Orange to red iridium(III) complexes possessing good electron mobility with a pyrimidine-4-carboxylic acid ligand for high-performance solution-processed OLEDs with an EQE over 31%†
Tao
Han‡
a,
Yan
Zhang‡
b,
Xin-yu
Zhang
a,
Yu-qiao
Tong
a,
Ming-yu
Teng
 *a,
Chong-yang
Shi
*a,
Long-wu
Ye
*a,
Chong-yang
Shi
*a,
Long-wu
Ye
 a,
Zhao
Chen
a,
Zhao
Chen
 *b,
Shuo-qi
Sun
c and
Guangzhao
Lu
*b,
Shuo-qi
Sun
c and
Guangzhao
Lu
 *d
*d
aFaculty of Chemistry and Chemical Engineering, Yunnan Normal University, Kunming 650500, PR China. E-mail: myteng@ynnu.edu.cn; chongyangshi@ynnu.edu.cn
bSchool of Applied Physics and Materials, Wuyi University, Jiangmen, 529020, P. R. China. E-mail: chenzhao2006@163.com
cHongzhiwei Technology (Shanghai) Co. Ltd., 1599 Xinjinqiao Road, Pudong, Shanghai 200137, PR China
dShenzhen Institute of Information Technology, Shenzhen 518172, PR China. E-mail: lugz@sziit.edu.cn
First published on 6th February 2024
Abstract
Three novel iridium(III) complexes with pyrimidine carboxylic acid as the auxiliary ligand were designed, showing adjustable emission peak wavelengths from 559 nm to 610 nm with relatively high photoluminescence quantum yields (PLQYs) of 58–89% in CH2Cl2. The structures of the title complexes were confirmed by single-crystal X-ray diffraction (XRD), nuclear magnetic resonance spectroscopy (NMR), and high-resolution mass spectrometry (HRMS). Their electronic states were investigated using time-dependent density functional theory (TD-DFT) calculations. When employing these emitters to fabricate solution-processed organic light-emitting diodes (OLEDs), the obtained orange and red OLEDs exhibit prominent performances with the maximum current efficiencies of 95.4 cd A−1 and 39.8 cd A−1, and the maximum external quantum efficiencies (EQEmax) of 31.3% and 24.7%, both of which are ranked among the highest values for orange and red solution-processed OLEDs.
Introduction
Organic light-emitting diodes (OLEDs) have the advantages of thin thickness, high brightness, high flexibility, fast response speed, etc., which are ideal for the new generation of flat panel displays and energy-saving solid-state light sources.1–10 Light-emitting materials are the core of OLEDs, especially phosphorescent metal complexes, which have high photoluminescence quantum yields (PLQYs) and suitable device performance.11–13 Among them, iridium(III) complexes are the essential emitters and have the advantages of easy chemical structure modification, flexible control of photophysical properties, high luminescence efficiency, and relatively low phosphorescence lifetimes.14In general, the hole-transporting capacity of most hole-transporting materials in OLEDs is much higher than the electron-transporting capacity of most electron-transporting materials, and in order to balance carrier injection and transport in the emissive layer, the introduction of electron-transporting groups into the ligand is an effective way to improve the electron mobility of a complex.15 Nitrogen heterocyclic pyrimidines can improve the electron transfer ability of complexes and increase the device efficiency, so we propose the use of pyrimidine carboxylic acid as an auxiliary ligand and the introduction of nitrogen heterocycles into primary ligands, which improve the device efficiency and reduce the efficiency roll-off.16
Since the invention of high-efficiency organic light-emitting diodes in 1987, the initial technique for fabricating each functional layer in a device has involved vapor deposition by thermal evaporation. However, high temperature and a vacuum are required in this process, so using evaporation treatments can be more expensive than other treatments.17 Solution processing has emerged as a new technology that utilizes liquid solutions of materials to deposit functional layers in devices at room temperature. This method opens the door to molecules with high molecular weights, high sublimation temperatures, or poor thermal stability. Due to their relatively low cost and ease of manipulating large-area displays, more and more attention has been given to developing these devices from both commercial and academic communities.18–22 For example, by using the solution-processed method, Liu et al. synthesized a boron-containing 2-phenylpyridine (ppy) type ligand as the primary ligand with an EQEmax of 28.1%.23 Gong et al. synthesized a dibenzo[b,d]thiophene-S,S-dioxide primary ligand, with an EQEmax of 16.6%.24 Sun et al. synthesized a 5-(dimesitylboranyl)-2-phenylpyridine primary ligand with an EQEmax of 23.2%.25 So far, solution-processed OLEDs with EQEmax over 30% have been rarely reported.
Combined with the above characteristics, we synthesized three new iridium complexes: (4-tfmpq)2Ir(4-pca), (4-tfmptp)2Ir(4-pca), and (4-pq)2Ir(4-pca). Their maximum emission wavelengths in CH2Cl2 ranged from 559 to 610 nm. Among them, (4-pq)2Ir(4-pca) showed a PLQY of 57.6% for the standard red CIE (0.67,0.33); (4-tfmpq)2Ir(4-pca) showed a better PLQY of 83.6%; (4-tfmptp)2Ir(4-pca) showed a maximum current efficiency (ηc,max) of 95.4 cd A−1 with the best PLQY of 88.5%, a maximum power efficiency (ηp,max) of 54.5 lm W−1, and an EQEmax of 31.3%.
Results and discussion
Synthesis and characterization
The structures of the three complexes are clearly shown in Scheme 1. The Suzuki cross-coupling reaction was employed to prepare three primary ligands. The ancillary ligand, 4-pca (pyrimidine-4-carboxylic acid), is available for direct purchase. The final products, (4-tfmpq)2Ir(4-pca), (4-tfmptp)2Ir(4-pca) and (4-pq)2Ir(4-pca), were synthesized based on a reported two-step method. In the first step, iridium chloride trihydrate reacts with 2.5 equivalents of the primary ligand in 2-ethoxyethanol solution to form a μ-chloro-bridged dimer, and in the second step, the μ-chloro-bridged dimer reacts with a mixture of 4-pca and Na2CO3 to give three Ir(III) complexes (Scheme S1†). The crude products of the three complexes were easily purified by column chromatography.The thermal properties of these Ir(III) complexes were investigated by thermogravimetric analysis (TGA) since the thermal stability of the emitters is crucial for the stability of OLEDs. The results show that the decomposition temperature Td (5%) values of (4-tfmpq)2Ir(4-pca) and (4-pq)2Ir(4-pca) are at excellent levels of 337 °C and 347 °C, respectively, while the Td value of (4-tfmptp)2Ir(4-pca) is at a moderate level of 243 °C (Table 1 and Fig. S1†). We speculate that this may be due to the relatively low coordination ability and stability of the thieno[2,3-d]pyrimidine unit.
| Complex |
T
d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (°C)
(°C) |
Absorptionb (λ nm) | Emissionb (λmax nm) |
τ
298 K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (μs)
(μs) |
Φ
p![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (%)
(%) |
E
gap![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (eV)
(eV) |
HOMO/LUMOe (eV) |
|---|---|---|---|---|---|---|---|
| a Decomposition temperature. b Measured in DCM (10−5 M) at 298 K. c Measured in DCM (10−5 M) under a N2 atmosphere. Φ: the quantum yields were calculated with the fac-Ir(ppy)3 standard in degassed CH2Cl2 solution (Φp = 40%). d Calculated from the UV-vis onset. e Calculated from cyclic voltammetry analysis. | |||||||
| (4-tfmpq)2Ir(4-pca) | 337 | 288/341/474/526 | 604 | 6.87 | 83.6 | 2.10 | −5.85/−3.75 |
| (4-tfmptp)2Ir(4-pca) | 243 | 282/441/496 | 558 | 5.45 | 88.5 | 2.31 | −5.85/−3.54 |
| (4-pq)2Ir(4-pca) | 347 | 291/339/475/530 | 610 | 7.03 | 57.6 | 2.03 | −5.60/−3.57 |
X-ray crystallography analysis
The three new complexes, (4-tfmpq)2Ir(4-pca), (4-tfmptp)2Ir(4-pca) and (4-pq)2Ir(4-pca), were characterized by single-crystal X-ray diffraction. The structures of these complexes are shown in Fig. 1, and the crystallographic data are summarized in Tables S1–S3 (ESI†). An approximate octahedral geometry around the iridium(III) center is observed. The bond lengths of Ir–C (1.987–2.030 Å), Ir–N (2.029–2.148 Å) and Ir–O (2.151–2.171 Å) match with those of other similar Ir(III) complexes.26–28 | ||
| Fig. 1 ORTEP drawing of (4-tfmpq)2Ir(4-pca), (4-tfmptp)2Ir(4-pca) and (4-pq)2Ir(4-pca) with CCDC numbers of 2302907, 2302905 and 2302906,† respectively. | ||
Electrochemical performance
The characterization of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels (EHOMO/ELUMO) is of great significance for the electrochemical properties of Ir(III) complexes. They can provide a theoretical basis for the design and fabrication of OLEDs. Cyclic voltammetry (CV) measurements were performed in degassed CH2Cl2 using ferrocene/ferrocene (Fc+/Fc) as an internal standard to investigate the redox properties and HOMO/LUMO levels of all Ir(III) complexes (Table 1 and Fig. 2†).29,30 Previous electrochemical tests with Ir(III) complexes have shown that the reduction process is generally believed to occur mainly in the heterocyclic portion of the C^ N ligand. In contrast, the oxidation process mainly involves the iridium center and the auxiliary ligand.The electrochemical properties of these complexes were examined using cyclic voltammetry in CH2Cl2 solution. Based on the formula EHOMO (eV) = −(Eox − EFc/Fc+ + 4.8) eV, ELUMO (eV) = EHOMO + Ebandgap, the EHOMO of four complexes was deduced, which should be ascribed to the oxidation process of the Ir (III) centers. The HOMO energy level of the Ir(III) complex is estimated from the above equation, and the LUMO energy level is estimated from the sum of the HOMO energy level and the energy bandgap, which is determined from the absorption edge in the absorption spectrum (MLCT). The HOMO/LUMO energy levels are within −5.60 eV–5.85 eV and −3.54 eV–3.75 eV, respectively. When the LUMO energy level is low, it exhibits more electron-transport property, which is favorable for electron trapping. These results demonstrate that an extra nitrogen atom can significantly improve the electron-withdrawing ability and modulate the electrochemical properties and HOMO/LUMO energy levels.31 The devices based on the three complexes would exhibit better EL performances.32–34
Theoretical calculations
To gain insight into the electronic states of the complexes, we performed the time-dependent density functional theory (TD-DFT) calculations. As shown in Fig. 2 and Table S4 (ESI†), the three complexes display a similar orbital charge density distribution profile. Besides the significant contribution (43.77–51.57%) from the primary ligands to the HOMOs, the Ir metal dπ orbitals also contribute considerably (41.19–48.72%) to the HOMOs. The LUMOs are composed of a significant contribution from the primary ligands (92.67–93.44%) and a negligible contribution from the Ir metal dπ orbitals (2.50–4.39%) and the ancillary ligands (2.43–4.06%). Usually, the HOMO–LUMO transitions contribute mainly to both the S1 and T1 states. Therefore, the S1 and T1 states of these complexes may show an MLCT character with LLCT features.35–39 The HOMO and LUMO energy levels were also calculated, which turned out to be −5.72/−2.67, −5.73/−2.52 and −4.94/−2.20 eV for (4-tfmpq)2Ir(4-pca), (4-tfmptp)2Ir(4-pca) and (4-pq)2Ir(4-pca), respectively. The frontier molecular orbital energy levels are significantly more deeper than their counterparts, suggesting that the electron-withdrawing capacity is improved with the increase in the number of nitrogen atoms.Photophysical properties
The ultraviolet-visible absorption (UV-vis) and photoluminescence (PL) spectra of Ir(III) complexes in dichloromethane (DCM, 10−5 M) at room temperature are shown in Fig. 3. The relevant data are listed in Table 1. All these complexes show intense absorption bands below 375 nm due to the spin-allowed ligand-centered singlet π–π* transitions. The weaker absorption bands between 375 and 600 nm can be assigned to the singlet and triplet metal–ligand charge transfer (1MLCT and 3MLCT) and triplet ligand-centered charge transfer (3LC) absorptions.40 Due to the strong spin–orbit coupling of iridium atoms, Ir(III) complexes undergo a mixture of singlet and triplet states, which may enhance their quantum efficiency, and differences in the primary ligands also affect the quantum efficiency of Ir(III) complexes.41,42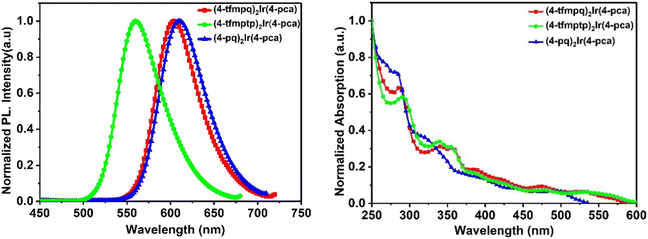 | ||
| Fig. 3 Photophysical properties of the three complexes measured in DCM solution at 298 K (5 × 10−5 M). | ||
Due to the primary ligand's relative rigidity and large planar structure, the three complexes show higher PLQYs in CH2Cl2 solution. They are 83.6% for (4-tfmpq)2Ir(4-pca), 88.5% for (4-tfmptp)2Ir(4-pca), and 57.6% for (4-pq)2Ir(4-pca), respectively. This result shows that OLEDs with these emitters can exhibit better performances. Introduction of –CF3 will enhance the electron-withdrawing ability of the primary ligands and weaken the conjugated system, thereby increasing the quantum yields. In contrast, the PLQYs of complexes without –CF3 will be reduced.18,43 In addition, the change of substituents on the primary ligands also affects the CIE properties of different emitters measured in CH2Cl2 solution. They include CIE (0.66, 0.34) for (4-tfmpq)2Ir(4-pca), CIE (0.67, 0.33) for (4-pq)2Ir(4-pca), and CIE (0.41, 0.58) for (4-tfmptp)2Ir(4-pca). All the complexes in degassed DCM solution exhibit quite short phosphorescence lifetimes, which are 6.87 μs for (4-tfmpq)2Ir(4-pca), 5.45 μs for (4-tfmptp)2Ir(4-pca), and 7.03 μs for (4-pq)2Ir(4-pca), respectively (Table 1).
Electroluminescent devices
By comparing the phosphorescence quantum efficiencies, the devices using (4-tfmpq)2Ir(4-pca), (4-tfmptp)2Ir(4-pca), and (4-pq)2Ir(4-pca) as the emitters for the solution-processed OLEDs are named D1, D2, and D3, respectively. The devices have a simple structure of ITO/PEDOT:PSS (35 nm)/PVK (5 nm)/Ir (1 wt%): CBP (25 nm)/TmPyPB (40 nm)/LiF (1 nm)/Al (120 nm), in which PEDOT is poly(3,4-ethylenedioxythiophene), PSS is polystyrene sulfonic acid, PVK is poly(N-vinylcarbazole), CBP is 4,4′-bis(N-carbazolyl)-1,1′-biphenyl, and TmPyPB is 1,3,5-tri[(3-pyridyl)phen-3-yl]benzene. Due to the carrier transport equilibrium, CBP was chosen as the hybrid host material, PEDOT: PSS as the hole-transport layer (HTL) and electron-blocking layer (EBL), and TmPyPB as the electron-transport layer (ETL) and hole-blocking layer (HBL) material (Scheme 2).44–46 The luminescence peaks of D1, D2, and D3 at 601 nm, 558 nm, and 607 nm, respectively, are close to those measured in CH2Cl2 solution, indicating that the EL emission of the devices originates from Ir(III) complexes. No prominent EL emission bands from the host material are observed in these devices, which suggests that the energy transfer from the host material to Ir(III) complexes in the emissive layer is efficient. | ||
| Scheme 2 Energy level diagram of the HOMO and LUMO levels of materials investigated and their chemical molecular structures. | ||
As can be seen from Fig. 4 and Table 2, device D1 based on (4-tfmpq)2Ir(4-pca) outperforms device D3 employing (4-pq)2Ir(4-pca), which correlates with the ΦPL of these phosphorescent emitters. Device D1 has a turn-on voltage of 5.5 V, Lmax of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 959 cd m−2, ηc,max of 39.8 cd A−1, ηp,max of 20.8 lm W−1, and EQEmax of 24.7%. In contrast, the device performance of D1 is superior to that of D3, mainly due to the introduction of –CF3 into the primary ligand, which improves the PLQY of (4-tfmpq)2Ir(4-pca) and also reduces the triplet–triplet annihilation (TTA) and triplet–polaron annihilation (TPA) effects.47,48 The device D2 has a start-up voltage of 5 V, the maximum luminance (Lmax) of 15
959 cd m−2, ηc,max of 39.8 cd A−1, ηp,max of 20.8 lm W−1, and EQEmax of 24.7%. In contrast, the device performance of D1 is superior to that of D3, mainly due to the introduction of –CF3 into the primary ligand, which improves the PLQY of (4-tfmpq)2Ir(4-pca) and also reduces the triplet–triplet annihilation (TTA) and triplet–polaron annihilation (TPA) effects.47,48 The device D2 has a start-up voltage of 5 V, the maximum luminance (Lmax) of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 673 cd m−2, ηc,max of 95.4 cd A−1, ηp,max of 54.5 lm W−1, and EQEmax of 31.3%. The excellent performance of the (4-tfmptp)2Ir(4-pca)-based device may stem from the fact that the heavy atom effect of S atoms can enhance the spin–orbital coupling (SOC) of the Ir(III) complexes, which can also improve the EL performance.49 The rigid structure of the primary ligand and a large number of nitrogen heterocyclic structures endow the three emitters with enhanced electron transport capabilities to achieve a balance between carrier injection and transport.6,27 The EQEmax values of 31.3% and 24.7% for orange and red OLEDs, respectively, are ranked among the highest values for solution-processed OLEDs employing iridium emitters (Fig. 5 and Table S5†).50–59
673 cd m−2, ηc,max of 95.4 cd A−1, ηp,max of 54.5 lm W−1, and EQEmax of 31.3%. The excellent performance of the (4-tfmptp)2Ir(4-pca)-based device may stem from the fact that the heavy atom effect of S atoms can enhance the spin–orbital coupling (SOC) of the Ir(III) complexes, which can also improve the EL performance.49 The rigid structure of the primary ligand and a large number of nitrogen heterocyclic structures endow the three emitters with enhanced electron transport capabilities to achieve a balance between carrier injection and transport.6,27 The EQEmax values of 31.3% and 24.7% for orange and red OLEDs, respectively, are ranked among the highest values for solution-processed OLEDs employing iridium emitters (Fig. 5 and Table S5†).50–59
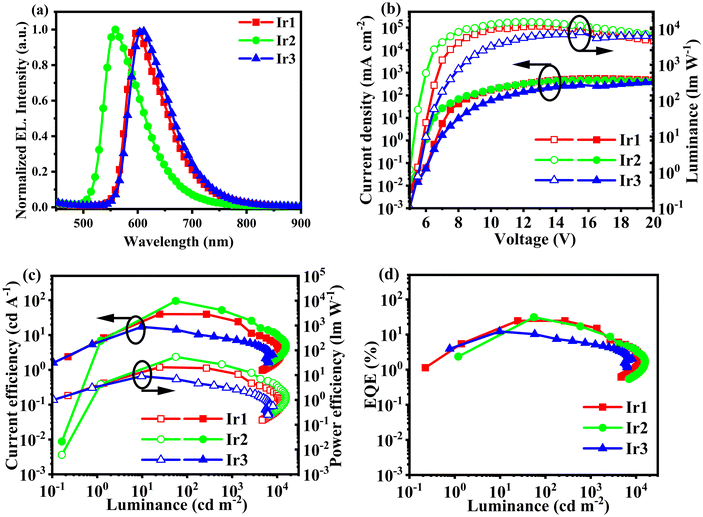 | ||
| Fig. 4 (a) EL spectra. (b) Current density–voltage–luminance characteristics. (c) ηc and ηp as a function of luminance. (d) Curves of EQE versus luminance. | ||
 | ||
| Fig. 5 The EQEs of solution-processed OLEDs using Ir complexes with emission at 510–630 nm are summarized, and their chemical names are summarized in Table S5.† | ||
| Device | ELmaxλ (nm) | V turn–on (V) | L max (cd m−2) | η c,max (cd A−1) | η p,max (lm W−1) | η ext,max (%) |
|---|---|---|---|---|---|---|
| a At 1 cd m−2. | ||||||
| D1 | 601 | 5.5 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 959 959 |
39.8 | 20.8 | 24.7 |
| D2 | 558 | 5.0 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 673 673 |
95.4 | 54.5 | 31.3 |
| D3 | 607 | 5.5 | 8094 | 17.2 | 8.90 | 12.3 |
Conclusions
Three phosphorescent bis-cyclometalated iridium complexes with rigid nitrogen heterocyclic structures have been successfully developed. Notably, varying the primary ligand moiety can modulate the iridium complexes’ color and PLQY properties. In addition, the high PLQY and charge carrier mobility endow these iridium complexes with excellent EL efficiencies to prepare OLEDs in solution. In particular, the solution-processed OLED based on (4-tfmptp)2Ir(4-pca) exhibits the most prominent performance with the peak ηc and EQE of 95.4 cd A−1 and 31.3%, respectively, which are among the highest values for orange solution-processed OLEDs. This research reveals the profound relationship between the iridium structure and optoelectronic properties. It provides an effective strategy for developing efficient iridium complexes and the corresponding solution-processed OLEDs.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We gratefully acknowledge HZWTECH for providing computation facilities and the financial support from the National Natural Science Foundation of China (52203231 and 22365035), the Guangdong Basic and Applied Basic Research Foundation (2021A1515110478), the Shenzhen University Stability Support Program Project (20220818172424009), the Applied Basic Research Projects of Yunnan Province (202101AT070217), and the Yunnan Normal University Postgraduate Research Innovation Fund (YJSJJ23-B128).References
- X. L. Yang, G. J. Zhou and W. Y. Wong, Functionalization of phosphorescent emitters and their host materials by main-group elements for phosphorescent organic light-emitting devices, Chem. Soc. Rev., 2015, 44, 8484–8575 RSC
.
- S. L. Gong, C. L. Yang and J. G. Qin, Efficient phosphorescent polymer light-emitting diodes by suppressing triplet energy back transfer, Chem. Soc. Rev., 2012, 41, 4797–4807 RSC
.
- J. F. Liang, L. Ying, F. Huang and Y. Cao, Recent advances in high performance solution processed WOLEDs for solid-state lighting, J. Mater. Chem. C, 2016, 4, 10993–11006 RSC
.
- Y. T. Tao, C. L. Yang and J. Q. Qin, Organic host materials for phosphorescent organic light-emitting diodes, Chem. Soc. Rev., 2011, 40, 2943–2970 RSC
.
- T. Chiba, Y. J. Pu and J. Kido, Solution-processable electron injection materials for organic light-emitting devices, J. Mater. Chem. C, 2015, 3, 11567–11576 RSC
.
- S. Reineke, F. Lindner, G. Schwartz, N. Seidler, K. Walzer, B. Lüssem and K. Leo, White organic light-emitting diodes with fluorescent tube efficiency, Nature, 2009, 459, 234–238 CrossRef CAS PubMed
.
- C. Fan and C. L. Yang, Yellow/orange emissive heavy-metal complexes as phosphors in monochromatic and white organic light-emitting devices, Chem. Soc. Rev., 2014, 43, 6439–6469 RSC
.
- D. D. Zhang, C. G. Zhao, Y. G. Zhang, X. Z. Song, P. C. Wei, M. H. Cai and L. Duan, Highly efficient full-color thermally activated delayed fluorescent organic light-emitting diodes: Extremely low efficiency roll-off utilizing a host with small singlet-triplet splitting, ACS Appl. Mater. Interfaces, 2017, 9, 4769–4777 CrossRef CAS PubMed
.
- C. Y. Kuei, W. L. Tong, B. Tsai, M. Jiao, W. K. Lee, Y. Chi, C. C. Wu, S. H. Liu, G. H. Lee and P. T. Chou, Bis-tridentate Ir(III) complexes with nearly unitary rgb phosphorescence and organic light-emitting diodes with external quantum efficiency exceeding 31%, Adv. Mater., 2016, 28, 2795–2800 CrossRef CAS PubMed
.
- C. Y. Kuei, S. H. Liu, P. T. Chou, G. H. Lee and Y. Chi, Room temperature blue phosphorescence: a combined experimental and theoretical study on the bis-tridentate, Ir(III) metal complexes, Dalton Trans., 2016, 45, 15364–15373 RSC
.
- Y. Chi and P. T. Chou, Transition-metal phosphors with cyclometalating ligands: fundamentals and applications, Chem. Soc. Rev., 2010, 39, 638–655 RSC
.
- J. Zhao, Y. Yu, X. L. Yang, X. G. Yan, H. M. Zhang, X. B. Xu, G. J. Zhou, Z. X. Wu, Y. X. Ren and W. Y. Wong, Phosphorescent iridium(III) complexes bearing fluorinated aromatic sulfonyl group with nearly unity phosphorescent quantum yields and outstanding electroluminescent properties, ACS Appl. Mater. Interfaces, 2015, 7, 24703–24714 CrossRef CAS PubMed
.
- X. B. Xu, H. R. Guo, J. Zhao, B. Liu, X. L. Yang, G. J. Zhou and Z. X. Wu, Asymmetric tris-heteroleptic iridium(III) complexes containing a 9-phenyl-9-phosphafluorene oxide moiety with enhanced charge carrier injection/transporting properties for highly efficient solution-processed organic light-emitting diodes, Chem. Mater., 2016, 28, 8556–8569 CrossRef CAS
.
- T. Y. Li, J. Wu, Z. G. Wu, Y. X. Zheng, J. L. Zou and P. Yi, Rational design of phosphorescent iridium(III) complexes for emission color tunability and their applications in OLEDs, Coord. Chem. Rev., 2018, 374, 55–92 CrossRef CAS
.
- W. Y. Wong, P. Y. Chiang, S. W. Lin, W. C. Tang, Y. T. Chen, S. H. Liu, P. T. Chou, Y. T. Hung and K. T. Wong, Balance the carrier mobility to achieve high performance exciplex OLED using a triazine-based acceptor, ACS Appl. Mater. Interfaces, 2016, 8, 4811–4818 CrossRef PubMed
.
- Q. M. Liu, L. Yuan, X. J. Liao, X. S. Zhong, H. X. Ni, Y. Wang, Y. Zhao and Y. X. Zheng, Efficient narrow green organic light-emitting diodes with low efficiency roll-offs based on iridium(III) complexes containing indolo[3,2,1-jk]carbazole unit, Mater. Chem. Front., 2023, 7, 4944–4951 RSC
.
- C. W. Tang and S. A. VanSlyke, Organic electroluminescent diodes, Appl. Phys. Lett., 1987, 51, 913–915 CrossRef CAS
.
- J. H. Burroughes, D. D. C. Bradley, A. R. Brown, R. N. Marks, K. Mackay, R. H. Friend, P. L. Burns and A. B. Holmes, Light-emitting diodes based on conjugated polymers, Nature, 1990, 347, 539–541 CrossRef CAS
.
- M. Vasilopoulou, A. Fakharuddin, F. P. García de Arquer, D. G. Georgiadou, H. Kim, A. R. B. Mohd Yusoff, F. Gao, M. K. Nazeeruddin, H. J. Bolink and E. H. Sargent, Advances in solution-processed near-infrared light-emitting diodes, Nat. Photonics, 2021, 15, 656–669 CrossRef CAS
.
- S. K. Zeng, C. Xiao, J. C. Zhou, Q. W. Dong, Q. Y. Li, J. Lim, H. L. Ma, J. Y. Lee, W. G. Zhu and Y. F. Wang, Deep Blue Emitter Based on Tris(triazolo)triazine Moiety with CIEy < 0.08 for Highly Efficient Solution-Processed Organic Light-Emitting Diodes Via Molecular Strategy of “Hot Excitons”, Adv. Funct. Mater., 2022, 32, 2113183 CrossRef CAS
.
- C. F. You, D. H. Liu, L. Wang, W. Q. Zheng, M. Li, P. Wang and W. G. Zhu, Rigid phenanthro[4,5-abc]phenazine-cored iridium(III) complexes for high-performance near-infrared emission at about 800 nm in solution-processed OLEDs, Chem. Eng. J., 2023, 452, 138956 CrossRef CAS
.
- C. F. You, D. H. Liu, M. B. Zhu, J. T. Yu, B. Zhang, Y. Liu, Y. F. Wang and W. G. Zhu, σ–π and p–π conjugation induced NIR-emitting iridium(III) complexes anchored by flexible side chains in a rigid dibenzo[a,c]phenazine moiety and their application in highly efficient solutionprocessable NIR-emitting devices, J. Mater. Chem. C, 2020, 8, 7079–7088 RSC
.
- B. Liu, F. Dang, Z. Feng, Z. Tian, J. Zhao, Y. Wu, X. Yang, G. Zhou, Z. Wu and W. Y. Wong, Novel iridium(III) complexes bearing dimesitylboron groups with nearly 100% phosphorescent quantum yields for highly efficient organic light-emitting diodes, J. Mater. Chem. C, 2017, 5, 7871–7883 RSC
.
- E. Gong, L. Liu, X. Deng, X. Chen, D. Zhong, B. Su, X. Yang, G. Zhou and B. Jiao, Ir(III) phosphorescent complexes with the ligands attaching pyridyl group to 4-position of dibenzo[b,d]thiophene-S,S-dioxide/dibenzo[b,d]thiophene unit and their efficient solution-processed OLEDs, J. Organomet. Chem., 2023, 984, 122562 CrossRef CAS
.
- Y. Sun, X. Yang, B. Liu, J. Dang, Y. Li, G. Zhou, Z. Wu and W. Y. Wong, Towards high performance solution-processed orange organic light-emitting devices: preciselyadjusting properties of Ir(III) complexes by reasonably engineering the asymmetric configuration with second functionalized cyclometalating ligands, J. Mater. Chem. C, 2019, 7, 8836–8846 RSC
.
- G. Z. Lu, N. Su, H. Q. Yang, Q. Zhu, W. W. Zhang, Y. X. Zheng, L. Zhou, J. L. Zuo, Z. X. Chen and H. J. Zhang, Rapid room temperature synthesis of red iridium(III) complexes containing a four-membered Ir-S-C-S chelating ring for highly efficient OLEDs with EQE over 30%, Chem. Sci., 2019, 10, 3535–3542 RSC
.
- G. Z. Lu, R. Wu, N. Li, X. Wang, L. Zhou and C. L. Yang, Naphthyridine-based iridium(III) complexes for green to red OLEDs with EQEs over 30% and low efficiency roll-off, J. Mater. Chem. C, 2022, 10, 17303–17308 RSC
.
- M. X. Mao, F. L. Li, Y. Shen, Q. M. Liu, S. Xing, X. F. Luo, Z. L. Tu, X. J. Wu and Y. X. Zheng, Simple synthesis of red iridium(III) complexes with sulfur-contained four-membered ancillary ligands for OLEDs, Molecules, 2021, 26, 2599 CrossRef CAS PubMed
.
