Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hydrothermal synthesis of dittmarite-group NH4(Co1−xMnx)PO4·H2O particles as inorganic violet pigments†
Hiroaki Uchiyama *a and
Saori Tachibanab
*a and
Saori Tachibanab
aDepartment of Chemistry and Materials Engineering, Kansai University, 3-3-35 Yamate-cho, Suita, 564-8680, Japan. E-mail: h_uchi@kansai-u.ac.jp; Tel: +81-6-6368-1121 ext. 6131
bKansai University, Japan
First published on 8th January 2024
Abstract
Dittmarite-group NH4(Co1−xMnx)PO4·H2O particles were prepared via a hydrothermal route. Single-phase platelike NH4(Co1−xMnx)PO4·H2O particles were obtained from aqueous solutions containing MnCl2·4H2O, CoCl2·6H2O, and (NH4)2HPO4, where the [Mn2+]/([Co2+] + [Mn2+]) mole ratios in the products were controlled by changing the MnCl2 and CoCl2 concentrations of the precursor solutions. The vivid violet colour of the ammonium cobalt phosphate (NH4CoPO4·H2O) particles was maintained upon substitution of Co2+ with Mn2+ ions up to x = 0.8, thus achieving an 80% saving of cobalt in the preparation of violet pigments.
Introduction
Dittmarite-group minerals, which have the general formula ABPO4·H2O (A = NH4+ or K+, B = Mn2+, Co2+, Mg2+, Ni2+), have received significant research attention for various applications, such as pigments, catalysts, fertilizers, and magnetic devices.1–3 The device properties of dittmarite-group materials are determined by the cation species, and thus synthetic routes that enable compositional control are strongly required.Cobalt phosphate materials, which exhibit various colour hues from pinkish purple to deep violet depending on their composition and crystal phase characteristics, are widely used as inorganic pigments.4–7 Ammonium cobalt phosphate monohydrate (NH4CoPO4·H2O), which is a member of the dittmarite group, has been identified as the main component in the pigment cobalt violet light (C.I. 77362 PV49).4 In recent years, NH4CoPO4·H2O has also been investigated as an electrocatalyst for the oxygen evolution reaction,8,9 a precursor of LiCoPO4 cathode materials for Li-ion batteries,10,11 and a high-performance supercapacitor.12–14 Accordingly, ammonium cobalt phosphate materials have drawn extensive research attention. However, cobalt is expensive, toxic, and susceptible to supply-chain instability, necessitating the development of alternative materials.15–17
Manganese phosphate minerals are also well known as inorganic violet pigments.18–21 Manganese violet is a typical commercial manganese phosphate pigment, the principal components of which have been identified as α- and β-NH4MnP2O7.18 Niahite (NH4MnPO4·H2O) is a dittmarite-group manganese material that occurs naturally in guano deposits.22 Synthetic routes to niahite have been studied recently owing to its great application potential for magnetic devices,23 supercapacitors,24,25 and as a precursor of olivine-type cathodes for Li-ion batteries.26,27 However, its potential applications to pigments and jewellery have not been fully investigated.
Because of their similarity in ionic radius, Mn2+ ions can partially replace Co2+ ions in the dittmarite crystal lattice, resulting in the formation of binary compounds represented by the formula NH4(Co1−xMnx)PO4·H2O. Manganese is less toxic and more available than cobalt.28–30 Therefore, the substitution of Co2+ by Mn2+ could provide a means of cobalt resource saving and cost reduction30,31 without loss of function. The compositional control of dittmarite-group NH4(Co1−xMnx)PO4·H2O phase would be useful for researchers in not only pigment but also many other material fields such as catalysts,8,9 capacitors,12–14,24,25 magnetic devises23 and electrodes for Li-ion batteries.10,11,26,27
In this work, we prepared NH4(Co1−xMnx)PO4·H2O particles via a hydrothermal route and evaluated their colour variation with the replacement of Co2+ ions by Mn2+ ions to different degrees. The binary dittmarite-group compounds were obtained from aqueous solutions containing MnCl2·4H2O, CoCl2·6H2O, and (NH4)2HPO4 by hydrothermal treatment. The effects of compositional changes on the colour characteristics of the products were quantitatively evaluated in terms of L*a*b* colour parameters, as calculated from UV-Vis absorption spectra.
Experimental
CoCl2·6H2O (∼99.0 mass%, Wako Pure Chemical Industries, Osaka, Japan) (0–0.48 g) and MnCl2·4H2O (∼99.0 mass%, Wako Pure Chemical Industries, Osaka, Japan) (0–0.40 g) were dissolved in 20 mL purified water at room temperature under stirring ([CoCl2·6H2O] = 0–0.10 M, [MnCl2·4H2O] = 0–0.10 M). The total amount of Co2+ and Mn2+ ions in the solutions was fixed at 0.10 M ([CoCl2·6H2O] + [MnCl2·4H2O] = 0.10 M), and the [Mn2+]/([Co2+] + [Mn2+]) mole ratios were denoted as x (x = 0–1.0). (NH4)2HPO4 solutions were prepared by addition of (NH4)2HPO4 (0.53 g) to 20 mL of purified water ([(NH4)2HPO4] = 0.20 M). The metal–salt solution containing CoCl2 and MnCl2 was poured into the (NH4)2HPO4 solution. The mixed solution immediately became cloudy. After stirring for 1 h, the cloudy suspension was treated hydrothermally at 150 °C in a Teflon-lined stainless steel autoclave (75 mL, Flon Industry, Tokyo, Japan) for 1 day. NH4(Co1−xMnx)PO4·H2O samples were obtained by washing the resulting precipitates with purified water and drying at 60 °C for 1 day.The samples were observed with an optical microscope (KH-1300, HiROX, Tokyo, Japan). The crystalline phases of the products were identified by powder X-ray diffraction (XRD) in normal 2θ/θ mode using an X-ray diffractometer (MiniFlex 600, Rigaku, Tokyo, Japan) with Cu Kα radiation at 40 kV and 15 mA. The morphologies of the products were investigated by scanning electron microscopy (SEM; JSM-6510, JEOL, Tokyo, Japan). The chemical compositions of the samples were determined by X-ray photoelectron spectroscopy (XPS; PHI5000 Versa Probe, ULVAC-PHI, Chigasaki, Japan) with a monochromatic Al Kα X-ray source. A charge neutralizer was used to counter surface charging during collection of the spectra. Diffuse-reflectance UV-Vis absorption spectra of the samples were obtained using an optical spectrometer (V-770, JASCO, Tokyo, Japan) with an integrating sphere, and the colour parameters in L*a*b* colour space were calculated from the absorption spectra. a*, b*, and L* values denote the red intensity (green-to-red axis), yellow intensity (blue-to-yellow axis), and luminosity, respectively, of a sample.
The heat resistance of the samples were evaluated by thermogravimetric and differential thermal analysis (TG-DTA) curves those were obtained at a heating rate of 10 °C min−1 in flowing air using a thermal analyzer (ThermoPlus 2, Rigaku, Tokyo, Japan). The chemical resistance was evaluated based on Japanese Industrial Standards (JIS); K5101-8:2004, where 0.1 g of the samples were dipped in 20 mL of 2 wt% HCl and 2 wt% NaOH aqueous solutions for 30 min at room temperature and then the colour change of the samples was visually checked.
Results and discussion
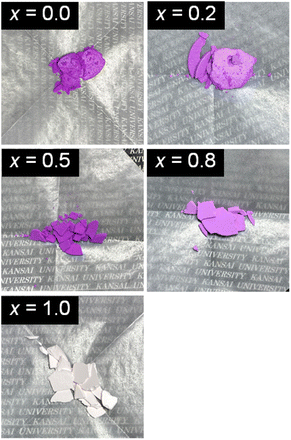
Fig. 1 shows the appearances of the NH4(Co1−xMnx)PO4·H2O products with x = 0–1.0. Violet-coloured precipitates were obtained for x = 0–0.8, while light-pink-coloured products were produced for x = 1.0. The oxidation states and the molar ratios of Co and Mn ions in the products were determined by XPS analysis. The XPS spectra are shown in ESI Fig. S1 and S2.† Only peaks attributed to bivalent Co2+ and Mn2+ ions are observed for the NH4(Co1−xMnx)PO4·H2O samples. The [Mn2+]/([Co2+] + [Mn2+]) mole ratios in the samples (x′) are shown in Table 1. The Mn2+ contents in the samples (x′) increase linearly with those in the precursor solutions (x), with the x′ values being slightly lower than x.| [Mn2+]/([Co2+] + [Mn2+]) mole ratio | |
|---|---|
| x (precursor solutions) | x′ (samples) |
| 0.0 | — |
| 0.2 | 0.18 |
| 0.5 | 0.46 |
| 0.8 | 0.65 |
| 1.0 | — |
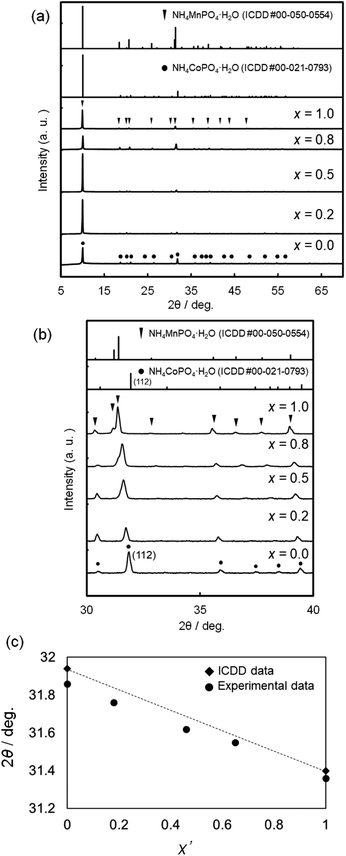
Fig. 2 shows the XRD patterns of NH4(Co1−xMnx)PO4·H2O samples with x = 0–1.0. Diffraction peaks attributed to ammonium cobalt phosphate (NH4CoPO4·H2O) are observed for x = 0, while single-phase ammonium manganese phosphate (NH4MnPO4·H2O) is observed for x = 1.0 (Fig. 2a). For the binary samples with x = 0.2–0.8, only the diffraction peaks attributed to single-phase dittmarite were also observed (Fig. 2a), where the peaks shift to lower angles with increasing x between NH4CoPO4·H2O (x = 0) and NH4MnPO4·H2O (x = 1.0) phases (Fig. 2b). The relationship between the Mn contents in the samples (x′) and the (112) diffraction angles are shown in Fig. 2c. The (112) diffraction angles decrease linearly with x′. These results could indicate the formation of NH4(Co1−xMnx)PO4·H2O solid-solution phases and the [Mn2+]/([Co2+] + [Mn2+]) mole ratios in the products changed with the MnCl2 and CoCl2 concentrations of the precursor solutions.
Fig. 3 shows the SEM images of the NH4(Co1−xMnx)PO4·H2O samples for x = 0–1.0. Platelike particles 10–20 μm in width were obtained for all the samples. The thickness of the platelike particles increases with x from 0 to 0.5, where the maximum thickness is ca. 1.5 μm (Fig. 3e and f). Further increase in x from 0.5 to 1.0 results in a decrease in thickness.
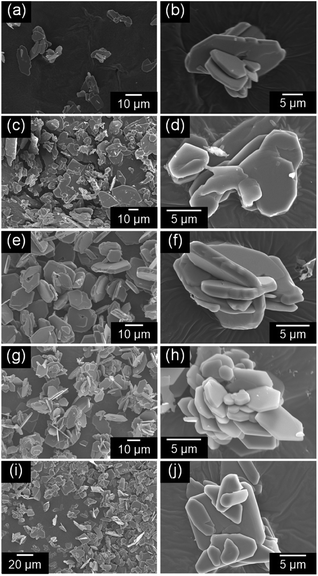 | ||
| Fig. 3 SEM images of NH4(Co1−xMnx)PO4·H2O samples with x = 0 (a and b), 0.2 (c and d), 0.5 (e and f), 0.8 (g and h), and 1.0 (i and j). | ||
The UV-Vis absorption spectra of the NH4(Co1−xMnx)PO4·H2O samples with x = 0–1.0 were measured, and the colour parameters in the L*a*b* colour space calculated from those spectra. Fig. 4a shows the UV-Vis absorption spectra of NH4(Co1−xMnx)PO4·H2O samples with x = 0–1.0. Absorption peaks at approximately 550 and 750 nm are observed for the NH4CoPO4·H2O sample (x = 0), which are assigned to the 4T1g → 4T1g(P) and 4T1g → 4A2g(F) transition of the Co2+ ions in MO6 octahedra.32 Conversely, for the NH4MnPO4·H2O sample (x = 1.0), weak absorption peaks around 360, 400, 440 and 530 nm are observed, which may be caused by 6A1g(S) → 4T1g(G), 6A1g(S) → 4T2g(G), 6A1g(S) → 4A1g(G) + 4Eg(G), and 6A1g(S) → 4T2g(D) transitions of Mn2+ ions with octahedral symmetry, respectively.33 All the photoabsorptions attributed to Co2+ and Mn2+ ions in the octahedral sites are also observed for NH4(Co1−xMnx)PO4·H2O samples with x = 0.2–0.8. The x = 0.5 sample exhibited the highest absorbance, which may be attributed to the higher thickness of the platelike particles (Fig. 3e and f).
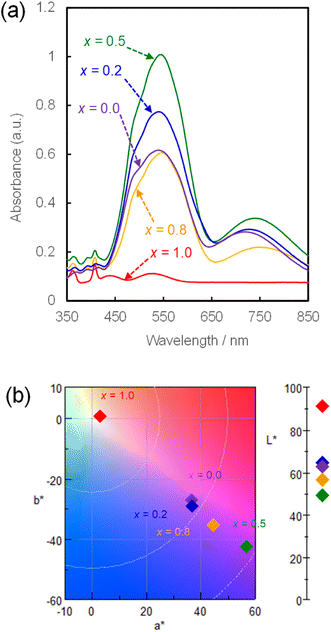 | ||
| Fig. 4 UV-Vis absorption spectra (a) and chromaticity diagram in L*a*b* colour space (b) for NH4(Co1−xMnx)PO4·H2O samples with x = 0–1.0. | ||
The L*a*b* colour parameters and the corresponding chromaticity diagram are shown in Table 2 and Fig. 4b, respectively. Increasing the Mn content from x = 0 to 0.5 increases a* and decreases b*. This means increases in the degrees of red and blue colouration, which results in a strong violet colour. A further increase in x from 0.5 to 1.0 weakens the violet colour, and the NH4MnPO4·H2O sample (x = 1.0) shows a very light pink colour. The colour characteristics, as determined by quantitative UV-Vis analysis, correspond well with the visual appearances of the samples (Fig. 1). Moreover, the NH4(Co1−xMnx)PO4·H2O sample with x = 0.8 has a stronger violet colour than that of the NH4CoPO4·H2O (x = 0) sample.
| x | Color parameters | ||
|---|---|---|---|
| L* | a* | b* | |
| 0.0 | 62.99 | 36.61 | −27.28 |
| 0.2 | 64.39 | 37.01 | −29.56 |
| 0.5 | 49.33 | 56.79 | −42.65 |
| 0.8 | 56.53 | 44.63 | −35.50 |
| 1.0 | 91.88 | 2.91 | 0.19 |
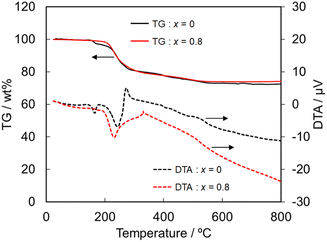
Next, the heat and chemical resistances were compared between the NH4(Co1−xMnx)PO4·H2O samples with x = 0 and 0.8. The heat resistance was evaluated by TG-DTA analysis. The TG-DTA curves of the samples with x = 0 and 0.8 are shown in Fig. 5. The weight loss and endothermic peaks are mainly observed at 200–300 °C for the both samples, and then the decrease in the weight gradually continued up to 500 °C. Moreover, the color of the both samples got light after the TG-DTA analysis. NH4CoPO4·H2O phase has been reported to be transformed to Co2P2O7 by the calcination.34 The total weight loss that detected in the present work was ca. 25% irrespective of samples, which well agreed with the theoretical value for the phase transition from NH4CoPO4·H2O to Co2P2O7. The TG-DTA curves suggest that the substitution of Co2+ by Mn2+ didn't affect the heat resistance.
The chemical resistance of the NH4(Co1−xMnx)PO4·H2O samples was evaluated based on the colour change by the soaking in acidic and alkaline solutions. In the case of acidic conditions, the both samples completely dissolved in the HCl solutions. On the other hand, the soaking in NaOH solutions resulted in the partial dissolution and the colour change of the residual samples. ESI Fig. S3† shows the appearances of the residual samples with x = 0 and 0.8 after soaking in NaOH solutions. The violet colour of the sample with x = 0 was bleached and partially changed to blue, while the sample with x = 0.8 showed dark red-brown color after the NaOH soaking. As the results, for the both samples, the violet colour couldn't keep in the acidic and alkaline solutions.
As mentioned above, the violet colour of the NH4CoPO4·H2O particles with x = 0 was maintained upon substitution of Co2+ with Mn2+ ions up to x = 0.8. On the other hand, the heat and chemical resistances of NH4CoPO4·H2O phase were not improved by the substitution by Mn2+ ions. However, the addition of Mn2+ ions didn't degrade the resistances. These results present an 80% cobalt resource saving with no decline in colour.
Conclusions
We hydrothermally prepared NH4(Co1−xMnx)PO4·H2O particles and investigated the effects of replacing Co2+ ions with Mn2+ ions on the colour of the resulting materials. Single-phase NH4(Co1−xMnx)PO4·H2O solid-solution particles were obtained by hydrothermal treatment at 150 °C, and the [Mn2+]/([Co2+] + [Mn2+]) mole ratios of the samples were controlled by changing the MnCl2 and CoCl2 concentrations in the precursor solutions. The violet colour of ammonium cobalt phosphate (NH4CoPO4·H2O) particles was maintained when their Mn content was increased to x = 0.8, thus achieving an 80% saving of cobalt in the preparation of violet pigments. These simple synthetic route for dittmarite-group solid-solution materials would be beneficial for many researchers in material fields.Author contributions
Hiroaki Uchiyama conceived of the study, designed the study, and drafted the manuscript; Saori Tachibana carried out the sample synthesis and characterization. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Nippon Sheet Glass Foundation for Materials Science and Engineering, 2022 Research Grant (No. 22-002). We thank Dr Jay Freeman at Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.References
- V. G. Koleva, Spectrochim. Acta, Part A, 2005, 62, 1196–1202 CrossRef PubMed.
- S. Phumying, T. Sichumsaeng, S. Sonsupap, P. Kidkhunthod, N. Chanlek, S. Pinitsoontorn, J. Khajonrit and S. Maensiri, Appl. Phys. A, 2021, 127, 352 CrossRef CAS.
- L. Torre-Fernandez, C. Trobajo, I. de Pedro, B. F. Alfonso, O. Fabelo, J. A. Blanco, J. R. Garcia and S. Garcia-Granda, J. Solid State Chem., 2013, 206, 75–84 CrossRef CAS.
- F. Casadio, A. Bezur, I. Fiedler, K. Muir, T. Trad and S. Maccagnola, J. Raman Spectrosc., 2012, 43, 1761–1771 CrossRef CAS.
- N. Gorodylova, V. Kosinova, Z. Dohnalova, P. Belina and P. Sulcova, Dyes Pigm., 2013, 98, 393–404 CrossRef CAS.
- S. Meseguer, M. A. Tena, C. Gargori, J. A. Badenes, M. Llusar and G. Monros, Ceram. Int., 2007, 33, 843–849 CrossRef CAS.
- M. A. Tena, R. Mendoza, C. Trobajo and S. Garcia-Granda, Materials, 2022, 15, 1111 CrossRef CAS.
- Q. D. Che, Q. Ma, J. P. Wang, Y. N. Zhu, R. X. Shi and P. Yang, Catal. Commun., 2020, 140, 106006 CrossRef CAS.
- Q. D. Che, X. B. Xie, Q. Ma, J. P. Wang, Y. N. Zhu, R. X. Shi and P. Yang, J. Energy Chem., 2020, 51, 167–174 CrossRef.
- A. Kondo, T. Kozawa, T. Ishii and M. Naito, Int. J. Appl. Ceram. Technol., 2023, 20, 700–707 CrossRef CAS.
- T. Kozawa, K. Fukuyama, A. Kondo and M. Naito, Mater. Res. Bull., 2021, 135, 111149 CrossRef CAS.
- H. Pang, Z. Z. Yan, W. Q. Wang, J. Chen, J. S. Zhang and H. H. Zheng, Nanoscale, 2012, 4, 5946–5953 RSC.
- S. M. Wang, H. Pang, S. S. Zhao, W. F. Shao, N. N. Zhang, J. S. Zhang, J. Chen and S. J. Li, RSC Adv., 2014, 4, 340–347 RSC.
- W. H. Wei, S. J. Luo, Y. Zhao, X. M. Li, B. Liang, J. Z. Fang and M. Luo, New J. Chem., 2021, 45, 11174–11182 RSC.
- S. Aso and H. Onoda, Materials, 2023, 16, 4132 CrossRef CAS PubMed.
- S. Lee and A. Manthiram, ACS Energy Lett., 2022, 7, 3058–3063 CrossRef CAS.
- M. H. Severson, R. T. Nguyen, J. Ormerod and S. Williams, Resour., Conserv. Recycl., 2023, 189, 106761 CrossRef CAS.
- Y. Begum and A. J. Wright, J. Mater. Chem., 2012, 22, 21110–21116 RSC.
- H. Onoda and S. Kanai, Phosphorus Res. Bull., 2020, 36, 023–028 CrossRef CAS.
- C. Anselmi, M. Vagnini, L. Cartechini, C. Grazia, R. Vivani, A. Romani, F. Rosi, A. Sgamellotti and C. Miliani, Spectrochim. Acta, Part A, 2017, 173, 439–1444 CrossRef CAS PubMed.
- R. R. Rajadurai and J. H. Lee, Materials, 2020, 13, 993 CrossRef CAS PubMed.
- G. V. Kiriukhina, O. V. Yakubovich and O. V. Dimitrova, Crystallogr. Rep., 2015, 60, 198–203 CrossRef CAS.
- W. W. Wu, Y. J. Fan, X. H. Wu, S. Liao and S. S. Li, J. Phys. Chem. Solids, 2009, 70, 584–587 CrossRef CAS.
- J. L. Liu, D. G. Hu, T. Huang and A. S. Yu, J. Alloys Compd., 2012, 518, 58–62 CrossRef CAS.
- T. A. Raja, P. Vickraman, A. S. Justin and B. J. Reddy, J. Mater. Sci., 2020, 55, 14447–14463 CrossRef CAS.
- T. Kozawa, K. Fukuyama, A. Kondo and M. Naito, ACS Omega, 2019, 4, 5690–5695 CrossRef CAS PubMed.
- C. Sronsri, P. Noisong and C. Danvirutai, Solid State Sci., 2014, 32, 67–75 CrossRef CAS.
- N. F. Yahya, N. Kamarulzaman, K. Elong, N. A. M. Mokhtar, A. F. M. Fadzil, N. H. Zainol and A. Azahidi, AIP Conf. Proc., 2017, 1877, 040008 CrossRef.
- K. M. Lee, H. J. Choi and J. G. Lee, J. Mater. Sci. Lett., 2001, 20, 1309–1311 CrossRef CAS.
- K. Fang, Q. Xie, C. Wang, X. Qiu, Y. Wang, W. Yang and X. W. Yu, ACS Appl. Energy Mater., 2021, 4, 7190–7200 CrossRef CAS.
- S. Ahmed, P. A. Nelson, K. G. Gallagher, N. Susarla and D. W. Dees, J. Power Sources, 2017, 342, 733–740 CrossRef CAS.
- L. Robertson, M. Duttine, M. Gaudon and A. Demourgues, Chem. Mater., 2011, 23, 2419–2427 CrossRef CAS.
- S. S. Sastry and B. R. V. Rao, Bull. Mater. Sci., 2015, 38, 475–482 CrossRef CAS.
- H. Pang, Z. Yan, Y. Ma, G. Li, J. Chen, J. Zhang, W. Du and S. Li, J. Solid State Electrochem., 2013, 17, 1383–1391 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra07387d |
| This journal is © The Royal Society of Chemistry 2024 |