Chemical doping-assisted shape transformation of block copolymer particles†
Zhengping
Tan‡
a,
Jinseok
Park‡
 a,
Sang Hoon
Han
a,
Tan Ngoc-Lan
Phan
a,
Younghyeon
Ahn
a,
Meng
Xu
a,
Shin-Hyun
Kim
a,
Sang Hoon
Han
a,
Tan Ngoc-Lan
Phan
a,
Younghyeon
Ahn
a,
Meng
Xu
a,
Shin-Hyun
Kim
 a,
Jaeman J.
Shin
a,
Jaeman J.
Shin
 *b and
Bumjoon J.
Kim
*b and
Bumjoon J.
Kim
 *a
*a
aDepartment of Chemical and Biomolecular Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 34141, Republic of Korea. E-mail: bumjoonkim@kaist.ac.kr
bDepartment of Materials Science and Engineering, Soongsil University, Seoul 06978, Republic of Korea. E-mail: jshin@ssu.ac.kr
First published on 24th July 2024
Abstract
Shape-tunable block copolymer (BCP) particles have attracted significant attention due to their applications as smart materials. Herein, we report iodine (I2)-mediated doping of polystyrene-block-polybutadiene (PS-b-PB) particles as a facile strategy to transform spherical BCP particles into nonspherical shaped particles. Upon introducing the I2 molecules to internally phase-separated spherical PS-b-PB particles, I2 selectively reacts with the double bonds in PB blocks while leaving the PS block unaffected. Monitoring the shape-transformation process reveals a gradual structural transition from spheres to oblates and ellipsoids as a function of reaction time, consistent with the increasing conversion of the double bonds into I2-doped cation radicals. Consequently, the interfacial tension of I2-doped PB blocks decreases, neutralizing the interfacial interaction of BCP and its surroundings, thereby restructuring the spherical particles into shape-anisotropic forms. Furthermore, the versatility of I2-mediated shape transition is demonstrated by applying the same chemistry to polystyrene-block-poly(4-vinylpyridine) BCP particles. Finally, spheroidal particles doped with I2 exhibit photothermal heating behavior, highlighting their potential application as photothermal agents.
Introduction
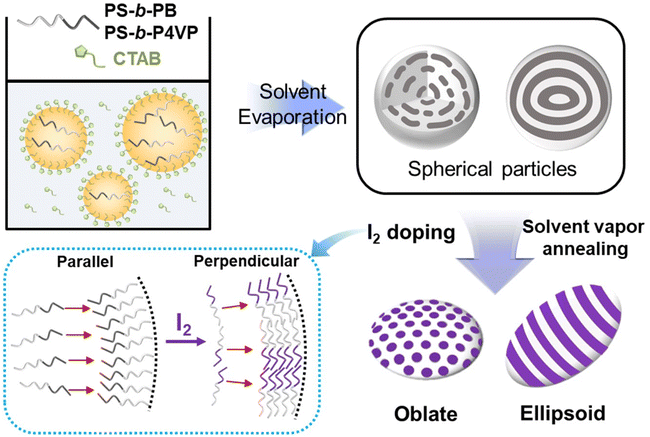
Colloidal polymer particles with controlled shapes and well-defined internal structures have attracted significant attention due to their unique shape-dependent physical and optical properties.1–4 Confined self-assembly of block copolymers (BCPs) in evaporative oil-in-water emulsions has been adopted as a robust and versatile method for producing anisotropic polymeric particles.5–9 In this approach, the BCP droplets act as soft and mobile templates that undergo spontaneous deformation driven by the minimization of interfacial energy and chain stretching/bending penalty.10,11 Recently, extensive efforts have been made to develop responsive BCP particles capable of shape transformation in response to external stimuli such as temperature, pH, or light.12–19 In these systems, BCP particles undergo a reversible transition from spheres to ellipsoids or oblates under external stimuli, and this capability establishes controllable shape-dependent physicochemical properties. Consequently, these smart BCP particles hold promise for a range of potential applications as smart nanomaterials including sensors, detection, and coating.20,21Surfactant engineering stands out as the most widely adopted strategy for generating nonspherical BCP particles by controlling the interfacial interaction between BCPs and the surrounding medium.10,22–26 The key to this strategy lies in the precise selection or design of dual surfactants that favorably interact with a specific BCP domain, allowing for tunable interfacial tensions between each block and the aqueous surrounding through a mixture of surfactants. Accordingly, a neutral wetting condition between the BCPs and the surrounding media can be established to form spheroidal BCP particles, including ellipsoids and oblates. For example, pioneering work from Hawker et al. used CTAB/CTAB–OH as dual surfactants to form ellipsoidal particles of polystyrene-block-poly(2-vinyl pyridine) (PS-b-P2VP), driven by the selective affinity of CTAB and CTAB–OH for the PS and P2VP blocks, respectively.25 Based on the preferential interaction of CTAB–OH and P2VP via hydrogen bonding of the hydroxyl group with pyridine units, other similar surfactants were designed and applied for controlling the particle shape in the scope of BCPs containing P2VP or poly(4-vinylpyridine) (P4VP) blocks.26–28 However, the applicability of this strategy is limited to BCPs consisting of highly immiscible blocks (i.e., high Flory–Huggins interaction parameter (χ)). Thus, examples of using a mixture of surfactants to non-selective low-χ BCPs are rarely reported.29 Alternatively, the shape transition of low-χ BCP particles has been achieved by carefully controlling the solvent evaporation rate from BCP-containing emulsions. However, as anisotropic particles in these works are kinetically controlled, and the obtained shapes and morphologies are meta-stable.30–34
Polystyrene-block-polybutadiene (PS-b-PB) represents an example of low-χ BCPs, which can serve as an important building block for generating self-assembled nanomaterials, including the PS-b-PB-b-PS (SBS) thermoplastic elastomers that are extensively utilized across various industries.35–37 In particular, chemical modification of PB has been utilized to tune the physicochemical properties of PS-b-PB BCPs in bulk or thin films,38–40 as the diene group in PB can be easily functionalized with various chemistries.41–44 Nevertheless, there has been less focus on establishing a reliable platform to fabricate shape-transforming PS-b-PB particles via diene group modification, potentially due to difficulty in particle shape transition through chemical modification. For example, thiol–ene chemistry45,46 was employed to modify PS-b-PB particles by selectively conjugating functional molecules on the PB block, however, the resulting shape transition was minimal or incomplete.47,48 Also, side reactions (e.g. bimolecular termination) can occur under typical thiol–ene reaction conditions, which can significantly hinder the particle shape transformation.49 In this regard, an effective chemical modification process should be developed to fabricate responsive PS-b-PB particles with controllable shapes and internal structures, considering the commercial significance of PS-b-PB BCPs.
Herein, we report shape transitions of PS-b-PB BCP particles based on selective I2-doping in PB domains. The spherical BCP particles are first prepared from an evaporative oil-in-water emulsion, followed by reconstructing colloidal particles using dichloromethane/iodine (DCM/I2) vapor annealing. During this process, I2 selectively reacts with the double bonds in PB domains and modulates the interfacial tension, allowing for the shape transition of BCP particles from spheres into oblate and prolate spheroids. Longer reaction time leads to a higher degree of double bond conversion into iodine-doped cation radicals, which is consistent with the gradual shape transformation of BCP spheres into oblates and prolate ellipsoids as a function of reaction time. We revealed that shape transition is driven by the decrease in interfacial tension between the PB domain and surroundings, achieving a neutral interaction of PS/surrounding and PB/surrounding at a certain level of I2 doping. The versatility of our method is demonstrated by applying the same strategy to PS-b-P4VP BCPs with various molecular weight and volume fractions. For all BCPs, the I2-doping has successfully demonstrated the shape transformation of spherical particles into oblates or prolate ellipsoids. Finally, I2-doped ellipsoidal particles exhibit excellent photothermal behavior compared to pristine spherical particles, highlighting the potential application of chemically modified BCP particles.
Experimental section
Materials
PS35k-b-PB11k (subscripts indicate the number-average molecular weight (Mn), polydispersity index (Đ) = 1.12), PS34k-b-PB25k, (Đ = 1.12), PS10k-b-P4VP10k (Đ = 1.08), PS15k-b-P4VP7k (Đ = 1.18), PS24k-b-P4VP51k (Đ = 1.15), PS30k (Đ = 1.15), PB11k (Đ = 1.12) were purchased from Polymer Source, Inc. Cetyltrimethylammonium bromide (CTAB) and iodine (I2) were purchased from Sigma-Aldrich and used without further purification.Preparation of spherical BCP particles
A DCM solution containing PS-b-PB or PS-b-P4VP (10 mg mL−1) was prepared and stirred at room temperature for 12 h. Then, the polymer solution (0.1 mL) was emulsified with 1 mL of surfactant-containing aqueous solution (10 mg mL−1, CTAB) by vortexing at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 min. Subsequently, DCM was evaporated at room temperature for 24 h. The residual surfactants were removed by repeated centrifugation at 12
000 rpm for 1 min. Subsequently, DCM was evaporated at room temperature for 24 h. The residual surfactants were removed by repeated centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and redispersion in water for 3 times, and the resulting particles were used for further characterization.
000 rpm and redispersion in water for 3 times, and the resulting particles were used for further characterization.
Iodine-mediated modification of BCP particles
0.5 mL suspensions of BCP spheres were re-dispersed into a surfactant-containing aqueous solution (1 mg mL−1, CTAB). The particle suspension was transferred into a 4 mL vial, which was placed inside a larger 20 mL vial containing 2 mL of DCM/I2 (2 mg mL−1) solution at the bottom. The 20 mL vial was sealed and kept at 40 °C to allow the reaction to proceed, where the presence of DCM vapors annealed the BCP particles to undergo shape restructuring. After annealing for a predetermined time, the 4 mL vial containing the particles was taken out and kept in the open air to evaporate residual DCM/I2.Characterization
Scanning electron microscopy (SEM, Magellan 400), transmission electron microscopy (TEM, Tecnai F20, 200 kV), and field-emission transmission electron microscopy (FE-TEM, Talos F200X) were used to observe the surface and internal structure of the BCP particles. SEM samples were prepared by drop-casting the BCP particle suspension on the silicon wafer. For TEM samples, 10 μL BCP particle suspension was transferred to the carbon-supported grid and dried in air. The PB domain was stained prior to TEM imaging by adding diluted OsO4 solution (0.2 wt%, 60 μL) to the BCP particle suspension (0.5 mL) after which the solution turned dark. For PS-b-P4VP particles, the carbon-supported grid with BCP particles was subjected to iodine vapor to selectively stain the P4VP domain. Fourier-transform infrared spectroscopy (FT-IR) measurement was performed on a Nicolet iS500 FT-IR spectrometer (Thermo Fisher Scientific Instrument). FT-IR samples were prepared by concentrating the particles by centrifugation, followed by drying in a vacuum at 40 °C. Proton nuclear magnetic resonance (1H NMR) spectra were obtained from a Bruker AVANCE III HD instrument at 400 MHz using CDCl3 as solvent. A pendant drop method was performed to analyze the interfacial tensions between the polymer solution (i.e., PS30k, PB11k in DCM, 10 mg mL−1) and the aqueous surfactant solution (i.e., CTAB, 10 mg mL−1 in water). The pendant drop images were taken with a CCD camera (WAT-902H Ultimate, Watec) and were analyzed by the MATLAB program to determine the interfacial tension values.Results and discussion
Scheme 1 shows our overall experimental system. First, spherical BCP particles are prepared by emulsifying a DCM solution containing PS-b-PB BCPs (10 mg mL−1) with an aqueous solution containing CTAB surfactant (10 mg mL−1). After evaporation of DCM, the spherical particles are obtained in which coiled PB cylinders and concentric lamellar layers are formed inside the particles for PS35k-b-PB11k and PS34k-b-PB25k, respectively. Then, the obtained BCP spheres are exposed to DCM/I2 vapors for two purposes: I2 vapors react with PB domains, and DCM vapors make the BCP chains mobile to allow the shape transitions of the spherical particles.22,50–53 After the reaction, the polarity of I2-doped PB chains increases, leading to the reduced interfacial tension between the modified PB domain and the aqueous surrounding. Accordingly, when the PS blocks and modified PB blocks exhibit comparable affinities to their surroundings, neutral interfacial interaction is attained to allow the transformation of spherical particles into oblate and prolate ellipsoids.
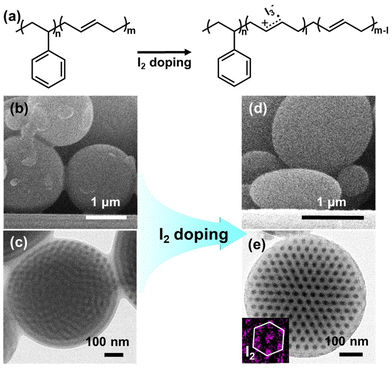
Fig. 1a shows the reaction scheme for the I2-doping of PS-b-PB BCPs. I2 serves as a strong oxidant undergoing reduction to I− or I3− while generating cation radicals in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds of the PB segment.54,55 In contrast, PS blocks have no reactivity toward the I2 molecules. Fig. 1b and c shows the shape and internal morphology of as-prepared spherical PS35k-b-PB11k particles. As CTAB is a typical PS-selective surfactant,11,25 outermost layer consists of PS domains while the cylindrical PB domains are internally coiled within the spherical confinement. After treating these particles with DCM/I2 vapors for 12 hours, the particles were transformed into well-defined oblates with hexagonally packed PB cylinders embedded in the PS matrix (Fig. 1d and e). Additionally, the increase in the aspect ratio (AR) of oblate particles to 1.29 after 12 h of reaction reflects the formation of anisotropic BCP particles (Table S1†). By performing the elemental mapping analysis of the dark-field STEM image, the hexagonal arrays of I2 clearly show (inset in Fig. 1e) that the I2 doping proceeded solely in PB chains while PS remained intact.
C double bonds of the PB segment.54,55 In contrast, PS blocks have no reactivity toward the I2 molecules. Fig. 1b and c shows the shape and internal morphology of as-prepared spherical PS35k-b-PB11k particles. As CTAB is a typical PS-selective surfactant,11,25 outermost layer consists of PS domains while the cylindrical PB domains are internally coiled within the spherical confinement. After treating these particles with DCM/I2 vapors for 12 hours, the particles were transformed into well-defined oblates with hexagonally packed PB cylinders embedded in the PS matrix (Fig. 1d and e). Additionally, the increase in the aspect ratio (AR) of oblate particles to 1.29 after 12 h of reaction reflects the formation of anisotropic BCP particles (Table S1†). By performing the elemental mapping analysis of the dark-field STEM image, the hexagonal arrays of I2 clearly show (inset in Fig. 1e) that the I2 doping proceeded solely in PB chains while PS remained intact.
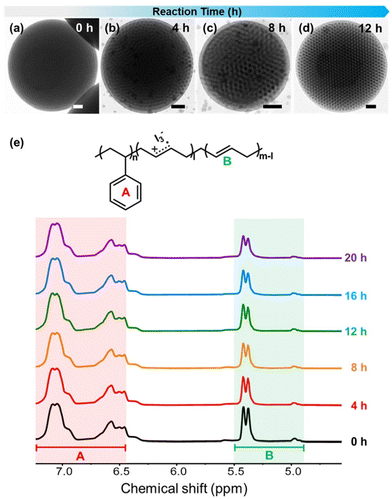
To better observe the shape transition process, the structural evolution of the particle shape and internal morphology of PS35k-b-PB11k was monitored as a function of reaction times (Fig. 2). First, the pristine spherical PS35k-b-PB11k particles with coiled PB cylinders having a domain spacing (D) of 33 ± 2.3 nm were initially formed (Fig. 2a). After exposing this particle to DCM/I2 vapors for 4 h, hexagonally packed structures started to form at the surface of the BCP particle, indicating that the PB cylinders are perpendicularly oriented to the particle surface (Fig. 2b). Such a feature became more prominent after 8 h of reaction, where perpendicularly oriented cylindrical PB domains span throughout the particle (Fig. 2c). After 12 h of reaction, complete transformation to oblate particles (D = 39 ± 1.5 nm) with well-organized, hexagonally-packed PB cylinders was observed (Fig. 2d). We attribute the gradual increase of D with an increasing reaction time to the swollen PB domains by solvent and the addition of I2 molecules within the modified PB domains.
The shape transition process from BCP spheres to oblates is further investigated by obtaining statistics that show the percentage of oblate particles in the particle batch as a function of reaction time (Fig. S1†). For the particles that reacted for 4 h and 8 h, 11% and 36% of oblate particles were observed, respectively (Fig. S1a–c†). The percentage of oblate particles was significantly increased to 85% at the reaction time of 12 h (Fig. S1d†). At longer reaction times of 16 and 20 h, slight increases in the percentage of oblate particles (88% and 89%, Fig. S1e and f†) indicate that the near-complete transformation into oblate particles can be attained after 12 h of reaction time. Similarly, the shape transition process of the lamellar forming PS34k-b-PB25k particles was monitored at different reaction times (Fig. S2 and S3†). First, the pristine PS34k-b-PB25k forms onion-like spherical particles with PS as the outermost layer (Fig. S2a†). After 4 h and 8 h of exposure to iodine vapors, particles with an intermediate morphology between onion and ellipsoid were observed, indicating a mixed orientation of parallel and perpendicular domains relative to the particle surface (Fig. S2b and c†). At 12 h of reaction, the particles were fully transformed into elongated ellipsoids with well-defined lamellae structures (Fig. S2d†). The percentage of PS34k-b-PB25k particles transition from spheres to ellipsoidal shapes was presented in Fig. S3† as a function of reaction time.
We quantitatively evaluated the degree of reaction as a function of reaction time by calculating the conversion of double bonds (R) in PB chains from 1H NMR spectra (Fig. 2e). In detail, the integration values of vinylic protons in PB (4.9–5.5 ppm) compared to that of aromatic signals (6.4–7.2 ppm) from intact PS chains were used to calculate the R values as a function of reaction time (Table S2†).47 Specifically, R increased gradually from 0 to 10.3, 15.2 and 17.9% after 4, 8, and 12 h of reaction time, respectively. As the majority of particles turned to oblates after 12 h of reaction time, we speculate that the critical conversion of double bonds by I2 to induce the switching of BCP orientation is around 17%. Further extension of the reaction time to 16 and 20 h led to the increase of R to 22% and 31%, respectively, although the oblate shape of the particle is maintained at these reaction times (Fig. S1†). A similar conclusion can be made by comparing the FT-IR spectra of pristine spherical and oblate particles obtained after the 20 h reaction. As shown in Fig. S4,† the decrease in peak intensity at 908 cm−1, which corresponds to the alkene ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H bending peak, indicates that the double bonds in PB reacted with the I2 molecules.
C–H bending peak, indicates that the double bonds in PB reacted with the I2 molecules.
The interfacial interaction of modified PB domains plays a key role in obtaining oblate BCP particles.6,9,25 The interfacial tensions (γ) between each polymer block and the aqueous surroundings were examined as a function of reaction time. The difference in the interfacial tension between PS and PB (Δγ = γPB − γPS) is plotted in Fig. 3. The measured values of γ between the PS block and the aqueous surroundings were kept nearly constant (γPS ∼ 4.12 mN m−1) for all reaction times while that of the PB block and the surroundings (γPB) gradually decreased from 4.95 to 3.96 mN m−1 after 20 h of reaction time (Table S3†). The constant γPS value is consistent with the intact PS block during the reaction as observed in the 1H NMR spectra, while the decrease in γPB can be attributed to the enhanced polarity of I2-doped PB domains.54 Notably, the Δγ value can serve as an indicator of neutral interfacial interactions, as the shape transformation of the BCP particles is expected to occur at the neutral condition of Δγ ∼ 0. Although PS-b-PBs are typical low-χ BCPs,56 a Δγ value of 0.83 before I2 doping indicated interfacial selectivity toward the PS block in the pristine spherical BCP particles, positioning the PS as the outermost layer. At a short reaction time of 4 and 8 h, Δγ decreased to 0.52 and 0.24, respectively. Further extension of the reaction time to 12 h led to the Δγ close to zero (Δγ = 0.01), indicating a neutralized interfacial interaction of PS and PB to surroundings. This is in good accordance with the emergence of oblate and prolate particles as observed by the electron microscopy tools.
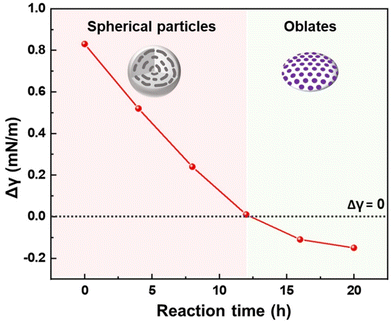 | ||
| Fig. 3 Interfacial tension difference (Δγ = γPB − γPS) as a function of reaction time. The dashed line indicates the Δγ = 0. | ||
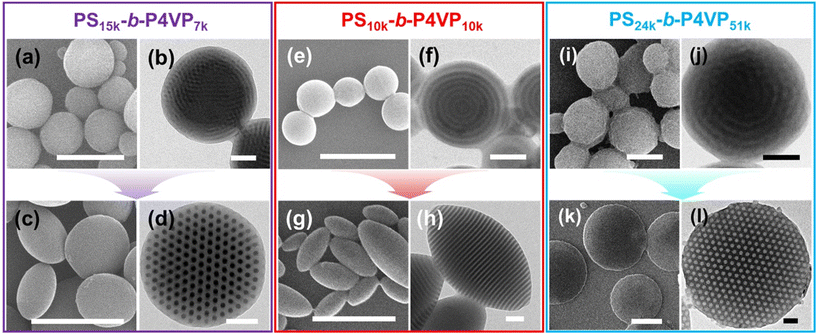
The versatility of I2-doping induced shape transition of BCP particles is further demonstrated by using PS-b-P4VP BCP particles with a wide range of volume fractions. While it is well-known that I2 molecules can physically bind to pyridine group (Pyr*I2),57–59 I2 can be further dissociated into ions (PyrI+ + I−) and form stronger bond with P4VP chains (Scheme S1†), similar to the oxidation of diene group in PB.60,61Fig. 4 shows the successful shape transformations of PS15k-b-P4VP7k, PS10k-b-P4VP10k, and PS24k-b-P4VP51k spheres into spheroids upon reacting with I2. Additionally, I2 doped PS-b-P4VP particles exhibited long-term stability (Fig. S5†), supporting the formation of strong ionic complex between iodine and P4VP. For PS15k-b-P4VP7k, pristine spherical particles with coiled P4VP cylinders were formed (Fig. 4a and b). After reacting with I2 for 12 h, the spheres transitioned into oblate particles with hexagonally packed cylinders having increased D from 21 ± 1.7 nm to 30 ± 2.4 nm while AR increased from 1.0 to 2.1. For PS10k-b-P4VP10k, onion-like particles transformed into ellipsoidal particles with axially stacked lamellae (Fig. 4e–h) having increased D from 20 ± 1.6 nm to 28 ± 2.1 nm and increased AR from 1.0 to 1.9. Finally, the PS24k-b-P4VP51k spherical particles having coiled PS cylinders (D = 60 ± 2.2 nm) transformed into oblate particles with perpendicular PS cylinders (D = 67 ± 3.1 nm) after the I2 reaction, and the average AR was significantly increased to 3.7 (Fig. 4(i–l)). We summarized the D and AR values of PS-b-P4VP particles before and after the I2 reaction in Table S4.† The increase in D can be attributed to the (i) swelling of ionized P4VP blocks by aqueous surroundings62–64 and (ii) the increase in interaction parameter (χ) between the PS and the modified P4VP block.65,66 Overall, I2 doping of P4VP blocks successfully modulated the interfacial interaction between each block and the surroundings, leading to the formation of well-defined anisotropic PS-b-P4VP particles (e.g., oblate or ellipsoid) similar to the shape transition observed in PS-b-PB BCP particles.
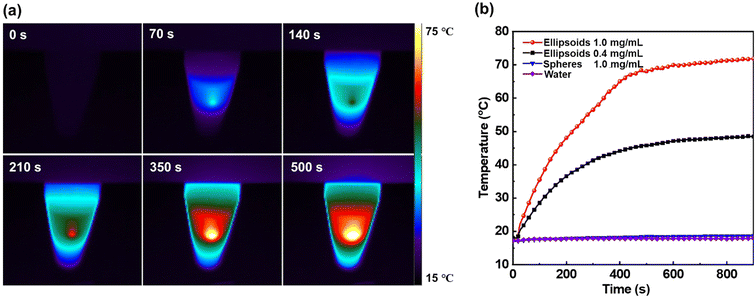
We examined the photothermal behavior of I2-doped nonspherical BCP particles as the I2-doping can lead to the materials for higher efficiency in light absorption.67,68 The color of the nonspherical PS34k-b-PB25k particles was changed from white to black after the reaction with I2 (Fig. S6†), and the photothermal behaviors were evaluated by recording the temperature change under the irradiation of an 808 nm laser source (Fig. 5). A rapid heating of the suspension containing I2-doped particles (1.0 mg mL−1) was visualized in the IR thermograph, particularly at the bottom of the tube where the particles are concentrated (Fig. 5a). Also, I2-doped ellipsoidal particles after IR heating retained their original shape and internal structure, indicating that the photothermal particles are stable in the observed range of temperatures (Fig. S7†). Additionally, the temperature change of the I2-doped particles was compared with various control samples (Fig. 5b). The suspension of pristine spheres and distilled water showed no change in temperature (17.4 °C, ΔT < 2.0 °C) until 900 s of irradiation. In contrast, the temperature of the suspension containing 0.4 and 1.0 mg mL−1 of I2-doped particles increased to 48.6 °C and 71.3 °C at 900 s, respectively. Overall, facile functionalization of PS-b-PB particles with I2 allowed the generation of nonspherical shapes, and the resulting I2-doped particles showed excellent photothermal effect.
Conclusions
In summary, we have developed a facile strategy to induce the shape transitions of spherical PS-b-PB BCP particles into anisotropic shapes using the I2-mediated reaction in combination with the solvent vapor annealing process. The I2 molecules selectively reacted with the double bonds in PB chains to form cation radicals, and the degree of I2 doping can be tuned by the reaction time. The incorporation of I2 enhanced the polarity of the modified PB blocks to decrease interfacial tension with the aqueous surroundings. Accordingly, neutralization of the interfacial interaction switched the orientation of BCP domains perpendicular to the surface, allowing the shape transformation from spherical BCP particles into oblates or ellipsoids. Furthermore, the versatility of our strategy was demonstrated by applying it to the PS-b-P4VP BCPs with various molecular weight and volume fractions. Finally, I2-doped ellipsoidal particles exhibited excellent photothermal behavior as compared to the pristine spherical particles, highlighting the potential application of these particles as a photothermal agent.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This research was supported by the Korea Research Foundation Grant, funded by the Korean Government (RS-2023-00244864). This work was supported by Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government (MOTIE) (20214000000650).References
- S. Mitragotri and J. Lahann, Nat. Mater., 2009, 8, 15–23 CrossRef CAS PubMed.
- P. J. Yunker, T. Still, M. A. Lohr and A. G. Yodh, Nature, 2011, 476, 308–311 CrossRef CAS PubMed.
- Q. He, K. H. Ku, H. Vijayamohanan, B. J. Kim and T. M. Swager, J. Am. Chem. Soc., 2020, 142, 10424–10430 CrossRef CAS PubMed.
- J. A. Champion and S. Mitragotri, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 4930–4934 CrossRef CAS PubMed.
- N. Yan, Y. Zhu and W. Jiang, Chem. Commun., 2018, 54, 13183–13195 RSC.
- K. H. Ku, J. M. Shin, H. Yun, G.-R. Yi, S. G. Jang and B. J. Kim, Adv. Funct. Mater., 2018, 28, 1802961 CrossRef.
- C. K. Wong, X. Qiang, A. H. Müller and A. H. Gröschel, Prog. Polym. Sci., 2020, 102, 101211 CrossRef CAS.
- M. Peng, D. Hu, X. Chang and Y. Zhu, J. Phys. Chem. B, 2022, 126, 9435–9442 CrossRef CAS PubMed.
- J. J. Shin, E. J. Kim, K. H. Ku, Y. J. Lee, C. J. Hawker and B. J. Kim, ACS Macro Lett., 2020, 9, 306–317 CrossRef CAS PubMed.
- K. H. Ku, Y. J. Lee, Y. Kim and B. J. Kim, Macromolecules, 2019, 52, 1150–1157 CrossRef CAS.
- S. G. Jang, D. J. Audus, D. Klinger, D. V. Krogstad, B. J. Kim, A. Cameron, S. W. Kim, K. T. Delaney, S. M. Hur, K. L. Killops, G. H. Fredrickson, E. J. Kramer and C. J. Hawker, J. Am. Chem. Soc., 2013, 135, 6649–6657 CrossRef CAS PubMed.
- J. Lee, K. H. Ku, M. Kim, J. M. Shin, J. Han, C. H. Park, G.-R. Yi, S. G. Jang and B. J. Kim, Adv. Mater., 2017, 29, 1700608 CrossRef PubMed.
- J. Kim, H. Yun, Y. J. Lee, J. Lee, S. H. Kim, K. H. Ku and B. J. Kim, J. Am. Chem. Soc., 2021, 143, 13333–13341 CrossRef CAS PubMed.
- J. Lee, K. H. Ku, J. Kim, Y. J. Lee, S. G. Jang and B. J. Kim, J. Am. Chem. Soc., 2019, 141, 15348–15355 CrossRef CAS PubMed.
- D. Hu, X. Chang, Y. Xu, Q. Yu and Y. Zhu, ACS Macro Lett., 2021, 10, 914–920 CrossRef CAS PubMed.
- J. Lee, K. H. Ku, C. H. Park, Y. J. Lee, H. Yun and B. J. Kim, ACS Nano, 2019, 13, 4230–4237 CrossRef CAS PubMed.
- J. Kim, J. Park, K. Jung, E. J. Kim, Z. Tan, M. Xu, Y. J. Lee, K. H. Ku and B. J. Kim, ACS Nano, 2024, 18, 8180–8189 CrossRef CAS PubMed.
- S. H. Kwon, M. Xu, J. Kim, E. J. Kim, Y. J. Lee, S. G. Jang, H. Yun and B. J. Kim, Chem. Mater., 2021, 33, 9769–9779 CrossRef CAS.
- Y. Wang, D. Hu, X. Chang and Y. Zhu, Macromolecules, 2022, 55, 6211–6219 CrossRef CAS.
- R. Deng, J. Xu, G.-R. Yi, J. W. Kim and J. Zhu, Adv. Funct. Mater., 2021, 31, 2008169 CrossRef CAS.
- C. Lu and M. W. Urban, Prog. Polym. Sci., 2018, 78, 24–46 CrossRef CAS.
- J. M. Shin, Y. J. Lee, M. Kim, K. H. Ku, J. Lee, Y. Kim, H. Yun, K. Liao, C. J. Hawker and B. J. Kim, Chem. Mater., 2019, 31, 1066–1074 CrossRef CAS.
- K. H. Ku, J. H. Ryu, J. Kim, H. Yun, C. Nam, J. M. Shin, Y. Kim, S. G. Jang, W. B. Lee and B. J. Kim, Chem. Mater., 2018, 30, 8669–8678 CrossRef CAS.
- J. Xu, K. Wang, J. Li, H. Zhou, X. Xie and J. Zhu, Macromolecules, 2015, 48, 2628–2636 CrossRef CAS.
- D. Klinger, C. X. Wang, L. A. Connal, D. J. Audus, S. G. Jang, S. Kraemer, K. L. Killops, G. H. Fredrickson, E. J. Kramer and C. J. Hawker, Angew. Chem., Int. Ed., 2014, 53, 7018–7022 CrossRef CAS PubMed.
- R. Deng, F. Liang, W. Li, Z. Yang and J. Zhu, Macromolecules, 2013, 46, 7012–7017 CrossRef CAS.
- B. Schmidt, J. Elbert, D. Scheid, C. J. Hawker, D. Klinger and M. Gallei, ACS Macro Lett., 2015, 4, 731–735 CrossRef CAS PubMed.
- C. Chen, Z. Xiao and L. A. Connal, Aust. J. Chem., 2016, 69, 741–745 CrossRef CAS.
- S. J. Jeon, G. R. Yi and S. M. Yang, Adv. Mater., 2008, 20, 4103–4108 CrossRef CAS.
- J. M. Shin, Y. Kim, H. Yun, G. R. Yi and B. J. Kim, ACS Nano, 2017, 11, 2133–2142 CrossRef CAS PubMed.
- J. M. Shin, Y. J. Kim, K. H. Ku, Y. J. Lee, E. J. Kim, G. R. Yi and B. J. Kim, Chem. Mater., 2018, 30, 6277–6288 CrossRef CAS.
- T. Higuchi, A. Tajima, K. Motoyoshi, H. Yabu and M. Shimomura, Angew. Chem., Int. Ed., 2008, 47, 8044–8046 CrossRef CAS PubMed.
- H. Yabu, T. Higuchi and M. Shimomura, Adv. Mater., 2005, 17, 2062–2065 CrossRef CAS.
- H. Li, X. Mao, H. Wang, Z. Geng, B. Xiong, L. Zhang, S. Liu, J. Xu and J. Zhu, Macromolecules, 2020, 53, 4214–4223 CrossRef CAS.
- L. S. Flosenzier and J. M. Torkelson, Macromolecules, 1992, 25, 735–742 CrossRef CAS.
- N. R. Legge, G. Holden and H. E. Schroeder, Thermoplastic Elastomers: A Comprehensive Review, Hanser, NewYork, 1987 Search PubMed.
- Kraton thermoplastic rubber. Technical Bulletin, SC: 971-87, Shell Chemical Company.
- H. Feng, M. Dolejsi, N. Zhu, P. J. Griffin, G. S. W. Craig, W. Chen, S. J. Rowan and P. F. Nealey, Adv. Funct. Mater., 2022, 32, 2206836 CrossRef CAS.
- B. Sutisna, G. Polymeropoulos, V. Musteata, R. Sougrat, D. M. Smilgies, K. V. Peinemann, N. Hadjichristidis and S. P. Nunes, Small, 2018, 14, e1701885 CrossRef PubMed.
- H. Fang, X. Gao, F. Zhang, W. Zhou, G. Qi, K. Song, S. Cheng, Y. Ding and H. H. Winter, Macromolecules, 2022, 55, 10900–10911 CrossRef CAS.
- C. M. Geiselhart, J. T. Offenloch, H. Mutlu and C. Barner-Kowollik, ACS Macro Lett., 2016, 5, 1146–1151 CrossRef CAS PubMed.
- A. Zhang and L. Chao, Eur. Polym. J., 2003, 39, 1291–1295 CrossRef CAS.
- R. Pandit, J. Giri, G. H. Michler, R. Lach, W. Grellmann, B. Youssef, J. M. Saiter and R. Adhikari, Macromol. Symp., 2012, 315, 152–159 CrossRef CAS.
- Y. Ren, T. P. Lodge and M. A. Hillmyer, Macromolecules, 2002, 35, 3889–3894 CrossRef CAS.
- D. P. Nair, M. Podgórski, S. Chatani, T. Gong, W. Xi, C. R. Fenoli and C. N. Bowman, Chem. Mater., 2014, 26, 724–744 CrossRef CAS.
- C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed., 2010, 49, 1540–1573 CrossRef CAS PubMed.
- J. J. Shin, Polymers, 2020, 12, 2804 CrossRef CAS PubMed.
- D. Varadharajan, H. Turgut, J. Lahann, H. Yabu and G. Delaittre, Adv. Funct. Mater., 2018, 28, 1800846 CrossRef.
- S. P. S. Koo, M. M. Stamenović, R. A. Prasath, A. J. Inglis, F. E. Du Prez, C. Barner-Kowollik, W. Van Camp and T. Junker, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 1699–1713 CrossRef CAS.
- L. Navarro, A. F. Thunemann and D. Klinger, ACS Macro Lett., 2022, 11, 329–335 CrossRef CAS PubMed.
- X. Mao, H. Li, J. Kim, S. Deng, R. Deng, B. J. Kim and J. Zhu, Nano Res., 2021, 14, 4644–4649 CrossRef CAS.
- L. Li, K. Matsunaga, J. Zhu, T. Higuchi, H. Yabu, M. Shimomura, H. Jinnai, R. C. Hayward and T. P. Russell, Macromolecules, 2010, 43, 7807–7812 CrossRef CAS.
- X. Qiang, X. Dai, A. Steinhaus and A. H. Gröschel, ACS Macro Lett., 2019, 8, 1654–1659 CrossRef CAS PubMed.
- R. Li, Z. Wang, X. Tao, J. Jia, X. Lian and Y. Wang, ACS Macro Lett., 2020, 9, 985–990 CrossRef CAS PubMed.
- R. Li, Z. Wang and Y. Wang, Innovation, 2021, 2, 100095 CAS.
- G. Kim and M. Libera, Macromolecules, 1998, 31, 2569–2577 CrossRef CAS.
- X. Zheng, M. Ren, H. Wang, H. Wang, Z. Geng, J. Xu, R. Deng, S. Chen, W. H. Binder and J. Zhu, Small, 2021, 17, 2007570 CrossRef CAS PubMed.
- G. Quintieri, M. Saccone, M. Spengler, M. Giese and A. H. Gröschel, Nanomaterials, 2018, 8, 1029 CrossRef PubMed.
- R. Milani, N. Houbenov, F. Fernandez-Palacio, G. Cavallo, A. Luzio, J. Haataja, G. Giancane, M. Saccone, A. Priimagi, P. Metrangolo and O. Ikkala, Chem, 2017, 2, 417–426 CAS.
- H. T. Nguyen, D. D. Nguyen and J. Spanget-Larsen, Chem. Phys. Lett., 2019, 716, 119–125 CrossRef CAS.
- T. Tassaing and M. Besnard, J. Phys. Chem. A, 1997, 101, 2803–2808 CrossRef CAS.
- A. Hanisch, A. H. Gröschel, M. Förtsch, M. Drechsler, H. Jinnai, T. M. Ruhland, F. H. Schacher and A. H. E. Müller, ACS Nano, 2013, 7, 4030–4041 CrossRef CAS PubMed.
- Z. Tan, E. J. Kim, T. N.-L. Phan, J. Kim, J. J. Shin, K. H. Ku and B. J. Kim, Macromolecules, 2022, 55, 9972–9979 CrossRef CAS.
- S. Lee, J. J. Shin, K. H. Ku, Y. J. Lee, S. G. Jang, H. Yun and B. J. Kim, Macromolecules, 2020, 53, 7198–7206 CrossRef CAS.
- A. Semenov, Zh. Eksp. Teor. Fiz., 1985, 88, 1242–1256 CAS.
- F. S. Bates and G. H. Fredrickson, Annu. Rev. Phys. Chem., 1990, 41, 525–557 CrossRef CAS PubMed.
- Z. Wang, X. Lian, R. Li, X. Tao and Y. Wang, Chem. – Eur. J., 2019, 25, 13811–13815 CrossRef CAS PubMed.
- R. Li, Z. Wang, P. Han, Y. He, X. Zhang and Y. Wang, Chem. – Eur. J., 2017, 23, 17889–17893 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Double bond conversion, SEM and TEM images of the particles. See DOI: https://doi.org/10.1039/d4py00524d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |