Open Access Article
Open Access ArticleLiOtBu-promoted synthesis of bis(3-indolyl)methanes by the alkylation of indoles with alcohols under air†
Hai Yen Nguyen‡
a,
Thu Hue Tran‡a,
Ha Nam Do‡a,
Dang Van Doa,
Quoc-Anh Ngobc,
Nguyen Quyet Tienb,
Truong Thi Thanh Ngab,
Hien Nguyend,
Tran Quang Hung *bc and
Tuan Thanh Dang
*bc and
Tuan Thanh Dang *a
*a
aFaculty of Chemistry, VNU-Hanoi University of Science, 19 Le Thanh Tong, Hanoi, 10000, Vietnam
bInsitute of Chemistry, Vietnam Academy of Science and Technology (VAST), 18 Hoang Quoc Viet, Cau Giay, Hanoi, 10000, Vietnam
cGraduate University of Science and Technology, Vietnam Academy of Science and Technology (VAST), 18 Hoang Quoc Viet, Cau Giay, Hanoi, 10000, Vietnam
dFaculty of Chemistry, Hanoi National University of Education, 136 Xuan Thuy, Cau Giay, Hanoi, 10000, Vietnam
First published on 11th January 2024
Abstract
Bis(3-indolyl)methanes (BIMs) are known for their important bioactivities, which include anti-cancer, anti-inflammatory, antibacterial, and antioxidant properties. In this study, we are disclosing a metal catalyst-free synthesis of BIMs in high yields via the alkylation reaction of indoles and alcohols in the presence of lithium tert-butoxide base. Notably, oxygen in air played an important role as an oxidant for the facilitation of this transformation. Interestingly, unactivated aliphatic alcohols could be successfully used as alkylating reagents in the alkylation reactions of indole. Especially, several chemical intermediates detected by GC-MS gave important information about the mechanism insights. This method demonstrated cost and environmental advantages for the development of green processes.
Introduction
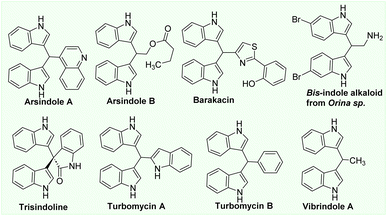
The indole structure is one of the most important heterocycles1–7 and is found in a variety of popular pharmaceuticals, agrochemicals, advanced organic materials, and bioactive alkaloids. The indole scaffold is responsible for constructing a highly important pharmacophore in medicinal chemistry found in over 40 pharmaceutical compounds and 3000 natural products.1 Notably, many bioactive natural alkaloids (arundine, arsindoline A, barakacin, turbomycins, vibrindole A etc.) contain bis(3-indolyl)methane derivatives (BIMs) in their core structures, making BIMs particularly significant molecules (Fig. 1). In addition, they played a crucial role in the development of novel bioactive compounds (anti-inflammatory, anticancer, antimetastatic, etc.).3–10The construction of indole structures from simple building blocks via cyclization reactions in the absence or presence of metal catalysts has been thoroughly established.11,12 Nevertheless, the construction of large molecules with multiple indole moieties from simple building blocks is a challenging problem. Due to the significance of BIM derivatives in the development of novel bioactive molecules, numerous new synthetic methods for preparing BIMs from indole derivatives have been disclosed.3,6,12 Most reports are based on the direct alkylation of indoles with aldehydes or ketones using Lewis or Brønsted acids.6,12 Bhaumik et al.13 reported in 2013 the preparation of a porous organic polymer with built-in CO2H groups and its use as an efficient heterogeneous catalyst for the alkylation of indoles with benzaldehyde and secondary benzylic alcohol derivatives to form BIMs at room temperature. Several new procedures for the preparation of BIMs by the direct coupling of indoles with a variety of alcohols (including aliphatic alcohols) have been demonstrated as a result of the development of green and sustainable processes.14–19 Grigg and colleagues isolated BIM for the first time as a by-product of the Ir-catalyzed alkylation of indoles with alcohols.14 In 2012, the Liu group reported the Ru-catalyzed reaction of indoles with benzylic alcohols as a method for the facile synthesis of BIM derivatives.15 A year later, Ohta et al.16 developed a practical Ru-catalyzed alkylation of indole with benzylic alcohols for 24 hours at 110 °C. The Srimani group has just disclosed the ruthenium pincer complex-catalyzed transformations of indoles with alcohols that yield either C3-alkylated indoles or BIMs in the year 2020.17 Hikawa and Yokoyama reported a Pd-catalyzed domino process for the synthesis of BIM derivatives involving C3–H benzylation of indoles and benzylic C–H functionalization in water.18 In 2014, the Sekar group reported the first FeCl2/BINAM catalyst in the use of dicumyl peroxide as an oxidant for the synthesis of BIMs based on the recent development of cheaper and greener catalysts based on base metals synthesising BIMs with modest yields.19 Recently, our group disclosed a facile method to prepare BIMs in high yield using Cu(OAc)2 catalyst. In this research, oxygen in air was found to be the oxidant for this transformation. Even though these homogeneous metal catalysts frequently offer higher yield and selectivity, their industrial applications are hampered by their complexity in separation and removal of catalysts following reactions.20,21 In the pharmaceutical and fine chemical industries, the contamination of desired products with transition metals may also be a major concern.20,21
To overcome these disadvantages, Babazadeh and colleagues demonstrated the preparation of BIMs in air using Ni nanoparticles supported on ionic liquid-functionalized magnetic silica as a recyclable heterogeneous catalyst.22 Very recently, a useful method for the synthesis of BIMs by the alkylation reaction of alcohols and indoles using blue LED light and the photocatalyst Fe3O4@SiO2@TPP-Cu was described.23 In general, these heterogeneous catalysts are only effective with benzylic alcohols, whereas aliphatic alcohols continue to be difficult substrates for the formation of desired BIM products. To solve this issue, in 2021, we reported an air-stable and easy-prepared CuFe2O4 catalyst that could be used as a convenient heterogeneous catalyst for the formation of BIMs in very high yields.24d
Recently, several metal-free alkylation processes using alcohols as electrophiles were reported.25 In 2022, Zhou et al. demonstrated a metal-free synthesis of BIMs by the alkylation of indoles with sodium alkoxides.26 Notably, 1-tetralone (1 equiv.) must be used as the hydrogen acceptor for the facilitation of this reaction. Very recently, during the preparation of this manuscript, Marques and coworkers disclosed a convenient synthesis of BIMs mediated by KOtBu in toluene.27 This study claimed that the alkylation of indoles with benzyl alcohols occurred via a radical mechanism, in which radical anion generated from benzyl alkoxide played a key role in the success of this reaction. However, this transformation only occurred with benzyl alcohol derivatives which are easily converted to benzyl alkoxide radicals. As a continuation of our interest in developing sustainable synthetic methodologies using alcohols to prepare N-heterocycles, such as quinolines, pyrroles, indoles, and others,24 we planned to investigate the synthesis of BIMs under metal catalyst-free conditions. Herein, we disclose, for the first time, a robust, metal catalyst-free method for the practical synthesis of bis(3-indolyl)phenylmethanes (BIMs) by the alkylation of indoles and alcohols including unactivated aliphatic alcohols under air. Notably, from several observed experimental evidence, we realized that the real mechanism did not undergo the formation of alkoxide radicals under our solvent-free condition. Especially, a plausible mechanism to explain the formation of BIM products and C-3 alkylated side products was also proposed in this research.
Results and discussion
We carried out our initial optimizations for the alkylation of indole 1a with benzyl alcohol 2a in the utilization of NaOtBu base. In general, the reaction mixture was stirred in 24 h with a magnetic stirrer and heated in the presence of atmospheric air. Firstly, toluene was chosen as a potential solvent. We did not obtain any product when the reaction was performed at 80 °C. Especially, the BIM product 3a was isolated in 51% yield when the reaction temperature was increased to 120 °C (entry 2, Table 1). Then, other common solvents (dioxane, DMSO) were investigated in this reaction which did not give better yields. Interestingly, when this reaction was carried out under solvent-free conditions, we obtained the product in a higher yield (65%) (entry 5, Table 1). Due to the very high boiling point of BnOH, we increased the reaction temperature to 140 °C, in fact, we could improve the product yield to 75% yield (entry 6, Table 1). In order to investigate the influence of base, a number of different bases are examined (Table 1). The bases KOtBu, KOH, CsOH, and KOAc exhibited unfavorable performance, whereas NaOtBu and NaOH only provided reasonable yields (entries 7–12, Table 1). Notably, LiOtBu under optimal conditions produced a 78% yield (entry 8, Table 1). In addition, when LiOtBu base with the highest product yield was chosen, the reaction temperature was increased to 150 °C, and the product yield slightly increased from 78% to 80%. Increasing the reaction time to 48 hours for the LiOtBu-mediated reaction did not result in a significant increase in yield. Then, the amount of LiOtBu base was the subject of investigation, in fact, we did not obtain a better yield with such conditions. Interestingly, when this reaction was performed under argon atmosphere with the optimized condition, the product yield significantly dropped to 28% (Entry 16, Table 1). This result has shown that oxygen in air may play a key role as an oxidant in this transformation.| Entry | Base (equiv.) | Solvent (mL) | Temp. (°C) | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: indole (35.1 mg, 0.3 mmol, 1 equiv.), BnOH (129.6 mg, 1.2 mmol, 4 equiv.), base (0.3 mmol, 1 equiv.), 24 h, 140 °C; product yield was determined by column chromatography.b Reaction was performed under argon atmosphere. | ||||
| 1 | NaOtBu (1) | Toluene (0.5) | 80 | — |
| 2 | NaOtBu (1) | Toluene (0.5) | 120 | 51 |
| 3 | NaOtBu (1) | Dioxane (0.5) | 120 | 36 |
| 4 | NaOtBu (1) | DMSO (0.5) | 120 | 49 |
| 5 | NaOtBu (1) | — | 120 | 65 |
| 6 | NaOtBu (1) | — | 140 | 75 |
| 7 | KOtBu (1) | — | 140 | 42 |
| 8 | LiOtBu (1) | — | 140 | 78 |
| 9 | KOH | — | 140 | 32 |
| 10 | NaOH | — | 140 | 71 |
| 11 | KOAc | — | 140 | 30 |
| 12 | CsOH | — | 140 | 50 |
| 13 | LiOtBu (1) | — | 150 | 80 |
| 14 | LiOtBu (0.5) | — | 140 | 73 |
| 15 | LiOtBu (2) | — | 140 | <20 |
| 16 | LiOtBu (1) | — | 140 | 28b |
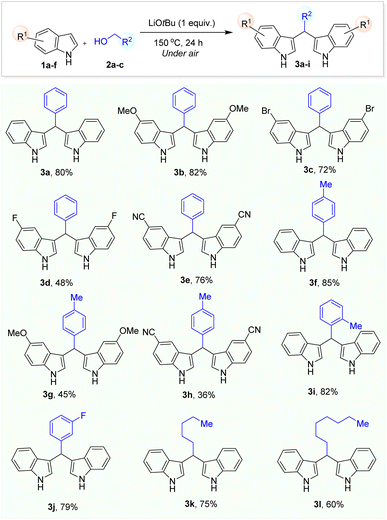
We then proceed to extend the reaction scope based on the optimization conditions by using indole and benzyl alcohol derivatives. First, indole derivatives containing electron-donating and electron-withdrawing groups were alkylated in up to 82% yield using benzylic alcohol (3b–e). In general, the yields of BIM derivatives were obtained in moderate to very good yields (Table 2). Then, the alkylation of indole with benzyl alcohol derivatives were carried out which resulted in up to 85% yield of desired products (3f–j). However, when the reaction of N-methyl indole with benzyl alcohol was carried out under optimized condition. Unfortunately, we did not observe the formation of the corresponding BIM product. Therefore, the deprotonation of HN-indole by a strong base is necessary for the success of this reaction. Especially, challenging unactivated aliphatic alcohols such as n-hexanol and n-octanol were successfully used as alkylating reagents in the reaction with indole which gave the corresponding BIM product 3k,l in 75% and 60% isolated yields, respectively. In the end, we tried scale up the model reaction in larger scale (using 3 mmol of indole) under air and we obtained product 3a in lower yield (72%) due to the lack of O2 in the volume of pressure tube. This issue would be solved by carrying out reaction under O2 atmosphere.
In order to prepare BIM products with different indole moieties, we carried out the reaction using a mixture of indole and 4-methylindole with 2 different ratios using benzyl alcohol under our optimized reaction. We realized that when the ratio of indole and 4-methylindole (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was employed, we obtained a mixture of 3 products (3,3′-(phenylmethylene)bis(1H-indole):3-((1H-indol-3-yl)(phenyl)methyl)-5-methyl-1H-indole:3,3′-(phenylmethylene)bis(5-methyl-1H-indole)) with the ratio 1.0
1) was employed, we obtained a mixture of 3 products (3,3′-(phenylmethylene)bis(1H-indole):3-((1H-indol-3-yl)(phenyl)methyl)-5-methyl-1H-indole:3,3′-(phenylmethylene)bis(5-methyl-1H-indole)) with the ratio 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8
2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3. When the ratio of indole and 4-methylindole (2
1.3. When the ratio of indole and 4-methylindole (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was used, we observed a mixture of 3 products (3,3′-(phenylmethylene)bis(1H-indole):3-((1H-indol-3-yl)(phenyl)methyl)-5-methyl-1H-indole:3,3′-(phenylmethylene)bis(5-methyl-1H-indole)) with the ratio 6.5
1) was used, we observed a mixture of 3 products (3,3′-(phenylmethylene)bis(1H-indole):3-((1H-indol-3-yl)(phenyl)methyl)-5-methyl-1H-indole:3,3′-(phenylmethylene)bis(5-methyl-1H-indole)) with the ratio 6.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.2
5.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0. However, only GC-MS analysis can reveal these BIM product combination ratios. In fact, column chromatography proved quite challenging for isolating these BIM products from the original crude mixtures.
1.0. However, only GC-MS analysis can reveal these BIM product combination ratios. In fact, column chromatography proved quite challenging for isolating these BIM products from the original crude mixtures.
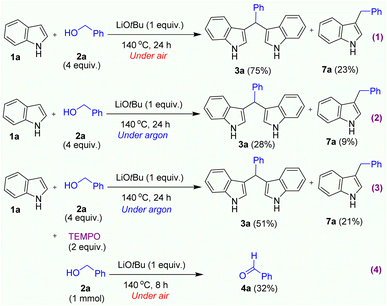
To investigate the reaction mechanism, we carried out several control experiments. Firstly, we were interested in understanding the real role of oxygen which would be the key oxidant in this reaction. We performed 2 reactions under our optimised condition in air and argon atmosphere (Scheme 1, reactions 1,2). The crude mixtures were carefully analysed by GC-MS instrument. Interestingly, we only obtained the formation of BIM product in high yield when reaction was carried out under air atmosphere. Notably, in both reactions, a C-3 alkylated indole product 7a was simultaneously formed (See SI). In order to understand clearly about the formation of C-3 alkylated indole products which could be generated along with BIM products in Table 2. The crude mixture of each reaction was sampled and analysed by GC-MS. Interestingly, we observed the formation of C3-alkylated indole products as side products (in 12–34% yield) along with the BIM products in all cases. During the latter stages of preparing our manuscript, Marques and coworkers reported similar research on the synthesis of BIMs mediated by KOtBu base in toluene solvent.27 According to the results of the mechanistic investigations, the C-3 alkylation of indoles occurred via a radical mechanism, and the KOtBu base played its role in the formation of benzylic radicals. In a similar approach, we added 2 equiv. of 2,2,6,6-tetramethylpiperidinooxy (TEMPO) (a radical-trapping reagent)25j into the reaction system under optimized condition in argon atmosphere (Scheme 1, reaction 3). Indeed, we still observed both products 3a and 7a in 51% and 21% yields, respectively. Interestingly, in all control experiments, a small amount of benzaldehyde was detected along with both products 3a and 7a. In a recent study on the synthesis of 3-arylpropanamides by radical condensation of benzylic alcohols and acetamides, Azizi and Madsen also disclosed a radical mechanism with the formation of benzylic radicals in the presence of KOtBu base.25l However, the authors also confirmed that only benzyl alcohol derivatives could be converted to corresponding benzylic radicals in the presence of KOtBu base. Especially, this alkylation did not work when unactivated aliphatic alcohols were employed.25l In our observation, the alkylation reaction of indole with n-hexanol resulted in the formation of product 3f in a high yield (75%). Then, the last control experiment using only benzyl alcohol as the starting material was performed to confirm the formation of benzaldehyde intermediate under air atmosphere. Notably, a significant amount of benzaldehyde was formed in 32% yield after running this reaction in 6h (Scheme 1, reaction 4). From these observations, we can conclude that this transformation did not go through the formation of a benzylic alkoxide radical intermediate.
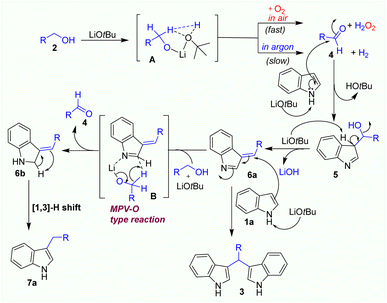
A plausible mechanism is proposed based on our experimental results (Scheme 1) and existing literature reports,25 as described in Scheme 2. Under solvent-free condition, LiOtBu base deprotonates alcohol to generate the corresponding lithium alkoxide which interacts with an in situ generated tert-butyl alcohol by hydrogen bonding and coordination with lithium cation, forming transition state A. In the presence of oxygen in air, the transition state A may well react with oxygen to form aldehyde 4 and a hydrogen peroxide molecule. On the other hand, under argon atmosphere, the dehydrogenation process of transition state A may slowly occur to generate aldehyde 4. When aldehyde 4 is formed, it easily reacts with indole to produce imine intermediate 5 by the addition reaction of an indole with aldehyde 4. Then, LiOtBu base take H-α of this imine intermediate 5 to generate 3-benzylidene-3H-indole 6a. The formation of key intermediate 6a would be important for further transformations via 2 pathways. Firstly, in the attention of LiOtBu base, another indole reacts with intermediate 6a to form BIM 3 as a main product by 1,4-addition reaction. This process was well established in several previous reports.16–23 In the second pathway, intermediate 6a may coordinate with lithium alkoxide to form six-membered ring transition state B which was converted to aldehyde 4 and intermediate 6b via Meerwein–Pondorf–Verley–Oppenauer-type (MPV–O) redox reaction.25 In the end, the intermediate 6b tautomerizes to side product 7a via the [1,3]-H shift process.
Conclusions
In this study, we have developed a convenient and practical method for preparing bis(3-indolyl)methane (BIM) derivatives by LiOtBu-promoted alkylation of indoles with alcohols under solvent-free condition in air, without the presence of any metal catalysts. In fact, we figured out that oxygen in air played an important role as an oxidant for the success of this transformation. Especially, we discovered that unactivated aliphatic alcohols (n-hexanol and n-octanol) could be successfully used as an alkylating reagent in the alkylation reaction of indole giving 75% and 60% yield of desired products, respectively. A series of control experiments were carried out, giving several important chemical intermediates. Based on these findings, a plausible mechanism for the LiOtBu-promoted formation of BIMs and C3-alkylated indoles in air was proposed. This procedure holds significant potential for application in the fields of medicinal chemistry and organic synthesis.Experimental
General procedure for synthesis of 3,3′-(phenylmethylene)bis(1H-indole) 3a
Indole (35.1 mg, 0.3 mmol, 1 eq.) was added to a pressure tube that was charged with benzyl alcohol (130 mg, 1.2 mmol, 4 eq.), LiOtBu (24 mg, 0.3 mmol, 1 eq.) under air. The reaction mixture was stirred with a magnetic stirrer and heated at 150 °C for 24 hours. After cooling, the reaction mixture was filtered through a pad of celite, which was washed three times with hot water (200 mL) to eliminate benzyl alcohol and three times with EtOAc to have the final filtrate. The filtrate was concentrated in vacuo. The crude product was further purified on silica gel column chromatography with a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 elution system of hexane/ethyl acetate to yield 3a (38.6 mg, 80%) as pale-yellow solid; mp = 86–87 °C; 1H NMR (600 MHz, chloroform-d) δ 7.87 (s, 1H), 7.40 (dd, J = 8.0, 1.1 Hz, 1H), 7.38–7.33 (m, 2H), 7.28 (dd, J = 8.4, 6.8 Hz, 1H), 7.25–7.20 (m, 0H), 7.17 (ddd, J = 8.2, 7.0, 1.2 Hz, 1H), 7.01 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H), 6.65 (dd, J = 2.4, 1.0 Hz, 1H), 5.90 (s, 1H). 13C NMR (151 MHz, CDCl3) δ 144.03, 136.72, 128.74, 128.22, 127.12, 126.14, 123.61, 121.94, 119.96, 119.77, 119.25, 111.02, 77.24, 77.03, 76.82, 40.23.
1 elution system of hexane/ethyl acetate to yield 3a (38.6 mg, 80%) as pale-yellow solid; mp = 86–87 °C; 1H NMR (600 MHz, chloroform-d) δ 7.87 (s, 1H), 7.40 (dd, J = 8.0, 1.1 Hz, 1H), 7.38–7.33 (m, 2H), 7.28 (dd, J = 8.4, 6.8 Hz, 1H), 7.25–7.20 (m, 0H), 7.17 (ddd, J = 8.2, 7.0, 1.2 Hz, 1H), 7.01 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H), 6.65 (dd, J = 2.4, 1.0 Hz, 1H), 5.90 (s, 1H). 13C NMR (151 MHz, CDCl3) δ 144.03, 136.72, 128.74, 128.22, 127.12, 126.14, 123.61, 121.94, 119.96, 119.77, 119.25, 111.02, 77.24, 77.03, 76.82, 40.23.
Author contributions
T. Q. Hung, T. T. Dang wrote this manuscript and conceived this project; H. Y. Nguyen, T. H. Tran, H. N. Do performed the experiments and analysed the data; D. V. Do, N. Q. Tien, T. T. T. Nga conducted GC-MS analysis; Q.-A. Ngo, H. Nguyen edited the manuscript and gave discussions about the mechanism.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is funded by the Institute of Chemistry, Vietnam Academy of Science and Technology under grant number CSCL06.03/22-23.Notes and references
- J. F. Austin and D. W. C. MacMillan, J. Am. Chem. Soc., 2002, 124, 1172 CrossRef CAS PubMed.
- Y. Wan, Y. Li, C. Yan, M. Yan and Z. Tang, Eur. J. Med. Chem., 2019, 183, 111691 CrossRef CAS PubMed.
- R. R. Jella and R. Nagarajan, Tetrahedron, 2013, 69, 10249 CrossRef CAS.
- G. Bifulco, I. Bruno, R. Riccio, J. Lavayre and G. Bourdy, J. Nat. Prod., 1995, 58, 1254 CrossRef CAS PubMed.
- S. Safe, S. Papineni and S. Chintharlapalli, Cancer Lett., 2008, 269, 326 CrossRef CAS PubMed.
- M. Shiri, M. A. Zolfigol, H. G. Kruger and Z. Tanbakouchian, Chem. Rev., 2010, 110, 2250 CrossRef CAS PubMed.
- M. Z. Zhang, Q. Chen and G. F. Yang, Eur. J. Med. Chem., 2015, 89, 421 CrossRef CAS PubMed.
- S. B. Bharate, J. B. Bharate, S. I. Khan, B. L. Tekwani, M. R. Jacob, R. Mudududdla, R. R. Yadav, B. Singh, P. R. Sharma, S. Maity, B. Singh, I. A. Khan and R. A. Vishwakarma, Eur. J. Med. Chem., 2013, 63, 435 CrossRef CAS PubMed.
- M. Marrelli, X. Cachet, F. Conforti, R. Sirianni, A. Chimento, V. Pezzi, S. Michel, G. A. Statti and F. Menichini, Nat. Prod. Res., 2013, 27, 2039 CrossRef CAS PubMed.
- J. Lee, Nutr. Cancer, 2019, 71, 992 CrossRef CAS PubMed.
- G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875 CrossRef CAS PubMed.
- M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9608 CrossRef CAS PubMed.
- A. Modak, J. Mondal and A. Bhaumik, ChemCatChem, 2013, 5, 1749 CrossRef CAS.
- S. Whitneys, R. Grigg, A. Derrick and A. Keep, Org. Lett., 2007, 9, 3299 CrossRef PubMed.
- S. Zhang, W. Fan, H. Qu, C. Xiao, N. Wang, L. Shu, Q. Hu and L. Liu, Curr. Org. Chem., 2012, 16, 942 CrossRef CAS.
- A. E. Putra, K. Takigawa, H. Tanaka, Y. Ito, Y. Oe and T. Ohta, Eur. J. Org Chem., 2013, 6344 CrossRef CAS.
- N. Biswas, R. Sharma and D. Srimani, Adv. Synth. Catal., 2020, 362, 2902 CrossRef CAS.
- H. Hikawa and Y. Yokoyama, RSC Adv., 2012, 3, 1061 RSC.
- S. Badigenchala, D. Ganapathy, A. Das, R. Singh and G. Sekar, Synthesis, 2014, 46, 101 CrossRef.
- V. Polshettiwar and R. S. Varma, Green Chem., 2010, 12, 743 RSC.
- C. Descorme, P. Gallezot, C. Geantet and C. George, ChemCatChem, 2012, 4, 1897 CrossRef CAS.
- R. Hosseinzadeh-Khanmiri, Y. Kamel, Z. Keshvari, A. Mobaraki, G. H. Shahverdizadeh, E. Vessally and M. Babazadeh, Appl. Organomet. Chem., 2018, 32, e4452 CrossRef.
- H. Mohammadi and H. R. Shaterian, ChemistrySelect, 2019, 4, 8700 CrossRef CAS.
- (a) M. T. Ha, N. T. Nguyen, N. H. Tran, Q. V. Ho, N. T. Son, V. H. Nguyen, H. Nguyen, D. V. Do, T. Q. Hung, B. K. Mai and T. T. Dang, Chem.–Asian J., 2022, 17, e202200909 CrossRef PubMed; (b) T. Q. Hung, T. T. Do, V. Q. Hoang, D. M. Tran, Q.-A. Ngo, T. A. L. Hoang, R. Eckelt, D. V. Do, T.T. Dang and X. H. Vu, Chem. Pap., 2022, 77, 89 CrossRef; (c) N. K. Nguyen, D. L. Tran, T. Q. Hung, T. M. Le, N. T. Son, Q. T. Trinh, T. T. Dang and P. Langer, Tetrahedron Lett., 2021, 68, 152936 CrossRef CAS; (d) N. K. Nguyen, M. T. Ha, H. Y. Bui, Q. T. Trinh, B. N. Tran, V. T. Nguyen, T. Q. Hung, T. T. Dang and X. H. Vu, Catal. Commun., 2021, 149, 106240 CrossRef CAS; (e) N. K. Nguyen, D. H. Nam, B. Van Phuc, V. H. Nguyen, Q. T. Trinh, T. Q. Hung and T. T. Dang, Mol. Catal., 2021, 505, 111462 CrossRef CAS; (f) H. N. Do, N. M. Quan, B. Van Phuc, D. Van Tinh, N. Q. Tien, T. T. T. Nga, V. T. Nguyen, T. Q. Hung, T. T. Dang and P. Langer, Synlett, 2021, 32, 611 CrossRef CAS; (g) B. V. Phuc, H. N. Do, N. M. Quan, N. N. Tuan, N. Q. An, N. V. Tuyen, H. L. T. Anh, T. Q. Hung, T. T. Dang and P. Langer, Synlett, 2021, 32, 1004 CrossRef.
- (a) S. Yao, K. Zhou, J. Wang, H. Cao, L. Yu, J. Wu, P. Qiu and Q. Xu, Green Chem., 2017, 19, 2945 RSC; (b) R. Radhakrishan, D. M. Do, S. Jaenicke, Y. Sasson and G. K. Chuah, ACS Catal., 2011, 1, 1631 CrossRef CAS; (c) G. Li, M. Li, Z. Xia, Z. Tan, W. Deng and C. Fang, J. Org. Chem., 2022, 87, 8884 CrossRef CAS PubMed; (d) H. X. Le, K. D. Nguyen, N. T. S. Phan, H. V. Le and T. T. Nguyen, ChemistrySelect, 2023, 8, e202204024 CrossRef CAS; (e) Q. Xu, J. Chen, H. Tian, X. Yuan, S. Li, C. Zhou and J. Liu, Angew. Chem., Int. Ed., 2014, 53, 225 CrossRef CAS PubMed; (f) T. Liu, K. Wu, L. Wang and Z. Yu, Adv. Synth. Catal., 2019, 361, 3958 CrossRef CAS; (g) M. B. Dambatta, J. Santos, R. R. A. Bolt and L. C. Morrill, Tetrahedron, 2020, 76, 131571 CrossRef CAS; (h) D. J. Dahatonde, A. Ghosh and S. Batra, Eur. J. Org Chem., 2021, 19, 2746 CrossRef; (i) F. Su, M. Lai, M. Zhao, M. Song, X. Hu and J. Zhang, ChemistrySelect, 2022, 7, e202104454 CrossRef CAS; (j) A. Banik, P. Datta and S. K. Mandal, Org. Lett., 2023, 25, 1305 CrossRef CAS PubMed; (k) Q. Xu, J. Chen and Q. Liu, Adv. Synth. Catal., 2013, 355, 697 CrossRef CAS; (l) K. Azizi and R. Madsen, Chem. Sci., 2020, 11, 7800 RSC.
- X. Chen, Y. Liu, H. Jin and B. Zhou, Synthesis, 2022, 54, 1347 CrossRef CAS.
- A. S. Santos, R. D. Ferro, N. Viduedo, L. B. Maia, A. M. S. Silva and M. M. B. Marques, ChemistryOpen, 2023, 12, e202200265 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra07115d |
| ‡ These authors contributed equally to this research. |
| This journal is © The Royal Society of Chemistry 2024 |