Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nano-enabled antimicrobial thin films: design and mechanism of action
Bilisuma Fekadu Finina
ac and
Anteneh Kindu Mersha
 *ab
*ab
aDepartment of Industrial Chemistry, Addis Ababa Science and Technology University, Addis Ababa, Ethiopia
bNanotechnology Center of Excellence, Addis Ababa Science and Technology University, Addis Ababa, Ethiopia
cDepartment of Chemistry, Kotebe University of Education, Addis Ababa, Ethiopia
First published on 9th February 2024
Abstract
Antimicrobial thin films are types of protective coatings that are applied to surfaces such as medical devices, food packaging materials, water-resistant coatings, and other systems. These films prevent and reduce the spread of microbial organisms, including bacteria, fungi, and viruses. Antimicrobial thin films can be prepared from a variety of nanostructured materials including metal nanoparticles, metal oxides, plant materials, enzymes, bacteriocins and polymers. Their antimicrobial mechanism varies mostly based on the types of active agents from which the film is made of. Antimicrobial thin films are becoming increasingly popular microbial treatment methods due to their advantages such as enhanced stability, reduced toxicity levels, extended effectiveness over time and broad spectrum antimicrobial action without side effects on human health or the environment. This popularity and enhanced performance is mainly due to the extended possibility of film designs. Thin films offer convenient formulation methods which makes them suitable for commercial practices aiming at high turnover rates along with residential applications requiring frequent application cycles. This review focuses on recent developments in the possible processing methods and design approaches for assembling the various types of antimicrobial materials into nanostructured thin film-based delivery systems, along with mechanisms of action against microbes.
1. Introduction
Microbes have become a major concern in health care sectors,1,2 water treatment,3 food industries,4,5 and textile industries.6 Establishing an efficient means to control these pathogenic microbes, therefore, has become a primary challenge among the research community.7 Use of thin films and other nanotechnology-assisted antimicrobial systems is a viable alternative either to kill bacteria or inhibit the bacterial adhesion to surfaces.8 Thin films are believed to be highly convenient drug delivery methods because of their capabilities to improve the onset of drug action, improve target drug delivery, reduce the dose frequency, enhance the drug efficacy, and minimize microbial resistance and side effects to the host.9 Membranes, a similar class of flat sheet materials with molecular permeation properties are also excellent platforms to incorporate nanostructured microbicidal agents.10Thin films (TFs), also known as thin-film coatings, are a thin layer of materials applied to a substrate. They typically have a thickness of less than a few hundred nanometers along one direction and extended length along another direction with a large surface area to volume ratio.11 TFs and membranes can be supported (i.e., coated on a substrate surface like adhesive coatings) or self-standing like wound dressing breathable films – depending on the preparation method and intended application. Various methods that have their own advantages and disadvantages are employed to prepare TFs and coatings; including simple methods (dip-coating, spin-coating, spray-coating, blade coating, and roll-coating) and those that require sophisticated equipment (such as physical and chemical vapour deposition techniques), which are reviewed elsewhere.12
Antimicrobial thin films (ATFs) are thin film structures that possess either inherent or nano-assisted microbicidal property. They are becoming increasingly popular because of their wide array of applications in various fields, mainly in wound dressing, food packaging, textile finishing, and anti-fouling water treatment membranes. ATFs can be used to prevent the growth of harmful microorganisms such as fungi and bacteria on a variety of surfaces through tailoring functionality to target specific microorganisms in various engineered designs.13 Designing an ideal antimicrobial thin film requires careful consideration into multitude factors including nature of active agent material, stability against external environmental triggers, manufacturing techniques, geometrical requirements for adherence to substrate material/surface, physical advantages e.g., breathability, suitability for application, release pattern, toxicity limits, exhaustive compatibility against interacting particles and host material.9
The mechanism of action behind nano-enabled antimicrobial thin film is that chemical biocides embedded in films are commonly used for anti-microbial action. For instance, cationic residues in thin film commonly first adhere to anionic lipid head groups in the negatively charged membrane surface of bacteria via the electrostatic interactions followed by the efficient insertion of their hydrophobic groups into the non-polar bacterial membrane, thereby leading to membrane permeance, cytoplasmic leakage, and bacterial death. Another mechanism of action could be penetration of active agents through microbial cell membranes by oxidation reaction or simply by dissolving into cells via electrochemical transduction processes.14
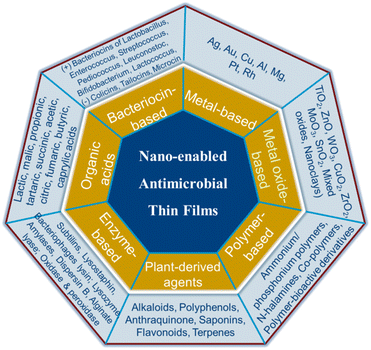
In this review, the various classes of nanostructured thin film materials (metallic, metal oxide, polymeric, plant-bioactives, enzymatic, organic acids and bacteriocin) with inherent or nano-enabled antimicrobial properties are discussed. In each class of materials, the research progress on the processing methods and design approaches for assembling antimicrobial agents into functional thin film based delivery systems are presented. Furthermore, the mechanisms of action against microbes, as well as their application in different areas where microbial prevalence is common are addressed.
2. General principle of antimicrobial action
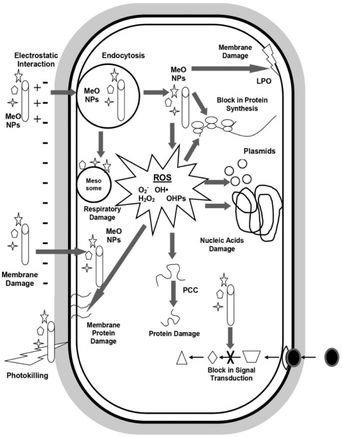
Antimicrobials are medicines which are used for treatment or prevention of infections caused by microbes.15 The activity of an antibacterial agent is mostly attributed to two mechanisms; interfering chemically with the production or function of essential bacterium components, and/or evading the conventional antibacterial resistance mechanisms. These mechanisms are depicted in Fig. 1, and as can be seen, the antibacterial agents have a variety of targets, including (I) bacterial protein biosynthesis; (II) bacterial cell-wall biosynthesis; (III) bacterial cell membrane destruction; (IV) bacterial DNA replication and repair, and (V) inhibition of a metabolic pathway.16 Table 1 also provides a summary of the several antimicrobial activity mechanisms along with examples of commercial antibiotics that work according to each mechanism.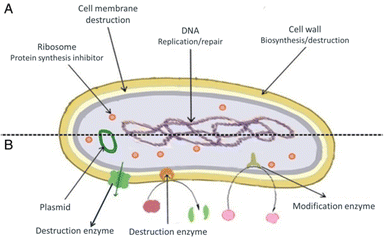 | ||
| Fig. 1 (A) Targets and mechanism for antibacterial action; (B) multiple antibiotic resistance mechanisms in bacteria (reproduced from ref. 16 used under Creative Commons CC-BY license). | ||
| Mechanism of action | Antimicrobial targets | Commercial antibiotics (active via same mechanism) | Ref. |
|---|---|---|---|
| Inhibition of protein synthesis | Bacterial ribosome | Macrolides, tetracyclines, aminoglycosides, and oxazolidinones | 17 |
| Inhibition of bacterial cell-wall biosynthesis | Transglycosylases and transpeptidases enzymes | Beta-lactams (like penicillins, ampicillins, cephalosporins) | 18 |
| Peptidoglycan layer within cell-wall | Vancomycin | 17 | |
| Bacterial cell membrane destruction | Bacterial outer membrane permeability | Polymyxins (polymyxin B and colistin) | 19 |
| Inhibition of DNA replication and repair | DNA gyrase enzyme | Fluoroquinolones, nalidixic acid | 20 |
| Inhibition of metabolic pathway | Folic acid pathway (dihydropteroate synthase enzyme) | Sulfonamides | 21 |
Although antibiotics have historically played a vital role in the prevention and treatment of bacterial infections, there is growing concern that many pathogens develop antimicrobial resistance (AMR), which poses a serious challenge to conventional antibiotic therapies.14 AMR can occur in two ways; intrinsic or acquired. Some types of bacteria have an intrinsic resistance to one or more classes of antimicrobial agents. In these situations, all strain of that bacterial species demonstrate resistance to every member of those classes of antimicrobials. The second and more pressing concern is that bacteria might acquire resistance, where previously vulnerable bacterial populations would start to develop resistance to a certain antibacterial agent.19 Acquired resistance may result from excessive or frequent use of antibiotics.
The possible mechanism for AMR development includes the following pathways;14,19,22 (a) reduced permeability of the bacterial cell wall, restricting antibiotics access to the target sites, (b) overexpression of efflux pumps that actively expels the ingested antibiotics from the bacteria, (c) enzymatic destruction (like amidases, epoxidases, and esterases which are secreted by the bacteria) of the antibiotics to inactivate the antibiotic molecule, (d) alteration of antibiotic targets to avoid the antibiotic binding with the target site or decrease the affinity of antibiotics toward target proteins, (e) altering metabolic pathways of bacteria to dampen the efficacy of antibiotics, and (f) transfer of AMR genes within components in a biofilm via quorum sensing.23–29 It should be noted that antibacterial resistance might relate to a single mechanism or a combination of the different mechanisms.19
3. Nano-enabled antimicrobial thin films: classes, design approaches and mechanisms of action
The growing concern of multidrug-resistant bacteria and subsequent diminished effectiveness of current antibiotics brought an urgent need to either develop new antibacterial agents that are less adaptable to microbial resistance or to restrain bacterial resistance using nano-enabled combinatorial therapy with existing antibiotics. Nanotechnology has proven to offer innovative tools in the design and fabrication of high-performance nanostructured delivery systems for antibacterial therapeutic applications.30,31Nano-enabled ATFs are materials incorporated with nanostructured formulations that have the ability to inhibit microbial growth by themselves or trigger the inhibition of bacterial growth by antibiotics. In the former case, nanostructured materials incorporated in thin films generate antimicrobial active agents such as reactive metal ions, photocatalysts and phytochemicals32,33 which can directly interact with bacteria. In the latter case, smart nanomaterials assembled in thin films (in the form of encapsulation coatings or precursor agents) improve potency of antibiotic drugs via effects like controlled release of active agents,34 synergistic efficacy enhancement,35 targeted antibacterial effects.36,37
On the basis of their active agents, antimicrobial nanocomposite thin film materials can be classified as: metal-based, metal oxide-based, plant extract-based, enzymes-based, organic acid-based, bacteriocin-based and polymer-based (Fig. 2).
Generally, from the view point of nanostructure design, microbicidal agents can be incorporated into thin films, membranes and surface coatings in three ways. (i) Active agents can be deposited on a porous or nonporous surfaces as a thin upper most layer (Fig. 3A). For example, thin-film nanocomposite membranes3,38 which involve the formation of a thin-film layer on a porous polymer matrix, are good examples of such designs. (ii) Blended nano-antimicrobial structures that involve the inclusion of active agents directly into the matrix material are another alternative designs (Fig. 3B). Such structures allow stable incorporation of nano-antimicrobials as the active agents are held in the matrix material leading to reduced leakage.39 Mix matrix membranes40 and tissue implants41 are suitable examples. (iii) The third type of design involves sandwich structures in which active agents are held between two layer structures (Fig. 3C). Such architectures enable controlled and prolonged release of antimicrobial agents, and are suitable to produce wound dressings42 and active food packing films.43
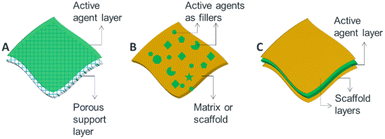 | ||
| Fig. 3 Schematic illustration showing options of incorporating antimicrobial active agents into ATFs: (A) layer or coating approach, (B) blending approach and (C) sandwich structure. | ||
In the following sections, the possible design alternatives of each class of ATFs from the view point of the way active agents are incorporated and immobilized into composite thin film materials and their antimicrobial mechanisms are summarized in Table 3. It should be noted that the discussions on ATFs also involve antimicrobial membranes-considering that the physical meaning of the term thin film encloses membranes as both materials belong to flat sheet structures.
