Open Access Article
Open Access ArticleIodine-catalyzed regioselective direct sulfenylation of uracil with sulfonyl hydrazide as sulfur source under solvent free conditions†
Cong Wang‡
ad,
Qi-Yun Peng‡ a,
Yi Wua,
Yan-Ling Heb,
Xiao-He Zhengb,
Hai-Bo Wangc,
Jia-Min Xind,
Qing Hea,
Jun Xie*a and
Lei Tang*ae
a,
Yi Wua,
Yan-Ling Heb,
Xiao-He Zhengb,
Hai-Bo Wangc,
Jia-Min Xind,
Qing Hea,
Jun Xie*a and
Lei Tang*ae
aSchool of Pharmacy, Guizhou Provincial Engineering Technology Research Center for Chemical Drug R&D, Guizhou Medical University, Guiyang, China. E-mail: tlei1974@163.com; 1099252587@qq.com
bZhejiang Hisun Pharmaceutical Co., Ltd., Taizhou, Zhejiang 318000, China
cZhejiang Hongyuan Pharmaceutical Co., Ltd., Linhai, Zhejiang 317016, China
dThe First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Formulation (R&D) Department, Guiyang 550001, China
eState Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medcial University, Guiyang, China
First published on 5th February 2024
Abstract
A facile method was developed for the selective thioetherification of uracils using sulfonyl hydrazide as the thioetherification reagent. This method offers advantages such as avoiding the use of additives and expensive metal catalysts, and providing good to excellent yields of various uracil thioethers. Experimental studies have demonstrated that the reaction follows a free radical pathway. Notably, the reaction can be carried out without solvent.
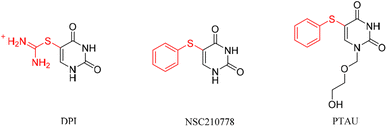
A diverse range of uracil-containing compounds show fascinating biological activity and many have been developed for clinical treatment in the field of chemotherapy and as nucleotide tools in molecular genetics.1 In particular, 5-sulfur-substituted uracil occupies an important place in pharmaceutical chemistry, and has widespread applications as a key structural moiety of antivirals and anticancer agents (Fig. 1).2 For example, 2,4-dihydroxy-5-pyrimidinyl imidothiocarbamate (DPI) is considered to be an antagonist of YB-1-like drugs for breast cancer treatment. 5-Phenylthioxypyrimidine (NSC210778) is thought to be effective in inhibiting mammalian thymidine phosphorylase, FUDR phosphorylase, DHUDase and UrdPase. 5-(Phenylthio)acyclouridine (PTAU) can efficiently and specifically inhibit uridine phosphorylase (UrdPase) to prevent its catabolism of uridine and improve the bioavailability of oral uridine. Hence, the development of novel efficient and green strategy for the direct construction of the 5-sulfur-substituted uracil is highly needed.
In literature, there are a large number of methods to construct C–S bonds, among which are coupling reactions with prefunctionalized uracil via thiols,3 disulfides,4 and Bunte salts.5 These processes frequently involve the use of oxidants and transition metal catalysts3,4 (Scheme 1a). Furthermore, C–S bonds can be formed through the presence of nucleophilic auxiliary groups, such as NH2 groups, and with the assistance of a metal-free environment containing I2 or NCS.6 (Scheme 1b). Although they are useful in forming C–S bonds, they also have obvious limitations. The use of toxic thiols, the need for oxidizing agents, large amounts of reaction solvents, and scaled promoters are all issues of concern. Given the limitations of current methods, it is imperative that we develop a more straightforward and environmentally-friendly approach for synthesizing C–S bonds.
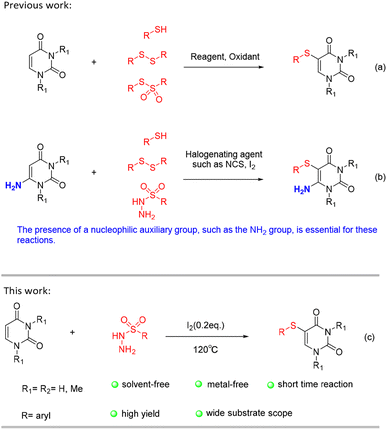 | ||
| Scheme 1 Previous protocols for the generation of 5-sulfur-substituted uracil derivatives (a and b) and our approach (c). | ||
In recent years, sulfonyl hydrazides have gained popularity as sulfenylating agents due to their numerous advantages.7 They are readily available, easy to handle, odorless, and typically present as stable solids. As a result, there has been an increasing number of examples of their use in this capacity. We are committed to using ecologically safe raw materials and catalysts in the production of 5-sulfur-substituted uracil derivatives. Herein, In this paper, we introduce a novel method for directly coupling sulfonyl hydrazide and uracil without the use of solvents. This method utilizes iodine catalysis to facilitate the reaction. Notably, the process does not require solvents or oxidants and produces high yields of the desired product (Scheme 1c).
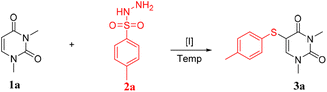
We found that a reaction of uracil (1a) and sulfonyl hydrazide (2a) in the presence of I2 (1 equiv.) and TFA at 120 °C under atmospheric air produced a 99% yield of the 5-thio-substituted product 3a (Table 1, entry 1). Subsequently, we investigate the impact of additives and catalysts on the reaction. To our satisfaction, we observed that even in the absence of additives, the yield of product 3a reached an impressive 99% (entry 2). It is shown that this iodine-catalyzed C–S bond formation is indeed necessary (entry 3).
| Entry | Additive | 2a 2(eq.) | [I] (equiv.) | Temp (°C) | Time | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: uracil 1a (0.2 mmol, 28 mg, 1 equiv.), sulfonyl hydrazide 2a (0.3 mmol, 56 mg, 1.5 equiv.), iodide source, designated temperature under open air for 0.5 h.b Isolated yield. | ||||||
| 1 | TFA | 1.5 | I2 (1) | 120 | 10 min | 99 |
| 2 | — | 1.5 | I2 (1) | 120 | 10 min | 99 |
| 3 | TFA | 1.5 | — | 120 | 0.5 h | Trace |
| 4 | — | 1.5 | KI (1) | 120 | 3 h | Trace |
| 5 | — | 1.5 | NIS (1) | 120 | 15 min | 91.7 |
| 6 | — | 1.5 | I2 (0.5) | 120 | 10 min | 97 |
| 7 | — | 1.5 | I2 (0.2) | 120 | 30 min | 99 |
| 8 | — | 1.5 | I2 (0.1) | 120 | 26 h | 99 |
| 9 | — | 1.5 | I2 (0.2) | 100 | 13 h | 80 |
| 10 | — | 1.5 | I2 (0.2) | 110 | 12 h | 95 |
| 11 | — | 1.5 | I2 (0.2) | 130 | 25 min | 99 |
| 12 | — | 1.2 | I2 (0.2) | 120 | 8 h | 95 |
| 13 | — | 1 | I2 (0.2) | 120 | 8 h | 91 |
Then, the effects of different iodine source on reaction were investigated. Among I2, KI, and NIS, Obviously, I2 produced the best yield 99% (entry 1 and entries 4, 5). Next, the effect of catalytic loading on the reaction was investigated. When the catalyst loading was decreased to 20 mol%, the product yield was 99% (Table 1, entry 7). However, when the catalyst loading decreased to 10 mol%, the yield did not decrease but the reaction time was extended to 26 h (Table 1, entry 8). The best option was 120 °C (entries 9–11) in the additional investigation of the effect of temperature. In avoiding waste of reagents, we screened the dosage of sulfonyl hydrazide 2a and finally determined 1.5 eq. sulfonyl hydrazide as the optimal equivalent (entries 12 and 13). On the basis of detailed investigations, the optimized reaction conditions were determined as entry 7 after careful study.
