 Open Access Article
Open Access ArticleMagneto-chiral dichroism of chiral lanthanide complexes
Fabrice
Pointillart
 *a,
Matteo
Atzori
*a,
Matteo
Atzori
 *b and
Cyrille
Train
*b and
Cyrille
Train
 b
b
aUniv Rennes, CNRS, ISCR (Institut des Sciences Chimiques de Rennes) – UMR 6226, 35000 Rennes, France. E-mail: fabrice.pointillart@univ-rennes1.fr
bLaboratoire National des Champs Magnétiques Intenses, CNRS, Univ. Grenoble Alpes, INSA Toulouse, Univ. Toulouse Paul Sabatier, EMFL, 38042 Grenoble, France. E-mail: matteo.atzori@lncmi.cnrs.fr
First published on 31st January 2024
Abstract
Magneto-Chiral Dichroism (MChD) is an enantioselective and polarization independent light–matter interaction shown by magnetized chiral molecules and materials. This phenomenon, predicted in 1984 and experimentally demonstrated in 1997 by studying the differential visible light emission of a chiral EuIII complex, is now attracting the interest of the chemical community working with transition metal and lanthanide-based chiral complexes. This is motivated by both the information on the magnetic, electronic and chiroptical properties that can be retrieved using this unconventional spectroscopic technique and the potential technological applications that can be foreseen, such as the optical readout of magnetic data without the need for polarization-based readout devices. In particular, chiral lanthanide complexes, which intrinsically have high spin–orbit coupling (a key factor to observe MChD), a variety of electronic configurations, a multitude of electronic transitions of different characteristics, variable coordination geometries and different degrees of magnetic anisotropy, represent ideal molecules to investigate MChD in both light absorption and emission in a wide spectral range. This perspective summarizes the studies reported so far in the literature on the MChD of chiral lanthanide complexes and provides some general conclusions that will help the chemical community in designing lanthanide-based systems highly responsive to MChD. Finally, we suggest prospective experiments and studies that are needed to push forward the understanding and the use of this fascinating phenomenon.
Introduction
Chirality represents the possibility for an object to exist as two non-superposable mirror images forming a pair of enantiomers.1 Such a structural property is extremely relevant for a multitude of scientific aspects in chemistry, biology and physics.1,2 One of the most known properties of chiral objects is their ability to rotate the plane of light polarization (Natural Optical Activity, NOA) with a rotatory sign (dextro or levo) dependent on the absolute configuration of the system.3 Optical manifestations related to NOA are Natural Circular Dichroism (NCD) and Birefringence (NCB), which represent a differential absorption and refraction of circularly polarized light, respectively.3 Another important manifestation of the light–matter interaction is represented by the Magnetic Optical Activity (MOA), which refers to a differential absorption of circularly polarized light of magnetized matter resulting in the well-known phenomenon of Magnetic Circular Dichroism (MCD).3–8 Although phenomenologically close, NOA is a consequence of the breaking of inversion symmetry by structural chirality, whereas MOA originates from the breaking of time reversal symmetry by magnetization.3,4 Moreover, one must keep in mind that both properties are dependent on the state of polarization of light.3There is another optical phenomenon that through the breaking of both time reversal and inversion symmetry allows chiral systems to differentially interact with light when magnetized. This phenomenon can be called Magneto-Chiral Activity (MChA) and manifests itself as a differential absorption or emission of light for Magneto-Chiral Dichroism (MChD) and as anisotropic refraction for Magneto-Chiral Birefringence (MChB).3,9–11 A key feature of MChA is that it is independent of the state of light polarization. In other words, it can be observed and studied by using unpolarized light sources, which is a practical and technological advantage.
MChD in emission should not be confused with Circularly Polarized Light (CPL) emission. In that case, a chiral system shows emission of CPL upon application of a nonpolarized excitation, even if this phenomenon is now also studied under a magnetic field.12–14 MChA refers to a modulation of the absorption or emission intensity of light collinear to an applied magnetic field without generation of CPL.
Besides the fascinating aspect of allowing chiral systems to interact with non-chiral unpolarized light, the intensity of MChD responses is proportional to the system magnetization,11,15,16 making MChD spectroscopy a powerful technique to probe both the optical and magnetic properties of chiral systems.
So far, MChD has been studied in a variety of chemical systems11,17–20 and metaobjects,21,22 but most of the studies have been undertaken on chiral complexes containing transition metals17,18,23–30 and lanthanide ions.23,31–35 The latter have attracted special attention as a consequence of their high angular momenta and spin–orbit coupling, a prerequisite to observing MChD, resulting in high magnetic anisotropy, as well as the magnetic-dipole allowed characteristic of certain f–f electronic transitions.
In this perspective, we first review the studies on the MChD of chiral lanthanide complexes reported in the literature in both light emission and absorption with light of different wavelengths, and then we provide some general hints that will help the chemical community in designing chiral lanthanide complexes responsive to MChD. Finally, we suggest prospective directions for this emerging and challenging research field.
Discussion
Magneto-chiral dichroism in visible light emission
The existence of the magneto-chiral anisotropy and its enantioselectivity were experimentally demonstrated for the first time by G. L. J. A. Rikken and E. Raupach10 realizing an experiment suggested by G. Wagnière.36 Tris(3-trifluoroacetyl-±-camphorato)europium(III), Eu((d/l)tfc)3, complexes ((d/l)1-Eu) were selected as ideal candidates to observe MChD9 due to the strong natural and magnetic optical activity of 5D0 → 7F1,2 luminescent transitions.37–39The magneto-chiral luminescence anisotropy was measured for a deuterated DMSO solution of (d/l)1-Eu pair of enantiomers under a 350 nm light irradiation and a 0–0.9 T magnetic field range. The highly luminescent electric-dipole allowed 5D0 → 7F2 electronic transitions displayed a weak MChD activity (gMChD = 0.03% at 0.9 T and room temperature) while the weakly luminescent magnetic-dipole allowed 5D0 → 7F1 transition displayed a stronger MChD activity (gMChD = 0.12% at 0.9 T and room temperature).
