Open Access Article
Open Access ArticleFacile synthesis of NiFe2O4-based nanoblocks for low-temperature detection of trace n-butanol
Xiujuan Qian,
Yanping Chen *,
Yuye Tao,
Jian Zhang,
Guangfeng Zhang and
Haoyang Xu
*,
Yuye Tao,
Jian Zhang,
Guangfeng Zhang and
Haoyang Xu
School of Science, Shandong Jianzhu University, Jinan 250100, China. E-mail: yanping_c@sdjzu.edu.cn
First published on 10th January 2024
Abstract
Fe2O3-loaded NiFe2O4 nanoblocks were successfully developed under a straightforward one-step hydrothermal synthesis method, aiming to detect trace amounts of n-butanol at the parts per billion (ppb) concentration range. The synthesized samples were comprehensively characterized using various techniques, including XRD, SEM, XPS, TEM and SAED. At a tantalizingly low temperature of 130 °C, the Ni/Fe-2 gas sensor demonstrated the optimum response (Ra/Rg = 29.747 @ 10 ppm) to n-butanol. Furthermore, Ni/Fe-2 sensor exhibited remarkable stability and reproducibility and an ultra-low detection limit. The enhanced gas sensitivity was primarily due to the assembly of Ni/Fe-2 nanoblocks from differently sized nanospheres, which exhibited a rich surface porosity conducive to gas adsorption. Besides, the formation of heterojunctions and the augmentation of oxygen vacancy content are also conducive to enhancing gas sensing capabilities. The Ni/Fe-2 sensor is expected to successfully detect trace amounts of n-butanol.
1 Introduction
N-butanol is commonly utilized as an industrial chemical and cleaning agent.1,2 Nevertheless, protracted contact with it may inflict harm upon human well-being and lead to a cascade of discomforting symptoms, such as headaches, dizziness, nausea, vomiting and diarrhea.3 Furthermore, n-butanol can pollute water and soil, thereby compromising the health of the ecological environment. Therefore, the development of extremely sensitive gas sensors in factories and laboratories is considerably imperative to monitor the n-butanol gas concentration.4Metal oxide semiconductor (MOS) gas sensors are widely used in industrial applications and household settings because of their benefits, including straightforward operation processes and real-time monitoring capabilities.5 The NiFe2O4 gas sensors have received significant attention in recent years. The structure exhibits a classic inverse spinel arrangement, with Ni2+ ions occupying the octahedral site and Fe3+ ions filling the tetrahedral site. The presence of Ni3+/Ni2+ and Fe3+/Fe2+ ions existing in NiFe2O4 leads to an increased carrier concentration, which manifests its great potential for development as a sensing material with high sensitivity. For instance, Pan et al.6 reported that a unique heterostructure of NiO nanoprisms modified Fe2O3 nanosheets to detect n-butanol concentration. Gas-sensing experiments demonstrated that the NiO/Fe2O3 sensor has an impressive response value of 4.2 (S = Ra/Rg) at 200 °C for 10 ppm n-butanol. Furthermore, the sensor demonstrates a minimum detectable concentration (48 ppb). The improved sensitivity results from the regulation of electron transport through p–n heterostructure, along with the increased specific surface area and adsorbed oxygen level. Zhang et al.7 successfully synthesized NiFe2O4 nanorods to detect toluene, which displayed the greatest sensitivity (Ra/Rg = 59.64 @ 500 ppm) when worked at 200 °C. The catalytic activity, large surface area, and porous structures of NiFe2O4 nanorods render it suitable for toluene detection. Liu et al.8 presented NiFe2O4 nanoboxes with a porous hollow structure prepared through annealing the self-sacrificed templates of hydrothermally synthesized metal–organic frameworks (MOFs) for the detection of ethyl acetate, which showed a high response value at 64.27 (S = Ra/Rg) to 200 ppm EtOAc at 120 °C. Additionally, the sensor based on NiFe2O4 exhibited a detection limit (LOD) of approximately 0.26 ppm. The unique porous hollow morphology and multiple surface element states result in excellent gas-sensitive performance. Based on the aforementioned analysis, it is evident that the majority of NiFe2O4 sensors are constrained by elevated operating temperatures. Additionally, the sensitivity of sensors also needs to be further improved to meet the demand for detecting low-concentration gases. Consequently, additional research is required to decrease sensors operating temperatures, enhance response speed and improve sensitivity.
In this work, the Fe2O3-loaded NiFe2O4 nanoblocks were successfully generated to realize the determination of low concentrations of n-butanol gas at ppb level. Ni/Fe-2 gas sensor was evidenced the most outstanding response (Ra/Rg = 29.747 @ 10 ppm) to n-butanol at 130 °C. The SEM images revealed that the prepared sample was composed of nanoblocks formed by the aggregation of nanospheres, and the abundant porosity may result in high response. Furthermore, the Ni/Fe-2 gas sensor exhibited an ultra-low limit. Therefore, it is reasonable to propose that the Ni/Fe-2 gas sensor has significant importance for the measurement of n-butanol gas.
2 Experimental
2.1 Materials
Iron nitrate nonahydrate (Fe(NO3)3·9H2O), nickel acetate tetrahydrate (Ni(CH3COO)2·4H2O), polyvinyl pyrrolidone (PVP), sodium hydroxide (NaOH), N,N-dimethylformamide (DMF), and other chemicals were procured from Sinopharm Chemical Reagent Co. Ltd. These analytical-grade reagents do not require any additional purification steps during the material preparation process.2.2 Preparation of NiFe2O4 composites with varying Ni/Fe atomic ratios
The synthesized NiFe2O4-based nanoblocks with varying Ni/Fe atomic ratios were obtained using the hydrothermal method. As illustrated in Fig. 1(a), solution A was prepared by dissolving 0.025 mol of Fe(NO3)3·9H2O and the specified dosage of Ni(CH3COO)2·4H2O in 60 mL of deionized water (DIW), followed by thorough stirring to obtain a brown–black solution. Concurrently, 60 mL of DMF were mixed briskly with 5.7 g of PVP to prepare a semi-transparent solution B. Afterward, solution B was gradually mixed with the previous solution with continuous stirring, while an appropriate amount of NaOH solution was poured into above solution until no new precipitate formed. After 5 minutes of sonication, the resultant precursor solution was put in an autoclave which was kept at 150 °C for a duration of 12 hours. The obtained precipitate underwent centrifugation, washing, and was dried overnight in a water bath at a temperature of 60 °C. Finally, the materials annealed at 500 °C for 2 h (5 °C min−1) to obtain the brown powders.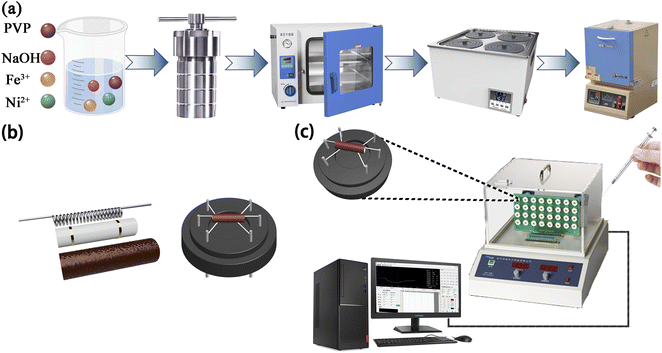 | ||
| Fig. 1 (a) Process flow chart of preparing Ni/Fe composite nanomaterials. (b) Composition diagram of gas sensor. (c) Gas sensitivity detection equipment. | ||
The obtained samples were named Ni/Fe-x (x = 1, 2, 4, 6, 8, 10), according to the different Ni/Fe atomic ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 2
10, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 4
10, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 6
10, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 8
10, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 10
10 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, respectively.
10, respectively.
2.3 Gas sensitivity test
A slight quantity of the acquired powder samples was blended with a suitable quantity of DIW in a mortar to grind into a paste. The paste obtained was subsequently spread onto the ceramic tube surface to produce a sensing film measuring approximately 0.1 mm in thickness. As shown in Fig. 1(b), a brace of golden electrodes and four platinum wires were pre-deposited at both ends of the ceramic tube. The nickel–chromium coil traversed through the ceramic tube for temperature regulation of the sensor. Gas-sensitive performance was assessed using WS-30A (Zhengzhou Weisheng Technology Co., Ltd., China) after 48 h of aging on the aging platform. During the entire gas sensing experiment, target gases of various concentrations were prepared via the static thermal evaporation method. As illustrated in Fig. 1(c), a micro-syringe was utilized to extract the necessary amount of liquid reagent. Subsequently, the reagent was injected onto the evaporation plate within the test apparatus. The volume of reagent required for the target concentration9 can be can be obtained by applying eqn (1):
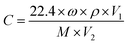 | (1) |
During the detection of gases with reducing properties, the gas sensor's sensitivity with n-type sensing material is determined by S = Ra/Rg. Conversely, for p-type material, the sensitivity is calculated according to the formula S = Rg/Ra. Here, Ra and Rg represent the resistance measured when exposed to air and reducing gases. Response–recovery time refers to the duration it takes for the sensor to demonstrate a resistance alteration of approximately 90% of the overall change following dynamic adsorption and desorption processes.
3 Result and discussion
3.1 Materials morphology
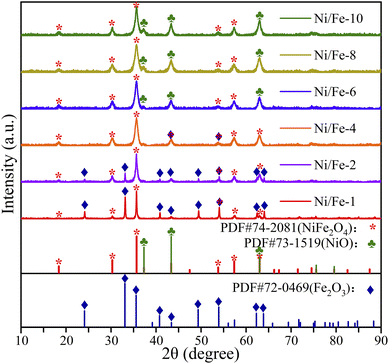
The crystalline characteristics were characterized determined via X-ray powder diffraction (XRD, Rigaku). According to Fig. 2, the XRD patterns clearly indicated that all samples belonged to inverse spinel structure of NiFe2O4, which perfectly matched the reference PDF#74-2081. The characteristic peaks observed at 2θ = 18.415°, 30.290°, 35.685°, 53.819° and 57.375° were assigned to the crystallographic planes (111), (220), (311), (422) and (511) of NiFe2O4, respectively. In addition, for samples with lower Ni element content (Ni/Fe-1 and Ni/Fe-2), the diffraction peaks of Fe2O3 at 2θ of 24.128° and 33.117° were identified as (012) and (104) planes, respectively (PDF#72-0469). As the increase of Ni element content, the diffraction peaks belonging to Fe2O3 gradually disappeared, and diffraction peaks attributed to NiO appeared in the XRD patterns. The peaks observed at 37.334°, 43.380° and 63.023° were identified as belonging to the NiO phase. These peaks were defined as the (111), (200) and (220) crystallographic planes of NiO according to PDF#73-1519. Sharp diffraction peaks indicates that the materials are well crystallized. No other peaks were found besides the diffraction peaks corresponding to NiFe2O4, Fe2O3 and NiO, implying the absence of other compounds in the samples.The nanomaterial's average diameters were determined using Scherrer's formula (D = 0.89λ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ).11 The approximate values for Ni/Fe-1, Ni/Fe-2, Ni/Fe-4, Ni/Fe-6, Ni/Fe-8 and Ni/Fe-10 samples were calculated at approximately 37.7 nm, 30.0 nm, 9.6 nm, 10.3 nm, 9.7 nm and 11.0 nm, respectively. For samples with lower Ni element content, their average crystal sizes were relatively large, around 30 nm or above. For samples with a Ni/Fe atomic ratio greater than 20%, the crystal size decreased to around 10 nm. The observed variation in crystal size can be attributed to the differences in grain size between Fe2O3 and NiO. The larger grain size of Fe2O3 compared to NiO may have contributed to the larger crystal sizes observed in samples with lesser Ni content. With increasing Ni content, the samples gradually transitioned from being predominantly Fe2O3-loaded to being NiO-loaded. So that, the transition in composition likely influenced the grain size of the material, resulting in the observed trend.
θ).11 The approximate values for Ni/Fe-1, Ni/Fe-2, Ni/Fe-4, Ni/Fe-6, Ni/Fe-8 and Ni/Fe-10 samples were calculated at approximately 37.7 nm, 30.0 nm, 9.6 nm, 10.3 nm, 9.7 nm and 11.0 nm, respectively. For samples with lower Ni element content, their average crystal sizes were relatively large, around 30 nm or above. For samples with a Ni/Fe atomic ratio greater than 20%, the crystal size decreased to around 10 nm. The observed variation in crystal size can be attributed to the differences in grain size between Fe2O3 and NiO. The larger grain size of Fe2O3 compared to NiO may have contributed to the larger crystal sizes observed in samples with lesser Ni content. With increasing Ni content, the samples gradually transitioned from being predominantly Fe2O3-loaded to being NiO-loaded. So that, the transition in composition likely influenced the grain size of the material, resulting in the observed trend.
The surface morphology and grain size characteristics were explored via scanning electron microscopy (SEM, Gemini SEM 300) analysis of the obtained materials. Representative SEM images observed that the prepared nanomaterials appeared as cubic nanoblocks, with a particle size of approximately 20 μm (as displayed in Fig. 3). For samples with lower Ni element content, two different sizes of nanospheres, 125 nm and 20 nm, can be distinctly discerned on the exterior of the prepared nanoblocks. Due to the nanoblocks being assembled from differently sized nanospheres, the nanomaterials exhibit a rich porosity, giving rise to a rough surface on the nanoblocks. However, for other samples with higher Ni element content, the nanoblocks were stacked by uniform nanospheres at approximately 10 nm. No larger nanospheres were observed. The uniform size of the nanospheres results in relatively small pores between them, thereby forming relatively dense and smooth nanoblocks. As seen by the sizes distribution map, the Ni/Fe-1, Ni/Fe-2, Ni/Fe-4, Ni/Fe-6, Ni/Fe-8 and Ni/Fe-10 samples were determined to have approximate average dimensions of 38.74 nm, 27.99 nm, 11.95 nm, 12.67 nm, 12.82 nm, 12.73 nm, respectively. The SEM characterization results are consistent with the XRD analysis results. For samples with lower Ni element content, the nanomaterials have larger crystal sizes, while as the Ni element content increases, the crystal size of the nanomaterials decreases. As we all know, higher porosity in sensing materials may facilitate gases diffusion. In accordance with the morphological characteristics of the material, it can be reasonably deduced that samples with lower Ni content possess better gas-sensing properties.
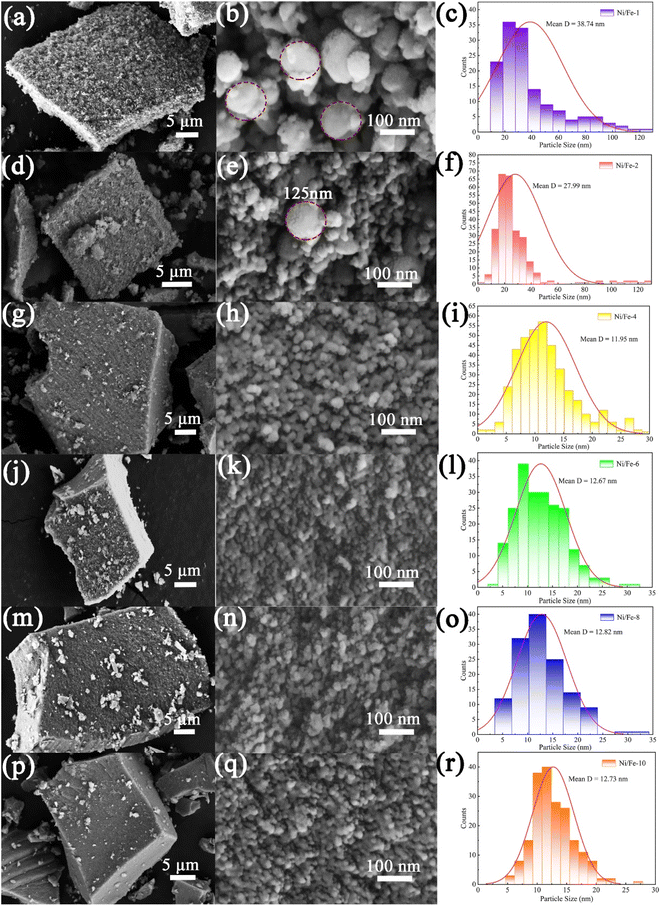 | ||
| Fig. 3 The SEM images and particle sizes distribution maps of the (a–c) Ni/Fe-1, (d–f) Ni/Fe-2, (g–i) Ni/Fe-4, (j–l) Ni/Fe-6, (m–o) Ni/Fe-8, (p–r) Ni/Fe-10. | ||
3.2 Gas sensing performances
Gas sensor's responses to 10 ppm n-butanol were gauged in the temperature interval of 100 to 160 °C to ascertain the optimum operating temperature. All sensors exhibit a “volcano-shaped” trend12 as presented in Fig. 4(a). With the rising temperature, the responses initially increase and subsequently decrease. At low temperatures, gas sensors typically exhibit low sensitivity since gas molecules do not possess sufficient energy to bind chemisorbed oxygen ions. As the operating temperature further rises, the sensitivity increases owing to enhanced reaction kinetics and increased gas diffusion. However, when the operating temperature exceeds a certain threshold, gas molecules do not fully react with the chemisorbed oxygen ions before escaping from the material.13 Consequently, excessively high temperatures can decrease the sensitivity of gas sensors. According to the data presented in Fig. 4(a), all gas sensors attain the maximum response at 130 °C. In addition to that, the Ni/Fe-2 gas sensor demonstrates the highest response (29.747), which is approximately three to sixfold greater than that of others. The increased gas sensitivity is mainly attributed to the Ni/Fe-2 composite composed of nanoblocks assembled from different sized nanospheres. The distinctive structure gives rise to a high porosity surface, which promotes gas adsorption and desorption. In conclusion, the Ni/Fe-2 sensor exhibits superior gas sensitivity towards n-butanol.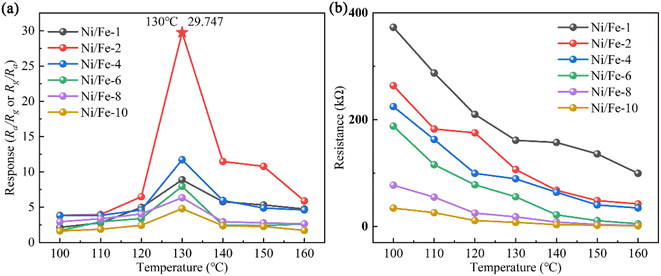 | ||
| Fig. 4 (a) The responses of sensors in the range of 100–160 °C. (b) Variation of sensor resistances (Ra) at various temperatures. | ||
The resistance variation of sensors with the operating temperature at 100–160 °C is depicted in Fig. 4(b). The results apparently indicate that with the ascending operating temperature, the sensor resistances noticeably decrease. The observed phenomenon aligns with the electrical characteristics of semiconductor materials.14 When the sensor is exposed to air, oxygen captures electrons within the nanomaterial and then adsorbs onto the surface as oxygen ions. Consequently, for n-type sensing materials, the decrease in carrier concentration increases material resistance. Conversely, for p-type sensing materials, oxygen adsorption raises hole concentration, reducing the material's resistance. Therefore, a deduction can be made that the resistance of the n-type sensor exceeds that of the p-type sensor. Furthermore, over the entire temperature range, the samples with low Ni element content exhibited higher resistances than those with higher content, which arises from the synergistic interplay between the material's oxygen adsorption capacity and electron mobility.10 These findings emphasize the potential for further enhancement of gas sensitivity in Ni/Fe-2 sensor.
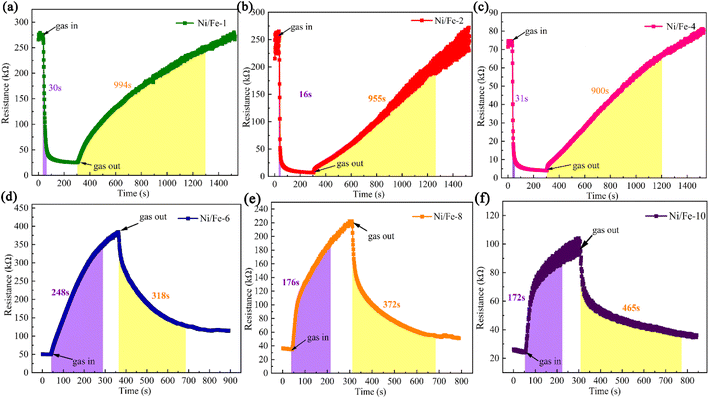
As indicated in Fig. 5, the response–recovery characteristics of all gas sensors were examined. Among them, Ni/Fe-2 gas sensor displays the shortest response time of 16 s. Fe2O3 is an n-type sensing material, while NiO is classified as a p-type sensing material.15 When the Ni content in the composite material is low, the presence of Fe2O3 causes the sensors to exhibit n-type sensing behavior. However, once the Ni/Fe atomic ratio surpasses 40%, the gradual increase in NiO content causes the sensors exhibit p-type sensing characteristics. In semiconductors, n-type semiconductors have a higher electron concentration, and electron mobility is typically higher than hole mobility.16,17 Such characteristics facilitate the faster transmission of electrons to the sensor surface. Consequently, n-type sensors tend to demonstrate shorter response times than p-type sensors. The SEM characterization images reveal that the Ni/Fe-2 nanoblocks surface exhibits abundant pores, which increase the channels for gas ingress and egress. Therefore, the response speed of the Ni/Fe-2 sensor is faster than that of other sensors. The longer recovery time of the n-type sensor when compared to the p-type sensor can be attributed to the effect of the barrier. At low temperatures, insufficient energy hinders the electron's ability to overcome the energy barrier, and the adsorbed n-butanol gas also struggles to desorb from the material surface.14,18 As a result, this leads to longer recovery times for the sensor. In summary, the Ni/Fe-2 sensor manifests superior response–recovery properties compared to others and is thus deemed the optimum device.
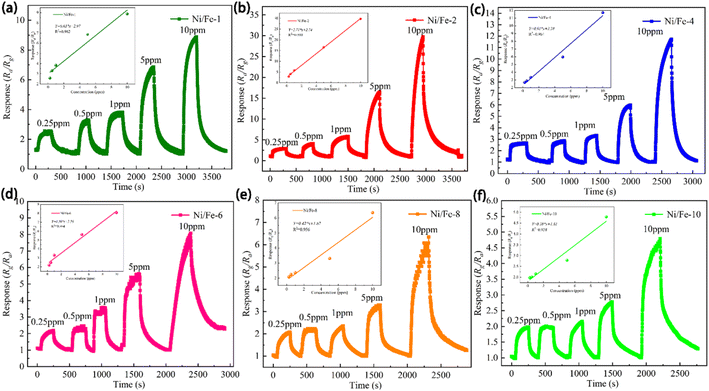
The sensors' dynamic sensing characteristics across varying concentrations of n-butanol at 130 °C, as revealed in Fig. 6. The nested figures within Fig. 6 clearly demonstrate that the sensors displayed favourable linear correlation, with linear fitting coefficients (R2) exceeding 0.95 for all sensors. In particular, Ni/Fe-2 sensor manifests linear fit function of Y = 2.71 × x + 2.74, with R2 of 0.999. The almost linear relationship indicates that the gas concentration of n-butanol in the environment can be estimated by inverse application of the sensor response values, with significant implications for environmental monitoring and air quality evaluation.
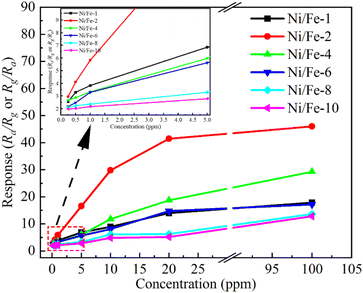
As evident in Fig. 7, the increase rate of sensor responses substantially diminishes when the gas concentration exceeds 10 ppm, which arises from the saturation of adsorption sites provided by the materials. The illustration apparently indicates the sensor responses are 2.569, 2.948, 2.676, 2.184, 2.081 and 1.981 even at a low n-butanol gas concentration of 250 ppb. Low detection limit demonstrates that the Ni/Fe-2 sensor is well-suited to detect n-butanol at trace levels.
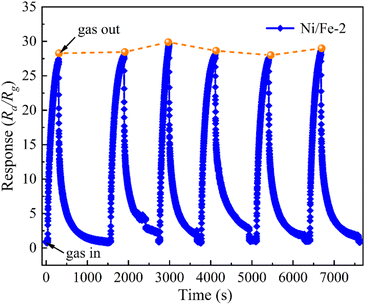
The repeatability of the Ni/Fe-2 gas sensor upon six reversible cycles of sensitivity tests to 10 ppm of n-butanol at 130 °C is presented in Fig. 8. The Ni/Fe-2 sensor exhibited consistent response and recovery characteristics during each reversible cycle, while the response values demonstrated approximately 29.7. The Ni/Fe-2 gas sensor consistently exhibits a commendable level of repeatability within an acceptable range of deviation.
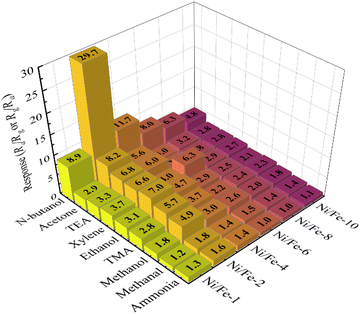
As illustrated in Fig. 9, 10 ppm various gases including n-butanol, acetone, triethylamine, xylene, ethanol, trimethylamine, methanol, formaldehyde, and ammonia have been chosen to evaluate the selectivity of the six sensors with different Ni/Fe atomic ratios at optimal operating temperature. The discriminative capability of the gas sensors can be ascribed to the impact of the alkyl chain length on the various gas molecules.19 As the length of the alkyl chain increases, so does the carrier concentration, resulting in a higher sensor response value.20–22 Evidently, the Ni/Fe-2 sensor exhibited significantly lower responses to both ammonia and methanol, while exhibiting notably higher responses to n-butanol gas. The observation indicates that the Ni/Fe-2 sensor has excellent selectivity and the potential to effectively differentiate multiple gases. Furthermore, sensor's responses to all target gases generally exhibits an initial increase followed by a decrease as the content of Ni content increased, further proving that the optimal doping ratio for Ni/Fe-based sensors is Ni/Fe = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.
10.
Table 1 compares the gas sensitivity characteristics among several n-butanol gas sensors. The result indicates a consistent observation that gas sensors functioning at lower operating temperatures generally demonstrate diminished response values. Conversely, sensors exhibiting higher response values tend to operate within the temperature range of approximately 300–400 °C. In comparison, the Ni/Fe-2 gas sensor prepared in this paper demonstrates the lowest operating temperature among the sensors listed in Table 1, reaching only 130 °C. In addition, the Ni/Fe-2 sensor also demonstrates a fast response characteristic, which can be ascribed to the notable augmentation in pore quantity, thereby increasing the transmission channel of gas molecules. Nevertheless, the Ni/Fe-2 sensor also exhibits a longer recovery time, which may be attributed to the insufficient energy for gas molecules to desorb from nanomaterials. Overall, Ni/Fe-2 sensor demonstrates exceptional potential and is suitable for the identification of n-butanol.
| Materials | T (°C) | Conc. (ppm) | Response (Ra/Rg or Rg/Ra) | tres/trec | Ref. |
|---|---|---|---|---|---|
| NiO nanoprisms/Fe2O3 nanosheets | 200 | 100 | 24.15 | 120 s/64 s | 6 |
| Ni–Co3O4 nanoflower | 165 | 100 | 8.34 | 59 s/63 s | 11 |
| 3D Sb-doped ZnFe2O4 spheres | 250 | 100 | 35.5 | 4 s/250 s | 18 |
| Tourmaline@ZnO | 320 | 100 | 120.84 | —/— | 19 |
| Fe2O3/rGO nanocube | RT | 100 | 2.71 | 53 s/42 s | 25 |
| Au–Pd decorated hierarchical WO3 nanowire bundles | 200 | 100 | 93 | 4 s/12 s | 26 |
| α-Fe2O3/ZnFe2O4 | 160 | 1 | 3.25 | 15 s/4 s | 27 |
| ZnO–NiO | 400 | 100 | 186 | 3 s/208 s | 28 |
| Au–LaFeO3 core–shell | 225 | 100 | 115 | 38 s/19 s | 29 |
| Co3O4–CuO–CuOHF | 180 | 100 | 120 | 160 s/250 s | 30 |
| Co–BiVO4 | 300 | 100 | 51.8 | 149 s/38 s | 31 |
| Al–CdIn2O4 nanofiber | 300 | 200 | 68.68 | 16 s/17 s | 32 |
| 1.5% In2O3–SnO2 | 140 | 10 | 5.96 | —/— | 33 |
| ZnO–In2O3 | 180 | 50 | 99.5 | —/— | 34 |
| Ni/Fe-2 | 130 | 0.25 | 2.948 | 30 s/115 s | This paper |
| 10 | 29.747 | 16 s/955 s |
3.3 Gas sensing mechanism
Given the analysis that came before, it can be reasonably inferred that the Ni/Fe-2 nanocomposite is primarily composed of Fe2O3 and NiFe2O4, which exhibit n-type sensing characteristics. Oxygen molecules absorb electrons in the conduction band23 when the Ni/Fe-2 nanocomposite contact ambient air. Depending on the environmental temperature, adsorbed oxygen ions (O2−, O−, O2−) are generated. The decreased carrier concentration and the electron depletion layer cause increased resistance.The reaction processes are apparent in eqn (2)–(5).24
| O2(gas) → O2(ads) | (2) |
| O2(gas) + e− → O2−, T < 100 °C | (3) |
| O2− + e− → 2O−(ads), 100 °C < T < 300 °C | (4) |
| O−(ads) + e− → O2−(ads), T > 300 °C | (5) |
| C2H10O + 12O− → 4CO2 + 5H2O + 12e− | (6) |
| e− + h+ → null | (7) |
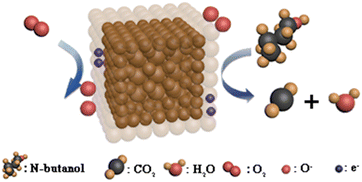
When the Ni/Fe-2 sensor is placed in an atmosphere of n-butanol, the gas molecules undergo an oxidation–reduction reaction with the adsorbed oxygen ions (O−) (eqn (6)).35 The electrons that were captured previously are freed into the Ni/Fe-2 nanocomposite conduction band and recombine with the electron vacancies situated in the valence band (eqn (7)).36 The electron depletion layer's thickness on the Ni/Fe-2 nanocomposite has reduced, causing a decrease in the Ni/Fe-2 sensor resistance when exposed to n-butanol. The potential gas detection mechanism for the Ni/Fe-2 sensor is illustrated in Fig. 10.
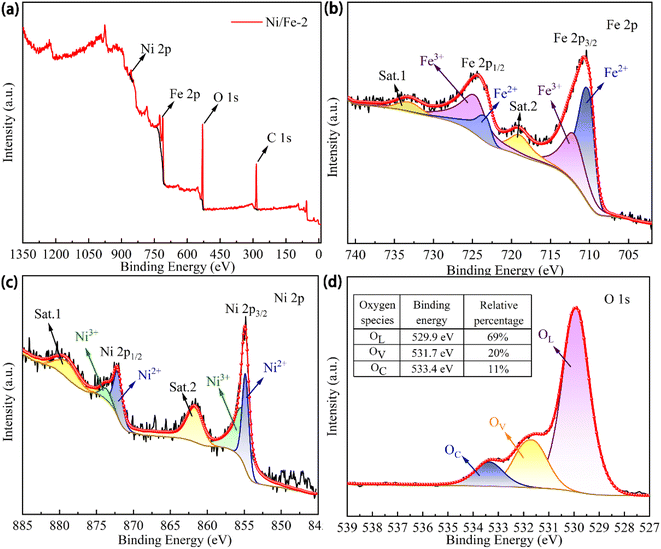
X-ray photoelectron spectroscopy (XPS, Thermo Scientific ESCALAB Xi+) was utilized for the characterization analysis to obtain additional insights into the chemistry valence and binding energy of the Ni/Fe-2 nanocomposite. The transition energies of various elements were calibrated utilizing the C 1s (284.8 eV) and the factors that enhance gas-sensing performance were further investigated. As illustrated in Fig. 11(a), only distinct peaks corresponding to Fe, Ni, and O elements are present in the XPS full scan spectrum, suggesting the absence of any additional impurities in the Ni/Fe-2 nanomaterial. Fig. 11(b) illustrates the Fe 2p fine scan spectrum of Ni/Fe-2. The Fe 2p1/2 (724.4 eV) is deconvoluted into two distinct peaks with binding energies corresponding to 725.1 ± 0.3 eV and 723.4 ± 0.1 eV, respectively. And the Fe 2p3/2 (710.7 eV) is deconvoluted into two individual peaks, characterized by binding energies of 712.3 ± 0.3 eV and 710.3 ± 0.2 eV,37 respectively. Among them, the peaks at 725.1 ± 0.3 eV and 712.3 ± 0.3 eV correspond to Fe3+, while the peaks at 723.4 ± 0.1 eV and 710.3 ± 0.2 eV are classified as Fe2+ state.14 The existence of Fe2+ indicates that the Ni/Fe-2 composite possesses more redox-active sites, which is favorable for surface reactions to occur. The fine scan spectrum of Ni 2p region for Ni/Fe-2 was illustrated in Fig. 11(c). Specifically, the primary peak of Ni 2p1/2 was identified at approximately 872.3 ± 1.7 eV, accompanied by a satellite peak at 879.7 ± 1.3 eV. While the primary peak of Ni 2p3/2 was detected at approximately 854.9 ± 0.1 eV, alongside a satellite peak at 861.8 ± 1.2 eV. Moreover, Ni3+ and Ni2+ peaks in Ni 2p1/2 were respectively placed at 873.8 ± 1.1 eV and 872.3 ± 0.1 eV. Similarly, Ni3+ and Ni2+ peaks in Ni 2p3/2 were located at 855.5 ± 1.7 eV and 854.8 ± 0.1 eV, respectively.38 According to the XPS characterization analysis, the Ni3+ content in Ni/Fe-2 is significantly higher than that of Ni2+. The higher concentration of Ni3+ ions would correlate with elevated levels of oxygen content.12 From the fine scan spectrum of O 1s (Fig. 11(d)), the three evident peaks at 529.9 ± 0.2 eV, 531.7 ± 0.2 eV and 533.4 ± 0.1 eV are corresponding to crystal lattice oxygen (OL), oxygen vacancies (OV) and chemisorbed oxygen (OC), respectively.39 The table presented in Fig. 11(d) displays the relative area percentages of different oxygen species. As widely acknowledged, the gas sensing characteristics are intricately linked to the OV content that exists at the nanomaterial's surface. The occurrence of OV may amplify the count of redox-active sites, hence facilitate redox reactions.40 A higher quantity of adsorption sites correlates with improved sensor performance.
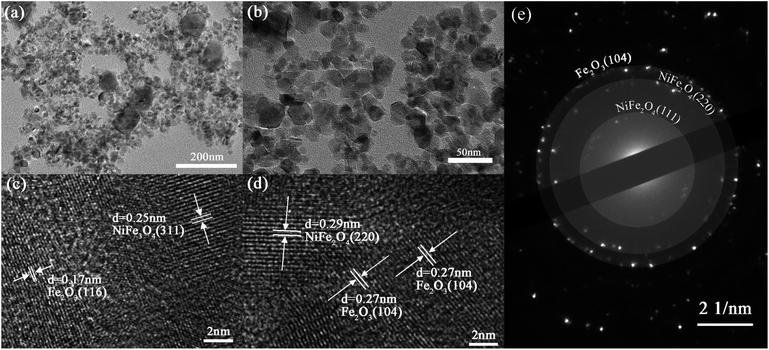
The transmission electron microscopy (TEM, JEM 2100 F) analysis in Fig. 12(a) and (b) displayed that two distinct sizes of nanospheres can be readily discerned on the surface of the Ni/Fe-2 nanoblocks, measuring approximately 125 nm and 20 nm, respectively. This finding aligns with the SEM images illustrated in Fig. 3(d) and (e). Lattice fringes corresponding to the crystal structures of Fe2O3 and NiFe2O4 are revealed in the high-resolution electron microscopy (HRTEM, JEM 2100 F) images of Ni/Fe-2 material (Fig. 12(c) and (d)), while no lattice fringes related to NiO are observed. The HRTEM analysis further suggests that the composition of Ni/Fe-2 comprises Fe2O3 and NiFe2O4, with no NiO in this sample. The selective area electron diffraction (SAED, JEM 2100 F) pattern of Ni/Fe-2 shown in Fig. 12(e) displays clear diffraction rings, indicating that the composite material has a well-crystallized polycrystalline structure.
In summary, the Ni/Fe-2 sensor's enhanced gas-sensing abilities are caused by the following factors:
First and foremost, gas-sensing performance is significantly influenced by the morphological characteristics of materials. Based on the SEM images, samples with low Ni content display a rough surface, while the nanospheres become more uniform in size on the surface of the nanoblocks as Ni content increases, resulting in an increase in surface flatness. From the SEM and TEM characterization images, Ni/Fe-2 nanocomposite exhibits nanoblocks formed by the aggregation of distinct sizes of nanospheres. The presence of nanospheres with varying sizes enhances the porosity of Ni/Fe-2 nanocomposite, leading to a roughened surface. The rough surface can enhance the number of redox active sites on the surface, leading to greater adsorption of gas molecules on the material. This significantly improves the gas-sensitive attributes of the material.41 The aforementioned characteristics create additional transmission channels for gas molecules to be adsorbed, thereby enabling the Ni/Fe-2 sensor to exhibit faster response times. However, gas molecules adsorbed within the material have difficulty desorbing from the material due to the low operating temperature. This is highly likely to be a significant contributing factor to the extended response time.
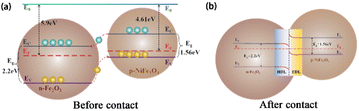
Additionally, the formation of heterojunctions can significantly improve sensing performance. The band diagrams of Fe2O3 and NiFe2O4 before contact are depicted in Fig. 13(a). P-n heterojunctions are established on the contact interfaces42 owing to the difference in work functions between n-type Fe2O3 (5.9 eV)25 and p-type NiFe2O4 (4.61 eV).9,43 During this process, electrons within the NiFe2O4 conduction band (EC) migrate towards the Fe2O3 EC until their Fermi level reaches equilibrium (Fig. 13). As depicted in Fig. 13(b), the electron depletion layer (EDL) and hole depletion layer (HDL) are located at the interface, which causes a reduction in carrier concentration and the formation of a high-resistance interface state in the sensor. Furthermore, the existence of a barrier restricts the free conduction of electrons. Electrons lack sufficient energy to overcome the barrier at lower temperatures, which is a contributing factor to the sensor's extended recovery time.
Finally, the content of oxygen vacancies at the nanomaterial is also a crucial factor influencing its gas sensing. The Ni3+/Ni2+ and Fe3+/Fe2+ ions existed in NiFe2O4, which increased the redox-active sites on nanomaterial surfaces, hence promoting the gas reaction. The presence of Fe2+ contributes to a higher number of redox-active sites, while Ni3+ further enhances the chemical oxygen content in the nanomaterial. Therefore, Ni/Fe-2 materials exhibit superior capacity to absorb oxygen, leading to an improvement in the oxidation reaction.44 With the catalytic action of the Ni3+ ions, the Ni/Fe-2 material exhibits improved gas sensing capabilities towards n-butanol gas.
4 Conclusions
In conclusion, the NiFe2O4-based nanoblocks were synthesized using hydrothermal method with varying Ni/Fe atomic ratios. The Ni/Fe-2 gas sensor exhibited excellent gas sensing performance (29.747) towards 10 ppm n-butanol at 130 °C. Compared to most n-butanol sensors, the Ni/Fe-2 sensor prepared in this paper demonstrated lower operating temperature, quick response (16 s) and extremely low detection limit. The improved gas sensing performance of the Ni/Fe-2 sensor can be attributed to the unique structure of the nanocubes, which increases the material's specific surface area. Additionally, the formation of heterojunctions and the increase in oxygen vacancy content also promote redox reactions. Therefore, the Ni/Fe-2 sensor has provided a highly feasible approach for achieving ppb-level trace detection of n-butanol.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Project supported by the Shandong Natural Science Foundation (ZR2019BF030) and the Development Plan of Youth Innovation Team of University in Shandong Province (Grant No. 2022KJJ005).Notes and references
- B. Yao, X. Cui, X. Tian, X. Xing, T. Chen and Y. Wang, SnO2 submicron porous cube derived from metal-organic framework for n-butanol sensing at room temperature, Ceram. Int., 2023, 49, 25477–25485 CrossRef CAS.
- O. M. Perrone, A. C. Roveda, D. A. de Moraes and D. P. Volanti, The enhanced n-butanol sensing performance of In2O3 loaded NiO cuboid heterostructure, J. Alloys Compd., 2023, 930, 167483–167490 CrossRef CAS.
- Q. Zhang, Q. Ma, X. Wang, Y. Wang and D. Zhao, Surface double oxygen defect engineering in button-shaped porous CeO2/WO2.9 heterostructures for excellent n-butanol detection at room temperature, Appl. Surf. Sci., 2023, 616, 156536–156545 CrossRef CAS.
- Y. Li, L.-X. Shan, R.-C. Wang, X.-X. Lian and Q.-J. Zhou, Enhanced n-butanol sensing performance of SnO2/ZnO nanoflowers fabricated via a facile solvothermal method, Ceram. Int., 2022, 48, 22426–22434 CrossRef CAS.
- C. Dai, M. Chen, Y. Lin, R. Qi, C. Luo, H. Peng and H. Lin, High performance gas sensors based on layered cobaltite nanoflakes with moisture resistance, Appl. Surf. Sci., 2022, 604, 154487–154496 CrossRef CAS.
- H. Pan, Z. Li, C. Lou, G. Lei, J. Xie, W. Zheng, X. Liu and J. Zhang, Anchoring Fe2O3 nanosheets on NiO nanoprisms to regulate the electronic properties for improved n-butanol detection, Sens. Actuators, B, 2022, 354, 131223–131232 CrossRef CAS.
- Y. Zhang, C. Jia, Q. Wang, Q. Kong, G. Chen, H. Guan and C. Dong, Highly sensitive and selective toluene sensor of bimetallic Ni/Fe-MOFs derived porous NiFe2O4 nanorods, Ind. Eng. Chem. Res., 2019, 58, 9450–9457 CrossRef CAS.
- N. Liu, X.-F. Wang, G. Zhang, H. Liang, T. Li, Y. Zhao, T. Zhang, Z. Tan and X.-Z. Song, Metal–organic framework-derived porous NiFe2O4 nanoboxes for ethyl acetate gas sensors, ACS Appl. Nano Mater., 2022, 5, 14320–14327 CrossRef CAS.
- J. Yang, B. Jiang, X. Wang, C. Wang, Y. Sun, H. Zhang, K. Shimanoe and G. Lu, MOF-derived porous NiO/NiFe2O4 nanocubes for improving the acetone detection, Sens. Actuators, B, 2022, 366, 131985–131995 CrossRef CAS.
- C. Wang, Y. Wang, P. Cheng, L. Xu, F. Dang, T. Wang and Z. Lei, In-situ generated TiO2/α-Fe2O3 heterojunction arrays for batch manufacturing of conductometric acetone gas sensors, Sens. Actuators, B, 2021, 340, 129926–129936 CrossRef CAS.
- P. Cheng, F. Dang, Y. Wang, J. Gao, L. Xu, C. Wang, L. Lv, X. Li, B. Zhang and B. Liu, Gas sensor towards n-butanol at low temperature detection: Hierarchical flower-like Ni-doped Co3O4 based on solvent-dependent synthesis, Sens. Actuators, B, 2021, 328, 129028–129039 CrossRef CAS.
- J. Yang, W. Han, J. Ma, C. Wang, K. Shimanoe, S. Zhang, Y. Sun, P. Cheng, Y. Wang, H. Zhang and G. Lu, Sn doping effect on NiO hollow nanofibers based gas sensors about the humidity dependence for triethylamine detection, Sens. Actuators, B, 2021, 340, 129971–129983 CrossRef CAS.
- U. T. Nakate, R. Ahmad, P. Patil, Y. T. Yu and Y.-B. Hahn, Ultra thin NiO nanosheets for high performance hydrogen gas sensor device, Appl. Surf. Sci., 2020, 506, 144971–144978 CrossRef CAS.
- Z. Lei, P. Cheng, Y. Wang, L. Xu, L. Lv, X. Li, S. Sun, X. Hao, Y. Zhang, Y. Zhang and Z. Weng, Pt-doped α-Fe2O3 mesoporous microspheres with low-temperature ultra-sensitive properties for gas sensors in diabetes detection, Appl. Surf. Sci., 2023, 607, 154558–154570 CrossRef CAS.
- N. Jayababu, M. Poloju, J. Shruthi and M. V. R. Reddy, NiO decorated CeO2 nanostructures as room temperature isopropanol gas sensors, RSC Adv., 2019, 9, 13765–13775 RSC.
- R. G. Kepler, P. M. Beeson, S. J. Jacobs, R. A. Anderson, M. B. Sinclair, V. S. Valencia and P. A. Cahill, Electron and hole mobility in tris(8hydroxyquinolinolatoN1,O8) aluminum, Appl. Phys. Lett., 1995, 66(26), 3618–3620 CrossRef CAS.
- Y. Takeda, A. Sasaki, Y. Imamura and T. Takagi, Electron mobility and energy gap of In0.53Ga0.47As on InP substrate, J. Appl. Phys., 2008, 47, 5405–5408 CrossRef.
- L. Lv, P. Cheng, Y. Wang, L. Xu, B. Zhang, C. Lv, J. Ma and Y. Zhang, Sb-doped three-dimensional ZnFe2O4 macroporous spheres for N-butanol chemiresistive gas sensors, Sens. Actuators, B, 2020, 320, 128384–128394 CrossRef CAS.
- L. Sun, Y. Guo, Y. Hu, S. Pan and Z. Jiao, Conductometric n-butanol gas sensor based on Tourmaline@ZnO hierarchical micro-nanostructures, Sens. Actuators, B, 2021, 337, 129793–129803 CrossRef CAS.
- X. Liu, J. Zhang, X. Guo, S. Wu and S. Wang, Enhanced sensor response of Ni-doped SnO2 hollow spheres, Sens. Actuators, B, 2011, 152, 162–167 CrossRef CAS.
- N. Swaminathana, A. Henninga, T. Jurca, J. Hayona, G. Shalevc and Y. Rosenwaks, Effect of varying chain length of n-alcohols and n-alkanes detected with electrostatically-formed nanowire sensor, Sens. Actuators, B, 2017, 248, 240–246 CrossRef.
- H. Park, J.-H. Kim, D. Vivod, S. Kim, A. Mirzaei, D. Zahn, C. Park, S. S. Kim and M. Halik, Chemical-recognition-driven selectivity of SnO2-nanowire-based gas sensors, Nano Today, 2021, 40, 101265–101277 CrossRef CAS.
- Q. Qin, Y. Zhang, W. Bu, N. Liu, Z. Zhou, C. Hu and X. Chuai, Hierarchical porous Fe2O3 derived from willow branch slices biotemplate with fast response and excellent selectivity for acetone, Sens. Actuators, B, 2023, 392, 134079–134088 CrossRef CAS.
- M. K. Tiwari, S. C. Yadav, A. Srivastava, A. Kanwade, J. A. K. Satrughna, S. S. Mali, J. V. Patil, C. K. Hong and P. M. Shirage, Enhancement of CO gas sensing performance by Mn-doped porous ZnSnO3 microspheres, RSC Adv., 2022, 12, 32249–32261 RSC.
- R. Mo, D. Han, C. Yang, J. Tang, F. Wang and C. Li, MOF-derived porous Fe2O3 nanocubes combined with reduced graphene oxide for n-butanol room temperature gas sensing, Sens. Actuators, B, 2021, 330, 129326–129335 CrossRef CAS.
- S. Zeb, X. Peng, Y. Shi, J. Su, J. Sun, M. Zhang, G. Sun, Y. Nie, Y. Cui and X. Jiang, Bimetal Au-Pd decorated hierarchical WO3 nanowire bundles for gas sensing application, Sens. Actuators, B, 2021, 334, 129584–129594 CrossRef CAS.
- W. Yan, W. Liu, Z. Zhao, J. Wang, G. B. Nam, S. Cui, X. Shen and H. W. Jang, Humidity-independent electronic nose of α-Fe2O3/ZnFe2O4 heterojunctions for trace detection of N-butanol exhalation in lung cancer screening, Sens. Actuators, B, 2023, 384, 133577–133586 CrossRef CAS.
- E. Wongrat, T. Ta-om, S. Khamprakaysit, N. Chanlek and S. Choopun, Effect of Cu or Ni addition to ZnO nanostructures on their n-butanol sensing performance, Thin Solid Films, 2023, 774, 139839–139849 CrossRef CAS.
- J. Shao, C. Sun, H. Liu, P. He, Q. Liu, J. Sun, J. Li, G. Pan and X. Yang, Insight into Au functionalization on core-shell LaFeO3 spheres for high-response and selectivity n-butanol gas sensors with DFT study, Sens. Actuators, B, 2023, 382, 133506–133515 CrossRef CAS.
- Z. Liao, Z. Yuan, H. Gao and F. Meng, Novel Co3O4-CuO-CuOHF porous sheet for high sensitivity n-butanol gas sensor at low temperature, Sens. Actuators, B, 2023, 384, 133619–133629 CrossRef CAS.
- W. Guo, Y. Shuai, X. Liu, J. Zhang, J. Wang, K. H. Mahmoud, Z. M. El-Bahy and N. M. Mubarak, A n-butanol gas sensor with enhanced gas sensing performance based on Co-doped BiVO4 polyhedrons, Sens. Actuators, B, 2022, 354, 131221–131232 CrossRef CAS.
- X. Tian, L. Yao, X. Cui, R. Zhao, X. Xiao and Y. Wang, Novel Al-doped CdIn2O4 nanofibers based gas sensor for enhanced low-concentration n-butanol sensing, Sens. Actuators, B, 2022, 351, 130946–130956 CrossRef CAS.
- D. An, N. Liu, H. Zhang, Q. Sun, C. Li, Y. Li, Q. Zhang and Y. Lu, Enhanced n-butanol sensing performance of SnO2-based gas sensors by doping In2O3 via co-precipitation method, Sens. Actuators, B, 2021, 340, 129944–129952 CrossRef CAS.
- D. An, Q. Wang, X. Tong, X. Lian, Y. Zou and Y. Li, ZnO-enhanced In2O3-based sensors for n-butanol gas, Ceram. Int., 2019, 45, 6869–6874 CrossRef CAS.
- L. Li, G. Wan, X. Cui and Y. Wang, Ultrasensitive sensing performances of amphiphilic block copolymer induced gyrus-like In2O3 thick films to low-concentration acetone, RSC Adv., 2023, 13, 20575–20583 RSC.
- X. Zhang, W. Du, Q. Li and C. Lv, Highly efficient ethanol vapour detection using g-C3N4/ZnO micro flower-like heterostructural composites, RSC Adv., 2022, 12, 20618–20627 RSC.
- P. Hao, G. Qiu, P. Song, Z. Yang and Q. Wang, Construction of porous LaFeO3 microspheres decorated with NiO nanosheets for high response ethanol gas sensors, Appl. Surf. Sci., 2020, 515, 146025–146032 CrossRef CAS.
- T. P. Mokoena, H. C. Swart, K. T. Hillie, Z. P. Tshabalala, M. Jozela, J. Tshilongo and D. E. Motaung, Enhanced propanol gas sensing performance of p-type NiO gas sensor induced by exceptionally large surface area and crystallinity, Appl. Surf. Sci., 2022, 571, 151121–151138 CrossRef CAS.
- T. Ai, J. Li, S. Nie, Y. Yin, J. Lu, S. Bao and L. Yan, High-performance H2 gas sensor based on Mn-doped α-Fe2O3 polyhedrons from N, N-dimethylformamide assisted hydrothermal synthesis, Int. J. Hydrogen Energy, 2022, 47, 20561–20571 CrossRef CAS.
- W. Li, Y. Guo, Y. Liu, W. Yang, J. Hu and J. Ma, A controllable surface etching strategy for MOF-derived porous ZnCo2O4@ZnO/Co3O4 oxides and their sensing properties, RSC Adv., 2023, 13, 24936–24943 RSC.
- K. Abdelkarem, R. Saad, A. M. El Sayed, M. I. Fathy, M. Shaban and H. Hamdy, Design of high-sensitivity La-doped ZnO sensors for CO2 gas detection at room temperature, Sci. Rep., 2023, 13, 18398 CrossRef CAS PubMed.
- Y. Xu, X. Tian, Y. Fan and Y. Sun, A formaldehyde gas sensor with improved gas response and sub-ppm level detection limit based on NiO/NiFe2O4 composite nanotetrahedrons, Sens. Actuators, B, 2020, 309, 127719–127728 CrossRef CAS.
- K. Karuppasamy, B. Sharma, D. Vikraman, E.-B. Jo, P. Sivakumar and H.-S. Kim, Switchable p–n gas response for 3D-hierarchical NiFe2O4 porous microspheres for highly selective and sensitive toluene gas sensors, J. Alloys Compd., 2021, 886, 161281–161290 CrossRef CAS.
- C. Zhou, F. Meng, K. Chen, X. Yang, T. Wang, P. Sun, F. Liu, X. Yan, K. Shimanoe and G. Lu, High sensitivity and low detection limit of acetone sensor based on NiO/Zn2SnO4 p–n heterojunction octahedrons, Sens. Actuators, B, 2021, 339, 129912–129923 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |