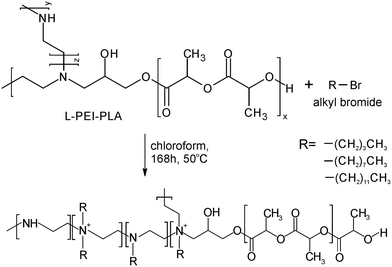
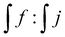Open Access Article
Open Access ArticleLactide oligomers modified with linear polyethyleneimine for antibacterial coatings†
Anna
Iuliano
 *,
Maksymilian
Kukuć
*,
Maksymilian
Kukuć
 ,
Julita
Pachla
,
Julita
Pachla
 ,
Dominik
Jańczewski
,
Dominik
Jańczewski
 ,
Jolanta
Mierzejewska
,
Jolanta
Mierzejewska
 and
Karolina
Drężek
and
Karolina
Drężek

Faculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664, Warsaw, Poland. E-mail: anna.iuliano@pw.edu.pl
First published on 7th August 2024
Abstract
Lactide oligomers were synthesized in the presence of glycidol via ring opening solution polymerization and used in the addition reaction with linear polyethyleneimine (L-PEI). Detailed analysis of the lactide oligomers was conducted using 1H NMR, GPC and MALDI-ToF. L-PEI with different molecular weight (2.5 and 4.0 kg mol−1) was introduced to the addition reaction with lactide oligomers. The obtained copolymers were modified with various 1-bromoalkanes and their percentage of grafting as well as antibacterial properties of the final product against Escherichia coli were studied. The product with the highest grafting level was obtained in the presence of bromobutane, regardless of the L-PEI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PLA ratio used, and displayed potent antimicrobial activity both as a polymer film and in the blend with commercial polylactide. Inhibition of bacterial growth was observed at a concentration of 30 wt% of the copolymer grafted with 1-bromooctane and 1-bromodecane in the polylactide blend. In this case the total wt% of PEI in the blend was 1.1%.
PLA ratio used, and displayed potent antimicrobial activity both as a polymer film and in the blend with commercial polylactide. Inhibition of bacterial growth was observed at a concentration of 30 wt% of the copolymer grafted with 1-bromooctane and 1-bromodecane in the polylactide blend. In this case the total wt% of PEI in the blend was 1.1%.
Introduction
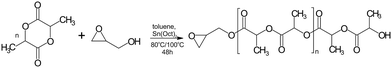
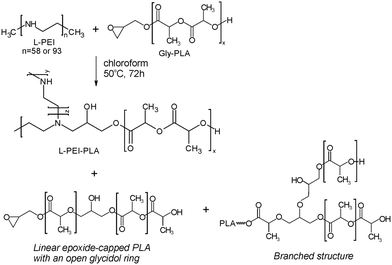
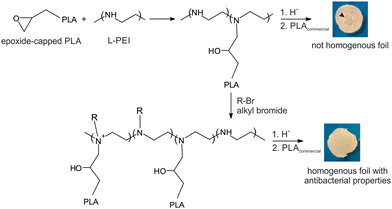
Currently, there is a great demand for functional materials with effective antibacterial properties.1 The global antimicrobial packaging market size is evaluated at USD 8 billion in 2022 with the prediction to be worth around USD 14.39 billion by 2032.2 The major players in the market like BASF SE, Dunmore Corporation, BioCote Limited, Microban International, DOW Chemical Company propose antibacterial products mainly as additives/antibacterial coating solutions produced using silver ion technology. Another group of products contains zinc pyrithione and isothiazolinone derivatives (Bioban 200, Bioban 045). Most of them are compatible with synthetic polymers of petroleum origin like SBR, PA, TPU, PET, BOPP, HDPE. The main concern of using such products is the migration of the metal ions which can be harmful for the humans. The European Food Safety Authority did provide upper limits of Ag migration from packaging. It is recommended that concentrations of active ingredient should not exceed 0.05 mg L−1 in water and 0.05 mg kg−1 in food.3 Therefore, one of the main challenges in the design of antibacterial polymer films is controlling the migration of antibacterial agents and balancing the compound's activity with its duration of action.4 The noncovalently bonded biocidal agents can be leach out reducing the duration of material activity, meanwhile covalently attached biocidal group alone can be not sufficient to exhibit antibacterial properties. There are limited investigations into the antibacterial activity of biocidal compounds incorporated into polylactide (PLA) structures. Among them are derivatives of vanillin compounds, guanidine, imidazoles, thiazoles, phenols and quaternary ammonium salts, which are one of the most effective compounds to counteract the growth of microorganisms.5,6 Their unique feature is high activity against a wide spectrum of bacteria, viruses with a bilayer membrane and fungi. El Habnouni et al. prepared a well-defined α-azido-functionalized poly(quaternary ammonium) which in the next step was covalently bounded to the propargylated PLA surface using “click” chemistry.7,8 The antibacterial properties of the tested PLA showed a strong reduction in the adherence of E. coli and S. aureus compared to neat PLA. The same strategy was used to prepare antibacterial copolymers of lactide and O-carboxyanhydrides (OCA). Alkyne–azide click reaction was conducted between propargylated OCA and 3-azido-N,N-dimethyl-N-propylpropan-1-aminium bromide.9 The strongest antibacterial activity against E. coli was observed with 40% and 70% of quaternary ammonium groups per chain, meanwhile for Gram-positive S. aureus, adhesion was already reduced when 14% of biocidal groups were introduced. The antibacterial activity was also exhibited by lactide copolymers substituted with pendant azide groups and functionalized by copper-catalyzed [3 + 2] cycloaddition reaction with N,N,N-trioctyl-N-propargylammonium bromide.10 5 mol% of the quaternary ammonium groups in the structure was sufficient to reduce the growth of Gram-negative bacteria, namely E. coli and P. aeruginosa.In this paper, we demonstrate an efficient strategy to prepare antibacterial polylactide film composed of a block copolymer of lactide and linear polyethyleneimine (L-PEI) which acts as biocidal agent covalently attached to PLA (Scheme 1). L-PEI can potentially be used as antibacterial agent in wound dressing application.11 It was chosen due to the high number of secondary amine groups in its structure which can be the source of quaternary ammonium centres. Moreover, due to the high positive charge, L-PEI exhibits strong antibacterial activity, but simultaneously is rather cytotoxic for the mammalian cells.12 In the presented studies, oligomers of L-lactide (L-LA) were prepared by ring opening polymerization (ROP) in the presence of glycidol and tin 2-ethylhexanoate (Sn(Oct)2) as a catalyst. Short chains of PLA were terminated with reactive epoxy group and subsequently attached to the secondary amino groups of L-PEI. Finally, alkyl bromide with different chain lengths was added to create quaternary ammonium centres and increase solubility in chloroform. L-PEI-g-PLA copolymers were characterized for their chemical structures and antibacterial properties in the mixture with commercial PLA. To the best of our knowledge, there are no studies on L-PEI-g-PLA copolymers with quaternary ammonium group incorporated to the PLA chain. Such antibacterial materials could be used for coating, packaging and biomedical applications.
 | ||
| Scheme 1 Synthesis pathway of L-PEI-g-PLA copolymer and blend preparation (a larger picture of the polymeric film is provided in ESI, Fig. S1†). | ||
Experimental section
Materials and methods
L,L-Lactide (Aldrich) was recrystallized from dry isopropanol, then toluene and vacuum dried before polymerization. Toluene, isopropanol and chloroform were dried with sodium and distilled. Linear polyethyleneimine (cat. number 764604) (Mn = 2.5 kg mol−1, ĐM < 1.3), tin 2-ethylhexanoate (95%), butyl bromide (99%), octyl bromide (99%), dodecyl bromide (≥95%) and chloramphenicol were purchased from Aldrich and used without further purification. Linear PEI of Mn = 4.0 kg mol−1 was synthesized according to the procedure described by Pachla et al.13 PLA with reference NW 2003D was manufactured by NatureWorks (Blair, NE, USA).Polymerization reaction
All reactions were carried out under nitrogen atmosphere in two neck round-bottom flasks with magnetic stirrer and water-cooled condenser. ROP of L-LA in the presence of glycidol was performed in a solution at 80 °C and 100 °C for 48 h. In the first step, monomer, initiator solution (C = 2.198 M) and toluene (4-fold excess (wt/vol) in relation to the monomer) were placed in the flask. Then, the mixture was heated until complete dissolution of L-LA and afterwards Sn(Oct)2 (0.02 mol% with respect to the monomer) was added. After polymerization completion, the reaction mixture was cooled to room temperature and dissolved in methylene chloride. The final product was precipitated by pouring the mixture into excess methanol, filtered off and dried in a vacuum oven at room temperature for 48 h.Addition reaction
Oligomers of L-LA were added to the two neck round-bottom flasks and dried under vacuum for 2 h at room temperature. After this time, L-PEI (2.5 kg mol−1 (L-PEI58) or 4.0 kg mol−1 (L-PEI93)) and chloroform were placed under nitrogen atmosphere, and a water-cooled condenser has been placed in one of the necks of the flask. The mixture was heated to 50 °C and the reaction time was counted from the moment of complete dissolution of the reagents. After 72 h of reaction, alkyl bromide was added and quaternization reaction was performed for 168 h. The alkyl bromide was added in a molar ratio L-PEI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alkyl bromide of 1
alkyl bromide of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 for products L-PEI58-PLA25 and L-PEI58-PLA25, and 1
25 for products L-PEI58-PLA25 and L-PEI58-PLA25, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 for products L-PEI58-PLA50 and L-PEI58-PLA50, respectively. The final product was precipitated using cold hexane and dried under vacuum for 24 h at 40 °C.
50 for products L-PEI58-PLA50 and L-PEI58-PLA50, respectively. The final product was precipitated using cold hexane and dried under vacuum for 24 h at 40 °C.
Protonation
Around 0.1 g of the final product was dissolved in 8 ml of ethyl acetate, and 3 ml of 30% HCl was added to the round-bottom flask. The reaction was performed for 3 h at room temperature. The solvents were removed under reduced pressure to afford the product as a white, wax-like solid.Antimicrobial assay
Antimicrobial effect against Escherichia coli ATCC8739 of the copolymers was performed by the disk diffusion and broth dilution method. Briefly, bacteria were grown in a Mueller-Hinton Broth (MHB; Biocorp, Poland) for about 24 h at 37 °C with shaking at 200 rpm (Incubated shaker IST-4075R, LabCompanion, USA). To assess the antimicrobial potential of the protonated products, 10 μL portions of each polymer solution in chloroform (or suspension, in the case of protonated L-PEI58-PLA25 copolymer without alkyl bromide) were spotted on the sterile paper disks located on a sterile Petri glass dish and left to dry for at least three days at room temperature (about 22 °C). Then, the saturated disks were moved to the Mueller-Hinton agar, (MHA; Merck, Germany) inoculated with bacteria (106 CFU mL−1). When the media solidified, the Petri dishes were incubated for 24 h at 37 °C. The growth inhibition zones in solid cultures were visible if the tested copolymer was toxic for microbial cells.The dilution method was used for the determining of minimum inhibition concentrations (MIC) of the L-PEI58-PLA25 copolymer. The tested sample has been prepared by dissolving 41.5 mg of copolymer in 1.2 mL of chloroform. A sample volume of: 640 μL, 320 μL, 160 μL and 80 μL was placed in a 4 sterile glass probe and dried at room temperature for at least 3 days. E. coli was inoculated into 50 mL of MHB and cultivated overnight at 37 °C. Overnight microbial strain was diluted with a sterile MHB solution to the cell density of 105 CFU mL−1. 2 mL of MHB and 2 mL of microorganism suspension were added to each glass probe and incubated at 37 °C for 24 h. After that time, the OD600 at a wavelength of 600 nm was determined. The MIC was recorded as the lowest concentration of the sample that inhibits growth of microorganism.
For copolymer/PLA blends additional test has been performed by quantifying the survival of bacteria held in intimate contact according to ISO 22196 standard.14,15 Blend solutions and commercial PLA were prepared in chloroform. All prepared solutions were casted onto the sterile glass plate with the dimension of 18 mm × 18 mm to obtain polymer layer with a thickness of 0.012 mm (±0.004) and left to dry for at least 3 days at room temperature. The polymer coated glass plates were placed into sterile petri dishes with the coated surface uppermost. An aliquot (30 μl) of test inoculum (2 × 105 CFU mL−1) were pipetted onto film samples and covered with the piece of sterilized glass plate. Petri dishes containing the inoculated film samples were incubated for 20 h at 35 °C under relative humidity of 90%. Microorganisms were recovered from film samples by shaking with 5 ml of saline solution and inoculated onto MHA agar plates. After incubation at 37 °C for 24 h, colonies grown on plates were counted and microorganism counts were calculated as log CFU mL−1. An additional control test using commercial polylactide and chloramphenicol was also performed. In that case 12 mg of PLA was dissolved in 0.9 mL of CHCl3 and 0.25 mL of acetone. After complete dissolution, 50 μL of chloramphenicol solution in acetone (C = 15.5 mg ml−1) was added. Reduction of the number of living and viable cells of tested bacteria (R) was calculated using the equation:
| R = (Ut − U0) − (At − U0) = Ut − At, | (1) |
According to ISO 22196, a reduction in the number of cells capable of growth by two orders of magnitude (R ≥ 2) was interpreted as a bactericidal effect of the investigated material.
Measurements
1H NMR measurement was performed on Varian Mercury 400 MHz spectrometer using CDCl3 as solvent. The molar mass and molar mass distribution were determined by GPC on a Viscotek system comprising GPCmax and TDA 305 triple detection unit (RI, IV, LS) equipped with one guard and two DVB Jordi gel columns (102–107, linear, mix bed) in CH2Cl2 as eluent at 30 °C at a flow rate of 1.0 mL min−1. MALDI-ToF mass spectrometry was performed on Bruker Daltonics UltrafleXtreme™ instrument. trans-2-[3-(4-tert-Butylphenyl)-2-methyl-2-propenylidene] malononitrile (DCTB) was used as MALDI matrix. Isotope distribution was estimated with freeware Molecular Weight Calculator ver. 3 WSearch Software65 for selected exponent molecules and then compared with mass spectra following the previously described method.16 The DSC measurements were performed using a TA Instruments DSC Q200 V24.2 Build 107 apparatus. The first heating run from 24 °C to 200 °C was performed at a heating rate of 10 °C min−1 in order to study crystallinity, then cooling at the rate of 20 °C min−1 was applied. The second heating run was measured at the rate of 20 °C min−1 to determine the glass transition temperature.Results and discussion
Solution polymerization of L-lactide in the presence of glycidol
First studies of lactide polymerization in the presence of glycidol were performed by Ptet.17 It has been demonstrated that to avoid epoxide ring opening, the low-temperature solution polymerization should be applied. In our case, the reactions were perfomed at 80 °C and 100 °C in toluene with low monomer to initiator molar ratio (Scheme 2). Low molar mass oligomers were desirable because they could enhanced miscibility between L-PEI-PLA copolymer and commercial PLA in the blend. The results for the series of seven reactions are presented in Table 1.| No. | ([Gly]/[LA])0 (mol/mol) | T (°C) | α (%) |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
M
n,GPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (g mol−1) c (g mol−1) |
Đ
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
M
n,MALDI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e (g mol−1) e (g mol−1) |
ECf (%) | %mol of PLA-GLYg |
|---|---|---|---|---|---|---|---|---|---|
a Conversion of L-lactide calculated from 1H NMR spectra.
b Expected Mn based on L-lactide conversion, according to the formula: Mn = [LA]0 × 144 × α + Mn,Gly.
c GPC with polystyrene calibration.
d
Đ
M = Mw/Mn.
e MALDI ToF analysis.
f EC – epoxide conversion, calculated using 1H NMR spectrum according to the formula: 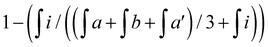 where where  is the integration of the methine protons from open glycidol, described on the Fig. 1b as “i”; is the integration of the methine protons from open glycidol, described on the Fig. 1b as “i”; 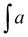 , , 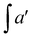 , , 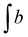 are the integrations of the methine protons coming from closed glycidol, described on the Fig. 1a as “a”, “a′” and “b”, respectively.
g %mol of PLA ended with epoxy ring closed calculated as are the integrations of the methine protons coming from closed glycidol, described on the Fig. 1a as “a”, “a′” and “b”, respectively.
g %mol of PLA ended with epoxy ring closed calculated as 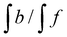 .
h Bimodal molar mass distribution; the value corresponds to the total distribution.
i Reaction conducted for 50 h. .
h Bimodal molar mass distribution; the value corresponds to the total distribution.
i Reaction conducted for 50 h.