The second study on MChD for visible light emission was published more than 20 years later19 after the seminal work of Rikken and Raupach. TbIII and EuIII analogues of the compounds [Ln(L1S,S/R,R)3](CF3SO3)3 (L1S,S/R,R = S or R-1-(2-naphthyl)ethyl amine, Ln = TbIII ((S,S/R,R)2-Tb), EuIII ((S,S/R,R)2-Eu)) (Fig. 1a) and [Ln((d/l)-tfc)3(phen)] (phen = 1,10-phenanthroline, Ln = TbIII ((d/l)3-Tb)), EuIII ((d/l)3-Eu) (Fig. 1d) were studied.19(S,S/R,R)2-Tb revealed a strong MChD signal for all the 5D4 → 7FJ (J = 6, 5, 4, 3) transitions with a maximum value of gMChD = 6% at 1 T and 4.3 K while this value increased to 16% at 14 T and 5 K (Fig. 1b). Similar measurements on the EuIII analogue showed that (S,S/R,R)2-Eu exhibited a much lower MChD signal for 5D0 → 7FJ (J = 1, 2) transitions with a maximum gMChD value of 0.3%. The significant difference in MChD activities between the two analogues demonstrated the crucial role of the paramagnetic nature with a large magnetic moment of TbIII (J = 6) compared to the weakly paramagnetic EuIII (J = 0). The first two analogues (S,S/R,R)2-Ln for which the lanthanide center adopts a symmetrical nona-coordinated geometry were compared with the TbIII and EuIII analogues of (d/l)3-Ln, where the lanthanide adopts a distorted octa-coordinated geometry. The aim was to evaluate the role of the coordination geometry in the MChD response. Under an applied magnetic field of 1 T, (d/l)3-Tb revealed a difference in the luminescence intensity of 1–2% while the MChD signal was almost undetectable for (d/l)3-Eu. For a given lanthanide ion, since the difference in the MChD activity could not be attributed to the difference in magnetization, the authors proposed to attribute it to the difference in coordination sphere, in other words, the degree of the inversion symmetry breaking at the lanthanide site. Such phenomena could be easily evaluated for EuIII using the R ratio between the emission intensities of the electric-dipole allowed transition (5D0 → 7F2) and the magnetic-dipole allowed transition (5D0 → 7F1).40 The R value is close to 1 for (S,S)2-Eu and 13.6 for (d)3-Eu leading to a degree of symmetry breaking lower for (S,S)2-Eu than the one for (d)3-Eu. Moreover, R = 1 is close to the ideal value expected for a strong MChD.41
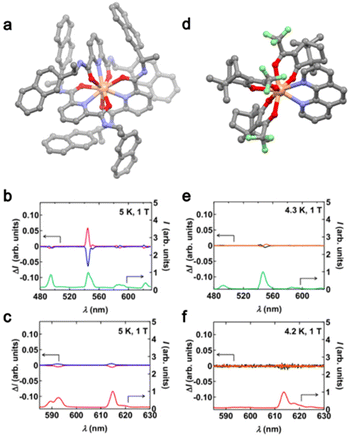 | ||
| Fig. 1 (a) Molecular structure of (S,S)2-Tb. Grey, C; red, O; blue, N and salmon, Tb. H atoms and CF3SO3− anions are omitted for clarity. (b) Average luminescence spectrum of (S,S)2-Tb (green line) and MChD spectra of (S,S)2-Tb (pink line) and (R,R)2-Tb (blue line). (c) Average luminescence spectrum of (S,S)2-Eu (red line) and MChD spectra of (S,S)2-Eu (pink line) and (R,R)2-Eu (blue line). (d) Molecular structure of (d)3-Tb. Grey, C; red, O; blue, N; green, F and salmon, Tb. H atoms are omitted for clarity. (e) Average luminescence spectrum of (d)3-Tb (green line) and MChD spectra of (d)3-Tb (black line) and (l)3-Tb (orange line). (f) Average luminescence spectrum of (d)3-Eu (red line) and MChD spectra of (d)3-Eu (black line) and (l)3-Eu (orange line). Adapted with permission from ref. 12. Copyright 2019, American Physical Society. | ||
In conclusion, the investigation of K. Taniguchi et al. concluded that the MChD signal is ascribed to a combination of strong magnetic moment (J ≠ 0) and small inversion symmetry breaking at the lanthanide center (high symmetry).
Magneto-chiral dichroism in hard X-ray absorption
The experimental demonstration of the existence of MChA in emission implies its existence in absorption because of the relationship between Einstein's coefficients in radiative processes.The motivation to investigate MChD in absorption takes its origin from the fact that large MChD effects are expected for the absorption bands of certain lanthanide complexes.9 Furthermore, MChD studies on light absorption can provide more quantitative information than those on light emission, which are intrinsically affected by the efficiency of the energy transfer processes, irradiation wavelength, etc.
The presence of MChD in the X-ray region (XMChD) was suggested by L. Barron.3 Indeed, five years after the discovery of the MChD in emission,10 J. Goulon et al.30 reported the first evidence of X-ray MChD for a magnetoelectric Cr2O3, and then ten years later, J. R. Galán-Mascarós et al. published a study on the observation of MChD in a chiral paramagnetic lanthanide-based complex.34 In the two investigations, MChD was probed by hard X-ray Absorption Spectroscopy (XAS).
At that time, such spectroscopy was selected because it is element-specific and because one can expect a good signal-to-noise ratio due to the higher extinction coefficients and penetration depths of X-rays than typical UV-vis-NIR absorption experiments.
The XMChD experiments were carried out on the {TbIII[NiII(pro)2]6}3+ cation (4) (pro = (L,D)-prolinate).42,43 The lanthanide center adopts an icosahedral coordination sphere surrounded by six NiII(pro)2 units. The first coordination sphere of the NiII centers formed a perfect octahedra with the (L,D)-prolinate linked at the equatorial positions. The difference between normalized XAS spectra obtained with magnetic field parallel and anti-parallel to the light wavevector led to a non-zero dichroic signal with image mirror when changing from (D)-prolinate to (L)-prolinate. The XMChD reached a maximum value of 1% of the total intensity at the TbIII L2 edge at room temperature and 1 T. Surprisingly, no significant XMChD signal was observed at the TbIII L3 edge even if the theory predicted a stronger dichroic phenomenon at the L2 edge.44,45 In contrast, the absence of XMChD at the NiII K-edge was attributed to the weaker spin–orbit coupling and electric quadrupolar contribution for transition metals than for lanthanides. Indeed, it is important to mention here that the electric-dipole/magnetic-dipole characteristic is the key to strong MChD intensity for investigations in the UV-Vis-NIR range while the electric-dipole/electric-quadrupole characteristic is crucial for working with X-rays.