- H. Y. Zhang, Y. J. Sun, Z. Chen, W. G. Wang, Q. W. Wang, S. M. Chen, Y. Q. Xu and W. Y. Wong, Efficient deep red and NIR OLEDs based on Ir(III) complexes fabricated by evaporation and solution processing, Chem. Eng. J., 2023, 451, 138632 CrossRef CAS
.
- P. B. Si, H. F. Zhe, A. H. Zhou, X. Q. Liu, M. Y. Teng, M. Z. Rong, Y. F. Wang, Q. Wang, Z. L. Wang and J. Zhang, Synthesis and photoelectric properties of IrIII complexes using fluorobenzylimidazole[2,1-b]thiazole derivatives as primary ligands, New J. Chem., 2021, 45, 18796–18804 RSC
.
- L. L. Wen, J. M. Zhang, Y. P. Han, Y. C. Duan, W. F. Xie, K. Z. Shao, G. G. Shan and Z. M. Su, Boosting the efficiency of deep-red Ir(III) complexes by modulating nitrogen atoms for high-performance OLEDs, Inorg. Chem. Front., 2024, 11, 133–141 RSC
.
- R. Kumaresan, H. Y. Park, A. Maheshwaran, H. Park, Y. Do, M. Song, J. Yoon, S. Ahn and S. H. Jin, High performance solution-processed deep-blue phosphorescence organic light-emitting diodes with EQE over 24% by employing new carbenic Ir(III) complexes, Adv. Opt. Mater., 2022, 10, 2101686 CrossRef CAS
.
- A. J. Pal, R. Österbacka, K. M. Källman and H. Stubb, Transient electroluminescence: Mobility and response time in quinquethiophene Langmuir-Blodgett films, Appl. Phys. Lett., 1997, 71, 228–230 CrossRef CAS
.
- X. Chang, K. Y. Lu, K. Jiang, B. Ma, J. W. Huang, X. Q. Gan, Y. Liu, J. T. Yu and W. G. Zhu, Extending rigid electron-deficient skeletons and appending electron-rich units to build high-efficiency red-emitting pyrene-derived TADF materials, J. Mater. Chem. C, 2023, 11, 14876 RSC
.
- W. J. Liu, G. Y. Hong, D. D. Dai, L. M. Li and M. Dolg, The Beijing four-component density functional program package (BDF) and its application to EuO, EuS, YbO and YbS, Theor. Chem. Acta, 1997, 96, 75–83 CrossRef CAS
.
- Y. Zhang, B. B. Suo, Z. K. Wang, N. Zhang, Z. D. Li, Y. B. Li, W. L. Zou, J. Gao, D. L. Peng, Z. C. Pu, Y. L. Xiao, Q. M. Sun, F. Wang, Y. T. Ma, X. P. Wang, Y. Gao and W. J. Liu, BDF: A relativistic electronic structure program package, J. Chem. Phys., 2020, 152, 064113 CrossRef PubMed
.
- W. J. Liu, F. Wang and L. M. Li, The Beijing density functional (BDF program package: Methodologies and applications, J. Theor. Comput. Chem., 2003, 2, 257–272 CrossRef CAS
.
-
W. J. Liu, F. Wang and L. M. Li, Relativistic Density Functional Theory: The BDF Program Package, chapter 9, World Scientific Publishing, 2004, 5, 257–282 Search PubMed
.
- Z. K. Wang, Z. D. Li, Y. Zhang and W. J. Liu, Analytic energy gradients of spin-adapted open-shell time-dependent density functional theory, J. Chem. Phys., 2020, 153, 164109 CrossRef CAS PubMed
.
- H. Y. Zhang, Y. L. Lei, H. T. Wang and W. Y. Wong, Supramolecular core-shell heterostructures with controllable multi-color-emitting properties, J. Mater. Chem. C, 2020, 8, 2669–2675 RSC
.
- M. X. Mao, S. Xing, Y. P. Zhang, Z. Z. Qu, L. Yuan, J. J. Hu, X. J. Liao, Y. Zhao and Y. X. Zheng, Iridium(III) complexes incorporating thieno[2,3-d]pyrimidine units for efficient orange-to-yellow electroluminescence with low efficiency roll-off, J. Mater. Chem. C, 2020, 10, 8650–8656 RSC
.
- E. Kabir, Y. Y. Wu, S. Sittel, B. L. Nguyen and T. S. Teets, Improved deep-red phosphorescence in cyclometalated iridium complexes via ancillary ligand modification, Inorg. Chem. Front., 2020, 7, 1362–1373 RSC
.
- J. N. Xue, S. N. Chen, J. Liang, H. Bi, Y. Liu and Y. Wang, Finely-tuned heteroleptic phosphorescent Ir(III) complexes for CIEy < 0.2-based pure blue organic light-emitting diodes with external quantum efficiencies > 30%, Chem. Eng. J., 2023, 463, 142493 CrossRef CAS
.
- C. F. You, D. H. Liu, L. Wang, W. Q. Zheng, M. Li, P. Wang and W. G. Zhu, Rigid phenanthro[4,5-abc]phenazine-cored iridium(III) complexes for high-performance near-infrared emission at about 800 nm in solution-processed OLEDs, Chem. Eng. J., 2023, 452, 138956 CrossRef CAS
.
- X. M. Li, J. Zhang, G. L. Huang, Y. F. Wang, M. Z. Rong, M. Y. Teng and J. Liu, A bipolar thiophene substituted 1,3,5-triazine host material for green phosphorescent OLEDs with low onset voltage and current efficiency roll-off, Dyes Pigm., 2017, 141, 1–4 CrossRef CAS
.
- S. Xu, Q. Q. Yang, Y. Zhang, H. Li, Q. Xue, G. H. Xie, M. Z. Gu, J. B. Jin, L. Huang and R. F. Chen, Solution-processed multi-resonance organic light-emitting diodes with high efficiency and narrowband emission, Chin. Chem. Lett., 2021, 32, 1372–1376 CrossRef CAS
.
- Z. Z. Qu, X. F. Luo, M. X. Mao, C. F. Yip, Y. X. Zheng and J. L. Zuo, Iridium(III) complexes based on indolo[3,2,1-jk]carbazole derivatives for narrowband organic light-emitting diodes with external quantum efficiency of 32.3% and low efficiency roll-off, Adv. Energy Sustainability Res., 2022, 3, 2200057 CrossRef CAS
.
- X. Chang, K. Y. Lu, S. K. Zeng, D. H. Liu, J. W. Huang, B. Ma, L. Wang, X. Q. Gan, J. T. Yu, Y. F. Wang, S. J. Su and W. G. Zhu, High-efficiency long wavelength NIR iridium complexes constructed by extending rigid coordination and optimizing peripheral donor position to break cocoon into butterfly, Chem. Eng. J., 2023, 475, 146031 CrossRef CAS
.
- H. B. Han, Z. L. Tu, Z. G. Wu and Y. X. Zheng, Green iridium complexes based on pyrimidine derivatives for efficient electroluminescence with EQE near 30%, Dyes Pigm., 2019, 160, 863–871 CrossRef CAS
.
- J. Liu, M. H. Jiang, S. G. Li, X. R. Chen, Y. Y. Gong and J. Y. Hu, A novel binuclear iridium complex with bis β-diketone and carbazole groups for efficient solution-processed yellow PLEDs, J. Organomet. Chem., 2023, 1001, 122850 CrossRef CAS
.
- M. Chen, Y. P. Xiao, Y. Wang, W. Cheng, J. Zhang, P. Wang, S. H. Ye and B. H. Tong, Highly efficient solution processed OLEDs based on iridium complexes with steric phenylpyridazine derivative, Inorg. Chim. Acta, 2021, 516, 120100 CrossRef CAS
.
- F. L. Zhang, C. F. Si, D. H. Wei, S. S. Wang, D. P. Zhang, S. Z. Li, Z. Y. Li, F. Q. Zhang, B. Wei, G. X. Cao and B. Zhai, Solution-processed organic light-emitting diodes based on yellow-emitting cationic iridium(III) complexes bearing cyclometalated carbene ligands, Dyes Pigm., 2016, 134, 465–471 CrossRef CAS
.
- B. H. Tong, Q. B. Mei, R. Q. Tian, M. Yang, Q. F. Hua, Y. J. Shi and S. H. Ye, High-brightness solution-processed phosphorescent OLEDs with pyrimidine-based iridium(III) complexes, RSC Adv., 2016, 6, 34970–34976 RSC
.
- H. R. Guo, J. Zhao, Z. Z. Tian, Y. Wu, B. A. Liu, F. F. Dang, X. L. Yang, G. J. Zhou, Z. X. Wu and W. Y. Wong, Homoleptic thiazole-based Ir(III) phosphorescent complexes for achieving both high EL efficiencies and an optimized trade-off among the key parameters of solution-processed WOLEDs, J. Mater. Chem. C, 2017, 5, 208–219 RSC
.
- X. L. Yang, Z. Feng, J. S. Dang, Y. H. Sun, G. J. Zhou and W. Y. Wong, High performance solution-processed organic yellow light-emitting devices and fluoride ion sensors based on a versatile phosphorescent Ir(iii) complex, Mater. Chem. Front., 2019, 3, 376–384 RSC
.
- M. W. Ha, M. H. Park, J. Y. Hwang, J. W. Kim, D. H. Kim, T. W. Lee and Y. H. Kim, Synthesis and characterization of homoleptic triply cyclometalated iridium(III) complex containing 6-(pyridin-2-yl)isoquinoline moiety for solution-processable orange-phosphorescent organic light-emitting diodes, Dyes Pigm., 2021, 185, 108880 CrossRef CAS
.
- L. C. Chen, S. M. Wang, Z. M. Yan, J. Q. Ding and L. X. Wang, An oligocarbazole-encapsulated heteroleptic red iridium complex for solution-processed nondoped phosphorescent organic light-emitting diodes with over 10% external quantum efficiency, J. Mater. Chem. C, 2017, 5, 5749–5756 RSC
.
- Z. M. Yan, Y. P. Wang, J. Q. Ding, Y. Wang and L. X. Wang, Solution processible yellow-emitting iridium complexes based on furo[3,2-c]pyridine ligand, Org. Electron., 2018, 53, 191–197 CrossRef CAS
.
- X. J. Liu, B. Yao, Z. L. Zhang, X. F. Zhao, B. H. Zhang, W. Y. Wong, Y. X. Cheng and Z. Y. Xie, Power-efficient solution-processed red organic light-emitting diodes based on an exciplex host and a novel phosphorescent iridium complex, J. Mater. Chem. C, 2016, 4, 5787–5794 RSC
.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC2302905–2302907. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qi02636a |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2024 |