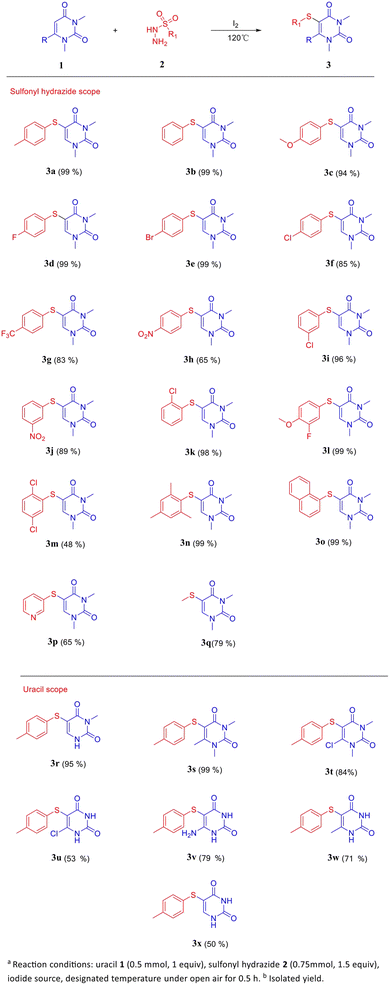
After the establishment of optimal reaction conditions, the substrate range and functional group tolerance of the procedure were explored (Scheme 2). Various substituted aryl sulfonyl hydrazides 2a–n, naphthyl sulfonyl hydrazide 2o, heteroaryl sulfonyl hydrazide 2p, and alkanesulfonyl hydrazide 2q were treated with uracil 1a, resulting in good yields. In general, substituted aryl sulfonyl hydrazides with electron-donating groups (–Me, –OMe) proceeded very well, and excellent yields of the products were obtained (3a and 3c).
Although the yield of para-NO2 sulfonyl hydrazide (3h) was only 65%, meta-NO2 substituted arylsulfonyl hydrazides and others electron-withdrawing groups (F/Cl/Br/CF3) showed excellent conversions (3d–3g, 3i–3k). The introduction of multiple electron-withdrawing groups on arylsulfonyl hydrazides leads to a decrease in the conversion rate. For example, the introduction of electron-withdrawing groups in both the neighbouring and interstitial positions of the sulfonyl hydrazide simultaneously leads to lower yield (3m 48%). Notably, the conversions of sulfonyl hydrazides with double steric hindrance and naphthyl sulfonyl hydrazides were also very high (3n, 99%, 3o, 99%). Despite a decrease in the conversion of alkylsulfonylhydrazides and heteroarylsulfonyl-hydrazides, they still yielded 5-thiouracil (3p, 65% and 3q, 79%) in moderate quantities. In addition, the scheme is applicable not only to N-substituted uracil but also to natural uracil and C6-substituted uracil. The N-substituted uracil and the single N-substituted uracil had excellent yields (3r–3t). C6-substituted uracil and natural uracil were also obtained in moderate to good yields (3u–3x).
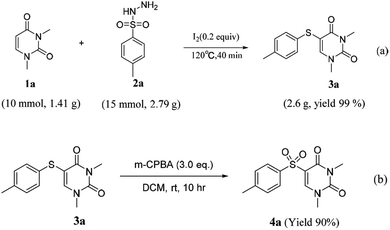
Considering the significant application value of 5-sulfur-substituted uracil, we proceeded with an gram scale experiment. To our satisfaction, the reaction yielded an exceptionally high amount (99%) of the product 3a [eq. (a)]. In addition, treatment of the compound 3a with 3-Chloroperbenzoic acid (m-CPBA) gave the sulfone compounds product 4a in high yield [eq. (b)] (Scheme 3).
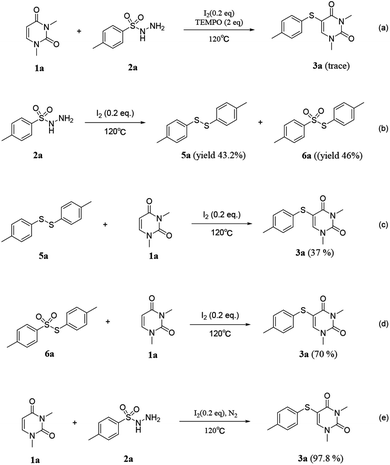
To clarify the reaction mechanism, several control experiments were performed (Scheme 4). The absence of product 3a [Scheme 4(a)] after adding 2 equiv. of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) to the standard reaction indicates that the free radical pathway is involved in the reaction. Subsequently, 2a was subjected to standard conditions, resulting in the formation of 5a (yield 43.2%) and 6a (yield 46%) [Scheme 4(b)]. Further, both compounds 5a and 6a were reacted with 1a, leading to the formation of the desired product 3a with yields of 37% and 70% respectively [Scheme 4(c) and (d)]. This observation suggests that 5 and 6 serve as the two key intermediates in the reaction. Additionally, we discovered that 3a can be obtained in high yield even under N2 protection, indicating that the involvement of air is not necessary for the reaction [Scheme 4(e)].
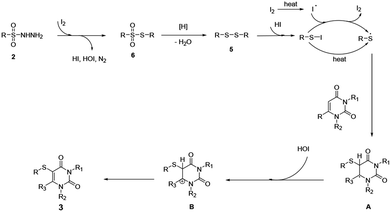
According to the above results and previous studies. A probable mechanism of free radical-mediated reactions is outlined in Scheme 5. Initially, substrate 2 is converted to intermediate 6 catalyzed by iodine. Meanwhile, N2, HOI and HI are released during this conversion process.8 Subsequently, the intermediate 5 was produced by the removal of H2O by reduction 6.9
Which then converted to the sulfur radical (RS˙) ether via direct radical cracking.10 Subsequently, the sulfur radical (RS˙) was added to the double bond of uracil to form the uracil free radical A. Then, The uracil free radical intermediate A could be oxidized by HOI to an uracil carbon cation B.3 At last, product 3 is obtained after the subsequent loss of a proton from B.11
Conclusions
In this study, we have successfully developed a solvent-free direct thioetherification reaction of sulfonylhydrazine with uracil. The reaction employs iodine as a catalyst and demonstrates high selectivity. The method is characterized by the absence of a metal catalyst and solvent, making it straightforward to execute. Furthermore, the reaction can be applied to a diverse array of substrates. Notably, the reaction yields 5-thioxopyrimidine derivatives in high quantities.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the Natural Science Foundation of China (no. 81560559), the Science and Technology Department of Guizhou Province (no. QKHSY[2015]3030), the Natural Science Research Fund of Guizhou Education Department (no. QJHKY[2021]161, QJHKY[2021]152), the Guizhou Provincial Natural Science Foundation [2020] no. 4Y237, the Science and Technology of Traditional Chinese Medicine and Ethnic Medicine in Guizhou Province QZYY-2023-105, the PhD Research Foundation of Guizhou Medical University (no. YBHJ[2014]029), the Qiankehejichu-ZK[2023]Yiban 288, the High-Level Innovative Talent Training Program “Ten” Level Talents of Guizhou Province, Platform Talent of Scientific Collaboration of Guizhou Province (No. GCC[2023]002).References
- (a) M. T. Nguyen, D. Moiani, Z. Ahmed, A. S. Arvai, S. Namjoshi, D. S. Shin, Y. Fedorov, E. J. Selvik, D. E. Jones, J. Pink, Y. Yan, D. J. Laverty, Z. D. Nagel, J. A. Tainer and S. L. Gerson, Prog. Biophys. Mol. Biol., 2021, 163, 143–159 CrossRef CAS PubMed; (b) D. Ramesh, B. G. Vijayakumar and T. Kannan, Eur. J. Med. Chem., 2020, 207, 112801 CrossRef CAS PubMed.
- (a) V. P. Gunasekaran, K. Nishi, D. Sivakumar, T. Sivaraman and G. Mathan, Eur. J. Pharm. Sci., 2018, 116, 2–14 CrossRef CAS PubMed; (b) P. W. Woodman, A. M. Sarrif and C. Heidelberger, Biochem. Pharmacol., 1980, 29, 1059–1063 CrossRef CAS PubMed; (c) O. N. Al Safarjalani, X. J. Zhou, R. H. Rais, J. Shi, R. F. Schinazi, F. N. Naguib and M. H. El Kouni, Cancer Chemother. Pharmacol., 2005, 55, 541–551 CrossRef CAS PubMed.
- (a) X. D. Li, Y. T. Gao, Y. J. Sun, X. Y. Jin, D. Wang, L. Liu and L. Cheng, Org. Lett., 2019, 21, 6643–6647 CrossRef CAS PubMed; (b) D. Beukeaw, M. Noikham and S. Yotphan, Tetrahedron, 2019, 75, 130537 CrossRef; (c) N. Probst, R. Lartia, O. Thery, M. Alami, E. Defrancq and S. Messaoudi, Chem.–Eur. J., 2018, 24, 1795–1800 CrossRef CAS PubMed.
- (a) M. Noikham and S. Yotphan, Eur. J. Org Chem., 2019, 2019, 2759–2766 CrossRef CAS; (b) F. Botha, M. Slavickova, R. Pohl and M. Hocek, Org. Biomol. Chem., 2016, 14, 10018–10022 RSC.
- F. Kopp and P. Knochel, Org. Lett., 2007, 9, 1639–1641 CrossRef CAS PubMed.
- (a) A. Jana, A. K. Panday, R. Mishra, T. Parvin and L. H. Choudhury, ChemistrySelect, 2017, 2, 9420–9424 CrossRef CAS; (b) G. Khalili, Mol. Diversity, 2016, 20, 963–968 CrossRef CAS PubMed; (c) J. Devi, N. Saikia, G. Choudhury and D. C. Deka, Tetrahedron Lett., 2021, 65, 152753 CrossRef CAS.
- (a) J. Zhang, Z. Wang, L. Chen, Y. Liu, P. Liu and B. Dai, RSC Adv., 2018, 8, 41651–41656 RSC; (b) W. Yueting, L. Yali, H. Jing, L. Xuezhen, L. Ping and Z. Jie, Tetrahedron, 2020, 76, 131646 CrossRef; (c) L. Chen, P. Liu, J. Wu and B. Dai, Tetrahedron, 2018, 74, 1513–1519 CrossRef CAS; (d) T. Sang, F. Jia, J. He, Y. Liu, J. C. Liu and P. Liu, J. Mol. Struct., 2023, 1292, 136165 CrossRef CAS; (e) P. J. L. Chen, P. Liu and B. Dai, J. Chem. Res., 2018, 42, 456–462 CrossRef.
- F. L. Yang and S. K. Tian, Angew. Chem., Int. Ed., 2013, 52, 4929–4932 CrossRef CAS PubMed.
- X. Kang, R. Yan, G. Yu, X. Pang, X. Liu, X. Li, L. Xiang and G. Huang, J. Org. Chem., 2014, 79, 10605–10610 CrossRef CAS PubMed.
- Y. Gao, Y. Gao, X. Tang, J. Peng, M. Hu, W. Wu and H. Jiang, Org. Lett., 2016, 18, 1158–1161 CrossRef CAS PubMed.
- Y. Zheng, Y. He, G. Rong, X. Zhang, Y. Weng, K. Dong, X. Xu and J. Mao, Org. Lett., 2015, 17, 5444–5447 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra07398j |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |