Diastereoselective cyclopropanation of α,β-unsaturated carbonyl compounds with vinyl sulfoxonium ylides†
Shalu
Deshwal
,
Dinesh Kumar
Gopalakrishnan
,
Alok
Purohit
,
Tarak
Karmakar
* and
Janakiram
Vaitla
 *
*
Department of Chemistry, Indian Institute of Technology Delhi, Hauz Khas, New Delhi, 110016, India. E-mail: tkarmakar@iitd.ac.in; vaitla@chemistry.iitd.ac.in
First published on 12th July 2024
Abstract
Herein, we report a three-component stereoselective cyclopropanation of vinyl sulfoxonium ylides with indane 1,3-dione and aldehydes under mild reaction conditions. In contrast to previous reports, the present work shows that electrophilic addition selectively takes place at the α-position of the vinyl sulfoxonium ylide. The interesting feature of this approach is that the multicomponent reaction selectively proceeds because of the difference in nucleophilic reactivity of vinyl sulfoxonium ylides and indane 1,3-dione with electrophilic partners, such as aldehydes and in situ generated arylidenes. Additionally, density functional theory (DFT) studies were conducted to investigate the difference in the reactivity of these reactants, as well as to unveil the mechanism of this three-component reaction. Furthermore, non-covalent interactions of selectivity-determining transition states explain the origin of the diastereoselectivity of cyclopropanation.
Introduction
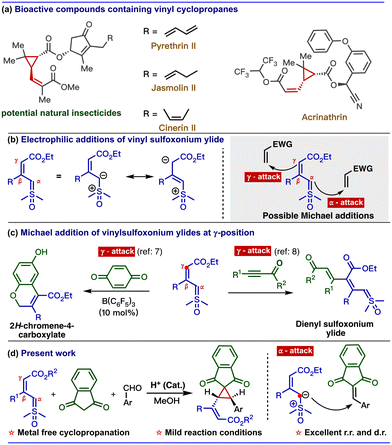
Vinyl cyclopropanes are important building blocks found in many pharmaceuticals and natural products. Some notable examples of medicinal compounds that include the vinylcyclopropane unit are Danoprevir (used in the treatment of COVID-19) and Simeprevir (used in the treatment of Hepatitis C).1 Additionally, natural insecticides, such as pyrethrines and chrysanthemic acid esters, also contain this unique structural framework.2 Inherent ring strain with the adjacent olefin makes the vinylcyclopropane a versatile synthetic precursor for various organic transformations, such as ring openings, cycloadditions, and rearrangements.3 Therefore, a significant effort has been made to develop efficient methods for synthesising vinylcyclopropane scaffolds. However, a major challenge in synthesising substituted vinylcyclopropanes is obtaining good diastereoselectivity at both the cyclopropane and the alkene units.4Our continuing interest in sulfur ylides prompted us to explore the reactivity of vinyl sulfoxonium ylides with electron-deficient alkynes and alkenes.5 Vinyl sulfoxonium ylides can undergo electrophilic addition at the α- or γ-carbon due to their dipole structure (Scheme 1b).6 Recently, our group developed a method to synthesize 2H-chromenes via catalyst-controlled regioselective [3 + 3] annulation of vinyl sulfoxonium ylides with quinones. In the absence of a metal catalyst, vinyl sulfoxonium ylide reacts with the quinone at the γ-position of the ylide, followed by cyclization to give 2H-chromene-4-carboxylate.7 Similarly, when we used propiolates as electrophiles, the reaction selectively underwent an addition reaction at the γ-position of ylides to give dienyl sulfoxonium ylide (Scheme 1c).8
Based on these results, we are interested in developing a multicomponent reaction involving a vinyl sulfoxonium ylide as a nucleophile with electrophiles, such as arylidenes that are generated in situ from aldehydes and compounds containing active methylene groups.9 However, the straightforward approach for the generation of vinylcyclopropane scaffolds from vinyl sulfoxonium ylide-mediated multicomponent reaction is challenging because the reaction can produce diverse products due to the presence of nucleophilic centers in both ylide and active methylene compounds (Scheme 2).10 The possible pathways are: (i) similar to the Corey–Chaykovsky reaction, whereby the addition of the α-carbon of the ylide to the aldehyde is followed by the elimination of DMSO to generate epoxide (path A). (ii) Due to the nucleophilicity at the γ -position, the ylide can attack the aldehyde via vinylogous addition (path B). (iii) Based on the well-known Knoevenagel condensation, active methylene compounds can react with the aldehyde to generate arylidenes (path C). (iv) Similar to our previous approach (Scheme 1c), the γ-attack of ylide at the arylidene gave the γ-substituted ylide (path D) and (v) α-attack of ylide at the arylidene gave the vinylcyclopropane (path E). Interestingly, the addition of these three reactive species in the presence of L-proline (20 mol%) in methanol exclusively produced vinylcyclopropane (Scheme 1d). Previously, vinylcyclopropanes were synthesized using carbenoids derived from diazo compounds11 or Simmons–Smith-like reactions,12 Michael-initiated ring closure reactions of ylides,13 and functional group interconversion of appropriately functionalized cyclopropanes.14 However, these methods often have drawbacks, such as poor diastereoselectivity,15 step economy,16 as well as the use of expensive metals17 or toxic reagents.13a Thus, the present mild diastereoselective cyclopropanation prompted us to develop a greener synthetic approach to generate complex cyclopropane scaffolds. In the present report, we have developed multi-component cyclopropanation from vinyl sulfoxonium ylides, aldehydes, and indane 1,3-dione. In addition, we have performed density functional theory (DFT) calculations to investigate the reaction mechanism and selectivity of the cyclopropanation.
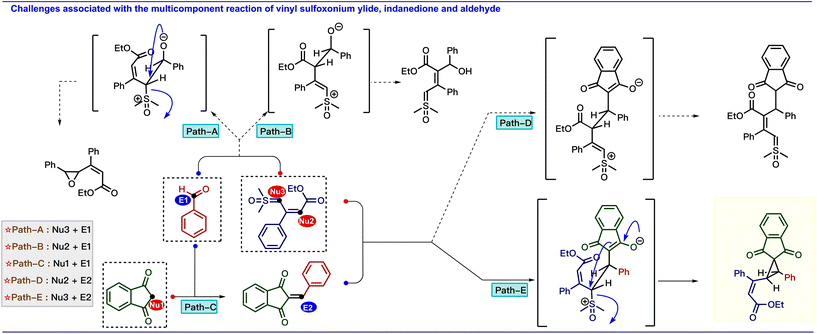 | ||
| Scheme 2 Possible reaction pathways between the vinyl sulfoxonium ylide, aldehyde, and indane 1,3-dione. | ||
We commenced our study by employing vinyl sulfoxonium ylide (1a), benzaldehyde (2a), and 1,3-indandione (3) as model substrates. The optimal conditions to afford substituted cyclopropane (4a) was determined to be the use of 20 mol% of (L)-proline as the catalyst and methanol as the solvent at room temperature for 30 min, resulting in an 87% isolated yield with >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Table 1, entry 1). Despite the reaction affording excellent yield and diastereoselectivity, we did not observe any enantioselectivity. Next, we investigated the reaction under different reaction conditions. A comparison of the changes introduced in each reaction parameter is summarized in Table 1. Lewis acids, such as In(OTf)3 and Sc(OTf)3, gave poor yields of cyclopropane 4a (entries 2 and 3). The reaction gave a low yield of product in the presence of a base, such as NEt3 (entry 4). Using a Brønsted acid, such as HBF4·OEt2, led to the formation of the desired product 4a in 85% yield (entry 5). However, using other Brønsted acids, such as acetic acid, failed to produce the product. Instead, the reaction resulted in the insertion of vinyl sulfoxonium ylides into the O–H bond of the carboxylic acid (entry 6).18 The reaction afforded a low yield of product 4a when TMSOTf was used as the catalyst. We noticed that protic solvents are critical for this transformation because other commonly used solvents in the laboratory, such as DMSO, acetonitrile, and EtOAc, resulted in a low yield of 4a (entries 8–10). Interestingly the reaction failed to afford the product in the absence of the proline catalyst (entry 11).
1 dr (Table 1, entry 1). Despite the reaction affording excellent yield and diastereoselectivity, we did not observe any enantioselectivity. Next, we investigated the reaction under different reaction conditions. A comparison of the changes introduced in each reaction parameter is summarized in Table 1. Lewis acids, such as In(OTf)3 and Sc(OTf)3, gave poor yields of cyclopropane 4a (entries 2 and 3). The reaction gave a low yield of product in the presence of a base, such as NEt3 (entry 4). Using a Brønsted acid, such as HBF4·OEt2, led to the formation of the desired product 4a in 85% yield (entry 5). However, using other Brønsted acids, such as acetic acid, failed to produce the product. Instead, the reaction resulted in the insertion of vinyl sulfoxonium ylides into the O–H bond of the carboxylic acid (entry 6).18 The reaction afforded a low yield of product 4a when TMSOTf was used as the catalyst. We noticed that protic solvents are critical for this transformation because other commonly used solvents in the laboratory, such as DMSO, acetonitrile, and EtOAc, resulted in a low yield of 4a (entries 8–10). Interestingly the reaction failed to afford the product in the absence of the proline catalyst (entry 11).
| Entry | Variation from the standard conditions | Yieldb (dr)c |
|---|---|---|
| a Standard conditions: 1a (0.14 mmol), 2a (0.11 mmol), 3 (0.10 mmol), catalyst (20 mol%), solvent (0.1 M), 30 minutes. b The yield was determined by 1H NMR analysis of crude products using dibromomethane as the internal standard. c Determined by crude NMR. d Isolated yield of the major diastereomer. | ||
| 1 | None | 92 (87)d (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 2 | Using In(OTf)3 instead of proline | 15 (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 3 | Using Sc(OTf)3 instead of proline | 22 (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 4 | Using NEt3 instead of proline | 34% (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 5 | Using HBF4·OEt2 instead of proline | 85 (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 6 | Using AcOH instead of proline | ND |
| 7 | Using TMSOTf instead of proline | 45 (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 8 | Using DMSO instead of MeOH | 80 (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 9 | Using EtOAc instead of MeOH | 65 (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 10 | Using MeCN instead of MeOH | 78 (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 11 | Without a proline catalyst | ND |
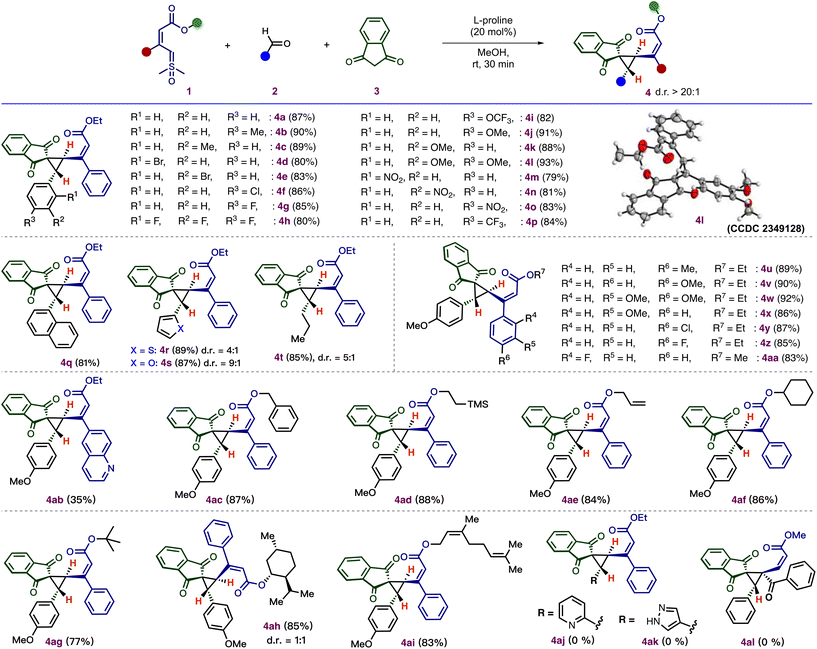
Following these optimization studies, we explored the scope of this cyclopropanation using a variety of aldehydes. As shown in Scheme 3, an electronically diverse set of aryl aldehydes was tolerated. Aryl aldehydes containing an alkyl group, such as methyl, on the para and meta positions, underwent smooth annulation to afford the desired cyclopropanes 4b–4c in good yield (89–90%) and excellent diastereoselectivity (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Also, the aryl aldehydes containing halogen substituents, such as –Br, –F, –Cl at ortho, meta, and para positions, gave the desired vinylcyclopropane 4d–4h in good to excellent yield (80–86%) with excellent diastereoselectivity (dr > 20
1). Also, the aryl aldehydes containing halogen substituents, such as –Br, –F, –Cl at ortho, meta, and para positions, gave the desired vinylcyclopropane 4d–4h in good to excellent yield (80–86%) with excellent diastereoselectivity (dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Electron-rich substituents, such as –OMe and –OCF3, and electron-withdrawing substituents, such as –NO2 and –CF3, at the ortho, meta, and para positions were compatible with this cyclopropanation and afforded corresponding annulated products 4i–4p in 79–93% yields. The relative stereochemistry of the two stereogenic centers of 4l was confirmed by X-ray crystallography (CCDC 2349128†), and the relative configurations of the other cyclopropane products were assigned by analogy. Furthermore, a poly-aromatic aldehyde, 2-naphthaldehyde, was tested in this reaction, which successfully produced 4q in 81% yield without the loss of selectivity. The efficiency of this method was further demonstrated by heteroaryl aldehydes, and the transformation was successful with thiophene-2-carboxaldehyde and furfural to afford the products, 4r (89% yield, 4
1). Electron-rich substituents, such as –OMe and –OCF3, and electron-withdrawing substituents, such as –NO2 and –CF3, at the ortho, meta, and para positions were compatible with this cyclopropanation and afforded corresponding annulated products 4i–4p in 79–93% yields. The relative stereochemistry of the two stereogenic centers of 4l was confirmed by X-ray crystallography (CCDC 2349128†), and the relative configurations of the other cyclopropane products were assigned by analogy. Furthermore, a poly-aromatic aldehyde, 2-naphthaldehyde, was tested in this reaction, which successfully produced 4q in 81% yield without the loss of selectivity. The efficiency of this method was further demonstrated by heteroaryl aldehydes, and the transformation was successful with thiophene-2-carboxaldehyde and furfural to afford the products, 4r (89% yield, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) and 4s (87% yield, 9
1 dr) and 4s (87% yield, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). The reaction is also successful with aliphatic aldehydes, giving the corresponding cyclopropane 4t in 85% with 5
1 dr). The reaction is also successful with aliphatic aldehydes, giving the corresponding cyclopropane 4t in 85% with 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr These observations indicate that the diastereoselectivity is significantly influenced by the aldehyde framework.
1 dr These observations indicate that the diastereoselectivity is significantly influenced by the aldehyde framework.
Next, we evaluated the scope of substituted vinyl sulfoxonium ylides. Initially, a variation of the aryl ring of the ylide was examined. The reaction demonstrated a high tolerance towards various substituents, such as –Cl, –F, –Me, and –OMe, located at the ortho-, meta-, and para-positions of the phenyl ring and afforded the corresponding cyclopropane products 4u–4aa in excellent yield (83–92%) with excellent diastereoselectivity (dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The ylide-containing heteroaromatic ring, such as quinoline, underwent annulations to afford 4ab in 35% yield with excellent diastereoselectivity (dr > 20
1). The ylide-containing heteroaromatic ring, such as quinoline, underwent annulations to afford 4ab in 35% yield with excellent diastereoselectivity (dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Subsequently, we assessed the variation in the ester moiety of the ylide. The reaction conditions were suitable for a variety of ester functional groups of ylides, including benzyl (4ac), ethyl trimethyl silyl (4ad), allyl esters (4ae), and cyclohexyl esters (4af). Next, the annulation was carried out in the presence of a ylide containing tertiary butyl ester, resulting in the formation of the annulated product 4ag in 77% yield with excellent dr, which shows that there is no steric influence of the ester group of the ylide on diastereoselectivity. Additionally, the incorporation of natural products, such as L-menthol and nerol, as ester moieties effectively afforded the corresponding cyclopropanes 4ah (85%, dr = 1
1). Subsequently, we assessed the variation in the ester moiety of the ylide. The reaction conditions were suitable for a variety of ester functional groups of ylides, including benzyl (4ac), ethyl trimethyl silyl (4ad), allyl esters (4ae), and cyclohexyl esters (4af). Next, the annulation was carried out in the presence of a ylide containing tertiary butyl ester, resulting in the formation of the annulated product 4ag in 77% yield with excellent dr, which shows that there is no steric influence of the ester group of the ylide on diastereoselectivity. Additionally, the incorporation of natural products, such as L-menthol and nerol, as ester moieties effectively afforded the corresponding cyclopropanes 4ah (85%, dr = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 4ai (83%, dr > 20
1) and 4ai (83%, dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Surprisingly, the reaction failed to afford the vinylcyclopropanes 4aj and 4ak using picolinaldehyde and 1H-pyrazole-4-carbaldehyde. When the reaction was carried out with α,α′-disubstituted vinyl sulfoxonium ylide, the reaction failed to produce the desired vinyl cyclopropane 4al under optimized conditions.
1). Surprisingly, the reaction failed to afford the vinylcyclopropanes 4aj and 4ak using picolinaldehyde and 1H-pyrazole-4-carbaldehyde. When the reaction was carried out with α,α′-disubstituted vinyl sulfoxonium ylide, the reaction failed to produce the desired vinyl cyclopropane 4al under optimized conditions.
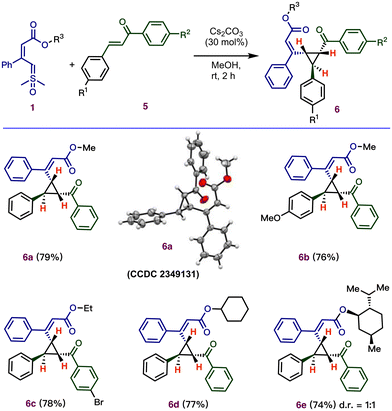
To expand the scope of the cyclopropanation, we performed the reaction using easily accessible chalcone derivatives, which can provide cyclopropanes with three stereogenic centers (Scheme 4). Surprisingly, the optimized acid-catalyzed conditions for the multicomponent reaction (Table 1) were unable to provide the desired cyclopropanes. However, using cesium carbonate as a base (30 mol%) afforded the desired product 6a in good yield and excellent stereoselectivity.19 The relative stereochemistry of the three stereogenic centers of 6a was confirmed by X-ray crystallography (CCDC 2349131†). Next, under these base-catalyzed conditions, various substituted chalcones with different electronic properties were investigated, which successfully produced cyclopropanes, 6b–6c, in good yield (76–78%) with excellent selectivity (dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
Further efforts were made to develop the scope of ylides as substrates by varying their ester moiety. The ylide containing ester functional groups, including cyclohexyl (6d) and L-menthol (6e), gave the desired cyclopropanes.
Notably, the base-catalyzed annulation (Scheme 4) reported here requires a longer reaction time compared to acid-catalyzed cyclopropanation (Scheme 3).
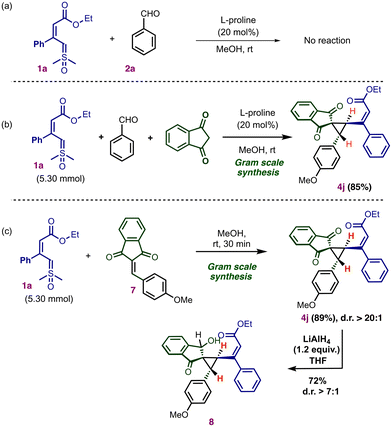
To investigate the mechanism for the multicomponent reaction, we treated the vinyl sulfoxonium ylide 1a with benzaldehyde in the presence of the L-proline catalyst. No conversion of the ylide was observed (Scheme 5a).
The present multicomponent reaction using 5.3 mmol of vinyl sulfoxonium ylide successfully afforded the vinylcyclopropane 4j in an 85% yield (Scheme 5b). Next, we performed gram-scale synthesis using vinyl sulfoxonium ylide 1a with arylidene 7 in the absence of the catalyst (Scheme 5c). The reaction afforded 89% of 4j without loss of selectivity. This observation indicates that the proline catalyst is necessary for the generation of the arylidene intermediate from indane 1,3-dione with aldehyde, but it is not required for (2 + 1) annulation.20 To further explore the utility of the vinyl cyclopropane, we performed selective ketone reduction in cyclopropane 4j using LiAlH4, which gave the product 8 in 72% yield without cleavage of the strained cyclopropane ring (Scheme 5c).
Next, we focused on the investigation of the regioselective electrophilic addition of vinyl sulfoxonium ylide on arylidenes. In our previous reports, vinyl sulfoxonium ylide underwent Michael addition selectively from the γ-position with benzoquinone and propiolate (as shown in Scheme 1b). In contrast to the previous reports, the present Michael addition selectively undergoes at the α-position of the ylide.
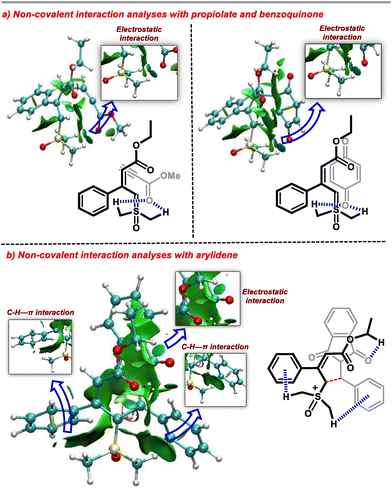
To rationalize this observation, we carried out a non-covalent interaction (NCI) analysis of the transition states using NCIplot software (Fig. 1). Interestingly, we observed that the methyl groups of the DMSO direct the regioselectivity of the ylide for the incoming electrophile. In the case of benzoquinone and propiolate as Michael acceptors, the methyl groups of DMSO exhibit a weak electrostatic attraction with the carbonyl group of electrophiles. Thus, the γ-carbon of the ylide remains in the vicinity of the β-carbon of the electrophile. This interaction favours the Michael addition from the γ-carbon of the ylide. We explored a similar reactivity with the arylidene generated from indane-1,3-dione and aldehyde. In the case of arylidene, the stable transition state possesses predominant C–H–π-interaction21 and electrostatic attraction between the two reactants. These interactions bring the β-carbon of electrophile close to the α-carbon of the ylide. Thus, in the present case, Michael addition occurs from the α-carbon of the ylide. In addition, we observed that the transition state corresponding to the γ-addition of ylide to the arylidine was less stable by 4.8 kcal mol−1 than the α-addition transition state (see the ESI for details†).
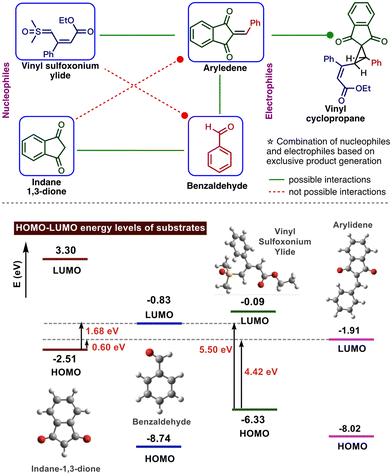
Then we turned our attention to investigating the selective reaction between nucleophilic reactants, such as sulfoxonium ylide and indane 1,3-dione, with electrophilic partners, such as aldehyde and in situ generated arylidene. In the present one-pot reaction, indane 1,3-dione selectively reacts with aldehyde, whereas in situ generated arylidene undergoes Michael addition with vinyl sulfoxonium ylide.
To rationalize this observation, we analyzed the HOMO–LUMO energy gap between the nucleophiles and electrophiles (Fig. 2). The results revealed that the energy gap between the HOMO of indane-1,3-dione and LUMO of aldehyde is less (1.68 eV) than the HOMO of ylide and LUMO of aldehyde (5.50 eV). This favours the path-C (Scheme 2) for the generation of arylidene intermediate. This arylidene can undergo Michael addition with either vinyl sulfoxonium ylide or indane-1,3-dione. Although the HOMO–LUMO energy gap is small between indane-1,3-dione and arylidene, experimentally, the vinyl sulfoxonium ylide attack generated cyclopropane. This could be due to the complete consumption of indane-1,3-dione for the generation of arylidene or the retro Michael reaction between indane-1,3-dione and arylidene.22
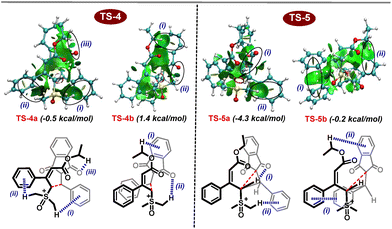
Further, DFT studies were performed at the M06-2X|def2-TZVPP|SMD (methanol) |M06-2X|def2-SVP|SMD (methanol) level of theory to provide more details on the mechanism of the present multi-component reaction (Scheme 6). Based on experimental observations and the HOMO–LUMO energy gap between nucleophiles and electrophiles (Fig. 2), it appears that proline-catalyzed Knoevenagel condensation initially takes place between aldehydes and indane 1,3-diones. Based on the previous observations, proline may act as a facilitator for condensation under protic solvents, rather than as a reactant to generate iminium ion species.23 Therefore, in the presence of a proline catalyst, indane-1,3-dione reacts with aldehyde to produce int-1. Further, the counter anion present in int-1 abstracts the acidic proton to generate the enolate ion int-2viaTS-2. Later, dehydration of int-2 generates the arylidene int-3. The reaction energy profile shows that dehydration is the rate-limiting step with a 15.2 kcal mol−1 energy barrier. As this is the slowest step in the reaction, indane-1,3-dione will be completely consumed to generate int-1 before generating arylidene, ruling out the possibility of indane-1,3-dione reaction with the arylidene (as previously explained with HOMO–LUMO energy gap in Fig. 2). Arylidene int-3 as Michael acceptor reacts with vinyl sulfoxonium ylide in four possible ways: (i) Si-face of ylide to Re-face of int-3 (Si–Re), (ii) Si-face of ylide to Si-face of int-3 (Si–Si), (iii) Re-face of ylide to Re-face of int-3 (Re–Re), (iv) Re-face of ylide to Si-face of int-3 (Re–Si). Among these possibilities, interaction (i) and (ii) generate diastereomers, whereas (iii) and (iv) generate enantiomers of (i) and (ii). The transition state corresponding to the Si–Re face attack (TS-4a) is better stabilized than the Si–Si face attack (TS-4b) by 1.9 kcal mol−1. Thus, the int-3 preferably generates the int-4a. Finally, the cyclopropanation of int-4 occurs through the elimination of the DMSO molecule through TS-5a or TS-5b to generate trans- or cis-cyclopropanes, respectively. Here, the TS-5a is more stabilized than TS-5b by 4.1 kcal mol−1. Thus, the reaction proceeds through TS-5a to deliver trans-cyclopropane as an exclusive product.
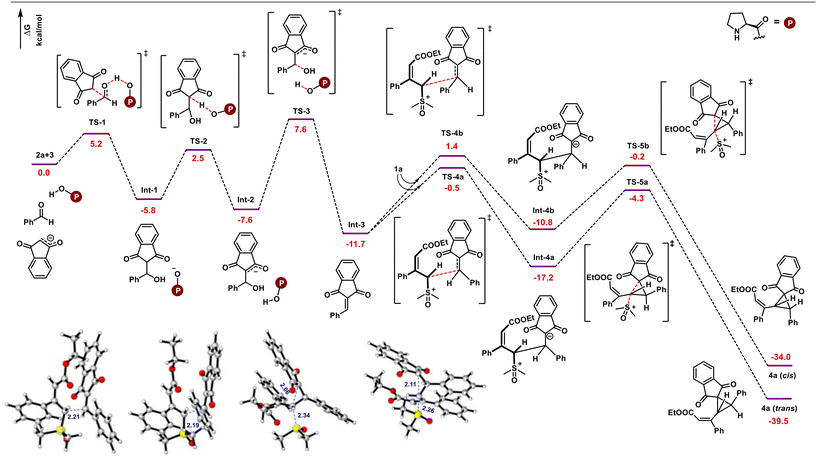 | ||
| Scheme 6 DFT computed the energy profile (in kcal mol−1) for the multicomponent reaction between vinyl sulfoxonium ylide, indane 1,3-dione, and aldehyde. | ||
We performed an NCI analysis to further understand the stability of TS-4a and TS-5a over TS-4b and TS-5b. As highlighted in Fig. 3, TS-4a is stabilized due to the presence of (i) C–H–π-interaction between the methyl group of DMSO and phenyl group of aldehydes, (ii) C–H–π-interaction between the methyl group of DMSO and phenyl group of ylide, and (iii) weak electrostatic attraction between oxygen of indane-1,3-dione with alkyl group of ylide. On the other hand, TS-4b possesses (i) the C–H–π-interaction between the alkyl group of ester and phenyl group of indane-1,3-dione and (ii) the electrostatic attraction between hydrogen of DMSO and oxygen of indane-1,3-dione. Similarly, in the case of TS-5a, (i) an electrostatic interaction develops between the C–H of ylidic carbon and oxygen of indane-1,3-dione during the cyclopropanation step and (ii) a C–H–π-interaction develops between the methyl group of DMSO and phenyl group of aldehydes. On the other hand, TS-5b possesses (i) a weak π–π stacking as a major stabilizing factor and (ii) C–H–π-interaction between the ester group of ylide and phenyl group of indane-1,3-dione. Thus, TS-4a and TS-5a are more stable than TS-4b and TS-5b.
Conclusions
In summary, we have developed stereoselective cyclopropanation using vinyl sulfoxonium ylides with arylidenes under acidic and basic conditions. The present multicomponent reaction selectively proceeds because of the difference in the nucleophilic reactivity of vinyl sulfoxonium ylide and indane 1,3-dione with electrophilic precursors, such as aldehydes and in situ generated arylidenes. This method affords various complex cyclopropane scaffolds in good yields with excellent diastereoselectivity under mild reaction conditions. The origin of excellent regioselectivity (addition at α- vs. γ-position of vinyl sulfoxonium ylide) and diastereoselectivity (cis/trans selectivity of cyclopropanation) was revealed by theoretical studies on non-covalent interactions.General procedure for the synthesis of spiro[cyclopropane-1,2′-indene]-1′,3′-dione
1,3-Indanedione 3 (0.30 mmol, 1 equiv.) and aldehyde 2 (0.33 mmol, 1.1 equiv.) solutions in methanol (0.1 M) were added to an oven-dried, 25 mL round bottom flask equipped with a magnetic stir bar. A catalytic amount of L-proline (7 mg, 20 mol%) and ylide 1 (0.42 mmol, 1.4 equiv.) was added to the stirring solution. After stirring for 30 minutes at room temperature, spiro[cyclopropane-1,2′-indene]-1′,3′-dione precipitated at the bottom of the round bottom flask. The precipitate was filtered and washed with methanol to yield a chromatographically and spectroscopically pure product.General procedure for the synthesis of (2-benzoyl-3-phenylcyclopropyl)-3-phenylacrylate
α,β-Unsaturated ketone 5 (0.30 mmol, 1 equiv.) solution in methanol (0.1 M) was added to an oven-dried, Ar-purged 25 mL round bottom flask equipped with a magnetic stir bar under argon atmosphere. A catalytic amount of cesium carbonate (28 mg, 30 mol%) and ylide 1 (0.40 mmol, 1.4 equiv.) were added to the stirring solution. After stirring for 2 hours at room temperature, the reaction mixture was concentrated under reduced pressure. The residue was purified using flash column chromatography (Biotage flash chromatography gradient purification: EtOAc/n-hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to give the product 6.
5) to give the product 6.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; yellow solid.
1; yellow solid.
IR (neat, cm −1 ): 3432, 3059, 2982, 1710, 1620, 1495, 1446, 1337, 1287, 1219, 1174, 1095, 1039, 875, 697, 617, 562, 534, 483. 1H NMR (500 MHz, Chloroform-D)δ 7.86 (s, 2H), 7.77–7.67 (m, 2H), 7.55–7.48 (m, 2H), 7.42–7.31 (m, 7H), 7.31–7.27 (m, 1H), 6.21 (s, 1H), 4.29 (d, J = 9.6 Hz, 1H), 3.84 (q, J = 7.1 Hz, 2H), 3.40 (d, J = 9.6 Hz, 1H), 0.99 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 195.5, 194.2, 164.9, 152.6, 142.0, 141.2, 140.3, 134.3, 134.0, 133.1, 128.7, 128.6, 128.1, 127.9, 127.4, 127.3, 122.3, 121.7, 121.7, 59.7, 50.5, 45.6, 37.9, 13.5. HRMS (ESI) m/z: [M + Na]+ calculated for C28H22O4Na, 445.1410; found 445.1412.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; yellow solid.
1; yellow solid.
IR (neat, cm −1 ): 3432, 2925, 2857, 1710, 1620, 1518, 1448, 1339, 1289, 1220, 1174, 1092, 1039, 876, 813, 774, 744, 699, 614, 562, 535, 485. 1H NMR (500 MHz, Chloroform-D)δ 7.87 (d, J = 1.9 Hz, 2H), 7.72 (dd, J = 6.0, 2.9 Hz, 2H), 7.54 (t, J = 3.6 Hz, 2H), 7.40–7.33 (m, 3H), 7.25–7.19 (m, 3H), 7.12 (s, 1H), 6.22 (s, 1H), 4.31 (d, J = 9.6 Hz, 1H), 3.85 (q, J = 7.1 Hz, 2H), 3.40 (d, J = 9.6 Hz, 1H), 2.35 (s, 3H), 1.00 (t, J = 7.1 Hz, 3H).13C NMR (101 MHz, Chloroform-D)δ 196.0, 194.7, 165.3, 153.1, 142.4, 141.6, 140.7, 137.5, 134.6, 134.3, 130.4, 129.0, 128.9, 128.9, 128.4, 127.6, 122.6, 122.1, 122.0, 60.0, 51.0, 46.0, 38.2, 21.2, 13.9. HRMS (ESI) m/z: [M + Na]+ calculated for C29H24O4Na, 459.1567; found 459.1570.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; white solid.
1; white solid.
IR (neat, cm −1 ): 3431, 2925, 2857, 1711, 1620, 1518, 1448, 1339, 1289, 1220, 1174, 1092, 1039, 876, 813, 774, 744, 700, 614, 562, 535, 485. 1H NMR (400 MHz, Chloroform-D)δ 7.86 (s, 2H), 7.74–7.67 (m, 2H), 7.53 (s, 2H), 7.36 (dd, J = 5.1, 2.0 Hz, 3H), 7.24–7.16 (m, 3H), 7.11 (s, 1H), 6.22 (s, 1H), 4.31 (d, J = 9.6 Hz, 1H), 3.85 (q, J = 7.1, 2H), 3.39 (d, J = 9.6 Hz, 1H), 2.34 (s, 3H), 0.99 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 196.0, 194.7, 165.4, 153.2, 142.5, 141.7, 140.7, 137.8, 134.7, 134.3, 133.4, 129.8, 129.0, 128.7, 128.5, 128.2, 127.7, 126.1, 122.7, 122.1, 122.1, 60.1, 51.0, 46.1, 38.3, 21.4, 13.9. HRMS (ESI) m/z: [M + Na]+ calculated for C29H24O4Na, 459.1567; found 459.1564.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; yellow solid.
1; yellow solid.
IR (neat, cm −1 ): 3432, 3060, 2982, 1710, 1622, 1598, 1446, 1407, 1341, 1278, 1220, 1175, 1094, 1039, 880, 767, 736, 698, 622, 567, 496. 1H NMR (500 MHz, Chloroform-D)δ 7.87 (s, 2H), 7.78–7.69 (m, 2H), 7.54–7.45 (m, 2H), 7.42–7.27 (m, 7H), 6.21 (d, J = 2.1 Hz, 1H), 4.24 (d, J = 9.5 Hz, 1H), 3.84 (q, J = 7.1 Hz, 2H), 3.33 (d, J = 9.5 Hz, 1H), 0.99 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 195.6, 194.6, 165.4, 152.6, 142.4, 141.7, 140.6, 135.9, 134.9, 134.6, 132.1, 131.0, 129.8, 129.1, 128.6, 127.7, 127.7, 122.9, 122.4, 122.3, 122.3, 60.2, 50.6, 44.8, 38.2, 14.0. HRMS (ESI) m/z: [M + Na]+ calculated for C28H21O4BrNa, 523.0515; found 523.0515.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; orange solid.
1; orange solid.
IR (neat, cm −1 ): 3432, 3060, 2982, 1710, 1621, 1598, 1446, 1407, 1341, 1278, 1218, 1175, 1094, 1039, 880, 767, 736, 698, 624, 567, 496. 1H NMR (500 MHz, Chloroform-D)δ 7.90–7.81 (m, 2H), 7.76–7.67 (m, 2H), 7.53–7.47 (m, 2H), 7.41–7.31 (m, 5H), 7.02 (t, J = 8.5 Hz, 2H), 6.21 (s, 1H), 4.25 (d, J = 9.6 Hz, 1H), 3.84 (q, J = 7.1 Hz, 2H), 3.36 (d, J = 9.6 Hz, 1H), 0.99 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 195.1, 194.6, 165.4, 152.1, 147.4, 142.3, 141.7, 141.3, 140.5, 135.1, 134.9, 130.0, 129.2, 128.7, 128.6, 127.6, 123.9, 123.5, 122.9, 122.5, 122.4, 60.3, 50.6, 44.2, 38.5, 14.0. HRMS (ESI) m/z: [M + Na]+ calculated for C28H21O4BrNa, 523.0515; found 523.0513.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; yellow solid.
1; yellow solid.
IR (neat, cm −1 ): 3427, 3060, 2987, 1708, 1605, 1494, 1446, 1400, 1339, 1294, 1214, 1165, 1091, 1036, 1016, 872, 817, 775, 735, 701, 622, 563, 530, 483. 1H NMR (500 MHz, Chloroform-D)δ 7.87 (s, 2H), 7.78–7.69 (m, 2H), 7.54–7.45 (m, 2H), 7.42–7.27 (m, 7H), 6.21 (d, J = 2.1 Hz, 1H), 4.24 (d, J = 9.5 Hz, 1H), 3.84 (q, J = 7.1 Hz, 2H), 3.33 (d, J = 9.5 Hz, 1H), 0.99 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 195.7, 194.8, 165.4, 152.7, 142.4, 141.7, 140.7, 134.9, 134.6, 133.7, 132.1, 130.4, 129.1, 128.6, 128.6, 127.6, 122.8, 122.3, 60.2, 50.7, 45.1, 38.4, 14.0. HRMS (ESI) m/z: [M + Na]+ calculated for C28H21O4ClNa, 479.1021; found 479.1018.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; orange solid.
1; orange solid.
IR (neat, cm −1 ): 3431, 3059, 2981, 2928, 2856, 1710, 1604, 1514, 1448, 1340, 1288, 1223, 1175, 1088, 1038, 878, 828, 738, 699, 612, 537. 1H NMR (500 MHz, Chloroform-D)δ 7.90–7.81 (m, 2H), 7.76–7.67 (m, 2H), 7.53–7.47 (m, 2H), 7.41–7.31 (m, 5H), 7.02 (t, J = 8.5 Hz, 2H), 6.21 (s, 1H), 4.25 (d, J = 9.6 Hz, 1H), 3.84 (q, J = 7.1 Hz, 2H), 3.36 (d, J = 9.6 Hz, 1H), 0.99 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 195.9, 194.9, 165.4, 161.1, 142.1 (d, J = 68.2 Hz), 140.7, 134.7 (d, J = 32.6 Hz), 130.7 (d, J = 8.1 Hz), 129.1, 128.6, 127.7, 122.8, 122.3, 115.4 (d, J = 21.7 Hz), 60.2, 50.8, 45.2, 38.6, 14.0. 19F NMR (376 MHz, Chloroform-D)δ −113.85. HRMS (ESI) m/z: [M + Na]+ calculated for C28H21O4FNa, 463.1316; found 463.1313.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; white solid.
1; white solid.
IR (neat, cm −1 ): 3436, 3059, 2981, 2928, 2856, 1711, 1604, 1514, 1448, 1340, 1288, 1223, 1175, 1088, 1038, 878, 828, 738, 699, 612, 537. 1H NMR (500 MHz, Chloroform-D)δ 7.85 (s, 2H), 7.79–7.72 (m, 2H), 7.50 (dd, J = 7.5, 2.0 Hz, 2H), 7.38 (dd, J = 5.0, 2.5 Hz, 3H), 7.31 (q, J = 8.1, 7.3 Hz, 1H), 7.08–6.99 (m, 1H), 6.21 (s, 1H), 4.12 (d, J = 9.3 Hz, 1H), 3.85 (q, J = 7.1 Hz, 2H), 3.28 (d, J = 9.3 Hz, 1H), 1.00 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 195.4, 194.8, 165.5, 152.4, 142.0 (d, J = 31.1 Hz), 140.7, 134.9 (d, J = 23.9 Hz), 129.2, 128.7, 127.7, 123.0, 122.9 (d, J = 22.6 Hz), 60.4, 48.9, 37.9, 29.8, 14.1. 19F NMR (376 MHz, Chloroform-D)δ −133.98 (dt, J = 21.9, 7.7 Hz), −135.88 (dt, J = 20.8, 7.4 Hz), −160.07 (td, J = 20.6, 7.0 Hz). HRMS (ESI) m/z: [M + Na]+ calculated for C28H19O4F3Na, 499.1128; found 499.1125.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; white solid.
1; white solid.
IR (neat, cm −1 ): 3436, 2927, 1711, 1621, 1447, 1409, 1326, 1291, 1220, 1171, 1123, 1069, 1037, 877, 772, 744, 698, 609, 531. 1H NMR (500 MHz, Chloroform-D)δ 7.87 (s, 2H), 7.78–7.69 (m, 2H), 7.54–7.45 (m, 2H), 7.42–7.27 (m, 7H), 6.21 (d, J = 2.1 Hz, 1H), 4.24 (d, J = 9.5 Hz, 1H), 3.84 (q, J = 7.1 Hz, 2H), 3.33 (d, J = 9.5 Hz, 1H), 0.99 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, Chloroform-D)δ 195.6, 194.8, 165.5, 152.6, 142.1 (d, J = 85.0 Hz), 140.7, 134.9 (d, J = 39.6 Hz), 129.5, 129.2, 128.2 (d, J = 126.5 Hz), 125.4 (q, J = 3.7 Hz), 123.0, 122.5 (d, J = 9.2 Hz).60.3, 50.7, 44.9, 38.5, 14.1. 19F NMR (376 MHz, Chloroform-D)δ −62.42. HRMS (ESI) m/z: [M + Na]+ calculated for C29H21O5F3Na, 529.1233; found 529.1229.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; white solid.
1; white solid.
IR (neat, cm −1 ): 3423, 3051, 2981, 2960, 2935, 2838, 1710, 1614, 1519, 1445, 1331, 1274, 1254, 1226, 1177, 1087, 1034, 869, 824, 775, 747, 696, 565, 541, 489. 1H NMR (500 MHz, Chloroform-D)δ1H NMR (500 MHz, Chloroform-D) δ 7.90–7.81 (m, 2H), 7.76–7.68 (m, 2H), 7.54–7.49 (m, 2H), 7.39–7.29 (m, 5H), 6.90–6.84 (m, 2H), 6.21 (d, J = 2.2 Hz, 1H), 4.26 (d, J = 9.6 Hz, 1H), 3.84 (q, J = 7.1 Hz, 2H), 3.80 (s, 3H), 3.35 (d, J = 9.6 Hz, 1H), 0.99 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 195.9, 194.8, 165.3, 159.1, 153.1, 142.3, 141.6, 140.7, 134.6, 134.3, 130.1, 128.9, 128.4, 127.6, 125.3, 122.6, 122.0, 122.0, 113.7, 60.0, 55.1, 51.1, 46.0, 38.4, 13.9. HRMS (ESI) m/z: [M + Na]+ calculated for C29H24O5Na, 475.1516; found 475.1517.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; white solid.
1; white solid.
IR (neat, cm −1 ): 3423, 3051, 2981, 2960, 2935, 2838, 1710, 1614, 1519, 1445, 1331, 1274, 1254, 1226, 1177, 1087, 1034, 869, 824, 775, 747, 696, 565, 541, 489. 1H NMR (500 MHz, Chloroform-D)δ 7.89–7.81 (m, 2H), 7.74–7.69 (m, 2H), 7.54–7.49 (m, 2H), 7.36 (dd, J = 5.2, 2.0 Hz, 3H), 7.33–7.30 (m, 2H), 6.87 (d, J = 8.7 Hz, 2H), 6.20 (d, J = 2.2 Hz, 1H), 4.25 (d, J = 9.7 Hz, 1H), 3.84 (q, J = 7.1 Hz, 2H), 3.80 (s, 3H), 3.35 (d, J = 9.7 Hz, 1H), 0.99 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 195.9, 194.8, 165.3, 159.1, 153.1, 142.3, 141.6, 140.7, 134.6, 134.3, 130.1, 128.9, 128.4, 127.6, 125.3, 122.6, 122.0, 122.0, 113.7, 60.0, 55.1, 51.1, 46.0, 38.4, 13.9. HRMS (ESI) m/z: [M + Na]+ calculated for C29H24O5Na, 475.1516; found 475.1516.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; white solid.
1; white solid.
IR (neat, cm −1 ): 3420, 3071, 3009, 2932, 2841, 1710, 1623, 1596, 1523, 1465, 1444, 1334, 1305, 1272, 1238, 1214, 1177, 1091, 1040, 873, 809, 768, 740, 703, 634, 500, 634, 463. 1H NMR (500 MHz, Chloroform-D)δ 7.87–7.78 (m, 2H), 7.74–7.64 (m, 2H), 7.52–7.47 (m, 2H), 7.33 (d, J = 1.5 Hz, 3H), 6.98 (d, J = 8.3 Hz, 1H), 6.91 (s, 1H), 6.86–6.81 (m, 1H), 6.20 (s, 1H), 4.26 (d, J = 9.6 Hz, 1H), 3.86–3.79 (m, 8H), 3.33 (d, J = 9.6 Hz, 1H), 0.96 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 195.9, 194.8, 165.3, 153.0, 148.6, 148.5, 142.4, 141.6, 140.6, 134.6, 134.3, 128.9, 128.4, 127.6, 125.8, 122.6, 122.0, 121.9, 121.3, 112.0, 110.6, 60.0, 55.8, 55.7, 51.3, 46.5, 38.5, 13.9. HRMS (ESI) m/z: [M + Na]+ calculated for C30H26O6Na, 505.1622; found 505.1624.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; yellow solid.
1; yellow solid.
IR (neat, cm −1 ): 3434, 3061, 2984, 1712, 1601, 1521, 1448, 1404, 1344, 1288, 1220, 1178, 1090, 1037, 920, 878, 852, 770, 735, 699, 631, 562, 533. 1H NMR (500 MHz, Chloroform-D)δ 8.18 (d, J = 8.7 Hz, 2H), 7.86 (s, 2H), 7.76 (dd, J = 5.9, 2.6 Hz, 2H), 7.58 (d, J = 8.5 Hz, 2H), 7.47 (dd, J = 6.7, 3.0 Hz, 2H), 7.38–7.32 (m, 3H), 6.22 (s, 1H), 4.29 (d, J = 9.5 Hz, 1H), 3.83 (q, J = 7.1 Hz, 2H), 3.41 (d, J = 9.5 Hz, 1H), 0.99 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 195.1, 194.6, 165.4, 152.1, 147.4, 142.3, 141.7, 141.3, 140.5, 135.1, 134.9, 130.0, 129.2, 128.7, 128.6, 127.6, 123.9, 123.5, 122.9, 122.5, 122.4, 60.3, 50.6, 44.2, 38.5, 14.0. HRMS (ESI) m/z: [M + Na]+ calculated for C28H21O6NNa, 490.1261; found 490.1263.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; yellow solid.
1; yellow solid.
IR (neat, cm −1 ): 3431, 3061, 2984, 1711, 1601, 1521, 1448, 1404, 1344, 1288, 1220, 1178, 1090, 1037, 920, 878, 852, 770, 735, 699, 631, 562, 533. 1H NMR (500 MHz, Chloroform-D)δ 7.90–7.81 (m, 2H), 7.76–7.67 (m, 2H), 7.53–7.47 (m, 2H), 7.41–7.31 (m, 5H), 7.02 (t, J = 8.5 Hz, 2H), 6.21 (s, 1H), 4.25 (d, J = 9.6 Hz, 1H), 3.84 (q, J = 7.1 Hz, 2H), 3.36 (d, J = 9.6 Hz, 1H), 0.99 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, Chloroform-D)δ 195.2, 194.8, 165.4, 152.2, 148.3, 142.3, 141.8, 140.6, 135.9, 135.2, 135.1, 134.9, 129.3, 129.2, 128.7, 127.6, 124.2, 123.0, 123.0, 122.6, 122.5, 60.3, 50.3, 44.1, 38.5, 14.0. HRMS (ESI) m/z: [M + Na]+ calculated for C28H21O6NNa, 490.1261; found 490.1260.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; orange solid.
1; orange solid.
IR (neat, cm −1 ): 3432, 3061, 2984, 1710, 1601, 1521, 1448, 1404, 1344, 1288, 1220, 1178, 1090, 1037, 920, 878, 852, 770, 735, 699, 631, 562, 533. 1H NMR (500 MHz, Chloroform-D)δ 8.18 (d, J = 8.7 Hz, 2H), 7.86 (s, 2H), 7.76 (dd, J = 5.9, 2.6 Hz, 2H), 7.58 (d, J = 8.5 Hz, 2H), 7.47 (dd, J = 6.7, 3.0 Hz, 2H), 7.38–7.32 (m, 3H), 6.22 (s, 1H), 4.29 (d, J = 9.5 Hz, 1H), 3.83 (q, J = 7.1 Hz, 2H), 3.41 (d, J = 9.5 Hz, 1H), 0.99 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 195.1, 194.6, 165.4, 152.1, 147.4, 142.3, 141.7, 141.3, 140.5, 135.1, 134.9, 130.0, 129.2, 128.7, 128.6, 127.6, 123.9, 123.5, 122.9, 122.5, 122.4, 60.3, 50.6, 44.2, 38.5, 14.0. HRMS (ESI) m/z: [M + Na]+ calculated for C28H21O6NNa, 490.1261; found 490.1253.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; white solid.
1; white solid.
IR (neat, cm −1 ): 3435, 2927, 1711, 1621, 1447, 1409, 1326, 1291, 1220, 1171, 1123, 1069, 1037, 877, 772, 744, 698, 609, 531. 1H NMR (500 MHz, Chloroform-D)δ 7.88 (s, 2H), 7.79–7.70 (m, 2H), 7.59 (s, 2H), 7.54 (s, 4H), 7.38–7.34 (m, 3H), 6.23 (s, 1H), 4.30 (d, J = 9.5 Hz, 1H), 3.84 (q, J = 7.1 Hz, 2H), 3.40 (d, J = 9.5 Hz, 1H), 0.99 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 195.5, 194.7, 165.4, 152.5, 142.0 (d, J = 67.9 Hz),140.6, 134.8 (d, J = 31.1 Hz), 129.5, 129.2, 128.1 (d, J = 100.7 Hz), 125.3 (q, J = 3.9 Hz), 122.9, 122.4 (d, J = 2.9 Hz),60.2, 50.6, 44.8, 38.4, 14.0. 19F NMR (376 MHz, Chloroform-D)δ −62.35. HRMS (ESI) m/z: [M + Na]+ calculated for C29H21O4F3Na, 513.1284; found 513.1290.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; white solid.
1; white solid.
IR (neat, cm −1 ): 3434, 2926, 1709, 1620, 1446, 1337, 1293, 1271, 1174, 1097, 1028, 867, 777, 738, 698.1H NMR (500 MHz, Chloroform-D)δ 7.93 (d, J = 7.5 Hz, 1H), 7.88–7.82 (m, 2H), 7.82–7.79 (m, 1H), 7.71 (d, J = 18.0 Hz, 3H), 7.62 (d, J = 7.3 Hz, 3H), 7.43–7.39 (m, 1H), 7.38–7.28 (m, 4H), 7.18–7.14 (m, 1H), 6.27 (s, 1H), 4.45 (d, J = 9.5 Hz, 1H), 3.84 (q, J = 7.1 Hz, 2H), 3.76 (d, J = 9.5 Hz, 1H), 0.99 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 196.6, 194.2, 165.6, 153.3, 142.2, 140.9, 134.9, 134.4, 133.6, 130.1, 129.1, 129.0, 128.9, 128.7, 127.8, 126.7, 126.6, 125.8, 125.5, 122.9, 122.8, 122.7, 122.3, 60.3, 50.6, 43.5, 38.4, 14.1. HRMS (ESI) m/z: [M + Na]+ calculated for C32H24O4Na, 495.1567; found 495.1568.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; orange solid.
1; orange solid.
IR (neat, cm −1 ): 3432, 3073, 2926, 1709, 1620, 1446, 1337, 1293, 1271, 1174, 1097, 1028, 867, 777, 738, 698. Major product:1H NMR (500 MHz, Chloroform-D)δ 7.94–7.90 (m, 1H), 7.86–7.83 (m, 1H), 7.76–7.73 (m, 2H), 7.53 (d, J = 1.7 Hz, 2H), 7.40–7.37 (m, 2H), 7.25–7.22 (m, 2H), 7.14 (d, J = 3.5 Hz, 1H), 7.01 (dd, J = 5.2, 3.5 Hz, 1H), 6.19 (s, 1H), 4.18 (d, J = 9.3 Hz, 1H), 3.84 (q, J = 7.1 Hz, 2H), 3.41 (d, J = 9.3 Hz, 1H), 0.99 (t, J = 7.1 Hz, 3H).13C NMR (101 MHz, Chloroform-D)δ 195.4, 194.0, 165.2, 152.4, 142.3, 141.4, 140.6, 136.7, 134.8, 134.4, 129.0, 128.5, 127.6, 127.4, 127.0, 125.5, 122.8, 122.1, 121.9, 60.1, 50.7, 40.3, 39.8, 13.9. HRMS (ESI) m/z: [M + Na]+ calculated for C26H20O4SNa, 451.0975; found 451.0970.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; white solid.
1; white solid.
IR (neat, cm −1 ): 3436, 2928, 1710, 1621, 1446, 1341, 1293, 1175, 1094, 1037, 879, 775, 739, 698, 598, 525. 1H NMR (500 MHz, Chloroform-D)δ 7.77 (s, 1H), 7.69 (d, J = 1.6 Hz, 1H), 7.63–7.55 (m, 2H), 7.41–7.37 (m, 2H), 7.26–7.18 (m, 4H), 6.35 (d, J = 3.3 Hz, 1H), 6.28–6.21 (m, 1H), 6.05 (d, J = 2.2 Hz, 1H), 3.99 (d, J = 9.3 Hz, 1H), 3.68 (q, J = 7.1 Hz, 2H), 3.08 (d, J = 9.3 Hz, 1H), 0.83 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 195.4, 194.2, 165.4, 152.2, 148.4, 142.7, 142.4, 141.8, 140.8, 134.9, 134.5, 129.1, 128.6, 127.7, 122.9, 122.3, 122.2, 110.8, 109.4, 60.2, 49.4, 37.6, 37.5, 14.0. HRMS (ESI) m/z: [M + Na]+ calculated for C26H20O5Na, 435.1203; found 435.1198.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; white solid.
1; white solid.
IR (neat, cm −1 ): 3428, 3059, 2961, 2928, 2870, 1709, 1620, 1449, 1368, 1334, 1276, 1209, 1173, 1103, 1038, 878, 769, 746, 699, 612, 562, 531. Major product:1H NMR (500 MHz, Chloroform-D)δ 7.98 (d, J = 7.4 Hz, 1H), 7.82–7.71 (m, 3H), 7.47–7.42 (m, 2H), 7.37 (dd, J = 11.6, 7.2 Hz, 3H), 6.07 (s, 1H), 3.83–3.77 (m, 2H), 3.60 (d, J = 7.0 Hz, 1H), 2.16 (dt, J = 9.1, 4.5 Hz, 1H), 2.07–1.99 (m, 1H), 1.95 (d, J = 5.8 Hz, 1H), 1.25 (s, 2H), 0.96 (t, J = 7.1 Hz, 3H), 0.80 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, Chloroform-D)δ 197.3, 197.1, 165.4, 153.8, 142.4, 141.6, 141.4, 134.6, 134.4, 128.8, 128.4, 128.4, 127.8, 126.9, 122.5, 122.1, 122.0, 60.1, 48.4, 43.5, 42.3, 28.6, 22.6, 14.0, 13.7. HRMS (ESI) m/z: [M + Na]+ calculated for C25H24O4Na, 411.1567; found 411.1566.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; yellow solid.
1; yellow solid.
IR (neat, cm −1 ): 3429, 2981, 2933, 2838, 1709, 1611, 1515, 1457, 1336, 1290, 1251, 1221, 1171, 1091, 1038, 876, 822, 740, 620, 579, 528, 501. 1H NMR (400 MHz, Chloroform-D)δ 7.90–7.83 (m, 2H), 7.73–7.67 (m, 2H), 7.43 (d, J = 8.2 Hz, 2H), 7.37–7.29 (m, 2H), 7.19–7.13 (m, 2H), 6.88 (d, J = 8.7 Hz, 2H), 6.21 (d, J = 2.1 Hz, 1H), 4.26 (d, J = 9.6, 2.1 Hz, 1H), 3.83 (q, J = 7.1 Hz, 2H), 3.77 (s, 3H), 3.38 (d, J = 9.6 Hz, 1H), 2.34 (s, 3H), 0.98 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 195.9, 194.8, 165.4, 159.1, 153.0, 142.3, 141.6, 139.0, 137.8, 134.6, 134.2, 130.1, 129.1, 127.5, 125.4, 122.5, 122.0, 121.1, 113.6, 59.9, 55.1, 51.1, 46.0, 38.4, 21.2, 13.9. HRMS (ESI) m/z: [M + Na]+ calculated for C30H26O5Na, 489.1672; found 489.1674.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; yellow solid.
1; yellow solid.
IR (neat, cm −1 ): 3429, 2960, 2935, 2838, 1708, 1604, 1514, 1460, 1336, 1292, 1251, 1169, 1091, 1037, 874, 833, 739, 535. 1H NMR (400 MHz, Chloroform-D)δ 7.89–7.79 (m, 2H), 7.71 (dd, J = 6.0, 2.8 Hz, 2H), 7.46 (d, J = 8.7 Hz, 2H), 7.33 (d, J = 8.6 Hz, 2H), 6.88 (dd, J = 8.8, 2.8 Hz, 4H), 6.16 (s, 1H), 4.22 (d, J = 9.6 Hz, 1H), 3.83–3.74 (m, 8H), 3.38 (d, J = 9.6 Hz, 1H), 0.97 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 196.1, 195.1, 165.6, 160.4, 159.3, 152.7, 142.5, 141.8, 134.7, 134.3, 133.2, 130.2, 129.1, 125.5, 122.7, 122.2, 120.4, 114.0, 113.8, 60.1, 55.4, 55.3, 51.2, 46.2, 38.6, 14.0. HRMS (ESI) m/z: [M + Na]+ calculated for C30H26O6Na, 505.1622; found 505.1621.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; yellow solid.
1; yellow solid.
IR (neat, cm −1 ): 3427, 3048, 2943, 2841, 2037, 1706, 1601, 1518, 1463, 1338, 1259, 1224, 1161, 1031, 844, 812, 750, 561, 539. 1H NMR (500 MHz, Chloroform-D)δ 7.88–7.80 (m, 2H), 7.73–7.66 (m, 2H), 7.11 (d, J = 2.1 Hz, 1H), 7.01 (d, J = 2.2 Hz, 1H), 6.89–6.81 (m, 3H), 6.20 (d, J = 2.2 Hz, 1H), 4.18 (d, J = 9.7 Hz, 1H), 3.87 (s, 3H), 3.83–3.79 (m, 2H), 3.78 (s, 3H), 3.71 (s, 3H), 3.36 (d, J = 9.7 Hz, 1H), 0.97 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 195.9, 195.0, 165.4, 159.1, 152.3, 149.8, 148.6, 142.3, 141.6, 134.6, 134.2, 133.3, 130.0, 125.3, 122.5, 122.0, 120.3, 120.1, 113.6, 110.8, 110.6, 59.9, 55.8, 55.6, 55.1, 51.1, 46.3, 38.3, 13.9. HRMS (ESI) m/z: [M + Na]+ calculated for C31H28O7Na, 535.727; found 535.720.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; orange solid.
1; orange solid.
IR (neat, cm −1 ): 3422, 2999, 2967, 2930, 2838, 1709, 1613, 1578, 1515, 1490, 1468, 1338, 1286, 1217, 1182, 1092, 1042, 871, 835, 785, 750, 689, 567, 541, 468. 1H NMR (500 MHz, Chloroform-D)δ 7.88–7.78 (m, 2H), 7.70 (dd, J = 5.9, 2.6 Hz, 2H), 7.31 (d, J = 8.5 Hz, 2H), 7.24 (s, 1H), 7.09 (d, J = 7.7 Hz, 1H), 6.85 (d, J = 8.5 Hz, 3H), 6.22 (s, 1H), 4.21 (d, J = 9.7 Hz, 1H), 3.81 (q, J = 7.1 Hz, 2H), 3.77 (s, 3H), 3.70 (s, 3H), 3.35 (d, J = 9.7 Hz, 1H), 0.97 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 195.9, 194.9, 165.4, 159.5, 159.2, 152.8, 142.4, 142.1, 141.7, 134.7, 134.3, 130.1, 129.5, 125.4, 122.6, 122.1, 122.0, 120.0, 115.1, 113.7, 112.7, 60.1, 55.2, 51.1, 46.1, 38.3, 13.9. HRMS (ESI) m/z: [M + Na]+ calculated for C30H26O6Na, 505.1622; found 505.1619.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; white solid.
1; white solid.
IR (neat, cm −1 ): 3427, 2969, 2841, 1710, 1614, 1517, 1451, 1331, 1285, 1256, 1174, 1092, 1036, 872, 829, 745, 671, 568, 500. 1H NMR (500 MHz, Chloroform-D)δ 7.85 (ddd, J = 8.5, 6.0, 3.7 Hz, 2H), 7.74–7.68 (m, 2H), 7.44 (d, J = 8.6 Hz, 2H), 7.35–7.28 (m, 4H), 6.87 (d, J = 8.6 Hz, 2H), 6.18 (s, 1H), 4.24 (d, J = 9.7 Hz, 1H), 3.84 (q, J = 7.1 Hz, 2H), 3.78 (s, 3H), 3.31 (d, J = 9.7 Hz, 1H), 0.98 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 195.9, 194.6, 165.2, 159.3, 151.9, 142.4, 141.6, 139.2, 135.0, 134.7, 134.4, 130.1, 129.0, 128.7, 125.1, 122.7, 122.3, 122.1, 113.8, 60.2, 55.2, 51.2, 45.9, 38.0, 13.9. HRMS (ESI) m/z: [M + Na]+ calculated for C29H23O5ClNa, 509.1126; found 509.1131.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; orange solid.
1; orange solid.
IR (neat, cm −1 ): 3423, 3066, 2975, 2842, 2051, 1894, 1711, 1607, 1513, 1454, 1329, 1280, 1171, 1092, 1032, 870, 832, 743, 572, 525. 1H NMR (500 MHz, Chloroform-D)δ 7.88–7.81 (m, 2H), 7.75–7.69 (m, 2H), 7.49 (dd, J = 8.4, 5.3 Hz, 2H), 7.31 (d, J = 8.2 Hz, 2H), 7.05 (t, J = 8.4 Hz, 2H), 6.87 (d, J = 8.3 Hz, 2H), 6.16 (s, 1H), 4.23 (d, J = 9.6 Hz, 1H), 3.84 (q, J = 7.1 Hz, 2H), 3.79 (s, 3H), 3.31 (d, J = 9.6 Hz, 1H), 0.99 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 196.0, 194.6, 165.2, 164.3, 161.8, 159.2, 152.0, 141.9 (d, J = 75.9 Hz), 136.8, 136.8, 134.5 (d, J = 36.2 Hz) 130.1, 129.5 (d, J = 8.2 Hz), 125.1, 122.4 (d, J = 58.2 Hz), 121.9, 115.5 (d, J = 21.9 Hz).113.7, 60.1, 55.1, 51.2, 45.9, 38.2, 13.9. 19F NMR (376 MHz, Chloroform-D)δ −111.94. HRMS (ESI) m/z: [M + Na]+ calculated for C29H23O5FNa, 493.1422; found 493.1426.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; orange solid.
1; orange solid.
IR (neat, cm −1 ): 3425, 3066, 2975, 2842, 2051, 1894, 1710, 1607, 1513, 1454, 1329, 1280, 1171, 1092, 1032, 870, 832, 743, 572, 525.1H NMR (400 MHz, Chloroform-D)δ 7.85 (s, 2H), 7.78–7.68 (m, 2H), 7.53–7.45 (m, 2H), 7.36 (td, J = 5.1, 3.2 Hz, 5H), 7.02 (t, J = 8.7 Hz, 2H), 6.22 (s, 1H), 4.23 (d, J = 9.5 Hz, 1H), 3.38 (s, 4H). 13C NMR (101 MHz, Chloroform-D)δ 195.8, 194.9, 165.9, 153.2, 142.12 (d, J = 64.1 Hz), 140.7, 134.73 (d, J = 28.3 Hz), 130.73 (d, J = 8.2 Hz),129.2, 127.7, 122.56 (d, J = 46.2 Hz), 121.9, 115.38 (d, J = 21.4 Hz), 51.4, 50.8, 45.3, 38.7. NMR (376 MHz, Chloroform-D)δ −113.88. HRMS (ESI) m/z: [M + Na]+ calculated for C28H21O5FNa, 479.1265; found 479.1262.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; green solid.
1; green solid.
IR (neat, cm −1 ): 3427, 3054, 2951, 2840, 1710, 1612, 1516, 1458, 1436, 1291, 1251, 1168, 1121, 1090, 1038, 888, 838, 800, 738, 615, 566, 530, 480. 1H NMR (400 MHz, Chloroform-D)δ 8.94–8.86 (m, 1H), 8.15–8.05 (m, 2H), 7.98 (s, 1H), 7.86 (dq, J = 4.9, 1.8, 1.4 Hz, 1H), 7.84–7.80 (m, 2H), 7.74–7.68 (m, 2H), 7.41–7.36 (m, 1H), 7.35–7.30 (m, 2H), 6.87 (d, J = 9.0 Hz, 2H), 6.33 (s, 1H), 4.31 (d, J = 9.7 Hz, 1H), 3.77 (s, 3H), 3.39 (s, 3H), 3.34 (d, J = 9.7 Hz, 1H). 13C NMR (101 MHz, Chloroform-D)δ 196.0, 194.7, 165.6, 159.3, 152.7, 151.1, 148.2, 142.4, 141.7, 138.9, 136.7, 134.8, 134.5, 130.1, 129.7, 128.8, 128.0, 127.2, 125.1, 122.8, 122.7, 122.2, 121.7, 113.8, 55.2, 51.5, 51.3, 46.1, 38.3. HRMS (ESI) m/z: [M + Na]+ calculated for C31H23O5NNa, 512.1468; found 512.1466.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; yellow solid.
1; yellow solid.
IR (neat, cm −1 ): 3428, 3060, 3033, 2935, 2838, 1709, 1613, 1516, 1452, 1341, 1291, 1251, 1160, 1089, 1036, 877, 826, 739, 699, 621, 564, 539. 1H NMR (400 MHz, Chloroform-D)δ 7.87 (s, 1H), 7.81 (s, 1H), 7.72 (s, 2H), 7.52 (d, J = 2.3 Hz, 2H), 7.38–7.34 (m, 4H), 7.33 (s, 1H), 7.25 (d, J = 2.3 Hz, 3H), 7.14 (d, J = 2.4 Hz, 2H), 6.89 (d, J = 8.7 Hz, 2H), 6.28 (s, 1H), 4.87 (s, 2H), 4.30 (d, J = 9.6 Hz, 1H), 3.80 (s, 3H), 3.38 (d, J = 9.6 Hz, 1H). 13C NMR (101 MHz, Chloroform-D)δ 196.0, 194.9, 165.1, 159.2, 154.3, 142.4, 141.7, 140.7, 135.9, 134.7, 134.3, 130.2, 129.1, 128.5, 128.4, 128.2, 128.1, 127.7, 125.4, 122.7, 122.2, 121.4, 113.8, 65.8, 55.2, 51.3, 46.1, 38.4. HRMS (ESI) m/z: [M + Na]+ calculated for C34H26O5Na, 537.1672; found 537.1670.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; orange solid.
1; orange solid.
IR (neat, cm −1 ): 3431, 2954, 2840, 1711, 1686, 1585, 1589, 1514, 1435, 1347, 1315, 1270, 1250, 1163, 1082, 1034, 862, 837, 775, 737, 698, 564, 532. 1H NMR (500 MHz, Chloroform-D)δ 7.86 (d, J = 1.2 Hz, 2H), 7.77–7.66 (m, 2H), 7.51 (dd, J = 7.1, 2.5 Hz, 2H), 7.40–7.28 (m, 5H), 6.87 (d, J = 8.7 Hz, 2H), 6.19 (d, J = 2.1 Hz, 1H), 4.26 (d, J = 9.6, Hz, 1H), 3.93–3.84 (m, 2H), 3.80 (s, 3H), 3.35 (d, J = 9.6 Hz, 1H), 0.75 (ddd, J = 13.7, 11.1, 5.9 Hz, 1H), 0.62 (ddd, J = 13.7, 11.1, 5.9 Hz, 1H), −0.08 (s, 9H). 13C NMR (101 MHz, Chloroform-D)δ 195.8, 194.8, 165.5, 159.1, 152.8, 142.4, 141.7, 140.8, 137.2, 135.0, 134.5, 134.2, 130.1, 128.9, 128.4, 127.6, 125.3, 123.0, 123.0, 122.6, 122.2, 122.0, 114.3, 113.7, 62.2, 55.1, 51.1, 46.0, 38.5, 17.0, −1.6. HRMS (ESI) m/z: [M + Na]+ calculated for C32H32O5SiNa, 547.1911; found 547.1909.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; white solid.
1; white solid.
IR (neat, cm −1 ): 3429, 2954, 2840, 1710, 1686, 1585, 1589, 1514, 1435, 1347, 1315, 1270, 1250, 1163, 1082, 1034, 862, 837, 775, 737, 698, 564, 532. 1H NMR (400 MHz, Chloroform-D)δ 7.91–7.80 (m, 2H), 7.71 (d, J = 8.5 Hz, 2H), 7.58–7.50 (m, 2H), 7.34 (dd, J = 12.0, 7.9 Hz, 5H), 6.88 (s, 2H), 6.26 (d, J = 2.1 Hz, 1H), 5.64 (dd, J = 17.2, 10.4 Hz, 1H), 5.14–4.97 (m, 2H), 4.35–4.26 (m, 3H), 3.78 (d, J = 0.9 Hz, 3H), 3.37 (d, J = 9.6 Hz, 1H). 13C NMR (101 MHz, Chloroform-D)δ 195.9, 194.7, 164.9, 159.2, 153.8, 142.4, 141.6, 140.7, 134.6, 134.3, 131.9, 130.1, 129.0, 128.5, 127.6, 125.3, 122.6, 122.1, 121.5, 118.0, 113.7, 64.7, 55.1, 51.1, 46.0, 38.3. HRMS (ESI) m/z: [M + Na]+ calculated for C30H24O5Na, 487.1516; found 487.1511.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; orange solid.
1; orange solid.
IR (neat, cm −1 ): 3429, 3058, 2936, 2858, 1708, 1614, 1516, 1450, 1346, 1290, 1251, 1176, 1121, 1090, 1038, 968, 928, 882, 824, 738, 699, 621, 564, 539. 1H NMR (400 MHz, Chloroform-D)δ 7.89–7.82 (m, 2H), 7.70 (d, J = 3.2 Hz, 2H), 7.56–7.51 (m, 2H), 7.37–7.31 (m, 5H), 6.87 (d, J = 8.7 Hz, 2H), 6.22 (d, J = 2.1 Hz, 1H), 4.45 (dt, J = 9.1, 4.9 Hz, 1H), 4.31 (d, J = 9.7 Hz, 1H), 3.78 (s, 3H), 3.36 (d, J = 9.7 Hz, 1H), 1.72–1.58 (m, 2H), 1.54–1.48 (m, 1H), 1.42 (s, 1H), 1.30 (s, 1H), 1.27–1.20 (m, 2H), 1.14–1.05 (m, 3H). 13C NMR (101 MHz, Chloroform-D)δ 196.0, 194.8, 164.8, 159.1, 152.7, 142.4, 141.6, 140.8, 134.6, 134.1, 130.1, 128.9, 128.4, 127.6, 125.4, 122.7, 122.4, 122.0, 113.7, 72.2, 55.1, 51.1, 46.0, 38.5, 31.4, 31.0, 25.2, 23.7, 23.6. HRMS (ESI) m/z: [M + Na]+ calculated for C33H30O5Na, 529.1985; found 529.1980.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; white solid.
1; white solid.
IR (neat, cm −1 ): 3430, 3058, 2975, 2930, 1708, 1613, 1517, 1454, 1348, 1251, 1220, 1150, 1090, 1037, 869, 824, 775, 739, 698, 564, 539. 1H NMR (400 MHz, Chloroform-D)δ 7.84 (d, J = 10.6 Hz, 2H), 7.69 (d, J = 1.9 Hz, 2H), 7.52 (s, 2H), 7.34 (d, J = 3.1 Hz, 5H), 6.88 (dd, J = 8.4, 1.4 Hz, 2H), 6.16 (s, 1H), 4.29 (d, J = 9.7 Hz, 1H), 3.80 (s, 3H), 3.32 (d, J = 9.7 Hz, 1H), 1.10 (s, 9H). 13C NMR (101 MHz, Chloroform-D)δ 196.3, 195.2, 164.9, 159.2, 151.8, 142.7, 141.7, 140.9, 134.6, 134.1, 130.2, 128.9, 128.6, 127.7, 125.7, 123.5, 122.7, 122.1, 113.8, 80.1, 55.3, 51.0, 45.9, 38.9, 27.7. HRMS (ESI) m/z: [M + Na]+ calculated for C31H28O5Na, 503.1829; found 503.1823.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; white solid.
1; white solid.
IR (neat, cm −1 ): 3428, 2952, 2869, 1709, 1621, 1517, 1453, 1350, 1331, 1290, 1251, 1221, 1173, 1089, 1040, 988, 882, 826, 775, 746, 697, 620, 564, 538. 1H NMR (500 MHz, Chloroform-D)δ7.82 (s, 2H), 7.77–7.67 (m, 2H), 7.53 (dt, J = 5.3, 2.8 Hz, 2H), 7.39–7.28 (m, 5H), 6.87 (d, J = 8.2 Hz, 2H), 6.20 (d, J = 20.1 Hz, 1H), 4.49–4.35 (m, 1H), 4.32 (d, J = 9.6 Hz, 1H), 3.80 (s, 3H), 3.33 (d, J = 9.6 Hz, 1H), 1.83–1.70 (m, 1H), 1.61–1.49 (m, 3H), 1.37–1.12 (m, 3H), 0.89–0.58 (m, 10H), 0.23 (d, J = 6.9 Hz, 1H). 13C NMR (101 MHz, Chloroform-D)δ 196.2, 195.0, 165.1, 165.0, 159.3, 152.9, 141.8, 140.9, 134.7, 134.3, 130.3, 129.1, 128.6, 127.9, 127.7, 125.6, 122.9, 122.3, 122.1, 113.8, 74.1, 55.3, 51.1, 46.7, 46.3, 40.0, 38.7, 34.3, 31.2, 26.4, 23.6, 22.1, 20.7, 16.5. HRMS (ESI) m/z: [M + Na]+ calculated for C37H38O5Na, 585.2611; found 585.2605.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; orange solid.
1; orange solid.
IR (neat, cm −1 ): 3432, 2927, 1709, 1614, 1516, 1447, 1335, 1252, 1222, 1165, 1089, 1037, 965, 879, 827, 749, 699, 618, 563, 539. 1H NMR (400 MHz, Chloroform-D)δ 7.86 (d, J = 1.8 Hz, 2H), 7.72 (s, 2H), 7.52 (s, 2H), 7.35 (s, 5H), 6.91–6.84 (m, 2H), 6.23 (s, 1H), 5.13–4.99 (m, 2H), 4.35–4.24 (m, 3H), 3.79 (s, 3H), 3.37 (d, J = 9.8 Hz, 1H), 1.97 (s, 3H), 1.66 (d, J = 4.9 Hz, 6H), 1.57 (s, 3H), 1.33–1.28 (m, 1H). 13C NMR (101 MHz, Chloroform-D)δ 196.0, 194.9, 165.4, 159.2, 153.3, 142.5, 142.2, 142.1, 141.8, 140.8, 134.6, 134.3, 132.0, 130.2, 129.0, 128.5, 127.7, 125.5, 123.7, 122.7, 122.1, 122.0, 119.1, 113.8, 60.8, 55.2, 51.2, 46.1, 38.4, 32.0, 26.6, 25.7, 23.5, 17.7. HRMS (ESI) m/z: [M + Na]+ calculated for C37H36O5Na, 583.2455; found 583.2459.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), yield: 92 mg (79%); dr > 20
5), yield: 92 mg (79%); dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; white solid.
1; white solid.
IR (neat, cm −1 ): 3406, 3061, 3029, 2944, 1711, 1660, 1609, 1492, 1446, 1429, 1374, 1337, 1275, 1226, 1170, 1078, 999, 914, 862, 772, 743, 697, 582, 529, 483. 1H NMR (500 MHz, Chloroform-D)δ 7.98 (d, J = 7.5 Hz, 2H), 7.44 (dd, J = 20.8, 7.7 Hz, 6H), 7.37–7.31 (m, 5H), 7.23 (d, J = 6.5 Hz, 2H), 6.11 (s, 1H), 3.67 (dd, J = 9.2, 5.3 Hz, 1H), 3.52 (ddd, J = 9.4, 7.7, 1.9 Hz, 1H), 3.46 (s, 3H), 2.82 (dd, J = 7.8, 5.3 Hz, 1H). 13C NMR (101 MHz, Chloroform-D)δ 196.4, 166.4, 156.3, 141.9, 139.7, 138.3, 132.7, 128.8, 128.8, 128.5, 128.4, 128.3, 127.8, 126.9, 126.4, 120.4, 51.1, 38.9, 35.9, 35.6. HRMS (ESI) m/z: [M + Na]+ calculated for C26H22O3Na, 405.1461; found 405.1459.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), yield: 96 mg (76%); dr > 20
5), yield: 96 mg (76%); dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; white solid.
1; white solid.
IR (neat, cm −1 ): 3404, 3061, 3029, 2944, 1705, 1660, 1609, 1492, 1446, 1429, 1374, 1337, 1275, 1226, 1170, 1078, 999, 914, 862, 772, 743, 697, 582, 529, 483. 1H NMR (500 MHz, Chloroform-D)δ 8.07–8.02 (m, 2H), 7.52 (s, 3H), 7.45 (s, 2H), 7.38 (s, 3H), 7.21 (s, 2H), 6.94–6.91 (m, 2H), 6.17 (d, J = 2.1 Hz, 1H), 3.81 (s, 3H), 3.70–3.65 (m, 1H), 3.54 (t, J = 8.5 Hz, 1H), 3.50 (s, 3H), 2.88–2.83 (m, 1H). 13C NMR (126 MHz, Chloroform-D)δ 196.2, 166.2, 158.6, 156.2, 141.8, 138.3, 132.5, 131.6, 128.6, 128.3, 128.3, 128.2, 127.7, 127.4, 120.2, 114.1, 55.2, 50.9, 38.8, 35.3, 35.2. HRMS (ESI) m/z: [M + Na]+ calculated for C27H24O4Na, 435.1567; found 435.1571.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), yield: 110 mg (78%); dr > 20
5), yield: 110 mg (78%); dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; orange solid.
1; orange solid.
IR (neat, cm −1 ): 3394, 3090, 3059, 2983, 1706, 1662, 1611, 1582, 1490, 1434, 1374, 1271, 1222, 1174, 1070, 1022, 860, 809, 766, 697, 621, 582, 533, 481, 582. 1H NMR (500 MHz, Chloroform-D)δ 7.86 (d, J = 8.4 Hz, 2H), 7.54 (d, J = 8.4 Hz, 2H), 7.43 (d, J = 1.9 Hz, 2H), 7.38–7.30 (m, 5H), 7.28–7.19 (m, 3H), 6.10 (s, 1H), 3.98 (d, J = 7.1 Hz, 1H), 3.91 (d, J = 7.1 Hz, 1H), 3.61 (dd, J = 9.2, 5.3 Hz, 1H), 3.53 (t, J = 7.8 Hz, 1H), 2.85–2.78 (m, 1H), 1.09 (t, J = 7.2 Hz, 3H). 13C NMR (126 MHz, Chloroform-D)δ 195.2, 165.8, 155.8, 141.7, 139.5, 137.0, 131.6, 130.0, 128.8, 128.7, 128.3, 127.8, 127.7, 127.0, 126.3, 120.9, 59.9, 38.8, 35.9, 35.7, 14.1. HRMS (ESI) m/z: [M + Na]+ calculated for C27H23O3BrNa, 497.0723; found 497.0720.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), yield: 120 mg (77%); dr > 20
5), yield: 120 mg (77%); dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; white solid.
1; white solid.
IR (neat, cm −1 ): 3441, 3060, 3029, 2933, 2857, 1706, 1668, 1614, 1495, 1449, 1371, 1266, 1219, 1173, 1078, 1018, 872, 750, 697, 534. 1H NMR (500 MHz, Chloroform-D)δ 7.91 (d, J = 7.2 Hz, 2H), 7.37 (d, J = 7.1 Hz, 3H), 7.29 (s, 2H), 7.23 (dd, J = 14.1, 7.2 Hz, 5H), 7.14 (s, 3H), 6.00 (s, 1H), 4.45 (s, 1H), 3.60 (ddd, J = 8.4, 5.3, 2.2 Hz, 1H), 3.47 (s, 1H), 2.74 (s, 1H), 1.68 (d, J = 5.7 Hz, 1H), 1.60–1.52 (m, 1H), 1.45–1.32 (m, 2H), 1.17 (s, 3H), 1.04–0.94 (m, 2H), 0.77 (dt, J = 15.1, 7.8 Hz, 1H). 13C NMR (126 MHz, Chloroform-D)δ 196.1, 165.3, 155.6, 141.9, 139.8, 138.1, 132.6, 128.7, 128.6, 128.5, 128.5, 128.5, 128.3, 128.3, 128.3, 128.2, 128.2, 127.7, 126.8, 126.3, 121.4, 72.2, 38.8, 35.8, 35.7, 31.7, 31.3, 25.4, 23.8, 23.7. HRMS (ESI) m/z: [M + Na]+ calculated for C31H30O3Na, 473.2087; found 473.2085.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), yield: 114 mg (74%); dr = 1
5), yield: 114 mg (74%); dr = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; white solid.
1; white solid.
HRMS (ESI) m/z: [M + Na]+ calculated for C35H38O3Na, 529.2713; found 529.2713.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), yield: 100 mg (71%); yellow liquid.
4), yield: 100 mg (71%); yellow liquid.
1 H NMR (400 MHz, Chloroform-D) δ 7.72 (d, J = 0.9 Hz, 1H), 7.62 (d, J = 1.7 Hz, 2H), 7.53–7.49 (m, 2H), 7.38–7.34 (m, 3H), 7.28–7.23 (m, 3H), 6.91 (d, J = 8.8 Hz, 2H), 6.18 (d, J = 2.2 Hz, 1H), 5.14 (d, J = 11.7 Hz, 1H), 4.71 (d, J = 11.7 Hz, 1H), 3.81 (s, 5H), 3.70–3.66 (m, 1H), 2.86 (d, J = 8.5 Hz, 1H), 0.98 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, Chloroform-D)δ 199.5, 166.9, 158.9, 156.9, 155.1, 141.5, 136.2, 134.8, 129.1, 129.0, 128.9, 128.6, 128.5, 128.0, 126.3, 122.3, 120.4, 114.3, 69.3, 60.6, 55.4, 53.8, 40.5, 34.6, 13.9. HRMS (ESI) m/z: [M + Na]+ calculated for C29H24O4Na, 459.1567; found 459.1570.
Author contributions
The manuscript was written with contributions from all authors. All authors have approved the final version of the manuscript. JV designed the project. SD and AP did all the experimental work reported in this manuscript. DKG performed the computation under the supervision of TK.Data availability
Electronic supplementary information (ESI) comprises experimental procedures, characterisation of new compounds, crystallographic data submitted as CCDC 2349128 (compound 4l) and CCDC 2349131 (compound 6a), and computational procedures.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the Science and Engineering Research Board (CRG/2023/000584). SD thanks IIT Delhi for the institutional fellowship. DKG thanks MHRD (Govt. of India) for a PMRF Fellowship. We also thank the IIT Delhi HPC facility for computer resources and CRF for providing the instrumentation facility. We gratefully acknowledge Haribrahma Singh for assisting with X-ray crystallography.References
- (a) H. Chen, Z. Zhang, L. Wang, Z. Huang, F. Gong, X. Li, Y. Chen and J. J. Wu, Medicine, 2020, 99, e23357 CrossRef CAS PubMed; (b) Z. Zhang, S. Wang, X. Tu, X. Peng, Y. Huang, L. Wang, W. Ju, J. Rao, X. Li, D. Zhu, H. Sun and H. Chen, J. Med. Virol., 2020, 92, 2631 CrossRef CAS PubMed.
- (a) A. R. Ravula and S. Yenugu, Crit. Rev. Toxicol., 2021, 51, 117 CrossRef CAS PubMed; (b) C. Hodosan, C. E. Gird, M. V. Ghica, C. E. Dinu-Pirvu, L. Istor, I. S. Barbuica, S. C. Marin, A. Mihalache and L. Popa, Plants, 2023, 12, 4022 CrossRef CAS PubMed; (c) D. B. Lybrand, H. Xu, R. L. Last and E. Pichersky, Trends Plant Sci., 2020, 25, 1240 CrossRef CAS PubMed; (d) R. Faust, Angew. Chem., Int. Ed., 2001, 40, 2251 CrossRef CAS.
- (a) M. Meazza, H. Guo and R. Rios, Org. Biomol. Chem., 2017, 15, 2479 RSC; (b) J. Wang, S. A. Blaszczyk, X. Li and W. Tang, Chem. Rev., 2021, 121, 110 CrossRef CAS PubMed; (c) K. N. Houk, M. Nendel, O. Wiest and J. W. Storer, J. Am. Chem. Soc., 1997, 119, 10545 CrossRef CAS; (d) M. Rubin, M. Rubina and V. Gevorgyan, Chem. Rev., 2007, 107, 3117 CrossRef CAS PubMed; (e) L. Jiao and Z. X. Yu, J. Org. Chem., 2013, 78, 6842 CrossRef CAS PubMed; (f) K. M. Allegre, N. Brennan and J. A. Tunge, Org. Lett., 2018, 20, 4191 CrossRef CAS PubMed; (g) Y. Cohen, A. U. Augustin, L. Levy, P. G. Jones, D. B. Werz and I. Marek, Angew. Chem., Int. Ed., 2021, 60, 11804 CrossRef CAS PubMed.
- J. Bruffaerts, D. Pierrot and I. Marek, Nat. Chem., 2018, 10, 1164 CrossRef CAS PubMed.
- (a) J. Vaitla, A. Bayer and K. H. Hopmann, Angew. Chem., Int. Ed., 2018, 57, 16180 CrossRef CAS; (b) J. Vaitla, A. Bayer and K. H. Hopmann, Synlett, 2019, 1377 CAS; (c) D. S. Davas, D. K. Gopalakrishnan, D. Kumar and J. Vaitla, Org. Lett., 2022, 24, 8359 CrossRef CAS PubMed; (d) D. K. Gopalakrishnan, S. Panigrahi, R. Sen and J. Vaitla, Org. Lett., 2023, 25, 1519 CrossRef CAS PubMed.
- R. Sen, S. Bhardwaj, K. Bar, S. Deshwal and J. Vaitla, Chem. Commun., 2023, 59, 12411 RSC.
- S. Deshwal, D. S. Davas, S. Bhardwaj and J. Vaitla, Org. Lett., 2024, 26, 809 CrossRef CAS PubMed.
- (a) S. Kumar, D. S. Davas, K. Bar, D. K. Gopalakrishnan, D. Kumar, T. Karmakar and J. Vaitla, Org. Lett., 2023, 25, 7906 CrossRef CAS PubMed; (b) D. S. Davas, D. K. Gopalakrishnan, K. Bar, S. Kumar, T. Karmakar and J. Vaitla, Org. Lett., 2023, 25, 8992 CrossRef CAS PubMed; (c) D. S. Davas, D. K. Gopalakrishnan, S. Kumar, Anmol, T. Karmakar and J. Vaitla, JACS Au, 2024, 4, 1073 CrossRef CAS PubMed.
- (a) A. Anand, J. Yenagi, J. Tonannavar and M. V. Kulkarni, Green Chem., 2016, 18, 2201 RSC; (b) Q. Chen, Y. Pan, D. Zhao, W. Yang and J. Zheng, RSC Adv., 2020, 10, 21895 RSC.
- B. Ganem, Acc. Chem. Res., 2009, 42, 463 CrossRef CAS PubMed.
- (a) W. Kirmse, P. van Chiem and P.-G. Henning, Tetrahedron, 1985, 41, 1441 CrossRef CAS; (b) H. M. L. Davies and D. K. Hutcheson, Tetrahedron Lett., 1993, 34, 7243 CrossRef CAS.
- M. J. Gonzalez, J. Gonzalez, L. A. Lopez and R. Vicente, Angew. Chem., Int. Ed., 2015, 54, 12139 CrossRef CAS PubMed.
- (a) H. Jiang, X. Deng, X. Sun, Y. Tang and L. X. Dai, J. Org. Chem., 2005, 70, 10202 CrossRef CAS PubMed; (b) X. L. Sun and Y. Tang, Acc. Chem. Res., 2008, 41, 937 CrossRef CAS PubMed.
- M. Mendel, L. Gnagi, U. Dabranskaya and F. Schoenebeck, Angew. Chem., Int. Ed., 2023, 62, e202211167 CrossRef CAS PubMed.
- A. Delbrassinne, M. Richald, J. Janssens and R. Robiette, Eur. J. Org. Chem., 2021, 2862 CrossRef CAS.
- A. Krief, L. Hevesi, G. Chaboteaux, P. Mathy, M. Sevrin and M. J. De Vos, J. Chem. Soc., Chem. Commun., 1985, 1693 RSC.
- (a) M. J. Johansson, D. J. Gorin, S. T. Staben and F. D. Toste, J. Am. Chem. Soc., 2005, 127, 18002 CrossRef CAS PubMed; (b) B. Herle, P. M. Holstein and A. M. Echavarren, ACS Catal., 2017, 7, 3668 CrossRef CAS PubMed.
- S. Bhardwaj, D. K. Gopalakrishnan, D. Garg and J. Vaitla, JACS Au, 2023, 3, 252 CrossRef CAS PubMed.
- F. Gao and Y. Huang, Adv. Synth. Catal., 2014, 356, 2422 CrossRef CAS.
- (a) Z. Karimi, X. Wei, J. Liu and B. Wang, Tetrahedron Lett., 2024, 137 Search PubMed; (b) P. Suresh, S. P. Kumari, S. M. K. Reddy, S. P. Anthony, S. Thamotharan and S. S. Ganesan, New J. Chem., 2022, 46, 20951 RSC.
- (a) Y. Wang, S.-R. Zhang, Y. Wang, L.-B. Qu and D. Wei, Org. Chem. Front., 2018, 5, 2065 RSC; (b) M. Faltracco, V. Sukowski, M. van Druenen, T. A. Hamlin, F. M. Bickelhaupt and E. Ruijter, J. Org. Chem., 2020, 85, 9566 CrossRef CAS PubMed; (c) B. K. Mishra and R. Venkatnarayan, Theor. Chem. Acc., 2018, 137, 72 Search PubMed; (d) A. Changotra and R. B. Sunoj, Org. Lett., 2016, 18, 3730 CrossRef CAS PubMed; (e) Y. Jin, B. Ramadoss, S. Asako and L. Ilies, Nat. Commun., 2024, 15, 2886 CrossRef CAS PubMed.
- S. Boncel, M. Mączka and K. Z. Walczak, Tetrahedron, 2010, 66, 8450 CrossRef CAS.
- Y. Nobakht and N. Arshadi, J. Mol. Model., 2018, 24, 334 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2349128 and 2349131. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ob00677a |
| This journal is © The Royal Society of Chemistry 2024 |