Solid-state polymer electrolytes in lithium batteries: latest progress and perspective
Jingbo
Mu
a,
Shimin
Liao
a,
Linlin
Shi
b,
Bihai
Su
b,
Feng
Xu
b,
Zengcai
Guo
 *a,
Hailing
Li
a and
Fangfang
Wei
*a
*a,
Hailing
Li
a and
Fangfang
Wei
*a
aKey laboratory of new energy development and energy storage technology of Handan, College of Materials Science and Engineering, Hebei University of Engineering, Handan 056038, People's Republic of China. E-mail: guozengcai@sina.com; fangfangwei@hebeu.edu.cn
bHebei Gellec New Energy Science&Technology Co., Ltd, Handan 057150, People's Republic of China
First published on 26th January 2024
Abstract
The increasing demands for battery performance in the new era of energy necessitate urgent research and development of an energy storage battery that offers high stability and a long service life. Among the various types of batteries available, the all-solid lithium battery emerges as the preferred choice because of its exceptional safety, stability, and sustainability features. The solid electrolyte plays a crucial role in facilitating efficient energy transmission within the structure of the lithium battery. Solid electrolytes based on polymer chemistry can be classified into different categories, such as ether-based, ester-based, nitrile-based, and polyvinylidene fluoride materials. This discussion also covers topics such as ion transport mechanisms, levels of ionic conductivity, techniques for modification, and analysis of cyclic stability specifically for lithium-ion batteries utilizing solid electrolytes. Finally, an outlook on the future research direction of solid-state polymer electrolytes is suggested for commercially large-scale production and application.
1. Introduction
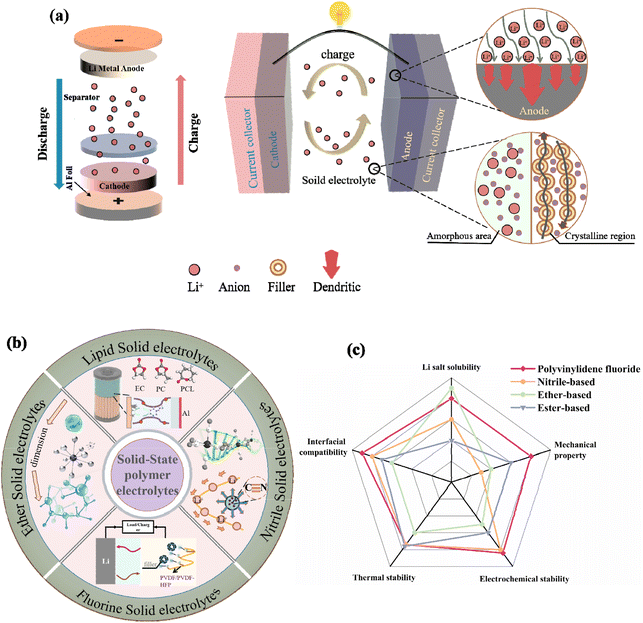
In recent times, significant advances have been made in the conversion and storage of energy batteries. Solid-state electrolytes (SSEs) have emerged as crucial components in the utilization of new-energy trams and everyday electronic devices due to their exceptional energy density, consistent power output, and extended lifespan. Compared to nickel metal hydride batteries, they find superior application prospects within the electric vehicle domain. As research progresses, there is an urgent need to enhance the energy density of rechargeable lithium-ion batteries. The utilization of lithium as an anode brings it closer to attaining its highest theoretical specific energy density (3862 mA h g−1).1–7 Researchers have devised various approaches to improve the performance of lithium ion batteries, such as incorporating modified electrolyte additives,8–12 implementing protective layers for the diaphragm,13,14 and using superior matrix materials.15–17 Some lithium batteries employ organic liquid electrolytes, which are prone to volatilization, leakage, and drying while exhibiting unstable chemical properties that increase the risk of explosions.18–20 Consequently, to ensure the safe utilization of lithium ions, we proposed a gel polymer electrolyte in which a small quantity of liquid organic solvent is added as a plasticizer and immobilized within the gel polymer. The most efficient technique involves substituting solid electrolytes for organic liquid. This method not only improves the energy efficiency of the battery, but also addresses issues related to short life expectancy and instability.21–23 Furthermore, due to their high thermal stability and low flammability characteristics along with their ability to prevent leakage or volatilization incidents, thus eliminating risks associated with fire or explosions; solid-state electrolytes facilitate the development of secure and stable solid-state batteries.24–28The conductivity of solid electrolytes has gradually approached or even exceeded that of a liquid electrolyte. However, the effective transport of lithium ions in batteries is hindered by interface impedance, which arises from element diffusion and interface reactions between the solid electrolyte and the cathode material. To address this problem, common approaches involve applying coatings to the cathode material and modifying the surface of the electrolyte.29,30
In addition, the presence of lithium dendrites is a common issue in lithium-ion batteries. However, the utilization of solid electrolytes can effectively mitigate the risk of short circuits between electrodes, making it more suitable for applications in microelectronics or portable equipment. Integrating the diaphragm with the electrolyte in solid electrolyte technology enhances ionic conductivity.31–33 The selection of electrolyte materials plays a crucial role in determining the operational mechanism of batteries, impacting factors such as specific energy, safety, cycle performance, rate charge–discharge performance, energy storage capabilities, and the cost of lithium batteries.34,35 As a result, future research can focused on the following directions: (1) design of single lithium-ion polymer electrolytes, in which the anions are immobilized by trapping agents or functional groups of polymers.36,37 (2) Decreasing the electrolyte/electrode interfacial resistance and interface impedance in the cathode.38(3) Improving the lithium ion conductivity of SPEs at room temperature or even low temperature.39,40(4) Prepare polymer electrolyte with special structure by template method and self-assembly method to optimize the lithium ion diffusion path.41,42 (5) In conjunction with in situ characterization of the lithium dendrite growth process, optimizing the interface composition and reducing the occurrence of side reactions.43,44
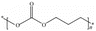
The modification of SSEs made from polymers has achieved remarkable success thus far. This paper initially explores the mechanism behind the growth of lithium dendrites in these electrolytes. Subsequently, it provides a comprehensive overview of various design approaches for SSEs with various chemical structures such as ether, ester, nitrile, and vinylidene fluoride (Fig. 1b). Generally, the use of different types of polymers is related to the stable use of batteries (Fig. 1c). The aim of this research is not only to offer guidance in developing polymer materials for solid electrolytes but also to assist in optimizing the interface reaction within all-solid-state lithium batteries. Ultimately, this optimization will lead to enhanced electrochemical performance and enable practical applications.
2. Interfacial diffusion of lithium ions
The solid electrolyte interface, commonly referred to as the SEI, facilitates the movement of lithium ions within its solid phase. The ion migration mechanism is intricately linked to the structural characteristics of the crystals.45–48 The conductivity is greatly influenced by the different transport mechanisms of lithium ions in different systems. Therefore, understanding the transportation process of lithium ions in solid electrolytes plays a crucial role in designing materials with enhanced performance.51Time correlation functions for the transportation of Li+ ions. The van Hove time correlation function characterizes the likelihood of locating a displaced Li+ ion at time t, with a displacement of r from its initial position. This van Hove function can be divided into two components, namely self and distinct parts, denoted as G(r, t) = Gs(r, t) + Gd(r, t), where the latter exhibits the following forms.
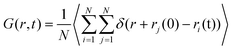 | (1) |
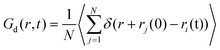 | (2) |
At time t = 0, the van Hove function simplifies the pair correlation function in a stationary state.
| G(r,0) = δ(r) + pg(r) | (3) |
The jump probability is determined by the self-component of the van Hove function, and it is commonly approximated using a Gaussian model as an initial estimation.
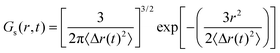 | (4) |
For fluids such as EC, the reliability of this Gaussian behavior can be expected. On the other hand, in the trapping regime of EDC, Gs(r,t) demonstrates a depletion of probability near the limits of the traps (Fig. 2, adapted from ref. 49), r > 〈Δr(t)2〉1/2 with a subsequent redistribution of that probability at shorter and longer distances. The departure from Gaussian behavior can be quantified by assessing the non-Gaussian parameter.50
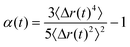 | (5) |
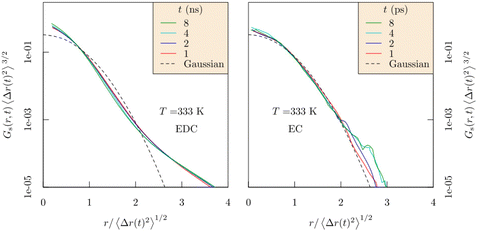 | ||
| Fig. 2 Dimensionally scaled Gs(r,t) for Li+ ions as it depends on displacement for increasing times in EDC (left) and EC (right). Though the Gaussian model (dashed curve) is reliable in EC solvent, the probability is depleted near the trap boundaries, r > 〈Δr(t)2〉1/2, and replaced at shorter and longer distances for EDC. Note that these correlations decay in a few ps for EC, but require ns for EDC ref. 49. Copyright 2017, The Electrochemical Society. | ||
2.1 Interfacial evolution of Li dendrites
Polymer electrolyte is a unique type of SSEs. Polymer electrolytes have limited mechanical strength, making them susceptible to penetration by lithium filaments (see Fig. 1a). The transportation of ions occurs through coordinated Li ions along the polymer chains. The propagation of dendrite in polymer SSEs resembles that in liquid electrolytes. Monroe et al. conducted simulations to study the dendrite formation process in polymer SSE.52 In summary, interfacial kinetics are primarily influenced by surface homogeneity and current density. The introduction of a lithium alloy helps reduce the overpotential for Li deposition and inhibits the penetration of Li dendrites. Even on rough surfaces, high current density is used to guide the deposition of Li to achieve high rate stability of the battery.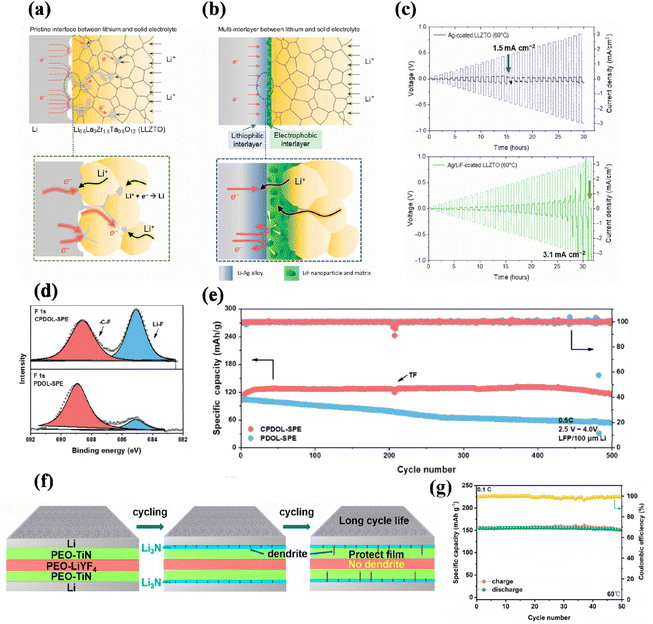 | ||
| Fig. 3 (a) Lithium dendrite formation at the conventional interface between lithium metal and the sintered solid electrolyte. (b) Interfacial design coupling with lithiophilic and electron-blocking interlayers. CCD of (c) Ag-coated and Ag/LiF-coated LLZTO in lithium symmetric cells at 60 °C with increasing current densities ranging from 0.1 to 3.2 mA cm−2 at a step size of 0.1 mA cm−2 (ref. 60). Copyright 2023, Amer Assoc Advancement Science. (d) XPS spectra of F 1s of cycled CPDOL-based SEI and PDOL-based SEI. (e) Cycling performance of LiFePO4/100 μm Li at 0.5 C (ref. 61). Copyright 2020, Wiley-VCH (f) schematic illustration of the cyclic evolution between sandwich-CPE and Li metal and the suppression of Li dendrite processes in a lithium symmetric cell. (g) Cycle performance of the pouch cell at 0.1 C under 60 °C (ref. 62). Copyright 2020, American Chemical Society. | ||
Based on this premise, Hantao Xu et al. aimed to enhance the mechanical strength and ion transport dynamics of solid-state electrolytes (SSEs). To achieve this, they employed a polymer design strategy and cross-linked poly1-3-dioxopentane to create a topological polymer known as CPDOL. This was then used to form an in situ topological polymer interface layer with lithium metal. As depicted in Fig. 3d, the structural system comprises a significant quantity of LiF components that effectively impede electron flow and suppress side reactions at the interface. As depicted in Fig. 3e, the CPLAR-SPE LFP/Li battery exhibits a consistent and enduring cycling performance, achieving a capacity of 116.5 mA h g−1 after undergoing 500 cycles at a rate of 0.5 C. Moreover, it consistently maintains an average Coulomb efficiency exceeding 99.78%, further validating the establishment of a steadfast electron-blocking layer that effectively hinders the proliferation of lithium dendrites.61
To emphasize the significance of interface stability, Long Hu et al. utilized an interlayer design (as depicted in Fig. 3f) to create an ion/electron conductive layer with the aid of fillers. By incorporating conductive TiN particles into a PEO-based solid electrolyte, we successfully obtained a polymer electrolyte with mixed ion/electronic properties. Additionally, an insulating LiYF4 filler was utilized. During the cycling process, the prepared PEO-TiN/PEO-LiYF4/PEO-TiN film formed a LiN interface. Moreover, the presence of PEO-TiN effectively prevented lithium dendrite penetration into the intermediate layer of PEO-LiYF4 by consuming it on its own. This remarkable electrochemical compatibility is demonstrated in Fig. 3g, where a capacity of 150 mA h g−1 was achieved after 50 cycles. These findings highlight the exceptional performance exhibited by soft pack batteries.62
3. Polyether-based solid polymer electrolytes
The presence of the chemical structure of –C–O–C in polyethers allows dissociation and complexation upon the addition of lithium salts, thus promoting relatively stable ion dynamics as a result of the flexible nature of the chain segments.63–68 In the course of polyether-based solid polymer electrolytes (SPEs) development over the years, poly(ethylene oxide) (PEO) has emerged as the most widely used polymer electrolyte. Within this category, polyethylene glycol (PEG) shares a structure similar to PEO but has a lower molecular weight (<20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). Despite its advantageous short-chain molecular structure that enhances molecular mobility, it does compromise the mechanical strength of SPEs. Consequently, PEG is frequently used as a preparatory precursor and modified inorganic filler to enhance its dispersion properties.69,70
000). Despite its advantageous short-chain molecular structure that enhances molecular mobility, it does compromise the mechanical strength of SPEs. Consequently, PEG is frequently used as a preparatory precursor and modified inorganic filler to enhance its dispersion properties.69,70
PEO is the most widely used polymer electrolyte matrix material. PEO exhibits excellent coordination with lithium ions, thus influencing the transportation of cations through its complex chain segment.71,72 At ambient temperature, the utilization of PEO polymer electrolyte results in the presence of three distinct phases: a crystalline phase (pure PEO phase), an amorphous phase (amorphous region) and a salt-rich phase. The conduction of lithium ions exclusively occurs within the amorphous region of the PEO polymer electrolyte, while exhibiting low ionic conductivity within the crystalline region.73–76 Given that the room-temperature conductivity of PEO polymer is considerably low, enhancing its conductivity necessitates an increase in temperature to expand the amorphous region. However, since most lithium-ion batteries operate at room temperature, the substrate must be modified to improve performance.77,78 PEO-based SPEs were first discovered by Wright et al. back in 1973.79 Reinforcing the polymer matrix with nanoscale fillers and nanostructures facilitated the development of non-crystalline sections in PEO, as depicted in (Fig. 1a). The inclusion of such nanostructures resulted in the enlargement of disordered regions within the polymer, thereby enhancing the extent of amorphous areas and ultimately improving ionic conductivity.
