Open Access Article
Open Access ArticleEnantioselective synthesis of α-tetrasubstituted (1-indolizinyl) (diaryl)-methanamines via chiral phosphoric acid catalysis†
Jialing Zhong,
Rihuang Pan and
Xufeng Lin *
*
Department of Chemistry, Zhejiang University, Hangzhou 310027, P. R. China. E-mail: lxfok@zju.edu.cn
First published on 2nd January 2024
Abstract
An enantioselective Friedel–Crafts reaction of cyclic α-diaryl N-acyl imines with indolizines catalyzed by a chiral spirocyclic phosphoric acid has been developed. The asymmetric transformation proceeds smoothly to afford α-tetrasubstituted (1-indolizinyl) (diaryl)methanamines in good yields with up to 98% ee under mild conditions.
Chiral α-tetrasubstituted methanamines are frequently distributed in diverse bioactive natural products,1 and extensive effort has been devoted to constructing these scaffolds in synthetic chemistry over the past decade.2 Some excellent chiral organocatalysts3 and chiral metal salt catalysts4 have been developed in the asymmetric synthesis of chiral α-tetrasubstituted (diaryl)alkyl or (triaryl) methanamines. Despite these notable advances, to the best of our knowledge, there are currently no versatile protocols for the asymmetric preparation of chiral α-(1-indolizinyl)-(diaryl)methanamines.
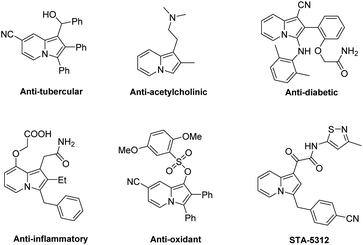
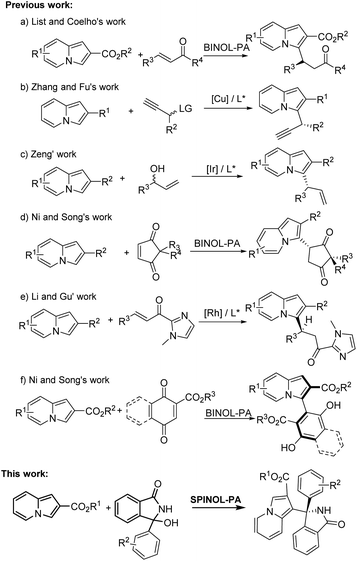
Indolizines as an important class of N-containing heterocycles can be found in organic synthesis and numerous pharmaceuticals (Fig. 1).5 Many versatile strategies for the direct functionalization of indolizines have been developed.6 Moreover, some elegant examples toward the asymmetric synthesis of enantioenriched indolizine derivatives have been reported,7 as shown in Scheme 1. For instance, List and Coelho reported the first organocatalyzed asymmetric conjugate addition of indolizines to enones using the chiral BINOL-derived phosphoric acid (BINOL-PA) as a catalyst (Scheme 1a).7a Zhang and Fu developed a copper-catalyzed enantioselective propargylation reaction of indolizines (Scheme 1b).7b Later, Zeng's group established a highly asymmetric allylic substitution reaction of indolizine derivatives catalyzed by chiral Ir complexes (Scheme 1c).7c Ni and Song described an organocatalytic highly diastereo- and enantioselective Friedel–Crafts conjugate addition of indolizines to prochiral cyclopentenediones catalyzed by BINOL-PA (Scheme 1d).7d
Recently, Li and Gu realized the catalytic asymmetric conjugate addition of indolizines to unsaturated ketones catalyzed by chiral Rh complexes (Scheme 1e).7e Very recently, Ni and Song reported BINOL-PA-catalyzed asymmetric atroposelective arylation of indolizines for the preparation of the axially chiral 3-arylindolizines (Scheme 1f).7f Although these strategies enable direct access to asymmetric synthesis of chiral indolizine derivatives, development of new class indolizines is still a formidable target. To continue our efforts8 on the advancement of chiral phosphoric acid catalysis,9 we here present the chiral spirocyclic phosphoric acid (SPINOL-PA) catalyzed enantioselective Friedel–Crafts of indolizines with in situ generated cyclic α-diaryl N-acyl imines10 for the synthesis of chiral α-(1-indolizinyl) (diaryl)methanamines.
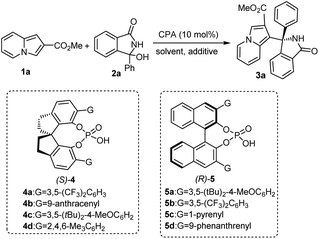
An initial investigation for the asymmetric Friedel–Crafts reaction was carried out with methyl indolizine-2-carboxylate (1a) and 3-phenyl 3-hydroxyisoindolinone (2a) in the presence of 10 mol% chiral phosphoric acid (CPA), as shown in Table 1. Chiral spirocyclic phosphoric acid (SPA) catalysts (4a–d) developed by our group8a were firstly screened in 1,2-dichloroethane (DCE) at room temperature to afford the desired chiral α-(1-indolizinyl) (diaryl)methanamine (3a) with acceptable yields but up to different enantioselectivities, and catalyst 4d gave the best reaction activity and enantioselectivity (89% yield, 74% ee) (entries 1–4). In addition, we also tested BINOL-derived phosphoric acids (5a–d) as catalysts, only low yields (37–72%) and poor enantioselectivities (12–53% ee) were observed (entries 5–8). We believe that the efficient catalytic activity of chiral spirocyclic phosphoric acid (SPA) is due to its special skeleton. Mostly the chiral backbone of the catalyst plays a vital role in attaining high stereoinduction by regulating electronic and structural properties of substrates. On the other hand, the dual hydrogen bonding network between the SPA and other two substrates plays a crucial role in terms of reactivity and selectivity. Moreover, the influence of solvent was investigated (entries 9–13). 1,2-Dichloroethane (DCE) was still the optimal solvent, and this reaction did not even proceed in toluene, THF or EtOAc. Next, the effect of additives was also investigated (entries 14–17). In the presence of 4 Å MS, the desired product 3a could be obtained in 91% yield with 87% ee (17). Lastly, we examined the temperature, and the reaction proceeded for 36 hours to afford the product 3a with 92% ee but in only 9% yield when the temperature was lowered to 0 °C (entry 18). Hence, the optimized reaction conditions employed 10 mol% (S)-4d in 1,2-dichloroethane at room temperature (entry 17).
| Entry | CPA | Solvent | Additive | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a Reactions were performed with 1a (0.05 mmol), 2a (0.05 mmol) and CPA catalyst (10 mol%) in the presence of additive (100 mg) in solvent (1 mL) for 24 hours at room temperature.b Isolated yields.c Determined by chiral HPLC analysis.d At 0 °C for 36 hours. | |||||
| 1 | (S)-4a | DCE | None | 49 | 15 |
| 2 | (S)-4b | DCE | None | 71 | 41 |
| 3 | (S)-4c | DCE | None | 78 | 6 |
| 4 | (S)-4d | DCE | None | 89 | 74 |
| 5 | (R)-5a | DCE | None | 55 | −12 |
| 6 | (R)-5b | DCE | None | 37 | −19 |
| 7 | (R)-5c | DCE | None | 61 | −23 |
| 8 | (R)-5d | DCE | None | 72 | −53 |
| 9 | (S)-4d | DCM | None | 91 | 37 |
| 10 | (S)-4d | MeCN | None | 55 | 23 |
| 11 | (S)-4d | Toluene | None | N.R. | — |
| 12 | (S)-4d | THF | None | N.R. | — |
| 13 | (S)-4d | EtOAc | None | N.R. | — |
| 14 | (S)-4d | MeOH | None | N.R. | — |
| 15 | (S)-4d | DCE | Na2SO4 | 82 | 78 |
| 16 | (S)-4d | DCE | MgSO4 | 79 | 74 |
| 17 | (S)-4d | DCE | 3 Å MS | 80 | 84 |
| 18 | (S)-4d | DCE | 4 Å MS | 91 | 87 |
| 19d | (S)-4d | DCE | 4 Å MS | 9 | 91 |
With the optimal conditions in hand, we set out to explore the substrate scope and limitations of this asymmetric transformation, as summarized in Table 2. In general, a range of 3-aryl 3-hydroxyisoindolinones 2 with different substituents were amenable to this strategy, and reacted efficiently with methyl indolizine-2-carboxylate 1a to provide good yields and high enantioselectivities (3a–j, up to 94% yield, up to 98% ee). When two methyl groups were placed around the 3-aryl ring, we observed a small drop in enantioselectivity as the corresponding product 3f was obtained in 82% yield and 78% ee. Interestingly, when methoxy group was introduced in ortho position of the 3-aryl, the enantioselectivity was dramatically improved (3e, 91% yield, 98% ee). Furthermore, CF3– group on the 3-aryl substituent proceeded smoothly to afford the desired product 3h in good yield with excellent enantioselectivity (94% ee). We found that 3-alkyl 3-hydroxyisoindolinones were not tolerated and the reactions did not run with 1a under the standard reaction conditions, such as methyl, allyl or benzyl substituent on the isoindolinone alcohol. We tried carrying out the reactions of 3-alkyl 3-hydroxyisoindolinones with substrate 1a in HFIP, and found that the reactions still could not run.
| a Reactions were performed with 1 (0.05 mmol), 2 (0.05 mmol) and (S)-4d (10 mol%) in the presence of 4 Å MS (100 mg) in DCE (1 mL) at room temperature for 24 hours. Isolated yield was given. The ee was determined by chiral HPLC analysis. |
|---|
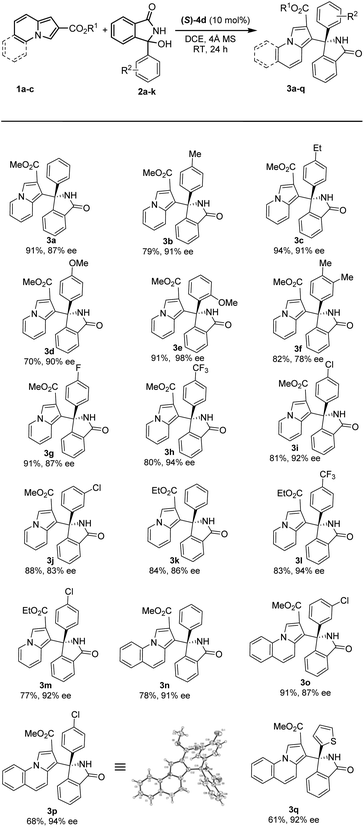 |
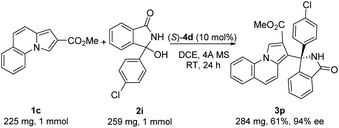
We next examined the scope of indolizines 1. Ethyl indolizine-2-carboxylate 1b also gave the desired products (3k–m) in good yields with excellent enantioselectivities (up to 94% ee). However, indolizine with a 5-methyl or 5-phenyl substituent depressed the reactivity and failed to provide the desired product under standard conditions because of the steric hindrance. Furthermore, methyl pyrrolo[1,2-a]quinoline-2-carboxylate 1c also gave the desired products (3n–q) with high enantioselectivities (up to 94% ee). The absolute configuration in product 3p (CCDC 2167990) was clearly determined to be (R) by X-ray diffraction analysis of a single crystal, and the absolute configuration of products 3 was assigned as (R) by analogy.
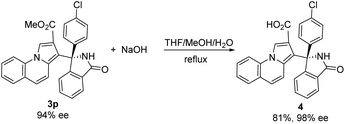
To further explore the synthetic practicality of the developed protocol, we investigated a 1 mmol scale reaction of this asymmetric Friedel–Crafts reaction, as shown in Scheme 2. Under the optimized reaction conditions, the reaction of methyl pyrrolo[1,2-a]quinoline-2-carboxylate 1c (1 mmol) and 3-(4-chlorophenyl)-3-hydroxyisoindolin-1-one 2i (1 mmol) afforded the desired product 3p in 61% yield with 94% ee.
We attempted to extend the reaction by treating product 3p with NaOH and obtained the corresponding product 4 in 81% yield and 98% ee, as shown in Scheme 3.
Conclusions
In summary, we have reported a metal free protocol for chiral spirocyclic phosphoric acid-catalyzed enantioselective Friedel–Crafts reaction of cyclic α-diaryl N-acyl imines with indolizines, providing convenient access to a range of α-tetrasubstituted (1-indolizinyl) (diaryl)methanamines in good yields with up to 98% ee under mild conditions.Experimental
General information
All reactions were carried out in oven-dried glassware with magnetic stirring under ambient conditions. Unless otherwise noted, all reagents, including the chiral phosphoric acid catalysts 4 and 5, were purchased from commercial supplies and used without further purification, and all solvents were dried and purified according to standard methods prior to use. Substrates 1 (ref. 11) and 2 (ref. 12) were synthesized according to the literature methods. 1H NMR and 13C NMR spectra were recorded on a Bruker AVANCE III 400 MHz spectrometer instrument at 400 MHz and 100 MHz spectrometer, respectively. The chemical shifts (δ) were quoted in parts per million (ppm) downfield relative to internal standard TMS (0.0 ppm) and referenced to solvent peaks in the NMR solvent (CDCl3 = δ 7.26 ppm; δ 77.00 ppm; D6-DMSO = δ 2.50 ppm; δ 40.00 ppm). Spin multiplicity were reported using the following abbreviations: s = singlet, d = doublet, t = triplet, dd = doublet of doublet, td = triplet of doublet, m = multiplet. Infrared spectra were recorded on an ATR-FTIR spectrometer. ESI-HRMS were recorded on a Water Micromass GCT Premier mass spectrometer. Optical rotations were measured on a PerkinElmer Model 341 polarimeter at 20 °C. Enantiomeric excess (ee) were measured by chiral HPLC analysis.General procedure for the asymmetric synthesis of 3 via chiral phosphoric acid-catalyzed reaction of indolizine-2-carboxylate 1 and quinone methyl ester 2
To a mixture of indolizine-2-carboxylate 1 (0.05 mmol), 3-hydroxyisoindolinones 2 (0.05 mmol, 1 equiv.) and catalyst (S)-4a (0.005 mmol, 10 mol%) in DCE (1 mL) was added 4 Å MS (100 mg). After stirring at room temperature for 24 hours, the residue was purified by flash column chromatography with acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (v/v) on silica gel to give the desired product 3.
2 (v/v) on silica gel to give the desired product 3.
Procedure for the 1.0 mmol scale reaction
To a mixture of indolizine-2-carboxylate 1c (225 mg, 1 mmol), 3-hydroxyisoindolinones 2i (259 mg, 1 mmol, 1 equiv.) and catalyst (S)-4a (0.1 mmol, 10 mol%) in DCE (20 mL) was added 4 Å MS (2 g). After stirring at room temperature for 24 hours, the residue was purified by flash column chromatography with acetate/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (v/v) on silica gel to give the desired product 3p (284 mg) in 61% yield with 94% ee.
2 (v/v) on silica gel to give the desired product 3p (284 mg) in 61% yield with 94% ee.
Procedure for the derivatization experiment
To a 100 mL round-bottomed flask, 3p (292 mg, 0.63 mmol), NaOH (125 mg, 3.2 mmol) and 30 mL mixed solvent [THF/MeOH/H2O 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 (v/v/v)] were added. After refluxing for 5 hours, adjusted the pH to 2 with 1 M HCl and extracted three times with ethyl acetate. The residue was purified by flash column chromatography with acetate/petroleum ether/formic acid 1
0.5 (v/v/v)] were added. After refluxing for 5 hours, adjusted the pH to 2 with 1 M HCl and extracted three times with ethyl acetate. The residue was purified by flash column chromatography with acetate/petroleum ether/formic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5% (v/v/v) on silica gel to give the desired product 4 (230 mg) in 81% yield with 98% ee.
1.5% (v/v/v) on silica gel to give the desired product 4 (230 mg) in 81% yield with 98% ee.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22071213), Leading Talents of Special Support Program of Zhejiang Province High-level Talents (2020R52008), the Fundamental Research Funds for the Central Universities (226-2022-00224) and Center of Chemistry for Frontier Technologies of Zhejiang University.Notes and references
- (a) P. M. Dewick, Medicinal Natural Products: A Biosynthetic Approach, Wiley, Chichester, U.K., 2009 CrossRef; (b) P. S. Sidhu, N. Nassif, M. M. McCallum, K. Teske, B. Feleke, N. Y. Yuan, P. Nandhikonda, J. M. Cook, R. K. Singh, D. D. Bikle and L. A. Arnold, ACS Med. Chem. Lett., 2014, 5, 199 CrossRef CAS PubMed; (c) J. Zhu, H. Chen, X.-E. Guo, X.-L. Qiu, C.-M. Hu, A. R. Chamberlin and W.-H. Lee, Eur. J. Med. Chem., 2015, 96, 196 CrossRef CAS PubMed.
- (a) S. Kobayashi, Y. Mori, J. S. Fossey and M. M. Salter, Chem. Rev., 2011, 111, 2626 CrossRef CAS PubMed; (b) S.-L. You, Q. Cai and M. Zeng, Chem. Soc. Rev., 2009, 38, 2190 RSC; (c) J. Vesely and R. Rios, Chem. Soc. Rev., 2014, 43, 611 RSC; (d) Z.-H. Kang, Y.-H. Wang, D. Zhang, R.-B. Wu, X.-F. Xu and W.-H. Hu, J. Am. Chem. Soc., 2019, 141, 1473 CrossRef CAS PubMed; (e) Z.-H. Kang, D. Zhang, J.-Y. Shou and W.-H. Hu, Org. Lett., 2018, 20, 983 CrossRef CAS PubMed; (f) J.-Y. Chen, H.-Y. Wu, Q.-W. Gui, S. Yan, J. Deng, Y.-W. Lin, Z. Cao and W.-M. He, Chin. J. Catal., 2021, 42, 1445 CrossRef CAS; (g) Y. Wu, J.-Y. Chen, J. Ning, X. Jiang, J. Deng, Y. Deng, R. Xu and W.-M. He, Green Chem., 2021, 23, 3950 RSC; (h) Z.-L. Wu, J.-Y. Chen, X.-Z. Tian, W.-T. Ouyang, Z.-T. Zhang and W.-M. He, Chin. Chem. Lett., 2022, 33, 1501 CrossRef CAS; (i) Q.-W. Gui, B. Wang, S. Zhu, F.-L. Li, M.-X. Zhu, M. Yi, J.-L. Yu, Z.-L. Wu and W.-M. He, Green Chem., 2021, 23, 4430 RSC.
- (a) M. Terada and K. Sorimachi, J. Am. Chem. Soc., 2007, 129, 292 CrossRef CAS PubMed; (b) Q. Kang, Z. Zhao and S.-L. You, J. Am. Chem. Soc., 2007, 129, 1484 CrossRef CAS PubMed; (c) G. B. Rowland, E. B. Rowland, Y. Liang, J. A. Perman and J. C. Antilla, Org. Lett., 2007, 9, 2609 CrossRef CAS PubMed; (d) S. Nakamura, N. Matsuda and M. Ohara, Chem.–Eur. J., 2016, 22, 9478 CrossRef CAS PubMed; (e) J. Feng, W. Yan, D. Wang, P. Li, Q. Sun and R. Wang, Chem. Commun., 2012, 48, 8003 RSC; (f) D. Glavac, C. Zheng, I. Dokli, S.-L. You and M. Gredicak, J. Org. Chem., 2017, 82, 8752 CrossRef CAS PubMed.
- (a) M. Johannsen, Chem. Commun., 1999, 2233 RSC; (b) S. Saaby, X. Fang, N. Gathergood and K. A. Jørgensen, Angew. Chem., Int. Ed., 2000, 39, 4114 CrossRef CAS; (c) S. Saaby, P. Bayon, P. S. Aburel and K. A. Jørgensen, J. Org. Chem., 2002, 67, 4352 CrossRef CAS PubMed; (d) L. Wu, R. Liu, G. Zhang, D. Wang, H. Wu, J. Gao and Y. Jia, Adv. Synth. Catal., 2015, 357, 709 CrossRef CAS; (e) K. Tsuchida, Y. Senda, K. Nakajima and Y. Nishibayashi, Angew. Chem., 2016, 128, 9880 CrossRef; (f) S. Dasgupta, J. Liu, C. A. Shoffler, G. P. A. Yap and M. P. Watson, Org. Lett., 2016, 18, 6006 CrossRef CAS PubMed.
- (a) J. Kluza, M. A. A. Gallego, J. C. Loyens, J. M. Beauvillain, F. Sousa-Faro, C. Cuevas, P. Marchetti and C. Bailly, Cancer Res., 2006, 66, 3177 CrossRef CAS PubMed; (b) G. S. Singh and E. E. Mmatli, Eur. J. Med. Chem., 2011, 46, 5237 CrossRef CAS PubMed; (c) T. Weide, L. Arve, H. Prinz, H. Waldmann and H. Kessler, Bioorg. Med. Chem. Lett., 2006, 16, 59 CrossRef CAS PubMed; (d) I. Macsari, Y. Besidski, G. Csjernyik, L. I. Nilsson, L. Sandberg, U. Yngve, K. Åhlin, T. Bueters, A. B. Eriksson, P. E. Lund, E. Venyike, S. Oerther, K. H. Blakeman, L. Luo and P. I. Arvidsson, J. Med. Chem., 2012, 55, 6866 CrossRef CAS PubMed.
- (a) X. Chen, X. Hu, Y. Deng, H. Jiang and W.-A. Zeng, Org. Lett., 2016, 18, 4742 CrossRef CAS PubMed; (b) S. A. Roy, J. Zgheib, C. Zhou and B. A. Arndtsen, Chem. Sci., 2021, 12, 2251 RSC; (c) I. V. Nechaev, G. V. Cherkaev, P. N. Solyev and N. V. Boev, J. Org. Chem., 2021, 86, 4220 CrossRef CAS PubMed; (d) K. Su, X. Guo, L. Zhu, Y. Liu, Y. Lu and B. Chen, Org. Chem. Front., 2021, 8, 4177 RSC; (e) D. Zhang, Z. Su, Q. He, Z. Wu, Y. Zhou, C. Pan, X. Liu and X. Feng, J. Am. Chem. Soc., 2020, 142, 15975 CrossRef CAS PubMed; (f) X. Huang and T.-X. Zhang, Tetrahedron Lett., 2009, 50, 208 CrossRef CAS; (g) J. Jaung and Y.-S. Jung, Bull. Korean Chem. Soc., 2003, 24, 1565 CrossRef CAS; (h) Y.-X. Yao, M. Alami, A. Hamze and O. Provot, Org. Biomol. Chem., 2021, 19, 3509 RSC; (i) C.-S. Xie, Y.-H. Zhang and P.-X. Xu, Synlett, 2008, 20, 3115 Search PubMed; (j) D. C. Rogness, N. A. Markina, J. P. Waldo and R. C. Larock, J. Org. Chem., 2012, 77, 2743 CrossRef CAS PubMed.
- (a) J. T. M. Corriea, B. List and F. Coelho, Angew. Chem., Int. Ed., 2017, 56, 7967 CrossRef PubMed; (b) L. Yang, X. Pu, D.-W. Niu, Z.-Y. Fu and X. Zhang, Org. Lett., 2019, 21, 8553 CrossRef CAS PubMed; (c) J.-M. Lu, M.-F. Wang, R.-G. Xu, H.-Z. Sun, X. Zheng, G.-F. Zhong and X.-F. Zeng, Asian J. Org. Chem., 2021, 10, 1500 CrossRef CAS; (d) Q.-J. Ni, Z.-M. Zhu, Y.-J. Fan, X.-Y. Chen and X.-X. Song, Org. Lett., 2021, 23, 9548 CrossRef CAS PubMed; (e) C. Huang, Z.-F. Zhao, S.-W. Li, J.-X. Zhao, L.-F. Wu and C.-Z. Gu, Org. Chem. Front., 2022, 9, 1932 RSC; (f) X.-X. Song, Y.-J. Fan, Z.-M. Zhu and Q.-J. Ni, Org. Lett., 2022, 24, 2315 CrossRef CAS PubMed.
- (a) F. Xu, D. Huang, C. Han, W. Shen, X.-F. Lin and Y.-G. Wang, Org. Chem., 2010, 75, 8677 CrossRef CAS PubMed; (b) D. Huang, X. Li, F. Xu, L. Li and X.-F. Lin, ACS Catal., 2013, 3, 2244 CrossRef CAS; (c) L. Wang, J.-L. Zhong and X.-F. Lin, Angew. Chem., Int. Ed., 2019, 58, 15824 CrossRef CAS PubMed; (d) J. Luo, T. Zhang, L. Wang, G. Liao, Q. Yao, Y. Wu, B. Zhan, Y. Lan, X.-F. Lin and B.-F. Shi, Angew. Chem., Int. Ed., 2019, 58, 6708 CrossRef CAS PubMed; (e) B. Zhan, L. Wang, J. Luo, X.-F. Lin and B.-F. Shi, Angew. Chem., Int. Ed., 2020, 59, 3568 CrossRef CAS PubMed; (f) A. B. Woldegiorgis, Z. Han and X.-F. Lin, Org. Lett., 2021, 23, 6606 CrossRef CAS PubMed; (g) L. Wang, J.-L. Zhong and X.-F. Lin, Synlett, 2021, 32, 417 CrossRef CAS; (h) Z. Chen, L. Wang, Y. Qian and X.-F. Lin, Synlett, 2021, 32, 1231 CrossRef CAS; (i) X.-F. Lin, L. Wang, Z. Han and Z.-L. Chen, Chin. J. Chem., 2021, 39, 602 Search PubMed.
- (a) T. Akiyama, J. Itoh, K. Yokota and K. Fuchibe, Angew. Chem., Int. Ed., 2004, 43, 1566 CrossRef CAS PubMed; (b) D. Uraguchi and M. Terada, J. Am. Chem. Soc., 2004, 126, 5356 CrossRef CAS PubMed; (c) For reviews, see: T. Akiyama, Chem. Rev., 2007, 107, 5744 CrossRef CAS PubMed; (d) M. Terada, Chem. Commun., 2008, 4097 RSC; (e) G. Adair, S. Mukherjee and B. List, Aldrichimica Acta, 2008, 41, 31 CAS; (f) S.-L. You, Q. Cai and M. Zeng, Chem. Soc. Rev., 2009, 38, 2190 RSC; (g) D. Parmar, E. Sugiono, S. Raja and M. Rueping, Chem. Rev., 2014, 114, 9047 CrossRef CAS PubMed; (h) A. B. Woldegiorgis and X.-F. Lin, Beilstein J. Org. Chem., 2021, 17, 2729 CrossRef CAS PubMed.
- (a) A. U. Rajshekhar, M. S. Milon, K. R. Sumit, G. B. Rayhan and K. S. A. Vinod, Chem. Commun., 2018, 54, 3516 RSC; (b) C.-X. Qian, W.-W. Liu, J.-W. Sun and P.-F. Li, Org. Chem. Front., 2022, 9, 1234 RSC; (c) L. Chen and Y. Zou, Adv. Synth. Catal., 2021, 363, 4159 CrossRef CAS; (d) M. Rong, J. Li, Y. Zhou, F. Zhang and J.-A. Ma, Org. Lett., 2020, 22, 9010 CrossRef CAS PubMed.
- Q.-J. Ni, Z.-M. Zhu, Y.-J. Fan, X.-Y. Chen and X.-X. Song, Org. Lett., 2021, 23, 9548 CrossRef CAS PubMed.
- M. M. Sadhu, S. K. Ray, R. A. Unhale and V. K. Singh, Org. Biomol. Chem., 2022, 20, 410 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2167990 (3p). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra07636a |
| This journal is © The Royal Society of Chemistry 2024 |