Open Access Article
Open Access ArticleAccelerated synthesis of 1,8-dioxo-octahydroxanthene and 1,8-dioxo-decahydroacridine derivatives using dendritic mesoporous nanosilica functionalized by hexamethylenetetramine: a novel nanocatalyst
Zahra Sabriab,
Nasrin Shadjou *ab and
Mehdi Mahmoudianab
*ab and
Mehdi Mahmoudianab
aDepartment of Nanotechnology, Faculty of Chemistry, Urmia University, Urmia, Iran. E-mail: n.shadjou@urmia.ac.ir; Tel: +98 44 32752741
bInstitute of Nanotechnology, Urmia University, Urmia, Iran
First published on 15th January 2024
Abstract
Xanthene and acridine derivatives are interesting organic compounds that are used in different research fields like biomedicine and pharmaceutical science. However, applied catalysts for their synthesis have some limitations such as long reaction times, the need for harsh conditions and low yield. So, discovery of novel catalysts for the synthesis of xanthene and acridine derivatives is highly demanded. To overcome the limitation of previous methods on the efficient synthesis of 1,8-dioxo-octahydroxanthene and 1,8-dioxo-decahydroacridine derivatives, a green heterogeneous organic nano-catalyst (Cu@KCC-1–nPr–HMTA) was synthesized by covalent attachment of hexamethylenetetramine to the cavities and channels of dendritic mesoporous nanosilica (KCC-1). The prepared nano-catalyst was identified using various spectroscopic and microscopic methods including scanning electron microscopy (SEM), Fourier transform infrared (FT-IR), X-ray energy diffraction (EDX), EDX mapping and nitrogen adsorption–desorption analysis (BET-BJH). The prepared green nano-catalyst showed a spherical and dendritic structure with a surface area of 65.699 m2 g−1, average pore size of 40.78 nm and pore volume of 0.66 cm3 g−1. Also, Cu@KCC-1–nPr–HMTA has many chemo-active sites for the condensation reaction and was used as an efficient nano-catalyst towards one-step synthesis of 1,8-dioxo-decahydroacridine and 1,8-dioxo-octahydroxanthene derivatives from the reaction of aromatic aldehydes, dimedone, and ammonium acetate under solvent-free conditions. Short reaction times of 1 to 5 minutes for 1,8-dioxo-decahydroacridine and 30 to 55 minutes for 1,8-dioxo-octahydroxanthene derivatives, high yields and mild reaction conditions are advantages of the proposed synthetic method. It is hoped that the engineered nano-catalyst will be used for the synthesis of other organic compounds in the future.
1. Introduction
Xanthenes are an important class of heterocyclic compounds that have shown a wide range of pharmacological properties such as antiviral,1 anti-cancer,2,3 antibacterial, antifungal,4,5 antiplasmodial,6 antinociceptive,7 anti-inflammatory,8 and DNA binding.9 Due to the specific structure of xanthene, these compounds have been used in laser technology,5,10 fluorescent materials for visualization of biomolecules,11 cosmetics and pigments.12Reaction of aldehydes with dimedone in the presence of various catalysts such as ionic liquids, silica sulfuric acid, resins, para dodecyl benzene sulfonic acid, sodium dodecyl sulfate, zinc oxide, acetyl chloride, zirconium, para toluene sulfonic acid, etc., has been reported for the synthesis of xanthenes.13–21 However, some of these methods have long reaction times, harsh conditions and undesirable yields. So, optimization of the methods for the efficient synthesis of xanthenes has been widely demanded by chemists.22–24
On the other hand, acridine derivatives are a nitrogen-containing heterocyclic organic compound with important biological activities25 such as antimalarial26 and chemotherapy of cancer.27 1,8-Dioxodihydroacridine derivatives can be prepared using quasi-four-component reaction between dimedone, aromatic aldehyde and amine or ammonium acetate in solvent-free thermal conditions in the presence of various catalysts.28–37 Advanced nanomaterials play an important role as heterogeneous active catalysts for the synthesis of organic compounds because of easy access to internal surfaces and active functional groups that are located inside the cavities and channels of the them.38
Recently, dendritic nanoparticles with homocentric radial cavities act as a very useful catalytic substrate, which creates an open three-dimensional structure that to block active surfaces in these catalysts impossible and this increases the catalytic activity of these nanostructure. Polshettiwar and co-workers39,40 reported a new nano-silica compound (KCC-1) with spherical, dendritic, fibrous and controlled morphology with novel physicochemical properties. These nanomaterials have been utilized for the synthesis of pharmaceutical compounds, photo-catalysts and superabsorbent for the removal of heavy metals, gas absorption, solar cells, self-cleaning materials, sensitive sensors, drug delivery and biomedical applications. Due to high surface area and accessible channels and pores, dendritic fibrous nanosilica (DFNS) is highly demanded as an advanced nanostructure for the technological approaches like organic synthesis. Also, the ability to loading various guest molecules, reactants, and solvents into their internal surface and create the available active catalytic centers, are the main advantages of these nanomaterials in different research areas.40–42
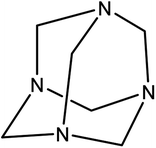
Hexamine,43 also known as methamine and hexamethylenetetramine (HMTA) (Scheme 1), is a white and biocompatible crystalline heterocyclic organic compound that is highly soluble in water and polar organic solvents. This flammable solid has a cage-like structure and it has been used in the synthesis of some chemical compounds like plastic, pharmaceuticals and rubber additives. In addition, HMTA has free and readily available amino groups which can be easily participate in substitution reactions. Hence, the modified nanostructures with this functional group are chemical active compound.44
Due to the importance of this functional group, Noori et al.,45 investigated the heterogeneous catalytic properties of hexamethylenetetramine functionalized magnetic silica in the N-benzylation of primary amines. Also, Ghorbani-Vaghei and Izadkhah44 prepared hexamethylenetetramines functionalized magnetic silica towards efficient synthesis of pyranopyrazole derivatives with high yields and short reaction time.
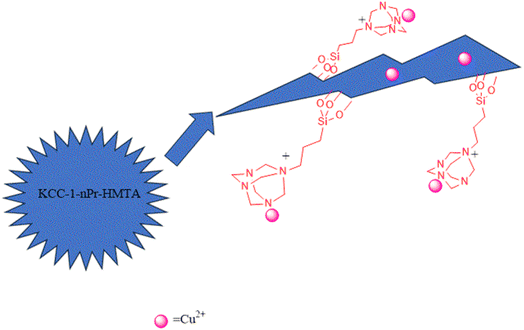
Considering the importance of xanthene and acridine derivatives and the scope of their application in various fields, we decided to propose a new method for the synthesis of these compounds in the presence of non-toxic, inexpensive, high-performance, and new nano-catalyst, by one-pot reaction of aromatic aldehydes, dimedone and ammonium acetate under solvent-free conditions. Dendritic nanoparticles have fibrous structure with three-dimensional radial channels which lead to the efficient synthesis of pharmaceutically important compounds. So, based on the openness of nanochannels, presence of accessible interior spaces and high volume of cavities, advanced nanomaterials are interesting for the chemists. Hence, in this research, hexamethylenetetramines along with copper ions were attached to the walls and channels of KCC-1 and its catalytic properties was studied in the synthesis of 1,8-dioxo-octahydroxanthene and 1,8-dioxo-decahydroacridine derivatives. The structure of functionalized catalyst (Cu@KCC-1–nPr–HMTA) is shown in Scheme 2.
2. Experimental
2.1. Materials and reagents
Cetyltrimethylammonium bromide (CTAB), HMTA, ammonium acetate, aromatic aldehydes, dimedone, and cyclohexane were purchased from Merck, Germany. CTAB was used as surfactant during the synthesis of KCC-1. Tetraethyl orthosilicate (TEOS) and (3-chloropropyl)triethoxysilane were bought from Sigma-Aldrich. Also, DMF, toluene, and hexanol were purchased from Sigma-Aldrich. Deionized water was produced in laboratory. No further purification was performed on materials and reagents and they were used as-purchased.2.2. Instruments
Philip Harris C4954718 was used to melting point measurement. Fourier transform infrared (FT-IR), spectras were obtained by Thermo-Nicolet Nexus 670 instrument. Thin layer chromatography (TLC) on Merck's silica plates were used to the monitoring of the reaction progress. The Field Emission Scanning Electron Microscopy (FE-SEM) images and Energy Dispersive X-ray (EDX) were recorded with FEG-SEM MIRA3 TESCAN, Czech Republic at 1000 kV. Brunauer–Emmett–Teller (BET) was recorded by Micromeritics NOVA 2000 (Florida, USA) apparatus at 77 K using nitrogen as the adsorption gas.2.3. Synthesis process KCC-1
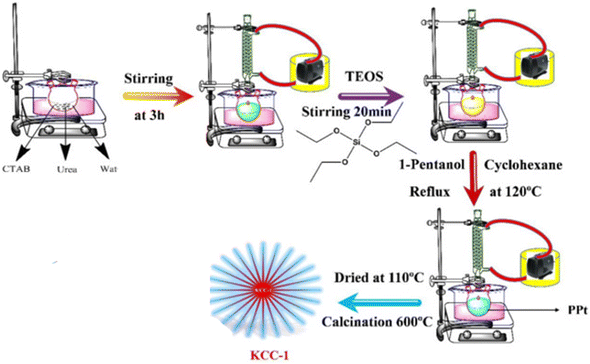
In this study, KCC-1 (bare dendritic fibrous nano-silica) was synthesized according to our previous report.46 For this purpose, CTAB was dissolved in 10 ml of distilled water and then 0.6 g of urea was added to this solution at room temperature and the mixture was stirred by stirrer for 3 hours to dissolve the whole CTAB. In the next step, 2.3 g of TEOS as a precursor of KCC-1, 80 ml cyclohexane and 1.3 ml hexanol added to the above-mentioned solution. The mixed solution was placed at room temperature for 80 minutes under sonication, and then it was refluxed at 120 °C. After this step, the mixture was cooled to room temperature and KCC-1 was collected as bare DFNS. The nano-silica was washed three times with distilled water and twice with acetone which is necessary for its purification. The product was dried at room temperature. Finally, to remove the template, the KCC-1 was calcined in 550 °C/6 h. It is important to point out that CTAB was used as a template in the KCC-1 synthesis process by the formation of reverse micelle and urea is used for hydrolysis and condensation of orthosilicate precursor. Fig. 1 shows the synthesis procedure of KCC-1.2.4. Functionalization of KCC-1 with n-propyl chloride functional group
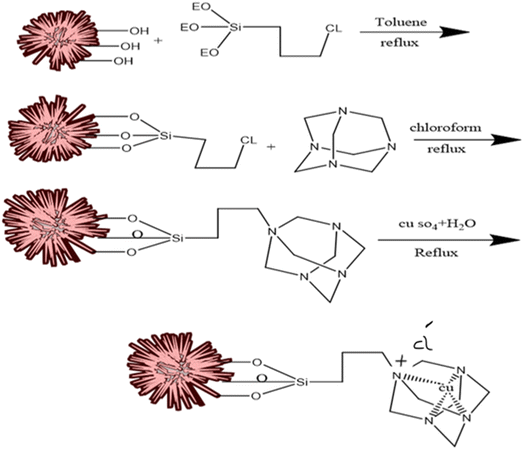
In this process, 0.02 g of KCC-1 was dispersed in 1.2 ml toluene and was exposed to ultrasound for 30 min. In the next step, 30 μl of 3-chloropropyltriethoxysilane was added to the solution and refluxed at 110 °C for 20 hours. Then, the modified KCC-1 was separated by centrifuge. It was washed with toluene several times and finally dried at 30 °C for 21 hours.2.5. Functionalization of KCC-1–nPr–Cl with HMTA
At first, 0.1 g of KCC-1–nPr–Cl were added to the 5.2 ml of chloroform and then, HMTA was added to 2.1 ml chloroform and completely dissolved. Then, the mixture was refluxed for 5 hours. Finally, the resulting precipitate was separated by centrifuge and washed several times with distilled water and chloroform toward its purification. The desired sediment was dried at 60 °C in oven.2.6. Doping of copper ions into the structure of KCC-1–nPr–HMTA
In the process of copper-doping on the structure of KCC-1–nPr–HMTA, the 5 g of the prepared nano-catalyst was added to the 1 M of copper sulfate solution with a volume of 25 ml (1 M in deionized water) and stirred for 15 h at 60 °C. Then, the obtained uniform mixture was filtered by centrifuge and the resulting sediment was dried in vacuum condition at room temperature. Finally, the blue powder was obtained as final nano-catalyst. Scheme 3 shows the schematic procedure of Cu@KCC-1–nPr–HMTA.2.7. General procedure of 1,8-dioxo-octahydroxanthene and 1,8-dioxo-decahydroacridine derivatives
The designed nano-catalyst Cu@KCC-1–nPr–HMTA (0.02 g) was applied for the synthesis of 1,8-dioxo-octahydroxanthene and 1,8-dioxo-decahydroacridine derivatives from the reaction of aromatic aldehydes (1 mmol), dimedone and ammonium acetate (1 mmol) under solvent free conditions at 120 °C. Also, reaction process was explored by TLC (ethyl acetate/diethyl ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). After completion of the reaction, hot ethanol was added to the reaction mixture and the nano-catalyst was separated. The obtained products were collected and then recrystallized in EtOH (Scheme 4).
2). After completion of the reaction, hot ethanol was added to the reaction mixture and the nano-catalyst was separated. The obtained products were collected and then recrystallized in EtOH (Scheme 4).
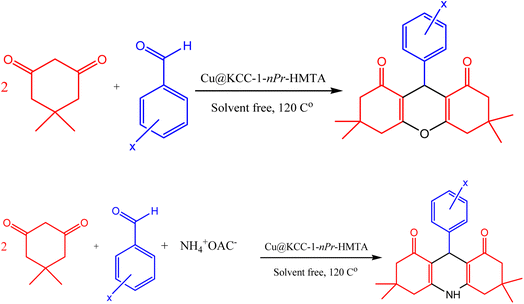 | ||
| Scheme 4 Synthesis procedure of 1,8-dioxo-octahydroxanthene and 1,8-dioxo decahydroacridine derivatives in the presence of Cu@KCC-1–nPr–HMTA. | ||
3. Results and discussion
3.1. Characterization of Cu@KCC-1–nPr–HMTA
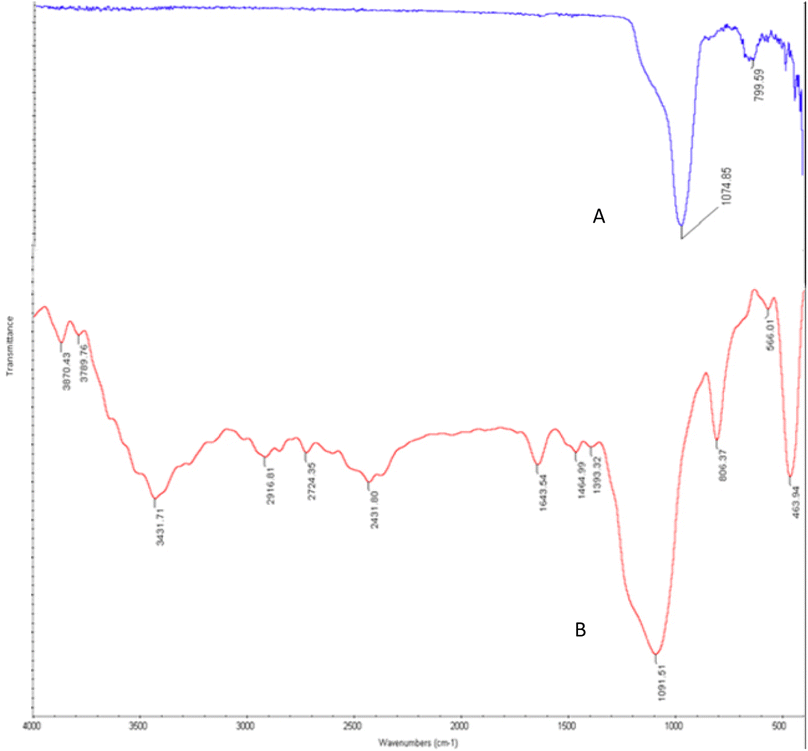
The morphology and chemical composition of functionalized and engineered nano-catalyst have been identified with a variety of spectroscopic and microscopic analyses including FE-SEM, FT-IR, EDX, mapping, and BET-BJH methods.The FT-IR spectrum of KCC-1 and Cu@KCC-1–nPr–HMTA are presented and compared in Fig. 2. KCC-1 has a sharp peak in 1074 cm−1 and 799 cm−1 which is related to the unsymmetric and symmetric Si–O–Si stretching, respectively (Fig. 2A).
The FT-IR spectra of KCC-1–nPr–HMTA was shown in Fig. 2B. The peak in 3431 cm−1 is related to the OH group in KCC-1. Also, the peaks in 2916 cm−1, 2724 cm−1 are related to the stretching vibration of aliphatic C–H in functional group which attached to the pores of KCC-1. Also, the peak in 1393 cm−1 is related to C–N bonds.
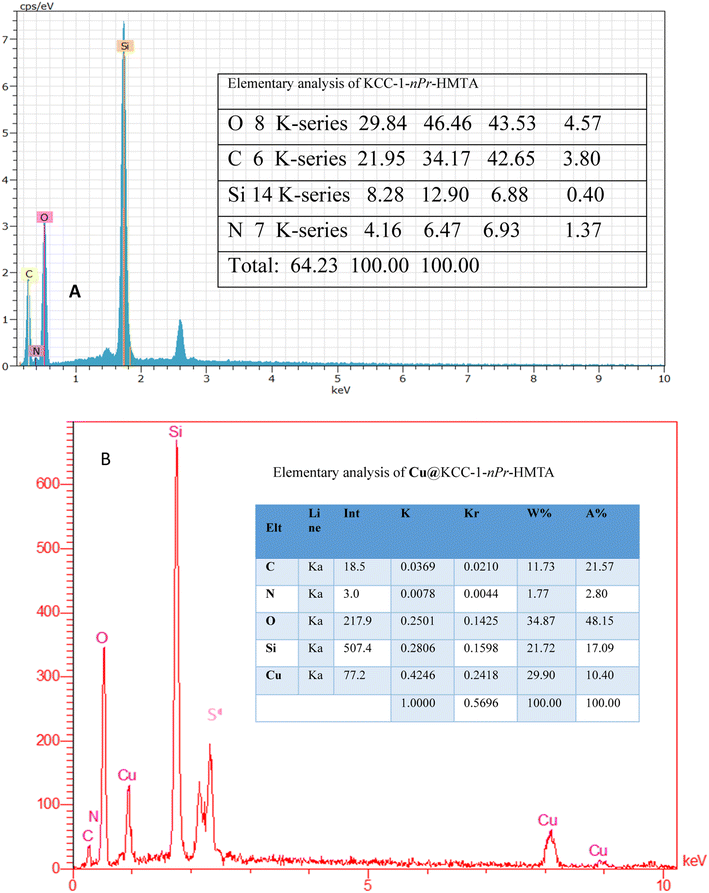
Elemental analysis of KCC-1–nPr–HMTA and Cu@KCC-1–nPr–HMTA was evaluated using EDX method which is shown in Fig. 3. These elemental analyses confirm the presence of N, C, Si, O, and Cu and confirmed the functionalization of KCC-1 with hexamethylenetetramine functional group and successful loading of copper cation on the structure of prepared nano-catalyst.
Also, mapping images of KCC-1–nPr–HMTA confirmed all of the obtained results by EDX method. As shown in Fig. 4, the scattering elements in the functionalized KCC-1 is including carbon, nitrogen, silicon and oxygen which shows successful functionalization of the KCC-1–nPr–Cl with HMTA.
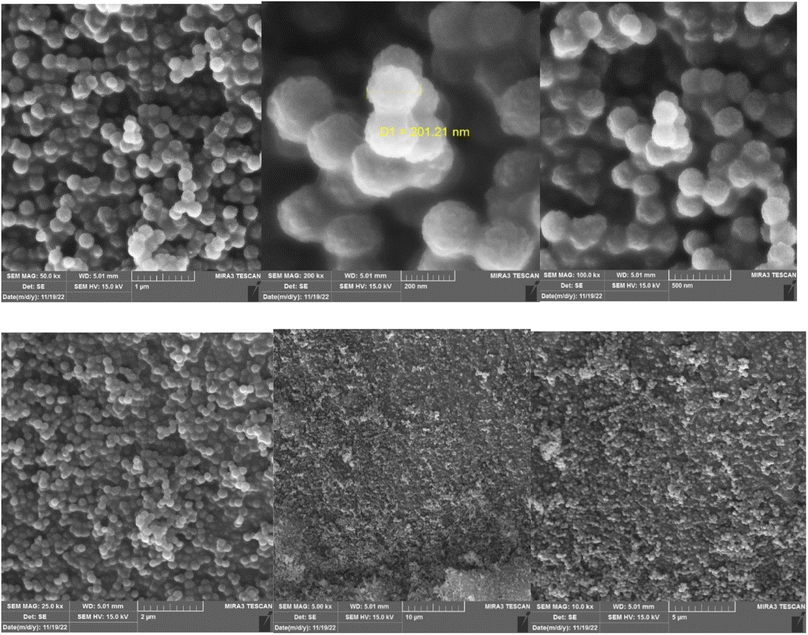
Using FE-SEM analysis, the morphology and surface properties of prepared nano-catalyst was surveyed. The spherical KCC-1–nPr–HMTA has fibrous and wrinkled structure (Fig. 5). Also, a relatively balanced distribution of size can be seen in synthesized nanoparticles.
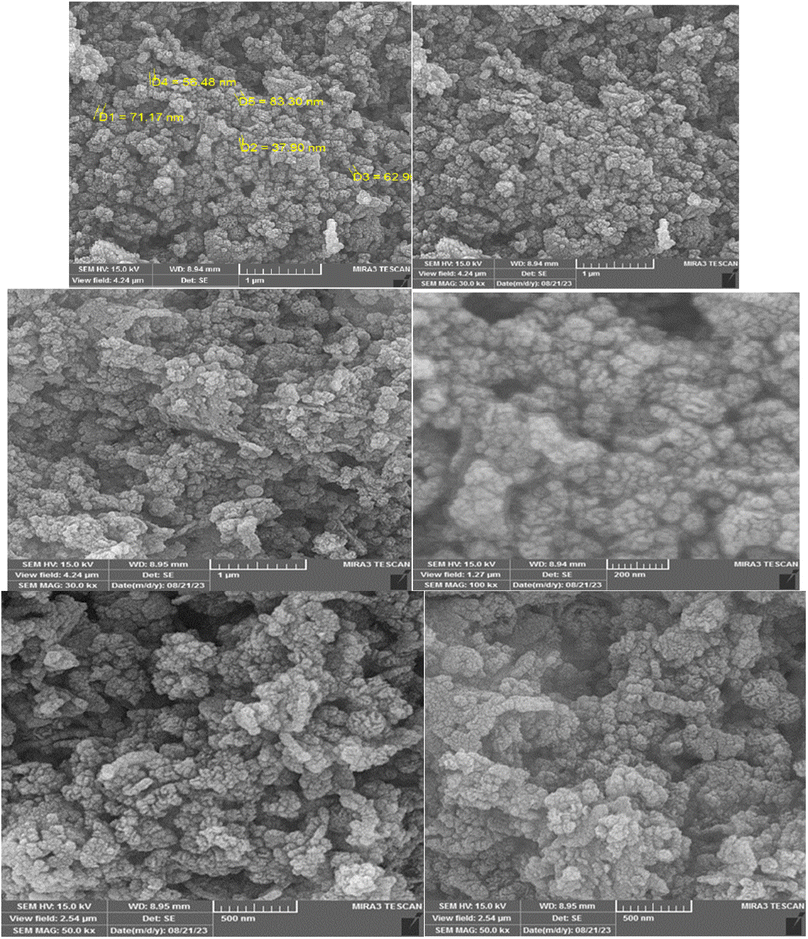
Also, the FE-SEM images of Cu@KCC-1–nPr–HMTA has been shown in Fig. 6. The spherical, fibrous and dendritic morphology of functionalized KCC-1 are obvious in all of images that recorded in different magnifications.
The results of nitrogen adsorption–desorption analysis of KCC1–nPr–HMTA were indicated in Fig. 7 and surface area and pore volume of this catalyst was compared with KCC-1 and KCC-1–nPr–Cl in Table 1 which shows a significant decrease in surface area and pore volume of Cu@KCC-1–nPr–HMTA than KCC-1 and KCC-1–nPr–Cl. So, it is confirmed the successful functionalization of KCC-1 by HMTA. The reason for the increase in the mean size of the channels can be related to the filling of channels from the central part of the KCC-1 spheres. They are pulled out of the center where the average size of the cavities is increased by functionalization. Also, according to Fig. 7, it's obvious that KCC1–nPr–HMTA is a mesoporous nano-silica with dendritic structure.
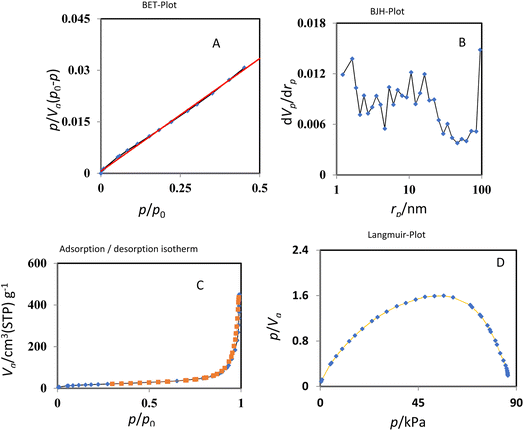 | ||
| Fig. 7 BET (A), BJH (B), absorption and desorption isotherms (C), and Langmuir-plot (D) of KCC1–nPr–HMTA. | ||
| Sample | Surface area (m2 g−1) | Average pore size (nm) | Pore volume (cm3 g−1) |
|---|---|---|---|
| KCC-1 | 617 | 9.9 | 1.5 |
| KCC-1–nPr–Cl | 386 | 12.4 | 1.1 |
| KCC-1–nPr–HMTA | 65.699 | 40.781 | 0.66 |
After characterization and confirm the successful synthesis of Cu@KCC-1–nPr–HMTA, its application as nano-catalyst for the efficient synthesis of 1,8-dioxo-octahydroxanthene and 1,8-dioxo-decahydroacridine derivatives were surveyed. For this purpose, the reaction of dimedone (2 mmol) and 4-chlorobenzaldehyde (1 mmol) was explored in the presence of KCC-1–nPr–HMTA (0.02 g) in ethanol (5 ml) as solvent. Also, the reaction process was investigated using TLC. The reaction was completed within 25 minutes. But the results of melting point and FT-IR analysis of the product shown that the production of the reaction is a tetraketone (2,2′-((4-chlorophenyl)methylene)bis(3-hydroxy-5,5-dimethylcyclohex-2-enone)) (Scheme 5).
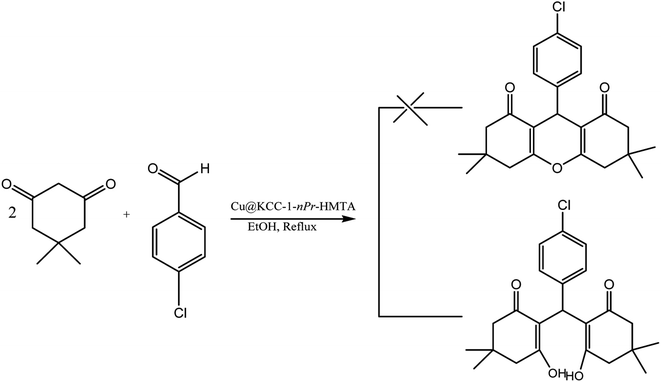 | ||
| Scheme 5 Synthesis of tetraketone in the presence of ethanol as solvent and under reflux conditions. | ||
FT-IR spectra of prepared tetraketone is shown in the Fig. 8. The presence of broad peak in 3447 cm−1 corresponds to the OH functional group confirms the formation of tetraketone product in this reaction. Also, the peak appeared in the 2957 cm−1 region is related to the stretching vibration of C–H and the peak in the 1489 cm−1 region is related to stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C. In addition, the peak observed in the 2871 cm−1 region is related to the stretching vibration of aliphatic C–H. Importantly, the peak in the 1596 cm−1 region corresponds to the stretching vibration of C
C. In addition, the peak observed in the 2871 cm−1 region is related to the stretching vibration of aliphatic C–H. Importantly, the peak in the 1596 cm−1 region corresponds to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and the peak in the 1253 cm−1 region indicate the stretching vibration of C–O bond that the resonance between oxygen and the double bond causes the peak absorption appear at lower frequency. Although the tetraketone was synthesized with high yield, but it is not our desired product, and the aim of our research was the synthesis of 1,8-dioxo-octahydroxanthene and 1,8-dioxo-decahydroacridine derivatives through one-pot three-component reaction of aromatic aldehydes, dimedone and ammonium acetate in the presence of designed nano-catalyst.
O and the peak in the 1253 cm−1 region indicate the stretching vibration of C–O bond that the resonance between oxygen and the double bond causes the peak absorption appear at lower frequency. Although the tetraketone was synthesized with high yield, but it is not our desired product, and the aim of our research was the synthesis of 1,8-dioxo-octahydroxanthene and 1,8-dioxo-decahydroacridine derivatives through one-pot three-component reaction of aromatic aldehydes, dimedone and ammonium acetate in the presence of designed nano-catalyst.
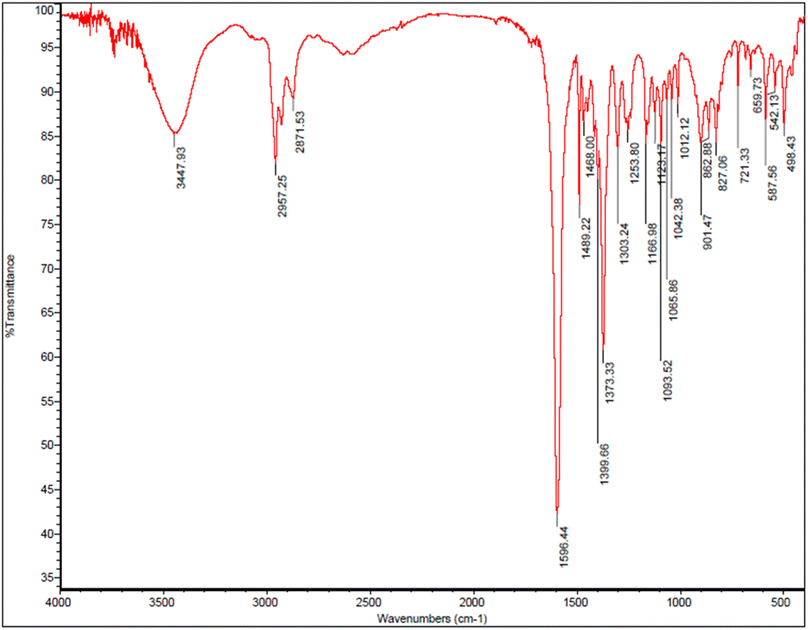 | ||
| Fig. 8 FT-IR spectra of tetraketone (2,2′-((4-chlorophenyl)methylene)bis(3-hydroxy-5,5-dimethylcyclohex-2-enone)). | ||
In this section, one-pot three-component reaction of dimedone (2 mmol) and 4-chlorobenzaldehyde (1 mmol) was investigated in the presence of nano-catalyst (KCC-1–nPr–HMTA), under solvent free condition and 120 °C. The reaction completed in 35 minutes with 98% efficiency.
Due to the FT-IR spectra of a sample a peak appeared in 2956 cm−1 is related to the C–H stretching vibration of C–H groups and the peaks in the range before 2956 cm−1 corresponds to the stretching vibration of aliphatic C–H groups (Fig. 9). Also, a peak appearing in 1578 cm−1 and 1490 cm−1 are related to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and the peak appeared in 1604 cm−1 is corresponds to the stretching vibration of C
C and the peak appeared in 1604 cm−1 is corresponds to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional group. In addition, the peak located in the 1245 cm−1 indicate the stretching vibration of C–O bond, which the resonance between oxygen and double bond caused to be appeared at lower frequency. Finally, the lack of broad peak of hydroxy functional group in the region 3447 cm−1 confirms the synthesis of 9-(4-chlorophenyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione as product in this reaction.
O functional group. In addition, the peak located in the 1245 cm−1 indicate the stretching vibration of C–O bond, which the resonance between oxygen and double bond caused to be appeared at lower frequency. Finally, the lack of broad peak of hydroxy functional group in the region 3447 cm−1 confirms the synthesis of 9-(4-chlorophenyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione as product in this reaction.
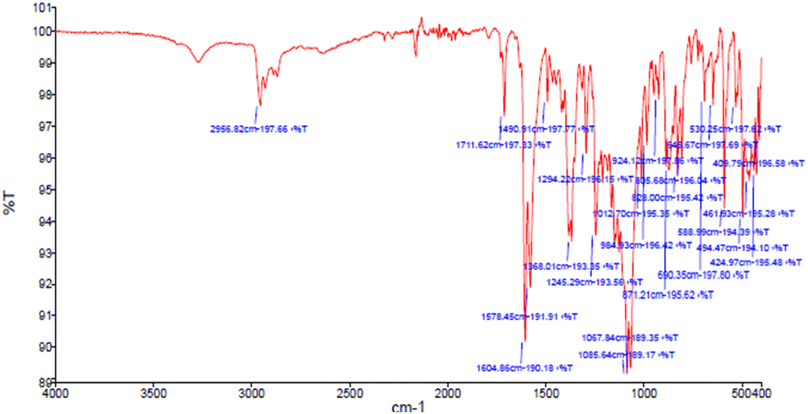 | ||
| Fig. 9 FT-IR spectra of 9-(4-chlorophenyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione. | ||
The important point in this study is the effective role of copper cation as an active center in catalytic activity. At first, in order to optimization of the reaction and investigate the role of basic functional group (HMTA) in the reaction process, different types of unfunctionalized substrate (KCC-1), chloropropyl functionalized KCC-1 (KCC-1–nPr–Cl) and HMTA functionalized KCC-1 (KCC-1–nPr–HMTA) were also used in reaction of dimedone (2 mmol) and 4-chlorobenzaldehyde (1 mmol) under solvent-free condition at 120 °C and in the presence of 0.02 g of nano-catalyst. The results showed that, in the presence of KCC-1 and KCC-1–nPr–Cl the reaction time was long (130 min) and the product with low yield (10%) was obtained (entries 1 and 2, Table 2). But in the presence of 0.02 g of the designed nano-catalyst (KCC-1–nPr–HMTA), the yield of the product was 90% and reaction time was reduced to 60 min (entry 3, Table 2). After loading the copper ions in the channels and pores of the catalyst (KCC-1–nPr–HMTA) and applying it in the synthesis of 1,8-dioxo-octahydroxanthene derivatives, the product was obtained in the lowest time (30 min) with the highest yield (98%) under solvent-free conditions (entry 4, Table 2). According to the results indicated in Table 2, the use of copper cation plays a significant role in increasing the yield of the reaction and shortens the reaction time. Therefore, Cu@KCC-1–nPr–HMTA (0.02 g) was selected as the optimal catalyst in the reaction of 1,8-dioxo-octahydroxanthene derivatives.
| Entry | Catalyst | Temperature (°C) | Time (min) | Yield (%) |
|---|---|---|---|---|
| 1 | KCC-1 (0.02 g) | 120 | 130 | 10 |
| 2 | KCC-1–nPr–Cl (0.02 g) | 120 | 130 | 10 |
| 3 | KCC-1–nPr–HMTA (0.02 g) | 120 | 60 | 90 |
| 4 | Cu@KCC-1–nPr–HMT (0.02 g) | 120 | 30 | 98 |
Also, different solvents such as ethanol, water/ethanol and dimethylformamide were used to investigate their effect in the time and yield of the reaction. According to the obtained results, (entry 1, Table 3), when the reaction of dimedone (2 mmol) and 4-chlorobenzaldehyde (1 mmol) was done in the presence of Cu@KCC-1–nPr–HMTA (0.02 g) and ethanol as a solvent under reflux conditions, the yield of reaction product was about 80% in 25 minutes. However, after crystallization of the obtained product, the formation of tetraketone was confirmed by IR spectra and melting point.
| Entry | Catalyst | Solvent | Temperature (°C) | Time (min) | Yield (%) |
|---|---|---|---|---|---|
| 1 | Cu@KCC-1–nPr–HMTA (0.02 g) | Ethanol | Reflux | 25 | 80 |
| 2 | Cu@KCC-1–nPr–HMTA (0.02 g) | Water and ethanol | Reflux | 90 | — |
| 3 | Cu@KCC-1–nPr–HMTA (0.02 g) | DMF (2 drops) | 70 | 70 | 60 |
| 4 | Cu@KCC-1–nPr–HMTA (0.025 g) | DMF (2 drops) | 120 | 40 | 60 |
| 5 | KCC-1–nPr–HMTA (0.02 g) | No solvent | 120 | 60 | 90 |
| 6 | KCC-1–nPr–HMTA (0.01 g) | No solvent | 120 | 90 | 90 |
| 7 | Cu@KCC-1–nPr–HMTA (0.02 g) | No solvent | 120 | 30 | 98 |
| 8 | No catalyst | No solvent | 120 | 60 | — |
In the next step, the yield of the reaction between dimedone (2 mmol) and 4-chlorobenzaldehyde (1 mmol) was negligible in the presence of Cu@KCC-1–nPr–HMTA (0.02 g) as catalyst and water/ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as solvent under reflux conditions in 90 minutes (entry 2, Table 3). Continuously, when DMF was used as solvent at 70 °C (entry 3, Table 3), the product of the reaction was obtained in 70 minutes with 60% yield. In the next step, the same reaction was investigated in DMF as solvent in the presence of Cu@KCC-1–nPr–HMTA as catalyst at 120 °C (entry 4, Table 3). The results showed that the reaction product was obtained in 40 min with 60% efficiency. In the next step, the same reaction was obtained in the presence of 0.02 g of KCC-1–nPr–HMTA as catalyst under solvent-free conditions at 120 °C (entry 5, Table 3). The results of TLC showed that the product was obtained in 60 min with 90% yield. After crystallization of obtained product in ethanol, the 1,8-dioxo-octahydroxanthene derivatives were confirmed by FT-IR spectroscopy and determination of melting point.
1) as solvent under reflux conditions in 90 minutes (entry 2, Table 3). Continuously, when DMF was used as solvent at 70 °C (entry 3, Table 3), the product of the reaction was obtained in 70 minutes with 60% yield. In the next step, the same reaction was investigated in DMF as solvent in the presence of Cu@KCC-1–nPr–HMTA as catalyst at 120 °C (entry 4, Table 3). The results showed that the reaction product was obtained in 40 min with 60% efficiency. In the next step, the same reaction was obtained in the presence of 0.02 g of KCC-1–nPr–HMTA as catalyst under solvent-free conditions at 120 °C (entry 5, Table 3). The results of TLC showed that the product was obtained in 60 min with 90% yield. After crystallization of obtained product in ethanol, the 1,8-dioxo-octahydroxanthene derivatives were confirmed by FT-IR spectroscopy and determination of melting point.
In order to achieve the optimum catalytic amount, the same experiment was carried out with the same conditions and the same reagents for xanthene synthesis in the presence of KCC-1–nPr–HMTA catalyst (entry 6, Table 3). The reaction was completed under solvent-free conditions and at 120 °C in 90 min with 90% efficiency and after crystallization of xanthene, the product was detected by FT-IR spectroscopy and its melting point was determined. Then, for the reaction of dimedone (2 mmol) and 4-chlorobenzaldehyde (1 mmol) for preparation of xanthene product in the presence of (0.01 g) KCC-1–nPr–HMTA, the reaction time was 90 min with 90% efficiency under solvent-free conditions and 120 °C. In the following, by loading copper ions in the structure of designed nano-catalyst (Cu@KCC-1–nPr–HMTA), the product was obtained in the shortest time (30 min) with the highest efficiency (98%) in solvent-free conditions (entry 7, Table 3). In the last step, the reaction was carried out at 120 °C under solvent-free conditions in the presence of catalyst. The reaction was completed in 60 min with negligible efficiency (entry 8, Table 3). Based on the obtained results indicated in Table 3, it can be concluded that solvent-free conditions are a green method and an appropriate option for advancing the reaction. On the other hand, costs will be reduced and we will have a green reaction that will not harm the environment. Accordingly, the above conditions play an effective role in this reaction. Therefore, 0.02 g of Cu@KCC-1–nPr–HMTA catalyst and solvent-free conditions and 120 °C were selected as optimum reaction conditions.
According to the data obtained from the condensation reaction of dimedone and aromatic aldehydes using the newly synthesized nano-catalyst, 1,8-dioxo-octahydroxanthene derivatives were produced with excellent yield and in a short reaction time. The products were synthesized in solvent-free conditions and within 30 to 45 minutes without by-products (Table 4).
| Entry | Products | Time (min) | Yield (%) | Melting point (reported) | Melting point (°C) | Ref. |
|---|---|---|---|---|---|---|
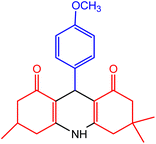
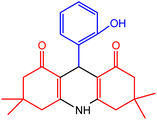
| 1 | 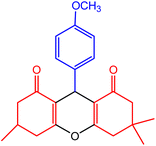 |
50 | 95 | 242–245 | 243–245 | 47 |
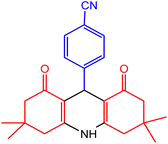
| 2 | 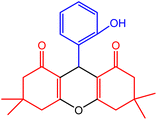 |
45 | 80 | 231–233 | 230–233 | 48 |
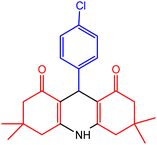
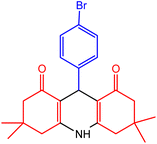
| 3 | 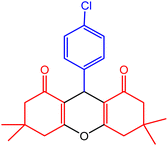 |
30 | 98 | 230–232 | 228–230 | 47 |
| 4 | 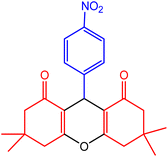 |
50 | 98 | 221–223 | 219–222 | 49 |
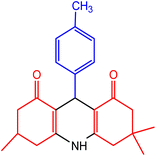
| 5 | 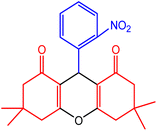 |
55 | 90 | 248–249 | 246–248 | 49 |
| 6 | 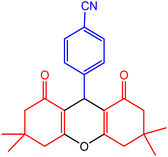 |
30 | 95 | 217–218 | 217–218 | 50 |
| 7 | 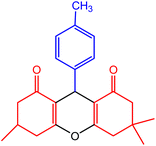 |
40 | 95 | 219–221 | 215–221 | 50 |
| 8 | 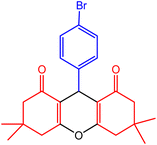 |
32 | 90 | 240–242 | 240–242 | 50 |
The Scheme 6 shows the mechanism for the synthesis of 1,8-dioxo-octahydroxanthene derivatives in the presence of Cu@KCC-1–nPr–HMTA as nano-catalyst. Loading of copper ions as a Lewis acid in the designed nano-catalyst have a significant impact on the catalytic performance. In the early stages of the reaction, reactants interact with copper ion as Lewis acid and HMTA as a basic group in the channels and pores of designed nano-catalyst. The copper ions interact with carbonyl of aromatic aldehydes and dimedone. Hence, the carbonyl groups are activated and the H-hydrogen that present in dimedone structure which becomes more acidic. It accelerates the Knoevenagel condensation between carbonyl of aromatic aldehydes and activated carbon of dimedone and led to formation of intermediate 1. Then, through Michael's addition, the other dimedone that has the catalyst-activated carbonyl group, is attached to the intermediate 1 and finally the product was synthesized. So, the reaction efficiency is increased and the synthesis time is shortened. Similar results were obtained by previous reports.17,51,52
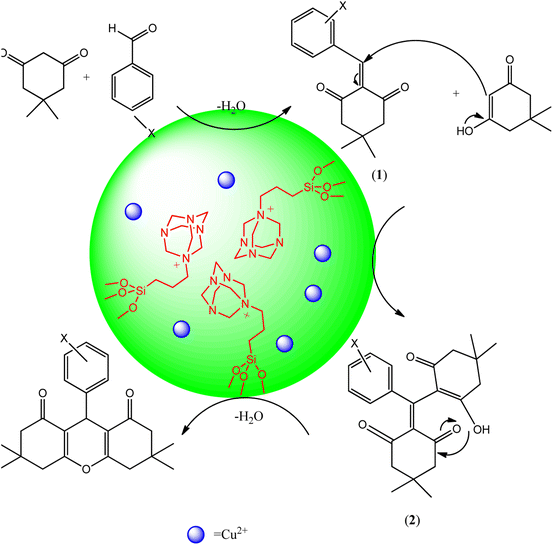 | ||
| Scheme 6 Synthesis mechanism of 1,8-dioxo-octahydroxanthene derivatives in the presence of Cu@KCC-1–nPr–HMTA as green nano-catalyst. | ||
In this section, the results of synthesis of 1,8-dioxo-octahydroxanthene derivatives are compared with other synthetic methods presented in the previous reports that indicated in the Table 5. High yields (80–98%) and short reaction times (30 to 55 min) are important advantages of designed nano-catalyst. These factors facilitate the synthesis of 1,8-dioxo-octahydroxanthene derivatives and highly efficient production.
| Catalyst | Solvent | Temperature (°C) | Time (min) | Yield (%) | Ref. |
|---|---|---|---|---|---|
| [Bmim]HSO4 | No solvent | 80 | 180 | 85 | 13 |
| Boric acid | No solvent | 20 | 120 | 98 | 53 |
| CuO NPs | No solvent | 100 | 14 | 89 | 54 |
| HClO4–SiO2 | No solvent | 140 | 180 | 90 | 33 |
| Fe3O4@SiO2@KCC-1@MPTMS@Cu | No solvent | 110 | 70 | 95 | 55 |
| [SO3H–pyrazine–SO3H]Cl | No solvent | 110 | 35 | 93 | 56 |
| [TMXH]FeCl | No solvent | 110 | 10 | 92 | 57 |
| Fe–X | No solvent | 90 | 15 | 95 | 58 |
| Cu@KCC-1–nPr–HMTA | No solvent | 120 | 30 | 98 | This work |
Due to the appropriate activity of the designed catalyst (Cu@KCC-1–nPr–HMTA) for the one-step synthesis of 1,8-dioxo-octahydroxanthene derivatives, we decided to investigate the reaction of dimedone, aromatic aldehydes and ammonium acetate salt for the synthesis of 1,8-dioxo-decahydroacridine derivatives in the presence of designed nano-catalyst. For this purpose, the reaction of dimedone (2 mmol), 4-chlorobenzaldehyde (1 mmol) and ammonium acetate (1 mmol) was tested in the presence of 0.02 g of catalyst (Cu@KCC-1–nPr–HMTA) and ethanol/water as solvent under reflux conditions. Also, the reaction process was investigated using TLC. Under these conditions, the reaction efficiency was negligible (Table 6, entry 1). Then, this reaction was tested in the presence of 0.02 g catalyst and ethanol as solvent under reflux conditions. After 120 minutes the reaction yield was 80% (Table 6, entry 2). In the following the reaction of dimedone (2 mmol), 4-chlorobenzaldehyde (1 mmol), and ammonium acetate (0.1 g) in the presence of 0.02 g catalyst, solvent-free conditions and 120 °C was investigated. The results showed that under these conditions and within 2 minutes, the reaction product was achieved with 98% of yield (Table 6, entry 3). In the last step, to compare the performance of the designed catalyst, the same reaction was investigated under solvent-free and without catalyst at 110 °C. Longer reaction time (60 minutes) and lower yield was associated with the results (Table 6, entry 4). Therefore, solvent-free conditions, temperature of 120 °C and 0.02 g of catalyst were selected as optimum conditions for the synthesis of 1,8-dioxo-decahydroacridine derivatives.
| Entry | Catalyst | Solvent | Temperature (°C) | Reaction time (min) | Yields% |
|---|---|---|---|---|---|
| 1 | Cu@KCC-1–nPr–HMTA (0.02 g) | Ethanol and water | Reflux, 70 | 50 | — |
| 2 | Cu@KCC-1–nPr–HMTA (0.02 g) | Ethanol | Reflux, 70 | 120 | 80 |
| 3 | Cu@KCC-1–nPr–HMTA (0.02 g) | Solvent free | 120 | 2 | 98 |
| 4 | No catalyst55 | Solvent free | 110 | 60 | 70 |
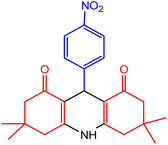
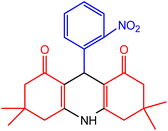
In order to synthesis of 1,8-dioxo-decahydroacridine derivatives, aldehydes with electron-donating groups such as methoxy, methyl, hydroxy and aldehydes with electron-withdrawing groups such as chloro- and nitro-groups on aromatic ring were used for the reaction. The data presented in Table 7 show that in the presence of nano-catalyst and under solvent-free conditions, 1,8-dioxo-decahydroacridine derivatives were synthesized in very short reaction times (1 to 5 minutes) with high yields without by-products.
The proposed mechanism of 1,8-dioxo-decahydroacridine synthesis in the presence of dendritic nanosilica Cu@KCC-1–nPr–HMTA is shown in Scheme 7. The copper which doped in the designed nano-catalyst acts as a Lewis acid and has a significant effect on the catalyst's performance which accelerate of the reaction kinetic. In other words, in the presence of dendritic structure of catalyst, with easier access and diffusion of reactants to the active sites (channels and pores), the intermediate can be formed inside the channels and the reaction efficiency is increased and the synthesis time is shortened. On the other hand, due to the Lewis acid properties of copper ions and basic properties of HMTA, the condensation occurs between activated carbonyl groups, dimedone and ammonium acetate which lead to the formation of intermediate 1. Then, by Michael addition of other dimedone to the intermediate 1 and lastly after cycloaddition reactions, the final product is formed. These results were confirmed by previous reports.60–64
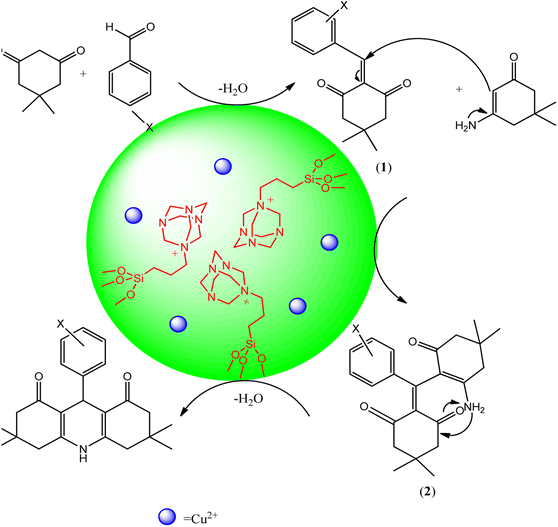 | ||
| Scheme 7 Synthesis mechanism of 1,8-dioxo-decahydroacridine derivatives in the presence of designed catalyst (Cu@KCC-1–nPr–HMTA). | ||
In summary, the performance of different catalysts were investigated for the reaction of 1,8-dioxo-decahydroacridine synthesis in comparison with designed catalyst (Table 8). Solvent-free conditions, stoichiometry use of reactants, high efficiency and short reaction time (1–5 min) are important features of applied nano-catalyst in synthesis.
| Entry | Catalyst | Solvent | Temperature (°C) | Time (min) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | Cu@KCC-1–nPr–HMTA | Solvent-free | 120 | 2 | 98 | This work |
| 2 | MCM-41–SO3H | Solvent-free | 110 | 10 | 95 | 64 |
| 3 | SiO2–Pr–SO3H | Solvent-free | 120 | 120 | 95 | 65 |
| 4 | Na+–MMT–[bip]-NH2+–H2SO4 | Solvent-free | 120 | 10 | 95 | 66 |
| 5 | Acetic acid | Solvent-free | 80 | 10 | 81 | 31 |
| 6 | Choline chloride | Ethanol | 120 | 180 | 98 | 67 |
| 7 | KCC-1–nPr–NH–Arg | Solvent-free | 110 | 30 | 98 | 68 |
In summary, the advantages this catalyst (Cu@KCC-1–nPr–HMTA) is its nanostructure, which by creating a very high surface area, allows the reaction to perform in a much more suitable condition than previous reports. In this method, instead of using solvents or toxic catalysts, solvent-free conditions in the presence of silica based mesoporous nano-material as a basic solid catalyst were applied which is environmentally friendly, along with easy and simple synthesis, high efficiency and very short time of reaction for the synthesis of 1,8-dioxo-decahydroacridin and 1,8-dioxo-octahydroxanthene derivatives.
4. Conclusion
In this study, Cu@KCC-1–nPr–HMTA was prepared and evaluated for the efficient synthesis of 1,8-dioxo-octahydroxanthene and 1,8-dioxo-decahydroacridin derivatives. Using this green dendritic nano-catalyst, the synthesis of xanthene and acridine derivatives was done in solvent-free and non-toxic reaction conditions which do not harm living organisms and environment. Also, one-step synthesis, low amounts of catalyst, high yields and shorter reaction times and purity of the products, are other advantages of this nano-catalyst.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the support of this work by Urmia University.References
- J. M. Jamison, K. Krabill, A. Hatwalkar, E. Jamison and C. C. Tsai, Cell Biol. Int. Rep., 1990, 1, 1075–1084 CrossRef PubMed.
- G. W. Rewcastle, G. J. Atwell, L. Zhuang, B. C. Baguley and W. A. Denny, J. Med. Chem., 1991, 11, 217–222 CrossRef PubMed.
- N. Mulakayala, P. V. Murthy, D. Rambabu, M. Aeluri, R. Adepu, G. R. Krishna, C. M. Reddy, K. R. Prasad, M. Chaitanya, C. S. Kumar and M. B. Rao, Bioorg. Med. Chem. Lett., 2012, 6, 2186–2191 CrossRef PubMed.
- T. Gu, M. Zhang, J. Chen and H. Qiu, Chem. Commun., 2015, 51, 9825–9828 RSC.
- A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2014, 2, 1063–1071 CrossRef CAS.
- A. G. B. Azebaze, M. Meyer, A. Valentin, E. L. Nguemfo, Z. T. Fomum and A. E. Nkengfack, Chem. Pharm. Bull., 2006, 54, 111–113 CrossRef CAS.
- E. F. Llama, C. Campo, M. Capo and M. Anadon, Eur. J. Med. Chem., 1989, 24, 391–396 CrossRef CAS.
- A. G. Banerjee, L. P. Kothapalli, P. A. Sharma, A. B. Thomas, R. K. Nanda, S. K. Shrivastava and V. V. Khatanglekar, Arabian J. Chem., 2016, 9, S480–S489 CrossRef CAS.
- R. Kakadiya, H. Dong, A. Kumar, D. Narsinh, X. Zhang, T. Chou, T. Lee, A. Shah and T. Su, Bioorg. Med. Chem., 2010, 18, 2285–2299 CrossRef CAS PubMed.
- M. B. Taysun, E. Sert and F. S. Atalay, J. Chem. Eng. Data, 2017, 62, 1173–1181 CrossRef CAS.
- C. G. Knight and T. Stephens, Biochem. J., 1989, 258, 683–689 CrossRef CAS PubMed.
- M. A. Zolfigol, R. Ayazi-Nasrabadi, S. Baghery, V. Khakyzadeh and S. Azizian, J. Mol. Catal. A: Chem., 2016, 418, 54–67 CrossRef.
- K. Niknam and M. Damya, J. Chin. Chem. Soc., 2009, 56, 659 CrossRef CAS.
- M. Salami and A. Ezabadi, Res. Chem. Intermed., 2019, 45, 3673 CrossRef CAS.
- A. N. Dadhania, K. P. Vaibhav and K. R. Dipak, J. Saudi Chem. Soc., 2017, 21, 163 CrossRef.
- F. A. Darweesh, S. K. Salama, I. A. Abdelhamid and A. H. M. Elwahy, Synth. Commun., 2021, 51, 471 CrossRef.
- A. M. Bhat, A. M. Naglah, S. A. Ansari, H. M. Al-Tuwajiri and A. A. Dhfyan, Molecules, 2021, 26, 3667 CrossRef PubMed.
- P. Mane, B. Shinde, P. Mundada, V. Gawade, B. Karale and A. Burungale, Res. Chem. Intermed., 2020, 46, 231 CrossRef CAS.
- M. Salam and A. Ezabadi, Res. Chem. Intermed., 2019, 45, 3673 CrossRef.
- H. A. Bhatt, V. R. Shah and R. M. Rawal, World Sci. News, 2019, 118, 100 Search PubMed.
- M. M. Hosseini, E. Kolvari, M. Vahidian and R. Bagheri, J. Appl. Chem., 2017, 11, 109 Search PubMed.
- M. Havaei, B. Karami and S. Khodabakhshi, Curr. Chem. Lett., 2014, 3, 167 CrossRef CAS.
- K. S. Kanakikodi, S. R. Churipard, A. B. Halgeri and S. P. Maradur, Sci. Rep., 2020, 10, 13103 CrossRef CAS.
- M. Akhtarkavian and Z. Rafiee, J. Appl. Chem., 2020, 15, 9 Search PubMed.
- R. Shan, C. Velazquez and E. E. Knaus, J. Med. Chem., 2004, 47(1), 254–261 CrossRef CAS PubMed.
- S. Girault, P. Grellier and A. Berecibar, et al., J. Med. Chem., 2000, 43(14), 2646–2654 CrossRef CAS PubMed.
- W. M. Cholody, B. Horowska, J. Paradziej-Lukowicz, S. Martelli and J. Konopa, J. Med. Chem., 1996, 39(5), 1028–1032 CrossRef CAS PubMed.
- N. Martin, M. Quinteiro and C. Seoane, Heterocycl. Chem., 1995, 32(1), 235–238 CrossRef CAS.
- X.-S. Wang, D.-Q. Shi, Y.-F. Zhang, S.-H. Wang and S.-J. Tu, Chin. J. Org. Chem., 2004, 24(4), 430–432 CAS.
- T.-S. Jin, J.-S. Zhang, T.-T. Guo, A.-Q. Wang and T.-S. Li, Synthesis, 2004, 2004(12), 2001–2005 CrossRef.
- K. Venkatesan, S. S. Pujari and K. V. Srinivasan, Synth. Commun., 2009, 39(2), 228–241 CrossRef CAS.
- B. Das, P. Thirupathi, I. Mahender, V. S. Reddy and Y. K. Rao, J. Mol. Catal. A: Chem., 2006, 247(1–2), 233–239 CrossRef CAS.
- S. Balalaie, F. Chadegani, F. Darviche and H. R. Bijanzadeh, Chin. J. Chem., 2009, 27(10), 1953–1956 CrossRef CAS.
- S.-J. Tu, C.-B. Miao, Y. Gao, Y.-J. Feng and J.-C. Feng, Chin. J. Chem., 2002, 20(7), 703–706 CrossRef CAS.
- X.-S. Wang, D.-Q. Shi, S.-H. Wang and S.-J. Tu, Chin. J. Org. Chem., 2003, 23(11), 1291–1293 CAS.
- Z.-Q. Tang, Y. Chen, C.-N. Liu, K.-Y. Cai and S.-J. Tu, Heterocycl. Chem., 2010, 47(2), 363–367 CrossRef CAS.
- G. M. Ziarani, A. Badiei, M. Hassanzadeh and S. Mousavi, Arabian J. Chem., 2021, 47, 2845–2855 Search PubMed.
- M. Khalil, G. T. M. Kadja and M. M. Ilmi, J. Ind. Eng. Chem., 2021, 93, 78–100 CrossRef CAS.
- V. Polshettiwar, D. Cha, X. Zhang and J. M. Basset, Angew. Chem., Int. Ed., 2010, 49, 9652 CrossRef CAS PubMed.
- A. Maity and V. Polshettiwar, ChemSusChem, 2017, 10, 3866 CrossRef CAS PubMed.
- H. Navay Baghban, M. Hasanzadeh, Y. Liu and F. Seidi, Biosensors, 2022, 12(10), 911–925 CrossRef PubMed.
- M. Baghal Behyar, F. Farshchi and M. Hasanzadeh, J. Mol. Recognit., 2022, 35(8), e2960 CrossRef PubMed.
- https://en.wikipedia.org/wiki/Hexamethylenetetramine.
- R. Ghorbani-Vaghei and V. Izadkhah, Appl. Organomet. Chem., 2018, 32, e4025 CrossRef.
- S. Noori, R. Ghorbani-Vaghei and M. Mirzaei-Mosbat, J. Mol. Struct., 2020, 1219, 128583 CrossRef CAS.
- M. Mahmudi, N. Shadjou and M. Hasanzadeh, J. Electroanal. Chem., 2019, 848, 113272 CrossRef CAS.
- B. Karami, Sh. Nejati and Kh. Eskandari, Curr. Chem. Lett., 2015, 4, 169 CrossRef.
- B. Mombaini, A. Kiasat and A. Ezabadi, Orient. J. Chem., 2015, 31, 483 CrossRef.
- S. K. Kantevari, R. Bantu and L. J. Nagarapu, J. Mol. Catal. A: Chem., 2007, 269, 53 CrossRef CAS.
- A. Zare, A. R. Moosavi-Zare, M. Merajoddin, M. A. Zolfigol, T. Hekmat-Zadeh, A. Hasaninejad, A. Khazaei, M. Mokhlesi, V. Khakyzadeh, F. Derakhshan-Panah, M. H. Beyzavi, E. Rostami, A. Arghoon and R. Roohandeh, J. Mol. Liq., 2012, 167, 69 CrossRef CAS.
- M. M. Heravi, H. Alinejhad, Kh. Bakhtiari, M. Saeedi, H. A. Oskooie and F. F. Bamoharram, Bull. Chem. Soc. Ethiop., 2011, 25, 399 CAS.
- F. Ghoreyshi Kahangi, M. Mehrdad, M. M. Heravi and S. Sadjadi, Sci. Rep., 2020, 10, 15285 CrossRef.
- S. Rezayati, R. Hajinasiri, Z. Erfani, S. Rezayati and S. Afsharisharifabad, Iran. J. Catal., 2014, 4, 157 Search PubMed.
- G. R. Chaudhary, P. Bansal, N. Kaur and S. Mehta, RSC Adv., 2014, 4, 16377 Search PubMed.
- M. Hasanpour Galehban, B. Zeynizadeh and H. Mousavi, J. Mol. Struct., 2023, 1271, 134017 CrossRef.
- S. E. Sadati Sorkhi, M. M. Hashemi and A. Ezabadi, Res. Chem. Intermed., 2020, 46, 2229 CrossRef CAS.
- M. Salami and A. Ezabadi, Res. Chem. Intermed., 2020, 46, 4611 CrossRef CAS.
- S. F. Hojati, M. Moosavifar and N. Moeinieghbali, J. Chem. Sci., 2020, 132, 38 CrossRef CAS.
- T. Josephrajan, V. T. Ramakrishnan, G. Kathiravan and J. Muthumary, ARKIVOC, 2005, xi, 124 Search PubMed.
- M. Bakherad, A. Keivanloo, A. H. Amin and A. Farkhondeh, J. Mex. Chem. Soc., 2019, 63, e2594 Search PubMed.
- N. Srividya, P. Ramamurthy, P. Shanmugasundaram and V. T. Ramakrishnan, J. Org. Chem., 1996, 61, 5083 CrossRef CAS.
- A. G. Swarna, A. S. Julie and J. A. Graham, J. Med. Chem., 1999, 42, 2383 CrossRef PubMed.
- H. H. Lee and W. R. William, J. Med. Chem., 1996, 39, 2508 CrossRef CAS PubMed.
- (a) T. Eicher and S. Hauptmann, The Chemistry of Heterocycles, Wiley-VCH, 2003 CrossRef; (b) G. M. Ziarani, A. Badiei, M. Hassanzadeh and S. Mousavi, Arabian J. Chem., 2014, 7, 335339 CrossRef.
- M. Kaya, Y. Yıldırır and G. Y. Celik, Med. Chem. Res., 2011, 20, 293 CrossRef CAS.
- M. Mazloumi and F. Shirini, J. Mol. Struct., 2022, 1217, 128326 CrossRef.
- Z. Cui, L. Zhang, C. Chen and Y. Jiang, Bioorg. Med. Chem., 2015, 24, 261 CrossRef PubMed.
- N. Hasannezhad and N. Shadjou, J. Mol. Recognit., 2022, 35, 2956 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2024 |