3.1 Polyether-based solid electrolyte modified by nanofiller
Compared to conventional inorganic additives, nanomaterials possess a greater specific surface area. By incorporating these nanomaterials into the electrolyte, it is possible to enhance the cell capacity and shorten the pathway for lithium transport, thus facilitating its movement.80–85 Nanofillers can be structured as ordered arrangements of nanoscale particles (0 dimensions), nanofibers (1 dimension), nanosheets (2 dimensions) or skeleton frame structures (3 dimensions) that are tailored for their mechanical and electrochemical properties. Research has indicated that the inclusion of inorganic nanofillers can mitigate the degradation of mechanical properties caused by low crystallinity in polyether-based solid polymer electrolytes. Moreover, these fillers have the potential to reduce interfacial impedance and enhance ionic conductivity, which are crucial factors in the design of high-performance electrolytes for energy storage systems.To prevent polymer crystallization and enhance the performance of solid-state electrolytes, Kondori et al. employed a physical approach by incorporating nanofillers into the electrolyte matrix to lower its glass transition temperature (Tg). They introduced Li10GeP2S12 (LGPS) nanoparticles into a PEO-based electrolyte, followed by chemical bonding using mPEO, which significantly increases the proportion of amorphous regions without phase separation and improves ionic conductivity.86 To better observe the introduction of chemical bonding by modifying functional groups on the surface of nanoparticles, Zhang and colleagues introduced the chemical bonding by grafting poly(ether amine) (PEA) onto zinc hydroxyalkanoate (ZHS) as shown in (Fig. 4a). The modified nanoparticles were evenly dispersed collectively, providing tiny pores that were friendly to contact with the cathode material, which resulted in excellent electrochemical stability of the material, as shown in (Fig. 4b). In a lithium plating stripping test at a constant current density of 0.2 mA cm−2 with a long-cycle stability of more than 1500 h and by testing the critical current (CCD), which reached 0.8 mA cm−2 as shown in (Fig. 4c), again demonstrating that the modified nanofillers of the functional group substantially improved electrochemical stability.87
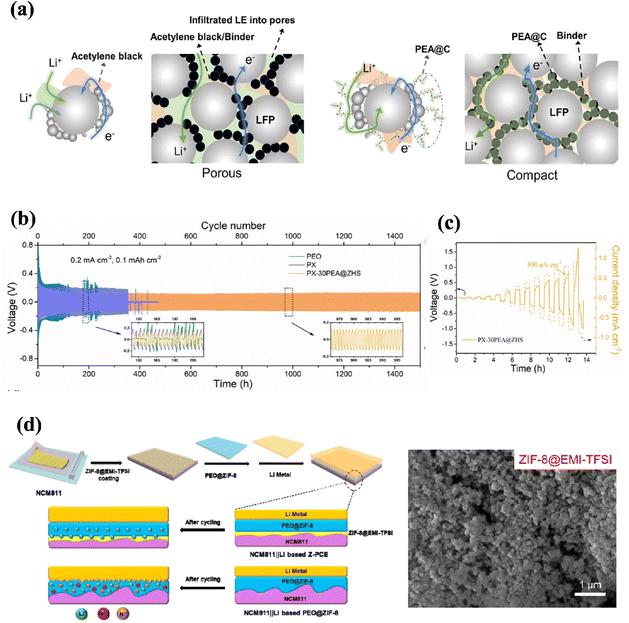 | ||
| Fig. 4 (a) Schematic illustration of the traditional cathode with a porous configuration for electrolyte percolation, (b) Galvanostatic cycling of the symmetric cells with PEO, PX, and PX-30PEA@ZHS SPEs at 0.2 mA cm−2 with a capacity of 0.1 mA h cm−2, (c) Galvanostatic cycling of the symmetric cells with PX-30PEA@ZHS SPEs at step-increased current densities ref. 87. Copyright 2022, Elsevier. (d) Schematic diagram of preparation and structure of NCM811/Li cell-based Z-PCE and PEO@ZIF-8 electrolytes ref. 89. Copyright 2022, Elsevier. | ||
Chen and colleagues incorporated zeolitic imidazolate framework-67 (ZIF-67) nanoparticles into LiNi0.5Co0.2Mn0.3O2 cells, resulting in a maintenance capacity of 123 mA h g−1 over 100 cycles at 1.0 C, through the active site of ZIF-67, the coordination of anions is restricted and the degree of dissociation degree of lithium salts is provided.88 In a related investigation, Shen et al. demonstrated that the addition of ZIF-8 nanofillers can effectively decrease the interfacial impedance of ionic liquids through microporous physisorption, more effectively limiting the concentration of anions in the system, leading to improved cycling stability as shown in (Fig. 4d).89
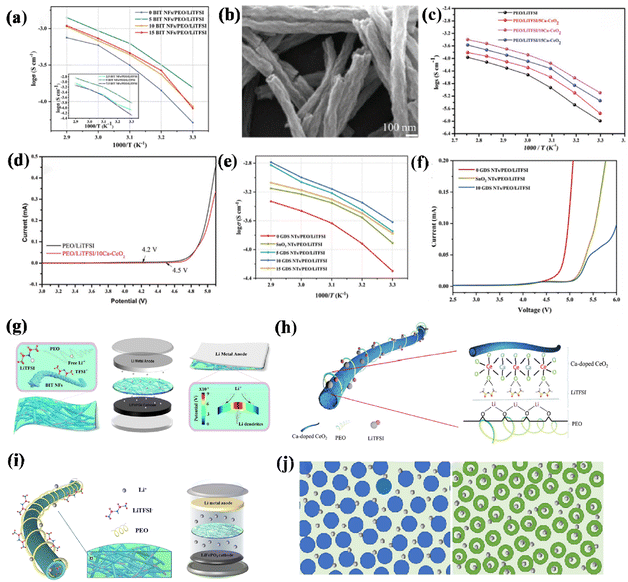 | ||
| Fig. 5 (a) Arrhenius plots (temperature increased from 30 °C to 70 °C) ref. 99. Copyright 2022, Elsevier. (b) Characterization of Ca–CeO2 SEM images, (c) Arrhenius plots of PEO/LiTFSI and PEO/LiTFSI/Ca-CeO2 electrolytes, (d) LSV curves of PEO/LiTFSI and PEO/LiTFSI/Ca–CeO2 films at a scanning rate of 1 mV s−1 at 60 °C (ref. 100). Copyright 2020, Wiley-VCH. (e) Arrhenius plots of several different electrolytes, (f) LSV curves of three different electrolytes ref. 101. Copyright 2023, Elsevier. (g) Schematic illustration of a mechanism for enhanced Li+ ions transport and dynamic regulation of Li+ ions deposition by BIT NFs ref. 99. Copyright 2022, Elsevier. (h) Schematic illustration of a mechanism for enhanced Li-ion transport in PEO-based electrolyte by Ca–CeO2 nanotubes ref. 100. Copyright 2020, Wiley-VCH. (i) Mechanism for enhanced Li+ transport by GDS NTs. (j) Comparison of the Li+ transport path in the cross-sectional direction of nanofibers and nanotubes ref. 101. Copyright 2023, Elsevier. | ||
Nanotubes (NTs) have unique properties in the inner space and are added to the electrolyte as fillers, reducing interfacial circuitry and discontinuity, enabling both the surface and interior of the material to act as efficient channels for the fast movement of free lithium ions. As a result, not only does this enhance the mechanical strength of the PEO electrolyte, but it also facilitates the migration of lithium ions.
The presence of numerous oxygen vacancies on the surface of CeO2, along with the addition of Ca, can enhance both the electrochemical and mechanical properties of the polymer. Chen et al. successfully synthesized hollow nanotubes enriched with vacant calcium in CeO2 (Ca–CeO2). The resulting structure facilitates a uniform lithium flux, as shown in (Fig. 5b), creating an efficient pathway for ion transport due to its larger contact area with the electrolyte. This leads to an impressive ionic conductivity of 1.33 × 10−4 S cm−1 at 60 °C, as demonstrated in (Fig. 5c). There is a positive correlation between the increase of oxygen vacancy and the ionic conductivity. Li+ cations move in the oxygen channel, accompanied by the change in coordination environment, starting from the process of ionic compounds being dissociated by polar solvent molecules and realizing rapid movement in the medium through the exchange of coordination molecules. To assess the ability to of the polymer electrolyte to withstand high voltages, linear scanning voltammetry (LSV) was performed at 60 °C using Li/SSE/SS cells to determine its electrochemical window, illustrated in (Fig. 5d). Interestingly, even under these conditions, the PEO/lithium bis(trifluoromethanesulfonyl)imide (LiTFSI)/Ca-CeO2 electrolyte exhibits oxidative stability above 4.5 V, a crucial factor in further enhancing the theoretical capacity of SSEs.100
To further increase the oxygen vacancy, an electrolyte with good interfacial compatibility and excellent ionic conductivity was developed. Zhang et al. proposed the utilization of a Gd–SnO2 nanotube filler to create long-range ion transport channels in PEO-based systems. This was achieved by taking advantage of its oxygen-rich vacancy mechanism, resulting in good interfacial compatibility and high ionic conductivity. When the positive charges carried by these oxygen vacancies were harnessed, Lewis acid sites were formed within the solid polymer, leading to strong interactions. Consequently, the composite electrolyte exhibited an elevated lithium ion conductivity of 2.41 × 10−4 S cm−1 at 30 °C (Fig. 5e). Furthermore, by combining the hollow structure with material modification techniques, there was a significant improvement in the enhancement of the electrochemical window (5 V), as shown in (Fig. 5f). In particular, this performance was close to that of commercially available liquid electrolytes.101
We explored the preparation of different one-dimensional nanomaterials (Fig. 5g–i) and compared the improvement of the polymer electrolyte performance by adding modified nanofibres and nanotube fillers, and found that nanofibres have a greater enhancement in mechanical strength, but the nanotube structure has a high specific surface while having a hollow structure to allow a uniform lithium flux, as shown in (Fig. 5j). Lithium ions can be transferred along the surface of the nanotube. At the same time, these particles quickly de-embedded through the hollow transfer tube, which has a greater improvement on the ion transfer efficiency.
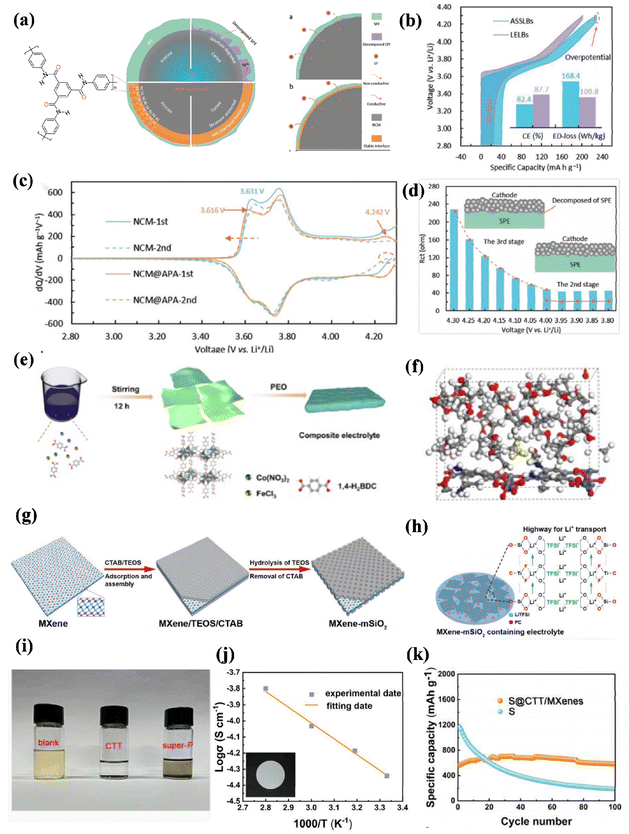 | ||
| Fig. 6 (a) Schematic illustration of solid–solid interface without nanolayer and with APA interfacial nanolayer between NCM and SPE in affecting the cycling stability of ASSLBs, (b) the initial charge/discharge profiles of NCM cathodes in the ASSLBs under a current density of 10 mA g−1, where the inset shows initial coulombic efficiency and energy density loss of the ASSLBs, (c) differential capacity curves of the 1st and 2nd cycle at 10 mA g−1, (d) the evolution of Rct in the NCM /SPE/Li ASSLBs during the initial charging ref. 103. Copyright 2022, Wiley-VCH. (e) Schematic diagram of the process for preparing MOFs and composite electrolyte membrane, (f) molecular dynamics simulation plots over time in the system of PEO/MOFs-NH2 ref. 106. Copyright 2023, Wiley-VCH. (g) Schematic illustration of fabricating sandwich-like MXene-based mesoporous silica (MXene–mSiO2) nanosheets, (h) schematic illustrating the fabrication of the MXene–mSiO2 containing solid polymer electrolyte ref. 104. Copyright 2020, Wiley-VCH. (i) The optical images of Li2S6 solution before and after mixed with CTT and super-P, (j) ionic conductivity of PEO/PE solid electrolyte and the EIS plot of PEO solid electrolyte at 27 °C and 60 °C, (k) cycling performance at 200 mA g−1 (ref. 107). Copyright 2022, Elsevier. | ||
As the two-dimensional layer structure prepared by the nanoframe itself can improve the mechanical strength of the solid electrolyte and promote the mobility of the polymer molecular chain, the perfect combination of matrix and filler can be realized; researchers used MXene as a filler to increase the amorphous region of the polymer.105 Shi et al. explored sandwich-structured silica nanosheets (MXene–mSiO2) with Lewis acid–base interaction depicted in (Fig. 6g), resulting in fast Li-ion transport at its interface and excellent resistance to deformation due to a Young's modulus of 10.5 MPa shown in (Fig. 6h). This can be attributed to the hydrogen bond between the rigid MXene–mSiO2 nanosheets and the poly(propylene oxide) elastomer (ePPO) polymer matrix.106 Furthermore, Zhang et al. improved the cycling stability of SSEs by incorporating a doped carbon–carbon composite transition metal carbide derived from the covalent organic framework (COF) and the 2D nitride MXene, along with the porous structure (CTT) and Mxene.107 As a result, when paired with a solid electrolyte, the S@CTT/MXene cathode exhibited enhanced cycling performance compared to the prepared CTT and super-p as shown in (Fig. 6i). Ionic conductivity was measured as 1.64 × 10−4 S cm−1 at 80 °C according to (Fig. 6j), indicating solution homogenization and improved lithium-ion transport efficiency. It is worth noting that during the first 20 cycles, a lower capacity was observed in this battery based on (Fig. 6k). This could be attributed to the use of a solid-state electrolyte, which only contacts the surface of the cathode, unlike a liquid electrolyte that allows for full infiltration. Consequently, the incomplete reaction between the internal active substances initially leads to reduced capacity within these cycles. However, the implementation of this design strategy significantly enhances the interface contact, thereby reducing the interface impedance considerably while ensuring more stable transportation of lithium ions. Using two-dimensional materials in the solid electrolyte can form a hydrogen bond network with polymer chain segments and on the other hand, accelerate the dissociation of lithium salt through a local polarization electric field effect, which effectively inhibits dendrites and side reactions, and achieve excellent cycling performance and significant service life.
