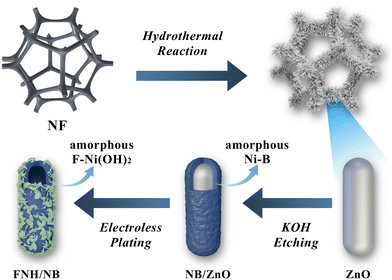
Amorphous heterojunction and fluoride-induced effects enable a F-Ni(OH)2/Ni–B electrocatalyst for efficient and stable alkaline freshwater/seawater hydrogen evolution at a high current density†
Shenyi
Chen
a,
Haoming
Chu
a,
Ziyin
Xie
a,
Lihui
Dong
ab,
Bin
Li
ab,
Minguang
Fan
 a,
Huibing
He
a,
Huibing
He
 a and
Zhengjun
Chen
a and
Zhengjun
Chen
 *a
*a
aGuangxi Key Laboratory of Electrochemical Energy Materials, School of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, P.R. China. E-mail: zjchen@gxu.edu.cn
bState Key Laboratory of Featured Metal Materials and Life-cycle Safety for Composite Structures, Guangxi University, Nanning 530004, P.R. China
First published on 28th August 2024
Abstract
The search for efficient, robust, and cost-effective catalysts for the hydrogen evolution reaction (HER) is highly desirable. However, the development of freshwater/seawater electrolysis for hydrogen production as a viable energy conversion technology remains a challenge. Herein, a fluorine-doped Ni(OH)2/Ni–B amorphous heterostructure (FNH/NB) was synthesized via simple hydrothermal, electroless plating, and alkaline etching methods. Our designed experiments demonstrate that the as-prepared catalyst benefiting from amorphous interfacial coupling and F-induced effects exhibits accelerated H2O dissociation kinetics and optimized adsorption of intermediates. As a result, the FNH/NB catalyst shows high alkaline HER activity, requiring low overpotentials of 23, 28, and 30 mV to drive a current density of 10 mA cm−2 in alkaline freshwater, simulated seawater, and real seawater, respectively. Particularly, the stability of the designed catalyst is effectively improved using a fluorine doping strategy. Specifically, FNH/NB could maintain excellent electrocatalytic performance over 50 hours at current densities of 10 and 1000 mA cm−2 in an alkaline solution containing KF. The current work reveals the superiority of integrating F doping and amorphous heterostructure engineering in developing efficient and robust catalysts.
1 Introduction
Electrocatalytic water splitting coupled with a green power source is one of the feasible and sustainable approaches for industrial hydrogen generation.1,2 However, the widespread deployment of this technology is still limited by the sluggish kinetics of the two half-reactions involved in this process; namely, the cathode electrochemical hydrogen evolution reaction (HER) and the anode electrochemical oxygen evolution reaction (OER).3–6 Meanwhile, owing to the increasingly limited freshwater resources, direct electrolysis in abundant seawater appears more practical. However, the development of seawater splitting is restricted by severe chlorine corrosion.7,8 To ameliorate the above limitations, it is critical to develop high-performance and erosion-resisting HER and OER catalysts. Platinum and its compounds represent the most excellent HER catalysts. However, their scarce resources and high cost limit their widespread application.9–12 Therefore, many non-noble transition metals and relevant compounds (such as borides, carbides, nitrides, sulfides, and phosphides) have been identified as potential HER catalysts.To boost the HER activity of non-precious metal electrocatalysts, many surface/interface/defect engineering strategies have been developed. Notably, heterostructured interface engineering is a particularly useful method for the alkaline HER, which benefits from the synergistic effects of different components to tune the electronic structure and provide additional active sites,13–15 as exemplified by the studies on NC@CrN/Ni,16 Ni/W5N4,17 and Ni2P/V2O3−x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 crystalline heterostructured catalysts. Additionally, the performance of heterostructured electrocatalysts can be further improved by employing crystal phase engineering to construct amorphous/crystalline heterostructures.19–22 Compared with crystalline components, amorphous materials possess more unsaturated coordination active sites and more flexible adaptive reaction structures, which boost their catalytic performance.23–25 This strategy has been successfully applied to Ni(OH)2–NiMoOx/NF,26 MoN/Ni@C,27 and NiWO4/Ni3S2
18 crystalline heterostructured catalysts. Additionally, the performance of heterostructured electrocatalysts can be further improved by employing crystal phase engineering to construct amorphous/crystalline heterostructures.19–22 Compared with crystalline components, amorphous materials possess more unsaturated coordination active sites and more flexible adaptive reaction structures, which boost their catalytic performance.23–25 This strategy has been successfully applied to Ni(OH)2–NiMoOx/NF,26 MoN/Ni@C,27 and NiWO4/Ni3S2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 catalysts. Nevertheless, the studies focusing on amorphous/amorphous heterostructures towards the HER have been rarely reported. The reason behind this may be related to the difficulties in the preparation of amorphous heterostructures29,30 and investigation of their structure–activity relationship31 as well as the poor stability of amorphous materials resulting from the high cation dissolution rate.25,32,33 In view of this, doping heteroatoms with highly electronegativity such as fluorine (F) may be beneficial to improve the stability of catalysts.34,35 The F doping will form more robust metal–F bonds, thereby resulting in higher lattice energies and a more stable phase structure.36,37 In addition, the surplus protonation in aqueous solution can be further balanced by the doped F atoms, thus stabilizing the structure of the electrocatalyst.37–39 In this regard, the rational design of a F-doped amorphous/amorphous heterostructured catalyst may provide an option toward achieving efficient and stable alkaline HER.
28 catalysts. Nevertheless, the studies focusing on amorphous/amorphous heterostructures towards the HER have been rarely reported. The reason behind this may be related to the difficulties in the preparation of amorphous heterostructures29,30 and investigation of their structure–activity relationship31 as well as the poor stability of amorphous materials resulting from the high cation dissolution rate.25,32,33 In view of this, doping heteroatoms with highly electronegativity such as fluorine (F) may be beneficial to improve the stability of catalysts.34,35 The F doping will form more robust metal–F bonds, thereby resulting in higher lattice energies and a more stable phase structure.36,37 In addition, the surplus protonation in aqueous solution can be further balanced by the doped F atoms, thus stabilizing the structure of the electrocatalyst.37–39 In this regard, the rational design of a F-doped amorphous/amorphous heterostructured catalyst may provide an option toward achieving efficient and stable alkaline HER.
Herein, we reported the preparation of a fluorine-doped Ni(OH)2/Ni–B (abbreviated as FNH/NB) amorphous/amorphous hollow rod heterostructure, and its outstanding and stable HER activity in alkaline freshwater/seawater. Specifically, the FNH/NB catalyst was prepared through simple hydrothermal, electroless plating, and alkaline etching methods. The as-prepared FNH/NB catalyst only requires a low overpotential of 23, 28, and 30 mV to drive a current density of 10 mA cm−2 in alkaline freshwater, simulated seawater, and real seawater, respectively. Our studies reveal that the superior HER activity arises from the high ECSA of the as-prepared catalyst, as well as the amorphous interfacial coupling and F-induced effects. More importantly, the overpotential of the FNH/NB catalyst shows negligible degradation after 50 h at current densities of 10 and 1000 mA cm−2 in an alkaline solution containing KF due to the F− stabilization, demonstrating its excellent stability for H2 production.
2 Experimental section
2.1. Synthesis of ZnO
Nickel foam (NF, 4 × 1.5 cm2) was washed ultrasonically in ethanol, 3 M HCl, and deionized (DI) water for 15 min, respectively. Then, the pretreated NF was transferred into a Teflon-lined stainless reactors containing 0.35 g Zn(NO3)2·6H2O, 0.14 g C6H12N4 and 40 mL DI water. After undergoing a hydrothermal reaction at 100 °C for 6 h, the ZnO hydrothermal precursor was obtained.2.2. Synthesis of NB/ZnO
0.30 g NaCl, 1.20 g NiCl2·6H2O, and 2.5 g C4H4Na2O4 were dissolved with 50 mL of DI water, followed by the addition 0.42 g DMAB to form an electroless plating (EP) solution under vigorous stirring. The hydrothermal sample was immersed in aqueous solution, and kept undisturbed for 90 min to obtain amorphous NB/ZnO.2.3. Synthesis of FNH/NB
Electroless plated samples were soaked in the 50 mL solution containing 5 M KOH and 0.5 g NaF for 8 h to obtain FNH/NB with an amorphous/amorphous heterostructure. The NH/NB comparative catalyst without F doping was synthesized in a similar procedure, except for the addition of NaF.2.4. Synthesis of NB
To synthesize NB for comparison, the pre-treated NF similarly underwent direct immersion into an EP solution with the same composition as mentioned above, and then set aside for 90 min.2.5. Synthesis of FNH
The FNH/NF comparative catalyst was synthesized following the reported method.40,41 0.238 g NiCl2·6H2O, 0.3 g urea, and 0.055 g ammonium fluoride (NH4F) were dissolved in 30 mL DI water. The mixed solution and a pretreated NF (4 × 1.5 cm2) were put into a 50 mL autoclave, and then heated at 120 °C for 10 h to obtain the FNH.3 Results
3.1 Synthesis and characterization
The FNH/NB amorphous/amorphous hollow rod heterostructure was synthesized by a three-step procedure (Fig. 1) using rod-like ZnO as a sacrificial template. First, the pretreated NF was loaded with the ZnO array by a hydrothermal reaction, as the substrate of the following electroless plating (EP) reaction. Second, nickel boride was deposited on ZnO templates by applying an auto-catalyzed EP method. In order to better control the reaction kinetics, DMAB was selected as the reducing agent and boron source, and C4H4Na2O4 as the complexing agent.42,43 The catalyst originally coated with a white surface completely turned black, thereby illustrating the successful completion of the electroless plating reaction. Third, the FNH/NB amorphous/amorphous heterostructure was finally synthesized after soaking the EP sample in 5 M KOH containing NaF to remove the ZnO template and concurrently achieve F doping and generation of Ni(OH)2 nanosheets on the Ni–B surface.The FE-SEM images of the hydrothermal sample (Fig. 2a and b) showed that the slick-surfaced microrods were uniformly distributed on the skeleton of NF, and had an average length of approximately 3–4 μm. Combined with the parallel XRD analysis in Fig. 2c, it can be confirmed that the microrods are indexed to the hexagonal ZnO phase (PDF #36-1451). Here, the signals of the metallic Ni phase (PDF #04-0850) should come from the NF substrate. After electroless plating, the FE-SEM image of the EP sample (Fig. 2d and e) showed that the microrods array was retained, and a crumpled and entangled surface layer was coated on the ZnO surface. The corresponding XRD pattern still retained the peaks belonging to the ZnO crystal phase without the appearance of the diffraction peaks detected for B or Ni–B, indicating the amorphous characteristic of the Ni–B component formed in the EP procedure. After etching, the uniform nanosheets were formed on the surface of the microrods, as shown in Fig. 2f and g. Meanwhile, the parallel XRD pattern shows that the diffraction peaks of the ZnO phase completely disappeared, leaving only the metallic Ni crystal phase derived from the NF substrate. To prevent the influence of the NF substrate, all catalyst powders scraped from NF was further investigated by XRD (Fig. S1†). A broad peak appeared at 40–50 degrees after KOH etching, which can be assigned to the amorphous nickel boride. The result demonstrated that the ZnO template was completely etched by KOH and amorphous Ni–B was formed. Significantly, Raman spectroscopy of the target catalyst recorded in Fig. 2h supported the existence of Ni(OH)2. The two peaks at 442 cm−1 and 469 cm−1 can be respectively assigned to the symmetric Ni–OH bond stretching vibration and Ni–O bond stretching vibration of Ni(OH)2,44,45 while the peak at 3567 cm−1 was attributed to the symmetric stretching vibration of O–H.46 The F doping and formation of the amorphous FNH/NB heterostructure were further illustrated by the following XPS and TEM analyses.
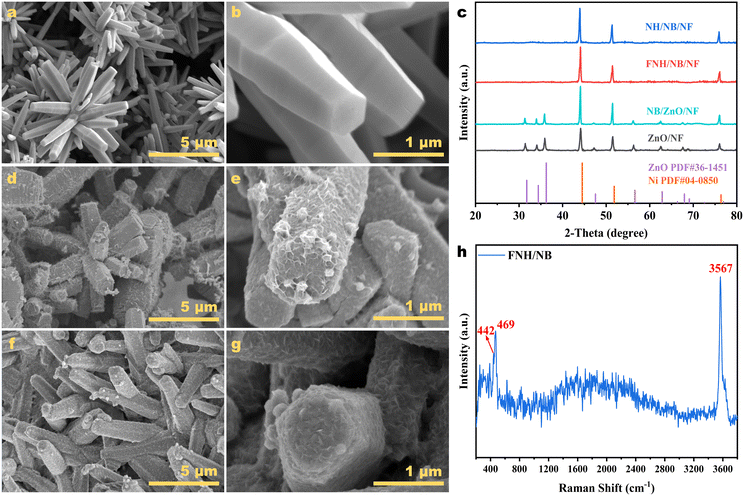 | ||
| Fig. 2 (a and b) SEM images of the ZnO template. (c) XRD patterns of a series of catalysts on the NF substrate. (d and e) SEM images of NB/ZnO. (f and g) SEM images and (h) Raman spectrum of FNH/NB. | ||
The XPS full spectrum of the etched sample (Fig. 3a) shows the presence of Ni, B, O, and F elements, and the disappearance of the Zn element, indicating the F doping and the complete removal of the ZnO templates, which is consistent with XRD analysis. The XPS full spectrum of the NB sample only shows the presence of Ni and B, and that of FNH only presents the F, Ni, and O elements. In the Ni 2p XPS spectrum of the etched sample (Fig. 3b), the peak located at a binding energy (BE) of 852.40 eV (Ni 2p3/2) can be attributed to Ni0 of the synthesized Ni–B.47 The other peaks were correlated to Ni2+ (855.60 eV for Ni 2p3/2 and 873.06 eV for Ni 2p1/2) and satellite peaks (861.35 eV for Ni 2p3/2 and 879.30 eV for Ni 2p1/2). It is noteworthy that the B 1s spectrum (Fig. 3c) corresponds perfectly to the signals of B0 (187.50 eV) and oxidic B (191.91 eV). The observed B–O signal was likely a result of the inevitable oxidation of the catalyst surface exposed to the air.48 Notably, the B0 binding energy was positively shifted by 0.20 eV compared to pure boron (187.3 eV),42 while the binding energy of Ni0 was negatively shifted by 0.90 eV in contrast to metallic Ni (853.3 eV). These results revealed that electrons flowed from B to metallic Ni, verifying the synthesis of Ni–B during the reduction process. Additionally, the high-resolution F 1s spectrum (Fig. 3d) clearly showed the peak at 684.5 eV corresponding to the M–F signal,49 demonstrating the successful doping of F. According to previous reports, F ions (1.31 Å) have similar radii to O ions (1.38 Å), and can take the place of OH− (1.35 Å) in hydroxides.40,50 It can be inferred that F was doped into the position of OH− in Ni(OH)2. Considering the analyses presented above, the final sample composition could be determined as F-doped Ni(OH)2/Ni-B. To better understand the charge interaction between different components, XPS surveys of pure NB and FNH were also recorded for comparison. After more meticulous inspection of the XPS findings, it is worth mentioning that the Ni0 signal (852.40 eV) and B0 signal (187.50 eV) of the etched sample had negative BE shifts of 0.58 eV and 0.88 eV compared to those of pure NB (852.98 eV and 188.38 eV), respectively, while the BEs of the Ni2+ peak (855.6 eV) and F peak (684.5 eV) of the etched sample were positively shifted by 0.25 eV and 0.52 eV compared to those of FNH (855.35 eV and 683.98 eV). These results clearly show the strong interfacial coupling between the NB and FNH phases, and that electrons were transported from boride to F-Ni(OH)2, evidencing the formation of the FNH/NB heterostructure.
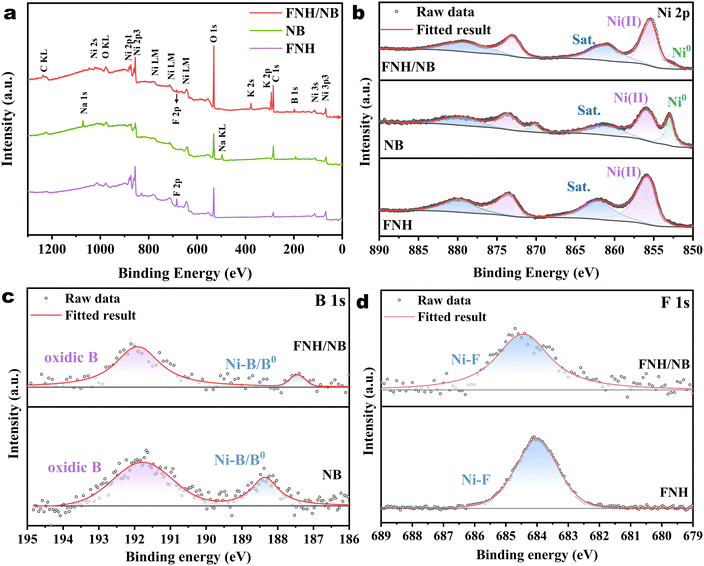 | ||
| Fig. 3 (a) XPS full spectra of FNH/NB, NB, and FNH. (b) Ni 2p XPS spectra of FNH/NB, pure NB, and FNH. (c) B 1s XPS spectra of FNH/NB, and pure NB. (d) F 1s XPS spectra of FNH/NB, and FNH. | ||
The structure of the target sample (FNH/NB) was investigated in depth, employing TEM technology. Under the low-resolution TEM in Fig. 4a, the contrast between the light and dark regions clearly showed the hollow rod-like structure of FNH/NB. The view at higher magnification (Fig. 4b) revealed that a thin layer of nanosheets appeared on the surface of the microrods. Considering that nickel hydroxide generated in alkaline solution usually appears in the structure of nanosheets according to previous reports,26,51,52 it can be inferred that this is FNH synthesized in the alkaline etching step. In the high-resolution TEM (HRTEM) image (Fig. 4c), several lattice fringes with a small distribution range were presented, which is in accordance with the property of the small-scale order and large-scale disorder in the amorphous phase. The fringe spacing of 0.233 nm corresponds to the (101) plane of Ni(OH)2 (PDF#14-0117), while the lack of an obvious lattice fringe can be attributed to the amorphous nickel boride. The amorphous nature of FNH/NB was further evidenced by the selected-area electron diffraction (SAED) image (Fig. 4d), which displayed typical amorphous diffraction rings and no crystalline diffraction spots. In the EDS mapping (Fig. 4e and f), the Ni, O, B, and F elements were uniformly distributed on the microrod shell, demonstrating the intimate contact of the FNH and NB phases. Therefore, the FNH/NB amorphous heterostructure was formed by hydrothermal, electroless plating, and alkaline etching methods.
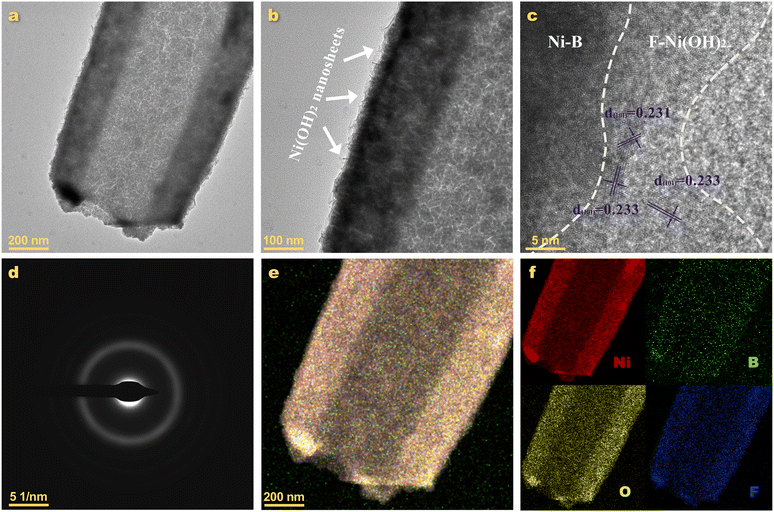 | ||
| Fig. 4 TEM characterization of FNH/NB. (a and b) TEM images, (c) HRTEM image, (d) SAED pattern, (e) overlap mapping image, and (f) elemental mapping images of Ni, B, O and F. | ||
3.2 Catalytic properties analysis
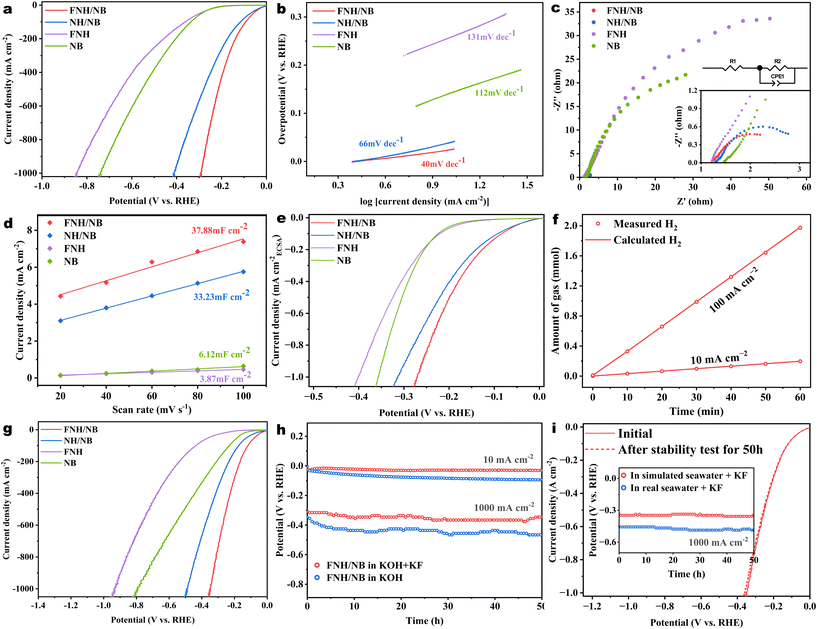
The HER catalyst activity of the target FNH/NB and a series of catalysts was assessed in 1 M KOH electrolyte using a three-electrode electrochemical setup. The LSV curves for the FNH/NB, NH/NB, NB, and FNH catalysts are compared in Fig. 5a. The FNH/NB catalyst was found to have a relatively low overpotential of only 23 mV in 1.0 M KOH to attain 10 mA cm−2, indicating its higher alkaline HER performance than that of NH/NB (38 mV), NB (141 mV), and FNH (206 mV). Impressively, the FNH/NB catalyst can generate a large current density of 1000 mA cm−2 while maintaining a much lower overpotential of 293 mV than that of the contrast samples, showing its potential for commercial use. It exhibits the best catalytic activity among the currently reported HER catalysts, superior to that of most reported non-noble transition metal catalysts (Table S4†). Here, the performance advantage of the FNH/NB catalyst over that of the NB and FNH samples should be attributed to the interfacial coupling of the constituent phase, while the superiority over the NH/NB sample should be attributed to the F-doping effect. All these impacts may concurrently optimize the fundamental processes involved in alkaline HER (H2O dissociation, OH* transfer, and H* associative desorption), thereby significantly improving the alkaline HER kinetics.For the purpose of better investigating the catalytic mechanism of the catalysts towards alkaline HER, Tafel slopes were fitted from the polarization curve in alkaline freshwater. As displayed in Fig. 5b, the Tafel slope of the FNH/NB catalyst was only 40 mV dec−1, which was smaller than that of NH/NB (66 mV dec−1), NB (112 mV dec−1) and FNH (131 mV dec−1). In accordance with the widely accepted HER mechanism, the above data indicated that the reaction process in alkaline electrolytes of the series of catalysts occurs via a Volmer–Heyrovsky route, and is kinetically restricted by the Volmer process, which can be accelerated by the FNH/NB catalyst. To interrogate the electrode kinetics of the catalysts, the Nyquist diagram (Fig. 5c) was recorded applying the EIS approach, and the experimental data was fitted by Zview software (Table S1†). The FNH/NB catalyst possessed a much lower Rct value (1.5 Ω) than NH/NB (2.1 Ω), NB (64.2 Ω) and FNH (117.5 Ω). The ultra-low Rct value indicates the exceptional charge-transfer ability, which is extremely advantageous in decreasing the overpotential of the HER process, particularly at high current densities. The improvement in the charge transfer characteristic may be attributed to the presence of a greater number of defective structures and disordered atomic arrangements within the amorphous heterostructure, which provides a favorable electron transfer path, thereby reducing the charge transfer resistance.25,53 Meanwhile, it has been reported that F doping with high electronegativity will affect the electron distribution of the catalyst, introduce a narrower band gap, and thus improve its conductivity.49,54 In addition, CV curves at different scan rates (Fig. S2†) were utilized to determine the Cdl. The Cdl of FNH/NB was calculated as 37.88 mF cm−2 (Fig. 5d), which is larger than that of NH/NB (33.23 mF cm−2), NB (6.12 mF cm−2), and FNH (3.87 mF cm−2), indicating a larger ECSA of FNH/NB for utilization in the catalytic process. This may arise from its hollow microrod structure and the amorphous substances. Nevertheless, the ECSA-normalized polarization curves (Fig. 5e) reveal that the FNH/NB catalyst still possessed greater catalytic performance than the reference catalysts, highlighting its outstanding intrinsic activity. In addition, the amounts of H2 generated under the same reaction durations were nearly consistent with the theoretically expected value at 10 and 100 mA cm−2 (Fig. 5f). These good matches suggested that almost all of the input charge was used to generate H2, illustrating an approximately 100% FE.
Given the outstanding HER activity of FNH/NB in 1.0 M KOH, its electrocatalytic performance was subsequently evaluated in simulated alkaline seawater (1.0 M KOH + 0.5 M NaCl) and real alkaline seawater (1.0 M KOH + seawater).8,49,55 As depicted in Fig. 5g and Fig. S3,† the FNH/NB catalyst retains its high HER activity in both simulated alkaline seawater and real alkaline seawater, despite the negative effect of the Cl ions. It only took 28 mV and 30 mV to achieve a current density of 10 mA cm−2 in simulated alkaline seawater and in real alkaline seawater, respectively, which is considerably lower than those of NH/NB (40 mV and 43 mV), NB (141 mV and 155 mV), and FNH (211 mV and 223 mV). Even under the current density of 1000 mA cm−2, the overpotential of the FNH/NB catalyst (349 mV in simulated alkaline seawater and 415 mV in real alkaline seawater) was only slightly larger than that in alkaline fresh water. According to the results in Fig. S4–S7 and Table S2,† the Tafel slope (43 mV dec−1), Cdl (35.48 mF cm−2), and Rct value (1.8 Ω) of the target catalyst in simulated alkaline seawater were close to those in 1.0 M KOH, indicating that its catalytic activity was well preserved.
Stability is also a crucial criterion when assessing the HER electrocatalysts. The chronopotentiometry (CP) method was applied to evaluate the stability of the FNH/NB catalyst. As seen in Fig. 5h, the overpotential of the FNH/NB catalyst exhibited an increase of around 68 and 120 mV after the 50 h test at 10 and 1000 mA cm−2, respectively. The decrease of stability may be related with the F-leaching, as the post-used FNH/NB showed similar activity to the NH/NB sample (Fig. S8†). To explore the effect of F doping on amorphous phase catalysts, we additionally added 0.01 M KF to the KOH solution to prevent F leaching from the catalyst.50 The CP curves (Fig. 5h) revealed that the overpotential at 10 mA cm−2 hardly decreases and only slightly fluctuates in a value of ±25 mV even at a high current density of 1000 mA cm−2, demonstrating the superior stability of the F-doped NH/NB amorphous/amorphous heterostructure. To ascertain the stability of FNH/NB, the post-reaction catalyst was carefully analyzed using SEM, XRD and XPS methods. The results showed that the FNH/NB catalyst retained its original morphology, microstructure and phase composition to a large extent after the 50-hour stability test. To be specific, the SEM images (Fig. S9†) demonstrated the good preservation of the microrod-like morphology of FNH/NB after long-term testing. The XRD pattern (Fig. S10†) only showed diffraction from the NF substrate, indicating that the catalyst remained amorphous after the stability test. Moreover, the XPS full survey (Fig. S11†) demonstrated that the elemental composition remained consistent before and after the stability test. The XPS semi-quantitative analysis (Table S3†) revealed that the element content exhibited minimal change, with a slight increase in the F element and a slight decrease in the B element. This suggests that the added KF effectively prevented the leakage of F during the stability test. However, the observed decrease in B may be attributed to the coverage of the Ni–B phase by the newly generated hydroxides (Fig. S12b†). The Ni 2p and F 1s fine spectra (Fig. S12a and c†) remained almost unchanged, suggesting the good stability of the catalyst composition. Furthermore, the target catalyst also displayed stable catalytic activity at a large current density of 1000 mA−2 in the stimulated and real alkaline seawater containing 0.01 M KF, as shown in the Fig. 5i inset. The LSV curve comparison of the target catalyst before and after the 50-hour stability test in simulated alkaline seawater (Fig. 5i) further illustrates the satisfactory stability of the phase structure and composition of the FNH/NB catalyst.
3.3 Mechanism exploration
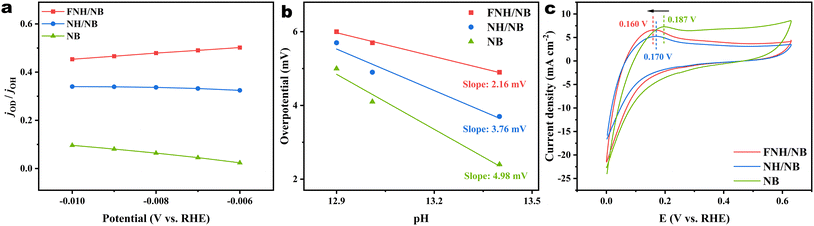
For the purpose of elucidating the origin of the excellent HER activity of the FNH/NB catalyst, we further conducted designed experiments to explore its capability for the H2O dissociation, OH* transfer, and H* coupling desorption. The H2O dissociation can be evaluated using the H/D kinetic isotope effect (KIE), which was determined by the ratio of H2 generation rates in H2O and D2O at a given potential. A KIE value closer to 1 typically means a stronger H2O dissociation ability, as the hydrogen-oxygen bonds in D2O have higher thermodynamic stability than that in H2O.56 As seen in Fig. 6a and Fig. S13,† FNH/NB exhibits a H/D KIE value of 0.46–0.51 in the micropolar region, higher than that of NH/NB (0.31–0.33) and NB (0.02–0.09), suggesting that faster water dissociation kinetics occurred on the FNH/NB catalyst due to the interfacial coupling and F-induced effects. Subsequently, the critical OH* transfer and H* adsorption behaviors involved in alkaline HER were experimentally investigated. As shown in Fig. 6b and Fig. S14,† the HER catalytic activity of the FNH/NB catalyst changes more slowly with increasing pH value than that of the NH/NB and NB catalysts, indicating an accelerated OH* transfer process on the FNH/NB catalyst. The faster OH* transfer kinetics would alleviate the effect of the OH− concentration on the slow Volmer step.57 Moreover, hydrogen underpotential deposition (HUPD) tests (Fig. 6c) revealed that the hydrogen desorption peak position of FNH/NB (0.160 V) was lower than that of NH/NB (0.170 V) and NB (0.187 V), manifesting an easier H* desorption on the FNH/NB surface.57 These results further highlight the key impact of heterojunction and F-induced engineering for accelerating the alkaline HER. The optimization of the H2O dissociation, OH* transfer, and H* binding desorption in FNH/NB might be attributed to the effective modulation of the electronic structure of the constituent phases by these two effects. According to previous research results,58–62 Ni-based hydroxides are effective in promoting the H2O dissociation, and borides are favorable in facilitating the associative desorption of molecular H2. The combination of Ni-based hydroxide and boride with different energy states at the heterogeneous interface will spontaneously form a built-in electric field due to the difference in the work function. This induces the transfer of charge between the constituent phases and further modulates the electronic structure of the constituent phases, thereby providing the possibility of optimizing the adsorption of reactants and intermediates on the catalyst surface.14,63 Additionally, many reported works49,54 have proved that F doping would induce a significant change in the charges of neighboring atoms, endowing the active sites with optimized adsorption of surface atoms and molecules. As a result of these two effects, the FNH/NB catalyst exhibits more favorable behavior in the H2O dissociation, OH* transfer, and H* binding desorption, resulting in higher HER activity than the contrast samples.4 Conclusion
In summary, a F-doped NH/NB amorphous/amorphous hollow rod heterostructure was prepared by simple hydrothermal, electroless plating, and alkaline etching methods. As a HER catalyst, FNH/NB exhibited a low overpotential of 23, 28, and 30 mV at a current density of 10 mA cm−2 in alkaline freshwater, simulated seawater, and real seawater, respectively. The superior catalytic performance benefits from its high ECSA, as well as the interfacial coupling and F-induced effects. Moreover, owing to the F− ion stabilization, the catalyst could stably and consistently produce H2 over 50 h at the current densities of 10, and 1000 mA cm−2 in an alkaline solution containing KF. This work demonstrates the feasibility of F-doping and amorphous heterostructure engineering in developing advanced and durable electrocatalysts, which may provide a new perspective for optimizing the catalytic performance of cost-effective electrocatalysts.Author contributions
Shenyi Chen: conceptualization, investigation, data curation, formal analysis, validation, writing – original draft. Haoming Chu: investigation. Ziyin Xie: investigation. Lihui Dong: resources, supervision. Bin Li: resources, supervision. Minguang Fan: resources. Huibing He: resources. Zhengjun Chen: project administration, resources, funding acquisition, writing – review & editing, supervision.Data available
Data will be made available on reasonable request.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors are grateful to Guangxi Science and Technology Program [No. Guike AD23026107], Natural Science Foundation of Guangxi Province of China [No. 2024GXNSFBA010234], and the National Natural Science Foundation of China [No. 22062002, 22265002] for financial support.References
- Q. Qian, Y. Zhu, N. Ahmad, Y. Feng, H. Zhang, M. Cheng, H. Liu, C. Xiao, G. Zhang and Y. Xie, Recent advancements in electrochemical hydrogen production via hybrid water splitting, Adv. Mater., 2024, 36, 2306108 CrossRef CAS PubMed.
- T. Capurso, M. Stefanizzi, M. Torresi and S. M. Camporeale, Perspective of the role of hydrogen in the 21st century energy transition, Energy Convers. Manage., 2022, 251, 114898 CrossRef CAS.
- S. Li, G. Xing, S. Zhao, J. Peng, L. Zhao, F. Hu, L. Li, J. Wang, S. Ramakrishna and S. Peng, Fe-N co-doped carbon nanofibers with Fe3C decoration for water activation induced oxygen reduction reaction, Natl. Sci. Rev., 2024, 11, nwae193 CrossRef.
- B. A. Yusuf, W. Yaseen, M. Xie, R. S. Zayyan, A. I. Muhammad, R. Nankya, J. Xie and Y. Xu, Recent advances in understanding and design of efficient hydrogen evolution electrocatalysts for water splitting: A comprehensive review, Adv. Colloid Interface Sci., 2023, 311, 102811 CrossRef CAS PubMed.
- Y. Zheng, Y. Jiao, A. Vasileff and S. Qiao, The hydrogen evolution reaction in alkaline solution: From theory, single crystal models, to practical electrocatalysts, Angew. Chem., Int. Ed., 2018, 57, 7568–7579 CrossRef CAS PubMed.
- Y. Hao, S. F. Hung, W. J. Zeng, Y. Wang, C. Zhang, C. H. Kuo, L. Wang, S. Zhao, Y. Zhang, H. Y. Chen and S. Peng, Switching the oxygen evolution mechanism on atomically dispersed Ru for enhanced acidic reaction kinetics, J. Am. Chem. Soc., 2023, 145, 23659–23669 CrossRef CAS PubMed.
- T. Cui, X. Zhai, L. Guo, J.-Q. Chi, Y. Zhang, J. Zhu, X. Sun and L. Wang, Controllable synthesis of a self-assembled ultralow Ru, Ni-doped Fe2O3 lily as a bifunctional electrocatalyst for large-current-density alkaline seawater electrolysis, Chin. J. Catal., 2022, 43, 2202–2211 CrossRef CAS.
- W. Ou, W. Zhang, H. Qin, W. Zhou, Y. Tang and Q. Gao, Enhancing anti-chlorine corrosion of Ni3S2 by Mo-doping for mimic seawater electrolysis, J. Colloid Interface Sci., 2024, 655, 852–862 CrossRef CAS PubMed.
- F. Guo, T. J. Macdonald, A. J. Sobrido, L. Liu, J. Feng and G. He, Recent advances in ultralow-Pt-loading electrocatalysts for the efficient hydrogen evolution, Adv. Sci., 2023, 10, 2301098 CrossRef CAS PubMed.
- H. Yang, Y. Ji, Q. Shao, W. Zhu, M. Fang, M. Ma, F. Liao, H. Huang, Y. Zhang, J. Yang, Z. Fan, Y. Li, Y. Liu, M. Shao and Z. Kang, Metastable-phase platinum oxide for clarifying the Pt–O active site for the hydrogen evolution reaction, Energy Environ. Sci., 2023, 16, 574–583 RSC.
- F. Li, D. H. Kweon, G.-F. Han, H.-J. Noh, W. Che, I. Ahmad, H. Y. Jeong, Z. Fu, Y. Lu and J. B. Baek, Merging platinum single atoms to achieve ultrahigh mass activity and low hydrogen production cost, ACS Nano, 2023, 17, 2923–2931 CrossRef CAS PubMed.
- A. A. Feidenhans’l, Y. N. Regmi, C. Wei, D. Xia, J. Kibsgaard and L. A. King, Precious metal free hydrogen evolution catalyst design and application, Chem. Rev., 2024, 124, 5617–5667 CrossRef PubMed.
- M. Lao, P. Li, Y. Jiang, H. Pan, S. X. Dou and W. Sun, From fundamentals and theories to heterostructured electrocatalyst design: An in-depth understanding of alkaline hydrogen evolution reaction, Nano Energy, 2022, 98, 107231 CrossRef CAS.
- G. Zhao, K. Rui, S. X. Dou and W. Sun, Heterostructures for electrochemical hydrogen evolution reaction: A review, Adv. Funct. Mater., 2018, 28, 1803291 CrossRef.
- H. Wang, W. Fu, X. Yang, Z. Huang, J. Li, H. Zhang and Y. Wang, Recent advancements in heterostructured interface engineering for hydrogen evolution reaction electrocatalysis, J. Mater. Chem. A, 2020, 8, 6926–6956 RSC.
- Z. Sun, B. Chu, S. Wang, L. Dong, Q. Pang, M. Fan, X. Zhang, H. He, B. Li and Z. Chen, Hydrogen-bond induced and hetero coupling dual effects in N-doped carbon coated CrN/Ni nanosheets for efficient alkaline freshwater/seawater hydrogen evolution, J. Colloid Interface Sci., 2023, 646, 361–369 CrossRef CAS PubMed.
- Y. Zhou, B. Chu, Z. Sun, L. Dong, F. Wang, B. Li, M. Fan and Z. Chen, Surface reconstruction and charge distribution enabling Ni/W5N4 Mott-Schottky heterojunction bifunctional electrocatalyst for efficient urea-assisted water electrolysis at a large current density, Appl. Catal., B, 2023, 323, 122168 CrossRef CAS.
- D. Li, J. Wang, S. Wang, B. Chu, R. Li, B. Li, L. Dong, M. Fan and Z. Chen, An interface engineering induced hierarchical NiCo/V2O3/C Schottky heterojunction catalyst for large-current-density hydrogen evolution reaction, J. Mater. Chem. A, 2023, 11, 23397–23404 RSC.
- J. Gu, F. Duan, S. Liu, W. Cha and J. Lu, Phase engineering of nanostructural metallic materials: Classification, structures, and applications, Chem. Rev., 2024, 124, 1247–1287 CrossRef CAS PubMed.
- H. Li, X. Zhou, W. Zhai, S. Lu, J. Liang, Z. He, H. Long, T. Xiong, H. Sun, Q. He, Z. Fan and H. Zhang, Phase engineering of nanomaterials for clean energy and catalytic applications, Adv. Energy Mater., 2020, 10, 2002019 CrossRef CAS.
- Q. Yun, Y. Ge, Z. Shi, J. Liu, X. Wang, A. Zhang, B. Huang, Y. Yao, Q. Luo, L. Zhai, J. Ge, Y. Peng, C. Gong, M. Zhao, Y. Qin, C. Ma, G. Wang, Q. Wa, X. Zhou, Z. Li, S. Li, W. Zhai, H. Yang, Y. Ren, Y. Wang, L. Li, X. Ruan, Y. Wu, B. Chen, Q. Lu, Z. Lai, Q. He, X. Huang, Y. Chen and H. Zhang, Recent progress on phase engineering of nanomaterials, Chem. Rev., 2023, 123, 13489–13692 CrossRef CAS PubMed.
- Y. Cao, Roadmap and direction toward high-performance MoS2 hydrogen evolution catalysts, ACS Nano, 2021, 15, 11014–11039 CrossRef CAS PubMed.
- X. Wang, C. Xing, Z. Liang, P. Guardia, X. Han, Y. Zuo, J. Llorca, J. Arbiol, J. Li and A. Cabot, Activating the lattice oxygen oxidation mechanism in amorphous molybdenum cobalt oxide nanosheets for water oxidation, J. Mater. Chem. A, 2022, 10, 3659–3666 RSC.
- H. Xu, B. Fei, G. Cai, Y. Ha, J. Liu, H. Jia, J. Zhang, M. Liu and R. Wu, Boronization–induced ultrathin 2D nanosheets with abundant crystalline–amorphous phase boundary supported on nickel foam toward efficient water splitting, Adv. Energy Mater., 2020, 10, 1902714 CrossRef CAS.
- Y. Zhang, F. Gao, D. Wang, Z. Li, X. Wang, C. Wang, K. Zhang and Y. Du, Amorphous/crystalline heterostructure transition-metal-based catalysts for high-performance water splitting, Coord. Chem. Rev., 2023, 475, 214916 CrossRef CAS.
- Z. Dong, F. Lin, Y. Yao and L. Jiao, Crystalline Ni(OH)2 /Amorphous NiMoOx, mixed–catalyst with Pt–like performance for hydrogen production, Adv. Energy Mater., 2019, 9, 1902703 CrossRef CAS.
- W. Peng, Z. Wang, R. Lu, Q. Li, Z. Wang, Y. Zhao, L. Xu and L. Mai, Artificial heterointerfaces of defect-rich Ni and amorphous/crystalline MoN enable efficient hydrogen evolution reaction, Chem. Eng. J., 2023, 457, 141173 CrossRef CAS.
- S. Huang, Y. Meng, Y. Cao, F. Yao, Z. He, X. Wang, H. Pan and M. Wu, Amorphous NiWO4 nanoparticles boosting the alkaline hydrogen evolution performance of Ni3S2 electrocatalysts, Appl. Catal., B, 2020, 274, 119120 CrossRef CAS.
- J. Nai, J. Kang and L. Guo, Tailoring the shape of amorphous nanomaterials: Recent developments and applications, Sci. China Mater., 2015, 58, 44–59 CrossRef CAS.
- S. Lan, L. Zhu, Z. Wu, L. Gu, Q. Zhang, H. Kong, J. Liu, R. Song, S. Liu, G. Sha, Y. Wang, Q. Liu, W. Liu, P. Wang, C.-T. Liu, Y. Ren and X. L. Wang, A medium-range structure motif linking amorphous and crystalline states, Nat. Mater., 2021, 20, 1347–1352 CrossRef CAS PubMed.
- J. Hu, S. Li, Y. Li, J. Wang, Y. Du, Z. Li, X. Han, J. Sun and P. Xu, A crystalline–amorphous Ni–Ni(OH)2 core–shell catalyst for the alkaline hydrogen evolution reaction, J. Mater. Chem. A, 2020, 8, 23323–23329 RSC.
- T. I. Singh, A. Maibam, D. C. Cha, S. Yoo, R. Babarao, S. U. Lee and S. Lee, High–alkaline water–splitting activity of mesoporous 3D heterostructures: An amorphous–shell@crystalline–core nano–assembly of Co–Ni–phosphate ultrathin–nanosheets and V–doped cobalt–nitride nanowires, Adv. Sci., 2022, 9, 2201311 CrossRef CAS PubMed.
- M. Liu, K. Xie, M. D. Nothling, L. Zu, S. Zhao, D. J. E. Harvie, Q. Fu, P. A. Webley and G. G. Qiao, Ultrapermeable composite membranes enhanced via doping with amorphous MOF nanosheets, ACS Cent. Sci., 2021, 7, 671–680 CrossRef CAS PubMed.
- X. Chen, M. Wei and J. Zhou, Fluorine-doping-assisted vacancy engineering for efficient electrocatalyst toward hydrogen production, J. Mater. Chem. A, 2021, 9, 22626–22634 RSC.
- C. Wang, S. Wei, F. Li, X. Long, T. Wang, P. Wang, S. Li, J. Ma and J. Jin, Activating a hematite nanorod photoanode via fluorine-doping and surface fluorination for enhanced oxygen evolution reaction, Nanoscale, 2020, 12, 3259–3266 RSC.
- Z. Liu, L. Qin, X. Chen, X. Xie, B. Zhu, Y. Gao, M. Zhou, G. Fang and S. Liang, Improving stability and reversibility via fluorine doping in aqueous zinc–manganese batteries, Mater. Today Energy, 2021, 22, 100851 CrossRef CAS.
- X. Li, Y. Tang, J. Zhu, H. Lv, L. Zhao, W. Wang, C. Zhi and H. Li, Boosting the cycling stability of aqueous flexible Zn batteries via F doping in nickel–cobalt carbonate hydroxide cathode, Small, 2020, 16, 2001935 CrossRef CAS PubMed.
- K. Xiao, Y. Wang, P. Wu, L. Hou and Z. Q. Liu, Activating lattice oxygen in spinel ZnCo2O4 through filling oxygen vacancies with fluorine for electrocatalytic oxygen evolution, Angew. Chem., Int. Ed., 2023, 62, e202301408 CrossRef CAS PubMed.
- L. F. Chen, Z. Y. Yu, J. J. Wang, Q. X. Li, Z. Q. Tan, Y. W. Zhu and S. H. Yu, Metal-like fluorine-doped β-FeOOH nanorods grown on carbon cloth for scalable high-performance supercapacitors, Nano Energy, 2015, 11, 119–128 CrossRef CAS.
- W. Liu, Q. Zhao, Y. Wang, Y. Chen and L. Chen, “Two birds with one stone”: F doping Ni–Co Hydroxide as high-performance cathode material for aqueous Zn batteries, Nanomaterials, 2022, 12, 1780 CrossRef CAS PubMed.
- S. J. Patil, N. R. Chodankar, S. K. Hwang, G. S. Rama Raju, Y. S. Huh and Y. K. Han, Fluorine engineered self-supported ultrathin 2D nickel hydroxide nanosheets as highly robust and stable bifunctional electrocatalysts for oxygen evolution and urea oxidation reactions, Small, 2022, 18, 2103326 CrossRef CAS PubMed.
- Y. Ren, J. Wang, W. Wang, H. Wen, M. Chen, Y. Qiu, G. Li, Z. Yang and P. Wang, Boride-mediated synthesis of a highly active cobalt-based electrocatalyst for alkaline hydrogen evolution reaction, J. Mater. Chem. A, 2023, 11, 13282–13288 RSC.
- V. Vitry, J. Hastir, A. Mégret, S. Yazdani, M. Yunacti and L. Bonin, Recent advances in electroless nickel-boron coatings, Surf. Coat. Technol., 2022, 429, 127937 CrossRef CAS.
- H. B. Li, M. H. Yu, F. X. Wang, P. Liu, Y. Liang, J. Xiao, C. X. Wang, Y. X. Tong and G. W. Yang, Amorphous nickel hydroxide nanospheres with ultrahigh capacitance and energy density as electrochemical pseudocapacitor materials, Nat. Commun., 2013, 4, 1894 CrossRef CAS PubMed.
- Y.-Z. Su, K. Xiao, N. Li, Z.-Q. Liu and S.-Z. Qiao, Amorphous Ni(OH)2 @ three-dimensional Ni core–shell nanostructures for high capacitance pseudocapacitors and asymmetric supercapacitors, J. Mater. Chem. A, 2014, 2, 13845–13853 RSC.
- M. S. Vidhya, G. Ravi, R. Yuvakkumar, D. Velauthapillai, M. Thambidurai, C. Dang and B. Saravanakumar, Nickel–cobalt hydroxide: A positive electrode for supercapacitor applications, RSC Adv., 2020, 10, 19410–19418 RSC.
- J. Wu, M. Hou, Z. Chen, W. Hao, X. Pan, H. Yang, W. Cen, Y. Liu, H. Huang, P. W. Menezes and Z. Kang, Composition engineering of amorphous nickel boride nanoarchitectures enabling highly efficient electrosynthesis of hydrogen peroxide, Adv. Mater., 2022, 34, 2202995 CrossRef CAS PubMed.
- X. Chen, Z. Yu, L. Wei, Z. Zhou, S. Zhai, J. Chen, Y. Wang, Q. Huang, H. E. Karahan, X. Liao and Y. Chen, Ultrathin nickel boride nanosheets anchored on functionalized carbon nanotubes as bifunctional electrocatalysts for overall water splitting, J. Mater. Chem. A, 2019, 7, 764–774 RSC.
- J. Zhu, J. Chi, T. Cui, L. Guo, S. Wu, B. Li, J. Lai and L. Wang, F doping and P vacancy engineered FeCoP nanosheets for efficient and stable seawater electrolysis at large current density, Appl. Catal., B, 2023, 328, 122487 CrossRef CAS.
- J. Wang, Y. Ren and P. Wang, (Fe, F) co-doped nickel oxyhydroxide for highly efficient oxygen evolution reaction, J. Mater. Chem. A, 2023, 11, 4619–4626 RSC.
- K. Lu, J. Zhang, Y. Wang, J. Ma, B. Song and H. Ma, Interfacial deposition of three-dimensional nickel hydroxide nanosheet-graphene aerogel on Ni wire for flexible fiber asymmetric supercapacitors, ACS Sustainable Chem. Eng., 2017, 5, 821–827 CrossRef CAS.
- F. Zhao, H. Liu, H. Zhu, X. Jiang, L. Zhu, W. Li and H. Chen, Amorphous/amorphous Ni–P/Ni(OH)2 heterostructure nanotubes for an efficient alkaline hydrogen evolution reaction, J. Mater. Chem. A, 2021, 9, 10169–10179 RSC.
- M. Moloudi, A. Noori, M. S. Rahmanifar, Y. Shabangoli, M. F. El-Kady, N. B. Mohamed, R. B. Kaner and M. F. Mousavi, Layered double hydroxide templated synthesis of amorphous NiCoFeB as a multifunctional electrocatalyst for overall water splitting and rechargeable Zinc–Air Batteries, Adv. Energy Mater., 2023, 13, 2203002 CrossRef CAS.
- W. He, L. Han, Q. Hao, X. Zheng, Y. Li, J. Zhang, C. Liu, H. Liu and H. L. Xin, Fluorine-anion-modulated electron structure of nickel sulfide nanosheet arrays for alkaline hydrogen evolution, ACS Energy Lett., 2019, 4, 2905–2912 CrossRef CAS.
- D. Zhang, H. Miao, X. Wu, Z. Wang, H. Zhao, Y. Shi, X. Chen, Z. Xiao, J. Lai and L. Wang, Scalable synthesis of ultra-small Ru2P@Ru/CNT for efficient seawater splitting, Chin. J. Catal., 2022, 43, 1148–1155 CrossRef CAS.
- L. Xiao, T. Yang, C. Cheng, X. Du, Y. Zhao, Z. Liu, X. Zhao, J. Zhang, M. Zhou, C. Han, S. Liu, Y. Zhao, Y. Yang, H. Liu, C. Dong and J. Yang, Coupled compressive-tensile stains boosting both activity and durability of NiMo electrode for alkaline water/seawater hydrogen evolution at high current densities, Chem. Eng. J., 2024, 485, 150044 CrossRef CAS.
- B. Zhang, J. Wang, G. Liu, C. M. Weiss, D. Liu, Y. Chen, L. Xia, P. Zhou, M. Gao, Y. Liu, J. Chen, Y. Yan, M. Shao, H. Pan and W. Sun, A strongly coupled Ru–CrOx cluster–cluster heterostructure for efficient alkaline hydrogen electrocatalysis, Nat. Catal., 2024, 7, 441–451 CrossRef CAS.
- J. Zhao, K. Bao, M. Xie, D. Wei, K. Yang, X. Zhang, C. Zhang, Z. Wang and X. Yang, Two-dimensional ultrathin networked CoP derived from Co(OH)2 as efficient electrocatalyst for hydrogen evolution, Adv. Compos. Hybrid Mater., 2022, 5, 2421–2428 CrossRef CAS.
- L. Wang, Y. Hao, L. Deng, F. Hu, S. Zhao, L. Li and S. Peng, Rapid complete reconfiguration induced actual active species for industrial hydrogen evolution reaction, Nat. Commun., 2022, 13, 5785 CrossRef CAS PubMed.
- Z. Zhu, H. Yin, C. T. He, M. Al-Mamun, P. Liu, L. Jiang, Y. Zhao, Y. Wang, H. G. Yang, Z. Tang, D. Wang, X. M. Chen and H. Zhao, Ultrathin transition metal dichalcogenide/3D Metal hydroxide hybridized nanosheets to enhance hydrogen evolution activity, Adv. Mater., 2018, 30, 1801171 CrossRef PubMed.
- F. Song, T. Zhang, Y. Qian, J. Shaw, S. Chen, G. Chen, Y. Sun and Y. Rao, Multifunctional electrocatalysts of nickel boride nanoparticles for superior hydrogen oxidation and water splitting, Mater. Today Energy, 2021, 22, 100846 CrossRef CAS.
- J. Song, Y. Chen, H. Huang, J. Wang, S. Huang, Y. Liao, A. E. Fetohi, F. Hu, H. Chen, L. Li, X. Han, K. M. El-Khatib and S. Peng, Heterointerface engineering of hierarchically assembling layered double hydroxides on cobalt selenide as efficient trifunctional electrocatalysts for water splitting and Zinc–Air battery, Adv. Sci., 2022, 9, 2104522 CrossRef CAS PubMed.
- D. Chen, R. Lu, R. Yu, Y. Dai, H. Zhao, D. Wu, P. Wang, J. Zhu, Z. Pu, L. Chen, J. Yu and S. Mu, Work-function-induced interfacial built-in electric fields in Os-OsSe2 heterostructures for active acidic and alkaline hydrogen evolution, Angew. Chem., Int. Ed., 2022, 61, e202208642 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qi01853b |
| This journal is © the Partner Organisations 2024 |