Open Access Article
Open Access ArticleSalophen chromium(III) complexes functionalized with pyridinium salts as catalysts for carbon dioxide cycloaddition to epoxides†
Karol Bester *a,
Agnieszka Bukowska
*a,
Agnieszka Bukowska a,
Aleksandra Kawka
a,
Aleksandra Kawka b,
Maciej Pytelc and
Wiktor Bukowski
b,
Maciej Pytelc and
Wiktor Bukowski a
a
aFaculty of Chemistry, Rzeszow University of Technology, Powstańców Warszawy 6, 35-959 Rzeszów, Poland. E-mail: bester_k@prz.edu.pl
bDoctoral School of Engineering and Technical Sciences at the Rzeszow University of Technology, Powstańców Warszawy 12, 35-959 Rzeszów, Poland
cFaculty of Mechanical Engineering and Aeronautics, Rzeszów University of Technology, Powstańców Warszawy 12, 35-959 Rzeszów, Poland
First published on 12th January 2024
Abstract
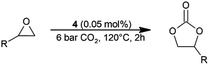
The catalytic properties of a series of novel chromium(III) salophen complexes having different pyridinium chloride units (pyridinium, 2,6-dimethylpyridinium or 4-(dimethylamino)pyridinium ones) have been studied in the reaction of carbon dioxide cycloaddition to phenyl glycidyl ether. The examined complexes were found to be capable of catalyzing cycloaddition under relatively mild reaction conditions without any additional nucleophilic co-catalyst. However, their catalytic activity depended strongly on the structure and number of pyridinium salt units in the ligand molecule. The complex with a single unit of 4-(dimethylamino)pyridinium chloride turned out to be the most active among the examined ones. A TOF of up to 1480 h−1 was obtained in the presence of this catalyst under the following conditions: 120 °C, 2 h, 6 bar, 0.05 mol% (74% epoxide conversion, and >99% selectivity). The most active complex has also been examined as a catalyst in the reactions of CO2 with a series of ten other terminal epoxides. High catalytic activity (TOF = 220–5045 h−1) was observed in most cases, except for the reaction of CO2 with allyl glycidyl ether.
Introduction
In recent decades, a dramatic increase in carbon dioxide emissions has been observed leading to increasing problems with global warming and climate changes. As a result, efforts to develop effective CO2 capture, storage, and chemical transformation technologies have become priority research areas.1–8 Since carbon dioxide is a nontoxic and non-flammable gas, it appears to be an interesting inexpensive and renewable green C1 carbon source that can be transformed into valuable organic chemicals. However, its thermodynamic and kinetic stability must be overcome by using appropriate substrates and/or catalysts. The conversion of CO2 to hydrocarbons for fuel purposes and to carbonates and bicarbonates for applications in the paper industry, plastics, rubber, and paint products appears to be the most attractive way of using CO2 as a chemical feedstock. The former would allow CO2 to be reconverted to the compounds from which it is mostly generated. This could lead to the closure of the carbon cycle and prevent the accumulation of anthropogenic CO2 in the atmosphere. However, alternative routes for the use of CO2 as an inexpensive, nontoxic, and renewable feedstock for the synthesis of valuable chemicals have also been extensively explored and reviewed in recent decades. One of them is the use of CO2 in reactions with epoxides, leading to two types of products: five-membered cyclic carbonates9–17 or polymeric carbonates.18–28 Cyclic carbonates can be used as precursors for polycarbonates, electrolytes, and other fine chemical intermediates.15 Aliphatic CO2-based polycarbonates are used as biodegradable polymers21,22 and in electrochemistry, as electrolytes exhibited exceptional interfacial stability with lithium metal and for ionic transportation.23–28 They promote salt dissociation and possess high thermal decomposition temperatures.The efficient and selective conversion of CO2 to cyclic carbonates or polycarbonates requires the presence of appropriate catalysts. Metal complexes that contain multidentate ligands, particularly those that contain salen/salophen ligands bound with Al,29–35 Co(III),36–39 Cr(III),40–44 or Zn ions,45–48 turned out to be one of the most effective groups of catalysts for CO2 reactions with epoxides. However, many other catalytic systems have been applied to CO2 reactions with epoxides in the last two decades.49–54
The activity of metal ions strongly depends on the structure of ligands which bind to them and can be tuned by modifying the ligand structure. However, to be effective catalysts, metal complexes require the presence of a proper nucleophilic cocatalyst.10,15 This role usually plays tertiary amines (e.g. DBU, DMAP, N-methylimidazole), ammonium (e.g. Et4NCl, Et4NBr), and phosphonium salts (e.g. PPNCl, PPNN3). The type of nucleophilic species used and the molar ratio of nucleophile to metal can decide whether the substrates are selectively converted to cyclic carbonate or linear polycarbonate, according to the cycloaddition or copolymerization routes, respectively. Cyclic carbonates are privileged products in the case of the use of a nucleophile that is a better leaving group (e.g. I−) and a large nucleophile-to-metal ratio, while polycarbonates are the main products when a nucleophile that is a worse leaving group (e.g. Cl−) and its low concentration in relation to a metal ion are applied.
The cocatalyst usually initiates a catalytic cycle interacting with an epoxide molecule activated by a metal ion, and it becomes an essential component of effective catalytic systems.16 Therefore, most catalysts used in the reactions of CO2 cycloaddition to epoxides are based on two-component systems composed of mixtures of a Lewis acid (or Brønsted acid) and a Lewis base.10–14,17,50 The use of catalysts that have only acidic or basic functionality is also possible, but requires much harsher conditions for cycloaddition reactions than in the case of catalytic systems containing both functions.9,10,55,56 Therefore, efforts are also being made to develop binary catalytic systems that contain the functions of a Lewis/Brønsted acid and a Lewis base within a single molecule. Numerous examples of organocatalysts and a few examples of metal complex catalysts have been previously described in the literature.11,53–55,57 An improvement in catalytic ability was found for some of them in relation to similar bicomponent systems.
It is well known that an improvement in catalytic activity of metal ions can be achieved by biding them with the ligands that have properly tuned structure.18,58–61
Thus, planning our experiments, it was expected that an improvement in the catalytic activity of salophen chromium(III) complexes in CO2 cycloaddition reactions to epoxides might be achieved by simple building into their structure the units of pyridinium chlorides. Furthermore, it was also expected that the complexes that have both metallic (Lewis acid) and nucleophilic (Lewis base) centres might serve as single component catalytic systems, i.e., the addition of any cocatalyst will not be required. In this work, the possibilities of synthesis and application of salophen complexes as single component catalysts have been presented. They base on new functionalized salophen ligands with pyridinium chloride units built into their structure. The ligands were derived from o-phenylenediamine and salicylaldehydes that contained onium salt units of pyridine, 2,6-lutidine, or 4-dimethylaminopyridine built into their molecules. The main goal of our research was to examine whether the presence of Lewis base within salophen molecules can improve the catalytic activity of salophen ligands and their chromium(III) complexes.
Experimental
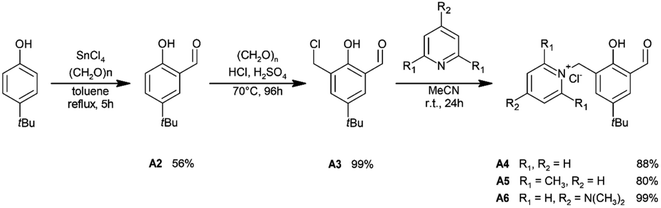
Phenols, epoxides, and other reagents and solvents used in this work were purchased from Sigma-Aldrich, Merck, Acros, and Alfa Aesar and applied as received, unless otherwise stated. 3,5-Di-t-butylsalicylaldehyde (A1) was prepared using the procedure developed by the Jacobsen group.60 5-t-Butylsalicylaldehyde (A2) was synthesized according to the procedure described by Casiraghi et al.61 Finally, 3-chloromethyl-5-t-butylsalicylaldehyde (A3) and salicylaldehydes with units of pyridinium chlorides (A4–A6) were synthesized using the similar methodology previously described by Peters et al.62–64 Experimental details of the preparation procedures are presented in the ESI.†General procedure for the synthesis of symmetrically substituted salophen ligands (L1–L3)
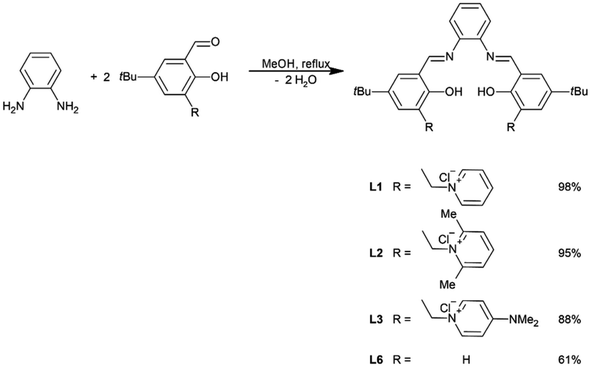
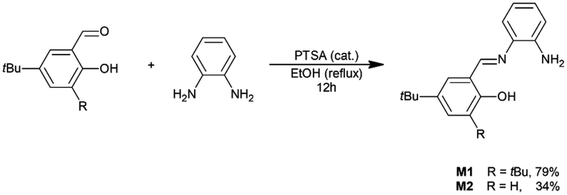
Salicylaldehyde with a pyridine chloride units (A4–A6, 3 mmol) was dissolved in methanol (20 ml). The resulting solution was heated to reflux and a solution of 1,2-phenylenediamine (162 mg; 1.5 mmol) in MeOH (10 ml) was added dropwise. The reaction mixture was vigorously mixed and refluxed first for 4 hours, and then overnight at ambient temperature. The post-reaction mixture was concentrated using a rotary evaporator to about 1/10 volume and diethyl ether (10 ml) was added. The precipitated solid was powdered using a magnetic stirrer for approximately 1 hour, filtered, rinsed with ethyl ether, and dried under reduced pressure (∼1 mbar). Ligands L1–L3 were in the form of yellow powders. Their structure was proved by 1H-NMR, 13C-NMR, HRMS and FTIR techniques (see ESI†).General procedure for the synthesis of unsymmetrically substituted salophen ligands
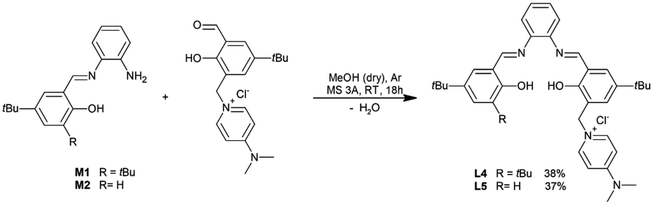
In a 100 ml Schlenk flask, molecular sieves 3A (1.5 g) were first placed. The flask was next equipped with a magnetic bar, sealed with a rubber septum, heated with a heating gun, and finally cooled to ambient temperature under argon atmosphere before adding reagents. Aldehyde A6 (1047 mg, 3 mmol) and monosalicylaldimine (M1 or M2, 3 mmol) was then placed in the flask after its short opening in an argon atmosphere and dry methanol (30 ml) was next added through the septum using a glass syringe. The mixture was stirred vigorously under argon atmosphere at 25 °C for 18 hours. The heterogeneous post-reaction mixture was filtered on a fritted glass funnel to remove molecular sieves. Methanol from the post-reaction mixture was removed at 40 °C under decreased pressure using a vacuum rotary evaporator. The residual was dissolved in 20 ml of dry methylene chloride and the crude product was purified chromatographically using SiO2 neutralized with triethylamine (2 ml TEA/10 g SiO2). The mixture of methylene chloride-methanol (9/1 v/v) with 1% TEA was applied as an eluent. The product isolated after removal of the eluent was pre-dried under vacuum (1 mbar, at 50 °C) for 2 hours, then redissolved in 50 ml of methylene chloride and washed with 25 ml of a saturated aqueous solution of NH4Cl. After removal of the drying agent and solvent, the residue was further dried under vacuum (1 mbar, 50 °C) for 2 hours. Ligands L4–L5 were obtained in the form of yellow powders. Their structure was proved by 1H-NMR, 13C-NMR, HRMS and FTIR techniques (see ESI†).General procedure for the synthesis of salophen chromium(II) complexes (1–5)
Anhydrous CrCl2 (65 mg of 0.525 mmol) was dissolved in 8 ml THF that was dried over sodium benzophenone and freshly distilled. A 25 ml Schlenk flask equipped with a magnetic stirrer and a rubber septum, previously heated with a heat gun and cooled under argon, was used. In the second 5 ml Schlenk flask, equipped and treated similarly, salophen ligand (0.5 mmol) was dissolved in the mixture of THF (3 ml) and methanol (2 ml, dried under molecular sieves 3A). The resulting solution was next transferred to the solution of CrCl2 using a steal canula. The canula and the empty flask were additionally rinsed with 2 ml methanol. The flask with the reaction mixture was placed in the heating block on a magnetic stirrer and mixed under an argon atmosphere at 40 °C for 4 hours. After that time, the heating block was removed, the flask opened and filled with air. The mixing under air atmosphere was continued at ambient temperature for 16 hours. The reaction mixture was concentrated to about 1/5 volume and 5 ml of dried diethyl ether was added. The precipitated solid was ground with a magnetic stirrer for 1 hour. The resulting powder was filtered using a fritted glass funnel and washed with an additional portion of diethyl ether, and finally dried under vacuum (1 mbar) at 50 °C for 4 hours. Complex 1–5 were obtained in the form of brown or red-brown powders. Their structure was proved by HRMS, elemental analysis and FTIR techniques (see ESI†).General procedure for catalyst screening
To perform catalytic studies, a laboratory glass pressure reactor (Büchi Tinyclave, 25 ml) was applied. The reactor was equipped with a magnetic bar, charged with a catalyst (0.01–0.05 mol%) and epoxide (1.5 ml), sealed, placed in the preheated oil bath, and finally pressurized to the required carbon dioxide pressure. The reaction mixture was gently stirred under the applied reaction conditions (2–8 bar CO2, 80–120 °C, 1–6 h). After the indicated time, the oil bath was removed, the CO2 flow was cut off, and the reactor was gently cooled, first in a water bath to room temperature, and then in an ice bath, before pressure was released, and the pressure vessel opened. The reaction mixture was solubilized with ca. 5–10 ml of acetone and 1H-NMR analysis was performed to determine the conversion of epoxide to cyclic carbonate. The products were isolated from post-reaction mixtures by flash chromatography after the previous removal of acetone. The spectrometric data for the isolated cyclic carbonates were consistent with those previously reported.65Results and discussion
Catalysts synthesis
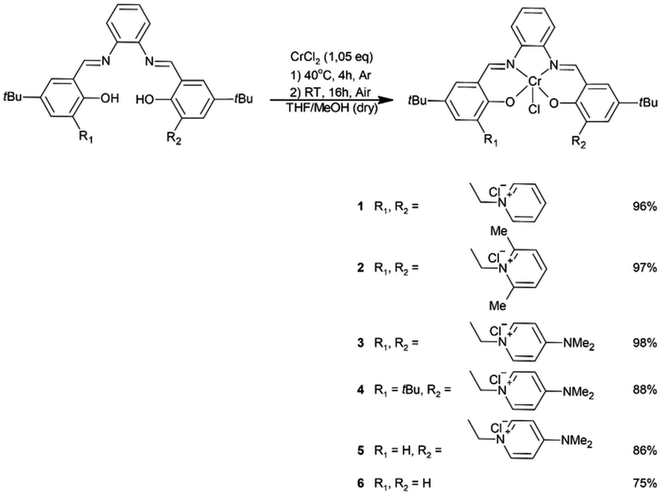
To obtain salophen-type ligands that have one or two units of pyridinium chlorides within their molecules, appropriate salicylaldehydes A4–A6 were first synthesized. The aldehydes were derived from 4-t-butylphenol in three-stage syntheses (Scheme 1; for details, see ESI†).Symmetrically substituted salophen ligands L1–L3 with yields of 88–98% were obtained in direct reactions between an appropriate salicylaldehyde (2 eq.) and 1,2-phenylenediamine (1 eq.) conducted in boiling methanol (Scheme 2). To obtain unsymmetrically substituted salophen derivatives L4–L5, monoimines M1 and M2 have to first be synthesized in the reaction of 1,2-phenylenediamine with 1 eq. (5)-t-butyl- and 3,5-di-t-butylsalicyladehyde, respectively. Syntheses were performed in ethanol in the presence of the catalytic amount of p-toluenesulfonic acid. The appropriate monoimines were isolated with good or moderate yields (79 and 34%, respectively) (Scheme 3). They were then reacted with salicylaldehyde A6 in dry methanol under an argon atmosphere (Scheme 4). Due to the high tendency to form symmetrically substituted salophens as the main products of condensation in the presence of water, molecular sieves 3A were also added to bind the water that is formed as a coproduct. Since the post-reaction mixtures contained both unsymmetrically and symmetrically substituted salophens in addition to the residues of substrates, the crude products had to be purified chromatographically on silica gel. Unsymmetrically substituted salophens L4–L5 were isolated with low yields (37–38%).
To obtain salophen chromium(III) complexes 1–6, the appropriate salophen ligands were first reacted with anhydrous chromium(II) chloride in an atmosphere of argon. Reactions were carried out in the mixed solvent consisting of dry THF and dry MeOH (L1–L3) or in dry THF (L4, L5 and L6). The final forms of the complexes were obtained by purging dry air through the reaction mixtures to oxidize, the preliminary formed chromium(II) complexes (Scheme 5). The structures of isolated complexes were confirmed using elementary analysis and spectral methods, including HRMS and FTIR. The results of analyses were presented in detail in the ESI.†
Catalysts activity evaluation
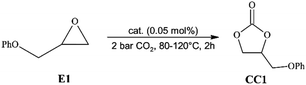
North et al.66 have previously shown that salophen ligand L6 can act on its own as an organocatalyst in CO2 cycloaddition reactions to various terminal epoxides under metal and halide-free conditions. It has also been shown that its catalytic activity is the result of the concerted and synergistic action of phenolate as a nucleophile and phenol as a H-donor involved in the activation of the CO2 and epoxide, respectively.67 However, to act as an efficient catalyst, L6 required rather harsh reaction conditions – 10 bar CO2, 120 °C and 1 mol% catalyst concentration.66 Therefore, it was expected that when salophen ligands with pyridinium salt units built into their molecules would be applied as a catalyst, a clear improvement in catalytic activity of the ligands could be observed in relation to L6 due to the presence of an additional basic centre in their molecules.The following reaction conditions were applied to examine the catalytic activity of the synthesized ligands in the model cycloaddition reaction: 2 bar CO2 (constant pressure), 120 °C, and 0.05 mol% L1–L5. The catalytic experiments lasted 2 hours. For comparison purposes, the activity of ligand L6 was also examined under the same reaction conditions. Phenyl glycidyl ether (E1) was used as a model epoxide due to its moderate reactivity, low volatility (bp. 254 °C) and good solubility of the examined catalysts in this compound. A disadvantage of its use was the possibility of solidification of reaction mixtures before achieving relatively high conversion of E1. Such situation had been observed particularly in the experiments performed later at lower temperatures, 80–100 °C, when the effect of reaction temperature on the CO2 cycloaddition was studied. It was the result of a relatively high melting point of the cyclic carbonate formed from E1 (ca. 102 °C).
Contrary to our expectations, the introduction of the units of pyridinium chloride at position 3 of the salicylaldehyde moieties did not influence sufficiently an improvement in the organocatalytic activity of L1 and L2 compared to L6 (Table 1 entries 2, 3, and 7). Ligand L1 being the derivative of pyridinium chloride, it was allowed to convert only 3% of E1 to CC1, while L2 that has two 2,6-dimethylpyridinium chloride units turned out to be nearly inactive under the applied reaction conditions, as L6 examined under the same reaction conditions, i.e. 0.05 mol% and 2 bar CO2 instead of 1 mol% and 10 bar CO2 as was used in ref. 66. Only traces of cyclic carbonate were detected in the post-reaction mixtures after 2 hours when the cycloaddition was performed in the presence of L2 or L6. However, a clear improvement in activity was observed when L3–L5, which have the units of 4-(dimethylamino)pyridinium chloride (DMAP-Cl) built into the salophen structure, were examined in a role of organocatalysts. Furthermore, ligand L3, which contains two units of DMAP-Cl, was found to be nearly twice as active as ligands L4 and L5 which have only a single moiety of DMAP-Cl. The values of E1 conversion amounted of approximately 21% for L4–L5 and 35% for L3 (Table 1 entries 4–6).
| No. | Catalyst | T [°C] | Conversion of E1a [%] | Yield of CC1a [%] | TONb [—] | TOFc [h−1] |
|---|---|---|---|---|---|---|
| a Determined based on 1H-NMR spectra of post-reaction mixtures with biphenyl as an internal standard.b Calculated as mol of carbonate/mol of catalyst.c Calculated as TON/time [h].d With 0.05 mol% DMAP as a co-catalyst.e With 0.05 mol% [BnDMAP]Cl as a co-catalyst. | ||||||
| 1 | [BnDMAP]Cl | 120 | 26 | 26 | 520 | 260 |
| 2 | L1 | 120 | 3 | 3 | 60 | 30 |
| 3 | L2 | 120 | <1 | <1 | — | — |
| 4 | L3 | 120 | 35 | 35 | 700 | 350 |
| 5 | L4 | 120 | 21 | 21 | 420 | 210 |
| 6 | L5 | 120 | 21 | 21 | 420 | 210 |
| 7 | L6 | 120 | <1 | <1 | — | — |
| 8 | 1 | 80 | <1 | <1 | — | — |
| 9 | 1 | 100 | 19 | 19 | 380 | 190 |
| 10 | 1 | 120 | 14 | 10 | 200 | 100 |
| 11 | 2 | 80 | <1 | <1 | — | — |
| 12 | 2 | 100 | 4 | 1 | 20 | 10 |
| 13 | 2 | 120 | 3 | 1 | 20 | 10 |
| 14 | 3 | 80 | 15 | 14 | 280 | 140 |
| 15 | 3 | 100 | 46 | 46 | 920 | 460 |
| 16 | 3 | 120 | 58 | 58 | 1160 | 580 |
| 17 | 4 | 80 | 8 | 7 | 140 | 70 |
| 18 | 4 | 100 | 34 | 34 | 680 | 340 |
| 19 | 4 | 120 | 71 | 70 | 1400 | 700 |
| 20 | 5 | 80 | 10 | 9 | 180 | 90 |
| 21 | 5 | 100 | 41 | 39 | 780 | 390 |
| 22 | 5 | 120 | 66 | 66 | 1320 | 660 |
| 23 | 6 | 120 | <1 | <1 | — | — |
| 24d | 6 | 120 | 39 | 36 | 720 | 360 |
| 25e | 6 | 120 | 63 | 61 | 1220 | 610 |
The difference in activity between L1–L2 and L3–L5 and between L3 and L4–L5 clearly indicated a key role of tertiary amine units in the activation of substrates in the cycloaddition of CO2 to E1. Similar findings have been previously discussed when organocatalytic systems composed of tertiary amines and salen ligands and other phenols,68 on the one hand, and a salophen ligand with a tertiary aromatic amine unit built in ref. 66 on the other hand, were examined. Two mechanisms of substrate activation by amine units have been proposed: the first included the interaction of amine with CO2, the second the addition of amine to the previously activated epoxide.68 Furthermore, different aliphatic monoamines and diamines have also been examined on their own as organocatalysts in CO2 cycloaddition to epoxides.9 Some of them, in particular, tertiary N,N,N′,N′-tetraethylethylenediamine, exhibited moderate to excellent efficiency in the studied cycloaddition reactions.
To gain additional proof of the participation of tertiary amines in the activation of substrates, there was also carried out an experiment in which 1-(benzyl)-4-(dimethylamino)pyridinium chloride ([BnDMAP]Cl) was used as a catalyst. The conversion of E1 comparable to those observed for L3–L5 was obtained (Table 1 entry 1). Thus, it can be concluded that the phenolic groups of L1–L5 do not play a meaningful role in the activation of E1 under the reaction conditions applied.
Next, a series of chromium(III) complexes 1–5 derived from the ligands L1–L5 were examined as catalysts in the cycloaddition of CO2 to E1. For comparison purposes, the same catalyst concentration, reaction time, temperature, and CO2 pressure were applied. The activity of the complexes was found to be clearly higher than that of the appropriate ligands. However, it is rather difficult to regard complex 2 bearing 2,6-pyridinium chloride units as a very active catalyst for CO2 cycloaddition due to the very low conversion of E1 observed in the presence of this complex (Table 1, entry 13).
Furthermore, like the experiments involving L1–L5, noticeable differences in catalytic activity of 1–5 were observed depending on their structure (Table 1 entries 10, 13, 16, 19, and 22). Complexes that were derivatives of pyridine (1) and 2,6-pyridine (2) showed clearly lower activity compared to complex 3 that contains two units of DMAP-Cl. For the first two, conversion E1 was 14 and 3%, respectively, while for complex 3 about 58%. In addition, 4 and 5 that have a single unit of DMAP-Cl showed higher catalytic activity compared to 3 that has two units of DMAP-Cl. This finding was in opposition to the relation described above for the salophen ligands. When catalytic experiments were performed in the presence of 4 and 5, 71% and 66% conversions of E1 were obtained, respectively, while for 3 – 58% (Table 1 entries 16, 19, and 22).
Unlike L4 and L5, a higher value of E1 conversion was observed for 4 compared to 5. This finding seems to point to the advantageous effect of the presence of the second tBu substituent on the activity of the salophen chromium(III) complex. In the case of metal complex catalysis, the benefit resulting from donor properties of the tBu group is probably greater than the spherical problems caused by its bulky nature, which can impede the access of substrates to the metallic centre in the salophen complexes.
For comparison purposes, complex 6 derived from an unsubstituted salophen ligand has also been examined as a catalyst of the cycloaddition of CO2 to E1 (Table 1, entry 23). It turned out to be nearly inactive catalytically under the reaction conditions applied. However, its activity increased clearly after the addition of an equivalent amount of DMAP as a co-catalyst, and about 39% conversion of E1 could be then obtained (Table 1, entry 24). These findings differ somewhat from those obtained previously by the North group, which examined 6 on its own (using 2.5 mol%) and with the addition of DMAP as a catalyst in the cycloaddition of CO2 to styrene oxide performed at room temperature under the pressure of 1 bar.42 No catalytic activity was then observed in both cases under the applied reaction condition.
To further examine a role of a pyridinium salt that has additional tertiary amine functionality as a co-catalyst, an equimolar mixture of 6 and [BnDMAP]Cl, which can be considered as a two-component substitute of complex 5, was also examined in the cycloaddition of CO2 to E1. In this case, the conversion comparable to that observed for 5 was obtained (Table 1, entries 22 and 25).
Numerous mechanistic studies shown that CO2 cycloaddition to epoxides can be initiated by the interaction of a catalyst with molecules of only one of the substrates or simultaneously with both.14,17,53,68–71
For instance, tertiary amines (Lewis bases) may interact with both reactants but they are on their own not among the most active catalysts of the cycloaddition when a source of halogen ions or acidic units is not available. When interacting with CO2 they form more reactive bicarbonate forms that can then serve as a nucleophile and react with epoxides providing the intermediate alcoholates. Tertiary amines can also participate directly in the ring opening reaction of epoxides to form the alcoholates which can act further as a nucleophiles in relation to CO2.68 Lewis or Brønsted acids and halide ions (e.g. as a part of ammonium, pyridinium, phosphonium, imidazolium, benzimidazolium salt units) can participate in the activation of epoxides. The acidic compounds by their interaction with an oxygen atom in the epoxide ring facilitate a further ring opening reaction with nucleophiles, while halide ions of onium salts initiate the epoxide ring opening by the nucleophilic attack on one of the carbon atoms of a epoxide ring. The intermediate alcoholates are formed as a result which react further with CO2. The presence only one type of catalytic centres (acidic or basic) do not allow the cycloaddition to be performed effectively under the mild reaction conditions, i.e. at a low temperature, under a low CO2 pressure, and using a low catalyst concentration. Therefore, to conduct the cycloaddition much more effectively, the catalytic systems consisting of both acidic and basic functionalities are mostly used epoxides.
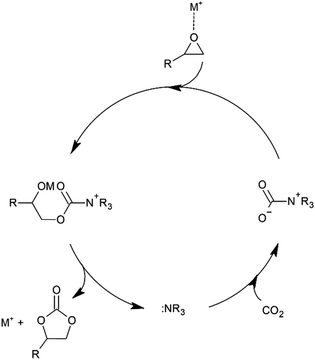
The results of the catalytic examination including 1–5 appear to point to the different pathways of substrate activation in the case of 1–2 and 3–5. The first two complexes have in their molecules two type centres (acidic Cr(III) ions and basic halide ions) that can simultaneously participate in the activation epoxide molecules according to the mechanism presented in Fig. 1. Complexes 3–5 that in addition to the mentioned two centres additionally possess one or two tertiary amine units can also participate in the activation of CO2 molecules increasing their reactivity and facilitating the epoxide ring opening to form an alcoholate according to the mechanism presented in Fig. 2. Consequently, due to parallelly occurring pathways of CO2 cycloaddition, complexes 3–5 as trifunctional catalytic species show clearly higher catalytic activity than binary systems 1–2. Since, [BnDMAP]Cl used as a catalyst on it own also show a certain activity in the CO2 cycloaddition under the same reaction conditions (Table 1, entry 1), it cannot be excluded that pyridinium cations in 1–5 can also participate in the activation of epoxide as Lewis acid centres.
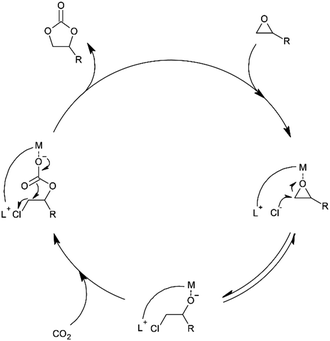 | ||
| Fig. 1 Possible mechanism of CO2 cycloaddition to epoxides in the presence of metal ion (and/or pyridinium) and chloride ion centres. | ||
Reaction conditions optimalization
The effect of reaction temperature on the conversion of E1 to cyclic carbonate (CC1) was next studied in the presence of complexes 1–5. Catalytic tests were performed at temperatures of 80, 100 and 120 °C, using a catalyst loading of 0.05 mol% and a pressure of 2–8 bar CO2. The results of the two-hour experiments are presented in Table 1 (entries 8–22). According to general kinetic relationships, it was expected to observe an increase in the conversion value when the reaction temperature increased, similarly as was described by Wang et al. in ref. 72 for the cycloaddition reaction performed in the presence of a chitosan-functionalized ionic liquid as a biopolymer-supported catalyst. Furthermore, an increase in reaction temperature should facilitate the selective conversion of E1 to cyclic carbonate CC1, as its formation required higher activation energy than an appropriate linear polycarbonate.10 However, exceeding a certain limit of the reaction temperature may result in a decrease in both epoxide conversion and cycloaddition reaction selectivity (the latter being due to occurring side reactions). Based on the results obtained (Table 1 entries 8–22), a selective course of CO2 cycloaddition to E1 was concluded for 1–5 in the entire range of the temperature studied (based on NMR analyses of post-reaction mixtures). However, the performed experiments revealed that an increase in E1 conversion within the entire temperature range was only observed for complexes 3–5. In the case of complexes 1 and 2, after an initial increase in E1 conversion with an increase in temperature from 80 to 100 °C, a decrease in its conversion was noted when the reaction temperature was elevated above 100 °C (Fig. 3). To exclude accidentality, the relevant experiments were repeated, and comparable results were obtained.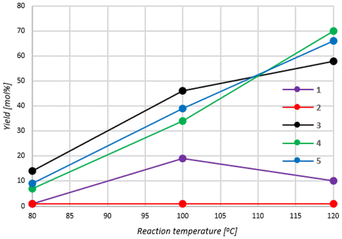 | ||
| Fig. 3 Effect of reaction temperature on the yield of CC1 in CO2 cycloaddition to E1 catalyzed by complexes 1–5. | ||
According to the dependences of Arrhenius or Eyring, when the temperature of reactions increases the values of reaction rate constants increase as well. However, in the case of gas–liquid reaction systems, the general rate of such transformations can decrease because of decreasing the solubility of a gaseous reactant in the liquid reaction mixture with increasing the reaction temperature. Furthermore, complexes 1–5, examined as catalysts of CO2 cycloaddition to E1, which have pyridinium chloride units within their structure, might undergo partial deactivation due to their side degradation, for instance, in nucleophilic substitution reactions, Hoffmann elimination, and/or Stevens rearrangement which can intensify with increasing reaction temperature.73 The last factors can be responsible for the differences in orders of catalytic activity observed for the complexes at a studied temperature range. The catalytic activity changed in the following order: 2 < 1 < 4 < 5 < 3 at 80 and 100 °C and 2 < 1 < 3 < 5 < 4 at 120 °C.
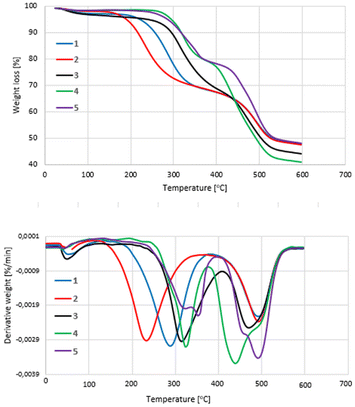
To examine the thermal stability of complexes 1–5, the additional thermogravimetric analyses were conducted in the temperature range of 30–600 °C. The obtained results (Fig. 4) revealed that when the samples were heated to 180 °C, i.e., to the temperature which is much higher than one applied in the catalytic experiments, their weight loss did not exceed 4%. This finding pointed rather on the desorption of residuals of solvents used for the synthesis of complexes and/or dissociation of THF molecules which is slightly bind to metal centres than the significant degradation of the complexes. However, it was noted that in the case of complex 2, that has two units of 2,6-dimethylpyridine chloride built into its molecule, the process of degradation accelerated clearly above 180 °C. Two maxima of the degradation rate were observed at approx. 229 and 492 °C, respectively, corresponding to a weight loss of approx. 32 and 52%, respectively. The first maximum can be attributed mainly to the dissociation of the units of 2,6-dimethylpyridine, the second is a result of gradual carbonization of salophen complex. Other two symmetrical substituted complexes, 1 and 3, with pyridinium and 4-(dimethylamino)pyridinium chloride units turned out to be clearly more stable. The degradation of 1 accelerated above approximately 200 °C and 3 above 240 °C, reaching the maxima of decomposition rate at temperatures of approximately – the first at around 286 and 312 °C and the second at about 490 °C for 1 and 470 °C for 3. Unsymmetrically substituted complexes 4 and 5 that have the single unit of 4-(dimethylamino)pyridinium chloride in their structure appeared to be yet more stable thermically compared to 1–3. Although the acceleration in their decomposition was recorded at similar temperature as for 3, the minima on DTGA curves corresponding to the maximum rate of primary degradation were observed respectively at higher temperature (323 and 353 °C, respectively for 4 and 5). Unexpectedly the second minimum on the DTGA curve obtained for of 4 was observed at temperature of 438 °C, although for 5 it was noted at 490 °C. The thermal stability of complexes 1–5 appeared to correlate roughly with their catalytic activity at 120 °C. Therefore, it cannot be ruled out that the thermal stability of the complexes influences their catalytic activity.
It cannot be also ruled out that the observed catalytical relationships are also a consequence of some other factors, for instance, relative changes in activity of the particular catalytic centres (Cr(III) ion as Lewis acid and chloride ion of an onium salt and tertiary amine as Lewis bases) depending on the reaction temperature applied. Pyridinium chloride and tertiary amine units may play a more meaningful role in the activation of substrates at lower temperatures, while the role of metal centres in activation of epoxide molecules can increase with an increase in reaction temperature. Furthermore, the presence of a bulky donor substituent (tBu group) can play a decisive role in the improvement in the activity of a Lewis acid centre when the reaction temperature increased. Due to the overlap of effects of many factors on the activity of complexes 1–5 dependently on the reaction temperature applied, similar orders of catalytic activity for the examined metal complexes and the appropriate ligands were observed exclusively at 80 and 100 °C.
To further optimize the cycloaddition condition, an effect of reaction time, CO2 pressure, and catalyst concentration on the conversion of E1 to CC1 was also studied. Using 4 in the amount of 0.05 mol%, a temperature of 120 °C, and a pressure of 2 bar CO2, approximately 50% conversion of E1 was obtained after 1 hour (Table 2, entry 1). The conversion increased by about 20% after the next hour, finally achieving the value of 94% within six hours (Table 2, entries 2 and 4). When the pressure of CO2 was increased, a clear improvement in epoxide conversion was observed, but only to a value of 6 bar. A further increase in CO2 pressure to 8 bar was rather irrelevant because it almost did not reflect the further increase in E1 conversion (Table 2, entries 2, 5, 6, and 9). Moreover, based on the results obtained, it was concluded that performing the cycloaddition under 6 bars, the conversion of E1 as that obtained in the 6 hour experiment at 2 bar could be obtained just after 2 hours.
| No. | Catalyst | Catalyst loading [mol%] | CO2 pressure [bar] | Reaction time [h] | Conversiona [%] | Yielda [%] | TONb [—] | TOFc [h−1] |
|---|---|---|---|---|---|---|---|---|
| a Determined based on 1H-NMR spectra of post-reaction mixtures with biphenyl as an internal standard.b Calculated as mol of carbonate/mol of catalyst.c Calculated as TON/time [h]. | ||||||||
| 1 | 4 | 0.05 | 2 | 1 | 49 | 47 | 940 | 940 |
| 2 | 4 | 0.05 | 2 | 2 | 71 | 70 | 1400 | 700 |
| 3 | 4 | 0.05 | 2 | 3 | 85 | 85 | 1700 | 567 |
| 4 | 4 | 0.05 | 2 | 6 | 94 | 94 | 1880 | 313 |
| 5 | 4 | 0.05 | 4 | 2 | 87 | 85 | 1400 | 700 |
| 6 | 4 | 0.05 | 6 | 2 | 96 | 96 | 1920 | 960 |
| 7 | 4 | 0.025 | 6 | 2 | 74 | 74 | 2960 | 1480 |
| 8 | 4 | 0.01 | 6 | 2 | 29 | 29 | 2900 | 1450 |
| 9 | 4 | 0.05 | 8 | 2 | 97 | 97 | 1940 | 970 |
| 10 | 1 | 0.05 | 2 | 2 | 14 | 10 | 200 | 100 |
| 11 | 1 | 0.05 | 4 | 2 | 60 | 55 | 1091 | 546 |
| 12 | 1 | 0.05 | 6 | 2 | 75 | 74 | 1475 | 738 |
| 13 | 1 | 0.05 | 8 | 2 | 89 | 89 | 1782 | 891 |
| 14 | 2 | 0.05 | 2 | 2 | 3 | 1 | 20 | 10 |
| 15 | 2 | 0.05 | 4 | 2 | 6 | 5 | 99 | 50 |
| 16 | 2 | 0.05 | 6 | 2 | 7 | 7 | 140 | 70 |
| 17 | 2 | 0.05 | 8 | 2 | 10 | 9 | 179 | 90 |
| 18 | 3 | 0.05 | 2 | 2 | 58 | 58 | 1160 | 580 |
| 19 | 3 | 0.05 | 4 | 2 | 89 | 89 | 1767 | 884 |
| 20 | 3 | 0.05 | 6 | 2 | 91 | 91 | 1828 | 914 |
| 21 | 3 | 0.05 | 8 | 2 | 94 | 94 | 1900 | 950 |
The 1H-NMR spectra of the post-reaction mixtures shown that an increase in CO2 pressure from 2 to 8 bar did not influence the worsening of selective conversion of E1 to CC1 in the presence of 4 as could be expected based on the data previously published by the Darensbourg group which observed the formation of linear polycarbonates at higher CO2 pressures.40 In the presence of complex 4, the selectivity to CC1 was almost quantitative, even at 8 bar. Probably, the relatively high temperature applied, 120 °C, favours the formation of CC1 as a thermodynamic product, making the formation of an appropriate linear polycarbonate hard (a kinetic product).18
To examine in more detail the effect of the structure of the pyridinium chloride units on the catalytic activity of the modified salophen complexes, a series of additional experiments using complexes 1–3 were also performed under the elevated pressure of CO2 (Table 2, entries 10–21). A strong dependence was found between the catalytic activity and the applied CO2 pressure for 1 and 3, similarly to that observed when examining the effect of the CO2 pressure on the activity of 4. For 1, when the pressure was elevated from 2 to 4 bar, the conversion of E1 increased more than four times, from 14% to 60%. The use of 3 under the pressure of 4 bars resulted in obtaining 89% conversion of E1, i.e. approximately by 30% higher than obtained under 2 bars and on the same level similar to that obtained for 1 under twice larger pressure.
The increase in CO2 pressure from 2 to 8 bar did not have a so spectacular effect on the activity of complex 2 for which the conversion of E1 increased from 3 to 10%.
As mentioned earlier, catalysts 1 and 2 have two types of active centers in their structure: acidic metallic centers and basic chloride ions that participate in the activation of the epoxide molecule. Catalysts 3–5, in addition to those two, have the third type of active centers in their structure, tertiary amine units. This future makes additional activation of CO2 molecules possible, which increases its reactivity toward epoxide and results in an improvement in catalytic activity compared to catalysts that do not have this functionality.
An effect of catalyst concentration on the conversion of CO2 in the reaction with E1 was also studied under a CO2 pressure of 6 bar. The concentration of 4 was changed in the range of 0.01–0.05 mol% since the use of more than 0.05 mol% was limited by the complex solubility in E1. The reduction in catalyst concentration twice resulted in a decrease in E1 conversion from 96 to 74% (Table 2, entries 6 and 7), while five times to 29% (Table 2, entry 8). The high TOF values obtained for the experiments with 0.025 and 0.01 mol% 4, which were, respectively, 1480 and 1450 h−1, proved the high catalytic activity of this complex in the cycloaddition of CO2 to E1 under the applied reaction conditions.
Having optimized reaction conditions, a series of twelve terminal epoxides were next applied to explore the scope of the use of 4 in the cycloaddition reaction of CO2. Epoxides that have both electron-withdrawing groups (EWG) and electron-donating groups (EDG) were examined. The appropriate two-hour experiments were performed using the following conditions: 0.05 mol% 4, 120 °C and 6 bar CO2. All cyclic carbonates obtained as products were isolated from the post-reaction mixtures by column chromatography and their structure was confirmed on the basis of 1H-NMR and 13C-NMR spectra (for more details, see ESI†). Generally, similar values of the yields of appropriate products were obtained based on the isolated carbonates to those evaluated by 1H-NMR spectra analysis (Table 3). Greater discrepancies between the values of epoxide conversion and the yields of isolated products were found for propylene and butylene oxide, that is, for the reactions that included epoxides characterized by low values of boiling points. However, these findings do not should be connected with occurring any side-transformations of these two epoxides since no additional signals of side products were found in the 1H-NMR spectra of the appropriate post-reaction mixtures.
| No. | R | Epoxide/cyclic carbonate | Conversion of epoxidea [%] | Yield of cyclic carbonatea,d [% (%)] | TONb [—] | TOFc [h−1] |
|---|---|---|---|---|---|---|
| a Determined based on 1H-NMR spectra of the reaction mixture with biphenyl as an internal standard.b Calculated as mol of carbonate/mol of catalyst.c Calculated as TON/time [h].d Isolated by column chromatography. | ||||||
| 1 | Me | E2/CC2 | 93 | 74 (71) | 1480 | 740 |
| 2 | Et | E3/CC3 | 80 | 62 (60) | 1240 | 620 |
| 3 | Bu | E4/CC4 | 46 | 40 (41) | 800 | 400 |
| 4 | Hex | E5/CC5 | 23 | 22 (19) | 440 | 220 |
| 5 | Ph | E6/CC6 | 44 | 36 (35) | 720 | 360 |
| 6 | CH2Cl | E7/CC7 | 95 | 94 (91) | 1880 | 940 |
| 7 | CH2OH | E8/CC8 | 95 | 94 (92) | 1880 | 940 |
| 8e | 88 | 84 (81) | 1680 | 5045 | ||
| 9 | CH2OtBu | E9/CC9 | 53 | 46 (46) | 920 | 460 |
| 10 | CH2OPh | E1/CC1 | 96 | 96 (92) | 1920 | 960 |
| 11 |  |
E10/CC10 | 0 | 0 | — | — |
| 12 | 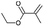 |
E11/CC12 | 86 | 81 (78) | 1620 | 810 |
When the reactivity of the epoxides was compared, some electronic and steric effects were noticed. First, the decrease in epoxide reactivity in order of propylene oxide (R = Me, yield 74%) to epoxy-1,2-octene (R = n-C6H13, yield 22%) appears to correlate with the decrease in donor properties of alkyl substituents, which results from weakening the induction effect of the CH3 group with increasing a number of CH2 groups (Table 3, entries 1–4). Second, the presence of EWG groups generally advantageously influences the reactivity of appropriate epoxides. For epoxides that have the –CH2Cl, –CH2OH, and –CH2OPh groups, yield values of 94–96% were observed (Table 3, entries 6, 7 and 10). In the case of glycidol, the 84% yield of cyclocarbonate (TOF = 5045 h−1) could be obtained just in the 20 minute experiment (Table 3, entry 8). The reactivity of glycidyl methacrylate (E6) appears to be only slightly lower compared to that observed for phenyl glycidyl ether (E1), epichlorohydrin (E7), and glycidol (E8) (Table 3, entry 12). However, the presence of substituents such as the –Ph (E6) and –OCH2tBu (E9) groups, clearly negatively influences the reactivity of the appropriate epoxides (Table 3, entries 5 and 9). Its bulky structure appears to be responsible for that. Finally, allyl glycidyl ether (E10) was found to be completely inactive in the CO2 cycloaddition carried out in the presence of 4 (Table 3, entry 11).
When comparing the activity of our catalysts with the activity of other homogeneous catalysts examined previously in the cycloaddition of CO2 to phenyl glycidyl ether,31,32,42,55,66,74–76 it was difficult to find ones that were used under similar reaction conditions (Table 4). Both metal complex catalysts (also bifunctional) and organocatalytic systems were taken into account. Generally, higher catalyst concentrations and higher CO2 pressure were required to obtain a yield of cyclic carbonates comparable to that obtained in the presence of 4 under the conditions applied in this work. Furthermore, it was noticed that to improve the activity of bifunctional catalytic systems a co-catalyst was used in some cases. For example, DMAP was also used in addition when the cycloaddition was conducted in the presence of a salen cobalt(III) complex that had two units of phosphonium chloride built into its molecule.74 Even then, the 95% yield of the proper cyclic carbonate was obtained in 4 hours when 1 mol% catalyst concentration, 100 °C, and 40 bar CO2 were applied (Table 4, entry 5). For comparison, in our work, a much lower catalyst concentration, 2 bar CO2 at 120 °C resulted in the 96% cyclic carbonate yield obtained after 2 hours.
| No. | Catalyst | T [°C] | P [bar] | t [h] | Conversion [%] | Yield [%] | TON | TOF [h−1] | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | NEt3–I2 (0.5 mol%) | 90 | 20 | 20 | 100 | 98 | 196 | 10 | 75 |
| 2 | 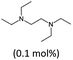 |
120 | 10 | 12 | 83 | — | 826 | 69 | 55 |
| 3 | 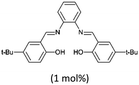 |
120 | 10 | 3.5 | 93 | 88 | 88 | 25 | 66 |
| 4 | 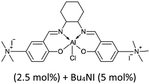 |
35 | 10 | 24 | 100 | 78 | 31 | 1 | 31 |
| 5 |  |
100 | 40 | 4 | — | 95 | 95 | 24 | 74 |
| 6 |  |
45 | 10 | 18 | 80 | 83 | 24 | 1 | 76 |
| 7 |  |
25 | 1 | 24 | 77 | 72 | 29 | 1 | 42 |
| 8 |  |
25 | 1 | 24 | 100 | 87 | 58 | 2 | 32 |
| 9 | 4 (0.05 mol%) | 120 | 2 | 2 | 71 | 70 | 1400 | 700 | This work |
| 10 | 4 (0.025 mol%) | 120 | 6 | 2 | 74 | 74 | 2960 | 1480 | This work |
Conclusions
Catalytic experiments carried out for a series of novel chromium(III) salophen complexes that have units of three different pyridinium chlorides built into their molecules showed that the structure of onium salts strongly influences the catalytic properties of the complexes in the cycloaddition reactions of CO2 to epoxides. It turned out that the introduction of the unit(s) of 4-(dimethylamino)pyridinium chloride (DMAP-Cl) into salophen molecules (complexes 3–5) provides the single component catalysts that are much more active than those that have units of pyridinium chloride (1) or 2,6-dimethylpyridinium chloride (2). A key role in an improvement in the catalytic activity of chromium(III) salophen complexes underwent the presence of an additional tertiary amine group introduced with 4-(dimethylamino)pyridine into the salicylaldehyde moiety. Tertiary amine units allow CO2 molecules to be activated additionally, in addition to the possible activation of epoxide molecules throughout the interaction with chromium(III) ions and chloride ions, thus increasing the rate of CO2 cycloaddition to epoxides. The most active of the catalysts examined, complex 4 with a single unit of 4-(dimethylamino)pyridinium chloride, showed a TOF value up to 1480 h−1 in the reaction of CO2 with phenyl glycidyl ether (E1 conversion 74%, CC1 selectivity >99%). This complex was also used successfully for the cycloaddition reactions that included nine other epoxides with the EWG or EDG substituents. It was characterized by relatively high catalytic activity (TOF = 220–5045 h−1) and high selectivity in the conversion of these epoxides to the appropriate cyclic carbonate under the reaction conditions applied.Author contributions
K. B.: conceptualization, methodology, investigation, writing – original draft, writing – review & editing. W. B.: conceptualization, writing – original draft, writing – review & editing. A. K.: investigation, writing – review & editing. A. B.: writing – review & editing. M. P.: investigation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Podkarpackie Innovation Center (PCI, Grant No. N2_161).References
- M. Peters, B. Köhler, W. Kuckshinrichs, W. Leitner, P. Markewitz and T. E. Müller, Chemical Technologies for Exploiting and Recycling Carbon Dioxide into the Value Chain, ChemSusChem, 2011, 4, 1216 CrossRef CAS PubMed.
- R. Martin and A. W. Kleij, Myth or Reality? Fixation of Carbon Dioxide into Complex Organic Matter under Mild Conditions, ChemSusChem, 2011, 4, 1259 CrossRef CAS PubMed.
- Q. Liu, L. Wu, R. Jackstell and M. Beller, Using carbon dioxide as a building block in organic synthesis, Nat. Commun., 2015, 6, 5933 CrossRef PubMed.
- M. D. Burkart, N. H. Cathy, L. Tway and E. L. Zeitler, Opportunities and Challenges for Catalysis in Carbon Dioxide Utilization, ACS Catal., 2019, 9, 7937 CrossRef CAS.
- A. Raza, R. Gholami, R. Rezaee, V. Rasouli and M. Rabiei, Significant aspects of carbon capture and storage - A review, Petroleum, 2019, 5, 335 CrossRef.
- I. Ghiat and T. Al-Ansari, A review of carbon capture and utilization as a CO2 abatement opportunity within the EWF nexus, J. CO2 Util., 2021, 45, 101432 CrossRef CAS.
- S. Sun, H. Sun, P. T. Williams and C. Wu, Recent advances in integrated CO2 capture and utilization: a review, Sustainable Energy Fuels, 2021, 5, 4546 RSC.
- T. Weidlich and B. Kamenická, Utilization of CO2-Available Organocatalysts for Reactions with Industrially Important Epoxides, Catalysts, 2022, 12, 298 CrossRef CAS.
- M. North, R. Pasquale and C. Young, Synthesis of cyclic carbonates from epoxides and CO2, Green Chem., 2010, 12, 1514 RSC.
- P. P. Pescarmona, M. Taherimehr, P. P. Pescarmona and M. Taherimehr, Challenges in the catalytic synthesis of cyclic and polymeric carbonates from epoxides and CO2, Catal. Sci. Technol., 2012, 2, 2169 RSC.
- C. Maeda, Y. Miyazaki and T. Ema, Recent progress in catalytic conversions of carbon dioxide, Catal. Sci. Technol., 2014, 4, 1482 RSC.
- C. Martín, G. Fiorani and A. W. Kleij, Recent Advances in the Catalytic Preparation of Cyclic Organic Carbonates, ACS Catal., 2015, 5, 1353 CrossRef.
- J. W. Comerford, I. D. V. Ingram, M. North and X. Wu, Sustainable metal-based catalysts for the synthesis of cyclic carbonates containing five-membered rings, Green Chem., 2015, 17, 1966 RSC.
- H. Büttner, L. Longwitz, J. Steinbauer, C. Wulf and T. Werner, Developments in the Synthesis of Cyclic Carbonates from Epoxides and CO2, Top. Curr. Chem., 2017, 375, 1 CrossRef PubMed.
- A. J. Kamphuis, F. Picchioni and P. P. Pescarmona, CO2-fixation into cyclic and polymeric carbonates: principles and applications, Green Chem., 2019, 21, 406 RSC.
- F. D. Monica and A. W. Kleij, Mechanistic guidelines in nonreductive conversion of CO2: the case of cyclic carbonates, Catal. Sci. Technol., 2020, 10, 3483 RSC.
- T. Yan, H. Liu, Z. X. Zeng and W. G. Pan, Recent progress of catalysts for synthesis of cyclic carbonates from CO2 and epoxides, J. CO2 Util., 2023, 68, 102355 CrossRef CAS.
- D. J. Darensbourg, Complexes as Catalysts for the Production of Polycarbonates from Epoxides and CO2, Chem. Rev., 2007, 107, 2388 CrossRef CAS PubMed.
- Y. Wang and D. J. Darensbourg, Carbon dioxide-based functional polycarbonates: Metal catalyzed copolymerization of CO2 and epoxides, Coord. Chem. Rev., 2018, 372, 85 CrossRef CAS.
- Y. Xu, L. Lin, M. Xiao, S. Wang, A. T. Smith, L. Su and Y. Meng, Synthesis and properties of CO2-based plastics: Environmentally-friendly, energy-saving and biomedical polymeric materials, Prog. Polym. Sci., 2018, 80, 163 CrossRef CAS.
- W. Yu, E. Maynard, V. Chiaradia, M. C. Arno and A. P. Dove, Aliphatic Polycarbonates from Cyclic Carbonate Monomers and Their Application as Biomaterials, Chem. Rev., 2021, 121, 10865 CrossRef CAS PubMed.
- J. Feng, R.-X. Zhuo and X.-Z. Zhang, Construction of functional aliphatic polycarbonates for biomedical applications, Prog. Polym. Sci., 2012, 37(2), 211 CrossRef CAS.
- X. Yu, M. Xiao, S. Wang, D. Han and Y. Meng, Fabrication and Properties of Crosslinked Poly(propylenecarbonate maleate) Gel Polymer Electrolyte for Lithium-Ion Battery, J. Appl. Polym. Sci., 2010, 118, 2078 CrossRef CAS.
- X.-Y. Yu, M. Xiao, S.-J. Wang, Q.-Q. Zhao and Y.-Z. Meng, Fabrication and Characterization of PEO/PPC Polymer Electrolyte for Lithium-Ion Battery, J. Appl. Polym. Sci., 2010, 115, 2718 CrossRef CAS.
- M. D. Konieczynska, X. Lin, H. Zhang and M. W. Grinstaff, Synthesis of Aliphatic Poly(ether 1,2-glycerol carbonate)s via Copolymerization of CO2 with Glycidyl Ethers Using a Cobalt Salen Catalyst and Study of a Thermally Stable Solid Polymer Electrolyte, ACS Macro Lett., 2015, 4, 533 CrossRef CAS PubMed.
- B. Sun, J. Mindemark, K. Edström and D. Brandell, Realization of high performance polycarbonate-based Li polymer batteries, Electrochem. Commun., 2015, 52, 71 CrossRef CAS.
- K. Kimura, J. Hassoun, S. Panero, B. Scrosati and Y. Tominaga, Electrochemical properties of a poly(ethylene carbonate)-LiTFSI electrolyte containing a pyrrolidinium-based ionic liquid, Ionics, 2015, 21, 895 CrossRef CAS.
- X. Huang, J. Huang, J. Wu, X. Yu, Q. Gao, Y. Luo and H. Hu, Fabrication and properties of polybutadiene rubber-interpenetrating cross-linking poly(propylene carbonate) network as gel polymer electrolytes for lithium-ion battery, RSC Adv., 2015, 5, 52978 RSC.
- M. North and C. Young, Bimetallic aluminium(acen) complexes as catalysts for the synthesis of cyclic carbonates from carbon dioxide and epoxides, Catal. Sci. Technol., 2011, 1, 93 RSC.
- M. North, P. Villuendas and C. Young, Inter- and intramolecular phosphonium salt cocatalysis in cyclic carbonate synthesis catalysed by a bimetallic aluminium(salen) complex, Tetrahedron Lett., 2012, 53, 2736 CrossRef CAS.
- Y. A. Rulev, Z. Gugkaeva, V. I. Maleev, M. North and Y. N. Belokon, Robust bifunctional aluminium-salen catalysts for the preparation of cyclic carbonates from carbon dioxide and epoxides, Beilstein J. Org. Chem., 2015, 11, 1614 CrossRef CAS PubMed.
- J. A. Castro-Osma, M. North and X. Wu, Synthesis of Cyclic Carbonates Catalysed by Chromium and Aluminium Salphen Complexes, Chem.–Eur. J., 2016, 22, 2100 CrossRef CAS PubMed.
- X. Wu and M. North, Bimetallic Aluminium(Salphen) Complex for the Synthesis of Cyclic Carbonates from Epoxides and Carbon Dioxide, ChemSusChem, 2017, 10, 74 CrossRef CAS PubMed.
- M. Strianese, D. Pappalardo, M. Mazzeo, M. Lamberti and C. Pellecchia, Salen-type aluminum and zinc complexes as two-faced Janus compounds: contribution to molecular sensing and polymerization catalysis, Dalton Trans., 2020, 49, 16533 RSC.
- H. Fish, S. Hart, K. J. Lamb, M. North, S. C. Z. Quek, A. C. Whitwood, B. Woodsa and X. Wua, Structural analysis of five-coordinate aluminium(salen) complexes and its relationship to their catalytic activity, Dalton Trans., 2021, 50, 587 RSC.
- X.-B. Lu and D. J. Darensbourg, Cobalt catalysts for the coupling of CO2 and epoxides to provide polycarbonates and cyclic carbonates, Chem. Soc. Rev., 2012, 41, 1462 RSC.
- Y. A. Rulev, V. A. Larionov, A. V. Lokutova, M. A. Moskalenko, O. L. Lependina, V. I. Maleev, M. North and Y. N. Belokon, Chiral Cobalt(III) Complexes as Bifunctional Brönsted Acid-Lewis Base Catalysts for the Preparation of Cyclic Organic Carbonates, ChemSusChem, 2016, 9, 216 CrossRef CAS PubMed.
- F. Zhou, S.-L. Xie, X.-T. Gao, R. Zhang, C.-H. Wang, G.-Q. Yin and J. Zhou, Activation of (salen)CoI complex by phosphorane for carbon dioxide transformation at ambient temperature and pressure, Green Chem., 2017, 19, 3908 RSC.
- J. Huang, Y. Xu, M. Wang and Z. Duan, Copolymerization of propylene oxide and CO2 catalyzed by dinuclear salcy-CoCl complex, J. Macromol. Sci., Part A: Pure Appl.Chem., 2020, 57(2), 131 CrossRef CAS.
- D. J. Darensbourg, R. M. Mackiewicz and D. R. Billodeaux, Pressure Dependence of the Carbon Dioxide/Cyclohexene Oxide Coupling Reaction Catalyzed by Chromium Salen Complexes. Optimization of the Comonomer-Alternating Enchainment Pathway, Organometallics, 2005, 24(1), 144 CrossRef CAS.
- X. Zhang, Y.-B. Jia, X.-B. Lu, B. Li, H. Wang and L.-C. Sun, Intramolecularly two-centered cooperation catalysis for the synthesis of cyclic carbonates from CO2 and epoxides, Tetrahedron Lett., 2008, 49, 6589 CrossRef CAS.
- J. A. Castro-Osma, K. J. Lamb and M. North, Cr(salophen) Complex Catalyzed Cyclic Carbonate Synthesis at Ambient Temperature And Pressure, ACS Catal., 2016, 6, 5012 CrossRef CAS.
- M. Balas, L. K. Bidi, F. Launay and R. Villanneau, Chromium-Salophen as a Soluble or Silica-Supported Co-Catalyst for the Fixation of CO2 Onto Styrene Oxide at Low Temperatures, Front. Chem., 2021, 9, 765108 CrossRef CAS PubMed.
- I. Grimaldi, F. Santulli, M. Lamberti and M. Mazzeo, Chromium Complexes Supported by Salen-Type Ligands for the Synthesis of Polyesters, Polycarbonates, and Their Copolymers through Chemoselective Catalysis, Int. J. Mol. Sci., 2023, 24, 7642 CrossRef CAS PubMed.
- M. Taherimehr, A. Decortes, S. M. Al-Amsyar, W. Lueangchaichaweng, C. J. Whiteoak, E. C. Escudero-Adán, A. W. Kleij and P. P. Pescarmona, A highly active Zn(salphen) catalyst for production of organic carbonates in a green CO2 medium, Catal. Sci. Technol., 2012, 2, 2231 RSC.
- C. Martín, C. J. Whiteoak, E. Martin, M. Martínez Belmonte, E. C. Escudero-Adána and A. W. Kleij, Easily accessible bifunctional Zn(salpyr) catalysts for the formation of organic carbonates, Catal. Sci. Technol., 2014, 4, 1615 RSC.
- M. A. Fuchs, S. Staudt, C. Altesleben, O. Walter, T. A. Zevaco and E. Dinjusa, A new air-stable zinc complex based on a 1,2-phenylene-diimino-2-cyanoacrylate ligand as an efficient catalyst of the epoxide-CO2 coupling, Dalton Trans., 2014, 43, 2344 RSC.
- S. A. Kuznetsova, Y. A. Rulev, V. A. Larionov, A. F. Smol'yakov, Y. V. Zubavichus, V. I. Maleev, H. Li, M. North, A. S. Saghyan and Y. N. Belokon, Self-Assembled Ionic Composites of Negatively Charged Zn(salen) Complexes and Triphenylmethane Derived Polycations as Recyclable Catalysts for the Addition of Carbon Dioxide to Epoxides, ChemCatChem, 2019, 11, 511 CrossRef CAS.
- A. C. Kathalikkattil, R. Babu, J. Tharun, R. Roshan and D.-W. Park, Advancements in the Conversion of Carbon Dioxide to Cyclic Carbonates Using Metal Organic Frameworks as Catalysts, Catal. Surv. Asia, 2015, 19, 223 CrossRef CAS.
- C. Calabrese, F. Giacalone and C. Aprile, Hybrid Catalysts for CO2 Conversion into Cyclic Carbonates, Catalyst, 2019, 9(4), 325 CrossRef.
- A. A. Marciniak, K. J. Lamb, L. P. Ozorio, C. J. A. Mota and M. North, Heterogeneous catalysts for cyclic carbonate synthesis from carbon dioxide and epoxides, Curr. Opin. Green Sustainable Chem., 2020, 26, 100365 CrossRef.
- Z.-J. Li, J.-F. Sun, Q.-Q. Xu and J.-Z. Yin, Homogeneous and Heterogeneous Ionic Liquid System: Promising "Ideal Catalysts" for the Fixation of CO2 into Cyclic Carbonates, ChemCatChem, 2021, 13, 1848 CrossRef CAS.
- L. Guo, K. J. Lamb and M. North, Recent developments in organocatalysed transformations of epoxides and carbon dioxide into cyclic carbonates, Green Chem., 2021, 23, 77 RSC.
- R. A. Shiels and C. W. Jones, Homogeneous and heterogeneous 4-(N,N-dialkylamino)pyridines as effective single component catalysts in the synthesis of propylene carbonate, J. Mol. Catal. A: Chem., 2007, 261, 160 CrossRef CAS.
- W. Cho, M. S. Shin, S. Hwang, H. Kim, M. Kim, J. G. Kim and Y. Kim, Tertiary amines: A new class of highly efficient organocatalysts for CO2 fixations, J. Ind. Eng. Chem., 2016, 44, 210 CrossRef CAS.
- M. North, P. Villuendas and C. Young, Inter- and intramolecular phosphonium salt cocatalysis in cyclic carbonate synthesis catalysed by a bimetallic aluminium(salen) complex, Tetrahedron Lett., 2012, 53, 2736 CrossRef CAS.
- T. Ema, Y. Miyazaki, J. Shimonishi, C. Maeda and J. Hasegawa, Bifunctional Porphyrin Catalysts for the Synthesis of Cyclic Carbonates from Epoxides and CO2: Structural Optimization and Mechanistic Study, J. Am. Chem. Soc., 2014, 136, 15270 CrossRef CAS PubMed.
- P. G. Cozzi, Metal-Salen Schiff base complexes in catalysis: practical aspects, Chem. Soc. Rev., 2004, 33, 410 RSC.
- A. W. Kleij, Nonsymmetrical Salen Ligands and Their Complexes: Synthesis and Applications, Eur. J. Inorg. Chem., 2009, 2, 193 CrossRef.
- J. F. Larrow, E. N. Jacobsen, Y. Gao, Y. Hong, X. Nie and C. M. Zepp, Practical Method for the Large-Scale Preparation of [N,N'-Bis(3,5-di-tertbutylsalicylidene)-1,2-cyclohexanediaminato(2-)]manganese(III) chloride, a Highly Enantioselective Epoxidation Catalyst, J. Org. Chem., 1994, 59(7), 1939 CrossRef CAS.
- G. Casiraghi, G. Casnati, G. Puglia, G. Sartori and G. Terenghi, Selective reactions between phenols and formaldehyde. A novel route to salicylaldehydes, J. Chem. Soc., Perkin Trans. 1, 1980, 1862 RSC.
- P. Meier, F. Broghammer, K. Latendorf, G. Rauhut and R. Peters, Cooperative Al(Salen)-Pyridinium Catalysts for the Asymmetric Synthesis of trans-Configured β-Lactones by [2+2]-Cyclocondensation of Acylbromides and Aldehydes: Investigation of Pyridinium Substituent Effects, Molecules, 2012, 17(6), 7121 CrossRef CAS PubMed.
- M. Mechler, K. Latendorf, W. Frey and R. Peters, Homo- and Heterobimetallic Pd-, Ag-, and Ni-Hybrid Salen-Bis-NHC Complexes, Organometallics, 2013, 32(1), 112 CrossRef CAS.
- K. Latendorf, M. Mechler, I. Schamne, D. Mack, W. Frey and R. Peters, Titanium Salen Complexes with Appended Silver NHC Groups as Nucleophilic Carbene Reservoir for Cooperative Asymmetric Lewis Acid/NHC Catalysis, Eur, J. Org. Chem., 2017, 28, 4140 Search PubMed.
- Á. Mesías-Salazar, Y. R. Yepes, J. Martínez and R. S. Rojas, Highly Active CO2 Fixation into Cyclic Carbonates Catalyzed by Tetranuclear Aluminum Benzodiimidazole-Diylidene Adducts, Catalysts, 2021, 11, 2 CrossRef.
- X. Wu, C. Chen, Z. Guo, M. North and A. C. Whitwood, Metal- and Halide-Free Catalyst for the Synthesis of Cyclic Carbonates from Epoxides and Carbon Dioxide, ACS Catal., 2019, 9(3), 1895 CrossRef CAS.
- C.-H. Guo, M. Lianga and H. Jiao, Cycloaddition mechanisms of CO2 and epoxide catalyzed by salophen - an organocatalyst free from metals and halides, Catal. Sci. Technol., 2021, 11, 2529 RSC.
- Y.-M. Shen, W.-L. Duan and M. Shi, Chemical Fixation of Carbon Dioxide Co-Catalyzed by a Combination of Schiff Bases or Phenols and Organic Bases, Eur. J. Org Chem., 2004, 3080 CrossRef CAS.
- T. Hirose, S. Qu, K. Kodama and X. Wang, Organocatalyst system for disubstituted carbonates from cycloaddition between CO2 and internal epoxides, J. CO2 Util., 2018, 24, 261 CrossRef CAS.
- Z. Guo, Y. Hu, S. Dong, L. Chen, L. Ma, Y. Zhou, L. Wang and J. Wang, “Spring-loaded’’ mechanism for chemical fixation of carbon dioxide with epoxides, Chem Catal., 2022, 2, 519 CrossRef CAS.
- R. Dalpozzo, N. Della Ca', B. Gabriele and R. Mancuso, Catalysts, 2019, 9, 511 CrossRef.
- J. Sun, J. Wang, W. Cheng, J. Zhang, X. Li, S. Zhang and Y. She, Chitosan functionalized ionic liquid as a recyclable biopolymer-supported catalyst for cycloaddition of CO2, Green Chem., 2012, 14, 654 RSC.
- M. B. Smith and J. March, March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, John Wiley & Sons, Inc., Hoboken, NJ, 6th edn, 2007 Search PubMed.
- C.-X. Miao, J.-Q. Wang, Y. Wu, Y. Du and L.-N. He, Bifunctional Metal-Salen Complexes as Efficient Catalysts for the Fixation of CO2 with Epoxides under Solvent-Free Conditions, ChemSusChem, 2008, 1, 236 CrossRef CAS PubMed.
- B. Chowdhury, A. A. Zvinchuk, R. R. Aysin, E. A. Khakina, P. V. Cherkasova and S. E. Lyubimov, Amine-Iodine Adducts as Simple but Effective Catalysts for the Synthesis of Organic Carbonates from Epoxides and CO2, Catal. Surv. Asia, 2021, 25, 419 CrossRef CAS.
- A. Decortes, M. M. Belmonte, J. Benet-Buchholza and A. W. Kleij, Chem. Commun., 2010, 46, 4580 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed information regarding all experimental procedures and spectral characteristics of the compounds obtained in this work is included therein. See DOI: https://doi.org/10.1039/d3ra07750k |
| This journal is © The Royal Society of Chemistry 2024 |