Open Access Article
Open Access ArticleStudy on dechlorination salt characteristics of pickling sludge by a water-washing process†
Yane Xu *ab,
Likui Xua,
Jie Yuan
*ab,
Likui Xua,
Jie Yuan ab,
Hongchao Luoab,
Chaochuang Yinab,
Yizhu Leiab,
Guoqi Lianab,
Aiyuan Ma*ab and
Xinqian Shu*c
ab,
Hongchao Luoab,
Chaochuang Yinab,
Yizhu Leiab,
Guoqi Lianab,
Aiyuan Ma*ab and
Xinqian Shu*c
aSchool of Chemistry and Materials Engineering, Liupanshui Normal University, Guizhou 553004, PR China. E-mail: YaneXu1986@126.com; may_kmust11@163.com
bGuizhou Provincial Key Laboratory of Coal Clean Utilization, Liupanshui, Guizhou 553004, PR China
cSchool of Chemistry and Environmental Engineering, China University of Mining and Technology Beijing, Beijing 100083, PR China. E-mail: sxq@cumtb.edu.cn
First published on 2nd January 2024
Abstract
Steel hydrochloric acid pickling sludge (SHPS), containing the heavy metals Fe, Zn, and Ni and a high chloride salt content, is considered a hazardous solid waste. With the gradual reduction of high-grade metal mineral resources such as Fe, Zn and Ni, it is particularly urgent to recycle valuable metals such as Fe, Zn and Ni in solid waste SHPS in order to realize the resource utilization of SHPS and reduce the environmental harm caused by SHPS. In addition, SHPS usually contains different amounts of alkali chloride, which will have a serious adverse impact on the subsequent extraction and smelting process of Fe, Zn and other metals. Therefore, the removal of chloride plays an important role in the resource utilization of valuable metals in SHPS. Thus, in this study, the effects of water washing dechlorination process parameters such as liquid–solid (L/S) ratio, SHPS particle size, washing time and washing frequency on the chloride removal rate were investigated. The best experimental parameters of SHPS washing were obtained. At the same time, the microscopic morphology and crystal phase composition of SHPS before and after washing were explored. The results showed that the optimized conditions were as follows: room temperature, a L/S ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, an SHPS particle size of 100 mesh, and 10 min of water washing, repeated two or three times; under these conditions, the removal rate of Cl, Na, Ca, K, Mg, and S reached 96.64–99.68%, 97.38–99.89%, 36.40–60.37%, 49.11–54.82%, 39.18–40.22%, and 36.98–42.13% respectively. The contents of Cl, K, and Na in filter residue (FR) meets the requirements in GB/T 36144-2018 and GB/T 32545-2016. Conversely, the contents of Fe, Zn, Mn and Ni in the FR are enriched, which is more conducive to the subsequent resource utilization of SHPS. The scanning electron microscope (SEM) image shows the particle size of the FR particles is reduced after washing. The X-ray diffractometer (XRD) results proved that the chlorine salt content in the FR after washing was significantly reduced, the diffraction peaks of Al2O3 appeared in the FR, and the diffraction peak intensity of CaCO3, Fe2O3 and SiO2 increased.
1, an SHPS particle size of 100 mesh, and 10 min of water washing, repeated two or three times; under these conditions, the removal rate of Cl, Na, Ca, K, Mg, and S reached 96.64–99.68%, 97.38–99.89%, 36.40–60.37%, 49.11–54.82%, 39.18–40.22%, and 36.98–42.13% respectively. The contents of Cl, K, and Na in filter residue (FR) meets the requirements in GB/T 36144-2018 and GB/T 32545-2016. Conversely, the contents of Fe, Zn, Mn and Ni in the FR are enriched, which is more conducive to the subsequent resource utilization of SHPS. The scanning electron microscope (SEM) image shows the particle size of the FR particles is reduced after washing. The X-ray diffractometer (XRD) results proved that the chlorine salt content in the FR after washing was significantly reduced, the diffraction peaks of Al2O3 appeared in the FR, and the diffraction peak intensity of CaCO3, Fe2O3 and SiO2 increased.
1. Introduction
Steel hydrochloric acid pickling sludge (SHPS) is usually produced during the surface treatment of carbon steel.1 SHPS contains Fe, Zn and Ni compounds, chloride and residual acid.1,2 SHPS exhibits leaching toxicity, pollutes soil and water, can cause chromosomal aberrations in plants, and causes potential harm to human health.1–4 In China, as per the National List of Hazardous Waste (2021 version),5 SHPS is a hazardous waste; special considerations must be made in the handling of SHPS. Commonly, the yield of SHPS is approximately 30–50 kg per ton of steel.6–8 According to statistics, the annual output of SHPS in China in recent years has exceeded 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons.9
000 tons.9
The conventional approach for treating SHPS is landfilling, which often follows stabilization/solidification. Increasingly stringent environmental and landfill regulations coupled with the increasing costs of raw materials make it imperative to find a feasible method of recycling and treating SHPS to replace the traditional landfill method. Generally, SHPS is an inevitable product in the carbon steel industry and contains a large amount of Fe2O3 and ZnFe2O4. Effectively utilizing the Fe and Zn contained in SHPS in the modern metal metallurgy industry is a highly attractive strategy to solve the problems related to the depletion of high-grade metallic ores.
However, if SHPS is directly used as a metal-smelting raw material, Na, K and Cl in SHPS are harmful elements because Na and K will be circularly enriched, resulting in blast furnace nodulation and furnace lining erosion; in addition, Na and K will also aggravate sinter reduction pulverization and coke dissolution loss,10 and chlorides will accelerate the corrosion of steel.11 Another aspect is high-temperature chloride cycling (sublimation + condensation), which is detrimental to the operating performance of high-temperature furnaces.12 Furthermore, chlorides provide convenient conditions to generate organic pollutants such as dioxins in the low-temperature regions of reaction furnaces.13 Therefore, chlorides must be removed before the SHPS is utilized as a resource. According to the standard requirements of limit of lead, arsenic, cadmium, mercury, fluorine and chlorine content in iron ore (GB/T 36144-2018),14 the chlorine content of raw materials shall be ≤0.40 wt%, so the chlorine salt in SHPS shall be removed to make it ≤0.40 wt%. Xu et al. found that SHPS mainly contains inorganic soluble chlorides, Fe2O3 and ZnFe2O4, and chlorides exists mainly in the form of sodium chloride (NaCl) and calcium chloride (CaCl2).1 Table 1 shows the requirements for the contents of Na, K, Si, Fe and other elements in iron concentrate powder in GB/T 32545-2016.15 Therefore, in addition to the content of Cl, the content of K2O + Na2O should also meet the requirements in the removal process of alkali metal chloride. Fortunately, Cl, K and Na usually exist in the form of soluble chlorine salt in SHPS. Table 2 shows the solubility of KCl, NaCl and CaCl2 at 20 °C under a standard atmospheric pressure. It can be seen from Table 2 that the solubility of these three soluble salts in water is large.
| Grade | Index (quality score) (%) | |||||
|---|---|---|---|---|---|---|
| TFe | SiO2 | P | S | F | K2O + Na2O | |
| Level 1 | ≥65.0 | ≤4.0 | ≤0.10 | ≤0.80 | ≤0.50 | ≤0.40 |
| Level 2 | 63.0 to <65.0 | ≤5.0 | ≤0.20 | ≤0.90 | ≤0.60 | ≤0.50 |
| Level 3 | 61.0 to <63.0 | ≤6.0 | ≤0.30 | ≤1.00 | ≤0.80 | ≤0.60 |
| Project | Solubility (g per 100 g water) |
|---|---|
| NaCl | 35.9 |
| KCl | 34.2 |
| CaCl2 | 74.5 |
At present, the methods for removing inorganic chlorides mainly include acid washing dechlorination,16 thermal dechlorination,17 microwave dechlorination,18 water washing dechlorination,19,20 etc., but the three former are mainly used to treat water-insoluble chlorides, and water-soluble chlorides are mostly used for water washing dechlorination. As an effective method to remove high contents of water-insoluble chlorides in solid waste, water washing pretreatment has been studied at home and abroad.21–23 Wang et al. studied the effects of water washing on the removal of chlorides from MSWI fly ash, and the results show that the removal ratio of Cl reached the optimum value of 75.33% when the L/S ratio was 10 and the washing time was 2 h.24 Chen et al. studied chloride removal and control by performing a water-washing process on MSWI fly ash, and the results show that with the optimal multi washing process, the removal rate of chlorine was beyond 99%.25 However, as far as we know, no scholar has studied the removal of soluble chloride in SHPS by water washing. Therefore, it is worth studying the removal of soluble chlorides in SHPS by water washing to facilitate the resource utilization of SHPS, which can create new environmental benefits for enterprises.
The objective of this article is to systematically investigate the chloride removal ability by water washing. The effects of the L/S ratio, SHPS particle size, washing time and washing frequency on the chloride removal rate in SHPS were investigated through single factor experiments to obtain the best experimental conditions. The morphological characteristics and crystal phase composition of SHPS before and after washing were analyzed. The results of this study can provide a preliminary basis for the resource utilization of SHPS and provide a reference for the dechlorination of other high chlorine solid wastes.
2. Experiment
2.1. Preparation of SHPS samples
A total of 3 typical SHPS samples were collected from the Jinghai District of Tianjin in China, as shown in Fig. 1. Sludges 1 was from a galvanized steel wire manufacturer, while sludges 2 was from a galvanized steel pipe manufacturer, and sludges 3 was from a galvanized steel sheet manufacturer. The SHPS generation of these three manufacturers was basically the same except for each processing condition. Fig. 1 presents photographs of the SHPS samples.The dried sample is placed in a sealed bag and stored in a dryer for use. To perform XRD and other analyses, the dried SHPS was ground into a powder and sieved by passing it through a 200 mesh (size approximately <74 μm).
2.2. Analytical methods and instruments
In the experiment, microwave digestion method was used to prepare samples for determining element content through inductive coupled plasma mass spectrometry (ICP-MS). A dry sample weighing 0.100–0.200 g was placed in a 50 mL Teflon digestion tube, and 5 mL of nitric acid, 2 mL of perchloric acid, and 2 mL of hydrofluoric acid were added. The mixture was covered until the resulting white smoke was exhausted, 0.5 mL of boric acid was added, the mixture was placed in a graphite digestion instrument at 160 °C overnight, and then it was diluted to 50 mL and transferred to a polypropylene bottle for storage. Then, the elemental content was tested three times by ICP-MS (an Agilent ICP-MS 7700 instrument made in America). The chlorine content in the powder samples was analyzed using an ARLAdvantX Intellipower TM3600 X-ray fluorescence spectrometer (Thermo Fisher, USA). Before the XRF test, an ultrahigh-pressure sample (UHPS) preparation system was used to directly press the sample powder into discs, and then the XRF test was carried out. X-ray diffraction (XRD) patterns of the samples were obtained with a Rigaku Ultimate-IV X-ray diffractometer utilizing a Cu Kα radiation source to determine the crystalline phases.X-ray diffraction (XRD) patterns of the samples between 10 and 90° (2θ) at a step size of 0.02° were obtained with a Rigaku Ultimate-IV X-ray diffractometer utilizing a Cu Kα radiation source operated at 40 kV and 100 mA to determine the crystalline phases. Before the XRD test, the sample was prepared by the tablet pressing method: the sample powder was sprinkled into the window of the sample preparation frame as evenly as possible, chopped gently with the knife edge of a small spatula to spread the powder evenly, and stacked inside the window hole. Then, the powder was pressed gently with a small spatula, and finally the excess protruding powder was scraped off with a safety blade. The sample preparation frame was then carefully picked up from the glass plane to obtain a very flat plane of sample powder.
Scanning electron microscopy (SEM) images were recorded by a Zeiss Sigma 300 scanning electron microscope operated at 2.0 kV to observe the morphology. Before the SEM test, a conductive adhesive was prepared, the sample powder was poured on the conductive adhesive, the base was held in the hand and vibrated on a table to disperse and spread the powder, and then the sample was purged to ensure that all particles were in full contact with the conductive adhesive and were distributed in a single layer without stacking. Finally, a thin layer of sample was obtained. In addition, the SHPS samples have good conductivity, so coating with a thin conductive layer was not necessary. All experiments were conducted in duplicate, and the averaged results were used for analysis. The variation between the replicates for each analysis, including the blank control, was below 5%.
2.3. SHPS water-washing dechlorination
In dechlorination experiments, four stages of water-washing were applied to investigate the optimal parameters. The first stage involved experiments with different liquid (milliliter)/solid (gram) (abbreviated L/S) L/S ratios of 2/1, 3/1, 4/1, and 5/1 to determine the optimal water intensity. The second stage involved experiments with different SHPS particle sizes of 60, 100, 140, and 180 mesh to determine the appropriate particle size. The third stage involved experiments with different washing times varying from 5 min to 70 min to determine the optimal contact time. In the fourth stage, double, triple and quadruple washing processes were conducted to investigate the effect of washing frequency on dissolution and to obtain the maximum dissolution level of SHPS chlorides in the water. During the process, 10 g of dried and ground SHPS was used in each single experiment, and the vibration speed was controlled at a constant of 200 rpm.During the washing experiments, the deionized water employed as washing solvent was mixed with SHPS in 100 mL beaker. Then, place the mixing rod of the electric mixer in the beaker and set the rotating speed to 200 rpm, and stop mixing after mixing for the required time. Then, the washing solution was removed with vacuum suction filtration using a 0.45 μm filter membrane. Fig. 2 shows a schematic diagram of the experimental device. An ICP-MS 7700 inductive coupling system was used for the determination of metal content in the filter residue (FR); and then, X-ray fluorescence equipment was used for the determination of chlorine content in the FR. Flow charts of chloride removal achieved by performing water washing on SHPS are shown in Fig. 3.
The element (such as Cl, Na, etc.) removal rate was calculated from the following:
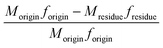 | (1) |
2.4. Analysis of SHPS characteristics
The main chemical compositions are shown in Table 3.| Main elements content of SHPS samples (wt%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Ca | Na | K | Mg | Si | Al | Cl | P | S | Fe | Zn | Mn |
| 1# | 3.18 | 3.31 | 0.05 | 0.03 | 1.99 | 0.17 | 8.76 | 0.02 | 0.02 | 37.32 | 13.89 | 0.38 |
| 2# | 11.11 | 0.38 | 0.13 | 0.19 | 4.78 | 0.21 | 12.13 | 0.01 | 0.37 | 29.80 | 4.21 | 0.29 |
| 3# | 0.32 | 3.21 | 0.01 | 0.14 | 3.74 | 0.78 | 5.21 | 0.01 | 0.08 | 54.16 | 0.02 | 0.03 |
Table 3 shows the main elements contents of SHPS samples respectively. As seen from Table 3 that the sample contains a certain amount of valuable metals Fe, Zn and Mn that should be recycled, and the Cl content in the samples is 5.21–12.13 and needs to be removed to less than 0.40%, the content of K2O + Na2O should to be removed to less than 0.60% to facilitate the further resource utilization of SHPS.
3. Results and discussion
3.1. Effects of different L/S ratios on chloride removal
L/S ratio is an important factor in the process of solid–liquid reaction. It directly affects the contact area between solid and liquid phase, and then affects the final reaction degree. The larger the L/S ratio is, per unit particle of SHPS in suspension will contact the more water solution, and the better the washing effect will be. However, too high L/S ratio will cause a waste of water resources. Therefore, while ensuring that the chlorine salt in SHPS can be fully dissolved, selecting the most water-saving L/S ratio can improve the economy of the washing process. Therefore, it is necessary to explore the effect of L/S ratio on the dechlorination effect. Ten grams of dried and ground SHPS was mixed with deionized water to achieve different L/S ratios: 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4
1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 5
1 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
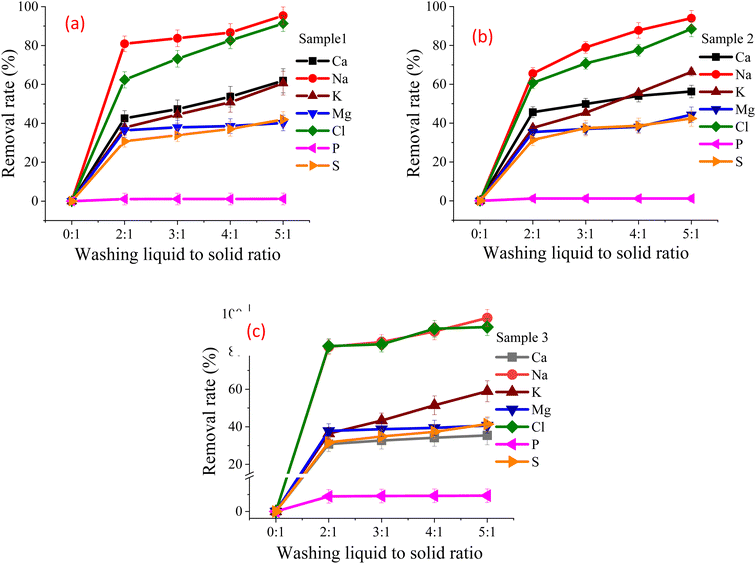
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The SHPS particle size was 60 mesh, and the washing time was set to 10 min at room temperature. After filtrating the washed mixture by vacuum suction filtration, the component contents of the filtrate and FR were tested. Fig. 4 shows the element removal rate in the FR. Table 1S† shows the main element content in the FR under different L/S ratios.
1. The SHPS particle size was 60 mesh, and the washing time was set to 10 min at room temperature. After filtrating the washed mixture by vacuum suction filtration, the component contents of the filtrate and FR were tested. Fig. 4 shows the element removal rate in the FR. Table 1S† shows the main element content in the FR under different L/S ratios.
As shown in Fig. 4, the soluble chloride in the sample can be removed to a certain extent after water washing, and the L/S ratio has a great impact on the removal rate of alkali chloride in the samples. As shown in Fig. 4, when the L/S ratio increased from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 5
1 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the removal rates of Ca, Na, K and Mg in sample 1 vary from 42.57% to 61.92%, 80.91% to 95.39%, 37.81% to 60.69% and 36.42% to 40.18% respectively; the removal rates of Cl and S vary from 62.39% to 91.33%, and 30.78% to 41.99% respectively. Obviously, when the L/S ratio is 5
1, the removal rates of Ca, Na, K and Mg in sample 1 vary from 42.57% to 61.92%, 80.91% to 95.39%, 37.81% to 60.69% and 36.42% to 40.18% respectively; the removal rates of Cl and S vary from 62.39% to 91.33%, and 30.78% to 41.99% respectively. Obviously, when the L/S ratio is 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the removal rate of Na and Cl in sample 1 can be more than 91%, the removal rate of Ca and K can be more than 60%, and the removal rate of Mg and S can be more than 40%. Based on the above experimental results, it can be preliminarily concluded that water washing has a good removal effect on Na, Cl, Ca, K, Mg and S in SHPS samples. However, the dissolution rate in water of Si, Al, P, Fe, Zn, Mn, Ni, Pb, Cr and As of sample 1 is low, the maximum dissolution rate does not exceed 1.98%, but is enriched in the FR. The effect of L/S ratio on the dechlorination of SHPS 2 and SHPS 3 is similar to that of SHPS 1. The water-washing process can not only remove the soluble chlorides in SHPS but also enrich the valuable metals in the FR, as shown in the data in Table 1S.†
1, the removal rate of Na and Cl in sample 1 can be more than 91%, the removal rate of Ca and K can be more than 60%, and the removal rate of Mg and S can be more than 40%. Based on the above experimental results, it can be preliminarily concluded that water washing has a good removal effect on Na, Cl, Ca, K, Mg and S in SHPS samples. However, the dissolution rate in water of Si, Al, P, Fe, Zn, Mn, Ni, Pb, Cr and As of sample 1 is low, the maximum dissolution rate does not exceed 1.98%, but is enriched in the FR. The effect of L/S ratio on the dechlorination of SHPS 2 and SHPS 3 is similar to that of SHPS 1. The water-washing process can not only remove the soluble chlorides in SHPS but also enrich the valuable metals in the FR, as shown in the data in Table 1S.†
The influence of L/S ratio on the amount of soluble chlorine salt can be divided into the following situations: when the L/S ratio is very small, due to the strong water absorption of SHPS, only part of the soluble chlorine salt (NaCl, KCl, CaCl2, CaClOH) is diffused and dissolved into the solution, at this time, the whole dissolution system is in a supersaturated state; with the increase of L/S ratio, the amount of soluble chloride in SHPS increased until some components reached saturation;26 continue to increase the L/S ratio, and the soluble chloride will continue to dissolve until it is almost completely dissolved; further increase the L/S ratio, since most of the soluble chlorine salts have been dissolved, the dissolved amount of chlorine salts has little change.
It can be seen from Fig. 4 that as the L/S ratio increases, the chloride removal rate also increases. However, the increase in L/S ratio will increase the extent of wastewater treatment, resulting in the waste of water resources and increase in project operation cost; but when the L/S ratio is too small, a large amount of leaching solution will be adsorbed around the solid particles of SHPS, which increases the difficulty of liquid–solid separation, the feasibility of filtration operation is poor, so it is not suitable for dechlorination. Therefore, the selection of SHPS washing L/S ratio should be considered from multiple perspectives. Under comprehensive consideration, the suitable L/S ratio for SHPS primary water washing is determined as 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
3.2. Effects of particle size on chloride removal
At a L/S ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
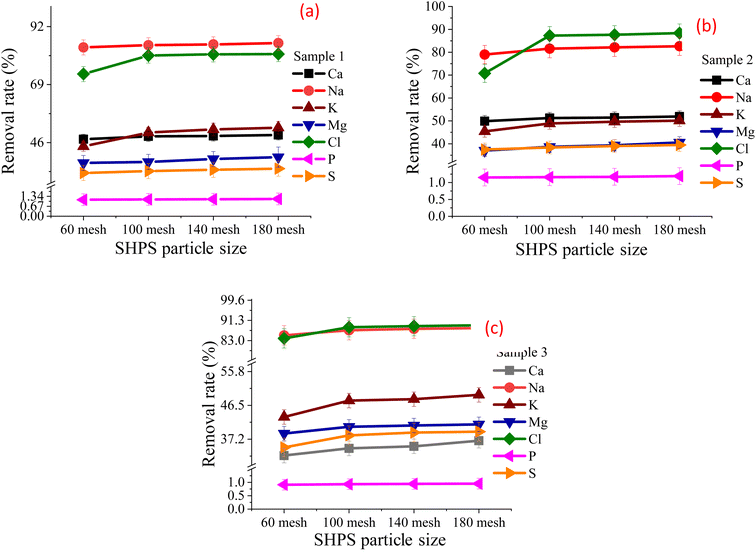
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and a washing time of 10 min, the effects of different SHPS particle sizes on the dechlorination effect were investigated. The particle sizes were 60 mesh, 100 mesh, 140 mesh and 180 mesh. Fig. 5 shows the element removal rate under SHPS different particle sizes. Table 2S† shows the main element content in the FR under SHPS different particle sizes.
1 and a washing time of 10 min, the effects of different SHPS particle sizes on the dechlorination effect were investigated. The particle sizes were 60 mesh, 100 mesh, 140 mesh and 180 mesh. Fig. 5 shows the element removal rate under SHPS different particle sizes. Table 2S† shows the main element content in the FR under SHPS different particle sizes.
As shown in Fig. 5, with SHPS particles become finer, the removal rate of chlorine salt first increases and then tends to be stable. When the particle size is greater than 100 mesh, the removal rate of chloride tends to be stable. When the particle size increased from 100 mesh to 180 mesh, the content of elements such as Cl, Ca, K, Na, Fe, Zn, Mn and Ni in FR remained almost unchanged. Based on the experimental results and cost-effectiveness, 100 mesh was selected as the optimal particle size.
When the particle size was 100 mesh and washed for 10 min, the Cl removal rates of the three samples were 80.53%, 87.29% and 88.48% respectively; the Cl content in the corresponding FR is 2.04%, 1.99% and 0.65% respectively; the contents of K2O + Na2O are 1.72%, 0.45% and 1.22% respectively. There is still a gap between meeting the requirements of K2O + Na2O content in GB/T 32545-2016 and Cl content limit in GB/T 36144-2018. Therefore, it is necessary to continue to explore the influence of washing time and washing frequency.
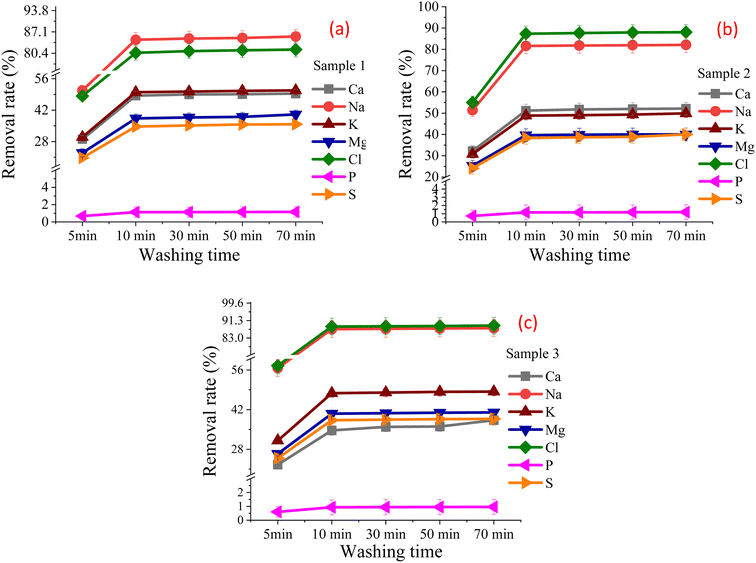
3.3. Effects of washing time on chloride removal
The washing time directly affects the dissolution and diffusion of salts and other components in SHPS. If the washing time is too short, the salt and other components cannot be fully dissolved. If the washing time is too long, the economy will be reduced. In order to investigate the effect of washing time on the dissolution of salts and other components in SHPS, and to determine the best time of washing process, based on the combination of 3.1, 3.2 sections and other relevant washing studies, the fixed stirring speed, L/S ratio and particle size at room temperature are 200 rpm, 3/1 and 100 mesh respectively. Different washing times, i.e., 5 min, 10 min, 30 min, 50 min and 70 min, were set to investigate the effect of time on the chlorine removal rate of SHPS.Fig. 6 shows the removal rate under different water-washing time. Table 3S† shows the main element content in the FR under different water-washing time. Fig. 6 and Table 3S† show that when the washing time is less than 10 min, the washing time has a significant impact on the removal rate of chloride salts, and at this stage, the removal rate of chloride salts increases with the extension of washing time. When the washing time exceeds 10 min, the chloride salt in SHPS reaches a solution equilibrium, the length of washing time has little effect on the removal of chloride in SHPS. Most of the chlorine salts rapidly dissolved into the liquid phase within 10 min. So, when the dissolution time exceeds 10 min, prolonging the water washing time will have little effect on the chloride removal rate. This is mainly because most of the chlorines in SHPS exist in the form of soluble chlorine salts such as NaCl and CaCl2. These chlorine salts have high solubility in water and can be dissolved in a short time (10 min).26 This is consistent with the time required for water washing to remove soluble chlorides from MSWI fly ash studied by Chen.25 Because after a certain period of time, the dissolution of chlorine salt reaches a relatively balanced state, so it will no longer dissolve.27 Eqn (2)–(5) is the reaction equation of chlorine salt dissolved into water.28 Moreover, in the actual project, with the washing time is prolonged, the operating cost of the whole set of washing equipment will also increase. Combined with the experimental results and time costs, 10 min was selected as the appropriate washing time.
| NaCl(s) → NaCl(aq) | (2) |
| KCl(s) → KCl(aq) | (3) |
| CaCl2(s) → CaCl2(aq) | (4) |
| 2CaCl[OH](s) → Ca(OH)2(s) + CaCl2(aq) | (5) |
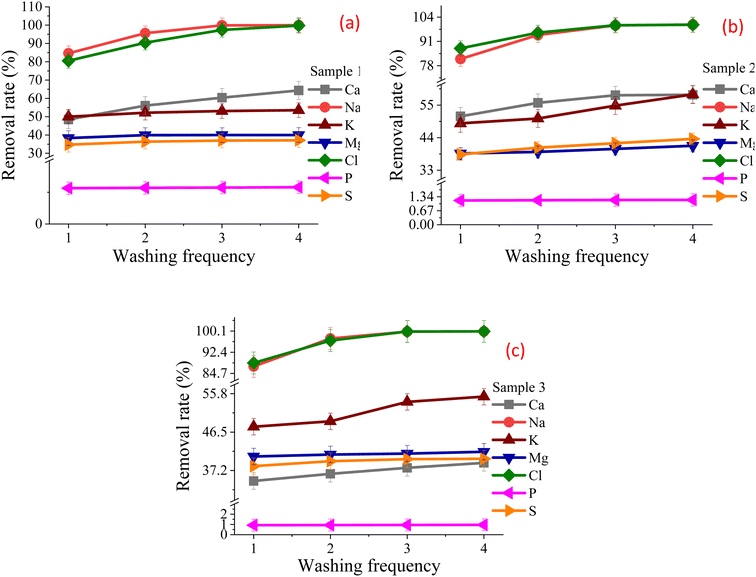
3.4. Effects of washing frequency on chloride removal
After one-time water washing, the content of Cl in the three SHPS FR is 2.04%, 1.99% and 0.65% respectively. However, GB/T 36144-2018 requires that the Cl content of raw materials shall be ≤0.40 wt%. And the contents of K2O + Na2O are 1.72%, 0.45% and 1.22% respectively, GB/T 32545-2016 requires that the contents of K2O + Na2O shall be ≤0.60%. It can be seen that one-time water washing can not make the FRs of the three samples both meet the limit requirements for K2O + Na2O and Cl contents in GB/T 32545-2016 and GB/T 36144-2018 respectively. Therefore, it is necessary to study the effect of water washing frequency on dechlorination efficiency to achieve the goal of Cl and K2O + Na2O content in FRs ≤ 0.40% and 0.60%.Based on the results of washing one time, the optimal washing conditions were selected: liquid–solid ratio of 3 mL g−1, SHPS particle size of 100 mesh, and washing time of 10 min. After filtration, the residue was washed with fresh deionized water to study the effect of washing frequency (including two, three and four times) on the dechlorination rate.
Fig. 7 shows the removal rate under different washing frequencies. Table 4S† shows the main element content in the FR under different washing frequency. Fig. 7 and Table 4S† shows that with the increase of water washing frequency, the removal of chlorine salt first increases rapidly and then tends to be stable. When the washing frequency are less than 3 times, the more times water washing is performed, the lower the content of Cl, Ca and Na in the FR; when the washing times are more than 3 times, increasing the washing times has little effect on the dechlorination effect. After three times of washing, the Cl contents in FR of SHPS 1 and SHPS 2 have been less than 0.40%, and the removal rate of Cl element has reached 97.41% and 99.68% respectively; the Na content in the FR is reduced to 0.005% and 0.002% respectively, and the removal rates are 99.89% and 99.58% respectively. After twice washing of SHPS 3, the Cl in the FR has been reduced to 0.20%, and the removal rate of Cl element has reached 96.64%; the Na content in the FR decreased to 0.094%, and the removal rate reached 97.38%. From the twice water washing effects, the elution rate of chlorine salt in SHPS 3 is higher than that in SHPS 1 and SHPS 2, which is mainly related to the particle properties, material content of SHPS and the content of chloride ion in the original SHPS (the chlorine content in SHPS 3 is lower than that in SHPS 1 and SHPS 2).
Under the condition of the same total L/S ratio, the elution rate of chlorine salt by multiple water washes is 4–7% higher than that by one water wash. The main reason may be that when SHPS performs multiple water washes, the chlorine in the residual water of the FR mainly exists on the surface of the FR in the form of adsorption, and the low concentration alkali metal chloride will continue to dissolve out, resulting in a slightly higher overall removal rate of chloride ions than that of one water wash.
To ensure the removal rate of Cl and Na and reduce cost, water washing was performed three times for SHPS 1 and SHPS 2, and two times for sample 3 to effectively reduce the contents of Cl, Na and K.
3.5. Characterization of the FR obtained under the optimum conditions
In summary, based on the above analysis and combining the actual economic foundation, the optimized conditions were L/S ratio of 3 mL g−1, SHPS particle size of 100 mesh, and washing time of 10 min two or three times. Under these conditions, the removal rate of Cl, Na, Ca, K, Mg, S reached 96.64–99.68%, 97.38–99.89%, 36.40–60.37%, 49.11–54.82%, 39.18–40.22%, 36.98–42.13% respectively. And Table 4 is the main element contents of the FR under the optimum conditions.| Project | TFe | SiO2 | P | S | Cl | K2O + Na2O | Zn | Mn | Ni |
|---|---|---|---|---|---|---|---|---|---|
| a FR 1 and 2 are washed for 3 times, and FR 3 is washed for 2 times. | |||||||||
| FR 1 | 46.97 | 5.35 | 0.025 | 0.016 | 0.29 | 0.046 | 17.47 | 0.475 | 0.062 |
| FR 2 | 40.89 | 14.01 | 0.014 | 0.294 | 0.05 | 0.112 | 5.76 | 0.394 | 0.004 |
| FR 3 | 61.94 | 9.12 | 0.011 | 0.054 | 0.20 | 0.121 | 0.02 | 0.03 | 0.022 |
As seen from Table 4 that the contents of Cl, K and Na in the FR are very low, and can meet the limit requirements of GB/T 36144-2018 for Cl content and GB/T 32545-2016 for K2O + Na2O content. Conversely, the content of Fe, Zn, Mn and Ni in the FR are enriched to a certain extent, but when the FR is to be recycled into iron concentrate powder, it is also necessary to separate Zn and Fe, improve the content of Fe, and reduce the content of SiO2 and other impurities.
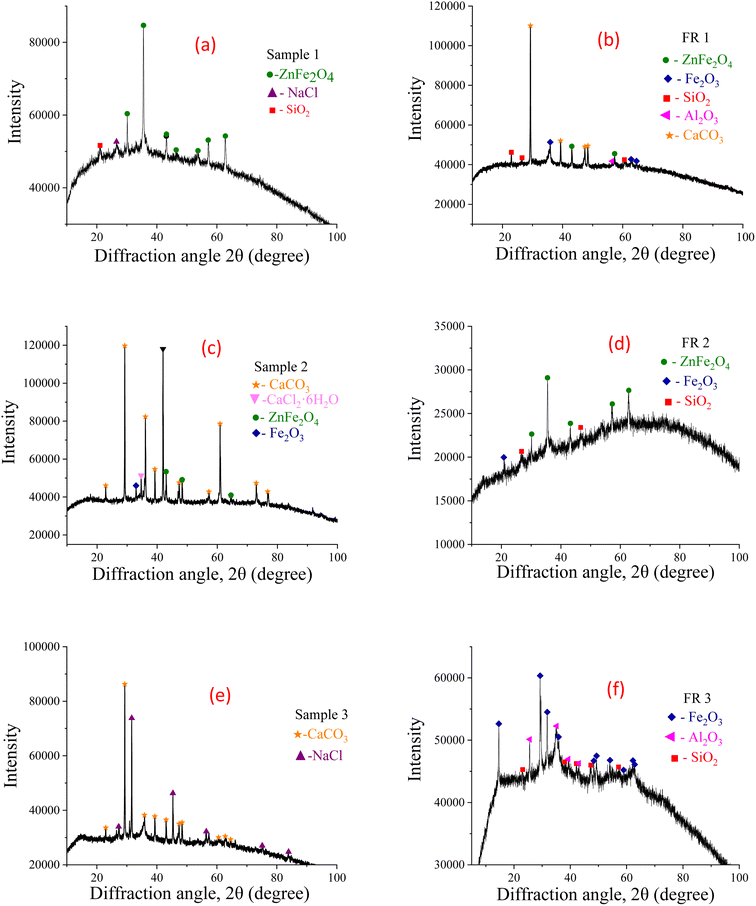
Under the optimal washing experiment operating conditions, after sieving and drying the original SHPS and the washed SHPS, the crystal phase composition of SHPS before and after the treatment is tested. The results are shown in Fig. 8. As seen from Fig. 8 that the crystalline phases that can be detected in the sample mainly include ZnFe2O4, NaCl, SiO2, CaCO3, CaCl2·6H2O, Fe2O3. This is consistent with the analysis results of the main elements in the SHPS samples in Table 3. Chlorine mainly forms water-soluble chlorine salt with alkali metal Ca and Na. The crystal phases of Fe, Zn, and Si mainly exist in the form of oxides. From the XRD spectrum of the FR from the optimized conditions, the mineral phase structure of the FR has changed significantly compared with the SHPS raw material. Firstly, no diffraction peaks of NaCl and CaCl2·6H2O were found in the FR, which indicates that the water washing dechlorination effect of SHPS is good, and most of Na, Ca and Cl in SHPS are dissolved in water; secondly, the diffraction peaks of Al2O3 appeared in the FR, and the diffraction peak intensity of CaCO3, Fe2O3 and SiO2 increased, which may be caused by the enrichment of insoluble components such as Al2O3, CaCO3 and SiO2 in the FR due to the large amount of soluble salts dissolved in water.20 And there are no diffraction peaks of low content elements such as S, P, Mn, Ni, etc. in the XRD spectra of SHPS and FR samples, due to their low content cannot be meet the detection limit of XRD.
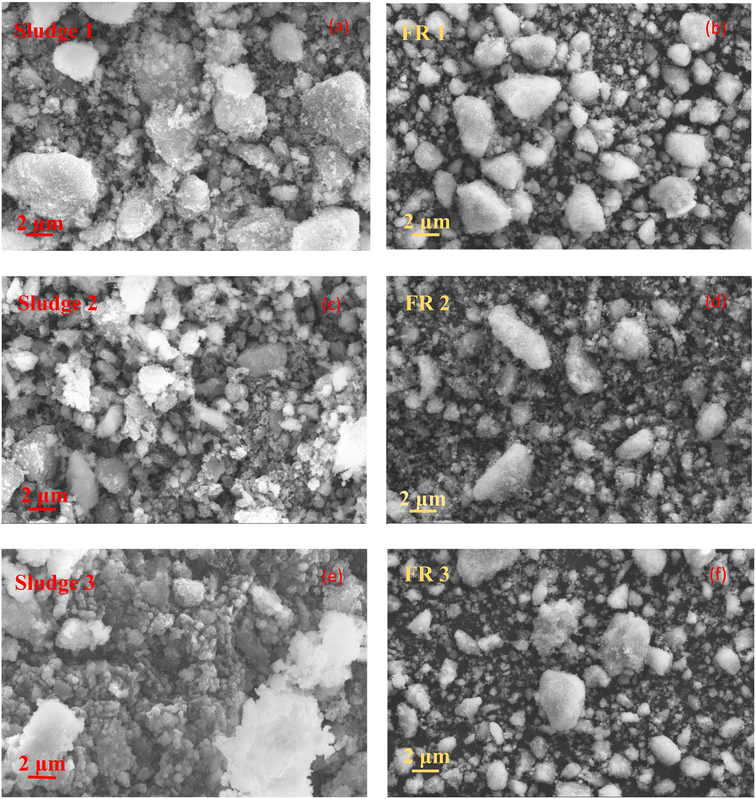
Under the optimal washing experiment operating conditions, the SEM images of SHPS before and after washing are shown in Fig. 9. As seen from Fig. 9 that the microstructure of the original SHPS is complex, the particle shape is irregular, and vary in size, with larger particles having a particle size of approximately 10 μm. The as-is the original SHPS is piled, with surface uneven and staggered, there is no fixed form and it mostly appears in the form of amorphous and polycrystalline aggregation. The particles are loosely piled up, with high porosity and large specific surface area, so soluble chloride salts and some heavy metals are easy to dissolve, which is harmful to the environment. After washing with water, most of the irregularly shaped square SHPS particles gradually transformed into regular spherical or elliptical shapes. The porosity becomes lower and the structure is more compact. Compared with the SHPS raw material, the particle size of the particles is reduced, which may be because the soluble salt on the original SHPS particles has been dissolved in water, resulting in the particle size reduction. It is also easy to observe from the SEM image of FR that the particle size of larger particles in FR has decreased to about 5 μm. The crystalline chemical substances originally attached to the surface of the SHPS particles are not reflected on the surface of the FR particles, which means that most of the salts in SHPS have been eluted after the washing treatment.
4. Conclusions
To systematically investigate SHPS chloride removal ability by water washing, the effects of the L/S ratio, SHPS particle size, washing time and washing frequency on the chloride removal rate in SHPS were investigated in this paper. Based on the test results, the following conclusions can be drawn:(1) The main chemical components of the original SHPS are ZnFe2O4, NaCl, SiO2, CaCO3, CaCl2·6H2O, Fe2O3, of which the content of Cl is as high as 5.21–12.13%. The high content of chloride salt has a serious effect on the resource utilization of SHPS. The water-washing dechlorination experimental results show that the optimum process parameters are as follows: 200 rpm, L/S ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, SHPS particle size of 100 mesh, and water washing for 10 min two or three times at room temperature. Under these conditions, a Cl removal rate of 96.64–99.68% can be reached, which proving that water-washing is a good way to remove the high concentration of chloride in SHPS. The L/S ratio, SHPS particle size, washing time and washing frequency are the main factors affecting the chloride removal rate, with the increase of L/S ratio, washing time and washing frequency, and the decrease of SHPS particle size, the dechlorination efficiency increased rapidly at first and then slowly.
1, SHPS particle size of 100 mesh, and water washing for 10 min two or three times at room temperature. Under these conditions, a Cl removal rate of 96.64–99.68% can be reached, which proving that water-washing is a good way to remove the high concentration of chloride in SHPS. The L/S ratio, SHPS particle size, washing time and washing frequency are the main factors affecting the chloride removal rate, with the increase of L/S ratio, washing time and washing frequency, and the decrease of SHPS particle size, the dechlorination efficiency increased rapidly at first and then slowly.
(2) According to the SEM images of SHPS before and after washing, it can be seen that after washing, most of the irregularly shaped square SHPS particles gradually transformed into regular spherical or elliptical shapes, and the particle size of the particles is reduced. According to the results of XRD detection, the mineral phases in SHPS mainly include ZnFe2O4, NaCl, SiO2, CaCO3, CaCl2·6H2O, Fe2O3 etc. Among them, NaCl and CaCl2·6H2O are soluble chloride salts and are easily soluble in water. After the water washing treatment, the content of chloride salt in SHPS is significantly reduced, and the diffraction peaks of Al2O3 appeared in the FR, and the diffraction peak intensity of CaCO3, Fe2O3 and SiO2 increased.
(3) After dechlorination the element composition of FR is transformed to the direction of beneficial resource utilization, the Fe, Zn, Mn, Ni and other elements are enriched. SHPS water washing dechlorination process is an effective pretreatment method for SHPS resource utilization. The process has strong applicability and operability, which provides a guarantee for the smooth development of subsequent SHPS resource utilization.
Abbreviations
| SHPS | Steel hydrochloric acid pickling sludge |
| FR | Filter residue |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Plan (2019YFC1904600), the Guizhou Provincial Key Laboratory of Coal Clean Utilization (qiankehepingtairencai [2020]2001), Liupanshui Science and Technology Plan Project (52020-2022-PT-04, 52020-2023-0-2-4), the Liupanshui Normal University high-level talent research project (LPSSYKYJJ202315), the Discipline Team of Liupanshui Normal University (LPSSY2023XKTD07), the Key Cultivation Disciplines of Liupanshui Normal University (LPSSYZDXK202001, LPSSY2023XKTD07), the Youth Talent Growth Project of Educational Department of Guizhou Province (qian jiao he KY [2022]044), and the Foundation of Liupanshui Normal University (LPSSYKYJJ202102). The authors express their thanks to all the scientists associated with this article for their encouragement and assistance.References
- Y. E. Xu, Y. C. Zhang, Y. F. Shu, H. Y. Song, X. Q. Shu, Y. X. Ma, L. L. Hao, X. Z. Zhang, X. L. Ren, Z. P. Wang and X. L. Zhang, ACS Omega, 2022, 7, 13826–13840 CrossRef CAS PubMed.
- Y. E. Xu, Y. F. Shu, Y. C. Wang, X. L. Ren, X. Q. Shu, X. Z. Zhang, H. Y. Song, H. X. Zhou, L. W. Dai, Z. P. Wang, X. Yuan and H. Y. Zhao, ACS Omega, 2022, 7, 17963–17975 CrossRef CAS PubMed.
- B. B. Fang, Z. Chu, Y. Yang, X. Y. Sun, W. P. Huang, X. F. Li and L. J. Wang, Adv. Mater. Res., 2013, 726–731, 2130–2134 Search PubMed.
- F. M. Pinto, R. A. Pereira, T. M. Souza, A. A. Saczk and Z. M. Magriotis, J. Environ. Manage., 2021, 280, 111706 CrossRef CAS PubMed.
- Ministry of Ecology and Environment of the People's Republic of China, China National List of Hazardous Waste (2021 Version), Order No. 15 of the Ministry of Ecology and Environment, Ministry of Ecology and Environment of the People's Republic of China, Beijing, China, 2020 Search PubMed.
- X. M. Li, S. J. Wang, J. X. Zhao, Y. R. Cui and S. B. Hou, Adv. Mater. Res., 2011, 194–196, 2072–2076 CAS.
- S. R. Zhang, X. G. Jiang, B. X. Liu, G. J. Lv, Y. Q. Jin and J. H. Yan, Energy Fuels, 2017, 31, 3019–3028 CrossRef CAS.
- J. X. Zhao, Z. Y. Zhao, R. M. Shi, X. M. Li and Y. R. Cui, JOM, 2018, 70, 2825–2836 CrossRef CAS.
- T. T. Liu, T. Zhao, J. Wang, Z. C. Huang and H. H. Fu, J. Environ. Eng. Technol., 2021, 11, 1027–1033 Search PubMed.
- W. Tang, L. Y. Tang, G. Zhao, W. P. Luo and Y. H. Zhou, Met. Mater. Metall. Eng., 2020, 48, 24–31 Search PubMed.
- B. Pradhan, Constr. Build. Mater., 2014, 72, 398–410 CrossRef.
- F. Zhu, M. Takaoka, K. Shiota, K. Oshita and Y. Kitajima, Environ. Sci. Technol., 2008, 42, 3932–3937 CrossRef CAS PubMed.
- Y. Chin, C. Lin, G. P. Chang-Chien and Y. M. Wang, Aerosol Air Qual. Res., 2012, 12, 228–236 CrossRef CAS.
- State Administration for Market Regulation and Standardization Administration of China, Limits on Element Content of Lead, Arsenic, Cadmium, Mercury, Fluorine, and Chlorine in Iron Ore, Chinese Standard GB/T36144-2018, State Administration for Market Regulation, Standardization Administration of China, Beijing, China, 2019 Search PubMed.
- General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China and Standardization Administration of China, Classification of Iron Ore Product Grades, Chinese Standard GB/T32545-2016, General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, Standardization Administration of China, Beijing, China, 2017 Search PubMed.
- X. N. Wang, M. L. Wang, D. Z. Zou, C. F. Wu, T. Li, M. Gao, S. Liu, Q. H. Wang and T. Shimaoka, Chemosphere, 2020, 261, 127754 CrossRef CAS PubMed.
- L. Li, T. Jiang, B. J. Chen and L. Wen, J. Sustain. Metall., 2021, 7, 1116–1127 CrossRef.
- B. B. Wang, Z. Q. Li, L. B. Zhang, J. H. Peng, J. Li, S. H. Yin and A. Y. Ma, J. Microwave Power, 2014, 48, 233–243 Search PubMed.
- W. S. Chen, F. C. Chang, Y. H. Shen, M. S. Tsai and C. H. Ko, J. Hazard. Mater., 2012, 237–238, 116–120 CrossRef CAS PubMed.
- S. Dontriros, S. Likitlersuang and D. Janjaroen, Waste Manage., 2020, 101, 44–53 CrossRef CAS PubMed.
- X. B. Gao, W. Wang, T. M. Ye, F. Wang and Y. X. Lan, J. Environ. Manage., 2008, 88, 293–299 CrossRef CAS PubMed.
- H. B. Guo, R. J. Li, J. H. Peng, G. G. Yan and W. L. Sun, Sustainable Min. Metall., 2021, 4, 49–53 Search PubMed.
- C. Q. Wu, S. Y. Cai, Q. Shao, X. L. Lv and L. Xu, China Water Wastewater, 2020, 22, 152–155 Search PubMed.
- X. X. Wang, A. M. Li and Z. K. Zhang, Procedia Environ. Sci., 2016, 31, 440–446 CrossRef.
- X. F. Chen, Y. F. Bi, H. B. Zhang and J. Wang, Procedia Environ. Sci., 2016, 31, 560–566 CrossRef.
- H. Huang, W. Liu, L. Zhang, J. M. Fang, F. Xu, S. Bu, W. G. Xu, C. Xu, H. Q. Yao and Z. L. Ma, Environ. Sci. Pollut. Res., 2022, 29, 36208–36215 CrossRef CAS PubMed.
- Y. L. Wang, Y. Deng, H. N. Zhang, W. Zhang and S. F. Zhang, Environ. Pollut. Control, 2020, 42, 1259–1262 Search PubMed.
- L. Wang, Y. Jin, Y. Nie and R. Li, Resour., Conserv. Recycl., 2010, 54, 1428–1435 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra05451a |
| This journal is © The Royal Society of Chemistry 2024 |