Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lithium extraction by metal–organic frameworks
Zongsu
Han
a,
Joshua
Rushlow
a,
Yihao
Yang
 a,
Jiatong
Huo
a,
Wei
Shi
a,
Jiatong
Huo
a,
Wei
Shi
 *b and
Hong-Cai
Zhou
*b and
Hong-Cai
Zhou
 *a
*a
aDepartment of Chemistry, Texas A&M University, College Station, Texas 77843, USA. E-mail: zhou@tamu.edu
bFrontiers Science Center for New Organic Matter, Key Laboratory of Advanced Energy Materials Chemistry (MOE), and State Key Laboratory of Advanced Chemical Power Sources, College of Chemistry, Nankai University, Tianjin 300071, China
First published on 4th November 2024
Abstract
The extraction of lithium has become increasingly critical due to the soaring demand for lithium-ion batteries, which power a wide range of products from smartphones to electric vehicles. Lithium, the lightest metal, boasts exceptional energy density, making it ideal for portable electronics and renewable energy storage solutions, addressing environmental pollution issues simultaneously. However, extracting and recycling lithium ions is highly challenging due to its low natural abundance, significant mining difficulties, and environmental concerns. Metal–organic frameworks (MOFs), with their intricate and designable structures as well as abundant and regulated pore environments, have emerged as promising candidates for lithium extraction. The tailored porous structures of MOFs could enable efficient lithium ion sieving and capture. This review aims to discuss the design principles of targeted MOFs and their lithium extraction capabilities, as well as the common analysis methods during these processes. We seek to provide a comprehensive summary of the field and promote the development of advanced materials for practical lithium extraction applications.
The 10th anniversary statementOver the past decade, Inorganic Chemistry Frontiers has quickly risen to become one of the premier platforms for inorganic chemists to showcase their top research, achieving a level of recognition and respect that is truly remarkable. Spearheaded by the dedicated efforts of Prof. Song Gao and the entire editorial team, the journal has consistently enhanced both its quality and its influence in the field, making it an essential publication for researchers worldwide. I regularly browse through its latest issues to stay up to date with significant advancements and trends within the inorganic chemistry community. The journal's rigor and dedication to excellence have made it a prestigious venue, and I am always eager to contribute my own work to its pages, knowing the impact it has on readers and researchers alike. With its strong foundation, I have no doubt that this journal will continue to achieve even greater success in the years to come. |
1. Introduction
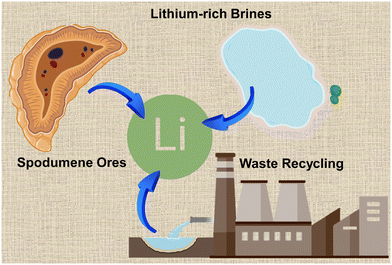
Lithium, a highly versatile and sought-after element, plays a pivotal role in modern technology and industry.1–3 As the lightest metal, lithium is renowned for its exceptional electrochemical properties, particularly its high energy density and low weight. These characteristics make it an ideal choice for various applications, most notably in the production of lithium-ion batteries.4–10 Batteries play a crucial role in modern life, serving as essential components in a wide array of devices and technologies. They provide portable and reliable power sources for everything from smartphones and laptops to medical devices and electric vehicles. As the world increasingly shifts towards greener energy solutions, the importance of batteries in reducing carbon emissions and enhancing energy security cannot be overstated.11–15 Beyond energy storage, lithium has significant applications in other fields. It is used in the production of high-strength glass and ceramics, lubricating greases, and as an alloying agent in lightweight metals. In the medical field, lithium compounds are employed in the treatment of bipolar disorder, showcasing its diverse utility.16–20The growing reliance on lithium to drive technological advancement and combat climate change has exponentially increased the demand for lithium. However, the process of obtaining lithium is fraught with challenges, involving environmental protection issues, safety considerations, and economic perspectives.21–23 Naturally occurring lithium is relatively scarce, and its extraction often involves complex and environmentally taxing mining operations. Traditional sources of lithium, such as spodumene ores and lithium-rich brines, require extensive processing to yield the metal in its usable form.24–27 These processes are not only energy-intensive but also pose significant environmental concerns, including water usage, habitat disruption, and chemical pollution, which simultaneously cause time and cost waste. In parallel with extraction from nature, the recycling of lithium from spent batteries has emerged as a crucial strategy to ensure the sustainable supply of this valuable resource.28–32 When improperly disposed of, lithium batteries can leak hazardous materials like lithium, cobalt, and nickel, contaminating soil and water, harming wildlife, and posing health risks to humans. Recycling not only helps mitigate the environmental impact associated with mining but also addresses the growing problem of electronic waste. Efficient recycling processes can recover a significant proportion of lithium from used batteries, thereby reducing dependence on virgin materials and contributing to a circular economy (Fig. 1).33–35
Amid these challenges, metal–organic frameworks (MOFs) have surfaced as promising materials for lithium extraction. MOFs represent an innovative class of materials that have garnered significant attention in the scientific community over the past few decades.36–42 Characterized by their unique structures, MOFs are composed of metal ions or clusters coordinated to organic ligands, forming porous multi-dimensional networks.43–50 These frameworks exhibit exceptional properties such as high surface area, variable porosity, and diverse chemical functionality, making them highly versatile for various applications, such as asymmetric catalysis,51–53 molecular recognition,54–56 optical devices,57,58 battery components,59,60 and especially in selective guest capture and separation processes, in both gases and liquids.61–65 Recent research has highlighted the potential of MOFs to enhance the efficiency and selectivity of lithium extraction from natural sources and to improve the recovery rates from recycled materials.66–75
This review explores the current state of lithium extraction from natural sources and recycling by MOFs, emphasizing the role of MOFs in these processes. In the literature, Konstas and coworkers have introduced the lithium extraction methods and summarized the advances in MOF-based membranes.76 Zhang's group focused on the MOF membrane separation of magnesium and lithium ions from salt lakes.77 Razmjou and coworkers discussed the MOF membranes for the lithium-ion extraction.78 We delve into the design principles and extraction capabilities of targeted MOFs with functional groups, and discuss the detection methods in detail used for recent advancements in this field. We aim to provide insights into the advancements and challenges in lithium extraction and recycling, thereby contributing to the development of sustainable and efficient methods for securing this vital resource.
2. Design principles
The design principles for lithium extraction using MOFs involve leveraging the specialized pore properties of these materials, particularly introducing functional groups with selective binding abilities in the pores of MOFs for lithium capture. For practical applications, the selective adsorption of Li+ ions over Na+, Mg2+, and Ca2+ ions are critical, depending on the factors such as binding strength, ionic radii, and charges. Targeted functional groups such as crown ethers, sulfonate/sulfonyl groups, thiol groups, hydroxyl/carboxyl groups, amino groups, and spiropyran, significantly alter the selective adsorption behavior in MOFs by providing binding sites through electrostatic interactions with Li+ ions. Besides, the porosity of MOFs, characterized by large surface areas and adjustable pore sizes, plays a key role in determining the adsorption capacity and influences the selectivity.Crown ethers are known for their effective cation anchoring capabilities due to their cyclic cavities and electron-donating properties, which have been exploited for selective separation of different cations. Inspired by protein channels in biofilms, which conditionally regulate ion transport by controlling the distribution of charged residues, Li and colleagues introduced benzo-12-crown-4-ether (BCE) into a classical Zn-MOF ZIF-7 to create BCE@ZIF-7, demonstrating a notable Li+/Mg2+ ion selectivity based on their different binding capacities.66 Various ions can bind with crown ethers embedded in the MOF cavity, acting as charged guests to modulate the channel's charge states. Experiments demonstrated that the positively charged channel exhibited improved Li+/Mg2+ selectivity, which was attributed to an increased difference in entry energy between Li+ and Mg2+ at the sub-nanoscale level, rather than a simple electrostatic effect. Compared to the negatively charged channel, the positively charged channel significantly enhanced Li+/Mg2+ selectivity due to stronger electrostatic repulsion between the ions and the channel (Fig. 2). Furthermore, theoretical calculations also indicated that transporting magnesium ions through the more positively charged channel necessitates overcoming a higher entry energy barrier than that of lithium ions.
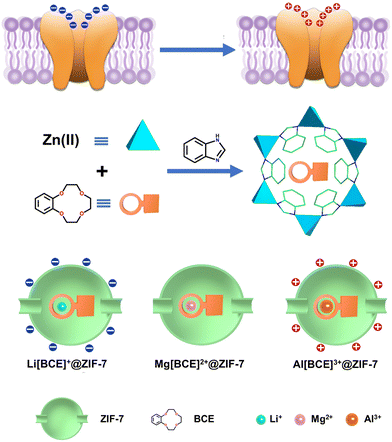 | ||
| Fig. 2 Scheme illustration for the construction of M[BCE]n+@ZIF-7 (M = Li+, Mg2+, Al3+).66 Copyright 2023 Wiley-VCH. | ||
Sulfonate and sulfonyl groups also exhibit strong binding affinity towards lithium ions. Chen et al. incorporated polystyrene sulfonate (PSS) into a classical Cu-MOF HKUST-1 at varying concentrations, to construct PSS@HKUST-1-6.7 with unique anchored three-dimensional sulfonate networks (Fig. 3), displaying high ion selectivities of 78, 99, and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 296 for Li+/Na+, Li+/K+, and Li+/Mg2+, respectively, and real binary ion selectivities of 35, 67, and 1815.67 This MOF possesses very high Li+ conductivity, outstanding selectivity for Li+ ion over Na+, K+, and Mg2+ ions, and low activation energy, which makes it exceptionally promising for efficient extraction of lithium ions from salt-lake brines. Liu's team introduced PSS into a two-dimensional Zr-MOF, where the differential affinity of Li+, Na+, K+, and Mg2+ ions to terminal sulfonate groups induced Li+ ion selectivity towards Na+, K+, and Mg2+ ions as 11.0, 16.5, and 37.8, respectively.68 They summarize that the ion transport across the material to occur through two main processes. First, metal cations undergo partial dehydration at the entrance of the channel and interact with the sulfonate groups due to their high affinity. In the second process, the cations hop between adjacent active sites, while anions are repelled by the negatively charged functional groups and bypass these regions due to electrostatic repulsion. The confined space interactions and the surface interactions possess a synergistic effect for the selective extraction of lithium in this case (Fig. 4). Li and coworkers employed a bis(sulfonyl)imide-based ligand 4,4′-((hydrosulfonylamino)sulfonyl)dibenzoic acid to construct a stable luminescent Zr-MOF LMOF-321.69 LMOF-321 not only has a maximum lithium-ion uptake capacity of 12.18 mg g−1, but also demonstrates high selectivity for lithium ions over other light metal ions, with the detection ratios of 6.2, 14.3, and 44.9 for Li+/Na+, Li+/Ca2+, and Li+/Mg2+, respectively. Also, this MOF can serve as the selective sensor for lithium ions with a 6549 M−1 quenching constant value. This MOF has been employed for not only the detection but also the extraction of lithium ions, advancing this MOF toward more efficient harvesting procedures.
296 for Li+/Na+, Li+/K+, and Li+/Mg2+, respectively, and real binary ion selectivities of 35, 67, and 1815.67 This MOF possesses very high Li+ conductivity, outstanding selectivity for Li+ ion over Na+, K+, and Mg2+ ions, and low activation energy, which makes it exceptionally promising for efficient extraction of lithium ions from salt-lake brines. Liu's team introduced PSS into a two-dimensional Zr-MOF, where the differential affinity of Li+, Na+, K+, and Mg2+ ions to terminal sulfonate groups induced Li+ ion selectivity towards Na+, K+, and Mg2+ ions as 11.0, 16.5, and 37.8, respectively.68 They summarize that the ion transport across the material to occur through two main processes. First, metal cations undergo partial dehydration at the entrance of the channel and interact with the sulfonate groups due to their high affinity. In the second process, the cations hop between adjacent active sites, while anions are repelled by the negatively charged functional groups and bypass these regions due to electrostatic repulsion. The confined space interactions and the surface interactions possess a synergistic effect for the selective extraction of lithium in this case (Fig. 4). Li and coworkers employed a bis(sulfonyl)imide-based ligand 4,4′-((hydrosulfonylamino)sulfonyl)dibenzoic acid to construct a stable luminescent Zr-MOF LMOF-321.69 LMOF-321 not only has a maximum lithium-ion uptake capacity of 12.18 mg g−1, but also demonstrates high selectivity for lithium ions over other light metal ions, with the detection ratios of 6.2, 14.3, and 44.9 for Li+/Na+, Li+/Ca2+, and Li+/Mg2+, respectively. Also, this MOF can serve as the selective sensor for lithium ions with a 6549 M−1 quenching constant value. This MOF has been employed for not only the detection but also the extraction of lithium ions, advancing this MOF toward more efficient harvesting procedures.
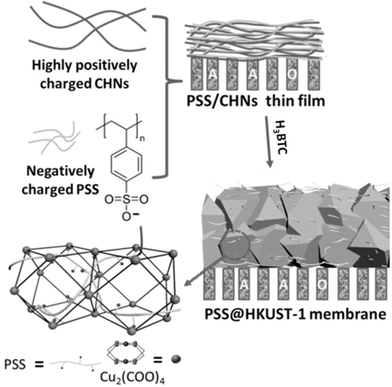 | ||
| Fig. 3 Preparation of PSS@HKUST-1 membranes.67 Copyright 2016 Wiley-VCH. | ||
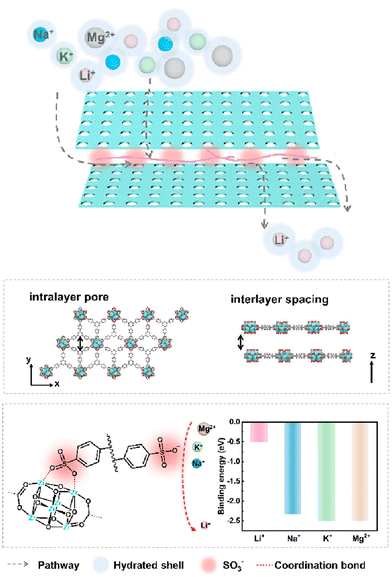 | ||
| Fig. 4 Design of the PSS modified Zr-MOF for lithium-ion sieving.68 Copyright 2024 American Chemical Society. | ||
Thiol groups were also introduced into a classical Zr-MOF UiO-66 by Zhang and coworkers, to construct UiO-66-(SH)2 for lithium extraction.70 UiO-66-(SH)2 demonstrates excellent monovalent metal ion selectivity over divalent metal ions, achieving Li+/Mg2+ ion selectivity up to 103. Experimental characterizations and theoretical simulations revealed that ion-sulfur interactions and ion-dehydration dynamics within the thiolated MOF channels collectively influence the overall energy barriers that determine ion sieving performance. Magnesium ions in this system must overcome a higher energy barrier than lithium ions during the transport through the pores, as confirmed by molecular dynamics simulations (Fig. 5).
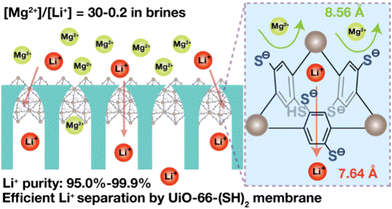 | ||
| Fig. 5 Selective binding of Li+ ions towards Mg2+ ions by UiO-66-(SH)2.70 Copyright 2024 American Chemical Society. | ||
Hydroxyl and carboxyl groups are employed to bind lithium ions in various MOFs. Dybtsev and coworkers reported the synthesis and characterization of a series of Zn12-cluster-based MOFs based on the dodecanuclear metallocyclic building block with the inner rim decorated by tuneable pendants.71 One of these MOFs, whose unit closely resembles α-cyclodextrin, the molecular macrocyclic cavitand characterized by a narrow portal surrounded by six –CH2OH groups with a highly diverse supramolecular chemistry, features highly size-selective adsorption of alkaline metal cations in the order of Li+ > Na+ > K+ > Cs+, in agreement with the increase of their ionic radii (Fig. 6). Wang and colleagues introduced additional carboxyl groups into UiO-66, with the heterogeneous structure and surface chemistry to achieve biological ion channels, which can achieve a mono/divalent ion selectivity of 103.72 At the same time, though the changes of the pH values from 3 to 8, the ion selectivity can be tuned further by a factor of 102 to 104. Theoretical simulations suggest that ion–carboxyl interactions significantly decrease the energy barrier for monovalent cations to traverse the MOF-based sub-nanochannel, resulting in exceptionally high ion selectivity.
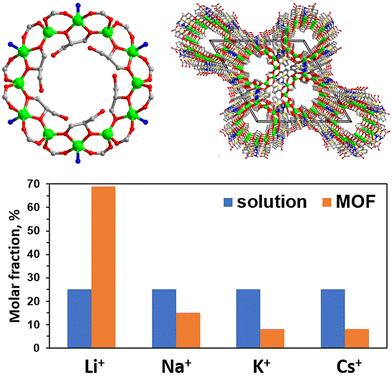 | ||
| Fig. 6 Structure of the Zn12-cluster-based MOF with its selective adsorption capacities.71 Copyright 2019 American Chemical Society. | ||
Amino groups have been studied for their selective binding with lithium ions. Choi and coworkers incorporated amino groups into UiO-66 to construct UiO-66-(NH2)2, emphasizing the crucial role of the robustness of ion hydrates in selective filtration properties.73 Lithium ions exhibit high permeability due to repeated cleavage and recombination of coordination water as they penetrate the MOF, while the hydrate formed by magnesium ions maintain its structure within the MOF due to the strong binding force of the coordination water. The presence of amine groups and the application of an electric field accelerate this jumping behavior, indicating that the difference in physicochemical behavior between Li+ and Mg2+ ions can be directly used to maximize the selective lithium extraction. The energy barriers hinder ionic movements, effectively retaining the target ion while facilitating the transport of competing ions. Razmjou and coworkers combined UiO-66-NH2 and ZIF-8 to construct MOF-on-MOF cavities.74 The resulting structure exhibits an unprecedented ionic current rectification ratio with exceptionally high ion selectivity for K+/Li+ and Na+/Li+ with ratios of 84 and 80, respectively. With the quantum mechanics/molecular mechanics calculations, the synergistic effect of spatial hindrance and nucleophilic entrapment in this MOF induces energy barriers that contribute to the ultrahigh selectivity and ion rectification (Fig. 7).
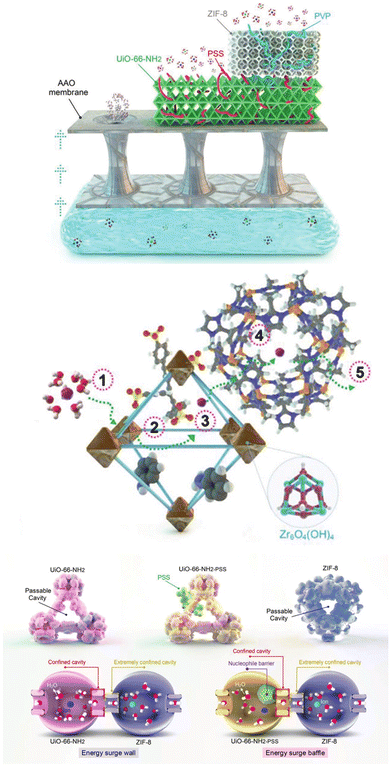 | ||
| Fig. 7 Conceptual design of the asymmetrical architecture for the combination of UiO-66-NH2 and ZIF-8.74 Copyright 2022 Wiley-VCH. | ||
Spiropyran was also employed to be introduced into UiO-66 by Ou and coworkers for selective lithium extraction.75 The as-synthesized MOF PSP-UiO-66 with the light-triggered function of polyspiropyran (PSP) possesses a narrowest 6.0 Å window size, which is well suited for lithium ions. Under dark, PSP-UiO-66 shows high LiCl adsorption capacity up to 10.17 mmol g−1 as well as great Li+/Mg2+ ion selectivity of 5.8 to 29 under different Li+/Mg2+ ion ratios from 1 to 10. Interestingly, PSP-UiO-66 can be further regenerated by sunlight based on its stimuli-triggered ligand, which provides convenience for the material regeneration (Fig. 8).
 | ||
| Fig. 8 Schematic illustration of the selective lithium adsorption by PSP-UiO-66.75 Copyright 2024 National Academy of Sciences. | ||
In conclusion, the design principles for lithium extraction using MOFs rely on their unique pore properties and selective binding abilities. Incorporating functional groups with N, O, and S atoms as binding sites, such as crown ethers, sulfonate, sulfonyl, thiol, hydroxyl, carboxyl, amino groups, and spiropyran significantly enhances the selective capture of lithium ions over other cations. These diverse strategies highlight the potential of MOFs to achieve efficient and selective lithium extraction, which is essential for the advancement of sustainable energy storage technologies.
Further, as discussed above, research on lithium extraction by MOFs primarily focuses on the differences in ionic radius and binding affinities with specific functional groups, which are influenced by the pore size, shape, and functionalization of MOFs. However, other parameters, such as redox potentials, conductivities in solvents, diffusion coefficients, and solubilities, also differ between lithium and other metal ions and could be explored for further applications in this area.
3. Analysis methods
At the same time, the instant and efficient quantitative analysis of the concentration of lithium and relative interfering metals is highly crucial in various fields. In the evaluation of materials, accurately determining lithium concentration is essential for evaluating and optimizing the properties of the advanced materials, where precise lithium levels directly impact the performance and longevity. In the discovery of lithium sources, the ability to swiftly and accurately measure the lithium concentration is vital for tracking its presence in natural water bodies and industrial effluents. Thus, advancements in rapid lithium quantification techniques are indispensable for both technological development and environmental stewardship.Inductively coupled plasma (ICP) is a classical and powerful analytical technique widely used in chemical analysis and elemental determination for solutions (Fig. 9).79 By subjecting a certain sample to a high-temperature plasma generated within an electromagnetic field, ICP excites atoms and ions, leading to their emission of characteristic radiation. This emitted radiation is then measured to identify and quantify the elements present in the sample. Known for its sensitivity, precision, and ability to handle complex samples, ICP has become indispensable in accurate elemental analysis.80–82 In most cases, the concentrations of lithium ions and other interferent metal ions are detected by ICP technology for its high sensitivity and precision, especially for the ICP-optical emission spectrometry (ICP-OES)68,75/ICP-atomic emission spectrometry (ICP-AES),71 while the emitted light is analysed using an optical spectrometer allowing for the precise identification and quantification of the elements. The high sensitivity, wide elemental coverage, high accuracy, and fast speed characteristics make ICP a widely-used technique for metal elemental analysis. However, this technology generally requires the expensive equipment and daily maintenance, interference issues, experimental testers and careful and cautious treatment with the samples. For lithium extraction by MOFs, the crucial issues are the difficulties for the complete dilution of the solid samples, the dilution processes, and the interferences of the widespread Na+, Mg2+, and Ca2+ ions, which extremely influence the property evaluation.
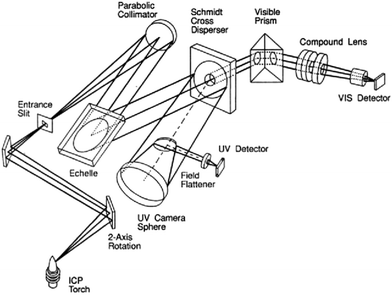 | ||
| Fig. 9 Schematic optical system of the ICP-OES equipment.79 Copyright 1993 American Chemical Society. | ||
Energy dispersive X-Ray spectroscopy (EDX or EDS) mapping is an efficient analytical technique used to determine the elemental composition of solid materials. This method leverages the interaction of X-ray with a sample to produce characteristic X-ray emissions that are unique to each element. By detecting and analysing these emissions, EDX mapping provides detailed information about the elements present in the sample and their spatial distribution. Key advantages of EDX mapping include its high spatial resolution, the ability to analyse a wide range of elements, and the non-destructive nature of the analytes. Additionally, EDX mapping is generally integrated with scanning electron microscopy (SEM) or transmission electron microscopy (TEM) (Fig. 10),83 providing a multi-faceted view of the sample's morphology and composition.84–87 These characteristics make the in situ detection of the changes of the ionic concentrations on the materials possible, which makes the evaluation of the selected materials more direct and efficient (Fig. 11).72 The rapid and non-destructive analysis process, wide elemental detection range, high spatial resolution for localized element distribution, facile sample preparation make EDX an attractive method for the rapid test. However, the limited sensitivity, overlapping peaks, surface limitation, and lower detection limits make EDX hard for high precision quantitative analysis. Besides, in general, EDX mapping can detect Na+, Mg2+, and Ca2+ ions, but cannot detect Li+ ion directly due to the limitation of this technology, which greatly restricts its application.
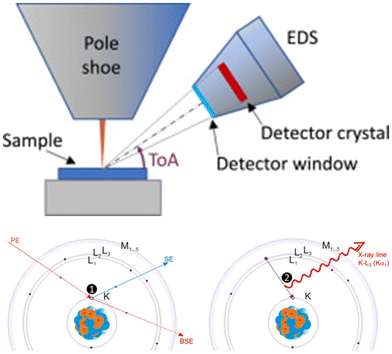 | ||
| Fig. 10 Schematic system of the SEM-EDS equipment and the process of the emission of characteristic X-rays.83 Copyright 2020 Elsevier. | ||
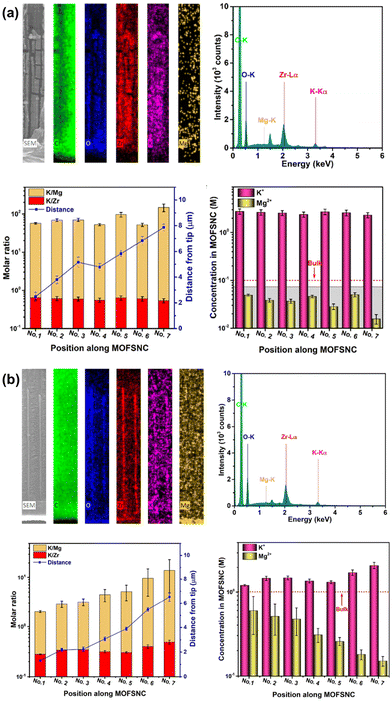 | ||
| Fig. 11 SEM-EDX analysis of MOFs with (a) 0.1 M KCl + 0.1 M MgCl2 and (b) 1.0 M KCl + 1.0 M MgCl2 mixture solution.72 Copyright 2020 Springer Nature. | ||
Luminescent quantitative detection is a cutting-edge analytical technique that leverages the emission of light from luminescent materials to measure and quantify various samples. This method has gained significant traction across multiple fields due to its high sensitivity, specificity, and the ability to provide real-time data (Fig. 12).88 The advancements in luminescent materials, detection instrumentation, and data analysis methods have significantly enhanced the performance and applicability of luminescent quantitative detection for a series of analytes, mainly including toxic, dangerous, or useful chemicals, like cations and anions, organic solvents, volatile organic compounds, persistent organic pollutants, explosives, and biomarkers. As the demand for more sensitive, accurate, and rapid detection methods continues to grow, luminescent quantitative detection stands at the forefront, offering unparalleled capabilities for researchers and professionals across diverse scientific disciplines.89–93 In lithium detection, luminescent quantitative recognition, possessing facile and instant characteristics, serves as a great candidate towards the equipment miniaturization for facile and daily detection of lithium ions. Through the combination of MOFs, the materials can not only achieve lithium extraction, but also reflect the lithium concentration in solutions (Fig. 13).69 Luminescent quantitative detection is attractive for its facile and convenient sensing equipment and rapid and sensitive response, which is highly adaptable to high-throughput screening platforms and automated systems. Nevertheless, the selectivity and sensitivity of luminescent quantitative recognition are generally not as well as other technologies, and the signals may decay over time. Besides, the absolute intensities are influenced by the external environment including the equipment, temperature, humidity, solvent, excitation light, and the detector, which limits its direct usage.
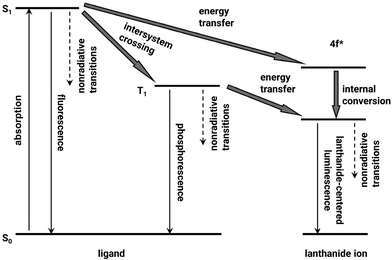 | ||
| Fig. 12 Simplified energy transformation processes for the luminescent mechanism in Ln-MOFs.88 Copyright 2011 American Chemical Society. | ||
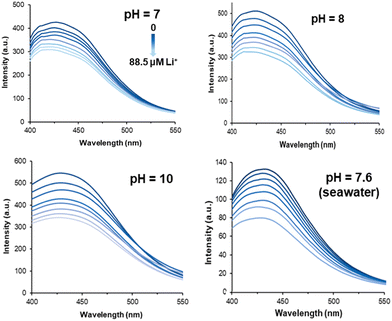 | ||
| Fig. 13 Luminescent emission spectra of LMOF-321 after incremental additions of a lithium-ion aqueous solution under different pH values.69 Copyright 2019 American Chemical Society. | ||
Besides, the ion selectivities can also be calculated by current–voltage (I–V) measurement. I–V measurement is a fundamental technique in electrical engineering and materials science used to characterize the electrical properties of various materials and devices (Fig. 14).94 This method involves measuring the current that flows through a device or material as a function of the applied voltage. By plotting the current versus voltage, researchers can gain insights into important parameters such as resistance, conductivity, and the presence of any non-linear behavior like that found in diodes or other non-ohmic devices. This technique is widely used in research and industry for the development and testing of electronic devices, ensuring they meet specific performance criteria and reliability standards.95–98 For the I–V measurement with single salt solutions or the mixed-ion diffusions, the selectivities of lithium compared with other metals can be calculated quantitatively by the relative current changes (Fig. 15).66,67,70,74 In summary, I–V measurement is versatile and widely applicable with simple setup and real-time monitoring characteristics. While it is also highly affected by the noise sensitivity, external factors, and safety precautions for high voltage/current limitations.
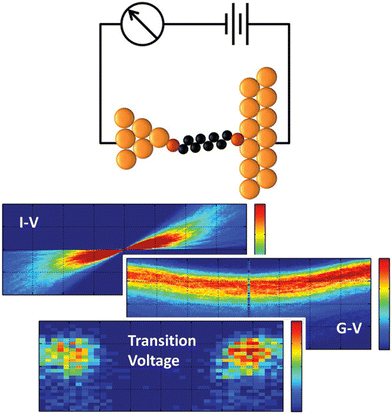 | ||
| Fig. 14 Typical curves for electrochemical testing.94 Copyright 2011 American Chemical Society. | ||
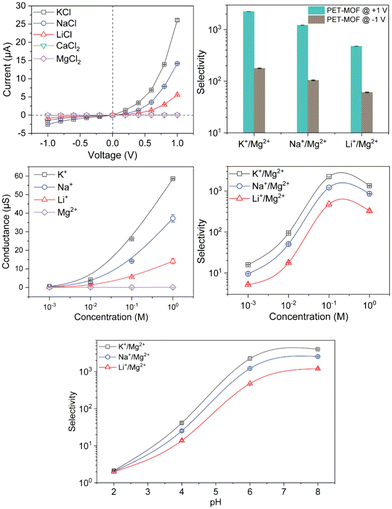 | ||
| Fig. 15 Ion transport, conductance, and selectivity performance of UiO-66-(SH)2 calculated by I–V measurement.70 Copyright 2024 American Chemical Society. | ||
The precise and efficient quantification of lithium concentrations is pivotal across various domains, including materials science, environmental monitoring, and industrial processes. Advanced techniques such as ICP-OES/AES, SEM-EDX mapping, luminescent quantitative detection, and I–V measurement are instrumental in this endeavour. These methods offer high sensitivity, precision, and the capability to handle complex samples, providing valuable insights into the quantitative analysis of lithium ion and its mixtures. Through these advanced techniques, researchers and professionals can achieve critical objectives, such as the thorough evaluation of advanced materials and the discovery and monitoring of lithium sources. These advancements not only enhance our understanding and optimization of material properties but also support sustainable practices and technological innovation in various fields. However, at the same time, these techniques possess respective problems which restricts the further development of the lithium extraction. More facile, precise, and efficient technologies are highly required for the future researches.
Based on the above, lithium extraction using MOFs presents exciting potential, fuelled by advancements in diverse pore structures and functional groups. Modern MOFs, featuring tailored porosity, high selectivity, and structural adaptability, are being designed to efficiently capture lithium ions from low-concentration sources like seawater and brine. Innovations in material synthesis are producing MOFs with improved stability and ion-exchange capabilities, crucial for effective lithium separation. This is paving the way for the discovery of new, highly selective binding interactions for lithium ions. Simultaneously, advanced testing technologies—such as in situ characterization tools and machine learning analysis—are empowering researchers to examine and optimize these MOFs with unprecedented scale and precision. These tools facilitate detailed monitoring of ion diffusion, binding interactions, and structural dynamics within MOFs during lithium extraction, accelerating the development of efficient and commercially viable MOF-based processes. Additionally, with advances in ionic detection, more robust and user-friendly detection methods are likely to emerge, streamlining quantitative analyses of metal ions and accelerating the selection and evaluation of novel materials.
4. Conclusions
MOFs offer significant potential in revolutionizing lithium extraction processes. Their high surface area, various pore structures, and chemical selectivity make them promising candidates for efficient lithium-ion extraction from natural sources or outlet water. These properties enable MOFs to selectively capture lithium ions from complex mixtures, enhancing the efficiency and purity of the extracted lithium. At the same time, based on the various chemical components and analysis technologies, the capacities of these materials can be well determined.However, lithium extraction using MOFs still presents several key challenges that require further research.
For the construction of MOFs, the major hurdle is the scalability of MOF production. While MOFs have demonstrated great promise in laboratory settings, scaling up their production for large-scale lithium extraction remains a significant challenge. Developing cost-effective and sustainable methods for synthesizing target ligands and corresponding MOFs at an industrial scale is essential for widespread adoption. Another critical issue is the stability of MOFs under operational conditions. To be effective in real-world applications, MOFs must maintain their structural integrity and performance over extended periods and under diverse environmental conditions typically encountered in industrial processes. Enhancing the stability and durability of MOFs will be crucial to ensuring their long-term viability. Cost-effectiveness is also a central concern. The economic feasibility of using MOFs for lithium extraction is dependent on the cost of raw materials, synthesis processes, and overall operational expenses. To make MOFs a viable option for large-scale lithium recovery, it is crucial to develop more affordable synthesis routes and improve the cost-efficiency of the entire extraction process.
On the extraction front, one of the foremost challenges is the selective capture of lithium ions from complex mixtures. Competing ions such as sodium, magnesium, and calcium are often present in much higher concentrations, and various chemicals in real-world environments may influence the extraction process. These real-world conditions introduce a level of complexity not encountered in controlled laboratory settings. Fine-tuning MOF structures to achieve high selectivity and affinity for lithium over these competing ions is a difficult task that requires advanced materials design. Additionally, significant differences may arise between lab-scale experiments and real-world, large-scale extraction processes, adding another layer of complexity. Overcoming these challenges will require continuous innovation in MOF design, improved manufacturing techniques, and a more sustainable approach to lithium recovery.
Based on the challenges above, the future researches for lithium extraction using MOFs holds significant promise, yet there are several key areas that require further exploration to unlock the full potential of this technology. Advancements in MOF design and synthesis, particularly in terms of pore size tuning and functionalization, could improve lithium-ion selectivity and enhance overall extraction efficiency. Additionally, scaling up MOF-based systems from laboratory to industrial levels also requires further research into the durability, regeneration, and cost-effectiveness of MOFs in real-world applications. Environmental considerations, such as reducing the energy consumption and environmental impact of lithium extraction processes, must also be addressed. Integrating MOF technologies with other extraction methods or renewable energy sources could further enhance their sustainability and practicality for large-scale lithium recovery, making them a vital tool in supporting the global shift towards electric vehicles and renewable energy storage systems. Besides, other differences in parameters between lithium and other metal ions may be considered for the future design in this area.
Further in the future, by addressing these challenges, researchers could unlock the full potential of MOFs for lithium extraction, paving the way for more sustainable and efficient lithium recovery methods. Overall, while MOFs present a promising avenue for sustainable lithium extraction, further advancements are essential to harness their full potential and facilitate their integration into commercial lithium production systems. By overcoming the challenges of scalability, stability, and cost-effectiveness, MOFs can play a pivotal role in meeting the growing demand for lithium in various high-tech applications, including batteries for electric vehicles and renewable energy storage systems.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Robert A. Welch Foundation through an endowed chair (A-0030) to H.-C. Z., Moselle Technologies, LLC through project M2304151, and National Natural Science Foundation of China (Grant No. 22471130). We thank the BioRender.com for the source materials for some figures.References
- J.-M. Tarascon and M. Armand, Issues and challenges facing rechargeable lithium batteries, Nature, 2001, 414, 359–367 CrossRef PubMed.
- X. Tan, Y. Wu, X. Lin, A. Zeb, X. Xu, Y. Luo and J. Liu, Application of MOF-derived transition metal oxides and composites as anodes for lithium-ion batteries, Inorg. Chem. Front., 2020, 7, 4939–4955 RSC.
- B. Dunn, H. Kamath and J.-M. Tarascon, Electrical energy storage for the grid: a battery of choices, Science, 2011, 334, 928–935 CrossRef PubMed.
- K. Xu, Nonaqueous liquid electrolytes for lithium-based rechargeable batteries, Chem. Rev., 2004, 104, 4303–4418 CrossRef PubMed.
- M. V. Reddy, G. V. S. Rao and B. V. R. Chowdari, Metal oxides and oxysalts as anode materials for Li ion batteries, Chem. Rev., 2013, 113, 5364–5457 CrossRef PubMed.
- F. Bonaccorso, L. Colombo, G. Yu, M. Stoller, V. Tozzini, A. C. Ferrari, R. S. Ruoff and V. Pellegrini, Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage, Science, 2015, 347, 1246501 CrossRef PubMed.
- A. Manthiram, X. Yu and S. Wang, Lithium battery chemistries enabled by solid-state electrolytes, Nat. Rev. Mater., 2017, 2, 16103 CrossRef.
- W.-J. Zhang and K.-J. Huang, A review of recent progress in molybdenum disulfide-based supercapacitors and batteries, Inorg. Chem. Front., 2017, 4, 1602–1620 RSC.
- M. Li, J. Lu, Z. Chen and K. Amine, 30 Years of lithium-ion batteries, Adv. Mater., 2018, 30, 1800561 CrossRef PubMed.
- A. Manthiram, Y. Fu, S.-H. Chung, C. Zu and Y.-S. Su, Rechargeable lithium–sulfur batteries, Chem. Rev., 2014, 114, 11751–11787 CrossRef CAS.
- T. H. Nguyen, A. Fraiwan and S. Choi, Paper-based batteries: A review, Biosens. Bioelectron., 2014, 54, 640–649 CrossRef CAS PubMed.
- A. Z. Weber, M. M. Mench, J. P. Meyers, P. N. Ross, J. T. Gostick and Q. Liu, Redox flow batteries: a review, J. Appl. Electrochem., 2011, 41, 1137–1164 CrossRef CAS.
- T. Hosaka, K. Kubota, A. S. Hameed and S. Komaba, Research development on K-ion batteries, Chem. Rev., 2020, 120, 6358–6466 CrossRef CAS PubMed.
- T. L. Kulova, V. N. Fateev, E. A. Seregina and A. S. Grigoriev, A brief review of post-lithium-ion batteries, Int. J. Electrochem. Sci., 2020, 15, 7242–7259 CrossRef.
- F. Cheng, J. Liang, Z. Tao and J. Chen, Functional materials for rechargeable batteries, Adv. Mater., 2011, 23, 1695–1715 CrossRef CAS PubMed.
- K. D. Johnson, S. T. Smith, J. D. Pouliot and L. N. Miller, Comparison of pharmacist- and provider-managed lithium in an inpatient medical center: A 6-month review, J. Am. Pharm. Assoc., 2021, 61, e103–e107 CrossRef CAS PubMed.
- J. K. Rybakowski, Lithium - past, present, future, Int. J. Psychiatry Clin., 2020, 24, 330–340 CrossRef CAS.
- E. Ferensztajn-Rochowiak and J. K. Rybakowski, Long-term lithium therapy: side effects and interactions, Pharmaceuticals, 2023, 16, 74 CrossRef CAS.
- R. D'Souza, T. K. Rajji, B. H. Mulsant and B. G. Pollock, Use of lithium in the treatment of bipolar disorder in late-life, Curr. Psychiatry Rep., 2011, 13, 488–492 CrossRef.
- J. T. Broome and C. C. Solorzano, Lithium use and primary hyperparathyroidism, Endocrinol. Pract., 2011, 17, 31–35 CrossRef.
- W. T. Stringfellow and P. F. Dobson, Technology for the recovery of lithium from geothermal brines, Energies, 2021, 14, 6805 CrossRef.
- X. Zhao, H. Yang, Y. Wang and Z. Sha, Review on the electrochemical extraction of lithium from seawater/brine, J. Electroanal. Chem., 2019, 850, 113389 CrossRef.
- E. A. Mends and P. Chu, Lithium extraction from unconventional aqueous resources - a review on recent technological development for seawater and geothermal brines, J. Environ., 2023, 11, 110710 Search PubMed.
- G. Liu, Z. Zhao and A. Ghahreman, Novel approaches for lithium extraction from salt-lake brines: a review, Hydrometallurgy, 2019, 187, 81–100 CrossRef.
- J. Sun, D. Liang, X. Meng and Z. Li, Recent advances in lithium extraction using electrode materials of Li-ion battery from brine/seawater, Processes, 2022, 10, 2654 CrossRef.
- R. Zhu, S. Wang, C. Srinivasakannan, S. Li, S. Yin, L. Zhang, X. Jiang, G. Zhou and N. Zhang, Lithium extraction from salt lake brines with high magnesium/lithium ratio: a review, Environ. Chem. Lett., 2023, 21, 1611–1626 CrossRef.
- Y. Zeng, W. Li, Z. Wan, S. Qin, Q. Huang, W. Cai, Q. Wang, M. Yao and Y. Zhang, Electrochemically mediated lithium extraction for energy and environmental sustainability, Adv. Funct. Mater., 2024, 34, 2400416 CrossRef.
- Y. Chen, A. Dou and Y. Zhang, A review of recycling status of decommissioned lithium batteries, Front. Mater., 2021, 8, 634667 CrossRef.
- H. Bae and Y. Kim, Technologies of lithium recycling from waste lithium ion batteries: a review, Mater. Adv., 2021, 2, 3234–3250 RSC.
- B. Huang, Z. Pan, X. Su and L. An, Recycling of lithium-ion batteries: Recent advances and perspectives, J. Power Sources, 2018, 399, 274–286 CrossRef.
- D. Werner, U. A. Peuker and T. Mütze, Recycling chain for spent lithium-ion batteries, Metals, 2020, 10, 316 CrossRef.
- J. Wang, J. Ma, Z. Zhuang, Z. Liang, K. Jia, G. Ji, G. Zhou and H.-M. Cheng, Toward direct regeneration of spent lithium-ion batteries: a next-generation recycling method, Chem. Rev., 2024, 124, 2839–2887 CrossRef PubMed.
- J. Zeng and S. Liu, Forecasting the sustainable classified recycling of used lithium batteries by gray graphical evaluation and review technique, Renewable Energy, 2023, 202, 602–612 CrossRef.
- Z. Takacova, D. Orac, J. Klimko and A. Miskufova, Current trends in spent portable lithium battery recycling, Materials, 2023, 16, 4264 CrossRef PubMed.
- Y. Yao, M. Zhu, Z. Zhao, B. Tong, Y. Fan and Z. Hua, Hydrometallurgical processes for recycling spent lithium-ion batteries: a critical review, ACS Sustainable Chem. Eng., 2018, 6, 13611–13627 CrossRef.
- H. Li, M. Eddaoudi, T. L. Groy and O. M. Yaghi, Establishing microporosity in open metal−organic frameworks: gas sorption isotherms for Zn (BDC) (BDC = 1,4-benzenedicarboxylate), J. Am. Chem. Soc., 1998, 120, 8571–8572 CrossRef.
- D. M. Young, U. Geiser, A. J. Schultz and H. H. Wang, Hydrothermal synthesis of a dense metal-organic layered framework that contains Cu(I)−olefinic bonds, Cu2(O2CCHCHCO2), J. Am. Chem. Soc., 1998, 120, 1331–1332 CrossRef.
- O. M. Yaghi, R. Jernigan, H. Li, C. E. Davis and T. L. Groy, Construction of a new open-framework solid from 1,3,5-cyclohexanetricarboxylate and zinc(II) building blocks, J. Chem. Soc., Dalton Trans., 1997, 14, 2383–2384 RSC.
- O. M. Yaghi and H. Li, Hydrothermal synthesis of a metal-organic framework containing large rectangular channels, J. Am. Chem. Soc., 1995, 117, 10401–10402 CrossRef.
- O. M. Yaghi, C. E. Davis, G. Li and H. Li, Selective guest binding by tailored channels in a 3-D porous Zinc(II)− benzenetricarboxylate network, J. Am. Chem. Soc., 1997, 119, 2861–2868 CrossRef.
- O. M. Yaghi, G. Li and H. Li, Selective binding and removal of guests in a microporous metal-organic framework, Nature, 1995, 378, 703–706 CrossRef.
- T. D. Bennett and S. Horike, Liquid, glass and amorphous solid states of coordination polymers and metal-organic frameworks, Nat. Rev. Mater., 2018, 3, 431–440 CrossRef.
- C. Zhang, W. Li and L. Li, Metal halide perovskite nanocrystals in metal-organic framework host: not merely enhanced stability, Angew. Chem., Int. Ed., 2021, 60, 7488–7501 CrossRef CAS PubMed.
- Z. Han, K. Wang, M. Wang, T. Sun, J. Xu, H.-C. Zhou, P. Cheng and W. Shi, Tailoring chirality and porosity in metal-organic frameworks for efficient enantioselective recognition, Chem, 2023, 9, 2561–2572 CAS.
- Z. Han, R. Zhang, J. Jiang, Z. Chen, Y. Ni, W. Xie, J. Xu, Z. Zhou, J. Chen, P. Cheng and W. Shi, High-efficiency lithium-ion transport in a porous coordination chain-based hydrogen-bonded framework, J. Am. Chem. Soc., 2023, 145, 10149–10158 CrossRef CAS.
- X. Li, K. Chen, R. Guo and Z. Wei, Ionic liquids functionalized MOFs for adsorption, Chem. Rev., 2023, 123, 10432–10467 CrossRef CAS.
- K.-Y. Wang, J. Zhang, Y.-C. Hsu, H. Lin, Z. Han, J. Pang, Z. Yang, R.-R. Liang, W. Shi and H.-C. Zhou, Bioinspired framework catalysts: from enzyme immobilization to biomimetic catalysis, Chem. Rev., 2023, 123, 5347–5420 CrossRef CAS.
- M. Gutiérrez, Y. Zhang and J.-C. Tan, Confinement of luminescent guests in metal-organic frameworks: understanding pathways from synthesis and multimodal characterization to potential applications of LG@MOF systems, Chem. Rev., 2022, 122, 10438–10483 CrossRef.
- Z. Han, K. Wang, Y. Chen, J. Li, S. J. Teat, S. Yang, W. Shi and P. Cheng, A multicenter metal-organic framework for quantitative detection of multicomponent organic mixtures, CCS Chem., 2022, 4, 3238–3245 CrossRef CAS.
- A. Knebel and J. Caro, Metal-organic frameworks and covalent organic frameworks as disruptive membrane materials for energy-efficient gas separation, Nat. Nanotechnol., 2022, 17, 911–923 CrossRef CAS PubMed.
- S. M. J. Rogge, A. Bavykina, J. Hajek, H. Garcia, A. I. Olivos-Suarez, A. Sepúlveda-Escribano, A. Vimont, G. Clet, P. Bazin, F. Kapteijn, M. Daturi, E. V. Ramos-Fernandez, F. X. Llabrés i Xamena, V. Van Speybroeck and J. Gascon, Metal-organic and covalent organic frameworks as single-site catalysts, Chem. Soc. Rev., 2017, 46, 3134–3184 RSC.
- R.-R. Liang, Z. Han, P. Cai, Y. Yang, J. Rushlow, Z. Liu, K.-Y. Wang and H.-C. Zhou, A robust pyrazolate metal-organic framework for efficient catalysis of dehydrogenative C–O cross coupling reaction, J. Am. Chem. Soc., 2024, 146, 14174–14181 CrossRef CAS PubMed.
- C. J. Doonan and C. J. Sumby, Metal-organic framework catalysis, CrystEngComm, 2017, 19, 4044–4048 RSC.
- Y. Zhao, H. Zeng, X.-W. Zhu, W. Lu and D. Li, Metal-organic frameworks as photoluminescent biosensing platforms: mechanisms and applications, Chem. Soc. Rev., 2021, 50, 4484–4513 RSC.
- Z. Han, T. Sun, R.-R. Liang, Y. Guo, Y. Yang, M. Wang, Y. Mao, P. R. Taylor, W. Shi, K.-Y. Wang and H.-C. Zhou, Chiral linker installation in a metal-organic framework for enantioselective luminescent sensing, J. Am. Chem. Soc., 2024, 146, 15446–15452 CrossRef CAS PubMed.
- M. Wang, Z. Han, K. Wang, B. Zhao, T. Sun, Y. Wu, P. Cheng and W. Shi, Confinement of p-xylene in the pores of a bilanthanide metal-organic framework for highly selective recognition, Angew. Chem., Int. Ed., 2024, 63, e202318722 CrossRef CAS PubMed.
- V. Stavila, A. A. Talin and M. D. Allendorf, MOF-based electronic and opto-electronic devices, Chem. Soc. Rev., 2014, 43, 5994–6010 RSC.
- H. He, H. Li, Y. Cui and G. Qian, MOF-based organic microlasers, Adv. Opt. Mater., 2019, 7, 1900077 CrossRef.
- C. Li, L. Liu, J. Kang, Y. Xiao, Y. Feng, F.-F. Cao and H. Zhang, Pristine MOF and COF materials for advanced batteries, Energy Storage Mater., 2020, 31, 115–134 CrossRef.
- Z. Ye, Y. Jiang, L. Li, F. Wu and R. Chen, Rational design of MOF-based materials for next-generation rechargeable batteries, Nano-Micro Lett., 2021, 13, 203 CrossRef CAS.
- Z. Han, J. Li, W. Lu, K. Wang, Y. Chen, X. Zhang, L. Lin, X. Han, S. J. Teat, M. D. Frogley, S. Yang, W. Shi and P. Cheng, A {Ni12}-wheel-based metal-organic framework for coordinative binding of sulphur dioxide and nitrogen dioxide, Angew. Chem., Int. Ed., 2022, 61, e202115585 CrossRef CAS PubMed.
- Z. Han, K. Wang, H. Min, J. Xu, W. Shi and P. Cheng, Bifunctionalized metal-organic frameworks for pore-size-dependent enantioselective sensing, Angew. Chem., Int. Ed., 2022, 61, e202204066 CrossRef CAS PubMed.
- Y. Peng, H. Huang, Y. Zhang, C. Kang, S. Chen, L. Song, D. Liu and C. Zhong, A versatile MOF-based trap for heavy metal ion capture and dispersion, Nat. Commun., 2018, 9, 187 CrossRef PubMed.
- S. Sharma, A. V. Desai, B. Joarder and S. K. Ghosh, A water-stable ionic MOF for the selective capture of toxic oxoanions of SeVI and AsV and crystallographic insight into the ion-exchange mechanism, Angew. Chem., Int. Ed., 2020, 59, 7788–7792 CrossRef CAS.
- J. Li, X. Wang, G. Zhao, C. Chen, Z. Chai, A. Alsaedi, T. Hayat and X. Wang, Metal-organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions, Chem. Soc. Rev., 2018, 47, 2322–2356 RSC.
- J. Li, Y. Shi, C. Qi, B. Zhang, X. Xing, Y. Li, T. Chen, X. Mao, Z. Zuo, X. Zhao, Z. Pan, L. Li, X. Yang and C. Li, Charging metal-organic framework membranes by incorporating crown ethers to capture cations for ion sieving, Angew. Chem., Int. Ed., 2023, 62, e202309918 CrossRef CAS PubMed.
- Y. Guo, Y. Ying, Y. Mao, X. Peng and B. Chen, Polystyrene sulfonate threaded through a metal-organic framework membrane for fast and selective lithium-ion separation, Angew. Chem., Int. Ed., 2016, 55, 15120–15124 CrossRef CAS.
- Y. Lv, Z. Dai, Y. Chen, Y. Lu, X. Zhang, J. Yu, W. Zhai, Y. Yu, Z. Wen, Y. Cui and W. Liu, Two-dimensional sulfonate-functionalized metal-organic framework membranes for efficient lithium-ion sieving, Nano Lett., 2024, 24, 2782–2788 CrossRef CAS.
- N. D. Rudd, Y. Liu, K. Tan, F. Chen, Y. J. Chabal and J. Li, Luminescent metal-organic framework for lithium harvesting applications, ACS Sustainable Chem. Eng., 2019, 7, 6561–6568 CrossRef CAS.
- C. Zhao, F. Feng, J. Hou, J. Hu, Y. Su, J. Z. Liu, M. Hill, B. D. Freeman, H. Wang and H. Zhang, Unlocking direct lithium extraction in harsh conditions through thiol-functionalized metal-organic framework subnanofluidic membranes, J. Am. Chem. Soc., 2024, 146, 14058–14066 CrossRef CAS PubMed.
- A. A. Lysova, D. G. Samsonenko, P. V. Dorovatovskii, V. A. Lazarenko, V. N. Khrustalev, K. A. Kovalenko, D. N. Dybtsev and V. P. Fedin, Tuning the molecular and cationic affinity in a series of multifunctional metal-organic frameworks based on dodecanuclear Zn(II) carboxylate wheels, J. Am. Chem. Soc., 2019, 141, 17260–17269 CrossRef CAS.
- J. Lu, H. Zhang, J. Hou, X. Li, X. Hu, Y. Hu, C. D. Easton, Q. Li, C. Sun, A. W. Thornton, M. R. Hill, X. Zhang, G. Jiang, J. Z. Liu, A. J. Hill, B. D. Freeman, L. Jiang and H. Wang, Efficient metal ion sieving in rectifying subnanochannels enabled by metal-organic frameworks, Nat. Mater., 2020, 19, 767–774 CrossRef CAS.
- Y. Lim, Y. Kim and J. Choi, Electro-mechanical factor affecting the Li+/Mg2+ selectivity performance of ion separation metal-organic frameworks, J. Mater. Chem. A, 2024, 12, 16657–16666 RSC.
- M. Abdollahzadeh, M. Chai, E. Hosseini, M. Zakertabrizi, M. Mohammad, H. Ahmadi, J. Hou, S. Lim, A. H. Korayem, V. Chen, M. Asadnia and A. Razmjou, Designing angstrom-scale asymmetric MOF-on-MOF cavities for high monovalent ion selectivity, Adv. Mater., 2022, 34, 2107878 CrossRef CAS.
- X. Wu, H. Zhang, X. Zhang, Q. Guan, X. Tang, H. Wu, M. Feng, H. Wang and R. Ou, Sustainable lithium extraction enabled by responsive metal-organic frameworks with ion-sieving adsorption effects, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2309852121 CrossRef CAS.
- J. Hou, H. Zhang, A. W. Thornton, A. J. Hill, H. Wang and K. Konstas, Lithium extraction by emerging metal–organic framework-based membranes, Adv. Funct. Mater., 2021, 31, 2105991 CrossRef CAS.
- Y. Liu, R. Zhu, C. Srinivasakannan, T. Li, S. Li, S. Yin and L. Zhang, Application of nanofiltration membrane based on metal-organic frameworks (MOFs) in the separation of magnesium and lithium from salt lakes, Separations, 2022, 9, 344 CrossRef CAS.
- S. Raggam, M. Mohammad, Y. Choo, G. Naidu, M. Zargar, H. K. Shon and A. Razmjou, Advances in metal organic framework (MOF) – based membranes and adsorbents for lithium-ion extraction, Sep. Purif. Technol., 2023, 307, 122628 CrossRef CAS.
- T. W. Barnard, M. I. Crockett, J. C. Ivaldi and P. L. Lundberg, Design and evaluation of an echelle grating optical system for ICP-OES, Anal. Chem., 1993, 65, 1225–1230 CrossRef CAS.
- D. Beauchemin, Inductively coupled plasma mass spectrometry, Anal. Chem., 2008, 80, 4455–4486 CrossRef CAS.
- J. Hopwood, Review of inductively coupled plasmas for plasma processing, Plasma Sources Sci. Technol., 1992, 1, 109 CrossRef CAS.
- J. W. Olesik, Fundamental research in ICP-OES and ICPMS, Anal. Chem., 1996, 68, 469A–474A CrossRef CAS.
- V.-D. Hodoroaba, Energy-dispersive X-ray spectroscopy EDS), Charact. Nanopart., 2020, 397–417 CAS.
- G. Zadora and Z. Brożek-Mucha, SEM-EDX-a useful tool for forensic examinations, Mater. Chem. Phys., 2003, 81, 345–348 CrossRef CAS.
- M. Scimeca, S. Bischetti, H. K. Lamsira, R. Bonfiglio and E. Bonanno, Energy dispersive X-ray (EDX) microanalysis: A powerful tool in biomedical research and diagnosis, Eur. J. Histochem., 2018, 62, 2841 Search PubMed.
- P. Lu, L. Zhou, M. J. Kramer and D. J. Smith, Atomic-scale chemical imaging and quantification of metallic alloy structures by energy-dispersive X-ray spectroscopy, Sci. Rep., 2014, 4, 3945 CrossRef.
- B. Shirley and E. Jarochowska, Chemical characterisation is rough: the impact of topography and measurement parameters on energy-dispersive X-ray spectroscopy in biominerals, Facies, 2022, 68, 7 CrossRef.
- Y. Cui, Y. Yue, G. Qian and B. Chen, Luminescent functional metal-organic frameworks, Chem. Rev., 2012, 112, 1126–1162 CrossRef CAS PubMed.
- Z. Han, K. Wang, H.-C. Zhou, P. Cheng and W. Shi, Preparation and quantitative analysis of multicenter luminescence materials for sensing function, Nat. Protoc., 2023, 18, 1621–1640 CrossRef CAS PubMed.
- Z. Du, B. Song, W. Zhang, C. Duan, Y.-L. Wang, C. Liu, R. Zhang and J. Yuan, Quantitative monitoring and visualization of hydrogen sulfide in vivo using a luminescent probe based on a ruthenium(II) complex, Angew. Chem., Int. Ed., 2018, 57, 3999–4004 CrossRef CAS PubMed.
- B. Yan, Lanthanide-functionalized metal-organic framework hybrid systems to create multiple luminescent centers for chemical sensing, Acc. Chem. Res., 2017, 50, 2789–2798 CrossRef CAS PubMed.
- A. Karmakar, P. Samanta, A. V. Desai and S. K. Ghosh, Guest-responsive metal-organic frameworks as scaffolds for separation and sensing applications, Acc. Chem. Res., 2017, 50, 2457–2469 CrossRef CAS.
- Z. Han, K. Wang, Y. Guo, W. Chen, J. Zhang, X. Zhang, G. Siligardi, S. Yang, Z. Zhou, P. Sun, W. Shi and P. Cheng, Cation-induced chirality in a bifunctional metal-organic framework for quantitative enantioselective recognition, Nat. Commun., 2019, 10, 5117 CrossRef PubMed.
- S. Guo, J. Hihath, I. Díez-Pérez and N. Tao, Measurement and statistical analysis of single-molecule current-voltage characteristics, transition voltage spectroscopy, and tunneling barrier height, J. Am. Chem. Soc., 2011, 133, 19189–19197 CrossRef CAS.
- J.-Y. Kim, B. J. Kang, Y.-M. Bahk, Y. S. Kim, J. Park, W. T. Kim, J. Rhie, S. Han, H. Jeon, C.-H. Park, F. Rotermund and D.-S. Kim, Tunnelling current-voltage characteristics of Angstrom gaps measured with terahertz time-domain spectroscopy, Sci. Rep., 2016, 6, 29103 CrossRef CAS PubMed.
- R. Nouchi, Extraction of the Schottky parameters in metal-semiconductor-metal diodes from a single current-voltage measurement, J. Appl. Phys., 2014, 116, 184505 CrossRef.
- J. Bylander, T. Duty and P. Delsing, Current measurement by real-time counting of single electrons, Nature, 2005, 434, 361–364 CrossRef CAS PubMed.
- S.-Y. Chung, I.-D. Kim and S.-J. L. Kang, Strong nonlinear current-voltage behaviour in perovskite-derivative calcium copper titanate, Nat. Mater., 2004, 3, 774–778 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2024 |