The first attempt to observe XMChD for molecular lanthanide-based complexes was limited to room temperature and low applied magnetic fields (1 T). Therefore, in 2020, S. Piligkos et al.35 reported an experimental observation of XMChD for a mononuclear lanthanide complex formulated as Na5[HoIII(ODA)3](BF4)2·6H2O (5-Ho) (ODA2− = oxydiacetate) (Fig. 2a).46 HoIII is coordinated to three virtually planar tridentate ODA ligands to form the two Λ and Δ enantiomers and the nine-coordinated HoIII ion in a distorted face-centered trigonal prismatic coordination sphere (D3). The XMChD experiments were performed at the L3 absorption edge of HoIII because the promotion of a 2p core electron into an empty 5d or 6s valence state via the electric-dipole allowed transition (Δl = ±1) and 2p into a 6p or 4f state (Δl = 0, +2) via electric-quadrupole transitions can be easily disentangled at the lanthanide L3-edge,47 while their contributions at the L2-edge is more difficult to disentangle.48 A XMChD signal of opposite sign for (Λ)5-Ho and (Δ)5-Ho was observed at the pre-edge (8069 eV) and the main (8078 eV) absorptions, but not in the extended region (Fig. 2b).35 Such observations confirmed that non-magnetic empty 6p and 6d states did not contribute to the XMChD signal while 4f and 5d did because of their orbital angular momentum contribution. The maximum XMChD activity was evaluated at 0.05% at 2.7 K under an applied magnetic field of 4 T. The authors attributed the weak XMChD activity of (Λ/Δ)5-Ho compared to (L/D)-4 to the strong localization of the 4f orbitals and weak hybridization with the 5d orbitals. Nevertheless, the difference in the lanthanide ion, local coordination symmetry and probed edge makes a direct comparison between the XMChD activities of these two compounds difficult.
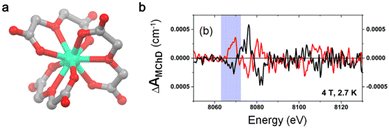 | ||
| Fig. 2 (a) Molecular structure of 5-Ho, Na+ cations, and BF4− anions; hydrogen atoms and water molecules of crystallization were omitted for clarity. Grey, C; red, O and green, Ho. (b) XMChD signal recorded for Λ (red line) and Δ (black line) at the Ho L3-edge at 2.7 K with an applied magnetic field of 4 T. The blueish zone corresponds to the pre-edge absorption. Adapted with permission from ref. 27. Copyright 2020, Royal Society of Chemistry. | ||
Magneto-chiral dichroism in visible light absorption
From the previous works, one can draw a few conclusions. It appears that the (X)MChD strongly depends on the angular moments of the lanthanide (L and J ≠ 0), the spin–orbit coupling and the symmetry of the coordination sphere (degree of inversion symmetry breaking). All these parameters are driven by the nature of the lanthanide and the crystal field in the complex leading to the magnetic anisotropy, which is indeed a key parameter in molecular magnetism to design Single-Molecule Magnets (SMMs). Thus, together with our colleagues, we decided to study both magnetic and (chiro)optical properties of enantiopure ytterbium(III) helicene-based compounds.23The helicoidal chirality of helicene comes from the ortho-fused (hetero) aromatic rings with extended π-conjugation, and they are well-known to display remarkable chiroptical activity.49–52 YbIII has been selected because it has one of the simplest electronic configurations among the lanthanide series with only one excited multiplet (2F5/2) leading to a unique magnetic-dipole allowed (|ΔJ| = 1) electronic transition (2F7/2 → 2F5/2).
First, the pair of enantiomers formulated as [Yb(L2(P,M))(hfac)3] (L2 = 3-(2-pyridyl)-4-aza[6]-helicene; hfac− = 1,1,1,5,5,5-hexafluoroacetylacetonate) ((P/M)6-Yb) was prepared as single crystals.31,53,54 The crystal structure revealed mononuclear complexes in which YbIII is surrounded by six oxygen atoms coming from three hfac− ancillary anions and two nitrogen atoms coming from the L2(P/M) ligand (Fig. 3a). A distorted D2d coordination sphere was identified and a Δ and Λ lanthanide-centered chirality driven by the L2(P) and L2(M) ligands, respectively, was observed. The direct current magnetic susceptibility measurements revealed a χMT value of 2.24 cm3 K mol−1 at room temperature and a magnetization of 1.73Nβ at 5 T in agreement with an YbIII (S = ½, L = 3 and gJ = 8/7). The alternating current susceptibility measurements highlighted a slow magnetic relaxation at 2 K and 1 kOe, revealing a significant magnetic anisotropy. Both enantiomers of 6-Yb showed the usual NIR YbIII centered luminescence at 77 K with additional bands due to hot bands and vibronic contributions. A combination of both chirality and emission opens the possibility to investigate their cross effects called Circularly Polarized Luminescence (CPL). Solid-state irradiation at 365 nm induced a strong mirror image CPL signal for (P/M)6-Yb with a maximum glum factor of 0.13 at 977 nm (Fig. 3b).33 By measuring the absorption spectra at 4 K on an oriented single crystal perpendicular to the [011] crystallographic face, it was possible to determine the energy splitting of the excited 2F5/2 multiplet. The complete experimental energy level diagram for both the 2F7/2 ground multiplet and the 2F5/2 excited multiplet under the crystal field effect was rationalized by ab initio calculations.
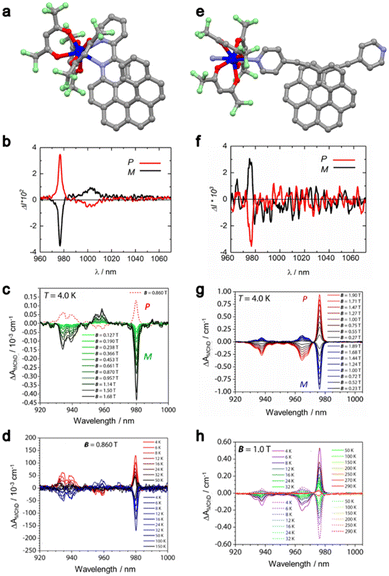 | ||
| Fig. 3 Molecular structure of (P)6-Yb (a) and [(P)7-Yb]n (e); H atoms and solvent molecules of crystallization were omitted for clarity. Grey, C; red, O; blue, N and dark blue, Yb. CPL spectra (at 298 K) of (P)6-Yb (red line)/(M)6-Yb (black line) (b) and [(P)7-Yb]n (red line)/[(M)7-Yb]n (black line) (f) under 365 nm excitation in solid-state. Field dependence of the difference in the absorption ΔAMChD of the 2F7/2 → 2F5/2 transition at 4 K for (P/M)6-Yb (c) and [(P/M)7-Yb]n (g). Thermal dependence of the difference in the absorption ΔAMChD of the 2F7/2 → 2F5/2 transition for (P/M)6-Yb under 0.86 T (d) and [(P/M)7-Yb]n under 1 T (h). Adapted with permission from ref. 23 and 25. Copyright 2021, American Chemical Society. Copyright 2023, Wiley-VCH Verlag GmbH & Co. KGaA Weinheim. | ||
These studies demonstrated that the helical ligands provided a strong-enough chiral environment around the YbIII center for MChD investigation.
Accordingly, the first experimental MChD measurements through visible-NIR light absorption were carried out at 4 K in the 0–1.68 T magnetic field range, allowing the observation of a fine-structured strong MChD signal associated with the 2F7/2 → 2F5/2 transition (Fig. 3c). The asymmetric factor gMChD was evaluated at 0.12% at 0.86 T and 4 K, which is in the same order of magnitude as that of (S,S/R,R)2-Tb (0.06% at 1 T and 4.3 K) determined by light emission. The MChD signal is strong enough to perform a temperature dependence study in the 4–150 K temperature range (Fig. 3d). Both field (Fig. 3c) and thermal (Fig. 3d) dependences of the MChD signal were compared to the thermal and field dependences of the magnetization determined by magnetometry with a perfect agreement. The thermal dependence of the MChD signal allowed us to verify the Barron and Vrbancich microscopic theory of MChD formulated on the basis of the Magnetic Circular Dichroism (MCD) theory. At low temperatures (T < 15 K), the so-called MChD C term (analogous to the Faraday C term in MCD), which is temperature dependent due to a variation of the population of the ground state and associated with an absorptive line-shape, dominates. For T > 20 K, the so-called MChD A term (analogous to the Faraday A term in MCD), which is due to the Zeeman energy splitting and hence temperature independent and associated with a derivative-type line-shape, dominates.
A similar magnetic and chiroptical investigation was carried out on a second pair of enantiomers using the 2,15-bis-(4-pyridyl)-ethynyl-carbo[6]helicene (L3(P/M)) ligand instead of L2(P/M). The reaction of L3(P/M) with the Yb(hfac)3(H2O)2 metallic precursor led to the formation of enantiopure coordination polymers of the formula [L3(P/M)Yb(hfac)3]n ([(P/M)7-Yb]n).33 A slightly distorted D2d N2O6 surrounding is found around the YbIII centers as found for the mononuclear complexes (P/M)6-Yb.
From a magnetic point of view, the main difference comes from the clear SMM behavior observed under an applied magnetic field of 1600 Oe with a magnetic relaxation occurring through a combination of Orbach and Raman processes. Such an enhancement of magnetic performances could be rationalized by the “trans” position of the nitrogen atoms in [(P/M)7-Yb]n while they have been observed in the “cis” position in (P/M)6-Yb leading to a smaller electronic repulsion for the prolate YbIII ion, i.e. a stronger spin–orbit coupling/axial magnetic anisotropy in the polymeric structures. Such qualitative analysis was confirmed by ab initio calculations which concluded that the ground Kramers doublet is mainly composed of MJ = ±5/2 (gz = 4.0) for (P/M)6-Yb and MJ = ±7/2 (gz = 7.0) for [(P/M)7-Yb]n.
Irradiation at 365 nm of [(P/M)7-Yb]n led to a strong NIR emission of the YbIII center with the total energy splitting similar to the one observed for the mononuclear systems but with a different shape (relative intensity and energy position of the emission lines) which is in agreement with a similar crystal field but different electronic distributions for both monomeric and polymeric systems. Attempts to measure CPL at room temperature in the solid state led to the observation of a weak mirror image signal for both enantiomers of [(P/M)7-Yb]n with a maximum dissymmetry factor glum of 0.007 at 978 nm (Fig. 3f). One could deduce from the CPL spectra that only a weak chiral environment is present around YbIII in [(P/M)7-Yb]n. In contrast, MChD measurements performed on single crystals of [(P/M)7-Yb]n at 4 K in the 0–1.90 T magnetic field revealed a remarkably high MChD signal (Fig. 3g) with gMChD = 0.19 T−1 at 1 T and 4 K. Such a value is the highest reported value for lanthanide complexes. The MChD activity was strong enough to observe a differential absorption ΔAMChD up to room temperature under an applied magnetic field of 1 T (Fig. 3h). In conclusion, despite the weaker chiral environment in [(P/M)7-Yb]n compared to (P/M)6-Yb, a stronger MChD activity was measured due to the stronger magnetic anisotropy determined by both magnetometry and ab initio calculations.
MChD measurements through light absorption were also performed for the heterobimetallic 3d–4f complexes of the formula [Ln5Ni6((R/S)-HL4)6(Ac)3(μ3-OH)9(H2O)6](ClO4)3(H2O)15 (H3L3 = (R/S)-(2-hydroxy-3-methoxybenzyl)-serine, Ln = DyIII(R/S)8-DyNi, YIII(R/S)8-YNi).23 The room temperature NCD of both DyIII and YIII analogues displayed two components at 670 nm and 1190 nm (Fig. 4a) which are associated with the 3T1/3T2/3A1/3E ← 3A2 transitions of the octahedral NiII.28,55,56 Since both NCD spectra are identical, one could conclude that the electronic transitions for DyIII do not contribute to the NCD. In contrast, the MCD spectra for (R/S)8-DyNi are composed of two weak and broad contributions of NiII and a series of intense and sharp signals associated with DyIII (Fig. 4c). Deep analysis of the MCD spectra of (R/S)8-DyNi highlighted a splitting of the MCD contributions, which has been attributed to the presence of the two crystallographically independent DyIII centers. The thermal dependence of the MChD was measured at 0.86 T in the 4–150 K temperature range (Fig. 4d). It displayed contributions coming from both the NiII and the DyIII centers, in contrast with what was observed in XMChD for the heterobimetallic complex 4.34
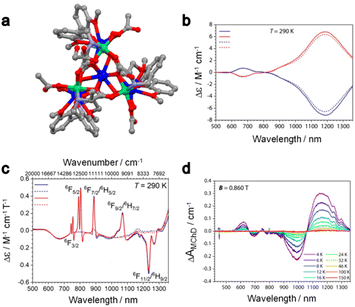 | ||
| Fig. 4 (a) Molecular structure of (R/S)8-DyNi; H atoms, ClO4− anions and solvent molecules of crystallization were omitted for clarity. Grey, C; red, O; blue, Dy and green, Ni. (b) Room temperature NCD spectra of (R)8-DyNi (blue line), (S)8-DyNi (red line), (R)8-YNi (dashed blue line) and (S)8-YNi (dashed red line) in MeOH solution. (c) Room temperature MCD spectra of (R)8-DyNi (blue line), (S)8-DyNi (red line), (R)8-YNi (dashed blue line) and (S)8-YNi (dashed red line) in MeOH solution. (d) Thermal variation of MChD spectra of (R)8-DyNi recorded on an oriented single crystal under an applied field of 0.86 T. Adapted with permission from ref. 16. Copyright 2022, American Chemical Society. | ||
Focusing on the MChD contributions of the DyIII centers, the two DyIII sites did not provide MChD signals of the same intensity. The most intense DyIII MChD signals were attributed to the three DyIII ions that occupy the external position of the clusters. Such attribution is in agreement with the fact that such eight-coordinated DyIII ions are closer to the chiral ligand than the two nine-coordinated DyIII ions, although the difference in the magnetic anisotropy between the two DyIII ions cannot be ruled out at this stage. In conclusion, the authors associated the NiII MChD as mainly driven by its NOA while the MChD of DyIII as mainly driven by its MOA.
Together with the magnetic and chiroptical investigations performed on (P/M)6-Yb and [(P/M)7-Yb]n, underlining that the magnetic anisotropy of the ground state was the leading factor of MChD in these species, this study confirms that engineering the magnetic anisotropy of the Ln systems is a more efficient strategy than optimizing the chirality at the metal center to enhance the MChD response in lanthanide systems. At the same time, although it is not the focus of this perspective, this result also highlights that the reverse is true for transition metal ions, confirming the observations performed on chiral Prussian blue analogues.24,25
Testing the potential use of MChD as a tool for the optical readout of the magnetic states without the need for light polarization motivated us to investigate the MChD properties of the DyIII analogue of [(P/M)7-Ln]n. The DyIII ion is undoubtedly the most used lanthanide ion for designing SMMs due to its high magnetic moment and strong magnetic anisotropy. Magnetic field dependent MChD signals for [(P/M)7-Dy]n were observed for each electronic transition detected in absorption at 4 K, i.e.6Fn/2/6Hm/2 ← 6H15/2 transitions with n = 3, 5, 7, 9 and 11 and m = 5, 7 and 9.32 The maximum gMChD factor was determined to be equal to 1.2% at 1 T and 4 K, which is one order of magnitude lower than that observed for the YbIII analogue. This difference can be rationalized on the basis of the nature of the investigated electronic transitions. Those of [(P/M)7-Dy]n are magnetic-dipole forbidden and only induced electric-dipole allowed, while for [(P/M)7-Yb]n the unique displayed transition is magnetic-dipole allowed.57,58 Although weak, the MChD activity for [(P/M)7-Dy]n was observed for all the electronic transitions up to 32 K under 1 T, which makes chiral DyIII-based SMMs good candidates to demonstrate the optical readout of magnetic states with unpolarized light.
Concluding remarks and perspectives
As examined in this perspective, chiral lanthanide complexes, which inherently show high spin–orbit coupling, a variety of electronic configurations, a multitude of electronic transitions of different characteristics, variable coordination geometries and different degrees of magnetic anisotropy, represent ideal molecules to investigate MChD in both light absorption and emission in a wide spectral range (from near-infrared to hard X-rays). Although only a limited number of studies have been undertaken so far, one can summarize some general findings that can help the chemical community in designing chiral lanthanide complexes responsive to MChD.1. For lanthanide complexes, it seems that a short distance between the chiral and the magnetic centers is not crucial to observe strong MChD responses. In turn, the leading parameter for intense MChD appears to be related to the electronic, thus magnetic, configuration of the ground state of the lanthanide center and not the chiral features of the coordination sphere. This was deduced from theoretical calculations as well as from experimental evidence such as the temperature dependence of the MChD signals and the determinant influence of the MCD intensity on the MChD one. To definitely establish this, NCD, MCD and MChD measurements have to be performed on the same sample and under the very same conditions of orientation, temperature and magnetic field (for MCD and MChD). It is challenging, from an instrumental point of view, to perform measurements at low temperatures under a magnetic field with polarized light. The complexity of the experiment is further increased by the linear birefringence shown by single crystals all the more since these effects can be more intense than those related to NOA and MOA and hinder quantitative determination. Indeed, most NCD and MCD studies at low temperature are performed on frozen solutions.
2. The higher the magnetic anisotropy, the higher the MChD response, with important differences in intensity expected if the probed orientation (wavevector and magnetic field) is along the magnetic anisotropy easy axis or perpendicular to it. This can be deduced by the similarity of the MChD response of lanthanide complexes with respect to the MCD theory, which also defines the angular dependency of the MCD response with respect to the anisotropy of the g-factor. This has not been experimentally demonstrated so far, but it can constitute a remarkable advancement to corroborate the MChD microscopic theory.
3. From a coordination chemistry point of view, one can consider that all the structural and electronic design criteria identified to develop SMMs with a high blocking temperature and energy barriers also apply to maximize MChD responses. The chemical challenge is to introduce chirality into the ligands while keeping the same structural and electronic parameters. It should be noted that chirality has been introduced in some SMMs to improve the energy barrier and/or to limit the relaxation pathways, but with no real interest in the optical enantiopurity of the resulting compounds, which is instead crucial for MChD.
4. The characteristics of the electronic transition have a crucial importance in determining the intensity of the MChD signals, regardless of the chirality of the ligands, the distortion of the coordination geometry and the magnetic anisotropy of the investigated systems: magnetic-dipole allowed transitions overall provide higher responses with respect to electric-dipole allowed as far as the visible-NIR range is concerned. Nonetheless, for a given lanthanide ion and a given electronic transition, the optimization of the above-mentioned parameters can be used to further maximize the MChD intensity.
Several experiments and studies are still needed to clearly elucidate this fascinating phenomenon and to fully understand how to maximize the MChD intensity for practical applications.
1. It can be clearly seen that the number of studies on MChD with light emission is very limited with respect to those with absorption. The fascinating properties of chiral lanthanide complexes as circularly polarized light emitters motivate further studies in this direction for comparing both the CPL and MChD properties in emission, including CPL under a magnetic field, and also the MChD properties in absorption and emission for the same system. With the same mindset, one can envisage MChD studies on the same system with light sources of very different energies, such as visible light and X-rays or visible light and microwaves. This will provide a better view of the microscopic parameters that have to be implemented and optimized as a function of the electronic transition and, from an application point of view, the light energy of interest.
2. So far, although extremely challenging, ab initio theoretical calculations of MChD have been reported only for a NiII-based complex with a relatively simple electronic configuration.28 The implementation of MChD theoretical calculations on lanthanide complexes, although more challenging as a consequence of their electronic properties and configurations with respect to those of transition metal ions, is expected to provide a substantial contribution to chemical design and experiment rationalization.
3. One can also envision to switch on and off the MChD properties by using chiral switchable ligands responsive to external stimuli (temperature, light, redox processes, electric field, etc.) and able to modify the coordination geometry around the lanthanide center or activate/deactivate the chiral influence of the ligand towards the lanthanide center.
4. Finally, as recently proposed but not experimentally demonstrated yet, MChD can be used to read out the magnetic state of SMMs by means of unpolarized light. This can be realized on chiral SMMs or chiral ferromagnets having an opened hysteresis cycle at a temperature viable for MChD measurements with an adapted measurement protocol able to follow the system magnetization dynamics. This extremely challenging demonstration will represent a breakthrough in the field of optical readout of magnetic data because novel polarization-free optical data readout technologies can result from this demonstration, contrary to current technologies.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The French National Research Agency (ANR) is acknowledged for the financial support through the SWITCH-MChD (ANR-23-CE07-0003) and MaChiNaCo (ANR-19-CE09-0018) projects.References
- G. H. Wagnière, On Chirality and the Universal Asymmetry, Wiley, Weinheim, Germany, 2007 Search PubMed.
- M. Yus and A. Guijarro, The Origin of Chirality in the Molecules of Life, Royal Society of Chemistry, Cambridge, 2008 Search PubMed.
- L. D. Barron, Molecular Light Scattering and Optical Activity, Cambridge University Press, 2004 Search PubMed.
- P. J. Stephens, Theory of Magnetic Circular Dichroism, J. Chem. Phys., 1970, 52, 3489 CrossRef CAS.
- P. Comba, L. J. Daumann, R. Klingeler, C. Koo, M. J. Riley, A. E. Roberts, H. Wadepohl and J. Werner, Correlation of Structural and Magnetic Properties in a Set of Mononuclear Lanthanide Complexes, Chem. – Eur. J., 2018, 24, 5319–5330 CrossRef CAS PubMed.
- J. Mack, M. J. Stillman and N. Kobayashi, Application of MCD spectroscopy to porphyrinoids, Coord. Chem. Rev., 2007, 251, 429–453 CrossRef CAS.
- W. R. Mason, A Practical Guide to Magnetic Circular Dichroism Spectroscopy, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2007 Search PubMed.
- Y. Kitagawa, S. Wada, K. Yanagisawa, T. Nakanishi, K. Fushimi and Y. Hasegawa, Molecular Design Guidelines for Large Magnetic Circular Dichroism Intensities in Lanthanide Complexes, ChemPhysChem, 2016, 17, 845–849 CrossRef CAS PubMed.
- L. D. Barron and J. Vrbancich, Magneto-chiral birefringence and dichroism, Mol. Phys., 1984, 51, 715–730 CrossRef CAS.
- G. L. J. A. Rikken and E. Raupach, Observation of magneto-chiral dichroism, Nature, 1997, 390, 493–494 CrossRef CAS.
- M. Atzori, G. L. J. A. Rikken and C. Train, Magneto–Chiral Dichroism: A Playground for Molecular Chemists, Chem. – Eur. J., 2020, 26, 9784–9791 CrossRef CAS PubMed.
- F. Zinna and L. Di Bari, Lanthanide Circularly Polarized Luminescence: Bases and Applications, Chirality, 2015, 27, 1–13 CrossRef CAS PubMed.
- O. G. Willis, F. Zinna and L. Di Bari, NIR–Circularly Polarized Luminescence from Chiral Complexes of Lanthanides and d–Metals, Angew. Chem., Int. Ed., 2023, 62, e202302358 CrossRef CAS PubMed.
- F. Zinna and G. Pescitelli, Magnetic Circularly Polarized Luminescence of Organic Compounds, Eur. J. Org. Chem., 2023, 26, e202300509 CrossRef CAS.
- C. Train, R. Gheorghe, V. Krstic, L.-M. Chamoreau, N. S. Ovanesyan, G. L. J. A. Rikken, M. Gruselle and M. Verdaguer, Strong magneto-chiral dichroism in enantiopure chiral ferromagnets, Nat. Mater., 2008, 7, 729–734 CrossRef CAS PubMed.
- M. Atzori, C. Train, E. A. Hillard, N. Avarvari and G. L. J. A. Rikken, Magneto–chiral anisotropy: From fundamentals to perspectives, Chirality, 2021, 33, 844–857 CrossRef CAS PubMed.
- R. Sessoli, M.-E. Boulon, A. Caneschi, M. Mannini, L. Poggini, F. Wilhelm and A. Rogalev, Strong magneto-chiral dichroism in a paramagnetic molecular helix observed by hard X-rays, Nat. Phys., 2015, 11, 69–74 Search PubMed.
- K. Taniguchi, S. Kishiue, S. Kimura and H. Miyasaka, Local-Site Dependency of Magneto-Chiral Dichroism in Enantiopure One-Dimensional Copper(II)–Chromium(III) Coordination Polymers, J. Phys. Soc. Jpn., 2019, 88, 93708 CrossRef.
- K. Taniguchi, M. Nishio, S. Kishiue, P.-J. Huang, S. Kimura and H. Miyasaka, Strong magnetochiral dichroism for visible light emission in a rationally designed paramagnetic enantiopure molecule, Phys. Rev. Mater., 2019, 3, 45202 CrossRef CAS.
- Y. Kitagawa, H. Segawa and K. Ishii, Magneto-Chiral Dichroism of Organic Compounds, Angew. Chem., Int. Ed., 2011, 50, 9133–9136 CrossRef CAS PubMed.
- S. Tomita, K. Sawada, A. Porokhnyuk and T. Ueda, Direct Observation of Magnetochiral Effects through a Single Metamolecule in Microwave Regions, Phys. Rev. Lett., 2014, 113, 235501 CrossRef PubMed.
- S. Tomita, K. Sawada, H. Kurosawa and T. Ueda, in Springer Series in Materials Science, 2019 Search PubMed.
- X. Wang, S.-Q. Wang, J.-N. Chen, J.-H. Jia, C. Wang, K. Paillot, I. Breslavetz, L.-S. Long, L. Zheng, G. L. J. A. Rikken, C. Train, X.-J. Kong and M. Atzori, Magnetic 3d–4f Chiral Clusters Showing Multimetal Site Magneto-Chiral Dichroism, J. Am. Chem. Soc., 2022, 144, 8837–8847 CrossRef CAS PubMed.
- M. Atzori, I. Breslavetz, K. Paillot, K. Inoue, G. L. J. A. Rikken and C. Train, A Chiral Prussian Blue Analogue Pushes Magneto-Chiral Dichroism Limits, J. Am. Chem. Soc., 2019, 141, 20022–20025 CrossRef CAS PubMed.
- M. Atzori, I. Breslavetz, K. Paillot, G. L. J. A. Rikken and C. Train, Role of structural dimensionality in the magneto-chiral dichroism of chiral molecular ferrimagnets, J. Mater. Chem. C, 2022, 10, 13939–13945 RSC.
- N. Nakagawa, N. Abe, S. Toyoda, S. Kimura, J. Zaccaro, I. Gautier-Luneau, D. Luneau, Y. Kousaka, A. Sera, M. Sera, K. Inoue, J. Akimitsu, Y. Tokunaga and T. Arima, Magneto-chiral dichroism of CsCuCl3, Phys. Rev. B, 2017, 96, 121102 CrossRef.
- B. Sun, X.-F. Liu, X.-Y. Li, Y. Zhang, X. Shao, D. Yang and H.-L. Zhang, Two-Dimensional Perovskite Chiral Ferromagnets, Chem. Mater., 2020, 32, 8914–8920 CrossRef CAS.
- M. Atzori, H. Ludowieg, M. Cortijo, I. Breslavetz, K. Paillot, P. Rosa, C. Train, J. Autschbach, E. A. Hillard and G. L. J. A. Rikken, Validation of Microscopic Magneto-Chiral Dichroism Theory, Sci. Adv., 2021, 7, eabg2859 CrossRef CAS PubMed.
- M. Atzori, F. Santanni, I. Breslavetz, K. Paillot, A. Caneschi, G. L. J. A. Rikken, R. Sessoli and C. Train, Magnetic Anisotropy Drives Magnetochiral Dichroism in a Chiral Molecular Helix Probed with Visible Light, J. Am. Chem. Soc., 2020, 142, 13908–13916 CrossRef CAS PubMed.
- J. Goulon, A. Rogalev, F. Wilhelm, C. Goulon-Ginet, P. Carra, D. Cabaret and C. Brouder, X-Ray Magnetochiral Dichroism: A New Spectroscopic Probe of Parity Nonconserving Magnetic Solids, Phys. Rev. Lett., 2002, 88, 237401 CrossRef CAS PubMed.
- M. Atzori, K. Dhbaibi, H. Douib, M. Grasser, V. Dorcet, I. Breslavetz, K. Paillot, O. Cador, G. L. J. A. Rikken, B. Le Guennic, J. Crassous, F. Pointillart and C. Train, Helicene-Based Ligands Enable Strong Magneto-Chiral Dichroism in a Chiral Ytterbium Complex, J. Am. Chem. Soc., 2021, 143, 2671–2675 CrossRef CAS PubMed.
- M. S. Raju, K. Dhbaibi, M. Grasser, V. Dorcet, I. Breslavetz, K. Paillot, N. Vanthuyne, O. Cador, G. L. J. A. Rikken, B. Le Guennic, J. Crassous, F. Pointillart, C. Train and M. Atzori, Magneto-Chiral Dichroism in a One-Dimensional Assembly of Helical Dysprosium(III) Single-Molecule Magnets, Inorg. Chem., 2023, 62, 17583–17587 CrossRef CAS PubMed.
- K. Dhbaibi, M. Grasser, H. Douib, V. Dorcet, O. Cador, N. Vanthuyne, F. Riobé, O. Maury, S. Guy, A. Bensalah-Ledoux, B. Baguenard, G. L. J. A. Rikken, C. Train, B. Le Guennic, M. Atzori, F. Pointillart and J. Crassous, Multifunctional Helicene–Based Ytterbium Coordination Polymer Displaying Circularly Polarized Luminescence, Slow Magnetic Relaxation and Room Temperature Magneto–Chiral Dichroism, Angew. Chem., 2023, 135, e202215558 CrossRef.
- M. Ceolín, S. Goberna-Ferrón and J. R. Galán-Mascarós, Strong Hard X-ray Magnetochiral Dichroism in Paramagnetic Enantiopure Molecules, Adv. Mater., 2012, 24, 3120–3123 CrossRef PubMed.
- D. Mitcov, M. Platunov, C. D. Buch, A. Reinholdt, A. R. Døssing, F. Wilhelm, A. Rogalev and S. Piligkos, Hard X-ray magnetochiral dichroism in a paramagnetic molecular 4f complex, Chem. Sci., 2020, 11, 8306–8311 RSC.
- G. Wagnière, Magnetochiral dichroism in emission. Photoselection and the polarization of transitions, Chem. Phys. Lett., 1984, 110, 546–551 CrossRef.
- H. G. Brittain and F. S. Richardson, Circularly polarized emission studies on the chiral nuclear magnetic resonance lanthanide shift reagent tris(3-trifluoroacetyl-d-camphorato) europium(III), J. Am. Chem. Soc., 1976, 98, 5858–5863 CrossRef CAS.
- P. H. Schippers, A. van den Buekel and H. P. J. M. Dekkers, An accurate digital instrument for the measurement of circular polarisation of luminescence, J. Phys. E: Sci. Instrum., 1982, 15, 945–945 CrossRef CAS.
- C. Görller-Walrand and J. Godemont, MCD of the Eu3+ ion in aqueous solution. Analysis of the 5D,0,1,2←7F0,1,2 transitions, J. Chem. Phys., 1977, 67, 3655–3658 CrossRef.
- Y. Hasegawa, M. Yamamuro, Y. Wada, N. Kanehisa, Y. Kai and S. Yanagida, Luminescent Polymer Containing the Eu(III) Complex Having Fast Radiation Rate and High Emission Quantum Efficiency, J. Phys. Chem. A, 2003, 107, 1697–1702 CrossRef CAS.
- Y. Tokura and N. Nagaosa, Nonreciprocal responses from non-centrosymmetric quantum materials, Nat. Commun., 2018, 9, 3740 CrossRef PubMed.
- Y. Yukawa, S. Igarashi, A. Yamano and S. Sato, Structure of the centred icosahedral samarium cluster formed by bis(l-prolinato)nickel(II) ligands, Chem. Commun., 1997, 711–712 RSC.
- Y. Yukawa, G. Aromí, S. Igarashi, J. Ribas, S. A. Zvyagin and J. Krzystek, [GdNi6] and [LaNi6]: High–Field EPR Spectroscopy and Magnetic Studies of Exchange–Coupled Octahedral Clusters, Angew. Chem., Int. Ed., 2005, 44, 1997–2001 CrossRef CAS PubMed.
- K. Fukui, H. Ogasawara, A. Kotani, I. Harada, H. Maruyama, N. Kawamura, K. Kobayashi, J. Chaboy and A. Marcelli, X-ray magnetic circular dichroism at rare-earth L2,3 edges in R2Fe14B compounds (R = La, Pr, Nd, Sm, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu), Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 104405 CrossRef.
- M. Nakazawa, K. Fukui and A. Kotani, Theory of X-ray absorption and resonant X-ray emission spectra by electric quadrupole excitation in light rare-earth systems, J. Solid State Chem., 2003, 171, 295–298 CrossRef CAS.
- C. Kremer, J. Torres and S. Domínguez, Lanthanide complexes with oda, ida, and nta: From discrete coordination compounds to supramolecular assemblies, J. Mol. Struct., 2008, 879, 130–149 CrossRef CAS.
- C. Dallera, M. Krisch, A. Rogalev, J. Goulon and F. Sette, Resonant inelastic X-ray scattering at the L3 edge of Eu2+, Gd3+, and Tb4+ compounds, Phys. B, 2002, 312–313, 850–852 CrossRef.
- M. Platunov, N. Kazak, V. Dudnikov, V. Temerov, I. Gudim, Y. Knyazev, S. Gavrilkin, V. Dyadkin, I. Dovgaliuk, D. Chernyshov, A. Hen, F. Wilhelm, A. Rogalev and S. Ovchinnikov, Element selective magnetism in Ho0.5Nd0.5Fe3(BO3)4 single crystal probed with hard X-ray magnetic circular dichroism, J. Magn. Magn. Mater., 2019, 479, 312–316 CrossRef CAS.
- C.-F. Chen and Y. Shen, Helicene Chemistry, Springer Berlin Heidelberg, Berlin, Heidelberg, 2017 Search PubMed.
- K. Dhbaibi, L. Favereau and J. Crassous, Enantioenriched Helicenes and Helicenoids Containing Main-Group Elements (B, Si, N, P), Chem. Rev., 2019, 119, 8846–8953 CrossRef CAS PubMed.
- M. Gingras, G. Félix and R. Peresutti, One hundred years of helicene chemistry. Part 2: stereoselective syntheses and chiral separations of carbohelicenes, Chem. Soc. Rev., 2013, 42, 1007–1050 RSC.
- J. Crassous, I. G. Stará and I. Starý, Helicenes – Synthesis, Properties and Applications, Wiley, 2022 Search PubMed.
- J.-K. Ou-Yang, N. Saleh, G. Fernandez Garcia, L. Norel, F. Pointillart, T. Guizouarn, O. Cador, F. Totti, L. Ouahab, J. Crassous and B. Le Guennic, Improved slow magnetic relaxation in optically pure helicene-based DyIII single molecule magnets, Chem. Commun., 2016, 52, 14474–14477 RSC.
- M. Galland, F. Riobé, J. Ouyang, N. Saleh, F. Pointillart, V. Dorcet, B. Le Guennic, O. Cador, J. Crassous, C. Andraud, C. Monnereau and O. Maury, Helicenic Complexes of Lanthanides: Influence of the f–Element on the Intersystem Crossing Efficiency and Competition between Luminescence and Oxygen Sensitization, Eur. J. Inorg. Chem., 2019, 2019, 118–125 CrossRef CAS.
- M. C. L. Yang and R. A. Palmer, Natural solid state optical activity of tris(ethylenediamine)metal(II) nitrates. II. Single-crystal circular and linear dichroism spectra of tris(ethylenediamine)cobalt(II) nitrate, J. Am. Chem. Soc., 1975, 97, 5390–5395 CrossRef CAS.
- M. C.-L. Yang and R. A. Palmer, The Natural Optical Activity of Tris(Ethylenediamine)Metal(II) Nitrates V. The Single Crystal Circular Dichroism Spectrum of Ni(en)3(NO3)2, J. Chin. Chem. Soc., 1978, 25, 195–201 CrossRef CAS.
- P. Comba, M. Großhauser, R. Klingeler, C. Koo, Y. Lan, D. Müller, J. Park, A. Powell, M. J. Riley and H. Wadepohl, Magnetic Interactions in a Series of Homodinuclear Lanthanide Complexes, Inorg. Chem., 2015, 54, 11247–11258 CrossRef CAS PubMed.
- F. S. Richardson, Selection rules for lanthanide optical activity, Inorg. Chem., 1980, 19, 2806–2812 CrossRef CAS.
| This journal is © the Partner Organisations 2024 |
