N-heterocyclic carbene-stabilized Cu9 clusters with combined thermally activated delayed fluorescence and phosphorescence†
Lin-Mei
Zhang‡
 a,
Mo
Xie‡
a,
Mo
Xie‡
 a,
Hui-Zhi
Wei
a,
Shang-Fu
Yuan
a,
Hui-Zhi
Wei
a,
Shang-Fu
Yuan
 *a,
Dong-Sheng
Li
*a,
Dong-Sheng
Li
 b and
Tao
Wu
b and
Tao
Wu
 *a
*a
aCollege of Chemistry and Materials Science, and Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou 510632, China. E-mail: sfyuan@jnu.edu.cn; wutao@jnu.edu.cn
bCollege of Materials and Chemical Engineering, Hubei Provincial Collaborative Innovation Centre for New Energy Microgrid, Key Laboratory of Inorganic Nonmetallic Crystalline and Energy Conversion Materials, China Three Gorges University, Yichang 443002, China
First published on 19th March 2024
Abstract
Metal clusters with both phosphorescence and thermally activated delayed fluorescence (TADF) at room temperature, allowing for efficient light emission and a short lifetime, are of particular interest. However, such clusters remain rare due to the challenge of achieving comparable and effective transitions T1 → S1 → S0 and T1 → S0 simultaneously. Herein, we report two copper clusters, [Cu9{(py)2im}2(PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)4(CF3CO2)4](PF6) (Cu9-Ph) and [Cu9{(py)2im}2(tBuC
C)4(CF3CO2)4](PF6) (Cu9-Ph) and [Cu9{(py)2im}2(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)4(CF3CO2)4](PF6) (Cu9-TBA), for which the ambient-temperature emission stems from both TADF from the S1 state and direct phosphorescence from the T1 state in thermal equilibrium. At 300 K, about 69% of the emission intensity of Cu9-TBA originates from the delayed fluorescence of the S1 state, and an additional 31% is contributed by the T1 → S0 emission. In comparison, Cu9-Ph exhibits nearly equal contributions from delayed fluorescence and phosphorescence at room temperature. As a result, the overall decay time is significantly shorter than that observed in the TADF-only situation of these clusters (e.g., reduced by 49% for Cu9-Ph). The combined harvesting mechanism arises from the simultaneous presence of a small ΔE(S1 − T1) gap and relatively strong spin–orbit coupling induced by the multi-metal centers. The combined use of both decay processes makes the low-cost Cu(I) clusters promising for applications in organic light-emitting diodes.
C)4(CF3CO2)4](PF6) (Cu9-TBA), for which the ambient-temperature emission stems from both TADF from the S1 state and direct phosphorescence from the T1 state in thermal equilibrium. At 300 K, about 69% of the emission intensity of Cu9-TBA originates from the delayed fluorescence of the S1 state, and an additional 31% is contributed by the T1 → S0 emission. In comparison, Cu9-Ph exhibits nearly equal contributions from delayed fluorescence and phosphorescence at room temperature. As a result, the overall decay time is significantly shorter than that observed in the TADF-only situation of these clusters (e.g., reduced by 49% for Cu9-Ph). The combined harvesting mechanism arises from the simultaneous presence of a small ΔE(S1 − T1) gap and relatively strong spin–orbit coupling induced by the multi-metal centers. The combined use of both decay processes makes the low-cost Cu(I) clusters promising for applications in organic light-emitting diodes.
Introduction
Coinage metal clusters with bright phosphorescence and/or thermally activated delayed fluorescence (TADF) have garnered increasing attention as promising organic light-emitting diode (OLED) emitters.1–6 In contrast to the triplet harvesting effect of phosphorescence, which employs the lowest T1 state to convert the harvested excitons into photons, TADF, referred to as a “singlet harvesting mechanism”, relies on thermally induced reverse intersystem crossing (RISC) from T1 to the S1 state followed by S1 to S0 emission. Given the inevitable cost issues of the triplet harvesting mechanism, which requires the application of scarce precious metals such as Ru(II), Ir(III), and Pt(II) complexes, the singlet harvesting effect has emerged as a preferable alternative.7 Nevertheless, the design of TADF materials is a nontrivial task since they should possess a small energy gap between the S1 and T1 states ΔE(S1 − T1) (<1500 cm−1 or 0.2 eV) to facilitate RISC (T1 → S1) at room temperature (RT). This criterion is met by several Cu(I) complexes with charge transfer character, attributed to the extensive metal-to-ligand charge transfer (MLCT) of the lowest excited states. The MLCT property induces distinct spatial separations among the involved orbitals, particularly the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbitals (LUMO), resulting in relatively small exchange integrals and consequently a reduced ΔE(S1 − T1).8–14 However, reducing ΔE(S1 − T1) implies a limited spatial overlap between the wave functions of S1 and S0, which often results in a small oscillator strength for the S1 → S0 fluorescent transition. Consequently, this leads to an undesirably prolonged singlet-state decay time, potentially causing pronounced saturation effects when applied in OLEDs. Therefore, improving TADF properties, particularly in terms of enhancing the radiative rate, poses a challenging optimization problem with inherent limitations.A promising approach to accelerating overall radiative decay is introducing an additional radiative decay pathway from the lowest triplet state. This can be achieved by utilizing polynuclear assemblies that exhibit high spin–orbit coupling (SOC) induced by the presence of metal centers. Previous efforts have developed strikingly emissive multicore Cu(I) clusters, which demonstrated a variety of supramolecular assemblies through Cu–Cu interactions.14–19 In this regard, cuprophilic interactions can greatly boost the radiative rate involving the T1 state by enhancing SOC strength, resulting in emission lifetimes comparable to those of TADF. Nevertheless, copper clusters that exhibit both TADF and phosphorescence are still relatively scarce, and unravelling this complex luminescence mechanism remains a challenging task.10,20
Recently, N-heterocyclic carbenes (NHCs) have been found to be valuable in the stabilization of metal clusters, endowing them with excellent reactivity and luminescent properties.21–25 For example, a copper acetylide cluster with ancillary NHC ligands has imparted outstanding catalytic activity in copper-catalyzed azide–alkyne cycloaddition reactions.25 Attributed to the strong bonding interaction between NHCs and the metal, the first NHC-stabilized copper-hydride cluster, Cu31(RS)25(NHC)3H6, has been isolated and demonstrated to possess good thermal and air stability.26 Herein, we report two interesting cases of NHC-stabilized Cu clusters that exhibited both efficient TADF and direct phosphorescence in thermal equilibrium.
With the stabilization of N-heterocyclic dipyridylcarbene (py)2im (H(py)2im = 1,3-di(pyridine-2-yl)-1H-imidazol-3-ium), two Cu9 clusters, [Cu9{(py)2im}2(PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)4(CF3CO2)4](PF6) (Cu9-Ph) and [Cu9{(py)2im}2(tBuC
C)4(CF3CO2)4](PF6) (Cu9-Ph) and [Cu9{(py)2im}2(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)4(CF3CO2)4](PF6) (Cu9-TBA), were isolated. The total structural determination of the clusters was carried out. To our surprise, both Cu9-Ph and Cu9-TBA exhibit the characteristics of combined triplet and singlet harvesting effects at ambient temperature. For Cu9-TBA, 69.2% of the emission stems from the S1 state as TADF, and 30.8% is contributed by T1 emission. In contrast, almost nearly half of phosphorescence and TADF account for the luminescence of Cu9-Ph. The combined emission paths distinctly shorten the overall decay time. In addition, the small activation energy ΔE(S1 − T1) is responsible for the delayed fluorescence process, while the additional phosphorescence pathway is attributed to strong SOC induced by the multi-metal centers.
