Open Access Article
Open Access ArticleRapid deterioration in buried leather: archaeological implications†
Halldórsdóttir H. H.ab,
Williams R. ab,
Greene E. M.
ab,
Greene E. M. c and
Taylor G.
c and
Taylor G. *ab
*ab
aSchool of Health and Life Sciences, Teesside University, Middlesbrough, Tees Valley, TS1 3BX, UK. E-mail: g.taylor@tees.ac.uk
bNational Horizons Centre, 38 John Dixon Lane, Darlington, DL1 1HG, UK
cFaculty of Arts and Humanities, Department of Classical Studies, Western University, 1151 Richmond St., London, Ontario N6A 5B8, Canada
First published on 25th January 2024
Abstract
Understanding archaeological leather degradation helps inform economies, crafts, and technologies of historic communities. However, archaeological leather is at high risk of degradation due to deterioration and changes within the burial conditions. This research applied non-destructive FTIR-ATR to experimentally buried vegetable-tanned leather and archaeological leather excavated at the Roman site of Vindolanda, UK to explore survival, destruction, and preservation processes of tanned leather. Analyses focused on observing and monitoring changes in chemical functional groups related to leather tannins, collagen and lipid components following burial. FTIR-ATR results highlighted rapid changes following experimental burial in wet soil, tentatively associated with early onset microbial activity, which targeted readily available lipids but not tightly bound collagen. Prior to burial, differences in structural composition were present in leather spectra based on manufacture; however, following burial in wet soil, FTIR-ATR spectra indicated de-tanning occurs rapidly, especially in waterlogged conditions, with archaeological leather becoming more uniform and similar to untanned leather. Therefore, the comparison of FTIR-ATR results from archaeological leather to experimentally buried leather samples was informative for showing the destructive de-tanning in waterlogged environments. The comparison of FTIR-ATR data from modern unburied leather cannot be compared against archaeological samples. Importantly, despite de-tanning occurring soon after burial, the vegetable-tanning method promoted long-term preservation of leather in wet soil. The observed changes could not be directly associated with the proportion of condensed to hydrolysable tannin, suggesting alternate variables impacted the preservation. Furthermore, mineral components introduced into the leather through the animal skin, tannin material and/or tannin liquid are suggested to contribute to these changes. Crucially a high degree of heterogeneity in error results within the experimentally buried sample material underlined that any changes in collagen ratios cannot be overinterpreted and must be considered within the context of larger datasets.
1 Introduction
Leather is a versatile material whose recovery from archaeological contexts can yield valuable insights into past societies. Vegetable-tanned leather is the only type of cured skin that routinely survives burial in wet soil environments in temperate climates.1,2 Vegetable-tanned leather is therefore the primary material available to archaeologists to understand past leather making technology and trade in regions such as Northern Europe, where vegetable-tanning technology became common following the expansion of the Roman Empire. In this paper, archaeological and experimentally buried leather samples were analysed using Fourier-transform infrared analysis with attenuated transmission reflectance (FTIR-ATR) to observe and monitor major changes in chemical functional groups related to leather tannins, collagen and lipid components following burial. FTIR spectra were also collected from the tannin material itself and untanned hide, capturing the major structural differences between four stages of the leather material life cycle: the raw material, the tanned leather, the decaying buried leather and the preserved archaeological leather. The respective impact of differences in leather manufacture and differences in the soil environment on FTIR spectra were investigated, as well as changes in FTIR peak ratios related to the preservation of collagen in skin-based material.1.1 Leather and its degradation
Vegetable tannins are a diverse group of polyphenolic compounds originating in plant material, most of which can be divided into condensed and hydrolysable tannins.3,4 Vegetable tannins are incorporated into the collagen matrix of animal hides during the vegetable-tanning process. The incorporation of tannins occurs through interaction with collagen in the skin at partially charged primary structure amine and amide sidechains, displacing tightly bound water between the collagen fibrils. The tanning process has been described by Covington5 as the ‘link–lock’ mechanism, where linking applies to the initial interaction of collagen and tannins and ‘locking’ applies to the displacement of tightly bound water. Carsote and Badea6 have suggested that in the degradation of vegetable-tanned leather, tannin removal must take place before the collagen complex can begin to degrade. Vyskočilová et al.,7 have further suggested that some extent of destabilisation must take place in the collagen-tannin matrix prior to de-tanning. Further work has indicated that vegetable-tanned leather degradation under atmospheric conditions (historical leather, as opposed to buried leather), and tannin type (hydrolysable or condensed) are the main catalysts behind patterns of degradation in vegetable-tanned leather.7–9 Thus, tannins are hypothesised to be the major variable to predict for its survival in soil as well. However, attempts at characterising vegetable-tannins in archaeological leather from wet soil environments have not been successful.1.2 FTIR and leather
Fourier-Transform Infrared spectroscopy (FTIR) is a rapid, versatile, analytical technique that allows archaeologists to screen for organic preservation in artefacts with minimal sample preparation.10,11 FTIR-ATR has been applied to archaeological research topics, including: damage assessments of lignin, hemicellulose and cellulose in wood,12 assessing sample purity of calcite, soil carbonates or humic acids in collagen prior to radiocarbon analysis,13 screening for collagen preservation in bone and skin using amide and hydrogen bond peaks,10,11,14,15 and the distinction of non-, low-, or high-intensity burning in bone using crystallinity index.16FTIR has been applied to historical and archaeological leather samples to identify bond structures associated with tanning compounds and organic dyes,8,17–21 and in damage assessments with focus on the integrity of the collagen backbone.22,23 Furthermore, FTIR-ATR spectra have been used on buried leather samples to detect collagen preservation and destabilisation.7 The FTIR spectra of vegetable-tanned leather are complex, making peak assignments highly complicated, but the key components that may be reflected are collagen (vegetable) tannins and lipids (specific peaks summarised in ESI, Table 1†).
This paper explores the relationship between leather structural composition and burial environment using FTIR-ATR. The focus was on early-onset interactions between internal components in vegetable tanned leather and external factors from the burial environment to provide insight into degradation and survival of leather.
2 Experimental
2.1 Experimental burial development
Experimentally buried leather samples constitute two types of dyed (Madder and Vinegaroon), undyed, oiled (neatsfoot oil) and unoiled vegetable-tanned leather (oak-tanned, mimosa-tanned, and chestnut-tanned), buried in a suite of laboratory-based wet soil microcosms under two main groups (Table 1). Chrome-tanned leather was not used because it was not representative of leather practices at Vindolanda. Group 1 consisted of a single type of oak-tanned leather samples buried in a suite of non-arid conditions, excavated in 2-month intervals over 8 months. Group 2 consisted of different types of leather samples in a single soil condition type, excavated at 4 and 8 months.| Group 1 | Group 2 | |
|---|---|---|
| Remit | Assess impact of differences in soil environment | Assess impact of differences in leather manufacturing |
| Soil types | TS, WL and LO (wet and dry) | TS only |
| Soil pH | Acidic, neutral and basic | Neutral only |
| Leather tannage | Oak tanned | Oak-, mimosa- and chestnut-tanned |
| Oiling | Oiled | Oiled and unoiled |
| Dyeing | Undyed | Madder-, vinegaroon-, or undyed |
| Excavation regime | 2, 4, 6 and 8 months | 4 and 8 months |
| Other | Cold control and No sample control | Iron inclusions near leather sample |
The microcosms were prepared in 500 ml Azlon bottles, modelled after three common soil conditions encountered in the wet soils at Vindolanda, where the archaeological material used in the study originated:
• Typical soil conditions (TS).
• Waterlogged soil conditions (WL).
• Low-oxygen soil conditions (LO).
TS and LO microcosms were modelled after the most common soil conditions encountered at Vindolanda: perennially wet or damp soil that is not visibly saturated or waterlogged, attempting to minimize access of oxygen to LO microcosms through routine flushing with nitrogen gas (N2).
2.2 Leather samples
Modern leather samples were provided by Thomas Ware & Sons. Samples included oak-, chestnut- and mimosa-tanned leather from cattle, untanned cowhide, and tannin material.Archaeological leather samples were excavated from Vindolanda with the help of the Vindolanda Trust and excavation volunteers during the summer excavation season of 2018. Leather samples were collected to be representative of the variety of soil layers encountered (see Table 2 in ESI†), while also targeting contexts rich in anaerobic sample material. Entire leather artefacts were not collected for this study, but rather material and scraps that were deemed suitable for the experimental approach undertaken.
2.3 FTIR analysis
Analysis was undertaken using a Nicolet iS5 FTIR, with a single bounce iD7 ATR component, ZnSe crystal and a KBr beam splitter was used, with OMNIC 9.8.372 software. Measurements were collected at 4000–400 cm−1 in absorbance mode, with 64 scans at 4 cm−1 resolution, collecting root-mean-squared (RMS) noise measurements from 2200–2000 cm−1. A performance test was run and passed on the FTIR before analysis, and the crystal stage was cleaned between samples using 2-propanol. Background scans were completed every 120 minutes. An automatic atmospheric moisture compensation was applied, and all spectra were normalised to 0–1 absorption during post processing.Freeze-dried skin and leather samples were gently scraped at the flesh (bottom) surface using a clean scalpel to create a smoother and cleaner surface on the leather for FTIR. Triplicate measurements were collected on the flesh side, and the sample was moved between scans to ensure heterogeneity. Powdered tannin material was also analysed in triplicate in direct contact with the crystal. Peak heights in leather samples were collected using a TQ Analyst EZ 9.8.208 quantitation file, allowing automatic calculation of all peaks identified in the leather spectra after analysis, taking into account possible shifts in wavenumber position caused by varying tannin material and crosslinking.7,33 Triplicate peak heights acquired from this process were averaged and used for statistical testing. The TQ Quant file is available in the ESI, Table 3.†
3 Results and discussion
3.1 Error ranges
High peak absorbance RSD values were expected due to the non-destructive analysis method and heterogeneous nature of the leather samples, which had varying fibre sizes, uneven sampling locations and soil adherence at various levels of decay. The % RSD values of unburied leather samples (n = 10) ranged from 0.1 to 30.0%, with a mean value of 10.4%, reflecting a high amount of variability even before burial. % RSD values above 30% were only present for 4% of the total datapoints, associated with specific samples or low absorbance values (<0.05 abs), but the TQ analyst file collected data for all peaks with no LOQ specified and low-value datapoints were removed at a later point during post-processing. The % RSD values highlighted that non-destructive FTIR-ATR analysis of buried vegetable tanned leather is most appropriate when the goal is to identify deviations or patterns within larger datasets. In archaeological leather samples, % RSD values ranged from 0.4 to 87.1%, with high values again associated with low collected values, averaging at 25.4%.Raw averaged FTIR peak measurements and error data are available in the ESI Tables† and Fig. 1. Root-mean-squared (RMS) noise ranged from 0.004 to 0.03 (abs), with a mean and median value of 0.012. In modern experimentally buried leather samples, relative standard deviations (% RSD) between triplicate measurements of the same sample ranged from 0.03% to 82.1%, with a mean of 11.9%, 1st and 3rd quartiles of 5.2% and 16.4%.
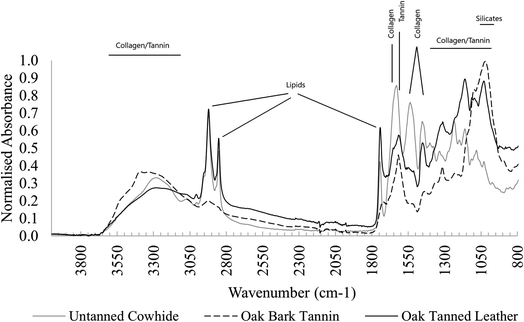
3.2 FTIR spectra of cowhide and vegetable-tanned leather
FTIR spectra of unburied leather samples show all major leather components (Fig. 1). Several changes occur after tanning in all types of leather, including restructuring of crosslinks and realignment of collagen fibrils.9,34 These changes include:• Amide I peak shifted to higher wavenumbers.
• Amide II peak shifted to lower wavenumbers.
• Amide I and II peaks broadened into a double-shouldered peak shape.
• Amide III peaks became less clear due to crosslinking and overlapping with tannin peaks.
• Peaks in the area 100–1150 cm−1 became more structured, impacted by strongly absorbing tannin peaks.
3.3 Rapid changes to leather FTIR spectra following experimental burial reflected in archaeological material
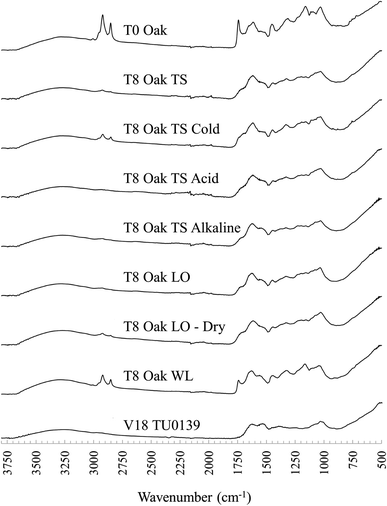
Overall, peaks in the FTIR spectra quickly became less structured following burial and in samples buried in ambient TS and LO microcosms, peaks associated with lipids (∼2955 cm−1, ∼2920 cm−1, ∼2850 cm−1, ∼1740 cm−1, and ∼1160 cm−1) decreased, often disappearing completely after only 2 months of burial, with minor further changes occurring until 8 months of burial (see Fig. 2).Vyskočilová et al.,22 also noted rapid changes in buried leather samples following burial and immediate soil reaction with vegetable tannins as well as absorption of mineral salts from the soil environment. The most likely cause of the difference to WL buried leather, which appears to maintain a structure more similar to pre-burial, is early microbe and fungal activity. Access of microbial organisms to easily degradable lipids will have been impeded by the cold environment in cold TS microcosms and oxygen-limited saturated conditions in WL microcosms.35–37 For all experimentally buried samples, changes to FTIR peaks at wavenumbers associated with crosslinked collagen material appeared were more gradual than of peaks related to lipids, possibly associated with succession to more slowly degrading microbial species in the soil.35,38
The broad N–H amide A band at ∼3300 cm−1 was present in all samples while the lipid peak at wavenumber 1740 cm−1 was not present in any archaeological samples. Lipid peaks at wavenumbers 2920 cm−1 and 2855 cm−1 persisted in a few archaeological samples, possibly representative of surface contamination. The FTIR spectra of archaeological leather samples lost definition between wavenumbers ∼1200–1500 cm−1 compared to experimentally buried samples, although the peak at 1450 cm−1 was present in most samples, with a small shoulder at ∼1235 cm−1 present in several samples. No new peaks were observed in experimentally buried leather samples, but in Vindolanda samples, the area between ∼1000 cm−1 and 1200 cm−1 became dominated by a double peak band, absorbing at ∼1000 cm−1 and 1030 cm−1, losing other peak structures related to tannin material in the experimentally buried samples. The large, double-banded peak is probably representative of Si–O bonding, arising from soil adherence or infiltration. Evidence of soil contamination is also indicated by the presence of peaks at 925 cm−1 in some samples, representative of Al–OH bonding, and a 1415–875 cm−1 peak doublet most likely representative of calcium carbonate (CaCO3).
3.4 De-tanning following burial in wet soil
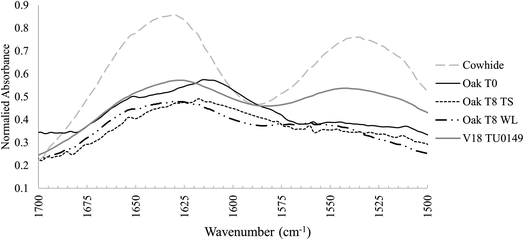
The FTIR spectra of waterlogged buried leather samples appeared to reflect that archaeological leather is best preserved in these conditions.The two primary characteristics observed were: (1) FTIR spectra of leather samples buried in waterlogged soil microcosms remained largely unchanged from their pre buried state, with slightly distorted peak structures at ∼1100–1030 cm−1 (see T8 Oak WL in comparison to T0 Oak – Fig. 2). The comparison between buried and unburied shows unchanged lipid peaks and no introduction of new peaks that could be related to the ingress of iron (expected because of their distinct black colour, induced by a ferric dye reaction). However, there was an indication of change in amide I peak structure after burial in waterlogged microcosms that was not noted in leather samples from other microcosms: the peak shoulder that had shifted from ∼1630 cm−1 to ∼1620 cm−1 following tanning crosslinking and the broadened amide I and II tanned peaks had narrowed and reverted towards their original position after burial (Fig. 2). This indicated a de-tanning process following burial in water saturated soil, which would be expected to leave collagen molecules vulnerable to decay and displacement, a pattern that was not observed in leather samples buried in typical soil cold control microcosms or waterlogged microcosms.
De-tanning has been associated with a destabilisation of the collagen network structure,6 but research into modern leather processing has shown that controlled removal of crosslinks from tanned leather does not have a bad impact.39 Furthermore, evidence of de-tanning was also observed within the archaeologically buried samples, where collagen peaks resembled non-crosslinked untanned skin spectra, not tanned leather spectra. Archaeological samples had no lipid peaks, indicating that whether or not they were subject to microbial degradation in clay sealed deposits at Vindolanda, there was probably clear microbial access at the onset of degradation (Fig. 3).22 Yet, leather is routinely recovered in good preservation states from Vindolanda. Thus, these results challenge claims that the leather tannins are the most important component for long term survival of vegetable-tanned leather, and that other mechanisms must be in place. Furthermore, the results highlight the importance of studying diagenetic processes at the onset of degradation which then informs long-term survival mechanisms.
3.5 FTIR peak ratios and collagen preservation assessment
Peak ratios are used to assess collagen stability and were calculated from the averaged triplicate peak absorbance values, to assess collagen degradation and crosslinking in the leather samples (Tables 2 and 3). All amide peak wavenumbers shifted and broadened after tanning, and therefore the wavenumbers traditionally used to calculate the ratios could not be used. Instead, the wavenumbers with the best internally linear fits of wavenumbers were ascribed to each peak in the experimentally buried leather samples, considered indicative of minimal inference from differences in the burial environment or manufacturing. Consequently, amide A was read at 3300 cm−1, amide I at 1620 cm−1, amide II at 1535 cm−1, and amide III at 1200 cm−1.| Peaks | Wavenumbers (cm−1) before and after tanning | ||
|---|---|---|---|
| Cowhide | Vegetable tanned leather | Shift | |
| Amide A | 3300 | 3300 | No |
| Lipid I | 2920 | 2920 | No |
| Lipid II | 2850 | 2850 | No |
| Lipid III | 1740 | 1740 | No |
| Amide I | 1630 | 1620, 1650 | Yes |
| Amide II | 1535 | 1535, 1515 | Yes |
| Hydroxyproline residue | 1450 | 1450 | No |
| Amide III | 1400, 1335, 1235 | 1365, 1335, 1315, 1225 | Yes |
| Lipid IV | 1160 | 1160 | No |
| Unassigned II | 1080 | — | No |
| Unassigned III | 1030 | — | No |
| Amide A/amide I | Amide I/amide II | 1450/amide III | 1620/1650 | 1630 | 1535 | ΔI/II | ||
|---|---|---|---|---|---|---|---|---|
| Unburied leather samples | Min | 0.6 | 3.1 | 0.9 | 1.1 | 1612 | 1530 | 58 |
| Mean | 0.8 | 7.2 | 2.0 | 1.2 | 1616 | 1539 | 77 | |
| Max | 1.1 | 20.4 | 4.9 | 1.4 | 1618 | 1560 | 88 | |
| Range | 0.5 | 17.3 | 4.0 | 0.3 | 6.0 | 30.0 | 30 | |
| Experimentally buried | Min | 0.4 | 1.7 | 0.4 | 1.0 | 1618 | 1503 | 57 |
| Mean | 0.7 | 8.8 | 1.2 | 1.2 | 1619 | 1540 | 79 | |
| Max | 0.9 | 30.7 | 3.7 | 1.7 | 1630 | 1560 | 112 | |
| Range | 0.5 | 29.0 | 3.3 | 0.7 | 12 | 55 | 55 | |
| Archaeological samples | Min | 0.6 | 3.1 | 0.0 | 1.0 | 1618 | 1517 | 58 |
| Mean | 0.8 | 4.2 | 0.8 | 1.1 | 1623 | 1543 | 79 | |
| Max | 1.5 | 8.9 | 3.2 | 1.1 | 1628 | 1560 | 105 | |
| Range | 0.9 | 5.8 | 3.2 | 0.1 | 10 | 43 | 47 | |
Peak ratio ranges in unburied samples were dependent on tannage and dye, showcasing that the ability of amide ratios to assess degradation in archaeological vegetable-tanned leather are likely to be dependent on past manufacturing. The results presented in this paper support previous results on leather degradation,22,40,41 where specific ratio values have been used to assess protein health in vegetable-tanned leather FTIR. For example, the amide I/amide II ratio in new leather lies between 1.25 and 1.3, whereas values above 1.8 indicate acid hydrolysis but below 1.0 demonstrate alkaline hydrolysis.7,9,42 Low values for the 1620/1650 ratio are tenuously associated with lower percentage leather shrinkage. Furthermore, the findings support those of Vyskočilová et al.,23 who observed a disassociation between published values used to assess leather health in modern/historical leather to those in buried leather. The range of unburied leather amide ratios was occasionally larger than the range in buried counterparts, and if de-tanning occurs soon after burial without clear impact on collagen ‘health’, then assessing the preservation of buried leather samples relative to unburied leather counterparts is not appropriate. Alternatively, it is suggested that a comparison to untanned skin, well-preserved and well-characterised buried archaeological leather samples, and/or samples from burial experiments, could give a clearer idea of the state of preservation for archaeological leather samples buried in wet soil environments.
3.6 Soil based degradation is dependent on tannin type
Although, de-tanning appears to occur early on following burial, amide ratios and ΔI/II values in experimentally buried leather samples were clearly associated with leather manufacture. Fig. 4 shows how most of the buried oak-tanned samples in group 2 had amide A/amide I ratios ranging from 0.50–0.65. Although, four samples had amide A/amide I ratios up to 0.9, indicating weaker crosslinks. No buried oak-tanned leather sample in group 2 had ΔI/II values above 65, despite unburied counterparts having ΔI/II values between 83 and 89 (Table 3). Three mimosa-tanned leather samples had ΔI/II values below 90, similar to unburied counterparts, but all other mimosa- and chestnut-tanned samples had ΔI/II values between 109 and 115, indicating collagen gelatinisation at the surface analysed by the FTIR, and amide A/amide I ratios ranging from 0.6–0.9, associated with crosslink dissolution. Chestnut- and mimosa-tanned leather samples with high ΔI/II values and amide A/amide I ratios above 0.6 were also associated with higher 1620/1650 ratios, which Godfrey and Kasi43,44 tenuously associated with collagen defibrillation, as well as higher 1450/amide III ratios, possibly indicating changes to the tertiary collagen structure. As seen in Fig. 4, archaeological leather sample ΔI/II values were generally higher than in experimentally buried oak-tanned leather samples, but lower than those of experimentally buried mimosa- and chestnut-tanned leather samples. Amide A/amide I ratios were similar to those in experimentally buried samples, with four samples showing greater signs of collagen dissolution (amide A/amide I) and hydrolysis (amide I/amide II).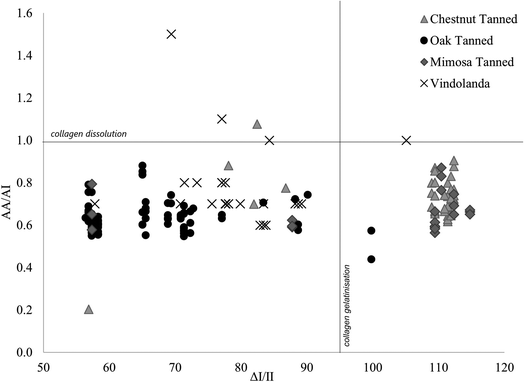 | ||
| Fig. 4 Scatterplot of amide A/amide I ratios against ΔI/II values for all experimentally buried (divided by tanning type) and archaeological Vindolanda samples. | ||
Fig. 4 demonstrates that differences in relative proportions of hydrolysable to condensed tannins had little impact on leather degradation in experimentally buried samples, whereas tannin species were more significant. This is shown by oak-tanned leather samples being clearly differentiated from chestnut- and mimosa-tanned leather samples, and mimosa and chestnut leathers grouping together. Mimosa and chestnut leather samples were tanned with different proportions of the same condensed and hydrolysable tannin material (mimosa, chestnut and myrobalans), leaving mimosa-tanned leather with ∼70% condensed tannin and chestnut-tanned leather with ∼70% hydrolysable tannins, while oak leather samples were tanned with only ∼60% condensed tannins from a different plant species (oak). During excavation and visual assessment of samples, it was clear early on that mimosa- and chestnut-tanned leather samples were not as well preserved as oak-tanned leather samples after burial. It is also interesting to see that only one archaeological leather sample showed signs of collagen gelatinisation with ΔI/II values lining more closely with those of oak-tanned leather samples. This clearly demonstrates that the method of vegetable-tanning does impact preservation of leather in soil, despite de-tanning occurring following burial. If tannin molecules in archaeological leather samples dissolute relatively soon after burial in wet soil, this raises the question as to why vegetable-tanned leather samples are continuously the main type of cured skin encountered in great quantities in wet archaeological soil environments.
4 Conclusions
FTIR spectra of unburied and buried leather samples were presented, peaks were related to structural components that were either skin collagen, lipids, tannins, or dye components and used to monitor major qualitative changes that occurred during leather tanning and later burial. Error results demonstrated high variability in analysed values and a need for caution when interpreting small-scale changes and indicated that FTIR-ATR analysis of buried vegetable-tanned leather is most appropriate when the goal is to identify deviations or patterns within larger datasets.After only two months of burial, considerable changes were observed in the leather FTIR spectra in ambient typical soil and low oxygen microcosms, as lipid peaks decreased quickly in most samples, appearing catalysed by microbial access, while other peaks degraded and/or distorted more gradually. No new peaks were identified in experimentally buried leather spectra, indicative of minimal participation of exogenous elements from the soil environment in organic structural components after burial. The difference between experimentally buried spectra and archaeological spectra was qualitatively not major, supporting that experimental burials are useful, and provided insight into the interpretation of the archaeological material. A greater variability of peak ratios was present between modern buried and unburied leather samples, as unburied sample ratios regularly covered similar or greater ranges than peak ratios collected after burial, indicating that unburied samples are not suitable proxies when the goal is to assess collagen preservation in buried leather samples. This was further confirmed by the observation that following burial, crosslinking in vegetable-tanned leather samples can revert rapidly. For example, FTIR spectra of samples buried in waterlogged microcosms revealed a de-tanning effect, a necessary precursor step preceding collagen denaturation, but seemingly without much further damage. This was particularly curious as archaeological leather samples are most commonly retrieved from waterlogged deposits and its impact on long term burial requires further examination. Amide peak ratios and their relative positions further revealed that despite an apparent de-tanning effect, the long-term recovery of leather artefacts from wet soil environments can still be subject to a manufacturing bias, especially at archaeological timescales.
The role of post-burial perimineralisation on long term survival of non-mineral organic material like plant remains and coprolites have been researched in detail22,23,45–48 and shown that elemental cementation can carry an important role for the preservation of vegetable-tanned leather. Possibly, a role for post-burial elemental accumulation in leather samples, impacted by the charge of the vegetable-tannin species present in the leather, could be important for long-term stability. However, well-established research has shown that a higher presence of calcium and phosphorous in carnivore faeces (scat), as opposed to mostly organic molecules in herbivore dung, has been associated with a better long-term preservation of scat biomolecules.47 The notion of a material role for manufacture-dependent elemental presence in vegetable-tanned leather prior to burial onset, introduced through either the tannin material elemental chemistry, tanning liquid or time spent in it, has however not been explored in detail.
Author contributions
Halldórsdóttir: conceptualization, writing, formal analysis and data curation. Williams: writing – review and editing. Greene: review and editing. Taylor: supervision, editing, funding acquisition, project administration, conceptualization, methodology.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was supported by The Vindolanda Trust.References
- W. G. V. Groenman-van Waateringe, M. Kilian and H. Van Londen, The curing of hides and skins in European prehistory, Antiquity, 1999, 73(282), 884–890 CrossRef.
- C. van Driel-Murray, Tanning and Leather, in Engineering and Technology in the Classical World, ed. J. P. Oleson, Oxford University Press, Oxford, 2008 Search PubMed.
- A. E. Hagerman, Tannin Handbook, Miami University, Oxford, 2002 Search PubMed.
- A. Ricci, K. J. Olejar, G. P. Parpinello, P. A. Kilmartin and A. Versari, Application of Fourier transform infrared (FTIR) spectroscopy in the characterization of tannins, Appl. Spectrosc. Rev., 2015, 50(5), 407–442 CrossRef CAS.
- A. D. Covington, Tanning Chemistry: the Science of Leather. Croydon, RS Publishing, 2009 Search PubMed.
- C. Carsote and E. Badea, Micro differential scanning calorimetry and micro hot table method for quantifying deterioration of historical leather, Heritage Sci., 2019, 7(1), 1–13 CrossRef.
- G. Vyskočilová, M. Ebersbach, R. Kopecká, L. Prokeš and J. Příhoda, Model study of the leather degradation by oxidation and hydrolysis, Heritage Sci., 2019, 7(1), 1–13 CrossRef.
- L. Falcão and M. E. M. Araújo, Vegetable tannins used in the manufacture of historic leathers, Molecules, 2018, 23(5), 1–12 CrossRef PubMed.
- C. Sendrea, C. Carsote, E. Badea, A. Adams, M. Niculescu and H. Iovu, Non-invasive characterisation of collagen based materials by NMR mouse and ATR-FTIR, UPB Sci. Bull. B: Chem. Mater. Sci., 2016, 78(3), 27–38 CAS.
- G. Pothier Bouchard, S. M. Mentzer, J. Riel-Salvatore, J. Hodgkins, C. E. Miller and F. Negrino, et al., Portable FTIR for on-site screening of archaeological bone intended for ZooMS collagen fingerprint analysis, J. Archaeol. Sci. Rep., 2019, 26(101862), 1–12 Search PubMed.
- M. Lebon, I. Reiche, X. Gallet, L. Bellot-Gurlet and A. Zazzo, Rapid quantification of bone collagen content by ATR-FTIR spectroscopy, Radiocarbon, 2016, 58(1), 131–145 CrossRef CAS.
- K. High and K. Penkman, Analytical Methods for Assessing Preservation in Waterlogged Archaeological Wood: Their Importance for Site Management Decisions, Swindon: Historic England, 2019 Search PubMed.
- M. D'Elia, G. Gianfrate, G. Quarta, L. Giotta, G. Giancane and L. Calcagnile, Evaluation of possible contamination sources in the 14C analysis of bone samples by FTIR spectroscopy, Radiocarbon, 2007, 49(2), 201–210 CrossRef.
- B. Demarchi, S. Hall, T. Roncal-Herrero, C. L. Freeman, J. Woolley and M. K. Crisp, et al., Protein sequences bound to mineral surfaces persist into deep time, eLife, 2016, 5(e17092), 1–50 Search PubMed.
- Y. C. Lee, C. C. Chiang, P. Y. Huang, C. Y. Chung, T. D. Huang and C. C. Wang, et al., Evidence of preserved collagen in an Early Jurassic sauropodomorph dinosaur revealed by synchrotron FTIR microspectroscopy, Nat. Commun., 2017, 8(14220), 1–8 CAS.
- S. T. D. Ellingham, T. J. U. Thompson, M. Islam and G. Taylor, Estimating temperature exposure of burnt bone - A methodological review, Sci. Justice, 2015, 55(3), 181–188 CrossRef PubMed.
- L. Falcão and M. E. M. Araújo, Tannins characterization in historic leathers by complementary analytical techniques ATR-FTIR, UV-Vis and chemical tests, J. Cult. Herit., 2013, 14(6), 499–508 CrossRef.
- L. Falcão and M. E. M. Araújo, Tannins characterisation in new and historic vegetable tanned leathers fibres by spot tests, J. Cult. Herit., 2011, 12(2), 149–156 CrossRef.
- A. G. Püntener and S. Moss, Ötzi, the iceman and his leather clothes, Chimia, 2010, 64(5), 315–320 CrossRef PubMed.
- A. Elnaggar, M. Leona, A. Nevin and A. Heywood, The Characterization of Vegetable Tannins and Colouring Agents in Ancient Egyptian Leather from the Collection of the Metropolitan Museum of Art, Archaeometry, 2017, 59(1), 133–147 CrossRef CAS.
- M. Iorio, V. Graziani, S. Lins, S. Ridolfi, P. Branchini and A. Fabbri, et al., Exploring manufacturing process and degradation products of gilt and painted leather, Appl. Sci., 2019, 9(3016), 1–15 Search PubMed.
- G. Vyskočilová, R. Kopecká, D. Pavliňák, M. Laichmanová, I. Sedláček and A. Orlita, et al., The influence of soil environment on the degradation of archaeological leather, Archaeometry, 2022, 64(2), 483–499 CrossRef.
- G. Vyskočilová, C. Carşote, R. Ševčík and E. Badea, Burial-induced deterioration in leather: a FTIR-ATR, DSC, TG/DTG, MHT and SEM study, Heritage Sci., 2022, 10(7), 1–14 Search PubMed.
- A. Barth and C. Zscherp, What vibrations tell us about proteins, Q. Rev. Biophys., 2002, 35(4), 369–430 CrossRef CAS PubMed.
- A. Barth, Infrared spectroscopy of proteins, Biochim. Biophys. Acta, Bioenerg., 2007, 1767(9), 1073–1101 CrossRef CAS PubMed.
- B. De Campos Vidal and M. L. S. Mello, Collagen type I amide I band infrared spectroscopy, Micron, 2011, 42(3), 283–289 CrossRef PubMed.
- S. Ye, H. Li, W. Yang and Y. Luo, Accurate determination of interfacial protein secondary structure by combining interfacial-sensitive amide i and amide III spectral signals, J. Am. Chem. Soc., 2014, 136(4), 1206–1209 CrossRef CAS PubMed.
- T. M. Greve, K. B. Andersen and O. F. Nielsen, ATR-FTIR, FT-NIR and near-FT-Raman spectroscopic studies of molecular composition in human skin in vivo and pig ear skin in vitro, Spectroscopy, 2008, 22(6), 437–457 CrossRef CAS.
- E. Wenting, H. Siepel and P. A. Jansen, Stoichiometric variation within and between a terrestrial herbivorous and a semi-aquatic carnivorous mammal, J. Trace Elem. Med. Biol., 2020, 62(126622), 1–7 Search PubMed.
- A. M. Neiva, M. A. Sperança, V. C. Costa, M. A. C. Jacinto and E. R. Pereira-Filho, Determination of toxic metals in leather by wavelength dispersive X-ray fluorescence (WDXRF) and inductively coupled plasma optical emission spectrometry (ICP OES) with emphasis on chromium, Environ. Monit. Assess., 2018, 190(10), 1–15 CrossRef CAS PubMed.
- M. Bardet, G. Gerbaud, L. Le Pape, S. Hediger, Q. K. Trân and N. Boumlil, Nuclear magnetic resonance and electron paramagnetic resonance as analytical tools to investigate structural features of archaeological leathers, Anal. Chem., 2009, 81(4), 1505–1511 CrossRef CAS PubMed.
- A. L. Lucius, J. M. Miller and B. Rajendar, Application of the sequential n-step kinetic mechanism to polypeptide translocases, Methods Enzymol., 2011, 488(1), 239–264 CAS.
- N. M. Puica, A. Pui and M. Florescu, FTIR spectroscopy for the analkysis of vegetable tanned ancient leather, Eur. J. Sci. Theol., 2006, 2(4), 49–53 Search PubMed.
- L. He, C. Mu, J. Shi, Q. Zhang, B. Shi and W. Lin, Modification of collagen with a natural cross-linker, procyanidin, Int. J. Biol. Macromol., 2011, 48(2), 354–359 CrossRef CAS PubMed.
- J. Rui, J. Peng and Y. Lu, Succession of bacterial populations during plant residue decomposition in rice field soil, Appl. Environ. Microbiol., 2009, 75(14), 4879–4886 CrossRef CAS PubMed.
- S. C. Voss, D. F. Cook and I. R. Dadour, Decomposition and insect succession of clothed and unclothed carcasses in Western Australia, Forensic Sci. Int., 2011, 211(1–3), 67–75 CrossRef PubMed.
- G. Turner-Walker, Early bioerosion in skeletal tissues: Persistence through deep time, Neues Jahrb. Geol. Paläontol., Abh., 2012, 265(2), 165–183 CrossRef.
- C. Bisker, G. Taylor, H. Carney and T. K. Ralebitso-Senior, A Combined Application of Molecular Microbial Ecology and Elemental Analyses Can Advance the Understanding of Decomposition Dynamics, Front. Ecol. Evol., 2021, 9(605817), 1–22 Search PubMed.
- K. H. Sizeland, R. L. Edmonds, M. M. Basil-Jones, N. Kirby, A. Hawley and S. Mudie, et al., Changes to collagen structure during leather processing, J. Agric. Food Chem., 2015, 63(9), 2499–2505 CrossRef CAS PubMed.
- C. Chahine, Changes in hydrothermal stability of leather and parchment with deterioration: A DSC study, Thermochim. Acta, 2000, 365(1–2), 101–110 CrossRef CAS.
- Z. Sebestyén, Z. Czégény, E. Badea, C. Carsote, C. Şendrea and E. Barta-Rajnai, et al., Thermal characterization of new, artificially aged and historical leather and parchment, J. Anal. Appl. Pyrolysis, 2015, 115, 419–427 CrossRef.
- M. G. Albu, M. V. Ghica, M. Leca, L. Popa, C. Borlescu and E. Cremenescu, et al., Doxycycline delivery from collagen matrices crosslinked with tannic acid, Mol. Cryst. Liq. Cryst., 2010, 523, 97–105 CAS.
- The analysis and treatment of waterlogged leather II – application of fourier transform infrared soectroscopy and articifical neural networks, Proceedings of the 9th ICOM Group on Wet Organic Archaeological Materials Conference, ed. I. M. Godfrey and K. Kasi, International Council of Museums (ICOM), Bremerhaven, 2005 Search PubMed.
- The analysis and treatment of waterlogged leather I: Denaturation and defibrilisation studies, Proceedings of the 7th ICOM-CC Working Group on Wet Organic Archaeological Materials Conference ARC-Nucleart, ed. I. M. Godfrey and K. Kasi, Grenoble, 1998 Search PubMed.
- D. E. G. Briggs and P. R. Wilby, The role of the calcium carbonate-calcium phosphate switch in the mineralization of soft-bodied fossils, J. Geol. Soc., 1996, 153(5), 665–668 CrossRef CAS.
- S. D. M. Allen, M. J. Almond, M. G. Bell, P. Hollins, S. Marks and J. L. Mortimore, Infrared spectroscopy of the mineralogy of coprolites from Brean Down: Evidence of past human activities and animal husbandry, Spectrochim. Acta, Part A, 2002, 58(5), 959–965 CrossRef PubMed.
- L. J. R. Marshall, M. J. Almond, S. R. Cook, M. Pantos, M. J. Tobin and L. A. Thomas, Mineralised organic remains from cesspits at the Roman town of Silchester: Processes and preservation, Spectrochim. Acta, Part A, 2008, 71(3), 854–861 CrossRef PubMed.
- L. M. Shillito and M. J. Almond, Comment on: Fruit and seed biomineralization and its effect on preservation by E. Messager et al.; in: Archaeological and Anthropological Sciences (2010) 2:25-34, Archaeol. Anthropol. Sci., 2010, 2(3), 225–229 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra07020d |
| This journal is © The Royal Society of Chemistry 2024 |