 Open Access Article
Open Access ArticleStereoselectivity in electrosprayed confined volumes: asymmetric synthesis of warfarin by diamine organocatalysts in microdroplets and thin films†
Martina De Angelis ,
Marta Managò,
Federico Pepi,
Chiara Salvitti
,
Marta Managò,
Federico Pepi,
Chiara Salvitti ,
Anna Troiani
,
Anna Troiani ,
Claudio Villani
,
Claudio Villani and
Alessia Ciogli
and
Alessia Ciogli *
*
Department of Chemistry and Drug Technologies, Sapienza University of Rome, Piazzale Aldo Moro 5, 00185 Roma, Italy. E-mail: alessia.ciogli@uniroma1.it
First published on 4th January 2024
Abstract
The asymmetric synthesis of warfarin in microdroplets and thin films generated by an electrospray ionization (ESI) source is reported. This is one of the first examples of an enantioselective organocatalyzed reaction in electrosprayed confined volumes. The optimal conditions in terms of system setting were established for this reaction.
Microdroplet reactions have drawn particular attention due to the unique reaction environment.1 The interest of the scientific community has been focused on the remarkable acceleration or, in some rare cases, to the different reactivity compared to the bulk.2 To date, microdroplet reactions have been performed for numerous reactions: formation of new carbon–carbon, carbon–oxygen and carbon–nitrogen bonds, dehydration as well as oxidation and reduction reactions.3–7 In addition, microdroplet synthesis has been scaled up at a preparative level with a production rate up to 30 mg min−1.3c,8 Moreover, the continuous deposition of the microdroplets onto a solid surface generates a thin film that retains the peculiar microdroplets confined volume needed for reaction acceleration but allows extension of the reaction time to any desired value.9 However, a field not even explored is the use of electrosprayed microdroplet reactions in the synthesis of chiral compounds by asymmetric organocatalysis. In fact, there are many examples of synthesis in microdroplets generated by ESI- or DESI-based techniques, including the production of compounds that possess stereogenic centers4,5b for which no data on stereoselectivity are reported. So, at the beginning of our study we wondered why the stereoselectivity issue had not investigated further before: do the asymmetric reactions in ESI microdroplets occur? Does stereoselectivity change compared to that in bulk conditions? Parallel to us, the research group of Sun and Cheng was wondering about the same issues.10 In their very recent publication, the authors analyzed the enantioselectivity in sonic sprayed microdroplets taking into account three different reaction cases. In the first one, the authors considered the retention of a chiral center in a C–N cleavage of a unimolecular reaction finding that pre-existing stereogenic centers of chiral starting materials are maintained in microdroplet conditions. Another analyzed case involved an enzymatic reduction by nicotinamide adenine dinucleotide phosphate (NADP+). Here the stereoselectivity achieved was the same as the one obtained in bulk conditions (99% ee). However, the authors found unfavorable results regarding the stereoselectivity when two asymmetric organocatalyzed reactions, a bimolecular Mannich reaction and a three-component Passerini reaction, were considered. In the latter examined cases, the authors have found a crash of stereoselectivity compared to the bulk either way (6% vs. 71% of ee for Mannich reaction and 15% vs. 91% of ee for Passerini reaction). According to these preliminary results, the authors have reached the conclusion that microdroplets conditions can harm stereospecificity in asymmetric catalysis by chiral catalysts and chiral organic ligands. In this work, we demonstrated that the confined volumes of ESI-microdroplets and of the thin film formed by their deposition can be used to perform asymmetric organocatalyzed reactions with stereoselectivity comparable to that obtained in bulk.
We chose to start exploring this field using a model reaction: the asymmetric synthesis of warfarin, an anticoagulant antagonist of vitamin K11 bearing a stereogenic center, present on the market as racemate, even though the S enantiomer is more active than the R one.12,13 The asymmetric synthesis of warfarin and of its analogues is a model reaction widely described in the evaluation of chiral amines and their derivatives as organocatalysts.14,15 In this work we selected the 1,2-diaminocyclohexane (DACH) 3 and 1,2-diphenylethylendiamine (DIPEDA) 4 as chiral catalysts (Scheme 1) without the addition of acidic cocatalysts. These diamines provide the warfarin through the formation of an iminium ion with the benzylideneacetone (2) followed by a nucleophilic attack of 4-hydroxycoumarin (1).
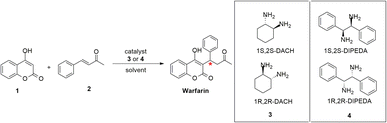 | ||
| Scheme 1 Asymmetric synthesis of warfarin by diamine catalysts 1S,2S- or 1R,2R-DACH and 1S,2S- or 1R,2R-DIPEDA. | ||
The first part of our work was dedicated to the optimization of conditions moving from the bulk to the ESI-microdroplet/thin film systems. In our previous studies, we found that the reaction between 1 and 2 (1 M in THF) by DACH 3 or DIPEDA 4 (10% mol) lead to the warfarin formation in 78(+)/22(−) or 86(+)/14(−) er (enantiomeric ratio) and yields >90% after 24 or 48 hours respectively.16 Starting from this, in order to find the optimal conditions for the ESI experiments, we first test the reaction at lower concentrations17 using DACH as catalyst in THF and in ACN at room temperature (r.t.) for 5 h, obtaining the results reported in Table 1 (entries 1–4). In addition to the room temperature, the effect of higher temperatures was also evaluated taking into account the availability to heat the ESI source. Then, reactions performed at 60 °C and at reflux led to a decrease of enantiomeric ratio as expected (Table 1, entries 5–6) and to an increase of yield from 46% to 63%.
| Entry | Bulk reactionsa | |||||
|---|---|---|---|---|---|---|
| Solvent | T (°C) | Conc.b | Catalyst | Time | er (+)/(−)c | |
| a All the reactions were performed in a round bottom flask under magnetic stirring.b Concentration 10−2 M: 0.12 mmol of 1, 0.15 mmol of 2 and 10% (0.01 mmol) of catalyst (3 or 4) in 10 mL of solvent. Concentration 10−4 M: 0.1 mL of mixture 10−2 M are diluted with 9.9 mL of solvent.c The er was calculated by enantioselective HPLC analysis (see ESI for more details).d The er was determined on reaction crude.e The er was determined on the isolated product (see ESI for more details): entry 3: yield 46%; entry 6: 63%; entry 7: yield 49%; entry 8: 68%. | ||||||
| 1 | THF | r.t. | 10−2 M | S,S-3 | 5 h | 20/80d |
| 2 | THF | r.t. | 10−4 M | S,S-3 | 5 h | 24/76d |
| 3 | ACN | r.t. | 10−2 M | S,S-3 | 5 h | 15/85e |
| 4 | ACN | r.t. | 10−4 M | S,S-3 | 5 h | 18/82d |
| 5 | ACN | 60 | 10−2 M | S,S-3 | 5 h | 20/80d |
| 6 | ACN | 82 | 10−2 M | S,S-3 | 5 h | 33/67e |
| 7 | ACN | r.t. | 10−2 M | R,R-4 | 5 h | 80/20e |
| 8 | ACN | 82 | 10−2 M | R,R-4 | 5 h | 64/36e |
We selected the concentration of 10−2 M in ACN to test the reaction with DIPEDA which gave the results reported in Table 1, entries 7–8. Starting from 19.6 mg of 1, after 5 hours of reaction time (entries 3 and 7), agree respectively with 17 mg and 18 mg of enantioenriched product (see ESI†), making them suitable at a preparative level. Once selected the suitable solvent and concentration, we then performed the reaction in microdroplets by injecting the 10−2 M ACN solutions into a suitably modified ESI Z-spray source.18 The ESI microdorplets were collected in an Eppendorf tip containing a “silica cap” in order to capture the neutral products of the reaction for their HPLC quantification. Initially, we tested the microdroplet reaction using System A set-up which involved one syringe containing the reaction mixture of 1, 2, and catalyst 3 (solution 10−2 M in ACN) in a single spray (Fig. 1a). The reaction mixture was filled in the syringe and infused at a flow rate of 15 μL min−1 for 5 hours in order to collect a manageable quantitative of crude (details of reaction setting and ESI-parameters are reported in ESI†). The crude of reaction was collected and analyzed by enantioselective HPLC. The value of found e.r. (entry 1, Table 2) was lower than that obtained from the reaction in bulk at room temperature but higher than the bulk reaction performed at reflux temperature, despite the measured temperature of ESI source is 85 °C (desolvation gas temperature (DSG) setting at 150 °C). Although the set-up of System A involving the reaction mixture with all the reactants placed in a single syringe is widely used for microdroplet reactions,2a,5a,19 we considered the possibility to make an error due to a not negligible mixing time of the reagents within the syringe. To avoid this, we employed the System B set-up (Fig. 1b). In the System B the solution of reagents is flushed separately from that of the catalyst in two capillary liners inserted into the same ESI spray needle. Unfortunately, higher quantities of unreacted 4-hydroxycoumarin (see chromatogram S16 in ESI†), and lower er (Table 2, entry 2) were recorded. This arrangement probably leads to inefficient microdroplets mixing. A good solution is represented by System C, where a “T junction” at zero void volume was introduced as feed of reagents/catalyst. In detail, one syringe contains the solution of 1 and 2 in ACN while the second syringe contains the solution of catalyst 3 or 4 (Fig. 1c). Once collected the reaction crude, a higher formation of warfarin has been highlighted by TLC monitoring, making possible its isolation by chromatographic purification. The chromatogram of isolated warfarin showed an er comparable to the bulk reaction at r.t. and higher respect to the er obtained in bulk at reflux temperature (Table 2, entry 3). Taking into account the high temperature involved, we checked the stereostability of warfarin by performing racemization studies on a scalemic mixture of pure warfarin using both bulk and microdroplets conditions (see ESI† for more informations). The enantiomeric excess did not change after 5 h of reaction attesting the stability of the warfarin produced in those conditions. System C was then tested increasing the distance of the ESI probe from 5 cm to 8.5 cm from the collecting system but lower formation of the product and lower e.r. were observed (Table 2, entry 4). In addition, we tested microdroplet reaction in absence of catalyst and no formation of warfarin was observed (Table 2, entry 5). The reaction was then performed in absence of the electric voltage applied to the ESI spray needle as proof of the beneficial effects of the ESI source. In this case, the formation of the product occurs with very low yields (traces) and er (Table 2, entry 6). Finally, best reaction conditions found using System C (5 cm, 85 °C, positive voltage) were also used with R,R-4 catalyst which confirmed the results obtained with catalyst 3 leading to 74/26 er (Table 2, entry 7). Afterwards, System D (Fig. 1d) was tested as clone of System C except for the reagents mixing mode. Here, the solution of 4-hydroxycoumarin 1 is placed in the syringe 1 while the solution of ketone with catalyst (2 + 3/4) is placed in the syringe 2. Before starting the microdroplet reaction, benzylideneacetone and catalyst were left in solution for 30 minutes in order to facilitate the iminium ion formation. The reaction gave better results in terms of warfarin formation for both the catalysts, even if the yields were still low. Concerning S,S-3 catalyst, the stereoselectivity remained comparable to that obtained in bulk conditions at r.t. (Table 2, entry 8), while using R,R-4 catalyst the er was slightly higher than that obtained using System C but lower than that obtained in bulk conditions at r.t. (Table 2, entry 9). The same reaction performed at room temperature gave higher yields, whereas the er remained almost unchanged (Table 2, entry 10). Further tests were carried out fixing the ESI source at the distance of 1 cm from the collection system. Unlike what is mostly reported for other microdroplet reactions,1,2a,b the use of a shorter distance led to considerably higher yields both at 25 °C and at 85 °C while no effect on the enantioselectivity was observed (Table 2, entries 11 and 12). The temperature decrease seems to be negligible at this distance if compared to the results obtained at the distance of 5 cm. The higher yields observed at the shorter distance can be explained by the formation of a naked-eye visible thin film onto the silica layer by microdroplets deposition. In this condition, the reaction may also proceed in the confined volume of the thin film leading to the increased yields observed. Noteworthy is that in bulk conditions these values of yields are obtained after about 24 hours while in microdroplet/thin film conditions were obtained in only 5 hours. Finally, taking into account that the arrangement where the sprays intersect is highly desirable,21 the last tested system involves two different ESI sources located at an angle of 60° and at 1 cm of distance from the silica cap (System E, Fig. 2) This assay led to the formation of the product with a 75% yield and 75/25 er (Table 2, entry 13). Results are comparable with those obtained with System D in entries 11 and 12. Overall, the use of System D at the distance of 1 cm from the collection system and at the temperature of 85 °C turned out to be the best solution both for the yields, the stereoselectivity, and the greater handless and ease of the experimental conditions which involve only one ESI spray probe.
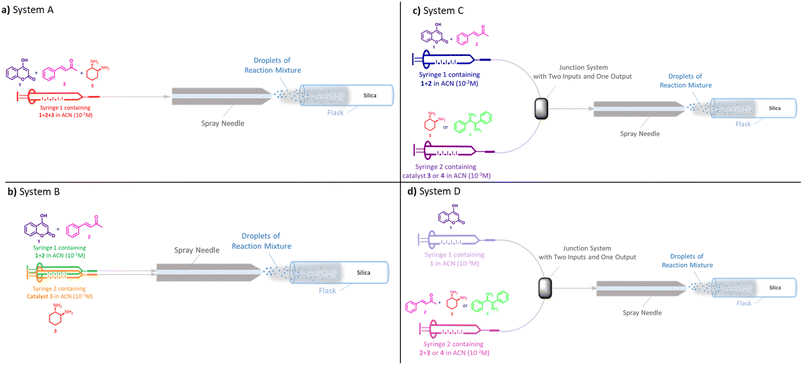 | ||
| Fig. 1 Simplified schemes of microdroplet reaction systems. Photograph of the apparatus involved is showed in Fig S1 of ESI.† | ||
| Microdroplet reactionsa | |||||||
|---|---|---|---|---|---|---|---|
| Entry | System | Catalyst | T (°C)b | Source distance | c.t.c | er (+)/(−)d | Yieldf |
| a General: all the reactions in microdroplet were performed using a modified Z-spray source (See ESI).b Measured temperatures of the microdroplets: when DSG = 150 °C (dSG, desolvation gas temperature), measured T = 85 °C; when DSG = 50 °C, measured T = 25 °C.c c.t.: collection time.d er was calculated by enantioselective HPLC.e er was determined on reaction crude.f Yield and er were determined on the isolated product.20g n.d: not determined. | |||||||
| 1 | A | S,S-3 | 85 | 5 cm | 5 h | 23/77e | n.d.g |
| 2 | B | S,S-3 | 85 | 5 cm | 5 h | 38/62e | n.d.g |
| 3 | C | S,S-3 | 85 | 5 cm | 5 h | 25/75f | 10% |
| 4 | C | S,S-3 | 85 | 8.5 cm | 5 h | 47/53e | n.d.g |
| 5 | C | None | 85 | 5 cm | 5 h | — | — |
| 6 | C (0 kV) | S,S-3 | 85 | 5 cm | 5 h | 31/69 | n.d.g |
| 7 | C | R,R-4 | 85 | 5 cm | 5 h | 74/26f | 16% |
| 8 | D | S,S-3 | 85 | 5 cm | 5 h | 25/75f | 35% |
| 9 | D | R,R-4 | 85 | 5 cm | 5 h | 78/22f | 23% |
| 10 | D | R,R-4 | 25 | 5 cm | 5 h | 76/24f | 45% |
| 11 | D | R,R-4 | 85 | 1 cm | 5 h | 77/23f | 79% |
| 12 | D | R,R-4 | 25 | 1 cm | 5 h | 75/25f | 71% |
| 13 | E | R,R-4 | 25 | 1 cm | 5 h | 75/25f | 75% |
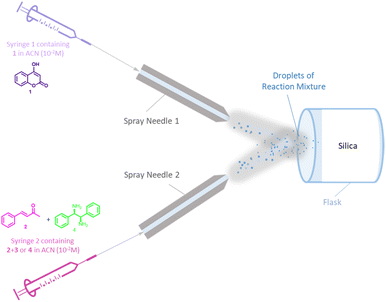 | ||
| Fig. 2 Simplified scheme of system E. The two spray needles are located at an angle of 60°, at the distance of 1 cm from the silica cap. | ||
To probe that the reactions occur in microdroplets environment, on-line reaction monitoring was performed by acquisition of the mass spectra of the solutions containing the DACH catalyst sprayed in the ESI source by using the same A, C and D systems. When the reaction with DACH is performed using System A, the mass spectrum shows the signal of the imine intermediate at m/z 243 and of the warfarin final product at m/z 309 (Fig. S29†). However, the formation of the imine intermediate and warfarin even in the syringe during the time required to spray 1 mL of the freshly prepared solutions (50 minutes) may not be negligible. Nevertheless, when the reaction is performed using System C, where the reactants and the catalyst are separated, this contribution is avoided. In spite of the low ion intensities, the fact that signals of the imine intermediate and of the product are measured under these conditions (Fig. S30†) demonstrates the formation of warfarin by microdroplets reaction. When System D is used (Fig. S31†), the higher imine intermediate ion intensity is justified by its concomitant formation in the syringe thus leading to a slightly higher ionic intensities of the final product. Similar results were obtained with catalyst 4. Nevertheless, the very high yields observed with System D can be explained by the generation of a thin film within which the reaction can proceed with further products formation. In conclusion, here we have reported the first example of an asymmetric organocatalyzed reaction in electrosprayed microdroplets/thin film confined volumes, proving that stereoselective syntheses are possible in such conditions. Concerning the warfarin synthesis, the achieved results about stereoselectivity are comparable to those obtained inbulk conditions at room temperature, and at the same time increase if compared to the bulk reaction performed at higher temperature. Notably, the use of the positive voltage of the ESI source enhances the yields and the stereoselectivity of the reaction underlying the role of the charged microdroplets to accelerate the reaction as also evidenced by the on-line MS reaction monitoring experiments. Yields up to 79% and 77/23 er were obtained when the reaction time was extended by the formation of a thin film from the microdroplets deposition at shorter distance from the silica layer giving the product in quantities comparable to those obtained in bulk after 24 hours of reaction, in only 5 hours of collection. Not withstanding this, our work represents the first example of an asymmetric organocatalyzed reaction in ESI-microdroplets/thin film confined volumes where the stereoselectivity is retained or higher compared to that in bulk conditions, enabling the possibility to merge the advantages of confined volumes reactions with those of the asymmetric organocatalysis.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Sapienza University of Rome to support the study (grant RM1221816BF705A8).Notes and references
- X. Yan, Int. J. Mass Spectrom., 2021, 468, 116639 CrossRef CAS.
- (a) X. Yan, R. M. Bain and R. G. Cooks, Angew. Chem., Int. Ed., 2016, 55, 12960 CrossRef CAS PubMed; (b) Z. Wei, Y. Li, R. G. Cooks and X. Yan, Annu. Rev. Phys. Chem., 2020, 71, 31 CrossRef CAS PubMed.
- (a) C. Y. Liu, J. Li, H. Chen and R. N. Zare, Chem. Sci., 2019, 10, 9367e9373 Search PubMed; (b) Y. Li, X. Yan, R. G. Cooks, Y. Li, X. Yan and R. G. Cooks, Angew. Chem., Int. Ed., 2016, 55, 3433 CrossRef CAS PubMed; (c) T. Müller, A. Badu-Tawiah and R. G. Cooks, Angew. Chem., Int. Ed., 2012, 51, 11832 CrossRef PubMed; (d) A. K. Badu-Tawiah, D. I. Campbell and R. G. Cooks, J. Am. Soc. Mass Spectrom., 2012, 23, 1461–1468 CrossRef CAS PubMed.
- W. Zhang, B. Zheng, X. Jin, H. Cheng and J. Liu, ACS Sustainable Chem. Eng., 2019, 7, 14389 CrossRef CAS.
- (a) K. H. Huang, Z. Wei and R. G. Cooks, Chem. Sci., 2021, 12, 2242e2250 Search PubMed; (b) E. Gnanamani, X. Yan and R. N. Zare, Angew. Chem., Int. Ed., 2020, 59, 3069–3072 CrossRef CAS PubMed; (c) N. Sahota, D. I. AbuSalim, M. L. Wang, C. J. Brown, Z. Zhang, T. J. El-Baba, S. P. Cook and D. E. Clemmer, Chem. Sci., 2019, 10, 4822 RSC.
- D. Gao, F. Jin, J. K. Lee and R. N. Zare, Chem. Sci., 2019, 10, 10974 RSC.
- J. K. Lee, D. Samanta, H. G. Nam and R. N. Zare, J. Am. Chem. Soc., 2019, 141, 10585 CrossRef CAS PubMed.
- (a) X. Yan, Y. H. Lai and R. N. Zare, Chem. Sci., 2018, 23, 5207 RSC; (b) Z. Wei, X. Zhang, J. Wang, S. Zhang, X. Zhang and R. G. Cooks, Chem. Sci., 2018, 9, 7779 RSC.
- (a) C. Salvitti, G. de Petris, A. Troiani, M. Managò, C. Villani, A. Ciogli, A. Sorato, A. Ricci and F. Pepi, J. Am. Soc. Mass Spectrom., 2022, 565–572 CrossRef CAS PubMed; (b) C. Salvitti, G. de Petris, A. Troiani, M. Managò, A. Ricci and F. Pepi, Molecules, 2022, 27, 5747 CrossRef CAS PubMed; (c) Z. Wei, M. Wleklinski, C. Ferreira and R. G. Cooks, Angew. Chem., Int. Ed., 2017, 56, 9386 CrossRef CAS PubMed.
- B. Zheng, Y. Wu, L. Xue, J. Sun, J. Liu and H. Cheng, J. Org. Chem., 2023, 88, 16186–16195 CrossRef CAS PubMed.
- N. Halland, T. Hansen and K. A. Jørgensen, Angew. Chem., Int. Ed., 2003, 42, 4955 CrossRef CAS PubMed.
- (a) R. A. O'Reilly, N. Engl. J. Med., 1976, 295, 354 CrossRef PubMed; (b) L. B. Wingard, R. A. O'Reilly and G. Levy, Clin. Pharmacol. Ther., 1978, 23, 212 CrossRef CAS PubMed.
- M. Ikawa, M. A. Stahmann and K. P. Link, J. Am. Chem. Soc., 1944, 66, 902 CrossRef CAS.
- (a) H. Kim, C. Yen, P. Preston and J. Chin, Org. Lett., 2006, 8, 5239 CrossRef CAS PubMed; (b) J. W. Xie, L. Yue, W. Chen, W. Du, J. Zhu, J. G. Deng and Y. C. Chen, Org. Lett., 2007, 9, 413 CrossRef CAS PubMed.
- (a) R. Q. Mei, X. Y. Xu, Y. C. Li, J. Y. Fu, Q. C. Huang and L. X. Wang, Tetrahedron Lett., 2011, 52, 1566 CrossRef CAS; (b) J. Dong and D. M. Du, Org. Biomol. Chem., 2012, 10, 8125 RSC; (c) S. V. Kochetkov, A. S. Kucherenko and S. G. Zlotin, Org. Biomol. Chem., 2018, 16, 642 RSC.
- A. Iazzetti, G. Mazzoccanti, G. Bencivenni, P. Righi, A. Calcaterra, C. Villani and A. Ciogli, Eur. J. Org Chem., 2022, e202101272 CrossRef CAS.
- Studies about reaction in bulk at lower concentration were necessary in order to switch to the microdroplet system where the internal diameter of the ESI capillary is of 0.150 mm.
- C. Salvitti, A. Troiani, F. Mazzei, C. D'Agostino, R. Zumpano, C. Baldacchini, A. R. Bizzarri, A. Tata and F. Pepi, Int. J. Mass Spectrom., 2021, 468, 116658–116665 CrossRef CAS.
- S. Banerjee and R. N. Zare, Angew. Chem., Int. Ed., 2015, 54, 14795 CrossRef CAS PubMed.
- The reported yields are calculated basing on the weight of the collected reaction crude which resulted lower than the theoretical one for each trial.
- D. T. Holden, N. M. Morato and R. G. Cooks, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2212642119 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra07975a |
| This journal is © The Royal Society of Chemistry 2024 |
