Open Access Article
Open Access ArticleHeliphyrin: a ring open porphyrinoid with helical chirality†
Briana R.
Schrage
 a,
Sanjay
Gaire
a,
Sanjay
Gaire
 a,
Kirsty
Mamattah
a,
Dustin E.
Nevonen
a,
Kirsty
Mamattah
a,
Dustin E.
Nevonen
 b,
Victor N.
Nemykin
b,
Victor N.
Nemykin
 b and
Christopher J.
Ziegler
b and
Christopher J.
Ziegler
 *a
*a
aDepartment of Chemistry, The University of Akron, Akron, Ohio 44312-3601, USA. E-mail: ziegler@uakron.edu
bDepartment of Chemistry, University of Tennessee – Knoxville, Knoxville, TN 37996-1600, USA
First published on 23rd May 2024
Abstract
Heliphyrin, a new porphyrinoid with a helicene-type structure, can be produced in two steps from commercially available reagents. Three macrocycle–like structures were produced via a template reaction involving a benzimidazolylylidene modified iminoisoindoline and divalent metal (Co, Ni, and Cu) salts. The heliphyrins are tetradentate, dianionic ligands that coordinate the central metal in a square planar configuration, while forming a helical structure around the metal due to the steric bulk of the benzimidazole units. These compounds are panchromatic chromophores, and we interrogated their electronic structures using DFT and TDDFT methods. We could also separate racemic heliphyrins into enantiomers using chiral chromatography, and axial ligation by tartaric acid induces formation of chiral species as detected by circular dichroism spectroscopy. Due to their chiral nature, these complexes may have uses in biological and sensor applications.
Normal porphyrins and phthalocyanines are highly symmetric aromatic macrocycles which typically do not exhibit chirality. Optical activity in porphyrinoids can be induced by incorporating chiral substituents or via functionalization at the periphery.1–4 Alternatively, porphyrin assemblies have also been shown to exhibit optical activities.4–6 To make the core ring itself chiral, one needs to break the symmetric reflection planes that lie in and orthogonal to the macrocycle. In this report, we present a new porphyrinoid where the reflection plane symmetry is eliminated due to its open ring structure which adopts a helical structure around a central metal atom. The helicity in these porphyrinoids is similar to that seen in the well-studied helicene class of compounds,7–10 and so we have named this new class of porphyrin inspired chelate as the heliphyrins (Hlp). The heliphyrins can be synthesized in two steps from commercially available reagents. We have produced cobalt, nickel, and copper metalated heliphyrins, and we can separate the enantiomers by chiral chromatography.
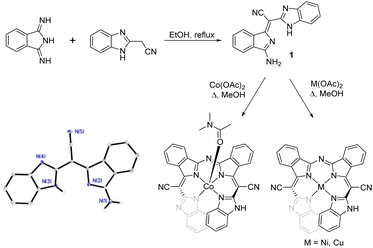
Over the past few years, we have been exploring the reactivity of 1,3-diiminoisoindoline (DII) with organic acids that possess a sp3 hybridized carbon atom with a pKa < 20.11–13 These organic acids can substitute the imine groups in DII with carbon–carbon double bonds to form ylidenes. Frequently, bis(ylidene)isoindolines are formed, but the reaction of equivalent amounts of 2-(benzimidazolyl)acetonitrile with DII produces a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct (compound 1, BzIm-DII) where just one of the two imine groups on the isoindoline is replaced with a carbon–carbon double bond (Fig. 1). We have fully characterized this compound, including via X-ray crystallography, and BzIm-DII is a planar bidentate chelate that can be considered as a meso carbon variant of the semihemiporphyrazine ligand. This compound also exhibits visible absorption bands that are similar to those observed in ylidene-modified isoindolines.13 Additionally, the structure of BzIm-DII is analogous to that of the pyrazole and indazole substituted DIIs which are precursors to the biliazine class of phthalocyanines analogues.14–16 We observe conjugation between both heterocycles in the BzIm-DII chelate as measured by the C–C bond distances about the cyano-modified sp2 hybridized carbon position (∼1.37 and ∼1.45 Å) as well as in the UV-visible spectrum (Fig. S15†), which shows a maxima at 438 nm.
1 adduct (compound 1, BzIm-DII) where just one of the two imine groups on the isoindoline is replaced with a carbon–carbon double bond (Fig. 1). We have fully characterized this compound, including via X-ray crystallography, and BzIm-DII is a planar bidentate chelate that can be considered as a meso carbon variant of the semihemiporphyrazine ligand. This compound also exhibits visible absorption bands that are similar to those observed in ylidene-modified isoindolines.13 Additionally, the structure of BzIm-DII is analogous to that of the pyrazole and indazole substituted DIIs which are precursors to the biliazine class of phthalocyanines analogues.14–16 We observe conjugation between both heterocycles in the BzIm-DII chelate as measured by the C–C bond distances about the cyano-modified sp2 hybridized carbon position (∼1.37 and ∼1.45 Å) as well as in the UV-visible spectrum (Fig. S15†), which shows a maxima at 438 nm.
When reacted with the transition metal ions Co(II), Ni(II), and Cu(II), two equivalents of BzIm-DII template at the metal centres to form an open porphyrinoid structure. The templating reactions are shown in Fig. 1, and the structures of the resultant metal adducts (Co(Hlp)(DMF)2, Ni(Hlp), and Cu(Hlp)) are shown in Fig. 2. These compounds are sparingly soluble in most organic solvents but can be dissolved in DMF, pyridine, and DMSO. The heliphyrins have a single meso nitrogen atom linking the two isoindoline units, and there are two nitrile modified meso carbon atoms connecting the benzimidazole groups to each isoindoline. Heliphyrin acts as a tetradentate ligand, binding each metal in a distorted square planar geometry, and the ligands act as dianions with the metals in the +2 oxidation state. In the Ni and Cu complexes, the metals are four coordinate overall, but in the Co complex there is an equivalent of solvent dimethylformamide present in the axial position. The M–N bonds around the metal are nearly equivalent in length, unlike that seen in the traditional hemiporphyrazine macrocycles, which exhibit asymmetric lengths about metal centres.17–20 In all three compounds, the M–N bond distances are relatively short as well, with all below 2.0 Å in length.
As in the biliazine porphyrinoid, the M(Hlp) macrocycles are not closed by a covalent bond. As a result, the steric bulk of the benzene ring of benzimidazoles are forced to adopt a slipped stack configuration. These open porphyrinoids thus adopt a helical configuration, as shown in the Fig. 2. Since the helicity is induced upon template reaction, the metal complexes of the heliphyrins are isolated as racemic mixtures. This can be seen in the crystal structures of the three metal complexes presented herein, as all the space groups have inversion centre symmetry. The spacing between the overlapping benzimidazole units, as measured by the distance between the 4 carbon positions, ranges between 3.4 and 3.6 Å, which also represents the rise in the helix for the single turn. Due to the limited size of the benzimidazole unit, the overlap occurs only at the 4 and 5 carbon atom positions on each terminal heterocycle. Previously, ring open hemiporphyrazines have been shown to adopt helical structures, but these ligand systems do not exhibit cross conjugation as observed in the heliphyrins.21,22 In addition to the similarities to the helicenes, this helicity has parallels to the chemistry of the bilinones and bilindiones.23–27 These open ring systems were produced as models for the metabolic byproducts of heme degradation, such as biliverdin. When coordinated to metals, the terminal carbonyls of the cleaved porphyrin ring create steric hindrance that forces the bilinones and bilindiones to adopt helical configurations.
The heliphyrins are strongly absorbing chromophores, with intense absorption bands across the enitre UV-visible spectrum (Fig. 3). Like many of ylidene-modified isoindolines,11,13,16 the precursor BzIm-DII chelate is a chromophore itself. Upon the template formation of the heliphyrins, the absorption bands extend through the visible out to the near-IR region. In the heliphyrins, the extinction coefficients are relatively large, on the order of 104 M−1 cm−1, although they are not as intense as in normal porphyrins due to the lack of ring closure to form an 18 electron annulene-type aromatic ring. Notably, the bands in all the heliphyrin complexes are very broad, such that we observe continuous absorbance from ∼300 nm to 750 nm, which includes the entire visible region. Thus, the heliphyrins are black in colour, and we can characterize this behaviour as panchromatic. In this regard, they are potentially useful for their light harvesting properties. As part of our characterization of these compounds, we investigated their cyclic voltammetry. We observed partially reductions of the ligand around −1 V as well as some metal-based redox events for the Co(Hlp) and Cu(Hlp) variants (Fig. S9–S11†).
In order to interrogate the optical properties of the biliazines, we carried out DFT and TDDFT calculations on Co(Hlp)(DMF)2, Ni(Hlp), and Cu(Hlp). The energies of the frontier orbitals for the heliphyrin complexes and compound 1 are shown in Fig. S13 and S14† along with the structures of the HOMO and LUMO orbitals in Fig. S12.† The frontier orbitals are predominantly π in character. Additionally, the LUMOs are relatively energetically isolated from the LUMO+n orbitals in these complexes. The TD-DFT calculations, shown in Fig. 3, are in good agreement with the experimental data (taking into consideration the presence of vibronic 0-n transitions associated with all major bands in the experimental spectra). Similar vibronic satellites can be seen in other isoindoline based chromophores.11–14 The lowest energy band observed around 700 nm in all complexes was predicted to be dominated by the HOMO → LUMO single-electron transition.
To isolate the different enantiomers of heliphyrin, we employed chiral chromatography. Using an HPLC equipped with a 5 µm CHIRALPAK IE (Daicel) column, we were able to separate the two enantiomers for all three products. Fig. 4 shows the separation between the two enantiomers of Ni(Hlp). However, due to the overall lack of overlap between the benzimidazole units and from the flexibility in the metal coordination environments, we observe re-isomerization in solution relatively quickly (<1 hour). However, we could drive the equilibrium toward the formation of a single enantiomer by axial ligation. Fig. 4 also shows the CD spectra of Co(Hlp)(DMF) in the presence of D- and L-tartaric acid. CD spectra of these enantiomers are clear mirror images of each other. In contrast, the Ni(Hlp) variant shows no such induction (based on CD spectroscopy data). Square planar Ni(II) complexes, unlike Co(II) analogues, do not readily bind ligands at their axial positions (see ESI† for more details) and thus the presence of the racemic mixture is still experimentally observed. We have carried out calculations on the axial induction of chirality in Co(II) complexes coordinated with one and three tartaric acids and observe good agreement with experimental data (Fig. S16†). Notably, the bilinones and bilindiones also exhibit induction of chirality by use of point modification, allosteric interactions, or solvent effects.25–27
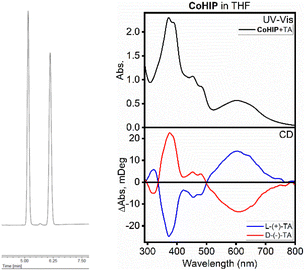 | ||
| Fig. 4 Chiral HPLC separation of the two enantiomers of Ni(Hlp) (left) and the CD spectra of Co(Hlp) in THF in the presence of D and L tartaric acid. | ||
In conclusion, we have synthesized a new ring open porphyrinoid using a simple metal mediated template reaction. The chelate precursor can be readily generated from commercially available reagents. The cobalt, nickel, and copper adducts of heliphyrin exhibit a helical structure similar to that seen in the helicenes. These ligands are fully conjugated and absorb light across the UV-visible spectrum so they can be considered panchromatic in character. The template synthesis reactions of the M(Hlp) compounds produce a racemic mixtures, and although enantiomers can be separated, they are only transiently stable to equilibration. However, axial binding by the chiral ligands D- or L- tartaric acid can be used to drive the equilibrium to a single enantiomer. We are continuing our work on developing new porphyrinoids with a focus on isoindoline chemistry as a starting point.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Generous support from the University of Akron, NSF (CHE-2153081), and the University of Tennessee to V. N. is greatly appreciated.References
- H. Lu and N. Kobayashi, Optically Active Porphyrin and Phthalocyanine Systems, Chem. Rev., 2016, 116, 6184–6261 CrossRef CAS PubMed
.
- G. Travagliante, M. Gaeta, R. Purrello and A. D'Urso, Recognition and Sensing of Chiral Organic Molecules by Chiral Porphyrinoids: A Review, Chemosensors, 2021, 9, 204 CrossRef CAS
.
- J. T. Groves and R. S. Myers, Catalytic asymmetric Epoxidations with Chiral Iron Porphyrins, J. Am. Chem. Soc., 1983, 105, 5791–5796 CrossRef CAS
.
- V. Borovkov, Supramolecular Chirality in Porphyrin Chemistry, Symmetry, 2014, 6, 256–294 CrossRef
.
- J. Ouyang, A. Swartjes, M. Geerts, P. J. Gilissen, D. Wang, P. C. P. Teeuwen, P. Tinnemans, N. Vanthuyne, S. Chentouf, F. P. J. T. Rutjes, J. V. Naubron, J. Crassous, J. A. A. W. Elemans and R. J. M. Nolte, Absolute Configuration and Host-Guest Binding of Chiral Porphyrin-Cages by a Combined Chiroptical and Theoretical Approach, Nat. Commun., 2020, 11, 4776C CrossRef PubMed
.
- C. Maeda, K. Ogawa, K. Sadanaga, K. Takaishi and T. Ema, Chiroptical and Catalytic Properties of Doubly Binaphthyl-Strapped Chiral Porphyrins, Chem. Commun., 2019, 55, 1064–1067 RSC
.
- R. H. Martin, The Helicenes, Angew. Chem., Int. Ed. Engl., 1974, 13, 649–660 CrossRef
.
- M. Gingras, One Hundred Years of Helicene Chemistry. Part 1, Chem. Soc. Rev., 2013, 42, 968–1006 RSC
.
- M. Gingras, G. Félix and R. Peresutti, One Hundred Years of Helicene Chemistry. Part 2, Chem. Soc. Rev., 2013, 42, 1007–1050 RSC
.
- G. Gingras, One Hundred Years of Helicene Chemistry. Part 3, Chem. Soc. Rev., 2013, 42, 1051–1095 RSC
.
- S. Gaire, B. R. Schrage, V. N. Nemykin and C. J. Ziegler, An Isoindoline Chelate with a Stretched Electronic Structure, Dalton Trans., 2021, 50, 826–829 RSC
.
- B. R. Schrage, C. A. Farmer, V. N. Nemykin and C. J. Ziegler, The Synthesis and Characterization of Ylideneisoindolinones, J. Porphyrins phthalocyanines, 2021, 25, 1048–1054 CrossRef CAS
.
- Y. V. Zatsikha, B. R. Schrage, J. Meyer, V. N. Nemykin and C. J. Ziegler, 1,3-Diylideneisoindolines: Synthesis, Structure, Redox, and Optical Properties, J. Org. Chem., 2019, 84, 6217–6222 CrossRef CAS PubMed
.
- B. R. Schrage, V. N. Nemykin and C. J. Ziegler, Biliazine: A Ring Open Phthalocyanine Analog with a Meso Hydrogen Bond, Chem. Commun., 2020, 56, 6628–6631 RSC
.
- B. R. Schrage, V. N. Nemykin and C. J. Ziegler, Subbiliazine: A Contracted Phthalocyanine Analog, Org. Lett., 2021, 23, 1076–1080 CrossRef CAS PubMed
.
- B. R. Schrage, V. N. Nemykin and C. J. Ziegler, BOSHPY Fluorophores: BODIPY Analogues with Single Atom Controlled Aggregation, Org. Lett., 2021, 23, 5246–5250 CrossRef CAS PubMed
.
- D. Attanasio, I. Collamati and E. Cervone, Inorg. Chem., 1983, 22, 3281–3287 CrossRef CAS
.
- I. Collamati, E. Cervone and R. Scoccia, Synthesis, Characterization, and Spectroscopic Studies of Some Metal Derivatives of Hemiporphyrazine, Inorg. Chim. Acta, 1985, 98, 11–17 CrossRef CAS
.
- S. M. Huber, G. Mata, A. Linden and N. W. Luedtke, Synthesis and Structure of a Hydrogenated Zinc Hemiporphyrazine, Chem. Commun., 2013, 49, 4280–4282 RSC
.
- S. Sripothongnak, A. M. Pischera, M. P. Espe, W. S. Durfee and C. J. Ziegler, Synthesis and Characterization of Lithium Hemiporphyrazines, Inorg. Chem., 2009, 48, 1293–1300 CrossRef CAS PubMed
.
- Y. Tanaka, T. Murayama, A. Muranaka, E. Imai and M. Uchiyama, Ring-Opened Hemiporphyrazines: Helical Molecules Exhibiting Circularly Polarized Luminescence, Chem. – Eur. J., 2020, 26, 1768–1771 CrossRef CAS PubMed
.
- Y. Tanaka, X. Ouyang, T. Murayama, A. Muranaka and M. Uchiyama, Formation of a Dinuclear Zinc Complex with Open-Ring Hemiporphyrazine, J. Porphyrins Phthalocyanines, 2023, 27, 184–189 CrossRef CAS
.
- J. F. Bonfiglio, R. Bonnett, M. B. Hursthouse and K. M. A. Malik, Crystal Structure of the Nickel Complex of 2,3,7,8,12,13,17,18-Octaethyl-1,19(21H,24H)-Bilindione (Octaethylbilatriene-abc), J. Chem. Soc., Chem. Commun., 1977, 83–84 RSC
.
- A. L. Bach, M. Mazzanti, B. C. Noll and M. M. Olmstead, Coordination Patterns for Biliverdin-Type Ligands. Helical and Linked Helical Units in Four-Coordinate Cobalt and Five-Coordinate Manganese(III) Complexes of Octaethylbilin,dione, J. Am. Chem. Soc., 1994, 116, 9114–9122 CrossRef
.
- T. Mizutani, S. Yagi, T. Morinaga, T. Nomura, T. Takagishi, S. Kitagawa and H. Ogoshi, Helical Chirality Induction by Point Chirality at Helix Terminal, J. Am. Chem. Soc., 1999, 121, 754–759 CrossRef CAS
.
- T. Mizutani, N. Sakai, S. Yagi, T. Takagishi, S. Kitagawa and H. Ogoshi, Allosteric Chirality Amplification in Zinc Bilinone Dimer, J. Am. Chem. Soc., 2000, 122, 748–749 CrossRef CAS
.
- S. Yagi, T. Morinaga, T. Nomura, T. Takagishi, T. Mizutani, S. Kitagawa and H. Ogoshi, Solvent Effect on Helicity Induction of Zinc Bilinone Bearing a Chiral Auxiliary at the Helix Terminal, J. Org. Chem., 2001, 66, 3848–3853 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, spectroscopic data, DFT data, and X-ray parameters. CCDC 2330320–2330323. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qi00743c |
| This journal is © the Partner Organisations 2024 |