|
|||||||||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
80 | 63 | 900 | 2600 | 1.21 | 1500 | 100 | 59 |
| 2a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
80 | 64 | 1900 | 3000 | 1.21 | 1900 | 100 | 91 |
| 2bi | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
80 | 95 | 2800 | 5000h | 1.28 | 2600 | 48 | 40 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
80 | 53 | 3900 | 2800h | 1.71 | 2500 | 18 | 6 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
100 | 84 | 1200 | 2700 | 1.22 | 1600 | 99 | 67 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
100 | 84 | 2500 | 5800h | 1.51 | 2400 | 22 | 3 |
| 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
100 | 94 | 6850 | 7400h | 1.59 | 4000 | 38 | 9 |
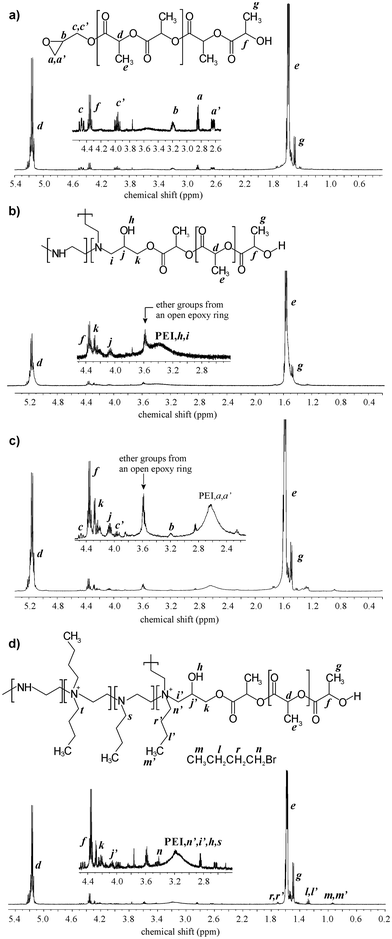
The polymerization reaction was monitored by analysing monomer conversion, the molar mass of oligomers, and the percentage of epoxide ring opening. Additionally, the content of PLA chains with closed epoxy ring was evaluated in relation to all products present in the mixture, including linear homopolymer terminated with hydroxyl and carboxyl groups and branched PLA. 1H NMR analysis of the reaction mixture demonstrated that, at a reaction temperature of 80 °C and the lowest [I]/[M] ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 1
10 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, PLA with closed epoxy ring was obtained (PLA-GLY) (Fig. 1a). However the product synthesised at lower molar ratio was rich in L-LA homopolymer chains. After 48 h of reaction, the monomer conversion was approximately 60%. For both products, GPC analysis revealed a monomodal distribution of molar mass (Fig. 2a). For the reaction no. 2a, MALDI-ToF analysis was performed to recognize the types of potassium ion series present in the polymerization product (Fig. 3a). It turned out that the main population, visible in the range of m/z 2120–2280 can be assigned to PLA capped with an epoxide group with an even number of lactic acid units (Fig. 3b). It means that under applied conditions the transesterification reaction of PLA is very limited. Above that range, small population of several products with an opened epoxy ring can be assigned, because the m/z value for those structures differ by at least 1 from the main product (Fig. 3c). For m/z range of 3130 to 3290 the highest intensity of the signal can be assigned to the reaction product between two linear epoxide-capped PLA (epoxide ring has been opened in the presence of PLA end group of the second linear PLA), described on the Fig. 3c as population GLY2-LAc42. In that case the PLA chain consisted of even number of lactic acid units. The same population, but with odd number of lactic acid units has been found (product described as GLY2-LAc41), however the intensity of the signal is much lower, and partially covered by signal of linear epoxide-capped PLA (GLY-LAc42). Additionally, we analysed small population of signals which could represents branched structures, obtained in the reaction between secondary hydroxyl group of the open epoxy ring with epoxide-capped PLA, described as GLY3-LAc41. Population of linear LAc homopolymer with even lactic acid units was observed as well, however cyclic homopolymer was not present in the full range of spectra. Molar mass determined from MALDI-ToF analysis correlates very well with the one calculated from 1H NMR spectra.
20, PLA with closed epoxy ring was obtained (PLA-GLY) (Fig. 1a). However the product synthesised at lower molar ratio was rich in L-LA homopolymer chains. After 48 h of reaction, the monomer conversion was approximately 60%. For both products, GPC analysis revealed a monomodal distribution of molar mass (Fig. 2a). For the reaction no. 2a, MALDI-ToF analysis was performed to recognize the types of potassium ion series present in the polymerization product (Fig. 3a). It turned out that the main population, visible in the range of m/z 2120–2280 can be assigned to PLA capped with an epoxide group with an even number of lactic acid units (Fig. 3b). It means that under applied conditions the transesterification reaction of PLA is very limited. Above that range, small population of several products with an opened epoxy ring can be assigned, because the m/z value for those structures differ by at least 1 from the main product (Fig. 3c). For m/z range of 3130 to 3290 the highest intensity of the signal can be assigned to the reaction product between two linear epoxide-capped PLA (epoxide ring has been opened in the presence of PLA end group of the second linear PLA), described on the Fig. 3c as population GLY2-LAc42. In that case the PLA chain consisted of even number of lactic acid units. The same population, but with odd number of lactic acid units has been found (product described as GLY2-LAc41), however the intensity of the signal is much lower, and partially covered by signal of linear epoxide-capped PLA (GLY-LAc42). Additionally, we analysed small population of signals which could represents branched structures, obtained in the reaction between secondary hydroxyl group of the open epoxy ring with epoxide-capped PLA, described as GLY3-LAc41. Population of linear LAc homopolymer with even lactic acid units was observed as well, however cyclic homopolymer was not present in the full range of spectra. Molar mass determined from MALDI-ToF analysis correlates very well with the one calculated from 1H NMR spectra.
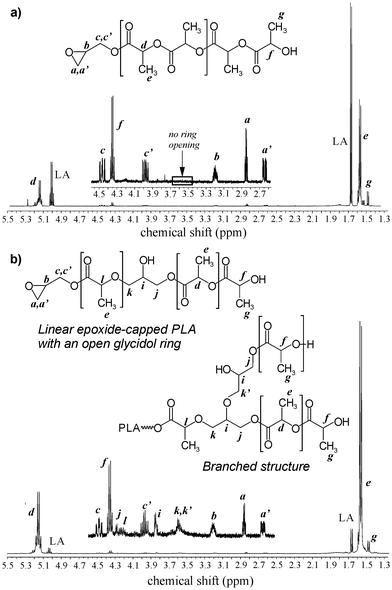 | ||
Fig. 1
1H NMR analysis of the L-LA polymerization product initiated by glycidol conducted at molar ratio: (a) GLY![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-LA = 1 L-LA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and temperature 80 °C for 48 h (Table 1, entry 1); (b) GLY 10 and temperature 80 °C for 48 h (Table 1, entry 1); (b) GLY![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L-LA = 1 L-LA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and temperature 80 °C for 50 h (Table 1, entry 2b). 20 and temperature 80 °C for 50 h (Table 1, entry 2b). | ||
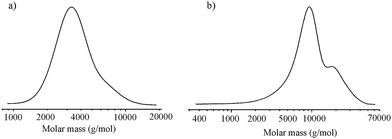 | ||
Fig. 2 Molar mass distribution of L-LA polymerization product obtained in the presence of glycidol after 48 h of the reaction at 80 °C with molar ratio [I]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [M] (a) 1 [M] (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and (b) 1 20 and (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. 50. | ||
In the next step, reaction no. 2a was extended to 50 h (2b) to increase monomer conversion. In that case about 50 mol% of epoxide ring opening has occurred, and the polymer mixture contained around 40 wt% of PLA-GLY (Fig. 1b). Similar observation was made also for the reaction with [I]/[M] molar ratio equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. The polymer mixture contained only 9 mol% of PLA-GLY and about 80% of glycidol ring has been opened. Molar mass distribution was bimodal with the main population composed of branched PLA with the small fraction of low molar mass linear PLA (Fig. 2b). Reactions conducted at 100 °C, resulted mainly with hyperbranched PLA except for the reaction carried out at the lowest molar ratio [I]/[M]. In that case we could see almost pure linear L-LA homopolymer and PLA capped with an epoxide group characterized by a narrow monomodal molar mass distribution. The presented results proved that linear PLA can be easily obtained under low temperature reaction conditions, using low molar ratio of reagents.
50. The polymer mixture contained only 9 mol% of PLA-GLY and about 80% of glycidol ring has been opened. Molar mass distribution was bimodal with the main population composed of branched PLA with the small fraction of low molar mass linear PLA (Fig. 2b). Reactions conducted at 100 °C, resulted mainly with hyperbranched PLA except for the reaction carried out at the lowest molar ratio [I]/[M]. In that case we could see almost pure linear L-LA homopolymer and PLA capped with an epoxide group characterized by a narrow monomodal molar mass distribution. The presented results proved that linear PLA can be easily obtained under low temperature reaction conditions, using low molar ratio of reagents.
Synthesis of L-PEI-g-PLA copolymer
Product 2a (Table 1, no. 2a) has been reacted with L-PEI58 at molar ratio L-PEI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PLA 1
PLA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 and 1
25 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (samples named as L-PEI58-PLA25 and L-PEI58-PLA50, respectively), and L-PEI93 at molar ratio 1
50 (samples named as L-PEI58-PLA25 and L-PEI58-PLA50, respectively), and L-PEI93 at molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 and 1
25 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 (samples named as L-PEI93-PLA25 and L-PEI93-PLA75, respectively) (Scheme 3).
75 (samples named as L-PEI93-PLA25 and L-PEI93-PLA75, respectively) (Scheme 3).
The assignment of the different signals was based on the 1H NMR analysis of 3 different products: 3-(dibutylamino)propane-1,2-diol, 3-(dibutylamino)-N-dodecylpropane-1,2-diol and L-PEI functionalized with glycidol (Fig. S2–S5, ESI†). The addition reaction progress of epoxide ring with secondary amine of L-PEI was monitored using 1H NMR analysis, based on the signals from methine group hydrogens of open epoxy ring close to nitrogen atom at 4.05 ppm (Fig. 4b, signal j) and methine end group protons of PLA at 4.35 ppm (Fig. 4b, signal f). In Table 2, column 4 the ratio is given as 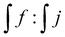 . In case of complete reaction between amine and epoxy ring, the ratio of integrated signal of protons j and f should be 1
. In case of complete reaction between amine and epoxy ring, the ratio of integrated signal of protons j and f should be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. As can be seen from the Table 2 column 4, the maximum ratio 1
1. As can be seen from the Table 2 column 4, the maximum ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 was obtained for the L-PEI with 93 repeating units and the highest ratio of L-PEI
0.5 was obtained for the L-PEI with 93 repeating units and the highest ratio of L-PEI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PLA (L-PEI93-PLA25). Furthermore, we calculated the theoretical molar percentage of epoxy ring opening in the presence of amine (SNth) expressed as nPLA/nN × 100%, where nPLA is the molar ratio of PLA used in the reaction, and nN is the number of nitrogen in the PEI structure (for reaction PEI58 with GLY-PLA at molar ratio 1
PLA (L-PEI93-PLA25). Furthermore, we calculated the theoretical molar percentage of epoxy ring opening in the presence of amine (SNth) expressed as nPLA/nN × 100%, where nPLA is the molar ratio of PLA used in the reaction, and nN is the number of nitrogen in the PEI structure (for reaction PEI58 with GLY-PLA at molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, SNth is equal to (25/58) × 100% = 43%). Based on AC ratio and SNth value, we calculated the percentage of epoxide substitution to the amine group (SN) using the equation:
25, SNth is equal to (25/58) × 100% = 43%). Based on AC ratio and SNth value, we calculated the percentage of epoxide substitution to the amine group (SN) using the equation: 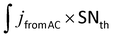 (for example: 0.3 × 43% = 13%, Table 2 column 6 no. 1). The results indicate the linking of linear PLA to L-PEI although the extent of linking is apparently limited to the maximum of 50% of the theoretical value. Moreover, for the reactions conducted at molar ratio L-PEI
(for example: 0.3 × 43% = 13%, Table 2 column 6 no. 1). The results indicate the linking of linear PLA to L-PEI although the extent of linking is apparently limited to the maximum of 50% of the theoretical value. Moreover, for the reactions conducted at molar ratio L-PEI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PLA 1
PLA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, regardless of the length of the L-PEI chain, the epoxy ring opened completely, meanwhile using twice as much PLA resulted in the presence of unreacted ring (Fig. 4c). It is worth mentioning that in every reaction product, we also observed a signal at 3.6 ppm, associated with the methylene group protons of the opened epoxy ring formed in the reaction between the epoxide and the hydroxyl group. This observation was surprising since the epoxide without L-PEI did not open under the applied condition (Fig. 4a). It seems that the amine in this reaction may acts as a catalyst for the ring opening initiated by hydroxyl groups. To conclude, the reaction product in every case was a mixture of PLA attached covalently to the L-PEI chain as well as linear and/or branched PLA created by the reaction of epoxide ring with hydroxyl groups. It should be also mentioned, that signals associated to the amidolysis reaction were not observed. To verify whether L-PEI can be involved in PLA degradation or can create a block copolymer by forming amide bonds, an additional reaction between L-PEI and commercial PLA was performed. In that case reaction was conducted at the molar ratio L-PEI
25, regardless of the length of the L-PEI chain, the epoxy ring opened completely, meanwhile using twice as much PLA resulted in the presence of unreacted ring (Fig. 4c). It is worth mentioning that in every reaction product, we also observed a signal at 3.6 ppm, associated with the methylene group protons of the opened epoxy ring formed in the reaction between the epoxide and the hydroxyl group. This observation was surprising since the epoxide without L-PEI did not open under the applied condition (Fig. 4a). It seems that the amine in this reaction may acts as a catalyst for the ring opening initiated by hydroxyl groups. To conclude, the reaction product in every case was a mixture of PLA attached covalently to the L-PEI chain as well as linear and/or branched PLA created by the reaction of epoxide ring with hydroxyl groups. It should be also mentioned, that signals associated to the amidolysis reaction were not observed. To verify whether L-PEI can be involved in PLA degradation or can create a block copolymer by forming amide bonds, an additional reaction between L-PEI and commercial PLA was performed. In that case reaction was conducted at the molar ratio L-PEI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PLA 1
PLA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in chloroform for 122 h at 30 °C, and additional 24 h at 60 °C. According to the GPC analysis, applied conditions did not affect the molar mass of PLA, (Fig. S6 and Table S1, ESI†).
2 in chloroform for 122 h at 30 °C, and additional 24 h at 60 °C. According to the GPC analysis, applied conditions did not affect the molar mass of PLA, (Fig. S6 and Table S1, ESI†).
| No. | Sample name | ([PEI]/[PLA])0 (mol/mol) | AC ratioa | SNth![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
SNc (%) | Alkyl bromide graftingd (%) | ||
|---|---|---|---|---|---|---|---|---|
| BuBr | OctBr | DodBr | ||||||
a Amine conversion calculated as ratio of integrated signal of protons j and f based on 1H NMR spectrum 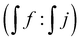 .
b Theoretical molar percentage of epoxy ring opening in the presence of amine.
c Calculated molar percentage of epoxy ring opening in the presence of amine.
d Percentage of general grafting to the polymer chain calculated by means of 1H NMR spectrum according to the formula .
b Theoretical molar percentage of epoxy ring opening in the presence of amine.
c Calculated molar percentage of epoxy ring opening in the presence of amine.
d Percentage of general grafting to the polymer chain calculated by means of 1H NMR spectrum according to the formula 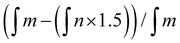 where where 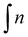 is the integration of the methylene protons in the immediate vicinity of the bromide atom in the unreacted alkyl bromide; is the integration of the methylene protons in the immediate vicinity of the bromide atom in the unreacted alkyl bromide; 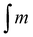 is the integration of the methyl protons in the reacted and unreacted alkyl bromide.
e Signals of the methylene protons present in the unreacted bromide (signal n) were covered by the signals of L-PEI.
f Too low intensity of the signal for the protons associated with methylene groups (signal n). is the integration of the methyl protons in the reacted and unreacted alkyl bromide.
e Signals of the methylene protons present in the unreacted bromide (signal n) were covered by the signals of L-PEI.
f Too low intensity of the signal for the protons associated with methylene groups (signal n).
|
||||||||
| 1 | L-PEI58-PLA25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3 |
43 | 13 | 72 | —e | 5.0 |
| 2 | L-PEI58-PLA50 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 0.4 |
86 | 34 | 20 | 22 | 8.0 |
| 3 | L-PEI93-PLA25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
27 | 13 | 76 | 29 | 6.8 |
| 4 | L-PEI93-PLA75 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
81 | 20 | —f | 28 | 8.0 |
In the last step the modification of obtained products was performed in the presence of alkyl bromide with different chain lengths: butyl bromide (BuBr), octyl bromide (OctBr) and dodecyl bromide (DodBr). This kind of modification was intended to enhance the solubility of copolymer in the chloroform, as well as to increase antibacterial properties.18 The reaction of alkyl bromide with L-PEI-g-PLA copolymer can lead to several products (Scheme 4). The grafting can occur either at the nitrogen atom of unreacted L-PEI resulting in a single- or double-substituted product, or at the nitrogen atom of L-PEI where PLA is attached through the open epoxide ring. The first possibility is sterically more favourable, however the quaternary ammonium salt formation cannot be excluded. As can be seen from Table 2 column 7 the highest grafting level was obtained for butyl bromide reacted with L-PEI58-PLA25 and L-PEI93-PLA25. It seems that the shorter alkyl chain and less oligomers of L-LA attached to the L-PEI chain, leads to the higher degree of alkyl bromide grafting. Additionally, for a series of samples (PEI58-PLA25 and the reaction product with alkyl bromide) we performed elemental analysis to calculate the C/N ratio (Table S2, ESI†), which confirmed that an alkyl bromide grafting leads to a higher C/N ratio in the final product.
Since our overall goal was to develop antimicrobial polymer films, and all the final products were in the form of wax-like solids, we prepared films of L-PEI58-PLA25-BuBr and L-PEI58-PLA25 with commercial PLA at 6 different concentrations: 90, 85, 80, 70, 50 and 15 wt%. All the L-PEI58-PLA25-BuBr/PLA blends created homogeneous films, however the sample consisting of 90 wt% of copolymer and 10 wt% of PLA was difficult to remove from the glass. L-PEI58-PLA25/PLA blends were not homogeneous, because copolymer L-PEI58-PLA25 was only partially soluble in chloroform. It seems that butyl bromide introduced into the copolymer structure, increase the solubility of copolymer in chloroform and as a consequence enables the formation of a homogeneous film.
Prepared blends, with 15 wt% and 30 wt% of copolymer PEI58-PLA25-BuBr, PLA capped with an epoxide group (PLA-Gly) and copolymer were additionally analysed by DSC analysis (Table 3). The obtained results revealed that PEI58-PLA25-BuBr copolymer was characterized by much lower Tg for about 28 °C and lower enthalpy of melting in comparison with LA oligomers. After mixing with commercial PLA, the Tg increased up to 33 °C and remained stable for both analysed blends. A single glass transition temperature point for the blends confirmed miscible behaviour of the two components of the blend. In addition, analysed blends exhibit triple melting endotherm. Different stability and size of crystallites or crystal reorganization can be responsible for the observed effect.19
| Sample name | T m (°C) | ΔHm (J g−1) | T g (°C) | T c (°C) | T m1 (°C) |
|---|---|---|---|---|---|
| T m and ΔHm were determined from the first heating cycle; Tg, Tc, and Tm1 were determined from the second heating cycle. | |||||
| PLA-Gly | 127 | 55.7 | 45.4 | 101 | 127/132 |
| PEI58-PLA25-BuBr | 106/110 | 40.5 | 17.5 | 90.6 | 111/116 |
| PEI58-PLA25-BuBr/PLA15% | 109/115/120 | 27.4 | 33.1 | 95.2 | 119/127 |
| PEI58-PLA25-BuBr/PLA30% | 111/121/127 | 23.6 | 33.7 | 95.5 | 127 |
| PLA | 153 | 34.3 | 64.3 | — | — |
Antibacterial properties
The antibacterial activity of the films was assessed as described in the Experimental section using Gram-negative bacteria (E. coli). The inhibition of bacteria growth was observed as clearance zones above and around the polymer. We evaluated the antibacterial effect of the copolymer L-PEI58-PLA25 with different alkyl bromide attached to the polymer chain. As the reference we used copolymer L-PEI58-PLA25 without any bromide grafting. For better solubility in water, all the tested products were protonated in the presence of hydrochloric acid. As can be seen from Fig. 5, the largest inhibition zone was observed for the reference sample which probably is related to its better solubility in water and partial diffusion. However, the MIC assay for that sample showed high value of 2.8 mg mL−1 which proves that migration of the compound to the water solution occurred minimally. Copolymers synthesized in the presence of alkyl bromide exhibited significantly smaller inhibition zone, as they are more hydrophobic and the migration is limited, especially for the copolymers with long alkyl bromide. As the last step, we performed antimicrobial activity test of L-PEI58-PLA25/PLA blends with different alkyl bromides attached to the L-PEI chain according to the ISO 22196 regulation. As a control sample we used PLA film and film consisted of commercial PLA with chloramphenicol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
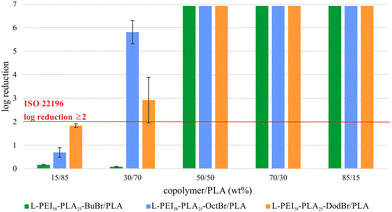
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.13 (w/w)). The detailed quantitative analysis showed (Table 4 and Fig. 6), that all the blends that contained more than 50 wt% of copolymer (which is equal to minimum 2 wt% of PEI in the sample) showed an inhibition growth of E. coli up to 1 log CFU mL−1. Antibacterial properties exhibited also 30/70 copolymer/PLA blend where copolymer was grafted with octyl bromide and dodecyl bromide.
0.13 (w/w)). The detailed quantitative analysis showed (Table 4 and Fig. 6), that all the blends that contained more than 50 wt% of copolymer (which is equal to minimum 2 wt% of PEI in the sample) showed an inhibition growth of E. coli up to 1 log CFU mL−1. Antibacterial properties exhibited also 30/70 copolymer/PLA blend where copolymer was grafted with octyl bromide and dodecyl bromide.
 | ||
| Fig. 5 The antibacterial effect of: L-PEI58-PLA25 copolymer without alkyl bromide (1) and grafted with BuBr (2), OctBr (3) and DodBr (4) against E. coli. | ||
| No. | Blend composition (copolymer/PLA) (wt%) | Content of PEI in the blend (wt%) | L-PEI58-PLA25-BuBr/PLA (log CFU ml−1) | L-PEI58-PLA25-OctBr/PLA | L-PEI58-PLA25-DodBr/PLA |
|---|---|---|---|---|---|
| a Commercial polylactide with 0.64% w/w of chloramphenicol. b Bacteria counted for the volume of 100 μL which means that above 1log growth of colonies on an agar plate was not observed. | |||||
| 1 | 0/100 | — | 6.93 ± 0.16 | ||
| 2 | 0/100/CHLa | — | 2.66 ± 0.18 | ||
| 3 | 15/85 | 0.70 | 6.77 ± 0.06 | 6.24 ± 0.20 | 5.10 ± 0.08 |
| 4 | 30/70 | 1.12 | 6.85 ± 0.08 | 1.43 ± 0.50 | 3.38 ± 0.29 |
| 5 | 50/50 | 1.99 | <1b | <1b | <1b |
| 6 | 70/30 | 2.74 | <1b | <1b | <1b |
| 7 | 85/15 | 3.31 | <1b | <1b | <1b |
L-PEI58-PLA25-BuBr/PLA blend prepared in the weight ratio 15/85 and 30/70 did not show antibacterial activity. Polylactide film with chloramphenicol showed antimicrobial properties with a reduction in colony forming units from 6.11 to 2.70 log CFU ml−1.
According to the presented results, we can conclude, that the most promising film was obtained for a PLA blend containing 30 wt% of L-PEI-g-PLA copolymer with octyl bromide grafting. In that case only 1.12 wt% of L-PEI was required to obtain antibacterial polylactide film.
Conclusions
Antibacterial polymer films based on L-PEI and covalently attached oligomers of lactide were successfully synthesized. Our approach involved preparation of lactide oligomers with an epoxy end group, for which detailed structural characterization was provided for the first time. The addition reaction of polyethyleneimine with the synthesized LA oligomers resulted in a mixture of products i.e. L-PEI-g-PLA copolymer as well as linear and branched polylactide. Antimicrobial activity test confirmed that protonated L-PEI-g-PLA copolymer exhibited antimicrobial properties, but it created a non-homogenous film with commercial polylactide. The miscibility of the copolymer in the PLA/chloroform solution improved after the incorporation of alkyl bromide into the copolymer structure. L-PEI-g-PLA copolymer synthesized in the presence of 1-bromooctane and 1-bromododecane enabled the formation of a homogeneous film with commercial PLA and demonstrated promising levels of antimicrobial activity against Gram-negative bacterial strain. Such material could potentially be used in food packaging; however, further research is needed to evaluate the diffusion and release kinetics of the copolymer from PLA films to ensure their suitability for food packaging or biomedical applications.Author contributions
A. Iuliano: conceptualization, methodology, investigation, analysis of results, writing – original draft, funding acquisition, project administration; M. Kukuć: investigation, analysis of results, writing – review and editing; J. Mierzejewska: analysis of results, writing – review and editing; K. Drężek: analysis of results, writing – review and editing; D. Jańczewski: analysis of results, writing – review and editing; J. Pachla: analysis of results, writing – review and editing.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research was funded by POB Technologie Materiałowe 2-START of the Warsaw University of Technology within the Excellence Initiative: Research University (IDUB) programme.References
- M. R. E. Santos, P. V. Mendonça, M. C. Almeida, R. Branco, A. C. Serra, P. V. Morais and J. F. J. Coelho, Biomacromolecules, 2019, 20, 1146–1156 CrossRef CAS PubMed
.
- precedenceresearch.com; report code: 2101; published August 2022.
- M. Carbone, D. T. Donia, G. Sabbatella and R. Antiochia, J. King Saud Univ., Sci., 2016, 28, 273–279 CrossRef
.
- D. Druvari, N. D. Koromilas, G. Ch. Lainioti, G. Bokias, G. Vasilopoulos, A. Vantarakis, I. Baras, N. Dourala and J. K. Kallitsis, ACS Appl. Mater. Interfaces, 2016, 8, 35593–35605 CrossRef CAS PubMed
.
- Z. Li, H. Bai, S. Jia, H. Yuan, L.-H. Gao and H. Liang, Mater. Chem. Front., 2021, 5, 1236–1252 RSC
.
- M. B. Harney, R. R. Pant, P. A. Fulmer and J. H. Wynne, ACS Appl. Mater. Interfaces, 2009, 1, 39–41 CrossRef CAS PubMed
.
- S. El Habnouni, V. Darcos, X. Garric, J.-P. Lavigne, B. Nottelet and J. Coudane, Adv. Funct. Mater., 2011, 21, 3321–3330 CrossRef CAS
.
- S. El Habnouni, J.-P. Lavigne, V. Darcos, B. Porsio, X. Garric, J. Coudane and B. Nottelet, Acta Biomater., 2013, 9, 7709–7718 CrossRef CAS PubMed
.
- Y. Huang, C. Hu, Y. Zhou, R. Duan, Z. Sun, P. Wan, C. Xiao, X. Pang and X. Chen, Angew. Chem., Int. Ed., 2021, 60, 9274–9278 CrossRef CAS PubMed
.
- P. P. Kalelkar, Z. Geng, M. G. Finn and D. M. Collard, Biomacromolecules, 2019, 20, 3366–3374 CrossRef CAS PubMed
.
-
A. Modarresi Chahardehi, M. Barati, M. Navaderi, Z. Velashjerdi, I. Zare and E. Mostafavi, in Antibacterial and Antiviral Functional Materials, Volume 1, American Chemical Society, 2023, vol. 1458, pp. 1–32 Search PubMed
.
- R. J. Kopiasz, D. Kozon, J. Pachla, Ł. Skórka and D. Jańczewski, Polymer, 2021, 217, 123452 CrossRef CAS
.
- J. Pachla, R. J. Kopiasz, G. Marek, W. Tomaszewski, A. Głogowska, K. Drężek, S. Kowalczyk, R. Podgórski, B. Butruk-Raszeja, T. Ciach, J. Mierzejewska, A. Plichta, E. Augustynowicz-Kopeć and D. Jańczewski, Biomacromolecules, 2023, 24, 2237–2249 CrossRef CAS PubMed
.
- E. Torlak and D. Sert, Int. J. Biol. Macromol., 2013, 60, 52–55 CrossRef CAS PubMed
.
- M. Walczak, M. S. Brzezinska, A. Richert and A. Kalwasińska, Int. Biodeterior. Biodegrad., 2015, 98, 1–5 CrossRef CAS
.
- A. Kundys, A. Plichta, Z. Florjańczyk, A. Frydrych and K. Żurawski, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 1444–1456 CrossRef CAS
.
- L. M. Pitet, S. B. Hait, T. J. Lanyk and D. M. Knauss, Macromolecules, 2007, 40, 2327–2334 CrossRef CAS
.
- D. Kozon, J. Mierzejewska, T. Kobiela, A. Grochowska, K. Dudnyk, A. Głogowska, A. Sobiepanek, A. Kuźmińska and T. Ciach, Macromol. Biosci., 2019, 19, 1900254 CrossRef CAS PubMed
.
- X. Kong, H. Qi and J. M. Curtis, J. Appl. Polym. Sci., 2014, 131, 40579 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4py00605d |
| This journal is © The Royal Society of Chemistry 2024 |