Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of anti-depressant molecules via metal-catalyzed reactions: a review
Aqsa Kanwala,
Uzma Afzala,
Muhammad Zubaira,
Muhammad Imran b and
Nasir Rasool
b and
Nasir Rasool *a
*a
aDepartment of Chemistry, Government College University Faisalabad, 38000, Pakistan. E-mail: aqsa6373@gmail.com; uzmaafzal520@gmail.com; zubairmkn@gcuf.edu.pk; nasirrasool@gcuf.edu.pk; Tel: +92-3085448384
bChemistry Department, Faculty of Science, King Khalid University, P.O. Box 9004, Abha 61413, Saudi Arabia. E-mail: imranchemist@gmail.com
First published on 26th February 2024
Abstract
Depression is one of the most mutilating conditions in the world today. It has been difficult to make advancements toward better, more effective therapies since the introduction of antidepressant medicines in the late 1950s. One important field of medicinal chemistry is the synthesis of antidepressant molecules through metal-catalyzed procedures. The important role that different transition metals, including iron, nickel, ruthenium, and others, serve as catalysts in the synthesis of antidepressants is examined in this review. Key structural motifs included in antidepressant drugs such as tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), and others can be synthesized in a variety of effective ways using metal-catalyzed steps. This review examines current developments in the catalytic synthesis of antidepressants and their potential application over the previous thirteen years.
Depression, the third major global health concern, is anticipated to escalate to the second most significant health challenge worldwide by 20301–3 According to WHO, 3.8% of the world's population is affected by depression; this includes 5% of adults (4% of men and 6% of women) and 5.7% of persons sixty years of age and over. The prevalence of depression affects roughly 280 million people worldwide. A 2011 survey conducted by the World Mental Health Survey across 17 countries revealed that one in 20 individuals went through a depressive episode. Depression can inflict significant distress, resulting in disability and even death for the affected individual. On a global scale, an estimated 700
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 annual suicide deaths are linked to depression.4,5 Depressive disorders still have a restricted range of treatments.6 Thus, it is particularly essential to develop novel antidepressants that have a quick onset, low side effects, with enhanced cognitive function. A significant area of study in the discipline is the development of novel dual- or multi-target antidepressants.7–9
000 annual suicide deaths are linked to depression.4,5 Depressive disorders still have a restricted range of treatments.6 Thus, it is particularly essential to develop novel antidepressants that have a quick onset, low side effects, with enhanced cognitive function. A significant area of study in the discipline is the development of novel dual- or multi-target antidepressants.7–9
Antidepressants have shown effectiveness in alleviating symptoms and enhancing the quality of life for individuals with moderate to severe depression. Approximately 50–60% of people with depression experience substantial improvement when using these medications.10 Depression is a common mood syndrome triggered by the improper release of monoamine neurotransmitters as noradrenaline, dopamine also serotonin in the CNS with the malfunction of noradrenergic, dopaminergic, and serotonergic systems.11–14
Anti-depressants are psychotropic drugs, primarily utilized to treat mental diseases characterized by depressed mood. They also can reduce nervousness, somatic symptoms, and anxiety. Tricyclic antidepressants (amitriptyline, imipramine, and nortriptyline), as well as oxidase inhibitors (such as moclobemide and phenelzine), and SSRIs, (such as citalopram, fluoxetine, and paroxetine), as well as SNRIs, (such as reboxetine), and reuptake inhibitors of serotonin–norepinephrine (desvenlafaxine and venlafaxine),15,16 and herbal remedies (St. John's Wort),17 tetracyclic antidepressants (mirtazapine) are all types of antidepressant medications that raise the levels of several monoamines in the synaptic clefts.18 Here's a concise way to summarize in Table 1.
| Classification of antidepressants | FDA-approved antidepressant drugs | Mechanism of action | References |
|---|---|---|---|
| Selective serotonin reuptake inhibitors (SSRIs) | Paroxetine, fluoxetine, sertraline, escitalopram | SSRIs, function by impeding the serotonin reuptake in the brain. By inhibiting the serotonin transporters, SSRIs prolong the presence of serotonin in the synaptic space between neurons. This prolonged serotonin activity enhances its effects on mood regulation and neuronal communication, potentially mitigating symptoms of depression and related mood disorders | 19 and 20 |
| Serotonin-norepinephrine reuptake inhibitors (SNRIs) | Milnacipran, venlafaxine, duloxetine, reboxetine | SNRIs, work by blocking the reuptake of both serotonin and norepinephrine in the brain. By inhibiting the transporters responsible for reabsorbing these neurotransmitters, SNRIs increase their availability in the synaptic space between neurons | 21 and 22 |
| Tricyclic antidepressant | Aripiprazole, desipramine, clomipramine | TCAs raise the levels of these neurotransmitters in the brain and prevent neurons from reabsorbing 5HT and NA by axonal absorption. These drugs are generally used for people suffering from migraine and chronic pain | 23 and 24 |
| Monoamine oxidase inhibitors | Toloxatone, diclofensine, selegiline | MAO inhibitors prevent the breakdown of serotonin, dopamine, and norepinephrine by binding to monoamine oxidase, elevating their levels in nerve endings. This action sustains these key neurotransmitters, potentially aiding antidepressant effects by prolonging their activity in the central nervous system | 25 and 26 |
| Atypical antidepressants | Rolipram, vilazodone, vortioxetine, lumateperone, agomelatine | These medications target different neurotransmitter systems, such as dopamine, norepinephrine, and serotonin, but their exact mechanisms can differ | 27 |
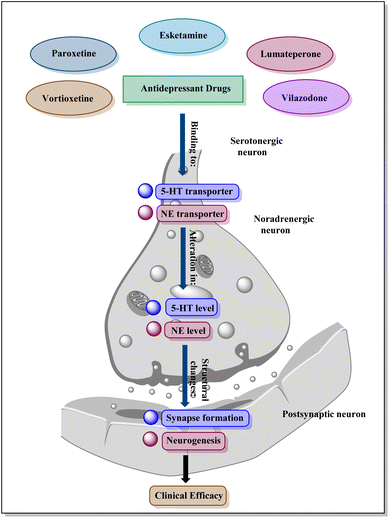
Antidepressants work through a variety of key receptors and neurotransmitter systems: SSRIs and some SNRIs primarily boost serotonin levels by affecting 5-HT receptors; others like SNRIs and TCAs target norepinephrine, impacting adrenergic receptors (Fig. 1).28
Atypical antidepressants like bupropion focus on dopamine reuptake. Ketamine influences glutamate receptors, particularly NMDA receptors, for its rapid-acting effect. While not directly targeted, GABA and cannabinoid receptors may be indirectly affected by antidepressants. Antidepressants often impact BDNF levels, influencing neuroplasticity and neuronal survival through TrkB receptors.29 These receptors and associated neurotransmitter systems are targeted by various classes of antidepressants, collectively impacting mood, emotions, and brain function to alleviate symptoms of depression.30,31
Metal-catalyzed transformations have been employed in the synthesis of antidepressants at several sites along the pathway, leading to the development of C–C, and C–N bonds and the functionalization of aromatic rings.32 In particular, SSRIs, including sertraline and fluoxetine, which are extensively used to treat depression, have been synthesized via couplings catalyzed by palladium.16,33
Moreover, the synthesis of other kinds of antidepressants, MO inhibitors, and tricyclic antidepressants has also been accomplished via metal-catalyzed reactions.34 These crucial compounds have been synthesized in vast quantities owing to the development of more effective and environmentally friendly synthetic pathways, which were greatly facilitated by the use of metal catalysts.35
Metal-mediated reactions have become integral in drug synthesis due to their adaptability, selectivity, mild reaction conditions, and compatibility with complex molecules, contributing significantly to the pharmaceutical industry's synthetic capabilities.36–40
This review provides a comprehensive analysis of synthetic pathways for a range of metal-catalyzed antidepressants, commercially available medications, and bioactive compounds with antidepressant properties. It offers valuable insights for synthetic chemists and pharmacists, elucidating the utilization of various metals and their complexes across different methodologies. By encompassing a broad spectrum of compounds, this review aims to enhance understanding within the field, serving as a guide for future chemists seeking to leverage these methodologies effectively.
1. Ruthenium-catalyzed reactions
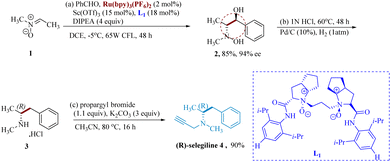
Selegiline when employed with L-DOPA is a highly successful treatment for both Parkinson's along Alzheimer's disease. It is a monoamine oxidase-B (MOB) antagonist that is specific and irreversible.41,42 Independent of MAO inhibitors, the propargylamine pharmacophore of selegiline and analogous drugs also seems to possess neuroprotective effects.43Ye et al. undertook the synthesis of selegiline using Ru photocatalyst & chiral N,N′-dioxide coordinated unique earth ion L1 which work synergistically to initiate the photocatalytic enantio-selective reductive coupling of aromatic aldehydes with nitrones. The asymmetric radical formation is sparked by chiral Lewis acid, which serves as a crucial framework for assembling the essential precursor and produces enantiopure vicinal hydroxyl amino alcohols in good to outstanding yields exhibiting great stereo-selectivity. Here, Sc(OTf)3 serves as the Lewis acid & Ru(bpy)3(PF6)2 (photocatalyst). Asymmetric reductive coupling of benzaldehyde with nitrone 1 gave the product 2 as the key diastereomer 11/1 dr with 94% enantiomeric excess (ee). Additionally, ®-methamphetamine hydrochloride 3 was produced by dehydroxylating vicinal hydroxyamino alcohol 2 in an aq. HCl at moderate Pd/C-catalyzed hydrogenolysis. Crude 3 was N-propargylated with K2CO3 in acetonitrile to get (−)-selegiline 4 (Scheme 1).
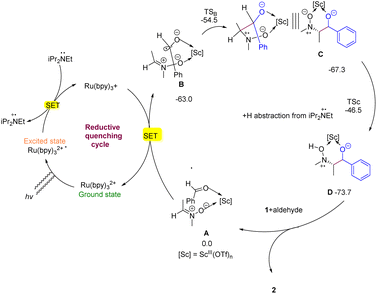
After Ru(bpy)32+ is photoexcited and reductively quenched by DIPEA, [iPr2(Et)N˙]+ and Ru(bpy)3+ (E1/2II/I = 1.33 V vs. SCE in MeCN) are generated. This is sufficiently to reduce complex A via intermolecular SET (onset potential Eop > −0.5 V vs. SCE) and yield the radical complex B. Indeed, DFT calculations confirmed that the electron affinity of A is much higher (∼63.0 kcal mol−1 in free energy) than that of nitrone 1 (∼23.4 kcal mol−1) and 4-fluorobenzaldehyde (∼45.1 kcal mol−1) in solvent, and the as-generated cross-coupling precursor B has spin density localized predominantly on the aldehyde moiety. Subsequently, N-radical intermediate C (or C′ of anti-configuration) is formed through an analogous 6-endo-trig radical annulation, and the transition state TSB leading to a syn-configuration is predicted to be by 1.9 kcal mol−1 favored over the anti-configuration transition state TSB′, C upon hydrogen abstraction from [iPr2(Et)N˙]+ affords regioselectively intermediate D (via TSC) other than D′ (via TSC′). Finally, protonation of D gives the desired vicinal hydroxyamino alcohol 2 as a major diastereomer. Moreover, DFT calculations also showed that the formation of cross-coupling precursor B is overwhelmingly favored over the formation of homocoupling precursors, accounting well for the reaction specificity towards cross-coupling rather than homocoupling. Based on this mechanism, the diastereoselectivity of vicinal hydroxyamino alcohols, such as 2, can be analyzed by comparing the energy of the six-member ring transition state TSB with that of TSB′. Chiral scandium complex I, which involves a Re-to-Re-facial assault of the ketyl radical to nitrone 1, exhibits enantioselectivity (Fig. 2).44,45
In medicinal chemistry, the polyethylene glycol scaffold has gained much significance. Rossi et al. described the hydrogen borrowing reductive amination method of PEG functionalization of amines was described. This was achieved by reacting the phosphorus-containing dppf or DPE with the catalyst [Ru(p-cymene)Cl2]2 to produce a range of 1° and 2° amine products. They were able to directly produce quetiapine 6 from 11-(piperazine-1-yl)-dibenzo[b,f][1,4]thiazepine 5 in 62% isolated yield (Scheme 2).46
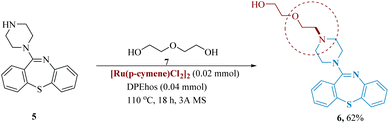 | ||
| Scheme 2 Synthesis of 2-(2-(4-(dibenzo[1,4]thiazepin-11-yl)piperazin-1-yl)ethoxy)ethan-1-ol (quetiapine). | ||
Recently, ketamine & its (S)-enantiomer, esketamine, were investigated for their immediate anti-depressant effects and have been proposed as a potential medication for depressive disorder, as well as resistant depression.47 In vitro, (S)-ketamine (esketamine) has a 3–4 fold higher affinity than (R)-ketamine for the glutamate N-methyl D-aspartate receptor.48 Esketamine has attracted more interest in the advancement of an antidepressant drug in short-term treatment.49,50 Chen & Lu synthesized Ketamine which primarily functions as a non-competitive NMDA receptor antagonist.51,52
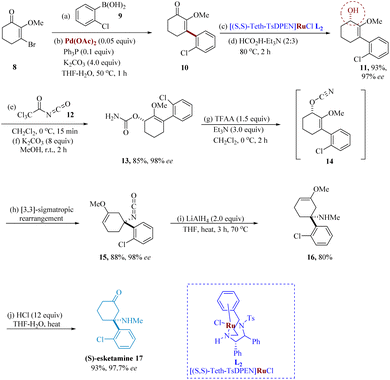
The Noyori catalytic AH of enone 10 was used to set up the stereogenic center in 11 and the [3,3]-sigmatropic re-arrangement of the allylic cyanate intermediate 14 to generate the quaternary stereogenic center in isocyanate 15 with exceptional stereochemical relay were two features of the small-scale asymmetric synthesis of esketamine. For the asymmetric reduction of enone 10, several ruthenium-based catalysts were investigated; however, at 0.1% loading, only [(S,S)-Teth-TsDPEN] RuCl afforded full conversion in 97–98% ee (Scheme 3).53,54
Other pharmaceutically useful substances, NK 1-receptor antagonist, antifungal amorofine, and SCH50911 GABA-antagonist, contain reboxetine, which is a SNRI.55
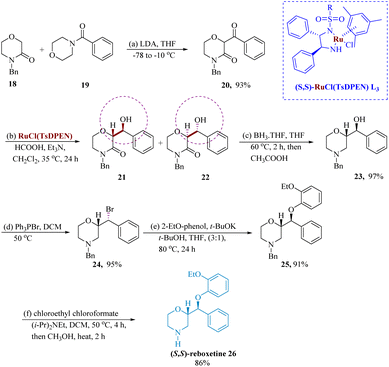
Son & Lee developed the dynamic kinetic resolution-mediated asymmetric transfer hydrogenation (ATH) of 2-benzoyl morpholine-3-ones served as a crucial step in the stereoselective synthesis of reboxetine 26. With a 93% yield, the N-benzyl-2-aroylmorpholin-3-one 20 was produced when the N-benzyl-3-morpholinone 18 was condensed with N-aroylmorpholines 19 in the presence of LDA. The alcohols (2R,3S)-21 and (2S,3R)-22 were produced in a combined yield of 90% by the ATH reaction of 20 with catalyst (S,S)–RuCl(TsDPEN) L3, which was mediated by dynamic kinetic resolution. After being reduced by BH3 THF, the lactam 21 produced the corresponding morpholine benzyl alcohol 23 in 97% yield, which was then processed by Ph3PBr2 to produce the respective morpholine bromide derivatives 24 in 95% yield. In the presence of t-BuOK, molecule 24 underwent bromide displacement with 2-ethoxyphenol to generate the N-benzyl-protected derivatives 25 91% of the time. After being treated with chloroethyl chloroformate and methanolysis, the compound 25 synthesized the target molecule (S,S)-reboxetine 26 with an 86% yield (Scheme 4).56
An SSRI antidepressant, nor-sertraline is a sertraline analog. Thalen et al. developed a new pathway to 33 using CALB, isopropyl acetate, and Na2CO3 in toluene and readily available 1, 2, 3, and 4-tetrahydro-1-naphthyl amine 27 and DKR of primary amines was developed. To start, the standard procedure was used to apply DKR to achieve 28 in 70% yield and 99% ee. KMnO4 had an impact on oxidation at the C-4 position to produce 29. Following the formation of the enolate and its trapping with N-phenyl-bis(trifluoromethanesulfonimide), the resultant compound 30, reacted with 3, 4-dichlorophenyl boronic acid to generate 31 with a 96% yield and 99% ee. It was possible to obtain 32 in 95% with a trans/cis ratio of >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using trans selectivity hydrogenation with the Crabtree catalyst. Following the deprotection of the acetamide in an acidic environment, nor-sertraline 33 produced a yield of 95% with full retention of dr and ee (99% ee, and trans/cis >99
1 using trans selectivity hydrogenation with the Crabtree catalyst. Following the deprotection of the acetamide in an acidic environment, nor-sertraline 33 produced a yield of 95% with full retention of dr and ee (99% ee, and trans/cis >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Scheme 5).57
1) (Scheme 5).57
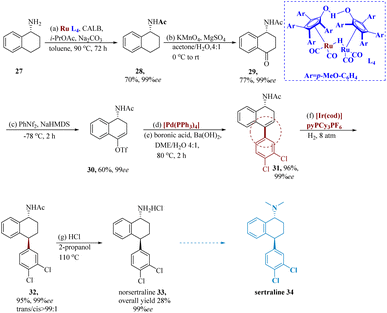 | ||
| Scheme 5 Synthesis of 4-(3,4-dichlorophenyl)-1,2,3,4-tetra hydro-naphthalene amine hydrochloride (norsertraline). | ||
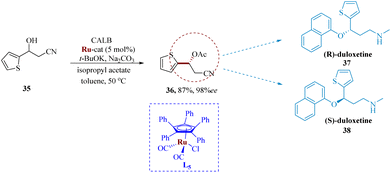
Träff et al. reported the lipase-catalyzation via kinetic resolution of a racemic β-hydroxy nitrile leading to the stereo-inversion to produce the eutomer (S)-duloxetine through Mitsunobu yields an enantiopure R diastereomer of duloxetine.58 By using the DKR process, the yields were significantly improved. Candida antarctica lipase as well as ruthenium catalyst (Shvo's catalyst) L5, were employed in the DKR of the starting molecule β-hydroxy nitrile 35 to produce the analogous β-cyano acetate 36 in yield of 87% & 98% ee. The production of both (R)-37 as well as (S)-duloxetine 38 was made possible by subsequent synthetic procedures (Scheme 6).59
2. Iron-catalyzed reactions
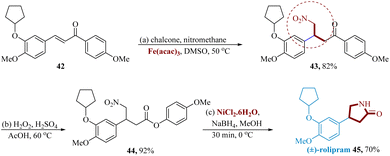
Allen et al. reported the synthesis of moclobemide 41 using Fe (NO3)3·9H2O, a low-cost catalyst, in a modest isolated yield by using readily available starting materials such as nitrile 39 and amine 40. When primary unbranched amines combine with nitriles that are not excessively electron-rich, the reaction is most favoured (Scheme 7).60Chopadea et al. reported an effective and convenient procedure for the Michael addition using Fe(acac)3 (5 mol%) as an effective catalyst, which catalyzes the Michael addition reaction of nitromethane to chalcone to produce corresponding γ-nitroketone derivatives with good yields under milder conditions.
Chalcone 42 as the starting material was added to iron-catalyzed Fe (acac)3 (5 mol%), and Michael's addition of nitromethane produced γ-nitro ketone 43 in 82% of the reactions. The Baeyer–Villiger reaction 43 using H2O2 in AcOH provided the analogous γ-nitro ester 44. Following a Ni-catalyzed reduction of the NO2 using sodium borohydride in combination with nickel chloride to generate cyclic amide as (±)-rolipram 45 in 3 steps with a total yield of 52.16%. (±)-Rolipram 45 acts as a neurotransmitter inhibitor drug molecule (Scheme 8).61,62
3. Nickel-catalyzed reactions
Illudalic acid is “the first potent, selective” MAOI, with an IC50 value of 18 ± 7.1 M in initial testing. Gaston et al. reported the alkaloid network that enables the formation of illudalic acid is illudinine. The synthesis of illudinine begins with known diyne 48, produced from isophorone 46 that underwent Eschenmoser–Tanabe fragmentation, was subsequently lithiated at a terminal (≡)-bonds and carboxylated to diester 49. Under MW irradiation in C6H5CH3 for two minutes at a power of 300 W, this molecule 49 was directed with alkyne 50 via a Ni(CO)2 (PPh3)2-catalyzed [2 + 2 + 2] cyclo-trimerization. The alkyne 50 was chosen as the PMB group may be effectively eradicated in an oxidation step to complete the whole synthesis. The cyclotrimerization product 51 was separated in an 84% yield. The phenolic OH was effectively introduced at C-7 in the subsequent steps. Remarkably, the 3° alcohol 52 was the single product produced at 84% when 51 was treated with an excess of CH3Li in CeCl3. Treatment with BF3OEt2/H2O2 in DCM at 0 °C mediates the successive carbenium ion rearrangement of 52 to 53. A 92% yield of phenol 53 is produced when the Boc group is simultaneously removed, and this prepares the way for the traditional Pictet–Spengler reaction to assemble the tricyclic skeleton. The corresponding tetrahydro iso-quinoline is produced by treating 53 with formaldehyde along with a sodium acetate buffer, and it is then immediately transformed to the methyl ether 54, treated with trimethylsilyl diazomethane (66% over two steps). Pd/C in mesitylene was used to oxidize 54 to iso-quinoline 55 at a temperature of 185 °C. In a combined yield of 58%, these conditions result in the simultaneous exclusion of the PMB-protecting group. This extremely selective and converged total synthesis of illudinine 56 is finished by quantitatively saponifying the ester by 40% aq. KOH in EtOH/H2O has a ratio of (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) (Scheme 9).63
5) (Scheme 9).63
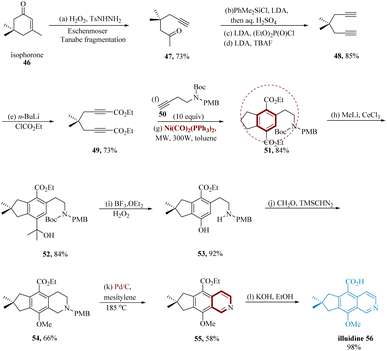 | ||
| Scheme 9 Synthesis of 9-methoxy-7,7-dimethyl-7,8-di-hydro-6H-cyclo penta iso-quinoline-5-carboxylic acid (illuidine). | ||
Serotonin reuptake and other anxiety-related illnesses are specifically blocked by sertraline hydrochloride.64 Sertraline 145 has one or more asymmetric centers, and as a result, the naturally dynamic 1S,4S-enantiomer, and sertraline, must be produced with great optical purity.
Poremba et al. developed Ni-catalyzed enantio-selective reductive coupling that produces 1,1-diarylalkanes with increased yields and enantioselectivity, using 4-heptyl-BiOX L6. Chiral tetrahydronaphthalene 149 is produced in 70% yield and 84% ee by cross-coupling 1-chloro-1,2,3,4-tetrahydro-naphthalene 57 with widely accessible iodobenzene 58. Tetralone 60 was produced in 51% yield by the benzylic oxidation of 59 utilizing CrO3 having 3 equiv. in AcOH/H2O. Tetralone 60 and N-methyl hydroxylamine are condensed to form nitrone 61, whose reduction yields the required amines (sertraline) 34 (Scheme 10).65
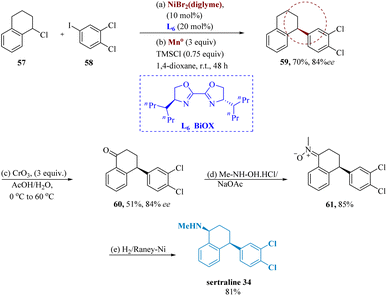 | ||
| Scheme 10 Synthesis of 4-(3,4-dichlorophenyl)-N-methyl-1,2,3,4-tetrahydro naphthalene amine (sertraline). | ||
Shen et al. described new lactam-fused chroman compounds with dual affinities for the 5-HT1A as well as the serotonin transporter.66,67 An effective pathway to intermediates 66 was necessary for the formation of des-fluoro lactam-chroman amines 76. Under the specified conditions, the aryl halide 63 was converted to the toluene derivative 64 at the start of the reaction. Aryl bromide and alkyl zinc undergo a cross-coupling that is accelerated by a transition metal. Bis-(triphenylphosphine) nickel(II) dichloride catalyzed the reaction of 63 at 50 °C using dimethylzinc in DMF, resulting in 64. The production of the five-membered lactam 66, which results in the regioselective isomer 74, was eventually made possible by the production of 64. Reductive amination was the strategy they had in mind for the synthesis of indoles to produce the necessary end products. The penultimate secondary amines 76 of new lactam-fused chroman compounds, primarily compound 77/78 having dual affinity at the 5-HT1A as well as the serotonin transporter in vitro cAMP turnover model, were produced by reductive amination of lactam-fused chroman amines via indole-substituted alcohols 75 (Scheme 11).68
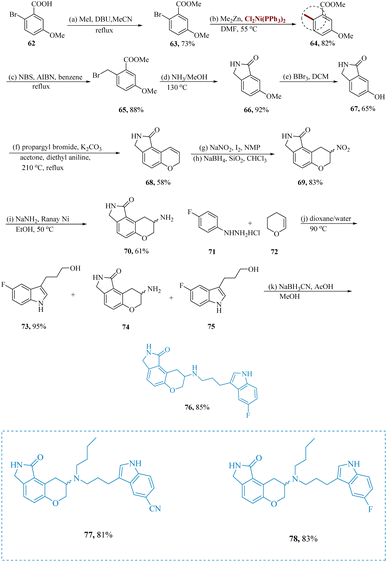 | ||
| Scheme 11 Synthesis of 8-((3-(1H-indol-3-yl)propyl)amino)-2,3,8,9-tetrahydro-pyrano[3,2-e]isoindol-1(7H)-one. | ||
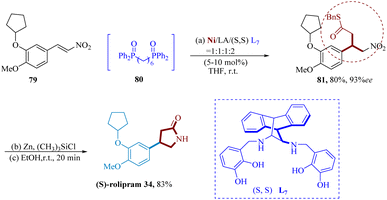
Furutachi et al. reported A hetero-bimetallic Ni/La-salan 2d complex of phosphine oxide was used in the catalytic decarboxylative 1,4-addition to 4-MeO-3-cyclopentyloxyC6H3-substituted nitro-alkene 79, which produced product 81 in 80% yield and 93% ee. By treating 81's nitro group with Zn and (CH3)3SiCl, the nitro group was converted to an amine, and subsequent cyclization occurred during workup to produce (S)-rolipram 34 in yield of 83% (Scheme 12).69
Bioactive compounds, pharmaceuticals, and organic functionalized materials all frequently contain C(sp2)–S bonds.70–75 As a result, metal-catalyzed C(sp2)–S formation is now receiving more and more attention.76–79 The direct C(sp2)–H thiolation of amides and sulfides was effectively used as the crucial step in the formation of quetiapine.
Li & wang reported the synthesis of quetiapine an atypical antipsychotic drug that has been licensed for treating bipolar disorder and schizophrenia.80,81 For accessing quetiapine this synthetic approach was constructed using direct C–H thiolation of benzamides as opposed to the conventional synthetic procedure, which involved cross-coupling of benzenethiols and aryl halides.80,82,83 The anticipated product 83 on a gram scale was produced by thiolating 1,2-diphenyldisulfane with 2-benzamidopyridine-1-oxide 82. Then, by hydrolyzing the amide, a derivative of benzoic acid 84 was produced. After that, the compound 84 underwent Curtius rearrangement and was treated with phenols to produce carbamate 85. After that, polyphosphoric acid facilitated cyclization, which was then followed by pseudohalogenation to produce triflate 86. Finally, the nucleophilic substitution of the piperazine 87 with triflate 86 led to the desired Quetiapine 88 product (Scheme 13).
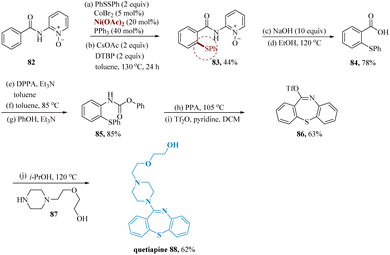 | ||
| Scheme 13 Synthesis of 2-(2-(4-(dibenzo[1,4]thiazepin-11-yl)piperazin-1-yl)ethoxy)ethan-1-ol (quetiapine). | ||
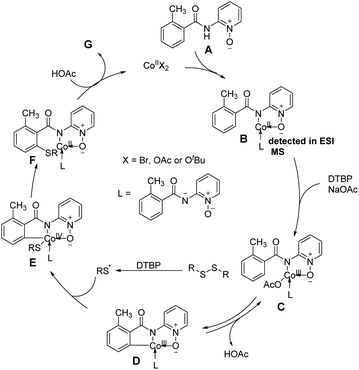
Based on the above-mentioned mechanistic investigations a proposed mechanism is illustrated in Fig. 3. Initially, the coordination of benzamide A with a cobalt(II) catalyst and a subsequent ligand exchange brought intermediate B, which was detected by ESI-MS. Next, the cobalt(II) complex is oxidized by DTBP yield cobalt(III) intermediate C, which undergoes a reversible C–H cobaltation to afford cobaltacycle species D. Subsequently, radical coupling of intermediate D with the thioether radical yielded from disulfides in the presence of DTBP provides cobalt(IV) intermediate E. Finally, reductive elimination of E following protonation furnishes the desired product G and cobalt(II) catalyst to finalized the catalytic cycle. The rate-determining progression could be the reductive elimination of intermediate E (Fig. 3).83
4. Palladium catalyzed reactions
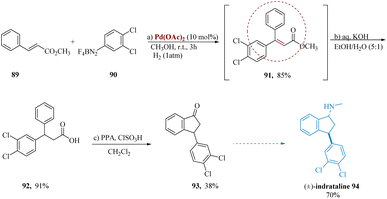
Indatraline is a potential psychoactive complex with significant binding as well as inhibitory activity aimed at monoamine reuptake neuronal sites, together with serotonin transporters & dopamine.84,85 Behavioral assays and in vivo, dialysis have suggested that indatraline has a strong dopaminergic mode of action with a prolonged half-life.86,87 Additionally, indatraline was found to decrease the self-administration of cocaine in monkey trials.88Pastre & Correia developed a synthesis of the psychoactive drug indatraline. To get a saturated β,β-di arylated product 91 with 85% yield, methyl cinnamate 89 was treated to Heck arylation with 3,4-dichloro benzene-diazonium tetra-fluoroborate 90, as it was accompanied through an in situ catalytic hydrogenation of adduct 91. Due to the generation of side products during the dehalogenation process, control of a hydrogenation phase is important. Next, the ester 91 was hydrolysed through aqueous KOH to produce the analogous acid 92 in a 91% yield. Successive cyclization via PPA and/or ClSO3H produced the well-known intermediate 93 of (±)-indatraline 94 in yields of 38% & 70% (Scheme 14).89,90
2-Oxazolidinones have attracted considerable attention owing to their significant heterocyclic scaffolds having a diverse range of pharmacological activities. Oxazolidinones are a significant class, naturally existing substances and promising medicinal frameworks with a variety of biological and pharmacological activities, including antimicrobial, antibacterial, antidepressant, anti-Parkinson's, anticancer, and anti-HIV activity.91,92 The usage of 2-oxazolidinones as chemical precursors in organic synthesis is also very common. Consequently, the synthesis of such therapeutic heterocyclic compounds has received a lot of attention.
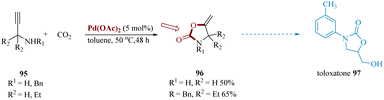
Arshadi et al. synthesized toloxatone with the brand name Humoryl, 33 is an antidepressant drug that is marketed in several different countries comprising 2-oxazolidinones moieties as a structural unit. It functions as a reversible selective MAO-A (RIMA) inhibitor.93 These valuable heterocyclic composites are also widely used in the construction of organic compounds.94
Palladium-catalyzed carboxylation of secondary α,α-disubstituted N-propargyl amines 95 with CO2 produced the highly substituted 2-oxazolidinones 96. Pd(OAc)2, finest effective for the conversion, including Pd2(dba)3, Pd(PPh3)4, NiBr2(PPh3)2, IrCl(CO) (PPh3)2 and RuH4(PPh3)2. Substituted oxazolidinones 96 were then converted to toloxatone 97 over subsequent steps (Scheme 15).95
Among the most effective tricyclic antidepressants for blocking serotonin and norepinephrine reuptake is clomipramine. Although it raises the risk of seizures at high dosages, it has been proven to be useful in curing obsessive-compulsive disorder.
Because of their remarkable biological activity, 5H-dibenzo[b,f]azepines are the primary pharmacophore in clomipramine.96–98 Casnati et al. reported the formal synthesis of clomipramine used commercially accessible 4-bromochlorobenzene 98, 2-bromoaniline 100, also norbornadiene 99, along with cesium carbonate and dimethyl formamide, to produce the anticipated 3-chloro-5H-dibenzo[b,f]azepine 101 in yield of 65%. This compound was easily transformed to the resultant dihydro compound 102 in 95% yield by a simple reduction of a conjugated (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )-bond using the multipurpose Mg in MeOH at 50 °C for 1.5 hours. Finally, 3-chloro-N,N-dimethylpropan-1-amine 103 can be employed in alkylation to obtain the desired Clomipramine 104 (Scheme 16).99–101
)-bond using the multipurpose Mg in MeOH at 50 °C for 1.5 hours. Finally, 3-chloro-N,N-dimethylpropan-1-amine 103 can be employed in alkylation to obtain the desired Clomipramine 104 (Scheme 16).99–101
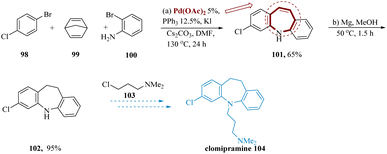 | ||
| Scheme 16 Synthesis of 3-(3-chloro-10,11-dihydro 5H-dibenzoazepin 5-yl)-N,N-dimethylpropane amine (clomipramine). | ||
Lumateperone, also known as ITI-007, is a strong 5-HT2A antagonist, post-synaptic D2 antagonist, and inhibitor of serotonin transport that was formed via Intra-Cellular Therapies.102 In 2017, the US FDA gave global approval for the single-dose oral administration of schizophrenia treatment in adults. The 5-HT2A, D2, D1/GluN2B, and SERT receptors exhibit a significant selectivity for the tetracyclic quinoxaline-type substance.103
Flick et al. described a simple and scalable pathway to lumateperone 119 and its structurally related compounds.104 Tricyclic indole 107 was produced using a Fischer indole with ketone 106 starting with hydrazine 105.105 Reduction with tri-ethylsilyl hydride, treating the resultant product with (R)-mandelic acid in MeOH, consequent synthesis of the (S)-mandelic acid diastereomeric salt, and free-basing with aq. NaOH yielded pure cis-indoline chirally 108. Protection of the amine and consequent Buchwald–Hartwig106,107 with 111 gave cyclization precursor 112. Tetracycle 114 was produced by spontaneous ring closure as a result of the N-alkylation of ethyl bromoacetate 113, following hydrolysis of the diphenylamine. Piperazine 115 was produced by again N-alkylation, following carbonyl reduction with borane in THF. The fully elaborated cis-tetracycle 118 was synthesized via hydrolysis of carbamate and N-alkylation using 4-chloro-1-(4-fluorophenyl)butane-1-one 117. Lumateperone tosylate 119 was produced by dissolving 118 in isopropanol and treating it with a p-toluene sulfonic acid solution (Scheme 17).108,109
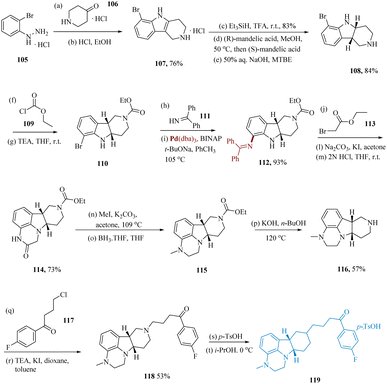 | ||
| Scheme 17 Synthesis of 1-(4-fluoro-phenyl)-4-((6b,10a)-3-methyl-2,3,6b,7,8,9,10,10a-octa-hydro-1H-pyrazino[3,2,1]carbazol-8-yl)butane-1-one (lumateperone). | ||
A new class of antipsychotic medication called aripiprazole (Abilify) is primarily utilized to cure schizophrenia and bipolar disorder.110 Because of the generation of difficult-to-remove isomers111 and the need for much more explosive Na azide as a nitrogen source and erosive TFA as the solvent, its conventional synthetic procedures usually gave poor yields. Yang et al. developed a faster synthetic pathway and milder reaction conditions allowed for a 77% total yield of aripiprazole using 120 as the initial substrate & current technology as the primary catalytic procedure. When the reaction was carried out in a Pd(TFA)2/BINAP/TsOH/H2O system, the NO2 group was completely deoxygenated and carbonylated to generate the isocyanate, which was then internally hydro-cyclized to produce aripiprazole 125 (Scheme 18).110
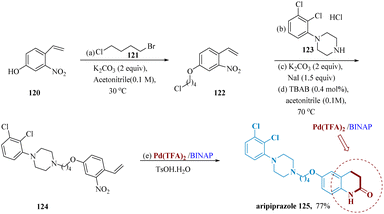 | ||
| Scheme 18 Synthesis of 7-(4-(4(2,3-dichloro-phenyl)piperazin-1-yl)-butoxy)3,4-dihydro quinolone 2(1H)-one (aripiprazole). | ||
Milnacipran is a serotonin noradrenaline reuptake inhibitor (SNRI), and its amine-containing cyclopropane moieties display a variety of biological activities.112–119
Ishizuka et al. reported an asymmetric production of milnacipran, initially, compound 129 was generated from radially available but2-yne-1,4-diol 126. By reacting with phenylboronic acid, compound 126 was transformed into (Z)-2-phenyl but-2-ene-1,4-diol 127. Using monoacetylation catalyzed by porcine pancreas lipase (PPL), the C4-hydroxy groups of 127 were regio-selectively preserved to provide 128. The C1-hydroxy group of 128 was protected with a t-butyldimethylsilyl group, and the C4-acetoxy group was then alkaline hydrolyzed to generate 129, which were then reacted with diiodomethane along with diethylzinc in 10 mol% of 130 to generate 131 with a yield of 87% (59% ee). Further primary hydroxy group of 131 was transformed into an azide; a t-butyldimethylsilyl group was then removed utilizing fluoride ions, and eventually, primary alcohol was oxidized utilizing Jones reagent to produce carboxylic acid 132. The carboxy group of 132 was transformed to amide 133, the azide group of 133 was hydrogenated, and the reaction with HCl provided the required optically active (−)-milnacipran hydrochloride 134 with 72% enantiomeric excess (Scheme 19).120
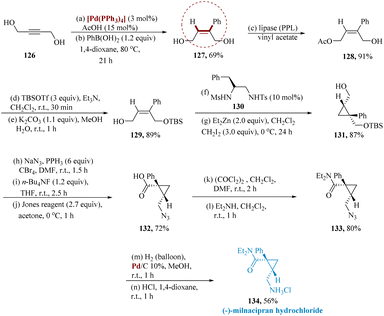 | ||
| Scheme 19 Synthesis of 2-((chloro-azanyl)methyl)-N,N-diethyl-1-phenyl cyclopropane carboxamide (milnacipran). | ||
The absorption of norepinephrine and serotonin is potently and specifically inhibited by the drug nafenodone. Rao et al. described the effective synthesis of an antidepressant (S)-nafenodone. It was made possible by sterically hindered enantio-selective α-arylation via palladium driven by chiral mono-phosphorus ligand BI-DIME L13. Pd-(S)-BI-DIME serving as a catalyst, tetralone 135 reacted with PhBr to produce 136 in an 80% efficient and 75% yield. (S)-nafenodone 137 was produced via ozonolysis, reductive amination, and subsequent reaction with HNMe2/Na(OAc)3BH (Scheme 20).121
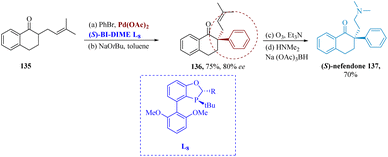 | ||
| Scheme 20 Synthesis of 2-(2-(dimethylamino) ethyl)-2-phenyl-3,4-dihydro naphthalen-1(2H)-one (nafenodone). | ||
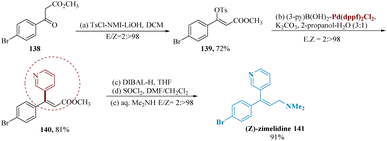
The universal α,β-esters were well-known as important olefin frameworks, and they were successfully used for parallel drug formation of (Z)-zimelidine. Ashida et al. reported the synthesis of an extremely selective serotonin reuptake inhibitor zimelidine, from readily available (Z)-stereo defined enol tosylates obtained from β-ketoesters 138 as well as α-formyl esters.122
Parallel and stereo-complementary enol tosylations were achieved via treating with starting material, undergoing Suzuki-couplings utilizing (3-Py)B(OH)2 which gave the compound 140. Our desired therapeutic molecule, (Z)-zimelidines 141, was then produced after being further treated with DIBAL-H, SOCl2, and aq. dimethyl amine (Scheme 21).123
3, 4-dihydro 2(1H)-quinolinones were marketed as an antipsychotic drug and exhibit promising antidepressant properties that are similar to those of aripiprazole.124–127 Triazole serves as the primary structural motif in a wide range of medicinal molecules, revealed to possess a variety of biological activities.128,129
In the FST and TST, the novel compound shows higher antidepressant efficacy than fluoxetine, as well as modest anticonvulsant action. These compounds could be employed as supplements to existing antidepressants to cure depression in epilepsy patients.
Deng et al. described the synthesis of triazole-containing quinolinones. The readily accessible 3, 4-dihydro-2(1H)-quinolinone 142 underwent successive nitration and catalytic hydrogenation to synthesize the compound 144. Then, compound 144 was reacted with dimethoxy-N,N-dimethylmethanamine (DMF-DMA) as well as formyl-hydrazine in acetonitrile to produce compound 145. In contrast to fluoxetine, the target compound 146 was produced by the successive alkylation 145 with a range of diverse alkylating agents (Scheme 22).130
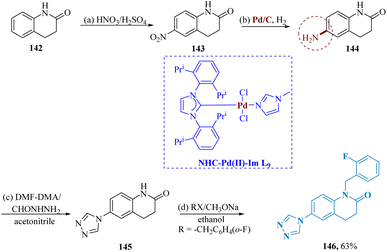 | ||
| Scheme 22 Synthesis of 1-(2-fluoro-benzyl)-6-(4H-1, 2,4-triazol-4-yl)-3,4-dihydro-quinolin-2(1H)-one. | ||
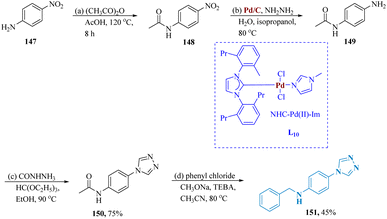
Song et al. described the synthesis of triazole containing quinolinones as a potent antidepressant. Starting with nitroaniline 147 and treating it with propionic anhydride & acetic anhydride in refluxed acetic acid to produce the compound 148. NO2 reducing conditions of Pd/C as well as hydrazine hydrates were used to reduce compound 148 to produce compound 149. After that, compound 149 was treated with formyl hydrazine and triethyl orthoformate in acetonitrile to produce compound 150. Finally, compound 151, which was produced by alkylating compound 150 with a range of diverse alkylating agents, demonstrated greater antidepressant ability than fluoxetine in the TST and FST studies (Scheme 23).131
Clinical research on psychiatric disorders and migraines has concentrated on NK1 receptor antagonists.132 SSRIs are currently employed to cure mental illnesses because they prevent the uptake of 5-HT, which increases levels of 5-HT inside synaptic cleft.133,134 A novel category of antidepressants with therapeutic potential may be produced by combining SR inhibition mostly with modification of 5HT activity through NK1 antagonist.135–137
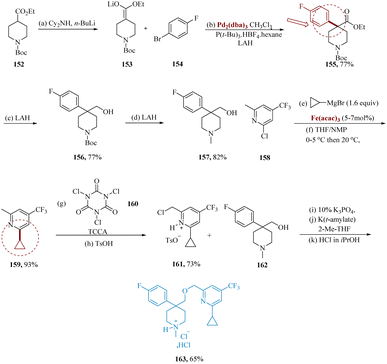
Risatti et al. reported the optimal α-arylation process which involves the formation of lithium enolate 153 using lithium dicyclohexyl amide accompanied via palladium with 154 utilizing tri-tert-butyl phosphonium tetra-fluoroborate as a ligand to provide ester 155, That was then reduced using lithium aluminum hydride to generate alcohol 156 in yields of 77%. LAH was also used to convert N-Boc to N-methyl, with a yield of 78–82%.
Through an iron-catalyzed coupling and TCCA chlorination, the 2,4,6-trisubstituted pyridine is synthesized selectively. However, after being treated with trichlorocyanuric acid 160, pyridine 159 was transformed into the necessary benzyl-chloride 161. Following the reaction, benzyl chloride 161 was produced as its p-toluene sulfonic acid (p-TSA) salt, including a dichloro impurity (5% LCAP) and leftover starting molecule 159. Finally, salt of p-toluenesulfonic acid 161 was synthesized from 158 with a yield of 68%, requiring just two synthetic transformations as opposed to the Boekelheide method's four stages, which resulted in a yield of 54%. Additionally, the method for coupling completely functionalized pyridine and piperidine components was extremely convergent, evaded the processing of non-crystalline products, and needed no chromatographic purifications. The crystalline HCl salt of 163, that were separated in 61–65% yield, was produced by etherifying the potassium alkoxide of 157 with a free base 162 (Scheme 24).138
 | ||
| Scheme 24 Synthesis of 4-(((6-cyclo-propyl-4-(trifluoro-methyl)-pyridine-2-yl)methoxy)methyl)4-(4-fluoro phenyl)-1-methyl piperidin-1-ium chloride (1 HCl) dual NK-1/serotonin receptor antagonist. | ||
5. Gold-catalyzed reactions
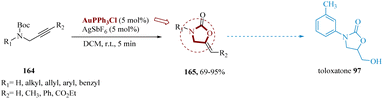
Vessally et al. reported a metal-catalyzed intra-molecular cyclization of N-Boc-protected propargyl amines using the AuPPh3Cl/AgSbF6 combination as the catalytic system to produce functionalized 2-oxazolidinones.139 Other catalysts, such as Pt(CH3CN)2(SbF6)2 and AuCl3, were discovered to increase the reaction in the optimization study, however, Au(PPh3)SbF6 provided the best results. N-Boc-protected propargyl amines 164 produced alkylidene 2-oxazolidinones 165 with fair to high yields and exceptional (Z)-selectivity under optimal conditions. Other merits of this synthetic methodology included simplicity, low reaction times, and a wide range of substrate scope. For instance, in the production of the antidepressant toloxatone 97 (Scheme 25).1406. Manganese-catalyzed reactions
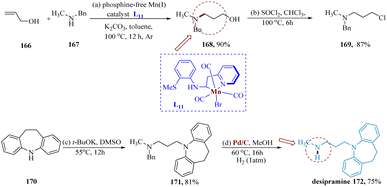
Desipramine is employed to treat depression, that works by enhancing the activity of a chemical called norepinephrine in the brain. This medication is a tricyclic anti-depressant. It might also be suitable to cure indications of attention-deficit hyperactivity disorder (ADHD).141Das et al. synthesized the precursor molecule 168 with exclusive anti-Markovnikov selectivity produced by hydrogenating allyl alcohol 166 with N-methylated aniline 167 and was transformed to chloro derivative 169 in yield of 87%, which was catalyzed via phosphine free Mn(I) L11 complex found abundantly in Earth and was carried out under hydrogen-borrowing conditions. Then, imino-dibenzyl treatment and debenzylation produced the antidepressant medication desipramine 172 in two steps with combined yields of 61% (Scheme 26).142
 | ||
| Scheme 26 Synthesis of 3-(10,11-di-hydro 5H-dibenzo azepin 5-yl)N-methyl propane amine (desipramine). | ||
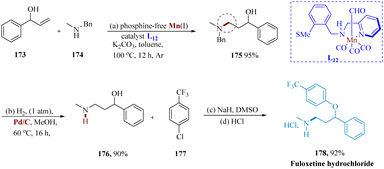
γ-Amino alcohols serve as efficient synthetic intermediates for a variety of drugs and bioactive compounds.143 Das et al. reported the synthesis of fluoxetine using a phosphine-free Earth's abundant Mn(I) catalyst. Under hydrogen-borrowing conditions, Mn(I) composite catalyzed the selective hydro-amination of allyl alcohols & 2° allylic alcohols with exceptional functional compatibility. 3-Benzyl(methyl)amino phenyl propane-1-ol 175, produced by treating 1-phenylprop-2-en-1-ol 173 with N-methyl-1-phenylmethanamine 174 and subjecting it to Mn, is then hydrogenated by using Pd/C in methanol at 60 °C for 16 hours. When the amine 176 was treated with 4-chlorobenzotrifluoride 177, it produced 178, as the hydrochloride salt (Scheme 27).142
Vortioxetine is a member of the bis-aryl-sulfanyl amines class and is chemically known as 1-[2-(2,4-dimethyl-phenylsulfanyl)-phenyl]-piperazine. Its main effects are the direct modulation of the 5-HT receptor and the selective blockade of SR (by inhibiting the SERT).144
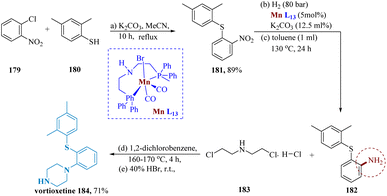
Mao et al. reported the synthesis of vortioxetine hydrobromide on a hectogram scale. Starting with 2,4-dimethylbenzenethiol 180 and 1-chloro-2-nitrobenzene 179, both of which are readily available in the market, the reaction with potassium carbonate in acetonitrile produced the desired intermediate 181 that was then purified through recrystallization in acetonitrile to yield the pure product in a total yield of 89%.145 The required the aniline derivative 182 is produced in 74% yield when the generated nitrophenylsulfane derivative 181 undergoes catalytic hydrogenation in the presence of Mn-1 under the optimal reaction conditions. It is worth noting that the activity of transition metals is frequently impeded by thio- and amino groups. Additionally, several Mn(0) species produced in late transition-metal catalyzation processes experience (C–S) oxidative additions which can be avoided by using Mn-L13. The required vortioxetine 184 is then produced by the reaction of 182 with 2-chloro-N-(2-chloro-ethyl)ethane amine hydrochloride 183 (Scheme 28).146,147
7. Copper-catalysed reactions
In many biological molecules, 1,5-benzo thiazepines are preferred heterocyclic pharmacophores.148 Ogawa et al. described speedy accessibility to 1,5-benzothiazepines using mesityl copper/(R)-DTBM segphos (DTBM = 3,5-di-tert-butyl-4 methoxy), pre-catalyst for conjugate addition of α,β-unsaturated thioamides 185 & thiophenol 186. The complex of mesitylcopper and (R)-DTBM segphos L1 may function as effective catalysts for direct enantio-selective production of C–S bonds. Several 1,4-conjugate addition compounds were produced by successfully using a range of electron-rich and deficient α,β-unsaturated thio-amides as electrophilic substrates in toluene at 0 °C. The second conversion required the utilization of methyl iodide for methylation of thio-amide functionalities, which produced a transitory thioester that was cyclized at 80 °C using a catalytic proportion of p-toluene sulfonic acid monohydrate (TsOHH2O). Following side chain addition, a 93% yield of (R)-thiazesim 190 was achieved (Scheme 29).149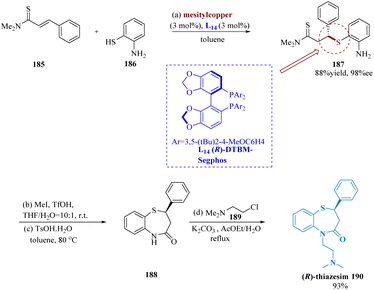 | ||
| Scheme 29 Synthesis of 5-(2-(dimethyl-amino)ethyl)2-phenyl-2,3-di-hydro benzo[1,4]thiazepin-4(5H)-one (thiazesim). | ||
Reboxetine, a selective norepinephrine reuptake inhibitor1 (SNRI), is used to treat depression, narcolepsy, cocaine dependence disorder, and hyperactivity disorder.150,151 In contrast to its (R,R) enantiomer, (S,S)-reboxetine is much more potent and specific for both nor-epinephrine transporters.152
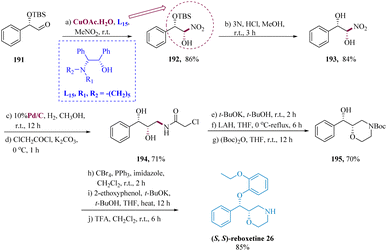
Liu et al. used Cu-L6 chiral amino alcohol-copper(II) catalyst to facilitate the diastereoselective nitro-aldol reactions of nitromethane with chiral aldehyde, which potentially leads to the privileged synthesis of specific stereoisomer for nitro-diol derivatives, Cu-L15 chiral amino alcohol-copper(II) catalyst was used. The nitro-aldol adduct (1S,2S) 192 was produced in 86% yield when the aldehyde (1S,2S) 191 was reacted with CH3NO2 in the presence of Cu-L6. The O-TBS-protected molecule 192 was first deprotected with 3 N HCl to generate the diol 193, which was then hydrogenated with Pd/C and subjected to a series of treatments with ClCH2COCl while being accompanied by a base to yield the chloroacetamide derivative 194 in 71% yield. The morpholine derivative (2S,3S) 195 was developed in 70% by cyclizing the amide derivative (2S,3S) 194 with t-BuOK, then reducing the amide with LAH and protecting it with N-Boc. Ultimately, derivative 195 was converted to (S,S) reboxetine 26 in 85% yield (Scheme 30).153
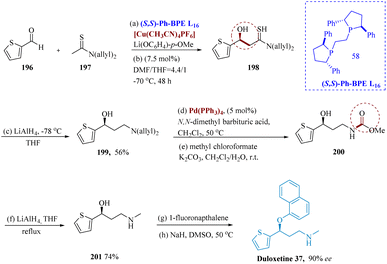
Duloxetine, a powerful antidepressant for the treatment of serious depressive disorders.59 Inhibitors of serotonin and nor-epinephrine reuptake for the treatment of several illnesses associated with depression.154,155 Larik et al. reported that thio-amides are employed as significant precursors for (C–C), leading to an aldol product having 92% ee for the enantio-selective direct asymmetric aldol reaction that produces (S)-duloxetine. The chirality has been produced by constructing a soft Lewis acid/hard Brønsted base co-ordinated catalyst consisting of [Cu(CH3CN)4]PF6, (S,S)-PhBPE L16, and Li(OC6H4-p-OMe), where thioamide was chemoselectively activated via soft–soft interaction of Cu+ and sulfur atom, resulting in the unique production of the thioamide enolate in aldehyde which undergoes reduction and synthesized compound 199. Molecule 200 was produced in two steps using 5 mol% of Pd(PPh3)4 and N,N-dimethyl of barbiturates in DCM at 50 °C. This molecule then underwent LiAlH4 reduction to produce molecule 201. The final target product 37 was then produced with a 65% yield by adding sodium hydride and 1-fluoro naphthalene (Scheme 31).156
Yang et al. reported the synthesis of triple reuptake inhibitor (TRI) ALB 109780, which prevents the reuptake of serotonin, norepinephrine, and dopamine, may help cure depression.157
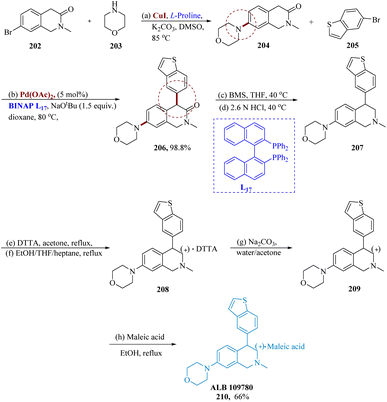
In the proximity of potassium carbonates, copper(I) iodide as well as L-proline in DMS (dimethyl sulfoxide), the reaction between compounds 202 and 203 produced the intended product 204 as the light-yellow limpid solid in 68% yield. A preliminary analysis of the reaction situations for the α-arylation of 204 showed that BINAP, NaOt-Bu, and Pd(OAc)2 were the best catalysts for this reaction. Compared to toluene, dioxane often offered superior adaptation and crude purity. Regardless of the quantity of catalyst utilized, increasing the addition of compound 205 harms the reaction's crude purity. Higher base concentrations resulted in improved conversion. With 1.2 equiv. of compound 205 at 80 °C, 5% Pd(OAc)2, BINAP as the ligand in dioxane, and 1.5 equiv. of NaOt-Bu, the best results were obtained.
Borane-dimethylsulfide (BMS) was used to reduce 206 in the presence of THF and 6 N HCl at 35 °C in a nitrogen environment. The resultant solution was then stirred at 40 °C till it was finished. Over the reduction and treatment with charcoal, compound 207 was separated also as a yellow solid with a 66% yield. After the treatment, purity rose to 95.4% from 87.5%.
By adding (+)-DTTA to a racemic 207 solution in acetone at reflux, 207 was resolved using (+)-di-p-toluoyl-D-tartaric acid. The resultant solution was chilled to 5 °C to yield the required product as the white solid via filtration in 81 to 88% ee. After the isolated product was recrystallized using heptane as an anti-solvent to speed up crystallization and 10% THF/EtOH at reflux (68 °C), the chiral limpidness was significantly improved to 98.8% enantiomeric excess. On a scale, 208 was reacted with 6 equiv. of Na2CO3 solution while being immersed in a mixture of aq. acetone. The resulting suspension underwent filtration to provide 97.1% HPLC purity and a quantitative yield of the target product. It was discovered that the chiral purity was 94.3%. The free base 209 was allowed to react with a solution of 1.1 equivalent to maleic acid after being heated to reflux in ethanol. It was filtered after cooling to 5 °C, yielding the anticipated product 210 as a white solid with a yield of 91% and HPLC purity of 98.1%. After isolation, the chiral purity was found to be greater than 99.9%. Additionally, small-scale investigations showed that during salt generation, from the starting substrate with only 86% ee, the chiral purity is sometimes increased to 99% (Scheme 32).157
Uwamori & Nakada synthesized Hyperforin, which was derived from Hypericum perforatum L., which prevents synapses from reabsorbing neurotransmitters.158,159 The compound known as hyperforin, which belongs to the family of poly-prenylated acyl phloroglucinols (PPAPs), is composed of a strongly oxygenated and highly substituted bi-cyclo[3.3.1]nonane or bicyclo[3.2.1]octane motif using geranyl or prenyl side chain derivatives prepared by intramolecular cyclopropanation.160,161
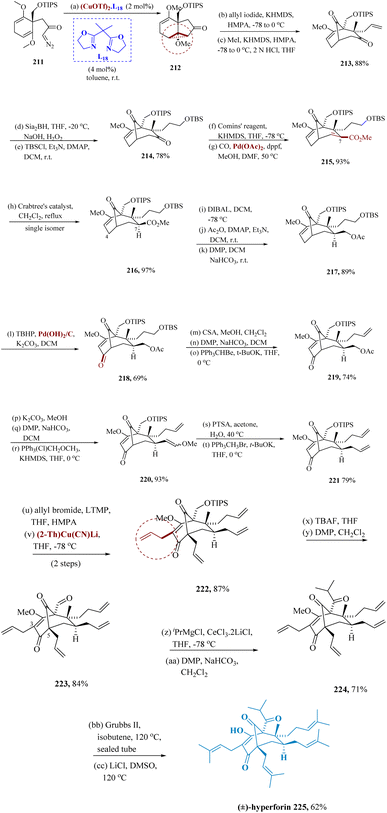
Intra-molecular cyclopropanation (IMCP) of 211, produced from methyl 2,6-dimethoxy benzoate by a series of steps, was accompanied by stereo-selective alkylation of the cyclopropane 212 via copper catalyzed complexes with 2,2′-(propane-2,2-diyl)bis(4,5-dihydro oxazole), and region-selective ring opening of a cyclo propane moiety to afford 213. Compound 213 was transformed to compound 217 through chemo-selective as well as stereo-selective hydroboration of 213 using disiamyl borane for further protection of the subsequent hydroxyl as TIPS ether generated 214. Synthesis of an enol triflate of 214 using Comins' reagent and Pd carbonylation provided 215. By hydrogenating the C6–C7 alkene in 215 using Crabtree's catalyst, chemo- and stereo-selective reduction of the C6–C7 alkene were accomplished, leading to the only product 216 after refluxing dichloroethane. The directing effect of a C2–C3 methoxy alkene may be responsible for this stereoselectivity. Preferential acetylation of a 1° hydroxyl and then Dess–Martin oxidation of a C-9 secondary hydroxyl produced product 217 from the reduction of 216 with DIBAL-H. Palladium-catalyzed oxidation of 217 was used to achieve allylic oxidation, yielding 218, which was then modified by Dess–Martin oxidation, Wittig reaction, and elimination of the TBS group to yield 219. As mentioned earlier, Wittig reactions were also used to synthesize the allyl group at the C-7 position. In other words, potassium carbonate in methanol removed the acetyl groups from 219, which were then proceeded via DMP and Wittig process to give 220. After compound 220 underwent acid hydrolysis, compound 221 with an allyl group at the C-7 position was successfully produced by the Wittig reaction. Lithium 2,2,6,6-tetra-methylpiperidide was necessary for allylation at the C-5 position of 221, as LDA reduced the C9 ketone. Under the same circumstances, subsequent allylation at a C-3 site did not occur, requiring the utilization of thienyl cuprate as an additive, thus giving compound 222. Compound 222 was exposed to a reaction with TBAF to construct the C-1 isopropyl ketone, and a Dess–Martin oxidation process followed to produce aldehyde 223.
The required product was successfully obtained with a trace amount of the reduced product from the treatment of molecule 223 with the isopropyl cerium, which was successfully prepared in situ from CeCl3·2LiCl and isopropyl magnesium chloride. Cross metathesis was subjected to 224, which was produced by the subsequent Dess–Martin oxidation. In the Grubbs II reagent at 60 °C, the reaction of 224 with isobutene produced a variety of products, some of which comprised compounds with cycloheptene rings that were obtained from the ring-closing metathesis among the C-7 and C-8 substituents.
Moreover, the target compound 225 was effectively produced in 93% yield at 120 °C. Under Krapcho's conditions, the C-2 methyl ether was finally cleaved (Scheme 33).162
8. Rhodium-catalysed reactions
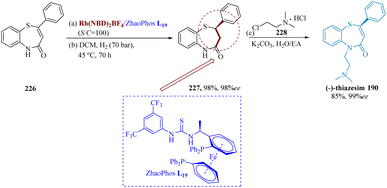
A lot of attention has been attracted to the asymmetric synthesis of optically active 1,5-benzo thiazepines that have chiral drugs in the form of a sulfur-substituted stereocenter. Yin et al. successfully developed asymmetric hydrogenation of the number of conjugate C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, using the Rh/ZhaoPhos catalytic system, which consists of the chiral ferrocene-based bis-phosphine & thiourea moiety as ligand. With an efficient S/C ratio of 100, asymmetric hydrogenation of 226 produced the compound (R)2-phenyl-2,3-dihydro benzo[b][1,4]thiazepin 4(5H)-one 227 having the yield of 98% (98% ee). The antidepressant drug (R)-(−)-thiazesim 190 could be easily converted from the hydrogenation product 227 with outstanding efficiency (Scheme 34).163
C, using the Rh/ZhaoPhos catalytic system, which consists of the chiral ferrocene-based bis-phosphine & thiourea moiety as ligand. With an efficient S/C ratio of 100, asymmetric hydrogenation of 226 produced the compound (R)2-phenyl-2,3-dihydro benzo[b][1,4]thiazepin 4(5H)-one 227 having the yield of 98% (98% ee). The antidepressant drug (R)-(−)-thiazesim 190 could be easily converted from the hydrogenation product 227 with outstanding efficiency (Scheme 34).163
Venlafaxine, a member of the phenylethylamine class of antidepressants with a unique structure, has a chiral center at a benzylic position, a tertiary amine & a tertiary hydroxy group.164,165
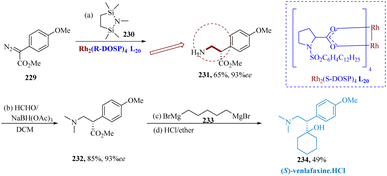
Preskorn described The C–H insertion reactions of bis-silylmethylamine 230 using different aryl diazo-acetates 229 were catalyzed via dirhodium-(II)-prolinate, Rh2(S-DOSP)4 L20 and produced β-amino esters in 62% yield and 93% ee. It was observed that HCHO/NaBH(OAc)3 was a viable substitute since it effectively converted 231 to 232 in a yield of 82% at room temperature without losing ee. Finally, (S)-venlafaxine 234 was synthesized by reacting 232 with pentyl-1,5 magnesium bromide 233. The Grignard reagent and the ester solutions have to be added slowly and simultaneously to the reaction vessel to achieve the best results. After working up the reaction, producing the HCl salt, and enriching by recrystallization, (S)-234 was produced in a yield of 49% and 99% ee (Scheme 35).166
 | ||
| Scheme 35 Synthesis of 1-(2-(dimethyl amino)-1-(4-methoxy phenyl)ethyl)cyclo-hexane-1-ol (venlafaxine). | ||
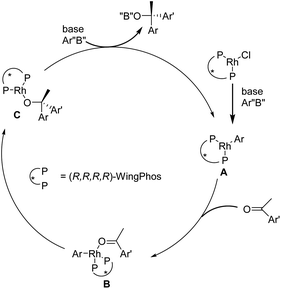
The antidepressant medicine escitalopram, also known as 3-(dimethyl amino)propyl-4-fluorophenyl-1,3-dihydro-isobenzofuran-5-carbonitrile, is one example of a pharmaceutical agent or natural product that contains chiral diaryl alkyl carbinol moieties.167–172 Huang et al. described for the first time addition of aryl-boroxines to modest aryl ketones in a highly enantioselective manner, using a Rh/(R,R,R,R)-WingPhos to produce chiral diaryl alkyl carbinols. It has been established that Rh/(R,R,R,R)-WingPhos is important for the significant reactivity and enantio-selectivity. Because of the two anthryl units in its composition, (R,R,R,R)-WingPhos can not only provide the necessary stereo-control but also help to bring two reactions together and increase reactivity.173
The targeted tertiary alcohol 236 was effectively produced in 70% yield (99% ee) by adding 4-fluoro-phenyl boroxine to 4-chloro-1-(2,4-dichloro phenyl)butan-1-one 235 using the Rh/(R,R,R,R)-WingPhos L21, which has excellent functional group compatibility. The lactone 237 was produced with a 73% overall yield by SN2 displacement of chloride in 236 with amines and then cyanation–lactonization under palladium catalysis. Escitalopram 238 was produced with a combined yield of 61% and enantiomeric excess of more than 98% after being reduced from 237 using DIBAL-H/NaBH4 and then having the ring closed with MsCl treatment. Thus, using this methodology, a fresh, brief, and extremely enantioselective synthesis of escitalopram was established (Scheme 36).
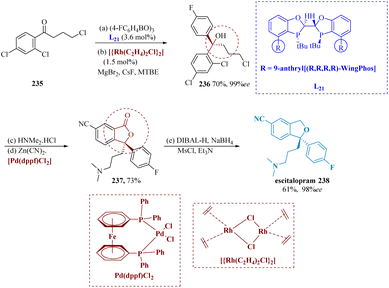 | ||
| Scheme 36 Synthesis of 1-(3-(dimethyl amino)propyl)-1-(4-fluoro phenyl)-1,3-dihydro-isobenzofuran-5-carbonitrile (escitalopram). | ||
A simplified mechanism for this rhodium-catalyzed asymmetric inclusion of arylboroxines to simple aryl ketones is proposed in Fig. 4. Transmetallation of the aryl boron with the [Rh(Cl){(R,R,R,R)-WingPhos}] species provides the aryl-Rh species A. This step is followed by the coordination of an aryl ketone to form the species B. The favorable conformer undergoes insertion and transmetallation with another aryl boron reagent to provide the chiral tertiary alcohol product with the ascertained stereochemistry and regenerates A (Fig. 4).173
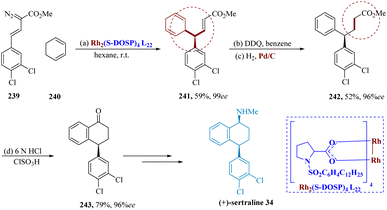
Davies et al. reported that cyclohexadienes can be successfully subjected intermolecularly to C–H insertion of phenyl diazoacetates via dirhodium tetrakis((S)-N-(dodecyl benzenesulfonyl)prolinate) (Rh2(S-DOSP)4) L22, resulting in the asymmetric production of diarylacetates. The 1,4-cyclohexadiene 240 was produced in 99% ee by the vinyl di-azoacetate 239 and 1,3-cyclohexadiene which was catalyzed via Rh2(S-DOSP)4 L22. The use of cyclohexadiene 241 as a precursor for the formal synthesis of (+)-sertraline 34 has many advantages. The 4,4-diaryl butanoate 242 was produced by oxidizing 241 with DDQ and catalytic hydrogenation, with only minimal racemization (96% ee). The tetralone 243 was produced by ester hydrolysis of 242 via an intra-molecular Friedel–Crafts acylation (79% yield for two processes), which was then transformed into (+)-sertraline 34 (Scheme 37).174
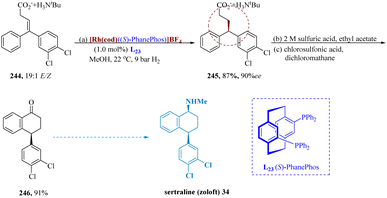
Boulton et al. reported a potent method to construct stereo-genic centers is the asymmetric hydrogenation of the olefin functional group using rhodium di-phosphine catalysts and an (S)-PhanePhos ligand L23.175 Z/E-olefin isomers produce different enantiomers in a given catalytic system, the compound 244 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E/Z mixture of olefins) needed to be employed as (almost) a single isomer to obtain excellent enantio-selectivity. The racemic form of 4,4-diaryl-3-butenoate 245 was produced by hydrogenating the tert-butylammonium salt 244 using a 1
1 E/Z mixture of olefins) needed to be employed as (almost) a single isomer to obtain excellent enantio-selectivity. The racemic form of 4,4-diaryl-3-butenoate 245 was produced by hydrogenating the tert-butylammonium salt 244 using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E/Z mixture of olefins. The hydrogenated product was cyclized by first treating it with 2 M H2SO4 to release a free acid, and then with chloro-sulfonic acid to produce tetralone 246 91% of the time. Tetralone 246 was then employed to develop sertraline 34 by reductive amination with methylamine (Scheme 38).176
1 E/Z mixture of olefins. The hydrogenated product was cyclized by first treating it with 2 M H2SO4 to release a free acid, and then with chloro-sulfonic acid to produce tetralone 246 91% of the time. Tetralone 246 was then employed to develop sertraline 34 by reductive amination with methylamine (Scheme 38).176
As a result of selective inhibition of the absorption of human synaptosomal serotonin, (+)-sertraline (Zoloft) has become the medication that is most usually used for the treatment of depression.177,178 It is frequently employed for treating depression, as well as occasionally OCD, PTSD, and panic attacks.
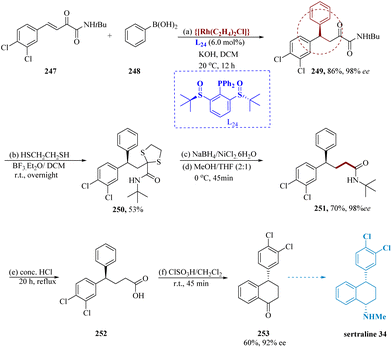
Wang et al. described the production of chiral gem-diaryl alkanes in significant yield and enantiomeric excess was enabled by chiral sulfinylphosphine ligands, which successfully promoted Rh-catalyzed arylation to chalcones. In aqueous KOH in dichloromethane (DCM), phenylboronic acid 248 has been introduced to the β,γ-unsaturated α-ketoamides & ligand L24. Rh(C2H4)2Cl2 served as the catalyst. Amide 247 yields the 1,4-adduct 249 over 86% while exhibiting outstanding 1,4-selectivity. Without losing the enantiomeric excess, the 1,4-adduct 249 (98% ee) was transformed into 1,3-dithiolane 250 and subsequently reduced to produce γ,γ-diarylamide 251. Using concentrated HCl heated under reflux for about 20 hours, the amides 251 were then hydrolyzed to generate 252. The tetralone 253 was then produced by subjecting product 252 to an acid-catalyzed cyclization179 which resulted in a 60% yield and a 92% enantiomeric excess. Tetralone 253 is used as a preliminary step in the production of sertraline 34 (Scheme 39).180
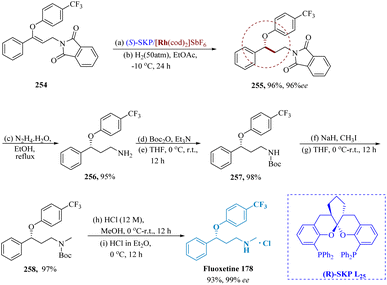
Zhang et al. described using the asymmetric hydrogenation of γ-branched N-phthaloyl allyl amines with bis-phosphine-Rh complex and (R)-SKP, [Rh((R)-SKP)(cod)]SbF6 bearing a high biting angle, it is possible to produce enantioselective γ-lochlorogenic amine derivatives with outstanding enantio-selectivity (up to >99% ee) and decent yields.
The intended product 255 could also be produced by hydrogenating 254 in EtOAc with L25 (S)-SKP/[Rh(cod)2]SbF6 at 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 S/C at room temperature and 50 atm H2. This method produced the intended product 255 in a good yield and with great enantioselectivity. The antidepressant medication Fluoxetine 178 was produced in various steps with >99% ee by varying the N-substituent from phthaloyl to methyl. This process yielded 83% of the desired product from the starting material 254 (Scheme 40).181
000 S/C at room temperature and 50 atm H2. This method produced the intended product 255 in a good yield and with great enantioselectivity. The antidepressant medication Fluoxetine 178 was produced in various steps with >99% ee by varying the N-substituent from phthaloyl to methyl. This process yielded 83% of the desired product from the starting material 254 (Scheme 40).181
9 Iridium-catalysed reactions
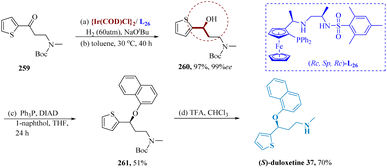
As essential intermediates in the transformation of organic material, enantiomerically enriched γ-amino alcohols, a group of widely used building blocks, plays a significant role.182–185 Liu et al. reported for (AH) of various γ-amino ketones. An effective catalytic system utilizing iridium and chiral tri-dentate ferrocene-based phosphine bearing unsymmetrical vicinal diamines were developed. Ir-(RC,SP,RC)-L26 catalyst efficiently hydrogenated 259, maintaining good results 97% yield and 99% ee. The respective amino alcohols result in the complete production of the desired compounds. It suggested that there was a great deal of potential for this Ir-catalyzed asymmetric conversion in industrialized applications. Additionally, N-Boc-N-methyl-(3-hydroxy)-3-(2-thienyl)propanamine 260 was obtained by Boc-protection of R-7, accompanied by Mitsunobu coupling to produce Boc-protected duloxetine 261, which was then deprotected to yield duloxetine 37 (Scheme 41).186,187SSRIs known as paroxetine are frequently prescribed to treat panic, obsessive, and depressive disorders.188 Krautwald et al. allylated aldehyde 263 and 4-fluorophenyl vinyl carbinol 262 were allylated by Ir((S)-L)/(S)-L27, resulting in γ,δ-unsaturated aldehyde 264 with a yield of 64% and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric ratio. After reduction to the analogous 1° alcohol, subsequently displaced to yield the respective aryl ether, separation of the diastereomers was accomplished. The terminal olefin 265 is provided by hydroboration/oxidation. (−)-Paroxetine 266 was produced through the phthalimide's cleavage and subsequent cyclization (Scheme 42).189–191
1 diastereomeric ratio. After reduction to the analogous 1° alcohol, subsequently displaced to yield the respective aryl ether, separation of the diastereomers was accomplished. The terminal olefin 265 is provided by hydroboration/oxidation. (−)-Paroxetine 266 was produced through the phthalimide's cleavage and subsequent cyclization (Scheme 42).189–191
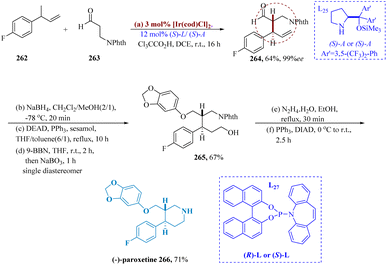 | ||
| Scheme 42 Synthesis of 3-((benzo[1,3]dioxol 5-yloxy)methyl)4-(4-fluoro phenyl)piperidine (paroxetine). | ||
The neuronal uptakes of norepinephrine (NE), serotonin, or dopamine are all inhibited to an equivalent extent by the triple monoamine reuptake inhibitor diclofensine. It is a molecular derivative of tetrahydroisoquinoline (THIQ).192,193
Tian et al. synthesized (S)-diclofensine Using Ir-(S,S,R) f-amPhox L28 catalyst, chiral α-α-di aryl acetamides. α-mesylates amide 268 was generated in 94% enantioselectivity and 96% by asymmetrically hydrogenating α-keto amide 267, accompanied by mesylation.194 The α,α-diaryl acetamide 270 was produced in 75% yield & 92% ee by stereospecific coupling of 268 and 269. (S)-diclofensine 272 was produced from 271 by amide reduction via DIBAL-H, proceeded through formylation, ring closure, as well as reduction (Scheme 43).195–197
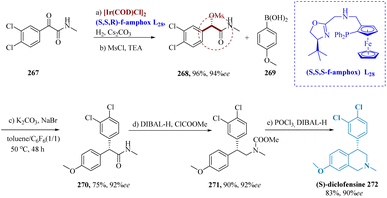 | ||
| Scheme 43 Synthesis of 4-(3,4-dichlorophenyl)7-methoxy 2-methyl-1,2,3,4tetra-hydroisoquinoline (diclofensine). | ||
10 Cobalt-catalysed reactions
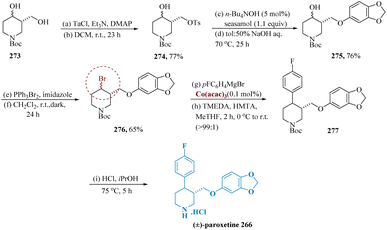
Paroxetine, a powerful inhibitor of serotonin reuptake, is frequently recommended to cure depression, social anxiety, post-traumatic stress, OCD, and panic disorder.198,199Despiau et al. reported cobalt-catalyzed cross-coupling reaction used to build the scaffold of the 3,4-disubstituted piperidine in a rapid way to produce (±)-paroxetine. Tri-ethyl amine (2.1 equiv.) was used as the base for the process of regioselective tosylation of diol 273, which resulted in a high yield and the generation of bromide 276. When the sesamol group was introduced with Cs2CO3 in DMF, adduct 275 was produced in 51% yield. By utilizing tetra-butyl ammonium hydroxide as a phase transfer catalyst and exposing a toluene solution of 274 and sesamol to aqueous NaOH, this yield increased to 76%. Finally, Bromo tri-phenyl phosphonium bromide was used to convert alcohol 275 into bromide 276 with a 65% yield.
Cross-coupling of 4-fluorophenyl magnesium bromide with bromides 276 using Co(III) acetyl-acetonate (acac) along with TMEDA and hexamethylenetetramine (HMTA) revealed cis-276 producing a 16% yield of coupling product 277 with enhanced selectivity for trans-277. After removing the Boc protecting group and undergoing recrystallization from 2-propanol, the synthesis of (±)-paroxetine 266 was finished (Scheme 44).200,201
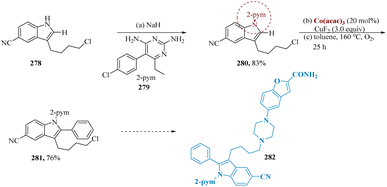
A wide range of C–H functionalizations has been achieved by cobalt catalysis with significant efficiency, and air-stable cobalt complexes abundantly present on earth have progressively emerged as stable and flexible catalysts.202–205 Lu et al. reported that this methodology has been utilized as the crucial step in the formation of the vilazodone derivative 282 from a readily accessible precursor 278. When the intermediate 280 was C–H arylated, the corresponding product 281 was produced in a 76% isolated yield and was easily transformed into a 2-arylated vilazodone derivative 282 (Scheme 45).206,207
Antidepressants, neuroleptics, and antiarrhythmics are only a few of the therapeutic classes represented by cationic amphiphilic drugs (CADs). Cationic amphiphilic drugs can enter cells and their organelles in their neutral, lipophilic form. These medications are effectively protonated and consequently confined in acidic cellular compartments, such as lysosomes. By making drugs more permeable to BBB in the brain, the antidepressant effect is enhanced.
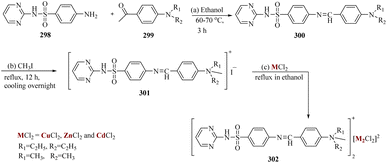
Hariprasath et al. reported by treating amines 298 with aromatic aldehydes 299 such as para diethyl and dimethyl amino benzaldehyde, Schiff's base of sulphadiazine was formed. By reacting with MeI, the synthesized Schiff's bases 300 were changed into their cationic amphiphilic bases 301. These bases were treated with metals such as ZnCl2, CdCl2, and CuCl2, to produce metal complexes 302. Both zinc and copper metal complexes demonstrated remarkable anti-inflammatory and antidepressant properties (Scheme 46).208,209
Vilazodone, an SSRI as well as a partial agonist of the serotonin 5-HT1A receptor, is utilized to cure major depressive disorder (MDD).210,211 Jin et al. synthesized the product 3-(4-chloro butyl)-1H-indole-5-carbonitrile 306, is produced by selectively deoxygenating the keto functionality of 3-(4-chlorobutanoyl)-1H-indole-5-carbonitrile 305 in 26% & the resultant solid 306 is problematic to purify via chromatographically. Finally, the intermediate 308 is produced with a yield of 32% from 306 and the readily available commercial 307 compounds. Although 308 could be made using the Mukaiyama reagent (1-methyl-2- chloro-pyridinium iodide), vilazodone 309 must be synthesized as the target molecule (Scheme 47).109
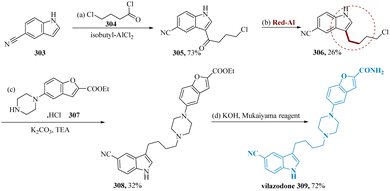 | ||
| Scheme 47 Synthesis of 5-(4-(4-(5-cyano-1H-indol-3-yl)butyl)piperazin-1-yl)benzo-furan 2-carboxamide (vilazodone). | ||
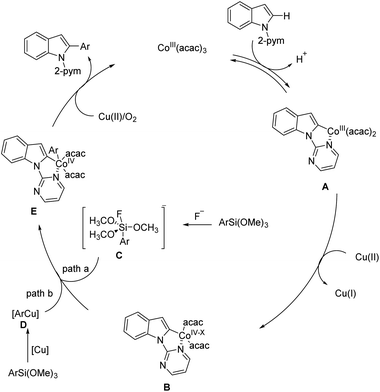
Initially, coordination of the nitrogen chelating group to the cobalt(III) catalyst facilitates the reversible C–H cobaltation through a concerted metalation deprotonation (CMD) process to afford the cobaltacycle species A, which may further proceed via a one-electron oxidation to deliver the key cobalt(IV) intermediate B. Subsequent transmetalation with the pentavalent silicate C generated in situ by the fluoride ion results in the formation of cobaltacycle E (path a). Alternatively, the arylsilicate probably proceeds via a transmetalation reaction to give the copper–aryl species D (path b), which may render the following transfer of an aryl group to the cobalt-metal center more readily. The cobaltacycle intermediate E undergoes the oxidatively induced reductive elimination step to afford the desired arylated product and release the Co(II) species. Finally, the Co(II) species is re-oxidized to regenerate the Co(III) catalyst by Cu(II) salt or O2 oxidant, thus sustaining the continuity of the proposed cycle (Fig. 5).212,213
Agomelatine is a melatonin bio-isosteric derivative in which the naphthalene core has taken the place of the indole core.214,215 As a 5-HT2C receptor antagonist and agonist of MT1/MT2 melatonergic receptors, agomelatine can resynchronize disrupted circadian rhythms, alleviating sleep disorders.216
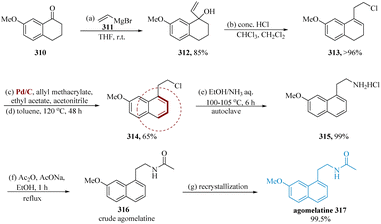
Stathakis et al. described that by combining vinylic organometallics with 7-methoxy-1-tetralone 310 the main building block for the synthesis of agomelatine carbinol 312 was obtained. In the next step, intermediate 313 could be obtained from carbinol 312 through allylic substitution, which is the crucial step, utilizing chlorination agents such as PCl3, SOCl2, or HCl as well as isomerization of the double bond. Next, chloride 313 had to be oxidized to produce the equivalent aromatic derivative 314; this was primarily done by consuming Pd in the manifestation of a hydrogen acceptor. The desired ammonium chloride 315 is produced in a decent yield by heating molecule 314 in equal volumes of EtOH and aq. NH3 at 100–105 °C in an auto-clave for 6 hours. By employing AcONa and Ac2O in EtOH heated to reflux, the acetylation of ammonium salt 315 to the analogous acetamide 316 completed the final API synthesis. After a simple re-crystallization, the pure molecule 317 was obtained with a purity of more than 99.5% (Scheme 48).217,218
11. Conclusion
Metal-catalyzed reactions have played a significant impact in the development of new pharmaceuticals, particularly antidepressants, and have transformed the field of organic synthesis. The development of more sustainable and effective routes for the synthesis of antidepressant compounds has been achieved via metal-catalysts. These reactions can provide the pharmaceutical industry with significant advantages, including benign reaction conditions, increased efficiency, and minimal waste output. It is estimated that metal-catalyzed reactions play a main role in the synthesis of antidepressant molecules and other therapeutics in the future owing to the ongoing advancement of novel catalysts and the continual improvement of synthetic methodologies.12. Future perspectives
Developments in the field of chemistry, particularly in drug discovery and synthesis, occur rapidly. The future outlook for metal-catalyzed reactions in antidepressant molecule synthesis is promising. Researchers are likely to continue exploring new methodologies, enhancing existing processes, and addressing challenges related to scalability and regulatory requirements. Through that comprehensive survey, synthetic chemists and pharmacists develop new ideas for the derivatization of these molecules. Depressant-related diseases led to the outcome of severe mortalities in the world especially in developing countries due to various factors. So, there is a desperate need to summarize the overall possible synthetic routes through the utilization of different metals and their complexes for the synthesis of antidepressants.Author contributions
All Authors have equal contributions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors express their appreciation to the Deanship of Scientific Research Muhammad Imran at King Khalid University, Saudi Arabia, for their support.References
- H. Hussain, S. Ahmad, S. W. A. Shah, A. Ullah, N. Ali, M. Almehmadi, M. Ahmad, A. A. K. Khalil, S. B. Jamal and H. Ahmad, Molecules, 2022, 27, 24–68 Search PubMed.
- S. Rubab, K. Naeem, I. Rana, N. Khan, M. Afridi, I. Ullah, F. A. Shah, S. Sarwar, F. ud Din and H.-I. Choi, Int. J. Pharm., 2021, 603, 120–670 CrossRef PubMed.
- J. C. Schaffer, B. Kuhns, J. Reuter, C. Sholtis, S. Karnyski, J. P. Goldblatt, R. D. Bronstein, M. D. Maloney, J. Baumhauer and S. Mannava, Arthroscopy, 2022, 38, 2863–2872 CrossRef PubMed.
- J.-P. Lépine and M. Briley, Neuropsychiatr. Dis. Treat., 2011, 7, 3–7 Search PubMed.
- R. Paul, T. Muhammad, R. Rashmi, P. Sharma, S. Srivastava and P. P. Zanwar, Sci. Rep., 2023, 13, 17651 CrossRef CAS PubMed.
- A. M. Fish, J. Vanni, F. N. Mohammed, D. Fedonni, K. B. Metzger, J. Shoop, C. L. Master, K. B. Arbogast and C. C. McDonald, Sports Health, 2023, 15, 185–191 CrossRef PubMed.
- Y.-N. Ni, X.-L. Du, T. Wang, Y.-Y. Chen, X.-Q. Xu, S. Zhao, J.-Q. Li and G. Wang, Pharmaceutical Fronts, 2021, 3, e183–e193 CrossRef.
- H.-U. Wittchen, F. Jacobi, J. Rehm, A. Gustavsson, M. Svensson, B. Jönsson, J. Olesen, C. Allgulander, J. Alonso and C. Faravelli, Eur. Neuropsychopharmacol., 2011, 21, 655–679 CrossRef CAS PubMed.
- A. Berger, E. Dukes, H.-U. Wittchen, R. Morlock, J. Edelsberg and G. Oster, Eur. J. Psychiatry, 2009, 23, 90–100 Search PubMed.
- G. I. Spielmans, M. I. Berman, E. Linardatos, N. Z. Rosenlicht, A. Perry and A. C. Tsai, Focus, 2016, 14, 244–265 CrossRef PubMed.
- J. O. McNamara, Goodman and Gilman's the pharmacological basis of therapeutics, 2006, Vol. 11, p. 501 Search PubMed.
- J. S. Lai, S. Hiles, A. Bisquera, A. J. Hure, M. McEvoy and J. Attia, Am. J. Clin. Nutr., 2014, 99, 181–197 CrossRef CAS PubMed.
- E. Dąbrowska, B. Galińska-Skok and N. Waszkiewicz, Life, 2021, 11, 10–56 CrossRef PubMed.
- X. Benarous, J. Renaud, J. J. Breton, D. Cohen, R. Labelle and J.-M. Guilé, J. Affective Disord., 2020, 265, 207–215 CrossRef PubMed.
- A. Özdemir, M. D. Altıntop, Z. A. Kaplancıklı, Ö. D. Can, Ü. D. Özkay and G. Turan-Zitouni, Molecules, 2015, 20, 2668–2684 CrossRef PubMed.
- C. Bolm, J. Legros, J. Le Paih and L. Zani, Chem. Rev., 2004, 104, 6217–6254 CrossRef CAS PubMed.
- Y. Zheng, X. Chang, Y. Huang and D. He, Biomed. Pharmacother., 2023, 157, 113985 CrossRef CAS PubMed.
- B.-K. Park, Y. R. Kim, Y. H. Kim, C. Yang, C.-S. Seo, I. C. Jung, I.-S. Jang, S.-H. Kim and M. Y. Lee, BioMed Res. Int., 2018, 2018, 5845491 Search PubMed.
- D. Lochmann and T. Richardson, Antidepressants: from Biogenic Amines to New Mechanisms of Action, 2019, pp. 135–144 Search PubMed.
- A. McGirr, P. A. Vöhringer, S. Nassir Ghaemi, R. W. Lam and L. N. Yatham, Focus, 2021, 19, 129–137 CrossRef PubMed.
- R. A. Sansone and L. A. Sansone, Innov. Clin. Neurosci., 2014, 11, 37–42 Search PubMed.
- K. Vardi, J. L. Warner and N. S. Philip, Ann. Clin. Psychiatry, 2014, 26, 207–216 Search PubMed.
- F. López-Muñoz, in Discoveries in Pharmacology, Elsevier, 2023, pp. 153–159 Search PubMed.
- N. Aarts, R. Noordam, A. Hofman, H. Tiemeier, B. H. Stricker and L. E. Visser, Int. J. Clin. Pharm., 2016, 38, 1311–1317 CrossRef CAS PubMed.
- G. Kaur, D. Goyal and B. Goyal, in Computational Modeling of Drugs against Alzheimer's Disease, Springer, 2023, pp. 325–353 Search PubMed.
- O. Bekircan, Ö. Danış, M. E. Şahin and M. Cetin, Bioorg. Chem., 2022, 118, 105–493 CrossRef PubMed.
- Y. S. Park, H. Oh and K.-W. Sung, J. Pharmacol. Sci., 2023, 151, 63–71 CrossRef CAS PubMed.
- S. M Stahl, C. Lee-Zimmerman, S. Cartwright and D. Ann Morrissette, Curr. Drug Targets, 2013, 14, 578–585 CrossRef PubMed.
- D. Cutuli and P. Sampedro-Piquero, Curr. Neuropharmacol., 2022, 20, 2202 CrossRef CAS PubMed.
- J. Orzelska-Górka, J. Mikulska, A. Wiszniewska and G. Biała, Int. J. Mol. Sci., 2022, 23, 10624 CrossRef PubMed.
- K. Sharma, A. Sundriyal, A. Loshali, M. Agrawal, C. G. Krishna and Y. Singh, in How Synthetic Drugs Work, Elsevier, 2023, pp. 255–273 Search PubMed.
- V. Dhayalan, D. Sharma, R. Chatterjee and R. Dandela, Eur. J. Org Chem., 2023, 26, e202300285 CrossRef CAS.
- M. Belal, Z. Li, X. Lu and G. Yin, Sci. China: Chem., 2021, 64, 513–533 CrossRef CAS.
- A. W. Sun, M. H. Wu, M. Vijayalingam, M. J. Wacker and X.-P. Chu, Biomolecules, 2023, 13, 229 CrossRef CAS PubMed.
- K. Kromm, P. L. Osburn and J. Gladysz, Organometallics, 2002, 21, 4275–4280 CrossRef CAS.
- J. Hafeez, M. Bilal, N. Rasool, U. Hafeez, S. A. A. Shah, S. Imran and Z. A. Zakaria, Arabian J. Chem., 2022, 104165 CrossRef CAS.
- S. Srinivas Kotha, S. Badigenchala and G. Sekar, Adv. Synth. Catal., 2015, 357, 1437–1445 CrossRef CAS.
- I. Kanwal, A. Mujahid, N. Rasool, K. Rizwan, A. Malik, G. Ahmad, S. A. A. Shah, U. Rashid and N. M. Nasir, Catalysts, 2020, 10, 443 CrossRef CAS.
- M. Bilal, M. Sharif, N. Rasool, I. Kanwal, G. Ahmad and P. Langer, Copper-Catalyzed Arylations And Heteroarylations, Royal Society of Chemistry, 2022, pp. 249–276 Search PubMed.
- H. Ahmad, M. Bilal, T. Maqbool, N. Rasool, S. A. A. Shah and Z. A. Zakaria, J. Saudi Chem. Soc., 2023, 101658 CrossRef CAS.
- I. Mizuta, M. Ohta, K. Ohta, M. Nishimura, E. Mizuta, K. Hayashi and S. Kuno, Biochem. Biophys. Res. Commun., 2000, 279, 751–755 CrossRef CAS PubMed.
- S. K. Talluri and A. Sudalai, Tetrahedron, 2007, 63, 9758–9763 CrossRef CAS.
- U. Chopade Manojkumar, U. Chopade Anil and M. Nikalje, Res. J. Chem. Sci., 2014, 2250, 9261 Search PubMed.
- C.-X. Ye, Y. Y. Melcamu, H.-H. Li, J.-T. Cheng, T.-T. Zhang, Y.-P. Ruan, X. Zheng, X. Lu and P.-Q. Huang, Nat. Commun., 2018, 9, 410 CrossRef PubMed.
- I. áO'Neil, Chem. Commun., 2014, 50, 7336–7339 RSC.
- F. V. Rossi, J. T. Starr, D. P. Uccello and J. A. Young, Org. Lett., 2020, 22, 5890–5894 CrossRef CAS PubMed.
- D. E. Potter and M. Choudhury, Drug Discovery Today, 2014, 19, 1848–1854 CrossRef CAS PubMed.
- S. Himmelseher and E. Pfenninger, Anasthesiol. Intensivmed. Notfallmedizin Schmerzther.: AINS, 1998, 33, 764–770 CrossRef CAS PubMed.
- S. Himmelseher and E. Pfenninger, Anasthesiol. Intensivmed. Notfallmedizin Schmerzther.: AINS, 1998, 33, 764–770 CrossRef CAS PubMed.
- L. A. Jelen, A. H. Young and J. M. Stone, J. Psychopharmacol., 2021, 35, 109–123 CrossRef CAS PubMed.
- D. Trauner and T. Ko, Synfacts, 2023, 19, 0416 CrossRef.
- Y.-T. Yen, S.-H. Tseng, S.-L. Zhou and Y.-L. Liu, Forensic Sci. Int., 2023, 349, 111776 CrossRef CAS PubMed.
- C.-y. Chen and X. Lu, Org. Lett., 2019, 21, 6575–6578 CrossRef CAS PubMed.
- P. Kocienski, Synfacts, 2019, 15, 1225 CrossRef.
- M. E. Kopach, U. K. Singh, M. E. Kobierski, W. G. Trankle, M. M. Murray, M. A. Pietz, M. B. Forst, G. A. Stephenson, V. Mancuso and T. Giard, Org. Process Res. Dev., 2009, 13, 209–224 CrossRef CAS.
- S.-M. Son and H.-K. Lee, J. Org. Chem., 2013, 78, 8396–8404 CrossRef CAS PubMed.
- L. K. Thalén, D. Zhao, J. B. Sortais, J. Paetzold, C. Hoben and J. E. Bäckvall, Chem.–Eur. J., 2009, 15, 3403–3410 CrossRef PubMed.
- A. Träff, R. Lihammar and J.-E. Bäckvall, J. Org. Chem., 2011, 76, 3917–3921 CrossRef PubMed.
- A. Kamal, G. R. Khanna, R. Ramu and T. Krishnaji, Tetrahedron Lett., 2003, 44, 4783–4787 CrossRef CAS.
- C. L. Allen, A. A. Lapkin and J. M. Williams, Tetrahedron Lett., 2009, 50, 4262–4264 CrossRef CAS.
- S. Davulcu, C. L. Allen, K. Milne and J. M. Williams, ChemCatChem, 2013, 5, 435–438 CrossRef CAS.
- M. U. Chopadea and A. U. Chopadeb, J. Chem. Pharm. Res., 2015, 7, 466–469 Search PubMed.
- R. Gaston Jr, W. J. Geldenhuys and G. B. Dudley, J. Org. Chem., 2020, 85, 13429–13437 CrossRef CAS PubMed.
- K. Vukics, T. Fodor, J. Fischer, I. Fellegvári and S. Lévai, Org. Process Res. Dev., 2002, 6, 82–85 CrossRef CAS.
- K. E. Poremba, N. T. Kadunce, N. Suzuki, A. H. Cherney and S. E. Reisman, J. Am. Chem. Soc., 2017, 139, 5684–5687 CrossRef CAS PubMed.
- G. Piñeyro and P. Blier, Pharmacol. Rev., 1999, 51, 533–591 Search PubMed.
- N. T. Hatzenbuhler, R. Baudy, D. A. Evrard, A. Failli, B. L. Harrison, S. Lenicek, R. E. Mewshaw, A. Saab, U. Shah and J. Sze, J. Med. Chem., 2008, 51, 6980–7004 CrossRef CAS PubMed.
- Z. Shen, P. S. Ramamoorthy, N. T. Hatzenbuhler, D. A. Evrard, W. Childers, B. L. Harrison, M. Chlenov, G. Hornby, D. L. Smith and K. M. Sullivan, Bioorg. Med. Chem. Lett., 2010, 20, 222–227 CrossRef CAS PubMed.
- M. Furutachi, S. Mouri, S. Matsunaga and M. Shibasaki, Chem.–Asian J., 2010, 5, 2351–2354 CrossRef CAS PubMed.
- A. R. Murphy and J. M. Frechet, Chem. Rev., 2007, 107, 1066–1096 CrossRef CAS PubMed.
- D. Nair, Chem. Mater., 2014, 26, 724 CrossRef CAS.
- T. Mori, T. Nishimura, T. Yamamoto, I. Doi, E. Miyazaki, I. Osaka and K. Takimiya, J. Am. Chem. Soc., 2013, 135, 13900–13913 CrossRef CAS PubMed.
- C. Bharathi, K. Prabahar, C. S. Prasad, M. Srinivasa Rao, G. Trinadhachary, V. Handa, R. Dandala and A. Naidu, Pharmazie, 2008, 63, 14–19 CAS.
- G. Thomas, R. Spandl, F. Glansdorp, M. Welch, A. Bender and J. Cock, Angew. Chem., Int. Ed., 2008, 47, 2808–2812 CrossRef CAS PubMed.
- N. Bontemps, F. Gattacceca, C. Long, O. P. Thomas and B. Banaigs, J. Nat. Prod., 2013, 76, 1801–1805 CrossRef CAS PubMed.
- C. Shen, P. Zhang, Q. Sun, S. Bai, T. A. Hor and X. Liu, Chem. Soc. Rev., 2015, 44, 291–314 RSC.
- Z. Li, J. Hong and X. Zhou, Tetrahedron, 2011, 67, 3690–3697 CrossRef CAS.
- S. Ranjit, R. Lee, D. Heryadi, C. Shen, J. E. Wu, P. Zhang, K.-W. Huang and X. Liu, J. Org. Chem., 2011, 76, 8999–9007 CrossRef CAS PubMed.
- C. Zhang, J. McClure and C. J. Chou, J. Org. Chem., 2015, 80, 4919–4927 CrossRef CAS PubMed.
- E. J. Warawa, B. M. Migler, C. J. Ohnmacht, A. L. Needles, G. C. Gatos, F. M. McLaren, C. L. Nelson and K. M. Kirkland, J. Med. Chem., 2001, 44, 372–389 CrossRef CAS PubMed.
- E. Vieta, G. Parramon, E. Padrell, E. Nieto, A. Martinez-Arán, B. Corbella, F. Colom, M. Reinares, J. M. Goikolea and C. Torrent, Bipolar Disord., 2002, 4, 335–340 CrossRef CAS PubMed.
- N. C. Niphade, A. C. Mali, B. S. Pandit, K. M. Jagtap, S. A. Jadhav, M. N. Jachak and V. T. Mathad, Org. Process Res. Dev., 2009, 13, 792–797 CrossRef CAS.
- M. Li and J. J. Wang, Org. Lett., 2018, 20, 6490–6493 CrossRef CAS PubMed.
- K. P. Bogeso, A. V. Christensen, J. Hyttel and T. Liljefors, J. Med. Chem., 1985, 28, 1817–1828 CrossRef CAS PubMed.
- J. Hyttel and J. J. Larsen, J. Neurochem., 1985, 44, 1615–1622 CrossRef CAS PubMed.
- J. Arnt, A. V. Christensen and J. Hyttel, Naunyn-Schmiedeberg's Arch. Pharmacol., 1985, 329, 101–107 CrossRef CAS PubMed.
- Y. L. Hurd and U. Ungerstedt, Eur. J. Pharmacol., 1989, 166, 251–260 CrossRef CAS PubMed.
- S. S. Negus, M. R. Brandt and N. K. Mello, J. Pharmacol. Exp. Ther., 1999, 291, 60–69 CAS.
- M. Froimowitz, K.-M. Wu, A. Moussa, R. M. Haidar, J. Jurayj, C. George and E. L. Gardner, J. Med. Chem., 2000, 43, 4981–4992 CrossRef CAS PubMed.
- J. C. Pastre and C. R. D. Correia, Adv. Synth. Catal., 2009, 351, 1217–1223 CrossRef CAS.
- K. L. Leach, S. J. Brickner, M. C. Noe and P. F. Miller, Ann. N. Y. Acad. Sci., 2011, 1222, 49–54 CrossRef CAS PubMed.
- G. J. Moran, E. Fang, G. R. Corey, A. F. Das, C. De Anda and P. Prokocimer, Lancet Infect. Dis., 2014, 14, 696–705 CrossRef CAS PubMed.
- I. Berlin, R. Zimmer, H. Thiede, C. Payan, T. Hergueta, L. Robin and A. Puech, Br. J. Clin. Pharmacol., 1990, 30, 805–816 CrossRef CAS PubMed.
- F. Moureau, J. Wouters, D. Vercauteren, S. Collin, G. Evrard, F. Durant, F. Ducrey, J.-J. Koenig and F.-X. Jarreau, Eur. J. Med. Chem., 1994, 29, 269–277 CrossRef CAS.
- S. Arshadi, E. Vessally, M. Sobati, A. Hosseinian and A. Bekhradnia, J. CO2 Util., 2017, 19, 120–129 CrossRef CAS.
- R. M. Hirschfeld and S. Kasper, Int. J. Neuropsychopharmacol., 2004, 7, 507–522 CrossRef CAS PubMed.
- J. Gomez-Arguelles, R. Dorado, J. Sepulveda, A. Herrera, F. G. Arrojo, E. Aragon, C. R. Huete, C. Terrón and B. Anciones, J. Clin. Neurosci., 2008, 15, 516–519 CrossRef CAS PubMed.
- H. V. Kumar and N. Naik, Eur. J. Med. Chem., 2010, 45, 2–10 CrossRef CAS PubMed.
- G. Lee, I. Youn, E. Choi, H. Lee, G. Yon, H. Yang and C. S. Pak, Curr. Org. Chem., 2004, 8, 1263–1287 CrossRef CAS.
- A. Casnati, M. Fontana, G. Coruzzi, B. M. Aresta, N. Corriero, R. Maggi, G. Maestri, E. Motti and N. Della Ca', ChemCatChem, 2018, 10, 4346–4352 CrossRef CAS.
- Q. P. B. Nguyen, J.-N. Kim and T.-H. Kim, Bull. Korean Chem. Soc., 2009, 30, 2093–2097 CrossRef CAS.
- H. A. Blair, Drugs, 2020, 80, 417–423 CrossRef CAS PubMed.
- R. E. Davis and C. U. Correll, Expert Rev. Neurother., 2016, 16, 601–614 CrossRef CAS PubMed.
- P. Li, Q. Zhang, A. J. Robichaud, T. Lee, J. Tomesch, W. Yao, J. D. Beard, G. L. Snyder, H. Zhu and Y. Peng, J. Med. Chem., 2014, 57, 2670–2682 CrossRef CAS PubMed.
- B. Robinson, Chem. Rev., 1963, 63, 373–401 CrossRef.
- G. Mann, J. F. Hartwig, M. S. Driver and C. Fernández-Rivas, J. Am. Chem. Soc., 1998, 120, 827–828 CrossRef CAS.
- J. P. Wolfe, J. Åhman, J. P. Sadighi, R. A. Singer and S. L. Buchwald, Tetrahedron Lett., 1997, 38, 6367–6370 CrossRef CAS.
- A. C. Flick, C. A. Leverett, H. X. Ding, E. McInturff, S. J. Fink, S. Mahapatra, D. W. Carney, E. A. Lindsey, J. C. DeForest and S. P. France, J. Med. Chem., 2021, 64, 3604–3657 CrossRef CAS PubMed.
- J. Langelaar, Appl. Phys., 1975, 6, 61–64 CAS.
- L. Yang, L. Shi, Q. Xing, K.-W. Huang, C. Xia and F. Li, ACS Catal., 2018, 8, 10340–10348 CrossRef CAS.
- F. Qu, G. Ye, W. Qiuye, Q. Falin and Y. Guangming, CN Pat., 1562973, 2004 Search PubMed.
- R. B. Silverman, J. Biol. Chem., 1983, 258, 14766–14769 CrossRef CAS PubMed.
- J. Alliot, E. Gravel, F. Pillon, D.-A. Buisson, M. Nicolas and E. Doris, Chem. Commun., 2012, 48, 8111–8113 RSC.
- J. Lopez-Ibor, J. Guelfi, Y. Pletan, A. Tournoux and J. Prost, Int. Clin. Psychopharmacol., 1996, 11, 41–46 CrossRef PubMed.
- M. Zhang, F. Jovic, T. Vickers, B. Dyck, J. Tamiya, J. Grey, J. A. Tran, B. A. Fleck, R. Pick and A. C. Foster, Bioorg. Med. Chem. Lett., 2008, 18, 3682–3686 CrossRef CAS PubMed.
- J. Tamiya, B. Dyck, M. Zhang, K. Phan, B. A. Fleck, A. Aparicio, F. Jovic, J. A. Tran, T. Vickers and J. Grey, Bioorg. Med. Chem. Lett., 2008, 18, 3328–3332 CrossRef CAS PubMed.
- B. Dyck, J. Tamiya, F. Jovic, R. R. Pick, M. J. Bradbury, J. O'Brien, J. Wen, M. Johns, A. Madan and B. A. Fleck, J. Med. Chem., 2008, 51, 7265–7272 CrossRef CAS PubMed.
- K. Vervisch, M. D'hooghe, K. W. Törnroos and N. De Kimpe, Org. Biomol. Chem., 2009, 7, 3271–3279 RSC.
- P. Zhang, E. A. Terefenko, J. Bray, D. Deecher, A. Fensome, J. Harrison, C. Kim, E. Koury, L. Mark and C. C. McComas, J. Med. Chem., 2009, 52, 5703–5711 CrossRef CAS PubMed.
- Y. Ishizuka, H. Fujimori, T. Noguchi, M. Kawasaki, M. Kishida, T. Nagai, N. Imai and M. Kirihara, Chem. Lett., 2013, 42, 1311–1313 CrossRef CAS.
- X. Rao, N. Li, H. Bai, C. Dai, Z. Wang and W. Tang, Angew. Chem., Int. Ed., 2018, 57, 12328–12332 CrossRef CAS PubMed.
- Y. Ashida, Y. Sato, T. Suzuki, K. Ueno, K. i. Kai, H. Nakatsuji and Y. Tanabe, Chem.–Eur. J., 2015, 21, 5934–5945 CrossRef CAS PubMed.
- Y. Ashida and Y. Tanabe, Chem. Rec., 2020, 20, 1410–1429 CrossRef CAS PubMed.
- J. N. Bauman, K. S. Frederick, A. Sawant, R. L. Walsky, L. M. Cox, R. S. Obach and A. S. Kalgutkar, Drug Metab. Dispos., 2008, 36, 1016–1029 CrossRef CAS PubMed.
- P. Zajdel, K. Marciniec, A. Maślankiewicz, K. Grychowska, G. Satała, B. Duszyńska, T. Lenda, A. Siwek, G. Nowak and A. Partyka, Eur. J. Med. Chem., 2013, 60, 42–50 CrossRef CAS PubMed.
- R. Yoshimura, T. Kishi, H. Hori, A. Ikenouchi-Sugita, A. Katsuki, W. Umene-Nakano, N. Iwata and J. Nakamura, Prog. Neuro-Psychopharmacol. Biol. Psychiatry, 2012, 39, 355–357 CrossRef CAS PubMed.
- D. Shin and S. Hong, Eur. Neuropsychopharmacol., 2012, S300 CrossRef.
- C. Radhika, A. Venkatesham and M. Sarangapani, Med. Chem. Res., 2012, 21, 3509–3513 CrossRef CAS.
- I. Nikitina, R. Gabidullin, E. Klen, L. Tyurina, E. Alekhin and F. Khaliullin, Pharm. Chem. J., 2012, 46, 213–218 CrossRef CAS.
- X.-Q. Deng, M.-X. Song, Y. Zheng and Z.-S. Quan, Eur. J. Med. Chem., 2014, 73, 217–224 CrossRef CAS PubMed.
- M.-X. Song, Y.-S. Huang, Q.-G. Zhou, X.-Q. Deng and X.-D. Yao, Bioorg. Chem., 2021, 106, 104505 CrossRef CAS PubMed.
- L. Quartara and M. Altamura, Curr. Drug Targets, 2006, 7, 975–992 CrossRef CAS PubMed.
- D. H. Barlow, V. M. Durand and S. G. Hofmann, Abnormal Psychology: an Integrative Approach, Cengage learning, 2016 Search PubMed.
- S. H. Preskorn, C. Y. Stanga, J. P. Feighner and R. Ross, Antidepressants: Past, Present and Future, Springer Science & Business Media, 2012 Search PubMed.
- T. Ryckmans, O. Berton, R. Grimée, T. Kogej, Y. Lamberty, P. Pasau, P. Talaga and C. Genicot, Bioorg. Med. Chem. Lett., 2002, 12, 3195–3198 CrossRef CAS PubMed.
- F. Chenu, B. Guiard, M. Bourin and A. Gardier, Behav. Brain Res., 2006, 172, 256–263 CrossRef CAS PubMed.
- G. Hache, F. Coudore, A. M. Gardier and B. P. Guiard, Pharmaceuticals, 2011, 4, 285–342 CrossRef CAS.
- C. Risatti, K. J. Natalie Jr, Z. Shi and D. A. Conlon, Org. Process Res. Dev., 2013, 17, 257–264 CrossRef CAS.
- R. Robles-Machín, J. Adrio and J. C. Carretero, J. Org. Chem., 2006, 71, 5023–5026 CrossRef PubMed.
- E. Vessally, M. Nikpasand, S. Ahmadi, P. Delir Kheirollahi Nezhad and A. Hosseinian, J. Iran. Chem. Soc., 2019, 16, 617–627 CrossRef CAS.
- A. Ghanizadeh, Curr. Drug Saf., 2013, 8, 169–174 CrossRef CAS PubMed.
- K. Das, K. Sarkar and B. Maji, ACS Catal., 2021, 11, 7060–7069 CrossRef CAS.
- H. Kakei, T. Nemoto, T. Ohshima and M. Shibasaki, Angew. Chem., Int. Ed., 2004, 43, 317–320 CrossRef CAS PubMed.
- A. R. Mahableshwarkar, P. L. Jacobsen and Y. Chen, Curr. Med. Res. Opin., 2013, 29, 217–226 CrossRef CAS PubMed.
- U. Slapšak, G. Salzano, L. Amin, R. N. Abskharon, G. Ilc, B. Zupančič, I. Biljan, J. Plavec, G. Giachin and G. Legname, J. Biol. Chem., 2016, 291, 21857–21868 CrossRef PubMed.
- Y. Mao, L. Jiang, T. Chen, H. He, G. Liu and H. Wang, Synthesis, 2015, 47, 1387–1389 CrossRef CAS.
- V. Zubar, A. Dewanji and M. Rueping, Org. Lett., 2021, 23, 2742–2747 CrossRef CAS PubMed.
- J. B. Bariwal, K. D. Upadhyay, A. T. Manvar, J. C. Trivedi, J. S. Singh, K. S. Jain and A. K. Shah, Eur. J. Med. Chem., 2008, 43, 2279–2290 CrossRef CAS PubMed.
- T. Ogawa, N. Kumagai and M. Shibasaki, Angew. Chem., 2012, 124, 8679–8682 CrossRef.
- K. E. Henegar, C. T. Ball, C. M. Horvath, K. D. Maisto and S. E. Mancini, Org. Process Res. Dev., 2007, 11, 346–353 CrossRef CAS.
- A. Ghanizadeh, Nord. J. Psychiatry, 2015, 69, 241–248 CrossRef PubMed.
- N. Benson, N. Snelder, B. Ploeger, C. Napier, H. Sale, N. JM Birdsall, R. P. Butt and P. H. van der Graaf, Br. J. Pharmacol., 2010, 160, 389–398 CrossRef CAS PubMed.
- C. Liu, Z.-W. Lin, Z.-H. Zhou and H.-B. Chen, Org. Biomol. Chem., 2017, 15, 5395–5401 RSC.
- J. C. Fournier, R. J. DeRubeis, S. D. Hollon, S. Dimidjian, J. D. Amsterdam, R. C. Shelton and J. Fawcett, Jama, 2010, 303, 47–53 CrossRef CAS PubMed.
- R. Pies, J. Clin. Psychopharmacol., 2010, 30, 101–104 CrossRef PubMed.
- F. A. Larik, A. Saeed, P. A. Channar and H. Mehfooz, Tetrahedron: Asymmetry, 2016, 27, 1101–1112 CrossRef CAS.
- Q. Yang, L. G. Ulysse, M. D. McLaws, D. K. Keefe, B. P. Haney, C. Zha, P. R. Guzzo and S. Liu, Org. Process Res. Dev., 2012, 16, 499–506 CrossRef CAS.
- S. T. Kaehler, C. Sinner, S. S. Chatterjee and A. Philippu, Neurosci. Lett., 1999, 262, 199–202 CrossRef CAS PubMed.
- C. Erdelmeier, E. Koch and R. Hoerr, Stud. Nat. Prod. Chem., 2000, 22, 643–716 CAS.
- R. Ciochina and R. B. Grossman, Chem. Rev., 2006, 106, 3963–3986 CrossRef CAS PubMed.
- M. Abe and M. Nakada, Tetrahedron Lett., 2006, 47, 6347–6351 CrossRef CAS.
- M. Uwamori and M. Nakada, Tetrahedron Lett., 2013, 54, 2022–2025 CrossRef CAS.
- C. Yin, T. Yang, Y. Pan, J. Wen and X. Zhang, Org. Lett., 2020, 22, 920–923 CrossRef CAS PubMed.
- S. P. Chavan and H. S. Khatod, Synthesis, 2017, 49, 1410–1418 CrossRef CAS.
- A. A. Aboelwafa and E. B. Basalious, AAPS PharmSciTech, 2010, 11, 1026–1037 CrossRef CAS PubMed.
- S. Preskorn, Eur. Psychiatry, 1997, 12, 285s–294s CrossRef PubMed.
- C. Garcia and V. S. Martin, Curr. Org. Chem., 2006, 10, 1849–1889 CrossRef CAS.
- O. Riant and J. Hannedouche, Org. Biomol. Chem., 2007, 5, 873–888 RSC.
- C.-L. Duan, Y.-X. Tan, J.-L. Zhang, S. Yang, H.-Q. Dong, P. Tian and G.-Q. Lin, Org. Lett., 2019, 21, 1690–1693 CrossRef CAS PubMed.
- P. Tian, H.-Q. Dong and G.-Q. Lin, ACS Catal., 2012, 2, 95–119 CrossRef CAS.
- D. Ameen and T. J. Snape, MedChemComm, 2013, 4, 893–907 RSC.
- S. Dhillon, L. J. Scott and G. L. Plosker, CNS Drugs, 2006, 20, 763–790 CrossRef CAS PubMed.
- L. Huang, J. Zhu, G. Jiao, Z. Wang, X. Yu, W. P. Deng and W. Tang, Angew. Chem., Int. Ed., 2016, 55, 4527–4531 CrossRef CAS PubMed.
- H. M. Davies, D. G. Stafford and T. Hansen, Org. Lett., 1999, 1, 233–236 CrossRef CAS PubMed.
- S. K. Armstrong, J. M. Brown and M. J. Burk, Tetrahedron Lett., 1993, 34, 879–882 CrossRef CAS.
- L. T. Boulton, I. C. Lennon and R. McCague, Org. Biomol. Chem., 2003, 1, 1094–1096 RSC.
- B. K. Koe, A. Weissman, W. M. Welch and R. G. Browne, J. Pharmacol. Exp. Ther., 1983, 226, 686–700 CAS.
- S. H. Lee, I. S. Kim, Q. R. Li, G. R. Dong, L. S. Jeong and Y. H. Jung, J. Org. Chem., 2011, 76, 10011–10019 CrossRef CAS PubMed.
- H. Ohmiya, Y. Makida, D. Li, M. Tanabe and M. Sawamura, J. Am. Chem. Soc., 2010, 132, 879–889 CrossRef CAS PubMed.
- J. Wang, M. Wang, P. Cao, L. Jiang, G. Chen and J. Liao, Angew. Chem., Int. Ed., 2014, 53, 6673–6677 CrossRef CAS PubMed.
- J. Zhang, T. Chen, Y. Wang, F. Zhou, Z. Zhang, I. D. Gridnev and W. Zhang, Nat. Sci., 2021, 1, e10021 CrossRef CAS.
- D. W. Robertson, J. H. Krushinski, R. W. Fuller and J. D. Leander, J. Med. Chem., 1988, 31, 1412–1417 CrossRef CAS PubMed.
- S. Chumpradit, M. P. Kung, C. Panyachotipun, V. Prapansiri, C. Foulon, B. P. Brooks, S. A. Szabo, S. Tejani-Butt, A. Frazer and H. F. Kung, J. Med. Chem., 1992, 35, 4492–4497 CrossRef CAS PubMed.
- E. Nowakowska, K. Kus and A. Chodera, Acta Physiol. Hung., 1996, 84, 445–447 CAS.
- J. L. Kirwin and J. L. Gören, Pharmacotherapy, 2005, 25, 396–410 CrossRef CAS PubMed.
- O. Tosic and J. Mattay, Eur. J. Org. Chem., 2011, 203–404 Search PubMed.
- C. Liu, L. Zhang, L. Cao, Y. Xiong, Y. Ma, R. Cheng and J. Ye, Commun. Chem., 2022, 5, 1–10 CrossRef PubMed.
- M. Kowalska, J. Nowaczyk, Ł. Fijałkowski and A. Nowaczyk, Int. J. Mol. Sci., 2021, 22, 1662 CrossRef CAS PubMed.
- M. A. Sultan, R. R. Pillai, E. Alzahrani, A. A. Alsofi, S. A. Al-Qadhi and R. A. Pashameah, J. Chem., 2022, 2022 Search PubMed.
- S. Krautwald, M. A. Schafroth, D. Sarlah and E. M. Carreira, J. Am. Chem. Soc., 2014, 136, 3020–3023 CrossRef CAS PubMed.
- A. J. Catino, R. E. Forslund and M. P. Doyle, J. Am. Chem. Soc., 2004, 126, 13622–13623 CrossRef CAS PubMed.
- H. Keller, R. Schaffner, M. Carruba, W. Burkard, M. Pieri, E. Bonetti, R. Scherschlicht, M. Da Prada and W. Haefely, Adv. Biochem. Psychopharmacol., 1982, 31, 249–263 CAS.
- L. Omer, Int. J. Clin. Pharmacol., Ther. Toxicol., 1982, 20, 320–326 CAS.
- G. Gu, T. Yang, O. Yu, H. Qian, J. Wang, J. Wen, L. Dang and X. Zhang, Org. Lett., 2017, 19, 5920–5923 CrossRef CAS PubMed.
- D. Tian, C. Li, G. Gu, H. Peng, X. Zhang and W. Tang, Angew. Chem., Int. Ed., 2018, 57, 7176–7180 CrossRef CAS PubMed.
- J. J. Gassensmith, J. M. Baumes, J. Eberhard and B. D. Smith, Chem. Commun., 2009, 2517–2519 RSC.
- M. Jarończyk and J. Walory, Molecules, 2022, 27, 533 CrossRef PubMed.
- A. J. Wagstaff, S. M. Cheer, A. J. Matheson, D. Ormrod and K. L. Goa, CNS Drugs, 2002, 16, 425–434 CrossRef PubMed.
- N. S. Gunasekara, S. Noble and P. Benfield, Drugs, 1998, 55, 85–120 CrossRef CAS PubMed.
- K. Nagayasu, J. Pharmacol. Sci., 2022, 295–299 CrossRef CAS PubMed.
- C. F. Despiau, A. P. Dominey, D. C. Harrowven and B. Linclau, Eur. J. Org. Chem., 2014, 2014, 4335–4341 CrossRef CAS PubMed.
- N. Yoshikai, Synlett, 2011, 2011, 1047–1051 CrossRef.
- N. Yoshikai, Bull. Chem. Soc. Jpn., 2014, 87, 843–857 CrossRef CAS.
- S. Prakash, R. Kuppusamy and C. H. Cheng, ChemCatChem, 2018, 10, 683–705 CrossRef CAS.
- P. Gandeepan, T. Müller, D. Zell, G. Cera, S. Warratz and L. Ackermann, Chem. Rev., 2018, 119, 2192–2452 CrossRef PubMed.
- P. J. Mease and E. H. Choy, Clin. Rheum. Dis., 2009, 35, 359–372 CrossRef PubMed.
- M.-Z. Lu, X. Ding, C. Shao, Z. Hu, H. Luo, S. Zhi, H. Hu, Y. Kan and T.-P. Loh, Org. Lett., 2020, 22, 2663–2668 CrossRef CAS PubMed.
- S. P. Chavan, K. P. Pawar and S. Garai, RSC Adv., 2014, 4, 14468–14470 RSC.
- K. Hariprasath, I. Sudheer Babu, P. Venkatesh and U. Upendra Rao, Global J. Inc, 2014, 14, 1 Search PubMed.
- T. P. Laughren, J. Gobburu, R. J. Temple, E. F. Unger, A. Bhattaram, P. V. Dinh, L. Fossom, H. J. Hung, V. Klimek and J. E. Lee, J. Clin. Psychiatry, 2011, 72, 22216 CrossRef PubMed.
- D. S. Robinson, D. K. Kajdasz, S. Gallipoli, H. Whalen, A. Wamil and C. R. Reed, J. Clin. Psychopharmacol., 2011, 31, 643–646 CrossRef CAS PubMed.
- H. Jin, C. Wu, S. Zhou, Y. Xin, T. Sun and C. Guo, Org. Process Res. Dev., 2021, 25, 1184–1189 CrossRef CAS.
- B. Hu, Q. Song and Y. Xu, Org. Process Res. Dev., 2012, 16, 1552–1557 CrossRef CAS.
- S. Yous, J. Andrieux, H. Howell, P. Morgan, P. Renard, B. Pfeiffer, D. Lesieur and B. Guardiola-Lemaitre, J. Med. Chem., 1992, 35, 1484–1486 CrossRef CAS PubMed.
- P. Depreux, D. Lesieur, H. A. Mansour, P. Morgan, H. E. Howell, P. Renard, D.-H. Caignard, B. Pfeiffer and P. Delagrange, J. Med. Chem., 1994, 37, 3231–3239 CrossRef CAS PubMed.
- L. H. H. A. D’haenen, Int. Clin. Psychopharmacol., 2002, 17, 239–247 CrossRef PubMed.
- C. Schäfer, F. Strübe, S. Bringmann and J. Mattay, Photochem. Photobiol. Sci., 2008, 7, 1457–1462 CrossRef PubMed.
- C. I. Stathakis, E. Neokosmidis and T. V. Koftis, Eur. J. Org Chem., 2014, 2014, 6376–6379 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |