Open Access Article
Open Access ArticlePyrrolo[2,1-a]isoquinoline scaffolds for developing anti-cancer agents
Leidy J. García Mazaa,
Arturo Mendoza Salgado a,
Vladimir V. Kouznetsov
a,
Vladimir V. Kouznetsov b and
Carlos M. Meléndez
b and
Carlos M. Meléndez *a
*a
aFacultad de Ciencias Básicas, Grupo de Investigación de Química Orgánica y Biomédica, Universidad del Atlántico, Barranquilla, Colombia. E-mail: carlosmelendez@mail.uniatlantico.edu.co
bLaboratorio de Química Orgánica y Biomolecular, Escuela de Química, Universidad Industrial de Santander, Piedecuesta 680002, Colombia
First published on 5th January 2024
Abstract
Fused pyrrolo[2,1-a]isoquinolines have emerged as compelling molecules with remarkably potent cytotoxic activity and topoisomerase inhibitors. This comprehensive review delves into the intricate world of this family of compounds, analyzing the natural marine lamellarins known for their diverse and complex chemical structures, exploring structure–activity relationships (SARs), and highlighting their remarkable versatility. The review emphasizes their fundamental role as topoisomerase inhibitors and cytotoxic agents, as well as some crucial aspects of the chemistry of pyrrolo[2,1-a]isoquinolines, exploring synthetic strategies in total synthesis and molecular diversification trends, highlighting their importance in the field of medicinal chemistry and beyond.
Introduction
Natural products from plants and marine organisms have become a hotspot of research over the past thirty-five years due to their remarkable capacity to produce diverse, complex chemical substances.1 Marine alkaloids called lamellarins are among the most promising families of natural marine products with various biological properties. As a result, there has been a growing interest in this alkaloid family, the bio-origin of which starts with 2-amino-3-(3′,4′-dihydroxyphenyl)propionic acid, known as DOPA.2 These DOPA-derived marine pyrrole alkaloids have been extensively studied.3 Since the discovery of the first lamellarins A–D in 1985, more than 70 lamellarins and their natural and synthetic analogs have been isolated and described to date.4 Having a pyrrolo[2,1-a]isoquinoline scaffold, the lamellarins and related alkaloids (lukianols, polycitrins, storniamides, and ningalins) exhibit a wide range of biological activities, including cytotoxicity against tumor cells, anti-cancer activity, multi-drug resistance reversal activity, and antibiotic and antioxidant properties, making these compounds a singularly important subject for research.5Cancer is the leading cause of death and represents a large and diverse group of diseases. The only effective therapy is chemotherapy, which frequently fails due to innate or acquired multi-drug resistance (MDR). Marine natural products represent an attractive source of bioactive chemical diversity.6 Recently, target-selective lamellarin analogs have been designed, synthesized, and evaluated as anti-cancer agents. Several lamellarins have been reported to display significant cytotoxicity, with IC50 values in the submicromolar range against a range of cancer cell lines.7 However, only a few showed interesting bioactive properties, whereas others (e.g., lamellarins D, K, and M) are highly potent cytotoxic compounds, which have been categorized as promising lead compounds for cancer therapy in a wide variety of cancer cells due to their potent inhibition of Topoisomerase I.8,9 For this reason, several efficient and versatile chemical routes to access diverse lamellarins and their pyrrolo[2,1-a]isoquinoline-based analogs have been portrayed.1 Besides, the number of reported marine alkaloids continues to grow at an increasing rate, allowing extensive biological studies on their potential applications.10
It is already clear that DNA-manipulating enzymes constitute privileged targets for lamellarins. The Structure–Activity Relationship (SAR) studies indicated that these marine alkaloids could be considered a multi-target effector. For instance, they target Topoisomerase I (Topo-I), protein kinases (PTKs), and act on mitochondria.4 Consequently, these crucial discoveries have encouraged the research community's studies on lamellarins and related alkaloids.
It is well known that various groups have provided an overview of the main achievements of lamellarin alkaloids and related pyrrole-derived alkaloids. Recently, Matveeva et al.11 performed a detailed and rigorous analysis as much they could, of the synthetic approaches, bioactivity, mechanism of action, pharmacophore and structure–activity relationships of synthetic analogs of the pyrrolo[2,1-a]isoquinoline (PIq) scaffold covering 2009 to 2019. However, the progress in the synthesis and biological characterization of small molecules built up on the PIq moiety has become necessary due to great interest of modern organic chemists in pursuing efficient synthetic routes for the development of lamellarin-derived bioactive compounds. The above focused mainly on the potential exploitation of the scaffold-like pyrrolo[2,1-a]isoquinolines in design and development of anti-cancer agents. We present an updated and comprehensive review of lamellarins alkaloids and related pyrrole-derived alkaloids summarizing their synthesis and anti-cancer activity and providing vital information essential to developing anti-cancer compounds of this series. Additionally, the total synthesis of selected lamellarins, and advanced synthetic methods of preparing for isoquinoline and pyrrolo[2,1-a] isoquinoline derivatives were briefly described. Moreover, the SAR between natural lamellarin alkaloids or alkaloid-like synthetic pyrrolo[2,1-a]isoquinolines and cytotoxic effects was examined.
Lamellarins and anticancer activity analysis
Lamellarins, pyrrolo[2,1-a]isoquinoline-based molecules (Type-I, Type-Ia and Type-Ib), and related pyrrole-derived alkaloids (Type-II) (Fig. 1) are frequently cytotoxic to a wide range of cancer cell lines, serving as a starting point in the design of anti-cancer compounds due to their interesting biological activity.12 The first compounds in the series of natural products alkaloids were called lamellarins A to D, and were identified by Faulkner in 1985 from the Palauan prosobranch mollusk Lamellaria sp.13 The lead compound in the family is lamellarin D (lam-D) (1), characterized as a potent inhibitor of both nuclear and mitochondrial Topo-I. Lam-D exhibited significant cytotoxicity against a large panel of cancer lines being capable of directly interfering with mitochondria to trigger cancer cell death.1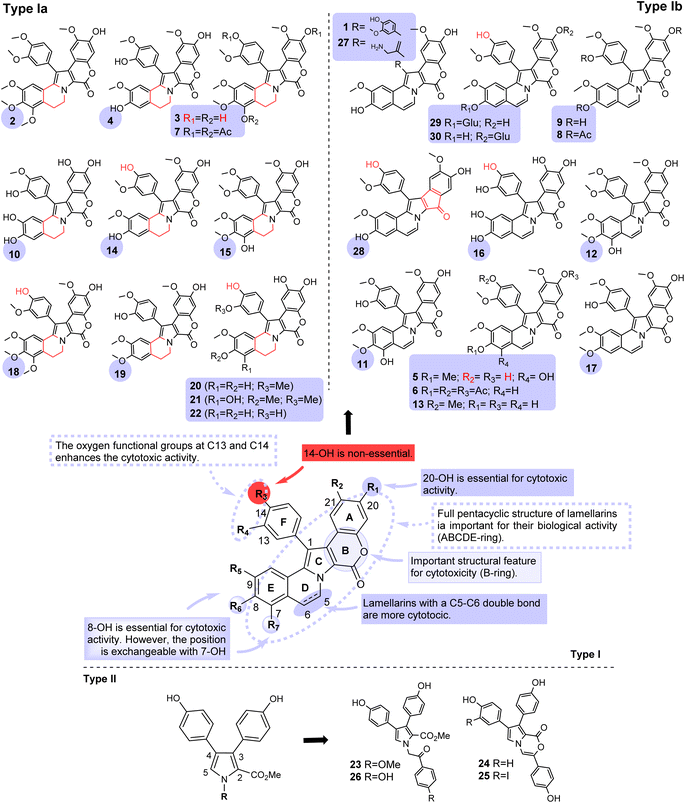 | ||
| Fig. 1 Scaffolds of fused type I and non-fused type II lamellarins with potent cytotoxic activity. The compound numbers correspond to the order of appearance throughout the text. | ||
In 1993, lamellarins I, J, K, L, and M (2–5) were isolated from an Australian colonial ascidian Didemnum sp.14 Bowden and coworkers reported that lamellarins 2–4 displayed significant cytotoxicity against P388 and A549 cell lines (IC50 ≈ 0.25 μg mL−1 against each cell line). In 1996, Quesada and coworkers found that lamellarins 3 and 5, lamellarins D triacetate (6), K triacetate (7), and N triacetate (8) exhibited high activity against a variety of cancer cell lines (P388, AUXB1, A549, HT29, and MEL28) in the nanomolar to the sub-nanomolar range.15 Lamellarins 6 and 7 showed the highest activity against the A549 cell line with IC50 values of 0.008 and 0.005 μM, respectively (Fukuda, Ishibashi, and Iwao, 2020). In 1997, Faulkner et al. showed that lamellarin N (9) exhibited some selectivity toward the melanoma cell line SK-MEL-5 (LC50 = 0.187 μM) and UACC-62 (LC50 = 9.88 μM).16 In 2002, Ham and Kang reported that lamellarin β (10) showed cytotoxicity against human promyelocytic leukemia HL-60 with an IC50 = 4.8 μg mL−1.17 Also, lam-I (2) at a concentration of 2 μM could reverse the resistance of those P388 murine leukemia cells resistant to anti-cancer drugs like doxorubicin.18 Furthermore, in 2010, a SAR (Structure–Activity Relationship) study on diverse lamellarins was reported.19 Performed SAR studies were the first to reveal the importance of having a C-7–OH group of lamellarin core for biological activity and also confirmed that the presence of the C–C double bond (C5![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C6) increased cytotoxicity; C-20- and C-8–OH groups are responsible for the cytotoxic activity and also for Topo-I inhibition while methylation of the C-7–OH- or 8–OH-groups decreased the cytotoxic activities of the lamellarins, especially those containing a C5
C6) increased cytotoxicity; C-20- and C-8–OH groups are responsible for the cytotoxic activity and also for Topo-I inhibition while methylation of the C-7–OH- or 8–OH-groups decreased the cytotoxic activities of the lamellarins, especially those containing a C5![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C6 double bond. Among the alkaloids tested, lamellarins D (1), X (11), ε (12), M (5), N (9), and dehydro-lam-J (13) were the most potent anti-tumor of the family with IC50 values from sub-nanomolar (0.08 nM) to micromolar (3.2 μM) showing selectivity against cancer cells: lung, breast, liver, and blood cells.20 The most promising compound was lamellarin 9.19 In 2013, Yoshida and co-workers reported its synthesis and cytotoxic activity on three cancer cell lines (IMR32, HeLa, and SH-SY5Y) disclosing its high IC50 values (0.019–0.040 μM) and the Topo-I inhibitory activity which was slightly less potent than that of lam-D.21
C6 double bond. Among the alkaloids tested, lamellarins D (1), X (11), ε (12), M (5), N (9), and dehydro-lam-J (13) were the most potent anti-tumor of the family with IC50 values from sub-nanomolar (0.08 nM) to micromolar (3.2 μM) showing selectivity against cancer cells: lung, breast, liver, and blood cells.20 The most promising compound was lamellarin 9.19 In 2013, Yoshida and co-workers reported its synthesis and cytotoxic activity on three cancer cell lines (IMR32, HeLa, and SH-SY5Y) disclosing its high IC50 values (0.019–0.040 μM) and the Topo-I inhibitory activity which was slightly less potent than that of lam-D.21
Alkaloids lamellarin χ (14), F (15), and lamellarin L triacetate (4) revealed excellent activities against colorectal cancer cells (COLO-205).22 Besides, lamellarin H (16) and α (17) exhibited good effectiveness against a panel of eight human tumor cell lines displaying mean IC50 = 4.0 μM, min/max IC50 ratio = 20, and mean IC50 = 2.9 μM, min/max IC50 ratio = 10, respectively. Lamellarins C (18) and U (19) also demonstrated potent cytotoxicity against ten human tumor cell lines (A549, HCT-116, LOX IMVI, MALME-3M, MCF-7, MOLT-4, OVCAR-3, PC-3, SF-295, UO-31) with IC50 ranging from 0.4 to 19.4 nM.10 In 2012, the SAR studies indicted interactions between lamellarins and P-glycoprotein (P-gp), an ABC transporter efflux pump, intending to reverse multidrug resistance (MDR) in a human colon cancer cell line (SW620 Ad300). The comparative cytotoxicity of lamellarins A1, A2, and S (20–22) indicated that they could be P-gp substrates. The SAR data also suggested that the P-gp inhibitory activity roughly correlated with higher methylation levels on rings A and F of these lamellarins. The hexamethylated lam-I (2), the only lamellarin with published data supportive of P-gp inhibitory activity, was consistent with this methylation hypothesis.7
In 1995, tri-substituted pyrrole open forms were found in the Australian marine sponge Dendrilla cactus, they were called lamerallin O and P.23 The pyrrole ring-closed analogs of lamellarin O, such as lukianol A and lukianol B (23–25), exhibited moderate activity against a cell line derived from human epidermoid carcinoma (KB) with a minimum inhibitory concentration (MIC) of 2.4 and 185.9 nM, respectively. In addition, alkaloids 23 and 24 proved to be effective cytotoxic agents in some leukemia and lymphoma screens and also active in human HeLa–S3 uterine. Moreover, lukianol A (24) showed a selective activity against colon adenocarcinoma (SW-480) growth10 and the 4′-O-dimethyl analog of lam-O identified as lamellarin O1 (26), resulted be more active (IC50 < 10 μM) than lamellarin O (23) (IC50 > 22 μM) against parental (SW620) and P-gp-overexpressing (SW620 Ad300) colon cancer cell lines6,24 Lately, it was found that alkaloid 23 and its 4′-O-dimethyl analog 26 act as P-gp inhibitors capable of reversing MDR.3 Structure–activity relationship revealed that the methoxy-acetophenone moiety of lamellarin 23 is a critical determinant of the inhibition of the breast cancer resistance protein (BCRP). These efflux transporters, P-gp and BCRP have been implicated to be the major efflux transporters responsible for MDR in cancer cells.
Several lamellarin derivatives exhibited potent anti-cancer activities, with promising IC50 values. Particularly, lamellarins D (1), K (3), and M (5) are usually classified among the most cytotoxic molecules in the series.25 These compounds exhibited cytotoxicity values in the nanomolar range (38–110 nM). Lam-D (1) presented a potent cytotoxic activity against a large panel of tumor cells types in vitro, especially in human prostate cancer cells (DU-145, LNCaP)10 and at doses in the micromolar range, exhibiting high apoptotic activities in leukemia cells (K562).26 Open lactone analogs of lam-D were evaluated against a panel of three human tumor cell lines, MDA-MB-231 (breast), A-549 (lung), and HT-29 (colon).27 The open chain lam-D analogs data concluded that more than 75% of the tested compounds showed cytotoxicity in a low micromolar GI50 range. In addition, the amino derivative PM031379 (27) displayed potent anti-cancer activities in vivo in a human colon tumor.8 Recently, Colligs demonstrated that a lam-D analog 28 with a five-membered cyclopentanone ring instead of the lactone ring (B-ring-contracted) displayed lower activity than camptothecin (CPT) in wild-type CCRF-CEM cell lines with sub-micromolar IC50 values.9 Consequently, another important structural feature for cytotoxicity is appearing in the presence of the lactone ring (B-ring) belonging to the pentacyclic system.
A new class of antineoplastic agents was generated by attaching different glycosyl groups on hydroxy groups at C-8, C-14, or C-20 positions of lam-D. Among the glycosylated derivatives, ZL1 (29) (IC50HCT116 = 14 nM; IC50HepG2 = 24 nM) and ZL3 (30) (IC50A549 = 3 nM; IC50HCT116 = 10 nM; IC50HepG2 = 15 nM) showed potent anti-proliferation activities against all the cell lines. The cytotoxic activities of ZL1 and ZL3 were even better than lam-D (IC50HCT116 = 25 nM; IC50HepG2 = 88 nM). However, the cytotoxicity of the lam-D-bearing glycosyl group at C-14-OH was decreased in the cell lines' cultures. The result demonstrated that most glycosylated derivatives kept the potent Topo-I inhibitory activity similar to lam-D.
The cytotoxicity was improved after glycosylation with glucose or galactose at the C-8- and C-20–OH positions of lam-D.4 The structure–activity relationships in the lam-D analogs (29, 30) revealed that small changes in their structure are sufficient to reduce, and in some cases to lose, the cytotoxicity of this parent alkaloid. The studies showed that hydroxyl groups at the C-8 and C-20 positions would be essential for their cytotoxicity. In contrast, the methoxy groups at C-13 and C-21 positions appeared to be less important for the cytotoxic activity.10 Notably, the high activity of alkaloids 1, 9, and 13, which possess two hydroxy groups at C-8 and C-20 positions, supported our previous SAR study described above.3 However, the increase in the number of methylations and/or methoxylations in lamellarins appears to cause a decrease in the anti-tumor activities.15
In most cases, the cytotoxic activity of lamellarins-type Ia was lower than of lamellarins-type Ib, as exemplified by the lamellarin M (5) (IC50 = 0.04 μM against A549) versus lamellarin K (3) (IC50 = 4.2 μM against A549) case, as well as the 5,6-double bond compared between lam-D (1) (GI50MCF-7/GI50HCT-116/GI50NCI-H522 < 0.01 μM) and lamellarin χ (14) (GI50MCF-7 = 0.98 μM; GI50HCT-116 = 0.78 μM; GI50NCI-H522 = 0.27 μM).3 Lamellarins 5, 11, and 12, possessing a C-7 hydroxy group, also showed high activity even when the methyl group blocked the hydroxy group at the C-8 position. These findings suggested that the hydroxy group could be placed at either C-7 or C-8 positions. The pioneer works8,14,18,19,27–33 have contributed to the discovery and synthesis of new lamellarins and then to the characterization of the modes of action of this group of marine alkaloids. Most of these natural and synthetic lamellarin derivatives have been characterized from a chemical and structural point of view, but until today their mechanisms of action remain incompletely understood.
Biochemical mechanisms of lamellarins
Our knowledge of the action mode of natural products is fragmentary, which is certainly also the case for lamellarins. Lamellarins are potent cytotoxic agents; however, their antiproliferative activity on cancer cells in vitro varies according to the structural characteristics of such alkaloidal molecules (Fig. 2). At the molecular level, the mechanism of action of lamellarins remains largely unknown. The only member extensively studied is lam-D which has been the subject of many pharmacological studies over the past 12 years.1 In 2003, Bailly and co-workers discovered that the lam-D functions as a poison of the Topo-I enzyme. Still, it is clear now that a pleiotropic mode of action is responsible for its antiproliferative activity in cancer cells.34 As detailed below, At least three types of effects have been described for this marine alkaloid and its related products.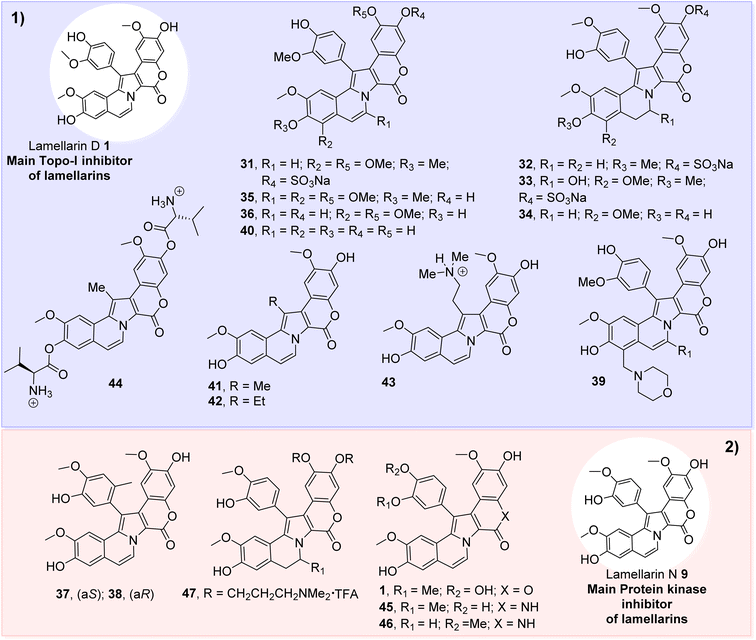 | ||
| Fig. 2 Some lamellarin compounds as promising inhibitors. (1) Lamellarins with potent anti-topoisomerase activity; (2) some lamellarins as potent protein kinase inhibitors. | ||
Inhibition of topoisomerase I
Topoisomerase I is a nuclear enzyme that facilitates the relaxation of supercoiled DNA for the duration of replication, transcription, and other nuclear processes via single-strand DNA cleavage and relegation.2 Human Topo-I forms a tightly closed clamp around the DNA, and modulation of this mechanism of transcriptional regulation is driven by different proteins, such as the tumor suppressor p53, which alters the enzyme's catalytic activity. This action of the inhibitors causes single-strand DNA cleavage, which is then transformed into double-strand DNA cleavage that is lethal for growing cells. The prototypic inhibitor of human Topo-I is the plant alkaloid camptothecin (CPT) which specifically stabilizes the intermediate covalent complexes between DNA and Topo-I. Specific binding of CPT at the Topo-I–DNA interface stabilizes the phosphotyrosyl linkage; whereby, the enzyme is linked to one strand of the double helix.18 CPT converted supercoiled plasmid DNA into the nicked DNA form in the presence of human recombinant Topo-I, and a similar effect is observed with lam-D.8,26 Although the drug concentration required to convert supercoiled DNA into simple form is approximately five times higher than CPT. In contrast to camptothecin, lamellarin D slows down the relaxation of mitochondrial Topo-I and strongly inhibits DNA relegation by this mitochondrial enzyme.35The initial study of Faulkner and coll. on the Topo-I inhibition by a series of ascidian alkaloids, i.e., the lamellarins involved HIV-1 integrase and the topoisomerase of the Molluscum contagiosum virus (MCV),36 indicated that whereas none of the studied compounds as 20-sulfate of lamellarin-α, U, and V (31–33), and non-sulfated forms of lamellarin N (9), T (34), and W (35) (Fig. 2) inhibited MCV topoisomerase at concentration <100 μM, only lamellarin-α 20-sulfate showed selective inhibitory activity against HIV-1 integrase and HIV-1 virus in cell culture (IC50 = 8 μM), being less toxic in HeLa cells (LD50 = 274 μM).
In contrast, lamellarin-A does not inhibit HIV-1 integrase but shows moderate cytotoxicity with good cell line selectivity. Subsequently, it was found that lam-H (16) had a potent anti-topoisomerase activity (IC50 = 0.23 μM) but lacked the specificity required to be medicinally useful given that it was quite cytotoxic toward HeLa cells (LD50 = 5.7 μM).37 In addition, lamellarins M (5), N (9), H (16), X (11), W (35), and B (36) (Fig. 1 and 2) also showed potent inhibition of human Topo-I.2 On the other hand, lam-N analogs were utterly inactive against Topo-I even at 50 μM and less potent than lam-D.
However, parental lam-N inhibited the action of Topo-I. This interesting result may be accounted for considering the unfavorable steric interactions between the 16-methyl group of alkaloids 37 or 38 and the base pairs of DNA, preventing the formation of a stable compound-DNA–Topo-I ternary complex.21 The phenotypic cytotoxic activities of 37 (IC50 2.0–4.1 μM) and 38 (IC50 0.16–0.79 μM) agree with no inhibition of Topo-I, observing that the lower cytotoxicity of these compounds may be considered by the lack of their Topo-I inhibitory activity.
Lamellarin D (1), a potent inhibitor of human Topo-I, breaks and rejoins DNA strands through a DNA-3′-phosphotyrosyl)-enzyme intermediate.18 Like other topoisomerase inhibitors, it stabilizes the DNA–Topo-I complex. Its main target is nuclear and mitochondrial Topo-I.32 The C-8–OH and C-20–OH groups of lam-D participate in hydrogen-bonding interactions with the enzyme's side chains of Glu356 and Asn722.1 Lam-D maintains a significant level of cytotoxicity in the Topo-I-mutated cells (P388CPT5) resistant to the camptothecin, a reference Topo-I poison. These results raise the possibility of “double hits” of lam-D, one directly on the mitochondria to trigger apoptosis and the other located in the cell nucleus, resulting in DNA damage, cell cycle arrest, and DNA repair.26 In other matters, the relative resistance index (RRI) is significantly reduced with lam-D (RRI = 21) compared to CPT (RRI = 103) against P388CPT5.8
Mannich derivatives of lam-D like heterocycle 39 were evaluated for their in vitro anti-cancer and Topo-I inhibitory activities at an equivalent level to that of lam-D, reporting that the compound SL-9 (39) showed a better Topo-I inhibitory activity than that of lamellarin D.38 Likewise, Colligs et al. synthesized new lam-D derivative (lamellarin G trimethyl ether) which displayed lower antiproliferative activity than CPT against drug-sensitive human leukemic lymphoblasts (CCRF-CEM cells) but with less resistance effect on multidrug-resistant CEM/ADR5000 cells.9 Lamellarin D and some related derivatives were effective in stabilizing the DNA-Topo-I covalent complex.11 Among these derivatives, lam-D and its analog 40 more effectively exhibited the lymphoblastic cells (CEM) and the camptothecin-resistant human T leukemia cells (CEM/C2) presenting the following values for 1 IC50 = 5 n/MIC50 = 720 nM and for 40 IC50 = 17 nM/MIC50 = 2740 nM. Molecular docking analysis of 1 into the Topo-I-binding site supported the role of hydrogen bonds between C-8–OH, C-20–OH groups, and carbonyl oxygen at the C-17 position with Asn722, Glu356, and Arg364, respectively. This study suggested that the planar pentacyclic lamellarin moiety, bearing hydroxyl groups at C-8 and C-20, is essential for inhibiting Topo-I. On the other hand, the aryl ring at C-1 (F-ring) is directed toward the major groove cavity and does not directly interact with the enzyme. This model suggests that the F-ring is probably not essential for Topo-I inhibition.2,11
A new class of Topo-I inhibitors with the benzo[g][1]benzopyrano[4,3-b]indol-6(13H)-one scaffold (BBPI) was generated by switching the positions of the pyrrole nitrogen and C-1 of lam-D. The Topo-I inhibitory activities of the selected BBPIs [N-methyl (41), N-ethyl (42), N-(2-dimethylamino)ethyl (43), and valine ester (44)] derivatives (Fig. 2) demonstrated higher activity than CPT and the parental.2 BBPI derivatives were designed to possess a planar polyaromatic structure similar to the type Ib lamellarin core, which enabled intercalation between the duplex DNA base pairs and the two hydroxy groups at positions corresponding to C-8 and C-20 in lamellarin to form hydrogen bonds with Asn722 and Glu356 of Topo-I.3 The inhibition of nuclear and mitochondrial Topo-I is not the only mechanism by which lam-D causes the decrease of the cancer cells' growth. Indeed, poisoning of mitochondrial Topo-I triggers oxidative stress and DNA damage.11 A link has now been established between the molecular action of lam-D on mitochondrial Topo-I and the mitochondrial cascade of events (inhibition of respiratory chain, swelling of the mitochondrial matrix).1 Due to the latter activity, lamellarin D retains cytotoxicity against CPT-resistant cancer cells.34
Structure–activity studies indicate a good correlation between Topo-I inhibition and cytotoxicity of lam-D derivatives, suggesting that one of the proapoptotic targets of lam-D is in the cell nucleus.26 The observed correlation between cytotoxicity and Topo-I inhibition indicated that Topo-I-mediated DNA cleavage assays could be used to guide the development of anti-cancer agents.
Inhibition of protein kinases
Apart from human Topo-I, other DNA interacting enzymes may contribute to the mechanism of action of the lamellarins. In 2008, Meijer and co-workers identified a new molecular target of anti-cancer lamellarins. They showed that certain lamellarins could interfere with the activity of a wide range of protein kinases relevant to cancer,39 including dual specificity tyrosine phosphorylation activated kinase 1A, glycogen synthase kinase-3 (GSK-3), casein kinase 1, PIM-1 and cyclin-dependent kinases (CDKs).20Lamellarin D displays unselective kinase inhibition (CDK1, CK5, GSK3, PIM1, and DYRK1A) in the sub-nanomolar range.32 A library of substituted chromeno[3,4-b]indoles as Lamellarin isosteres was achieved, allowing the identification of two lead compounds as new nanomolar inhibitors of the kinase DYRK1A (dual-specificity tyrosine-(Y)-phosphorylation-regulated kinase, that is a potential drug target for neurodegenerative diseases and cancer.40 It was noted that lam-D was a modest kinase inhibitor, with IC50 values in the low μM range, whereas lam-N proved to be much more potent with IC50 values (against glycogen synthase kinase 3, GSK-3) in the nM range.41 But it also affected many other kinases to a lesser extent.1 Furthermore, Ruchirawat and co-workers evaluating the GSK-3β inhibitory activity of lam-D, lam-N, and azalamellarins D (45) and N (46), showed that following the order of inhibition: azalamellarin N (IC50 = 0.008 μM) > azalamellarin D (IC50 = 0.018 μM) > lam-N (IC50 = 0.036 μM) > lam-D (IC50 = 0.32 μM). Therefore, replacing the lactone moiety of the lam-D ring skeleton with a lactam ring of the azalamellarin structure markedly increased the kinase inhibitory activity.42
The kinase inhibition may contribute, at least to some extent, to the cytotoxic and pro-apoptotic properties of lam-N. A series of A-ring-modified lamellarin N analogs were synthesized and evaluated as potential inhibitors of the epidermal growth factor receptor, EGFR T790M/L858R, a causal factor in drug-resistant non-small cell lung cancer. It was found that lam-N alkaloid and the most promising analog 47 displayed an excellent inhibitory profile against the T790M/L858R mutant (IC50 = 31.8 nM).43 In addition, the kinase inhibitory activities of the lam-N synthetic analogs were also evaluated on eight protein kinases relevant to cancer and neurodegenerative diseases (CDK1/cyclin B, CDK2/cyclin A, CDK5/p25, GSK-3α/β, PIM1, DYRK1A, CLK3, and CK1). Whereas isomer (aR) (38) exhibited potent but nonselective inhibition on all protein kinases except CK1 (IC50 = 0.024–0.21 μM), its isomer (aS) (37) selectively inhibited only GSK-3α/β, PIM1, and DYRK1A (IC50 = 0.22–0.44 μM). A good correlation was observed between the effects of lamellarins on protein kinases and their action on cell death, suggesting that inhibition of specific kinases may contribute to their potent cytotoxicity.21 Synthetic analogs showed inhibitory effects toward protein kinases but were inactive toward the Topo-I enzyme. This is attributed to the unfavorable steric interaction of the methyl group and the DNA base pairs.11 Notably, lam-N showed selectivity for a few kinases on a Cerep kinase panel, some of which were identified as major cancer targets, such as VEGFR1/2, Flt-3, PDGFR, LcK, and Lyn.3
The above-discussed results suggested that protein kinases that transfer a phosphate group to a protein while phosphatases remove a phosphate group from protein may contribute to the drug's cytotoxicity. Consequently, the structural requirements for protein kinase inhibition are as follows: (1) the hydroxy groups at C-8 and C-13 are essential for inhibition, whereas the hydroxy groups at C-14 and C-20 are less critical; (2) the C5![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C6-double bond, i.e., the planar structure of the pentacyclic core, is essential for a high activity. These requirements are somewhat different from those required for the Topo-I inhibition and cytotoxic activity that suggests the possibility of producing selective kinase inhibitors according to the needs at the molecular level.3
C6-double bond, i.e., the planar structure of the pentacyclic core, is essential for a high activity. These requirements are somewhat different from those required for the Topo-I inhibition and cytotoxic activity that suggests the possibility of producing selective kinase inhibitors according to the needs at the molecular level.3
Inhibition of mitochondrial function
In recent years, mitochondria have emerged as a promising target for cancer therapy.26 Mitochondria play a crucial role in the apoptotic process induced by chemotherapeutic agents.44 These organelles can represent an attractive primary anti-cancer drug target. They may act on different mitochondrial locations, including the Bcl-2 protein family, the respiratory chain complexes, and the mitochondrial permeability transition (MPT) pore constituents, representing attractive pro-apoptotic targets for new anti-cancer drugs.45Lamellarin D (1) acts on cancer cell mitochondria to induce apoptosis through early disruption of the inner mitochondrial transmembrane potential (Δψm) in the P388 leukemia cell line. The direct mitochondrial effect of lam-D accounts for the sensitivity of Topo-I-mutated P388CPT5 cells resistant to CPT. Interestingly, the effects of 1 on Δψm occurred over the same concentration range (5 μM L−1), suggesting that the proapoptotic effect of lam-D depends on the functional alterations of mitochondria.8 Furthermore, lam-D alone cannot promote apoptosis of the isolated core. These data suggested that lam-D induces apoptosis of Topo-I-mutated cells via its effect on MPT, a process used by mitochondria to activate cell death.26 In 2009, Bailly et al., reported extensive studies using various tumor cell lines; their results indicated that lam-D exerts its cytotoxicity for all cell types tested primarily by inducing mitochondrial apoptosis independently of nuclear signaling.26
Interestingly, a tumor-active lam-D analog, titled PM031379 (27) exerted a direct proapoptotic action on mitochondria via the generation of reactive oxygen species and up-regulation of the apoptosis-inducing factor, which have been seen in the non-small cell lung cancer cell line, U1810.1 The amino derivative PM031379 did not stabilize Topo-I–DNA covalent complexes. However, this derivative produced a dose-dependent increase in tumor cell death through a mitochondrial-dependent pathway. These effects are equally potent and rapid with lam-D analog compared with its parental, suggesting that mitochondria are the target of both compounds.8 Other studies examined the apoptosis induction of lam-D and related synthetic products (compound analogs lacking the 20-OH or 8-OH group) at 5 μM in Jurkat leukemia cells.45 These derivatives induced nuclear apoptosis by acting directly on mitochondria. However, its analogs with various substitutions at positions C-8, C-14, and C-20 failed to induce mitochondrial apoptosis. The analysis of the structure–apoptosis relationships points to a critical role of the OH groups at positions C-8 and C-20 for this effect. These results were consistent with previous reports demonstrating that hydroxyl groups are essential structural requirements for cytotoxic activity. Thus, lam-D and analogs appear to have unique mitochondrial mechanisms of action, leading to cancer cell death.
Synthetic strategies in the total synthesis of lamellarins
Lamellarins and related pyrrole-derived alkaloids have attracted great interest as targets for total synthesis. Several research groups have reported the synthesis of lamellarins and related pyrrole-derived alkaloids, including extended/condensed structures. Consequently, extensive efforts have been devoted to creating novel structures and improving synthetic methods, leading to these natural marine alkaloids.1Several elegant synthetic strategies have been employed in which highly functionalized precursors were assembled into the pyrrole core through intramolecular ylide cycloaddition,46 azadiene Diels–Alder cycloaddition,47 oxidative dimerization,28 among others. Owing to their interesting structural features and promising biological activities, many synthetic chemistry groups have developed efficient strategies for the total synthesis of lamellarins and related analogs.
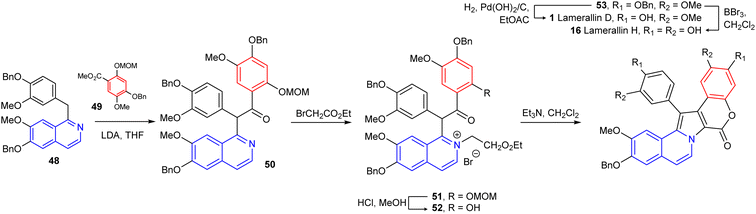
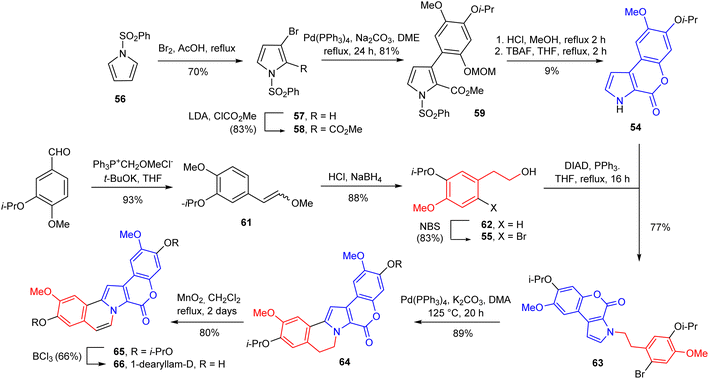
In 1997, Ishibashi's group achieved the first total synthesis of these marine alkaloids, i.e., lam-D and lam-H by N-ylide-mediated cyclization using benzylisoquinoline derivative as the critical ring construction procedure in five steps; yields of lam-D (1) and lam-H (16) were 18% and 15%, respectively46 (Scheme 1). The synthesis of 1-dearyllamellarin D was reported in 2006.33 The main precursor, N-alkaryl pyrrollo-coumarin derivative 63 was obtained by the use of the Mitsunobu reaction of the lactone 54 with alcohol 55 in the presence of diisopropyl azodicarboxylate (DIAD) and triphenylphosphine. Its palladium-catalyzed intramolecular direct arylation afforded pentacyclic derivative 64 in 89% yield which then, was converted into 65 via the dehydrogenation reaction under mild conditions (MnO2/CH2Cl2, reflux) in good yield. Synthesis of 1-dearyllamellarin D (66) ended with a simple procedure, selective deprotection of the isopropyl groups (BCl3) (Scheme 2).
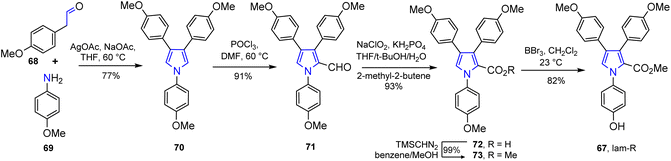
The total synthesis of lamellarin R (67), belonging to non-fused 3,4-diarylpyrrole marine alkaloids was performed by Jia and co-workers.48 The lineal synthesis in 5 steps started with an aldehy-de 68 and p-methoxyaniline 69; their oxidative coupling reaction smoothly afforded a pyrrole derivative 70 and, consequently, its traditional Vilsmeier–Haack reaction (POCl3/DMF) produced pyrrole-aldehyde 71. The Lindgren oxidation of 71 under the optimized conditions allowed to prepare pyrrole-acid 72 whose treatment with TMSCHN2 gave methyl ester 73, and finally, the demethylation reaction of 73 provided marine alkaloid of Type-II, lamellarin R (67) (Scheme 3).
On the other hand, the total synthesis of lam-D, lam-H, and lam-D trimethyl ether has also been accomplished using Ru(II)-catalyzed [3 + 2] annulation strategy to construct the central pyrrole ring.49 The striking features of this synthesis were the use of PEG-400 as a green solvent for various Ru(II)-catalyzed C–H activations. The synthetic sequence begins with the annulation reaction of enamide 74 and diarylalkyne 78a to give 2,3-diarylpyrrole-5-carboxylate 75a. Its bromination reaction easily provided bromide derivative 77a, a suitable precursor for the Suzuki reaction with boronic acid 76a. Thus, triaryl-substituted pyrrole 79a was obtained using catalytic Pd(dba)2/1,1′-bis(diphenylphosphino)ferrocene (dppf) system in DME-H2O medium (Scheme 4).
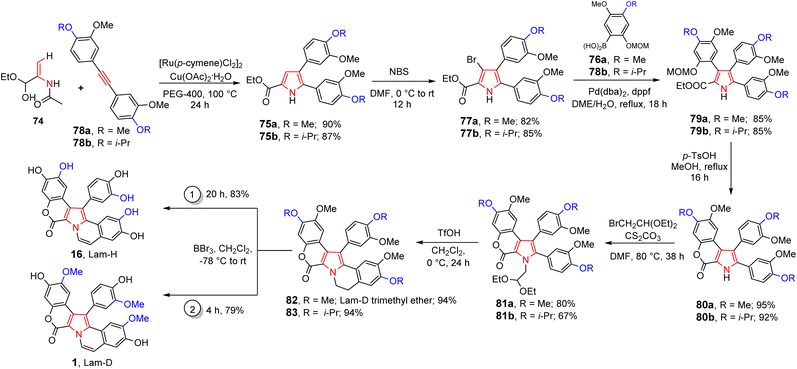 | ||
| Scheme 4 Total synthesis of lam-D (1), lam-H (16), and lam-D trimethyl ether (82), through various Ru(II)-catalyzed C–H activations. | ||
Then, one-step lactonization process by methoxymethyl ether (MOM)-deprotection catalyzed by p-TsOH in MeOH afforded the lactone ring of 80a. Its N-alkylation with bromoacetaldehy de diethyl acetal allowed to prepare 81a and its cyclization under mild reaction conditions (TfOH/CH2Cl2, 0 °C) gave lam-D trimethyl ether (82) in 94% yield.
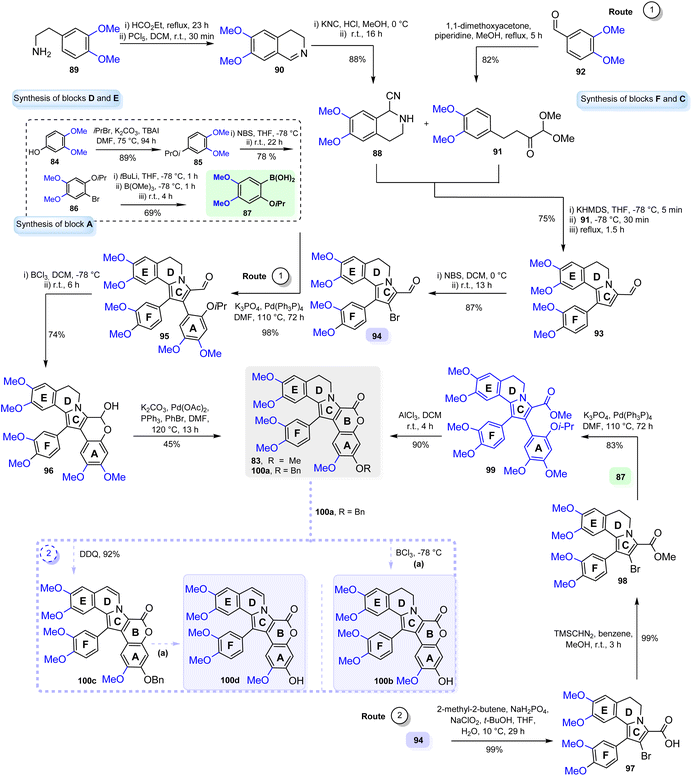
The global deprotection of the methyl groups with BBr3 yielded lam-H (16) n 83% yield. Finally, using the lam-D trimethyl ether (82) obtained, lam-D was easily prepared in 79% yield after the treatment with BCl3 (Scheme 4). Colligs and coworkers also reported the synthesis of marine alkaloid 82 based on von Miller–Plöchl cyclocondensation of a deprotonated α-amino nitrile with an α,β-unsaturated ketone as a critical step, where this alkaloid was accessed by two different synthetic routes (Scheme 5, route 1).50
In general, block A (arylboronic acid 87) was built in three steps from 3,4-di-methoxy phenol 84. Blocks D and E (α-amino nitrile 88) were made from homoveratrylamine 89, which was converted into dihydroisoquinoline 90 through the subsequent Bischler–Napieralski-reaction. Blocks F and C (dimethoxybutan-2-one derivative 91) were built by aldol condensation of veratraldehyde 92 with 1,1-dimethoxypropan-2-one.
Thus, the modified von Miller–Plöchl reaction of the deprotonated α-amino nitrile 88 and enone 91 afforded aryl-pyrrolo[2,1-a]isoquinoline F-EDC skeleton 93. To introduce the aryl substituent in a 2-position (ring A), the bromination reaction with NBS was carried out affording 94. In the first route, diaryl-pyrrolo[2,1-a]isoquinoline 95 was formed for coupling the brominated dihydroisoquinoline 94 and boronic acid 87. Eventually, the cyclization's hemiacetal product 96 must be oxidized to give the desired lamellarin 83. In the second route, the acid 97 was obtained via the Pinnick oxidation to form the soluble ester 98 leading to the synthesis of desired lamellarin 83 in a few steps. Indeed, this route was more efficient than the first route. Imbri et al. reported the synthesis of dihydro-lamellarin ƞ (100b) and lamellarin ƞ (100d) from an intermediate of lam-G trimethyl ether 100a 51 (Scheme 5, route 2).
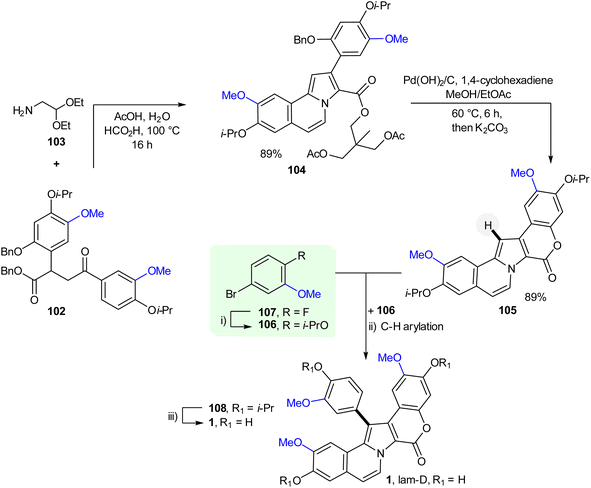
To get lamellarin ƞ (100d) from alkaloid 100a, the generation of the 5,6-double bond was necessary that was achieved using 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) to furnish intermediate 100c. Its post-debenzylation with BCl3 gave marine alkaloid 100d. Concurrently, dihydro-lamellarin ƞ (100b) was obtained from 100a under the same debenzylation reaction conditions. Recently, the first late-stage pyrrole C–H arylation in a lamellarin alkaloid synthesis to obtain lam-D (1) using ortho-ester-masked α was reported; traditional routes to lamellarins have almost universally used pyrrole halogenation followed by C–C cross-coupling reactions52 (Scheme 6). Lam-D was obtained from 1,4-dicarbonyl derivative 102 previously synthesized (not described below). The lam-D synthesis starts with the cyclocondensation of 102 and 103.
Thus, treating 102 with aminoacetaldehyde diethyl acetal 103, led to the formation of the desired pyrroloisoquinoline product 104. Its subsequent treatment with Pd-catalyst on carbon in a MeOH/AcOEt mixture gave a pentacyclic alkaloidal system 105. Notably, this reaction allowed the construction of the fused coumarin ring in a single step. The key C–H arylation of 105 with aryl halide fragment 106 was accomplished by utilizing nucleophilic aromatic substitution of 5-bromo-2-fluoroanisole 107 at C-2 with iso-PrOK, and eventually, all O-isopropyl protected lam-D 108 was isolated. Finally, the synthesis accessed the lam-D alkaloid using BCl3 to remove all isopropyl groups.
The pentacyclic skeleton diversification has provided an attractive template to incorporate other functional or protective groups. Moreover, the hydroxyl groups around the lamellarin core can be easily substituted with labile moieties to build pro-drugs.18 The variation of the substituents on this pentacyclic core and saturated or unsaturated D-ring has allowed for obtaining a large panoply of natural and unnatural derivatives.1 Due to the fascinating novel structures and biological activities, more and more researchers have devoted themselves to the synthetic studies of lamellarins and related pyrrolo[2,1-a]isoquinoline analogs.
Approach to the synthesis and diversification of isoquinoline and pyrrolo[2,1-a] isoquinoline core
Pyrrolo[2,1-a]isoquinoline is a fusion of two privileged frameworks. Furthermore, it is a crucial molecule present in a large number of natural products and synthetic molecules. Notably, most lamellar alkaloids isolated from marine organisms possess the pyrrolo[2,1-a]isoquinoline scaffold as their structure. As a result, significant efforts have been made to develop target-oriented and diversity-oriented synthesis strategies for constructing pyrrolo[2,1-a]isoquinoline derivatives. The evolution of new catalysts, reaction conditions, and reagents has increased the ability to create efficient, regioselective, stereospecific, and robust methods.53–55 Recent advances in the methodologies employed in the synthesis of isoquinolines as precursors and pyrrolo[2,1-a]isoquinolines are presented.Strategies in the construction of isoquinolines
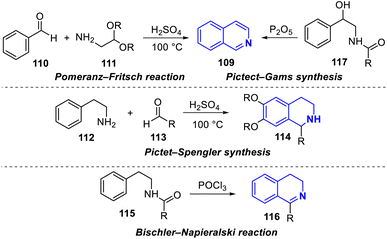
The construction of the isoquinoline core uses classic synthetic strategies such as the Pomeranz–Fritsch synthesis, which shows the acid-promoted synthesis of isoquinoline 109 from benzaldehyde 110 and a 2,2-dialkoxyethylamine 111 in two steps.Condensation of aryl aldehyde with amino acetal to form an aryl-aldimine and then cyclization of aldimine, or the Pictet–Spengler synthesis which uses β-arylethylamines 112 and carbonyl compounds 113 followed by cyclization reaction in strong acids media (hydrochloric acid, trifluoroacetic acid),56 the Bischler–Napieralski reaction employs β-arylethylamindes 115 in phosphorus oxychloride to obtain dihydroisoquinolines 116,57 whereas the Pictect-Gams synthesis uses acetoaminomethyl phenylcarbinols 117 phosphorus pentoxide as a reagent to obtain isoquinolines58 (Scheme 7).
New routes to access the isoquinoline core are still highly desirable, particularly ones with the ability to directly access the isoquinoline moiety in a range of oxidation levels that do not require highly-specialized starting materials (Scheme 8).
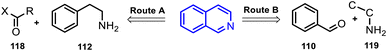
These synthetic strategies are based on the type of substrates used, which has a marked influence on the reactivity and conditions; route A involves the use of phenylethylamines 112 and aldehydes (or acid derivatives) 118 as the initial substrate leading to the interaction with electrophilic agents 118 and subsequent cyclization reactions, whereas route B employs synthetic strategies based on the coupling annulation of aromatic aldehydes 110 and amine derivatives 119.
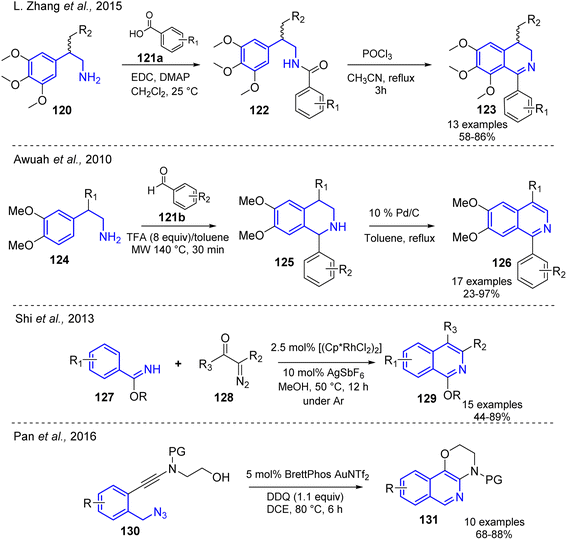
Route A (see, Scheme 8). The aryl-methylamines combined with diverse electrophilic agents can undergo intramolecular cyclization reactions, including C–C bond formation. New 1,4-disubstituted-3,4-dihydroisoquinoline derivatives 123 were synthesized using the amidation reaction of substituted 2-arylethylamines 120 and benzoic acids derivatives 121a to give substituted benzamides 122 and their subsequent Bischler–Napieralski cyclization which afforded the desired isoquinoline products 123 in good yields (Scheme 9).59
A similar synthetic sequence involving Pictet–Spengler cyclization but under microwave irradiation conditions (MW, 140 °C, 30 min) was reported by Awuah and Capretta. Diversely substituted 1-arylisoquinolines 126 were quickly prepared using the cyclization reaction of substituted 2-arylethylamines 124 and benzaldehydes 121b and subsequent oxidation of intermediate tetrahydroisoquinolines 125 which were converted into the isoquinoline analogs.60 An approach that provided direct access to multi-substituted isoquinoline 129 is based on the catalytic tandem C–H metalation [(Cp*RhCl2)2] and cyclization–condensation processes (AgSbF6) of ketoximes based on acetophenones 127 and diazo compounds 128 under mild conditions61,62 (Scheme 9).
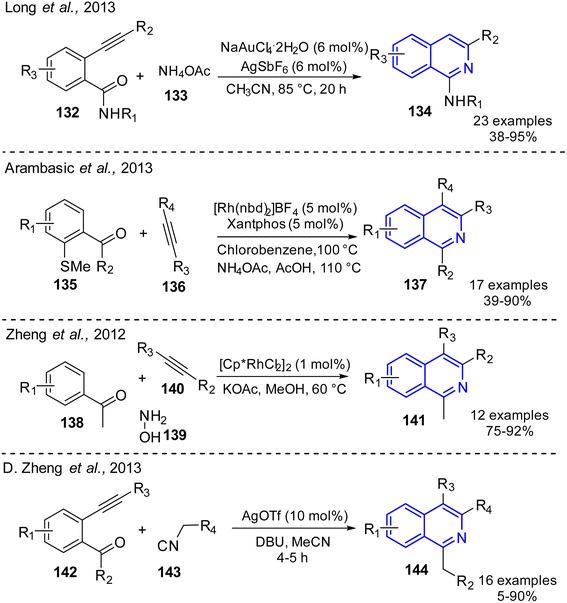
Route B (see, Scheme 8). The strategy usually employs a Brønsted and Lewis acid-catalyzed tandem reactions that simultaneously involve a Michael addition reaction of diverse N-nucleophiles to electrophiles (aryl-alkyne(alkene) compounds) with a subsequent cyclization process (annulation, cyclization, or electrophilic substitution) to obtain the isoquinoline core (Xing et al. 2016).63 For example, gold, a soft Lewis acid, mediated domino reaction of readily available 2-alkynylbenzamides 132 and ammonium acetate 133 as a source of NH3 molecules to afford substituted 1-aminoisoquinolines 134 in good to excellent yields (Long et al. 2013) (Scheme 10).64 In this reaction, a catalytic NaAuCl4·2H2O–AgSbF6 system worked well under mild reaction conditions and are compatible with various functional groups.
Arambasic and co-workers also reported a one-pot route to isoquinoline compounds 137 which is based on the Rh-catalyzed alkyne carbothiolation reaction of alkynes 136 and acetophenone-containing methyl sulfide group 135 which is achieved by simply adding NH4OAc and acetic acid directly to the reaction upon completion.65 The developed method involves the use of the commercially available precursor [Rh(nbd)2]BF4 and Xantphos phosphine ligand to form in situ [Rh(Xantphos)(nbd)][BF4] complex which presented remarkable activity with full conversion to the isoquinoline product achieved in less than 2 h (Scheme 10). Noteworthy that this one-pot, regioselective synthesis of isoquinolines was due to the presence of the activating 2-SMe groups of acetophenone derivatives.
There are different catalytic systems proposed for isoquinoline synthesis. As an example, [Cp*RhCl2]2/KOAc catalytic system was developed for the synthesis of polysubstituted isoquinolines 141 using three-component condensation reaction of acetophenones 138, hydroxylamine 139, and internal alkynes 140 in a one-pot manner (Scheme 10).66 The condensation process starts the formation of aryl-ketone oximes under mild conditions, then [Cp*RhCl2]2/KOAc system generates the active Cp*Rh(OAc)n species triggering ortho-C–H bond of intermediate acetophenone-oximes and their intermolecular cyclization with alkynes proceeds to give diverse isoquinolines. A highly efficient procedure based on silver triflate catalysis was also proposed for the isoquinoline preparation (Scheme 10).
It involves the cascade addition/6-exo cyclization reaction of 2-alkynylbenzaldehydes 141 and 2-isocyanoacetates 143 in the presence of AgOTf and DBU in MeCN at 80 °C for 5 h which allowed to provide isoquinolines 144. It was believed that isocyanoacetates 142 could attack 2-alkynylbenzaldehydes 141 first in the presence of a base (DBU) to generate an intermediate, a product of the nucleophilic addition to the CHO group, and then it could be transformed into desired isoquinoline products via subsequent 6-exo cyclization with a loss of carbon monoxide.66 Recently, Sestelo and co-workers reported the cycloisomerization reaction of imines derived from o-(alkynyl)benzaldehydes using InI3 (5 mol%) and the Hantzsch ester (120 mol%), under milder reaction conditions, can condu-ce to the formation of diverse functionalized 1,2-dihydroisoquinolines through a domino cycloisomerization/reduction approach.67
The essential biological properties and the difficulty in obtaining large quantities from the natural sources of lamellarins and related pyrrole-derived alkaloids have attracted great interest as challenging natural product targets for total synthesis.
Strategies in the construction of pyrrolo[2,1-a]isoquinolines
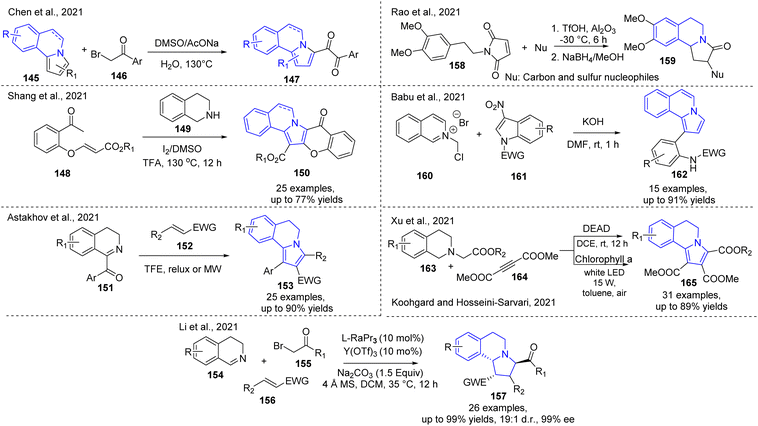
Several synthetic strategies for constructing pyrrolo[2,1-a]isoquinolines have been developed. These strategies are classified by pyrrole ring and isoquinoline-based formation (Fig. 3). Recently, various groups performed a detailed and rigorous analysis of these methodologies covering 2010 to 2021,11,68,69 which revealed the great interest of modern organic chemists in pursuing efficient synthetic routes for the development of lamellarin-derived bioactive compounds.Developing the pyrroloisoquinoline chemistry, an important modification of pyrrolo[2,1-a]isoquinolines 145 with arylacyl bromides 146 in the presence of DMSO as oxidant was made via the dicarbonylation reaction which allowed the preparation of 1,2-dicarbonylated pyrroloisoquinolines 145 in acceptable to good yields (12–73%).70 (Scheme 11). On the other hand, tetrahydroisoquinoline ring is a suitable precursor in the pyrroloisoquinoline chemistry. Thereby, a metal-free approach based on the I2/DMSO system and a Brønsted acid as catalyst was recently employed for the synthesis of chromone-fused pyrrolo[2,1-a]isoquinolines 150 using o-acetyl-phenoxy acrylates 148, tetrahydroisoquinolines 149, and with iodine in heated DMSO in the presence of TFA for 12 h. This iodine-promoted one-pot cascade oxidative annulation process consists of diverse sequential reactions, i.e., α-halogenation, oxidation, nucleophilic addition, 1,3-dipolar cycloaddition.71 Likewise, recently, Cui and Chen used simple tetrahydroisoquinoline, terminal alkyne, and aldehyde precursors for the synthesis of pyrrolo[2,1-a]isoquinolines based on a copper-catalyzed three component reaction (A3 type) in the presence of CuCl2/PhCOOH in DMF at 130 °C. The developed procedure permitted generating various pyrroloisoquinoline derivatives with 17–69% yield.68 1-Aroyl-3,4-dihydro isoquinolines 151 were also utilized as simple starting materials in the synthesis of functionalized 5,6-dihydropyrrolo[2,1-a]isoquinolines 153 with diverse electron-withdrawing substituents (EWG) at the C-2 position. Reported a convenient procedure involves the treatment of 151 with α,β-unsaturated compounds 152 through the microwave-assisted two-component domino reaction in TFE under reflux.72
Feng and co-workers also reported a three-component [3 + 2] cycloaddition of 3,4-dihydroisoquinolines 154, bromoacetates 155 and α,β-unsaturated pyrazole amide 156 in the presence of a chiral N,N′-dioxide-Y(OTf)3 complex as the catalyst which allowed the preparation of hexahydropyrrolo-isoquinolines 157 in moderate to good yields with excellent diastereo- and enantioselectivities.73 Similar tetrahydropyrrolo[2,1-a] isoquinolin-3(2H)-ones 159 were obtained using a tandem Michael addition/carbo-cyclization of 3,4-dimethoxyphenethyl maleimides 158 with carbon, amine, and sulfur nucleophiles (Nu) in the presence of γ-Al2O3/TfOH binary system (0.1/1 ratio) at −30 °C followed by reduction using NaBH4/MeOH (Scheme 11). It was found that the active species involved in the binary system were Al(O)–OH which facilitated the Michael addition of nucleophiles such as amines and thiols to maleimides.74
Although the use of catalysts is feasible, the vision of the organic chemist is focused on generating methodologies without metals and oxidizing agents. It was also found that the domino reaction of isoquinolinium ylides 160 and electrophilic indoles 161 with KOH in dry DMF at room temperature gave smoothly functionalized pyrrolo[2,1-a]isoquinolines 162 in good yields.75 Zhen and coll use diethyl azodicarboxylate (DEAD) as a dual-functional reagent with both oxidation and dehydrogenation functions. developed a novel metal-free methodology that describes a reliable pathway for synthesizing a range of 5,6-dihydropyrrolo[2,1-a]isoquinolines 165 through [3 + 2] cycloaddition/aromatization tandem reactions of alkyl (e.g., benzyl, methyl, ethyl, and tert-butyl) 2-(3,4-dihydroisoquinolin-2-(1H)-yl) acetates 163 and dimethyl but-2-ynedioate 164 in the presence of DEAD (1.2 equiv.) as the oxidant in dichloroethane (DCE) at room temperature for 12 h.
An iodine–H2O2 catalytic system in MeCN under reflux conditions also stimulated the preparation of such pyrrolo[2,1-a]isoquinolines.6 Such green-like reaction conditions allowed to prepare easily pyrroloisoquinoline products in good yields.76 Early, it was developed the first visible-light-driven dipolar [3 + 2] cycloadditions report for the synthesis of pyrrolo[2,1-a]isoquinoline via [Ru(bpy)3]Cl2 as photosensitizer (Zou et al. 2011).77 After ten years, Koohgard and Hosseini-Sarvari employed chlorophyll-a, a natural pigment in the synthesis of pyrrolo[2,1-a]isoquinolines 16577 (Scheme 11).
Their procedure involves chlorophyll-a-catalyzed dipolar [3 + 2] cycloaddition reaction of 163 as dipoles and 164 as a dipolarophile (among other dipolarophiles 1,4-anthraquinone, acrylonitrile, nitroolefin, activated alkynes, and N-arylmaleimides were successfully employed) which carried out in toluene under irradiation conditions with 15 W white LED for 30 h at rt. Although this photocatalytic approach cannot be considered a green strategy because toluene was used as a solvent. However, it is an excellent starting point for further research.
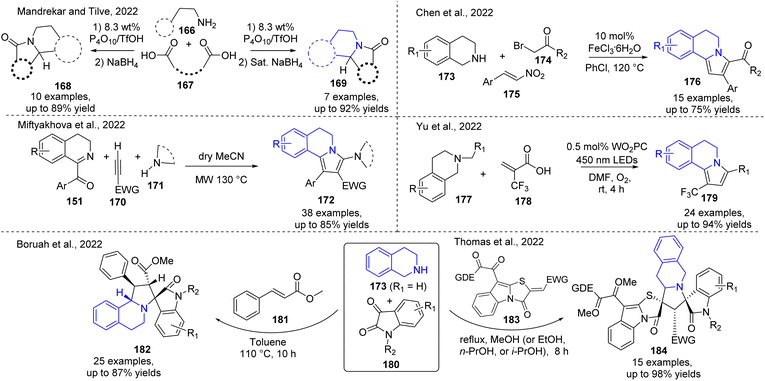
Indolizidine alkaloids, related to the pyrroloisoquinolines are also structurally significant molecules that belong to the broader class of natural products and so, their synthesis has been extensively studied. Recently, it was reported the direct condensation–cyclization reaction of diverse primary amines 166 and dicarboxylic acids 167 for the construction of various indolizidine compounds like 168 and 169.78 The condensation was carried out in the presence of an 8.3 wt% mixture of phosphorus pentaoxide (P4O10) and TfOH at 100 °C followed by reduction (sodium borohydride) of the subsequent iminium ion to produce the final products 168 and 169 (Scheme 12). The use of diverse aliphatic and aromatic dicarboxylic acids with various primary amines makes this method suitable also for synthesizing pyrrolo-, pyrido-, and isoindolo[2,1-a]isoquinolines in excellent yields.
Due to the continuous interest in pseudo-natural products, modifications of known molecular scaffolds are one of the important directions employed in organic and medicinal chemistry. Recently, a microwave-assisted three-component domino metal-oxidizing agent-free reaction of 1-aroyl-3,4-dihydroisoquinolines 151, terminal alkynes 170, and cyclic NH-acids 171 (cyclic NH-amides and NH-azoles) in dry acetonitrile at 130 °C makes it possible to obtain quickly C-3-N-functionalized pyrrolo[2,1-a]isoquinoline derivatives 172.79 On the other hand, metal-catalyzed reactions for preparing new functionalized pyrrolo[2,1-a]isoquinolines are still relevant because it make easy the diversification of pyrroloisoquinoline skeleton
Thus, Cui's Group found that an iron catalysis (FeCl3) in air as the terminal oxidant allowed the synthesis of 5,6-dihydropyrrolo[2,1-a]isoquinoline derivatives 176 using three-component condensation reaction of easily available tetrahydroquinolines 173, arylacyl bromides 174, and nitroolefins 175 in chlorobenzene at 120 °C.68 The formation of final products carried out through the consecutive N-alkylation/oxidative 1,3-dipolar cycloaddition/elimination/aromatization process. Analogous trifluoromethyled 5,6-dihydro-pyrrolo[2,1-a]isoquinolines 179, which are problematic to get ready via traditional methods, were promptly obtained in one-pot manner through a tungsten-catalyzed decarboxylative [3 + 2] cycloaddition aromatization process, in which N-substituted tetrahydroquinolines 177 reacted with the commercially accessible 2-(trifluoromethyl)acrylic acid 178 in the presence of W-complex WO2PC in DMF under visible light irradiation using 450 nm LEDs (3W × 4) under oxygen atmosphere for 4 h.80 This photocatalytic reaction was found tolerant to multiple functional groups, including ester, nitrile, ketone, and alkenes. Noteworthy that the trifluoromethyl moiety exhibited unique properties such as enhanced lipophilicity, metabolic stability, and the ability for non-covalent interactions with biological targets that could be pharmaceutically useful for the trifluoromethyl substituted lamellarins research. Spiroheterocyclane- and dispiroheterocyclane- [2,1-a]isoquinolines are also complex structures related to lamellarin alkaloids and of great importance for the development of medicinally active drugs.81,82 Accordingly, the highly diastereoselective construction of the pyrrolo[2,1-a]isoquinoline scaffold and its modifications with spiro-oxindole cores 182 and 184 are attractive due to their versatile biological properties. Although their structures look very complex, efficient three-component approaches for the stereoselective preparation of these spiro-heterocycles do not need a metal catalyst or additive.83–85 They are based on the 1,3-dipolar cycloadditions of tetrahydrosoquinolium ylides, appropriately produced in situ from hydrogenated isoquinolines 173 and isatins 180, and the third component such as chalcones 181 or thiazolo[3,2-a]indole derivatives 183 (Scheme 12). Excellent yields with high regio- and stereoselectivity added to the easy purification of these spiro heterocyclic products make an attractive and valuable method for synthesizing complex pseudo-natural spiro-heterocycles based on pyrrolo[2,1-a]isoquinoline core.
In a brief manner, it can be concluded that current research remains on the synthesis of the pyrrolo[2,1-a]isoquinoline moiety and its modifications toward diversity-oriented organic synthesis. Therefore, finding suitable reaction conditions is a never-ending task.
Structure–activity relationships of pyrrolo[2,1-a]isoquinolines compounds
Like lamellarin alkaloids, some synthetic pyrrolo[2,1-a]isoquinolines (186–188) possess cytotoxic properties (Scheme 13) and act as Topoisomerase inhibitors. The pyrrolo[2,1-a]isoquinolines 185a–e were synthesized and tested against MCF-7 (human breast cancer), Hep-G2 (human liver carcinoma), A549 (human lung cancer), T47D (human breast cancer), and HeLa (human cervical cancer) cell lines, showing weak to moderate cytotoxic activity towards A549 and HeLa).86,87 Also, it was found that the cytotoxic activity of 2-phenyl-5,6-dihydropyrrolo[2,1-a]isoquinolines 185a–d was very similar to that of 5,6-dihydropyrrolo[2,1-a]isoquinoline-1-carboxylates 186a–e, suggesting that the phenyl ring at the C-1 position is not essential for the activity. Noteworthy that 2-(p-methoxyphenyl) pyrroloisoquinoline 186b displayed a good activity against MCF-7 (IC50 = 9.4 μM) and Hep-G2 (IC50 = 4.2 μM) cell lines, whereas its analogs 186c, 186d were less active. Docking calculations revealed a good overlap of 186b with topotecan at the Topo-I binding site. This study emphasizes the importance of the electronic effects of meta-methoxy substituent in the C-2-phenyl group. The role of these organic frameworks on the cytotoxic effects were also evaluated on six tumor cell lines, PC-3 (human prostatic adenocarcinoma), U-251 (human glioblastoma), K-562 (human chronic myelogenous leukemia), HCT-15 (human colorectal adenocarcinoma), MCF-7 (human mammary adenocarcinoma) and SKLU-1 (human lung adenocarcinoma).86,87 Analyzing the results obtained, it was noted that the C-2 phenyl substituted 186b showed an increase of cytotoxic activity in U-251 (IC50 = 4.86 μM), HCT-15 (IC50 = 0.14 μM), SKLU-1 (IC50 = 0.59 μM), PC-3 (IC50 = 18.15 μM) and MCF-7 (IC50 = 25.2 μM) cell lines. Interestingly, replacement of phenyl ring (186a) by the thiophene ring (186c) increased the activity against PC-3 prostate cells (IC50 = 8.47 μM) compared with another series of pyrroloisoquinoline carboxylates 187a–d.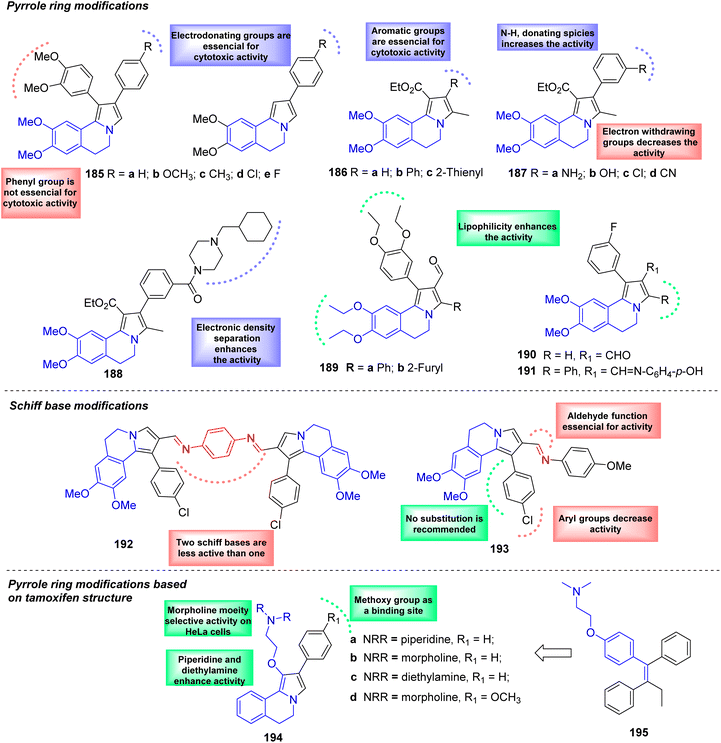 | ||
| Scheme 13 Pyrrolo[2,1-a]isoquinoline analogs as anti-cancer agents, structure–activity relationship analysis. | ||
On other hand, in this series, meta-aminophenyl ring 187a improved the inhibitory activity in U-251 (IC50 = 5.96 μM), K-562 (IC50 = 2.5 μM), MCF-7 (IC50 = 1.3 μM), and SKLU-1 (IC50 = 0.10 μM) cell lines compared with its analogs 187b–d. Thus, these results could suggest also the importance of the 2-phenyl ring-bearing meta-electron-donating groups group such as –NH2 or –OH (187a and 187b), i.e., modifying the electronic properties and aromaticity of the C-2 substituent could considerably influence on the antiproliferative activity. Remarkably, the derivative 187a was more potent than topotecan or camptothecin drugs in HCT-15 (colon) cell lines (IC50 = 0.01 μM vs. IC50 = 0.50 μM or IC50 = 0.13 μM), while its hydroxy analog 187b resulted to be more effective than cisplatin drug in PC-3 (prostate) cell lines (IC50 = 0.76 μM vs. IC50 = 8.30 μM). These results confirmed that the cytotoxic activity depends upon the chemical nature of 2-aryl substituents.86
Notable, the synthesized pyrroloisoquinoline with meta-(cyclohexylmethylpiperazinamide)phenyl fragment 188 exhibited an inhibitory activity in the nanomolar range in U-251 (IC50 = 50 nM), HCT-15 (IC50 = 20 nM) and SKLU-1 (IC50 = 20 nM) cell lines. Moreover, this derivative displayed inhibitory activity in K-562 (IC50 = 0.16 μM) and SKLU-1 (IC50 = 20 nM) cell lines even stronger than camptothecin (IC50 = 0.59 and 0.15 μM, respectively). This is much better than the above-mentioned series of 5,6-dihydropyrrolo[2,1-a]isoquinoline-1-carboxylates 186, 187.87
Synthetic lamellarin analogs were evaluated for their ability to inhibit P-gp and MRP1 efflux pumps in MDCKMDR1 (overexpressing P-gp protein) and MDCK-MRP1 (overexpressing MRP1 protein) cell lines and screened for their cytotoxic effects in drug combination assays with doxorubicin. In particular, the 3-phenyl-5,6-dihydropyrrolo[2,1-a]isoquinoline-2-carbaldehydes 189a, 189b, which inhibited P-gp with micromolar potency (IC50 = 0.24 and 0.19 μM, respectively), reversed in vitro MDR of tumor cells to doxorubicin at no cytotoxic concentrations. Regarding the MRP1 inhibitory effect, only C-3-unsubsituted dihydropyrroloisoquinoline-2-carbaldehyde 190 and its Schiff base 191 were slightly more potent than verapamil, which was MRP1-selective positive control. SAR studies emphasized lipophilicity's role in increasing compounds' biological potency.88 Novel Schiff compounds based on the pyrrolo[2,1-a]isoquinoline skeleton 192, 193 were synthesized and the cytotoxicity was tested against RD (rhabdomyosarcoma), HCT116 (intestinal carcinoma), HeLa (adenocarcinoma of the cervix uterus), and A549 (lung adenocarcinoma) cell lines, some of the synthesized compounds were non-cytotoxic in the low micromolar range (<30 μM). Moreover, it could be noticed that (i) pyrrolo[2,1-a]isoquinoline' Schiff bases were mostly less cytotoxic than the parent aldehydes, (ii) the adducts bearing a phenyl ring at C-3 were generally less cytotoxic than the corresponding unsubstituted compounds, and (iii) homobivalent Schiff base derivatives were significantly less cytotoxic than the corresponding mono Schiff bases.89
The compounds 200a–d were designed by combining the structure of lamellarin analogs and pharmacophore tamoxifen 201, which prevents estradiol binding to estrogen receptor (ER) in breast cancer cells and consequently slows down the estrogen-induced cell growth. Also, tamoxifen drug and other ER modulators induce apoptosis. The line cells ERα-positive MCF7 and T47D, ERα-negative MDA-MB-231, and two different cancer cell lines, namely A549 (adenocarcinoma human alveolar basal epithelial cells) and HeLa were used in an MTT-based cell viability assay to determine their effects on the viability of breast cancer cells in vitro. Derivatives 200a and 200c disclosed cytotoxic activity against all cancer cell lines showing the highest potency against T47D cell lines with IC50 values of 5.18 μM for 200a and 2.34 μM for 200c, contrary to the their analogs 200b and 200d; both with morpholine moiety groups, were proved cytotoxic only against HeLa and A549 cell lines.90
Until now, information about the anti-cancer properties of pyrrolo[2,1-a]isoquinolines is still a bit limited. Few studies covered a complete analysis of their cytotoxic properties, and few were known about their absorption, distribution, metabolism, and excretion characteristics as promising agents in the fight against cancer. However, the available knowledge provides an idea of how valuable and diverse these chemical systems are, and they could undoubtedly be the starting point for future research.
Conclusions
In this comprehensive review, we have analyzed the biology and chemistry of the fused pyrrolo[2,1-a]isoquinolines, highlighting the relevance of the marine natural product Lamellarins. These compounds have shown exceptional cytotoxic activity and have proven to be convincing topoisomerase inhibitors, highlighting their importance in the field of medicinal chemistry. In addition, several studies on the possible mechanisms of action of pyrrolo[2,1-a]isoquinolines highlight the intricacies of how these compounds exert their cytotoxic effects and open the door to innovative therapeutic interventions. Their diverse structural features have made them promising candidates for further exploration in drug discovery. In-depth studies of their biological activity have become a fundamental step in the search for new therapeutic agents.Furthermore, the dynamic overview of synthetic strategies in the total synthesis and molecular diversification of pyrrolo[2,1-a]isoquinolines shows the contribution to the expansion of structural diversity and provides valuable insights into structure–activity relationships (SARs). These efforts offer a promising avenue for developing more potent and selective derivatives.
An in-depth study of the various biological and chemical characteristics of pyrrolo[2,1-a]isoquinolines highlights the profound synergy between biological chemistry and medicinal chemistry in the search for new pharmaceutical agents. The importance of collaboration between these disciplines cannot be overemphasized, as it catalyzes innovation and scientific progress.
Author contributions
Leidy J. García. Conceptualization, formal analysis, and writing. Arturo Mendoza Salgado. Conceptualization, supervision, review, writing and editing. Vladimir V. Kouznetsov. Supervision, review, and editing. Carlos Mario Melendez. Conceptualization, supervision, formal analysis, review, writing and editing.Conflicts of interest
The authors declare nonfinancial interests/personal relationships, which may be considered as potential competing interests.Acknowledgements
Leidy J. García, Arturo Mendoza Salgado, and Carlos Mario Meléndez are grateful to Universidad del Atlántico and MinCiencias (SGR. BPIN 2020000100161).References
- C. Bailly, Mar. Drugs, 2015, 13, 1105–1123 CrossRef CAS PubMed.
- T. Fukuda, Y. Nanjo, M. Fujimoto, K. Yoshida, Y. Natsui, F. Ishibashi, F. Okazaki, H. To and M. Iwao, Bioorganic Med. Chem., 2019, 27, 265–277 CrossRef CAS PubMed.
- T. Fukuda, F. Ishibashi and M. Iwao, Lamellarin Alkaloids: Isolation, Synthesis, and Biological Activity, Elsevier Inc., 1st edn, 2020, vol. 83 Search PubMed.
- L. Zheng, T. Gao, Z. Ge, Z. Ma, J. Xu, W. Ding and L. Shen, Eur. J. Med. Chem., 2021, 214, 113226 CrossRef CAS PubMed.
- M. Chittchang, M. Paul Gleeson, P. Ploypradith and S. Ruchirawat, Eur. J. Med. Chem., 2010, 45, 2165–2172 CrossRef CAS PubMed.
- X. C. Huang, X. Xiao, Y. K. Zhang, T. T. Talele, A. A. Salim, Z. S. Chen and R. J. Capon, Mar. Drugs, 2014, 12, 3818–3837 CrossRef CAS PubMed.
- F. Plisson, X. C. Huang, H. Zhang, Z. Khalil and R. J. Capon, Chem.–An Asian J., 2012, 7, 1616–1623 CrossRef CAS PubMed.
- J. Kluza, M. A. Gallego, A. Loyens, J. C. Beauvillain, J. M. F. Sousa-Faro, C. Cuevas, P. Marchetti and C. Bailly, Cancer Res., 2006, 66, 3177–3187 CrossRef CAS PubMed.
- V. Colligs, S. P. Hansen, D. Imbri, E. J. Seo, O. Kadioglu, T. Efferth and T. Opatz, Bioorganic Med. Chem., 2017, 25, 6137–6148 CrossRef CAS PubMed.
- H. Fan, J. Peng, M. T. Hamann and J. F. Hu, Chem. Rev., 2008, 108, 264–287 CrossRef CAS PubMed.
- M. D. Matveeva, R. Purgatorio, L. G. Voskressensky and C. D. Altomare, Future Med. Chem., 2019, 11, 2735–2755 CrossRef CAS PubMed.
- A. L. Wang, S. H. Liao, D. H. Hu and D. P. Li, Complement. Altern. Med., 2011, 2011, 3–8 CrossRef PubMed.
- R. J. Andersen, D. J. Faulkner, C. H. He, G. D. Van Duyne and J. Clardy, J. Am. Chem. Soc., 1985, 107, 5492–5495 CrossRef CAS.
- J. C. Coll, B. F. Bowden and J. C. Coll, Aust. J. Chem., 1993, 46, 489–501 CrossRef.
- A. R. Quesada, M. D. García Grávalos and J. L. Fernández Puentes, Br. J. Cancer, 1996, 74, 677–682 CrossRef CAS PubMed.
- M. V. R. Reddy, D. J. Faulkner, Y. Venkateswarlu and M. R. Rao, Tetrahedron, 1997, 53, 3457–3466 CrossRef CAS.
- J. Ham and H. Kang, Bull. Korean Chem. Soc, 2002, 23, 205 CrossRef.
- C. Bailly, Curr. Med. Chem., 2004, 4, 363–378 CAS.
- M. Chittchang, P. Batsomboon, S. Ruchirawat and P. Ploypradith, ChemMedChem, 2009, 4, 457–465 CrossRef CAS PubMed.
- D. Pla, F. Albericio and M. Álvarez, MedChemComm, 2011, 2, 689–697 RSC.
- K. Yoshida, R. Itoyama, M. Yamahira, J. Tanaka, N. Loaëc, O. Lozach, E. Durieu, T. Fukuda, F. Ishibashi, L. Meijer and M. Iwao, J. Med. Chem., 2013, 56, 7289–7301 CrossRef CAS PubMed.
- S. M. Reddy, M. Srinivasulu, N. Satyanarayana, A. K. Kondapi and Y. Venkateswarlu, Tetrahedron, 2005, 61, 9242–9247 CrossRef CAS.
- S. Urban, L. Hobbs, J. Hooper and R. Capon, Aust. J. Chem., 1995, 48, 1491 CrossRef CAS.
- H. Zhang, M. M. Conte, X. C. Huang, Z. Khalil and R. J. Capon, Org. Biomol. Chem., 2012, 10, 2656–2663 RSC.
- C. Bailly, Curr. Med. Chem. Agents, 2005, 4, 363–378 CrossRef PubMed.
- C. Ballot, J. Kluza, A. Martoriati, U. Nyman, P. Formstecher, B. Joseph, C. Bailly and P. Marchetti, Mol. Cancer Ther., 2009, 8, 3307–3317 CrossRef CAS PubMed.
- D. Pla, M. Martí, J. Farrera-Sinfreu, D. Pulido, A. Francesch, P. Calvo, C. Cuevas, M. Royo, R. Aligué, F. Albericio and M. Álvarez, Bioconjug. Chem., 2009, 20, 1112–1121 CrossRef CAS PubMed.
- A. Heim, A. Terpin and W. Steglich, Angew. Chem., Int. Ed. Engl., 1997, 36, 155–156 CrossRef CAS.
- S. Urban and R. J. Capon, Aust. J. Chem., 1996, 49, 711–713 CrossRef CAS.
- R. A. Davis, A. R. Carroll, G. K. Pierens and R. J. Quinn, J. Nat. Prod., 1999, 62, 419–424 CrossRef CAS PubMed.
- A. Hamasaki, J. M. Zimpleman, I. Hwang and D. L. Boger, J. Am. Chem. Soc., 2005, 127, 10767–10770 CrossRef CAS PubMed.
- E. Marco, W. Laine, C. Tardy, A. Lansiaux, M. Iwao, F. Ishibashi, C. Bailly and F. Gago, J. Med. Chem., 2005, 48, 3796–3807 CrossRef CAS PubMed.
- N. Fujikawa, T. Ohta, T. Yamaguchi, T. Fukuda, F. Ishibashi and M. Iwao, Tetrahedron, 2006, 62, 594–604 CrossRef CAS.
- M. Facompré, C. Tardy, C. Bal-Mahieu, P. Colson, C. Perez, I. Manzanares, C. Cuevas and C. Bailly, Cancer Res., 2003, 63, 7392–7399 Search PubMed.
- S. Khiati, Y. Seol, K. Agama, I. D. Rosa, S. Agrawal, K. Fesen, H. Zhang, K. C. Neuman and Y. Pommier, Mol. Pharmacol., 2014, 86, 193–199 CrossRef PubMed.
- M. V. R. Reddy, M. R. Rao, D. Rhodes, M. S. T. Hansen, K. Rubins, F. D. Bushman, Y. Venkateswarlu and D. J. Faulkner, J. Med. Chem., 1999, 42(11), 1901–1907 CrossRef CAS PubMed.
- C. P. Ridley, M. V. R. Reddy, G. Rocha, F. D. Bushman and D. J. Faulkner, Bioorganic Med. Chem., 2002, 10, 3285–3290 CrossRef CAS PubMed.
- L. Shen, N. Xie, B. Yang, Y. Hu and Y. Zhang, Eur. J. Med. Chem., 2014, 85, 807–817 CrossRef CAS PubMed.
- D. Skropeta, N. Pastro and A. Zivanovic, Mar. Drugs, 2011, 9, 2131–2154 CrossRef CAS PubMed.
- J.-Y. Mérour, C. Neagoie, S. Routier, A. Lansiaux, F. Buron, E. Vedrenne, L. Meijer, B. Baldeyrou, S. Bourg, S. Rosca and O. Lozach, Eur. J. Med. Chem., 2012, 49, 379–396 CrossRef PubMed.
- D. Baunbæk, N. Trinkler, Y. Ferandin, O. Lozach, P. Ploypradith, S. Rucirawat, F. Ishibashi, M. Iwao and L. Meijer, Mar. Drugs, 2008, 6(4), 514–527 CrossRef PubMed.
- A. Theppawong, P. Ploypradith, P. Chuawong, S. Ruchirawat and M. Chittchang, Chem.–An Asian J., 2015, 10, 2631–2650 CrossRef CAS PubMed.
- T. Fukuda, T. Umeki, K. Tokushima, G. Xiang, Y. Yoshida, F. Ishibashi, Y. Oku, N. Nishiya, Y. Uehara and M. Iwao, Bioorganic Med. Chem., 2017, 25, 6563–6580 CrossRef CAS PubMed.
- G. Kroemer, L. Galluzzi and C. Brenner, Physiol. Rev., 2007, 87, 99–163 CrossRef CAS PubMed.
- C. Ballot, J. Kluza, S. Lancel, A. Martoriati, S. M. Hassoun, L. Mortier, J. C. Vienne, G. Briand, P. Formstecher, C. Bailly, R. Nevière and P. Marchetti, Apoptosis, 2010, 15, 769–781 CrossRef CAS PubMed.
- F. Ishibashi, Tetrahedron, 1997, 53, 5951–5962 CrossRef CAS.
- D. L. Boger, C. W. Boyce, M. A. Labroli, C. A. Sehon and Q. Jin, J. Am. Chem. Soc., 1999, 121, 54–62 CrossRef CAS.
- Q. Li, J. Jiang, A. Fan, Y. Cui and Y. Jia, Org. Lett., 2011, 13, 312–315 CrossRef CAS PubMed.
- D. M. Lade, A. B. Pawar, P. S. Mainkar and S. Chandrasekhar, J. Org. Chem., 2017, 82, 4998–5004 CrossRef CAS PubMed.
- V. C. Colligs, C. Dialer and T. Opatz, Eur. J. Org. Chem., 2018, 2018, 4064–4070 CrossRef CAS.
- D. Imbri, J. Tauber and T. Opatz, Chem.–A Eur. J., 2013, 19, 15080–15083 CrossRef CAS PubMed.
- H. J. Shirley, M. Koyioni, F. Muncan and T. J. Donohoe, Chem. Sci., 2019, 10, 4334–4338 RSC.
- M. Leonardi, M. Villacampa and J. C. Menéndez, J. Org. Chem., 2017, 82, 2570–2578 CrossRef CAS PubMed.
- M. Lautens and K. Yamamoto, Synfacts, 2016, 12, 506 CrossRef.
- J.-C. Castillo, A. Tigreros, Y. Coquerel, J. Rodríguez, M. A. Macías and J. Portilla, ACS Omega, 2019, 4, 17326–17339 CrossRef CAS PubMed.
- K. Zaman, F. Rahim, M. Taha, A. Wadood, S. A. A. Shah, Q. U. Ahmed and Z. A. Zakaria, Sci. Rep., 2019, 9, 16015 CrossRef PubMed.
- C. E. Puerto Galvis and V. V Kouznetsov, Studies in Natural products Chemistry, ed. H. E. Atta-ur-Rahman, 2018, vol. 56, pp. 1–51 Search PubMed.
- R. Gujjarappa, N. Vodnala and C. C. Malakar, Adv. Synth. Catal., 2020, 362, 4896–4990 CrossRef CAS.
- L. Zhang, Y. Song, J. Huang, J. Liu, W. Zhu, Y. Zhou, J. Lv, C. Zheng and J. Zhu, Int. J. Mol. Sci., 2015, 16, 10173–10184 CrossRef CAS PubMed.
- E. Awuah and A. Capretta, J. Org. Chem., 2010, 75, 5627–5634 CrossRef CAS PubMed.
- Z. Shi, D. C. Koester, M. Boultadakis-Arapinis and F. Glorius, J. Am. Chem. Soc., 2013, 135, 12204–12207 CrossRef CAS PubMed.
- X. Yang, J. Jie, H. Li and M. Piao, RSC Adv., 2016, 6, 57371–57374 RSC.
- S. Xing, J. Ren, K. Wang, H. Cui, H. Yan and W. Li, Adv. Synth. Catal., 2016, 358, 532–538 CrossRef CAS.
- Y. Long, Z. She, X. Liu and Y. Chen, J. Org. Chem., 2013, 78(6), 2579–2588 CrossRef CAS PubMed.
- M. Arambasic, J. F. Hooper and M. C. Willis, Org. Lett., 2013, 15, 5162–5165 CrossRef CAS PubMed.
- L. Zheng, J. Ju, Y. Bin and R. Hua, J. Org. Chem., 2012, 77, 5794–5800 CrossRef CAS PubMed.
- F. A.-M. Seoane-Carabel Lorena, L. A. Sarandeses and J. P. Sestelo, Synthesis, 2022, 55, 1714–1723 Search PubMed.
- X.-H. Chen, Y.-Y. Pan, W.-X. Wang and H.-L. Cui, Synlett, 2022, 33(16), 1645–1654 CrossRef CAS.
- C. Mohan, R. B. Krishna, S. T. Sivanandan and I. Ibnusaud, Eur. J. Org. Chem., 2021, 2021, 4911–4926 CrossRef CAS.
- X.-H. Chen, X. Xiao, J.-Q. Li, W.-Z. Li and H.-L. Cui, Tetrahedron Lett., 2021, 87, 153548 CrossRef CAS.
- Z.-H. Shang, X.-J. Zhang, Y.-M. Li, R.-X. Wu, H.-R. Zhang, L.-Y. Qin, X. Ni, Y. Yan, A.-X. Wu and Y.-P. Zhu, J. Org. Chem., 2021, 86, 15733–15742 CrossRef CAS PubMed.
- G. S. Astakhov, R. R. Shigaev, T. N. Borisova, A. A. Ershova, A. A. Titov, A. V Varlamov, L. G. Voskressensky and M. D. Matveeva, Mol. Divers., 2021, 25, 2441–2446 CrossRef CAS PubMed.
- Z. Li, N. Xu, N. Guo, Y. Zhou, L. Lin and X. Feng, Chem.–A Eur. J., 2021, 27, 14841–14845 CrossRef CAS PubMed.
- R. S. Rao, A. Sahani, S. H. Ali, S. Pradhan and C. R. Ramanathan, J. Heterocycl. Chem., 2021, 58, 1415–1428 CrossRef CAS.
- S. A. Babu, A. R. Rajalekshmi, P. R. Nitha, V. K. Omanakuttan, P. Rahul, S. Varughese and J. John, Org. Biomol. Chem., 2021, 19, 1807–1817 RSC.
- Y.-W. Xu, J. Wang, G. Wang and L. Zhen, J. Org. Chem., 2021, 86, 91–102 CrossRef CAS PubMed.
- M. Koohgard and M. Hosseini-Sarvari, J. Photochem. Photobiol., A, 2021, 404, 112877 CrossRef CAS.
- K. S. Mandrekar and S. G. Tilve, RSC Adv., 2022, 12, 17701–17705 RSC.
- A. R. Miftyakhova, M. B. Sidakov, T. N. Borisova, V. V Ilyushenkova, A. N. Fakhrutdinov, E. A. Sorokina, A. V Varlamov and L. G. Voskressensky, Tetrahedron Lett., 2022, 103, 153991 CrossRef CAS.
- D. Yu, Y. Liu and C.-M. Che, Org. Chem. Front., 2022, 9, 2779–2785 RSC.
- A. Toumi, S. Boudriga, K. Hamden, I. Daoud, M. Askri, A. Soldera, J.-F. Lohier, C. Strohmann, L. Brieger and M. Knorr, J. Org. Chem., 2021, 86, 13420–13445 CrossRef CAS PubMed.
- S. Boudriga, S. Haddad, M. Askri, A. Soldera, M. Knorr, C. Strohmann and C. Golz, RSC Adv., 2019, 9, 11082–11091 RSC.
- N. V. M. Thomas, S. S. Leena and A. Deepthi, Synthesis, 2022, 54, 2885–2893 CrossRef CAS.
- D. J. Boruah, D. Kathirvelan, S. Borra, R. A. Maurya and P. Yuvaraj, New J. Chem., 2022, 46, 792–797 RSC.
- Y. Huang, Y.-X. Huang, J. Sun and C.-G. Yan, RSC Adv., 2018, 8, 23990–23995 RSC.
- R. M. Chávez-Santos, P. E. Reyes-Gutiérrez, R. O. Torres-Ochoa, M. T. Ramírez-Apan and R. Martínez, Chem. Pharm. Bull., 2017, 65, 973–981 CrossRef PubMed.
- P. E. Reyes-Gutiérrez, J. R. Camacho, M. T. Ramírez-Apan, Y. M. Osornio and R. Martínez, Org. Biomol. Chem., 2010, 8, 4374–4382 RSC.
- A. A. Nevskaya, M. D. Matveeva, T. N. Borisova, M. Niso, N. A. Colabufo, A. Boccarelli, R. Purgatorio, M. de Candia, S. Cellamare, L. G. Voskressensky and C. D. Altomare, ChemMedChem, 2018, 13, 1588–1596 CrossRef CAS PubMed.
- A. A. Nevskaya, L. V Anikina, R. Purgatorio, M. Catto, O. Nicolotti, M. de Candia, L. Pisani, T. N. Borisova, A. R. Miftyakhova, A. V Varlamov, E. Y. Nevskaya, R. S. Borisov, L. G. Voskressensky and C. D. Altomare, Molecules, 2021, 26, 259 CrossRef PubMed.
- S. Kakhki, S. Shahosseini and A. Zarghi, Iran. J. Pharm. Res., 2016, 15, 743–751 CAS.
| This journal is © The Royal Society of Chemistry 2024 |