altered cell cycle profiles with reduced percentage of cells observed in the G1 and S/G2M phases with a significant increase in the G0 population in 1a treated MCF-7 cells (Fig. 4D and S21D†). To further evaluate the presence of oxidative stress, mitochondrial ROS levels in MCF-7 cells were quantified using the probe MitoSOX Red, a compound known to rapidly oxidize to a highly fluorescent product in the presence of ROS. Increasing dosage of 1a (0–20 μM) in MCF-7 yielded higher readout of MitoSOX fluorescence, thereby confirming the generation of ROS species (Fig. 4E). Increased ROS levels lead to alteration of mitochondrial membrane potential (MMP), a hallmark of cells experiencing cell death through the intrinsic apoptotic pathway. Next, we investigated the possibility of mitochondrial membrane depolarization using MMP-sensitive JC-1 dye, which forms red fluorescent J-aggregates in healthy mitochondria. Loss of MMP results in release of dye molecules into the cytoplasmic medium, which gives green intracellular fluorescence. Following treatment of MCF-7 cells with 1a (0–20 μM) for 24 h, a decrease in red fluorescence of the JC-1 dye along with a subsequent increase in green fluorescence was observed on imaging under fluorescence microscope (Fig. 4F), indicating mitochondrial membrane depolarization as a result of bisindole facilitated disruption of chloride ion homeostasis. The quantification of red/green fluorescence showed an increase in MMP depolarization with increasing concentrations of 1a (Fig. 4G). The depolarization of MMP causes an interruption in the electron transport chain of the mitochondrial respiratory cycle, leading to the generation of reactive oxygen species (ROS). In addition to this, an increase in the lysosomal pH (measured by acridine orange staining) was observed in cells exposed to 1a. As the concentration of 1a was increased, a decrease in red fluorescence with a concomitant increase in green fluorescence was observed, thereby indicating towards increase in the lysosomal pH (Fig. 4H). Thus, it can be concluded that 1a mediates cytotoxicity in MCF-7 cells by disruption of chloride ion homeostasis, which is associated with increased oxidative stress, mitochondrial membrane depolarization and lysosomal pH disruption.
Conclusions
In summary, we have demonstrated the bisindole system to be an efficient transmembrane anion antiporter capable of inducing cytotoxicity in MCF-7 breast cancer cells via destabilization of cellular chloride ion homeostasis. Tuning of the ion transport property was achieved through variation of alkyl chain length of the substituted ester group. This perturbation of the cellular ion homeostasis by the bisindole transporter resulted in mitochondrial membrane depolarization, enhanced ROS generation and alteration in lysosomal pH in MCF-7 cells.Author contributions
S. B. S. and P. T. designed the project and established research plans. S. B. S. synthesized and characterized compounds, and conducted chemical studies. S. N. S. conducted biological studies under the supervision of S. S. N. J. R. performed the computational studies. R. N. and N. J. R. characterized some compounds, performed some vesicle assays and ion titration studies. N. J. R., S. B. S. and S. N. S. wrote the manuscript. P. T. and S. S. revised the manuscript. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
P. T. acknowledges the financial support of the Science and Engineering Research Board, Government of India (Grant No. CRG/2022/001640) and IISER Pune. S. S. acknowledges funding from University Grants Commission, Government of India (Grant No. F.4-5(18-FRP) (IV-Cycle)/2017(BSR)) and RUSA 2.0 to SPPU. S. B. S. thanks DST, India for the Inspire Fellowship. S. N. S. has been supported by Mahatma Jyotiba Phule Research Fellowship, Government of Maharashtra (Mahajyoti/Nag./Fellowship/2021-22/1042(418)). N. J. R. acknowledges the Council of Scientific & Industrial Research (CSIR), Government of India, for research fellowship. This research used the resources of the PARAM Brahma supercomputing facility at IISER Pune.References
- D. C. Gadsby, Nat. Rev. Mol. Cell Biol., 2009, 10, 344 CrossRef CAS PubMed.
- S. Y. Chiu and G. F. Wilson, J. Physiol., 1989, 408, 199–222 CrossRef CAS PubMed.
