Open Access Article
Open Access ArticleFunctionalization of Shorea faguetiana biochar using Fe2O3 nanoparticles and MXene for rapid removal of methyl blue and lead from both single and binary systems†
Aysha Bukharib,
Irfan Ijaz *b,
Ammara Nazirb,
Sajjad Hussain
*b,
Ammara Nazirb,
Sajjad Hussain ab,
Hina Zainc,
Ezaz Gilanib,
Ahmad A. lfseisid and
Hijaz Ahmadefg
ab,
Hina Zainc,
Ezaz Gilanib,
Ahmad A. lfseisid and
Hijaz Ahmadefg
aSchool of Physics, Henan Key Laboratory of Photovoltaic Materials, Henan Normal University, Xinxiang, 453007, China
bSchool of Chemistry, Faculty of Basic Sciences and Mathematics, Minhaj University Lahore, Lahore 54700, Pakistan. E-mail: iffichemixt266@gmail.com
cDepartment of Biological Sciences, Superior University Lahore, Lahore 54700, Pakistan
dDepartment of Chemistry, College of Science, King Saud University, P.O. Box 2455, Riyadh 11451, Saudi Arabia
eCenter for Applied Mathematics and Bioinformatics, Gulf University for Science and Technology, Kuwait
fDepartment of Computer Science and Mathematics, Lebanese American University, Beirut, Lebanon
gNear East University, Operational Research Center in Healthcare, TRNC Mersin 10, Nicosia, 99138, Turkey
First published on 29th January 2024
Abstract
The synthesis of polymeric magnetic composites is a promising strategy for the rapid and efficient treatment of wastewater. Lead and methyl blue are extremely hazardous to living organisms. The sorption of Pb2+ and the dye methyl blue (MB) by biochar is an ecologically sustainable method to remediate this type of water pollution. We functionalized Shorea faguetiana biochar with Fe2O3 and MXene, resulting in Fe2O3/BC/MXene composites with an efficient, rapid, and selective adsorption performance. Based on X-ray photoelectron and Fourier transform infrared spectrometry, we found that the Fe2O3/BC/MXene composites had an increased number of surface functional groups (F−, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, CN, NH, and OH−) compared with the original biochar. The batch sorption findings showed that the maximum sorption capacities for Pb2+ and MB at 293 K were 882.76 and 758.03 mg g−1, respectively. The sorption phenomena obeyed a pseudo-second-order (R2 = 1) model and the Langmuir isotherm. There was no competition between MB and Pb2+ in binary solutions, indicating that MB and Pb2+ did not influence each other as a result of their different adsorption mechanisms (electrostatic interaction for Pb2+ and hydrogen bonding for MB). This illustrates monolayer sorption on the Fe2O3/BC/MXene composite governed by chemical adsorption. Thermodynamic investigations indicated that the sorption process was spontaneous and exothermic at 293–313 K, suggesting that it is feasible for practical applications. Fe2O3/BC/MXene can selectively adsorb Pb2+ ions and MB from wastewater containing multiple interfering metal ions. The sorption capacities were still high after five reusability experiments. This work provides a novel Fe2O3/BC/MXene composite for the rapid and efficient removal of Pb2+ and MB.
O, CN, NH, and OH−) compared with the original biochar. The batch sorption findings showed that the maximum sorption capacities for Pb2+ and MB at 293 K were 882.76 and 758.03 mg g−1, respectively. The sorption phenomena obeyed a pseudo-second-order (R2 = 1) model and the Langmuir isotherm. There was no competition between MB and Pb2+ in binary solutions, indicating that MB and Pb2+ did not influence each other as a result of their different adsorption mechanisms (electrostatic interaction for Pb2+ and hydrogen bonding for MB). This illustrates monolayer sorption on the Fe2O3/BC/MXene composite governed by chemical adsorption. Thermodynamic investigations indicated that the sorption process was spontaneous and exothermic at 293–313 K, suggesting that it is feasible for practical applications. Fe2O3/BC/MXene can selectively adsorb Pb2+ ions and MB from wastewater containing multiple interfering metal ions. The sorption capacities were still high after five reusability experiments. This work provides a novel Fe2O3/BC/MXene composite for the rapid and efficient removal of Pb2+ and MB.
1. Introduction
Human efforts to fulfill the demands and needs of our growing population—resulting in market growth, technological innovations, capital accumulation, and advancements in living standards—have led to the expansion of many different industries worldwide.1–5 The heavy metal lead is non-biodegradable in water and accumulates in living organisms, sludges, and sediments, from where it can enter the food chain.6–8 Human exposure to lead through the consumption of lead-contaminated food and water may affect the nervous system, lungs, and kidneys, and has been linked to hypertension, anemia, peripheral neuropathy, miscarriage, low fertility, cognitive disorders, depressive disorders, and renal damage.9–14 Organic dyes are used in many different industries, such as the plastics, leather, paper, and textile industries.15–18 These industries use a lot of water and generate a substantial quantity of colored wastewater. The substance most often used to dye cotton, silk, paper, and leather is methyl blue (MB).19–21 Positively charged MB can injure human eyes and cause respiratory issues on inhalation; ingesting MB through the mouth can cause vomiting, mental confusion, a burning sensation, profuse sweating, and nausea.22,23Different technologies and approaches are used to remove heavy metals from wastewater, such as ion exchange, evaporation, chemical precipitation, adsorption, reverse osmosis, membrane filtration, flotation, electrochemical deposition, and coagulation–flocculation.24–33 These approaches have some flaws, such as high operating costs, hazardous byproducts, insufficient removal efficiency, sludge formation, high energy requirements, and difficulties in disposal. Sorption strategies offer advantages over other techniques, such as ease of operation, an outstanding elimination efficiency, affordability, and the ability to regenerate the sorbent.34 These issues can be resolved by creating an adsorbent with a large surface area, high porosity, greater dispensability, and the correct functional groups.35–37 The removal of MB from aqueous media before discharge into the environment is important to lessen its influence on living organisms.
A porous, stable, and carbon-rich substance known as biochar (BC) has been prepared and used for various applications, such as soil amelioration, enhancing the biodegradation of organic pollutants or contaminants, and the adsorption of heavy metals.38–41 For instance, it has been reported that straw or pristine biochar effectively adsorbs Pb2+ from water. Biochar synthesized from canola straw was used for the selective elimination of lead from aqueous media; the qm of lead was 65 mg g−1 at a temperature of 500 °C.42 However, pristine or straw BC shows a lower adsorption of toxic heavy metals, including lead, than modified BC.43 Consequently, different engineering or modification systems have been used to increase the adsorption capacity of virgin BC.44 For instance, the binding of BC with nano-materials or metal oxide particles increases the sorption capacity of pristine BC.45,46 Wang, Yan et al. increased the efficiency of BC using an H2O2 treatment for lead elimination. The number of functional groups (oxygen) on the BC was enhanced after oxidation by hydrogen peroxide, leading to an increase in the lead sorption capacity (60.87 mg g−1). In other studies, nanoparticles (NPs) or nano-sized materials (e.g., iron, AlOOH, MnOx, MOS2, CeO2, Fe2O3, ZrO2, La(OH)3, and hydroxyapatite) were loaded into BC for use in wastewater remediation.47–54
Iron oxide (magnetic) particles or adsorbents are considered to be suitable for solving these issues because they have a high surface area with excellent physiochemical characteristics for metal adsorption and can also be readily isolated using an external magnetic field.55 However, the direct use of pure or naked magnetic particles or adsorbents may be unsuitable for the removal of metals due to their low surface area, fewer active sites, and low stability without the assistance of covalent linkages. The synergistic coupling of magnetic particles with other adsorbents is considered to be the best way forward for this area of research. A chitosan Schiff base and its magnetic composite have been prepared and applied to the adsorption of pollutants from wastewater.56,57 Shen, Li, et al. reported a highly efficient Fe2O3@microalgae composite for the elimination of lead from aqueous media.58 In another study, a PANI/starch/Fe2O3 biocomposite was prepared and used for the adsorption of heavy metals from wastewater.59
Since graphene or graphene oxide was prepared from fine graphite powder in 2004,60,61 2D materials have attracted attention due to their specific physicochemical and structural features and have been broadly studied in different applications.62–66 One of the recently developed 2D layered substances is known as MXene, which not has only a large surface area, but also has a more complex chemical composition than graphene67. Various researchers have used MXene and its composites for the removal of different environmental contaminants and pollutants.68 MXene has a rapid and effective adsorption capacity due to the existence of O−, OH−, and F− groups, which provide adsorption sites for positively charged dyes and heavy metals.
An Fe2O3/BC/MXene composite has been shown to efficiently, selectively, and rapidly adsorb Pb2+ from water, demonstrating that it could be used practically as a new and novel composite to remove ecological contaminants and pollutants. The primary objectives of our research were as follows. First, to functionalize BC using MXene and Fe2O3 NPs. MXene provides O, OH, and F cationic groups that enhance the rapid adsorption of anionic lead and MB. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, CN, and OH groups from BC also rapidly absorb Pb2+ and MB. Materials belonging to three different categories can therefore be used to design a composite that has a vital role as a novel adsorbent for the rapid, efficient, and selective adsorption of heavy metals, with a >99% removal rate of Pb2+ from wastewater. We investigated the influence of the initial MB and lead concentrations, the adsorption time, the initial pH, and the temperature on the adsorption characteristics of the adsorbent. The adsorption was fitted by kinetic, isothermal, and thermodynamic models. The selectivity and reusability of the Fe2O3/BC/MXene composite for the adsorption of lead and MB were investigated and the sorption mechanisms were explored.
O, CN, and OH groups from BC also rapidly absorb Pb2+ and MB. Materials belonging to three different categories can therefore be used to design a composite that has a vital role as a novel adsorbent for the rapid, efficient, and selective adsorption of heavy metals, with a >99% removal rate of Pb2+ from wastewater. We investigated the influence of the initial MB and lead concentrations, the adsorption time, the initial pH, and the temperature on the adsorption characteristics of the adsorbent. The adsorption was fitted by kinetic, isothermal, and thermodynamic models. The selectivity and reusability of the Fe2O3/BC/MXene composite for the adsorption of lead and MB were investigated and the sorption mechanisms were explored.
2. Materials and methods
2.1. Chemicals
The chemicals used are discussed in Text S1 (ESI†).2.2. Synthesis of Fe2O3/BC/MXene
Multi-layered Ti3C2 (MXene) materials were prepared by etching the Al from MAX powders using the approach reported by Kong F. et al.69 In a typical synthesis, 4 g of MAX powder were successfully etched using 80 mL of hydrofluoric solution with magnetic stirring at 40 °C for 16 h. The collected suspension was washed with water and subjected to centrifugation at 1500 rpm, producing a dark-colored suspension. Iron oxide NPs were synthesized as reported by Paulson E. et al.70 Shorea faguetiana branches were purchased from the native market in Lahore city in Punjab province, Pakistan. The branches of S. faguetiana were ground using a grinder, washed with deionized water, and then dried. The biomass was pyrolyzed at 523 K in a muffle furnace for 2 h. The S. faguetiana BC was then crushed and passed through a 0.045–0.089 mm sieve and then saved and referred to as virgin BC. The Ti3C2 dispersion was sonicated for 1 h. Fe2O3 NPs and BC powder were then dropped into the MXene dispersion. To obtain Fe2O3/BC/MXene, the resultant product was once more sonicated for 35 min. Fe2O3/BC/MXene was isolated by an external magnetic field, rinsed several times with deionized water to remove impurities, and then dried at 100 °C.2.3. Characterization
Characterization of the materials is discussed in Text S2 (ESI†).2.4. Adsorption experiment
The sorption capability and rate of lead and MB removal were determined using eqn (1) and (2) as discussed in Text S3 (ESI†).
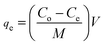 | (1) |
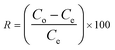 | (2) |
2.5. Isothermal study
The Langmuir isotherm equation is expressed as:
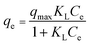 | (3) |
The Freundlich isotherm equation is expressed as:
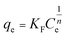 | (4) |
The Dubinin–Radushkevich isotherm model is expressed as:
qe = qs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Bε2) exp(−Bε2)
| (5) |
The determination and calculation of the Temkin parameters are given as:
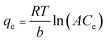 | (6) |
These equations are discussed in Text S4 (ESI†).
2.6. Kinetics analysis
The sorption rate (%) was gauged via a pseudo-first-order (PFO) reaction, a pseudo-second-order (PSO) reaction, intra-particle diffusion (IPD), and the Elovich and Bangham diffusion models, as defined in eqn (7), (8), (9), (10), and (11), respectively. The kinetics analysis is discussed in detail in Text S5 (ESI†).PFO reaction:
qe = (qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − K1t qe − K1t
| (7) |
PSO reaction:
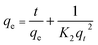 | (8) |
IPD model:
| qe = K3t0.5 + C | (9) |
Elovich diffusion model:
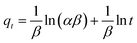 | (10) |
Bangham diffusion model:
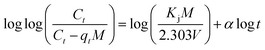 | (11) |
2.7. Error functions
To select a suitable kinetic and isothermal model for the Pb2+ and MB sorption processes, the sum of the squares of the errors and χ2 were determined using the following equations:
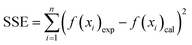 | (12) |
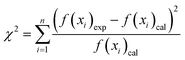 | (13) |
2.8. Thermodynamic study
The thermodynamic parameters were calculated using eqn (14)–(16) and are discussed in detail in the ESI.†
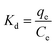 | (14) |
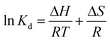 | (15) |
| ΔG = ΔH + TΔS | (16) |
3. Results and discussion
3.1. Characterization
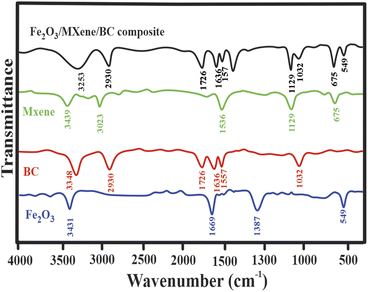
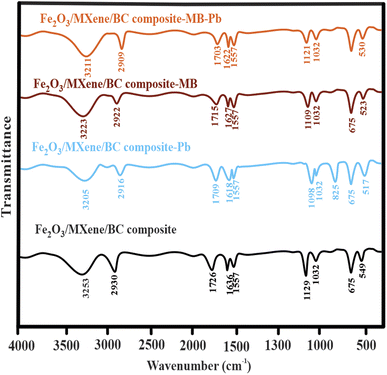
Fig. 1 shows the FTIR spectra of the Fe2O3 NPs, BC, MXene, and the Fe2O3/BC/MXene composite. FT-IR was used to explore the essential surface functional groups of the synthesized materials. The Fe–O stretching vibration of the iron oxide NPs is attributed to the peak at 549 cm−1. The OH and C–O–C groups were assigned to bands around 3431 and 1387 cm−1, respectively. The FTIR results for Fe2O3 were well matched with a previous study.71 For BC, there were four bands around 3348, 2930, 1726, 1636, 1557, and 1032 cm−1, which were indexed with the vibration modes of OH, CH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, CN, NH, and CO, respectively. For MXene, there were two typical bands around 675 and 1129 cm−1, indexed to the TiO and F groups. The bands around 3439 and 1641 cm−1 were attributed to the hydroxyl and aromatic groups, respectively. Fig. 1 shows the existence of all the major peaks of Fe2O3, BC, and MXene in the Fe2O3/BC/MXene composite. Fig. 1 also shows a broad peak of OH, which may be due to a combination of Fe2O3, BC, and MXene.
O, CN, NH, and CO, respectively. For MXene, there were two typical bands around 675 and 1129 cm−1, indexed to the TiO and F groups. The bands around 3439 and 1641 cm−1 were attributed to the hydroxyl and aromatic groups, respectively. Fig. 1 shows the existence of all the major peaks of Fe2O3, BC, and MXene in the Fe2O3/BC/MXene composite. Fig. 1 also shows a broad peak of OH, which may be due to a combination of Fe2O3, BC, and MXene.
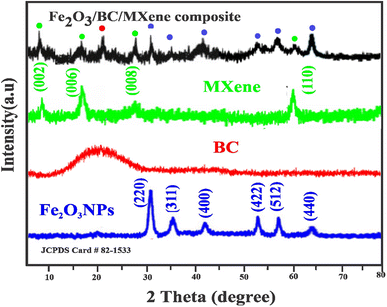
Fig. 2 shows the XRD patterns of the Fe2O3 NPs, BC, MXene, and the Fe2O3/BC/MXene composite. For the Fe2O3 NPs, the 220, 311, 400, 422, 512, and 440 crystal planes correspond to 2θ values of 30.1, 36.4, 42.90, 53.6, 57.4, and 63.19°, respectively. The XRD pattern indexed well with JCPDS Card #82-1533. Fig. 2 shows that no apparent peak for BC was observed, except for an XRD peak counterpart of an interlayer distance of 0.39 nm at 23°, suggesting that the BC was an amorphous phase. A similar broad peak of BC was also reported by Shan H., et al.72 Specifically, using chemical etching strategies, MXene sheets were obtained via the removal of Al layers from the MAX phase with HF. This was confirmed by the substantial shifts found in the XRD peaks. The peak (002) shifted to the left with the disappearance of the characteristic peak of the Ti3AlC2 phase at roughly 39° 2θ (Fig. S1†). The typical peaks of MXene at 2θ = 8.026, 18.632, 27.458, and 60.821° are attributed to the (002), (006), (008), and (110) crystal planes, respectively.73 The typical bands of Ti3C2 were better in agreement with the result reported by Qi M., et al.74 The composites showed all the characteristic bands of Fe2O3 NPs, BC, and MXene (Fig. 2).
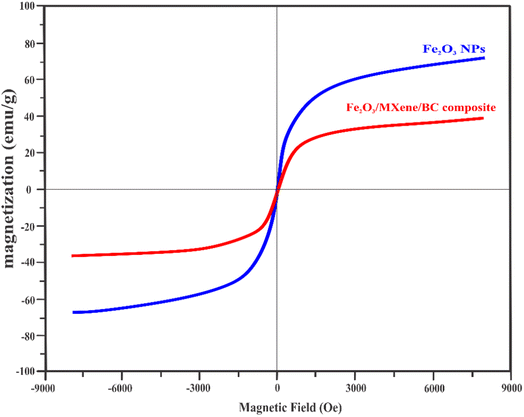
Fig. 3 shows that the saturation magnetization of the composite was 40.24, suggesting paramagnetic behavior. The decrease in the magnetization of the composite could be attributed to the presence of Fe3O4 coupled with the non-magnetic features of both MXene and BC. The magnetization features of the composite were sufficient for rapid isolation under an external magnetic field.
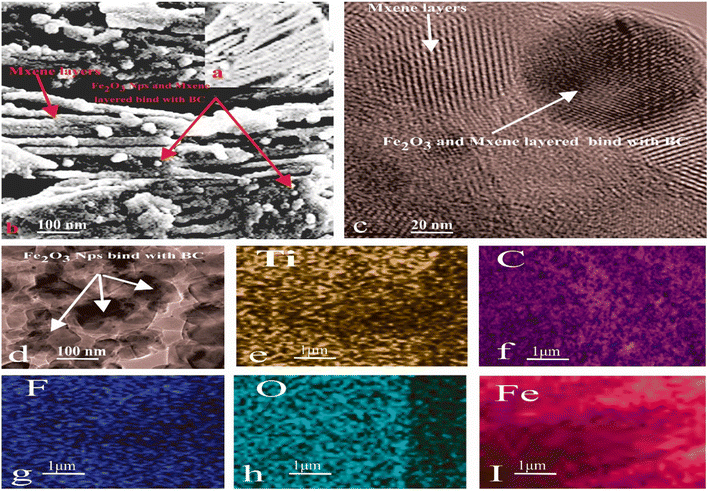
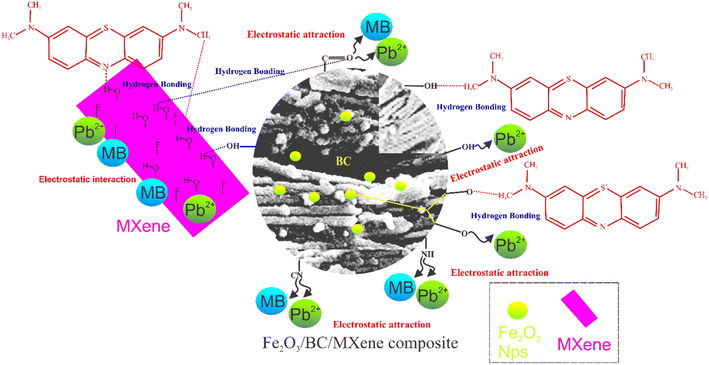
Fig. 4(a–i) shows the morphological traits and distribution of elemental species in BC, the Fe2O3 NPs, MXene, and the composite determined using SEM, TEM, and EDS. Fig. 4(a) shows the layered structure of MXene. The SEM micrograph in Fig. 4(b) shows the binding of the MXene layers and the Fe2O3 NPs with BC. The TEM micrograph in Fig. 4(d) shows iron oxide NPs attached to the surface of the BC. The TEM images in Fig. 4(c) indicate that the MXene layered structure and the Fe2O3 NPs bind with BC, in agreement with the SEM results. The EDS patterns in Fig. 4(e–i) show the existence of F, Ti, OH, C, O, and Fe and the homogeneous distribution of elements.
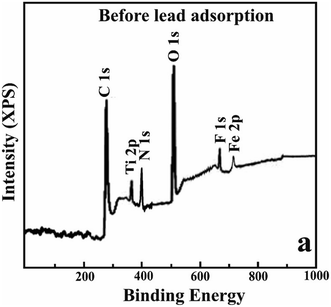
To investigate the sorption process, the binding energies and elemental composition of the Fe2O3/BC/MXene composite were determined using the XPS patterns before and after sorption. The Fe2O3/BC/MXene composite consists of O, C, H, F, N, Fe, and Ti and confirmed the synthesis of an adsorbent (Fig. 5).
The sorbents exhibited mesoporous characteristics based on the IUPAC classification, as indicated by the type IV isotherms with H3 hysteresis loops (Fig. S1†). The Fe2O3/BC/MXene, BC, MXene, and Fe3O4 materials had surface areas of 135.38, 77.091, 51.72, and 49.95 cm3 g−1, respectively. Fig. S2† indicates that the loading of Fe3O4 and MXene increased the surface area of the Fe2O3/BC/MXene composite. This increased surface area promoted the diffusion of contaminants during the adsorption process, allowing the more rapid and efficient adsorption of contaminants.75
3.2. Adsorption analysis
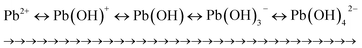 | (17) |
 | ||
| Fig. 6 Influence of the initial pH on the adsorption of (a) Pb2+, (b) MB, and (c) the zeta potential of Fe2O3/BC/MXene. | ||
Precipitates were formed in an aqueous solution of lead above pH 6, suggesting that elimination above pH 6 was due to both the precipitation of lead as Pb(OH)2 and adsorption as Pb2+ and Pb(OH)+ ions. The decrease in the elimination of lead above pH 10 was caused by an increase in the concentration of Pb(OH)42− and Pb(OH)3− due to repulsion between the anionic composite and Pb(OH)3− and Pb(OH)4− at high pH values. The pH-based zeta potential of the composite is shown in Fig. 6(b). The Fe2O3/BC/MXene composite had a positive charge at pH values <2.2 and the surface charge changed to negative. As a result, a change in pH from 2.2 to 9 caused an increase in the removal rate of MB.
The zeta potential is zero at the iso-electronic point. Fig. 6(c) shows that the pHzpc of the composite was 2.2, suggesting that the Fe2O3/BC/MXene composite had no surface charge at pH 2.2. The surface of the composite was positively charged below pH 2.2, but changed to a negative charge at pH values above 2.2.
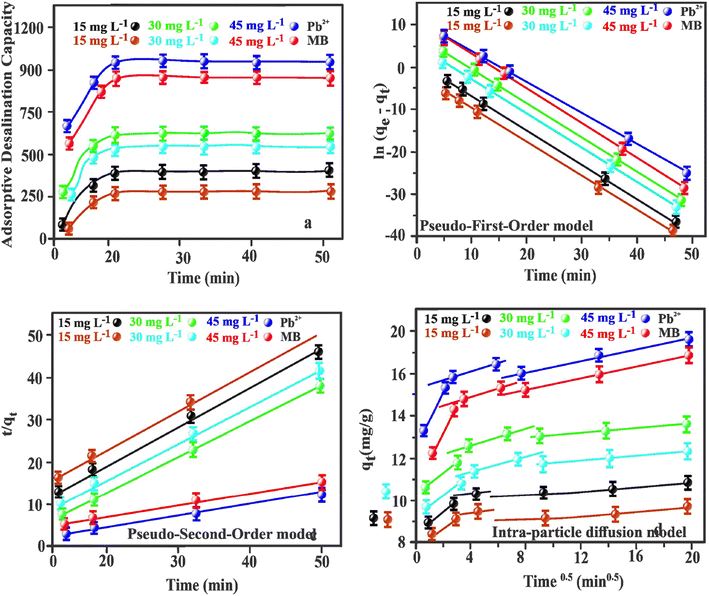
The kinetic sorption of MB and Pb2+ by the Fe2O3/BC/MXene composite could be fitted by the PFO, PSO, and IPD kinetics models (Fig. 7(b–d)) using eqn (7), (8), and (9). Table S1† shows that the correlation coefficients (Pb2+: R2 = 1; MB: R2 = 0.997) of the PSO model for the composite were greater than those of the PFO model (Pb2+: R2 = 0.9326; MB: R2 = 0.9183). Higher values of the correlation coefficients and lower values for χ2 and the SSEs showed the better suitability of the PSO model. The qe values of the composite derived from the PSO kinetic model were comparable with the experimental values. Hence the sorption process of the composite for Pb2+ and MB could be explained by the PSO kinetic model, suggesting that chemical adsorption occurred between the prepared Fe2O3/BC/MXene composite and the Pb2+ ions and MB.78
The Elovich kinetic model is an efficient strategy for elucidating the mechanism of sorption of gases onto the surface of solids. It is widely used in a number of different applications, especially the adsorption of pollutants from aqueous solutions.79 The Elovich model exhibited practical suitability in fitting the kinetic experimental data for Pb2+ and MB (R2 = 0.996 and 0.991, respectively) (Fig. S3(a)†). The greater αEl values acquired for the Fe2O3/BC/MXene composite demonstrate rapid chemisorption due to the porous nature of the synthesized composite.
The Bangham kinetic model was formulated under the hypothesis that the rate-limiting step in the sorption phenomenon is due to pore diffusion. Using eqn (11), the double logarithmic plot (Fig. S3(b)†) produced linear curves showing significant correlation coefficients (R2) of 0.981 and 0.969 for Pb2+ and MB, respectively. This shows that pore diffusion greatly affects the rate-controlling step.
The IPD model was also used to investigate intra-particle diffusion between Pb2+ and MB and Fe2O3/BC/MXene composite. Fig. 7(d) shows the association between qt and t1/2 of the Pb2+ and MB dose by the Fe2O3/BC/MXene composite, which was non-linear throughout the time range. The outcome obtained suggests that the adsorption mechanism is not impacted via monolayered diffusion parameters.80 The entire diffusion process is divided into three linear areas that demonstrate surface sorption, membrane diffusion, and IPD. By observing the fundamental parameters of IPD at different concentrations (Table S2†), we found that KI and CI were larger than KII and KIII and CII and CIII, respectively. After three sorption stages, the sorption sites on the adsorbent gradually decrease and the sorption rate (%) also gradually decreases. KI for both pollutants was greater than KII and KIII due to the reduced number of active sites on the Fe2O3/BC/MXene with increasing time. The steadily decreasing K readings showed that the diffusion rate had slowed and that chemisorption was becoming balanced. The effect of the boundary layer increased with increasing c values.
 | ||
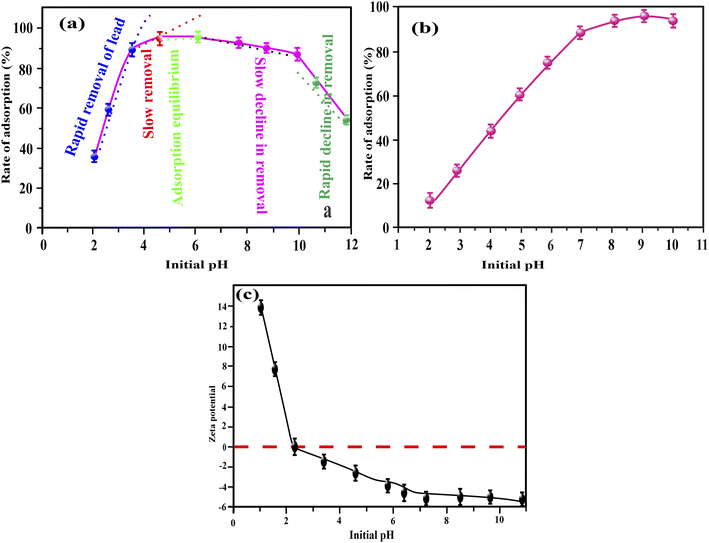
| Fig. 8 Influence of Pb2+ dosages on the adsorption capability of Fe2O3/BC/MXene (a) using Langmuir (b), Freundlich (c), and Temkin (d) models. | ||
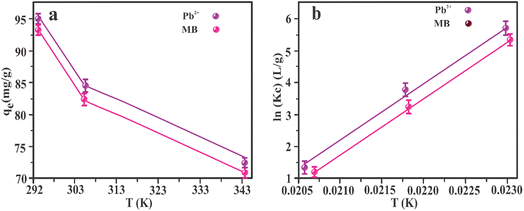
The adsorption isotherms of MB and Pb2+ by Fe2O3/BC/MXene using non-linear fitting are shown in Fig. 8(a and b) and the associated parameters are listed in Table S3.† These findings show that the Langmuir model gave a better fit than the Freundlich and Temkin isothermal models, with higher R2 values for both Pb2+ and MB. The fitting of the Langmuir model defines the single-layer, homogeneous adsorption of Pb2+ ions and MB onto Fe2O3/BC/MXene composite.81 The maximum sorption capacities of the Fe2O3/BC/MXene composite toward Pb2+ and MB were 882.76 and 758.03 mg g−1, respectively, which are higher than those for previously reported adsorbents. Furthermore, the calculated sorption capacity at 293, 303, and 313 K (882.76, 613.37, and 305.06 mg g−1, respectively) for Pb2+ from the Langmuir model was close to the experimental adsorption capacity (885, 620, and 310 mg g−1). The calculated sorption capacities for MB (758.03, 562.91, and 296.88 mg g−1, respectively) obtained from the Langmuir isotherm model were also close to the experimental adsorption capacities (760, 570, and 300 mg g−1) at 293, 303, and 313 K, respectively.
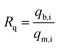 | (18) |
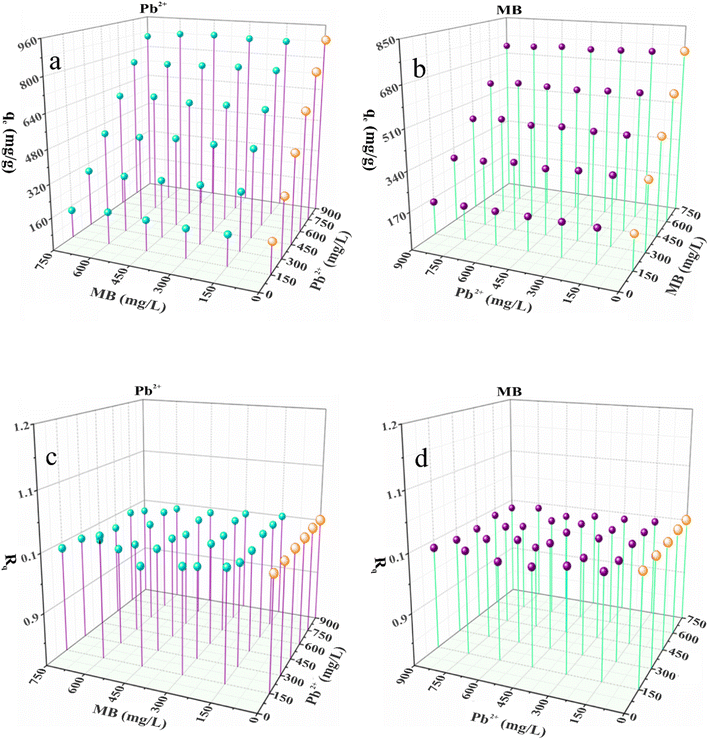
Fig. 9(a–d) shows the results of the simultaneous examination of Pb–MB adsorption on the Fe2O3/BC/MXene composite. The contaminant adsorption curves in the single-pollutant system are also shown as a reference (orange spheres). These outcomes indicate that the elimination of MB and Pb from the binary solution was analogous to that from the single-pollutant systems (MB and Pb2+) with Rq values close to 1 (Fig. 9(c and d)). The synergistic interaction is probably explained as follows. According to the adsorption results in the single-pollutant system, the MB and Pb2+ absorption modes were different (electrostatic interaction for Pb2+ and hydrogen bonding for MB). Consequently, there is no competition between MB and Pb2+ in the binary solution. These outcomes indicate that MB and Pb2+ did not influence each other as a result of their different adsorption mechanisms (Fig. 10(a and b)).
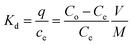 | (19) |
 | (20) |
The results are displayed in Table S5 and Fig. S4.† For the BC/MXene, BC/Fe2O3, and Fe2O3/BC/MXene adsorbents, the Kd values of Pb2+ were higher than for other heavy metal ions in simulated wastewater and showed the strongest interaction between Pb2+ ions and the adsorbent. The percentage removal rate was higher than for other metal ions. The tables also suggest that BC/MXene and BC/Fe2O3 showed a higher value of K than Fe2O3/BC/MXene for interfering ions, suggesting that the functional groups have less affinity for mixed ions. We conclude that Pb2+ ions can be selectively and efficiently eliminated from wastewater using the Fe2O3/BC/MXene composite.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, CN, and F interact (electrostatic interactions) with Pb2+, MB, and Pb2+–MB+ due to differences in polarity (Fig. 12). In the third area (500–900 cm−1), the band at 549 cm−1 corresponding to the FeO stretching vibration of iron oxide NPs moved to a lower wavenumber for Pb2+ (517 cm−1), MB (523 cm−1), and Pb2+–MB+ (530 cm−1) and became weaker, which indicates the interaction (oxido-reduction) of FeO with Pb2+, MB, and Pb2+–MB. A further band appeared at 825 cm−1 corresponding to PbO, similar to the band also reported previously.83
O, CN, and F interact (electrostatic interactions) with Pb2+, MB, and Pb2+–MB+ due to differences in polarity (Fig. 12). In the third area (500–900 cm−1), the band at 549 cm−1 corresponding to the FeO stretching vibration of iron oxide NPs moved to a lower wavenumber for Pb2+ (517 cm−1), MB (523 cm−1), and Pb2+–MB+ (530 cm−1) and became weaker, which indicates the interaction (oxido-reduction) of FeO with Pb2+, MB, and Pb2+–MB. A further band appeared at 825 cm−1 corresponding to PbO, similar to the band also reported previously.83
 | ||
| Fig. 11 FTIR patterns of the Fe2O3/BC/MXene–Pb and Fe2O3/BC/MXene–MB composites before and after the adsorption of Pb2+, MB, and Pb2+–MB. | ||
The presence of Pb2+ in the XPS pattern of Fe2O3/BC/MXene–Pb verified the successful adsorption of Pb2+ (Fig. S6(a)†). The existence of Pb2+ in the XPS pattern was confirmed at a binding energy of about 143.17 (Pb4f 5/2) and 139.23 eV (Pb4F 7/2) (Fig. S6(b)†). Similarly, the presence of S in the XPS pattern of Fe2O3/BC/MXene–MB showed the successful adsorption of MB (Fig. S6(c)†). The O 1s XPS bands at 532.3 and 533.5 eV correspond to C–OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively, before adsorption (Fig. S7(a)†). After the adsorption of Pb2+, MB, and Pb2+–MB (simultaneous removal), there was a slight movement of the bands at 532.3 eV (C–OH) to 532.5 eV (Pb2+), 533.8 eV (MB), and 533.0 eV (Pb2+–MB), which indicated the interaction of the hydroxyl group with Pb2+ and MB. After the adsorption of Pb2+, MB, and Pb2+–MB (simultaneous removal), there was a slight movement of the bands at 533.5 eV (carbonyl oxygen) to 533.7 eV (Pb2+), 533.9 eV (MB), and 533.6 eV (Pb2+–MB), respectively, which indicated the interaction of the carbonyl oxygen with Pb2+ and MB and the simultaneous removal of lead and MB (Fig. S7(a)†).
O, respectively, before adsorption (Fig. S7(a)†). After the adsorption of Pb2+, MB, and Pb2+–MB (simultaneous removal), there was a slight movement of the bands at 532.3 eV (C–OH) to 532.5 eV (Pb2+), 533.8 eV (MB), and 533.0 eV (Pb2+–MB), which indicated the interaction of the hydroxyl group with Pb2+ and MB. After the adsorption of Pb2+, MB, and Pb2+–MB (simultaneous removal), there was a slight movement of the bands at 533.5 eV (carbonyl oxygen) to 533.7 eV (Pb2+), 533.9 eV (MB), and 533.6 eV (Pb2+–MB), respectively, which indicated the interaction of the carbonyl oxygen with Pb2+ and MB and the simultaneous removal of lead and MB (Fig. S7(a)†).
Three peaks appeared at 284.3, 285.3, 287.5, and 288.6 eV, correlating to the C–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–O, and C–F groups of the composite, respectively (Fig. S7(b)†). The peaks at 284.3, 285.3, 287.5, and 288.6 eV correspond to C–C, C
C, C–O, and C–F groups of the composite, respectively (Fig. S7(b)†). The peaks at 284.3, 285.3, 287.5, and 288.6 eV correspond to C–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–O, and C–F shifting to the binding energies for Pb2+ (284.5, 285.6, 287.7, and 288.8 eV), MB (284.9, 285.5, 287.9, and 288.9 eV), and Pb2+–MB (284.7, 285.8, 287.8, and 289.0 eV), suggesting that the C–C, C
C, C–O, and C–F shifting to the binding energies for Pb2+ (284.5, 285.6, 287.7, and 288.8 eV), MB (284.9, 285.5, 287.9, and 288.9 eV), and Pb2+–MB (284.7, 285.8, 287.8, and 289.0 eV), suggesting that the C–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–O, and C–F of the composites provided a binding site for Pb2+ and MB adsorption as well as the simultaneous removal of lead and MB (Fig. S7(b)†).
C, C–O, and C–F of the composites provided a binding site for Pb2+ and MB adsorption as well as the simultaneous removal of lead and MB (Fig. S7(b)†).
The N 1s XPS bands at 398.0, 398.7, and 399.6 eV correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C–N, and NH groups before adsorption (Fig. S7(i)†). After the adsorption of Pb2+, MB, and Pb2+–MB (simultaneous removal), there was a slight shift from 398.0 eV (C
N, C–N, and NH groups before adsorption (Fig. S7(i)†). After the adsorption of Pb2+, MB, and Pb2+–MB (simultaneous removal), there was a slight shift from 398.0 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) to 398.4 eV (Pb2+), 398.2 eV (MB), and 398.5 eV (Pb2+–MB), indicating the interaction of the C
N) to 398.4 eV (Pb2+), 398.2 eV (MB), and 398.5 eV (Pb2+–MB), indicating the interaction of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group of Fe2O3/BC/MXene with both pollutants. After the adsorption of Pb2+, MB, and Pb2+–MB (simultaneous removal), there was a slight shift from 398.7 eV (C
N group of Fe2O3/BC/MXene with both pollutants. After the adsorption of Pb2+, MB, and Pb2+–MB (simultaneous removal), there was a slight shift from 398.7 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) to 398.9 eV (Pb2+), 399.1 eV (MB), and 399.3 eV (Pb2+–MB), which indicates the interaction of the C–N group of Fe2O3/BC/MXene with both pollutants. Similarly, after the adsorption of Pb2+, MB, and Pb2+–MB (simultaneous removal), there was a slight shift from 399.6 eV (NH) to 399.8 eV (Pb2+), 400.0 eV (MB), and 400.2 eV (Pb2+–MB), which indicates the interaction of the NH group of Fe2O3/BC/MXene with Pb2+ and MB as well as the simultaneous removal of lead and MB (Fig. S7(c)†).
N) to 398.9 eV (Pb2+), 399.1 eV (MB), and 399.3 eV (Pb2+–MB), which indicates the interaction of the C–N group of Fe2O3/BC/MXene with both pollutants. Similarly, after the adsorption of Pb2+, MB, and Pb2+–MB (simultaneous removal), there was a slight shift from 399.6 eV (NH) to 399.8 eV (Pb2+), 400.0 eV (MB), and 400.2 eV (Pb2+–MB), which indicates the interaction of the NH group of Fe2O3/BC/MXene with Pb2+ and MB as well as the simultaneous removal of lead and MB (Fig. S7(c)†).
| Adsorbent | Adsorption capacity (mg g−1) | Temperature | pH | Reference |
|---|---|---|---|---|
| Pb2+ | ||||
| KMnO4-modified BC | 37.51 | 22 °C | 5 | 84 |
| MoO3–BC | 229.87 | 315 K | 4.0 | 85 |
| MnSO4·4H2O peanut shell BC | 68 | 45 °C | 5.0 | ·86 |
| Magnetic oak bark BC | 30 | 25 °C | 7.7 | 87 |
| CeO2–MoS2 hybrid wood BC (600 °C) | 263.6 | — | 4.0–4.2 | 48 |
| Fe2O3/BC/MXene | 992 | 293 K | 4.5 | This work |
| MB | ||||
| Olive pomace boiler ash | 149.11 | 30 °C | 3–7 | 88 |
| Ultrasonic acid modification of raw olive pomace | 25.64 | 303 K | 3–12 | 89 |
| Manganese-modified lignin BC | 248.96 | — | 3–11 | 90 |
| Magnetic BC synthesized with waterwork sludge and sewage sludge | 186.003 | — | — | 91 |
| Fe2O3/BC/MXene | 899.03 | 293 K | 3–9 | This work |
4. Conclusions
We prepared and characterized magnetic BC resulting from the modification of S. faguetiana BC with Fe2O3 NPs and MXene. The characterization showed that the targeted engineered BC was prepared successfully and further investigations showed its high adsorption and rapid sorption rate for the removal of lead and MB from wastewater. The qm value was 992 and 899.03 mg g−1 for Pb2+ and MB, respectively, at 293 K. The removal of Pb2+ and MB was enhanced by surface electrostatic forces and hydrogen bonding. The simulation of the interaction between the composite and Pb2+ and MB using sorption isotherms and kinetics showed that the sorption process fitted a PSO kinetic model and the Langmuir isothermal model, suggesting the single-layer sorption of Pb2+ and MB onto the Fe2O3/BC/MXene composite.Author contributions
Aysha Bukhari: conceptualization and supervision, writing original draft, writing reviewing and editing, Irfan Ijaz: conceptualization, methodology, writing original draft, writing reviewing and editing, Ammara Nazir: data curation and investigation, Sajjad Hussain: data curation and methodology, Hina Zain: data curation and investigation, Ezaz Gilani: resource, Ahmad A. lfseisi: conceptualization and methodology, and Hijaz Ahmad: methodology and investigation.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors are grateful to the Researchers Supporting Project Number RSPD2024R669, King Saud University, Riyadh, Saudi Arabia for financial support.References
- R. M. J. T. economic, history review Hartwell, The causes of the industrial revolution: an essay in methodology, Econ. Hist. Rev., 1965, 18, 164–182 Search PubMed.
- R. Szostak, Role of Transportation in the Industrial Revolution: A Comparison of England and France, McGill-Queen’s Press-MQUP, 1991 Search PubMed.
- M. Das, M. K. Ahmed, M. S. Islam, M. M. Islam, M. S. J. T. Akter and A. E. Toxicology, Heavy metals in industrial effluents (tannery and textile) and adjacent rivers of Dhaka city, Bangladesh, Terr. Aquat., 2011, 5, 8–13 Search PubMed.
- V. Kumar, A. K. Chopra, S. Srivastava, V. Tomar, R. K. Thakur and J. Singh, Impact of glass industry effluent disposal on soil characteristics in Haridwar region, J. Environ. Heal. Sci., 2016, 2, 1–10 CrossRef.
- V. Kumar and A. K. Chopra, Heavy metals accumulation in soil and agricultural crops grown in the province of Asahi India Glass Ltd., Haridwar (Uttarakhand), India, Adv. Crop Sci. Technol., 2015, 1–6 Search PubMed.
- M. M. Ali, M. L. Ali, M. S. Islam and M. Z. Rahman, Preliminary assessment of heavy metals in water and sediment of Karnaphuli River, Bangladesh, Environ. Nanotechnol., Monit. Manage., 2016, 5, 27–35 CrossRef.
- A. E. Duncan, N. de Vries and K. B. Nyarko, Assessment of heavy metal pollution in the sediments of the River Pra and its tributaries, Water, Air, Soil Pollut., 2018, 229, 1–10 CrossRef PubMed.
- J. Ifthikar, T. Wang, A. Khan, A. Jawad, T. Sun, X. Jiao, Z. Chen, J. Wang, Q. Wang, H. Wang and A. Jawad, Highly Efficient Lead Distribution by Magnetic Sewage Sludge Biochar: Sorption Mechanisms and Bench Applications, Bioresour. Technol., 2017, 238, 399–406 CrossRef PubMed.
- C. Winder, Lead, reproduction and development, Neurotoxicology, 1993, 14, 303–317 Search PubMed.
- G. Assennato, C. Paci, M. E. Baser, R. Molinini, R. G. Candela, B. M. Altamura and R. Giorgino, Sperm count suppression without endocrine dysfunction in lead-exposed men, Arch. Environ. Health, 1987, 42, 124–127 CrossRef PubMed.
- M. S. Barats, H. C. Gonick, S. Rothenberg, M. Balabanian and W. I. Manton, Severe lead-induced peripheral neuropathy in a dialysis patient, Am. J. Kidney Dis., 2000, 35, 963–968 CrossRef PubMed.
- M. F. Bouchard, D. C. Bellinger, J. Weuve, J. Matthews-Bellinger, S. E. Gilman, R. O. Wright, J. Schwartz and M. G. Weisskopf, Blood lead levels and major depressive disorder, panic disorder, and generalized anxiety disorder in US young adults, Arch. Gen. Psychiatry, 2009, 66, 1313–1319 CrossRef PubMed.
- B. C. Campbell, H. L. Elliott and P. A. Meredith, Lead exposure and renal failure: does renal insufficiency influence lead kinetics, Toxicol. Lett., 1981, 9, 121–124 CrossRef PubMed.
- C. Fenga, S. Gangemi, A. Alibrandi, C. Costa and E. Micali, Relationship between lead exposure and mild cognitive impairment, G. Ig. Med. Prev., 2016, 57, E205 Search PubMed.
- A. Alvera, E. Baştürka, L. Altaşb and M. Işıkb, A solution of taste and odor problem with activated carbon adsorption in drinking water: detailed kinetics and isotherms, Desalin. Water Treat., 2022, 252, 300–318 CrossRef.
- E. Baştürk and A. Alver, Modeling azo dye removal by sono-fenton processes using response surface methodology and artificial neural network approaches, J. Environ. Manage., 2019, 248, 109300 CrossRef PubMed.
- E. Basturk and M. Karatas, Advanced oxidation of reactive blue 181 solution: A comparison between fenton and sono-fenton process, Ultrason. Sonochem., 2014, 21, 1881–1885 CrossRef PubMed.
- E. Basturk and M. Karatas, Decolorization of antraquinone dye Reactive Blue 181 solution by UV/H2O2 process, J. Photochem. Photobiol., A, 2015, 299, 67–72 CrossRef.
- E. Baştürk, M. Işık and M. Karakaş, Usage of Titanium Nanomaterial for the Decolorization of Methylene Blue and Reactive Red 198 Dyes by Sonocatalysis, Desalin. Water Treat., 2021, 224, 389–394 CrossRef.
- A. Ebrahimian Pirbazari, E. Saberikhah, M. Badrouh and M. S. Emami, Alkali treated Foumanat tea waste as an efficient adsorbent for methylene blue adsorption from aqueous solution, Water Resour. Ind., 2014, 6, 64–80 CrossRef.
- A. Ngambia, J. Ifthikar, I. I. Shahib, A. Jawad, A. Shahzad, M. Zhao, J. Wang, Z. Chen and Z. Chen, Adsorptive purification of heavy metal contaminated wastewater with sewage sludge derived carbon-supported Mg(II) composite, Sci. Total Environ., 2019, 691, 306–321 CrossRef PubMed.
- A. Alver, E. Baştürk, Ş. Tulun and İ. Şimşek, Adaptive neuro-fuzzy inference system modeling of 2, 4-dichlorophenol adsorption on wood-based activated carbon, Environ. Prog. Sustainable Energy, 2020, 39, e13413 CrossRef.
- E. Basturk, M. Işık and M. Karatas, Removal of aniline (Methylene Blue) and azo (Reactive Red 198) dyes by photocatalysis via nano TiO2, Desalin. Water Treat., 2019, 143, 306–313 CrossRef.
- M. Agarwal and W. R. Singh, Desalination, Heavy metal removal from wastewater using various adsorbents: a review, J. Water Reuse Desalin., 2017, 7, 387–419 CrossRef.
- G. Al-Enezi, M. F. Hamoda and N. Fawzi, Ion exchange extraction of heavy metals from wastewater sludges, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2004, 39, 455–464 CrossRef PubMed.
- C. Blöcher, J. Dorda, V. Mavrov, H. Chmiel, N. K. Lazaridis and K. A. Matis, Hybrid flotation—membrane filtration process for the removal of heavy metal ions from wastewater, Water Res., 2003, 37, 4018–4026 CrossRef PubMed.
- J. P. Chen and L. L. Lim, Recovery of precious metals by an electrochemical deposition method, Chemosphere, 2005, 60, 1384–1392 CrossRef PubMed.
- Z. Djedidi, M. Bouda, M. A. Souissi, R. Ben Cheikh, G. Mercier, R. D. Tyagi and J.-F. Blais, Metals removal from soil, fly ash and sewage sludge leachates by precipitation and dewatering properties of the generated sludge, J. Hazard. Mater., 2009, 172, 1372–1382 CrossRef PubMed.
- A. Jakob, S. Stucki and R. P. Struis, Complete heavy metal removal from fly ash by heat treatment: influence of chlorides on evaporation rates, Environ. Sci. Technol., 1996, 30, 3275–3283 CrossRef.
- H. Ozaki, K. Sharma and W. J. D. Saktaywin, Performance of an ultra-low-pressure reverse osmosis membrane (ULPROM) for separating heavy metal: effects of interference parameters, Desalination, 2002, 144, 287–294 CrossRef.
- J. Rubio, M. L. Souza and R. W. Smith, Overview of flotation as a wastewater treatment technique, Miner. Eng., 2002, 15, 139–155 CrossRef.
- D. Sakhi, Y. Rakhila, A. Elmchaouri, M. Abouri, S. Souabi and A. Jada, Optimization of coagulation flocculation process for the removal of heavy metals from real textile wastewater, in Int. Conf. Adv. Intell. Syst. Sustain. Dev., Springer, 2018, pp. 257–266 Search PubMed.
- Y. Vasseghian, E.-N. Dragoi, F. Almomani and M. Berkani, Innovation, Graphene-based membrane techniques for heavy metal removal: A critical review, Environ. Technol. Innovation, 2021, 24, 101863 CrossRef.
- B. Renu, M. Agarwal and K. Singh, Methodologies for removal of heavy metal ions from wastewater: an overview, Interdiscip. Environ. Rev., 2017, 18, 124–142 CrossRef.
- F. Marrakchi, B. H. Hameed and M. Bouaziz, Mesoporous and high-surface-Area activated carbon from defatted olive cake by-products of olive mills for the adsorption kinetics and isotherm of methylene blue and acid blue 29, J. Environ. Chem. Eng., 2020, 8, 104199 CrossRef.
- B. Wang, B. Gao and Y. Wan, Comparative study of calcium alginate, ball-milled biochar, and their composites on aqueous methylene blue adsorption, Environ. Sci. Pollut. Res., 2019, 26, 11535–11541 CrossRef PubMed.
- J. Ifthikar, X. Jiao, A. Ngambia, T. Wang, A. Khan, A. Jawad, Q. Xue, L. Liu and Z. Chen, Facile One-Pot Synthesis of Sustainable Carboxymethyl Chitosan – Sewage Sludge Biochar for Effective Heavy Metal Chelation and Regeneration, Bioresour. Technol., 2018, 262, 22–31 CrossRef PubMed.
- Y. Zhang, G. Shi, W. Wu, A. Ali, H. Wang, Q. Wang, Z. Xu, W. Qi, R. Li, Z. J. C. Zhang, S. A. Physicochemical and E. Aspects, Magnetic biochar composite decorated with amino-containing biopolymer for phosphorus recovery from swine wastewater, Colloids Surf., A, 2022, 634, 127980 CrossRef.
- Y. Sun, S. M. Shaheen, E. F. Ali, H. Abdelrahman, B. Sarkar, H. Song, J. Rinklebe, X. Ren, Z. Zhang and Q. J. E. P. Wang, Enhancing microplastics biodegradation during composting using livestock manure biochar, Environ. Pollut., 2022, 306, 119339 CrossRef PubMed.
- M. Azeem, S. M. Shaheen, A. Ali, P. G. S. A. Jeyasundar, A. Latif, H. Abdelrahman, R. Li, M. Almazroui, N. K. Niazi and A. K. Sarmah, Removal of potentially toxic elements from contaminated soil and water using bone char compared to plant-and bone-derived biochars: a review, J. Hazard. Mater., 2021, 128131 Search PubMed.
- Q. Wang, B. Wang, X. Lee, J. Lehmann and B. Gao, Sorption and desorption of Pb (II) to biochar as affected by oxidation and pH, Sci. Total Environ., 2018, 634, 188–194 CrossRef PubMed.
- C. Nzediegwu, M. A. Naeth and S. X. Chang, Lead (II) adsorption on microwave-pyrolyzed biochars and hydrochars depends on feedstock type and production temperature, J. Hazard. Mater., 2021, 412, 125255 CrossRef PubMed.
- S. M. Shaheen, A. Mosa, H. Abdelrahman, N. K. Niazi, V. Antoniadis, M. Shahid, H. Song, E. E. Kwon and J. J. B. Rinklebe, Removal of toxic elements from aqueous environments using nano zero-valent iron-and iron oxide-modified biochar: a review, Biochar, 2022, 4, 1–21 CrossRef.
- X. Yang, S. M. Shaheen, J. Wang, D. Hou, Y. S. Ok, S.-L. Wang, H. Wang and J. Rinklebe, Elucidating the redox-driven dynamic interactions between arsenic and iron-impregnated biochar in a paddy soil using geochemical and spectroscopic techniques, J. Hazard. Mater., 2022, 422, 126808 CrossRef PubMed.
- N. Puri, A. Gupta and A. Mishra, Recent advances on nano-adsorbents and nanomembranes for the remediation of water, J. Cleaner Prod., 2021, 322, 129051 CrossRef.
- J. Xiao, R. Hu and G. Chen, Micro-nano-engineered nitrogenous bone biochar developed with a ball-milling technique for high-efficiency removal of aquatic Cd(II), Cu(II) and Pb(II), J. Hazard. Mater., 2020, 387, 121980 CrossRef PubMed.
- R. Li, J. J. Wang, L. A. Gaston, B. Zhou, M. Li, R. Xiao, Q. Wang, Z. Zhang, H. Huang and W. J. C. Liang, An overview of carbothermal synthesis of metal–biochar composites for the removal of oxyanion contaminants from aqueous solution, Carbon, 2018, 129, 674–687 CrossRef.
- R. Li, H. Deng, X. Zhang, J. J. Wang, M. K. Awasthi, Q. Wang, R. Xiao, B. Zhou, J. Du and Z. Zhang, High-efficiency removal of Pb (II) and humate by a CeO2–MoS2 hybrid magnetic biochar, Bioresour. Technol., 2019, 273, 335–340 CrossRef PubMed.
- R. Li, Y. Zhang, H. Deng, Z. Zhang, J. J. Wang, S. M. Shaheen, R. Xiao, J. Rinklebe, B. Xi and X. He, Removing tetracycline and Hg (II) with ball-milled magnetic nanobiochar and its potential on polluted irrigation water reclamation, J. Hazard. Mater., 2020, 384, 121095 CrossRef PubMed.
- Y. Luo, K. Xie, Y. Feng, Q. He, K. Zhang, S. Shen, F. J. C. Wang, S. A. Physicochemical and E. Aspects, Synthesis of a La(OH)3 nanorod/walnut shell biochar composite for reclaiming phosphate from aqueous solutions, Colloids Surf., A, 2021, 610, 125736 CrossRef CAS.
- Y. Peng, M. Azeem, R. Li, L. Xing, Y. Li, Y. Zhang, Z. Guo, Q. Wang, H. H. Ngo and G. Qu, Zirconium hydroxide nanoparticle encapsulated magnetic biochar composite derived from rice residue: Application for As(III) and As(V) polluted water purification, J. Hazard. Mater., 2022, 423, 127081 CrossRef CAS PubMed.
- Z. Shen, J. Zhang, D. Hou, D. C. W. Tsang, Y. S. Ok and D. Alessi, Synthesis of MgO-coated corncob biochar and its application in lead stabilization in a soil washing residue, Environ. Int., 2019, 122, 357–362 CrossRef CAS PubMed.
- X. Su, Y. Guo, L. Yan, Q. Wang, W. Zhang, X. Li, W. Song, Y. Li and G. Liu, MoS2 nanosheets vertically aligned on biochar as a robust peroxymonosulfate activator for removal of tetracycline, Sep. Purif. Technol., 2022, 282, 120118 CrossRef CAS.
- P. Wu, P. Cui, Y. Zhang, M. E. Alves, C. Liu, D. Zhou and Y. J. Wang, Unraveling the molecular mechanisms of Cd sorption onto MnOx-loaded biochar produced from the Mn-hyperaccumulator Phytolacca americana, J. Hazard. Mater., 2022, 423, 127157 CrossRef CAS PubMed.
- A. Briso, G. Quintana, V. Ide, C. Basualto, L. Molina, G. Montes and F. Valenzuela, Integrated use of magnetic nanostructured calcium silicate hydrate and magnetic manganese dioxide adsorbents for remediation of an acidic mine water, J. Water Process Eng., 2018, 25, 247–257 CrossRef.
- A. Foroughnia, A. D. Khalaji, E. Kolvari and N. Koukabi, Synthesis of new chitosan Schiff base and its Fe2O3 nanocomposite: Evaluation of methyl orange removal and antibacterial activity, Int. J. Biol. Macromol., 2021, 177, 83–91 CrossRef CAS PubMed.
- J. Ifthikar, Z. Chen, Z. Chen and A. Jawad, A self-gating proton-coupled electron transfer reduction of hexavalent chromium by core-shell SBA-Dithiocarbamate chitosan composite, J. Hazard. Mater., 2020, 384, 121257 CrossRef CAS PubMed.
- L. Shen, J. Wang, Z. Li, L. Fan, R. Chen, X. Wu, J. Li and W. Zeng, A high-efficiency Fe2O3@ Microalgae composite for heavy metal removal from aqueous solution, J. Water Process Eng., 2020, 33, 101026 CrossRef.
- E. Ragab, M. Shaban, A. A. Khalek and F. Mohamed, Design and characterization of PANI/starch/Fe2O3 bio composite for wastewater remediation, Int. J. Biol. Macromol., 2021, 181, 301–312 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, The rise of graphene, Nat. Mater., 2010, 11–19 Search PubMed.
- C. N. R. Rao, A. K. Sood, K. S. Subrahmanyam and A. Govindaraj, Graphene: the new two-dimensional nanomaterial, Angew. Chemie., 2009, 48, 7752–7777 CrossRef CAS PubMed.
- F. Cao, Y. Zhang, H. Wang, K. Khan, A. K. Tareen, W. Qian, H. Zhang and H. J. A. M. Ågren, Recent Advances in Oxidation Stable Chemistry of 2D MXenes, Adv. Mater., 2022, 34, 2107554 CrossRef CAS PubMed.
- P. Ma, D. Fang, Y. Liu, Y. Shang, Y. Shi and H. Y. Yang, MXene-Based Materials for Electrochemical Sodium-Ion Storage, Adv. Mater., 2021, 8, 2003185 CAS.
- L. Qin, Q. Tao, X. Liu, M. Fahlman, J. Halim, P. O. Å. Persson, J. Rosen and F. Zhang, Polymer-MXene composite films formed by MXene-facilitated electrochemical polymerization for flexible solid-state microsupercapacitors, Nano Energy, 2019, 60, 734–742 CrossRef CAS.
- H. Shin, W. Eom, K. H. Lee, W. Jeong, D. J. Kang and T. H. Han, Highly Electroconductive and Mechanically Strong Ti3C2Tx MXene Fibers Using a Deformable MXene Gel, ACS Nano, 2021, 15, 3320–3329 CrossRef CAS PubMed.
- S. P. Sreenilayam, I. U. Ahad, V. Nicolosi and D. Brabazon, MXene materials based printed flexible devices for healthcare, biomedical and energy storage applications, Mater. Today, 2021, 43, 99–131 CrossRef CAS.
- M. Malaki, A. Maleki and R. S. Varma, MXenes and ultrasonication, J. Mater. Chem. A, 2019, 7, 10843–10857 RSC.
- X. Wu, M. Ding, H. Xu, W. Yang, K. Zhang, H. Tian, H. Wang and Z. Xie, Scalable Ti3C2Tx MXene interlayered forward osmosis membranes for enhanced water purification and organic solvent recovery, ACS Nano, 2020, 14, 9125–9135 CrossRef CAS PubMed.
- F. Kong, X. He, Q. Liu, X. Qi, D. Sun, Y. Zheng, R. Wang and Y. Bai, Further surface modification by carbon coating for in-situ growth of Fe3O4 nanoparticles on MXene Ti3C2 multilayers for advanced Li-ion storage, Electrochim. Acta, 2018, 289, 228–237 CrossRef CAS.
- E. Paulson and M. Jothibas, Significance of thermal interfacing in hematite (α-Fe2O3) nanoparticles synthesized by sol-gel method and its characteristics properties, Surf. Interfaces, 2021, 26, 101432 CrossRef CAS.
- C. N. C. Hitam and A. A. Jalil, A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants, J. Environ. Manage., 2020, 258, 110050 CrossRef CAS PubMed.
- H. Shan, C. Zeng, C. Zhao and H. Zhan, Iron oxides decorated graphene oxide/chitosan composite beads for enhanced Cr(VI) removal from aqueous solution, Int. J. Biol. Macromol., 2021, 172, 197–209 CrossRef CAS PubMed.
- W. Feng, H. Luo, Y. Wang, S. Zeng, L. Deng, X. Zhou, H. Zhang and S. Peng, Ti3C2 MXene: a promising microwave absorbing material, RSC Adv., 2018, 8, 2398–2403 RSC.
- M. Qi, L. Lin, L. Wang, Z. Bai, Y. Yu, J. Gu and Y. Liu, Spindle MnCO3 tightly encapsulated by MXene nanoflakes with strengthened interface effect for lithium-ion battery, Surf. Coat. Technol., 2021, 417, 127192 CrossRef CAS.
- Y. Shen, Q. Fang and B. Chen, Environmental applications of three-dimensional graphene-based macrostructures: adsorption, transformation, and detection, Environ. Sci. Technol., 2015, 49, 67–84 CrossRef CAS PubMed.
- Q. Wu, Y. Xian, Z. He, Q. Zhang, J. Wu, G. Yang, X. Zhang, H. Qi, J. Ma, Y. Xiao and L. Long, Adsorption characteristics of Pb(II) using biochar derived from spent mushroom substrate, Sci. Rep., 2019, 9, 1–11 CrossRef PubMed.
- C. A. Guerrero-Fajardo, L. Giraldo and J. C. Moreno-Piraján, Preparation and characterization of graphene oxide for Pb(II) and Zn(II) ions adsorption from aqueous solution: Experimental, thermodynamic and kinetic study, Nanomaterials, 2020, 10, 1022 CrossRef CAS PubMed.
- T. Bahadir, İ. Şimşek, Ş. Tulun and H. Çelebi, Use of different food wastes as green biosorbent: isotherm, kinetic, and thermodynamic studies of Pb2+, Environ. Sci. Pollut. Res., 2023, 30, 103324–103338 CrossRef CAS PubMed.
- E. Mosaffa, A. Banerjee and H. Ghafuri, Sustainable high-efficiency removal of cationic and anionic dyes using new super adsorbent biochar: performance, isotherm, kinetic and thermodynamic evaluation, Environ. Sci.: Water Res. Technol., 2023, 9, 2643–2663 RSC.
- Y. Li, J. Shao, X. Wang, H. Yang, Y. Chen, Y. Deng, S. Zhang and H. Chen, Upgrading of bio-oil: Removal of the fermentation inhibitor (furfural) from the model compounds of bio-oil using pyrolytic char, Energy Fuels, 2013, 27, 5975–5981 CrossRef CAS.
- S. A. Dzuba, D. V. Leonov and N. V. Surovtsev, Membrane-sugar interactions probed by low-frequency raman spectroscopy: The monolayer adsorption model, Langmuir, 2020, 36, 11655–11660 CrossRef PubMed.
- M. Shafiee, R. Foroutan, K. Fouladi, M. Ahmadlouydarab, B. Ramavandi and S. Sahebi, Application of oak powder/Fe3O4 magnetic composite in toxic metals removal from aqueous solutions, Adv. Powder Technol., 2019, 30, 544–554 CrossRef CAS.
- Y. Huang, H. Zheng, X. Hu, Y. Wu, X. Tang, Q. He and S. Peng, Enhanced selective adsorption of lead (II) from complex wastewater by DTPA functionalized chitosan-coated magnetic silica nanoparticles based on anion-synergism, J. Hazard. Mater., 2022, 422, 126856 CrossRef CAS PubMed.
- M. F. El-Banna, A. Mosa, B. Gao, X. Yin, Z. Ahmad and H. Wang, Sorption of lead ions onto oxidized bagasse-biochar mitigates Pb-induced oxidative stress on hydroponically grown chicory: Experimental observations and mechanisms, Chemosphere, 2018, 208, 887–898 CrossRef CAS PubMed.
- Y. Li, S. M. Shaheen, M. Azeem, L. Zhang, C. Feng, J. Peng, W. Qi, J. Liu, Y. Luo and Y. Peng, Removal of lead (Pb2+) from contaminated water using a novel MoO3-biochar composite: Performance and mechanism, Environ. Pollut., 2022, 308, 119693 CrossRef CAS.
- S. Wan, J. Wu, S. Zhou, R. Wang, B. Gao and F. He, Enhanced lead and cadmium removal using biochar-supported hydrated manganese oxide (HMO) nanoparticles: Behavior and mechanism, Sci. Total Environ., 2018, 616, 1298–1306 CrossRef PubMed.
- D. Mohan, H. Kumar, A. Sarswat, M. Alexandre-Franco and C. U. Pittman Jr, Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars, Chem. Eng. J., 2014, 236, 513–528 CrossRef CAS.
- F. Marrakchi, M. Bouaziz and B. H. Hameed, Adsorption of acid blue 29 and methylene blue on mesoporous K2CO3-activated olive pomace boiler ash, Colloids Surf., A., 2017, 535, 157–165 CrossRef CAS.
- E. Kalipci, Removal of methylene blue from aqueous solutions with natural olive pomace modified with ultrasounds and acid, Environ. Prot. Eng., 2016, 42, 5–17 Search PubMed.
- X. J. Liu, M. F. Li and S. K. Singh, Manganese-modified lignin biochar as adsorbent for removal of methylene blue, J. Mater. Res. Technol., 2021, 12, 1434–1445 CrossRef CAS.
- H. Zeng, W. Qi, L. Zhai, F. Wang, J. Zhang and D. Li, Magnetic biochar synthesized with waterworks sludge and sewage sludge and its potential for methylene blue removal, J. Environ. Chem. Eng., 2021, 9, 105951 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra07250a |
| This journal is © The Royal Society of Chemistry 2024 |