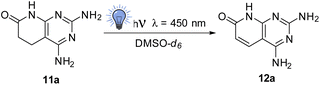Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Autocatalytic photoinduced oxidative dehydrogenation of pyrido[2,3-d]pyrimidin-7(8H)-ones: synthesis of C5–C6 unsaturated systems with concomitant formation of a long-lived radical†
Claudi
de Rocafiguera
 a,
Vega
Lloveras
a,
Vega
Lloveras
 b,
José
Vidal-Gancedo
b,
José
Vidal-Gancedo
 b,
Jordi
Teixidó
b,
Jordi
Teixidó
 a,
Roger
Estrada-Tejedor
a,
José I.
Borrell
a,
Roger
Estrada-Tejedor
a,
José I.
Borrell
 a and
Raimon
Puig de la Bellacasa
a and
Raimon
Puig de la Bellacasa
 *a
*a
aGrup de Química Farmacèutica, Institut Químic de Sarrià, Universitat Ramon Llull, Via Augusta, 390, E-08017 Barcelona, Spain. E-mail: raimon.puig@iqs.url.edu
bInstitut de Ciència de Materials de Barcelona (ICMAB-CSIC) and CIBER de Bioingeniería, Biomateriales y Nanomedicina (CIBER-BBN), Instituto de Salud Carlos III, Campus UAB, E-08193 Bellaterra, Spain
First published on 2nd November 2023
Abstract
5,6-Dihydropyrido[2,3-d]pyrimidin-7(8H)-ones are readily accessed by a variety of methods and are a good scaffold for the development of biologically active compounds. However, they are very reluctant to dehydrogenate to give C5–C6 unsaturated compounds, usually with higher activity. A serendipitous discovery has allowed us to develop an autocatalytic photochemical dehydrogenation process by irradiating at 450 or 365 nm in DMSO, in the presence of air, and at room temperature the corresponding 5,6-dihydro derivative (with a variety of substituents at C2, C4, C5, C6, and N8) without adding any external photosensitizer. A complete study including reactions in DMSO-d6 followed by NMR spectroscopy, EPR experiments, the use of radical quenchers, spin-trapping techniques, and reaction with methyl viologen, complemented with ab initio calculations has allowed us to propose a mechanistic rationalization for such a process.
Introduction
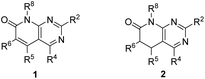
Our research group has been actively working in the field of Tyrosine Kinase Inhibitors (TKIs) with the aim of developing drug candidates against diverse types of cancers such as breakpoint cluster region protein (BCR) kinase inhibitors for B lymphoid malignancies,1 discoidin domain-containing receptor 2 (DDR2) inhibitors for the treatment of lung cancer,2 MAP kinase interacting kinase (MNK1/2) inhibitors for the treatment of breast or prostate cancer,3 zeta-chain-associated protein kinase 70 kDa (ZAP-70) inhibitors,4 and more recently a promising candidate for the treatment of pancreatic cancer (unpublished results).Most of such developments are based on pyrido[2,3-d]pyrimidin-7(8H)-ones 1 (Fig. 1), a scaffold for which we have described several straightforward synthetic strategies, allowing the decoration of all the five centers of diversity present in 1.1,5–7
However, some of the most versatile methodologies afford the corresponding 5,6-dihydro derivatives 2 (Fig. 1), such as the one that starts from α,β-unsaturated esters (3). Thus, 2-methoxy-6-oxo-1,4,5,6-tetrahydropyridin-3-carbonitriles (5) are obtained by the reaction of an α,β-unsaturated ester (3) and malononitrile (4) in NaOMe/MeOH.
Treatment of pyridones 5 with guanidine systems (6, R2 = H, alkyl, aryl) affords 4-amino-5,6-dihydropyrido[2,3-d]pyrimidines (7, R4 = H, alkyl, aryl). The synthesis of 2-arylamino substituted 4-amino-5,6-dihydropyrido[2,3-d]pyrimidin-7(8H)-ones (7, R2 = aryl) occurred via the corresponding 3-aryl substituted pyridopyrimidines 8, formed upon the treatment of pyridones 5 with an aryl-substituted guanidine 6 in 1,4-dioxane, which underwent the Dimroth rearrangement to the desired 4-aminopyridopyrimidines (7, R2 = aryl) with NaOMe/MeOH. The overall yields of such a two-step protocol are in general higher than those of the reaction between pyridones 5 and an aryl-substituted guanidine 6 (Scheme 1).5
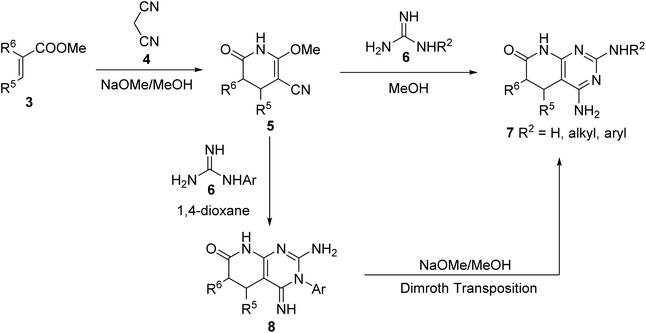 | ||
| Scheme 1 Synthesis of 4-amino-5,6-dihydropyrido[2,3-d]pyrimidin-7(8H)-ones 7 from α,β-unsaturated esters 3. | ||
In those cases in which the compound obtained does not bear C5–C6 unsaturation, critical for having biological activity as TKIs, it is precise to proceed to a dehydrogenation process that has never been an easy step to be performed. We have developed useful protocols for the dehydrogenation of 2 to 1 using NaH in DMSO when an aryl substituent is present at C-6, Na2SeO3 in DMSO,8 or 10% palladium on carbon in decalin at 175 °C, but these methods were highly dependent on the nature of the substituents at C5 and C6.
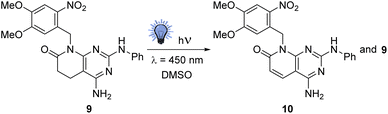
On the other hand, compounds 1 show a very low solubility in water and other common solvents because they are associated with a self-complementary ADAD–DADA quadruple hydrogen-bonding centrosymmetric motif. A way to increase such solubility is breaking such association by alkylation (usually methylation) of the nitrogen atom at the pyridone ring of 1. However, in connection with a project focused on the design of TKIs against pancreatic cancer, we were interested in obtaining candidate compounds bearing an NH group at such a position. Consequently, we selected a photocleavable protecting group to ensure deprotection under smooth conditions of molecules presenting several functionalities.9,10 More precisely, starting from 2-amino-4-phenylamino-5,6-dihydropyrido[2,3-d]pyrimidin-7(8H)-one, easily obtained by a multicomponent reaction of methyl acrylate (3, R5 = R6 = H), malononitrile (4), and N-phenylguanidine (6, R2 = Ph) in MeOH under microwave irradiation for 10 min,11 we obtained the protected compound 9 using 4,5-dimethoxy-2-nitrobenzyl bromide and NaH in DMSO.
When we tested the deprotection of 9 at 450 nm in DMSO, corresponding to the blue region of the spectrum recommended for the deprotection of this photolabile group,12 surprisingly, we did not observe the elimination of the photocleavable group but the partial creation of a double bond at C5–C6, affording a mixture of 9 and 10 (Scheme 2).
Such an unexpected result, which can solve one of our main problems with our pyridopyrimidine scaffold, impelled us to study this photochemical dehydrogenation in detail. The present paper deals with the results obtained in this study.
Results and discussion
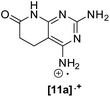
In order to determine the factors governing such dehydrogenation, we selected 2,4-diamino-5,6-dihydropyrido[2,3-d]pyrimidin-7(8H)-one (11a) as the model compound, which was synthesized by a multicomponent reaction of methyl acrylate (3a, R5 = R6 = H), malononitrile (4), and guanidine carbonate (6, R2 = H) in MeOH under microwave (MW) irradiation at 140 °C for 10 min.6The study was carried out in a Penn PhD Photoreactor M2 (Penn Photon Devices, LLC), initially provided with a 450 nm LED module, using 12 mL vials, and DMSO-d6 as a solvent to allow the direct register of the 1H-NMR spectrum of the reaction mixture. The experiments carried out are summarized in Table 1.
| Entry | Atmosphere | LED intensity (%) | Time (h) | % of 12ab |
|---|---|---|---|---|
| a Reaction conditions: pyridopyrimidine 11a (50 mg, 0.28 mmol), solvent (DMSO-d6, 5 mL), atmosphere (as stated), LED intensity (% as stated), fan speed (6800 rpm), stirrer speed (400 rpm), time (as stated), room temperature. b Percentage (%) of 12a formed, determined by relative integration of appropriate 1H-NMR signals of 11a and 12a. c Isolated yield of 12a. d Experiment carried out using 2 mL of DMSO-d6. | ||||
| 1 | Air, sealed vial | 100 | 1 | 13 |
| 2 | Air, sealed vial | 100 | 2 | 22 |
| 3 | Air, sealed vial | 100 | 8 | 33 |
| 4 | Argon, sealed vial | 100 | 2 | 0 |
| 5 | Oxygen, sealed vial | 100 | 2 | 48 |
| 6 | Oxygen, bubbling | 100 | 1 | >95 |
| 7 | Oxygen, bubbling | 100 | 2 | 100 + imp |
| 8 | Air, open vial | 100 | 4 | 100 (76%)c |
| 9 | Air, open vial | 0 | 4 | 0 |
| 10d | Air, open vial | 100 | 2 | 100 |
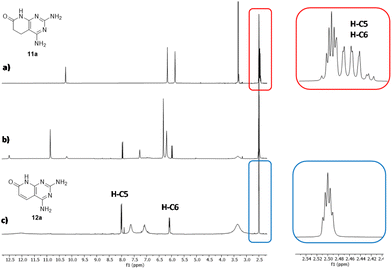
In the first three experiments (entries 1–3), we used a sealed vial containing 11a and DMSO-d6 under an air atmosphere (the air initially included in the sealed vial) for 1, 2, and 8 h. The presence of 12a in the crude reaction mixture was easily established by the presence of two doublets at 5.90 (1H) and 7.89 ppm (1H) in the 1H-NMR spectrum, corresponding to H-C6 and H-C5, and a signal at 11.5 ppm (1H) corresponding to H-N8 (Fig. 2). The relative integration of these signals with respect to those of compound 11a allows to determine the approximate percentage of 12a in the mixture, because the reaction mixtures are extremely clean. Although the amount of 12a in the mixture is increased with the irradiation time (from 13% in 1 h to 33% in 8 h), it seems that the conversion is limited by the amount of air (most precisely oxygen) present in the vial.
To confirm the role of oxygen in the reaction, we repeated the experiment of entry 2, but by using a sealed vial, in which the mixture was previously bubbled with argon (entry 4), observing no dehydrogenation of 11a.
So, we decided to repeat the experiment using a solution saturated with O2 in a sealed vial (entry 5) also for 2 h, obtaining 48% of 12a instead of 22% obtained when an air atmosphere was used. Consequently, we decided to carry out the experiments of entries 6 and 7 using continuous bubbling of pure O2 for 1 h and 2 h, respectively. In both cases, the starting material was totally consumed and 12a was formed almost quantitatively. However, in the 1H-NMR spectrum of the reaction mixture at 1 h (entry 6), there were some extra signals not corresponding either to 11a or 12a, which could correspond to a possible reaction intermediate. These signals disappeared after two hours of irradiation (entry 7), but a plethora of small signals were formed that seemed to correspond to the overreaction terms.
Once the critical role of the oxygen was proved, we decided to continue the experiments with a vial open to the atmosphere but provided with a CaCl2 tube to prevent the entry of moisture (entries 8–10). The first experiment carried out using this approach (entry 8) afforded a total and clean conversion to 12a after 4 h of irradiation at 450 nm. 12a was isolated by precipitation with acetone to afford 76% yield. To confirm the need for blue light during this dehydrogenation, the previous experiment was repeated but with the LED system switched off (entry 9) and, convergently, no dehydrogenation of 11a was observed. Finally, the amount of 11a was doubled in entry 10 and a total conversion was obtained in only 2 h.
At this point, it is necessary to highlight that this photoinduced oxidative dehydrogenation of 5,6-dihydropyrido[2,3-d]pyrimidin-7(8H)-one 11a is, to the best of our knowledge, the first case in which the dehydrogenation takes place without the presence of an extra photosensitizer component or metals.13,14
In fact, according to the work of Sadhu et al.,15 most of the dehydrogenating methods use strong oxidants (H2SO4, HNO3, CrO3–acetic acid, DDQ, or MnO2), NBS-peroxide assisted oxidation, or metal catalysts (Cu(OAc)2–Pb(OAc)4 and Pd–C). Their photochemical dehydrogenation using oxygen and a photoinduced electron transfer (PET) sensitizer (9-cyanoanthracene, 9-cyanophenanthrene, or 1-cyanonaphthalene) is the only precedent of our autocatalytic process.
Once the best reaction conditions were found and the key role of oxygen and light was established in the dehydrogenation of 11a, we decided to evaluate the effect of the presence of substituents at positions C5 and C6 and the impact of changing the wavelength of the used light by using a complementary 365 nm LED module. Consequently, we synthesized compounds 11b–e (Scheme 3), starting from the corresponding α,β-unsaturated esters (3b–e), malononitrile (4), and guanidine carbonate (6·H2CO3, R2 = H), using the multicomponent protocol previously developed by our group.11
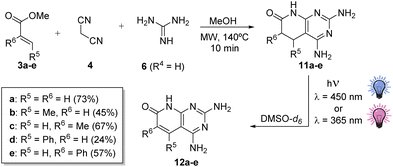 | ||
| Scheme 3 Synthesis of 5,6-dihydropyridopyrimidines 11b–e with substituents at C5 or C6 and subsequent photochemical dehydrogenation to 12b–e and their isolated yields. | ||
We decided to start the study using 11c (R5 = H, R6 = Me), the compound presenting a methyl at the α-carbonyl position, because substitution at position C6 is usually connected with better TKI activities. The assay was carried out by using 0.26 mmol of 11c in 2 mL of DMSO-d6, at 450 nm with 100% LED and a fan at 6800 rpm, in an open-air vial provided with a CaCl2 tube as in the case of 11a.
After 4 h of irradiation, the conversion to 12c (R5 = H, R6 = Me) was complete, as proved in the 1H-NMR spectrum (see the ESI†) by the presence of a singlet (1H) at 7.86 ppm corresponding to H-C5 and a singlet (3H) at 1.98 ppm of Me-C6. The isolated yield upon precipitation with acetone was 64%. The results were similar at 365 nm. In contrast, in the case of 11b (R5 = Me, R6 = H), after 2 h of irradiation at 450 and 365 nm, the conversions to 12b were 1% and 22%, respectively, seeming to indicate faster kinetics at 365 nm. After reducing the initial amount of 11b to 0.051 mmol to ensure the completion of the reaction, the total conversion to 12b was possible only after 3 h of irradiation at 365 nm, although with minor impurities, while at 450 nm, there was still 11b without any reaction. The isolated yield of 12b at 365 nm upon precipitation with acetone was 45% (1H-NMR spectrum: 5.77 ppm (s, 1H) H-C6; 2.5 ppm (s, 3H) Me-C5).
These results seemed to indicate that the kinetics of the reaction were favored at 365 nm. To better compare the results at both wavelengths excluding a possible different diffusion process of the air into the vial between experiments, we decided to bubble compressed air into the vial in the forthcoming reactions. Using in all cases 0.26 mmol of the corresponding 11a–c, the reactions were carried out at 450 and 365 nm, taking samples each hour until a maximum of 4 h. The percentage of the resulting dehydrogenation compounds 12a–c was obtained by relative integration in the corresponding 1H-NMR spectrum. The kinetic curves for the dehydrogenation process conducted in this way (Fig. 3) show that the dehydrogenation proceeds faster at 365 nm than at 450 nm (this aspect will be discussed later in this paper).
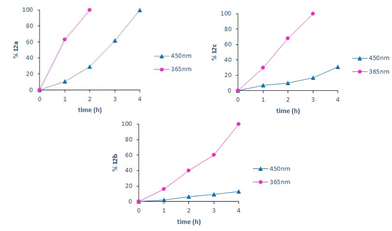 | ||
| Fig. 3 Kinetic curves for the dehydrogenation of 11a–c at 450 and 365 nm using bubbled air in an open vial. | ||
Taking this result into account, we carried out the dehydrogenation of 11e (R5 = H, R6 = Ph) and 11d (R5 = Ph, R6 = H) using 0.26 mmol in DMSO-d6 and 100% LED intensity at 365 nm and bubbling air into the sample. Under these conditions, 11e needed 7 h for the complete conversion in 12e (characterized by a singlet, 1H at 8.25 ppm corresponding to the H-C5 proton), while 11d needed 10 h to achieve the same result (in this case, H-C6 appears at 5.69 ppm), but the impurity level was clearly higher. Such results clearly showed that the ease of dehydrogenation depends on the nature and position of the substituents present on the pyridone ring, being better when smaller in size and when the substituent is present at C6.
With all the preceding results in hand, we began to consider proposing a mechanistic rationalization able to explain the above observations. We took the mechanism proposed by Schilling et al.16 for the visible-light-induced oxidation of benzyl alcohol to benzaldehyde with oxygen in DMSO and blue light in the presence of 9-fluorenone as a referable and plausible starting point for our proposal.
Consequently, we evaluated the effect of several quenchers in the conversion of 11a (R5 = R6 = H) to 12a (Table 2) to recognize the reactive oxygen species. The use of sodium azide does not affect the conversion to 12a, thus revealing that singlet oxygen is not formed or at least has no role in the dehydrogenation. In contrast, the strong effect of benzoquinone clearly indicates that the superoxide radical anion (O2˙−) plays a major role in this process. Spin trapping studies (discussed later in this paper, see the ESI, pages S42 and S43†) confirmed such an assumption. The quenching effect on the conversion to 12a of BHT, and to a lesser extent of CuCl2, suggests the interplay of a radical in this process. However, the fact that the addition of t-BuOH has no effect on the transformation excludes the participation of the hydroxyl radical in the transformation of 11a to 12a.
| Quencher | Equivalent | % of 12ab | Type of scavenger |
|---|---|---|---|
| a Reaction conditions: 0.14 mmol of 11a, DMSO-d6 (1.0 mL), air bubbling in an open vial. b Percentage (%) of 12a formed determined by relative integration of appropriate 1H-NMR signals of 11a and 12a. | |||
| NaN3 | 10 | 100 | Singlet oxygen scav. |
| BHT | 1.0 | 7 | Radical scav. |
| Benzoquinone | 1.0 | 4 | Superoxide radical anion scav. |
| CuCl2 | 1.0 | 20 | Electron scav. |
| t-BuOH | 1.0 | 100 | Hydroxyl radical scav. |
Complementarily, we studied the effect of TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy, free radical) on the dehydrogenation of 13 to 14 (Scheme 4). 13 (obtained following the protocol described by Galve et al.6) was selected as a substrate to increase the solubility, and to allow the analysis of the resulting crude mixture by HPLC-MS, as the use of 1H-NMR is not possible due to the presence of a radical species. When the reaction was carried out in the absence of TEMPO using blue light (λ = 450 nm) and DMSO-d6 as a solvent in the presence of air, the dehydrogenation took place in 1 h, affording 73% of 14 (structure confirmed by MS). In contrast, in the presence of TEMPO, the conversion to 14 was reduced to 6% after 1 h of irradiation, proving once more the radical character of such dehydrogenation.
Once the key role of the superoxide radical anion (O2˙−) in the process was established and the formation of H2O2 was expected during the dehydrogenation, we tested the presence of such a compound by adding NaI/AcOH during the irradiation of 11e (R5 = H, R6 = Ph) and observed the evolution of a brownish color corresponding to the I2 formed that was decolorized by the addition of sodium thiosulfate. Knowing that the evolution of H2O2 in DMSO causes the oxidation of the latter to dimethyl sulfone (CH3SO2CH3), we concentrated the DMSO of one of the experiments and recorded both the 1H- and 13C-NMR spectra, observing the characteristic signals of dimethyl sulfone at 2.99 and 42.1 ppm, respectively, thus confirming the formation of such an oxidation compound of DMSO.
To evaluate if the starting pyridopyrimidine can form one of the radicals involved in the process, we decided to use methyl viologen dichloride hydrate to test, in the absence of oxygen, whether the pyridopyrimidine structure can give electrons to such dyestuff, reducing it to the blue colored form. When the reaction was carried out with 11e (R5 = H, R6 = Ph) in DMSO degasified with argon at 450 nm for 30 min, the initially pale-yellow solution changed to dark blue, confirming the transference of electrons from 11e to methyl viologen. No color was observed in the presence of air. Such a result could be considered an indirect confirmation of the Single Electron Transfer (SET) from the excited state [11e]* to methyl viologen, forming the corresponding cation-radical [11e]˙+.
At this point of the mechanistic study, we decided to use electron paramagnetic resonance (EPR) spectroscopy, a specific technique to characterize radicals,17,18 to gain more insight into the radicals involved in this process. The experiment carried out consisted of recording the EPR spectrum of a sample of the dehydrogenation of 11a (R5 = R6 = H) to 12a, at a concentration of 25 mg mL−1 in DMSO-d6 after 15 min of irradiation at 450 nm with bubbling of air. The result was the spectrum included in Fig. 4a, corresponding to a highly stable radical species that can be directly detected without the use of a spin trap, which presents eleven groups of lines. The EPR spectrum was simulated by using Bruker WIN-EPR SimFonia software (Fig. 4b) and it is compatible with the experimental one considering 5 quasi-equivalent N atoms (I = 1), 3 N atoms with a hyperfine coupling constant (hfcc) of 1.67 G and 2 N atoms with a hfcc of 1.69 G, to simulate the basic EPR pattern.
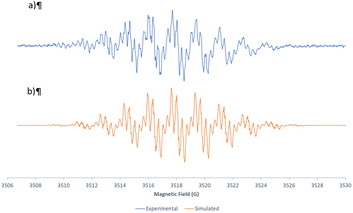 | ||
| Fig. 4 Experimental (a) and simulated (b) EPR spectra corresponding to the radical species formed during the dehydrogenation of 11a to 12a at 450 nm using bubbled air in an open vial. | ||
This could indicate an important delocalization of the spin density over the 5 nitrogen atoms of 11a. The fine structure of each group of the eleven lines was compatible with 4 equivalent H with a hfcc of 0.32 G and another H with a constant of 0.15 G. The g-factor and linewidth were g = 2.00445 and ΔHpp = 0.1 G, respectively. The spectrum obtained was identical if non-deuterated DMSO was used.
When the spectrum was recorded at 15, 30, 45, 60, and 75 min of irradiation, a decrease in the EPR signal was observed. However, such a radical was still observed after 1 month, showing its great stability. Such a radical could be the cation radical of the starting material 11a ([11a]˙+). The transference of a hydrogen radical and a proton from the first to the second would explain the formation of the C5–C6 double bond and the concomitant production of H2O2.
With the aim of obtaining more information about the nature of the radicals involved in the process, we decided to use the spin trapping technique using DMPO (5,5-dimethyl-1-pyrroline N-oxide).19 Such a spin trap reacts with radical species to form a stable radical adduct that allows to characterize the atom that has been bonded to the DMPO β-carbon atom (with respect to the oxygen atom) based on the Hβ coupling constant. If such a coupling constant is smaller than 20 G, the atom linked to the DMPO carbon is an oxygen atom (OCR, oxygen centered radical) and if it is greater than 20 G, the linked atom is a carbon atom (CCR, carbon centered radical).
When we irradiated a mixture of 11a and DMPO in DMSO-d6 with bubbling of air at 450 nm for 30 min, the EPR spectrum (see the ESI, page S42†) showed 2 groups of lines: a group of 3 main lines, which corresponded to a DMPO impurity that was already present in the blank spectrum of the DMPO used, and a second group composed of 4 groups of lines, whose coupling constants obtained from the simulation were aN = 13 G, aHβ = 10.4 G and aHγ = 1.35 G with a linewidth of 0.7 G and a g-factor of 2.0056.
This DMPO radical adduct spectral pattern was compatible with the trapping of the superoxide radical anion (O2˙−), thus confirming the main role of such species in the dehydrogenation process.
In contrast, when we carried out such an experiment by adding an excess of DMPO to the radical already formed from 11a after 30 min of irradiation at 450 nm with bubbling of air, we obtained an EPR signal (see the ESI, page S43†) with a Hβ coupling constant of 20.8 G, compatible with an adduct of DMPO, and the cation radical [11a]˙+ through a carbon atom.
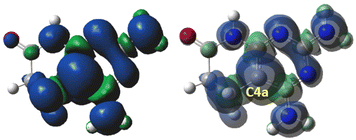
DFT calculations using Gaussian 16 at the B3LYP/6-311++G(d,p) theory level of the cation-radical [11a]˙+ of 11a were carried out (Fig. 5).
Such calculations showed that in the case of the cation-radical [11a]˙+, the radical was centered on the C4a bridgehead carbon and the spin density delocalized on the five nitrogen atoms, in accordance with the EPR data (Fig. 6).
Having apparently identified all the pieces of the reaction mechanism, that is, the starting product 11a, its excited state [11a]*, the cation-radical [11a]˙+, oxygen in the ground state (3O2) and the superoxide radical anion (O2˙−), the final dehydrogenated product 12a, and H2O2, before proposing such a mechanism, the DFT calculations of all species involved using Gaussian 16 were performed at the B3LYP/6-311++G(d,p) theory level (TD-DFT for the excited state [11a]*) with DMSO as an implicit solvent (Table 3). As a result of such calculations, the reaction mechanism included in Scheme 5 was proposed.
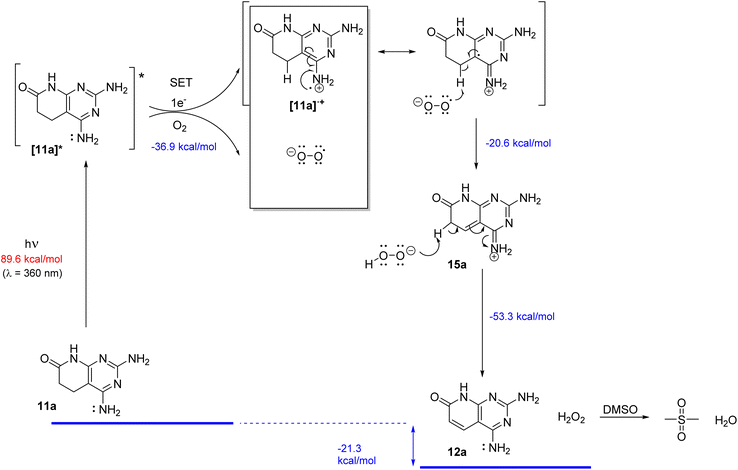 | ||
| Scheme 5 Proposed reaction mechanism for the dehydrogenation of 11a to 12a in DMSO irradiated with light (450 or 365 nm) in the presence of oxygen. | ||
| Structure | Role | Implicit DMSOb |
|---|---|---|
| a DFT calculations using Gaussian 16 were performed at the B3LYP/6-311++G(d,p) theory level (TD-DFT for the excited state [11a]*). b An implicit DMSO solvent model calculated using the CPCM method. | ||
| 11a | Starting compound | −621.410320 |
| 3 O 2 | Oxygen | −150.370631 |
| [11a]* | Excited state | −621.277098 |
| [11a]˙+ | Cation-radical | −621.197657 |
| O 2 ˙ − | Superoxide radical anion | −150.504930 |
| 1 O 2 | Singlet oxygen | −150.310000 |
| HOO − | Hydrogen peroxide anion | −151.117206 |
| 15a | Intermediate tautomer | −620.616069 |
| 12a | Final compound | −620.202291 |
| H 2 O 2 | Hydrogen peroxide | −151.610302 |
We propose the excitation of 11a (R5 = R6 = H) to the excited state [11a]*. The excited state could be achieved at 450 nm (which corresponds to an energy contribution of 63.5 kcal mol−1), but was certainly easier at 365 nm (corresponding to 78.3 kcal mol−1), thus explaining the observed faster kinetics at this wavelength. Such an excited state [11a]* would suffer a Single Electron Transfer (SET) to oxygen with the corresponding formation of the cation-radical [11a]˙+ and superoxide radical anion (O2˙−). Such direct transfer is energetically favorable when the calculation includes DMSO as an implicit solvent (−36.9 kcal mol−1).
Although the formation of the superoxide radical anion (O2˙−) seems to be clear, taking into account the quenching experiments included in Table 2 and the favorable energy associated with the SET between the excited state of 11a and oxygen obtained in the DFT calculations, we cannot exclude the concomitant formation of other Reactive Oxygen Species (ROS) such as singlet oxygen (1O2), although it was not detected in our experiments.
The resulting radical-cation [11a]˙+ and superoxide radical anion (O2˙−) pair would evolve by a radical hydrogen abstraction at C5 with the formation of the HOO− anion and a C4a–C5 double bond affording 15a. The predicted high spin density at C4a of [11a]˙+ would favor the abstraction of the hydrogen radical and the concomitant formation of the C4a–C5 double bond. The final tautomerization through the abstraction of a proton at C6 of 15a by the HOO− anion would afford the final dehydrogenated compound 12a and H2O2. Such tautomerization is favored by a decrease of the pKa value of the α-carbonyl methylene group due to the presence of the C5–C4a formed double bond (from around 20–25 to 9.6, value predicted for 15a with Marvin version 21.18.0, ChemAxon, https://www.chemaxon.com). The generated H2O2 oxidizes DMSO to dimethyl sulfone.
Such a mechanistic proposal is also useful to understand the dehydrogenation of compounds 11b–e that present substituents at R5 or R6 and is also capable of rationalizing why the reaction is slower when there is a substituent at R5 either by steric hindrance that it can cause or by the fact that there is only one hydrogen atom to be abstracted from such a position. However, all the compounds 12a–e studied to date have two amino groups at C2 and C4 and an unsubstituted nitrogen atom at N8. In order to determine if such dehydrogenation can take place in other pyrido[2,3-d]pyrimidine scaffolds, we decided to synthesize the compounds included in Chart 1.
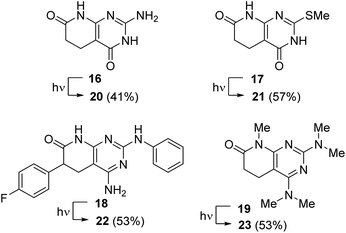 | ||
| Chart 1 Complementary compounds 16–19 evaluated in the dehydrogenation with light (450 or 365 nm) in the presence of air. The corresponding C5–C6 unsaturated compounds are 20–23. | ||
Thus, compound 16 was synthesized in 10% yield from methyl acrylate and guanidine using the procedure previously described by our group.11 Compound 17 was obtained from methyl acrylate and 6-amino-2-(methylthio)pyrimidin-4(3H)-one in 25% yield using an extension of the protocol described also by our group.718 was obtained in 49% yield following the protocol described in Scheme 1, starting from methyl 2-(4-fluorophenyl)acrylate.5,6 Finally, compound 19 was obtained in 91% yield from 11a upon treatment with 10 equiv. of NaH and 10 equiv. of MeI.
Compounds 16–19 were dehydrogenated at 365 nm to the corresponding compounds 20–23 bearing a C5–C6 double bond after 2.5, 5 and 10 h starting from 0.13, 0.13, and 0.07 mmol, respectively, thus showing an increased difficulty in such dehydrogenation. Although the conversions were almost quantitative, the isolated yields upon precipitation were 41%, 57%, and 53%. The use of a 450 nm lamp in the case of 16 increased the irradiation time to 8 h, also increasing the impurities present.
On the other hand, with the aim of evaluating whether DMSO is the only solvent adequate for such a process, we selected compounds 11e (R5 = H, R6 = Ph) and 13 to be irradiated at 365 nm in DMF and acetone, respectively, with bubbling air. 11e was converted into 12e in DMF in 2 h, affording an isolated 45% yield. Similarly, 0.2 mmol of 13 in acetone was dehydrogenated to 14 in 2 h, affording the final isolated compound in 74% yield. In contrast, no dehydrogenation was achieved in a proton-active solvent such as MeOH. These results seem to confirm that DMSO used in our study has no key role in the process either as a photocatalyst or a stabilizer.
Finally, the pentamethyl substituted compound 19 was dehydrogenated in DMSO at 365 nm in 1 h to afford compound 23 in 53% yield. The dehydrogenation achieved with 19 is especially relevant in supporting the proposed mechanism, in particular, the suggested formation of the C4a–C5 double bond by the radical abstraction of the H-C5 hydrogen, because in this case all nitrogen atoms are methylated, so without having any hydrogen bonded to an heteroatom, the mechanism must necessarily pass through the abstraction of the aforementioned H-C5.
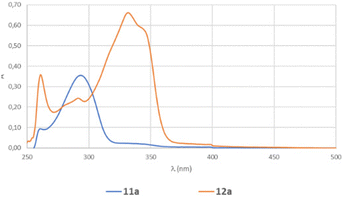
A last aspect to be considered for our process was the difference in the kinetics at 450 and 365 nm. Although the energy calculated for the excitation of 11a to [11a]* (79.3 kcal mol−1) seems to adequately fit with the energy given by the irradiation at 365 nm (78.3 kcal mol−1), we wanted to analyze if there could be another hidden factor. Consequently, we recorded the UV-visible spectra of 11a and 12a (Fig. 7) and we realized that the absorption both at 450 and 365 nm for 11a was extremely low, but apparently enough for the reaction to proceed.
We determined the molar absorptivity of each compound, by measuring the absorption at three different concentrations, obtaining 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 857 L mol−1 cm−1 for 12a and 6856 L mol−1 cm−1 for 11a. These results agree with the higher conjugation observed in 12a due to the introduction of the extra double bond. The fact that the two absorption spectra crossed at certain wavelengths could indicate the presence of isosbestic points during the dehydrogenation process.
857 L mol−1 cm−1 for 12a and 6856 L mol−1 cm−1 for 11a. These results agree with the higher conjugation observed in 12a due to the introduction of the extra double bond. The fact that the two absorption spectra crossed at certain wavelengths could indicate the presence of isosbestic points during the dehydrogenation process.
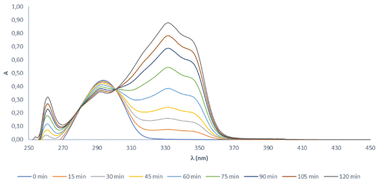
Consequently, the UV-visible spectrum of the reaction mixture was recorded each 15 min from 0 to 120 min (Fig. 8), showing two isosbestic points at 280 and 301 nm. The existence of isosbestic points in a chemical process indicates that there are no secondary reactions, and the absence of new absorption maxima, different from that of the starting product and that of the final compound, also indicates that there are no reaction intermediates that coexist long enough to absorb.
However, the fact that the absorption of 12a at 365 nm is extremely high led us to consider the possible formation of the radical of such a compound during the dehydrogenation process at such a wavelength and its possible role as a photosensitizer, thus increasing the kinetics of the reaction. Consequently, we irradiated 12a in DMSO-d6 in the presence of bubbling air and at 450 nm for 30 min and we registered the EPR spectrum obtaining an EPR signal (not as well resolved as in the case of 11a due to the lower solubility of 12a in DMSO), which proved that 12a can also form a radical with enough stability to be detected.
On the other hand, we conducted the normal dehydrogenation of 11a in DMSO at 365 nm, adding a 10% molar amount of 12a to see if there is a possible role of the latter compound as a photosensitizer, which increases the kinetics of the reaction, but we did not see a significant reduction of the reaction time.
Finally, we have also demonstrated that this methodology can be scaled-up. Starting from 1.0 g (5.6 mmol) of 11a in 40 mL of DMSO and irradiating the solution at 365 nm for 43 h, we achieved complete dehydrogenation and the isolation of 12a with a 76% yield.
Conclusions
In conclusion, a serendipitous discovery has allowed us to develop a general and clean method for the dehydrogenation of 5,6-dihydropyrido[2,3-d]pyrimidin-7(8H)-ones with a wide range of different substituents at C2, C4, C5, C6, and N8 by using the irradiation in DMSO (or other non-protic solvents) at 450 or 365 nm (the latter with shorter reaction times) in the presence of bubbling air and at room temperature. This reaction occurs without the addition of any photosensitizer because the pyridopyrimidine scaffold acts like it in an autocatalytic process that, to the best of our knowledge, is the first ever described in the literature for a dehydrogenation of a pyridone ring.A mechanistic proposal has been established based on experiments carried out in DMSO-d6, followed by NMR spectroscopy, EPR experiments, use of radical quenchers, spin-trapping techniques, and reaction with methyl viologen. Such experiments allowed us, in the case of the dehydrogenation of the C5–C6 unsubstituted compound 11a to 12a, to establish the radical nature of the process and detect using EPR a radical extended to the 5 nitrogen atoms of the pyridopyrimidine structure that was assigned to be the corresponding radical-cation [11a]˙+ on the basis of the DFT calculations. Computational predictions also indicated that such a radical was mainly centered at C4a (the bridgehead carbon between C5 and N4). Convergently, the treatment of this radical with DMPO yielded a radical adduct, which indicated the trapping of a carbon centered radical, probably by binding of C4a to DMPO. The studies carried out with radical quenchers have clearly indicated the key role of the superoxide radical anion (O2−) in such a process, and its conversion in hydrogen peroxide that later transform DMSO to the corresponding dimethyl sulfone (DMSO2).
All these pieces of experimental evidence and the DFT calculations carried out in implicit DMSO have allowed us to propose a mechanism that starts with the absorption of one photon by 11a molecules to yield the corresponding excited state [11a]*, which suffers a SET process with oxygen to yield the corresponding radical-cation [11a]˙+ and the superoxide radical anion (O2˙−), which abstracts a hydrogen radical probably from C5 of the radical-cation [11a]˙+ to give hydrogen peroxide anion (HOO−) and a C5–C4a unsaturated intermediate that finally undergoes a proton abstraction at C6 to yield hydrogen peroxide (H2O2) and the final C5–C6 unsaturated compound 12a through a tautomeric process.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. C. d. R., investigation; V. L., investigation; J. V.-G., investigation and methodology; J. T., formal analysis; R. E.-T., investigation; J. I. B., conceptualization and writing – original draft; R. P. d. l. B., conceptualization and supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by Ministerio de Ciencia, Innovación y Universidades, Proyectos de I + D + I “Retos Investigación” del Programa Estatal de I + D + I orientada a los Retos de la Sociedad, grant number RTI2018-096455-B-I00. We thank Dr Alexandr Shafir of the Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) and Dr Ana Belén Cuenca of Institut Químic de Sarrià, Universitat Ramon Llull for giving us the possibility of carrying out the first experiment using Penn PhD Photoreactor M2. We thank one of the referees for very interesting and fruitful suggestions and discussion about the mechanistic proposal.References
- R. Puig de la Bellacasa, G. Roué, P. Balsas, P. Perez-Galan, J. Teixido, D. Colomer and J. I. Borrell, 4-Amino-2-arylamino-6-(2,6-dichlorophenyl)-pyrido[2,3-d]pyrimidin-7-(8H)-ones as BCR kinase inhibitors for B lymphoid malignancies, Eur. J. Med. Chem., 2014, 86, 664–675 CrossRef CAS PubMed.
- M. A. Molina, S. García-Román, J. I. Borrell, J. Teixidó, R. Estrada-Tejedor and R. Puig de la Bellacasa, (2,6-dichlorophenyl)-8-methyl-2-(phenylamino)-pyrido[2,3-d]pyrimidin-7(8H)-one for treatment of solid cancers, EP3120851A1, 2017 Search PubMed.
- E. Bou-Petit, S. Hümmer, H. Alarcon, K. Slobodnyuk, M. Cano-Galietero, P. Fuentes, P. J. Guijarro, M. J. Muñoz, L. Suarez-Cabrera, A. Santamaria, R. Estrada-Tejedor, J. I. Borrell and S. R. y. Cajal, Overcoming Paradoxical Kinase Priming by a Novel MNK1 Inhibitor, J. Med. Chem., 2022, 65, 6070–6087 CrossRef CAS PubMed.
- V. Masip, Á. Lirio, A. Sánchez-López, A. B. Cuenca, R. Puig de la Bellacasa, P. Abrisqueta, J. Teixidó, J. I. Borrell, A. Gibert and R. Estrada-Tejedor, Expanding the Diversity at the C-4 Position of Pyrido[2,3-d]pyrimidin-7(8H)-ones to Achieve Biological Activity against ZAP-70, Pharmaceuticals, 2021, 14, 1311 CrossRef PubMed.
- I. Galve, R. Puig de la Bellacasa, D. Sanchez-Garcia, X. Batllori, J. Teixido and J. I. Borrell, Synthesis of 2-arylamino substituted 5,6-dihydropyrido[2,3-d]pyrimidine-7(8H)-ones from arylguanidines, Mol. Diversity, 2012, 16, 639–649 CrossRef CAS.
- I. Galve, R. Ondoño, C. de Rocafiguera, R. Puig de la Bellacasa, X. Batllori, C. Puigjaner, M. Font-Bardia, O. Vallcorba, J. Teixidó and J. I. Borrell, A captured room temperature stable Wheland intermediate as a key structure for the orthogonal decoration of 4-amino-pyrido[2,3-d]pyrimidin-7(8H)-ones, Org. Biomol. Chem., 2020, 18, 9810–9815 RSC.
- M. Camarasa, C. Barnils, R. Puig de la Bellacasa, J. Teixido and J. I. Borrell, A new and practical method for the synthesis of 6-aryl-5,6-dihydropyrido[2,3-d]pyrimidine-4,7(3H,8H)-diones, Mol. Diversity, 2013, 17, 525–536 CrossRef CAS PubMed.
- I. Perez-Pi, X. Berzosa, I. Galve, J. Teixido and J. I. Borrell, Dehydrogenation of 5,6-dihydropyrido[2,3-d]pyrimidin-7(8H)-ones: A convenient last step for a synthesis of pyrido[2,3-d]pyrimidin-7(8H)-ones, Heterocycles, 2010, 82, 581–591 CrossRef CAS.
- C. G. Bochet and A. Blanc, Photolabile protecting groups in organic synthesis, in Handbook of Synthetic Photochemistry, Wiley, 2010, vol. 13, pp. 417–447 Search PubMed.
- P. Klán, T. Šolomek, C. G. Bochet, A. Blanc, R. Givens, M. Rubina, V. Popik, A. Kostikov and J. Wirz, Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy, Chem. Rev., 2013, 113, 119–191 CrossRef.
- N. Mont, J. Teixido, C. O. Kappe and J. I. Borrell, A one-pot microwave-assisted synthesis of pyrido[2,3-d]pyrimidines, Mol. Diversity, 2003, 7, 153–159 CrossRef CAS PubMed.
- M. J. Hansen, W. A. Velema, M. M. Lerch, W. Szymanski and B. L. Feringa, Wavelength-selective cleavage of photoprotecting groups: strategies and applications in dynamic systems, Chem. Soc. Rev., 2015, 44, 3358–3377 RSC.
- W.-L. Yu, Z.-G. Ren, K.-X. Ma, H.-Q. Yang, J.-J. Yang, H. Zheng, W. Wu and P.-F. Xu, Cobalt-catalyzed chemoselective dehydrogenation through radical translocation under visible light, Chem. Sci., 2022, 13, 7947–7954 RSC.
- W. Jin and S. Yu, Photoinduced and palladium-catalyzed remote desaturation of amide derivatives, Org. Lett., 2021, 23(17), 6931–6935 CrossRef CAS PubMed.
- P. S. Sadhu, M. Ravinder, P. A. Kumar and V. J. Rao, Photochemical dehydrogenation of 3,4-dihydro-2-pyridones, Photochem. Photobiol. Sci., 2009, 8, 513–515 CrossRef CAS.
- W. Schilling, D. Riemer, Y. Zhang, N. Hatami and S. Das, Metal-Free Catalyst for Visible-Light-Induced Oxidation of Unactivated Alcohols Using Air/Oxygen as an Oxidant, ACS Catal., 2018, 8, 5425–5430 CrossRef CAS.
- V. Lloveras, P. Elías-Rodríguez, L. Bursi, E. Shirdel, A. R. Goñi, A. Calzolari and J. V. -Gancedo, Multifunctional Switch Based on Spin-Labeled Gold Nanoparticles, Nano Lett., 2022, 22, 768–774 CrossRef CAS PubMed.
- S. Zhang, V. Lloveras, S. Lope-Piedrafita, P. Calero-Pérez, S. Wu, A. P. Candiota and J. Vidal-Gancedo, Metal-Free Radical Dendrimers as MRI Contrast Agents for Glioblastoma Diagnosis: Ex Vivo and In Vivo Approaches, Biomacromolecules, 2022, 23, 2767–2777 CrossRef CAS PubMed.
- F. A. Villamena, EPR Spin Trapping, in Reactive Species Detection in Biology, Elsevier, 2017, vol. 5, pp. 163–202 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterization data, and spectra for the synthesized compounds, and the EPR and DFT calculation details. See DOI: https://doi.org/10.1039/d3qo01358h |
| This journal is © the Partner Organisations 2024 |