- K. Lange, J. Cell. Physiol., 2000, 185, 21–35 CrossRef CAS PubMed.
- P. A. Gale, J. T. Davis and R. Quesada, Chem. Soc. Rev., 2017, 46, 2497–2519 RSC.
- T. Saha, M. S. Hossain, D. Saha, M. Lahiri and P. Talukdar, J. Am. Chem. Soc., 2016, 138, 7558–7567 CrossRef CAS PubMed.
- N. Busschaert, S.-H. Park, K.-H. Baek, Y. P. Choi, J. Park, E. N. W. Howe, J. R. Hiscock, L. E. Karagiannidis, I. Marques, V. Félix, W. Namkung, J. L. Sessler, P. A. Gale and I. Shin, Nat. Chem., 2017, 9, 667 CrossRef CAS PubMed.
- N. J. Roy, S. N. Save, V. K. Sharma, B. Abraham, A. Kuttanamkuzhi, S. Sharma, M. Lahiri and P. Talukdar, Chem. – Eur. J., 2023, 29, e202301412 CrossRef CAS PubMed.
- S.-K. Ko, S. K. Kim, A. Share, V. M. Lynch, J. Park, W. Namkung, W. Van Rossom, N. Busschaert, P. A. Gale, J. L. Sessler and I. Shin, Nat. Chem., 2014, 6, 885 CrossRef CAS PubMed.
- H. Konno, H. Matsuya, M. Okamoto, T. Sato, Y. Tanaka, K. Yokoyama, T. Kataoka, K. Nagai, H. H. Wasserman and S. Ohkuma, J. Biochem., 1998, 124, 547–556 CrossRef CAS PubMed.
- A. Fürstner, Angew. Chem., Int. Ed., 2003, 42, 3582–3603 CrossRef PubMed.
- G. A. Islan, B. Rodenak-Kladniew, N. Noacco, N. Duran and G. R. Castro, Bioengineered, 2022, 13, 14227–14258 CrossRef CAS PubMed.
- N. Darshan and H. K. Manonmani, J. Food Sci. Technol., 2015, 52, 5393–5407 CrossRef CAS PubMed.
- J. L. Sessler, L. R. Eller, W.-S. Cho, S. Nicolaou, A. Aguilar, J. T. Lee, V. M. Lynch and D. J. Magda, Angew. Chem., Int. Ed., 2005, 44, 5989–5992 CrossRef CAS PubMed.
- N. J. Knight, E. Hernando, C. J. E. Haynes, N. Busschaert, H. J. Clarke, K. Takimoto, M. García-Valverde, J. G. Frey, R. Quesada and P. A. Gale, Chem. Sci., 2016, 7, 1600–1608 RSC.
- I. Carreira-Barral, M. Mielczarek, D. Alonso-Carrillo, V. Capurro, V. Soto-Cerrato, R. Pérez Tomás, E. Caci, M. García-Valverde and R. Quesada, Chem. Commun., 2020, 56, 3218–3221 RSC.
- P. A. Gale, C. C. Tong, C. J. E. Haynes, O. Adeosun, D. E. Gross, E. Karnas, E. M. Sedenberg, R. Quesada and J. L. Sessler, J. Am. Chem. Soc., 2010, 132, 3240–3241 CrossRef CAS PubMed.
- D. S. Kim and J. L. Sessler, Chem. Soc. Rev., 2015, 44, 532–546 RSC.
- P. A. Gale, M. E. Light, B. McNally, K. Navakhun, K. E. Sliwinski and B. D. Smith, Chem. Commun., 2005, 3773–3775 RSC.
- N. J. Roy, P. L. Pujari and P. Talukdar, Org. Biomol. Chem., 2023, 21, 3323–3329 RSC.
- F. G. Bordwell, G. E. Drucker and H. E. Fried, J. Org. Chem., 1981, 46, 632–635 CrossRef CAS.
- N. M. Koropatkin, H. B. Pakrasi and T. J. Smith, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 9820–9825 CrossRef CAS PubMed.
- J. J. He and F. A. Quiocho, Science, 1991, 251, 1479–1481 CrossRef CAS PubMed.
- N. M. Koropatkin, D. W. Koppenaal, H. B. Pakrasi and T. J. Smith, J. Biol. Chem., 2007, 282, 2606–2614 CrossRef CAS PubMed.
- K. H. G. Verschueren, F. Seljée, H. J. Rozeboom, K. H. Kalk and B. W. Dijkstra, Nature, 1993, 363, 693–698 CrossRef CAS PubMed.
- K. J. Chang, D. Moon, M. S. Lah and K. S. Jeong, Angew. Chem., Int. Ed., 2005, 44, 7926–7929 CrossRef CAS PubMed.
- P. A. Gale, Chem. Commun., 2008, 4525–4540 RSC.
- P. A. Gale, J. R. Hiscock, C. Z. Jie, M. B. Hursthouse and M. E. Light, Chem. Sci., 2010, 1, 215–220 RSC.
- C. Caltagirone, P. A. Gale, J. R. Hiscock, M. B. Hursthouse, M. E. Light and G. J. Tizzard, Supramol. Chem., 2009, 21, 125–130 CrossRef CAS.
- G. W. Bates, Triyanti, M. E. Light, M. Albrecht and P. A. Gale, J. Org. Chem., 2007, 72, 8921–8927 CrossRef CAS PubMed.
- S. J. Moore, M. Wenzel, M. E. Light, R. Morley, S. J. Bradberry, P. Gómez-Iglesias, V. Soto-Cerrato, R. Pérez-Tomás and P. A. Gale, Chem. Sci., 2012, 3, 2501–2509 RSC.
- L. A. Jowett, E. N. W. Howe, V. Soto-Cerrato, W. Van Rossom, R. Pérez-Tomás and P. A. Gale, Sci. Rep., 2017, 7, 9397 CrossRef PubMed.
- S. B. Salunke, J. A. Malla and P. Talukdar, Angew. Chem., Int. Ed., 2019, 58, 5354–5358 CrossRef CAS PubMed.
- ChemAxon, 5.8.0 Marvin, (https://chemaxon.com), 2012.
- L. Xiong, X. L. Zhu, H. W. Gao, Y. Fu, S. Q. Hu, L. N. Jiang, W. C. Yang and G. F. Yang, J. Agric. Food Chem., 2016, 64, 4830–4837 CrossRef CAS PubMed.
- D. Bastos-González, L. Pérez-Fuentes, C. Drummond and J. Faraudo, Curr. Opin. Colloid Interface Sci., 2016, 23, 19–28 CrossRef.
- X. Wu and P. A. Gale, Chem. Commun., 2021, 57, 3979–3982 RSC.
- A. Kerckhoffs and M. J. Langton, Chem. Sci., 2020, 11, 6325–6331 RSC.
- R. L. Biltonen and D. Lichtenberg, Chem. Phys. Lipids, 1993, 64, 129–142 CrossRef CAS.
- H. Goto and E. Osawa, J. Am. Chem. Soc., 1989, 111, 8950–8951 CrossRef CAS.
- H. Goto, S. Obata, N. Nakayama and K. Ohta, CONFLEX8, CONFLEX Corporation, Tokyo, Japan, 2017 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2013 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS PubMed.
- P. C. Hariharan and J. A. Pople, Theor. Chim. Acta, 1973, 28, 213–222 CrossRef CAS.
- M. M. Francl, W. J. Pietro, W. J. Hehre, J. S. Binkley, M. S. Gordon, D. J. DeFrees and J. A. Pople, J. Chem. Phys., 1982, 77, 3654–3665 CrossRef CAS.
- BindFit, v0.5, https://app.supramolecular.org/bindfit/ Search PubMed.
- M. Tsukimoto, H. Harada, A. Ikari and K. Takagi, J. Biol. Chem., 2005, 280, 2653–2658 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of synthetic protocols and characterization of compounds, crystallographic parameters, experimental protocols, including vesicle preparation, ion transport assays and biological studies, additional data for ion transport studies, details of geometry optimized structures and additional biological data. CCDC 2306377. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ob00554f |
| This journal is © The Royal Society of Chemistry 2024 |