3.1 Metal-based ATFs
Metal nanoparticles are among widely reported materials of promising antimicrobial activity due to their small size and the high surface area-to-volume ratio which gives them a relatively large reactive surface area with which to interact with microbial molecules.44,45 They can be easily complexed with other biomaterials to exert enhanced antibacterial activity.46 For example, thin films based on silver nanoparticles (AgNPs) and gold nanoparticles (AuNPs) are reported to have high antibacterial activity against different bacterial species.47 Copper is another metal known for its good antimicrobial activity with as high as 99.9% bactericidal efficacy.48 Cobalt, indium, tungsten, tin, aluminum, chromium, zinc, manganese, tantalum, and titanium-based thin films are also reported to have good antimicrobial activity.49 Some of the aforementioned metal-based ATFs are discussed in this section.Silver nanoparticle based thin film nanocomposite (TFN) membranes prepared by incorporating AgNPs in high surface area graphene oxide (GO) quantum dot and integrated with highly stable polyamide layer.3 In this design, suitable amount of AgNPs and graphene oxide quantum dot mixture is sonicated to get homogenized mixture followed by deposition of the mixture on the previously prepared polyamide membrane surface to undergo polymerization. The prepared thin film membrane experienced high antibacterial activity against both Gram-negative (98.6%) and Gram-positive (96.5%) bacteria due to synergetic effect and high surface area of graphene oxide quantum dot (GOQD) occupied by AgNPs. It was demonstrated that antibacterial activity appeared in the order of TFC < TFN-GO < TFN-GOQD < TFN-GOQD/Ag membrane, signifying the importance of Ag loading and nanostructure size, which subsequently increased surface area and amount of reactive oxygen species (ROS) generation.
The authors also studied the bactericidal mechanism for GOQD/Ag, where the restriction to respiratory enzymes by AgNPs induced the release of ROS through the oxygenolysis of the cellular components. ROS could oxidize the lipids in the bacterial cell membrane, disturbing the cell metabolism and leading to cell death. They further justified that the high oxidase-like catalytic activity of GOQD/Ag induced an outstanding antibacterial property of the TFN-GOQD/Ag membrane via increased ROS generation.
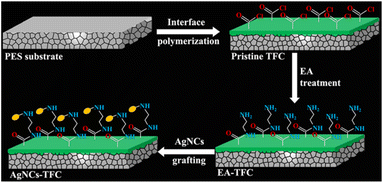
In another study, Qingquan. G et al.50 designed polymeric thin film membrane decorated with ultra-small silver nano-cluster (AgNCs) encapsulated in layer of thiolate ligands. Fig. 4 shows the fabricated thin film with highly stable AgNCs-thiolate ligands complex deposited on polymeric membrane surface. The strong bonding between thiolate ligands and AgNCs could be responsible for slow-release of Ag ions, which would further regulate the concentration of Ag ions on the film surface at a constant level. This process resulted in the long life time, tunable and sustainable antibacterial behaviour of the AgNC-modified thin-film composite membrane.
 | ||
| Fig. 4 Formation of AuNPs/AgNPs-TiO2 thin film (reproduced from ref. 47 used under Creative Commons CC-BY license). | ||
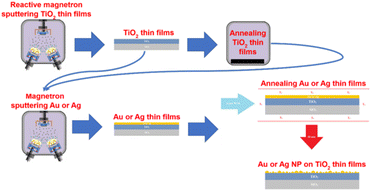
Gold is another noble metal used to prepare bactericidal thin film on the surface of different materials including medical devices due to its low toxicity and a great bio-affinity. Villa Garcia L. et al.51 reported coating (via magnetron sputtering) of gold nanoparticles on polyethylene, a versatile polymeric material used in surgical instruments to temporary and permanent biomedical devices. The gold coated polyethylene demonstrated strong biofilm inhibition activity. In another work, titania thin films decorated with AuNPs and AgNPs also demonstrated higher antibacterial activity. Briefly, amorphous TiO2 thin film is prepared by using magnetron sputtering method and crystallized by annealing at high temperature. Consequently, AuNPs and AgNPs are deposited onto the crystallized TiO2 thin film under vacuum condition followed by annealing at high temperature.47 Fig. 5 shows the designed titania thin film coated with AuNPs and AgNPs.
 | ||
| Fig. 5 Schematic illustration of the fabrication of AgNC-modified thin-film composite (AgNC-TFC) membrane (reproduced from ref. 50 with permission from American Chemical Society). | ||
T. Kruk, et al.32 reported copper nanoparticles (CuNPs) suspension coated poly(diallyldimethylammonium chloride) multilayer antibacterial film. Their result indicated the developed multilayer composite film coated with CuNPs showed significant decrease in the cell viability of S. aureus bacteria after 6 hours of incubation.
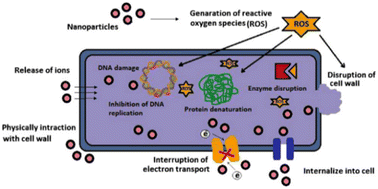
The primary mechanism by which metal-based thin films can inhibit microbial growth is through the release of ions such as Ag+, Zn2+, Au+ and Cu2+.33 These ions directly interact with cell walls of microorganisms to create pores or holes that disrupt normal functioning. In addition, they also form complexes with enzymes or other cellular components that can further inhibit biofilm growth or cause membrane damage and cell death. For example, silver ions have been found to be able to penetrate bacterial cell membranes rapidly, leading to disruption of oxidative processes such as respiration and denaturation of proteins and then damage the integrity of microbial cells. In another work, T. Kruk, et al.32 confirmed the antibacterial mechanism of CuNPs is through depolymerisation of bacterial cell membrane when in contact with CuNPs and generation of ROS which in turn cause cellular lipid peroxidation, protein oxidation, DNA degradation; and finally bacterial cell death.
Fig. 6 demonstrates antimicrobial mechanisms of metal nanoparticles based thin film. Accordingly, (1) release of metal ions from the metal nanoparticles and (2) direct interaction of the metal ions and/or (3) metal nanoparticles with the cell wall through electrostatic interactions, leading to impaired membrane function and impaired nutrient assimilation; (4) formation of extracellular and intracellular reactive oxygen species (ROS), and damage of lipids, proteins and DNA by oxidative stress; (5) high-levels of metal-binding to the cell envelope and high ROS levels can cause damage to the plasma membrane and thus lead to the leakage of the cell content; (6, 7) upon metal uptake, metal nanoparticles and metal ions can directly interfere with both proteins and DNA, impairing their function and disturbing the cellular metabolism in addition to metal-mediated ROS production are reported antimicrobial mechanisms of metal nanoparticles.52
 | ||
| Fig. 6 Various mechanisms of antimicrobial activity of metal nanoparticles (reproduced from ref. 45 with permission from Elsevier). | ||
3.2 Metal oxide-based ATFs
Metal oxide (MOx) semiconductors are among the widely employed antimicrobial materials due to their photocatalytic properties.53 Various metal oxide nanomaterials including CuO, ZnO, titania (TiO2), alumina (Al2O3), iron oxides, ZrO2, silica (SiO2), and others used in the development of antibacterial thin films and membranes.54 They are processed into nanocomposite films in various designs and architectures; distributed in a polymer matrix, layered film structures and hybrids.55 The frequently reported approach to fabricate antimicrobial MOx nanocomposite films is using a MOx/polymer layered structure,56 including layer-by-layer (LBL) alternate deposition of MOx and polymer structures.57 Due to the versatility of MOx nanomaterials, MOx-based antimicrobial thin films and membranes are extensively applicable in areas such as medical disinfection coatings, water treatment membranes and self-cleaning surfaces.MOx coating materials which have antibacterial property and prevent bacterial adhesion are desirable in implant technology to prevent bacterial infection associated trauma and high cost.58 For example, antibacterial agents incorporated mesoporous titania thin films characterized by controlled drug release, high pore volume and high surface area which make them ideal drug loading site is among the promising materials which can be coated onto the medical devices.59 Atefyekta et al. reported titanium oxide-based mesoporous titania thin film loaded with the antimicrobial agents (vancomycin, gentamicin, and daptomycin) in its pore volume. The thin film is characterized by decreased biofilm attachment to its surface and tunable antibacterial loading observed to be increased with increasing pore volume; thus highly contribute to host tissue growth and reduced biomaterial associated infections.60
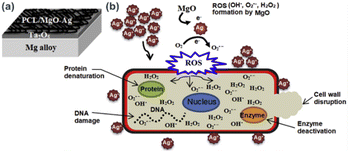
In another study, orthopedic implant has been developed by subsequent deposition of tantalium dioxide (Ta2O5) and poly(ε-caprolactone)/magnesium oxide–silver (PCL/MgO–Ag) nanofiber porous layers on Mg alloys.61 In this work Ta2O5 was coated on Mg alloys via magnetron sputtering using argon gas as the sputtering gas. Then, homogenised PCL/MgO–Ag film forming solution is deposited onto the previously prepared Mg alloy-Ta2O5 layers (Fig. 7a). Antibacterial efficacy test of the developed thin film indicated high antibacterial activity which is supposed to be due to synergetic antibacterial activity of MgO and Ag in the thin film. The antibacterial mechanism of PCL/MgO–Ag is illustrated in Fig. 7b. MgO reacts with intracellular oxygen and generate ROS, such as OH˙, O2˙−, and H2O2, which may dissolve AgNPs and subsequently generate Ag+ ions into the medium. This synergetic process causes further inhibition of proliferation and bacterial growth via cell wall disruption, protein denaturation, enzyme deactivation and DNA damage. The MgO-induced oxidative dissolution of AgNPs into Ag+ and successive increase in bactericidal effect was further confirmed by other reports.62,63
 | ||
| Fig. 7 A schematic representations of (a) electrospun nanofiber coatings and (b) antimicrobial mechanisms of PCL/MgO–Ag (reproduced from ref. 61 with permission from Elsevier). | ||
Thomas D. et al.64 reported microwave assisted successive layer of iodine doped ZnO thin film. The film was developed by depositing ZnO on indium titanium oxide glass substrate followed by UV irradiation and the process repeated many times. The prepared ZnO is doped by adding iodine solution and annealed at high temperature. The antibacterial test of the thin film indicated that antibacterial activity of thin film is through splitting of the bacterial membrane. This disruption of bacterial membrane results from high surface oxygen species generation from ZnO which leads to the bacterial death. Another report also confirmed that visible light contacted ZnO NPs results in increased H2O2 which disturb cellular homeostasis and DNA lysis that eventually prompt premature bacterial cell death.65
Silver nanoparticle doped TiO2 and SiO2 double layer thin films deposited on self-adhesive polyurethane foil has been developed. AgNPs doped and double layer thin film showed improved antibacterial activity when compared with non-doped and single layer thin films respectively. In this work, UV light exposure is reported to affect antibacterial efficacy of the thin film, which UV light stimulated thin film experienced enhanced antibacterial activity when compared with non-UV light treated film.1
Another prominent area of application for MOx-based nanostructures is in the development of antimicrobial membranes and surfaces for photocatalytic water disinfection systems.66,67 We also have recently reviewed cellulose supported photocatalytic membranes where photoactive MOx nanomaterials are discussed. In view of nanostructure design, MOx are incorporated as fillers to polymeric membranes to minimize biofilm formation, to kill pathogenic microorganisms, to increase water flux via imparting membrane hydrophilicity and pore formation, and to improve mechanical properties.
Chaudhary et al.40 developed cellulose acetate (CA) based mixed matrix membrane (MMM) using mixed metal oxides nanoparticles-polymer composite (Fe–Al–Mn@chitosan) as nanofiller. The excellent antibacterial property of the MMM was due to the stable incorporation of nano-Fe–Al–Mn@chitosan inside the CA matrix.
