Open Access Article
Open Access ArticleA simple ‘turn-on’ fluorescence chemosensor for Al(III) detection in aqueous solution and solid matrix†
Cuiping Yang and
Jianbo Zhao
and
Jianbo Zhao *
*
School of Chemistry and Chemical Engineering, Tarim University, Alar 843300, P. R. China. E-mail: zjb1102@outlook.com
First published on 3rd January 2024
Abstract
A simple fluorescence chemosensor of FHS-OH based on salicylaldehyde Schiff base was developed via a one-step reaction, which achieved a fast and highly selective response for Al(III). Mechanism studies showed that when FHS-OH was exposed to Al(III) with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding stoichiometry in an aqueous solution at neutral pH, C
2 binding stoichiometry in an aqueous solution at neutral pH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and PET processes were limited, resulting in a ‘turn-on’ fluorescence response with a low detection limit of 63 nmol L−1 and a satisfying linear range of 0.0–20.0 μmol L−1. Compared to traditional detection methods for Al(III), fluorometry using FHS-OH has several advantages, including simplicity, quick response, and capability of real-time detection. More importantly, the detection of Al(III) on a solid matrix (test paper) was successfully achieved. After the addition of Al(III), a significant emission colour change from green to bright blue was observed by the naked eye owing to the intrinsic aggregation-induced emission (AIE) characteristic of FHS-OH.
N isomerization and PET processes were limited, resulting in a ‘turn-on’ fluorescence response with a low detection limit of 63 nmol L−1 and a satisfying linear range of 0.0–20.0 μmol L−1. Compared to traditional detection methods for Al(III), fluorometry using FHS-OH has several advantages, including simplicity, quick response, and capability of real-time detection. More importantly, the detection of Al(III) on a solid matrix (test paper) was successfully achieved. After the addition of Al(III), a significant emission colour change from green to bright blue was observed by the naked eye owing to the intrinsic aggregation-induced emission (AIE) characteristic of FHS-OH.
1 Introduction
Aluminium is the third most abundant element in the earth's crust and the most abundant metallic element, constituting about 8% of the total mineral composition. It is a highly toxic metal element for the human body. The accumulation of excessive aluminium can result in serious effects on the brain, liver, and reproductive system. Aluminium is usually found in the form of Al(III) in nature and living organisms. The World Health Organization (WHO) recommends an average dietary intake of Al(III) of around 3–10 mg day−1 and limits the concentration in drinking water to 7.41 μmol L−1.1 Owing to its noteworthy effect on human health, research investigating reliable methods for the detection of Al(III) is gaining much attention.2,3 Fluorometry for the detection of Al(III), which offers simplicity, sensitivity, selectivity, and real-time nondestructive detection, has attracted significant attention compared to traditional instrumental analytical methods, such as atomic absorption spectrometry and potentiometric titration.4–9However, most reported Al(III) fluorescent probes have been used in pure organic solvents or organic/H2O mixed solutions.10–17 This is because of the aggregation-caused quenching (ACQ) of most conventional fluorescent molecules. They typically exhibit strong fluorescence in the dispersed state but weak or no fluorescence in the aggregated state, which limits their applications. In addition, Al(III) is susceptible to hydrolysis, has a weak coordination ability, and is prone to interference from trivalent ions, such as Fe(III) and Cr(III), with similar electronic structures during the detection process.8 These factors limit the detection of Al(III) in pure water under neutral conditions. Unfortunately, physiological processes typically occur in environments with high water content and neutral pH, making the detection of Al(III) in drinking water and biological samples challenging. Furthermore, the development of Al(III) chemosensors is limited by intricate synthesis procedures, low yields, and high expenses.
Aggregation-induced emission is a luminescence characteristic that is opposite to ACQ.18–21 AIE luminogens emit little or no light when dissolved in a benign organic solvent, but emit significantly more light when aggregated or solidified (usually in a poor solvent, such as water). AIE offers a basic remedy for the ACQ effect of the traditional probes and has been highly valued in the design and fabrication of fluorescent probes in physiological samples owing to their typically high signal-to-noise ratio and reliable photostability.
Herein, we report a novel fluorescent ‘turn-on’ probe FHS-OH with salicylaldehyde moieties, which could be used to recognize Al(III) in pure water aqueous solution at pH 7.4. FHS-OH is rich in N and O atoms, which favours coordination with Al(III), resulting in intense fluorescence enhancement. Moreover, FHS-OH was successfully used to detect Al(III) in real water samples and fluorescent test papers.
2 Experimental
2.1. Reagents
All the raw materials used in this work were of analytical grade and used without further purification. 2,4-Dihydroxybenzaldehyde and 2-furoic hydrazide were purchased from Energy Chemical Co., Anhui, China. Unless otherwise noted, all the other materials were purchased from Sinopharm Chemical Reagent Beijing Co., Beijing, China. Deionized water (distilled) was used throughout the experiments. A phosphate buffer solution (PBS, 10 mmol L−1) was obtained by adding an appropriate amount of HCl/NaOH, which was modulated using the pH meter to monitor the pH values.2.2. Instruments
NMR spectra were recorded on a Bruker 600 Avance NMR spectrometer operated at 600 MHz. Fluorescence spectra were obtained on an F-7000 fluorescence spectrophotometer with a 1 cm cuvette. Absorption spectra were obtained on a UV/vis spectrophotometer with a 1 cm cuvette. Dynamic Light Scattering (DLS) experiments were performed using a NanoPlus-3 dynamic light scattering particle size/zeta potential analyser. All the pH measurements were carried out using a PHS-3C pH meter. The photographs were taken on a Nikon Z5 camera.2.3. Analytical procedure
2.4. Synthesis and characterization
The synthetic routes for FHS-OH are shown in Scheme 1.2,4-Dihydroxybenzaldehyde (0.28 g, 2 mmol) and 2-furoic hydrazide (0.25 g, 2 mmol) were dissolved in 20 mL absolute ethanol. Then, the mixture was stirred and refluxed at 80 °C for 30 minutes. After cooling the mixture to room temperature, the solvent was removed under reduced pressure. After that, the crude product was recrystallized from ethanol, obtaining a light yellow solid (393 mg, yield 80%). 1H NMR (DMSO-d6) δ (ppm): 11.94 (s, 1H), 11.33 (s, 1H), 9.97 (s, 1H), 8.51 (s, 1H), 7.95 (s, 1H), 7.28 (dd, J = 14.4 Hz, 2H), 6.70 (s, 1H), 6.37 (d, J = 12.0 Hz, 1H), 6.32 (s, 1H). 13C NMR (DMSO-d6) δ (ppm): 161.22, 159.80, 154.19, 149.64, 137.66, 136.54, 135.61, 134.22, 133.09, 133.04, 131.76, 129.99, 129.22, 129.07, 128.65, 122.04, 121.87, 119.66, 113.02, 110.63. Detailed characterization data can be found in the ESI.†
2.5. Preparation of test paper
The filter paper was placed into an ethanol solution of FHS-OH (50 mmol L−1) for 20 minutes. Then, the filter paper was dried in air for 30 minutes at room temperature. In this way, a test paper was obtained.3 Results and discussion
3.1. Fluorescence response of FHS-OH toward Al(III)
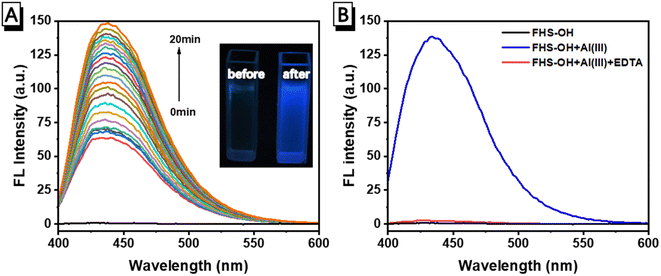
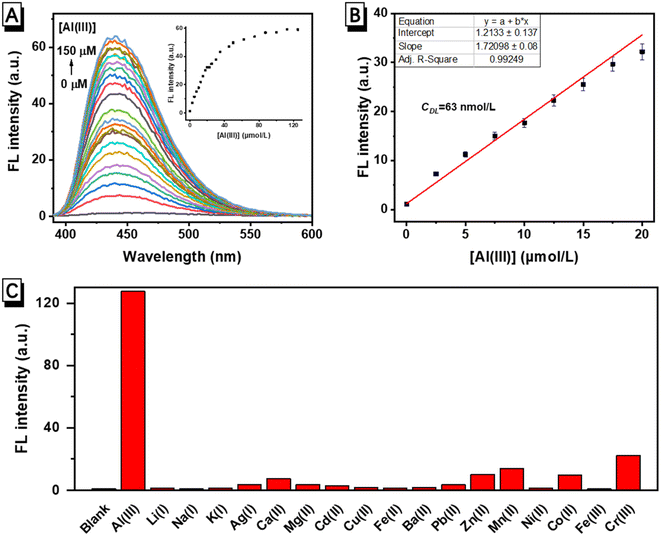
The fluorescence response of FHS-OH to Al(III) in a neutral aqueous solution buffered using 10 mmol L−1 of the phosphate buffer solution (PBS, pH 7.4) at 25 °C was first carried out. As seen in Fig. 1A, there was no fluorescence emission of FHS-OH when Al(III) was not present. Al(III) caused a brilliant blue fluorescence to arise, with the emission peak occurring at 438 nm. After the addition of Al(III), the emission rose progressively, reaching a 106-fold enhancement in about 20 minutes. The fluorescence ‘turn-on’ response could be clearly observed by the naked eye under UV light (Fig. 1A inset), indicating that FHS-OH has the potential to be used as a ‘turn-on’ fluorescence probe for the detection of Al(III) in the aqueous solution at pH 7.4. More importantly, when EDTA was added to the solution of FHS-OH and Al(III), the fluorescence emission at 438 nm quenched, again leaving a characteristic fluorescence emission of FHS-OH (Fig. 1B), which suggested that the combination between FHS-OH and Al(III) was reversible.To verify whether FHS-OH can be used for the quantitative detection of Al(III), a fluorescence titration experiment was performed. When more than 2.0 equiv. Al(III) was added, the fluorescence intensity at 438 nm reached the maximum and varied very slightly, indicating that the binding ratio of FHS-OH to Al(III) might be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Fig. 2A). Furthermore, the calibration curve for the determination of Al(III) was constructed (Fig. 2B). A satisfying linear range of 0.0–20.0 μmol L−1 was achieved with a correlation coefficient of R2 = 0.99 (n = 3). The detection limit was calculated to be 63 nmol L−1 based on the equation of CDL = 3Sb/m (Sb is the standard deviation from 10 blank solutions while m represents the slope of the calibration curve), which is defined by IUPAC. The detection limit (LOD) of FHS-OH is in the normal range compared to some reported molecular probes for the detection of Al(III) in recent years. These results suggested that FHS-OH has the potential to be used in the quantitative detection of Al(III) in aqueous physiological samples. Based on the Benesi–Hildebrand equation, the binding constant K of FHS-OH with Al(III) was determined as 3.32 × 104 M−1 (Fig. S3†).
2 (Fig. 2A). Furthermore, the calibration curve for the determination of Al(III) was constructed (Fig. 2B). A satisfying linear range of 0.0–20.0 μmol L−1 was achieved with a correlation coefficient of R2 = 0.99 (n = 3). The detection limit was calculated to be 63 nmol L−1 based on the equation of CDL = 3Sb/m (Sb is the standard deviation from 10 blank solutions while m represents the slope of the calibration curve), which is defined by IUPAC. The detection limit (LOD) of FHS-OH is in the normal range compared to some reported molecular probes for the detection of Al(III) in recent years. These results suggested that FHS-OH has the potential to be used in the quantitative detection of Al(III) in aqueous physiological samples. Based on the Benesi–Hildebrand equation, the binding constant K of FHS-OH with Al(III) was determined as 3.32 × 104 M−1 (Fig. S3†).
The ability to achieve a highly selective response to analytes is an important characteristic of a good chemical sensor. The selectivity of FHS-OH to Al(III) was further investigated. Fluorescence intensities of FHS-OH at 438 nm in the presence of different metal ions including Al(III), Li(I), Na(I), K(I), Mg(II), Ca(II), Ba(II), Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Ag(I), Zn(II), Cd(II), and Pb(II) exhibited a weak fluorescence emission. As can be seen in Fig. 2C, only Al(III) caused a significant enhancement compared to other metal ions, which proves that Al(III) showed a strong binding affinity towards FHS-OH, indicating an excellent selectivity of FHS-OH to Al(III).
3.2. Response mechanism of FHS-OH toward Al(III)
The superior selectivity of FHS-OH can be attributed to the higher charge density and smaller ionic radius of Al(III), which allows the formation of a stable coordination structure between FHS-OH and Al(III).17,22 Salicylaldehyde Schiff base compounds are generally considered to be poorly fluorescent, partly due to the isomerization of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond in the excited state and partly due to the presence of a photo-induced electron transfer (PET) process within the molecule. A possible mechanism to explain the ‘turn-on’ fluorescence response of FHS-OH towards Al(III) is proposed in Scheme 2. The lone pair electron of nitrogen from C
N double bond in the excited state and partly due to the presence of a photo-induced electron transfer (PET) process within the molecule. A possible mechanism to explain the ‘turn-on’ fluorescence response of FHS-OH towards Al(III) is proposed in Scheme 2. The lone pair electron of nitrogen from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N can be freely transferred to the excited state fluorophore, resulting in fluorescence quenching.23–25 When the nitrogen and oxygen atoms of FHS-OH coordinate with Al(III), the electron-donating ability decreases, and the PET effect is constrained, leading to a notable enhancement of fluorescence emission at 438 nm.26–33 Besides PET and C
N can be freely transferred to the excited state fluorophore, resulting in fluorescence quenching.23–25 When the nitrogen and oxygen atoms of FHS-OH coordinate with Al(III), the electron-donating ability decreases, and the PET effect is constrained, leading to a notable enhancement of fluorescence emission at 438 nm.26–33 Besides PET and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization could also be involved in the fluorescence enhancement. In compounds with an unbridged C
N isomerization could also be involved in the fluorescence enhancement. In compounds with an unbridged C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N structure, C
N structure, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization is the dominant excited state decay process. As a result, the free sensor FHS-OH has very weak fluorescence. In contrast, compounds with a covalently bridged C
N isomerization is the dominant excited state decay process. As a result, the free sensor FHS-OH has very weak fluorescence. In contrast, compounds with a covalently bridged C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N structure are known to show fluorescence intensity due to the restriction of C
N structure are known to show fluorescence intensity due to the restriction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization in the excited states. Likewise, Al(III) binding can lock the C
N isomerization in the excited states. Likewise, Al(III) binding can lock the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of FHS-OH and inhibit the C
N bond of FHS-OH and inhibit the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization. The molecular structure changed from flexible to rigid, resulting in a dramatic increase in the fluorescence intensity.34–41
N isomerization. The molecular structure changed from flexible to rigid, resulting in a dramatic increase in the fluorescence intensity.34–41
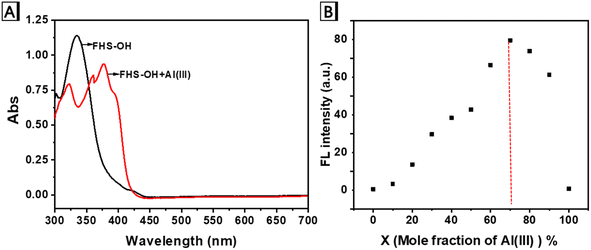
To support the hypothesis, a series of experiments were carried out. As shown in the absorption spectra (Fig. 3A), FHS-OH showed a significant absorption peak at 336 nm. With the addition of Al(III), the absorption at 336 nm gradually decreased, accompanied by three new absorption peaks at 323, 359, and 377 nm, respectively. An isosbestic point was observed at 353 nm, potentially indicating the formation of novel coordination between FHS-OH and Al(III). In addition, the Job's plot analysis was performed to evaluate the binding of FHS-OH and Al(III) (Fig. 3B), with the highest fluorescence intensity achieved when Al(III) accounted for 70%, consistent with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 metal-to-ligand ratio.
2 metal-to-ligand ratio.
3.3. Analytical performance of FHS-OH toward Al(III) in real water samples
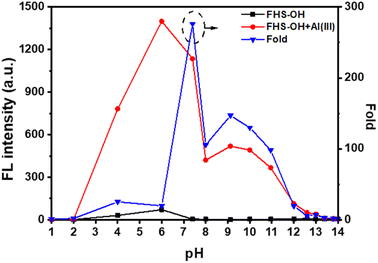
In order to determine the optimum pH for Al(III) detection, the fluorescence spectra of FHS-OH before and after the addition of Al(III) at different pH levels were examined.42 As shown in Fig. 4, the fluorescence intensity of FHS-OH was very weak in pH 1.0–14.0. Interestingly, when Al(III) was added, fluorescence intensity was not affected under strongly acidic conditions (pH = 1.0–2.0). This phenomenon could be attributed to the protonation of oxygen and nitrogen atoms of FHS-OH, which inhibited the coordination with Al(III).43 On the contrary, an intense fluorescence enhancement can be observed under weak acid and neutral conditions, which can be attributed to the formation of FHS-OH and Al(III) chelate polymers (pH = 4.0–8.0). At pH > 8.0, the fluorescence intensity of the system again showed an overall decreasing trend with increasing pH, which may be due to two possible reasons. One is the deprotonation of FHS-OH under alkaline conditions. The other is the formation of Al(OH)3 precipitates, which can prevent the formation of chelate polymers.44,45 These results indicate that the neutral environment is favorable for the chelation of FHS-OH with Al(III). In particular, the maximum signal-to-noise ratio is around pH 7.4, indicating the optimal detection conditions for Al(III), which offers the possibility of achieving bio-detection.Based on the above experimental results, the optimal conditions were obtained as: 50 μmol per L FHS-OH was used as a probe in a 10 mmol per L PBS at pH 7.4. The fluorescence intensity was measured at the excitation/emission wavelengths of 374 nm/438 nm after the reaction with Al(III) for 20 minutes at room temperature. Under optimized conditions, FHS-OH was applied to detect Al(III) in real drinking water and tap water samples by the proposed method, which showed satisfactory recovery and R.S.D. values for all the water samples, demonstrating the potential applications of FHS-OH in real sample analysis (Table 1).
| Sample | Al(III) added (μmol L−1) | Al(III) found (μmol L−1) | Recovery (%) | R.S.D. (n = 3) (%) |
|---|---|---|---|---|
| Drinking water | 0.00 | 0.56 | — | 1.21 |
| 10.00 | 10.63 | 100.7 | 0.98 | |
| Tap water | 0.00 | 6.73 | — | 0.79 |
| 10.00 | 17.01 | 102.8 | 1.11 | |
| Laker water | 0.00 | 9.96 | — | 0.87 |
| 10.00 | 19.85 | 98.9 | 1.02 |
Owing to the advantages of rapid detection and good selectivity, test papers based on FHS-OH for Al(III) detection were successfully fabricated. Interestingly, FHS-OH on the filter paper exhibited green fluorescence rather than no emission under UV-light irradiation. When exposed to Al(III) with certain concentrations, the fluorescence emission changed from green to blue. When the Al(III) concentration was higher than 5 mmol L−1, strong blue fluorescence could be directly observed by the naked eye upon UV-light irradiation (Fig. 5A). More importantly, as illustrated in Fig. 5B, strong blue fluorescence was only observed with the addition of Al(III), indicating that the test papers exhibited good selectivity for Al(III) over other tested metal ions.
3.4. AIE properties of FHS-OH
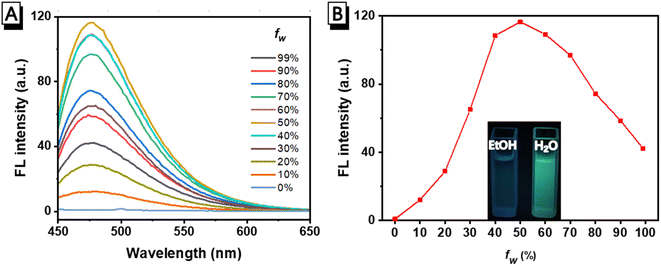
It has been reported that luminogens based on salicylaldehyde Schiff bases usually exhibit AIE properties.46–49 In order to understand whether the green fluorescence of FHS-OH on test papers is derived from its AIE property, the fluorescence intensity of FHS-OH in H2O/EtOH (water fraction fw: 0–99%, v/v) was firstly investigated. Normally, H2O is a poor solvent, whereas EtOH is a good solvent for organic compounds. As can be seen in Fig. 6A and B, there was no fluorescence emission of FHS-OH in pure EtOH, whereas a green fluorescence with an emission peak at about 478 nm increased sharply up to fw = 50% with maximum fluorescence intensity and a 151-fold enhancement compared to that of the EtOH solution. Upon further addition of H2O to the mixture, the fluorescence emission slowly decreased, indicating its AIE property. In addition, dynamic light scattering (DLS) data were collected, which revealed the presence of nanoscale particles in the FHS-OH solution (Fig. S4†). The above results showed that the intense fluorescence of FHS-OH in the solid matrix was attributed to aggregation, i.e., AIE fluorescence.3.5. Comparison with other reported Al(III) chemosensors
To further evaluate the detection performance of FHS-OH, a brief comparison of the sensing performance of FHS-OH with other reported fluorescence probes based on Schiff base for Al(III) recognition is listed in Table 2. As shown in Table 2, despite numerous investigations on sensing Al(III) in organic solvents or organic/H2O, probes that function in aqueous solutions are still limited. In this work, the FHS-OH can be used to detect Al(III) in a pure aqueous medium. In addition, in comparison with other reported probes, FHS-OH possessed other outstanding superiorities in terms of the detection limit, and pH application range, indicating that FHS-OH can act as a promising fluorescence probe for Al(III) detection.4 Conclusions
In summary, a ‘turn-on’ fluorescent probe FHS-OH for Al(III) detection based on salicylaldehyde Schiff base was facilely prepared via a one-step condensation reaction with inexpensive raw materials. FHS-OH exhibited a highly sensitive and selective fluorescence response towards Al(III) with a LOD of 63 nmol L−1. Fluorescence experiments and Job's plot data showed that FHS-OH and Al(III) formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex with an obvious color change from colorless to blue in an aqueous solution. More importantly, FHS-OH was successfully used to establish an effective method for the rapid detection of Al(III) on test paper with an obvious color change from green to blue by the naked eye due to the intrinsic AIE characteristic of FHS-OH. This work provides a new strategy for the trace analysis of Al(III) in a pure water system at neutral conditions.
2 complex with an obvious color change from colorless to blue in an aqueous solution. More importantly, FHS-OH was successfully used to establish an effective method for the rapid detection of Al(III) on test paper with an obvious color change from green to blue by the naked eye due to the intrinsic AIE characteristic of FHS-OH. This work provides a new strategy for the trace analysis of Al(III) in a pure water system at neutral conditions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22268038 and 21865026), the Presidential Research Fund of Tarim University (No. TDZKSS202129).References
- WHO, Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda (who.int), 2022 Search PubMed.
- G. Khairy, A. Amin, S. Moalla, A. Medhat and N. Hassan, Anal. Sci., 2023, 39, 1307–1316 CrossRef CAS PubMed.
- Y. Liu, A. Bi, T. Gao, X. Cao, F. Gao, P. Rong, W. Wang and W. Zeng, Talanta, 2019, 194, 38–45 CrossRef CAS PubMed.
- R. Shanmugapriya, P. Kumar, C. Nandhini, K. Satheeshkumar, K. Vennila and K. Elango, Methods Appl. Fluoresc., 2022, 10, 034005–034018 CrossRef PubMed.
- H. Silva, A. Mageste, S. Silva, G. Dias Ferreira and G. Ferreira, Carbohydr. Polym., 2020, 230, 115679–115686 CrossRef CAS PubMed.
- E. Oliveira, H. Santos, J. Capelo and C. Lodeiro, Inorg. Chim. Acta, 2012, 381, 203–211 CrossRef CAS.
- V. Jisha, K. Arun, M. Hariharan and D. Ramaiah, J. Phys. Chem. B, 2010, 114, 5912–5919 CrossRef CAS PubMed.
- Q. Wang, X. Wen and Z. Fan, J. Photochem. Photobiol., A, 2018, 358, 92–99 CrossRef CAS.
- N. Chereddy, P. Nagaraju, M. Niladri Raju, V. Krishnaswamy, P. Korrapati, P. Bangal and V. Rao, Biosens. Bioelectron., 2015, 68, 749–756 CrossRef CAS PubMed.
- H. Wang, B. Wang, Z. Shi, X. Tang, W. Dou, Q. Han, Y. Zhang and W. Liu, Biosens. Bioelectron., 2015, 65, 91–96 CrossRef CAS PubMed.
- H. Peng, Y. Liu, J. Huang, S. Huang, X. Cai, S. Xu, A. Huang, Q. Zeng and M. Xu, J. Mol. Struct., 2021, 1229, 129866–129871 CrossRef CAS.
- Q. Wang, X. Wen and Z. Fan, J. Photochem. Photobiol., A, 2018, 358, 92–99 CrossRef CAS.
- Y. Jiang, L. Sun, G. Ren, X. Niu, W. Hu and Z. Hu, ChemistryOpen, 2015, 4, 378–382 CrossRef CAS PubMed.
- Y. Zhu, X. Gong, Z. Li, X. Zhao, Z. Liu, D. Cao and R. Guan, Spectrochim. Acta, Part A, 2019, 219, 202–205 CrossRef CAS PubMed.
- I. Song, P. Torawane, J. Lee, S. Warkad, A. Borase, S. Sahoo, S. Nimse and A. Kuwar, Mater. Adv., 2021, 2, 6306–6314 RSC.
- N. Kshirsagar, R. Sonawane, P. Patil, J. Nandre, P. Sultan, S. Sehlangia, C. Pradeep, Y. Wang, L. Chen and S. Sahoo, Inorg. Chim. Acta, 2020, 511, 119805–119811 CrossRef CAS.
- Y. Huang, F. Wang, S. Mu, X. Sun, Q. Li, C. Xie and H. Liu, Spectrochim. Acta, Part A, 2020, 243, 118754–118761 CrossRef CAS PubMed.
- K. Li, Q. Feng, G. Niu, W. Zhang, Y. Li, M. Kang, K. Xu, J. He, H. Hou and B. Tang, ACS Sens., 2018, 3, 920–928 CrossRef CAS PubMed.
- Y. He, Y. Li, H. Su, Y. Si, Y. Liu, Q. Peng, J. He, H. Hou and K. Li, Mater. Chem. Front., 2019, 3, 50–56 RSC.
- Y. Li, Q. Peng, S. Li, Y. Cai, B. Zhang, K. Sun, J. Ma, C. Yang, H. Hou, H. Su and K. Li, Sens. Actuators, B, 2019, 301, 127139–127149 CrossRef CAS.
- C. Yang, Y. Li, J. Wang, J. He, H. Hou and K. Li, Sens. Actuators, B, 2019, 285, 617–624 CrossRef CAS.
- Y. Huang, F. Wang, S. Mu, X. Sun, Q. Li, C. Xie and H. Liu, Spectrochim. Acta, Part A, 2020, 243, 118754–118761 CrossRef CAS PubMed.
- G. Park, M. Lee, G. You, Y. Choi and C. Kim, Tetrahedron Lett., 2014, 55, 2517–2522 CrossRef CAS.
- J. Wang, Y. Li, K. Li, X. Meng and H. Hou, Chem. – Eur. J., 2017, 23, 5081–5089 CrossRef CAS PubMed.
- D. Xiu, J. Shi, M. Deng, H. Song, Z. Hao, Q. Feng and H. Yu, J. Mol. Struct., 2021, 1243, 130761–130767 CrossRef CAS.
- Z. Liu, W. He and Z. Guo, Chem. Soc. Rev., 2013, 42, 1568–1600 RSC.
- H. Wang, B. Wang, Z. Shi, X. Tang, W. Dou, Q. Han, Y. Zhang and W. Liu, Biosens. Bioelectron., 2015, 65, 91–96 CrossRef CAS PubMed.
- N. Kshirsagar, R. Sonawane, P. Patil, J. Nandre, P. Sultan, S. Sehlangia, C. Pradeep, Y. Wang, L. Chen and S. Sahoo, Inorg. Chim. Acta, 2020, 511, 119805–119811 CrossRef CAS.
- Y. Wu, W. Lan, S. He, X. Guo, C. Hai, X. Zhao, H. Chen, W. Long, Y. She and H. Fu, Spectrochim. Acta, Part A, 2023, 298, 122760–122769 CrossRef CAS PubMed.
- T. Zhou, J. Wang, J. Xu, C. Zheng, Y. Niu, C. Wang, F. Xu, L. Yuan, X. Zhao, L. Liang and P. Xu, Anal. Chem., 2020, 92, 5064–5072 CrossRef CAS PubMed.
- C. Li, W. Hu, J. Wang, X. Song, X. Xiong and Z. Liu, Analyst, 2020, 145, 6125–6129 RSC.
- J. Zhu, L. Hu, X. Meng, F. Li, W. Wang, G. Shi and Z. Wang, Molecules, 2023, 28, 3650–3662 CrossRef CAS PubMed.
- F. Bu, B. Zhao, W. Kan, L. Ding, T. Liu, L. Wang, B. Song, W. Wang and Q. Deng, J. Photochem. Photobiol., A, 2020, 387, 112165–112173 CrossRef CAS.
- D. Ray and P. Bharadwaj, Inorg. Chem., 2008, 47, 2252–2254 CrossRef CAS PubMed.
- H. Jung, K. Ko, J. Lee, S. Kim, S. Bhuniya, J. Lee, Y. Kim, S. Kim and J. Kim, Inorg. Chem., 2010, 49, 8552–8557 CrossRef CAS PubMed.
- Z. Li, M. Yu, L. Zhang, M. Yu, J. Liu, L. Wei and H. Zhang, Chem. Commun., 2010, 46, 7169–7171 RSC.
- J. Wu, W. Liu, J. Ge, H. Zhang and P. Wang, Chem. Soc. Rev., 2011, 40, 3483–3495 RSC.
- N. Zhao, Y. Wu, R. Wang, L. Shi and Z. Chen, Analyst, 2011, 136, 2277–2282 RSC.
- J. Wu, W. Liu, X. Zhuang, F. Wang, P. Wang, S. Tao, X. Zhang, S. Wu and S. Lee, Org. Lett., 2007, 9, 33–36 CrossRef CAS PubMed.
- S. Banjan, B. Mondal, G. Vijaykumar and A. Thakur, Inorg. Chem., 2017, 56, 11577–11590 CrossRef PubMed.
- A. Gupta and N. Kumar, RSC Adv., 2016, 6, 106413–106434 RSC.
- Q. Yan, Y. Wang, Z. Wang, G. Zhang, D. Shi and H. Xu, Spectrochim. Acta, Part A, 2022, 279, 121384–121392 CrossRef CAS PubMed.
- Y. Li, K. Xu, Y. Si, C. Yang, Q. Peng, J. He, Q. Hu and K. Li, Dyes Pigm., 2019, 171, 107682–107686 CrossRef CAS.
- J. Wang, Y. Li, K. Li, X. Meng and H. Hou, Chem. – Eur. J., 2017, 23, 5081–5089 CrossRef CAS PubMed.
- J. Wang, L. Feng, J. Chao, Y. Wang and S. Shuang, Anal. Methods, 2019, 11, 5598–5606 RSC.
- J. Kwon and S. Park, Adv. Mater., 2011, 23, 3615–3642 CrossRef CAS PubMed.
- W. Ding, W. Cao, X. Zheng, D. Fang, W. Wong and L. Jin, Inorg. Chem., 2013, 52, 7320–7322 CrossRef CAS PubMed.
- K. Keshav, M. Kumawat, R. Srivastava and M. Ravikanth, Mater. Chem. Front., 2017, 1, 1207–1216 RSC.
- L. Peng, S. Xu, X. Zheng, X. Cheng, R. Zhang, J. Liu, B. Liu and A. Tong, Anal. Chem., 2017, 89, 3162–3168 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra06558h |
| This journal is © The Royal Society of Chemistry 2024 |