Open Access Article
Open Access ArticleAnderson-type polyoxometalate-based metal–organic framework as an efficient heterogeneous catalyst for selective oxidation of benzylic C–H bonds†
Hong-Ru Tana,
Xiang Zhoub,
Tengfei Gongc,
Hanqi Youa,
Qi Zheng b,
Sheng-Yin Zhao
b,
Sheng-Yin Zhao *a and
Weimin Xuan
*a and
Weimin Xuan *a
*a
aState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Chemistry and Chemical Engineering, Donghua University, Shanghai 201620, P. R. China. E-mail: syzhao8@dhu.edu.cn; weiminxuan@dhu.edu.cn
bState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Materials Science and Engineering, Donghua University, Shanghai 201620, P. R. China. E-mail: qi.zheng@dhu.edu.cn
cJiaxing Jiayuan Inspection Technology Service Co., Ltd, Building 2, No. 1403, Hongbo Road, Economic and Technological Development Zone, Jiaxing City, Zhejiang Province, P. R. China
First published on 2nd January 2024
Abstract
Oxidative transformation of benzylic C–H bonds into functional carbonyl groups under mild conditions represents an efficient method for the synthesis of aromatic carboxylic acids and ketones. Here we report a high-efficiency catalyst system constructed from an Anderson-type polyoxometalate-based metal–Organic framework (POMOF-1) and N-hydroxyphthalimide (NHPI) for selective oxidation of methylarenes and alkylarenes under 1 atm O2 atmosphere. POMOF-1 exerted a synergistic effect originating from the well-aligned Anderson {CrMo6} clusters and Cu centers within the framework, and this entailed good cooperation with NHPI to catalyze the selective oxidation. Accordingly, the reactions exhibit good tolerance and chemical selectivity for a wide range of substrates bearing diverse substituent groups, and the corresponding carboxylic acids and ketones were harvested in good yields under mild conditions. Mechanism study reveals that POMOF-1 worked synergistically with NPHI to activate the benzylic C–H bonds of substrates, which are sequentially oxidized by oxygen and HOO˙ to give rise to the products. This work may pave a way to design high-efficiency catalysts by integration of polyoxometalate-based materials with NPHI for challenging C–H activation.
Introduction
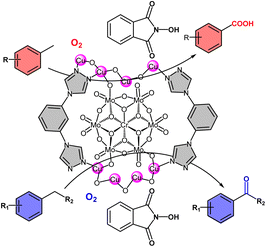
The selective oxidation of alkylaromatic hydrocarbons is of great fundamental and practical importance to manufacture value-added alcohols, aldehydes, ketones and carboxylic acids.1–5 Evolving from conventional Cr(VI) and Mn(VII)-based reagents, the application of air or molecular oxygen as a green oxidant has aroused constant attention.6–11 The most impressive demonstration of this methodology includes the industrial production of benzoic acid using a Co/Mn/Br/AcOH system via selective oxidation of toluene with O2, which manifests the high-efficiency by combination of catalytic metal centers with bromide as a promoter.12–16 However, the system suffers from strong corrosion owing to the presence of bromide ions and intermediate hydroperoxides in acidic medium.17 In this context, metal/N-hydroxyphthalimide (NHPI) systems such as Co(OAc)2/NHPI and Co(OAc)2/Mn(OAc)2/NHPI have been developed as highly effective alternatives that facilitate oxidative conversion of alkylaromatic hydrocarbons into benzoic acid and terephthalic acid under more environmentally benign conditions.18–23 In view of the high-efficiency of the above systems, combination of solid supports containing metal sites with NHPI has emerged as a versatile approach to build effective catalyst systems.22,24,25 The confined surfaces and well-defined active sites of the solid support can on one hand improve the activity and stability of NHPI, while on the other hand promote the catalysis in a cooperative manner with NHPI absorbed on the surface.26,27 As such, the activity and selectivity can be modulated so that higher catalysis efficiency is achieved as compared with the homogeneous counterparts. For example, employing metal–organic frameworks (MOFs) as porous platform to accommodate NHPI, a variety of oxidative transformations were catalyzed by MOF/NHPI systems, including oxidative coupling of benzylamines, aerobic oxidation of alkanes and alkenes, and selective oxidation of benzylic C–H bonds.28–32 In all these cases, the integrated catalyst systems showed catalytic performance superior to the homogeneous mixture of metal salts and NHPI.Polyoxometalates (POMs), a class of versatile and discrete anionic metal oxides with unique properties, have been applied in catalysis, materials science and medicine.33–38 POMs have been well established as high-efficiency catalysts for oxidative transformation due to their inherent strong oxidizing ability, reversible multi-electron redox property, and high resistance to oxidative decomposition.39–44 Integrated with NHPI, POM clusters such as Mo72V30 can facilely catalyze the oxidation of benzylic alcohols and hydrocarbons to corresponding aldehydes or ketones.45,46 Going beyond the homogeneous system, Installation of Cu-containing POM clusters within crystalline porous all-inorganic framework, the resulted heterogeneous catalyst can cooperate well with NHPI to promote biomimetic aerobic oxidation of aliphatic alcohols into carboxylic acids.47 Alternatively, employing Cu-porphyrin functionalized MOF as solid support to work synergistically with POM cluster and NHPI as co-catalyst platform has also furnished the production of phenylketones via aerobic oxidation of arylalkanes.48 As demonstrated above, combination of NHPI with catalytic transition metal sites and POM clusters which are incorporated within robust frameworks facilitates the catalytic oxidation. Along with these lines, we postulate that direct assembly of metal nodes, POM cluster and organic linkers to build polyoxometalate-based metal–organic frameworks (POMOFs) can offer multifunctional supports to function with NHPI for other advanced oxidative transformations.
Herein, we reported that the design and synthesis of a 2D POMOF H2[Cu4(OH)2(H2O)7L2(CrMo6)2]·6H2O (POMOF-1, L = 1,3-di-(1,2,4-triazol-4-yl)benzene, CrMo6 = [CrMo6O18(OH)5] for selective oxidation of benzylic C–H bonds to carbonyl compounds (Scheme 1). POMOF-1 is constructed from Anderson-type POM Na3[CrMo6O18(OH)5], L and Cu2+, and features a 2D layered structure via the connection of CrMo6 clusters and 1D metal–organic chains built from {Cu4O3} units and L. The presence of catalytically active POM clusters and abundant Cu2+ sites make POMOF-1 an excellent platform for catalytic oxidation of benzylic C–H bonds with NHPI as co-catalyst. Owing to the synergistic effect of functional components, POMOF-1 showed high activity and selectivity towards the oxidative transformation of methylarenes into aryl carboxylic acids. In particular, dimethyl and trimethyl-substituted substrates can also be selectively and facilely converted into aromatic monocarboxylic acid. Besides, the high-efficiency system can further be applied for oxidation of alkylarenes to afford ketones in good yields. POMOF-1 is stable and could be recycled for five times without noticeable loss of catalytic activity.
Experimental
Unless otherwise noted, all the materials were purchased from commercial suppliers and used without further purification. L and Na3[CrMo6O18(OH)6] are prepared according to literature with some modifications. The detailed synthetic process and characterization is described in ESI.†Preparation of POMOF-1
CuCl2·2H2O (5.38 mg, 0.04 mmol), L (4.24 mg, 0.02 mmol) and Na3[CrMo6O18(OH)6] (17.96 mg 0.015 mmol) were added into H2O (5 mL). The solution was stirred and pH was adjusted to 3.6 by 1 M HCl. Then the mixture was heated in a Teflon-lined stainless steel reactor at 140 °C for 3 days. After cooling to room temperature, green bulk crystals were obtained by filtration and washed with DMSO, H2O, EtOH and Et2O. Yield: 14.3 mg. 32.4% based on Cr. Elemental analysis for POMOF-1 (H2[Cu4(OH)2(H2O)7L2(CrMo6)2]·6H2O): C 8.16%, H 1.56%, N 5.71%, Cr 3.54%, Mo 39.16%, Cu 8.65%; found: C 8.01%, H 1.77%, N 5.62%, Cr 3.56%, Mo 39.03%, Cu 8.77%. FT-IR (cm−1): 3369, 2960, 2872, 1637, 1479, 1381, 1116, 1026, 935, 911, 894, 638, 563.General methods for oxidation reaction of methylarenes
POMOF-1 (0.1 mol%), NHPI (0.04 mmol), methylarenes 1 (0.2 mmol) and CH3CN (1 mL) were added into seal tube, which was subsequently charged with 1 atm O2. Then the reaction was heated at 85 °C and kept stirring for 24 h. The reactor was cooled to room temperature, the catalyst was centrifuged and the supernatant was diluted with ethyl acetate. The conversion of methylarenes 1 and yields of product aromatic acid 2 were determined by gas chromatography-mass spectrometry (GC-MS) with hexadecane as internal standard. Then, the reaction was quenched with H2O and extracted with ethyl acetate. Filtering out the insoluble substances, the organic phases were washed with H2O, and dried over anhydrous Na2SO4. The organic phases were filtered and concentrated under reduced pressure and the crude products were purified by silica gel column chromatography (eluent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate–petroleum ether, 1
ethyl acetate–petroleum ether, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10–5
10–5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield the pure products. The products were characterized by 1H NMR and 13C NMR.
1) to yield the pure products. The products were characterized by 1H NMR and 13C NMR.
General methods for oxidation reaction of alkylarenes
POMOF-1 (0.1 mol%), alkylarenes 4 (0.2 mmol) and CH3CN (1 mL) were added into seal tube, which was subsequently charged with 1 atm O2. Then the reaction was heated at 85 °C and kept stirring for 6 h. The reactor was cooled to room temperature, the catalyst was centrifuged and the supernatant was diluted with ethyl acetate. The conversion of alkylarenes 4 and yields of product aromatic ketones 5 were determined by gas chromatography-mass spectrometry (GC-MS) with hexadecane as internal standard. Then, the reaction was quenched with H2O and extracted with ethyl acetate. Filtering out the insoluble substances, the organic phases were washed with H2O, and dried over anhydrous Na2SO4. The organic phases were filtered and concentrated under reduced pressure and the crude products were purified by silica gel column chromatography (eluent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate–petroleum ether, 1
ethyl acetate–petroleum ether, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100–500) to yield the pure products. The products were characterized by 1H NMR and 13C NMR.
100–500) to yield the pure products. The products were characterized by 1H NMR and 13C NMR.
Results and discussion
Crystal structure of POMOF-1
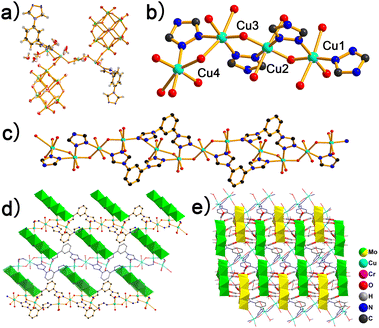
Hydrothermal reaction of L, CuCl2 and Na3[CrMo6O18(OH)6] in acidified aqueous solution at 140 °C resulted in green bulk crystals of H2[Cu4(OH)2(H2O)7L2{CrMo6}2]·6H2O (POMOF-1). The crystallographic data and structural refinement parameters are summarized in Table S1.† The Single-crystal X-ray diffraction structure analysis reveals that POMOF-1 crystallizes in the triclinic P1 space group. The asymmetric unit of POMOF-1 consists of four Cu2+, two L, two {CrMo6} clusters, two μ3-OH atoms and seven coordination water (Fig. 1a). With connection of two μ3-OH atoms one O from coordination water, the four Cu2+ aggregate into a rod-like {Cu4O3} unit (Fig. 1b). Besides, three triazole moieties alternatively bridge the adjacent Cu centers, further reinforcing the rigidity of {Cu4O3} unit (Fig. 1b). All the four Cu2+ adopt octahedral coordination environments. To fulfill the six coordination sites, along with μ3-OH atoms and N atoms from μ2-triazoles, coordination water, terminal O atoms from {CrMo6} clusters and N atoms from L occupy the residual coordination sites around Cu1–Cu4 (Fig. 1b). With such binding mode, each {Cu4O3} unit thus connects with four L while every L links with two {Cu4O3} units, leading to the formation of 1D metal–organic chain (Fig. 1c). The adjacent 1D chains are further pillared by {CrMo6} clusters and therefore giving rise to a 2D layered structure along ac plane (Fig. 1d). Owing to the presence of three μ3-OH groups on each surface of disk-like {CrMo6} cluster, multiple O⋯H–O hydrogen bonds (2.6094(1)–2.8806(1) Å) are generated between {CrMo6} clusters lined on neighbouring layers, which assist the layer-by-layer stacking and hence the formation of a 3D supramolecular framework (Fig. 1e). In fact, Anderson-type clusters are well known for the facile formation of strong hydrogen bonding between terminal oxygen atoms and μ3-OH groups from adjacent clusters in crystalline solids; this has resulted in supramolecular structures from 1D zigzag chain to 3D porous framework.49,50The experimental PXRD pattern of POMOF-1 agrees well with the simulated data (Fig. S2†), demonstrating its high phase purity. As shown in TGA curve (Fig. S3†), the first weight loss of 7.24% occurs from 50 to 240 °C, which is corresponding to the release of six lattice H2O molecules and six coordinated water molecules. Afterwards, the organic ligands decompose in the temperature range from 240 to 420 °C with the weight loss of 14.42%. Finally, the collapse of {CrMo6} skeleton happens between 420 and 700 °C. The residue can be attributed to a mixture of CuO, MoO3 and CrO3. The FT-IR spectrum of POMOF-1 clearly shows the characteristic peaks of both L and {CrMo6} (Fig. S4†). According to the literature, the bands centered at 935, 911, 894, 638 and 563 cm−1 are ascribed to ν(Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), ν(Mo–O–Mo), ν(Mo–O–Cr), ν(Mo–O), ν(Cr–O) vibrations of {CrMo6}, respectively.51,52 The peaks at 2960 and 2872 cm−1 are corresponding to C–H stretching of the aromatic rings from L. Moreover, the characteristic peaks of C
O), ν(Mo–O–Mo), ν(Mo–O–Cr), ν(Mo–O), ν(Cr–O) vibrations of {CrMo6}, respectively.51,52 The peaks at 2960 and 2872 cm−1 are corresponding to C–H stretching of the aromatic rings from L. Moreover, the characteristic peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C
N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C can be clearly identified from 1350 to 1700 cm−1, further confirming the presence of L.
C can be clearly identified from 1350 to 1700 cm−1, further confirming the presence of L.
Catalytic oxidation of methylarenes by POMOF-1
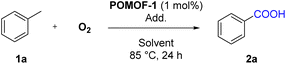
As an archetypal POM cluster, Anderson POMs are widely used for a variety of catalytic oxidative transformations.53–56 Meanwhile, as one of the most utilized transition metals, copper is also extensively applied for synthetic organic chemistry.57–60 Very recently, Copper-based catalyst has showed enzymatic activity for C–H oxidation with NHPI as co-catalyst.61 By integration of Anderson-type {CrMo6} cluster and {Cu4O3} unit in the framework of POMOF-1, we thus intend to use it as a catalyst for the synthesis of aryl carboxylic acids via oxidation of methylarenes.The investigation was initialized by using toluene (1a) as a model substrate (Table 1). The oxidative reaction was carried out under 1 atm O2 atmosphere in CH3CN at 85 °C for 24 h using 1 mol% POMOF-1 as catalyst and 0.2 equivalent NHPI as co-catalyst, benzoic acid (2a) can be obtained in a yield of 91% (Table 1, entry 1). Increase of the amount of NHPI afforded similar yield (Table 1, entry 2) while decrease of NHPI to 0.1 and 0.05 equivalent significantly reduced the yield (Table 1, entries 3 and 4). Further optimization of the reaction temperature and time showed 85 °C and 24 h were the optimal conditions (Table 1, entries 5–8). In addition, screening the scope of solvents showed that CH3CN was the best solvent (Table 1, entries 9–14). Control experiments showed that NHPI and O2 were essential for the oxidation. As indicated by entry 15, the reaction hardly happened in the absence of NHPI. Performing the catalysis in air atmosphere also led to the production of 2a albeit in much lower yield (Table 1, entry 16), and the reaction could not proceed in N2 atmosphere (Table 1, entry 17). After extensive optimization with reaction parameters, it was found that 1.0 mol% POMOF-1 along with 0.2 equivalent of NHPI in CH3CN at 85 °C for 24 h was the most optimized reaction condition (Table 1, entry 1).
| Entry | Add. | Solvent | Yields% |
|---|---|---|---|
| a Reaction condition: POMOF-1 (1 mol%), add., solvent (1 mL), toluene 1a (0.2 mmol) in O2 atmosphere (1 atm) at 85 °C. Yields were determined by GC-MS and hexadecane as internal standard.b Reaction temperature: 95 °C.c Reaction temperature: 65 °C.d Reaction time: 12 h.e Reaction time: 36 h.f Under air atmosphere.g Under N2 atmosphere. | |||
| 1 | 0.2 equiv. NHPI | CH3CN | 91 |
| 2 | 0.5 equiv. NHPI | CH3CN | 90 |
| 3 | 0.1 equiv. NHPI | CH3CN | 42 |
| 4 | 0.05 equiv. NHPI | CH3CN | 20 |
| 5b | 0.2 equiv. NHPI | CH3CN | 85 |
| 6c | 0.2 equiv. NHPI | CH3CN | 51 |
| 7d | 0.2 equiv. NHPI | CH3CN | 68 |
| 8e | 0.2 equiv. NHPI | CH3CN | 95 |
| 9 | 0.2 equiv. NHPI | EtOH | Trace |
| 10 | 0.2 equiv. NHPI | Acetone | 18 |
| 11 | 0.2 equiv. NHPI | THF | Trace |
| 12 | 0.2 equiv. NHPI | DCM | Trace |
| 13 | 0.2 equiv. NHPI | DMF | Trace |
| 14 | 0.2 equiv. NHPI | DMSO | Trace |
| 15 | None | CH3CN | Trace |
| 16f | 0.2 equiv. NHPI | CH3CN | 11 |
| 17g | 0.2 equiv. NHPI | CH3CN | Trace |
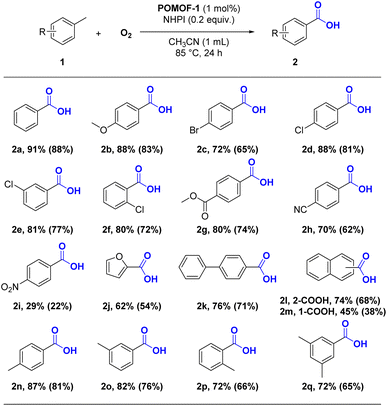
With the optimized reaction condition, the scope of oxidation reaction of methylarenes was then investigated. As shown in Table 2, the reaction showed good functional group tolerance for a series of substituted toluene in good to excellent yields ranging from 72% to 91% (2b–2g). Both the electron-donating methoxy and weak electron-withdrawing bromo/chloro/ester substituted toluene can be facilely converted to corresponding aryl carboxylic acids (2b–2g). For strong electron-withdrawing cyan group, targeted products could also be harvested in 70% yield (2h). Nitro group severely deactivated the substrate, 2i was only acquired in 29% yield. As revealed by the Hammett plot (Fig. S11†), the log of relative reaction rates for para-substituted methylarenes correlated very well with Hammett parameter (R2 = 0.937), affording a ρ value of −0.90, which indicated electron-rich methylarences are favored for oxidative conversion with higher rates.62,63 Meanwhile, methyl-substituted pyrrole could be smoothly transformed to 2j in a yield of 62%. Regarding the substrates with bigger size, the linear 4-phenyltoluene and 2-methyl naphthalene can be converted to 2k and 2l in a good yield of 76% and 74%, while the conversion of more sterically hindered 1-methyl naphthalene to 2m was much lower. Next, we pay attention to the selective oxidation of dimethyl and trimethyl-substituted benzene. To our delight, all kinds of xylenes can be selectively oxidized to aromatic monocarboxylic acids in satisfied yields (2n–2p). Compared with m- and p-xylene (2n, 2o), the less effective conversion of o-xylene may be caused by the steric effect (2p). In a similar way, 2q can be acquired in a yield of 72% from mesitylene. Overall, POMOF-1 demonstrated general applicability towards a variety of methylarenes.
| a Reaction condition: POMOF-1 (1 mol%), NHPI (0.2 equiv.), methylarenes 1 (0.2 mmol) and CH3CN (1 mL) in O2 (1 atm) at 85 °C for 24 h. Yields were determined by GC-MS. Hexadecane as internal standard. Values in parentheses are the isolated yields. |
|---|
 |
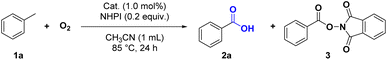
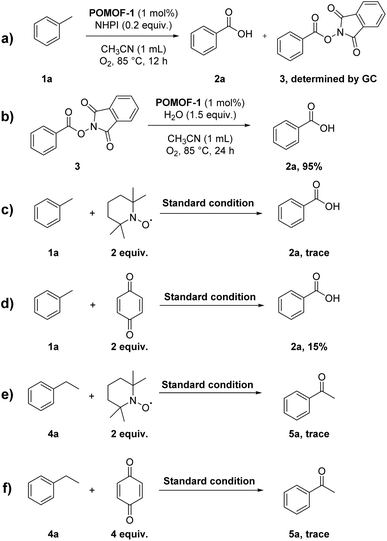
To confirm the synergistic effect of POMOF-1, a series of control experiments were carried out by employing the precursors of POMOF-1 and their mixtures as catalysts (Table 3). The catalytic result demonstrated that NHPI itself can promote the oxidation but with a low yield of 53% for 2a (Table 3, entry 2). Using CuCl2 or {CrMo6} as catalyst, each can enhance the conversion of toluene up to 70% as compared with NHPI alone (Table 3, entries 3 and 4). However, the adduct (3) of benzoic acid and NHPI was obtained as side product in the yield of 26% for CuCl2 (Table 3, entry 3). On the contrary, utilization of L together with NHPI resulted in dramatic decrease of the yield (Table 3, entry 5). In view of the binary mixtures of the precursors, 3 was always observed in the presence of CuCl2 while the yield of 2a is generally lower than that of NHPI when the mixtures contained L (Table 3, entry 6–8). In the case of tertiary mixture of CuCl2 + L + {CrMo6}, the overall yield is 64% with a ratio of 47/17 for 2a/3 (Table 3, entry 9). In summary, the control experiments ambiguously demonstrated the synergistic effect from the rational assembly of multiple components of POMOF-1, which not only furnished the best yield of 2a but also avoided the formation of 3.
| Entry | Cat. | 2a yield% | 3 yield% |
|---|---|---|---|
| a Reaction condition: cat. (1 mol%), NHPI (0.2 equiv.), toluene 1a (0.2 mmol) and CH3CN (1 mL) in O2 (1 atm) at 85 °C for 24 h. Yields were determined by GC-MS. Hexadecane as internal standard. | |||
| 1 | POMOF-1 | 91 | Trace |
| 2 | None | 53 | Trace |
| 3 | CuCl2 | 44 | 26 |
| 4 | {CrMo6} | 75 | Trace |
| 5 | L | 23 | Trace |
| 6 | CuCl2 + L | 32 | 20 |
| 7 | CuCl2 + {CrMo6} | 51 | 17 |
| 8 | {CrMo6} + L | 45 | Trace |
| 9 | CuCl2 + L + {CrMo6} | 47 | 17 |
Catalytic oxidation of alkylarenes by POMOF-1
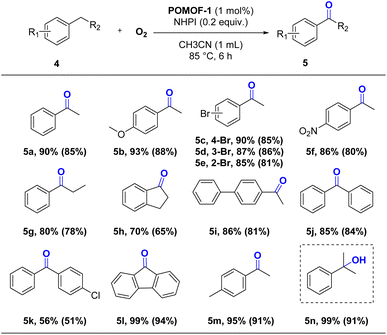
Encouraged by the successful oxidation of methylarenes, we try to use POMOF-1 to study the oxidation reaction of other alkylarenes. The catalysis was probed with ethylbenzene (4a) as a model substrate (Table S2†). Adopting the same conditions as oxidation of methylarenes, acetophenone (5a) was afforded in high yield of 91% (Table S2,† entry 1). Reduction of the reaction time from 24 h to 6 h, 5a can also be obtained in a yield of 90% (Table S2,† entry 3). The subsequent screening of the amount of NHPI, reaction temperature and solvents indicated that the best yield can be achieved by performing the reaction at 85 °C in CH3CN with 0.2 equivalent of NHPI as co-catalyst (Table S2,† entries 4–11). By combination of 1 mol% POMOF-1 and the reaction time of 6 h, the optical conditions are thus established (Table S2,† entry 3).After determining the optical conditions (Table S2,† entry 3), the general applicability for oxidation reaction of alkylarenes was then investigated. As shown in Table 4, the reaction showed good functional group tolerance for ethyl benzene derivatives. Ethyl benzenes containing both electron-donating and electron-withdrawing substituents can be efficiently converted into corresponding ketones in excellent yields ranging from 85% to 93% (5b–5f). Extending alkyl group from ethyl to propyl and cycloalkane, 5g and 5h were afforded in 80% and 70% yields, respectively. The catalytic system was also tolerant with substrate bearing bulky substituent on aromatic ring, 4-phenylethyl benzene was converted to 5i in a good yield of 86%. The method can be facilely extended to substrates containing arylmethylene groups, the oxidation of diphenylmethane and fluorene proceeded smoothly to give 5j–5l in good yields. When unsymmetrically substituted 4-methyl ethylbenzene was subjected to the oxidation, 5m could be afforded in high yield of 95%, indicating that the oxidation preferred to occur on the secondary C–H bond. As expected, the oxidation of tertiary C–H bond can only result in alcohol 5n as the sole product. Similar to the oxidation of methylarenes, control experiments also demonstrated the synergistic effect of POMOF-1 during catalysis, which exhibited remarkably improved activity than the precursors or the mixture of the individual components (Table S3†).
| a Reaction condition: POMOF-1 (1 mol%), NHPI (0.2 equiv.), alkylarenes 4 (0.2 mmol) and CH3CN (1 mL) in O2 (1 atm) at 85 °C for 6 h. Yields were determined by GC-MS. Hexadecane as internal standard. Values in parentheses are the isolated yields. |
|---|
 |
Recyclability and heterogeneity of POMOF-1
To test the recyclability and stability of POMOF-1, it was reused for 5 cycles towards oxidation of toluene and ethylbenzene, respectively. In each case, the catalytic activity was maintained without appreciable loss (Fig. S5†). The PXRD patterns and FT-IR spectra of the recovered catalysts matched well with pristine sample, indicative of the preservation of structural integrity (Fig. S6 and S7†). Moreover, ICP analysis of POMOF-1 before and after catalysis showed little change, further confirming the structural stability of POMOF-1 (Table S4†). Additionally, ICP analysis of the filtered solution after catalysis indicated negligible amounts of metal ions (<0.01%), demonstrating no leaking of catalyst and heterogeneous nature during the reaction (Table S4†). Hot filtration tests revealed the reactions didn't completely stop but proceeded in much lower conversion (Fig. S8†). This was probably caused by the catalysis of residual NHPI in the filtrate.Mechanism study
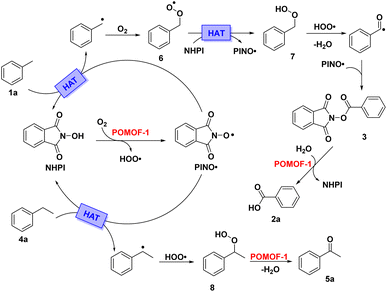
With toluene and ethylbenzene as model substrates, a series of experiments are performed to reveal the reaction mechanisms for oxidative transformation (Scheme 2). For the oxidation of toluene, detection of the reaction mixture after 12 h by GC-MS showed both the targeted 2a and intermediate 3 are presented in solution (Scheme 2a and Fig. S9†). Extension of the reaction time to 24 h at which point the catalysis was complete, 2a was the sole product detected by GC-MS (Fig. S9†). This implied 2a was probably generated by disassociation of 3. To verify this point, 3 was synthesized and used as reactant for hydrolysis under the same conditions as oxidation of toluene. With POMOF-1 as catalyst, 3 could be hydrolyzed to benzoic acid in 95% yield (Scheme 2b and Fig. S10†). The above results ambiguously indicated 3 was the key intermediate during catalysis, which underwent hydrolysis to produce 2a. On the other hand, radical scavengers were introduced into the catalytic system to probe other active and intermediate species. The reaction was totally suppressed by adding 2 equiv. TEMPO, indicating that the reaction proceeded in a radical pathway (Scheme 2c). Meanwhile, benzoquinone could suppress the reaction as well, suggesting HOO˙ was probably the active species that furnish the oxidation (Scheme 2d). Adopting the same procedure for the oxidation of ethylbenzene, both TEMPO and benzoquinone can suppress the transformation (Scheme 2e and f). Therefore, HOO˙ was proposed as the reactive oxygen species to promote the formation of ketone.Based on the experimental results and well-established reaction pathways in literature,61,64–66 the plausible mechanisms for catalytic oxidation of methylarenes and alkylarenes are proposed in Scheme 3. Initially, O2 and NHPI interact with POMOF-1 to afford HOO˙ and PINO˙. The benzylic C–H bonds of toluene and ethylbenzene are then activated by PINO˙ via hydrogen atom transfer (HAT) process to generate toluene radical and ethylbenzene radical, respectively. Upon oxidation by O2, the toluene radical is converted into intermediate 6. Afterwards, 6 undergoes HAT with NHPI to produce 7, which is further oxidized by HOO˙ and then coupled with PINO˙ to give the key intermediate 3. Finally, 3 could be hydrolyzed by POMOF-1 and thus give rise to benzoic acid (2a). In view of the ethylbenzene radical, it undergoes direct oxidation by HOO˙ to get intermediate 8, which is then transformed to 5a via the dehydration catalyzed by POMOF-1.
Conclusion
In conclusion, we demonstrate that the self-assembly of a 2D POMOF-1 consisted of orderly distributed Anderson-type {CrMo6} clusters and Cu2+ centers for catalytic oxidation of benzylic C–H bonds to functional carbonyl groups. Coupled with NHPI as co-catalyst, POMOF-1 efficiently promote the oxidative conversion of methylarenes and alkylarenes into aryl carboxylic acids and aromatic ketones, respectively. The high efficiency is ascribed to the synergistic effect derived from the rational combination of multiple functional components in POMOF-1 and the cooperative catalysis with NHPI on the confined surface of POMOF-1. The catalyst is robust and can be reused for five cycles with little loss of activity while maintaining the structural integrity. Mechanism study reveals that POMOF-1 works together with NHPI to activate the benzylic C–H bonds of substrates, which are sequentially oxidized by oxygen and HOO˙ and resulted in targeted carboxylic acids or ketones. This work manifests that combination of multifunctional POM-based frameworks with NHPI proves to be an efficient way for challenging C–H bond oxidation under mild condition, which can further promote the development of high-performance catalytic systems for advanced oxidation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 92161111, 21901037, 21901038 and 52372040), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, and International Cooperation Fund of Science and Technology Commission of Shanghai Municipality (No. 21130750100) and the Fundamental Research Funds for the Central Universities (No. 2232023D-01), Shanghai Science & Technology Committee (No. 22ZR1403300) and Graduate Student Innovation Fund of Donghua University (CUSF-DH-D-2019074). We also thank the staff from college of materials science and engineering at Donghua University for X-ray data collection using Bruker D8 Venture.Notes and references
- J. H. Teles, I. Hermans, G. Franz and R. A. Sheldon, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2015, pp. 1–103 Search PubMed.
- S. Caron, R. W. Dugger, S. G. Ruggeri, J. A. Ragan and D. H. B. Ripin, Large-scale oxidations in the pharmaceutical industry, Chem. Rev., 2006, 106, 2943–2989 CrossRef CAS.
- T. Newhouse and P. S. Baran, If C-H bonds could talk: selective C-H bond oxidation, Angew. Chem., Int. Ed., 2011, 50, 3362–3374 CrossRef CAS.
- L. Kesavan, R. Tiruvalam, M. H. A. Rahim, M. I. bin Saiman, D. I. Enache, R. L. Jenkins, N. Dimitratos, J. A. Lopez-Sanchez, S. H. Taylor, D. W. Knight, C. J. Kiely and G. J. Hutchings, Solvent-free oxidation of primary carbon-hydrogen bonds in toluene using Au-Pd alloy nanoparticles, Science, 2011, 331, 195–199 CrossRef CAS PubMed.
- H. Sterckx, B. Morel and B. U. W. Maes, Catalytic aerobic oxidation of C(sp3)−H bonds, Angew. Chem., Int. Ed., 2019, 58, 7946–7970 CrossRef CAS PubMed.
- J. Piera and J.-E. Bäckvall, Catalytic oxidation of organic substrates by molecular oxygen and hydrogen peroxide by multistep electron transfer—a biomimetic approach, Angew. Chem., Int. Ed., 2008, 47, 3506–3523 CrossRef CAS PubMed.
- Z. Shi, C. Zhang, C. Tang and N. Jiao, Recent advances in transition-metal catalyzed reactions using molecular oxygen as the oxidant, Chem. Soc. Rev., 2012, 41, 3381–3430 RSC.
- Z. Guo, B. Liu, Q. Zhang, W. Deng, Y. Wang and Y. Yang, Recent advances in heterogeneous selective oxidation catalysis for sustainable chemistry, Chem. Soc. Rev., 2014, 43, 3480–3524 RSC.
- M. Liu and C.-J. Li, in Green Oxidation in Organic Synthesis, Wiley-VCH, 2019, pp. 159–180 Search PubMed.
- H. Gu, X. Liu, X. Liu, C. Ling, K. Wei, G. Zhan, Y. Guo and L. Zhang, Adjacent single-atom irons boosting molecular oxygen activation on MnO2, Nat. Commun., 2021, 12, 5422 CrossRef CAS.
- S. Li, N. Li, G. Li, Y. Ma, M. Huang, Q. Xia, Q. Zhao and X. Chen, Silver-modified polyniobotungstate for the visible light-induced simultaneous cleavage of C–C and C–N bonds, Polyoxometalates, 2023, 2, 9140024 CrossRef.
- W. Partenheimer, Methodology and scope of metal/bromide autoxidation of hydrocarbons, Catal. Today, 1995, 23, 69–158 CrossRef CAS.
- S. A. Chavan, S. B. Halligudi, D. Srinivas and P. Ratnasamy, Formation and role of cobalt and manganese cluster complexes in the oxidation of p-xylene, J. Mol. Catal. A: Chem., 2000, 161, 49–64 CrossRef CAS.
- W. Partenheimer, The structure of metal/bromide catalysts in acetic acid/water mixtures and its significance in autoxidation, J. Mol. Catal. A: Chem., 2001, 174, 29–33 CrossRef CAS.
- W. Partenheimer, The high yield synthesis of benzaldehydes from benzylic alcohols using homogeneously catalyzed aerobic oxidation in acetic acid, Adv. Synth. Catal., 2006, 348, 559–568 CrossRef CAS.
- R. A. F. Tomás, J. C. M. Bordado and J. F. P. Gomes, p-Xylene oxidation to terephthalic acid: a literature review oriented toward process optimization and development, Chem. Rev., 2013, 113, 7421–7469 CrossRef PubMed.
- D. Lisicki, A. Maciej and B. Orlińska, Selective aerobic oxidation of toluene in the presence of Co2+ and task-specific organic salts, including ionic liquids, Ind. Eng. Chem. Res., 2021, 60, 11579–11589 CrossRef CAS.
- Y. Yoshino, Y. Hayashi, T. Iwahama, S. Sakaguchi and Y. Ishii, Catalytic oxidation of alkylbenzenes with molecular oxygen under normal pressure and temperature by N-hydroxyphthalimide combined with Co(OAc)2, J. Org. Chem., 1997, 62, 6810–6813 CrossRef CAS.
- N. Sawatari, S. Sakaguchi and Y. Ishii, Oxidation of nitrotoluenes with air using N-hydroxyphthalimide analogues as key catalysts, Tetrahedron Lett., 2003, 44, 2053–2056 CrossRef CAS.
- R. A. Sheldon and I. W. C. E. Arends, Organocatalytic oxidations mediated by nitroxyl radicals, Adv. Synth. Catal., 2004, 346, 1051–1071 CrossRef CAS.
- F. Recupero and C. Punta, Free radical functionalization of organic compounds catalyzed by N-hydroxyphthalimide, Chem. Rev., 2007, 107, 3800–3842 CrossRef CAS PubMed.
- M. Caruso, S. Navalón, M. Cametti, A. Dhakshinamoorthy, C. Punta and H. García, Challenges and opportunities for N-hydroxyphthalimide supported over heterogeneous solids for aerobic oxidations, Coord. Chem. Rev., 2023, 486, 215141 CrossRef CAS.
- I. K. Goncharova, K. P. Silaeva, A. V. Arzumanyan, A. A. Anisimov, S. A. Milenin, R. A. Novikov, P. N. Solyev, Y. V. Tkachev, A. D. Volodin, A. A. Korlyukov and A. M. Muzafarov, Aerobic Co-/N-hydroxysuccinimide-catalyzed oxidation of p-tolylsiloxanes to p-carboxyphenylsiloxanes: synthesis of functionalized siloxanes as promising building blocks for siloxane-based materials, J. Am. Chem. Soc., 2019, 141, 2143–2151 CrossRef CAS PubMed.
- B. Dutta, R. Clarke, S. Raman, T. D. Shaffer, L. Achola, P. Nandi and S. L. Suib, Lithium promoted mesoporous manganese oxide catalyzed oxidation of allyl ethers, Nat. Commun., 2019, 10, 655 CrossRef CAS PubMed.
- X. Huang, G. Lu, K. Zhang, J. Liu, H. Zhang, Z. Guo and J. Tao, Selective synthesis of imines by direct oxidative coupling of amines on Cu-doped CeO2 catalysts, Appl. Surf. Sci., 2020, 514, 145948 CrossRef CAS.
- C. Zhang, Z. Huang, J. Lu, N. Luo and F. Wang, Generation and confinement of long-lived N-oxyl radical and its photocatalysis, J. Am. Chem. Soc., 2018, 140, 2032–2035 CrossRef CAS PubMed.
- R. Ma, W. Chen, L. Wang, X. Yi, Y. Xiao, X. Gao, J. Zhang, X. Tang, C. Yang, X. Meng, A. Zheng and F.-S. Xiao, N-oxyl radicals trapped on Zeolite surface accelerate photocatalysis, ACS Catal., 2019, 9, 10448–10453 CrossRef CAS.
- A. Dhakshinamoorthy, A. M. Asiri and H. Garcia, Metal–organic frameworks as catalysts for oxidation reactions, Chem.–Eur. J., 2016, 22, 8012–8024 CrossRef CAS PubMed.
- A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Aerobic oxidation of benzyl amines to benzyl imines catalyzed by metal–organic framework solids, ChemCatChem, 2010, 2, 1438–1443 CrossRef CAS.
- A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Aerobic oxidation of cycloalkenes catalyzed by iron metal organic framework containing N-hydroxyphthalimide, J. Catal., 2012, 289, 259–265 CrossRef CAS.
- A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Atmospheric-pressure, liquid-phase, selective aerobic oxidation of alkanes catalysed by metal–organic frameworks, Chem.–Eur. J., 2011, 17, 6256–6262 CrossRef CAS.
- Y. Wang, L. Zhao, S. Liu, G. Ji, C. He, Y. Tang and C. Duan, Mixed-component metal–organic framework for boosting synergistic photoactivation of C(sp3)–H and oxygen, ACS Appl. Mater. Interfaces, 2023, 15, 16744–16754 CrossRef CAS PubMed.
- D.-L. Long, R. Tsunashima and L. Cronin, Polyoxometalates: building blocks for functional nanoscale systems, Angew. Chem., Int. Ed., 2010, 49, 1736–1758 CrossRef CAS PubMed.
- H. N. Miras, J. Yan, D.-L. Long and L. Cronin, Engineering polyoxometalates with emergent properties, Chem. Soc. Rev., 2012, 41, 7403–7430 RSC.
- H. N. Miras, L. Vilà-Nadal and L. Cronin, Polyoxometalate based open-frameworks (POM-OFs), Chem. Soc. Rev., 2014, 43, 5679–5699 RSC.
- S.-S. Wang and G.-Y. Yang, Recent advances in polyoxometalate-catalyzed reactions, Chem. Rev., 2015, 115, 4893–4962 CrossRef CAS PubMed.
- A. Bijelic, M. Aureliano and A. Rompel, Polyoxometalates as potential next-generation metallodrugs in the combat against cancer, Angew. Chem., Int. Ed., 2019, 58, 2980–2999 CrossRef CAS PubMed.
- M. R. Horn, A. Singh, S. Alomari, S. Goberna-Ferrón, R. Benages-Vilau, N. Chodankar, N. Motta, K. Ostrikov, J. MacLeod, P. Sonar, P. Gomez-Romero and D. Dubal, Polyoxometalates (POMs): from electroactive clusters to energy materials, Energy Environ. Sci., 2021, 14, 1652–1700 RSC.
- I. A. Weinstock, R. E. Schreiber and R. Neumann, Dioxygen in polyoxometalate mediated reactions, Chem. Rev., 2018, 118, 2680–2717 CrossRef CAS PubMed.
- N. Mizuno and K. Kamata, Catalytic oxidation of hydrocarbons with hydrogen peroxide by vanadium-based polyoxometalates, Coord. Chem. Rev., 2011, 255, 2358–2370 CrossRef CAS.
- X. Chen, G. Zhang, B. Li and L. Wu, An integrated giant polyoxometalate complex for photothermally enhanced catalytic oxidation, Sci. Adv., 2021, 7, eabf8413 CrossRef CAS PubMed.
- J. Liu, N. Jiang, J.-M. Lin, Z.-B. Mei, L.-Z. Dong, Y. Kuang, J.-J. Liu, S.-J. Yao, S.-L. Li and Y.-Q. Lan, Structural evolution of giant polyoxometalate: from “Keplerate” to “Lantern” type Mo132 for improved oxidation catalysis, Angew. Chem., Int. Ed., 2023, 62, e202304728 CrossRef CAS PubMed.
- J. Song, M. Hua, X. Huang, J. Ma, C. Xie and B. Han, Robust bio-derived polyoxometalate hybrid for selective aerobic oxidation of benzylic C(sp3)–H bonds, ACS Catal., 2023, 13, 4142–4154 CrossRef CAS.
- J. Li, D. Zhang, Y. Chi and C. Hu, Catalytic application of polyoxovanadates in the selective oxidation of organic molecules, Polyoxometalates, 2022, 1, 9140012 CrossRef.
- A. Khoshyan, M. Pourtahmasb, F. Feizpour, M. Jafarpour and A. Rezaeifard, Aerobic {Mo72V30} nanocluster-catalysed heterogeneous one-pot tandem synthesis of benzimidazoles, Appl. Organomet. Chem., 2019, 33, e4638 CrossRef.
- A. Rezaeifard, A. Khoshyan, M. Jafarpour and M. Pourtahmasb, Selective aerobic benzylic C–H oxidation co-catalyzed by N-hydroxyphthalimide and Keplerate {Mo72V30} nanocluster, RSC Adv., 2017, 7, 15754–15761 RSC.
- M. Zhao, X.-W. Zhang and C.-D. Wu, Structural transformation of porous polyoxometalate frameworks and highly efficient biomimetic aerobic oxidation of aliphatic alcohols, ACS Catal., 2017, 7, 6573–6580 CrossRef CAS.
- M. Zhao and C.-D. Wu, Biomimetic activation of molecular oxygen with a combined metalloporphyrinic framework and co-catalyst platform, ChemCatChem, 2017, 9, 1192–1196 CrossRef CAS.
- P. Wu, P. Yin, J. Zhang, J. Hao, Z. Xiao and Y. Wei, Single-side organically functionalized Anderson-type polyoxometalates, Chem.–Eur. J., 2011, 17, 12002–12005 CrossRef CAS PubMed.
- X.-X. Li, X. Ma, W.-X. Zheng, Y.-J. Qi, S.-T. Zheng and G.-Y. Yang, Composite hybrid cluster built from the integration of polyoxometalate and a metal halide cluster: synthetic strategy, structure, and properties, Inorg. Chem., 2016, 55, 8257–8259 CrossRef CAS.
- X. Wang, Z. Chang, H. Lin, A. Tian, G. Liu and J. Zhang, Assembly and photocatalysis of two novel 3D Anderson-type polyoxometalate-based metal–organic frameworks constructed from isomeric bis(pyridylformyl)piperazine ligands, Dalton Trans., 2014, 43, 12272–12278 RSC.
- X. Wang, Z. Chang, H. Lin, A. Tian, G. Liu, J. Zhang and D. Liu, Two novel Anderson-type polyoxometalate-based metal–organic complexes with high-efficiency photocatalysis towards degradation of organic dyes under UV and visible light irradiation, RSC Adv., 2015, 5, 14020–14026 RSC.
- J. Zhang, Y. Huang, G. Li and Y. Wei, Recent advances in alkoxylation chemistry of polyoxometalates: from synthetic strategies, structural overviews to functional applications, Coord. Chem. Rev., 2019, 378, 395–414 CrossRef CAS.
- H. Yu, S. Ru, G. Dai, Y. Zhai, H. Lin, S. Han and Y. Wei, An efficient iron(III)-catalyzed aerobic oxidation of aldehydes in water for the green preparation of carboxylic acids, Angew. Chem., Int. Ed., 2017, 56, 3867–3871 CrossRef CAS PubMed.
- Z. Wei, J. Wang, H. Yu, S. Han and Y. Wei, Recent advances of Anderson-type polyoxometalates as catalysts largely for oxidative transformations of organic molecules, Molecules, 2022, 27, 5212 CrossRef CAS.
- J. Wang, H. Yu, Z. Wei, Q. Li, W. Xuan and Y. Wei, Additive-Mediated Selective Oxidation of Alcohols to Esters via Synergistic Effect Using Single Cation Cobalt Catalyst Stabilized with Inorganic Ligand, Research, 2020, 2020, 3875920 CAS.
- C. P. Delaney, E. Lin, Q. Huang, I. F. Yu, G. Rao, L. Tao, A. Jed, S. M. Fantasia, K. A. Püntener, R. D. Britt and J. F. Hartwig, Cross-coupling by a noncanonical mechanism involving the addition of aryl halide to Cu(II), Science, 2023, 381, 1079–1085 CrossRef CAS PubMed.
- S. Bhunia, G. G. Pawar, S. V. Kumar, Y. Jiang and D. Ma, Selected copper-based reactions for C−N, C−O, C−S, and C−C bond formation, Angew. Chem., Int. Ed., 2017, 56, 16136–16179 CrossRef CAS PubMed.
- S. Nakai, T. Yatabe, K. Suzuki, Y. Sasano, Y. Iwabuchi, J.-y. Hasegawa, N. Mizuno and K. Yamaguchi, Methyl-selective α-oxygenation of tertiary amines to formamides by employing copper/moderately hindered nitroxyl radical (DMN-AZADO or 1-Me-AZADO), Angew. Chem., Int. Ed., 2019, 58, 16651–16659 CrossRef CAS PubMed.
- R. Trammell, K. Rajabimoghadam and I. Garcia-Bosch, Copper-promoted functionalization of organic molecules: from biologically relevant Cu/O2 model systems to organometallic transformations, Chem. Rev., 2019, 119, 2954–3031 CrossRef CAS PubMed.
- H. Chen, L. Wang, S. Xu, X. Liu, Q. He, L. Song and H. Ji, Selective functionalization of hydrocarbons using a ppm bioinspired molecular tweezer via proton-coupled electron transfer, ACS Catal., 2021, 11, 6810–6815 CrossRef CAS.
- C. Hansch, A. Leo and R. W. Taft, A survey of Hammett substituent constants and resonance and field parameters, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
- G. A. DiLabio, P. Franchi, O. Lanzalunga, A. Lapi, F. Lucarini, M. Lucarini, M. Mazzonna, V. K. Prasad and B. Ticconi, Hydrogen atom transfer (HAT) processes promoted by the quinolinimide-N-oxyl radical. A kinetic and theoretical study, J. Org. Chem., 2017, 82, 6133–6141 CrossRef CAS PubMed.
- M. A. Hoque, J. Twilton, J. Zhu, M. D. Graaf, K. C. Harper, E. Tuca, G. A. DiLabio and S. S. Stahl, Electrochemical PINOylation of methylarenes: improving the scope and utility of benzylic oxidation through mediated electrolysis, J. Am. Chem. Soc., 2022, 144, 15295–15302 CrossRef CAS.
- P. Li, J. Sun, X. Xu, Z. Mi, Y. Lin, J. Cheng, R. Bai and Y. Xie, Ferric nitrate-promoted oxidative esterification of toluene with N-hydroxyphthalimide: synthesis of N-hydroxyimide esters, Tetrahedron Lett., 2018, 59, 2640–2643 CrossRef CAS.
- K. Singha, S. C. Ghosh and A. B. Panda, Visible light-driven efficient synthesis of amides from alcohols using Cu−N−TiO2 heterogeneous photocatalyst, Eur. J. Org Chem., 2021, 2021, 657–662 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2300360. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra07120k |
| This journal is © The Royal Society of Chemistry 2024 |