Blue light-excited broadband NIR-II-emitting Li2ZnSn3O8:Cr3+,Ni2+ phosphor for multifunctional optical applications†
Zhexuan
Gao
a,
Yi
Zhang
a,
Yinyan
Li
 a,
Shilong
Zhao
a,
Shilong
Zhao
 a,
Peng
Zhang
a,
Xiaolong
Dong
b,
Degang
Deng
a,
Peng
Zhang
a,
Xiaolong
Dong
b,
Degang
Deng
 *a and
Shiqing
Xu
*a
*a and
Shiqing
Xu
*a
aKey Laboratory of Rare Earth Optoelectronic Materials and Devices of Zhejiang Province, Institute of Optoelectronic Materials and Devices, China Jiliang University, Hangzhou 310018, China. E-mail: dengdegang@cjlu.edu.cn; Fax: +86-571-86835781; Tel: +86-571-86835781
bFujian Institute of Metrology, Fuzhou,350003, China
First published on 31st May 2024
Abstract
The second near-infrared window (NIR-II, 1000–1700 nm) emitting phosphor is a key component for the phosphor-converted light-emitting diode (pc-LED), which has garnered significant attention. However, it remains a major challenge to discover efficient NIR-II broadband phosphors that can be excited by blue LEDs. Herein, Cr3+ and Ni2+ ions were co-doped into the Li2ZnSn3O8 (LZSO) host. LZSO:Cr3+,Ni2+ phosphor achieves a broadband NIR-II emission peak at 1465 nm with a large full width at half maximum (FWHM) of 300 nm and internal quantum efficiency (IQE) of 43.31% under excitation at 426 nm because of the efficient energy transfer (ET) from Cr3+ to Ni2+. In addition, a dual-emissive doped phosphor across the NIR-I (700–1000 nm) and NIR-II regions with a higher sensitivity at physiological temperatures was obtained. The relative sensitivity value (Sr) reaches 1.44% K−1 at 293 K, indicating that the LZSO:Cr3+,Ni2+ phosphor is a potential candidate for NIR optical thermometers in biotechnological applications. The manufactured NIR-II pc LED provides a radiative flux of 8.26 mW with a photoelectric conversion efficiency of 2.71% at a drive current of 100 mA and has promising potential in night vision, medical imaging, and spectral analysis applications.
1 Introduction
NIR light sources have various applications in non-invasive biological imaging, optical communication, health monitoring, and night vision.1–4 The pc-LED based on energy down-conversion technology serves as a novel type of NIR light source, offering advantages such as low cost, high efficiency, portability, and long lifetime, which is also known as a replacement for traditional NIR light sources such as incandescent lamps, tungsten halogen lamps, and NIR LEDs.5,6 A reliable method to obtain this NIR light source is by combining NIR phosphor with inexpensive high-efficiency blue light chips. The NIR phosphor determines the characteristics of NIR pc-LED, including the emission peak, FWHM, and luminous efficiency. Typically, the emission spectra of NIR phosphors are located in NIR-I and NIR-II regions.7,8 In comparison to NIR-I, NIR-II has less background interference, less tissue scattering, deeper tissue penetration depth, and high imaging contrast.9,10 In addition, dipole moments and bond anharmonicities play a significant role in the typical NIR absorptions that arise from overtones and combinations of fundamental vibrations. However, the behavior of C–H, O–H, N–H, and S–H bonds varies in different substances and corresponds to significant absorptions mainly located in the broad wavelength range above 1000 nm.11–13 Therefore, NIR-II light is more suitable for achieving promising night vision, biomedical and spectral analysis applications.Cr3+ with a 3d3 electron configuration typically exhibits intrinsic broad emission when entering octahedral sites, which has attracted widespread attention.14–16 In recent years, a group of excellent NIR emission performance Cr3+-doped phosphors excited by blue light has been reported, such as Ba5La3MgAl3O15:Cr3+ (λem = 925 nm, IQE = 66%), Ga2GeO5:Cr3+ (λem = 923 nm, IQE = 71.25%), NaInSi2O6:Cr3+ (λem = 936 nm, IQE = 68.9%), and LiScGeO4:Cr3+(λem = 1120 nm, IQE = 26%).5,17–19 Nevertheless, the maximum peak position at around 1000 nm and the limited spectral bandwidth are insufficient to meet various practical application requirements. Co-doping of Yb3+ for ET from Cr3+ to increase spectral bandwidth and improve NIR emission performance is a good choice, such as KScP2O7:Cr3+,Yb3+, CaZnGe2O6:Cr3+,Yb3+, LiScP2O7:Cr3+,Yb3+, LiIn2SbO6:Cr3+,Yb3+, LiGaP2O7:Cr3+,Yb3+, Lu0.2Sc0.8BO3:Cr3+,Yb3+, and Y3Ga5O12:Cr3+,Yb3+.12,20–25 However, considering the emission characteristics of Yb3+ ions, their emission wavelength are still difficult to embrace the NIR-II region.26 Therefore, exploring novel infrared phosphors with efficient emission in the NIR-II region by doping with other optically active ions is necessary.
Ni2+-activated phosphors display broadband emission in the NIR-II range. Unfortunately, these phosphors are typically excited by UV light, which has drawbacks of weak blue light absorption and low efficiency (IQE, EQE < 10%).27–29 To address this issue, Cr3+ can be introduced as a sensitizer in the material system to effectively transfer energy to Ni2+. In consideration of the broad absorption band of Cr3+ in the blue region and the perfect overlap between the absorption of Ni2+ and the emission of Cr3+. Therefore, when Cr3+ and Ni2+ are simultaneously introduced into a suitable host, the emission in the NIR-II range of Ni2+ can be achieved through sensitization by Cr3+ excited by blue LEDs.30
Furthermore, the progress of dual-emissive materials for thermometers relies on visible light (VIS). The optical thermometer emission wavelength in the biological window (NIR-I, NIR-II) could make it suitable for applications such as biomedical thermal imaging or more sensitive temperature sensing for the development and application of biotechnology.31,32 However, the progress of thermometers based on dual-emissive materials that originate from NIR-I and NIR-II light has been minimal. Accordingly, developing dual-emissive doped phosphors that cover both NIR I and NIR II regions and have higher sensitivity at physiological temperatures remains a challenge.
Herein, we report a blue LED-excited and highly efficient NIR-II phosphor LZSO:Cr3+,Ni2+. The photoluminescence spectra, luminescence decay curves, ET efficiency, quantum efficiency, and thermal stability of LZSO:0.03Cr3+,0.03Ni2+ phosphor were investigated. A fabricated NIR-II pc-LED was applied in night vision, biomedical imaging, and spectroscopic analysis fields. Furthermore, the broadband NIR dual-emissive properties of LZSO:0.03Cr3+,0.03Ni2+ are noteworthy and provide insights for optical thermometers applicable in biotechnology.
2 Experimental
2.1 Materials and synthesis
Reagents: lithium carbonate (Li2CO3, 99.99%), zinc oxide (ZnO, 99.99%), stannic oxide (SnO2, 99.99%), chromium sesquioxide (Cr2O3, 99.95%), and niobium pentaoxide (NiO, 99.99%) were supplied by Aladdin Reagent Co., Ltd.Synthesis: stoichiometric raw materials were weighed based on stoichiometric ratios. The mixtures were combined completely homogeneous in an agate mortar. Subsequently, they were placed in a muffle furnace sintered at 1200 °C for 3 h, and the heating rate was maintained at 6.5 °C min−1, which contributes to a full reaction. Then, the samples were cooled naturally to room temperature and ground with the aim of subsequent testing.
2.2 Characterization
Power X-ray diffraction (XRD) patterns of the phosphor were acquired by employing Bruker Axs D2 PHASER diffractometer with Cu Kα radiation (λ = 0.15405 nm) at 30 kV and 10 mA. Rietveld refinement was performed using the software the General Structural Analysis System (GASA). The field-emission scanning electron microscopy (SEM) and energy-dispersive spectroscopy (EDS) were performed using the HITACHI, SU8010, instrument, Japan. Field-emission transmission electron microscopy (TEM) was conducted at an acceleration voltage of 200 kV (FEI, SU 8010, America). The diffuse reflectance spectra (DRS) of the phosphors were collected using an ultraviolet spectrophotometer (Agilent Cay 5000, America) with BaSO4 as a standard. The fluorescence decay curve was obtained using a 460 nm LED pulse Nd: YAG laser with a fl3-211 fluorescence spectrophotometer (HORIBA, JOBIN YVON, France). The photoluminescence (PL) and photoluminescence excitation (PLE) spectra were recorded on a modular fluorescence spectrometer (HORIBA, Quanta Master 8000, Canada). The corresponding temperature-dependence PL spectra were recorded on the same spectrophotometer equipped with a TAP-02 high-temperature fluorescence instrument (Tian Jin Orient – KOJI Instrument Co., Ltd). A quantum yield (QY) measurement system (Hamamatsu, Quantaurus-QY plus C13684-01) was used to measure the internal and external quantum efficiency (IQE, EQE); the electroluminescence (EL) spectra were collected using a FL3-211 fluorescence spectrophotometer (HORIBA, JOBIN YVON, France). An LED photoelectric test system (HAAS-2000, EVER FINE) was employed to analyze the performance of the fabricated LED. Visible and NIR-II photographs were captured by an industrial camera (EOS R50, Canon, China) and an NIR-II camera (Raptor Owl 640 S).2.3 Fabrication of the pc-LED device
The optimal LZSO:0.03Cr3+,0.03Ni2+ phosphor was used to package the NIR-II pc-LED, which was sufficiently mixed with glue A and glue B in conformity with the weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 on a blue chip (460 nm, 3 W) and then cured at 150 °C for 3 h.
1 on a blue chip (460 nm, 3 W) and then cured at 150 °C for 3 h.
3 Results and discussion
It is reported that LZSO in the orthorhombic system with space group Cmc21 (No. 36), as shown in Fig. 1a.33 Li+ ions are coordinated to six oxygen ions to form [Li1O6], [Li2O6] octahedra and four oxygen ions to form [Li3O6], [Li4O6] tetrahedra. Sn4+ ions have five different crystallographic sites coordinated with six oxygen ions to form five different kinds of SnO6 octahedra. While the Zn2+ ion is linked to four oxygen ions to form two [ZnO4] tetrahedra. These coordinated polyhedra are orderly connected to form the LZSO unit. Fig. 1b shows XRD patterns of the representative LZSO, LZSO:0.03Cr3+, LZSO:0.03Ni2+, and LZSO:0.03Cr3+,0.03Ni2+ samples, as well as the XRD patterns of LZSO:xCr3+ and LZSO:0.03Cr3+,yNi2+ (0.005 ≤ x ≤ 0.07, 0.003 ≤ y ≤ 0.07) phosphors (Fig. S1, ESI†). All diffraction peaks agree with the standard phase Li2ZnSn3O8 in the Inorganic Crystal Structure Database (ICSD: 59772), demonstrating that the introduction of Cr3+ and Ni2+ dopants does not generate impurity phases. Considering that the valence and ionic radius of Cr3+ (coordination number, CN = 6, r = 0.615 Å) are very close to those of Sn4+ (CN = 6, r = 0.69 Å), the Cr3+ dopant prefers to substitute the Sn4+ ions in the LZSO host. From the perspective of charge balance, the substitution of Ni2+ (CN = 6, r = 0.69 Å) for two different Li+ (CN = 6, r = 0.76 Å) can avoid imbalance. Detailed crystallographic information was obtained by Rietveld's refinement of the XRD pattern of the prepared sample. It can be seen from Fig. 1c that the refined result of LZSO with Rp = 5.85%, Rwp = 9.28%, χ2 = 1.584. The refinement result demonstrates reliable results and phase purity. The specific crystallographic data and the atomic positions of LZSO are listed in Tables S1 and S2† respectively. In addition, the morphology and elemental mapping distribution of the obtained phosphors were characterized from SEM images and EDS of LZSO:0.03Cr3+,0.03Ni2+ phosphors, as shown in Fig. 1d. The SEM image indicates the irregular morphology of the synthesized LZSO:0.03Cr3+,0.03Ni2+ phosphor. The smooth surface of the particles also indicates its high crystallinity. The EDS reveals the existence of Zn, Sn, O, and Cr, Ni elements in the LZSO:0.03Cr3+,0.03Ni2+ phosphor. The atomic percentages of Ni, Zn, Sn, Cr, and O in LZSO:0.03Cr3+,0.03Ni2+ were determined as 0.40%, 9.34%, 31.90%, 0.34% and 58.02% (Fig. S2†), respectively. However, the absence of Li is due to its low atomic number. The results approach an actual chemical formula of Li1.97Ni0.03ZnSn3Cr0.03O8 (the atomic percentages neglecting Li are 0.25%, 8.31%, 24.69%, 0.25%, and 66.5%, respectively). Furthermore, TEM and HR-TEM images of LZSO:0.03Cr3+,0.03Ni2+ are displayed in Fig. 1e. The lattice fringes are clearly visible, and an estimated crystal plane spacing of 0.52 nm matches well with the (020) plane. These results confirm the good crystallinity of the synthesized phosphors.The PL and PLE spectra of LZSO:0.03Cr3+, LZSO:0.03Ni2+, and LZSO:0.03Cr3+,0.03Ni2+ are presented in Fig. 2a. The broadband of LZSO:0.03Cr3+, which peaks at 959 nm and spans a range of 700 to 1300 nm with a FWHM of 176 nm, is caused by the spin-allowed 4T2 → 4A2 transition of Cr3+ ions when excited at 426 nm. Under the monitoring at 959 nm emission, the sample exhibits a wide excitation band from ultraviolet to red light, with the peak located in the blue light region, indicating that the LZSO:0.03Cr3+ phosphor can be effectively excited by the blue light chip. The excitation band centered at 288 nm could arise from charge transfer between the O ligand and the central Sn atom in the SnO6 octahedral group, while the excitation bands at 350, 426, 617 and 676 nm are ascribed to the 4A2 → 4T1 (4P), 4A2 → 4T1 (4F), 4A2 → 4T2 (4F) and 4A2 → 2E(2G) transitions of Cr3+ ions, respectively.34,35 For LZSO:0.03Ni2+ phosphor, a broad emission centered at 1465 nm ranging from 1200–1750 nm (FWHM = 300 nm) was observed under excitation at 400 nm, derived from the 3T2 → 3A2 transition of Ni2+ ions. At the same time, the sample has four peaks at 400, 426, 623, and 676 nm consisting of a continuous PLE spectrum monitored at 1465 nm, which originated from the 3A2 (3F) → 3T1 (3P), 3A2 (3F) → 1T2 (1D), 3A2 (3F) → 3T1 (3F) and 3A2(3F) → 1E(1D) transitions of Ni2+ ions, respectively.36 The LZSO:0.03Cr3+,0.03Ni2+ phosphor has two broad emission bands corresponding to the 4T2 → 4A2 transition of Cr3+ and the 3T2 → 3A2 transition of Ni2+ excited by 426 nm blue light. The PLE spectra of the LZSO:0.03Cr3+,0.03Ni2+ monitored at 959 and 1465 nm are similar to those of LZSO:0.03Cr3+, indicating the ET from Cr3+ to Ni2+. Compared to Ni2+ single-doped phosphor, which cannot be effectively excited by blue light irradiation, the co-doped Cr3+, Ni2+ phosphor greatly broadens the excitation spectral range, especially in the blue light region, solving the problem of inefficient excitation of Ni2+ activator by blue light.
The DRS of LZSO:0.03Cr3+, LZSO:0.03Ni2+, and LZSO:0.03Cr3+,0.03Ni2+ samples correspond well with their respective PLE spectra (Fig. 2b). Moreover, no characteristic absorption band of Cr4+ around 1000 nm was observed in the LZSO:0.03Cr3+ sample, indicating that the emission center is Cr3+ ions rather than Cr4+.37 Meanwhile, the PL spectrum of LZSO:0.03Cr3+ and the PLE spectrum of LZSO:0.03Ni2+ show a significant overlap, as shown in Fig. S3,† demonstrating that the ET between Cr3+ and Ni2+ is possible. The Tanabe–Sugano diagrams demonstrate the splitting degree of the Cr3+ and Ni2+ ions located in octahedral coordination are presented in Fig. S4.† The online calculation program developed by Song et al. was used to calculate the Dq/B values related to the matrix for the 3d3 electronic configuration of Cr3+.38 The calculated intersection points for the 2E and 4T2 levels were found to be at 1.94, with the sample's Dq/B value at 2.13, close to the intersection point value. The crystal field strength in octahedrally coordinated approximation of Ni2+ can be calculated using eqn (S1) and (S2).† Consequently, the Dq/B value for Ni2+ can be given as 1.09. This indicates that the host provides a relatively weak crystal field for Ni2+ (Dq/B < 1.8). When Ni2+ ions are in a weak octahedral crystal field, they usually exhibit a broad emission band.36
The PL spectra of LZSO:xCr3+ (x = 0.005–0.07) phosphors under 426 nm excitation are shown in Fig. 2c. The highest PL intensity was obtained at x = 0.03, as shown in the inset of Fig. 2c. As the possibility of nonradiative transitions between Cr3+ ions increases, quenching will occur beyond this concentration.39Fig. 2d presents the PL spectra of LZSO:0.03Cr3+,yNi2+ (y = 0.003–0.07) under 426 nm excitation. The inset illustrates the relative PL intensity of Cr3+ and Ni2+ in the LZSO:0.03Cr3+,yNi2+ system. The PL intensity of Cr3+ monotonically decreases with increasing Ni2+ concentration, while the emission intensity of Ni2+ is correspondingly enhanced and then declines. When y is 0.03, the PL intensity of Ni2+ is strongest owing to the concentration quenching effect. According to the PL intensity, the efficiency (ηET) from Cr3+ to Ni2+ is obtained:40
 | (1) |
 | (2) |
| I = I0 + A1exp(−t/τ1) + A2exp(−t/τ2) | (3) |
 | (4) |
 | (5) |
We also investigated the thermal properties of the LZSO:0.03Cr3+,0.03Ni2+ phosphor. Fig. 3a presents the temperature-dependent PL spectra of LZSO:0.03Cr3+,0.03Ni2+ upon 426 nm excitation. The emissions of both Cr3+ and Ni2+ decrease with increasing temperature. The relative PL intensity of Cr3+ can maintain 75.34% of its initial intensity at room temperature when the temperature is elevated from 303 to 373 K, while Ni2+ remains at 30.14% with increasing temperature. Temperature-dependent emission spectra and relative intensity of LZSO:Ni2+ are shown in Fig. S7.† The emission intensity of LZSO:Ni2+ is maintained at 27.63% at 373 K compared to that at 293 K. Thus, the thermal stability is improved with the introduction of Cr3+. Generally speaking, the thermal quenching of Cr3+ and Ni2+ can occur through non-radiative transitions stemming from the crossing of excited state and ground state parabolas in the configurational coordination model (Fig. S8†).47 The longer emission wavelength of the Ni2+ ion indicates a narrower band gap, which leads to a more severe thermal quenching effect.26 The FWHM values of Cr3+ and Ni2+ in Fig. S9† extend from 498 and 317 to 960 nm and 655 nm, respectively, resulting from the increased electron–phonon coupling at high temperatures.24 Meanwhile, the peak position of the PL spectra remains almost constant The emission-integrated intensity histogram of Cr3+ and Ni2+ ions is shown in Fig. 3c. The emissions of Cr3+ and Ni2+ ions exhibit different responses with increasing temperature in the range of 293–423 K, which especially shows significant changes in the physiological temperature range (293–323 K) and cover a biological window, indicating its potential application in biomedical research. In general, the fluorescence intensity ratio (FIR) represents the temperature more accurately. FIR is based on the integral intensity of each emission peak, which denotes the ratio of luminescence intensities between two different emission wavelengths under the same excitation wavelength.43,48 To study the temperature sensing performance, the following equation can represent the relationship between FIR (I959 nm/I1465 nm) and temperature:31
 | (6) |
 | ||
| Fig. 3 (a and b) Temperature dependence of the PL spectra of LZSO:0.03Cr3+,0.03Ni2+. The inset shows the intensity curves of different emission bands as a function of temperature. (c) Temperature-dependent histogram of the PL intensity for Cr3+ and Ni2+ of LZSO:0.03Cr3+,0.03Ni2+ (d) (I959 nm/I1465 nm) as functions of temperature. (e) Calculated Sa and Sr values. (f) Sr values at the physiological temperatures and NIR emission range of LZSO:Cr3+,Ni2+, compared to other NIR optical thermometers under blue light excited, such as LaZnGa11O9:Cr3+,Ni2+, α-Ga2O3:Cr3+, β-Ga2O3:Cr3+, Ba2SrSc4O9:Ce3+,Yb3+, YScGaSbO7:Cr3+, YAl3(BO3)O4:Cr3+, Mg2GeO4:Cr3+,Yb3+, BaIn2(P2O7)2:Cr3+, Sc2O3:Cr3+.2,31,42,50–53 | ||
Fig. 3d indicates the FIR datapoints fitted using eqn (6), which can be fitted by the  . The following equations outline the fitting curves for the optical thermometer's absolute sensitivity (Sa) and its relative sensitivity (Sr):49
. The following equations outline the fitting curves for the optical thermometer's absolute sensitivity (Sa) and its relative sensitivity (Sr):49
 | (7) |
 | (8) |
The values of Sa and Sr at different temperatures (298–423 K) are shown in Fig. 3e. Sa reaches its peak value of 0.34% K−1 at 423 K. The Sr value decreases from 1.44% K−1 to 1.08% K−1 as the physiological temperature range increases from 293 K to 323 K. Fig. 3f provides a performance comparison of LZSO:0.03Cr3+,0.03Ni2+ and a number of reported NIR optical thermometers at physiological temperatures. It is worth noting that LZSO:0.03Cr3+,0.03Ni2+ exhibits a high Sr value with an ultra-broadband spanning from 700 to 1750 nm, covering the NIR-I and NIR-II windows. As a consequence, these results demonstrate that the LZSO:0.03Cr3+,0.03Ni2+ system has great potential in the biological application field of optical thermometers.
Fig. 4a displays the EL spectra of the prepared NIR-II pc-LED under different driving currents. It is observed that all the EL spectra consist of a dominant NIR-II broadband NIR emission at ≈1465 nm and a weak blue emission ≈at 460 nm, indicating that LZSO:Cr3+,Ni2+ phosphors can effectively convert blue light into NIR light. In addition, when the driving current is increased from 20 to 200 mA, the PL intensity gradually increases. At the same time, the operating temperature of the NIR pc-LED device increased from 28 °C to 49 °C, as induced by the heat buildup that occurs with higher drive currents (Fig. 4b). Such a small temperature change is acceptable in applications. Fig. 4c shows the relationship between NIR-II output power and photoelectric conversion efficiency of the NIR-II pc-LED as a function of driving current. Moreover, with an increase in the drive current from 10 to 200 mA, the output power of the prepared NIR-II pc-LED escalates from 1.98 to 9.96 mW, while the photoelectric conversion efficiency shows the opposite trend from 4.43 to 1.81%. The primary cause of this phenomenon is the efficiency drop of the blue light chip.23 Under the illumination of the NIR-II pc-LED light source in darkness, the NIR-II camera is able to capture shapes and positions of fruits and toys, as well as finger veins (Fig. 4d). This fully demonstrates the potential applications of the prepared NIR-II pc-LED in night vision and medical imaging.
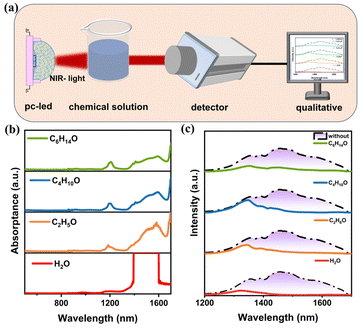
To further demonstrate that the fabricated NIR-II pc-LED can be applied to non-destructive testing, a spectral experimental setup is shown in Fig. 5a. Fig. 5b displays the absorption spectra of four frequently used organic solvents, which include H2O, C2H5OH, C4H10O, and C6H14O. Interestingly, NIR-II PL spectra can cover more information about the molecule and contribute to higher detection sensitivity compared to the NIR-I PL spectra. There are characteristic absorption bands toward the NIR-II region for four solvents, such as H2O (∼1390 nm), C2H5OH (∼1185 and ∼1585 nm), C4H10O (∼1195 and ∼1595 nm), C6H14O (∼1210 and ∼1595 nm). Chemical analysis was performed using the original PL spectrum of LZSO:0.03Cr3+,0.03Ni2+ and the PL spectrum of NIR light penetrating different solvents (Fig. 5c). Therefore, the setup can distinguish differences between solvents by detecting the PL intensity and peak position.
4 Conclusions
To summarize, we synthesized a novel Cr3+–Ni2+ co-doped LZSO phosphor using a conventional high-temperature solid-phase method. The prepared LZSO:Cr3+,Ni2+ phosphor utilizes the sensitization effect of Cr3+ ions on the Ni2+, achieving blue-light excitation and efficient broadband emission in the NIR-II region. The obtained LZSO:Cr3+,Ni2+ exhibited broadband emission peaking at 1465 nm, with a large FWHM of 300 nm and an IQE of 43.3% under 426 nm excitation. More intriguingly, using the prepared NIR-II pc-LED as the light source for night vision, medical imaging, and non-destructive analysis were realized. Furthermore, the phosphor has the characteristics of ultra-wideband emission with dual emission peaks in the NIR-I and NIR-II regions, as well as high sensitivity, which hold promise for the application of NIR thermometry in biotechnology. This research not only obtains a prospective phosphor for future NIR-II light sources but also highlights the vast application prospects of NIR technology.Author contributions
Zhexuan Gao: investigation, visualization, methodology, formal analysis, writing – original draft. Yi Zhang: supervision, formal analysis. Yinyan Li: investigation, validation. Peng Zhang: formal analysis. Xiaolong Dong: formal analysis. Shilong Zhao: investigation, conceptualization. Degang Deng: conceptualization, writing – review and editing, funding acquisition. Shiqing Xu: methodology, formal analysis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is supported by Zhejiang Provincial Natural Science Foundation of China (Grant No. LZ22F050002), National Key Research and Development Program of China (SQ2023YFE0201285), National Natural Science Foundation of China (62075205) and Zhejiang Provincial Key Research and Development Program (Grant No. 2021C01027, 2021C01024 and 2022C01133).References
- F. Zhao, Z. Song and Q. Liu, Advances in Chromium–Activated Phosphors for Near–Infrared Light Sources, Laser Photonics Rev., 2022, 16, 2200380 CrossRef CAS
.
- Q. Pang, Y. Wang, L. Yan, G. Zhu, S. Xu, J. Zhang, X. Zhang, Y. Cao and B. Chen, Cr3+-Cr3+ Ion Pair Induced Fast Energy Migration in Cr3+ Doped Na-β-Al2O3 Ultra-Wide Near-Infrared Phosphors for NIR Spectroscopy Application, Laser Photonics Rev., 2024, 18, 2301039 CrossRef CAS
.
- T. Yang, L. Chu, Y. Qin, Q. Zhou, J. Wan, H. Tang, Y. Ye and Z. Wang, An efficient and thermally stable source with Cr3+ near-infrared luminescence for non-destructive testing applications, Mater. Today Chem., 2024, 35, 101918 CrossRef CAS
.
- M. Zhao, S. Liu, H. Cai, F. Zhao, Z. Song and Q. Liu, Cr3+-Doped double perovskite antimonates: efficient and tunable phosphors from NIR-I to NIR-II, Inorg. Chem. Front., 2022, 9, 4602–4607 RSC
.
- M. Xu, Z. Li, Q. Li, T. Mou, X. Wang, D. Yan, Y. Li, X. Zhou, Z. Fang and L. Ning, Thermally Stable NIR Emission from Disordered Ba5La3MgAl3O15:Cr3+ with Peak Wavelength Beyond 900 nm, Adv. Opt. Mater., 2024, 2303144, DOI:10.1002/adom.202303144
.
- S. Wei, Z. Lyu, D. Sun, S. Shen, X. Zhang, Z. Lu, P. Luo, H. Hu and H. You, A NIR phosphor with ultra-broadband emission enabled by dual energy transfer within two Cr3+ emitters and Cr3+ → Yb3+, J. Mater. Chem. C, 2024, 12, 4977–4985 RSC
.
- X. Wang, Z. Wang, J. Cui, Y. Yao, M. Zheng, M. Zhang, L. Cao, Z. Yang, H. Suo and P. Li, Multifunctional Near-Infrared (NIR) Phosphors with NIR I and NIR II Luminescence for Biological Detection, ACS Appl. Electron. Mater., 2021, 4, 432–442 CrossRef
.
- A. Satpathy, W. T. Huang, M. H. Chan, T. Y. Su, M. Kamiński, N. Majewska, S. Mahlik, G. Leniec, S. M. Kaczmarek, M. Hsiao and R. S. Liu, Near–Infrared I/II Nanophosphors with Cr3+/Ni2+ Energy Transfer for Bioimaging, Adv. Opt. Mater., 2023, 11, 2300321 CrossRef CAS
.
- L. Jiang, L. Zhang, X. Jiang, J. Xie, G. Lv and Y. Su, Cr3+–Yb3+–Ni2+ Tri–Doped NIR Phosphors with Spectral Output Covering NIR–I and NIR–II Regions, Adv. Mater. Technol., 2024, 9, 2301495 CrossRef CAS
.
- B. M. Liu, X. X. Guo, L. Huang, R. F. Zhou, R. Zou, C. G. Ma and J. Wang, A Super–Broadband NIR Dual–Emitting Mg2SnO4:Cr3+,Ni2+ Phosphor for Ratiometric Phosphor–Converted NIR Light Source Applications, Adv. Mater. Technol., 2023, 8, 2201181 CrossRef CAS
.
- L. Yuan, Y. Jin, D. Zhu, Z. Mou, G. Xie and Y. Hu, Ni2+-Doped Yttrium Aluminum Gallium Garnet Phosphors: Bandgap Engineering for Broad-Band Wavelength-Tunable Shortwave-Infrared Long-Persistent Luminescence and Photochromism, ACS Sustainable Chem. Eng., 2020, 8, 6543–6550 CrossRef CAS
.
- M. Lin, Z. Gao, C. Xu, Y. Yuan, Y. Tang, T. Liu and L. Sun, Enhanced and Broadened NIR Emission of CaZnGe2O6:Cr3+ by Yb3+ Codoping for NIR Applications, ACS Appl. Opt. Mater., 2023, 1, 724–735 CrossRef CAS
.
- L. Jiang, L. Zhang, X. Zhao, X. Jiang, P. Gao and Y. Su, Awakening the Dumb Site to Realize Ultra–Broadband NIR Phosphor, Laser Photonics Rev., 2024, 18, 2301226 CrossRef CAS
.
- J. Wang, X. Han, Y. Zhou, Z. Wu, D. Liu, C. Zeng, S. Cao and B. Zou, Ion Substitution Strategy toward High-Efficiency Near-Infrared Photoluminescence of Cs2KIn1−yAlyF6:Cr3+ Solid Solutions, J. Phys. Chem. Lett., 2023, 14, 1371–1378 CrossRef CAS PubMed
.
- Q. Zhang, X. Wei, J. Zhou, B. Milićević, L. Lin, J. Huo, J. Li, H. Ni and Z. Xia, Thermal Stability Improvement of Cr3+–Activated Broadband Near–Infrared Phosphors via State Population Optimization, Adv. Opt. Mater., 2023, 11, 2300310 CrossRef CAS
.
- Z. Jia, C. Yuan, Y. Liu, X.-J. Wang, P. Sun, L. Wang, H. Jiang and J. Jiang, Strategies to approach high performance in Cr3+-doped phosphors for high-power NIR-LED light sources, Light: Sci. Appl., 2020, 9, 86 CrossRef CAS PubMed
.
- C.-Y. Peng, B. Wang, L.-F. Yuan, K.-G. Hu, G. Chen, H.-Y. Wu, Y.-H. Hu and Y.-H. Jin, Six- and five-coordinated Cr3+ in Ga2GeO5 invokes tunable broadband near-infrared emission toward night-vision applications, Rare Met., 2023, 42, 3787–3796 CrossRef CAS
.
- S. Chen, M. Han, J. Li, Y. Li, Z. Gao, Y. Zhang, M. Meng, Q. Zhang, D. Deng and L. Chen, Cr3+-activated NaInSi2O6: An efficient broadband near-infrared phosphor with its applications in LED, Ceram. Int., 2023, 49, 36360–36367 CrossRef CAS
.
- S. Miao, Y. Liang, Y. Zhang, D. Chen and X.-J. Wang, Broadband Short-Wave Infrared Light-Emitting Diodes Based on Cr3+-Doped LiScGeO4 Phosphor, ACS Appl. Mater. Interfaces, 2021, 13, 36011–36019 CrossRef CAS PubMed
.
- G.-H. Li, J.-B. Huang, Q.-H. Yang, J. Wang, G.-M. Cai and X.-J. Wang, Composition–structure–luminescence and enhancement of Cr3+ activated broadband near infrared phosphors for night vision, bio-imaging, and noninvasive detection, J. Mater. Chem. C, 2023, 11, 16578–16586 RSC
.
- L. Yao, Q. Shao, S. Han, C. Liang, J. He and J. Jiang, Enhancing Near-Infrared Photoluminescence Intensity and Spectral Properties in Yb3+ Codoped LiScP2O7:Cr3+, Chem. Mater., 2020, 32, 2430–2439 CrossRef CAS
.
- Z. Lu, S. Chen, Y. Liu, C. Yuan, R. Li, P. Sun, Z. Luo, Z. Liu and J. Jiang, LiGaP2O7:Cr3+, Yb3+ phosphors for broadband NIR LEDs toward multiple applications, J. Alloys Compd., 2023, 956, 170311 CrossRef CAS
.
- G. Liu, T. Hu, M. S. Molokeev and Z. Xia, Li/Na substitution and Yb3+ co-doping enabling tunable near-infrared emission in LiIn2SbO6:Cr3+ phosphors for light-emitting diodes, iScience, 2021, 24, 102250 CrossRef CAS PubMed
.
- Y. Zhang, S. Miao, Y. Liang, C. Liang, D. Chen, X. Shan, K. Sun and X.-J. Wang, Blue LED-pumped intense short-wave infrared luminescence based on Cr3+-Yb3+-co-doped phosphors, Light: Sci. Appl., 2022, 11, 136 CrossRef CAS PubMed
.
- R. Shi, S. Miao, X. Lv, D. Chen, Y. Zhang and Y. Liang, High–Efficiency Short–Wave Infrared Emitter Enabled by Cr3+–Yb3+ Co–Doped Phosphor, Adv. Opt. Mater., 2024, 2303221, DOI:10.1002/adom.202303221
.
- Q. Jia, L. Yao, S. Yu, S. Gong, J. Jiang and Q. Shao, Efficient and ultra-broadband Cr3+/Ni2+ co-doped phosphors for light-emitting diodes with spectral output over NIR-I and NIR-II regions, J. Mater. Chem. C, 2023, 11, 11046–11054 RSC
.
- S. Miao, Y. Liang, R. Shi, W. Wang, Y. Li and X.-J. Wang, Broadband Short-Wave Infrared-Emitting MgGa2O4:Cr3+, Ni2+ Phosphor with Near-Unity Internal Quantum Efficiency and High Thermal Stability for Light-Emitting Diode Applications, ACS Appl. Mater. Interfaces, 2023, 15, 32580–32588 CrossRef CAS PubMed
.
- J. Chen, Y. Gao, J. Chen, X. Lu, M. Tan and J. Qiu, Designing and controlling the Ni2+-activated (Zn,Mg)Al2O4 spinel solid-solution for phosphor-converted broadband near-infrared illumination, J. Mater. Chem. C, 2023, 11, 2217–2228 RSC
.
- M. Jin, F. Li, J. Xiahou, L. Zhu, Q. Zhu and J.-G. Li, A new persistent luminescence phosphor of ZnGa2O4:Ni2+ for the second near-infrared transparency window, J. Alloys Compd., 2023, 931, 167491 CrossRef CAS
.
- S. Miao, Y. Liang, Y. Zhang, D. Chen and X. J. Wang, Blue LED–Pumped Broadband Short–Wave Infrared Emitter Based on LiMgPO4:Cr3+,Ni2+ Phosphor, Adv. Mater. Technol., 2022, 7, 2200320 CrossRef CAS
.
- Q. Zhang, G. Li, G. Li, D. Liu, P. Dang, L. Qiu, H. Lian, M. S. Molokeev and J. Lin, Optical Thermometer Based on Efficient Near–Infrared Dual–Emission of Cr3+ and Ni2+ in Magnetoplumbite Structure, Adv. Opt. Mater., 2023, 12, 2301429 CrossRef
.
- M. Han, S. Chen, J. Li, Z. Gao, Y. Zhang, Y. Shen, Y. Tian and D. Deng, Sr2(Ga/Al)TaO6:Cr3+ phosphor with tunable near-infrared emitting for light-emitting diodes and optical thermometer, J. Alloys Compd., 2024, 973, 172927 CrossRef CAS
.
- D. Kovacheva, T. Trendafilova, K. Petrov and A. Hewat, Cation ordering in Li2M(II)Sn3O8,M(II)=Mn,Zn, J. Solid State Chem., 2002, 169, 44–52 CrossRef CAS
.
- S. Qing, J. Wan, T. Yang, Q. Zhou, Y. Zhou, Z. Wang, D. Wen and M. Wu, Rational design for broad near-infrared emission from a two-sited Rb2LiAlF6:Cr3+ phosphor with high efficiency and thermal stability for spectroscopic applications, Inorg. Chem. Front., 2024, 11, 2718–2725 RSC
.
- Z. Lu, Y. Liu, S. Chen, P. Sun, Z. Luo, X.-J. Wang and J. Jiang, Improved Near-Infrared Luminescence Properties of LiScSi2O6:Cr3+,Yb3+ Phosphors via Efficient Energy Transfer, ACS Appl. Opt. Mater., 2023, 1, 1097–1103 CrossRef CAS
.
- T. Suzuki, G. S. Murugan and Y. J. J. O. L. Ohishi, Spectroscopic properties of a novel near-infrared tunable laser material Ni : MgGa2O4, J. Lumin., 2005, 113, 265–270 CrossRef CAS
.
- Y. Zhang, Z. Gao, Y. Li, S. Chen, M. Han, J. Li, Q. Zhang, Y. Shen, D. Deng and S. Xu, Broadband La2LiSbO6: Cr3+ Phosphors with Double Luminescent Centers for NIR pc-LEDs, Inorg. Chem., 2023, 62, 17371–17381 CrossRef CAS PubMed
.
- Z. Song, P. A. Tanner and Q. Liu, Host Dependency of Boundary between Strong and Weak Crystal Field Strength of Cr3+ Luminescence, J. Phys. Chem. Lett., 2024, 15, 2319–2324 CrossRef CAS PubMed
.
- H. Zhang, J. Zhong, F. Du, L. Chen, X. Zhang, Z. Mu and W. Zhao, Efficient and Thermally Stable Broad-Band Near-Infrared Emission in a KAlP2O7:Cr3+ Phosphor for Nondestructive Examination, ACS Appl. Mater. Interfaces, 2022, 14, 11663–11671 CrossRef CAS PubMed
.
- C. Wang, Y. Zhang, X. Han, D. Hu, D. He, X. Wang and H. Jiao, Energy transfer enhanced broadband near-infrared phosphors: Cr3+/Ni2+ activated ZnGa2O4−Zn2SnO4 solid solutions for the second NIR window imaging, J. Mater. Chem. C, 2021, 9, 4583–4590 RSC
.
- Q. Zhang, D. Liu, Z. Wang, P. Dang, H. Lian, G. Li and J. Lin, LaMgGa11O19:Cr3+,Ni2+ as Blue–Light Excitable Near–Infrared Luminescent Materials with Ultra–Wide Emission and High External Quantum Efficiency, Adv. Opt. Mater., 2023, 11, 2202478 CrossRef CAS
.
- Y. Wang, M. Shang, S. Huang, Y. Sun, Y. Zhu, X. Xing, P. Dang and J. Lin, Continuous Ultra–Broadband Near–Infrared Sc2O3−Based Nanophosphor Realized by Spectral Bridge of Cr3+–Yb3+–Cr4+ for Multiple Optical Applications, Adv. Opt. Mater., 2023, 11, 2300517 CrossRef CAS
.
- Y. Wang, M. Shang, Y. Sun, Y. Zhu, X. Xing, P. Dang and J. Lin, Small Stokes Shift and Two–Site Occupation in the ANb2O6:Cr3+ (A = Zn/Mg) Phosphors Toward Highly Efficient Ultra–Broadband Near–Infrared Emission for Multifunctional Applications, Adv. Opt. Mater., 2023, 12, 2302611 CrossRef
.
- S. Wang, Z. Lyu, D. Sun, S. Shen and H. You, Tailoring the Cr3+ Emission via Combinatorial Management of Crystal–Field and Energy Transfer for Multiple NIR LEDs, Adv. Opt. Mater., 2024, 2303140, DOI:10.1002/adom.202303140
.
- Y. Zhuo, F. Wu, Y. Niu, Y. Wang, Q. Zhang, Y. Teng, H. Dong and Z. Mu, Super Broadband Emission Across NIR–I and NIR–II Under Blue Light Excitation of Cr3+, Ni2+ Co–Doped Sr2GaTaO6 Phosphor Achieved by Two–Site Occupation and Effective Energy Transfer, Laser Photonics Rev., 2024, 2400105, DOI:10.1002/lpor.202400105
.
- M. U. Dumesso, W. Xiao, G. Zheng, E. T. Basore, M. Tang, X. Liu and J. Qiu, Efficient, Stable, and Ultra–Broadband Near–Infrared Garnet Phosphors for Miniaturized Optical Applications, Adv. Opt. Mater., 2022, 10, 2200676 CrossRef CAS
.
- W.-T. Huang, V. Rajendran, M.-H. Chan, M. Hsiao, H. Chang and R.-S. Liu, Near-Infrared Windows I and II Phosphors for Theranostic Applications: Spectroscopy, Bioimaging, and Light-Emitting Diode Photobiomodulation, Adv. Opt. Mater., 2023, 11, 2202061 CrossRef CAS
.
- R. Shi, S. Miao, Y. Zhang, X. Lv, D. Chen and Y. Liang, Blue-light-excitable pure and efficient short-wave infrared luminescence via Cr3+ → Yb3+ energy transfer in a KYbP2O7:Cr3+ phosphor, J. Mater. Chem. C, 2023, 11, 2748–2755 RSC
.
- Z. Chen, S. Du, K. Zhu, Z. Tian, J. Zhang, F. Li, S. Zhang, S. Zhao, W. Cui, X. Yuan, K. Chen, G. Yuan and G. Liu, Mn4+-Activated Double-Perovskite-Type Sr2LuNbO6 Multifunctional Phosphor for Optical Probing and Lighting, ACS Appl. Mater. Interfaces, 2023, 15, 28193–28203 CrossRef CAS PubMed
.
- M. Back, J. Ueda, H. Nambu, M. Fujita, A. Yamamoto, H. Yoshida, H. Tanaka, M. G. Brik and S. Tanabe, Boltzmann Thermometry in Cr3+–Doped Ga2O3 Polymorphs: The Structure!. Matters, Adv. Opt. Mater., 2021, 9, 2100033 CrossRef CAS
.
- K. Elzbieciak-Piecka and L. Marciniak, Optical heating and luminescence thermometry combined in a Cr3+-doped YAl3(BO3)4, Sci. Rep., 2022, 12, 16364 CrossRef CAS PubMed
.
- Y. Huo, H. Cai, Y. Shao, Z. Song and Q. Liu, Enabling Yb3+ Luminescence with Visible Light Response in Mg2GeO4 via Energy Transfer, Inorg. Chem., 2023, 62, 14402–14410 CrossRef CAS PubMed
.
- Q. Wang, S. Wang, R. Pang, T. Tan, T. Tan, H. Wen, S. Zhang, H. You, C. Li and H. Zhang, Two-site occupation in Cr3+-activated BaIn2(P2O7)2 phosphor for broadband near-infrared thermometry and LED applications, Mater. Res. Bull., 2023, 163, 112222 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qi00896k |
| This journal is © the Partner Organisations 2024 |