C)4(CF3CO2)4](PF6) (Cu9-TBA), were isolated. The total structural determination of the clusters was carried out. To our surprise, both Cu9-Ph and Cu9-TBA exhibit the characteristics of combined triplet and singlet harvesting effects at ambient temperature. For Cu9-TBA, 69.2% of the emission stems from the S1 state as TADF, and 30.8% is contributed by T1 emission. In contrast, almost nearly half of phosphorescence and TADF account for the luminescence of Cu9-Ph. The combined emission paths distinctly shorten the overall decay time. In addition, the small activation energy ΔE(S1 − T1) is responsible for the delayed fluorescence process, while the additional phosphorescence pathway is attributed to strong SOC induced by the multi-metal centers.
Experimental section
Synthesis of [Cu9{(py)2im}2(PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e002.gif) C)4(CF3CO2)4](PF6) (Cu9-Ph)
C)4(CF3CO2)4](PF6) (Cu9-Ph)
[H(py)2im](PF6) (Fig. S1†) was synthesized following a previously reported procedure.27–29 To a 5 mL solution of CH2Cl2/MeOH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), Cu(CF3CO2)2·H2O (15 mg, 0.050 mmol), copper powder (6.3 mg, 0.10 mmol), PhC
2), Cu(CF3CO2)2·H2O (15 mg, 0.050 mmol), copper powder (6.3 mg, 0.10 mmol), PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (44 μL, 0.40 mmol), and [H(py)2im](PF6) (15 mg, 0.040 mmol) were added. The mixture was stirred overnight, resulting in a color change from green to yellow. The resulting solution was centrifuged for 2 min at 10
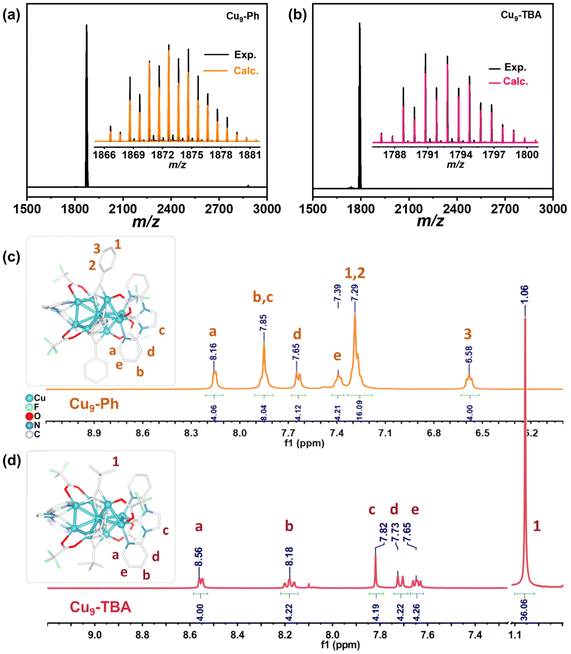
CH (44 μL, 0.40 mmol), and [H(py)2im](PF6) (15 mg, 0.040 mmol) were added. The mixture was stirred overnight, resulting in a color change from green to yellow. The resulting solution was centrifuged for 2 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 r min−1. After filtration, the solution was rotary evaporated to dryness. This crude yellow solid was then dissolved in 2 mL of CH2Cl2. The filtrate was subject to the diffusion of about 8 mL of n-hexane at 4 °C to afford yellow crystals of Cu9-Ph after about three days. (10 mg, yield 44% based on Cu). 1H NMR (400 MHz, CD2Cl2, Fig. 2c): δ = 8.16 (s, 4H), 7.85 (t, 8H), 7.65 (d, 4H), 7.39 (t, 4H), 7.29 (m, 16H), 6.58 (t, 4H). ESI-MS (m/z): calcd for Cu9(C13H10N4)2(C8H5)4(CF3COO)4 [M+], 1872.64; found, 1872.64. Elemental analysis (%) for C66H40F18N8O8P1Cu9, found (calcd): C, 39.88 (39.28); H, 1.85 (2.00); N, 5.54 (5.55).
000 r min−1. After filtration, the solution was rotary evaporated to dryness. This crude yellow solid was then dissolved in 2 mL of CH2Cl2. The filtrate was subject to the diffusion of about 8 mL of n-hexane at 4 °C to afford yellow crystals of Cu9-Ph after about three days. (10 mg, yield 44% based on Cu). 1H NMR (400 MHz, CD2Cl2, Fig. 2c): δ = 8.16 (s, 4H), 7.85 (t, 8H), 7.65 (d, 4H), 7.39 (t, 4H), 7.29 (m, 16H), 6.58 (t, 4H). ESI-MS (m/z): calcd for Cu9(C13H10N4)2(C8H5)4(CF3COO)4 [M+], 1872.64; found, 1872.64. Elemental analysis (%) for C66H40F18N8O8P1Cu9, found (calcd): C, 39.88 (39.28); H, 1.85 (2.00); N, 5.54 (5.55).
Synthesis of [Cu9{(py)2im}2(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e002.gif) C)4(CF3CO2)4](PF6) (Cu9-TBA)
C)4(CF3CO2)4](PF6) (Cu9-TBA)
Following the procedures described for Cu9-Ph, Cu9-TBA can be obtained by using tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (42 μL, 0.40 mmol) instead of PhC
CH (42 μL, 0.40 mmol) instead of PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH. The yellow filtrate was subject to the diffusion of 8 mL of n-hexane at 4 °C to afford yellow crystals of Cu9-TBA after about three days. (8 mg, yield 37% based on Cu). 1H NMR (400 MHz, CD2Cl2, Fig. 2d): δ = 8.56 (d, 4H), 8.18 (t, 4H), 7.82 (s, 4H), 7.73 (d, 4H), 7.65 (t, 4H), 1.08 (s, 36H). ESI-MS (m/z): calcd for Cu9(C13H10N4)2(C6H9)4(CF3COO)4 [M+], 1792.76; found, 1792.78. Elemental analysis (%) for C58H56F18N8O8P1Cu9, found (calcd): C, 35.55 (35.95); H, 2.89 (2.91); N, 5.50 (5.78).
CH. The yellow filtrate was subject to the diffusion of 8 mL of n-hexane at 4 °C to afford yellow crystals of Cu9-TBA after about three days. (8 mg, yield 37% based on Cu). 1H NMR (400 MHz, CD2Cl2, Fig. 2d): δ = 8.56 (d, 4H), 8.18 (t, 4H), 7.82 (s, 4H), 7.73 (d, 4H), 7.65 (t, 4H), 1.08 (s, 36H). ESI-MS (m/z): calcd for Cu9(C13H10N4)2(C6H9)4(CF3COO)4 [M+], 1792.76; found, 1792.78. Elemental analysis (%) for C58H56F18N8O8P1Cu9, found (calcd): C, 35.55 (35.95); H, 2.89 (2.91); N, 5.50 (5.78).
Physical measurements
Mass spectra were recorded on a Bruker MicroFlex Matrix-assisted laser desorption ionization-time of flight mass spectrometer (MALDI-TOF-MS). An Elementar vario Micro Cube was used to collect the element analysis data. Fourier transform (FT-IR) spectra were obtained on a Thermo Scientific FT-IR Nicolet iS10 spectrophotometer in the range of 4000–400 cm−1. UV-visible absorption spectra were recorded on a Shimadzu UV3600 plus. 1H NMR (400 MHz) spectra were recorded on a Bruker AVANCE III HD 400 spectrometer. Steady-state photoluminescence spectra (PL) for all samples were tested on a PTI Quanta Master Model QM/TM fluorescence spectrometer (Birmingham, NJ, USA), and the detector was calibrated using a calibration file in FelixGX software. The temperature change spectrum test used the Janis Research Model VPF-100 temperature change system with a liquid nitrogen Dewar; the temperature range was 77–400 K, and the interval was 25 K. During the temperature-variable steady-state spectrum test, it was ensured that the instrument parameters remained consistent. The luminescence lifetime was tested by an Edinburgh FLS-920 fluorescence spectrometer. The absolute photoluminescence quantum yield was measured with a Hamamatsu C11347-01 absolute PL quantum yield spectrometer at RT. X-ray photoelectron spectroscopy (XPS) data were collected on a Thermo ESCALAB 250XI system. All binding energies were calibrated using the C (1s) carbon peak (284.8 eV), which was applied as an internal standard.X-Ray crystallography
Intensity data for Cu9-Ph and Cu9-TBA were collected on an Oxford Cryo stream system on an XtaLAB PRO MM007-DW diffractometer system equipped with a HyPix-6000HE Hybrid Photon Counting (HPC) X-ray detector (Rigaku, Japan, Cu Kα, graphite monochromator, λ = 1.54 Å) at 100 K. Absorption corrections were applied using the program CrysAlis (multi-scan) and all the structures were solved by direct methods. The hydrogen atoms of the organic ligands were generated geometrically. Nonhydrogen atoms except solvent molecules were refined anisotropically by least-squares on F2 using the SHELXTL program. Crystal data and structural refinement parameters are summarized in Table S2.†Decay time fitting based on the Boltzmann equation

| (1) |
Fractional emission intensities
According to the literature,31 the fractional intensities of phosphorescence and TADF-only, respectively, can be deduced according to the following considerations. The total emission intensity Itot is composed of the emission from the triplet state I(T1) and the singlet emission I(S1), which can be written as:| I(i) = α·kr(i)·N(i) | (2) |
 | (3) |
 | (4) |
Under the assumption of equal photoluminescence quantum yields for the phosphorescence and the TADF (ΦPL(S1) = ΦPL(T1)), eqn (4) can be simplified to:
 | (5) |
 | (6) |
Computational details
All calculations were performed with the Gaussian09 suite of programs32 employing density functional theory (DFT) and time-dependent density functional theory (TDDFT). The hybrid functional PBE0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 and double zeta basis set (LanL2DZ34 for Cu and 6-31G(d)35 for other atoms) were applied here. The geometries of Cu9-Ph and Cu9-TBA in the ground state were fully optimized based on the X-ray crystal structures. The singlet vertical excitation energy and corresponding electron transitions as well as the frontier molecular orbital analysis were based on the ground-state geometry. Based on the excitation energy, En→m, and oscillator strength f, the absorption spectra were simulated using Gaussian functions. A full-width at half-maximum (FWHM), that is, the broadening of each peak (individual transition), of 0.30 eV was applied. The geometries of Cu9-Ph and Cu9-TBA in the S1 states were fully optimized as well. The vertical/adiabatic excitation energy for emission was calculated based on the S1 state geometries and S1/S0 state geometries of Cu9-Ph and Cu9-TBA, respectively (Table S1†).
33 and double zeta basis set (LanL2DZ34 for Cu and 6-31G(d)35 for other atoms) were applied here. The geometries of Cu9-Ph and Cu9-TBA in the ground state were fully optimized based on the X-ray crystal structures. The singlet vertical excitation energy and corresponding electron transitions as well as the frontier molecular orbital analysis were based on the ground-state geometry. Based on the excitation energy, En→m, and oscillator strength f, the absorption spectra were simulated using Gaussian functions. A full-width at half-maximum (FWHM), that is, the broadening of each peak (individual transition), of 0.30 eV was applied. The geometries of Cu9-Ph and Cu9-TBA in the S1 states were fully optimized as well. The vertical/adiabatic excitation energy for emission was calculated based on the S1 state geometries and S1/S0 state geometries of Cu9-Ph and Cu9-TBA, respectively (Table S1†).
Results and discussion
Crystal structure and characterization
Single-crystal X-ray diffraction (SCXRD) analysis reveals that Cu9-Ph crystallizes in the orthorhombic space group Pbcn, while Cu9-TBA belongs to the monoclinic crystal system with a P21/c space group (Table S2†). Both clusters comprise a monocationic cluster [Cu9{(py)2im}2(RC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)4(CF3CO2)4]+ (R = Ph for Cu9-Ph; R = tBu for Cu9-TBA) and one PF6− counteranion. The structure of Cu9-TBA is analogous to Cu9-Ph, except for the substitution of PhC
C)4(CF3CO2)4]+ (R = Ph for Cu9-Ph; R = tBu for Cu9-TBA) and one PF6− counteranion. The structure of Cu9-TBA is analogous to Cu9-Ph, except for the substitution of PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C− ligands with tBuC
C− ligands with tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C− (Fig. 1a and b). For brevity, the structural analysis of Cu9-Ph is presented here as an example. Cu9-Ph consists of a Cu9 core protected by two (py)2im ligands, four PhC
C− (Fig. 1a and b). For brevity, the structural analysis of Cu9-Ph is presented here as an example. Cu9-Ph consists of a Cu9 core protected by two (py)2im ligands, four PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C−, and four trifluoroacetate groups (Fig. 1a). As depicted in Fig. 1c, the Cu9 skeleton can be viewed as the fusion of two distorted triangular biconical Cu5 units via the sharing of the central pink atom. The two Cu5 units join together orthogonally, resulting in a Cu9 core with an ideal symmetry of S4 (Fig. 1d). In the Cu9 kernel, the Cu–Cu distances for Cu9-Ph are in the range of 2.497(1)–2.741 (1) Å (average 2.574 Å), while for Cu9-TBA, they fall in the range of 2.462(1)–2.768(1) Å (average 2.583 Å). These distances are slightly shorter than twice the van der Waals radius of copper (2.8 Å), suggesting strong bonding between the copper atoms in the Cu9 core.
C−, and four trifluoroacetate groups (Fig. 1a). As depicted in Fig. 1c, the Cu9 skeleton can be viewed as the fusion of two distorted triangular biconical Cu5 units via the sharing of the central pink atom. The two Cu5 units join together orthogonally, resulting in a Cu9 core with an ideal symmetry of S4 (Fig. 1d). In the Cu9 kernel, the Cu–Cu distances for Cu9-Ph are in the range of 2.497(1)–2.741 (1) Å (average 2.574 Å), while for Cu9-TBA, they fall in the range of 2.462(1)–2.768(1) Å (average 2.583 Å). These distances are slightly shorter than twice the van der Waals radius of copper (2.8 Å), suggesting strong bonding between the copper atoms in the Cu9 core.
As illustrated in Fig. 1e, each dipyridylcarbene ligand bridges two Cu atoms, with the C atom of the carbene ligand adopting the μ coordination mode, while two pyridine N atoms each ligate a Cu atom. The Cu–Ccarbene bond lengths range from 2.019(5) to 2.078(6) Å. Similar values with the same motif have previously been found in trinuclear Cu clusters.36,37 Additionally, four terminal alkynes and four CF3COO− ligands are distributed into two groups along the S4 axis. All four PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C− ligands adopt μ3-η1,η1,η2 modes (Fig. 1f), while each CF3COO− bridges two copper centers in the μ-κ1,κ1 motif, providing further protection to the obtained clusters.
C− ligands adopt μ3-η1,η1,η2 modes (Fig. 1f), while each CF3COO− bridges two copper centers in the μ-κ1,κ1 motif, providing further protection to the obtained clusters.
The compositions of Cu9-Ph and Cu9-TBA were firstly determined by electrospray ionization mass spectrometry (ESI-MS) (Fig. 2a and b). Positive mode ESI-MS of Cu9-Ph dissolved in DCM was carried out, which showed a dominant signal at m/z = 1872.64, corresponding to the molecular ion [Cu9{(py)2im}2(PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)4(CF3CO2)4]+ (calcd 1872.64). The spectrum of Cu9-TBA gave the signal of a monocation [Cu9{(py)2im}2(tBuC
C)4(CF3CO2)4]+ (calcd 1872.64). The spectrum of Cu9-TBA gave the signal of a monocation [Cu9{(py)2im}2(tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)4(CF3CO2)4]+ at m/z = 1792.76 (Fig. 2b). The observed isotopic patterns of the clusters are both in perfect agreement with their simulated ones. FT-IR (Fig. S2†) gives a stretching vibration peak 1685 cm−1 related to the C
C)4(CF3CO2)4]+ at m/z = 1792.76 (Fig. 2b). The observed isotopic patterns of the clusters are both in perfect agreement with their simulated ones. FT-IR (Fig. S2†) gives a stretching vibration peak 1685 cm−1 related to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, and bands at 1148 cm−1 and 1195 cm−1 assigned to CF3, which are the characteristic peaks of CF3COO−. In addition, the presence of PF6− is confirmed by the 843 cm−1 peak, which corresponds to the P–F stretching vibration mode. The Cu 2p1/2 binding energies are 932.7 eV for Cu9-Ph and 933.1 eV for Cu9-TBA (Fig. S3†), confirming their copper atoms are present in monovalent Cu+.
O group, and bands at 1148 cm−1 and 1195 cm−1 assigned to CF3, which are the characteristic peaks of CF3COO−. In addition, the presence of PF6− is confirmed by the 843 cm−1 peak, which corresponds to the P–F stretching vibration mode. The Cu 2p1/2 binding energies are 932.7 eV for Cu9-Ph and 933.1 eV for Cu9-TBA (Fig. S3†), confirming their copper atoms are present in monovalent Cu+.
1H NMR analysis of Cu9-Ph and Cu9-TBA revealed two distinct sets of signals that can be assigned to (py)2im and alkynyl ligands (Fig. 2c and d). For Cu9-Ph, four peaks at 8.16, 7.85, 7.65, and 7.39 ppm, with an integral ratio of 4.06![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.04
8.04![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.12
4.12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.21 correspond to the (py)2im ligands. Additionally, two peaks at 7.29 and 6.58 ppm, with an integral ratio of 16.09
4.21 correspond to the (py)2im ligands. Additionally, two peaks at 7.29 and 6.58 ppm, with an integral ratio of 16.09![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.00, are characteristic of PhC
4.00, are characteristic of PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C−. The results verify the purity of the samples and indicate that the cluster remains intact in solution. Remarkably, both Cu9-Ph and Cu9-TBA demonstrate exceptional chemical stability, whether in the solid state or in solution. The DCM solutions of Cu9-Ph and Cu9-TBA under ambient conditions were monitored by 1H NMR (Fig. S4†) and UV-vis analysis (Fig. S5†). In both cases, the curves of the clusters did not display noticeable changes for at least one week. Furthermore, Cu9-Ph crystals remained intact after being heated in air at 50 °C for 6 hours. No new peaks were observed in the 1H NMR and 19F NMR spectra of Cu9-Ph (Fig. S6†), suggesting its high stability throughout the process. The TGA curve showed that Cu9-Ph decomposed at approximately 210 °C (Fig. S7†), implying the moderate thermal stability of the cluster.
C−. The results verify the purity of the samples and indicate that the cluster remains intact in solution. Remarkably, both Cu9-Ph and Cu9-TBA demonstrate exceptional chemical stability, whether in the solid state or in solution. The DCM solutions of Cu9-Ph and Cu9-TBA under ambient conditions were monitored by 1H NMR (Fig. S4†) and UV-vis analysis (Fig. S5†). In both cases, the curves of the clusters did not display noticeable changes for at least one week. Furthermore, Cu9-Ph crystals remained intact after being heated in air at 50 °C for 6 hours. No new peaks were observed in the 1H NMR and 19F NMR spectra of Cu9-Ph (Fig. S6†), suggesting its high stability throughout the process. The TGA curve showed that Cu9-Ph decomposed at approximately 210 °C (Fig. S7†), implying the moderate thermal stability of the cluster.
Ambient-temperature photoluminescence
Interestingly, both copper clusters exhibit bright photoluminescence under ambient conditions. Cu9-Ph demonstrates bright yellow emission peaking at 570 nm, with excitation wavelengths ranging from 300 to 500 nm, and the maximum excitation wavelength is 470 nm (Fig. S8†). The PL quantum yield (PLQY) is measured as 16.5%, and the observed lifetime (τ) is 18.3 μs under ambient conditions (Fig. S9 and Tables S3, 4†). In contrast, Cu9-TBA shows a red-shifted emission peak at 605 nm, with excitation wavelengths ranging from 300 to 540 nm, and an observed τ of 12.3 μs (Fig. S10†). It is remarked that Cu9-TBA in the solid state displays a broad UV-vis absorption with an absorption edge at 550 nm, whereas Cu9-Ph crystals exhibit an extended absorption edge up to 600 nm (Fig. S11†). By extrapolating the absorbance to zero, both Cu9 analogs have a comparable HOMO–LUMO gap (∼2.3 eV) (Fig. S12†). This similarity arises from the shared Cu9 kernel, which largely dictates the optical spectrum.Similarly, the luminescence of Cu9-TBA in a dilute solution also exhibits a red-shift compared to that of Cu9-Ph. The emission spectra (Fig. S13†) of Cu9-Ph in DCM exhibit a maximum wavelength of 580 nm, while Cu9-TBA shows a PL peak at 610 nm under the same conditions. This suggests that the emission may originate from the internal Cu9 core, with a slight influence from the peripheral alkynyl group. Moreover, the emissions of Cu9-Ph and Cu9-TBA at RT are independent of the excitation wavelength (Fig. S13†), indicating that the emission originates from the lowest excited state. Photophysical data of the two clusters in DCM and in solid at ambient temperature are summarized in Table 1.
| Complex | Solvent | PLQY (%) | E x (nm) | E m (nm) | τ av (μs) | k r (s−1) | k nr (s−1) |
|---|---|---|---|---|---|---|---|
| τ av, average lifetime, measured under ambient conditions; kr, radiative decay rate; knr, nonradiative decay rate; PLQY = kr/(knr + kr); τ = 1/(knr + kr). | |||||||
| Cu9-Ph | Solid | 16.5 | 470 | 570 | 18.30 | 9.01 × 103 | 45.6 × 103 |
| DCM | 1.00 | 440 | 580 | 0.326 | 3.06 × 104 | 3.03 × 106 | |
| Cu9-TBA | Solid | 14.4 | 460 | 605 | 12.30 | 11.7 × 103 | 69.6 × 103 |
| DCM | 0.30 | 470 | 610 | 0.614 | 4.88 × 103 | 1.62 × 106 | |
In comparison, the emission intensity in the solid state is not only more intense but also at a higher energy level than in solution. This may be attributed to the interactions between clusters in the solid state, which can rigidify the cluster conformation and enhance emission by suppressing nonradiative decay pathways. On the other hand, these cluster interactions also affect the electronic structure of the clusters, leading to a certain degree of blue-shift. In this work, multiple O/F⋯H–C hydrogen bonds and π–π interactions were observed in the crystals of Cu9-Ph and Cu9-TBA clusters (Fig. S14†), and we speculate that these interactions contribute to the enhancement of their solid-state luminescence.
TADF–phosphorescence dual-channel emission processes
To gain a better understanding of the origin of this solid-state emission, we conducted temperature-dependent measurements of the luminescence properties for Cu9-Ph and Cu9-TBA in the temperature range of 77 to 400 K (Fig. 3 and 4). As the temperature increased from 100 to 400 K, the emission intensity of Cu9-Ph decreased (Fig. 3a), accompanied with a blue-shift of the emission maximum from 595 nm (100 K) to 570 nm (400 K) under excitation at 470 nm (Fig. 3b). A similar temperature-dependent trend in emission properties was also observed for Cu9-TBA (Fig. 4a and b). The blue-shift of the emission peaks of Cu9-Ph (25 nm) and Cu9-TBA (17 nm) with increasing temperature can be explained by the thermally activated delayed fluorescence (TADF) process. This delayed process arises from effective reverse intersystem crossing (RISC) from the T1 state to the S1 state, activated by thermal energy. With increasing temperature, the contribution of TADF (shorter wavelength) increases, while that of phosphorescence (longer wavelength) decreases. As a result, a blue-shift in the emission is observed.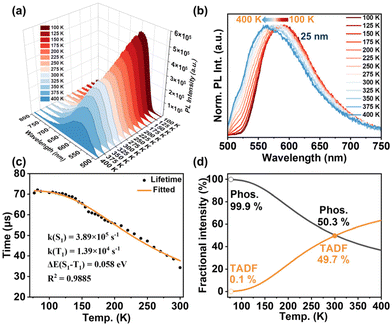 | ||
| Fig. 3 (a) Temperature-dependent PL intensity of Cu9-Ph crystals from 100 K to 400 K in a vacuum environment. (b) Normalized temperature-dependent solid-state emission of Cu9-Ph crystals in the range of 100 to 400 K in a vacuum environment. (c) Plot of emission decay lifetime at 570 nm against temperature (77 to 300 K); the orange line represents the fit according to the Boltzmann equation (eqn (1)). (d) Simulation of the fractional emission intensities of TADF and phosphorescence dependent on temperature based on the experimental data in Fig. 3c; the hollow circles and solid circles characterize the properties of Cu9-Ph at 77 K and 300 K, respectively. | ||
 | ||
| Fig. 4 (a) Temperature-dependent PL intensity of Cu9-TBA crystals in the range of 77 K to 400 K in a vacuum environment. (b) Normalized temperature-dependent solid-state PL intensity of Cu9-TBA crystals changes from 77 K to 400 K in a vacuum environment. (c) Plot of emission lifetime at 605 nm against temperature for Cu9-TBA and the fitting of the data using the Boltzmann equation (eqn (1)). (d) Simulation of temperature dependence of the fractional contributions of TADF and phosphorescence to the total emission based on the experimental data in Fig. 4c; the hollow circles and solid circles characterize the properties of Cu9-TBA at 77 K and 300 K, respectively. | ||
To further confirm the hypothesis, the temperature-dependent emission decay curves of Cu9-Ph and Cu9-TBA from 77 to 400 K were recorded. For Cu9-Ph, a relatively constant value of about 70 μs was observed at lower temperatures (77 to 105 K), primarily attributed to the PL decay time τ(T1) of the T1 → S0 transition. However, as the temperature exceeded 105 K, the decay lifetime gradually decreased, ultimately reaching 34.5 μs at 300 K (Fig. S15†). The temperature-dependent PL decay lifetime τ (T, 77 to 300 K) for Cu9-Ph, represented in Fig. 3c, was fitted to the modified Boltzmann equation (eqn (1)). This analysis gave an activation energy of ΔE(S1 − T1) = 0.058 eV, a phosphorescence radiative rate k(T1) = 1.39 × 104 s−1, and a fluorescence radiative rate k(S1) = 3.89 × 105 s−1. Based on the emission quantum yield and decay time, the radiative decay rate kr = ΦPL/τ(300 K) = 9.01 × 103 s−1 can be determined, indicating the contribution of delayed fluorescence and direct phosphorescence to the RT emission.
Upon substituting PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C− with tBuC
C− with tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C−, Cu9-TBA exhibited a similar trend of temperature-dependent lifetime decay to Cu9-Ph (Fig. 4c). Fitting the plots of the emission lifetime of Cu9-TBA against temperature with eqn (1) determined ΔE(S1 − T1) = 0.018 eV, which is significantly smaller than that of Cu9-Ph (0.058 eV). In addition, for Cu9-TBA, k(T1) was found to be 1.62 × 104 s−1, slightly higher than the 1.39 × 104 s−1 of Cu9-Ph; k(S1) = 2.28 × 105 s−1, much lower than the 3.89 × 105 s−1 of Cu9-Ph. Typically, a small ΔE(S1 − T1) and a long-lived T1 state are crucial for a compound to exhibit efficient TADF. In this case, the reduced activation energy ΔE(S1 − T1) and slightly increased k(T1) rate from T1 to S0 in Cu9-TBA contribute to a lower activation temperature for delayed fluorescence and a higher proportion of TADF at RT. Notably, the k(S1) rates of both Cu9-Ph and Cu9-TBA are extraordinarily lower than other TADF metal complexes and clusters hitherto reported.2,20,38,39 The limited singlet harvesting rate (k(S1)) is largely responsible for the combined emission paths of TADF and phosphorescence at ambient temperature (vide infra).
C−, Cu9-TBA exhibited a similar trend of temperature-dependent lifetime decay to Cu9-Ph (Fig. 4c). Fitting the plots of the emission lifetime of Cu9-TBA against temperature with eqn (1) determined ΔE(S1 − T1) = 0.018 eV, which is significantly smaller than that of Cu9-Ph (0.058 eV). In addition, for Cu9-TBA, k(T1) was found to be 1.62 × 104 s−1, slightly higher than the 1.39 × 104 s−1 of Cu9-Ph; k(S1) = 2.28 × 105 s−1, much lower than the 3.89 × 105 s−1 of Cu9-Ph. Typically, a small ΔE(S1 − T1) and a long-lived T1 state are crucial for a compound to exhibit efficient TADF. In this case, the reduced activation energy ΔE(S1 − T1) and slightly increased k(T1) rate from T1 to S0 in Cu9-TBA contribute to a lower activation temperature for delayed fluorescence and a higher proportion of TADF at RT. Notably, the k(S1) rates of both Cu9-Ph and Cu9-TBA are extraordinarily lower than other TADF metal complexes and clusters hitherto reported.2,20,38,39 The limited singlet harvesting rate (k(S1)) is largely responsible for the combined emission paths of TADF and phosphorescence at ambient temperature (vide infra).
Importantly, the emission properties of Cu9-Ph and Cu9-TBA are determined by both the lowest excited T1 and S1 states at ambient temperature. Fig. 3d and 4d present the simulated fractional emission intensities of TADF and phosphorescence dependent on temperature based on the experimental data in Fig. 3c and 4c, respectively. It is worth noting that nearly 100% of the emission intensity of Cu9-Ph stems from phosphorescence at 77 K. The calculated time constant τ(T1) = 71.7 μs, according to the corresponding k(T1) = 1.39 × 104 s−1 (eqn (1)), deviates only slightly from the experimentally measured value of τ(77 K) = 70.5 μs, thus supporting the presented model. Additionally, for Cu9-TBA, 69.2% of the emission intensity stems from the S1 state as TADF, while the remaining 30.8% is contributed by the energetically lower lying triplet state at 300 K. In contrast, phosphorescence and TADF accounted for nearly half of each other for Cu9-Ph at 300 K. Previously reported Cu(I) complexes displayed a limited ratio of phosphorescence at RT, almost below 20%, owing to their relatively weak spin–orbit coupling (SOC).10,40,41
In OLED emitters, utilizing both singlet and triplet excitons significantly enhances the performance of the device, potentially achieving up to 100% internal efficiency. Triplet harvesting relies on strong SOC, while singlet harvesting benefits from a small energy gap ΔE(S1 − T1), resulting in a TADF process. In the present work, Cu9-Ph combines the advantages of both triplet and singlet harvesting effects. (i) It demonstrates relatively strong spin–orbit coupling, resulting in a short triplet emission decay time of 71.7 μs. (ii) The energy gap between the first excited singlet and triplet states is only 0.058 eV, enabling efficient TADF. At ambient temperature, 50.3% of the emission intensity arises from the triplet state and 49.7% from the singlet state. Consequently, deactivation via these two radiative decay pathways leads to a higher overall radiative decay rate. This dual-channel emission mechanism yields an effective decay time of τ = 34.3 μs, which is shorter than that of the individual processes (τTADF = 67.0 μs, τPhos = 71.7 μs) (Fig. 5a). While our materials possess lifetimes in the tens of microseconds, it is acknowledged that for certain applications in OLEDs, shorter photoluminescence decay times in the lower single-digit microsecond range may be better. Nevertheless, the singlet-plus-triplet harvesting character exhibited by such materials holds promise for applications that benefit from reduced photoluminescence decay.
Density functional theory calculations
To gain more insights into the complex photophysical properties of Cu9-Ph and Cu9-TBA, time-dependent density functional theory (TD-DFT) calculations were performed. The UV-vis absorption spectra of Cu9-Ph and Cu9-TBA were fitted by the calculated vertical excitation energies and oscillator strengths based on the fully optimized ground-state geometries of Cu9-Ph and Cu9-TBA. The simulated absorption curve reasonably matched the experimental spectra in DCM (Fig. 5b and Fig. S16†). The energy levels and patterns of the corresponding frontier molecular orbitals (FMOs) are illustrated in Fig. 5c and Fig. S17, S18.† The highest occupied molecular orbitals (HOMO) of Cu9-Ph and Cu9-TBA were found to be mainly distributed on the copper and coordinated alkyne groups, whereas the unoccupied molecular orbitals, similar to the lowest unoccupied molecular orbitals (LUMO), were mainly derived from the N-heterocyclic carbene ligands. In the case of Cu9-Ph, the lowest-lying excitation (peak I) can be primarily ascribed to the HOMO → LUMO transition. This excitation is a typical metal-to-ligand charge transfer (MLCT)/ligand-to-ligand charge transfer (LLCT) state from the Cu9 core and PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C− ligands to the carbene ligands. Peak II primarily involves the transitions from HOMO-2 and HOMO−3 to LUMO+1 dominated by the carbene moieties. Peak III originates from the HOMO → LUMO+5 transition, while peak IV results from the HOMO−1 → LUMO+4 and HOMO−1 → LUMO+5 transitions (Fig. S17†). The simulated absorption spectrum of Cu9-TBA features a broad band at around 400 nm, resulting from the mixture of HOMO → LUMO transition and HOMO−3 → LUMO transition (Fig. S18†). It is important to highlight that the key orbitals involved in significant transitions consist primarily of copper atoms at the core acting as electron donors and coordinated carbene ligands at the interface serving as electron acceptors. In this regard, the distinctive electronic transition responsible for luminescence can be attributed to metal-to-ligand charge transfer (MLCT).
C− ligands to the carbene ligands. Peak II primarily involves the transitions from HOMO-2 and HOMO−3 to LUMO+1 dominated by the carbene moieties. Peak III originates from the HOMO → LUMO+5 transition, while peak IV results from the HOMO−1 → LUMO+4 and HOMO−1 → LUMO+5 transitions (Fig. S17†). The simulated absorption spectrum of Cu9-TBA features a broad band at around 400 nm, resulting from the mixture of HOMO → LUMO transition and HOMO−3 → LUMO transition (Fig. S18†). It is important to highlight that the key orbitals involved in significant transitions consist primarily of copper atoms at the core acting as electron donors and coordinated carbene ligands at the interface serving as electron acceptors. In this regard, the distinctive electronic transition responsible for luminescence can be attributed to metal-to-ligand charge transfer (MLCT).
Based on the aforementioned experiments and theoretical analysis, we proposed an energy diagram based on Cu9-Ph (Fig. 5a) to illustrate the interplay of S1-TADF and T1-phosphorescence. Below 105 K, the emission with a plateau decay time of 71.7 μs originates from the lowest triplet state, T1. From 105 to 400 K, TADF becomes active, surpassing non-radiative decay, resulting in enhanced emission intensity. As the temperature increases, TADF gradually becomes dominant with a decay time of approximately 67.0 μs. The combined emission processes result in an overall decay time of 34.3 μs (reduced by about 49% compared to the TADF-only situation) at RT.
To ensure efficient RISC, TADF materials require a small singlet–triplet energy gap, which corresponds to the efficient separation of their HOMO and LUMO orbitals.42 Seen from the calculated FMO patterns, the occupied orbitals (HOMO and HOMO minus orbitals) all located on the Cu9 core and PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C−/tBuC
C−/tBuC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C− ligands, while all the unoccupied orbitals (LUMO and LUMO plus orbitals) were distributed on carbene ligands. This results in complete charge separation between the initial and final states of the excitation transition of Cu9-Ph and Cu9-TBA. Achieving a small energy gap ΔE(S1 − T1) can be accomplished through intramolecular charge transfer between spatially separated donor and acceptor moieties. In the present work, the Cu9 cores act as the donors, while N-heterocyclic carbenes serve as the acceptors, resulting in a remarkably small ΔE(S1 − T1) and reasonable radiative decay rates to overcome competitive non-radiative decay pathways. This leads to the development of luminescent TADF materials.
C− ligands, while all the unoccupied orbitals (LUMO and LUMO plus orbitals) were distributed on carbene ligands. This results in complete charge separation between the initial and final states of the excitation transition of Cu9-Ph and Cu9-TBA. Achieving a small energy gap ΔE(S1 − T1) can be accomplished through intramolecular charge transfer between spatially separated donor and acceptor moieties. In the present work, the Cu9 cores act as the donors, while N-heterocyclic carbenes serve as the acceptors, resulting in a remarkably small ΔE(S1 − T1) and reasonable radiative decay rates to overcome competitive non-radiative decay pathways. This leads to the development of luminescent TADF materials.
Cu(I) complexes usually exhibit limited radiative processes from T1 to S0 at room temperature, primarily due to their relatively weak SOC.43 Thus, the development of Cu(I) complexes that can simultaneously display phosphorescence and TADF at room temperature remains a challenge.40 In general, SOC is dominantly induced by the metal d-orbitals. The high SOC efficiency observed in the present polynuclear copper clusters with MLCT states can be attributed to the energy separations between the occupied d-orbitals in the HOMO range. As proposed by Yersin et al., the energies of the corresponding states can be roughly determined from the energy differences between the HOMO and HOMO−1 states, which possess distinct d-orbital characters.31,40,44 Indeed, the DFT calculations reveal an approximate energy separation of ≈0.13 eV for Cu9-TBA but only ≈0.078 eV for Cu9-Ph between the HOMO and HOMO−1 states. In this regard, the coordination of the PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C− moieties results in smaller d-orbital splitting of Cu9-Ph, thereby facilitating a stronger direct phosphorescent radiative pathway.
C− moieties results in smaller d-orbital splitting of Cu9-Ph, thereby facilitating a stronger direct phosphorescent radiative pathway.
Conclusions
In summary, we managed to prepare two Cu(I) clusters, Cu9-Ph and Cu9-TBA, with the stabilization of N-heterocyclic dipyridylcarbene. These clusters exhibited both novel triplet and singlet emission effects at room temperature. In particular, Cu9-TBA demonstrated 69% emission from the S1 state via TADF, with the remaining 31% originating from the T1 state. In contrast, Cu9-Ph displayed nearly half of its emission from phosphorescence and TADF. This is due to the effective separation of their HOMO and LUMO, which are mainly located on the Cu9 core and carbene ligands, respectively, resulting in a small activation energy gap ΔE(S1 − T1). Furthermore, the additional phosphorescence path was attributed to strong SOC induced by the multi-metal centers. The comparable decay rates between the S1 and T1 states not only provides an innovative avenue for efficient light emission but also empowers low-cost Cu(I) clusters with a shorter lifetime for future OLED applications.Author contributions
Tao Wu and Shang-Fu Yuan: funding acquisition, project administration, resources, writing – reviewing, editing and supervision. Dong-Sheng Li: funding acquisition. Mo Xie: DFT research, writing and discussion. Lin-Mei Zhang: conceptualization, data curation, formal analysis, investigation, methodology, writing – reviewing and editing. Hui-Zhi Wei: formal analysis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by National Natural Science Foundation of China (22071165, 92261205, 22201103, and U22A20432) and the 111 Project (D20015).References
- X. Kang and M. Zhu, Tailoring the photoluminescence of atomically precise nanoclusters, Chem. Soc. Rev., 2019, 48, 2422–2457 RSC.
- Z. Han, X.-Y. Dong and S.-Q. Zang, Crystalline Metal-Organic Materials with Thermally Activated Delayed Fluorescence, Adv. Opt. Mater., 2021, 9, 2100081 CrossRef CAS.
- Z. Lei, X.-K. Wan, S.-F. Yuan, Z.-J. Guan and Q.-M. Wang, Alkynyl Approach toward the Protection of Metal Nanoclusters, Acc. Chem. Res., 2018, 51, 2465–2474 CrossRef CAS PubMed.
- Y. Xiao, Z. Wu, Q. Yao and J. Xie, Luminescent metal nanoclusters: Biosensing strategies and bioimaging applications, Aggregate, 2021, 2, 114–132 CrossRef CAS.
- J. Zheng, Z. Lu, K. Wu, G.-H. Ning and D. Li, Coinage-Metal-Based Cyclic Trinuclear Complexes with Metal–Metal Interactions: Theories to Experiments and Structures to Functions, Chem. Rev., 2020, 120, 9675–9742 CrossRef CAS PubMed.
- W.-D. Si, C. Zhang, M. Zhou, W.-D. Tian, Z. Wang, Q. Hu, K.-P. Song, L. Feng, X.-Q. Huang, Z.-Y. Gao, C.-H. Tung and D. Sun, Two triplet emitting states in one emitter: Near-infrared dual-phosphorescent Au20 nanocluster, Sci. Adv., 2023, 9, eadg3587 CrossRef CAS PubMed.
- G. Li, Z.-Q. Zhu, Q. Chen and J. Li, Metal complex based delayed fluorescence materials, Org. Electron., 2019, 69, 135–152 CrossRef CAS.
- C. Chen, R.-H. Li, B.-S. Zhu, K.-H. Wang, J.-S. Yao, Y.-C. Yin, M.-M. Yao, H.-B. Yao and S.-H. Yu, Highly Luminescent Inks: Aggregation-Induced Emission of Copper–Iodine Hybrid Clusters, Angew. Chem., Int. Ed., 2018, 57, 7106–7110 CrossRef CAS PubMed.
- Q. Benito, X. F. Le Goff, S. Maron, A. Fargues, A. Garcia, C. Martineau, F. Taulelle, S. Kahlal, T. Gacoin, J.-P. Boilot and S. Perruchas, Polymorphic Copper Iodide Clusters: Insights into the Mechanochromic Luminescence Properties, J. Am. Chem. Soc., 2014, 136, 11311–11320 CrossRef CAS PubMed.
- M. Olaru, E. Rychagova, S. Ketkov, Y. Shynkarenko, S. Yakunin, M. V. Kovalenko, A. Yablonskiy, B. Andreev, F. Kleemiss, J. Beckmann and M. Vogt, A Small Cationic Organo–Copper Cluster as Thermally Robust Highly Photo- and Electroluminescent Material, J. Am. Chem. Soc., 2020, 142, 373–381 CrossRef CAS PubMed.
- M.-M. Zhang, X.-Y. Dong, Z.-Y. Wang, H.-Y. Li, S.-J. Li, X. Zhao and S.-Q. Zang, AIE Triggers the Circularly Polarized Luminescence of Atomically Precise Enantiomeric Copper(I) Alkynyl Clusters, Angew. Chem., Int. Ed., 2020, 59, 10052–10058 CrossRef CAS PubMed.
- H. Li, H. Zhai, C. Zhou, Y. Song, F. Ke, W. W. Xu and M. Zhu, Atomically Precise Copper Cluster with Intensely Near-Infrared Luminescence and Its Mechanism, J. Phys. Chem. Lett., 2020, 11, 4891–4896 CrossRef CAS PubMed.
- C. Dutta, S. Maniappan and J. Kumar, Delayed luminescence guided enhanced circularly polarized emission in atomically precise copper nanoclusters, Chem. Sci., 2023, 14, 5593–5601 RSC.
- T. Jia, Z.-J. Guan, C. Zhang, X.-Z. Zhu, Y.-X. Chen, Q. Zhang, Y. Yang and D. Sun, Eight-Electron Superatomic Cu31 Nanocluster with Chiral Kernel and NIR-II Emission, J. Am. Chem. Soc., 2023, 145, 10355–10363 CrossRef CAS PubMed.
- W.-Y. Lo, C.-H. Lam, V. W.-W. Yam, N. Zhu, K.-K. Cheung, S. Fathallah, S. Messaoudi, B. Le Guennic, S. Kahlal and J.-F. Halet, Synthesis, Photophysics, Electrochemistry, Theoretical, and Transient Absorption Studies of Luminescent Copper(I) and Silver(I) Diynyl Complexes. X-ray Crystal Structures of [Cu3(μ-dppm)3(μ3-η1-C⋮CC⋮CPh)2]PF6 and [Cu3(μ-dppm)3(μ3-η1-C⋮CC⋮CH)2]PF6, J. Am. Chem. Soc., 2004, 126, 7300–7310 CrossRef CAS PubMed.
- Y.-J. Li, Z.-Y. Deng, X.-F. Xu, H.-B. Wu, Z.-X. Cao and Q.-M. Wang, Methanol triggered ligand flip isomerization in a binuclear copper(I) complex and the luminescence response, Chem. Commun., 2011, 47, 9179–9181 RSC.
- J. Nitsch, F. Lacemon, A. Lorbach, A. Eichhorn, F. Cisnetti and A. Steffen, Cuprophilic interactions in highly luminescent dicopper(I)–NHC–picolyl complexes – fast phosphorescence or TADF?, Chem. Commun., 2016, 52, 2932–2935 RSC.
- B. Hupp, J. Nitsch, T. Schmitt, R. Bertermann, K. Edkins, F. Hirsch, I. Fischer, M. Auth, A. Sperlich and A. Steffen, Stimulus-Triggered Formation of an Anion–Cation Exciplex in Copper(I) Complexes as a Mechanism for Mechanochromic Phosphorescence, Angew. Chem., Int. Ed., 2018, 57, 13671–13675 CrossRef CAS PubMed.
- J.-P. Xu, W. Zou, S.-Z. Zhan, J. Zheng, K. Wu, G.-H. Zhang, J.-H. Li, M. Li, G.-H. Ning and D. Li, Visible-light excited luminescent trigonal prismatic metallocages from a template-directed assembly, Inorg. Chem. Front., 2021, 8, 3222–3229 RSC.
- L. L.-M. Zhang, G. Zhou, G. Zhou, H.-K. Lee, N. Zhao, O. V. Prezhdo and T. C. W. Mak, Core-dependent properties of copper nanoclusters: valence-pure nanoclusters as NIR TADF emitters and mixed-valence ones as semiconductors, Chem. Sci., 2019, 10, 10122–10128 RSC.
- H. Shen, S. Xiang, Z. Xu, C. Liu, X. Li, C. Sun, S. Lin, B. K. Teo and N. Zheng, Superatomic Au13 clusters ligated by different N-heterocyclic carbenes and their ligand-dependent catalysis, photoluminescence, and proton sensitivity, Nano Res., 2020, 13, 1908–1911 CrossRef CAS.
- H. Yi, K. M. Osten, T. I. Levchenko, A. J. Veinot, Y. Aramaki, T. Ooi, M. Nambo and C. M. Crudden, Synthesis and enantioseparation of chiral Au13 nanoclusters protected by bis-N-heterocyclic carbene ligands, Chem. Sci., 2021, 12, 10436–10440 RSC.
- H. Shen, Z. Xu, M. S. A. Hazer, Q. Wu, J. Peng, R. Qin, S. Malola, B. K. Teo, H. Häkkinen and N. Zheng, Surface Coordination of Multiple Ligands Endows N-Heterocyclic Carbene-Stabilized Gold Nanoclusters with High Robustness and Surface Reactivity, Angew. Chem., Int. Ed., 2021, 60, 3752–3758 CrossRef CAS PubMed.
- P. Luo, S. Bai, X. Wang, J. Zhao, Z.-N. Yan, Y.-F. Han, S.-Q. Zang and T. C. W. Mak, Tuning the Magic Sizes and Optical Properties of Atomically Precise Bidentate N-Heterocyclic Carbene-Protected Gold Nanoclusters via Subtle Change of N-Substituents, Adv. Opt. Mater., 2021, 9, 2001936 CrossRef CAS.
- A. Makarem, R. Berg, F. Rominger and B. F. Straub, A Fluxional Copper Acetylide Cluster in CuAAC Catalysis, Angew. Chem., Int. Ed., 2015, 54, 7431–7435 CrossRef CAS PubMed.
- H. Shen, L. Wang, O. López-Estrada, C. Hu, Q. Wu, D. Cao, S. Malola, B. K. Teo, H. Häkkinen and N. Zheng, Copper-hydride nanoclusters with enhanced stability by N-heterocyclic carbenes, Nano Res., 2021, 14, 3303–3308 CrossRef CAS.
- J. C. C. Chen and I. J. B. Lin, Palladium Complexes Containing a Hemilabile Pyridylcarbene Ligand, Organometallics, 2000, 19, 5113–5121 CrossRef CAS.
- J. Agarwal, T. W. Shaw, C. J. Stanton Iii, G. F. Majetich, A. B. Bocarsly and H. F. Schaefer Iii, NHC-Containing Manganese(I) Electrocatalysts for the Two-Electron Reduction of CO2, Angew. Chem., Int. Ed., 2014, 53, 5152–5155 CrossRef CAS PubMed.
- I. I. E. Markovits, M. H. Anthofer, H. Kolding, M. Cokoja, A. Pöthig, A. Raba, W. A. Herrmann, R. Fehrmann and F. E. Kühn, Efficient epoxidation of propene using molecular catalysts, Catal. Sci. Technol., 2014, 4, 3845–3849 RSC.
- M. J. Leitl, F.-R. Küchle, H. A. Mayer, L. Wesemann and H. Yersin, Brightly Blue and Green Emitting Cu(I) Dimers for Singlet Harvesting in OLEDs, J. Phys. Chem. A., 2013, 117, 11823–11836 CrossRef CAS PubMed.
- T. Hofbeck, U. Monkowius and H. Yersin, Highly Efficient Luminescence of Cu(I) Compounds: Thermally Activated Delayed Fluorescence Combined with Short-Lived Phosphorescence, J. Am. Chem. Soc., 2015, 137, 399–404 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Inc, Wallingford CT, 2009 Search PubMed.
- C. Adamo and V. Barone, Toward reliable density functional methods without adjustable parameters: The PBE0 model, J. Chem. Phys., 1999, 110, 6158–6170 CrossRef CAS.
- L. E. Roy, P. J. Hay and R. L. Martin, Revised basis sets for the LANL effective core potentials, J. Chem. Theory Comput., 2008, 4, 1029–1031 CrossRef CAS PubMed.
- P. C. Hariharan and J. A. Pople, The influence of polarization functions on molecular orbital hydrogenation energies, Theor. Chim. Acta, 1973, 28, 213–222 CrossRef CAS.
- B. Liu, Y. Zhang, D. Xu and W. Chen, Facile synthesis of metal N-heterocyclic carbene complexes, Chem. Commun., 2011, 47, 2883–2885 RSC.
- T. Jia, Y.-X. Li, X.-H. Ma, M.-M. Zhang, X.-Y. Dong, J. Ai and S.-Q. Zang, Atomically precise ultrasmall copper cluster for room-temperature highly regioselective dehydrogenative coupling, Nat. Commun., 2023, 14, 6877 CrossRef CAS PubMed.
- Z.-R. Yuan, Z. Wang, B.-L. Han, C.-K. Zhang, S.-S. Zhang, Z.-Y. Zhu, J.-H. Yu, T.-D. Li, Y.-Z. Li, C.-H. Tung and D. Sun, Ag22 Nanoclusters with Thermally Activated Delayed Fluorescence Protected by Ag/Cyanurate/Phosphine Metallamacrocyclic Monolayers through In-Situ Ligand Transesterification, Angew. Chem., Int. Ed., 2022, 61, e202211628 CrossRef CAS PubMed.
- X.-S. Han, X. Luan, H.-F. Su, J.-J. Li, S.-F. Yuan, Z. Lei, Y. Pei and Q.-M. Wang, Structure Determination of Alkynyl-Protected Gold Nanocluster Au22(tBuC
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)18 and Its Thermochromic Luminescence, Angew. Chem., Int. Ed., 2020, 59, 2309–2312 CrossRef CAS PubMed.
C)18 and Its Thermochromic Luminescence, Angew. Chem., Int. Ed., 2020, 59, 2309–2312 CrossRef CAS PubMed. - R. Czerwieniec, M. J. Leitl, H. H. H. Homeier and H. Yersin, Cu(I) complexes–Thermally activated delayed fluorescence. Photophysical approach and material design, Coord. Chem. Rev., 2016, 325, 2–28 CrossRef CAS.
- A. Schinabeck, N. Rau, M. Klein, J. Sundermeyer and H. Yersin, Deep blue emitting Cu(i) tripod complexes. Design of high quantum yield materials showing TADF-assisted phosphorescence, Dalton Trans., 2018, 47, 17067–17076 RSC.
- P. K. Samanta, D. Kim, V. Coropceanu and J.-L. Brédas, Up-Conversion Intersystem Crossing Rates in Organic Emitters for Thermally Activated Delayed Fluorescence: Impact of the Nature of Singlet vs Triplet Excited States, J. Am. Chem. Soc., 2017, 139, 4042–4051 CrossRef CAS PubMed.
- S. Fraga, J. Karwowski and K. M. S. Saxena, Handbook of Atomic Data, Elsevier, 1976 Search PubMed.
- H. Yersin, A. F. Rausch, R. Czerwieniec, T. Hofbeck and T. Fischer, The triplet state of organo-transition metal compounds. Triplet harvesting and singlet harvesting for efficient OLEDs, Coord. Chem. Rev., 2011, 255, 2622–2652 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional figures and tables. CCDC 2308966 for Cu9-Ph and 2308967 for Cu9-TBA. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qi00160e |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2024 |