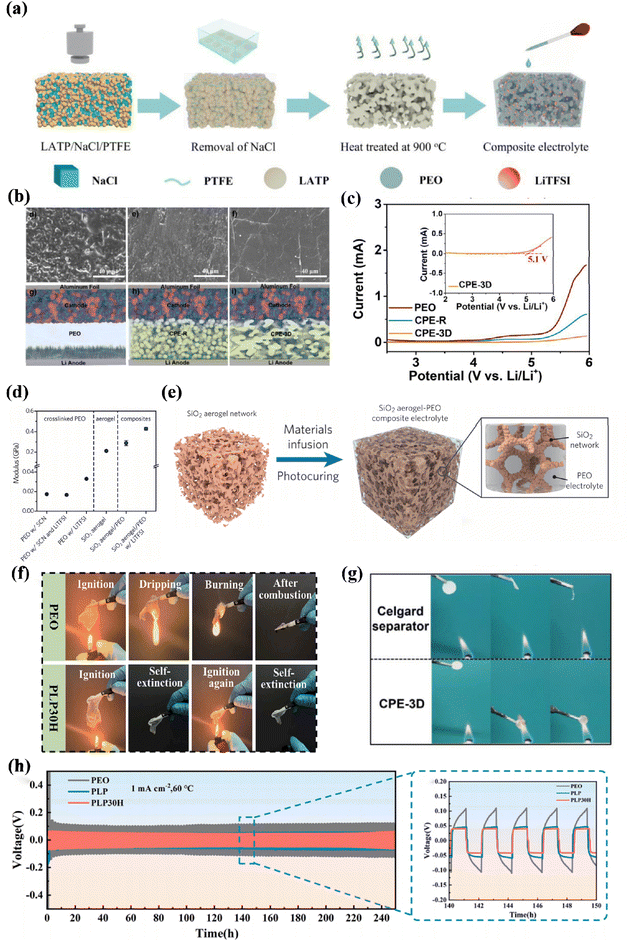 | ||
| Fig. 7 (a) Schematic diagram of the fabrication of CPE-3D, (b) LFP/PEO/Li, LFP/CPE-R/Li, and LFP/CPE-3D/Li cells after charge/discharge cycling testes under 1 C, (c) voltage profiles of Li/PEO/Li, Li/CPE-R/Li, and Li/CPE-3D/Li symmetric batteries at different current density with 1 h stripping and 1 h alternating step ref. 108. Copyright 2022, Elsevier. (d) Elastic modulus of the crosslinked PEO, (e) schematic showing the synthetic procedures of the SiO2-aerogel-reinforced CPE ref. 110. Copyright 2018, Elsevier. (f) The combustion behavior of SSEs ref. 109. Copyright 2023, Elsevier. (g) The combustion behavior of SSEs ref. 108. Copyright 2022, Elsevier. (h) Galvanostatic voltage test for Li–Li symmetric-cell for PEO (containing lithium salt), PLP, and PLP30H SSE (1 mA cm−2) ref. 109. Copyright 2023, Elsevier. | ||
In order to pursue the ultimate safety of SSEs, there is no risk of burning, explosion etc., in any case. The combination of aerogel makes it flame retardant, heat insulation, and has excellent electrochemical energy storage properties. This electrolyte utilizes aerogel as a carrier and absorbs ionic liquid or electrolyte into the aerogel to form a nonflowing solid electrolyte. It exhibits excellent performance under extreme conditions, such as high ionic conductivity at low temperatures. However, because it is a nonfluid solid electrolyte, it is susceptible to polarization during high-current discharge, negatively impacting device performance. Lin et al. introduced rigid mesoporous silica aerogels as the framework for polymer-based electrolytes (Fig. 7e). This structure provides sites for anion adsorption, resulting in a high modulus of 0.43 Gpa (Fig. 7d) and an impressive ionic conductivity of ≈6 × 10−4 S cm−1, effectively inhibiting the growth of Li dendrites.118
More significantly, the multifunctional three-dimensional framework not only facilitates a continuous pathway for ion transport but also mitigates agglomeration and precipitation, thereby amplifying the synergistic effect of 3D framework formation and enhancing battery safety and stability.
In general, the incorporation of zero Vinamil particles into polymer-based solid electrolytes can facilitate polymer segment rearrangement, expand the amorphous region, and enhance ionic conductivity. However, addressing the issue of uneven diffusion remains challenging. One-dimensional materials (nanofibers, nanowires) and two-dimensional nanomaterials (nanosheets) possess non-continuous and tortuous ion transport channels that circumvent particle-induced “nodes” while facilitating the formation of high-quality continuous interfaces with polymers. Nevertheless, there is still a possibility of agglomeration. Therefore, to prevent isolated distribution of nano-fillers within the polymer matrix, it is necessary to establish a three-dimensional interconnected network for ion transport that effectively controls filler agglomeration and inhibits lithium dendrite formation. However, constructing solid electrolytes with such a three-dimensional framework is costly and complex; thus further exploration and improvement are required.
4. Polyester-based solid polymer electrolytes
Polyester-based solid-state polymeric electrolytes (SPEs) have received significant attention due to their robust polar functional groups [–O–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–O]. Notable examples include caprolactone (CL), ethylene ester (EC), and propylene carbonate (PC) (Table 1). These polyester electrolytes exhibit excellent compatibility with alkali metal salts, while their strong polar functional groups effectively enhance the dielectric constant and ionic conductivity of the electrolyte; linear block copolymers or grafted copolymers with various topologies are constructed to destroy the crystallinity of polyesters. Consequently, extensive research is being conducted to develop lithium-ion batteries base on high-performance polyester-based SPE.119–122
O)–O]. Notable examples include caprolactone (CL), ethylene ester (EC), and propylene carbonate (PC) (Table 1). These polyester electrolytes exhibit excellent compatibility with alkali metal salts, while their strong polar functional groups effectively enhance the dielectric constant and ionic conductivity of the electrolyte; linear block copolymers or grafted copolymers with various topologies are constructed to destroy the crystallinity of polyesters. Consequently, extensive research is being conducted to develop lithium-ion batteries base on high-performance polyester-based SPE.119–122
4.1. Solid polymer electrolytes (PCL)
The use of ε-caprolactone (PCL) in solid-state electrolytes (Fig. 8a) serves a dual purpose as a carrier and a matrix. PCL possesses specific structural properties that improve its ductility and mechanical strength, along with a relatively low glass transition temperature (Tg < 60 °C). Additionally, the weak interaction between the carbon group and lithium ions within PCL facilitates improved migration of lithium ions.123,124 Mindemark et al. initially developed a solid-state electrolyte using PCL material; however, its high crystallinity at room temperature limited its potential applications.125 To address this issue, Zhang and colleagues introduced inorganic fillers to facilitate Li+ transport within the electrolyte matrix. By incorporating 45 wt% LiTFSI and 75% LAGP into PCL, they achieved an impressive ionic conductivity of approximately 1.75 × 10−4 S cm−1 at a temperature of 30 °C (as depicted in Fig. 8b). Furthermore, the introduction of LAGP into the electrolyte demonstrated improved compatibility between the PCL and the Li anode during lithium plating stripping tests conducted over multiple cycles. This enhancement did not compromise the mechanical properties of the electrolyte (Fig. 8c).126 Sångeland et al. successfully inhibited PCL crystallization by incorporating bis(methylene carbonate) (TMC), leading to substantial improvements in cell performance. They also discussed the deposition of interfacial by-products and the growth of lithium dendrites (Fig. 8g), which resulted in a reduced interfacial impedance. Additionally, three cycles of Open Circuit Voltage (OCV) to −0.5 V and OCV to 5 V were performed to observe irreversible interfacial species build-up during reduction and oxidation, respectively (Fig. 8f).127 To study the interfacial contact, Chen et al. developed a poly(ether-ester)-based poly(pyrrolidone chloride) (PDCL-SPE) with adjustable composition and structure through direct bulk of cyclic monomers CL and poly(1,5-dioxepan-2-one) (PDXO). The amorphous structure, lower glass transition temperature, as well as synergistic effects from the ether and carbonyl groups facilitated the dissociation of lithium salts and transport of lithium ions even after long-term cycling (600 h). The charge/discharge voltage curves for Li/PDCL40-SPE/Li remained stable with a low polarization potential (0.04 V).128 P. Nkosi et al. added LLZO at different levels to improve composite electrolyte performance with LiFePO4 cathode interfacial contact, achieving a conductivity value of 1.31 × 10−4 S cm−1 at 60 °C (Fig. 8d). This was attributed to the formation of random connectivity among ceramic particles, as well as long-range connectivity at the polymer–ceramic interface (Fig. 8e).129 Since the conductivity of PCL is an order of magnitude larger than that of PEO-based “ceramic polymers”, the introduction of ceramic particles does not reduce ionic conductivity, has excellent interfacial contact, and has stable electrochemical performance at 5 V voltage, offering prospects for the production of high energy-density solid lithium batteries.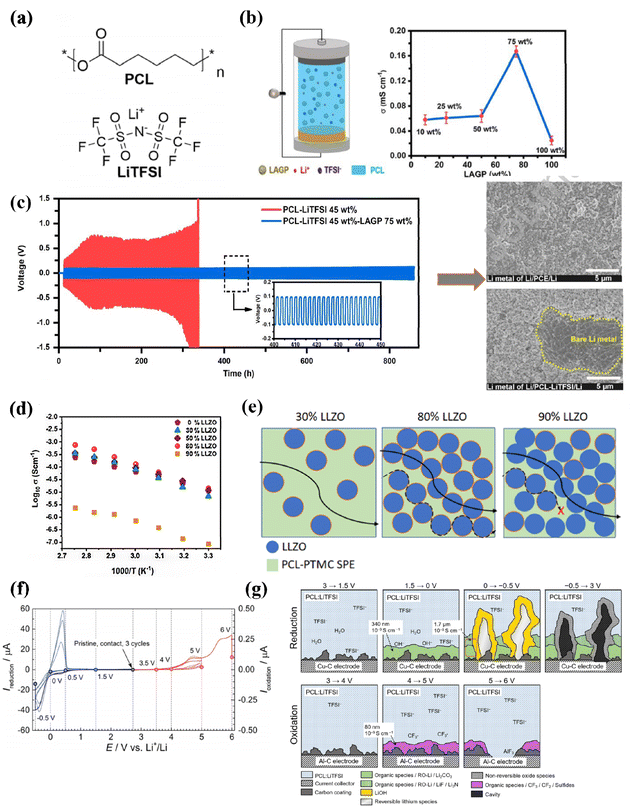 | ||
| Fig. 8 (a) Structure of poly(ε-caprolactone) ref. 127. Copyright 2015, Elsevier. (b) Ionic conductivity of the composite electrolyte, (c) Galvanostatic tests of Li/PCL-LITFSI 45 wt%-LAGP 75 wt% Li and Li/PCL-LiTFSI 45 wt% Li cells at a constant current density of 0.1 mA cm−2 (ref. 126). Copyright 2021, Elsevier. (d) Comparison of Arrhenius plots for the ionic conductivity of the polymer and composite electrolytes with different LLZO loadings, (e) schematic representation of the Li-ion transport in composite electrolytes at 30 wt% LLZO, 80 wt% LLZO, and 90 wt% LLZO loadings ref. 129. Copyright 2021, Elsevier. (f) Current profiles of Li/PCL: LiTFSI/Cu–C or Al–C cells taken apart for postmortem morphological and compositional analysis, current response during linear sweep to different cutoff potentials followed by a potential hold for 3 h (solid line) and during the first three cycles, (g) schematic of solid polymer electrolyte–electrode interface at different potentials. Interfacial layer thickness and ionic conductivity are based on calculations that assume that the interfacial layer covers 50% of the electrode area ref. 127. Copyright 2015, Elsevier. | ||
4.2. Solid polymer electrolytes (PEC)
An EC is a member of the aliphatic group, and research has indicated that the aliphatic backbone possesses enhanced flexibility, allowing for more frequent movement of chain segments. Its synthesis involves the copolymerization of carbon dioxide and epoxide.130–133 Additionally, its thermodynamic stability can be attributed to its five-membered ring structure. Elmér et al. conducted a study on the coordination behavior of Li+ in the PEC/LiClO4 system using Fourier infrared (FTIR) spectroscopy, the concentration of lithium ions increased by decreasing solvent polarization.134PEC, as a low-donor-concentration molecule, can reduce the coordinate bonding between the polymer chains and lithium ions in the polymer framework, thus increasing the ionic conductivity. Wang and his team successfully developed a new solid polymer electrolyte by incorporating a carbonate substrate with a unique functional group (4-vinyltrifluorotoluene) through a precise molecular design, as shown in (Fig. 9a). This non-covalent bonding effect greatly enhances lithium ion migration and can also be used as a composite cathode bonding agent, leading to improved active mass loading and enhanced interfacial chemical stability.135 It is worth noting that the impact of anion conduction on lithium ion mobility is mainly based on the receptor chain segment within the polymer matrix, as illustrated in (Fig. 9b). The molecular orbital HOMO and LUMO energy levels are depicted, providing further information on the chemical structure of this modified fluorinated polymer electrolyte (MFPE) in (Fig. 9c). To achieve a wider electrochemical window, higher-energy bands are required. This phenomenon explains why there exists such a strong interaction between polymer-incorporated lithium salts.136
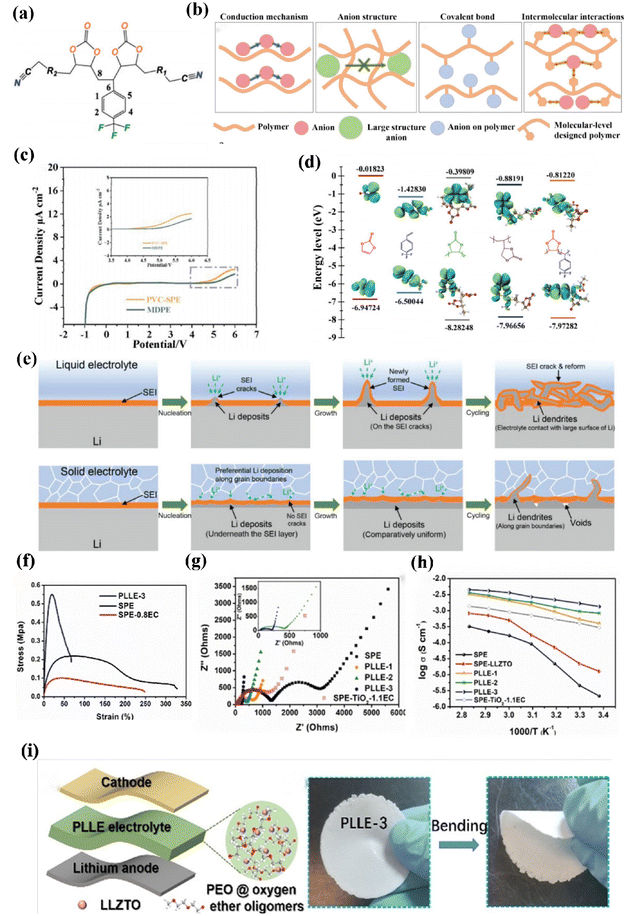 | ||
| Fig. 9 (a) Schematic of novel molecular-level designed polymer structures, (b) scheme of conduction mechanism of anion in polymer and different separate measures of increase lithium-ion transport, (c) linear voltammetry curve of Li/PVC-SPE/SS and Li/MDPE/SS, (d) the HOMO and LUMO of Vinyl Chloride (VC), PVC unit, poly(vinyl ethylene carbonate) ref. 127. Copyright 2023, Wiley-VCH. (e) Schematic illustration of Li deposition behavior in liquid electrolytes and schematic illustration of Li deposition behavior in solid-state electrolytes ref. 128. Copyright 2023, Wiley-VCH. (f) H NMR spectra showing EC structural changes under the influence of LLZTO, (g) impedance spectra of Li/SPE or SCEs/LiFeO4 cells, (h) Arrhenius plots of PEO and its composite electrolytes, (i) composite electrolyte (PLLE) ref. 131. Copyright 2021, German Chemical Society. | ||
To improve the ionic conductivity of solid electrolytes and reach a level of that of liquid electrolytes, we incorporated an additive called ethylene carbonate (EC). Liu et al. devised a method to enhance the interaction between active Li6.4La3Zr1.4Ta0.6O12 (LLZTO) doped with EC and Ta using PEO and LiTFSI (PLLE), resulting in a composite electrolyte with an ionic conductivity of 1.43 × 10−3 S cm−1 at 25 °C (Fig. 9g). The inclusion of EC had a synergistic effect by reducing the crystallinity of PEO while also acting as a plasticizer compared to other SSEs doped with LLZTO, thus maintaining a higher ion transport efficiency (Fig. 9h). Compared to liquid electrolytes and PLLE, it is evident that PLLE demonstrates better inhibition against lithium dendrites due to the formation of a solid electrolyte interfacial layer on its surface through vinyl carbonate decomposition (Fig. 9e).137 Cyclic monomers are widely recognized to undergo decomposition when exposed to Lewis acids or bases as initiators.138 Therefore, we propose that at high temperatures (>100 °C), there is an interaction between EC and LLZTO that triggers ring-opening reactions in the reactive structural state of EC based on the findings of our tests using 1H NRM (Fig. 9f).139
4.3. Solid polymer electrolytes (PPC)
Propylene carbonate (PPC) exhibits excellent resistance oxidation and demonstrates stable cycling performance at high voltage, offering an electrochemical window of 4.6 V.140–144 Moreover, PPC is readily dissolvent in a wide range of organic solvents. However, its mechanical properties are relatively poor. To address this limitation, Cui et al. investigated PPC-based SSEs and successfully developed LFP batteries with enhanced capacity within the temperature range of 0–160 °C. In another study by Chen et al., a composite electrolyte composed of PPC/LZTO was designed, incorporating inorganic fillers to modify the rate capacity and cycling stability of PPC.145The stable SEI interface formed by the PPC-based solid electrolyte is a key factor for the long-cycle stability of lithium batteries. Jia and his colleagues conducted a study on the chemical decomposition pathway of PPC to alkaline LLZTO particles to investigate (Fig. 10a). According to the literature, when hydroxide and glycerol are present along with alkali and water, PPC decomposes into cyclic propylene carbonate (PC). In (Fig. 10b), different temperatures were used for the EIS analysis of PPC.146 Sung et al., on the other hand, incorporated LAGP as an active filler into the propylene carbonate matrix, which exhibited good compatibility with the lithium metal as shown in (Fig. 10e). Additionally, the LAGP particles showed favorable wettability with PPC as illustrated in (Fig. 10c). The optimization of the performance of this composite electrolyte was evaluated using different LAGP contents represented by (Fig. 10g). Interestingly, the LFP/PPC-LAGP/Li cell achieved an impressive discharge specific capacity of 50 mA h g−1 with 63% capacity retention over 1000 cycles and nearly 100% crossover efficiency, as shown in (Fig. 10f). These outstanding results indicate that, compared to the LFP cathode, PPC-LAGP also exhibits exceptional stability at high currents.147 To enhance the thermal stability of batteries, Luo et al. introduced a polyimide (PI)-PPC substrate doped with LLZTO as reported in the literature. This resulted in an excellent thermal stability of the composite electrolyte. XRM analysis demonstrated that the LLZTO filler was evenly distributed within the matrix shown in (Fig. 10d), this rational doping reduces the agglomeration phenomenon, makes lithium deposition uniform, and reduces lithium dendrites puncture.148
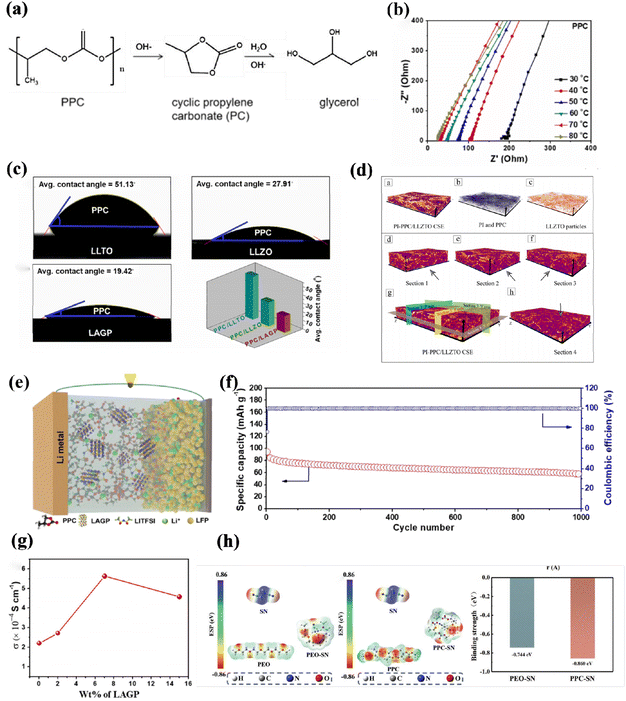 | ||
| Fig. 10 (a) Possible chemical decomposition pathways of PPC against the LLZTO particles, (b) Nyquist plots of PPC at various temperatures ref. 146. Copyright 2020, American Chemical Society. (c) Histogram of average contact angle for comparison among PPC and LLTO, LLZO, and LAGP ref. 147. Copyright 2021, Elsevier. (d) 3D XRM analysis of PI-PPC/LLZTO SPEs ref. 148. Copyright 2021, Elsevier. (e) Schematic of a LFP/PPC-LAGP/Li full cell, (f) corresponding cyclability at 60 °C, (g) LSV curve of DSPE at 25 °C ref. 147. (h) Optimized geometrical configurations and calculated potential potentials were used to obtain the optimal configurations of PEO-SN and PPC-SN and to calculate their binding energies ref. 149. Copyright 2023, Wiley-VCH. | ||
The addition of active filler can substantially improve lithium-ion transport capacity, but still needs to improve its interface engineering, Wang et al. designed a bilayer solid polymer by putting polypropylene carbonate (PPC)/succinonitrile (SN) in contact with the cathode (DSPE), while the anode uses PEO/LiLa3Zr2O12, and for further study of the interaction diagram of PPC, PEO, SN, PPC (Fig. 10h) synergizes stronger with PEO than PEO, so this structure has a high critical current density of 1.3 mA cm−2, the onset decomposition potential of DSPE at 25 °C is 5.6 V, which proves its excellent electrochemical performance.149
5. Nitrile-based solid polymer electrolytes
Nitriles, which possess the NC group that is polar in nature and electron-withdrawing, exhibit high dielectric constants of approximately (ε = 30). Their wide electrochemical window may be attributed to their low LUMO energy. Recently, there has been significant interest in nitrile-based SPEs due to their excellent electrochemical properties and strong coordination capabilities.150–1535.1. Butylene dinitrile solid polymer electrolytes
The plastic phase of succinonitrile (N–C–CH2–CH2–N, SN) spans from −35 °C to its melting point at 62 °C, with a boiling point of 265 °C. At 25 °C, the dielectric constant (ε = 55) of SN was measured, indicating its strong solvent capabilities for various lithium salts. The high oxidation potential of SN can be attributed to its ability to accept electrons due to the lower LUMO energy level.154It is widely acknowledged that SN-based solid electrolytes with oxidation resistance exhibit good compatibility with high-voltage electrode materials. However, the presence of residual SN molecules, while capable of reducing the interfacial impedance by infiltrating the cathode material, also poses a risk of corroding the lithium–metal anode. To enhance the connection with the high-voltage cathode, Zuo and colleagues have implemented an in situ curing process between PEO and the cathode by introducing SN plastic crystals in between. This approach forms an ion-conducting network within the cathode and at its interface, as shown in (Fig. 11a), effectively preventing the oxidative decomposition of PEO during high-voltage charging and discharging. As a result, they were able to extend the electrochemical window to 5.2 V (as depicted in Fig. 11b), demonstrating excellent electrochemical stability.155
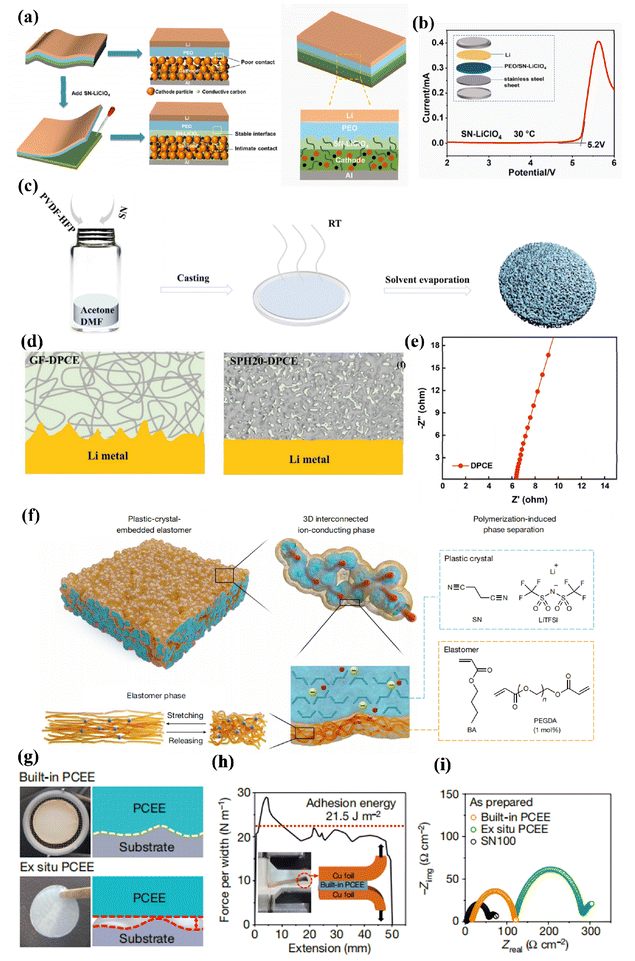 | ||
| Fig. 11 (a) Schematic diagram of NCM/PEO/Li cells with interfacial modification layer, (b) LSV curve of PEO/SN-LiClO4 ref. 155. Copyright 2023, Elsevier. (c) Schematic illustration of the fabrication process of porous polymer host, (d) schematic diagrams of Li deposition in Li/Li cells assembled with GF-DPCE and eSPH20-DPCE, (e) DPCE EIS impedance mapping ref. 156. Copyright 2023, Wiley-VCH. (f) Schematic illustration of the design and structure for PCEE. The structure of PCEE shows that the 3D interconnected plastic crystal phase (cyan) is surrounded by the elastomer phase (orange), (g) photo images and schemes showing the ex situ and the built-in PCEEs, (h) interfacial adhesion test between the built-in PCEE and the Cu foil. (i) Nyquist plots of as-prepared symmetric Li cells configured with various electrolytes ref. 157. Copyright 2023, Springer Nature. | ||
In order to enhance the overall performance of cells, the bilayer structure can be utilized. Additionally, the stability at the interface between SN and lithium metal anodes can be improved by incorporating double salts or suitable additives such as fluoroethylene carbonate (FEC). Bao et al. proposed a simple method of phase separation using SN to modulate the preparation of porous membranes made from poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) (SPH). They employed a double salt system consisting of LiTFSI and lithium bis(oxalate)borate (LiBOB) to provide Li+ ions (DPCE) (Fig. 11c). In contrast, the dense pore structure of SPH20 plays a crucial role in impeding dendrite growth during this process. Furthermore, continuous plating/stripping operations were maintained for more than 1200 hours in the case of the Li|SPH20-DPCE|Li cell (Fig. 11d). The resulting ionic conductivity at room temperature reached 8.21 × 10−4 S cm−1 (Fig. 11e). Consequently, when assembling a LFP/SPH20-DPCE/Li cell, it exhibited superior cycling stability compared to pure PVDF-HFP and commercially available glass fiber materials. This led to a relatively smooth surface morphology for the Li–metal electrode. When identical DPCE is used employed in two symmetric lithium cells, it can be concluded that the loose and brittle fiber structure of GF would inevitably facilitate dendrite growth during the plating/stripping process of lithium deposition/dissolution as shown in (Fig. 11d).156
The dispersion function component of elastomers, such as SN, has been found to exhibit excellent properties in terms of its ability to disperse materials effectively. Lee et al. have reported on a group of solid-state electrolytes made from elastomers that possess a three-dimensional interconnected plastic crystal phase (PCEE). These elastomeric electrolytes demonstrate a combination of mechanical strength, high conductivity for ions, low resistance at interfaces, and a high transference number for lithium ions (Fig. 11f). The in situ generated elastomer electrolyte on copper foils effectively accommodates volume changes during prolonged lithium plating and stripping processes, achieving a remarkable coulombic efficiency of 100%. Furthermore, these elastomer electrolytes enable stable operation of full cells even under constrained conditions such as limited lithium availability, thin electrolyte layers, and high-loading LiNi0.83Mn0.06Co0.11O2 cathodes operating at a high voltage of 4.5 V at room temperature. By establishing uniform ion transport channels, the impedance is significantly reduced, resulting in a high specific energy exceeding watt hours per kilogram of electrode plus electrolyte, while effectively preventing the formation of lithium dendrites (Fig. 11g). The implementation of an elastomeric electrolyte system presents a robust approach to ensure the stable performance of high-energy solid state lithium batteries.157 Furthermore, (Fig. 11h) shows an impressive adhesion energy value of 21.5 J m−2 for this system that surpasses that observed in most commercially available polymer electrolytes, thus showing excellent interfacial stability (adhesion energy greater than or equal to 5 J m−2 is considered crucial to create durable interfaces capable of withstand mechanical stresses associated with battery manufacturing and operation).158
5.2 Solid polyacrylonitrile electrolytes
The PAN is formed through the polymerization of acrylonitrile monomer radicals and exhibits excellent thermal stability. It lacks oxygen atoms, and the nitrogen atom in PAN does not strongly interact with lithium ions. Its ionic conductivity can reach 3 mS cm−1 while possessing an electrochemical window exceeding 4.5 V.159–163 However, due to its low strength and poor mechanical properties, PAN has been used predominantly utilized as a gel polymer electrolyte (GPE) for a long period.164This is primarily attributed to uncontrolled passivation reactions that occur between the nitrile group and the lithium-metal anode, resulting in increased interfacial resistance between the electrolyte and electrode, which deteriorates electrochemical performance. Consequently, PAN alone is not suitable for polymer electrolyte-based materials.165
To enhance the stability at the interface, we incorporated PAN fibers into a three-dimensional structure. This structural modification greatly improved the overall performance of the solid electrolyte and facilitated stable long-term cycling.166 In order to address the low activity and ionization ability of MOFs in electrochemical reactions, Zhang et al. introduced ionic liquids (IL) to enhance their activity. When efficient ionic liquids are incorporated, Li+ ions are transported along the chain of MOFs rather than through conduction at the interface. The preparation process is depicted in (Fig. 12a). In particular, the MOF@PAN/PEO/IL composite exhibits an impressive ionic conductivity of 2.57 × 10−4 S cm−1 at 25 °C (Fig. 12b), which is two orders of magnitude higher than other composites used as solid-state LMB operating at room temperature (RT). The stability test shown in (Fig. 12c), conducted for 600 hours with repeated lithium plating and stripping cycles, demonstrates that densely packed MOF nanoparticles grown on PAN fibers form continuous pathways for Li+ transport. Furthermore, the encapsulating of an ionic liquid (IL) within MOFs not only reduces energy barriers for fast Li+ migration but also enhances the interfacial compatibility between various MOF/PEO and electrode/electrolyte interfaces.167
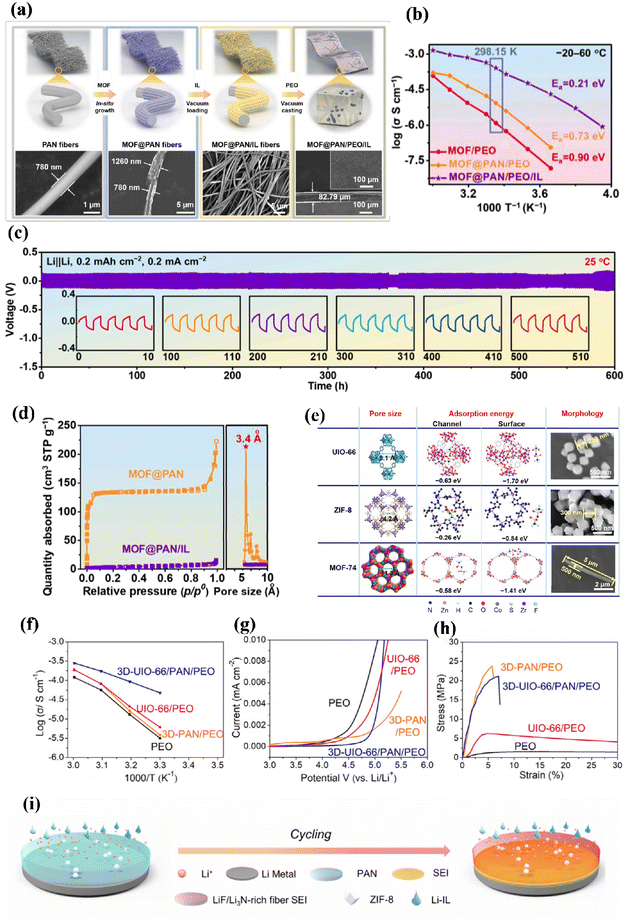 | ||
| Fig. 12 (a) Schematic diagram of the fabrication process of the MOF@PAN/PEO/IL CPE and their corresponding SEM images. (b) Ionic conductivity of different CPEs as well as their activation energy at the temperature range of 20–60 °C. (c) Long-term cycling stabilities of Li/Li symmetric cell using MOF@PAN/PEO/IL CPE at a current density of 0.2 mA cm−2 for 0.2 mA h cm−2. (d) Li+ distributions in MOF@PAN/PEO/IL CPE at pristine (0 s), intermediate (60 s), and steady (10 h) ref. 167. Copyright 2022, Elsevier. (e) The crystalline structures and SEM images of UIO-66, ZIF-8, and MOF-74, clearly show their differences in pore sizes, adsorption energies with TFSI−, and morphologies. (f) Temperature-dependent ionic conductivities. (g) Oxidative stabilities ref. 169. (h) Stress–strain curves of the PEO/LiTFSI, 3D-PAN/PEO/LiTFSI, UIO-66/PEO/LiTFSI and 3D-UIO-66/PAN/PEO/LiTFSI SSEs ref. 169. Copyright 2022, Elsevier. (i) Schematic drawing of suppressing Li dendrites ref. 170. Copyright 2023, Wiley-VCH. | ||
Polyacrylonitrile nanofiber mesh with a strong three-dimensional skeleton can provide support for the ionic conductive matrix and significantly improve the overall mechanical properties. Bandyopadhyay et al. has reported the synthesis of a unique one oligomer of type A–B–A–B from a diamine, 1,4-diazabicyclo [2.2.2] octane dihalide diethylene glycol bis (2 chloroethyl) ether, with three units of oxyethylene (–CH2CH2O–) in the backbone through the Menschutkin reaction. Subsequently, this oligomer was blended with PAN and subjected to heat treatment to form a vein-like network structure. In this structure, PAN serves both as a supporting skeleton and as a pathway for lithium-ion transport. This innovative approach provides an alternative design strategy for the preparation of high-performance pan-based electrolytes.168 To bind the active site of organometallic particles and make them have good dispersion. Li and his team took an innovative approach by creating a connected three-dimensional network structure using MOFs, effectively establishing continuous channels for rapid transport of Li+ ions within the MOF particles (Fig. 12e). Among the commonly used MOF particles (Fig. 12d), the université Catholique de Louvain-66 (UIO-66) exhibits superior porosity and anion adsorption energy, thus limiting anion transport in the cell while improving the number of lithium ion transfer (tLi+). The ionic conductivity, mechanical properties, and electrochemical stability of uio-66 are significantly better than other 3D polymer electrolyte frameworks (Fig. 12f–h). Simulation results also demonstrate a uniform distribution of lithium flux throughout (Fig. 12d), dendrite formation due to effective modulation of the 3D framework structure that restricts anion transportation.169 In terms of the SEI interface (as depicted in Fig. 12i), dense LiF/Li3 formation promotes efficient transfer kinetics for Li+ ions and improves the overall stability of the structural system, ultimately prolonging the useful life of solid-state Li-ion batteries.170
6. Polyvinylidene difluoride-based solid polymer electrolytes
PVDF is a partially crystalline polar polymer that displays a high dielectric constant, which enhances the interaction between the matrix and lithium salts to speed up their dissolution and transmission of Li+ ions. Furthermore, PVDF has excellent heat resistance and mechanical deformation resistance, along with outstanding compatibility at the interfaces. These properties have recently made PVDF an attractive option for solid polymer electrolytes in extensive research studies.170–173To develop a flexible all-solid-state lithium metal battery with a high-density three-dimensional structure, Wang et al. utilized PVDF as a supporting framework filled with PEG electrolyte through an in situ thermal curing method (Fig. 13a). This approach not only accelerates ion transport but also ensures both the electrochemical stability and the flexibility of the system. Additionally, it was observed that at higher current densities up to 0.3 mA cm−2, PEG and PVDF polymer electrolytes gradually become less stable while demonstrating the long cycle stability of PVDF@PEG electrolytes. The construction of this three-dimensional structure allowed us to achieve an ionic conductivity value of 1.06 × 10−3 S cm−1 at 100 °C (Fig. 13b), indicating its potential for practical applications.174
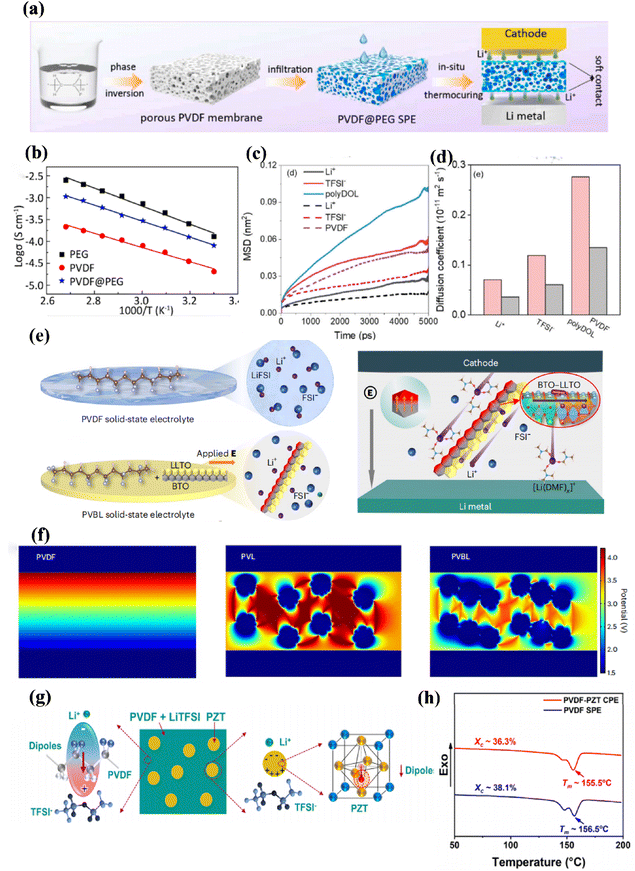 | ||
| Fig. 13 (a) Preparation schematic of PVDF@PEG electrolyte, (b) Arrhenius plots of PEG, PVDF, and PVDF@PEG electrolytes at different temperatures ref. 174. Copyright 2022, Elsevier. (c) MSD versus time for the PVDF-based polymer electrolyte (dotted line) and polyDOL-based polymer electrolyte, (d) corresponding diffusion coefficients of the PVDF-based polymer electrolyte (grey) and the poly DOL-based polymer electrolyte (pink) ref. 175. Copyright 2022, Wiley-VCH. (e) Illustration of the Li salt dissociation and Li+ transport by the coupled BTO-LLTO in the PVBL electrolyte, (f) COMSOL Multiphysics simulations of the potential distribution in PVDF ref. 178. Copyright 2022, Springer Nature. (g) Schematic diagram of the dipoles in PVDF and PZT in dissociating LiTFSI, (h) DSC spectra of the PVDF-PZT CPE and PVDF SPE ref. 180. Copyright 2022, Elsevier. | ||
In another study by Yu et al., they examined the higher diffusion coefficients of species such as Li+, TFSI− and PVDF in polyol-based polymer electrolytes compared to those of their PVDF-based counterparts (Fig. 13c). Mean square displacement (MSD) analysis was used to determine their respective diffusion coefficients (Fig. 13d). In particular, the diffusion coefficient of Li+ increased to 7.04 × 10−13 m2 s−1 at room temperature, surpassing that observed in PVDF-based electrolytic systems, thus ensuring excellent compatibility between organic–inorganic combinations for positive electrodes.175
By coupling the ceramic medium with the PVDF-based electrolyte, more mobile Li+ can be generated synchronously to establish an effective directional surface charge density; this addresses the challenge of low ionic conductivity. Shi et al. created a composite solid-state electrolyte (PVBL) by incorporating BaTiO3–Li0.33La0.56TiO3−x nanowires into a poly(vinylidene difluoride) matrix, forming a side-by-side heterojunction structure (Fig. 13e). The presence of polarized dielectric BaTiO3 promotes the dissociation of Li salt, facilitating the movement of Li+ ions across the interface to facilitate efficient transport through coupled Li0.33La0.56TiO3−x regions. Furthermore, this combination suppresses the formation of the space charge layer with poly(vinylidene difluoride), resulting in an impressive ionic conductivity (8.2 × 10−4 S cm−1) and the lithium transference number (0.57) for PVBL at 25 °C. In particular, when used as an interlayer between Li metal and PVL, PVBL reduces the overpotential due to the partial reduction of LLTO (from Ti4+ to Ti3+) in PVL by Li metal, creating a mixed conductor interface that minimizes interfacial resistance.176,177 COMSOL multiphysics simulations demonstrate that, compared to PVDF and PVL (Fig. 13f), PVBL achieves a more uniform potential distribution and negligible concentration polarization of Li+ ions, while also homogenizing the interfacial electric field with electrodes. Solid state LiNi0.8Co0.1Mn0.1O2/PVBL/Li batteries cycle stably 1500 times at a current density of 180 mA h g−1.178
Furthermore, Kang et al. reported another strategy involving salt polarization to fabricate highly ion-conductive SPEs using a high-dielectric polymer capable of strong interaction with lithium salts. Such a polymer with large dipole moments can guide lithium cations (Li+) to align along the chain, forming a continuous pathway for Li+ hopping within SSEs.179 Similarly, Kang et al. enriched the dense thin layer on the anode side by incorporating highly dielectric asymmetrical poly(vinylidene fluoride)(PVDF)-PbZrxTi1−xO3 (PZT) nanoparticles with strong electronegativity at the dipole end. As shown in (Fig. 13g), this phenomenon attracts lithium ions (Li+) at the PVDF–PZT interface and facilitates their transport through the dipole channel, promoting the dissociation of lithium salts into free Li+ Furthermore, it somewhat increases the proportion of the amorphous zone as shown in (Fig. 13h).180
6.1 PVDF-HFP-based solid polymer electrolytes
A PVDF-HFP produced by the copolymerization of PVDF, which takes advantage of the increase in the proportion of the amorphous region of the copolymer to give it excellent electrochemical stability (Fig. 14f). Zhai et al. conducted a study on the challenges in polymer electrolytes by designing fluorinated graphene-reinforced PVDF-HFP-LiTFSI (FPH-Li) polymer electrolytes in a 2D structure. The incorporation of uniformly dispersed fluorinated graphene resulted in improved mechanical properties without significantly increasing the thickness of the polymer electrolyte (Fig. 14a). This was achieved through a unique grain refinement effect induced by fluorinated graphene, which enhanced the transport of interfacial lithium-ion (Li-ion) and homogenized Li ion flux. As a result, there was an enhancement in Li-ion conductivity and a promotion of uniform Li plating/stripping. Furthermore, extensive characterizations revealed that fluorinated graphene played a crucial role in the construction of a stable artificial interface, effectively preventing unwanted reactions between the lithium–metal anode and solvated molecules. Consequently, the use of thin FPH-Li polymer electrolytes with approximately 45 μm thickness facilitated long-term Li plating/stripping with minimal overpotential in symmetrical Li/Li cells and ensured stable cycling of full cells consisting of Li/LiNi0.6Co0.2Mn0.2O2 with a high average coulombic efficiency reaching 99.5% at 1.0 C.181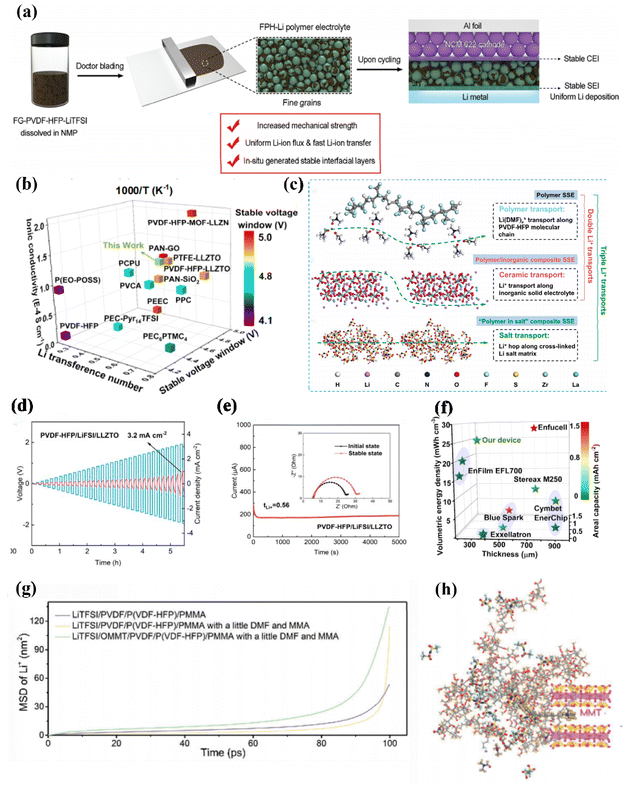 | ||
| Fig. 14 (a) Schematic illustration of the influence of fluorinated graphene on the properties of polymer electrolytes ref. 181. Copyright 2022, Wiley-VCH. (b) Comparison of the reported conductivity of electrolyte ions ref. 182. Copyright 2021, Wiley-VCH. (c) Li+ transport mechanism of bare polymer SSEs (single Li+ transport path), polymer/inorganic composite SSEs (double Li+ transport paths). “polymers in salt” composite SSE (triple Li+ transport paths). (d) Critical current density test of PVDF-HFP/LiFSI/LLZTO electrolyte, (e) chronoamperometry curve of Li/FPH-Li/Li cell under 10 mV polarization ref. 183. Copyright 2023, Elsevier. (f) Compare the properties of various polymer matrices ref. 182. Copyright 2021, Wiley-VCH. (g) MD-simulated mean square displacement (MSD) of Li+, (h) MD-simulated model curves of LOPPM PE ref. 184. Copyright 2023, Wiley-VCH. | ||
In PVDF-HFP-based electrolytes, there is interfacial instability caused by REDOX reactions between the electrolyte and the electrode, especially at high charge voltage or high temperature, which also leads to a large interfacial impedance generated by limited solid–solid rigid contact. Liu et al. developed a solid electrolyte called polymer-in-salt (PISSE) using PVDF-HFP. They used an integrated TiO2/Li SSEs 3D fully permeable solid electrolyte model to construct it. PISSE exhibited faster Li+ transport compared to conventional polymer electrolytes due to the formation of unique ion channels through aggregated ionic clusters, as shown in (Fig. 14b). Among a group of polymer electrolytes, this PISSE demonstrated the most favorable overall performance.182
The formation of a homogenized electrolyte. Zhang et al. designed impressive composite SSEs that incorporate a PVDF-HFP matrix for good mechanical properties and Li salt solubility, a high content of lithium bis(fluorosulfonyl) imide (LiFSI) for an additional Li+ hopping transmission path, and LLZTO filler for improved electrochemical stability, as shown in (Fig. 14c). As a result, this polymer-in-salt composite SSEs has an outstanding ionic conductivity of 1.67 × 10−3 S cm−1 and a superior critical current density (CCD) of 3.2 mA cm−2 at room temperature (25 °C), as illustrated in (Fig. 14d). Furthermore, the symmetric Li/Li battery displayed consistent polarization for up to 240 h at low current density and low capacity, as shown in (Fig. 14e).183
The researchers, Zhao et al. aimed to enhance the ionic conductivity at room temperature and optimize the charge/discharge performance for developing reusable polymer electrolytes (PEs). To achieve this, they utilized polyvinylidene fluoride PVDF and a copolymer of PVDF-HFP, along with polymerized methyl methacrylate (MMA) monomers, as substrates in the preparation of the LiTFSI/OMMT/PVDF/PVDF-HFP/PMMA. The resulting LOPPM exhibited interconnected lithium-ion 3D network channels. The incorporation of organic-modified montmorillonite, which is rich in Lewis acid centers, facilitated the dissociation of lithium salt. (Fig. 14g) demonstrated how lithium ions could move within the composite system consisting of polymer-combined montmorillonite (OMMT). Further analysis revealed an excess of negatively charged adsorbed cations on the surface of each lamella, as depicted in (Fig. 14h). Through compatibility with PVDF, OMMT was introduced into the PVDF-HFP block PVDF, leading to a material that was uniformly dispersed with unique multi-interfacial channels.184
7. Conclusion and prospect
In recent times, researchers have been increasingly focused on advancing the research of safe SSEs because of the inherent limitations of liquid electrolytes, such as their susceptibility to leakage, flammability, and toxicity. SSEs-based batteries are considered promising contenders for replacing current liquid batteries in the next generation. The key driver for the promotion of the applications of advanced ASSLBs lies in the development of high-performance materials specifically designed for solid-state electrolytes. In this regard, polymer electrolytes with exceptional processability can be seen as potential candidates for ASSLBs because of their remarkable chemical stability, optimal working range, and ionic conductivity.This paper presents a comprehensive examination of the application of polymers with various chemical compositions in solid-state lithium batteries. The addition of inorganic fillers enhances the noncrystalline phase, although it poses challenges in terms of agglomeration and the achievement of uniform electrolytes. However, by incorporating a small quantity of liquid plasticizer modification, there is a significant improvement in ionic conductivity at the expense of the meticulous stability. Consequently, functional structures are devised through meticulous selection of polymers with distinct chemical architectures, such as one-dimensional nanofibers, two-dimensional nanolayers, and three-dimensional cross-linked skeletal frameworks.
Secondly, to increase the ionic conductivity of solid polymer electrolytes to that of liquid electrolytes, it is essential to establish extensive pathways for the transport of ion within the filler material. Regulation of ion transport is heavily dependent on factors such as the degree of crystallinity in the polymer matrix, considerations of free energy, and interactions among chain segments. Additionally, optimizing the interaction between the polymer electrolyte and the lithium salt can also significantly enhance cell performance. This objective can be accomplished through various approaches including incorporating various types of lithium salts and employing strategies involving polar salting.
Finally, the research progress of SPE in recent years is reviewed, including the types of polymer matrix, the corresponding modification methods, the interface detection, and the factors affecting the interface between solid polymer electrolyte and anode. We hope that this review provides an effective way to predict important breakthroughs in the development of new polymers, the addition of new functional polymers, or the use of additives in the near future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by S&T Program of Hebei (216Z4405G, 226Z4303G), the Science and Technology Project of Hebei Education Department (ZD2022080), the Program for the Young Top Talents of Hebei Province.References
- J. B. Goodenough and Y. Kim, Chem. Mater., 2010, 22, 587–603 CrossRef CAS.
- J. Janek and W. G. Zeier, Nat. Energy, 2016, 1, 16141 CrossRef.
- C.-M. Park and H.-J. Sohn, Adv. Mater., 2007, 19, 2465–2468 CrossRef CAS.
- T. Hasegawa, F. Bai, D. Mori, S. Taminato, Y. Takeda, O. Yamamoto, H. Izumi, H. Minami and N. Imanishi, ChemElectroChem, 2023, 10, e202201043 CrossRef CAS.
- Y. Yao, X. Zhang, B. Li, C. Yan, P. Chen, J. Huang and Q. Zhang, Materials, 2020, 2, 379–388 CAS.
- Q. Kang, Z. Zhuang, Y. Liu, Z. Liu, Y. Li, B. Sun, F. Pei, H. Zhu, H. Li, P. Li, Y. Lin, K. Shi, Y. Zhu, J. Chen, C. Shi, Y. Zhao, P. Jiang, Y. Xia, D. Wang and X. Huang, Adv. Mater., 2023, 2303460 CrossRef CAS PubMed.
- Z. Wang, L. Shen, S. Deng, P. Cui and X. Yao, Adv. Mater., 2021, 33, 2100353 CrossRef CAS PubMed.
- J. Hu, P. He, B. Zhang, B. Wang and L.-Z. Fan, Energy Storage Mater., 2020, 26, 283–289 CrossRef.
- Q. Guo, F. Xu, L. Shen, Z. Wang, J. Wang, H. He and X. Yao, J. Power Sources, 2021, 498, 229934 CrossRef CAS.
- Z. Gao, H. Sun, L. Fu, F. Ye, Y. Zhang, W. Luo and Y. Huang, Promises, Adv. Mater., 2018, 30, 1705702 CrossRef PubMed.
- Q. Guo, F. Xu, L. Shen, S. Deng, Z. Wang, M. Li and X. Yao, Energy Mater. Adv., 2022, 2022, 9753506 Search PubMed.
- S. Tang, W. Guo and Y. Fu, Adv. Energy Mater., 2021, 11, 2000802 CrossRef CAS.
- T. K. Schwietert, V. A. Arszelewska, C. Wang, C. Yu, A. Vasileiadis, N. J. J. De Klerk, J. Hageman, T. Hupfer, I. Kerkamm, Y. Xu, E. Van Der Maas, E. M. Kelder, S. Ganapathy and M. Wagemaker, Nat. Mater., 2020, 19, 428–435 CrossRef CAS PubMed.
- J. Mindemark, M. J. Lacey, T. Bowden and D. Brandell, Prog. Polym. Sci., 2018, 81, 114–143 CrossRef CAS.
- J. Wu, L. Shen, Z. Zhang, G. Liu, Z. Wang, D. Zhou, H. Wan, X. Xu and X. Yao, Electrochem. Energy Rev., 2021, 4, 101–135 CrossRef CAS.
- P. Fan, H. Liu, V. Marosz, N. T. Samuels, S. L. Suib, L. Sun and L. Liao, Adv. Funct. Mater., 2021, 31, 2101380 CrossRef CAS.
- N. Piao, S. Liu, B. Zhang, X. Ji, X. Fan, L. Wang, P.-F. Wang, T. Jin, S.-C. Liou, H. Yang, J. Jiang, K. Xu, M. A. Schroeder, X. He and C. Wang, ACS Energy Lett., 2021, 6, 1839–1848 CrossRef CAS.
- F. Xu, S. Deng, Q. Guo, D. Zhou and X. Yao, Small Methods, 2021, 5, 2100262 CrossRef CAS PubMed.
- N. Lu, X. Jing, Y. Xu, W. Lu, K. Liu and Z. Zhang, ACTA PHYS. SIN.-CH ED, 2023, 2207045 Search PubMed.
- J. Wu, S. Liu, F. Han, X. Yao and C. Wang, Adv. Mater., 2021, 33, 2000751 CrossRef CAS PubMed.
- J. Chen, J. Wu, X. Wang, A. Zhou and Z. Yang, Energy Storage Mater., 2021, 35, 70–87 CrossRef.
- N. Lu, X. Jing, J. Zhang, P. Zhang, Q. Qiao and Z. Zhang, Chem. Eng. J., 2022, 431, 134001 CrossRef CAS.
- Y. Huang, K. Dai, J. Zhang and G. Dawson, Chin. J. Catal., 2022, 43, 2539–2547 CrossRef CAS.
- L. Ye and X. Li, Nature, 2021, 593, 218–222 CrossRef CAS PubMed.
- M. Jiang, G. Liu, Q. Zhang, D. Zhou and X. Yao, ACS Appl. Mater. Interfaces, 2021, 13, 18666–18672 CrossRef CAS PubMed.
- W. Li, Y. Li, X. Liu, Z. Gu, H. Liang, X. Zhao, J. Guo and X. Wu, Adv. Funct. Mater., 2022, 32, 2201038 CrossRef CAS.
- N. Zhang, J. He, W. Han and Y. Wang, J. Mater. Sci., 2019, 54, 9603–9612 CrossRef CAS.
- P. Dhatarwal and R. J. Sengwa, Compos. Commun., 2020, 17, 182–191 CrossRef.
- W. Liu, S. W. Lee, D. Lin, F. Shi, S. Wang, A. D. Sendek and Y. Cui, Nat. Energy, 2017, 2, 17035 CrossRef CAS.
- Q. Zhou, Q. Li, S. Liu, X. Yin, B. Huang and M. Sheng, J. Power Sources, 2021, 482, 228929 CrossRef CAS.
- V. Augustyn, J. Come, M. A. Lowe, J. W. Kim, P.-L. Taberna, S. H. Tolbert, H. D. Abruña, P. Simon and B. Dunn, Nat. Mater., 2013, 12, 518–522 CrossRef CAS PubMed.
- Y. Lu, X. Huang, Z. Song, K. Rui, Q. Wang, S. Gu, J. Yang, T. Xiu, M. E. Badding and Z. Wen, Energy Storage Mater., 2018, 15, 282–290 CrossRef.
- X. Jiang, J. Huang, Z. Bi, W. Ni, G. Gurzadyan, Y. Zhu and Z. Zhang, Adv. Mater., 2022, 34, 2109330 CrossRef CAS PubMed.
- H. Song, S. Xue, S. Chen, Z. Wang, Y. Song, J. Li, Z. Song, L. Yang and F. Pan, Chin. J. Struct. Chem., 2022, 41, 2205048–2205054 CAS.
- K. Nie, X. Wang, J. Qiu, Y. Wang, Q. Yang, J. Xu, X. Yu, H. Li, X. Huang and L. Chen, ACS Energy Lett., 2020, 5, 826–832 CrossRef CAS.
- J. Bae, Y. Li, J. Zhang, X. Zhou, F. Zhao, Y. Shi, J. B. Goodenough and G. Yu, Angew. Chem., Int. Ed., 2018, 57, 2096–2100 CrossRef CAS PubMed.
- X. Xu, L. Wang, H. Fei and L. Ci, J. Mater. Sci.: Mater. Electron., 2019, 30, 19119–19125 CrossRef CAS.
- Z. Bi, S. Mu, N. Zhao, W. Sun, W. Huang and X. Guo, Energy Storage Mater., 2021, 35, 512–519 CrossRef.
- S. Yi, T. Xu, L. Li, M. Gao, K. Du, H. Zhao and Y. Bai, Solid State Ionics, 2020, 355, 115419 CrossRef CAS.
- B. Ziebarth, M. Klinsmann, T. Eckl and C. Elsässer, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 174301 CrossRef.
- A. Muralidharan, M. I. Chaudhari, L. R. Pratt and S. B. Rempe, Sci. Rep., 2018, 8, 10736 CrossRef PubMed.
- A. Muralidharan, M. Chaudhari, S. Rempe and L. R. Pratt, ECS Trans., 2017, 77, 1155–1162 CrossRef CAS.
- B. Vorselaars, A. V. Lyulin, K. Karatasos and M. A. J. Michels, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2007, 75, 011504 CrossRef PubMed.
- X. Meng, Y. Liu, Y. Ma, Y. Boyjoo, J. Liu, J. Qiu and Z. Wang, Adv. Mater., 2023, 35, 2212039 CrossRef CAS PubMed.
- B. Zhao, R. Ran, M. Liu and Z. Shao, Mater. Sci. Eng., R, 2015, 98, 1–71 CrossRef.
- J. Jung, J. Ku, Y. S. Park, C.-H. Ahn, J.-H. Lee, S. S. Hwang and A. S. Lee, Polym. Rev., 2022, 62, 789–825 CrossRef CAS.
- W. Li, Y. Li, X. Liu, Z. Gu, H. Liang, X. Zhao, J. Guo and X. Wu, Adv. Funct. Mater., 2022, 32, 2201038 CrossRef CAS.
- M. Basappa, H. Ganesha, S. Veeresh, Y. S. Nagaraju, M. Vandana, H. Vijeth and H. Devendrappa, Chem. Phys. Lett., 2022, 799, 139609 CrossRef CAS.
- A. Muralidharan, M. I. Chaudhari, S. B. Rempe and L. R. Pratt, ECS Trans., 2017, 77, 1155–1162 CrossRef CAS.
- B. Vorselaars, A. V. Lyulin, K. Karatasos and M. A. Michels, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2007, 75, 011504 CrossRef PubMed.
- A. Muralidharan, M. I. Chaudhari, L. R. Pratt and S. B. Rempe, Sci. Rep., 2018, 8, 10736 CrossRef PubMed.
- C. Monroe and J. Newman, J. Electrochem. Soc., 2003, 150, 10–A1377 CrossRef.
- Y. Lu, X. Huang, Z. Song, K. Rui, Q. Wang, S. Gu, J. Yang, T. Xiu and M. E. Badding, Energy Storage Mater., 2018, 15, 282–290 CrossRef.
- P. Barai, K. Higa, A. T. Ngo, L. A. Curtiss and V. Srinivasan, J. Electrochem. Soc., 2019, 166, A1752–A1762 CrossRef CAS.
- S. Yu and D. J. Siegel, ACS Appl. Mater. Interfaces, 2018, 10, 38151–38158 CrossRef CAS PubMed.
- X. Huang, Y. Lu, Z. Song, T. Xiu, M. E. Badding and Z. Wen, J. Energy Chem., 2019, 39, 8–16 CrossRef.
- Y. Lu, X. Huang, Y. Ruan, Q. Wang, R. Kun, J. Yang and Z. Wen, J. Mater. Chem. A, 2018, 6, 18853–18858 RSC.
- X. Huang, Y. Lu, Z. Song, K. Rui, Q. Wang, T. Xiu, M. E. Badding and Z. Wen, Energy Storage Mater., 2019, 22, 207–217 CrossRef.
- W. Ping, C. Wang, Z. Lin, E. Hitz, C. Yang, H. Wang and L. Hu, Adv. Energy Mater., 2020, 10, 2000702 CrossRef CAS.
- S. Lee, K. Lee, S. Kim, K. Yoon, S. Han, M. H. Lee, Y. Ko, J. H. Noh, W. Kim and K. Kang, Sci. Adv., 2022, 8, eabq0153 CrossRef CAS PubMed.
- H. Xu, J. Zhang, H. Zhang, J. Long, L. Xu and L. Mai, Adv. Energy Mater., 2023, 13, 2204411 CrossRef CAS.
- L. Hu, X. Gao, Z. Li, Y. Liu, H. Wang, J. Liu and R. Hu, ACS Appl. Mater. Interfaces, 2023, 15, 38485–38495 CrossRef CAS PubMed.
- M. Ge, X. Zhou, Y. Qin, Y. Liu, J. Zhou, X. Wang and B. Guo, Chin. Chem. Lett., 2022, 33, 3894–3898 CrossRef CAS.
- X. Zhang, Y. Sun, C. Ma, N. Guo, H. Fan, J. Liu and H. Xie, J. Power Sources, 2022, 542, 231797 CrossRef CAS.
- Z. Bi, S. Mu, N. Zhao, W. Sun, W. Huang and X. Guo, Energy Storage Mater., 2021, 35, 512–519 CrossRef.
- R. Kumar, S. Sharma, D. Pathak, N. Dhiman and N. Arora, Solid State Ionics, 2017, 305, 57–62 CrossRef CAS.
- J. Chen and S. Han, Chem. Eng. J., 2023, 470, 144150 CrossRef CAS.
- J. Wei, X. Zheng, W. Lin, Y. Si, K. Ji, C. Wang and M. Chen, J. Alloys Compd., 2022, 909, 164825 CrossRef CAS.
- Y. G. Choi, J. C. Shin, A. Park, Y. M. Jeon, J. I. Kim, S. Kim, S. Kim, W. B. Lee, M. Lee and J. H. Park, Adv. Energy Mater., 2021, 11, 2102660 CrossRef CAS.
- S. Li, K. Jiang, J. Wang, C. Zuo, Y. H. Jo, D. He, X. Xie and Z. Xue, Macromolecules, 2019, 52, 7234–7243 CrossRef CAS.
- S. Y. An, X. Wu, Y. Zhao, T. Liu, R. Yin, J. H. Ahn, L. M. Walker, J. F. Whitacre and K. Matyjaszewski, Adv. Sci., 2023, e2302932 CrossRef PubMed.
- F. Fu, Y. Zheng, N. Jiang, Y. Liu, C. Sun, A. Zhang, H. Teng, L. Sun and H. Xie, Chem. Eng. J., 2022, 450, 137776 CrossRef CAS.
- Y. Xu, J. Li and W. Li, Colloids Surf., A, 2022, 632, 127773 CrossRef CAS.
- Q. Ye, H. Liang, S. Wang, C. Cui, C. Zeng, T. Zhai and H. Li, J. Energy Chem., 2022, 70, 356–362 CrossRef CAS.
- S. J. Wen, T. J. Richardson, D. I. Ghantous, K. A. Striebel, P. N. Ross and E. J. Cairns, J. Electroanal. Chem., 1996, 408, 113–118 CrossRef.
- Y. Mallaiah, V. R. Jeedi, R. Swarnalatha, A. Raju and S. Narender Reddy, J. Phys. Chem. Solids, 2021, 155, 110096 CrossRef CAS.
- J. Qiu, X. Liu, R. Chen, Q. Li, Y. Wang, P. Chen, L. Gan, S. J. Lee, D. Nordlund, Y. Liu, X. Yu, X. Bai, H. Li and L. Chen, Adv. Funct. Mater., 2020, 30, 1909392 CrossRef CAS.
- S. Wang, Y. Chen, Q. Fang, J. Huang, X. Wang, S. Chen and S. Zhang, Energy Storage Mater., 2023, 54, 596–604 CrossRef.
- D. E. Fenton, J. M. Parker and P. V. Wright, Polymer, 1973, 14, 589 CrossRef CAS.
- D. Cai, D. Wang, Y. Chen, S. Zhang, X. Wang, X. Xia and J. Tu, Chem. Eng. J., 2020, 394, 124993 CrossRef CAS.
- V. Selvanathan, M. N. A. Halim, A. D. Azzahari, M. Rizwan, N. Shahabudin and R. Yahya, Ionics, 2018, 24, 1955–1964 CrossRef CAS.
- F. Li, B. Su, L. Shi, J. Mu, F. Xu, J. Wang, H. Yang and Z. Guo, Ceram. Int., 2023, 49, 26604–26615 CrossRef CAS.
- X. Cai, J. Ding, Z. Chi, W. Wang, D. Wang and G. Wang, ACS Nano, 2021, 15, 20489–20503 CrossRef CAS PubMed.
- H. Zhuang, W. Ma, J. Xie, X. Liu, B. Li, Y. Jiang, S. Huang, Z. Chen and B. Zhao, J. Alloys Compd., 2021, 860, 157915 CrossRef CAS.
- J. Yin, X. Xu, S. Jiang, Y. Lei and Y. Gao, J. Power Sources, 2022, 550, 232139 CrossRef CAS.
- A. Kondori, M. Esmaeilirad, A. M. Harzandi, R. Amine, M. T. Saray, L. Yu and M. Asadi, Science, 2023, 379, 499–505 CrossRef CAS PubMed.
- M. Zhang, K. Zhou, D. Ma, H. Wang, X. Tang, M. Bai, F. Liu, Z. Wang and Y. Ma, Mater. Today, 2022, 56, 53–65 CrossRef CAS.
- P. Chen, J. Shen, T. Wang, M. Dai, C. Si, J. Xie, M. Li, X. Cong and X. Sun, J. Power Sources, 2018, 400, 325–332 CrossRef CAS.
- J. Shen, Z. Lei and C. Wang, Chem. Eng. J., 2022, 447, 137503 CrossRef CAS.
- L. Gao, S. Luo, J. Li, B. Cheng, W. Kang and N. Deng, Energy Storage Mater., 2021, 43, 266–274 CrossRef.
- L. Gao, J. Li, J. Ju, B. Cheng, W. Kang and N. Deng, J. Energy Chem., 2021, 54, 644–654 CrossRef CAS.
- Q. Yang, G. Li, D. Shi, L. Gao, N. Deng, W. Kang and B. Cheng, Small, 2023, 2301521 CrossRef CAS PubMed.
- Y. Zhao, J. Yan, W. Cai, Y. Lai, J. Song, J. Yu and B. Ding, Energy Storage Mater., 2019, 23, 306–313 CrossRef.
- J. Shin, W.-H. Ryu, K.-S. Park and I.-D. Kim, ACS Nano, 2013, 7, 7330–7341 CrossRef CAS PubMed.
- Y. Li, Z. Fu, S. Lu, X. Sun, X. Zhang and L. Weng, Chem. Eng. J., 2022, 440, 135816 CrossRef CAS.
- H. Y. Sun, Y. Takeda, N. Imanishi, O. Yamamoto and H.-J. Sohn, J. Electrochem. Soc., 2000, 147, 2462 CrossRef CAS.
- T. Itoh, Solid State Ionics, 2003, 156, 393–399 CrossRef CAS.
- T. Itoh, Y. Miyamura, Y. Ichikawa, T. Uno, M. Kubo and O. Yamamoto, J. Power Sources, 2003, 119–121, 403–408 CrossRef CAS.
- J. Kang, Z. Yan, L. Gao, Y. Zhang, W. Liu, Q. Yang, Y. Zhao, N. Deng, B. Cheng and W. Kang, Energy Storage Mater., 2022, 53, 192–203 CrossRef.
- H. Chen, D. Adekoya, L. Hencz, J. Ma, S. Chen, C. Yan, H. Zhao, G. Cui and S. Zhang, Adv. Energy Mater., 2020, 10, 2000049 CrossRef CAS.
- L. Zhang, N. Deng, J. Kang, X. Wang, H. Gao, Y. Liu, H. Wang, G. Wang, B. Cheng and W. Kang, J. Energy Chem., 2023, 77, 326–337 CrossRef CAS.
- B. Xu, X. Li, C. Yang, Y. Li, N. S. Grundish, P.-H. Chien, K. Dong, I. Manke, R. Fang, N. Wu, H. Xu, A. Dolocan and J. B. Goodenough, J. Am. Chem. Soc., 2021, 143, 6542–6550 CrossRef CAS PubMed.
- X. Wang, Y. Song, X. Jiang, Q. Liu, J. Dong, J. Wang, X. Zhou, B. Li, G. Yin, Z. Jiang and J. Wang, Adv. Funct. Mater., 2022, 32, 2113068 CrossRef CAS.
- L. Xu, X. Xiao, H. Tu, F. Zhu, J. Wang, H. Liu, W. Huang, W. Deng, H. Hou, T. Liu, X. Ji, K. Amine and G. Zou, Adv. Mater., 2023, 2303193 CrossRef CAS PubMed.
- Q. Pan, Y. Zheng, S. Kota, W. Huang, S. Wang, H. Qi, S. Kim, Y. Tu, M. W. Barsoum and C. Y. Li, Nanoscale Adv., 2019, 1, 395–402 RSC.
- Y. Shi, B. Li, Q. Zhu, K. Shen, W. Tang, Q. Xiang, W. Chen, C. Liu, J. Luo and S. Yang, Adv. Energy Mater., 2020, 10, 1903534 CrossRef CAS.
- Y. Zhang, Y. Wu, Y. Liu and J. Feng, Chem. Eng. J., 2022, 428, 131040 CrossRef CAS.
- Z. Li, W.-X. Sha and X. Guo, ACS Appl. Mater. Interfaces, 2019, 11, 26920–26927 CrossRef CAS PubMed.
- H. Zhai, P. Xu, M. Ning, Q. Cheng, J. Mandal and Y. Yang, Nano Lett., 2017, 17, 3182–3187 CrossRef CAS PubMed.
- J. Bae, Y. Li, F. Zhao, X. Zhou, Y. Ding and G. Yu, Energy Storage Mater., 2018, 15, 46–52 CrossRef.
- X. Zuo, K. Chang, J. Zhao, Z. Xie, H. Tang, B. Li and Z. Chang, J. Mater. Chem. A, 2016, 4, 51–58 RSC.
- Y. Yan, J. Ju, S. Dong, Y. Wang, L. Huang, L. Cui, F. Jiang, Q. Wang, Y. Zhang and G. Cui, Adv. Sci., 2021, 8, 2003887 CrossRef CAS PubMed.
- X. Wang, H. Zhai, B. Qie, Q. Cheng, A. Li, J. Borovilas, B. Xu, C. Shi, T. Jin, X. Liao, Y. Li, X. He, S. Du, Y. Fu, M. Dontigny, K. Zaghib and Y. Yang, Nano Energy, 2019, 60, 205–212 CrossRef.
- S. Cheng, D. M. Smith and C. Y. Li, Macromolecules, 2015, 48, 4503–4510 CrossRef CAS.
- X. Tian and B. Xu, Small Methods, 2021, 5, 2100877 CrossRef CAS PubMed.
- G. Wang, H. Liu, Y. Liang, C. Wang and L.-Z. Fan, Energy Storage Mater., 2022, 45, 1212–1219 CrossRef.
- Y. Liu, L. Han, C. Liao, H. Yu, Y. Kan and Y. Hu, Chem. Eng. J., 2023, 468, 143222 CrossRef CAS.
- D. Lin, P. Y. Yuen, Y. Liu, W. Liu, N. Liu, R. H. Dauskardt and Y. Cui, Adv. Mater., 2018, 30, 1802661 CrossRef PubMed.
- H. Yang, X. Chen, N. Yao, N. Piao, Z. Wang, K. He, H.-M. Cheng and F. Li, ACS Energy Lett., 2021, 1413–1421 CrossRef CAS.
- S.-J. Yang, N. Yao, X.-Q. Xu, F.-N. Jiang, X. Chen, H. Liu, H. Yuan, J.-Q. Huang and X.-B. Cheng, J. Mater. Chem. A, 2021, 9, 19664–19668 RSC.
- N. Piao, S. Liu, B. Zhang, X. Ji, X. Fan, L. Wang, P.-F. Wang, T. Jin, S.-C. Liou, H. Yang, J. Jiang, K. Xu, M. A. Schroeder, X. He and C. Wang, ACS Energy Lett., 2021, 6, 1839–1848 CrossRef CAS.
- D. Xiao, Q. Li, D. Luo, G. Li, H. Liu, L. Shui, S. Gourley, G. Zhou, X. Wang and Z. Chen, Small, 2020, 16, 2004688 CrossRef CAS PubMed.
- J. Mindemark, E. Törmä, B. Sun and D. Brandell, Polymer, 2015, 63, 91–98 CrossRef CAS.
- M. P. Rosenwinkel, R. Andersson, J. Mindemark and M. Schönhoff, J. Phys. Chem. C, 2020, 124, 23588–23596 CrossRef CAS.
- J. Mindemark, B. Sun, E. Törmä and D. Brandell, J. Power Sources, 2015, 298, 166–170 CrossRef CAS.
- B. Zhang, Y. Liu, J. Liu, L. Sun, L. Cong, F. Fu, A. Mauger, C. M. Julien, H. Xie and X. Pan, J. Energy Chem., 2021, 52, 318–325 CrossRef CAS.
- C. Sångeland, G. Hernández, D. Brandell, R. Younesi, M. Hahlin and J. Mindemark, ACS Appl. Mater. Interfaces, 2022, 14, 28716–28728 CrossRef PubMed.
- Y. Chen, Y. Zhang, J. Niu, H. Xu, Z. Dong, J. Xu and C. Lei, ACS Appl. Energy Mater., 2023, 6, 3113–3125 CrossRef CAS.
- F. P. Nkosi, M. Valvo, J. Mindemark, N. A. Dzulkurnain, G. Hernández, A. Mahun, S. Abbrent, J. Brus, L. Kobera and K. Edström, ACS Appl. Energy Mater., 2021, 4, 2531–2542 CrossRef CAS.
- S.-B. Hong, Y.-J. Lee, U.-H. Kim, C. Bak, Y. M. Lee, W. Cho, H. J. Hah, Y.-K. Sun and D.-W. Kim, ACS Energy Lett., 2022, 7, 1092–1100 CrossRef CAS.
- S. T. Oyakhire, H. Gong, Y. Cui, Z. Bao and S. F. Bent, ACS Energy Lett., 2022, 7, 2540–2546 CrossRef CAS.
- E. Markevich, G. Salitra and D. Aurbach, ACS Energy Lett., 2017, 2, 1337–1345 CrossRef CAS.
- W. Huang, H. Wang, D. T. Boyle, Y. Li and Y. Cui, ACS Energy Lett., 2020, 5, 1128–1135 CrossRef CAS.
- A. M. Elmér and P. Jannasch, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 2195–2205 CrossRef.
- C. Wang, H. Liu, Y. Liang, D. Li, X. Zhao, J. Chen, W. Huang, L. Gao and L. Fan, Adv. Funct. Mater., 2023, 33, 2209828 CrossRef CAS.
- O. B. Chae and B. L. Lucht, Adv. Energy Mater., 2023, 13, 2203791 CrossRef CAS.
- K. Vignarooban, M. A. K. L. Dissanayake, I. Albinsson and B.-E. Mellander, Solid State Ionics, 2014, 266, 25–28 CrossRef CAS.
- X. Wang, Y. Zhang, X. Zhang, T. Liu, Y.-H. Lin, L. Li, Y. Shen and C.-W. Nan, ACS Appl. Mater. Interfaces, 2018, 10, 24791–24798 CrossRef CAS PubMed.
- K. He, S. H. Cheng, J. Hu, Y. Zhang, H. Yang, Y. Liu, W. Liao, D. Chen, C. Liao, X. Cheng, Z. Lu, J. He, J. Tang, R. K. Y. Li and C. Liu, Angew. Chem., Int. Ed., 2021, 60, 12116–12123 CrossRef CAS PubMed.
- X. Zuo, K. Chang, J. Zhao, Z. Xie, H. Tang, B. Li and Z. Chang, J. Mater. Chem. A, 2016, 4, 51–58 RSC.
- Y. Zheng, X. Li and C. Y. Li, Energy Storage Mater., 2020, 29, 42–51 CrossRef.
- J. Zhang, J. Yang, T. Dong, M. Zhang, J. Chai, S. Dong, T. Wu, X. Zhou and G. Cui, Small, 2018, 14, 1800821 CrossRef PubMed.
- Z. Li, H. Zhang, X. Sun and Y. Yang, ACS Energy Lett., 2020, 5, 3244–3253 CrossRef CAS.
- L. Zhu, J. Li, Y. Jia, P. Zhu, M. Jing, S. Yao, X. Shen, S. Li and F. Tu, Int. J. Energy Res., 2020, 44, 10168–10178 CrossRef CAS.
- H. Chen, M. Jing, C. Han, H. Yang, S. Hua, F. Chen, L. Chen, Z. Zhou, B. Ju, F. Tu, X. Shen and S. Qin, Int. J. Energy Res., 2019, 43, 5912–5921 CrossRef CAS.
- M. Jia, N. Zhao, Z. Bi, Z. Fu, F. Xu, C. Shi and X. Guo, ACS Appl. Mater. Interfaces, 2020, 12, 46162–46169 CrossRef CAS PubMed.
- B.-J. Sung, P. N. Didwal, R. Verma, A.-G. Nguyen, D. R. Chang and C.-J. Park, Electrochim. Acta, 2021, 392, 139007 CrossRef CAS.
- S. Luo, E. Zhao, Y. Gu, J. Huang, Z. Zhang, L. Yang and S. Hirano, Chem. Eng. J., 2021, 421, 127771 CrossRef CAS.
- S. Wang, Q. Sun, Q. Zhang, C. Li, C. Xu, Y. Ma, X. Shi, H. Zhang, D. Song and L. Zhang, Adv. Energy Mater., 2023, 13, 2204036 CrossRef CAS.
- Q. Lu, J. Fang, J. Yang, G. Yan, S. Liu and J. Wang, J. Membr. Sci., 2013, 425–426, 105–112 CrossRef CAS.
- S. Xu, Z. Sun, C. Sun, F. Li, K. Chen, Z. Zhang, G. Hou, H. Cheng and F. Li, Adv. Funct. Mater., 2020, 30, 2007172 CrossRef CAS.
- Q. Zhou, J. Ma, S. Dong, X. Li and G. Cui, Adv. Mater., 2019, 31, 1902029 CrossRef CAS PubMed.
- Q. Zhang, K. Liu, F. Ding, W. Li, X. Liu and J. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 29820–29828 CrossRef CAS PubMed.
- F. Fu, Y. Liu, C. Sun, L. Cong, Y. Liu, L. Sun and H. Xie, Energy Environ. Mater., 2023, 6, e12367 CrossRef CAS.
- M. Zuo, Z. Bi and X. Guo, Chem. Eng. J., 2023, 463, 142463 CrossRef CAS.
- D. Bao, Y. Tao, Y. Zhong, W. Zhao, M. Peng, H. Zhang and X. Sun, Adv. Funct. Mater., 2023, 33, 2213211 CrossRef CAS.
- M. J. Lee, J. Han, K. Lee, Y. J. Lee, B. G. Kim, K.-N. Jung, B. J. Kim and S. W. Lee, Nature, 2022, 601, 217–222 CrossRef CAS PubMed.
- F. Urbain, V. Smirnov, J.-P. Becker, A. Lambertz, F. Yang, J. Ziegler, B. Kaiser, W. Jaegermann, U. Rau and F. Finger, Energy Environ. Sci., 2016, 9, 145–154 RSC.
- L. N. Sim, F. C. Sentanin, A. Pawlicka, R. Yahya and A. K. Arof, Electrochim. Acta, 2017, 229, 22–30 CrossRef CAS.
- X. Wang, X. Hao, Y. Xia, Y. Liang, X. Xia and J. Tu, Science, 2019, 582, 37–47 CAS.
- N. K. Jyothi, K. K. V. Ratnam, P. N. Murthy and K. V. Kumar, Mater. Today: Proc., 2016, 3, 21–30 Search PubMed.
- R. Rojaee, S. Cavallo, S. Mogurampelly, B. K. Wheatle, V. Yurkiv, R. Deivanayagam, T. Foroozan, M. G. Rasul, S. Sharifi-Asl, A. H. Phakatkar, M. Cheng, S. Son, Y. Pan, F. Mashayek, V. Ganesan and R. Shahbazian-Yassar, Adv. Funct. Mater., 2020, 30, 1910749 CrossRef CAS.
- S. Chai, Z. Chang, Y. Zhong, Q. He, Y. Wang, Y. Wan, M. Feng, Y. Hu, W. Li, W. Wei and A. Pan, Adv. Funct. Mater., 2023, 33, 2300425 CrossRef CAS.
- P. Raghavan, X. Zhao, C. Shin, D.-H. Baek, J.-W. Choi, J. Manuel, M.-Y. Heo, J.-H. Ahn and C. Nah, J. Power Sources, 2010, 195, 6088–6094 CrossRef CAS.
- X. Wang, Y. Fang, X. Yan, S. Liu, X. Zhao and L. Zhang, Polymer, 2021, 230, 124038 CrossRef CAS.
- D. Li, L. Chen, T. Wang and L.-Z. Fan, ACS Appl. Mater. Interfaces, 2018, 10, 7069–7078 CrossRef CAS PubMed.
- X.-L. Zhang, F.-Y. Shen, X. Long, S. Zheng, Z. Ruan, Y.-P. Cai, X.-J. Hong and Q. Zheng, Energy Storage Mater., 2022, 52, 201–209 CrossRef.
- S. Bandyopadhyay, A. Gupta, R. Srivastava and B. Nandan, Chem. Eng. J., 2022, 440, 135926 CrossRef CAS.
- Z. Li, S. Wang, J. Shi, Y. Liu, S. Zheng, H. Zou, Y. Chen, W. Kuang, K. Ding, L. Chen, Y. Lan, Y. Cai and Q. Zheng, Energy Storage Mater., 2022, 47, 262–270 CrossRef.
- X. Zhang, Q. Su, G. Du, B. Xu, S. Wang, Z. Chen, L. Wang, W. Huang and H. Pang, Angew. Chem., Int. Ed., 2023, e202304947 CAS.
- J. Li, K. Zhu, Z. Yao, G. Qian, J. Zhang, K. Yan and J. Wang, Ionics, 2020, 26, 1101–1108 CrossRef CAS.
- S. Yi, T. Xu, L. Li, M. Gao, K. Du, H. Zhao and Y. Bai, Solid State Ionics, 2020, 355, 115419 CrossRef CAS.
- J. Xi, X. Qiu and L. Chen, Solid State Ionics, 2006, 177, 709–713 CrossRef CAS.
- Z. Wang, Q. Guo, R. Jiang, S. Deng, J. Ma, P. Cui and X. Yao, Chem. Eng. J., 2022, 435, 135106 CrossRef CAS.
- J. Yu, G. Zhou, Y. Li, Y. Wang, D. Chen and F. Ciucci, Small, 2023, 2302691 CrossRef CAS PubMed.
- K. Yang, L. Chen, J. Ma, Y. He and F. Kang, InfoMat, 2021, 3, 1195–1217 CrossRef CAS.
- W. Guo, S. Liu, X. Guan, X. Zhang, X. Liu and J. Luo, Adv. Energy Mater., 2019, 9, 1900193 CrossRef.
- P. Shi, J. Ma, M. Liu, S. Guo, Y. Huang, S. Wang, L. Zhang, L. Chen, K. Yang, X. Liu, Y. Li, X. An, D. Zhang, X. Cheng, Q. Li, W. Lv, G. Zhong, Y.-B. He and F. Kang, Nat. Nanotechnol., 2023, 18, 602–610 CrossRef CAS PubMed.
- B.-H. Kang, S.-F. Li, J. Yang, Z.-M. Li and Y.-F. Huang, ACS Nano, 2023, 17, 14114–14122 CrossRef CAS PubMed.
- Q. Kang, Y. Li, Z. Zhuang, D. Wang, C. Zhi, P. Jiang and X. Huang, J. Energy Chem., 2022, 69, 194–204 CrossRef CAS.
- P. Zhai, Z. Yang, Y. Wei, X. Guo and Y. Gong, Adv. Energy Mater., 2022, 12, 2200967 CrossRef CAS.
- W. Liu, C. Yi, L. Li, S. Liu, Q. Gui, D. Ba, Y. Li, D. Peng and J. Liu, Angew. Chem., Int. Ed., 2021, 60, 12931–12940 CrossRef CAS PubMed.
- J. Zhang, Y. Zeng, Q. Li, Z. Tang, D. Sun, D. Huang, L. Zhao, Y. Tang and H. Wang, Energy Storage Mater., 2023, 54, 440–449 CrossRef.
- Y. Zhao, L. Li, Y. Shan, D. Zhou, X. Chen, W. Cui and H. Wang, Small, 2023, 2301572 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |