A review on covalent organic frameworks: exploration of their growing potential as porous materials in photocatalytic applications
Kamal
Prakash
a,
Rakesh
Deka
a and
Shaikh M.
Mobin
 *abc
*abc
aDepartment of Chemistry, Indian Institute of Technology Indore, Khandwa Road, Simrol, Indore 453552, India. E-mail: xray@iiti.ac.in; Tel: +917316603336
bCenter for Electric Vehicle and Intelligent Transport System (CEVITS), Indian Institute of Technology Indore, Khandwa Road, Simrol, Indore 453552, India
cCenter of Advanced Electronics (CAE), Indian Institute of Technology Indore, Khandwa Road, Simrol, Indore 453552, India
First published on 19th August 2024
Abstract
Photocatalysis powered by unlimited solar energy is an effective strategy to resolve energy and environmental issues. To achieve an efficient photocatalytic system, photocatalysts need to be highly crystalline and porous with excellent photostability under extreme conditions. In this case, covalent organic frameworks (COFs) have shown immense potential for photocatalytic applications owing to their unique structure as well as electronic and photophysical characteristics. COFs possess a crystalline porous network with light absorption capabilities and excellent stability. Furthermore, functionalized COFs can be developed through organic unit variation to obtain broader absorption, narrow bandgap, effective charge separation, and transportation. Furthermore, high photocatalytic efficiency can be achieved via the formation of heterostructures through anchoring or post-synthetic modification. Our review is focused on the recent advancements in COFs as photocatalysts for various photocatalytic applications. Initially, we emphasize the topological design, linkage chemistry, and functionalization of COFs, underscoring the principles and requirements for high photocatalytic efficiency. This provides deep insights into the capabilities of COFs in different photocatalytic applications, covering areas such as hydrogen and oxygen evolution, carbon dioxide reduction, organic transformation, and organic pollutant degradation. Finally, we summarize the pivotal points that need urgent attention and outline future avenues, offering fresh perspectives and contributing to revolutionary innovations in this rapidly evolving field.
1. Introduction
From the last decade, fossil fuels have been the primary source of energy playing an important role in enhancing the quality of human life, starting from the laboratory to the industrial scale. However, the high and rapid consumption of fossil fuels not only poses the threat of an energy crisis in the near future but also affects the environment.1 Furthermore, due to pollution and global warming, fossil fuel consumption has become a concern for the global environment.2,3 Thus, it is necessary to replace fossil fuels with greener and eco-friendly energy technology to fulfil the world's energy demand while maintaining a safe environment.4–6 In our Solar System, the sun is an unlimited source of energy for our planet, and solar energy is considered the best green alternative to fossil fuels. However, producing solar energy as fuels on a large scale is challenging owing to the low solar energy conversion efficiency.7 Accordingly, researchers are applying different techniques to utilize solar energy with a high conversion rate, and among them, photocatalysis has emerged as a promising technology to convert solar energy with high efficiency.8,9 In natural systems, photosynthesis in plants is an efficient photocatalysis process, where the green leaves of plants utilize sunlight to form oxygen and carbohydrates from water and carbon dioxide.10 Chlorophyll, a porphyrin derivative in leaves, is responsible for the photosynthesis process, which converts solar energy into chemical energy for various chemical reactions.11 Inspired by this, researchers have focused on developing efficient photocatalysts that can mimic the photosynthetic mechanism and utilize sunlight for various applications such as hydrogen or oxygen evolution, CO2 reduction, and organic transformation reactions.12–15 The understanding of the mechanism of a particular photocatalytic conversion reaction is key to developing an efficient photocatalyst. Generally, a photocatalyst should have light absorption ability, chemical or thermal stability, and high solar-to-power conversion efficiency to achieve efficient photocatalytic performance.16,17Fujishima and Honda first reported a type of photocatalyst for photocatalytic water splitting, where TiO2, an inorganic semiconductor, produced hydrogen under light illumination.18 Their works inspired researchers in this field to develop efficient photocatalysts for light-driven applications. Subsequently, many inorganic photocatalysts such as oxides (ZnO, TiO2, and SnO2), sulfides (CdS, ZnS and MoS2), and nitrides have been explored as photocatalysts due to their high abundance, appropriate band structure, high stability, and anticorrosive properties.19–21 However, these materials cannot utilize solar energy effectively because of their lower absorption capability in the visible region, which hinders their large-scale applicability. Similarly, carbon-based materials having high π–π conjugated and layered structures have also been investigated for photocatalysis but their fabrication and functionalization are expensive and difficult.22–24
Over the last decade, porous organic materials such as covalent organic frameworks (COF), metal–organic frameworks (MOF), and conjugated organic polymers (COP) have emerged as new crystalline materials consisting of reticular structures and porous networks.25–28 Among the porous materials explored for photocatalytic applications because of their distinctive properties, covalent organic frameworks (COF) have attracted wide recognition considering their regularly ordered network, highly porous surface, and excellent stability.29,30 COFs are highly crystalline materials with strong covalent bonding between two or more organic moieties. In contrast to MOFs, which consist of metal centres in their frameworks, COFs are generally metal-free networks and have a lower density due to the presence of lightweight elements such as C, H, B, O, and N in their structure.31 The first COF was synthesized by Yaghi and his group in 2005 using the solvothermal method.32 Their pioneering work led the way for the synthesis of COFs, with hundreds of new COFs developed within the next 10–15 years for different applications such as energy storage, catalysis, sensing, optoelectronics and biomedical.33,34
In recent years, COFs have attracted significant attention for various photocatalytic applications such as hydrogen evolution reaction (HER), CO2 reduction/conversion, organic transformation, oxygen evolution reaction (OER), and pollutant degradation35 due to their unique structure and photophysical properties. Jiang and his group first reported a photo-responsive COF in 2008 that behaves as a semiconductor upon light irradiation. Lotsch and co-workers prepared a triazine COF with hydrazone linkage for photocatalytic HER application in 2014, opening the way for the utilization of COFs in photocatalytic applications.36 Later, photoactive COFs were developed as photocatalysts for organic transformations and decomposition of organic pollutants.37,38 In 2018, a rhenium (Re) complex was incorporated on the surface of a 2D COF for visible-light-driven CO2 conversion into CO, which showed a higher photocatalytic performance compared to the pristine Re catalyst.39 Similarly, covalent triazine frameworks, a subclass of COFs, were investigated for photocatalytic water splitting to produce H2 and O2, simultaneously.40 COFs possess a well-defined polymer network with permanent porosity, strong light absorption, and high stability, which boost their performances as photocatalysts for particular photocatalytic applications. Especially, their π-conjugated network arranged directionally contributes to facile charge migration and transportation in photocatalytic processes.41 Thus, COFs have gained popularity as promising materials for energy and environmental applications.42
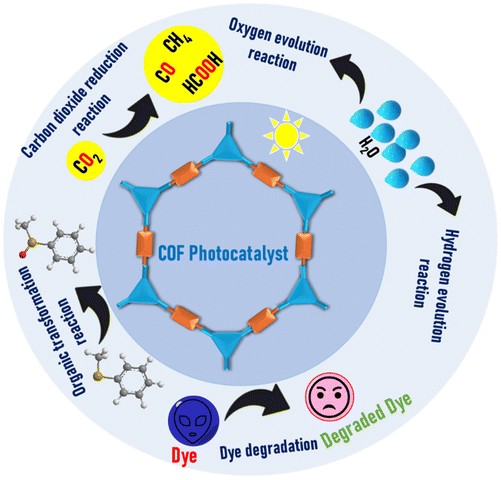
As the field of COFs is expanding at a rapid pace, particularly in the case of their photocatalytic application, many reviews have been published on COFs, discussing their application in the field of photocatalysis. However, most reviews only focused on COFs for energy-related applications such as hydrogen evolution, CO2 reduction, and water splitting,43–46 while a few comprehensive reviews thoroughly covered these applications.35,47 Thus, a detailed review concerning the design and structural manipulation of COFs for the development of photocatalysts for various photocatalytic applications is still lacking. Considering this, our review presents a brief introduction to the design, functionalization and desirable properties of COFs as efficient photocatalysts, and further systematically summarizes the recent development of COFs for different photocatalytic applications, focusing on hydrogen evolution, CO2 reduction, oxygen evolution, organic transformation, and organic pollutant degradation (Fig. 1). Also, the development of hybrid COFs as highly efficient photocatalysts is briefly discussed in each section. Finally, future challenges and perspectives are discussed to develop efficient COF photocatalysts. Thus, this review will provide a systematic study on COFs in photocatalytic applications to promote their development as highly efficient photocatalysts.
2. Topological design, linkage chemistry, and functionalization of COFs
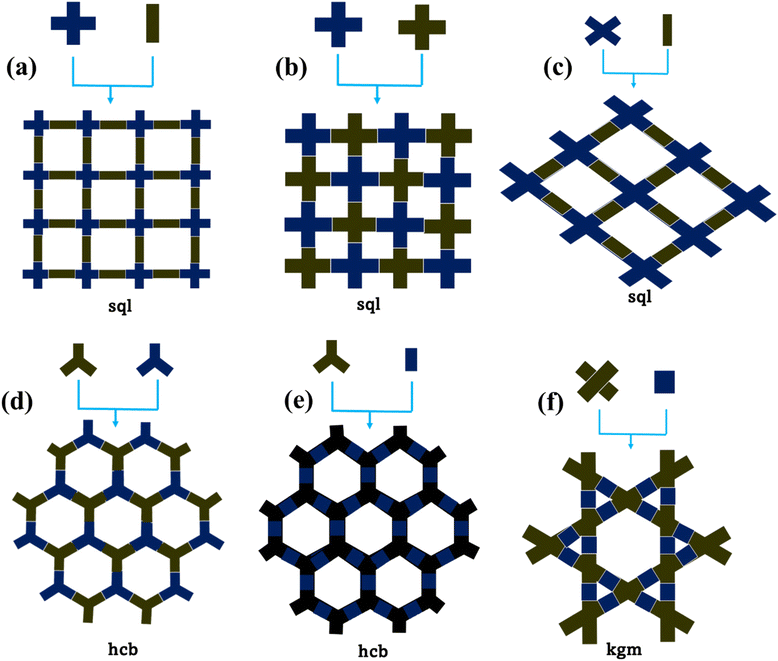
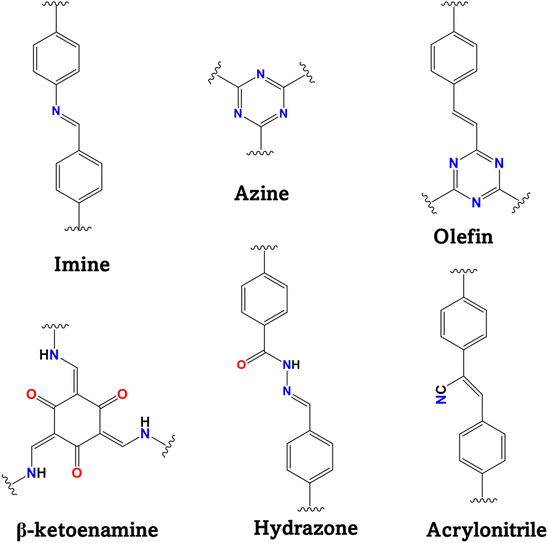
COFs are composed of a crystalline layered network of organic building blocks known as knots and linkers and the geometry of these organic units determines the topology of a COF system. The main advantage of COFs is their topological design and structural tunability using different organic building blocks.34 By varying the linkers and knots based on different organic moieties, the topology of COFs can be defined into one-dimensional to three-dimensional (3D) networks with a variety of pore channels. Presently, the formation of a 3D network is not very popular given that optimization of a 3D layered crystalline structure is quite challenging. In contrast, the development of two-dimensional (2D) COFs is well-known and has been mostly explored for photocatalytic applications. Generally, 2D COFs can be formed from organic building blocks that have various symmetrical geometries known as C2, C3, C4, and C6 geometries. Most 2D COFs are formed by the combination of [C3 + C2], [C3 + C3], [C4 + C2], [C4 + C4], and [C6 + C2] building blocks.48,49Fig. 2 represents the different topologies according to the symmetry combinations. The [C2 + C3] or [C3 + C3] combination forms the hexagonal hcb topology, whereas the [C4 + C2] or [C4 + C4] combination results in the tetragonal sql topology.50–52 The topology of COFs dictates their pore network with a uniform pore size and shape, but it has been observed that the pore size and shape vary for the same type of topology originating from different combinations. [C6 + C2] and [C6 + C3] construct a rhombic topology with dual pore sizes.53,54 One of the fascinating combinations is [C2 + C2], which gives two different topologies, kgm and sql, with different pore structures depending on the reaction conditions and building block functionality.55,56 Another way to develop a structural skeleton in COFs is multicomponent COF synthesis, for example, designing a COF with one knot and two different linkers provides two different topological structures in one COF.57 This can open the way for the synthesis of complex COFs with unique properties by the integration of two different COFs. The important topological designs for 2D COFs are trigonal, tetragonal, rhombic, and hexagonal forms. Thus, based on the topology of COFs, their pore structure, surface area, active sites, and interlayer structure can be varied, which can have a great effect on their photophysical properties. In this case, the appropriate selection of organic moieties enables precise control of the pore network in COFs to be achieved. Topology has a significant impact on the formation of COFs with different structures and is crucial in the reticular chemistry of COFs.Generally, COFs are formed by two or more monomers consisting of different reactive groups. The organic moieties are coordinated via a covalent linkage, which has a great impact on the effective π-delocalization and stability of the reticular COF network. Different types of linkages have been explored to synthesize COFs such as boron-ester, imine, azine, hydrazone, vinyl, β-ketoenamine, and amide linkages (Fig. 3).58 The boronic ester linkage is formed via the reaction between the boronic acid and hydroxy group, while the imine linkage results from the Schiff base reaction between amine and formyl groups. This indicates that appropriate functional groups are necessary to acquire COFs with a particular linkage. To perform efficient photocatalytic reactions, COFs should possess excellent chemical stability in aqueous and non-aqueous solutions, different pH, and harsh environments.59 Boronic-ester-linked COFs are highly sensitive toward water and transform into their parent organic units, inhibiting their application in aqueous solution. Hence, imine-linked COFs have been largely explored for photocatalytic applications. The first imine-based COF was introduced as COF-300 formed by the Schiff base reaction of tetra(p-aminophenyl)methane amino and terephthalaldehyde, which opened the way for developing numerous imine-linked COFs using different building blocks.60 To date, β-ketoenamine COFs are the most explored COFs for different applications because of their keto–enol tautomerization, which improves their stability.61 Imine, azine, and olefinic-linked COFs have better crystallinity, higher stability, and are easier to design compared to hydrazone, hydrazine, dioxine, and ester-linked COFs.58 Imine and β-ketoenamine-linked COFs can undergo protonation or bind with metal atoms, metal nanoparticles, and inorganic compounds to form hybrid composites and have been further explored in photocatalytic applications.62,63
Functionalization is an important factor in modulating the properties of COFs. As discussed previously, various building blocks can have different types of symmetries to develop specific topological designs, affecting the structural properties of COFs. The functionalization of COFs can be developed by modulating their organic moieties as knots and linkers, post-synthetic modification, or in situ formation of hybrid materials.64 Consequently, COFs have become more advantageous compared to semiconductors and noble metals, where their electronic and photophysical properties can easily be tuned using different organic moieties as building units for their synthesis. Based on the linkage chemistry, several organic moieties have been explored to develop COFs for photocatalysis (Fig. 4). The absorption region of COFs can be expanded by applying organic moieties having a broad absorption in the visible region. The inclusion of chromophore moieties offers broader absorption with extended conjugation in the 2D network. Pyrene or porphyrin moieties provide strong absorption in the range of 400 to 800 nm upon integration in COFs, thus increasing their light-harvesting ability.65,66 A porphyrin-containing 2D COF was reported for the light-activated transformation of amines into imines, where the generation of light-activated electron–hole pairs was responsible for the oxidation reaction.66 COF structures with the triazine moiety possess high crystallinity and porous surface, given that the rigid and planar triazine units stacked to form orderly arranged COF layers.67 Also, the band structure such as band positions or band gap of COFs can be optimized by varying the organic moiety for their formation. A series of vinylene-linked 2D COFs displayed different band gap values by modifying their building block with a benzobisthiazole linker, affecting their photocatalytic activity.68 Extended conjugation in the organic moiety can be very effective in enhancing the photocatalytic activity of COFs. Kuo et al. enhanced the HER performance of a carbazole-based COF by changing the degree of conjugation in its organic linkers.69 Further, the introduction of donor and acceptor groups as functional groups in COF networks is very effective to induce efficient charge separation and migration during photo-excitation.70 Thus, a library of COFs can be developed by modulating their organic moieties, topologies, and linkages, which can have diverse applications based on their properties and functionalization.
 | ||
| Fig. 4 Representative structures of different types of building units (knots and linkers) of COF photocatalysts. | ||
Further, post-synthetic modification of COFs and the formation of hybrid COFs are alternative ways to introduce functional groups, active sites, and heterogeneous structures in the COF network.43 Post-synthetic modification can provide new active sites by attaching metal and metal nanoparticles to the COF periphery or transforming the functional groups into active groups in the precursor COF.48,71,72 To anchor metal atoms or metal nanoparticles (NPs) on the surface of COFs, the pore wall of COFs should have anchoring sites, where the metal or metal NPs can coordinate. COFs with bipyridine groups or nitrogen heteroatoms (imine or β-ketoenamine linkage) are capable of binding metal atoms or metal NPs effectively, while maintaining the integrity of the original COFs.73,74 The surface area of COFs is reduced upon the introduction of new active sites but these active sites improve their uptake capacity for reactant molecules and lower the energy barrier for photocatalytic reactions, resulting in better charge separation and charge migration for electron–hole pairs, subsequently improving their photocatalytic activity.75 Hybrid COFs can be developed by incorporating metal oxides, metal sulfides, inorganic compounds, metal–organic frameworks, or carbon-based materials in COFs via in situ solvothermal reaction.64,71,76 The combination of two different materials generates a synergetic effect, constructing a Z-scheme or S-scheme-based heterojunction system, improving the charge separation and charge transfer capacity of COFs for photocatalytic activity.
3. Basic principle of photocatalytic reaction
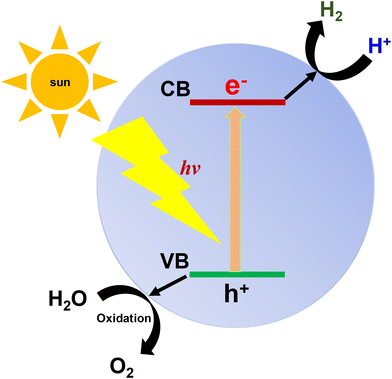
Photocatalysis is a light-driven process, where solar energy transforms into chemical energy to carry out a chemical reaction.77 Photocatalysis has three main steps, as follows (Fig. 5): (i) absorption of visible light for photoexcitation of electrons, (ii) excited electrons move from the valence band (VB) to conduction band (CB), leaving holes behind and generating electron–hole pairs; and (iii) the photogenerated charges further reach to the surface of the photocatalyst to assist in the corresponding reaction. Photocatalytic reactions will be feasible when the VB is more positive or the CB is more negative than the redox potential of the corresponding half-reactions. Thus, the band positions are very important for photocatalytic reactions to proceed, where a smaller band gap is more favourable for increasing the light absorption, subsequently producing a high efficiency. Light-activated processes such as HER, CO2 reduction, and organic transformations generally proceed through the capture of photo-excited electrons, whereas the oxygen evolution reaction involves the reaction of photogenerated holes with water molecules.78–80 Many COF photocatalysts exhibit overall water splitting activity, where electrons and holes simultaneously yield hydrogen and oxygen as products, respectively.Generally, COF-based photocatalytic systems carry out photocatalysis in the presence of co-catalysts and sacrificial donors.81 In HER or CO2 photoreduction, electrons are received by a co-catalyst, and then transferred to H+ and CO2 for H2 evolution and CO/CH4 production, respectively. A sacrificial donor is utilized as an electron donor to replace the oxygen evolution reaction and regenerate the photosensitizer. Mostly, the noble metal platinum is used as a co-catalyst given that it forms an efficient heterojunction system with the photocatalyst, while triethylamine, triethanolamine, and ascorbic acid are used as sacrificial donors.82 The use of co-catalysts and sacrificial donors also inhibits photo corrosion during photocatalysis and limits the OER process.
The major factor causing low photocatalytic activity is the charge recombination process, where excited electrons can combine with holes again, reducing the number of photogenerated electrons for chemical reactions. Thus, the recombination process must be reduced and effective charge separation and efficient charge transfer promoted to achieve the maximum efficiency. Therefore, the rational design of COFs as photocatalysts with appropriate band edges, narrow bandgap, and high charge separation efficiency can make them efficient for photocatalytic applications. Further, COFs can be integrated with other materials such as metals, metal complexes, and carbon-based entities to develop hybrid COF photocatalysts, which can form different heterojunction structures such as type-II-heterojunction and Z- and S-scheme heterojunction systems via the synergetic effect between COFs and other materials.83,84 Due to the formation of these heterojunction systems, the charge separation efficiency and charge carrier transportation are greatly enhanced, resulting in high photocatalytic performances.
4. Required properties of COFs as photocatalysts
To act as an efficient photocatalyst, COFs must possess a crystalline structure, light-harvesting ability, narrow bandgap, large surface area, excellent stability and enriched π-conjugation for feasible charge separation and migration. It is necessary to satisfy many factors to realize the crystalline nature of photocatalysts with fewer defects given that this significantly reduces the charge recombination process, provides longer lifetimes, and enhances the charge transfer efficiency, eventually contributing to the photocatalytic efficiency. The primary requirement of a photocatalyst is that it should have high absorption in the visible region of 400 to 800 nm.85 Organic moieties with large conjugation aromatic systems are capable of harnessing light in the visible region due to their effective π–π delocalization. Thus, to consider this aspect, many building blocks such as triphenylamine, anthracene, pyrene, and porphyrin have been employed for the development of COFs.86–89Further, a crystalline structure provides a long-range ordered arrangement of the organic precursor employed for the formation of COFs. Amorphous polymers have short-range ordered structures, which limit the delocalization of π-conjugated electrons, and suffer from weak charge dynamics and high charge recombination rate. In contrast, the ordered network of crystalline COFs minimizes the defect sites and provides efficient charge separation and charge mobility by inhibiting the charge recombination process.90 Dong Jiang reported the use of porphyrin-based COFs as efficient photocatalysts for HER. It was found that high-crystalline ZnP-PZ-PEO-COF produced an excellent HER rate of 11 mmol g−1 h−1 with a high apparent quantum yield (AQY) efficiency, which was five-times higher than its amorphous counterpart ZnP-PZ-PEO-POP.91 Crystalline COFs also exhibit a uniform distribution of pore channels, increasing their surface area compared to amorphous polymers. ZnP-PZ-PEO-COF possessed a large porous surface area of 557 m2 g−1, while ZnP-PZ-PEO-POP showed a smaller surface area of 474 m2 g−1, respectively. It is evident that highly ordered crystalline COFs achieve wide absorption in the visible region, increased porosity with the maximum number of accessible active sites, and effective electron–hole pair generation with efficient charge transfer, leading to high photocatalytic performances.92 A reduced crystallinity not only results in diminished properties, but the surface area is also effectively reduced, leading to a lower HER performance.93
The most established method to achieve high crystalline COFs is the solvothermal method, where reactions are performed under vacuum-pressure at a fixed temperature and pressure.32 However, the solvothermal process has many limitations such as small-scale reactions and longer reaction times. Several other methods such as microwave synthesis, mechanochemical synthesis, and atmospheric solution method have been reported with fast reaction and mild conditions.94 However, the research on these methods is still in its infancy and they result in structures with low crystallinity and reduced surface area, restraining their application in photocatalysis.
Another important factor is the stability of COFs, which should have excellent thermal and chemical stability under extreme conditions to work as efficient photocatalysts.95–97 Most photocatalytic applications are carried out in different aqueous solutions or at different pH solutions. It is very well known in the literature that boronic ester-linked COFs are unstable in water and can easily decompose into precursor monomers due to their sensitive B–O bonds.98 Thereon, COFs with different linkages such as β-ketoenamine, azine, imine, olefin, and cyanovinyl have been explored for photocatalytic applications, which show high stability in acid, base, and neutral media.99 COFs with long-term stability and recyclability can be considered highly efficient photocatalysts by producing a high yield of products without degrading after use in multiple cycles. A pyrene-based COF with amide linkage showed remarkable recyclability for visible-light-driven uranyl reduction for up to 5 cycles without degradation.100 It is also necessary that COFs should be stable after post-synthetic treatment to incorporate active sites or functional groups and should not lose their structural properties. Many imine and β-ketoenamine COFs have been anchored with metal atoms or metal nanoparticles, exhibiting enhanced photocatalytic performances with excellent stability.101 A palladium-decorated sp2-carbon-linked COF, PD-COF, showed extreme stability after 8 cycles, exhibiting remarkable photodegradation and HER activity.102 The excellent structural stability of COFs during photocatalytic experiments is a necessity for their large-scale practical application.
Porosity is a significant property of COFs for catalytic activity, where highly porous COFs have more accessible sites for catalytic reactions.103 A well-designed pore network with a uniform pore size and shape is an ideal platform for gas adsorption, charge transportation, and functionalization.104,105 Mostly, the pore size of COFs depends on the topological design of their monomers. The size and length of their building units regulate their pore size, i.e. larger or longer building units will form larger pore sizes. Pang et al. synthesized two COFs with different pores by simply utilizing one C4 monomer with two C2 monomers having different lengths.106 COFs can have microporous and mesoporous structures according to their pore size.107,108 It was observed that hexagonal, tetragonal, and rhombic types of pores produce mesoporous COFs, while trigonal pores form microporous COFs. Microporous COFs possess a large surface area with high stability but mesoporous COF structures are more prominent given that π-organic building units generally have large molecular sizes.109 Mesoporous COFs are also more compatible with pore surface engineering compared to microporous COFs because larger pores can easily adsorb large molecules.48 A series of mesoporous and microporous COFs has been developed by modulating the size and length of the monomers, where small organic moieties formed microporous COFs, while large organic units with longer linkers produced microporous COFs.110 The size and distribution of pores are essential factors in photocatalysts, and modifying the surface of these pores plays a pivotal role in enhancing the accessibility of the reactants to the active surfaces.49 The development of COFs with large pores is very important to realize high adsorption and surface area for photocatalytic activity. Further, the tailored construction of the pore wall interface can greatly improve the capability of functional design and properties.
The high efficiency of a photocatalyst toward photocatalytic activity is dependent on its absorption of photon energy from sunlight, which generates electron–hole pairs to participate in catalytic reactions on its surface. The photoexcitation of an electron is related to the band gap energy. If the band gap is narrower, photon absorption increases, and subsequently more electrons can undergo photo-excitation and easily transfer from the VB to CB.111 Thus, by varying the band positions or band edges of a photocatalyst, its band gap can be optimized for a particular catalytic reaction. Large conjugation systems can elevate the HOMO (highest occupied molecular orbital) level, resulting in a reduction in the HOMO–LUMO (lowest unoccupied molecular orbital) gap, which is commonly known as the band gap. Diacetylene-functionalized COFs possess a comparatively smaller band gap than that of acetylene-functionalized COFs due to their extended conjugation, resulting in a high HER performance.112 Another effective strategy to tune the band gap is to append functional groups to the building blocks. The addition of various functional groups such as amino, nitro, hydroxyl, and halogen to organic linkers has been observed to alter their electronic densities. This modification results in a smaller band gap and a redshift in the light absorption edge.113,114 One of the simple strategies for rearranging the band structure of COF photocatalysts is to anchor metal atoms, metal complexes, or metal nanoparticles on their porous surface. Metal ions can shift the band positions due to ligand-to-metal charge transfer, improving the light absorption and increasing the photocatalytic activity. Guo and co-workers appended nickel (Ni) atoms on a bipyridine-containing COF to achieve high photocatalytic efficiency. The coordination of Ni negatively shifted the conduction band, increasing the reducing ability for H2 evolution and allowing metal-to-ligand charge transfer, which led to broader absorption and high charge mobility, thus leading to a high HER performance.115
The separation of electron–hole pairs and charge transfer are the decisive effects of photocatalytic reactions. The excited electrons tend to recombine with holes, which is known as the charge recombination process, and decrease the number of electrons reaching the surface of the catalyst for chemical reactions, thus decreasing the photocatalytic activity. To enhance the separation of electron–hole pairs and their transfer rate, some strategies can be applied in COFs, as follows: (i) extending their π-conjugation system, (ii) preparation of highly conjugated aromatic systems, (iii) functional group modification, and (iv) use of donor–acceptor (D–A) system. The introduction of extended conjugation in the COF network can be very effective to facilitate π-electron delocalization and charge carrier mobility. Two metal-free sp2c-COFs were investigated for CO2 photoreduction by modulating intrinsic the π-conjugation in their structure. The ethylene moiety in the COF skeleton significantly enhanced CO production due to its extensive π-conjugation, facilitating charge separation and migration.116 The use of highly π-conjugated aromatic systems such as triphenylamine, pyrene, porphyrin, and carbazole moieties as building blocks for COFs can enhance the charge transfer for photocatalysis.61,65,117,118 Additionally, it has also been found that the presence of functional groups such as methoxy, hydroxyl, and halogen groups is very effective in improving photocatalytic performance. Fan and his group prepared β-ketoenamine-linked COFs using different functional groups to analyse their effect on photocatalytic CO2 reduction. The presence of electron-donor groups in COFs has a positive impact on CO2 photoreduction compared to electron-acceptor groups, resulting in enhanced photocatalytic activity.119 Chen and co-workers prepared a series of pyrene-based COFs consisting of substituted benzothiadiazole units as linkers and studied their HER performance. The fluorine-substituted benzothiadiazole-linked COF achieved high HER activity due to the electron-withdrawing effect of the fluorine moiety, inducing effective charge separation, which increased the charge migration rate for excited electrons.120
The photocatalytic performance of COFs can be greatly enhanced by developing donor–acceptor-based (D–A) COFs as photocatalysts.121 Many benzothiadiazole, thiazole, and other electron-withdrawing organic units have been utilized to develop donor–acceptor COF systems to enhance their photocatalytic performances.28,122 By introducing donor and acceptor groups, donor–acceptor COF systems can be achieved, which suppress the charge recombination process and generate effective charge separation with charge species possessing a longer lifetime. The photogenerated electrons readily move from the donor to acceptor moiety through the π-conjugation system and their spatial arrangement in the COF allows the flow of holes and electrons in opposite directions, and thus a stable charge separation system with reduced charge recombination exists for a longer period, enabling smooth charge transfer for the photocatalytic process.
Further, other materials can be incorporated in COFs such as metal oxides, inorganic complexes, carbon nitride, and metal–organic frameworks to prepare COF hybrids as photocatalysts to enhance their photocatalytic activity. These hybrid COF composites such as MOF–COF hybrids, COF–CNT hybrids, and COFs often construct Z-scheme or S-scheme heterojunction systems, which enhance the charge separation efficiency and charge migration rate, leading to a high photocatalytic performance.
5. Photocatalytic applications of COFs
5.1 Photocatalytic hydrogen evolution reaction
In recent times, the energy crisis has become one of the main issues to address for economic development. Thus, hydrogen energy from water splitting can be a promising source of clean and sustainable energy. However, the light-driven water splitting process to produce H2 is a challenging target to achieve and needs an effective photocatalyst. The energy barrier for light-driven water splitting is 1.23 eV given that the redox potential for hydrogen and oxygen evolution is zero and 1.23 eV against NHE (normal hydrogen electrode), respectively. Photocatalysts need to overcome the energy barrier to perform the water-splitting process. They must possess a band gap of 1.5–3.2 eV and their conduction band and valence band should be more negative and more positive, corresponding to their redox potential, respectively. The structural diversity of COFs through skeleton modulation allows them to exhibit light absorption in the visible region, optimized band structure, and large porous surface, which can be advantageous for photocatalytic activity. Owing to these properties, COFs have been largely explored for solar-driven hydrogen generation with excellent stability and recyclability.Extended conjugation can be very effective in improving photocatalytic efficiency. Thomas and co-workers synthesized two β-ketoenamine COFs, TP-BDDA and TP-EDDA, consisting of a diacetylene and acetylene functionality, respectively, and utilized them for HER. Notably, TP-BDDA COF achieved an HER rate of 324 μmol h−1 g−1, which was 10 times greater than that of TP-EDDA COF. The extended conjugation due to diacetylene functionalization provided a narrow band gap for photoexcitation and enhanced charge mobility for photogenerated electrons, resulting in an improved hydrogen evolution rate.125 Likewise, three novel pyrene-based COFs, A-TEXPY-COF, were reported with extended alkynes, which were modulated by peripheral phenyl units with nitrogen atoms. Interestingly, A-TEBPY-COF without nitrogen at the peripheral phenyl ring exhibited the highest HER rate of 98 μmol h−1 g−1 among the A-TEXPY-COFs. This trend of HER rate for A-TEXPY-COF suggested that the photocatalytic HER process proceeded via a radical cation mechanism, which is opposite to the radical anion mechanism by the Nx-COF series. The high electron-rich A-TEBPY-COF greatly stabilized the radical cation, resulting in high photocatalytic activity.126
The crystalline and hydrophilic nature of COFs can drastically affect the photocatalysis process. Two sulfone-based COFs, FS-COF and S-COF, were prepared by incorporating two different sulfone moieties to achieve higher photocatalytic performance than N3-COF. Owing to the parallel C–N bonds in the FS-COF linker and their effective π–π stacking, FS-COF possessed a highly crystalline and stable COF structure compared to S-COF and TP-COF (without the sulfone moiety). FS-COF and S-COF exhibited HER rates of 16.3 and 4.4 mmol h−1 g−1 and outperformed N3-COF by exhibiting 22 times higher HER rates, respectively. Sulfone moieties are hydrophilic and increase the wettability of COFs in aqueous solution, which results in excellent HER performances. Additionally, FS-COF exhibited long-term stability under light irradiation and HER activity for up to 50 h.127
A donor–acceptor (D–A)-based COF system can be very advantageous for HER application. Effective intermolecular charge transfer in the COF system can generate effective charge separation and further enhance the charge transport ability. Many donor organic units such as porphyrin, pyrene, carbazole, and triphenylamine have been employed to form D–A COFs using electron-withdrawing moieties such as thiazole, benzothiadiazole, and substituted phenylenediamine as linkers. Dong et al. developed two donor–acceptor COFs, BT-TAPT-COF and BDF-TAPT-COF, using the triazine moiety as the acceptor unit, whereas benzothiadiazole and benzodifurane moieties as donor units. Both COFs possessed a highly crystalline structure with strong light absorption and excellent stability, exhibiting impressive HER rates of 949 μmol h−1 g−1 and 1390 μmol h−1 g−1, respectively.128,129
L. Chen and his team also reported a set of D–A-based COFs featuring electron-donating pyrene as the main core with different substituted benzothiadiazole moieties as linkers. To enhance the charge separation efficiency, chloro- or fluoro-groups were attached to the benzothiadiazole organic units for the formation of COFs, where X represents the substituted group. The average lifetimes of excited electrons of the halogenated COFs were drastically improved compared to the non-substituted COFs, representing the high charge separation of excitons and inhibition of charge-recombination phenomena. It also impacted the HER efficiency of COFs, where Py-FTP-BT-COF and Py-ClTP-BT-COF produced hydrogen at the rate of 177 μmol h−1 g−1 and 57 μmol h−1 g−1, which are comparatively higher than that of Py-HTP-BT-COFs (21 μmol h−1 g−1).130 Similarly, Li and his group constructed PyTz-COF by employing thiazole (Tz) as an acceptor moiety with pyrene as the donor. PyTz-COF exhibited a highly crystalline structure with an ordered arrangement of thiazole and pyrene groups, which subsequently generated fast intermolecular charge transfer within the COF and effectively extended its π-conjugation. Consequently, PyTz-COF displayed remarkable optoelectronic properties such as broad absorption, photogenerated electrons with a long lifetime, and a high cathodic photocurrent, reaching up to 100 μA cm−2. Accordingly, PyTz-COF demonstrated an impressive HER rate of 2072 μmol h−1 g−1, outperforming many reported COFs.131
Lee et al. introduced 1H-phenanthro[9,10-d]imidazole-5,10-diamine (PIDA) as a linker with triformylbenzene and triformylphloroglucinol moieties to develop two new highly crystalline COFs, PIm-COF1 and PIm-COF2, which produced H2 evolution rates of 528.5 and 7417.5 μmol g−1 h−1, respectively. The photocatalytic performance of PIm-COF2 surpassed that of PIm-COF1 due to the donor–acceptor characteristics facilitated by the strong conjugation between the PIDA moiety and β-ketoenamine linkage. This resulted in increased light absorption, a suitable bandgap, and effective charge separation, leading to high HER activity.132
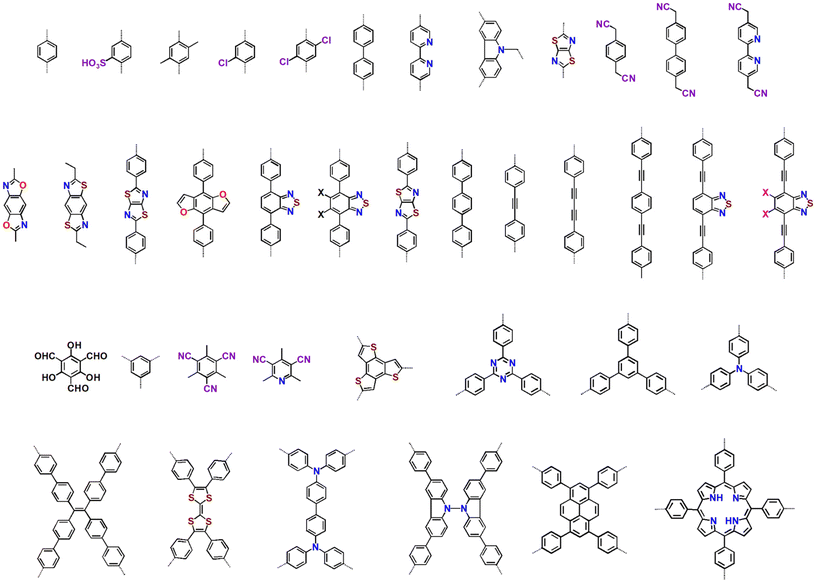
Further, band gap engineering via structural modification significantly changes the photophysical properties of COFs. To understand this, Y. Chen and group synthesized four different β-ketoenamine-linked COFs, represented as COF-OH-n (n = 0–3), using the triazine moiety as the core with four trialdehyde-benzene units with a varying number of hydroxyl groups (Fig. 6). The presence of the –OH group determined the proton tautomerism in COFs given that COF-OH-0 was unable to undergo tautomerization, whereas COF-OH-1 and COF-OH-2 exhibited reversible keto–enol tautomerization (Fig. 6a). COF-OH-n exhibited proton tautomerism, influencing its band edge positions, band structure, and light absorption, which are critical factors in water splitting. Notably, the HER rate of COF-OH-3 was found to be 9.89 mmol g−1 h−1, which is the highest among the COFs (Fig. 6c). COF-OH-1 did not exhibit any HER activity under similar photocatalytic reaction conditions. COF-OH-3 exhibiting irreversible keto–enol tautomerisation featured moderate visible absorption, high stability, and a suitable optical bandgap of 2.28 eV (Fig. 6b), and therefore exhibited the highest photocatalytic efficiency among them.133 Similar types of results were obtained for Tz-COF-X developed by the same group using a benzothiazole-diamine (Tz) derivative as the donor unit with similar hydroxyl-substituted triarylaldehydes. As the number of –OH groups increased, the donor–acceptor interaction was enhanced for Tz-COF-3, triggering exciton dissociation for efficient charge separation with a longer lifetime, enforcing better photocatalytic activity. Thus, Tz-COF-3 produced an excellent HER rate of 43.2 mmol g−1 h−1 with a quantum efficiency of 6.9% at 420 nm.134
 | ||
| Fig. 6 (a) Keto–enol tautomerism in salicylidene anilines and synthesis and structures of all COFs; (b) band structure of COFs vs. the standard hydrogen electrode (SHE) and (c) plot of H2 evolution versus time for all COFs under solar simulator irradiation. Reproduced from ref. 133 with permission from the Royal Society of Chemistry. | ||
Vinylene-linked COFs have been explored for photocatalytic hydrogen evolution because their organic units are linked via C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C linkages, providing a highly stable and fully π-conjugated structure. Imine, azine, and hydrazone-linked COFs possess partial π-conjugation between building blocks, which limits extended conjugation in the COF system, while vinylene-linkage forms a fully conjugated system, which leads to strong absorption, effective charge transfer, and charge migration, benefitting their photocatalytic activity. Jiang and his group first reported the synthesis of vinylene-linked sp2c-COF using pyrene and phenylenediacetonitrile (PDAN) as building units. Surprisingly, sp2c-COF exhibited a highly crystalline, porous network with excellent chemical stability under ambient conditions. sp2c-COF further transformed sp2c-COFERDN by anchoring the electron-deficient ERDN group (3-ethylrhodanine) on its edges. This generated a push–pull effect, developing a heterojunction structure to facilitate charge separation of excitons to increase H2 generation. Thus, sp2c-COFERDN produced hydrogen at the rate of 2120 μmol g−1 h−1 and showed enhanced photocatalysis compared to sp2c-COF (1360 μmol g−1 h−1).135 Similarly, X. Liu and co-workers prepared a highly crystalline, porous, and durable sp2-carbon-linked COF, COF-JLU100, using triazine as the core moiety. The vinylene-linked triazine moieties formed a large π-conjugated structure, promoting charge separation and limiting charge recombination, which resulted in bulk conductivity and high charge mobility. Additionally, COF-JLU100 achieved high surface hydrophilicity, which increased its affinity towards water molecules, thus improving photocatalytic water reduction into hydrogen. Combining these features, COF-JLU100 achieved a high photocatalytic performance, achieving an HER rate of over 100
C linkages, providing a highly stable and fully π-conjugated structure. Imine, azine, and hydrazone-linked COFs possess partial π-conjugation between building blocks, which limits extended conjugation in the COF system, while vinylene-linkage forms a fully conjugated system, which leads to strong absorption, effective charge transfer, and charge migration, benefitting their photocatalytic activity. Jiang and his group first reported the synthesis of vinylene-linked sp2c-COF using pyrene and phenylenediacetonitrile (PDAN) as building units. Surprisingly, sp2c-COF exhibited a highly crystalline, porous network with excellent chemical stability under ambient conditions. sp2c-COF further transformed sp2c-COFERDN by anchoring the electron-deficient ERDN group (3-ethylrhodanine) on its edges. This generated a push–pull effect, developing a heterojunction structure to facilitate charge separation of excitons to increase H2 generation. Thus, sp2c-COFERDN produced hydrogen at the rate of 2120 μmol g−1 h−1 and showed enhanced photocatalysis compared to sp2c-COF (1360 μmol g−1 h−1).135 Similarly, X. Liu and co-workers prepared a highly crystalline, porous, and durable sp2-carbon-linked COF, COF-JLU100, using triazine as the core moiety. The vinylene-linked triazine moieties formed a large π-conjugated structure, promoting charge separation and limiting charge recombination, which resulted in bulk conductivity and high charge mobility. Additionally, COF-JLU100 achieved high surface hydrophilicity, which increased its affinity towards water molecules, thus improving photocatalytic water reduction into hydrogen. Combining these features, COF-JLU100 achieved a high photocatalytic performance, achieving an HER rate of over 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μmol g−1 h−1.136,137
000 μmol g−1 h−1.136,137
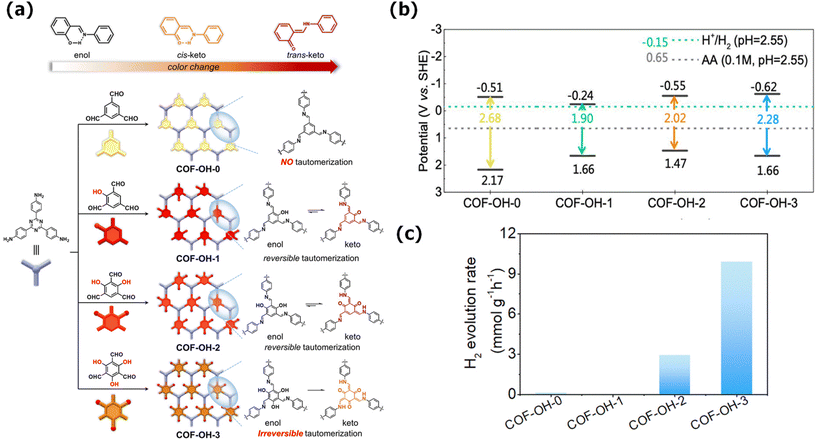
To highlight the importance of the vinylene linkage, COF-JLU35 and COF-JLU36, representing sp2 carbon and imine linkages were synthesized via a multicomponent synthetic method using benzotrithiophene and triazine moieties as electron-deficient and electron-rich sites, respectively (Fig. 7a). Despite their similar COF frameworks, COF-JLU35, as the sp2-carbon-linked COF, displayed broader absorption in the visible region, higher charge separation efficiency, and efficient charge transfer than the imine-linked COF. Thus, COF-JLU35 demonstrated a better photocatalytic performance with an HER rate of 70.8 mmol g−1 h−1 than COF-JLU36 and many reported COFs (Fig. 7b). The HER rate of COF-JLU36 was effectively maintained for five cycles without losing its initial properties (Fig. 7c).137 To signify the impact of the cyano group in the olefin linkage, Cai and his group designed a series of COFs (TTI-COF, TTV-COF, and TTAN-COF) with identical frameworks but different linkages. Among them, TTAN-COF displayed a significant enhancement in photoluminescence and photocatalytic properties such as a strong emission, improved photo-response, and high conductance of charged species. Consequently, the HER rates of these COF were found to follow the order of TTI-COF (0.46 mmol g−1 h−1) < TTV-COF (5.50 mmol g−1 h−1) < TTAN-COF (11.94 mmol g−1 h−1), where the TTAN-COF consisting of a vinylene linkage with cyano group displayed the highest photocatalytic performance. This work established that cyano functionalization in vinylene-linked COFs increases their chemical and photostability and enhances their optoelectronic properties.138
 | ||
| Fig. 7 (a) Synthetic route for COF-JLU35 and COF-JLU36. (b) Photocatalytic H2 evolution by COF-JLU36 under light irradiation (λ > 420 nm) and (c) hydrogen evolution curve as a function of time for COF-JLU36. Reproduced with permission from ref. 137. Copyright 2023, the American Chemical Society. | ||
Later, Zhang et al. developed two novel vinylene-linked COFs, g-C18N3-COF and g-C33N3-COF, via the Knoevenagel condensation of a triazine derivative with 1,3,5-tris(4-formylphenyl)benzene (TFPB) and 1,4-diformylbenzene (DFB) for sunlight driven H2 evolution, respectively. Although both COFs possessed strong light absorption ability, g-C18N3-COF displayed a superior HER rate of 14.6 μmol h−1 compared to g-C33N3-COF (14.6 μmol h−1). The longer conjugated backbone of g-C18N3-COF effectively suppressed the charge recombination and enhanced the charge separation and transfer ability for photocatalysis, which was also reflected in the longer lifetimes of its excitons and higher photocurrent value compared to g-C33N3-COF.139 Similarly, g-C31N3-COF, g-C37N3-COF, and g-C40N3-COF, with –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– linkages were prepared using dicyano-pyridine derivatives with different aldehydic organic moieties. Among them, g-C40N3-COF with the longest polyphenylene chain possessed a highly crystalline structure and porosity with large pore size, demonstrating an HER rate of 153 μmol h−1 with an apparent quantum yield of 4.84% (±0.27%) under 420 nm. Notably, the HER rates of g-C31N3-COF (27.1 μmol h−1) and g-C37N3-COF (19.8 μmol h−1) were relatively lower than g-C40N3-COF, suggesting that the crystalline nature of these COFs play an important role in enhancing their photocatalytic efficiency.140
C– linkages were prepared using dicyano-pyridine derivatives with different aldehydic organic moieties. Among them, g-C40N3-COF with the longest polyphenylene chain possessed a highly crystalline structure and porosity with large pore size, demonstrating an HER rate of 153 μmol h−1 with an apparent quantum yield of 4.84% (±0.27%) under 420 nm. Notably, the HER rates of g-C31N3-COF (27.1 μmol h−1) and g-C37N3-COF (19.8 μmol h−1) were relatively lower than g-C40N3-COF, suggesting that the crystalline nature of these COFs play an important role in enhancing their photocatalytic efficiency.140
Porphyrins are macrocyclic compounds with highly conjugated π-electron systems, which possess strong visible absorption, redox properties, and high thermal stability. Further, the porphyrin core can be metalated with different metal centres for the appropriate application. Porphyrin as the building block for COF networks can be beneficial for photoactivity given that it will enhance the absorption of light and develop unique photophysical and optoelectronic properties for COFs. R. Chen reported the synthesis of a series of porphyrin-based COFs, MPor-DETH-COF (M = 2H, Co, Ni, and Zn) for application in HER for the first time. MPor-DETH-COF was constructed via the Adler–Longo reaction between metalloporphyrinic aldehydes, p-MPor-CHO, and 2,5-diethoxyterephthalohydrazide (DETH). All the porphyrinic-based COFs exhibited a highly crystalline porous structure, as evidenced by the sharp peaks in their PXRD patterns and large BET surface. The HER performances of MPor-DETH-COF were observed to follow the order of CoPor-DETH-COF (25 μmol g−1 h−1) < H2Por-DETH-COF (80 μmol g−1 h−1) < NiPorDETH-COF (211 μmol g−1 h−1) < ZnPor-DETH-COF (413 μmol g−1 h−1) as the highest HER rate for a Zn-containing COF system. During photocatalytic hydrogen evolution, photoexcited electrons transfer through metal-on-metal channels to the catalytic surface for chemical reaction, while holes migrate through macrocycle-on-macrocycle channels. However, cobalt ions have a great tendency to undergo the ligand-to-metal transfer (LMCT) process, restraining hole migration, resulting in weak photocatalytic activity. The Ni ion showed a partial LMCT process, while the free base displayed charge transfer via macrocycle-on-macrocycle channels, producing hydrogen with lower HER rates. The LMCT process is strictly forbidden for Zn ions, which allows the free migration of holes and electrons, resulting in the best photocatalytic performance.141
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
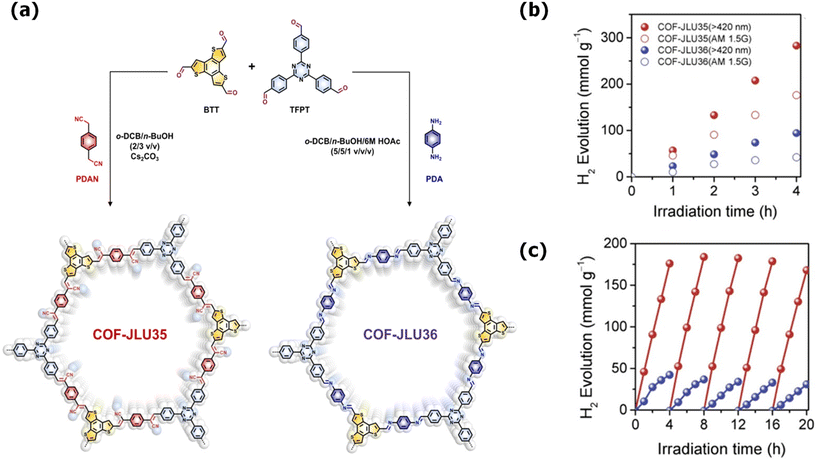
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) achieved an increased HER rate of 3678 μmol g−1 h−1, exceeding that of the pristine COF and CdS materials.142 Recently, Liu and his group fabricated T-COF with CdS to prepare a T-COF@CdS hybrid via an in situ reaction. T-COF was obtained using 2,4,6-tris(4-aminophenyl)-1,3,5-triazine as a building block with 1,3,5-benzenetricarboxaldehyde as the linker. CdS and T-COF formed a shell–core structure, where T-COF protected the CdS nanoparticles from deactivation and photo-corrosion during photocatalysis. T-COF@COF developed a Z-scheme heterojunction through strong interfacial interaction and gained a suitable band structure, which enhanced its charge separation and transfer capability. Thus, T-COF@CdS-3 achieved an AQY of 37.8% at 365 nm and exhibited a higher photocatalytic performance than CdS and T-COF.143 Further, Zhang et al. integrated MoS2 into a model TpPa-2 COF to develop a noble metal-free MoS2/TpPa-1 COF composite for photocatalytic HER application given that MoS2 can be a more effective co-catalyst than platinum. Among the different loadings of MoS2, MoS2-3%/TpPa-1-COF showed the highest HER activity, producing hydrogen at a rate of 55.85 μmol h−1. Notably, its HER performance is approximately similar to Pt/TpPa-1-COF (54.79 μmol h−1) with Pt loading {3 wt% (weight percentage)}, which can inspire the development of noble metal-free COFs as photocatalysts for HER. The photocurrent studies revealed that MoS2 behaves as a co-catalyst and enhances the charge transport capacity and reduces the charge recombination process, and thus the maximum electrons participate in H2 evolution.144 Likewise, D. Shang prepared an SnS2/TpPa-1-COF hybrid to produce a maximum HER rate of 37.11 μmol h−1 without the addition of a co-catalyst (Fig. 8a and b). SnS2/TpPa-1-COF formed a 2D–2D heterojunction, where the CB potential of TpPa-1-COF is more negative than that of SnS2 and the conduction band of SnS2 is higher compared to the potential for H2 generation (Fig. 8c). Thus, SnS2 displayed not only a broader absorption up to 600 nm, maximizing the photon absorption but also sped up the charge separation and migration, which resulted in a higher HER performance.145
10) achieved an increased HER rate of 3678 μmol g−1 h−1, exceeding that of the pristine COF and CdS materials.142 Recently, Liu and his group fabricated T-COF with CdS to prepare a T-COF@CdS hybrid via an in situ reaction. T-COF was obtained using 2,4,6-tris(4-aminophenyl)-1,3,5-triazine as a building block with 1,3,5-benzenetricarboxaldehyde as the linker. CdS and T-COF formed a shell–core structure, where T-COF protected the CdS nanoparticles from deactivation and photo-corrosion during photocatalysis. T-COF@COF developed a Z-scheme heterojunction through strong interfacial interaction and gained a suitable band structure, which enhanced its charge separation and transfer capability. Thus, T-COF@CdS-3 achieved an AQY of 37.8% at 365 nm and exhibited a higher photocatalytic performance than CdS and T-COF.143 Further, Zhang et al. integrated MoS2 into a model TpPa-2 COF to develop a noble metal-free MoS2/TpPa-1 COF composite for photocatalytic HER application given that MoS2 can be a more effective co-catalyst than platinum. Among the different loadings of MoS2, MoS2-3%/TpPa-1-COF showed the highest HER activity, producing hydrogen at a rate of 55.85 μmol h−1. Notably, its HER performance is approximately similar to Pt/TpPa-1-COF (54.79 μmol h−1) with Pt loading {3 wt% (weight percentage)}, which can inspire the development of noble metal-free COFs as photocatalysts for HER. The photocurrent studies revealed that MoS2 behaves as a co-catalyst and enhances the charge transport capacity and reduces the charge recombination process, and thus the maximum electrons participate in H2 evolution.144 Likewise, D. Shang prepared an SnS2/TpPa-1-COF hybrid to produce a maximum HER rate of 37.11 μmol h−1 without the addition of a co-catalyst (Fig. 8a and b). SnS2/TpPa-1-COF formed a 2D–2D heterojunction, where the CB potential of TpPa-1-COF is more negative than that of SnS2 and the conduction band of SnS2 is higher compared to the potential for H2 generation (Fig. 8c). Thus, SnS2 displayed not only a broader absorption up to 600 nm, maximizing the photon absorption but also sped up the charge separation and migration, which resulted in a higher HER performance.145
 | ||
Fig. 8 (a) Schematic synthesis of the 2D–2D SnS2/TpPa-1-COF hybrid, (b) photocatalytic H2 evolution of TpPa-1-COF, SnS2, S/C (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7), and mixed (3 7), and mixed (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) and (c) plausible mechanism of photocatalytic H2 evolution for 2D–2D SnS2/TpPa-1-COF. Reproduced with permission from ref. 145. Copyright 2021, the American Chemical Society. 7) and (c) plausible mechanism of photocatalytic H2 evolution for 2D–2D SnS2/TpPa-1-COF. Reproduced with permission from ref. 145. Copyright 2021, the American Chemical Society. | ||
Zhang and his co-workers incorporated hematite (α-Fe2O3) on the surface of a COF to form a Z-scheme-based hybrid, α-Fe2O3/TpPa-2 COF, for effective water splitting. The α-Fe2O3/TpPa-2 COF hybrid was synthesized via an in situ method under solvothermal conditions by varying the ratio of α-Fe2O3 and COF. The highest HER activity was found to be an HER rate of 3.77 mmol h−1g−1 by the α-Fe2O3/TpPa-2 COF (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 ratio). The excited electrons of α-Fe2O3 transfer to the VB of the COF and recombine with the photo-generated holes, inducing efficient charge separation on the COF surface. Thus, the photogenerated electrons in the COF at its CB are effectively involved in the reduction of protons to hydrogen.146 Similarly, SA Bao and his group constructed a core–shell structure of TpBD-COF@ZnIn2S4 (ZIS) nanosheets via the in situ growth of ZIS on the COF surface. Firstly, TpBD-COF was synthesized via the solvothermal reaction of triformylphloroglucinol (Tp) and benzidine (BD) and its hydrothermal treatment with ZIS formed the TpBD-COF@ZIS composite. The mass ratio of COF and ZIS was varied in the H2 evolution studies, where TpBD-COF@ZIS-10 with a mass ratio of 1
7 ratio). The excited electrons of α-Fe2O3 transfer to the VB of the COF and recombine with the photo-generated holes, inducing efficient charge separation on the COF surface. Thus, the photogenerated electrons in the COF at its CB are effectively involved in the reduction of protons to hydrogen.146 Similarly, SA Bao and his group constructed a core–shell structure of TpBD-COF@ZnIn2S4 (ZIS) nanosheets via the in situ growth of ZIS on the COF surface. Firstly, TpBD-COF was synthesized via the solvothermal reaction of triformylphloroglucinol (Tp) and benzidine (BD) and its hydrothermal treatment with ZIS formed the TpBD-COF@ZIS composite. The mass ratio of COF and ZIS was varied in the H2 evolution studies, where TpBD-COF@ZIS-10 with a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 exhibited an excellent performance under visible light, showing an HER rate of 2304 μmol g−1 h−1 with an AQE of 5.02% at 420 nm. The H2 evolution mechanism involves an S-scheme charge transfer, in which the charged electrons on the CB of COF transfer to VB of ZIS and are accompanied by holes, which provide efficient charge separation on the ZIS surface and retarded charge recombination.147
10 exhibited an excellent performance under visible light, showing an HER rate of 2304 μmol g−1 h−1 with an AQE of 5.02% at 420 nm. The H2 evolution mechanism involves an S-scheme charge transfer, in which the charged electrons on the CB of COF transfer to VB of ZIS and are accompanied by holes, which provide efficient charge separation on the ZIS surface and retarded charge recombination.147
MOF–COF hybrids have attracted significant attention for various applications given that they provide an enhanced ordered structure with high porosity and unique optoelectronic properties. In this regard, Lan and his group reported the synthesis of an MOF–COF hybrid, NH2-UiO-66/TpPa-1-COF, by appending NH2-UiO-66 MOF on the surface of the TpPa-1 COF via in situ solvothermal reaction. The MOF–COF hybrid possessed a crystalline porous network of both MOF and COF with a higher surface area than its parent COF. Notably, the CB position of TpPa-1-COF was found to be more negative than MOF, developing an n-type heterojunction system, where photogenerated electrons transfer from the CB of COF to the CB of MOF, increasing the charge separation efficiency on the COF surface. The photocatalytic performance of the NH2-UiO-66/TpPa-1 COF was greatly improved compared to its parent COF, producing hydrogen at an HER rate of 23.41 mmol g−1 h−1, which was 20 times higher than that of TpPa-1 COF under the same conditions.148 Likewise, Yan and his co-workers fabricated NH2-UiO-66 with TAPT-TP-COF to prepare the NH2-UiO-66/TAPT-TP-COF-X composite, where x represents the weight ratio of COF. NH2-UiO-66/TAPT-TP-COF-50 achieved an H2 evolution rate of 8.44 mmol h−1 g−1 under visible light irradiation using triethanolamine as a sacrificial agent.149 Additionally, a conventional Zr-MOF, MOF-808, was modified with an –NH2 ligand, resulting in the development of a core–shell MOF–COF hybrid, MOF-808@TpPa-1-COF, through an in situ solvothermal reaction with suitable COF organic units. The HER rate of MOF-808@TpPa-1-COF reached 11.88 mmol h−1 g−1, a notable increase of 5.6 times compared to its parent COF. Notably, the CB position of TpPa-1-COF was observed to be more negative than that of MOF, establishing an n-type heterojunction system. In this system, photogenerated electrons transfer from the CB of COF to the CB of MOF, enhancing the charge separation efficiency at the COF surface. The increased photocatalytic activity of the MOF/COF hybrid materials was observed due to their exceptional stability, effective charge separation/migration, and efficient inhibition of the charge recombination process for excitons.150
Graphitic carbon nitride (g-C3N4) is an emerging material as a metal-free photocatalyst due to its facile synthesis, light absorption capability, and optimized band structure for catalytic reactions. However, its high charge recombination of excited electrons and holes rapidly inhibits its photocatalytic activity. Therefore, the construction of COF hybrid materials with carbon nitride can improve its photocatalytic efficiency. H. Yan and his group first reported a g-C3N4-modified CN–COF hybrid via the in situ solvothermal reaction of g-C3N4, triformylphloroglucinol (TP) and triazine moieties. The ratio of COF was varied to prepare different COF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CN composites for the HER study. The photocatalytic water splitting experiment showed that CN–COF with 0.3 wt% COF produced the best HER activity with an HER rate of 10.1 mmol g−1 h−1, which was higher than that of g-C3N4 (1.05) and COF (0.16) under similar conditions.151 Further, Li and his group synthesized COF/g-C3N4 composites, represented as COF–CN, by varying the mass ratio of COF and g-C3N4 (1
CN composites for the HER study. The photocatalytic water splitting experiment showed that CN–COF with 0.3 wt% COF produced the best HER activity with an HER rate of 10.1 mmol g−1 h−1, which was higher than that of g-C3N4 (1.05) and COF (0.16) under similar conditions.151 Further, Li and his group synthesized COF/g-C3N4 composites, represented as COF–CN, by varying the mass ratio of COF and g-C3N4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x; x represents the mass ratio of COF, x = 2.5, 5, 10, 15, 20). COF/g-C3N4 developed a 1D/2D heterojunction system, where 1D COF extended its absorption area to 560 nm. Interestingly, COF–CN (1
x; x represents the mass ratio of COF, x = 2.5, 5, 10, 15, 20). COF/g-C3N4 developed a 1D/2D heterojunction system, where 1D COF extended its absorption area to 560 nm. Interestingly, COF–CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) exhibited a hydrogen evolution rate of 12.8 mmol g−1 h−1 with an AQY of 15.09% under 500 nm light illumination.152 In this context, g-C3N4 enhances the light absorption in the visible spectrum, facilitating efficient charge carrier transfer from g-C3N4 to COF through the imine linkage. This reduction in charge recombination of electron–hole pairs resulted in significantly improved charge separation and transfer. The substantial and close interface contact between the materials played a critical role in this, ultimately contributing to the outstanding visible photocatalytic performance exhibited by these composites.
10) exhibited a hydrogen evolution rate of 12.8 mmol g−1 h−1 with an AQY of 15.09% under 500 nm light illumination.152 In this context, g-C3N4 enhances the light absorption in the visible spectrum, facilitating efficient charge carrier transfer from g-C3N4 to COF through the imine linkage. This reduction in charge recombination of electron–hole pairs resulted in significantly improved charge separation and transfer. The substantial and close interface contact between the materials played a critical role in this, ultimately contributing to the outstanding visible photocatalytic performance exhibited by these composites.
Metalation of covalent organic frameworks (COFs) is another strategy to develop hybrid COFs for photocatalytic hydrogen evolution. COFs can have many functional groups that can bind with a single metal effectively without losing their crystallinity such as 2,2-bipyridine, phenanthroline, porphyrin, β-ketoenamine, and phosphine. In this regard, P. Dong et al. anchored single-atom platinum on the surface of β-ketoenamine-linked TpPa-1-COF to enhance its HER performance. Single-atom platinum atoms were highly dispersed on the COF surface and formed a six-coordinated species, C3N–Pt–Cl2, to gain stability. 3% Pt1@TpPa-1 displayed the optimal hydrogen evolution with a rate of 99.86 mmol gPt−1 h−1, which was found to be 3.9 and 48-times greater than that of 3% Pt NPs/TpPa-1 and pristine COF under similar conditions, respectively. The coordinated Pt single atom acts as the photocatalytic active site and reduces the energy barrier for the formation of H*, which becomes a key factor in the improved catalytic activity.153 Recently, Guo and co-workers prepared a nickel-metalated COF, TpBpy-NiX, by binding Ni(II) on a bipyridine-containing COF via a room temperature and solvothermal method (Fig. 9a). The solvothermally prepared metalated TpBpy-NiX (X = 1%, 2%, 3%, 10%, 20%) showed stable hydrogen evolution under visible light, and the HER rate increased as the content of Ni(II) ions increased. Among them TpBpy-Ni2% exhibited high photocatalytic activity, producing the highest HER rate of 51300 μmol h−1 g−1 (Fig. 9c).154 The emission studies revealed that the interaction between the nickel ion and bipyridine decreased the bandgap between the HOMO and LUMO compared to the metal-free COF (Fig. 9b). This prolonged the lifetimes of the photoexcited electrons and improved the electron–hole separation, which enhanced the photocatalytic performance.
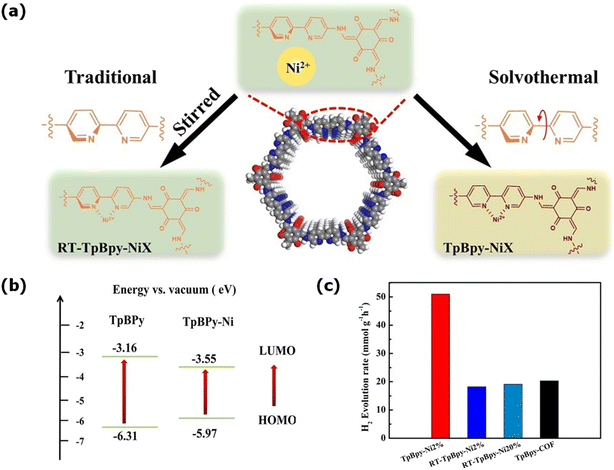 | ||
| Fig. 9 (a) Synthesis of TpBpy-NiX COF by traditional and solvothermal methods. (b) Comparison of the band gap of TpBpy and TpBpy-Ni COFs and (c) H2 evolution rates for TpBpy-Ni2% with other COFs. Reproduced with permission from ref. 154. Copyright 2023, Wiley Online Library. | ||
5.2 Photocatalytic CO2 reduction reaction
Presently, global warming and climate change have become the main environmental problems due to the high concentration of carbon-dioxide in the atmosphere.155 In this case, although many CO2 capture/conversion technologies have been developed, the photocatalytic conversion of CO2 into valuable chemicals has emerged as green technology to address environmental issues.156 The breaking of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in CO2 requires high energy, making CO2 conversion a kinetic and thermodynamically unfavourable process.157 The conversion of CO2 into CO occurs at −0.53 V vs. NHE, while other products such as formic acid, methane, and methanol can also be produced at different potentials depending on the nature of the photocatalyst. Hence, efficient photocatalysts are being employed to reduce CO2 by utilizing sunlight as an energy source.158 Among them, COFs have attracted worldwide attention as promising candidates for the photocatalytic reduction of CO2. In this case, the COFs should possess high CO2 adsorption ability, light-harvesting capacity, and appropriate band structure for CO2 photoreduction.159 Although metal-free COFs have been applied as photocatalysts, metal-supported COFs are highly effective for CO2 reduction given that CO2 molecules can coordinate with the metal site, enhancing the CO2 adsorption capacity and lowering the activation energy for CO formation.
O bond in CO2 requires high energy, making CO2 conversion a kinetic and thermodynamically unfavourable process.157 The conversion of CO2 into CO occurs at −0.53 V vs. NHE, while other products such as formic acid, methane, and methanol can also be produced at different potentials depending on the nature of the photocatalyst. Hence, efficient photocatalysts are being employed to reduce CO2 by utilizing sunlight as an energy source.158 Among them, COFs have attracted worldwide attention as promising candidates for the photocatalytic reduction of CO2. In this case, the COFs should possess high CO2 adsorption ability, light-harvesting capacity, and appropriate band structure for CO2 photoreduction.159 Although metal-free COFs have been applied as photocatalysts, metal-supported COFs are highly effective for CO2 reduction given that CO2 molecules can coordinate with the metal site, enhancing the CO2 adsorption capacity and lowering the activation energy for CO formation.
Later, Lie et al. designed a 2D-COF, CT-COF, which produced CO with 98% selectivity upon CO2 photoreduction without the use of any sacrificial donor or co-catalyst. CT-COF, having carbazole and triazine moieties, formed a donor–acceptor COF, which displayed strong light absorption, optimal band gap, and high nitrogen content. The donor–acceptor COF system generated effective charge separation and charge transfer of electron–hole pairs, which resulted in good photocatalytic activity. CT-COF achieved a CO production rate of 102.7 μmol g−1 h−1 with high selectivity.161
Wang et al. proposed that the modulation of the band structure of COFs can provide effective CO2 conversion upon light irradiation. They modified a porphyrin-based COF, COF-366, into TAPBB-COF by introducing a bromo group. Consequently, the valence band position of TAPBB-COF shifted to 1.10 V compared to COF-366 (0.86 V), making its band energy gap more suitable for CO2 photoreduction. TAPBB-COF achieved a CO production rate of 295.2 μmol g−1 h−1, which was significantly higher compared to that of COF-366 (3.9 μmol g−1 h−1). Notably, the porphyrin ring activated the CO2 molecules and the bromo group increased the adsorption of water molecules, thus achieving high CO2 conversion into CO.162 Similarly, Gao and co-workers utilized porphyrin-based covalent organic nanosheets for visible light-driven CO2 reduction. Two porphyrin-based COFs, 2,3-DhaTph, and 2,3-DmaTph, were first exfoliated into CONs (covalent organic nanosheets) with a high yield via a simple and efficient method. The photocatalytic experiments revealed that the CO yield of CONs was 132.2 and 115.5 μmol g−1 h−1, which are much higher than that of their parent COFs, 2,3-DhaTph (56.6 μmol g−1 h−1) and 2,3-DmaTph (115.5 μmol g−1 h−1) under similar conditions, respectively. The formation of CONs produced more active sites for CO2 uptake, exhibiting excellent electron–hole separation and high charge transfer during photocatalysis. Overall, CONs exhibited a superior CO production rate compared to bulk COF photocatalysts, providing a new direction to develop novel photocatalysts for CO2 reduction.163
To demonstrate the effect of functionalization on the photocatalytic activity of COFs, four different COFs, TpBD-H2, TpBD-(CH3)2, TpBD-(OCH3)2, and TpBD-(NO2)2, were developed by varying different functionalized diamines with 1,3,5-triformylphloroglucinol as the building block and further explored for CO2 reduction. All TpBD-X COFs transformed CO2 into formic acid under light illumination without any side products. Among them, TpBD-(OCH3)2 and TpBD-(CH3)2 produced formic acid at a rate of 108.3 and 86.3 μmol g−1 h−1, respectively, which are comparatively higher than that of TpBD-H2 and TpBD-(NO2)2. The electron-donating effect of methoxy and methyl groups exhibits strong conjugation within the COFs, leading to the maximum light absorption, rapid charge transfer, and enhanced charge separation compared to electron-withdrawing groups, and thus a high photocatalytic performance was observed. The results demonstrated that the photophysical properties of COFs can be tuned by the functionalization of their organic units to achieve high photocatalytic performances.164 Islam and his group designed a novel 2D triphenylamine-triazine COF, Tta-TFPA COF, via a hydrothermal reaction between triazine and triphenylamine organic moieties. Tta-TFPA COF exhibited a highly crystalline structure with wide light absorption and produced solar fuels such as formic acid and methanol upon photocatalytic CO2 reduction. It formed HCOOH at a rate of 48 mol g−1 h−1 using TEOA as a sacrificial donor and a blue LED (445 nm) as the light source. It also converted CO2 into MeOH at a rate of 8.3 mmol g−1 h−1 using triethylamine (TEA) as a sacrificial agent under similar conditions (Fig. 10). Notably, Tta-TFPA COF was also capable of performing CO2 photoreduction under sunlight irradiation, producing HCOOH and MeOH at the rate of 41 mol g−1 h−1 and 0.73 mmol g−1 h−1, respectively.165 Likewise, Zhu and co-workers prepared two imine-linked COFs, PDA-TTA and PDA-TAB, using diphenylamine as the linker and 4,4′,4′-(1,3,5-triazin-2,4,6-triyl)triphenylamine (TTA) and 1,3,5-tris(4-aminophenyl)benzene (TAB) as building blocks and explored their activity as photocatalysts for CO2 reduction. PDA-TTA showed better photocatalytic efficiency than PDA-TAB for the conversion of CO2 into HCCOH with a rate of 65.7 μmol g−1 h−1 under visible light. PDA-TTA COF exhibited high light absorption and efficient charge transfer due to its planar triazine units, which increased its photocatalytic performances.166
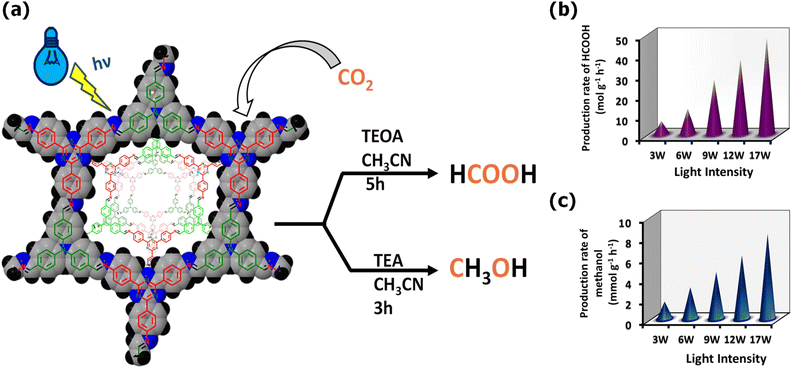 | ||
| Fig. 10 (a) Photocatalytic generation of HCOOH and MeOH by Tta-TFPA COFs, (b) HCOOH production rate and (c) MeOH generation rate using the Tta-TFPA COFs under visible light. Reproduced with permission from ref. 165. Copyright 2022, the American Chemical Society. | ||
Likewise, Goa and his group developed a series of COF membranes, XN-COF, using an ionic–liquid interface and mimicked an artificial leaf system for efficient light-driven CO2 reduction. In the XN-COF membranes, X represents the number (0, 1, and 2) of triazine units present in the parent amine and aldehyde monomers. The 2N-COF membrane exhibited a highly crystalline network and larger surface area compared to 1N-COF and 0N-COF given that its high number of triazine units forms an ordered stacking pattern. Thus, the 2N-COF membrane exhibited excellent visible light CO2 reduction, giving the highest CO production rate of 310 μmol g−1 h−1 without the use of any photosensitizer or donor. The 2N-COF membrane outperformed the 2N-COF powder as a photocatalyst, which produced CO at a rate of 98 μmol g−1 h−1 under similar conditions. The remarkable photocatalytic activity of the leaf-like COF membrane was based on its triazine–imine–triazine framework, where the triazine moieties functioned as electron reservoirs, the imine bond acted as a photocatalytic centre and the leaf-like structure enhanced the charge/mass transfer. This study suggests that artificial leaf systems based on COFs can be very effective as photocatalysts for CO2 reduction to CO.167
Yang and his group reported the synthesis of two novel sp2c-COFs, TFPB-COF and BTE-TBD-COF, for improved light-driven CO2 reduction to CO. BTE-TBD-COF possessed an ethylene bridge in its COF network, which enhanced the conjugation effect, significantly resulting in improved charge separation of photogenerated electrons and efficient charge migration. This is also supported by the photocurrent responses from both COFs, where the average lifetime of the excited electrons for BTE-TBD-COF (8.3 ns) was much longer compared to TFPB-COF. Further, the band gap of BTE-TBD-COF became more favorable for the CO2 reduction process, producing CO at a rate of 382.03 μmol h−1 g−1, while TFPB-COF showed a CO production rate of 109.8 μmol h−1 g−1. This demonstrated that the extended conjugation in the COF framework can facilitate charge separation and migration to enhance its photocatalytic activity.168
Tang and co-workers explored hydrazone-linked COFs for photocatalytic CO2 reduction and synthesized two new COFS, BTT-Hz-1 and BTT-Hz-2 COFs, using a conjugated aldehyde (benzo[1,2-b:3,4-b′:5,6-b′′]trithiophene-2,5,8-tricarbaldehyde, BTT) with two different hydrazone monomers. BTT-Hz-2 exhibited a CO evolution rate of 774.3 μmol g−1 h−1 with CoCl2/Bpy as a co-catalyst and TEOA as a sacrificial donor, while BTT-Hz-1 produced CO at a rate of 251.4 μmol g−1 h−1 under similar conditions. To signify the importance of the BTT unit in the COF, TFB-1 and TFB-2 COFs were prepared by applying a benzene-containing aldehyde (1,3,5-triformylbenzene, TFB) monomer with the corresponding hydrazones, and these COFs were unable to produce CO under similar conditions. The conjugated nature of the “benzotrithiophene” moiety in BTT than the “benzene” ring of TFB increased the electron delocalization in the BTT-based COFs and decreased their bandgap, making them suitable for photocatalysis. The better photocatalytic performance of BTT-Hz-2 compared to BTT-Hz-1 can be ascribed to its broader visible-light absorption range due to its extended conjugation, large porous surface, and efficient charge separation within its COF network.169 Jin and co-workers enhanced the photocatalytic CO2 reduction by introducing a hydrophilic group in the COF photocatalyst. They developed QL-COF by carboxylquinoline linkages, which possessed hydrophilic carboxylic acid in its porous surface. Due to the presence of carboxy groups, QL-COF exhibited a low binding energy toward water and CO2 molecules, leading to the high adsorption of water and CO2 on the surface of the COF. Thus, QL-COF showed a CO production rate of 156 μmol g−1 h−1 with 99.3% selectivity, which is much higher than that of LZU1-COF (25 μmol g−1 h−1) lacking a carboxy group in its structure.170
 | ||
| Fig. 11 (a) Route for the synthesis of viCOF-bpy-Re. (b) Schematic representation of bipyridine and triazine ring units in viCOF-bpy. (The purple balls indicate bipyridine units while yellow balls indicate triazine units and vinyl linkages.) (C) Band gap diagram of viCOF-bpy-Re and viCOF-bpy and (d) photocatalytic CO2 reduction by Re(CO)6Cl, viCOF-by and viCOF-by-Re. Reproduced with permission from ref. 172. Copyright 2023, Wiley Online Library. | ||
The incorporation or loading of a single metal atom on the surface of COFs can also be beneficial for solar-driven CO2 reduction because a metal site can act as an active centre for photocatalytic CO2 conversion. The coordination of metal sites on COFs effectively enhances the rate of CO2 reduction with high selectivity for the products. In this regard, Lan et al. reported the modification of a series of COFs by appending transition metals such as cobalt, nickel, and zinc atoms on their porous surface. The DQTP-COF-M (M = Co/Ni/Zn) COFs were prepared via a facile and simple post-treatment method with metal complexes. The metalated COFs showed high crystallinity given that their PXRD patterns were similar to that of the non-metalated DQTP-COF. The photocatalytic experiments were performed in acetonitrile solution using triethanolamine (TEOA) as a sacrificial agent and Ru(bpy)3Cl2·6H2O (bpy = 2,2-bipyridine) as a photosensitizer. Surprisingly, the different metals produced different products with high selectivity. DQTP COF-Co produced CO at a rate of 1.02 × 103 μmol h−1 g−1, while DQTP COF-Zn produced formic acid at a rate of 152.5 μmol h−1 g−1 with 90% selectivity over CO. The adsorbed CO2 activated upon photocatalysis and formed intermediate CO2, which can produce the final product via two pathways. Based on the experiments, it was suggested that Co(II) has good π–donor ability and converts CO2 to CO, while Zn(II) as a poor π–donor produces HCOOH over CO.174 Additionally, this work demonstrated that metal centres on COFs as active sites can be very effective for CO2 reduction.
Porphyrin-based COFs have emerged as efficient catalysts for the catalytic reduction of CO2. A series of single-atom porphyrin-based COFs, TTCOF-M (M = 2H, Zn, Ni, Cu), was synthesized using the tetrathiafulvalene (TTF) metalloporphyrin as the building block, which was utilized as photocatalysts for reducing CO2 with water. Porphyrin moieties have strong absorption in the visible region, while sulfur-rich TTF moieties possess strong electron-donating nature with fast electron transfer, thus forming a donor–acceptor COF system. Therefore, this drives the effective separation of photogenerated electrons and holes on the porphyrins and TTF sites, respectively. TTCOF-M was developed as an artificial photosynthetic system, where its porphyrin unit participates in CO2 reduction to CO, while its TTF unit transforms H2O into oxygen. Among them, TTCOF-Zn showed the best CO production rate of 12.33 μmol over a period of 60 h, which is double the oxygen evolution rate.175 Similarly, Zhu et al. also applied a nickel-metalated porphyrin-based COF, PD-COF-23-Ni, for the photocatalytic transformation of CO2 into CO and produced CO at a rate of 40.0 μmol g−1 h−1 without any photosensitizers or noble metal catalyst. Compared to PD-COF-23 without any metal site, PD-COF-23-Ni showed an effective charge separation efficiency and CO2 reduction process at the Ni(II) centre, enhancing its activity for the photoreduction process.176 The incorporation of metal nanoparticles (NPs) is the most effective way to overcome the charge recombination process during photocatalytic reactions. Using this approach, Fan and co-workers deposited ruthenium nanoparticles on a bipyridine-containing COF to develop a ruthenium-decorated COF, Ru@TpBpy. Ruthenium nanoparticles were deposited on the COF via the solution infiltration technique with different loading amounts. The photocatalytic experiments revealed that Ru@TpBpy with 0.7 wt% Ru NPs displayed a higher photocatalytic CO2 conversion compared to TpBpy and yielded formic acid at a rate of 172 mmol gcat−1 h−1. The ruthenium nanoparticles in the COF widened its absorption region and enhanced the charge separation and migration by reducing the charge recombination process, leading to an increased photocatalytic performance.177
Z-scheme heterojunction systems can be developed by covalently connecting COFs with inorganic semiconductors, which can be effective for photocatalytic applications. Zhang and co-workers developed a Z-scheme-based heterojunction system of COF-semiconductor composites as photocatalysts. Three different inorganic semiconductors, TiO2, Bi2WO6, and α-Fe2O3, were combined with COF-316 and COF-318 and employed for photocatalytic CO2 reduction. The photocatalytic CO2-reduction process was carried out under visible light without the use of any photosensitizer, sacrificial agent, or co-catalyst. Among them, the COF-318-TiO2 heterojunction COF system exhibited the highest photocatalytic performance, achieving a CO production rate of 69.67 μmol g−1 h−1, which was six-times higher than that of the pristine COF-318, TiO2 powder, and their physical mixture. The covalent linkage in the Z-scheme heterojunction COF-semiconductor facilitates effective charge transfer between the COF and semiconductor, which leads to the accumulation of excited electrons on the COF and holes at the semiconductor for the reduction and oxidation process, respectively, forming an artificial photosynthetic system.178 Further, Do and co-workers integrated single-atom Co-1T-MoS2 on a β-ketoenamine-linked 2D COF TpPa-1 COF for the selective reduction of CO2 into CO under visible light irradiation. The single-atom Co-1T-MoS2 was prepared via the hydrothermal reaction of a molybdenum complex ((NH4)6Mo7O24·4H2O) with thiourea and cobalt acetate and incorporated it in a COF via a physical method (Fig. 12a). The TpPa-1/Co-1T-MoS2 composite exhibited increased CO2 reduction, giving a CO production rate of 196 μmol g−1 h−1 with 93% selectivity (Fig. 12b). The photocatalytic efficiency of CO generation by the composite was 1.23 and 1.6-times higher than that of the bare COF and Co-1T-MoS2, respectively. The mechanistic pathway for the composite depicted that the energy band of MoS2 lies below the conduction band of the COF and the photogenerated electrons in the COF effectively transfer to the CB of MoS2 under light irradiation, which further reach the surface of the photocatalyst to participate in the catalytic reaction.179
 | ||
| Fig. 12 (a) Schematic representation of the formation of the TpPa-1/Co-1T-MoS2 composite. (b) Photocatalyzed CO2 reduction of (a) MoS2, (b) Co-1T-MoS2, (c) TpPa-1 (hollow), and (d) TpPa-1/Co-1T-MoS2. Reproduced with permission from ref. 179. Copyright 2023, the American Chemical Society. | ||
Graphene as a carbon-based material can increase the photocatalytic activity of COFs for CO2 photoreduction given that its extended conjugated network hampers the charge recombination of excitons, enhancing the adsorption of CO2, activation, and stability under visible light. Do and co-workers synthesized a hybrid composite of COF and reduced graphene oxide, rGO@TpPa-1, to improve the CO2 reduction process under light illumination. The rGO@TpPa-1 composites were synthesized via the in situ reaction of rGO (content of 5%, 10%, 15%, and 20%), triformylphloroglucinol (Tp), and diaminobenzene under solvothermal conditions. The formation of the hybrid composite was confirmed through PXRD analysis based on the appearance of the characteristic peaks for COF and rGO. The CO2 photoreduction experiments for rGO15@TpPa-1 exhibited a CO yield of 198.97 μmol g−1 h−1 with 89% selectivity over HER, which is higher than that of the parent TpPa-1 COF (126.36 μmol g−1 h−1) and bare rGO (28.51 μmol g−1 h−1). The integration of rGO with the COF improved the light-absorption capacity, inhibited the charge recombination process, and enhanced the charge mobility, consequently increasing its photocatalytic performance.180 Likewise, Ye and his group developed a 2D/2D heterojunction COF hybrid (g-C3N4(NH)/COF) as a photocatalyst by combining g-C3N4 with TpTta-COF, which was synthesized using triazine and trifomylphloroglucinol moieties. g-C3N4(NH)/CO achieved high photocatalytic CO2 reduction, yielding CO at a rate of 11.25 μmol h−1 with 90% selectivity. The CO production rate for the COF hybrid was 45-times higher compared to that of the bare g-C3N4 (0.25 μmol h−1). The g-C3N4(NH)/COF hybrid works as an S-scheme heterojunction system, where the photo-excited electrons in the CB of COF combine with the photogenerated holes in the VB of g-C3N4, effectively reducing the charge recombination rate, while the excited electrons from the CB of g-C3N4 reaches the photosensitizer for the conversion of adsorbed CO2 molecules.181
Further, Wu and co-workers proposed a novel MOF/COF hybrid as a photocatalyst using olefin-linked TTCOF and NUZ MOF (NH2–UiO-66 (Zr)) as precursors to achieve high photocatalytic activity for CO2 reduction to CO. TTCOF/NUZ hybrids were prepared via the in situ reaction of TTCOF with NH2-BDC and ZrCl4 by varying the ratio of TTCOF (x = 5, 10, 15, 20, 30). The optimized TTCOF/NUZ was an S-scheme heterojunction system and displayed a higher photocatalytic performance than the bare COF and MOF. TTCOF/NUZ produced CO at a rate of 6.56 μmol g−1 h−1 in a gas–solid system under visible light. The increased photocatalytic activity can be ascribed to the effective charge carrier separation in the heterojunction system, which enhances the charge transportation and redox properties.182
5.3 Photocatalytic oxygen evolution reaction
The photocatalytic oxygen evolution reaction (OER) is a half-reaction of photocatalytic overall water splitting, where photogenerated holes produce oxygen as the product from water molecules. The redox potential for oxygen evolution is up to 1.23 eV, which is a four-electron process consisting of two water molecules.183 Thus, the OER process is a thermodynamically and kinetically unfavorable process. In this case, the design of a photocatalyst for OER not only requires strong light absorption and a highly porous surface but also needs an appropriate valence band potential to successfully produce oxygen from water splitting. Thus, very few COFs have been reported as photocatalysts for the OER process.184Metal-free COFs can not facilitate the photocatalytic OER process. Thus, COFs have been combined with suitable metals, noble metals or photosensitizers or modified with them to develop hybrid COFs to perform this reaction. These hybrid COFs exhibit activity for overall water splitting, producing oxygen and hydrogen in high yield. In 2018, Yang et al. and co-workers synthesized a cobalt-incorporated bipyridine-containing COF, BpCo-COF, for visible light-driven oxygen evolution. Bp-COF was prepared via the solvothermal reaction between tris(4-aminophenyl)benzene and 2,2-bipyridine-5,5′-dicarboxaldehyde organic moieties and further incorporated cobalt metal through coordination with the bipyridine unit via post-synthetic treatment. The HOMO level of Bp-COF appeared at +1.46 V vs. RHE and positively shifted at 1.54 V for BpCo-COF, providing a feasible oxidation potential for oxygen evolution. Also, BpCo-COF showed a higher water uptake capacity than Bp-COF due to the high affinity of water molecules toward the Co atom, which will be beneficial for ORR activity. BpCo-COF exhibited superior oxygen evolution at a rate of 152 μmol g−1 h−1 under visible light with an AQY value of 0.46%, while Bp-COF was unable to produce oxygen under similar conditions. BpCo-COF was also tested for long-term stability, exhibiting excellent ORR activity over 31 h. It was evidenced that cobalt serves as a co-catalyst in BpCo-COF and increases its photocatalytic activity by lowering the activation energy for redox reactions by facilitating charge separation and migration. This was the first report in which a COF was successfully employed for visible-light-driven oxygen evolution.185
Likewise, Li et al. incorporated cobalt ions in a triazine-based COF, TAPT-Bpy-COF, to enhance its photocatalytic performance for oxygen evolution. The absorption profile of TAPT-Bpy-COF-Co was red-shifted compared to that of the parent COF, indicating its increased light absorption capability. The wettability of the COF also improved after the integration of cobalt, depicting a high water intake capacity. TAPT-Bpy-COF-Co showed an impressive oxygen evolution rate of 483 μmol g−1 h−1 upon light irradiation. TAPT-Bpy-COF-Co displayed a superior oxygen evolution rate compared to the similar BpCo-COF with an increased AQY of 7.6%. This improved photocatalytic activity of TAPT-Bpy-COF-Co can be ascribed to its high crystallinity, porosity, good wettability, and better charge separation.186
Further, He and his group designed a novel COF (BtB-COF) with N,S-chelated atomic Co catalytic sites in its pore channel for photocatalytic water splitting. BtB-COF was synthesized via the condensation reaction of benzotrithiophene tricarbaldehyde and benzothiadiazole-diamine building units (Fig. 13a). A strong peak was observed in the EPR study, corresponding to the unpaired electrons, which indicated that the electron-deficient benzothiadiazole and electron-rich benzothiophene groups developed a push–pull effect, increasing the optical absorption with a smaller band gap and enhancing the photoinduced charge separation and transfer (Fig. 13b). The light-driven oxygen evolution by BtB-COF was investigated using Co as co-catalyst and AgNO3 as an electron acceptor, achieving an oxygen evolution rate of 6.65 μmol h−1 (Fig. 13d). The FT-IR and XPS analyses revealed that the cobalt ions chelated with nitrogen and sulfur atoms under light illumination to form active metal sites for oxygen evolution activity. The chelated cobalt sites adsorbed water molecules through a hydration process, which increased the water dispersibility and lowered the energy barrier for the oxygen evolution process, overall promoting the water oxidation process (Fig. 13c). Under similar photocatalytic experiments, the analogous BtD-COF without a benzothiadiazole unit produced an O2 evolution rate of 2.2 μmol h−1, which was less than that of BtB-COF, implying the advantages of functionality and structural modulation of COFs for photocatalytic application. Further, a heterostructure CdS/BtB-COF was developed by immobilizing CdS nanoparticles on BtB-COF for overall water splitting. CdS/BtB-COF displayed high photocurrent responses and decreased resistance compared to the bare BtB-COF and CdS due to the effective charge separation and migration in the hybrid composite. Thus, the photocatalytic efficiency of CdS/BtB-COF was greatly improved for water splitting and it achieved an O2 evolution rate of 212 μmol g−1 h−1, which is comparatively higher than that of many inorganic semiconductor-based photosystems for water splitting.187
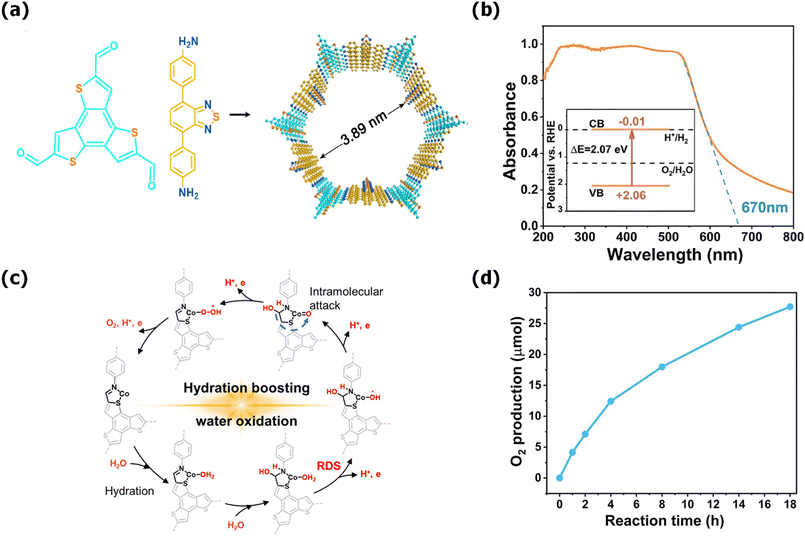 | ||
| Fig. 13 (a) Synthesis and (b) absorption profile and bandgap of BtB-COFs. (c) Plausible mechanism of light-driven O2 evolution of BtB-COFs and (d) O2 evolution over BtB-COFs over a period of 18 h under AM 1.5G light irradiation. Reproduced with permission from ref. 187. Copyright 2023, the American Chemical Society. | ||
Han and his group designed two metal covalent organic frameworks as photocatalysts for the water oxidation process by employing a ruthenium complex as one of the building units for the formation of COF. A solvothermal reaction was performed between a tetradentate aldehyde-functionalized Ru(II) complex, Ru(bpy-CHO)2Cl2, and the amino-functionalized organic units ETTA and ETTBA to synthesize novel MCOFs, RuCOF-100 and Ru-COF101, respectively. Both COFs exhibited highly crystalline networks, forming three-dimensional frameworks with high porosity and easily accessible, orderlyarranged Ru sites. The coordinated Cl ligands of the Ru sites were replaced by water molecules, forming Ru-100′ and Ru-101′, which enhanced the hydrophilicity of the COFs and promoted their water dispersibility. The photocatalytic water oxidation reaction of Ru-COFs was investigated using Ru(bpy)32+ as a photosensitizer. RuCOF-100′ and RuCOF-101′ showed excellent oxygen evolution rates of 2305 and 2830 nmol g−1 s−1, respectively. The plausible mechanism of the water oxidation process involves the oxidation of Ru(II) into high-valent Ru(V)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species, which is transformed into the hydroperoxo intermediate Ru(III)–O–OH by H2O coordination. This is further converted to Ru(V)–OO via a proton-coupled electron transfer process, which releases dioxygen as the final product.188
O species, which is transformed into the hydroperoxo intermediate Ru(III)–O–OH by H2O coordination. This is further converted to Ru(V)–OO via a proton-coupled electron transfer process, which releases dioxygen as the final product.188
Similarly, S. Karak synthesized a novel 3D COF, Ru(bda)COF, for improved photocatalytic oxygen evolution via the condensation reaction of aldehyde-functionalized ruthenium complex and tetrakis(4-aminophenyl)methane. The electrochemical studies for Ru(bda)COF showed three oxidation peaks versus NHE at +0.67, +0.89, and +1.08 V, corresponding to the formation of Ru(III), Ru(IV), and Ru(V) in aqueous solution, while oxygen evolution generally proceeds at a lower potential. Upon light-driven water oxidation, Ru(bda)COF exhibited an oxygen evolution rate of 1072 μmol L−1 s−1. The water oxidation catalyst (WOC) mechanism of Ru(bda)COF generally involves second-order rate kinetics, which suggests that the rate-determining step involves two COF particles. However, its amorphous counterpart, Ru(bda)COF, showed much lower activity, exhibiting an O2 evolution rate of 3.0 μmol L−1 s−1, which demonstrated that the orderly arrangement in the crystalline form is effective for efficient photocatalysis.189
Xu and his group developed a Z-scheme heterostructure-based piezoelectric material-decorated COF photocatalyst to achieve photocatalytic water splitting. BiFeO3@TpPa-1-COF was prepared under in situ solvothermal conditions, where the precursor of TpPa-1-COF underwent Schiff base condensation with BiFeO3 nanosheets that were amino-functionalized by 3-aminopropyltriethoxysilane (APTES) (Fig. 14a). The combination of COF and BiFeO3 formed a synergistic Z-scheme heterostructure, where electrons accumulated at the COF site, while holes collected at BiFeO3. Thus, BiFeO3@TpPa1-COF (BFO@COF20-C) demonstrated the excellent H2 and O2 production rates of 1416.4 and 708.2 μmol g−1 h−1 under the combined effect of light and ultrasound, respectively (Fig. 14b). The piezo-photocatalytic effect of the BiFeO3@TpPa-1-COF heterojunction increased the transfer of photogenerated electrons and holes in the opposite sites, resulting in efficient charge separation and mass transportation, respectively, which subsequently improved the photocatalytic performances (Fig. 14c). The BiFeO3@TpPa-1-COF hybrid COF showed a superior oxygen evolution rate compared to the reported BaTiO3 NPs and ZnS nanosheet material, demonstrating the superiority of piezo-catalysis (Fig. 14d).190
 | ||
| Fig. 14 (a) Schematic synthetic route for the BiFeO3@TpPa-1-COF hybrid. (b) O2 evolution of piezo-photocatalysis. (c) Mechanism of O2 evolution of piezo-photocatalysis for the BiFeO3@TpPa-1-COF heterojunction and (d) COFs and C3N4-based photocatalysts. Reproduced with permission from ref. 190. Copyright 2022, Wiley Online Library. | ||
A series of β-ketoamine COFs with and without a bipyridine moiety, denoted as TpBD-COF, TpBpy-2-COF, and TpBpy-COF, was exfoliated to form COF nanosheets (COF-NS) and their pore channels were encapsulated with Pt nanoparticles for photocatalytic water splitting. The smooth surface of COF-NS increased the dispersion of NPs throughout the pore channel of COF-NS, as evidenced by TEM and AFM studies. Pt@TpBpy-NS displayed excellent photocatalytic water splitting activity, producing H2 and O2 evolution at the rate of 9.9 and 4.8 μmol over a period of 5 h, which is comparably higher relative to Pt@TpBpy-2-NS, producing H2 and O2 at the evolution rate of 3.1 and 4.1 μmol, respectively. The engineering of the nitrogen site in the COF framework greatly affects the photocatalytic activity of Pt@COF-NS given that bipyridine acts as the binding site for COF. Pt@Tp-BD-COF could not perform the water-splitting process due to the absence of the bipyridine moiety and only exhibited hydrogen evolution, half of the reaction of the water-splitting process. Pt@TpBpy-2-NS possessed differently positioned nitrogen sites, which could not coordinate with Pt NPs, lowering its photocatalytic activity. Pt@TpBpy-NS with adjacent nitrogen positions exhibited the effective accumulation of Pt NPs, which promoted photoinduced charge separation and transfer, thus achieving a high photocatalytic performance. This work demonstrated that the existence and rational position of the nitrogen sites are crucial to inducing charge transfer and reaction potential barriers for water splitting activity.191
Later, Shen and his co-workers constructed a heterojunction system via the in situ deposition of 2D COF on WO3 nanosheets (Ov-WO3) to boost the photocatalytic performance for overall water splitting. The COF precursor, triformylphloroglucinol (TP), and 3,7-diaminodibenzo[b,d]thiophene-5,5-dioxide underwent in situ photo-assisted reaction with WO3 nanosheets and coordinated to the oxygen vacancy of WO3via a W–O–C covalent bond to form TSCOFW (COF–WO3) as a hybrid composite. The combination of WO3 and COF developed a synergistic Z-heterojunction system via the increased built-in electric field of the W–O–C bond, which is beneficial for the effective charge separation of photoinduced electrons and holes and maximizes the utilization efficiency of charge carriers. This led to the high photocatalytic performance of the TSCOFW hybrid for OWS, which exhibited hydrogen and oxygen evolution at the rate of 146 and 68 μmol h−1 g−1, respectively. Under photo-irradiation, the photoinduced electrons of TSCOF and WO3–Ov reached the CB level, leaving holes in the VB level. The photoexcited electrons in the CB of WO3–Ov recombine with the holes in the VB of TSCOF due to the internal electrical field. The electrons in the CB of COF participate in HER, while the photogenerated holes in the VB of WO3 are involved in the water oxidation into oxygen. Thus, the 2D/2D Z-scheme heterojunction of TSCOFW provides a new way for efficient overall water splitting (OWS).192
In another report, a fluorenone-based COF, COF-SCAU-2, was modified into ultrathin three-layer nanosheets (UCOF-SCAU-2) for an efficient OWS process. COF-SCAU-2 was synthesized using tris(4-formylphenyl)benzene (TPB) and diamino-fluorenone moieties and further reduced to an ultrathin structure by mechanical delamination treatment. Interestingly, UCOF-SCAU-2 served as an effective photocatalyst for OWS under visible light illumination and produced hydrogen and oxygen at rates of 0.046 and 0.021 mmol h−1 g−1, respectively. The bulk COF-SCAU-2 did not show any activity for photocatalytic OWS under similar conditions. The charge-carrier kinetic calculation and DFT (density functional theory) studies revealed that the ultrathin structure of COF increases the accessibility to the active site, generates a short electron migration distance, enhances the average lifetime of excitons, and lowers the activation energy photocatalytic reaction compared to the parent COF, leading to high photocatalytic activity.193
5.4. Photocatalytic organic transformations
COFs with high porosity can be very effective in the field of organic synthesis by acting as photocatalysts in heterogeneous catalysis. Traditional photocatalysts such as transition metal complexes, dyes, and semiconductors have been efficiently utilized as heterogeneous organocatalysts.194 However, these photocatalysts generally suffer from many problems such as low photostability, high cost, poor recyclability, and laborious separation techniques, which restrict their large-scale application. COFs have a highly conjugated π-network with wide absorption in the visible-region, large surface area, and high chemical stability, which are the primary requirements for photocatalytic organic transformations.195 Thus, COFs have attracted increasing attention as effective photocatalysts for organic synthesis. The photocatalytic mechanism of organic conversions involves the activation of oxygen into O2˙− and 1O2 intermediates via photogenerated electrons, which perform the reaction with the organic compound. The great advantage of using COFs as photocatalysts is that their photocatalytic properties can be optimized through different types of building blocks according to the corresponding organic reactions. Moreover, hybrid COFs can be developed using metal or metal complexes or other materials to increase photo-activated organic transformation. COFs have been explored in many types of organic transformation reactions such as oxidation, reduction, and coupling.Reactive oxygen species (ROS) are another interesting aspect of photocatalytic activity. Employing efficient photocatalysts has emerged as a practical method to induce ROS generation. Wu and his team developed a benzoxazole-linked COF, LZU-191, via photocatalytic polymerization using natural sunlight as the energy source and utilized it for photocatalytic investigations (Fig. 15). Due to the presence of irreversible oxazole linkages in the COF framework, LZU-191 possessed high crystallinity with a BET surface area of 1314 m2 g−1 and excellent chemical stability in water, acid, base, and common organic solvents. Furthermore, it demonstrated high photocatalytic activity for the light-driven aerobic oxidation of sulfides to sulfoxides because of its unique morphological properties.198
 | ||
| Fig. 15 (a) Synthesis of LZU-191 and (b) model photooxidation of methylphenylsulfide. Reproduced with permission from ref. 198. Copyright 2022, the American Chemical Society. | ||
In continuation of studies on the photo-oxidation of sulfides to sulfoxides, Li et al. crafted a photosensitive COF by strategically integrating photoactive triphenylamine moieties into its architecture. The EPR (electron paramagnetic resonance) studies demonstrated the effectiveness of the COF as a photocatalyst for generating ROS, specifically the superoxide radical anion (O2˙−), featuring one unpaired electron.199 This underscores the potential of COFs as promising photocatalysts for ROS-involved reactions. Trenker and co-workers synthesized FEAx-COF, a groundbreaking photocatalyst inspired by natural flavin cofactors. This work showed the successful incorporation of alloxazine chromophores into the COF backbone, preserving their functionality. This enabled the efficient oxidation of benzylic alcohols to aldehydes under low-energy visible light. It displayed superior versatility across various solvents compared to molecular alloxazines. FEAx-COF can act as a promising and sustainable choice for heterogeneous photocatalysis due to its simple and eco-friendly conversion of organic substrates.200
Lang and co-workers prepared an sp2 carbon-conjugated porphyrin-based COF, Por-sp2c-COF, using porphyrin and PDAN as building units. The porphyrin macrocycle in COF forms an extended π-conjugation network, which leads to strong light absorption, while the vinyl linkage ensures the stability of the COF in high concentrations of amine. Por-sp2c-COF with DMPO (5,5-dimethyl-1-pyrroline N-oxide) was explored for the oxidative coupling of amines under light illumination. It readily converted the amine into imine in up to 99% yield within 15–20 min. The cooperation of DMPO with porphyrin established efficient charge separation and migration of photogenerated electrons, resulting in high photocatalytic activity.201
Further, COFs were also explored for reduction and coupling reactions. In 2019, Liu and co-workers explored a donor–acceptor COF having pyrene and benzothiadiazole units in its structure for the photocatalytic reductive dehalogenation of phenacyl bromide. COF-JLU22 was synthesized via the Schiff base condensation between amino-functionalized pyrene and formyl-containing benzothiadiazole moieties. It exhibited wide absorption ranging from the visible region to NIR region and possessed a large surface area (954 m2 g−1) and pore volume (1.03 cm3 g−1). Upon light irradiation, COF-JLU22 facilitated the effective reductive dehalogenation of phenacyl bromide derivatives, yielding >99% conversion. Its high photocatalytic efficiency can be rationalized in terms of its high crystallinity, porosity, excellent stability, and donor–acceptor structure forming extended π-conjugation, resulting in a broader absorption region and unique photoelectric properties. This work highlights that metal-free COFs can be strategically designed for less feasible reduction reactions.202
An innovative approach was reported for the synthesis of photoactive COFs as effective heterogeneous photocatalysts for visible-light-driven organic transformations. This method exploited the photocatalytic activity of hydrazone-based 2D-COF-1 in generating molecular oxygen, facilitating the selective aerobic oxidation of several small organic molecules. 2D-COF-1 demonstrated high photo-efficiency and functional group tolerance in the synthesis of various compounds, including heterocyclic compounds, quinolones, sulfoxides, and amides. Noteworthy achievements in large-scale reactions include the selective production of key compounds such as the drug molecule modafinil and an oxidized mustard gas simulant (2-chloroethyl ethyl sulfoxide).203 Porphyrin-based COFs are highly interesting due to their robust nature and high porosity. A novel porphyrin-based COF (TA-Por-sp2-COF) was developed through a self-polymerization reaction. The porphyrin units with two cyano groups at the opposite peripheral positions formed a highly organized crystalline COF structure. The presence of electron-donating porphyrin and electron-withdrawing triazine units led to efficient photo-induced carrier separation, while the ample porosity of the system facilitated mass transportation. This hyper-conjugated system exhibited outstanding photocatalytic activity toward various organic transformations, particularly excelling in the aerobic coupling of benzylamine and the selective oxidation of thioanisole.204
A donor–acceptor-based COF was developed using the phenothiazine (PTZ) and triazine (TTA) moieties, which served as the donor and acceptor units, respectively. Unlike its triphenylamine (TPA)-based counterpart, which had a smaller contrast in donor–acceptor properties, PTZ–TTA-COF exhibited a reduced exciton binding energy and superior charge separation/transfer. Consequently, it exhibited markedly improved photocatalytic efficacy under light irradiation in the presence of air, specifically in facilitating amine coupling reactions and cyclization of thioamide to 1,2,4-thiadiazole.205 Similarly, Feng et al. synthesized TpTt-COF using triformylphloroglucinol (Tp) and triazine (Tt) moieties to perform the photo-oxidative coupling of amines in the presence of air within 1 h.206 Moreover, Li et al. synthesized a donor–acceptor COF with the benzothiadiazole unit, which could generate super-oxide radical anions under visible-light irradiation. This COF showed the capability to efficiently photocatalyze the oxidative coupling of amines and the cyclization of thioamide, resulting in the formation of 1,2,4-thiadiazole with yields ranging from moderate to high.207
In another investigation, Jati et al. introduced a dual metalation strategy in TpBpy COF for photocatalytic C–N cross-coupling reactions. They inserted iridium and nickel metal into the COF structure using [Ir(ppy)2(CH3CN)2]PF6 [ppy = 2-phenylpyridine] and NiCl2 as metal precursors, respectively (Fig. 16a). The attachment of iridium units to the COF pore walls resulted in a synergistic enhancement in photosensitization for dual photocatalysis. Further optimization occurred by appending nickel units in the COF structure. The Ni–Ir@TpBpy catalyst showed impressive catalytic activity and stability, substantially boosting its reusability. The simulated study revealed that the intramolecular electron transfer occurred from the Ir-site to Ni-site within the COF system. The developed Ni–Ir@TpBpy photocatalyst efficiently catalyzed coupling reactions involving sulfonamides, amines (aryl, heteroaryl, and alkyl), and carbamides with neutral, electron-rich, and electron-poor (hetero)aryl iodides, respectively, yielding up to 94% isolated yield (Fig. 16b).209
 | ||
| Fig. 16 (a) Schematic of the synthesis of Ni–Ir@Tp-Bpy and coordination of Ni complexes in TpBpy COF and (b) diagram of different substrates utilized for the C–N cross-coupling strategy. Reproduced with permission from ref. 209. Copyright 2022, the American Chemical Society. | ||
In another aspect of synthesizing a hybrid COF structures for photocatalytic organic reactions, Yang and his group reported the synthesized two heterostructures, namely QH-COF@TiO2 and TiO2@QH-COF, with a focus on the photocatalytic oxidation of alcohols. This study marks a significant advancement by meticulously orchestrating the spatial arrangement of two semiconductors, QH-COF (tetrahydroquinoline-linked COFs), either in the core or on the shell and thoroughly evaluating their respective photocatalytic performances. In the realm of benzyl alcohol oxidation, QH-COF@TiO2 exhibited an outstanding threefold increase in activity compared to TiO2@QH-COF, boasting a reaction rate of 1.19 vs. 0.44 mmol g−1 h−1 (Fig. 17). Interestingly, despite their similar capabilities in light harvesting and charge separation, the superior performance of QH-COF@TiO2 is attributed to its heightened electron donation to O2. This pattern continues in the realm of visible-light photocatalytic aerobic cross-dehydrogenative coupling reactions, underscoring the effectiveness of spatially engineered semiconductor heterojunctions, where graphitic carbon nitride (g-C3N4) containing a heptazine moiety showed excellent photocatalytic activity.210
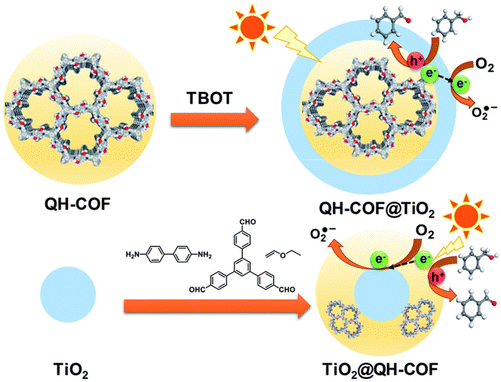 | ||
| Fig. 17 Formation of QH-COF@TiO2 and its utilization in photocatalytic organic conversions. Reproduced from ref. 210 with permission from the Royal Society of Chemistry. | ||
Furthermore, Chen et al. synthesized a heptazine-based COF to increase the photocatalytic activity of the COF due to the presence of the heptazine unit. Two distinct N-rich COFs, namely HEP-TAPT-COF and HEP-TAPB-COF, were obtained by reacting the 4-(methylenediacetate)phenyl substituted heptazine monomer (HEP-OAc) with two C3-symmetric aromatic amines. HEP-TAPT-COF significantly improved the benzylic C–H oxidation and selective sulfoxidation compared to pristine g-C3N4.211 In another investigation, Li et al. employed interlinked rigid macrocyclic struts, particularly pillar arenes in different proportions featuring electron-rich cavities. This arrangement established a restricted molecular space, facilitating exciton migration and streamlined carrier transport. Consequently, novel interfaces were formed, engaging effectively with photogenerated charge carriers and showing outstanding efficacy in catalyzing the oxidation of amines to imines.212
5.5. Photocatalytic degradation of organic pollutants
Presently, the rapid industrialization has generated many environmental problems. One of the main problems is the increasing amount of industrial waste being released into the environment, which is harmful to human health and the ecosystem. Industrial waste contains organic pollutants, heavy metals, and antibiotics, which have to be eliminated immediately. The major organic pollutants are phenol, methylene blue, methylene orange, tetracycline, rhodamine B, chlorinated biphenyl, etc. Photocatalytic degradation of organic pollutants presents simple, economical, and eco-friendly technology to remove them from the environment. The mechanism for the photocatalytic degradation of pollutants involves the generation of hydroxyl and superoxide radicals via photogenerated electrons, which react with organic compounds and degrade them. In this case, COFs have emerged as potential photocatalysts for the degradation of pollutants and many COFs have been applied in the photocatalytic degradation of pollutants.Further, Qiu and his group synthesized three vinylene-linked COFs, BDA-TMT, EDATMT, and TDA-TMT, for environmental application, which were prepared via the aldol condensation reaction of four different triazine moieties (Fig. 18a). These vinylene-linked COFs possessed highly crystalline structures and large porous surfaces following the order of TDA-TMT < EDA-TMT < BDA-TMT. The valence band (VB) positions of these three COFs appeared at 1.15, 1.37, and 1.45 V, respectively. The lower oxidation potential of BDA-TMT generated a suitable band gap for photocatalytic application. These three COFs were investigated for the photocatalytic degradation of various organic pollutants such as methylene blue, phenol, and norfloxacin. BDA-TMT exhibited excellent photocatalytic activity, resulting in the degradation of organic pollutants up to 96% within 15 min, which was much faster than the other COF (Fig. 18b–d, respectively). The photocatalytic degradation of phenol and norfloxacin reached up to 100 and 96%, respectively, achieving an ultrafast photocatalytic degradation efficiency. Additionally, BMD-TMT COF also possessed higher antibacterial activity under light irradiation. The high photocatalytic performance of BDA-COF can be rationalized in terms of its effective π-conjugation delocalization due to the presence of diacetylene moieties, leading to an optimized band gap and efficient charge separation of photogenerated electrons.214
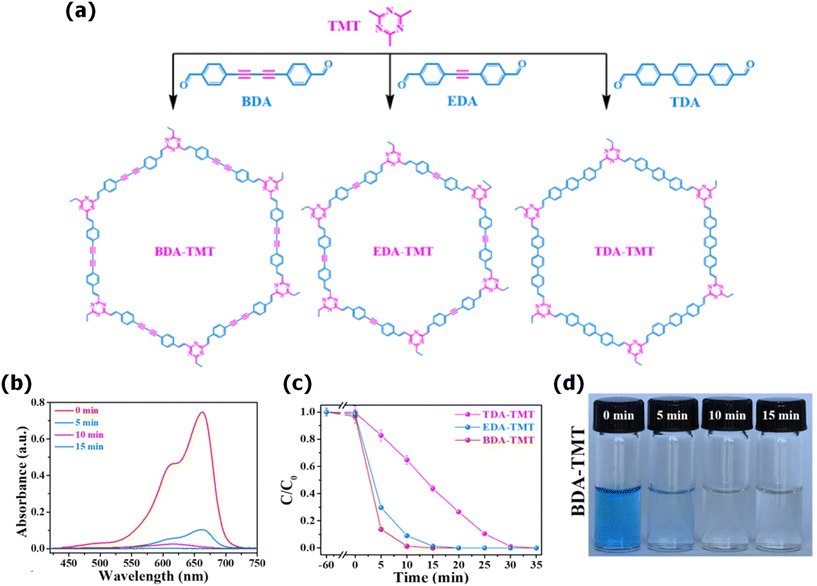 | ||
| Fig. 18 (a) Method for the synthesis of BDA-TMT, EDA-TMT, and TDA-TMT COFs. (b) Absorption changes in MB aqueous solution upon the addition of BDA-TMT. (c) Reaction kinetic curves of all COFs for the degradation of MB and (d) colour change upon the degradation of MB aqueous solution in the presence of BDA-TMT with time. Reproduced with permission from ref. 214. Copyright 2021, the American Chemical Society. | ||
Similarly, Ma and his group developed five different COFs and tuned their photocatalytic properties by modulating the number and position of nitrogen atoms on p-phenylenediamine as a building block. These five COFs were named COF-1 (without N), COF-PD (one N), COF-PZ (two N at 1,5 position), COF-PMD (two N at 1,6 position), and COF-PDZ (two N at 1,3 position) with respect to p-phenylenediamine. The assessment covered three distinct photocatalytic applications of Cr(VI) photoreduction, Escherichia coli inactivation, and degradation of paracetamol across all COFs subjected to testing. Remarkably, COF-PDZ demonstrated exceptional efficacy in Cr(VI) reduction, achieving an impressive 95% removal within a mere 20-minute timeframe. Conversely, the other COFs exhibited removal rates varying from 49% to 99% over a 120-minute duration. Further, COF-1 and COF-PD did not show any activity for bacterial disinfection, while COF-PZ and COF-PMD exhibited weak capacity, deactivating ∼0.97 log CFU mL−1 of cells within 90 min under visible-light irradiation. In contrast, COF-PDZ showed complete deactivation of 6.2 log CFU mL−1 with the same time, achieving the best antibacterial property. COF-1 and COF-PD also showed low activity for the photocatalytic degradation, resulting in 26% and 68% degradation of paracetamol over 60 min, while the other COFs gave 100% photodegradation rate for paracetamol within 30 min. These results suggest that the number and position of heterocyclic N atoms in the COF structure have a great effect on the photocatalytic properties of COFs. The Cr(VI) reduction mechanism suggests that high adsorption and quantum efficiency play an important role in effective photocatalytic performances. This work rationalized the structure–property relation for the formation of COFs as photocatalysts.215
Cui and co-workers designed two pyrene-based covalent organic frameworks, sp2c-COF and Py-NH2–COF, which were synthesized via the Schiff base condensation of a pyrene precursor with 1,4-phenylene diacetonitrile and 1,4-p-phenylenediamine organic moieties respectively, for the photocatalytic degradation of tetracycline hydrochloride (TC) in water. Different types of linkages can vary the conjugation in the COF structure, which may have an impact on their photocatalytic performances. The photocatalytic experiments for the COFs under visible light evidenced that sp2c-COF was the better photocatalyst compared to Py-NH2-COF. Sp2c-COF degraded 73.5% of initial TC within 90 min, while Py-NH2-COF showed the negligible efficiency of 3.1% under visible light. The XPS and absorption data revealed that sp2c-COF showed a narrow band gap of 1.98 eV compared to 2.28 eV of Py-NH2-COF, resulting in a high charge transport efficiency. Sp2c-COF also had a high photocurrent efficiency, which indicates the efficient charge separation of photogenerated electrons, leading to great photocatalytic performance. Additionally, sp2c-COF possessed good recyclability, exhibiting 70.3% degradation efficiency after a five-cycle run. This work implied the importance of the type of linkage in the formation of COFs given that the COF with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds achieved strong light absorption, high catalytic sites, and improved charge separation and migration rate in comparison to the COF with C
C bonds achieved strong light absorption, high catalytic sites, and improved charge separation and migration rate in comparison to the COF with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds, which are beneficial for the photo-degradation of tetracycline.216 It is expected that the donor–acceptor COF system generates intermolecular charge transfer, which can improve the photocatalytic efficiency.
N bonds, which are beneficial for the photo-degradation of tetracycline.216 It is expected that the donor–acceptor COF system generates intermolecular charge transfer, which can improve the photocatalytic efficiency.
Considering this, Tong et al. synthesized two donor–acceptor COFs, COF-TD1 and COF-TD2, via the solvothermal reaction of thiadiazole and quinine-based organic moieties with triformylphloroglucinol and investigated their photocatalytic properties for the removal of paracetamol. In photocatalytic degradation under visible light, COF-TD1 displayed better efficiency by degrading >98% of paracetamol in 60 min, while COF-TD2 only managed 59.3% degradation of paracetamol over 120 min. Due to the electron-donating nature of the thiadiazole ring, effective intermolecular transfer occurs, generating a strong push–pull effect within a COF system, which leads to the efficient electron–hole separation and high photocatalytic efficiency observed for COF-TD1 compared to COF-TD2. Remarkably, COF-TD1 also showed efficient photocatalytic degradation of other water pollutants such as diclofenac, bisphenol A, naproxen, and tetracycline hydrochloride and it can be utilized for degradation of paracetamol in complicated water obtain from river, lake water, and sewage. Importantly, COF-TD1 can be used in a powder form or immobilized onto a glass slide for the efficient removal of paracetamol to achieve large-scale application in a scaled-up reactor under sunlight irradiation.217
A. Bhaumik and his group developed a new metal-free COF for the efficient removal of organic pollutants from contaminated water. They prepared a donor–acceptor COF, C6-TRZ-TPA, using the triphenylamine moiety as the electron donor and triazine moiety as the electron acceptor building blocks. This COF possessed a highly crystalline structure due to the effective π-stacking of the triazine units and a large surface area of 1058 m2 g−1 with a pore size of 0.73 cc g−1. The optical absorption profile of the COF had a broader absorption region from 400 to 800 nm, depicting its wide visible light absorption capacity. Having a donor–acceptor motif, C6-TRZ-TPA possessed an extensive π-conjugation system and planar triazine moieties with an abundance of nitrogen heteroatoms, which generate effective charge separation for photogenerated electrons with a smaller band gap of 2.2 eV. Leveraging its remarkable photophysical properties, C6-TRZ-TPA demonstrated high efficacy in the photocatalytic degradation of organic pollutants. The investigation focused on the photodegradation of model pollutants, namely Rose Bengal (RB) and methylene blue (MB), due to their considerable toxicity, health hazards, and bioaccumulative tendencies. The C6-TRZ-TPA COF catalyst displayed outstanding catalytic efficiency, achieving a degradation rate of over 97% for both Rose Bengal (RB) and methylene blue (MB) within 80 and 120 min under light irradiation, respectively. Notably, the C6-TRZ-TPA COF also showed the adsorption of 99% radioactive iodine from iodine/cyclohexane solution in a period of 24 h. Due to its large surface area, it demonstrated a high iodine uptake capacity of 4832 mg g−1. This work explored the structure–property relation for the synthesis of crystalline and porous COFs for photocatalytic application.218
Zhao reported a simple and efficient method to synthesize ultrathin 2D CONs for the photocatalytic degradation of organic contaminants. This new approach for the formation of COFs was carried out by the ultrasound treatment of monomers in a solvent system for longer hours to achieve gram-scale synthesis. Two different CONs, P-CON and T-CON, were prepared by applying triphenylamine as the core moiety and varying the triformylbenzene and triazine moieties as benzenetricarbaldehyde, respectively. Although the BET surface area of P-CON and T-CON was reduced compared to their crystalline COF counterparts, these ultrathin 2D CONs have great potential for photocatalytic activity because of the large number of accessible active sites within their porous surface, enabling the greater participation of reactants in catalytic reactions. This was further proven by the investigation of the photocatalytic activity of P-CONs and T-CONs for the degradation of methylene blue in aqueous solution. P-CON and T-CON achieved 96.2% and 99.8% degradation efficiency toward MB in 90 min, which were much higher than that of their P-COF and T-COF counterparts, respectively.219
Similarly, Cai and co-workers designed a Z-scheme-based 2D/2D CON heterojunctions system for the photocatalytic degradation of antibiotics. Firstly, the olefin-linked pyrene-based PTO-COF and triazine unit containing TpMa-COF were synthesized and further developed into covalently linked 2D/2D CONs by connecting with remaining linker groups via a mechanochemical grinding method. 2D/2D CONs exhibited improved absorption and photophysical properties compared to their parent COFs. The construction of a Z-scheme heterojunction system in CONs enhanced their charge separation and conduction efficiency, which led to increased photocatalytic performances. 2D/2D CONs were evaluated for the degradation of the sulfamethazine antibiotic under visible light, where CONs displayed 98.4% degradation of sulfamethazine within 50 min, which was almost double that of PTO-CON and TpMa-CON. Thus, the above-mentioned work establishes an approach for the facile and scalable synthesis of COF nanosheets as photocatalysts for pollutant degradation and paves the way for practical applications.220
Qiu prepared a silver-doped COF, Ag@TAPP-TEPT, for the selective degradation of sulfur mustard [bis(2-chloroethyl)]sulfide, named HD. TAPP-TFPT COF was constructed via the Schiff base reaction of 5,10,15,20-tetrakis(4-aminophenyl)porphyrin (TAPP) and 2,4,6-tris(4-formyl phenyl)-1,3,5-triazine (TFPT) and modified by the deposition of silver nanoparticles on COF via a simple post-synthetic treatment. TAPP-TFPT COF possessed high crystallinity and the SEM and HR-TEM data revealed the uniform dispersion of the Ag NPs on the COF surface. Ag@TAPP-TFPT successfully degraded HD by oxidizing CEES into 2-chloroethyl sulfone under simulated sunlight in an O2 environment. This COF formed a non-toxic sulphoxide product of CEESO and avoided the formation of toxic CEESO2 products by over-oxidation. Due to the enhanced separation of charge carriers and the effective energy transfer/electron migration process from Ag@TAPP–TFPT to oxygen, visible light irradiation enabled the selective degradation of CEES into CEESO without the formation of harmful oxidants, leading to the efficient production of 1O2 and O2˙−. The photocatalytic studies suggested that Ag coordination improved the charge carrier transportation to oxygen, forming the 1O2 and O2˙− intermediates, which selectively transform CEES into CEESO without the formation of any toxic product. This work demonstrates that the metal coordination in COFs is beneficial for the selective and efficient degradation of organic contaminants.222
Khaing and co-workers developed a heterojunction 2D–2D COF composite as a photocatalyst for the degradation of organic pollutants. TaPa-1-COF was fabricated using molybdenum sulfide (MoS2) via a facile hydrothermal method. The X-ray analysis depicted peaks corresponding to MOS2 and COF, indicating the successful formation of the MoS2/COF composite. The MoS2/COF composite demonstrated superior efficiency compared to its parent MoS2 and COF, displaying the degradation of RhB and TC of about 98% and 85.9% under simulated sunlight irradiation in a period of 30 and 60 min, respectively. The enhancement in the photocatalytic activity of the COF composites is attributed to the formation of a 2D–2D heterostructure, which increases its absorption in the visible region and surface area and facilitates the separation and transfer of excitons upon solar illumination. This work indicated that the integration of inorganic substances in COFs in the form of a single composite can be an effective strategy for developing highly efficient photocatalysts.223
Inspired by this, a novel hybrid photocatalyst, Cu2O-ACOF-1@Pd, was developed by embedding an azine-based COF in Cu2O cubes and further anchoring Pd nanoparticles on it. A-COF was synthesized via the condensation reaction of 1,3,5-triformylbenzene and hydrazine. In the hybrid material, the COF provides a stable, crystalline, and porous surface, Cu2O has semiconductor properties, enhancing light absorption, and Pd nanoparticles can enhance the charge separation of photoexcited electron–hole pairs, reducing the charge recombination process. Thus, their combined synergistic effect in the hybrid material can result in superior photocatalytic performances. The light-induced degradation of chlorinated biphenyls was examined using a hybrid COF, with a focus on three distinct monochlorinated PCB (polychlorinated biphenyl) congeners, where chlorine was positioned at the ortho, meta, or para positions. Cu2O-ACOF-1@Pd showed a better photocatalytic performance than A-COF, Cu2O-ACOF-1, and ACOF1@Pd, resulting in the complete degradation of chlorinated biphenyl in a period of 30–40 min. The photocatalytic activity of all the tested photocatalysts followed the order of Cu2O-ACOF-1@Pd > Cu2O-ACOF-1 > ACOF1@Pd > ACOF-1. The difference in photocatalytic performance indicated that Cu2O and Pd nanoparticles are important for the photocatalytic degradation of PCB and their combination with COF achieved the rapid decomposition of PCB.224
Xu and co-workers prepared a Fe3O4-decorated MOF/COF hybrid with magnetic properties for photocatalytic dye degradation from polluted water. To prepare the Fe3O4@MOFUIO-66@TzDa-COF, Fe3O4 nanoparticles were decorated on UIO-66-MOF to form Fe3O4@MOFUIO-66, which was further treated with the precursor of TzDa-COF under solvothermal conditions to grow the COF on the modified surface (Fig. 19). The MOF and COF possessed a highly crystalline structure with a large porous surface area, excellent stability, and photocatalytic properties. The structure of the MOF/COF hybrid revealed a uniform layer of COF dispersed on the surface of Fe3O4@MOFUIO-66@TzDa-COF, which was stable under different pH conditions. The hybrid material had a specific BET surface area of 4144 m2 g−1 with a pore volume of 1.2186 cm3 g−1, and thus high adsorption of dyes can be achieved for a fast degradation process. This study explored the photocatalytic degradation of various dyes using Fe3O4@MOFUIO-66@TzDa-COF, revealing its effectiveness in rapidly degrading anionic dyes, particularly malachite green (MG) and Congo red (CR). The hybrid completely degraded MG and CR dyes within 10–120 min irrespective of their concentration and the removal rate was found to be approx. 97–99% (Fig. 19). Additionally, it also enabled the degradation of methylene blue and methyl orange dyes with a removal rate of 73% and 97%, respectively. This work can inspire the construction of hybrid photocatalysts with increased magnetic and functionality separability by combining the properties of COF, MOF, and nanoparticles.225
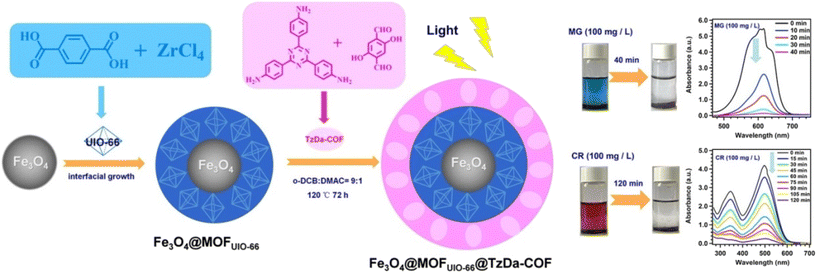 | ||
| Fig. 19 Schematic representation of Fe3O4@MOFUIO-66@TzDa-COFs and photocatalytic dye degradation of methylene blue and Congo red. Reproduced with permission from ref. 225. Copyright 2020, the American Chemical Society. | ||
Furthermore, Xue and his group fabricated carbon nanotubes (CNTs) on the surface of a COF to form a COF/CNT membrane to enhance their photocatalytic application for dye degradation. TpBD COF was grown on the carbon nanotubes via in situ treatment. The CNT/COF membrane emerged as a hybrid photocatalyst, which has a large surface area and photocatalytic properties. The contact angle of the COF/CNT surface decreased to 54° with the formation of the COF layer, which was lower than the initial 126° for the CNT membrane. The hydrophilic nature of the CNT membrane was significantly enhanced, improving the adsorption of water during photocatalysis. Consequently, the COF/CNT hybrid achieved an excellent degradation efficiency against mordant black 17 (MB17), exhibiting a total degradation capacity of 708.2 mg g−1. Notably, the mechanical properties of CNTs significantly increased, and the efficiency of COF/CNT decreased only 10.6% in degradation capacity after seven cycles. This work employed the benefits of COF and CNT integration and described a new strategy to prepare hybrid materials for dye degradation application.226
6. Summary
In summary, this review focused on the recent advancements in COFs as photocatalysts for various photocatalytic applications. COFs have been widely investigated as promising photocatalyst candidates due to their highly crystalline structure, porosity, light absorption ability, and excellent stability. The structure of COFs can be predesigned by tuning the topology of their organic building blocks, linkage chemistry, and functionalization. The topology of COFs can be modulated by varying their precursor organic building blocks. The topology of COFs is the best way to predetermine their morphology and pore channel networks. The pore size and shape of COFs can be modulated by using different topological designs. The possibilities of using different types of organic monomers for the development of COFs have been investigated extensively. However, there are many combinations of organic precursors in the form of knots and linkers that have to be explored to gain topological advantages for photo-based applications.Subsequently, crystallinity is associated with strong absorption, large surface area, and efficient charge separation, improving the photocatalytic performance. Long-range ordered COFs possess superior structural and photoelectrical properties compared to their amorphous polymer. The challenge of obtaining crystalline COFs can be overcome by applying the solvothermal method for their synthesis. Solvothermal synthesis yields COFs with controlled dehydration and slow diffusion of monomers, leading to a highly crystalline structure. However, the solvothermal method has many drawbacks such as harsh reaction conditions, long reaction time, and low yield, which need to be overcome. Furthermore, several other synthetic methods such as microwave synthesis and room temperature synthesis have been developed but they produce COFs with low crystallinity and porosity. Thus, there is still scope for developing facile and cost-effective synthetic methods for highly crystalline COFs with large scalability.
In addition, the stability of COFs is a major concern for photo-based applications. Stable COFs are not only capable of avoiding photo-corrosion and degradation under different reaction conditions but are also necessary for long-term stability and large-scale application. COFs with β-ketoenamine, imine, and azine linkages have been explored for photocatalytic activity considering their facile synthesis and highly stable nature. It has also been revealed that the covalent linkage between organic units in COFs dictates their thermal and chemical stability under different conditions. In this regard, olefin-linked COFs display feasible delocalization of π-electrons through the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bond, forming a strong conjugated COF network with high crystallinity.
C– bond, forming a strong conjugated COF network with high crystallinity.
Moreover, COFs must have broad absorption in the visible region to develop effective light harvesting ability, enhancing their photocatalytic efficiency. The utilization of highly π-conjugated aromatic systems as building blocks generates high photon absorption capacity in the visible region. Additionally, the fabrication of COFs with photoactive materials can also increase their light-harvesting in the broad range of UV (ultraviolet) to NIR (near infrared) regions. Simultaneously, the availability of a large number of catalytic sites maximizes the photocatalytic performances, and thus the high porosity of COFs becomes an important factor. The high porous surface of COFs enhances their adsorption capacity and provides numerous active sites for catalytic reactions, increasing their photocatalytic activity. Microporous COFs have small pore sizes and large surface areas and can perform efficient photocatalysis for light-driven water splitting, CO2 reduction/conversion, etc. On the contrary, large aromatic system-based organic monomers form mesoporous COFs, demonstrating high molecular adsorption for trapping large molecules, enabling efficient organic transformations or dye degradation. It has been shown that the bigger or longer the building units, the larger the pore size and shape, subsequently enhancing the surface area.
Furthermore, band gap engineering, charge separation efficiency, and high charge migration rate are key factors to improve the photocatalytic efficiency of COFs. COFs with narrow bandgaps are preferred as photocatalysts due to their increased light absorption, resulting in the photo-excitation of more electrons for catalytic activity. The bandgap of COFs can be engineered by employing highly aromatic π-conjugated organic units, functional groups, and extended conjugation in the COF. The incorporation of metal atoms or metal complexes also reduces the HOMO–LUMO gap and activation energy of COFs for photocatalytic reactions. Accordingly, the appropriate band structure of COFs also controls the charge separation and migration mechanism of photoexcited electron–hole pairs, which leads to higher efficiency. In this context, several donor–acceptor-based COF systems have been developed, where electron-rich and electron-withdrawing moieties are aligned perfectly in the crystalline COF structures. The charge transfer from donor to acceptor moieties in the COF network contributes to effective charge separation. Many donor–acceptor systems were briefly discussed, where electron-rich moieties such as pyrene, triphenylamine, triazine, and porphyrin are applied as donors, while thiazole, benzothiadiazole, and halogen-containing organic moieties serve as the acceptor. Also, the presence of extended conjugation in the COF network through organic monomers is another strategy to enhance the charge separation and migration of photogenerated electrons. Additionally, the formation of a heterojunction system by incorporating COFs with other materials that consist of the required optoelectronic properties can also improve the charge separation and mass transportation of charge carriers by inhibiting the charge recombination process. In this regard, many hybrid COFs have been developed by the in situ incorporation of metal oxides, inorganic compounds, carbon-based materials, metal–organic frameworks, etc.
The judicious choice of organic building blocks for the structural design of COFs can play a decisive role in developing COF-based photocatalysts with high crystallinity, porosity, light harvesting ability, optimal band gap tuning, efficient charge separation, and migration for effective photocatalytic activity. Although the crystallinity and stability of COFs depend on their synthetic process and linkage chemistry, large π-array organic units are more effective in forming long columnar structures due to the π–π interaction between them and enhance the COF network crystallinity. Besides, organic moieties with planar π-conjugation systems produce photoactive properties, lower the band gap, and facilitate charge transportation in COFs, thus developing robust COFs for photocatalytic activity. In addition, donor–acceptor COFs have been studied for several photo-based applications such as CO2 conversion, HER and OER. In the case of CO2 photoreduction, organic monomers consisting of active sites for metal coordination such as bipyridine units have great importance given that single metal atoms/metal complexes can readily be incorporated in COF systems to enhance their efficiency. Current research revealed that COFs consisting of triformylphloroglucinol, triazine, diethoxy-terephthalohydrazide, and sulphone moieties as building blocks have shown great promise in terms of efficiency, photostability, and long-term durability for photocatalysis.
Finally, COFs as photocatalysts have been widely investigated within the last few years and shown great improvement but their large-scale application is still far away. The methods for the synthesis of COFs need to be optimized at least to the gram-scale level for large-scale application. The in-depth analysis of fundamentals of COF-based photocatalytic systems can lead to strategically design COFs with high crystallinity, porous surface, appropriate band gap, and effective charge separation to improve their photocatalytic performance. Regarding H2 evolution and CO2 reduction, the dependence on noble metal catalysts or co-catalysts must be reduced to develop cost-effective COF photocatalysts. Further, the development of overall water-splitting photocatalytic systems or artificial photosynthetic systems based on COFs needs to be encouraged for efficient H2 or O2 evolution with CO2 reduction. Regarding light-driven organic transformations and degradation, COFs need to be functionalized to be highly porous and more selective toward organic transformations or the degradation of organic contamination. Overall, the development of COFs as photocatalysts with ideal properties has great potential for solar-driven applications, and the progress in this field will lead to a green and sustainable economy.
Data availability
The data generated and/or analyses during in this review are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. M. M. sincerely thanks SERB-DST, New Delhi, India (Project CRG/2020/001769), BRNS, Mumbai, India (Project 58/14/17/2020-BRNS/37215), and Ministry of Textiles {Project 2/1/2021-NTTM(Pt.)}, India for financial assessment. K. P. thanks Science and Engineering Board (SERB), India for National Postdoctoral Fellowship (PDF/2022/000110). R. D. thanks UGC, New Delhi for the fellowship.References
- A. Gani, Fossil Fuel Energy and Environmental Performance in an Extended STIRPAT Model, J. Cleaner Prod., 2021, 297, 126526 CrossRef.
- M. Höök and X. Tang, Depletion of Fossil Fuels and Anthropogenic Climate Change—A Review, Energy Policy, 2013, 52, 797–809 CrossRef.
- G. Lopez, D. Keiner, M. Fasihi, T. Koiranen and C. Breyer, From Fossil to Green Chemicals: Sustainable Pathways and New Carbon Feedstocks for the Global Chemical Industry, Energy Environ. Sci., 2023, 16, 2879–2909 RSC.
- G. Z. S. Ling, J. J. Foo, X.-Q. Tan and W.-J. Ong, Transition into Net-Zero Carbon Community from Fossil Fuels: Life Cycle Assessment of Light-Driven CO 2 Conversion to Methanol Using Graphitic Carbon Nitride, ACS Sustainable Chem. Eng., 2023, 11, 5547–5558 CrossRef CAS.
- A. I. Osman, L. Chen, M. Yang, G. Msigwa, M. Farghali, S. Fawzy, D. W. Rooney and P.-S. Yap, Cost, Environmental Impact, and Resilience of Renewable Energy under a Changing Climate: A Review, Environ. Chem. Lett., 2023, 21, 741–764 CrossRef CAS.
- V. Ş. Ediger, An Integrated Review and Analysis of Multi-Energy Transition from Fossil Fuels to Renewables, Energy Procedia, 2019, 156, 2–6 CrossRef.
- V. Balzani, A. Credi and M. Venturi, Photochemical Conversion of Solar Energy, ChemSusChem, 2008, 1, 26–58 CrossRef CAS PubMed.
- L. Wang, X. Xu, Q. Cheng, S. X. Dou and Y. Du, Near–Infrared–Driven Photocatalysts: Design, Construction, and Applications, Small, 2021, 17, 1904107 CrossRef CAS PubMed.
- J. Gong, C. Li and M. R. Wasielewski, Advances in Solar Energy Conversion, Chem. Soc. Rev., 2019, 48, 1862–1864 RSC.
- A. Stirbet, D. Lazár, Y. Guo and G. Govindjee, Photosynthesis: Basics, History and Modelling, Ann. Bot., 2020, 126, 511–537 CrossRef CAS PubMed.
- J. H. Alstrum-Acevedo, M. K. Brennaman and T. J. Meyer, Chemical Approaches to Artificial Photosynthesis, Inorg. Chem., 2005, 44, 6802–6827 CrossRef CAS PubMed.
- X. Wang, F. Wang, Y. Sang and H. Liu, Full–Spectrum Solar–Light–Activated Photocatalysts for Light–Chemical Energy Conversion, Adv. Energy Mater., 2017, 7, 1700473 CrossRef.
- J. L. White, M. F. Baruch, J. E. Pander, Y. Hu, I. C. Fortmeyer, J. E. Park, T. Zhang, K. Liao, J. Gu, Y. Yan, T. W. Shaw, E. Abelev and A. B. Bocarsly, Light-Driven Heterogeneous Reduction of Carbon Dioxide: Photocatalysts and Photoelectrodes, Chem. Rev., 2015, 115, 12888–12935 CrossRef CAS PubMed.
- M. Z. Rahman, M. G. Kibria and C. B. Mullins, Metal-Free Photocatalysts for Hydrogen Evolution, Chem. Soc. Rev., 2020, 49, 1887–1931 RSC.
- J. Xiao, X. Liu, L. Pan, C. Shi, X. Zhang and J.-J. Zou, Heterogeneous Photocatalytic Organic Transformation Reactions Using Conjugated Polymers-Based Materials, ACS Catal., 2020, 10, 12256–12283 CrossRef CAS.
- X. Yang and D. Wang, Photocatalysis: From Fundamental Principles to Materials and Applications, ACS Appl. Energy Mater., 2018, 1, 6657–6693 CrossRef CAS.
- S. Zhu and D. Wang, Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities, Adv. Energy Mater., 2017, 7, 1700841 CrossRef.
- A. Fujishima and K. Honda, Electrochemical Photolysis of Water at a Semiconductor Electrode, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- R. Medhi, M. D. Marquez and T. R. Lee, Visible-Light-Active Doped Metal Oxide Nanoparticles: Review of Their Synthesis, Properties, and Applications, ACS Appl. Nano Mater., 2020, 3, 6156–6185 CrossRef CAS.
- F. Wang, Q. Li and D. Xu, Recent Progress in Semiconductor–Based Nanocomposite Photocatalysts for Solar–to–Chemical Energy Conversion, Adv. Energy Mater., 2017, 7, 1700529 CrossRef.
- W.-K. Chong, B.-J. Ng, L.-L. Tan and S.-P. Chai, Recent Advances in Nanoscale Engineering of Ternary Metal Sulfide-Based Heterostructures for Photocatalytic Water Splitting Applications, Energy Fuels, 2022, 36, 4250–4267 CrossRef CAS.
- J. Zhang, M. Dai, S. Zhang, M. Dai, P. Zhang, S. Wang and Z. He, Recent Progress on Carbon–Nanotube–Based Materials for Photocatalytic Applications: A Review, Sol. RRL, 2022, 6, 2200243 CrossRef CAS.
- N. Zhang, M.-Q. Yang, S. Liu, Y. Sun and Y.-J. Xu, Waltzing with the Versatile Platform of Graphene to Synthesize Composite Photocatalysts, Chem. Rev., 2015, 115, 10307–10377 CrossRef CAS PubMed.
- N. Mate, D. Khandelwal, K. Nabeela and S. M. Mobin, Portable and Non-Invasive Fluorescent Thin Films from Photocatalytically Active Carbon Dots for Selective and Trace-Level Detection of Picric Acid, J. Mater. Chem. C, 2023, 11, 16201–16213 RSC.
- V. F. Yusuf, N. I. Malek and S. K. Kailasa, Review on Metal–Organic Framework Classification, Synthetic Approaches, and Influencing Factors: Applications in Energy, Drug Delivery, and Wastewater Treatment, ACS Omega, 2022, 7, 44507–44531 CrossRef CAS PubMed.
- K. T. Tan, S. Ghosh, Z. Wang, F. Wen, D. Rodríguez-San-Miguel, J. Feng, N. Huang, W. Wang, F. Zamora, X. Feng, A. Thomas and D. Jiang, Covalent Organic Frameworks, Nat. Rev. Methods Primers, 2023, 3, 1 CrossRef CAS.
- T. Zhang, G. Xing, W. Chen and L. Chen, Porous Organic Polymers: A Promising Platform for Efficient Photocatalysis, Mater. Chem. Front., 2020, 4, 332–353 RSC.
- S. Li, R. Ma, S. Xu, T. Zheng, G. Fu, Y. Wu, Z. Liao, Y. Kuang, Y. Hou, D. Wang, P. S. Petkov, K. Simeonova, X. Feng, L.-Z. Wu, X.-B. Li and T. Zhang, Direct Construction of Isomeric Benzobisoxazole–Vinylene-Linked Covalent Organic Frameworks with Distinct Photocatalytic Properties, J. Am. Chem. Soc., 2022, 144, 13953–13960 CrossRef CAS PubMed.
- T. Zhang, G. Zhang and L. Chen, 2D Conjugated Covalent Organic Frameworks: Defined Synthesis and Tailor-Made Functions, Acc. Chem. Res., 2022, 55, 795–808 CrossRef CAS PubMed.
- K. Nabeela, R. Deka, Z. Abbas, P. Kumar, M. Saraf and S. M. Mobin, Covalent Organic Frameworks (COFs)/MXenes Heterostructures for Electrochemical Energy Storage, Cryst. Growth Des., 2023, 23, 3057–3078 CrossRef CAS.
- R. Freund, O. Zaremba, G. Arnauts, R. Ameloot, G. Skorupskii, M. Dincă, A. Bavykina, J. Gascon, A. Ejsmont, J. Goscianska, M. Kalmutzki, U. Lächelt, E. Ploetz, C. S. Diercks and S. Wuttke, The Current Status of MOF and COF Applications, Angew. Chem., Int. Ed., 2021, 60, 23975–24001 CrossRef CAS PubMed.
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Porous, Crystalline, Covalent Organic Frameworks, Science, 2005, 310, 1166–1170 CrossRef PubMed.
- S.-Y. Ding and W. Wang, Covalent Organic Frameworks (COFs): From Design to Applications, Chem. Soc. Rev., 2013, 42, 548–568 RSC.
- R. Liu, K. T. Tan, Y. Gong, Y. Chen, Z. Li, S. Xie, T. He, Z. Lu, H. Yang and D. Jiang, Covalent Organic Frameworks: An Ideal Platform for Designing Ordered Materials and Advanced Applications, Chem. Soc. Rev., 2021, 50, 120–242 RSC.
- S. Liu, M. Wang, Y. He, Q. Cheng, T. Qian and C. Yan, Covalent Organic Frameworks towards Photocatalytic Applications: Design Principles, Achievements, and Opportunities, Coord. Chem. Rev., 2023, 475, 214882 CrossRef CAS.
- L. Stegbauer, K. Schwinghammer and B. V. Lotsch, A Hydrazone-Based Covalent Organic Framework for Photocatalytic Hydrogen Production, Chem. Sci., 2014, 5, 2789–2793 RSC.
- W. Liu, Q. Su, P. Ju, B. Guo, H. Zhou, G. Li and Q. Wu, A Hydrazone–Based Covalent Organic Framework as an Efficient and Reusable Photocatalyst for the Cross–Dehydrogenative Coupling Reaction of N –Aryltetrahydroisoquinolines, ChemSusChem, 2017, 10, 664–669 CrossRef CAS PubMed.
- S. He, Q. Rong, H. Niu and Y. Cai, Construction of a Superior Visible-Light-Driven Photocatalyst Based on a C3N4 Active Centre-Photoelectron Shift Platform-Electron Withdrawing Unit Triadic Structure Covalent Organic Framework, Chem. Commun., 2017, 53, 9636–9639 RSC.
- S. Yang, W. Hu, X. Zhang, P. He, B. Pattengale, C. Liu, M. Cendejas, I. Hermans, X. Zhang, J. Zhang and J. Huang, 2D Covalent Organic Frameworks as Intrinsic Photocatalysts for Visible Light-Driven CO2 Reduction, J. Am. Chem. Soc., 2018, 140, 14614–14618 CrossRef CAS PubMed.
- J. Xie, S. A. Shevlin, Q. Ruan, S. J. A. Moniz, Y. Liu, X. Liu, Y. Li, C. C. Lau, Z. X. Guo and J. Tang, Efficient Visible Light-Driven Water Oxidation and Proton Reduction by an Ordered Covalent Triazine-Based Framework, Energy Environ. Sci., 2018, 11, 1617–1624 RSC.
- H. V. Babu, M. G. M. Bai and M. Rajeswara Rao, Functional π-Conjugated Two-Dimensional Covalent Organic Frameworks, ACS Appl. Mater. Interfaces, 2019, 11, 11029–11060 CrossRef CAS PubMed.
- H. Wang, H. Wang, Z. Wang, L. Tang, G. Zeng, P. Xu, M. Chen, T. Xiong, C. Zhou, X. Li, D. Huang, Y. Zhu, Z. Wang and J. Tang, Covalent Organic Framework Photocatalysts: Structures and Applications, Chem. Soc. Rev., 2020, 49, 4135–4165 RSC.
- J. Chen, D. Yuan and Y. Wang, Covalent Organic Frameworks Based Heterostructure in Solar–To–Fuel Conversion, Adv. Funct. Mater., 2023, 33, 2304071 CrossRef CAS.
- Y. Deng, Y. Wang, Y. Chen and Z. Zhang, Strategies for Improving the Catalytic Performance of 2D Covalent Organic Frameworks for Hydrogen Evolution and Oxygen Evolution Reactions, Chem. – Asian J., 2021, 16, 1851–1863 CrossRef CAS PubMed.
- H. L. Nguyen and A. Alzamly, Covalent Organic Frameworks as Emerging Platforms for CO2 Photoreduction, ACS Catal., 2021, 11, 9809–9824 CrossRef CAS.
- G.-B. Wang, K.-H. Xie, H.-P. Xu, Y.-J. Wang, F. Zhao, Y. Geng and Y.-B. Dong, Covalent Organic Frameworks and Their Composites as Multifunctional Photocatalysts for Efficient Visible-Light Induced Organic Transformations, Coord. Chem. Rev., 2022, 472, 214774 CrossRef CAS.
- Y.-N. Gong, X. Guan and H.-L. Jiang, Covalent Organic Frameworks for Photocatalysis: Synthesis, Structural Features, Fundamentals and Performance, Coord. Chem. Rev., 2023, 475, 214889 CrossRef CAS.
- Y. Yuan, K. Bang, R. Wang and Y. Kim, Macrocycle–Based Covalent Organic Frameworks, Adv. Mater., 2023, 35, 2210952 CrossRef CAS PubMed.
- K. Geng, T. He, R. Liu, S. Dalapati, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang and D. Jiang, Covalent Organic Frameworks: Design, Synthesis, and Functions, Chem. Rev., 2020, 120, 8814–8933 CrossRef CAS PubMed.
- W. Chen, Z. Yang, Z. Xie, Y. Li, X. Yu, F. Lu and L. Chen, Benzothiadiazole Functionalized D–A Type Covalent Organic Frameworks for Effective Photocatalytic Reduction of Aqueous Chromium(VI), J. Mater. Chem. A, 2019, 7, 998–1004 RSC.
- A. F. M. EL-Mahdy, C. Young, J. Kim, J. You, Y. Yamauchi and S.-W. Kuo, Hollow Microspherical and Microtubular [3 + 3] Carbazole-Based Covalent Organic Frameworks and Their Gas and Energy Storage Applications, ACS Appl. Mater. Interfaces, 2019, 11, 9343–9354 CrossRef CAS PubMed.
- A. F. M. EL-Mahdy, M.-Y. Lai and S.-W. Kuo, A Highly Fluorescent Covalent Organic Framework as a Hydrogen Chloride Sensor: Roles of Schiff Base Bonding and π-Stacking, J. Mater. Chem. C, 2020, 8, 9520–9528 RSC.
- S.-Q. Xu, T.-G. Zhan, Q. Wen, Z.-F. Pang and X. Zhao, Diversity of Covalent Organic Frameworks (COFs): A 2D COF Containing Two Kinds of Triangular Micropores of Different Sizes, ACS Macro Lett., 2016, 5, 99–102 CrossRef CAS.
- B. Zhang, H. Mao, R. Matheu, J. A. Reimer, S. A. Alshmimri, S. Alshihri and O. M. Yaghi, Reticular Synthesis of Multinary Covalent Organic Frameworks, J. Am. Chem. Soc., 2019, 141, 11420–11424 CrossRef CAS PubMed.
- S. Dalapati, E. Jin, M. Addicoat, T. Heine and D. Jiang, Highly Emissive Covalent Organic Frameworks, J. Am. Chem. Soc., 2016, 138, 5797–5800 CrossRef CAS PubMed.
- S. Bhunia, A. Peña-Duarte, H. Li, H. Li, M. F. Sanad, P. Saha, M. A. Addicoat, K. Sasaki, T. A. Strom, M. J. Yacamán, C. R. Cabrera, R. Seshadri, S. Bhattacharya, J.-L. Brédas and L. Echegoyen, [2,1,3]-Benzothiadiazole-Spaced Co-Porphyrin-Based Covalent Organic Frameworks for O2 Reduction, ACS Nano, 2023, 17, 3492–3505 CrossRef CAS PubMed.
- H. L. Nguyen, C. Gropp and O. M. Yaghi, Reticulating 1D Ribbons into 2D Covalent Organic Frameworks by Imine and Imide Linkages, J. Am. Chem. Soc., 2020, 142, 2771–2776 CrossRef CAS PubMed.
- A. López-Magano, S. Daliran, A. R. Oveisi, R. Mas-Ballesté, A. Dhakshinamoorthy, J. Alemán, H. Garcia and R. Luque, Recent Advances in the Use of Covalent Organic Frameworks as Heterogenous Photocatalysts in Organic Synthesis, Adv. Mater., 2023, 35, 2209475 CrossRef PubMed.
- Q. Yang, M. Luo, K. Liu, H. Cao and H. Yan, Covalent Organic Frameworks for Photocatalytic Applications, Appl. Catal., B, 2020, 276, 119174 CrossRef CAS.
- F. J. Uribe-Romo, J. R. Hunt, H. Furukawa, C. Klöck, M. O'Keeffe and O. M. Yaghi, A Crystalline Imine-Linked 3-D Porous Covalent Organic Framework, J. Am. Chem. Soc., 2009, 131, 4570–4571 CrossRef CAS PubMed.
- J. Zhang, Y. Cao, W. Liu, T. Cao, J. Qian, J. Wang, X. Yao, A. Iqbal and W. Qin, Structural Engineering of Covalent Organic Frameworks Comprising Two Electron Acceptors Improves Photocatalytic Performance, ChemSusChem, 2022, 15, e202101510 CrossRef CAS PubMed.
- J. Hu, H. Mehrabi, Y.-S. Meng, M. Taylor, J.-H. Zhan, Q. Yan, M. Benamara, R. H. Coridan and H. Beyzavi, Probe Metal Binding Mode of Imine Covalent Organic Frameworks: Cycloiridation for (Photo)Catalytic Hydrogen Evolution from Formate, Chem. Sci., 2021, 12, 7930–7936 RSC.
- H. Wang, C. Qian, J. Liu, Y. Zeng, D. Wang, W. Zhou, L. Gu, H. Wu, G. Liu and Y. Zhao, Integrating Suitable Linkage of Covalent Organic Frameworks into Covalently Bridged Inorganic/Organic Hybrids toward Efficient Photocatalysis, J. Am. Chem. Soc., 2020, 142, 4862–4871 CrossRef CAS PubMed.
- K. Prakash, B. Mishra, D. D. Díaz, C. M. Nagaraja and P. Pachfule, Strategic Design of Covalent Organic Frameworks (COFs) for Photocatalytic Hydrogen Generation, J. Mater. Chem. A, 2023, 11, 14489–14538 RSC.
- J. Sun, H. S. Jena, C. Krishnaraj, K. S. Rawat, S. Abednatanzi, J. Chakraborty, A. Laemont, W. Liu, H. Chen, Y. Liu, K. Leus, H. Vrielinck, V. Van Speybroeck and P. V. D. Voort, Pyrene–Based Covalent Organic Frameworks for Photocatalytic Hydrogen Peroxide Production, Angew. Chem., Int. Ed., 2023, 62, e202216719 CrossRef CAS PubMed.
- J. Shi, R. Chen, H. Hao, C. Wang and X. Lang, 2D Sp2 Carbon–Conjugated Porphyrin Covalent Organic Framework for Cooperative Photocatalysis with TEMPO, Angew. Chem., Int. Ed., 2020, 59, 9088–9093 CrossRef CAS PubMed.
- V. S. Vyas, F. Haase, L. Stegbauer, G. Savasci, F. Podjaski, C. Ochsenfeld and B. V. Lotsch, A Tunable Azine Covalent Organic Framework Platform for Visible Light-Induced Hydrogen Generation, Nat. Commun., 2015, 6, 8508 CrossRef CAS PubMed.
- Y. Wang, W. Hao, H. Liu, R. Chen, Q. Pan, Z. Li and Y. Zhao, Facile Construction of Fully Sp2-Carbon Conjugated Two-Dimensional Covalent Organic Frameworks Containing Benzobisthiazole Units, Nat. Commun., 2022, 13, 100 CrossRef CAS PubMed.
- A. F. M. EL-Mahdy, A. M. Elewa, S. Huang, H. Chou and S. Kuo, Dual–Function Fluorescent Covalent Organic Frameworks: HCl Sensing and Photocatalytic H2 Evolution from Water, Adv. Opt. Mater., 2020, 8, 2000641 CrossRef CAS.
- J. Zhao, J. Ren, G. Zhang, Z. Zhao, S. Liu, W. Zhang and L. Chen, Donor–Acceptor Type Covalent Organic Frameworks, Chem. – Eur. J., 2021, 27, 10781–10797 CrossRef CAS PubMed.
- M. Lu, M. Zhang, J. Liu, Y. Chen, J. Liao, M. Yang, Y. Cai, S. Li and Y. Lan, Covalent Organic Framework Based Functional Materials: Important Catalysts for Efficient CO2 Utilization, Angew. Chem., Int. Ed., 2022, 61, e202200003 CrossRef CAS PubMed.
- Q. Guan, L.-L. Zhou and Y.-B. Dong, Metalated Covalent Organic Frameworks: From Synthetic Strategies to Diverse Applications, Chem. Soc. Rev., 2022, 51, 6307–6416 RSC.
- J.-X. Cui, Y.-M. Fu, B. Meng, J. Zhou, Z.-Y. Zhou, S.-M. Liu and Z.-M. Su, A Novel Cobalt-Anchored Covalent Organic Framework for Photocatalytic Conversion of CO2 into Widely Adjustable Syngas, J. Mater. Chem. A, 2022, 10, 13418–13427 RSC.
- J. Qiu, Y. Zheng, L. Wang, M. Liu, L. Tian, X. Yu, X. An and G. Lv, Highly Dispersed Co-Modified Covalent Organic Frameworks as Bridging Cocatalysts for Boosting CO2 Photoreduction over Defective Carbon Nitride, J. Mater. Chem. A, 2023, 11, 4572–4578 RSC.
- L. Chen, M. Huang, B. Chen, C. Gong, N. Li, H. Cheng, Y. Chen, Y. Peng and G. Xu, Two-Dimensional Covalent Organic Framework Nanosheets: Synthesis and Energy-Related Applications, Chin. Chem. Lett., 2022, 33, 2867–2882 CrossRef CAS.
- L. Kong, M. Liu, H. Huang, Y. Xu and X. Bu, Metal/Covalent–Organic Framework Based Cathodes for Metal–Ion Batteries, Adv. Energy Mater., 2022, 12, 2100172 CrossRef CAS.
- J. You, Y. Zhao, L. Wang and W. Bao, Recent Developments in the Photocatalytic Applications of Covalent Organic Frameworks: A Review, J. Cleaner Prod., 2021, 291, 125822 CrossRef CAS.
- T. Banerjee, K. Gottschling, G. Savasci, C. Ochsenfeld and B. V. Lotsch, H2 Evolution with Covalent Organic Framework Photocatalysts, ACS Energy Lett., 2018, 3, 400–409 CrossRef CAS PubMed.
- J. Kosco, F. Moruzzi, B. Willner and I. McCulloch, Photocatalysts Based on Organic Semiconductors with Tunable Energy Levels for Solar Fuel Applications, Adv. Energy Mater., 2020, 10, 2001935 CrossRef CAS.
- H. Chen, H. S. Jena, X. Feng, K. Leus and P. V. D. Voort, Engineering Covalent Organic Frameworks as Heterogeneous Photocatalysts for Organic Transformations, Angew. Chem., Int. Ed., 2022, 61, e202204938 CrossRef CAS PubMed.
- J. Yang, D. Wang, H. Han and C. Li, Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis, Acc. Chem. Res., 2013, 46, 1900–1909 CrossRef CAS PubMed.
- S. Wu, Y. Pan, H. Lin, L. Li, X. Fu and J. Long, Crystalline Covalent Organic Frameworks with Tailored Linkages for Photocatalytic H2 Evolution, ChemSusChem, 2021, 14, 4958–4972 CrossRef CAS PubMed.
- N. Fajrina and M. Tahir, A Critical Review in Strategies to Improve Photocatalytic Water Splitting towards Hydrogen Production, Int. J. Hydrogen Energy, 2019, 44, 540–577 CrossRef CAS.
- Q. Wang and K. Domen, Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies, Chem. Rev., 2020, 120, 919–985 CrossRef CAS PubMed.
- B. V. Kramar, N. C. Flanders, W. Helweh, W. R. Dichtel, J. T. Hupp and L. X. Chen, Light Harvesting Antenna Properties of Framework Solids, Acc. Mater. Res., 2022, 3, 1149–1159 CrossRef CAS.
- S. Haldar, D. Chakraborty, B. Roy, G. Banappanavar, K. Rinku, D. Mullangi, P. Hazra, D. Kabra and R. Vaidhyanathan, Anthracene-Resorcinol Derived Covalent Organic Framework as Flexible White Light Emitter, J. Am. Chem. Soc., 2018, 140, 13367–13374 CrossRef CAS PubMed.
- J. Yang, A. Acharjya, M. Ye, J. Rabeah, S. Li, Z. Kochovski, S. Youk, J. Roeser, J. Grüneberg, C. Penschke, M. Schwarze, T. Wang, Y. Lu, R. van de Krol, M. Oschatz, R. Schomäcker, P. Saalfrank and A. Thomas, A. Protonated Imine–Linked Covalent Organic Frameworks for Photocatalytic Hydrogen Evolution, Angew. Chem., Int. Ed., 2021, 60, 19797–19803 CrossRef CAS PubMed.
- A. F. M. EL-Mahdy, H. A. E. Omr, Z. A. Alothman and H. Lee, Design and Synthesis of Metal-Free Ethene-Based Covalent Organic Framework Photocatalysts for Efficient, Selective, and Long-Term Stable CO2 Conversion into Methane, J. Colloid Interface Sci., 2023, 633, 775–785 CrossRef CAS PubMed.
- L.-J. Gong, L.-Y. Liu, S.-S. Zhao, S.-L. Yang, D.-H. Si, Q.-J. Wu, Q. Wu, Y.-B. Huang and R. Cao, Rapid Charge Transfer in Covalent Organic Framework via Through-Bond for Enhanced Photocatalytic CO2 Reduction, J. Chem. Eng., 2023, 458, 141360 CrossRef CAS.
- B. Mishra, A. Alam, B. Kumbhakar, D. Díaz Díaz and P. Pachfule, Impact of the Crystallinity of Covalent Organic Frameworks on Photocatalytic Hydrogen Evolution, Cryst. Growth Des., 2023, 23, 4701–4719 CrossRef CAS.
- T. He, W. Zhen, Y. Chen, Y. Guo, Z. Li, N. Huang, Z. Li, R. Liu, Y. Liu, X. Lian, C. Xue, T. C. Sum, W. Chen and D. Jiang, Integrated Interfacial Design of Covalent Organic Framework Photocatalysts to Promote Hydrogen Evolution from Water, Nat. Commun., 2023, 14, 329 CrossRef CAS PubMed.
- W. Huang, W. Luo and Y. Li, Two-Dimensional Semiconducting Covalent Organic Frameworks for Photocatalytic Solar Fuel Production, Mater. Today, 2020, 40, 160–172 CrossRef CAS.
- A. M. Elewa, A. F. M. EL-Mahdy, A. E. Hassan, Z. Wen, J. Jayakumar, T.-L. Lee, L.-Y. Ting, I. M. A. Mekhemer, T.-F. Huang, M. H. Elsayed, C.-L. Chang, W.-C. Lin and H.-H. Chou, Solvent Polarity Tuning to Enhance the Crystallinity of 2D-Covalent Organic Frameworks for Visible-Light-Driven Hydrogen Generation, J. Mater. Chem. A, 2022, 10, 12378–12390 RSC.
- Q. Guan, G.-B. Wang, L.-L. Zhou, W.-Y. Li and Y.-B. Dong, Nanoscale Covalent Organic Frameworks as Theranostic Platforms for Oncotherapy: Synthesis, Functionalization, and Applications, Nanoscale Adv., 2020, 2, 3656–3733 RSC.
- S. Pasricha, A. Chaudhary and A. Srivastava, Evolving Trends for C−C Bond Formation Using Functionalized Covalent Organic Frameworks as Heterogeneous Catalysts, ChemistrySelect, 2022, 7, e202200576 CrossRef CAS.
- C. Xia, K. O. Kirlikovali, T. H. C. Nguyen, X. C. Nguyen, Q. B. Tran, M. K. Duong, M. T. Nguyen Dinh, D. L. T. Nguyen, P. Singh, P. Raizada, V.-H. Nguyen, S. Y. Kim, L. Singh, C. C. Nguyen, M. Shokouhimehr and Q. Van Le, The Emerging Covalent Organic Frameworks (COFs) for Solar-Driven Fuels Production, Coord. Chem. Rev., 2021, 446, 214117 CrossRef CAS.
- I. Ahmed and S. H. Jhung, Covalent Organic Framework-Based Materials: Synthesis, Modification, and Application in Environmental Remediation, Coord. Chem. Rev., 2021, 441, 213989 CrossRef CAS.
- H. Li, H. Li, Q. Dai, H. Li and J. Brédas, Hydrolytic Stability of Boronate Ester–Linked Covalent Organic Frameworks, Adv. Theory Simul., 2018, 1, 1700015 CrossRef.
- M. S. Lohse and T. Bein, Covalent Organic Frameworks: Structures, Synthesis, and Applications, Adv. Funct. Mater., 2018, 28, 1705553 CrossRef.
- J. Kang, J. Hang, B. Chen, L. Chen, P. Zhao, Y. Xu, Y. Luo and C. Xia, Amide Linkages in Pyrene-Based Covalent Organic Frameworks toward Efficient Photocatalytic Reduction of Uranyl, ACS Appl. Mater. Interfaces, 2022, 14, 57225–57234 CrossRef CAS PubMed.
- D. Jiang, Covalent Organic Frameworks: A Molecular Platform for Designer Polymeric Architectures and Functional Materials, Bull. Chem. Soc. Jpn., 2021, 94, 1215–1231 CrossRef CAS.
- F. Xu, B. Liang, L. Liu, X. Hu and B. Weng, Pd Nanoparticle-Decorated Covalent Organic Frameworks for Enhanced Photocatalytic Tetracycline Hydrochloride Degradation and Hydrogen Evolution, Chem. Commun., 2023, 59, 6387–6390 RSC.
- R.-R. Liang, S.-Y. Jiang, A. Ru-Han and X. Zhao, Two-Dimensional Covalent Organic Frameworks with Hierarchical Porosity, Chem. Soc. Rev., 2020, 49, 3920–3951 RSC.
- S. Karak, K. Dey and R. Banerjee, Maneuvering Applications of Covalent Organic Frameworks via Framework–Morphology Modulation, Adv. Mater., 2022, 34, 2202751 CrossRef CAS PubMed.
- Y. Liu, L. Chen, L. Yang, T. Lan, H. Wang, C. Hu, X. Han, Q. Liu, J. Chen, Z. Feng, X. Cui, Q. Fang, H. Wang, L. Li, Y. Li, H. Xing, S. Yang, D. Zhao and J. Li, Porous Framework Materials for Energy & Environment Relevant Applications: A Systematic Review, Green Energy Environ., 2024, 9, 217–310 CrossRef CAS.
- Z.-F. Pang, S.-Q. Xu, T.-Y. Zhou, R.-R. Liang, T.-G. Zhan and X. Zhao, Construction of Covalent Organic Frameworks Bearing Three Different Kinds of Pores through the Heterostructural Mixed Linker Strategy, J. Am. Chem. Soc., 2016, 138, 4710–4713 CrossRef CAS PubMed.
- L. Liao, X. Guan, H. Zheng, Z. Zhang, Y. Liu, H. Li, L. Zhu, S. Qiu, X. Yao and Q. Fang, Three-Dimensional Microporous and Mesoporous Covalent Organic Frameworks Based on Cubic Building Units, Chem. Sci., 2022, 13, 9305–9309 RSC.
- X. Zhao, P. Pachfule, S. Li, T. Langenhahn, M. Ye, C. Schlesiger, S. Praetz, J. Schmidt and A. Thomas, Macro/Microporous Covalent Organic Frameworks for Efficient Electrocatalysis, J. Am. Chem. Soc., 2019, 141, 6623–6630 CrossRef CAS PubMed.
- N. Arora, C. Flores, M. C. Senarathna, C. M. Thompson and R. A. Smaldone, Design, Synthesis, and Applications of Mesoporous Covalent Organic Frameworks, CCS Chem., 2024, 6, 57–68 CrossRef CAS.
- A. P. Côté, H. M. El-Kaderi, H. Furukawa, J. R. Hunt and O. M. Yaghi, Reticular Synthesis of Microporous and Mesoporous 2D Covalent Organic Frameworks, J. Am. Chem. Soc., 2007, 129, 12914–12915 CrossRef PubMed.
- P. Cheng and Y. Yang, Narrowing the Band Gap: The Key to High-Performance Organic Photovoltaics, Acc. Chem. Res., 2020, 53, 1218–1228 CrossRef CAS PubMed.
- P. Pachfule, A. Acharjya, J. Roeser, T. Langenhahn, M. Schwarze, R. Schomäcker, A. Thomas and J. Schmidt, Diacetylene Functionalized Covalent Organic Framework (COF) for Photocatalytic Hydrogen Generation, J. Am. Chem. Soc., 2018, 140, 1423–1427 CrossRef CAS PubMed.
- L. Yin, Y. Zhao, Y. Xing, H. Tan, Z. Lang, W. Ho, Y. Wang and Y. Li, Structure-Property Relationship in β-Keto-Enamine-Based Covalent Organic Frameworks for Highly Efficient Photocatalytic Hydrogen Production, J. Chem. Eng., 2021, 419, 129984 CrossRef CAS.
- J. Sheng, H. Dong, X. Meng, H. Tang, Y. Yao, D. Liu, L. Bai, F. Zhang, J. Wei and X. Sun, Effect of Different Functional Groups on Photocatalytic Hydrogen Evolution in Covalent–Organic Frameworks, ChemCatChem, 2019, 11, 2313–2319 CrossRef CAS.
- H. Zhang, Z. Lin, P. Kidkhunthod and J. Guo, Stable Immobilization of Nickel Ions on Covalent Organic Frameworks for Panchromatic Photocatalytic Hydrogen Evolution, Angew. Chem., Int. Ed., 2023, 62, e202217527 CrossRef CAS PubMed.
- J. Jeon, Y. J. Kim, S. H. Joo, H. Noh, S. K. Kwak and J. Baek, Benzotrithiophene–based Covalent Organic Framework Photocatalysts with Controlled Conjugation of Building Blocks for Charge Stabilization, Angew. Chem., Int. Ed., 2023, 62, e202217416 CrossRef CAS PubMed.
- X. Wang, X. Ding, T. Wang, K. Wang, Y. Jin, Y. Han, P. Zhang, N. Li, H. Wang and J. Jiang, Two-Dimensional Porphyrin-Based Covalent Organic Framework with Enlarged Inter-Layer Spacing for Tunable Photocatalytic CO2 Reduction, ACS Appl. Mater. Interfaces, 2022, 14, 41122–41130 CrossRef CAS PubMed.
- K. Lei, D. Wang, L. Ye, M. Kou, Y. Deng, Z. Ma, L. Wang and Y. Kong, A Metal–Free Donor–Acceptor Covalent Organic Framework Photocatalyst for Visible–Light–Driven Reduction of CO2 with H2O, ChemSusChem, 2020, 13, 1725–1729 CrossRef CAS PubMed.
- L. Peng, S. Chang, Z. Liu, Y. Fu, R. Ma, X. Lu, F. Zhang, W. Zhu, L. Kong and M. Fan, Visible-Light-Driven Photocatalytic CO2 Reduction over Ketoenamine-Based Covalent Organic Frameworks, Catal. Sci. Technol., 2021, 11, 1717–1724 RSC.
- Z. Zhao, Y. Zheng, C. Wang, S. Zhang, J. Song, Y. Li, S. Ma, P. Cheng, Z. Zhang and Y. Chen, Fabrication of Robust Covalent Organic Frameworks for Enhanced Visible-Light-Driven H2 Evolution, ACS Catal., 2021, 11, 2098–2107 CrossRef CAS.
- Y. Xia, W. Zhang, S. Yang, L. Wang and G. Yu, Research Progress in Donor−Acceptor Type Covalent Organic Frameworks, Adv. Mater., 2023, 35, 2301190 CrossRef CAS PubMed.
- H. Yu, J. Zhang, X. Yan, C. Wu, X. Zhu, B. Li, T. Li, Q. Guo, J. Gao, M. Hu and J. Yang, Donor–Acceptor Covalent Organic Framework Hollow Submicrospheres with a Hierarchical Pore Structure for Visible-Light-Driven H2 Evolution, J. Mater. Chem. A, 2022, 10, 11010–11018 RSC.
- L. Stegbauer, K. Schwinghammer and B. V. Lotsch, A Hydrazone-Based Covalent Organic Framework for Photocatalytic Hydrogen Production, Chem. Sci., 2014, 5, 2789–2793 RSC.
- V. S. Vyas, F. Haase, L. Stegbauer, G. Savasci, F. Podjaski, C. Ochsenfeld and B. V. Lotsch, A Tunable Azine Covalent Organic Framework Platform for Visible Light-Induced Hydrogen Generation, Nat. Commun., 2015, 6, 8508 CrossRef CAS PubMed.
- P. Pachfule, A. Acharjya, J. Roeser, T. Langenhahn, M. Schwarze, R. Schomäcker, A. Thomas and J. Schmidt, Diacetylene Functionalized Covalent Organic Framework (COF) for Photocatalytic Hydrogen Generation, J. Am. Chem. Soc., 2018, 140, 1423–1427 CrossRef CAS PubMed.
- L. Stegbauer, S. Zech, G. Savasci, T. Banerjee, F. Podjaski, K. Schwinghammer, C. Ochsenfeld and B. V. Lotsch, Tailor–Made Photoconductive Pyrene–Based Covalent Organic Frameworks for Visible–Light Driven Hydrogen Generation, Adv. Energy Mater., 2018, 8, 1703278 CrossRef.
- X. Wang, L. Chen, S. Y. Chong, M. A. Little, Y. Wu, W.-H. Zhu, R. Clowes, Y. Yan, M. A. Zwijnenburg, R. S. Sprick and A. I. Cooper, Sulfone-Containing Covalent Organic Frameworks for Photocatalytic Hydrogen Evolution from Water, Nat. Chem., 2018, 10, 1180–1189 CrossRef CAS PubMed.
- G.-B. Wang, F.-C. Zhu, Q.-Q. Lin, J.-L. Kan, K.-H. Xie, S. Li, Y. Geng and Y.-B. Dong, Rational Design of Benzodifuran-Functionalized Donor–Acceptor Covalent Organic Frameworks for Photocatalytic Hydrogen Evolution from Water, Chem. Commun., 2021, 57, 4464–4467 RSC.
- G.-B. Wang, S. Li, C.-X. Yan, Q.-Q. Lin, F.-C. Zhu, Y. Geng and Y.-B. Dong, A Benzothiadiazole-Based Covalent Organic Framework for Highly Efficient Visible-Light Driven Hydrogen Evolution, Chem. Commun., 2020, 56, 12612–12615 RSC.
- W. Chen, L. Wang, D. Mo, F. He, Z. Wen, X. Wu, H. Xu and L. Chen, Modulating Benzothiadiazole–Based Covalent Organic Frameworks via Halogenation for Enhanced Photocatalytic Water Splitting, Angew. Chem., 2020, 132, 17050–17057 CrossRef.
- W. Li, X. Huang, T. Zeng, Y. A. Liu, W. Hu, H. Yang, Y.-B. Zhang and K. Wen, Thiazolo [5,4-d]Thiazole-Based Donor-Acceptor Covalent Organic Framework for Sunlight-Driven Hydrogen Evolution, Angew. Chem. Int. Ed., 2021, 60, 1869–1874 CrossRef CAS PubMed.
- C.-X. Liu, D.-L. Pan, Y. Seo, S. Park, J.-L. Kan, J. Y. Koo, W. Choi and E. Lee, Phenanthroimidazole-Based Covalent Organic Frameworks with Enhanced Activity for the Photocatalytic Hydrogen Evolution Reaction, ACS Appl. Energy Mater., 2023, 6, 1126–1133 CrossRef CAS.
- Y. Chen, X. Luo, J. Zhang, L. Hu, T. Xu, W. Li, L. Chen, M. Shen, S.-B. Ren, D.-M. Han, G.-H. Ning and D. Li, Bandgap Engineering of Covalent Organic Frameworks for Boosting Photocatalytic Hydrogen Evolution from Water, J. Mater. Chem. A, 2022, 10, 24620–24627 RSC.
- F. Liu, Y. He, X. Liu, Z. Wang, H.-L. Liu, X. Zhu, C.-C. Hou, Y. Weng, Q. Zhang and Y. Chen, Regulating Excitonic Effects in Covalent Organic Frameworks to Promote Free Charge Carrier Generation, ACS Catal., 2022, 12, 9494–9502 CrossRef CAS.
- E. Jin, Z. Lan, Q. Jiang, K. Geng, G. Li, X. Wang and D. Jiang, 2D Sp2 Carbon-Conjugated Covalent Organic Frameworks for Photocatalytic Hydrogen Production from Water, Chem, 2019, 5, 1632–1647 CAS.
- S. Ma, T. Deng, Z. Li, Z. Zhang, J. Jia, Q. Li, G. Wu, H. Xia, S. Yang and X. Liu, Photocatalytic Hydrogen Production on a Sp 2 –Carbon–Linked Covalent Organic Framework, Angew. Chem., 2022, 134, e202208919 CrossRef.
- Z. Li, T. Deng, S. Ma, Z. Zhang, G. Wu, J. Wang, Q. Li, H. Xia, S.-W. Yang and X. Liu, Three-Component Donor–π–Acceptor Covalent–Organic Frameworks for Boosting Photocatalytic Hydrogen Evolution, J. Am. Chem. Soc., 2023, 145, 8364–8374 CrossRef CAS PubMed.
- Y. Yang, N. Luo, S. Lin, H. Yao and Y. Cai, Cyano Substituent on the Olefin Linkage: Promoting Rather than Inhibiting the Performance of Covalent Organic Frameworks, ACS Catal., 2022, 12, 10718–10726 CrossRef CAS.
- S. Wei, F. Zhang, W. Zhang, P. Qiang, K. Yu, X. Fu, D. Wu, S. Bi and F. Zhang, Semiconducting 2D Triazine-Cored Covalent Organic Frameworks with Unsubstituted Olefin Linkages, J. Am. Chem. Soc., 2019, 141, 14272–14279 CrossRef CAS PubMed.
- S. Bi, C. Yang, W. Zhang, J. Xu, L. Liu, D. Wu, X. Wang, Y. Han, Q. Liang and F. Zhang, Two-Dimensional Semiconducting Covalent Organic Frameworks via Condensation at Arylmethyl Carbon Atoms, Nat. Commun., 2019, 10, 2467 CrossRef PubMed.
- R. Chen, Y. Wang, Y. Ma, A. Mal, X.-Y. Gao, L. Gao, L. Qiao, X.-B. Li, L.-Z. Wu and C. Wang, Rational Design of Isostructural 2D Porphyrin-Based Covalent Organic Frameworks for Tunable Photocatalytic Hydrogen Evolution, Nat. Commun., 2021, 12, 1354 CrossRef CAS PubMed.
- J. Thote, H. B. Aiyappa, A. Deshpande, D. Díaz Díaz, S. Kurungot and R. Banerjee, A Covalent Organic Framework–Cadmium Sulfide Hybrid as a Prototype Photocatalyst for Visible–Light–Driven Hydrogen Production, Chem. – Eur. J., 2014, 20, 15961–15965 CrossRef CAS PubMed.
- Y. Wang, Z. Hu, W. Wang, H. He, L. Deng, Y. Zhang, J. Huang, N. Zhao, G. Yu and Y.-N. Liu, Design of Well-Defined Shell–Core Covalent Organic Frameworks/Metal Sulfide as an Efficient Z-Scheme Heterojunction for Photocatalytic Water Splitting, Chem. Sci., 2021, 12, 16065–16073 RSC.
- M.-Y. Gao, C.-C. Li, H.-L. Tang, X.-J. Sun, H. Dong and F.-M. Zhang, Boosting Visible-Light-Driven Hydrogen Evolution of Covalent Organic Frameworks through Compositing with MoS2: A Promising Candidate for Noble-Metal-Free Photocatalysts, J. Mater. Chem. A, 2019, 7, 20193–20200 RSC.
- D. Shang, D. Li, B. Chen, B. Luo, Y. Huang and W. Shi, 2D–2D SnS2 /Covalent Organic Framework Heterojunction Photocatalysts for Highly Enhanced Solar-Driven Hydrogen Evolution without Cocatalysts, ACS Sustainable Chem. Eng., 2021, 9, 14238–14248 CrossRef CAS.
- Y.-P. Zhang, H.-L. Tang, H. Dong, M.-Y. Gao, C.-C. Li, X.-J. Sun, J.-Z. Wei, Y. Qu, Z.-J. Li and F.-M. Zhang, Covalent-Organic Framework Based Z-Scheme Heterostructured Noble-Metal-Free Photocatalysts for Visible-Light-Driven Hydrogen Evolution, J. Mater. Chem. A, 2020, 8, 4334–4340 RSC.
- S. Bao, Q. Tan, S. Wang, J. Guo, K. Lv, S. A. C. Carabineiro and L. Wen, TpBD COF@ZnIn2S4 Nanosheets: A Novel S-Scheme Heterojunction with Enhanced Photoreactivity for Hydrogen Production, Appl. Catal., B, 2023, 330, 122624 CrossRef CAS.
- F. Zhang, J. Sheng, Z. Yang, X. Sun, H. Tang, M. Lu, H. Dong, F. Shen, J. Liu and Y. Lan, Rational Design of MOF/COF Hybrid Materials for Photocatalytic H2 Evolution in the Presence of Sacrificial Electron Donors, Angew. Chem., 2018, 130, 12282–12286 CrossRef.
- Y. Wang, Q. Yang, F. Yi, R. Lu, Y. Chen, C. Liu, X. Li, C. Wang and H. Yan, NH2-UiO-66 Coated with Two-Dimensional Covalent Organic Frameworks: High Stability and Photocatalytic Activity, ACS Appl. Mater. Interfaces, 2021, 13, 29916–29925 CrossRef CAS PubMed.
- H.-Y. Zhang, Y. Yang, C.-C. Li, H.-L. Tang, F.-M. Zhang, G.-L. Zhang and H. Yan, A New Strategy for Constructing Covalently Connected MOF@COF Core–Shell Heterostructures for Enhanced Photocatalytic Hydrogen Evolution, J. Mater. Chem. A, 2021, 9, 16743–16750 RSC.
- M. Luo, Q. Yang, K. Liu, H. Cao and H. Yan, Boosting Photocatalytic H2 Evolution on g-C3N4 by Modifying Covalent Organic Frameworks (COFs), Chem. Commun., 2019, 55, 5829–5832 RSC.
- Y. Xing, L. Yin, Y. Zhao, Z. Du, H.-Q. Tan, X. Qin, W. Ho, T. Qiu and Y.-G. Li, Construction of the 1D Covalent Organic Framework/2D g-C3N4 Heterojunction with High Apparent Quantum Efficiency at 500 Nm, ACS Appl. Mater. Interfaces, 2020, 12, 51555–51562 CrossRef CAS PubMed.
- P. Dong, Y. Wang, A. Zhang, T. Cheng, X. Xi and J. Zhang, Platinum Single Atoms Anchored on a Covalent Organic Framework: Boosting Active Sites for Photocatalytic Hydrogen Evolution, ACS Catal., 2021, 11, 13266–13279 CrossRef CAS.
- H. Zhang, Z. Lin, P. Kidkhunthod and J. Guo, Stable Immobilization of Nickel Ions on Covalent Organic Frameworks for Panchromatic Photocatalytic Hydrogen Evolution, Angew. Chem., 2023, 135, e202217527 CrossRef.
- N. Shehzad, M. Tahir, K. Johari, T. Murugesan and M. Hussain, A Critical Review on TiO2 Based Photocatalytic CO2 Reduction System: Strategies to Improve Efficiency, J. CO2 Util., 2018, 26, 98–122 CrossRef CAS.
- S. Vaz, A. P. Rodrigues de Souza and B. E. Lobo Baeta, Technologies for Carbon Dioxide Capture: A Review Applied to Energy Sectors, Clean. Eng. Technol., 2022, 8, 100456 CrossRef.
- Y. Dai, Z. Niu, Y. Wang, S. Zhong, P. Mu and J. Li, Recent Advances and Prospect of Emerging Microporous Membranes for High-Performance CO2 Capture, Sep. Purif. Technol., 2023, 318, 123992 CrossRef CAS.
- Ž. Kovačič, B. Likozar and M. Huš, Photocatalytic CO2 Reduction: A Review of Ab Initio Mechanism, Kinetics, and Multiscale Modeling Simulations, ACS Catal., 2020, 10, 14984–15007 CrossRef.
- W. Huang, W. Luo and Y. Li, Two-Dimensional Semiconducting Covalent Organic Frameworks for Photocatalytic Solar Fuel Production, Mater. Today, 2020, 40, 160–172 CrossRef CAS.
- Y. Fu, X. Zhu, L. Huang, X. Zhang, F. Zhang and W. Zhu, Azine-Based Covalent Organic Frameworks as Metal-Free Visible Light Photocatalysts for CO2 Reduction with H2O, Appl. Catal., B, 2018, 239, 46–51 CrossRef CAS.
- K. Lei, D. Wang, L. Ye, M. Kou, Y. Deng, Z. Ma, L. Wang and Y. Kong, A Metal–Free Donor–Acceptor Covalent Organic Framework Photocatalyst for Visible–Light–Driven Reduction of CO2 with H2O, ChemSusChem, 2020, 13, 1725–1729 CrossRef CAS PubMed.
- L. Wang, R. Wang, X. Zhang, J. Mu, Z. Zhou and Z. Su, Improved Photoreduction of CO2 with Water by Tuning the Valence Band of Covalent Organic Frameworks, ChemSusChem, 2020, 13, 2973–2980 CrossRef CAS PubMed.
- Y. Guo, Q. Zhang, S. Gao, H. Wang, Z. Li, J. Qiu, Y. Zhao, Z. Liu and J. Wang, Bi-Functional Ionic Liquids Facilitate Liquid-Phase Exfoliation of Porphyrin-Based Covalent Organic Frameworks in Water for Highly Efficient CO2 Photoreduction, Green Chem., 2022, 24, 9530–9541 RSC.
- L. Peng, S. Chang, Z. Liu, Y. Fu, R. Ma, X. Lu, F. Zhang, W. Zhu, L. Kong and M. Fan, Visible-Light-Driven Photocatalytic CO2 Reduction over Ketoenamine-Based Covalent Organic Frameworks: Role of the Host Functional Groups, Catal. Sci. Technol., 2021, 11, 1717–1724 RSC.
- S. Das, I. H. Chowdhury, A. H. Chowdhury, N. Singh, M. Sarkar and Sk. M. Islam, Metal-Free Covalent Organic Framework for Facile Production of Solar Fuel via CO2 Reduction, Ind. Eng. Chem. Res., 2022, 61, 17044–17056 CrossRef CAS.
- H. Jia, B. Zhu, X. Zhi, Y. Du, J. Liu, G. Jie, Y. Fu, R. Ma, F. Zhang and W. Zhu, Terephthalaldehyde-Based Covalent Organic Frameworks as Photocatalysts for CO2 Reduction under Visible Light, FlatChem, 2023, 38, 100492 CrossRef CAS.
- S. Gao, Q. Zhang, X. Su, X. Wu, X.-G. Zhang, Y. Guo, Z. Li, J. Wei, H. Wang, S. Zhang and J. Wang, Ingenious Artificial Leaf Based on Covalent Organic Framework Membranes for Boosting CO2 Photoreduction, J. Am. Chem. Soc., 2023, 145, 9520–9529 CrossRef CAS PubMed.
- L. Yang, W. Yan, N. Yang, G. Wang, Y. Bi, C. Tian, H. Liu and X. Zhu, Regulating Π–Conjugation in Sp2 –Carbon–Linked Covalent Organic Frameworks for Efficient Metal–Free CO2 Photoreduction with H2O, Small, 2023, 19, e202217527 Search PubMed.
- Q. Tang, Y.-Y. Gu, J. Ning, Y. Yan, L. Shi, M. Zhou, H. Wei, X. Ren, X. Li, J. Wang, C. Tang, L. Hao and J. Ye, Boosting Photocatalysis of Hydrazone-Linked Covalent Organic Frameworks through Introducing Electron-Rich Conjugated Aldehyde, J. Chem. Eng., 2023, 470, 144106 CrossRef CAS.
- X. Yu, K. Gong, S. Tian, G. Gao, J. Xie and X.-H. Jin, A Hydrophilic Fully Conjugated Covalent Organic Framework for Photocatalytic CO2 Reduction to CO Nearly 100% Using Pure Water, J. Mater. Chem. A, 2023, 11, 5627–5635 RSC.
- Z. Fu, X. Wang, A. M. Gardner, X. Wang, S. Y. Chong, G. Neri, A. J. Cowan, L. Liu, X. Li, A. Vogel, R. Clowes, M. Bilton, L. Chen, R. S. Sprick and A. I. Cooper, A Stable Covalent Organic Framework for Photocatalytic Carbon Dioxide Reduction, Chem. Sci., 2020, 11, 543–550 RSC.
- Y. Cheng, W. Ji, P. Hao, X. Qi, X. Wu, X. Dou, X. Bian, D. Jiang, F. Li, X. Liu, D. Yang, X. Ding and B. Han, A Fully Conjugated Covalent Organic Framework with Oxidative and Reductive Sites for Photocatalytic Carbon Dioxide Reduction with Water, Angew. Chem., Int. Ed., 2023, 62, e202308523 CrossRef CAS PubMed.
- M. Kou, W. Liu, Y. Wang, J. Huang, Y. Chen, Y. Zhou, Y. Chen, M. Ma, K. Lei, H. Xie, P. K. Wong and L. Ye, Photocatalytic CO2 Conversion over Single-Atom MoN2 Sites of Covalent Organic Framework, Appl. Catal., B, 2021, 291, 120146 CrossRef CAS.
- M. Lu, Q. Li, J. Liu, F.-M. Zhang, L. Zhang, J.-L. Wang, Z.-H. Kang and Y.-Q. Lan, Installing Earth-Abundant Metal Active Centers to Covalent Organic Frameworks for Efficient Heterogeneous Photocatalytic CO2 Reduction, Appl. Catal., B, 2019, 254, 624–633 CrossRef CAS.
- M. Lu, J. Liu, Q. Li, M. Zhang, M. Liu, J. Wang, D. Yuan and Y. Lan, Rational Design of Crystalline Covalent Organic Frameworks for Efficient CO2 Photoreduction with H2O, Angew. Chem., Int. Ed., 2019, 58, 12392–12397 CrossRef CAS PubMed.
- N. Xu, Y. Diao, X. Qin, Z. Xu, H. Ke and X. Zhu, Donor–Acceptor Covalent Organic Frameworks of Nickel(II) Porphyrin for Selective and Efficient CO2 Reduction into CO, Dalton Trans., 2020, 49, 15587–15591 RSC.
- Z. Liu, Y. Huang, S. Chang, X. Zhu, Y. Fu, R. Ma, X. Lu, F. Zhang, W. Zhu and M. Fan, Highly Dispersed Ru Nanoparticles on a Bipyridine-Linked Covalent Organic Framework for Efficient Photocatalytic CO2 Reduction, Sustainable Energy Fuels, 2021, 5, 2871–2876 RSC.
- M. Zhang, M. Lu, Z. Lang, J. Liu, M. Liu, J. Chang, L. Li, L. Shang, M. Wang, S. Li and Y. Lan, Semiconductor/Covalent–Organic–Framework Z–Scheme Heterojunctions for Artificial Photosynthesis, Angew. Chem., Int. Ed., 2020, 59, 6500–6506 CrossRef CAS PubMed.
- V. N. Gopalakrishnan, J. Becerra, S. Mohan, J. M. E. Ahad, F. Béland and T.-O. Do, Cobalt-Doped MoS2 -Integrated Hollow Structured Covalent Organic Framework Nanospheres for the Effective Photoreduction of CO2 under Visible Light, Energy Fuels, 2023, 37, 2329–2339 CrossRef.
- V. N. Gopalakrishnan, D.-T. Nguyen, J. Becerra, M. Sakar, J. M. E. Ahad, J. J. Jautzy, L. M. Mindorff, F. Béland and T.-O. Do, Manifestation of an Enhanced Photoreduction of CO2 to CO over the In Situ Synthesized RGO–Covalent Organic Framework under Visible Light Irradiation, ACS Appl. Energy Mater., 2021, 4, 6005–6014 CrossRef CAS.
- J. Wang, Y. Yu, J. Cui, X. Li, Y. Zhang, C. Wang, X. Yu and J. Ye, Defective G-C3N4/Covalent Organic Framework van Der Waals Heterojunction toward Highly Efficient S-Scheme CO2 Photoreduction, Appl. Catal., B, 2022, 301, 120814 CrossRef CAS.
- Q. Niu, S. Dong, J. Tian, G. Huang, J. Bi and L. Wu, Rational Design of Novel COF/MOF S-Scheme Heterojunction Photocatalyst for Boosting CO2 Reduction at Gas–Solid Interface, ACS Appl. Mater. Interfaces, 2022, 14, 24299–24308 CrossRef CAS PubMed.
- C. Lai, N. An, B. Li, M. Zhang, H. Yi, S. Liu, L. Qin, X. Liu, L. Li, Y. Fu, F. Xu, Z. Wang, X. Shi, Z. An and X. Zhou, Future Roadmap on Nonmetal-Based 2D Ultrathin Nanomaterials for Photocatalysis, J. Chem. Eng., 2021, 406, 126780 CrossRef CAS.
- Y. Deng, Y. Wang, Y. Chen and Z. Zhang, Strategies for Improving the Catalytic Performance of 2D Covalent Organic Frameworks for Hydrogen Evolution and Oxygen Evolution Reactions, Chem. – Asian J., 2021, 16, 1851–1863 CrossRef CAS PubMed.
- J. Chen, X. Tao, C. Li, Y. Ma, L. Tao, D. Zheng, J. Zhu, H. Li, R. Li and Q. Yang, Synthesis of Bipyridine-Based Covalent Organic Frameworks for Visible-Light-Driven Photocatalytic Water Oxidation, Appl. Catal., B, 2020, 262, 118271 CrossRef CAS.
- H. Chen, A. M. Gardner, G. Lin, W. Zhao, X. Wang, M. Bahri, N. D. Browning, X. Xu and X. Li, Triazine-Based Covalent Organic Framework for Photocatalytic Water Oxidation: The Role of Bipyridine Ligand and Cobalt Coordination, J. Phys. Chem. C, 2023, 127, 14137–14145 CrossRef CAS.
- Y. He, G. Liu, Z. Liu, J. Bi, Y. Yu and L. Li, Photoinduced Hydration Boosts O2 Evolution on Co-Chelating Covalent Organic Framework, ACS Energy Lett., 2023, 8, 1857–1863 CrossRef CAS.
- W.-K. Han, Y. Liu, J.-D. Feng, X. Yan, H. Pang and Z.-G. Gu, Engineering a Molecular Ruthenium Catalyst into Three-Dimensional Metal Covalent Organic Frameworks for Efficient Water Oxidation, Chem. Sci., 2023, 14, 11768–11774 RSC.
- S. Karak, V. Stepanenko, M. A. Addicoat, P. Keßler, S. Moser, F. Beuerle and F. Würthner, A Covalent Organic Framework for Cooperative Water Oxidation, J. Am. Chem. Soc., 2022, 144, 17661–17670 CrossRef CAS PubMed.
- M. Xu, M. Lu, G. Qin, X. Wu, T. Yu, L. Zhang, K. Li, X. Cheng and Y. Lan, Piezo–Photocatalytic Synergy in BiFeO3@COF Z–Scheme Heterostructures for High–Efficiency Overall Water Splitting, Angew. Chem., Int. Ed., 2022, 61, e202210700 CrossRef CAS PubMed.
- Y. Yang, X. Chu, H.-Y. Zhang, R. Zhang, Y.-H. Liu, F.-M. Zhang, M. Lu, Z.-D. Yang and Y.-Q. Lan, Engineering β-Ketoamine Covalent Organic Frameworks for Photocatalytic Overall Water Splitting, Nat. Commun., 2023, 14, 593 CrossRef CAS PubMed.
- R. Shen, G. Liang, L. Hao, P. Zhang and X. Li, In Situ Synthesis of Chemically Bonded 2D/2D Covalent Organic Frameworks/O–Vacancy WO3 Z–Scheme Heterostructure for Photocatalytic Overall Water Splitting, Adv. Mater., 2023, 35, 2303649 CrossRef CAS PubMed.
- R. Shen, C. Qin, L. Hao, X. Li, P. Zhang and X. Li, Realizing Photocatalytic Overall Water Splitting by Modulating the Thickness–Induced Reaction Energy Barrier of Fluorenone–Based Covalent Organic Frameworks, Adv. Mater., 2023, 35, 2305397 CrossRef CAS PubMed.
- T. Luo, L. Gilmanova and S. Kaskel, Advances of MOFs and COFs for Photocatalytic CO2 Reduction, H2 Evolution and Organic Redox Transformations, Coord. Chem. Rev., 2023, 490, 215210 CrossRef CAS.
- R. K. Sharma, P. Yadav, M. Yadav, R. Gupta, P. Rana, A. Srivastava, R. Zbořil, R. S. Varma, M. Antonietti and M. B. Gawande, Recent Development of Covalent Organic Frameworks (COFs): Synthesis and Catalytic (Organic-Electro-Photo) Applications, Mater. Horiz., 2020, 7, 411–454 RSC.
- W. Wang, K. Cai, W. Zhou, F. Tao, Z. Li, Q. Lin, L. Wang, Z. Yu, J. Zhang and H. Zhou, Nanoporous Vinylene-Linked 2D Covalent Organic Frameworks for Visible-Light-Driven Aerobic Oxidation, ACS Appl. Nano Mater., 2023, 6, 8396–8403 CrossRef CAS.
- R. Paul, S. C. Shit, H. Mandal, J. Rabeah, S. S. Kashyap, Y. Nailwal, D. B. Shinde, Z. Lai and J. Mondal, Benzothiazole-Linked Metal-Free Covalent Organic Framework Nanostructures for Visible-Light-Driven Photocatalytic Conversion of Phenylboronic Acids to Phenols, ACS Appl. Nano Mater., 2021, 4, 11732–11742 CrossRef CAS.
- C.-J. Wu, X.-Y. Li, T.-R. Li, M.-Z. Shao, L.-J. Niu, X.-F. Lu, J.-L. Kan, Y. Geng and Y.-B. Dong, Natural Sunlight Photocatalytic Synthesis of Benzoxazole-Bridged Covalent Organic Framework for Photocatalysis, J. Am. Chem. Soc., 2022, 144, 18750–18755 CrossRef CAS PubMed.
- Y. Li, T.-X. Luan, K. Cheng, D. Zhang, W. Fan, P.-Z. Li and Y. Zhao, Effective Photocatalytic Initiation of Reactive Oxygen Species by a Photoactive Covalent Organic Framework for Oxidation Reactions, ACS Mater Lett., 2022, 4, 1160–1167 CrossRef CAS.
- S. Trenker, L. Grunenberg, T. Banerjee, G. Savasci, L. M. Poller, K. I. M. Muggli, F. Haase, C. Ochsenfeld and B. V. Lotsch, A Flavin-Inspired Covalent Organic Framework for Photocatalytic Alcohol Oxidation, Chem. Sci., 2021, 12, 15143–15150 RSC.
- R. Chen, J. Shi, Y. Ma, G. Lin, X. Lang and C. Wang, Designed Synthesis of a 2D Porphyrin–Based Sp2 Carbon–Conjugated Covalent Organic Framework for Heterogeneous Photocatalysis, Angew. Chem., Int. Ed., 2019, 58, 6430–6434 CrossRef CAS PubMed.
- H. Cheng and T. Wang, Covalent Organic Frameworks in Catalytic Organic Synthesis, Adv. Synth. Catal., 2021, 363, 144–193 CrossRef CAS.
- S. Liu, M. Tian, X. Bu, H. Tian and X. Yang, Covalent Organic Frameworks toward Diverse Photocatalytic Aerobic Oxidations, Chem. – Eur. J., 2021, 27, 7738–7744 CrossRef CAS PubMed.
- X. Liu, R. Qi, S. Li, W. Liu, Y. Yu, J. Wang, S. Wu, K. Ding and Y. Yu, Triazine–Porphyrin-Based Hyperconjugated Covalent Organic Framework for High-Performance Photocatalysis, J. Am. Chem. Soc., 2022, 144, 23396–23404 CrossRef CAS PubMed.
- Y. Liu, X. Jiang, L. Chen, Y. Cui, Q.-Y. Li, X. Zhao, X. Han, Y.-C. Zheng and X.-J. Wang, Rational Design of a Phenothiazine-Based Donor–Acceptor Covalent Organic Framework for Enhanced Photocatalytic Oxidative Coupling of Amines and Cyclization of Thioamides, J. Mater. Chem. A, 2023, 11, 1208–1215 RSC.
- J. Feng, J. Cheng, J. Pang, M. Tang, Z. Liu, C. Rong and R. Tan, Donor–Acceptor Covalent Organic Framework Promotes Visible Light-Induced Oxidative Coupling of Amines to Imines in Air, Catal. Sci. Technol., 2022, 12, 6865–6874 RSC.
- S. Li, L. Li, Y. Li, L. Dai, C. Liu, Y. Liu, J. Li, J. Lv, P. Li and B. Wang, Fully Conjugated Donor–Acceptor Covalent Organic Frameworks for Photocatalytic Oxidative Amine Coupling and Thioamide Cyclization, ACS Catal., 2020, 10, 8717–8726 CrossRef CAS.
- Y.-X. Chen, M. Zhang, S.-Z. Zhang, Z.-Q. Hao and Z.-H. Zhang, Copper-Decorated Covalent Organic Framework as a Heterogeneous Photocatalyst for Phosphorylation of Terminal Alkynes, Green Chem., 2022, 24, 4071–4081 RSC.
- A. Jati, K. Dey, M. Nurhuda, M. A. Addicoat, R. Banerjee and B. Maji, Dual Metalation in a Two-Dimensional Covalent Organic Framework for Photocatalytic C–N Cross-Coupling Reactions, J. Am. Chem. Soc., 2022, 144, 7822–7833 CrossRef CAS PubMed.
- H. Li, H. Liu, C. Li, J. Liu, J. Liu and Q. Yang, Micro-Scale Spatial Location Engineering of COF–TiO2 Heterojunctions for Visible Light Driven Photocatalytic Alcohol Oxidation, J. Mater. Chem. A, 2020, 8, 18745–18754 RSC.
- D. Chen, W. Chen, G. Zhang, S. Li, W. Chen, G. Xing and L. Chen, N-Rich 2D Heptazine Covalent Organic Frameworks as Efficient Metal-Free Photocatalysts, ACS Catal., 2022, 12, 616–623 CrossRef CAS.
- M.-H. Li, Z. Yang, Z. Li, J.-R. Wu, B. Yang and Y.-W. Yang, Construction of Hydrazone-Linked Macrocycle-Enriched Covalent Organic Frameworks for Highly Efficient Photocatalysis, Chem. Mater., 2022, 34, 5726–5739 CrossRef CAS.
- H. Lv, X. Zhao, H. Niu, S. He, Z. Tang, F. Wu and J. P. Giesy, Ball Milling Synthesis of Covalent Organic Framework as a Highly Active Photocatalyst for Degradation of Organic Contaminants, J. Hazard. Mater., 2019, 369, 494–502 CrossRef CAS PubMed.
- X.-R. Chen, W.-R. Cui, R.-P. Liang, C.-R. Zhang, R.-H. Xu, W. Jiang and J.-D. Qiu, Band Gap Engineering in Vinylene-Linked Covalent Organic Frameworks for Enhanced Photocatalytic Degradation of Organic Contaminants and Disinfection of Bacteria, ACS Appl. Bio Mater., 2021, 4, 6502–6511 CrossRef CAS PubMed.
- F. Liu, Z. Ma, Y. Deng, M. Wang, P. Zhou, W. Liu, S. Guo, M. Tong and D. Ma, Tunable Covalent Organic Frameworks with Different Heterocyclic Nitrogen Locations for Efficient Cr(VI) Reduction, Escherichia Coli Disinfection, and Paracetamol Degradation under Visible-Light Irradiation, Environ. Sci. Technol., 2021, 55, 5371–5381 CrossRef CAS PubMed.
- Z. Hu, Y. Luo, L. Wang, Y. Wang, Q. Wang, G. Jiang, Q. Zhang and F. Cui, Synthesis of Pyrene-Based Covalent Organic Frameworks for Photocatalytic Tetracycline Degradation, ACS Appl. Polym. Mater., 2023, 5, 9263–9273 CrossRef CAS.
- Y. Hou, F. Liu, B. Zhang and M. Tong, Thiadiazole-Based Covalent Organic Frameworks with a Donor–Acceptor Structure: Modulating Intermolecular Charge Transfer for Efficient Photocatalytic Degradation of Typical Emerging Contaminants, Environ. Sci. Technol., 2022, 56, 16303–16314 CrossRef CAS PubMed.
- S. Ruidas, A. Chowdhury, A. Ghosh, A. Ghosh, S. Mondal, A. D. D. Wonanke, M. Addicoat, A. K. Das, A. Modak and A. Bhaumik, A. Covalent Organic Framework as a Metal-Free Photocatalyst for Dye Degradation and Radioactive Iodine Adsorption, Langmuir, 2023, 39, 4071–4081 CrossRef CAS PubMed.
- S.-X. Gan, C. Jia, Q.-Y. Qi and X. Zhao, A Facile and Scalable Synthetic Method for Covalent Organic Nanosheets: Ultrasonic Polycondensation and Photocatalytic Degradation of Organic Pollutants, Chem. Sci., 2022, 13, 1009–1015 RSC.
- Y. Yang, W. Zhao, H. Niu and Y. Cai, Mechanochemical Construction 2D/2D Covalent Organic Nanosheets Heterojunctions Based on Substoichiometric Covalent Organic Frameworks, ACS Appl. Mater. Interfaces, 2021, 13, 42035–42043 CrossRef CAS PubMed.
- F. Xu, B. Liang, L. Liu, X. Hu and B. Weng, Pd Nanoparticle-Decorated Covalent Organic Frameworks for Enhanced Photocatalytic Tetracycline Hydrochloride Degradation and Hydrogen Evolution, Chem. Commun., 2023, 59, 6387–6390 RSC.
- L. Zhang, C. Sun, S.-J. Xiao, Q.-G. Tan, G.-P. Yang, J.-Q. Fan, Y.-T. Luo, R.-P. Liang and J.-D. Qiu, Deposition of Silver Nanostructures on Covalent Organic Frameworks for Photocatalytic Degradation of Sulfur Mustard Simulants, ACS Appl. Nano Mater., 2023, 6, 17083–17091 CrossRef CAS.
- K. K. Khaing, D. Yin, Y. Ouyang, S. Xiao, B. Liu, L. Deng, L. Li, X. Guo, J. Wang, J. Liu and Y. Zhang, Fabrication of 2D–2D Heterojunction Catalyst with Covalent Organic Framework (COF) and MoS2 for Highly Efficient Photocatalytic Degradation of Organic Pollutants, Inorg. Chem., 2020, 59, 6942–6952 CrossRef CAS PubMed.
- A. E. ElMetwally, E. Zeynaloo, D. Shukla, B. Surnar, S. Dhar, J. L. Cohn, M. R. Knecht and L. G. Bachas, Cu2O Cubes Decorated with Azine-Based Covalent Organic Framework Spheres and Pd Nanoparticles as Tandem Photocatalyst for Light-Driven Degradation of Chlorinated Biphenyls, ACS Appl. Nano Mater., 2021, 4, 2795–2805 CrossRef CAS.
- M. Zheng, C. Yao and Y. Xu, Fe3O4 Nanoparticles Decorated with UIO-66 Metal–Organic Framework Particles and Encapsulated in a Triazine-Based Covalent Organic Framework Matrix for Photodegradation of Anionic Dyes, ACS Appl. Nano Mater., 2020, 3, 11307–11314 CrossRef CAS.
- H. Xue, Z. Bi, J. Cheng, S. Xiong and Y. Wang, Coupling Covalent Organic Frameworks and Carbon Nanotube Membranes to Design Easily Reusable Photocatalysts for Dye Degradation, Ind. Eng. Chem. Res., 2021, 60, 8687–8695 CrossRef CAS.
| This journal is © the Partner Organisations 2024 |