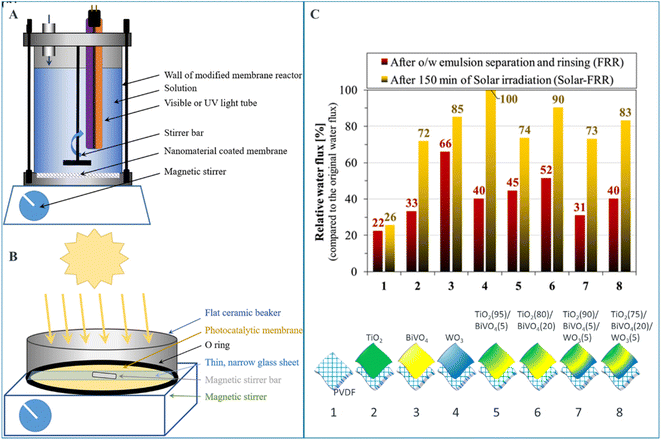
Santos et al. investigated the photocatalytic activities and subsequent oily water purification performance of PVDF ultrafiltration membrane modified by different metal oxides TiO2, BiVO4, and WO3.68 As it can be seen in Fig. 8, upon solar irradiation, the membranes disinfect themselves from biofilms in a chemical-free manner, and sufficiently recover the membrane flux. The authors claim TiO2 (80%)/BiVO4 (20%) composite coating demonstrated a good compromise between flux and self-cleaning properties.
MOx nanocoatings are also employed in thin film supercapacitors to impart antimicrobial surface property coupled with water resistance and transparency. Choi et al.69 developed zinc aluminate films for smartphone panel glass that is resistant to water and acidic water while demonstrating self-disinfecting and hydrophobic behaviours. The MOx thin film with good stability and strong antibacterial activity was prepared by depositing ZnAl2O4 layer onto gorilla glass as a substrate using radio frequency magnetron sputtering technique. The strong antibacterial activity of the thin film is due to electrostatic interaction between negatively charged bacterial cell wall and high positive surface potential of the ZnAl2O4 layer onto gorilla glass where the reaction could result in an effective bacterial death.
In general, the principal antimicrobial mechanisms of metal oxides is generation of ROS like H2O2, O2˙− and OH˙ which increase oxidative stress and cause microbial cell damage.70 Another mechanism behind microbial cell damage is due to the electrostatic interaction between metal cation generated from metal oxides and negatively charged microbial membrane which cause destruction of the membrane that allow metal oxides nanoparticles entry to the cell. Once metal oxides nanoparticles entered the microbial cells, they react with biomolecules and cause cell disruption which ends up with microbial death.53 Fig. 9 shows general antimicrobial mechanisms of metal oxide nanoparticles. Metal oxides also facilitate the controlled release of metal ions from metal nanoparticles, which leads to synergetic bactericidal effect.71 It is worth noting that the mechanism of microbial action by MOx nanoparticle thin films and membranes vary considerably, requiring further investigations which would help in systematically designing antimicrobial nanostructures of a particular MOx on surfaces and functional systems.
 | ||
| Fig. 9 Overview of antimicrobial mechanisms by metal oxide nanoparticles (reproduced from ref. 53 with permission from Elsevier). | ||
3.3 Metal organic framework and nanoclay based ATFs
Metal organic frameworks (MOFs) and nanoclays (NCs) are versatile ceramic materials with well-defined porous structures. Thin films and membranes of MOF and NC materials possess inherent antimicrobial properties. Nejad et al. reported the preparation of anti-fouling and anti-biofouling zeolitic imidazolate framework-7 on the surface of functionalized porous polyethersulfone substrate.72 The inhert antibacterial properties of MOF and clay nanomaterials vary with their nanoparticle size and shape, type of metal atoms and ligands present, and their crystalline structures. Nevertheless, their antibacterial activity is generally a result of their structural collapse,73 and subsequent release of metal ions and ligands.Metal-doped and other functionalized MOF membranes are also widely researched microbicidal materials, with Ag-based MOFs becoming very prominent in the area.38,74,75 In such nanocomposite designs, the incorporated metal ions introduce additional activity against microbes. Seyedpour et al.38 fabricated a high antibacterial and antifouling Ag-MOF nanorod modified thin film nanocomposite (TFN) forward osmosis membranes. They developed the TFN-FO membranes in two steps: synthesis of Ag-MOF nanorods followed by interfacial polymerization of polyamide in the presence of 2-aminoterephthalic acid treated Ag-MOF. Even though the nanorods were buried within the polyamide matrix, they could still produce high bacterial inactivation in a short bacteria-membrane contact time of 1 h.
MOFs and NCs also serve as charge carriers in the development of nanocomposite photocatalysts with semiconductors, resulting in enhanced photocatalytic performance.76,77 For example, palygorskite (Pal), the environmentally friendly nanoclay, based membrane has been prepared for reverse osmosis (RO) water desalination. The thin film nanocomposite (TFN-Pal/TiO2) RO membrane was designed by incorporating Pal-TiO2 nanocomposite in the polyamide (PA) selective layer via interfacial polymerization. The membranes exhibited strong photocatalytic antibacterial activity while maintaining high water flux and anti-fouling property in the order of TFN-Pal/TiO2 > TFN-Pal > TFC for all antibacterial, water flux and fouling resistance tests. UV treatment of the thin film membrane generated ROS like OH˙ and H2O2 and these ROS could damage bacterial membrane and block bacterial growth.76
3.4 Plant-derived ATFs
Long time, synthetic antibiotics were used to treat and prevent microbial invasion to overcome microbial associated risks in different sectors. However, synthetic antibiotics are limited to rapid rise of antimicrobial resistance, their undesired side effect and even death.78,79 In this regard, bioactive phytochemicals are being discovered as the viable alternative to overcome or minimize these associated risks.79,80 For instance, antimicrobial thin films prepared from plant extracts, essential oils, and active compounds are widely used to control biofilm formation on the contact surfaces. Such activity is contributed from plant-derived secondary metabolites such as alkaloids, polyphenols, flavonoids, tannins, anthraquinone, saponins and terpenoids embedded in a matrix of sterile polymers.80,81 Even though there is no clear antimicrobial mechanism of bioactive phytochemicals reported yet, different phytochemicals are suggested to act through different mechanism82 as shown in Table 2.| Bioactive compounds | Suggested predominant antimicrobial mechanism of action | Reported plant origin | Ref. |
|---|---|---|---|
| Alkaloids | Efflux pump (bacterial transmembrane protein complexes) inhibition followed by cell membrane destruction | Callistemon citrinus, Vernonia adoensis and Peganum harmala | 82 and 83 |
| Papaver rhoeas | 84 | ||
| Inhibition of bacterial cell wall and intercalating into bacterial DNA | Papaver glaucum and Papaver decaisnei | 83 and 85 | |
| Polyphenols | Inhibition of bacterial protein biosynthesis | Rhus coriaria, Viscum cruciatum Sieb and Quercus infectoria olive | 86 |
| Interaction with the bacterial cell wall and cell membrane | Cloudberry, black currant, cranberry, and blueberry extracts | 87 | |
| Grape seed, apple, cinnamon bark, and rosemary leaves | 88 | ||
| Inhibition of metabolic pathway | Mentha longifolia, Gentian lutea, Nigella sativa, Chamomilla recutita, Murraya koenigii, and Terminalia chebula | 89 | |
| Anthraquinone | Cell wall and membrane disruption | Curtisia dentate | 90 |
| Vismia laurentii | 91 | ||
| Binding to bacterial DNA | Cassia nodosa | 92 | |
| Saponins | Cell membrane disruption | Chenopodium quinoa | 93 |
| Camellia oleifera | 94 | ||
| Cell wall and cell membrane damaging | Green tea seed | 95 | |
| Flavonoids | Cell membrane disruption | Anemarrhenae rhizome, Gardeniae fructus, and Mangosteen peel | 80 and 96 |
| Category | Nano-structure design | Type of material | Film preparation method | Predominant antimicrobial mechanism | Ref. |
|---|---|---|---|---|---|
| a Abbreviations: PC – polycarbonate, LE – leaf extract, PCL – polycaprolactone, ZO – Zingiber officinale, CNC – cellulose nanocrystal, AZO-Al-doped ZnO, PET – polyethylene terephthalate, NS – not specified, AVO – aloe vera essential oil, RO – Rosemary essential oil, CA – cellulose acetate, CitA – citric acid, CMC – carboxymethyl cellulose, BA – benzoic acid, LA – lactic acid, MA – malic acid, API – anchovy protein isolate. | |||||
| Metal-based thin film | Layer-by-layer | Thiolete/AgNCs/thiolete | Soaking | Electrostatic interaction and membrane penetration | 50 |
| Single layer | Au/polyethylene | Surface coating | 51 | ||
| Multilayer deposition | Ag/TiO2/polyurethane | Doping | 1 | ||
| Metal oxide-based thin film | Layer-by-layer | ZnO/ZnO/I | UV irradiation | ROS generation & electrostatic attraction | 65 |
| Multilayer deposition | Ta2O5/(PCL/MgO–Ag)/Mg alloy | Magnetron sputtering (1st layer) and electro-spinning (2nd layer) | 61 | ||
| Multilayer deposition | (AZO/Ag/AZO)/ZnAl2O4/PET/(AZO/Ag/AZO) | 69 | |||
| Surface deposition | WO3/stainless steel | Magnetron sputtering | 70 | ||
| Plant-based thin film | Dispersion | CH/ZO | Solution casting | Cell wall/membrane interaction | 98 |
| Dispersion | PC/LE | Solution casting | 81 | ||
| Dispersion | AVO/CA & RO/CA | Solution casting | 100 | ||
| Dispersion | GEO/Starch | Solution casting | 99 | ||
| Enzyme-based thin film | Layer-by-layer | Endolysin/SiO2 | Solution casting | Attack DNA | 113 |
| Organic acids-based thin film | Dispersion | API/BA | Solution casting | Lowering intracellular pH | 119 |
| Dispersion | LA or MA/Zein | Solution casting | 118 | ||
| Dispersion | LA/PVA | Solution casting | 120 | ||
| Dispersion | CitA/CMC | Solution casting | 117 | ||
| Bacteriocin-based thin film | Dispersion | M35/CH | Solution casting | Cell membrane disruption | 130 |
| Dispersion | Bacteriocin/CNC | Solution casting | 132 | ||
| Dispersion | Bacteriocin/agar–agar polysaccharide | NS | 129 | ||
| Polymer-based thin film | Layer-by-layer | Zn@CuO/polydopamine | Coating | Prevent adhesion/cell wall interaction/penetration | 144 |
Zingiber officinale is among bioactive constituent rich medicinal plant with high antibacterial effect.97 Z. officinale essential oil incorporated chitosan film with enhanced antibacterial activity has been reported.98 Z. officinale essential oil is dispersed in chitosan containing solution to facilitate essential oil–chitosan interaction. The film forming solution is casted on Petri dish and allowed to dry to form the thin film. The interaction holding the film components together is reported to be interaction between the chitosan hydroxyl and amine group with the phenolic compounds Z. officinale essential oil. Garlic is another antibacterial rich plant processed in to active packaging film. Sihombing N. et al.99 prepared garlic essential oil-based edible active packaging by coating essential oil film onto the previously plasticized and moulded cassava starch. The film demonstrated high antibacterial activity against Escherichia coli and extended shelf-life. Rosemary and aloe vera oil based cellulose acetate film is another plant extract-based antibacterial thin film. El Fawal et al.100 developed bioactive packaging membrane from rosemary and aloe vera essential oils emulsified separately into cellulose acetate polymer matrix. The portions of incorporated essential oils are reported to move to the membrane surface during the drying process. Thus the thin film membrane showed strong bactericidal activity against tested bacteria; whereas bare cellulose acetate used as a control does not show any antibacterial activity.
Aaliya B. et al.101 developed plant extract incorporated active food packaging thin film materials. Plant extracts from neem tree, tulsi, Mexican mint, and curry leaves is mixed with gelatinized carboxymethyl cellulose and glycerol and further homogenized prior to casting. The developed thin film demonstrated excellent antibacterial activity against Staphylococcus aureus and Escherichia coli.101 Polyethylene surface (PE) decorated with different plant extract using surface coating techniques also has been reported. In this work, suitable amount of rosemary, raspberry, and pomegranate CO2 extracts is mixed with fixing agent followed by homogenization. PE plate is inserted in the film forming solution, stirred and extruded by twin-screw extruder. The resulted thin film is again extruded through a flat die to obtain films with a uniform thickness. The developed thin film is reported to inhibit the growth of some bacterial strains.102 Ali et al.103 fabricated antimicrobial food packaging thin films incorporated with different medicinal plants (Acontium heterophyllum, Artemisia annua, and Thymus serpyllum) and tested their bactericidal activity against S. aureus, and Salmonella. The result demonstrated good zone of inhibition against both bacterial strain. Increment in zone of inhibition with increased plant extract concentration was reported in this work.
The interaction between the active molecules of plant-based thin film and microbial cell is the key mechanism by which plant extract exhibit increased antimicrobial efficacy against various classes of microbes. Synergistic activities of plant bioactive constituents also play a major role in providing great protection not only against single species but even cocktail species thus making it more effective compared with individual independent compound approaches.104 To the point, these molecules exhibit inhibition mechanisms such as reducing membrane permeability, increasing osmotic pressure, inhibition of nucleic acid and protein synthesis, inhibition of energy metabolism within microbial cells and inhibition of bacterial efflux pumps.16,105 Similarly, antifungal properties may be exhibited by trapping fungal spores in mucilage or wax secretions or preventing germination through secretion of sesquiterpenes amongst other physiological processes.16
Among the reported plant's secondary metabolites; antimicrobial mechanisms of alkaloids is thought to be through inhibition of bacterial cell wall synthesis, change in cell membrane permeability, inhibition of bacterial metabolism, and inhibition of nucleic acid and protein synthesis.83 Different mechanisms for antimicrobial activity of polyphenols are also reported. These includes enzyme inhibition by the oxidized compounds, possibly through reactions with proteins through SH− groups or through nonspecific interactions. Furthermore, some phenolics such as quinones act as a source of stable free radicals and bind irreversibly with microbial proteins leading to its loss of function. Other targets are inactivating enzymes, binding to adhesins on the microbial cell surface, binding to cell wall proteins, and interacting with substrates rendering them unavailable to the microorganism, complexing with metal ions and others.86 In another work, plant flavonoids rich materials are also reported to inhibit bacterial growth by increasing bacterial cell membrane permeability, reduction of ATP production, lowering mobility, and stimulation of host immune system.80
3.5 Enzyme-based ATFs
Antimicrobial enzymes are other important types of bioactive molecules that can be used to combat microbial infections. They can be incorporated or grafted into polymers or on the polymers surface to prevent microbial colonization.106 Enzymes are also used in the development of nanobiocomposite ATFs with nanomaterials.107 Recent researches show the fabrication of biomimetic antimicrobial nano-enzymes, called nanozymes.108 Nanozymes are immerging as potential substituents to scares natural enzymes, especially for industrial scale applications.Some antimicrobial enzymes like antimicrobial peptides are reported to be found in natural defensive (immune) system of wide range of organisms including plants.109,110 Leitgeb et al.,111 for example, confirmed the presence of enzymes such as α-amylase, cellulase, lipase, peroxidase, protease, and transglutaminase in the Aloe arborescens and Aloe barbadensis ethanolic extract. The obtained enzymatic fractions are reported to have microbial growth inhibition activity.111
Lysozyme enzyme deposited pyridinium-based zwitterionic copolymer antibacterial thin film with strong antibacterial and antifouling activity has been reported. The film is prepared by depositing lysozyme onto poly(4-vinylpyridine-co-pentaflurophenyl methacrylate-co-divinyl benzene) surface by nucleophilic substitution of the pentafluorophenyl group and lysozyme enzyme which result amide bond formation between them.112 The surface nucleophilic substitution of lysozyme and bactericidal action of lysozyme based thin film is as shown in Fig. 10.
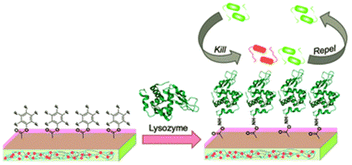 | ||
| Fig. 10 Nucleophilic substitution of lysozyme and bactericidal action of lysozyme based thin film (reproduced from ref. 112). | ||
In another work, Solanki K. et al.113 developed listeria bacteriophage endolysin activated silica nanoparticles thin film observed to inhibit microbial growth. The covalently bonded thin film is prepared by copolymerization between endolysin and surface modified silica nanoparticles in the presence of polyethylene glycol dimethacrylate as a fixing agent.
Antimicrobial enzymes acts by different mechanisms; some of which include disrupting the cell membrane, degrading proteins and DNA, inhibiting growth, and inducing antibacterial responses from the host.106 Among mechanistic action of different class of antimicrobial enzymes, Blackman et al.114 reported that proteolytic enzymes act as antibiofilm by degrading essential microbial proteins and peptides, polysaccharide depolymerases by destructing the microbial polysaccharides and DNA, quenching enzymes by interfering cell–cell signalling, proteases and nucleases by degrading the extra polymeric substances secreted by the biofilm. Baek et al.115 confirmed that the mechanism of action of antimicrobial peptides (specifically cecropin P1, antibacterial peptide isolated from the pig stomach) is through electrostatic interaction of positively charged peptides ends (C-terminal) and lipopolysaccharide, which is the main component of the outer membrane of Gram-negative bacteria.
Furthermore, the same enzymes having different structure acts differently against suspected microbes.106 For example, two of protease enzyme subtilisins and lysostaphin inhibit microbial growth through different mechanism; subtilisins hydrolyse bacterial proteins essential for attachment onto solid supports and other bacteria whereas lysostaphin cleaves bacterial cell walls on the third and fourth glycine residues of the pentaglycine cross-bridge. Another class of antimicrobial enzyme, polysaccharide-degrading enzymes, attack bacteria by hydrolyzing 1,4-beta-linkages in the bacterial cell walls. Oxidative enzyme acts by generating ROS against invading pathogens. Fig. 11 shows antibacterial mechanisms of protease enzyme lysostaphin.
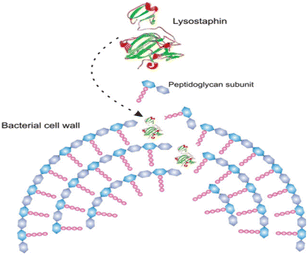 | ||
| Fig. 11 Simplified scheme of the hydrolysis of bacterial cell walls by lysostaphin (reproduced from ref. 106 with permission from John Wiley and Sons). | ||
3.6 ATFs from organic acids
Organic aids are among widely used antimicrobial agent especially in food industry and they can be originated from oxidation of alcohols, fruits, plants and microorganisms among others.116,117 Lactic acid, malic acid, propionic acid, tartaric acid, succinic acid, acetic acid, citric acid, fumaric acid, butyric acid and caprylic acid are among the commonly used organic acids.116Previously, natural organic acids decorated zein edible active packaging has been reported with improved antimicrobial activity and flexibility. The film is developed following uniform dispersion of lactic acid, malic acid and tartaric acids in zein solution.118 Rocha M. et al. also reported sorbic acid and bezoic acid incorporated argentine anchovy protein isolate (API) thin film. API as a film matrix, glycerol as a plasticizer and antimicrobial sorbic acid (SA) or benzoic acid (BA) is mixed prior to homogenization. The film forming solution is poured onto Petri dish and dried to result API-AS and API-BA antimicrobial thin film. The prepared API-organic acids thin film demonstrated strong antibacterial activity against the tested bacteria, escherichia coli, salmonella enteritidis and listeria monocytogenes.119
In another work, Suganthi et al.120 reported PVA polymer film functionalized with different organic acids for food packaging application. Their result confirmed that PVA-malic acid; PVA-tartaric acid and PVA-lactic acid containing packaging films showed better zone of inhibition against S. aureus and E. coli bacteria when compared with bare PVA film. Romainor et al.121 also synthesised effective antimicrobial food packaging film from premixed starch, PVA, and citric acid using solution casting method. In contrast to unmodified starch polymer film, the prepared starch-citrate packaging film demonstrated complete growth inhibition against test microorganisms (pathogenic food borne bacteria; Salmonella thypimurium, E. coli, and Listeria monocytogenes and food fungus; Aspergillus species, and Rhizopus species) and thus enhanced shelf life of some foods under study.
Mechanistic antimicrobial action of organic acids are ability to penetrate into bacterial cells122 due to their lipophilic properties which allow them to easily penetrate into microbial plasma membrane, which in turn cause lowering the pH of the medium. Such change in cell medium cause alteration in proteins and phospholipid structures of microbial cell membrane followed by cell growth limit and death.116,117,123
The detailed mechanism behind the lowering of the cell pH is that; as organic acids penetrate bacterial cell membrane they undergo dissociation at pH neutral, thus H+ produced and intracellular pH decreased.121,124 The decreased intracellular pH leads to the protonation of the carboxyl and phosphate groups of lipopolysaccharides on the bacterial cell membrane, thus cell stability disturbed and cause inhibited cell growth. Second, the lower intracellular pH also affects the enzymatic activities and inhibits DNA replication and transcription as well as protein expression. To stabilize this intracellular pH, bacteria must release hydrogen ions via active transport, but this process consumes adenosine triphosphate (ATP) and affects the normal growth of bacteria.124 Fig. 12 demonstrate the general antimicrobial mechanisms of organic acids.
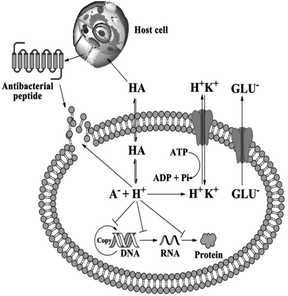 | ||
| Fig. 12 Antimicrobial mechanisms of organic acids (reproduced from ref. 124 with permission from John Wiley and Sons). | ||
Suganthi et al.120 reported another possible antibacterial mechanisms of organic acid-based film are; generation of reactive oxygen species, deposition of carboxyl groups on the surface of the bacteria, and tuning of the film surface to prevent bacterial surface adhesion. Organic acids are also reported to enhance bactericidal activity of the materials through increasing the osmotic stress of the cell and disrupting the biomolecule synthesis by releasing their anion and proton, reduction of ATP production by uncoupling electron transport, and disturbance of regulation of bacterial signalling pathways; finally, these all mechanistic cause microbial death.125
3.7 Bacteriocin-based ATFs
Bacteriocin is bacterial peptide produced by bacterial ribosome which is used as antimicrobial agent.126 Like other antibacterial agents they can be incorporated into edible polymers such as thin film to enhance controlled and continuous flow of active agents to maintain high concentration for long period127 with additional the advantages that Bacteriocin are stable at higher temperature, work over wide pH range, and have lower minimum inhibitory concentration (MIC) when compared with plant extracts and the other protein-based antimicrobial preservatives.128For instance, antimicrobial thin film from lactobacillus sakei extracted bacteriocin and polysaccharide (agarose and agaropectin) as a biopolymer matrix has been prepared and demonstrated strong antibacterial activity. The film is prepared by homogenization of film forming solution followed by casting technique to obtain uniformly dispersed bacteriocin in polysaccharide mixture matrix.129 In another work, divergicin M35 (bacteriocin from carnobacterium divergens M35strain) based thin film has been prepared by incorporating the bactriocin in chitosan support matrix. This prepared thin film showed high antibacterial activity due to its synergetic effect with chitosan.130
La Storia A. et al.131 reported three dimensional thin films from whey protein matrix and Lactobacillus curvatus 54M16 bacteriocin using gelatin and inuline to form gels and to create synergy among the components to enable the formation of three dimensional networks. High zone of inhibition is observed for bacteriocin incorporated thin film when compared with the control. P. Bagde and V. Nadanathangam132 also developed antimicrobial film from Bacteriocin (antibacterial peptides which is extracted from the lactic acid bacteria) immobilized crystalline nanocellulose (BIN) and corn starch. As reported in this work, all the films containing BIN revealed inhibited growth against pathogenic S. aureus, E. coli and this growth inhibition comes from the incorporated antibacterial peptides.
The general mechanism of bacteriocin (antimicrobial peptides) is through disruption of anionic bacterial cell membrane due to the electrostatic forces between positively charged amino acids and the negatively charged cell surface. Fig. 13 demonstrates the detailed bactericidal mechanism of bacteriocin; (A) disruption of cell membrane integrity: (1) random insertion into the membrane, (2) alignment of hydrophobic sequences, and (3) removal of membrane sections and formation of pores. (B) Inhibition of DNA synthesis. (C) Blocking of RNA synthesis. (D) Inhibition of enzymes necessary for linking of cell wall structural proteins. (E) Inhibition of ribosomal function and protein synthesis. (F) Blocking chaperone proteins necessary for proper folding of proteins. (G) Targeting mitochondria: (1) inhibition of cellular respiration and induction of ROS formation and (2) disruption of mitochondrial cell membrane integrity and efflux of ATP and NADH.133
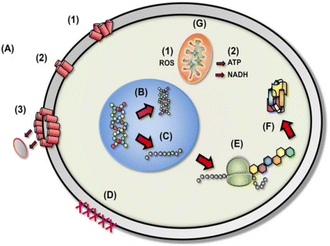 | ||
| Fig. 13 Diverse mechanistic modes of action for antimicrobial bacteriocin in microbial cells (reproduced from ref. 132, used under Creative Commons CC-BY license). | ||
3.8 Polymer-based ATFs
The development of advanced nanomaterial offers multiple opportunities in a variety of engineering applications including biomedical ones.134 Polymer-based anti-microbial thin film is one such example which is gaining immense importance lately since they can act as a physical barrier to prevent the growth, spread or entry of microorganisms like bacteria and fungi into the critical parts inside a device or substrate.135 The increasing demand for products that prevent microbial contamination has made polymer-based antimicrobial thin film an attractive option due to its advantages like flexibility, strength and durability as well as its ability to sustain antifungal protection for prolonged periods without compromising performances.136Polymer-based antimicrobial coating design usually consists of three components namely; polymer backbone, adhesive layer and active layer.137 Through a variety of surface treatment techniques, the physical and chemical states of the substrate have been induced in order to investigate the mechanism of how the substrate state affects the adhesive. The adhesive layer helps in adhering the film while active layers such as lysozyme, chitosan etc., impart antibacterial/antifungal property by specific killing mechanisms like enzyme blockade.138
Polymer materials may demonstrate intrinsic antimicrobial activity in the ATF systems or they can serve as a matrix media to accommodate other active agents. Some polymers with intrinsic antimicrobial activity are shown in Fig. 14.139 In the latter case, polymers serve as slow release nanocomposite vehicles for the delivery of metal ion or other active agents.140,141
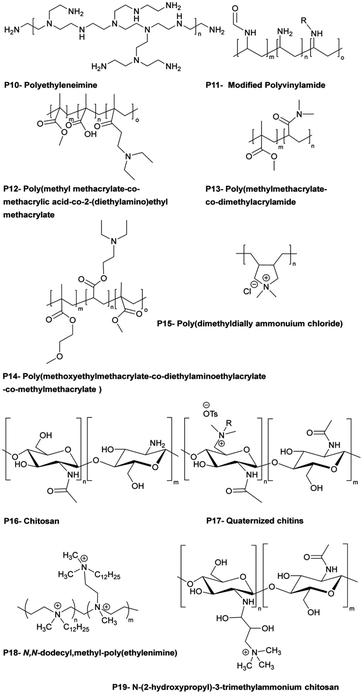 | ||
| Fig. 14 Examples of bactericidal polymers used in layer-by-layer films and as antibacterial coatings (reproduced from ref. 139 with permission from American Chemical Society). | ||
For example, antimicrobial polydopamine layer decorated with Zn@CuO under ultrasonication treatment has been developed through complexation and coordination formation between polydopamine and metal oxides. The synergy between the polymer matrix and metal oxides brought about increased bactericidal effect of the developed thin film.140 Another illustration is the development of a regenerable silver slow-releasing thin film nanocomposite membrane.142 As carriers for Ag+ or Ag0, nanozeolites were covalently bound to the surface of a polyamide nanofiltration membrane. The findings have shown that the slow release of biocides from porous nanoparticles can act as a long-lasting mechanism for bio-fouling control.
Polymer-based antimicrobial thin films act through three main mechanisms143 shown in Fig. 15; physical barriers (non-leaching), contact killing (leaching), and diffusion killing (semi-leaching). Physical barriers prevent bacterial attachment by using contact angles and surface roughness characteristics, while contact killing biofilms provide direct killing by leachate release often via ion exchange reactions when dampened and illuminated. Lastly, diffusion killing systems rely on preformed pores and capillaries within the film matrix that enable small amounts of chemicals scattered throughout the layer to slowly oozes out using thermally induced polymer chain motions over time upon exposure to humidity or light triggers contributing localized & prolonged efficacy while reducing transport away from site (reducing environmental impact).144
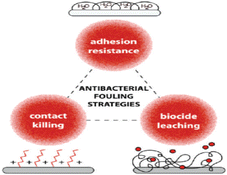 | ||
| Fig. 15 Polymer-based thin film antimicrobial mechanisms (reproduced from ref. 143 with permission from American Chemical Society). | ||
In addition to the above classes of nano-assisted materials, there also exist other emerging antimicrobial materials such as quantum dots,145 graphene oxides,146 metal sulphides,147 and MXenes,148 which are reviewed elsewhere. As discussed in this review, these nanostructured materials are also used as modifiers or functionalizing agents of other nanomaterials and nano-assisted thin films for synergetic antimicrobial properties.
4. Summary and future outlook
Interactions between surface treatment methodologies continues to play a fundamental role in today's product development process – hence having solutions such as antimicrobial thin films will prove beneficial in this regard in providing effective prevention against microbial contamination. While traditional approaches continue to remain relevant today, there is no doubt that antimicrobial thin film technology has come a long way over recent years owing its success primarily due its high efficacy levels combined with fewer risks associated with its use when compared with chemical solutions typically used for similar purposes. Thin films possess both physical and chemical properties which contribute to their biocidal action; antimicrobial thin films often contain small particle sizes that can physically disrupt bacterial cell walls or act as physical barriers, preventing microbial attachment and proliferation on treated surfaces or chemical biocides primarily penetrate cell membranes by oxidation reaction or simply by dissolving into cells via electrochemical transduction processes and cause microbial cell damage.The robust nature of thin films allows the development and scale up of practical nano-assisted antimicrobial systems by immobilizing various active ingredients, including metals, metal oxides, plant bioactives, enzymes, organic acids, bacteriocins and polymers. Accordingly, these systems pave the way for the realization of large scale advanced antimicrobial systems for applications like wound dressings, tissue engineering, membrane-based water treatments, active food packaging, surface disinfection, self-healing surfaces, blocking of UV and other damaging radiations. The multitude options in design, development and large-scale processing of nanostructured antimicrobial thin films still provides multitude of research innovation opportunities to realize affordable and efficient treatment systems.
Author contributions
Bilisuma Finina wrote the main manuscript. Anteneh Mersha conceptualized the idea, reviewed and edited the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The work was supported by Nanotechnology Center of Excellence at Addis Ababa Science and Technology University.References
- C. Adochite, C. Vitelaru, A. Parau, A. Kiss, I. Pana, A. Vladescu, S. Costinas, M. Moga, R. Muntean, M. Badea and M. Idomir, Synthesis and Investigation of Antibacterial Activity of Thin Films Based on TiO(2)-Ag and SiO(2)-Ag with Potential Applications in Medical Environment, Nanomaterials, 2022, 12(6), 1–11 CrossRef PubMed.
- R. Gharibi, A. Shaker, A. Rezapour-Lactoee and S. Agarwal, Antibacterial and Biocompatible Hydrogel Dressing Based on Gelatin- and Castor-Oil-Derived Biocidal Agent, ACS Biomater. Sci. Eng., 2021, 7(8), 3633–3647 CrossRef CAS PubMed.
- L. Yu, W. Zhou, Y. Li, Q. Zhou, H. Xu, B. Gao and Z. Wang, Antibacterial Thin-Film Nanocomposite Membranes Incorporated with Graphene Oxide Quantum Dot-Mediated Silver Nanoparticles for Reverse Osmosis Application, ACS Sustain. Chem. Eng., 2019, 7(9), 8724–8734 CrossRef CAS.
- R. Raybaudi-Massilia, J. Mosqueda-Melgar, R. Soliva-Fortuny and O. Martín-Belloso, Combinational Edible Antimicrobial Films and Coatings, Antimicrobial Food Packaging, 2016, pp. 633–646 Search PubMed.
- A. K. Mersha, B. F. Finina and G. Gebreslassie, Applications, Opportunities and Challenges of Nanotechnology in the Food Industry, Smart Nanomaterials Technology, 2023 Search PubMed.
- D. Sanders, A. Grunden and R. R. Dunn, A review of clothing microbiology: the history of clothing and the role of microbes in textiles, Biol. Lett., 2021, 17(1), 20200700 CrossRef CAS PubMed.
- A. Nagaraja, Y. M. Puttaiahgowda, A. Kulal, A. M. Parambil and T. Varadavenkatesan, Synthesis, Characterization, and Fabrication of Hydrophilic Antimicrobial Polymer Thin Film Coatings, Macromol. Res., 2019, 27(3), 301–309 CrossRef CAS.
- P. Stahel, V. Mazankova, D. Podzemna, E. Podzemna, V. Pizurova, J. Jurmanova, L. Prokes, M. Lehocky, K. Ozaltin, H. Pistekova and D. Trunec, Antibacterial Thin Films Deposited from Propane-Butane Mixture in Atmospheric Pressure Discharge, Int. J. Mol. Sci., 2023, 24(2), 1–9 CrossRef PubMed.
- S. Karki, H. Kim, S.-J. Na, D. Shin, K. Jo and J. Lee, Thin films as an emerging platform for drug delivery, Asian J. Pharm. Sci., 2016, 11(5), 559–574 CrossRef.
- P. S. Goh, K. C. Wong and A. F. Ismail, Nanocomposite Membranes for Liquid and Gas Separations from the Perspective of Nanostructure Dimensions, Membranes, 2020, 10(10), 1–29 CrossRef PubMed.
- O. Oluwatosin Abegunde, E. Titilayo Akinlabi, O. Philip Oladijo, S. Akinlabi and A. Uchenna Ude, Overview of thin film deposition techniques, AIMS Mater. Sci., 2019, 6(2), 174–199 Search PubMed.
- M. A. Butt, Thin-Film Coating Methods: A Successful Marriage of High-Quality and Cost-Effectiveness—A Brief Exploration, Coatings, 2022, 12(8), 1–22 CrossRef.
- K. J. Moor, C. O. Osuji and J. H. Kim, Dual-Functionality Fullerene and Silver Nanoparticle Antimicrobial Composites via Block Copolymer Templates, ACS Appl. Mater. Interfaces, 2016, 8(49), 33583–33591 CrossRef CAS PubMed.
- Y. Zhao, L. Chen, Y. Wang, X. Song, K. Li, X. Yan, L. Yu and Z. He, Nanomaterial-based strategies in antimicrobial applications: Progress and perspectives, Nano Res., 2021, 14(12), 4417–4441 CrossRef CAS.
- N. Puvaca, Antimicrobial Resistance and Treatment in Companion, Food and Exotic Animals, Antibiotics, 2022, 11(10), 1–3 CrossRef PubMed.
- B. Khameneh, M. Iranshahy, V. Soheili and B. S. Fazly Bazzaz, Review on plant antimicrobials: a mechanistic viewpoint, Antimicrob. Resist. Infect. Control, 2019, 8, 118 CrossRef PubMed.
- H. Cho, T. Uehara and T. G. Bernhardt, Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery, Cell, 2014, 159(6), 1300–1311 CrossRef CAS PubMed.
- T. Schneider and H. G. Sahl, An oldie but a goodie - cell wall biosynthesis as antibiotic target pathway, Int. J. Med. Microbiol., 2010, 300(2–3), 161–169 CrossRef CAS PubMed.
- F. C. Tenover, Mechanisms of Antimicrobial Resistance in Bacteria, Am. J. Med., 2006, 119(6), S3–S10 CrossRef CAS PubMed.
- A. Maxwell, DNA gyrase as a drug target, Trends Microbiol., 1997, 5(3), 102–109 CrossRef CAS PubMed.
- A. Ovung and J. Bhattacharyya, Sulfonamide drugs: structure, antibacterial property, toxicity, and biophysical interactions, Biophys. Rev., 2021, 13(2), 259–272 CrossRef CAS PubMed.
- B. Khameneh, R. Diab, K. Ghazvini and B. S. Fazly Bazzaz, Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them, Microb. Pathog., 2016, 95, 32–42 CrossRef CAS PubMed.
- S. B. Levy, Active efflux mechanisms for antimicrobial resistance, Antimicrob. Agents Chemother., 1992, 36(4), 695–703 CrossRef CAS PubMed.
- G. P. Tegos, M. Haynes, J. J. Strouse, M. M. Khan, C. G. Bologa, T. I. Oprea and L. A. Sklar, Microbial efflux pump inhibition: tactics and strategies, Curr. Pharm. Des., 2011, 17(13), 1291–1302 CrossRef CAS PubMed.
- E. De, A. Basle, M. Jaquinod, N. Saint, M. Mallea, G. Molle and J. M. Pages, A new mechanism of antibiotic resistance in Enterobacteriaceae induced by a structural modification of the major porin, Mol. Microbiol., 2001, 41(1), 189–198 CrossRef CAS PubMed.
- J. M. Blair, M. A. Webber, A. J. Baylay, D. O. Ogbolu and L. J. Piddock, Molecular mechanisms of antibiotic resistance, Nat. Rev. Microbiol., 2015, 13(1), 42–51 CrossRef CAS PubMed.
- C. Walsh, Molecular mechanisms that confer antibacterial drug resistance, Nature, 2000, 406(6797), 775–781 CrossRef CAS PubMed.
- J. K. Savjani, A. K. Gajjar and K. T. Savjani, Mechanisms of resistance: useful tool to design antibacterial agents for drug - resistant bacteria, Mini-Rev. Med. Chem., 2009, 9(2), 194–205 CrossRef CAS PubMed.
- S. Zhao, J. W. Adamiak, V. Bonifay, J. Mehla, H. I. Zgurskaya and D. S. Tan, Defining new chemical space for drug penetration into Gram-negative bacteria, Nat. Chem. Biol., 2020, 16(12), 1293–1302 CrossRef CAS PubMed.
- H. A. Hemeg, Nanomaterials for alternative antibacterial therapy, Int. J. Nanomed., 2017, 12, 8211–8225 CrossRef CAS PubMed.
- M. A. Sani, M. Tavassoli, M. Azizi-Lalabadi, K. Mohammadi and D. J. McClements, Nano-enabled plant-based colloidal delivery systems for bioactive agents in foods: Design, formulation, and application, Adv. Colloid Interface Sci., 2022, 305, 102709 CrossRef CAS PubMed.
- T. Kruk, M. Golda-Cepa, K. Szczepanowicz, L. Szyk-Warszynska, M. Brzychczy-Wloch, A. Kotarba and P. Warszynski, Nanocomposite multifunctional polyelectrolyte thin films with copper nanoparticles as the antimicrobial coatings, Colloids Surf., B, 2019, 181, 112–118 CrossRef CAS PubMed.
- Y. N. Slavin, J. Asnis, U. O. Hafeli and H. Bach, Metal nanoparticles: understanding the mechanisms behind antibacterial activity, J. Nanobiotechnol., 2017, 15(1), 65 CrossRef PubMed.
- Y. Wu, Z. Song, H. Wang and H. Han, Endogenous stimulus-powered antibiotic release from nanoreactors for a combination therapy of bacterial infections, Nat. Commun., 2019, 10(1), 4464 CrossRef PubMed.
- A. I. Ribeiro, A. M. Dias and A. Zille, Synergistic Effects Between Metal Nanoparticles and Commercial Antimicrobial Agents: A Review, ACS Appl. Nano Mater., 2022, 5(3), 3030–3064 CrossRef CAS PubMed.
- N. Huang, X. Chen, X. Zhu, M. Xu and J. Liu, Ruthenium complexes/polypeptide self-assembled nanoparticles for identification of bacterial infection and targeted antibacterial research, Biomaterials, 2017, 141, 296–313 CrossRef CAS PubMed.
- S. J. Richards, K. Isufi, L. E. Wilkins, J. Lipecki, E. Fullam and M. I. Gibson, Multivalent Antimicrobial Polymer Nanoparticles Target Mycobacteria and Gram-Negative Bacteria by Distinct Mechanisms, Biomacromolecules, 2018, 19(1), 256–264 CrossRef CAS PubMed.
- S. F. Seyedpour, M. Dadashi Firouzjaei, A. Rahimpour, E. Zolghadr, A. Arabi Shamsabadi, P. Das, F. Akbari Afkhami, M. Sadrzadeh, A. Tiraferri and M. Elliott, Toward Sustainable Tackling of Biofouling Implications and Improved Performance of TFC FO Membranes Modified by Ag-MOF Nanorods, ACS Appl. Mater. Interfaces, 2020, 12(34), 38285–38298 CrossRef CAS PubMed.
- W. Lu, R. Cui, B. Zhu, Y. Qin, G. Cheng, L. Li and M. Yuan, Influence of clove essential oil immobilized in mesoporous silica nanoparticles on the functional properties of poly(lactic acid) biocomposite food packaging film, J. Mater. Res. Technol., 2021, 11, 1152–1161 CrossRef CAS.
- M. Chaudhary and A. Maiti, Fe–Al–Mn@chitosan based metal oxides blended cellulose acetate mixed matrix membrane for fluoride decontamination from water: Removal mechanisms and antibacterial behavior, J. Membr. Sci., 2020, 611, 118372 CrossRef CAS.
- M. A. Chowdhury, N. Hossain, M. G. Mostofa, M. R. Mia, M. Tushar, M. M. Rana and M. H. Hossain, Green synthesis and characterization of zirconium nanoparticlefor dental implant applications, Heliyon, 2023, 9(1), e12711 CrossRef CAS PubMed.
- H. Wang, W. Duan, Z. Ren, X. Li, W. Ma, Y. Guan, F. Liu, L. Chen, P. Yan and X. Hou, Engineered Sandwich-Structured Composite Wound Dressings with Unidirectional Drainage and Anti-Adhesion Supporting Accelerated Wound Healing, Adv. Healthcare Mater., 2023, 12(8), e2202685 CrossRef PubMed.
- B. Gu, Q. Jiang, B. Luo, C. Liu, J. Ren, X. Wang and X. Wang, A sandwich-like chitosan-based antibacterial nanocomposite film with reduced graphene oxide immobilized silver nanoparticles, Carbohydr. Polym., 2021, 260, 117835 CrossRef CAS PubMed.
- E. Sanchez-Lopez, D. Gomes, G. Esteruelas, L. Bonilla, A. L. Lopez-Machado, R. Galindo, A. Cano, M. Espina, M. Ettcheto, A. Camins, A. M. Silva, A. Durazzo, A. Santini, M. L. Garcia and E. B. Souto, Metal-Based Nanoparticles as Antimicrobial Agents: An Overview, Nanomaterials, 2020, 10(2), 1–39 CrossRef PubMed.
- V. Chiriac, D. N. Stratulat, G. Calin, S. Nichitus, V. Burlui, C. Stadoleanu, M. Popa and I. M. Popa, Antimicrobial property of zinc based nanoparticles, IOP Conf. Ser.: Mater. Sci. Eng., 2016, 133, 012055 Search PubMed.
- A. Evans and K. A. Kavanagh, Evaluation of metal-based antimicrobial compounds for the treatment of bacterial pathogens, J. Med. Microbiol., 2021, 70(5), 1–18 CrossRef PubMed.
- M. Sriubas, K. Bockute, P. Palevicius, M. Kaminskas, Z. Rinkevicius, M. Ragulskis, S. Simonyte, M. Ruzauskas and G. Laukaitis, Antibacterial Activity of Silver and Gold Particles Formed on Titania Thin Films, Nanomaterials, 2022, 12(7), 1–21 CrossRef PubMed.
- D. Mitra, E. T. Kang and K. G. Neoh, Antimicrobial Copper-Based Materials and Coatings: Potential Multifaceted Biomedical Applications, ACS Appl. Mater. Interfaces, 2020, 12(19), 21159–21182 CrossRef CAS PubMed.
- E. M. Cazalini, W. Miyakawa, G. R. Teodoro, A. S. S. Sobrinho, J. E. Matieli, M. Massi and C. Y. Koga-Ito, Antimicrobial and anti-biofilm properties of polypropylene meshes coated with metal-containing DLC thin films, J. Mater. Sci.: Mater. Med., 2017, 28(6), 97 CrossRef PubMed.
- G. Qingquan, L. Jingguo, C. Tiankai, Y. Qiaofeng and X. Jianping, Antimicrobial thin-film composite membranes with chemically decorated ultrasmall Silver nanoclusters, ACS Sustain. Chem. Eng., 2019, 7(17), 14848–14855 CrossRef.
- L. D. Villa-Garcia, R. Marquez-Preciado, M. Ortiz-Magdaleno, O. A. Patron-Soberano, M. A. Alvarez-Perez, A. Pozos-Guillen and L. O. Sanchez-Vargas, Antimicrobial effect of gold nanoparticles in the formation of the Staphylococcus aureus biofilm on a polyethylene surface, Braz. J. Microbiol., 2021, 52(2), 619–625 CrossRef CAS PubMed.
- M. Godoy-Gallardo, U. Eckhard, L. M. Delgado, Y. J. D. de Roo Puente, M. Hoyos-Nogues, F. J. Gil and R. A. Perez, Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications, Bioact. Mater., 2021, 6(12), 4470–4490 CAS.
- A. Raghunath and E. Perumal, Metal oxide nanoparticles as antimicrobial agents: a promise for the future, Int. J. Antimicrob. Agents, 2017, 49(2), 137–152 CrossRef CAS PubMed.
- A. Agrawal, A. Sharma, K. K. Awasthi and A. Awasthi, Metal oxides nanocomposite membrane for biofouling mitigation in wastewater treatment, Mater. Today Chem., 2021, 21, 100532 CrossRef CAS.
- P. Latko-Duralek, M. Misiak, M. Staniszewska, K. Rosloniec, M. Grodzik, R. P. Socha, M. Krzan, B. Bazanow, A. Pogorzelska and A. Boczkowska, The Composites of Polyamide 12 and Metal Oxides with High Antimicrobial Activity, Polymers, 2022, 14(15), 1–21 CrossRef PubMed.
- S. Sinha Ray and M. Okamoto, Polymer/layered silicate nanocomposites: a review from preparation to processing, Prog. Polym. Sci., 2003, 28(11), 1539–1641 CrossRef.
- A. Mersha, R. Selyanchyn and S. Fujikawa, Preparation of large, ultra-flexible and free-standing nanomembranes of metal oxide–polymer composite and their gas permeation properties, Clean Energy, 2017, 1(1), 80–89 CrossRef.
- S. Wu, J. Xu, L. Zou, S. Luo, R. Yao, B. Zheng, G. Liang, D. Wu and Y. Li, Long-lasting renewable antibacterial porous polymeric coatings enable titanium biomaterials to prevent and treat peri-implant infection, Nat. Commun., 2021, 12(1), 3303 CrossRef CAS PubMed.
- B. Sartori, H. Amenitsch and B. Marmiroli, Functionalized Mesoporous Thin Films for Biotechnology, Micromachines, 2021, 12(7), 1–17 CrossRef PubMed.
- S. Atefyekta, B. Ercan, J. Karlsson, E. Taylor, S. Chung, T. J. Webster and M. Andersson, Antimicrobial performance of mesoporous titania thin films: role of pore size, hydrophobicity, and antibiotic release, Int. J. Nanomed., 2016, 11, 977–990 CAS.
- H. R. Bakhsheshi-Rad, A. F. Ismail, M. Aziz, Z. Hadisi, M. Omidi and X. Chen, Antibacterial activity and corrosion resistance of Ta2O5 thin film and electrospun PCL/MgO-Ag nanofiber coatings on biodegradable Mg alloy implants, Ceram. Int., 2019, 45(9), 11883–11892 CrossRef CAS.
- B. Le Ouay and F. Stellacci, Antibacterial activity of silver nanoparticles: A surface science insight, Nano Today, 2015, 10(3), 339–354 CrossRef CAS.
- I. Fernando and Y. Zhou, Impact of pH on the stability, dissolution and aggregation kinetics of silver nanoparticles, Chemosphere, 2019, 216, 297–305 CrossRef CAS PubMed.
- D. Thomas, J. Abraham and K. K. Sadasivuni, A scrutiny of antibacterial activity of pure and iodine doped ZnO thin films synthesized by mSILAR method, in Proceedings of the International Conference on Advanced Materials: Icam 2019, 2019 Search PubMed.
- L. K. Ruddaraju, S. V. N. Pammi, P. Pallela, V. S. Padavala and V. R. M. Kolapalli, Antibiotic potentiation and anti-cancer competence through bio-mediated ZnO nanoparticles, Mater. Sci. Eng., C, 2019, 103, 109756 CrossRef CAS PubMed.
- U. Chakraborty, G. Kaur, H.-G. Rubahn, A. Kaushik, G. R. Chaudhary and Y. K. Mishra, Advanced metal oxides nanostructures to recognize and eradicate water pollutants, Prog. Mater. Sci., 2023, 139, 101169 CrossRef CAS.
- D. Nunes, A. Pimentel, R. Branquinho, E. Fortunato and R. Martins, Metal Oxide-Based Photocatalytic Paper: A Green Alternative for Environmental Remediation, Catalysts, 2021, 11(4), 1–30 CrossRef.
- E. Nascimben Santos, Á. Ágoston, S. Kertész, C. Hodúr, Z. László, Z. Pap, Z. Kása, T. Alapi, S. A. G. Krishnan, G. Arthanareeswaran, K. Hernadi and G. Veréb, Investigation of the applicability of TiO2, BiVO4, and WO3 nanomaterials for advanced photocatalytic membranes used for oil-in-water emulsion separation, Asia-Pac. J. Chem. Eng., 2020, 15(5), 1–15 Search PubMed.
- H.-J. Choi, S.-Y. Seo, J.-S. Jung and S.-G. Yoon, Water-resistant and antibacterial zinc aluminate films: Application of antibacterial thin film capacitors, ACS Appl. Electron. Mater., 2021, 3(3), 1429–1436 CrossRef CAS.
- G.-L. Tan, D. Tang, D. Dastan, A. Jafari, Z. Shi, Q.-Q. Chu, J. P. B. Silva and X.-T. Yin, Structures, morphological control, and antibacterial performance of tungsten oxide thin films, Ceram. Int., 2021, 47(12), 17153–17160 CrossRef CAS.
- S. M. Dizaj, F. Lotfipour, M. Barzegar-Jalali, M. H. Zarrintan and K. Adibkia, Antimicrobial activity of the metals and metal oxide nanoparticles, Mater. Sci. Eng., C, 2014, 44, 278–284 CrossRef CAS PubMed.
- S. Mohammad Nejad, S. F. Seyedpour, S. Aghapour Aktij, M. Dadashi Firouzjaei, M. Elliott, A. Tiraferri, M. Sadrzadeh and A. Rahimpour, Loose nanofiltration membranes functionalized with in situ-synthesized metal organic framework for water treatment, Mater. Today Chem., 2022, 24, 100909 CrossRef CAS.
- Y. Li, X. Xia, W. Hou, H. Lv, J. Liu and X. Li, How Effective are Metal Nanotherapeutic Platforms Against Bacterial Infections? A Comprehensive Review of Literature, Int. J. Nanomed., 2023, 18, 1109–1128 CrossRef CAS PubMed.
- M. Pejman, M. Dadashi Firouzjaei, S. Aghapour Aktij, E. Zolghadr, P. Das, M. Elliott, M. Sadrzadeh, M. Sangermano, A. Rahimpour and A. Tiraferri, Effective strategy for UV-mediated grafting of biocidal Ag-MOFs on polymeric membranes aimed at enhanced water ultrafiltration, Chem. Eng. J., 2021, 426, 130704 CrossRef CAS.
- M. Pejman, M. Dadashi Firouzjaei, S. Aghapour Aktij, P. Das, E. Zolghadr, H. Jafarian, A. Arabi Shamsabadi, M. Elliott, M. Sadrzadeh, M. Sangermano, A. Rahimpour and A. Tiraferri, In Situ Ag-MOF Growth on Pre-Grafted Zwitterions Imparts Outstanding Antifouling Properties to Forward Osmosis Membranes, ACS Appl. Mater. Interfaces, 2020, 12(32), 36287–36300 CrossRef CAS PubMed.
- T. Zhang, Z. Li, W. Wang, Y. Wang, B. Gao and Z. Wang, Enhanced antifouling and antimicrobial thin film nanocomposite membranes with incorporation of Palygorskite/titanium dioxide hybrid material, J. Colloid Interface Sci., 2019, 537, 1–10 CrossRef CAS PubMed.
- G. Hu, J. Yang, X. Duan, R. Farnood, C. Yang, J. Yang, W. Liu and Q. Liu, Recent developments and challenges in zeolite-based composite photocatalysts for environmental applications, Chem. Eng. J., 2021, 417, 129209 CrossRef CAS.
- M. I. Hutchings, A. W. Truman and B. Wilkinson, Antibiotics: past, present and future, Curr. Opin. Microbiol., 2019, 51, 72–80 CrossRef CAS PubMed.
- R. Cristescu, A. Visan, G. Socol, A. V. Surdu, A. E. Oprea, A. M. Grumezescu, M. C. Chifiriuc, R. D. Boehm, D. Yamaleyeva, M. Taylor, R. J. Narayan and D. B. Chrisey, Antimicrobial activity of biopolymeric thin films containing flavonoid natural compounds and silver nanoparticles fabricated by MAPLE: A comparative study, Appl. Surf. Sci., 2016, 374, 290–296 CrossRef CAS.
- D. Stan, A. M. Enciu, A. L. Mateescu, A. C. Ion, A. C. Brezeanu, D. Stan and C. Tanase, Natural Compounds With Antimicrobial and Antiviral Effect and Nanocarriers Used for Their Transportation, Front. Pharmacol, 2021, 12, 723233 CrossRef CAS PubMed.
- R. Mahendran, D. Sridharan, C. Arunmozhidevan, T. A. Selvakumar and P. Rajasekar, Fabrication and Antibacterial Effects of Polycarbonate/Leaf Extract Based Thin Films, J. Mater., 2016, 2016, 1–7 Search PubMed.
- W. Huang, Y. Wang, W. Tian, X. Cui, P. Tu, J. Li, S. Shi and X. Liu, Biosynthesis Investigations of Terpenoid, Alkaloid, and Flavonoid Antimicrobial Agents Derived from Medicinal Plants, Antibiotics, 2022, 11(10), 1–32 CrossRef PubMed.
- Y. Yan, X. Li, C. Zhang, L. Lv, B. Gao and M. Li, Research Progress on Antibacterial Activities and Mechanisms of Natural Alkaloids: A Review, Antibiotics, 2021, 10(3), 1–30 CrossRef PubMed.
- F. G. Avci, N. A. Sayar and B. Sariyar Akbulut, An OMIC approach to elaborate the antibacterial mechanisms of different alkaloids, Phytochemistry, 2018, 149, 123–131 CrossRef CAS PubMed.
- H. J. Jafaar, O. Isbilen, E. Volkan and G. Sariyar, Alkaloid profiling and antimicrobial activities of Papaver glaucum and P. decaisnei, BMC Res. Notes, 2021, 14(1), 348 CrossRef CAS PubMed.
- L. Othman, A. Sleiman and R. M. Abdel-Massih, Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants, Front. Microbiol., 2019, 10, 911 CrossRef PubMed.
- C. Papuc, G. V. Goran, C. N. Predescu, V. Nicorescu and G. Stefan, Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms, Compr. Rev. Food Sci. Food Saf., 2017, 16(6), 1243–1268 CrossRef CAS PubMed.
- L. Bouarab Chibane, P. Degraeve, H. Ferhout, J. Bouajila and N. Oulahal, Plant antimicrobial polyphenols as potential natural food preservatives, J. Sci. Food Agric., 2019, 99(4), 1457–1474 CrossRef CAS PubMed.
- S. Atta, D. Waseem, I. Naz, F. Rasheed, A. R. Phull, T. Ur-Rehman, N. Irshad, P. Amna and H. Fatima, Polyphenolic characterization and evaluation of multimode antioxidant, cytotoxic, biocompatibility and antimicrobial potential of selected ethno-medicinal plant extracts, Arabian J. Chem., 2023, 16(2), 1–21 Search PubMed.
- J. H. Doughari, P. A. Ndakidemi, I. S. Human and S. Benade, Antioxidant, antimicrobial and antiverotoxic potentials of extracts of Curtisia dentata, J. Ethnopharmacol., 2012, 141(3), 1041–1050 CrossRef CAS PubMed.
- G. A. Kemegne, P. Mkounga, J. J. Essia Ngang, S. L. Sado Kamdem and A. E. Nkengfack, Antimicrobial structure activity relationship of five anthraquinones of emodine type isolated from Vismia laurentii, BMC Microbiol., 2017, 17(1), 41 CrossRef PubMed.
- A. Yadav, R. Bhardwaj and R. A. Sharma, Phytochemical Screening and Antimicrobial Activity of Anthraquinones Isolated from Different Parts of Cassia nodosa, Res. J. Med. Plant, 2013, 7(3), 150–157 CrossRef CAS.
- S. Dong, X. Yang, L. Zhao, F. Zhang, Z. Hou and P. Xue, Antibacterial activity and mechanism of action saponins from Chenopodium quinoa Willd. husks against foodborne pathogenic bacteria, Ind. Crops Prod., 2020, 149, 112350 CrossRef CAS.
- Z. Yu, X. Wu and J. He, Study on the antifungal activity and mechanism of tea saponin from Camellia oleifera cake, Eur. Food Res. Technol., 2022, 248(3), 783–795 CrossRef CAS.
- M. I. Khan, A. Ahhmed, J. H. Shin, J. S. Baek, M. Y. Kim and J. D. Kim, Green Tea Seed Isolated Saponins Exerts Antibacterial Effects against Various Strains of Gram Positive and Gram Negative Bacteria, a Comprehensive Study In Vitro and In Vivo, J. Evidence-Based Complementary Altern. Med., 2018, 2018, 3486106 Search PubMed.
- L. Song, X. Hu, X. Ren, J. Liu and X. Liu, Antibacterial Modes of Herbal Flavonoids Combat Resistant Bacteria, Front. Pharmacol, 2022, 13, 873374 CrossRef CAS PubMed.
- Y. Liu, J. Liu and Y. Zhang, Research Progress on Chemical Constituents of Zingiber officinale Roscoe, BioMed Res. Int., 2019, 2019, 5370823 Search PubMed.
- S. A. Al-Hilifi, R. M. Al-Ali and A. T. Petkoska, Ginger essential oil as an active addition to composite chitosan films: Development and characterization, Gels, 2022, 8(6), 1–14 CrossRef PubMed.
- N. Sihombing, M. Elma, R. N. Thala’ah, F. A. Simatupang, E. A. Pradana and A. Rahma, Garlic essential oil as an edible film antibacterial agent derived from Nagara sweet potato starch applied for packaging of Indonesian traditional food - Dodol, IOP Conf. Ser.: Mater. Sci. Eng., 2022, 999(1), 1–13 Search PubMed.
- G. F. El Fawal, A. M. Omer and T. M. Tamer, Evaluation of antimicrobial and antioxidant activities for cellulose acetate films incorporated with Rosemary and Aloe Vera essential oils, J. Food Sci. Technol., 2019, 56(3), 1510–1518 CrossRef CAS PubMed.
- B. Aaliya, K. V. Sunooj and P. Krina, Determining the Effect of Plant Extracts on the Development and Characterization of Biodegradable Composite Films from Corypha umbraculifera L. Stem Starch, Biol. Life Sci. Forum, 2022, 20(13), 1–10 Search PubMed.
- M. Ordon, M. Zdanowicz, P. Nawrotek, X. Stachurska and M. Mizielinska, Polyethylene Films Containing Plant Extracts in the Polymer Matrix as Antibacterial and Antiviral Materials, Int. J. Mol. Sci., 2021, 22, 13438 CrossRef CAS PubMed.
- A. Ali, A. Basit, A. Hussain, S. Sammi, A. Wali, G. Goksen, A. Muhammad, F. Faiz, M. Trif, A. Rusu and M. F. Manzoor, Starch-based environment friendly, edible and antimicrobial films reinforced with medicinal plants, Front. Nutr., 2022, 9, 1066337 CrossRef PubMed.
- N. Vaou, E. Stavropoulou, C. C. Voidarou, Z. Tsakris, G. Rozos, C. Tsigalou and E. Bezirtzoglou, Interactions between Medical Plant-Derived Bioactive Compounds: Focus on Antimicrobial Combination Effects, Antibiotics, 2022, 11, 1014 CrossRef CAS PubMed.
- J. Liang, X. Huang and G. Ma, Antimicrobial activities and mechanisms of extract and components of herbs in East Asia, RSC Adv., 2022, 12(45), 29197–29213 RSC.
- B. Thallinger, E. N. Prasetyo, G. S. Nyanhongo and G. M. Guebitz, Antimicrobial enzymes: an emerging strategy to fight microbes and microbial biofilms, Biotechnol. J., 2013, 8(1), 97–109 CrossRef CAS PubMed.
- K. Yuan, X. Liu, J. Shi, W. Liu, K. Liu, H. Lu, D. Wu, Z. Chen and C. Lu, Antibacterial Properties and Mechanism of Lysozyme-Modified ZnO Nanoparticles, Front. Chem., 2021, 9, 762255 CrossRef CAS PubMed.
- Z. Lian, C. Lu, J. Zhu, X. Zhang, T. Wu, Y. Xiong, Z. Sun and R. Yang, Mo@ZIF-8 nanozyme preparation and its antibacterial property evaluation, Front. Chem., 2022, 10, 1093073 CrossRef CAS PubMed.
- M. R. Felicio, O. N. Silva, S. Goncalves, N. C. Santos and O. L. Franco, Peptides with Dual Antimicrobial and Anticancer Activities, Front. Chem., 2017, 5, 5 CrossRef PubMed.
- R. Nawrot, J. Barylski, G. Nowicki, J. Broniarczyk, W. Buchwald and A. Gozdzicka-Jozefiak, Plant antimicrobial peptides, Folia Microbiol., 2014, 59(3), 181–196 CrossRef CAS PubMed.
- M. Leitgeb, K. Kupnik, Z. Knez and M. Primozic, Enzymatic and Antimicrobial Activity of Biologically Active Samples from Aloe arborescens and Aloe barbadensis, Biology, 2021, 10, 765 CrossRef CAS PubMed.
- A. Khlyustova, M. Kirsch, X. Ma, Y. Cheng and R. Yang, Surfaces with antifouling-antimicrobial dual function via immobilization of lysozyme on zwitterionic polymer thin films, J. Mater. Chem. B, 2022, 10(14), 2728–2739 RSC.
- K. Solanki, N. Grover, P. Downs, E. E. Paskaleva, K. K. Mehta, L. Lee, L. S. Schadler, R. S. Kane and J. S. Dordick, Enzyme-based listericidal nanocomposites, Sci. Rep., 2013, 3, 1584 CrossRef PubMed.
- L. D. Blackman, Y. Qu, P. Cass and K. E. S. Locock, Approaches for the inhibition and elimination of microbial biofilms using macromolecular agents, Chem. Soc. Rev., 2021, 50(3), 1587–1616 RSC.
- M. H. Baek, M. Kamiya, T. Kushibiki, T. Nakazumi, S. Tomisawa, C. Abe, Y. Kumaki, T. Kikukawa, M. Demura, K. Kawano and T. Aizawa, Lipopolysaccharide-bound structure of the antimicrobial peptide cecropin P1 determined by nuclear magnetic resonance spectroscopy, J. Pept. Sci., 2016, 22(4), 214–221 CrossRef CAS PubMed.
- H. B. Coban, Organic acids as antimicrobial food agents: applications and microbial productions, Bioprocess Biosyst. Eng., 2020, 43(4), 569–591 CrossRef CAS PubMed.
- P. Kaur, T. Alam, H. Singh, J. Jain, G. Singh and A. A. Broadway, Organic Acids Modified Starch–CMC Based Biodegradable Film: Antibacterial Activity, Morphological, Structural, Thermal, and Crystalline Properties, J. Pure Appl. Microbiol., 2023, 17(1), 241–257 CrossRef.
- G. S. Sözbilen, E. Çavdaroğlu and A. Yemenicioğlu, Incorporation of organic acids turns classically brittle zein films into flexible antimicrobial packaging materials, Packag. Technol. Sci., 2021, 35(1), 81–95 CrossRef.
- M. d. Rocha, M. R. Loiko, E. C. Tondo and C. Prentice, Physical, mechanical and antimicrobial properties of Argentine anchovy (Engraulis anchoita) protein films incorporated with organic acids, Food Hydrocolloids, 2014, 37, 213–220 CrossRef.
- S. Suganthi, S. Vignesh, J. Kalyana Sundar and V. Raj, Fabrication of PVA polymer films with improved antibacterial activity by fine-tuning via organic acids for food packaging applications, Appl. Water Sci., 2020, 10, 100 CrossRef CAS.
- A. N. Romainor, S. F. Chin and S. Lihan, Antimicrobial Starch-Based Film for Food Packaging Application, Starch/Staerke, 2021, 74, 2100207 CrossRef.
- M. Gumienna and B. Gorna, Antimicrobial Food Packaging with Biodegradable Polymers and Bacteriocins, Molecules, 2021, 26, 3735 CrossRef CAS PubMed.
- R. U. Khan, S. Naz, F. Raziq, Q. Qudratullah, N. A. Khan, V. Laudadio, V. Tufarelli and M. Ragni, Prospects of organic acids as safe alternative to antibiotics in broiler chickens diet, Environ. Sci. Pollut. Res. Int., 2022, 29(22), 32594–32604 CrossRef CAS PubMed.
- H. Chen, T. Chen, P. Giudici and F. Chen, Vinegar Functions on Health: Constituents, Sources, and Formation Mechanisms, Compr. Rev. Food Sci. Food Saf., 2016, 15(6), 1124–1138 CrossRef CAS PubMed.
- L. Kovanda, W. Zhang, X. Wei, J. Luo, X. Wu, E. R. Atwill, S. Vaessen, X. Li and Y. Liu, In Vitro Antimicrobial Activities of Organic Acids and Their Derivatives on Several Species of Gram-Negative and Gram-Positive Bacteria, Molecules, 2019, 24(20), 1–14 CrossRef PubMed.
- C. Kranjec, K. V. Ovchinnikov, T. Gronseth, K. Ebineshan, A. Srikantam and D. B. Diep, A bacteriocin-based antimicrobial formulation to effectively disrupt the cell viability of methicillin-resistant Staphylococcus aureus (MRSA) biofilms, npj Biofilms Microbiomes, 2020, 6(1), 58 CrossRef CAS PubMed.
- A. W. Negash and B. A. Tsehai, Current Applications of Bacteriocin, Int. J. Microbiol., 2020, 2020, 4374891 Search PubMed.
- W. Woraprayote, Y. Malila, S. Sorapukdee, A. Swetwiwathana, S. Benjakul and W. Visessanguan, Bacteriocins from lactic acid bacteria and their applications in meat and meat products, Meat Sci., 2016, 120, 118–132 CrossRef CAS PubMed.
- C. R. Contessa, N. B. de Souza, G. B. Goncalo, C. M. de Moura, G. S. da Rosa and C. C. Moraes, Development of Active Packaging Based on Agar-Agar Incorporated with Bacteriocin of Lactobacillus sakei, Biomolecules, 2021, 11(12), 1–9 CrossRef PubMed.
- R. Benabbou, M. Subirade, M. Desbiens and I. Fliss, Divergicin M35-Chitosan Film: Development and Characterization, Probiotics Antimicrob. Proteins, 2020, 12(4), 1562–1570 CrossRef CAS PubMed.
- A. La Storia, F. A. Di Giuseppe, S. Volpe, V. Oliviero, F. Villani and E. Torrieri, Physical properties and antimicrobial activity of bioactive film based on whey protein and Lactobacillus curvatus 54M16 producer of bacteriocins, Food Hydrocolloids, 2020, 108, 105959 CrossRef CAS.
- P. Bagde and V. Nadanathangam, Mechanical, antibacterial and biodegradable properties of starch film containing bacteriocin immobilized crystalline nanocellulose, Carbohydr. Polym., 2019, 222, 115021 CrossRef CAS PubMed.
- M. Amerikova, I. Pencheva El-Tibi, V. Maslarska, S. Bozhanov and K. Tachkov, Antimicrobial activity, mechanism of action, and methods for stabilisation of defensins as new therapeutic agents, Biotechnol. Biotechnol. Equip., 2019, 33(1), 671–682 CrossRef CAS.
- J. D. Simpson, S. A. Smith, K. J. Thurecht and G. Such, Engineered Polymeric Materials for Biological Applications: Overcoming Challenges of the Bio-Nano Interface, Polymers, 2019, 11(9), 1–33 CrossRef PubMed.
- A. C. Pinho and A. P. Piedade, Polymeric Coatings with Antimicrobial Activity: A Short Review, Polymers, 2020, 12(11), 1–14 CrossRef PubMed.
- L. Floroian, C. Ristoscu, N. Mihailescu, I. Negut, M. Badea, D. Ursutiu, M. C. Chifiriuc, I. Urzica, H. M. Dyia, C. Bleotu and I. N. Mihailescu, Functionalized Antimicrobial Composite Thin Films Printing for Stainless Steel Implant Coatings, Molecules, 2016, 21(6), 1–18 CrossRef PubMed.
- Y. Huan, Q. Kong, H. Mou and H. Yi, Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields, Front. Microbiol., 2020, 11, 582779 CrossRef PubMed.
- A. F. van Tol, J. E. Tibballs, N. Roar Gjerdet and P. Ellison, Experimental investigation of the effect of surface roughness on bone-cement-implant shear bond strength, J. Mech. Behav. Biomed. Mater., 2013, 28, 254–262 CrossRef CAS PubMed.
- M. Haktaniyan and M. Bradley, Polymers showing intrinsic antimicrobial activity, Chem. Soc. Rev., 2022, 51(20), 8584–8611 RSC.
- M. Maruthapandi, A. Saravanan, P. Das, M. Natan, G. Jacobi, E. Banin, J. H. T. Luong and A. Gedanken, Antimicrobial Activities of Zn-Doped CuO Microparticles Decorated on Polydopamine against Sensitive and Antibiotic-Resistant Bacteria, ACS Appl. Polym. Mater., 2020, 2(12), 5878–5888 CrossRef CAS.
- V. Kudryavtseva, M. Otero, J. Zhang, A. Bukatin, D. Gould and G. B. Sukhorukov, Drug-Eluting Sandwich Hydrogel Lenses Based on Microchamber Film Drug Encapsulation, ACS Nanosci. Au, 2023, 3(3), 256–265 CrossRef CAS PubMed.
- J. Wu, C. Yu and Q. Li, Regenerable antimicrobial activity in polyamide thin film nanocomposite membranes, J. Membr. Sci., 2015, 476, 119–127 CrossRef CAS.
- J. A. Lichter, K. J. Van Vliet and M. F. Rubner, Design of Antibacterial Surfaces and Interfaces: Polyelectrolyte Multilayers as a Multifunctional Platform, Macromolecules, 2009, 42(22), 8573–8586 CrossRef CAS.
- Y. Ahmadi, N. Moeini, M. Yadav and S. Ahmad, Antimicrobial polymer nanocomposite films and coatings, in Handbook of Polymer Nanocomposites for Industrial Applications, 2021, pp. 379–397 Search PubMed.
- K. Rajendiran, Z. Zhao, D. S. Pei and A. Fu, Antimicrobial Activity and Mechanism of Functionalized Quantum Dots, Polymers, 2019, 11(10), 1–13 CrossRef PubMed.
- V. Srimaneepong, H. E. Skallevold, Z. Khurshid, M. S. Zafar, D. Rokaya and J. Sapkota, Graphene for Antimicrobial and Coating Application, Int. J. Mol. Sci., 2022, 23(1), 1–17 Search PubMed.
- F. Jamal, A. Rafique, S. Moeen, J. Haider, W. Nabgan, A. Haider, M. Imran, G. Nazir, M. Alhassan, M. Ikram, Q. Khan, G. Ali, M. Khan, W. Ahmad and M. Maqbool, Review of Metal Sulfide Nanostructures and their Applications, ACS Appl. Nano Mater., 2023, 6(9), 7077–7106 CrossRef CAS.
- M. Khatami, P. Iravani, G. Jamalipour Soufi and S. Iravani, MXenes for antimicrobial and antiviral applications: recent advances, Mater. Technol., 2021, 37(11), 1890–1905 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |