Open Access Article
Open Access ArticleLiquid–liquid extraction of boron from continental brines by 2-butyl-1-octanol diluted in kerosene†
Abdoul Fattah Kiemdea,
Jérôme Marina,
Victoria Flexer b and
Alexandre Chagnes
b and
Alexandre Chagnes *a
*a
aUniversité de Lorraine, CNRS, GeoRessources, F-54000 Nancy, France. E-mail: alexandre.chagnes@univ-lorraine.fr
bCIDMEJu, CONICET – Universidad Nacional de Jujuy, Jujuy, Argentina
First published on 10th January 2024
Abstract
Lithium production from brines generates significant quantities of salts, including boron, that are not effectively utilized and end up being stored in landfills. This study delves into a novel approach for directly extracting boron from native brines without performing solar evaporation as an alternative to traditional methods based on boron extraction from ores, offering a sustainable route to producing boric acid or borax. By exploring factors such as 2-butyl-1-octanol concentration, phase volume ratio, temperature, and pH, the research scrutinizes boron extraction efficiency from two native brines sourced from the salar de Hombre Muerto in Argentina, alongside a synthetic brine simulating these native compositions. Notably, the extractant demonstrates exceptional promise due to its limited solubility in the brine, measuring at just 18 mg L−1. Optimal conditions—2 mol L−1 2-butyl-1-octanol, O/A ratio of 4, 25 °C temperature, and pH of 5.5—resulted in a remarkable 98.2% and 94.2% recovery of boron from synthetic and native brines, respectively. Importantly, this extraction process showcased minimal co-extraction of lithium, calcium, magnesium, potassium, and sodium. Leveraging these findings, a proposed flowsheet outlines a highly selective method for extracting boron from brines, presenting an alternative avenue to conventional borax production from boron ores.
1. Introduction
Agriculture, glass, ceramic and detergent industries use 86% of the worldwide production of boron as boric acid (H3BO3) or borax (Na2B4O7·10H2O).1 Boron is also used in nuclear,2 steel,3 pharmaceutical,4 catalysis,5 hydrogen storage,6 concrete,7 textile8 and military sectors.9 Therefore, boron is classified as a strategic commodity in many countries, including the U.S. and the EU. Boron is mainly extracted from ores containing various minerals such as colemanite (Ca2B6O11·5H2O), tincal (Na2B4O7·10H2O), kernite (Na2B4O7·4H2O) and ulexite (NaCaB5O9·8H2O) in which boron concentration can reach up to 15 wt%.10 However, brines could be an alternative to minerals for boron production as they usually contain large amounts of boron. For instance, it is estimated that the salar de Uyuni (Bolivia) contains 3.2 million tons of boron.11 Thus, boron extraction from brine could be a good alternative to ores as the exploitation of boron ores implies high energy consumption for crushing and grinding steps and the use of huge amounts of chemicals to leach ore concentrates.12 Furthermore, the huge demand of lithium in the next decades due to the exponential increase of lithium-ion battery production for electric vehicles will be responsible for the co-extraction of a considerable amount of boron from brines, which should be valorised as co-product.Many technologies were tested to extract boron from different types of aqueous solutions including precipitation,13 (electro)-membrane operations,14,15 solid–liquid extraction,16 or liquid–liquid extraction.17–20 Among them, liquid–liquid extraction is the most used technology in hydrometallurgy for the recovery of many metals including boron.21,22 Alcohols23–25 diluted in kerosene,12 toluene,26 chloroform,19,25 benzene27 or sulfonated kerosene23,24,28,29 were extensively reported in the literature as extractants to recover boron from brines.
Brine evaporation in open air ponds is the most common technology in use today for lithium salts production from aqueous sources.30 Boron needs to be removed from the concentrated brines in order to produce high purity lithium salts. Table 1 gathers the extraction and stripping conditions of boron processing from brines concentrated by solar evaporation for three companies. In 1998, the company Chemetall performed boron extraction from concentrated brine of the salar de Atacama (Chile) in lithium production plant.31 Four mixers-settlers were implemented to extract boron at pH 2 by using 20% (vol.) isooctanol or 2-ethyl-1-hexanol diluted in kerosene. The pH of the brine was adjusted by adding hydrochloric acid and the phase volume ratio between the organic phase (O) and the aqueous phase (A) was O/A = 4. Boron extraction efficiency reached 99% and the residual boron concentration was less than 2 mg L−1, although 5–10% lithium was lost during boron extraction (see the brine composition before and after boron extraction in Table 2). Thereafter, boron was stripped from the loaded solvent with water or diluted sodium hydroxide by means of four mixers-settlers.
| (a) Processes | Extractants | Diluents | T (°C) | O/A | EB (%) | pH | Number of stages | Brines | Li loss (%) |
|---|---|---|---|---|---|---|---|---|---|
| (1) Chemetall | Isooctanol or 2-ethyl-1-hexanol, 20% (vol.) | Kerosene | — | 4 | 99.0 | 2 | 4 | Salar de Atacama concentrated | 5–10 |
| (2) Minsall | Isooctanol, 50% (vol.) | Kerosene | 25 | 1 | 99.9 | 2 | 4 | Salar de Atacama concentrated | 10 |
| (3) Anon et al. | 2-Chloro-4(1,1,3,3)-tetramethylbutyl-6-methylol-phenol, 25% (vol.) | Kerosene | 34 | 1.6 | 99.4 | 10 | 2 | Searles lake | — |
| (b) Process | Stripping solution | T (°C) | O/A | SEB (%) | Stage |
|---|---|---|---|---|---|
| (1) Chemetall | Water | — | — | 100 | 4 |
| (2) Minsall | 0.02 mol L−1 NaOH or water | — | 6 | 100 | 3 |
| (3) Anon et al. | 0.5 mol L−1 H2SO4 | 38 | 1 | 100 | 5 |
| Concentration (g L−1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Li | ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca Ca |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg Mg |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B B |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na Na |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K K |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl− Cl− |
SO42− | |
| (1) Before boron extraction | 63 | 0.53 | 12.9 | 7.3 | 0.8 | 0.19 | 358.6 | 0.16 |
| (2) After boron extraction | 60.3 | 0.51 | 12.9 | 0.001 | 0.73 | 0.18 | 344.6 | 0.19 |
In 2002, boron was also extracted from concentrated brine containing 8 g L−1 boron from the salar de Atacama (Chile) by the Minsal company.31 The extraction was performed with 50% (vol.) isooctanol in kerosene at O/A = 1 and 25 °C. The extraction efficiency of boron reached 99.9% by implementing four mixers-settlers but 10% lithium was lost.31 The extraction solvent was regenerated in three stages with water or 0.02 mol L−1 sodium hydroxide (NaOH) at phase volume ratio O/A = 6.
The solubility of the extraction solvent in the brine and in the stripping solution were 200 mg L−1 and 2000 mg L−1, respectively.
In the flowsheet reported in Fig. 1, boron was recovered and valorised as boric acid from the Searles lake brine (California, USA, composition reported in Table 3). The boron extraction plant was designed in 1996 to produce 150 tons per day of boric acid.32 Boron processing started with boron extraction in two stages at 34 °C and O/A = 1.6. The extraction efficiency of boron reached 99.4% by contacting the extraction solvent containing 25% (vol.) 2-chloro-4-(1,1,3,3)tetramethylbutyl-6-methylol-phenol diluted in kerosene (flowrate of the extraction solvent = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 L min−1, flowrate of the native brine = 7380 L min−1).
100 L min−1, flowrate of the native brine = 7380 L min−1).
 | ||
| Fig. 1 Flowsheet implemented to extract glaserite and boron from the Searles lake brine in U.S. (adapted from Garrett).32 | ||
| Mineral | Content (wt%) |
|---|---|
| KCl | 3.5 |
| Na2CO3 | 6.5 |
| Na2B4O7 | 1.55 |
| Na2B2O4 | 0.75 |
| Na2SO4 | 6 |
| Na2S | 0.3 |
| Na3AsO4 | 0.05 |
| Na3PO4 | 0.1 |
| NaCl | 15.5 |
| H2O | 65.72 |
| WO3 | 0.005 |
| Br | 0.071 |
| Li2O | 0.009 |
| I | 0.002 |
| F | 0.001 |
After extraction, the organic–aqueous phase mixture was sent to three lines of settlers where the two phases were separated. The solubility of the extraction solvent in the brine was 100 mg L−1 (pH = 8.9). The boron-depleted brine was sent to three parallel lines at 2460 L min−1 each, where the extraction solvent was removed as a froth or vapor in order to reduce the solvent solubility below 17 mg L−1 as recommended by the government,32 before returning the boron-depleted brine to the lake. The extraction solvent recovered from the brine was collected and reused.
After extraction, 0.5 mol L−1 sulfuric acid was used to strip boron at O/A = 1 and 38 °C by using five mixer-settlers in counter-current. After stripping, the extraction solvent was washed with water so that it could be reused. The plant consumed about 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 L per day of concentrated sulfuric acid. Furthermore, 15
700 L per day of concentrated sulfuric acid. Furthermore, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 L of alcohol and 22
100 L of alcohol and 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 L of kerosene were needed each week to makeup the extraction solvent.
700 L of kerosene were needed each week to makeup the extraction solvent.
The boron-loaded stripping solution contained 6.1 wt% H3BO3, 2.8 wt% Na2SO4, 5.2 wt% K2SO4 as well as 10–15 mg L−1 extraction solvent due to solubility losses. The stripping solution was first sent through an adsorption column, filled with activated carbon, to remove the extraction solvent.
The activated carbon was regenerated in six-tray furnace at 650–760 °C. The boron-loaded stripping solution without extraction solvent was sent to two hemispherically-domed evaporator-crystallizers where it was joined by recycle sulfuric acid solution and circulated through titanium heat-exchanger tubes at 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L min−1. Water was evaporated at 65 °C, and salt cake and glaserite (K3Na(SO4)2) were crystallized. They were removed, settled, and dewatered in solid bowl centrifuge. After the first stage of evaporation–crystallization, the overflow was then cooled at 35 °C in the second crystallization stage (flowrate = 83
000 L min−1. Water was evaporated at 65 °C, and salt cake and glaserite (K3Na(SO4)2) were crystallized. They were removed, settled, and dewatered in solid bowl centrifuge. After the first stage of evaporation–crystallization, the overflow was then cooled at 35 °C in the second crystallization stage (flowrate = 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 L min−1). Boric acid was crystallized, and the slurry withdrawn, settled, and dewatered in a four-stage pusher centrifuge. The solid was washed in two stages, and the overflow stripping solution depleted of boron was returned to the circuit. The boric acid cake was dried in a gas-fired rotary dryer and pneumatically transported to a storage silo. The purity of boric acid was 99.9% and contained only 0.05% SO3 and 0.029% Na.
300 L min−1). Boric acid was crystallized, and the slurry withdrawn, settled, and dewatered in a four-stage pusher centrifuge. The solid was washed in two stages, and the overflow stripping solution depleted of boron was returned to the circuit. The boric acid cake was dried in a gas-fired rotary dryer and pneumatically transported to a storage silo. The purity of boric acid was 99.9% and contained only 0.05% SO3 and 0.029% Na.
The nature of the extractant is particularly important in these processes as the extractant will control the efficiency of the process and the purity of the final product. In this work, preliminary experiments were performed to determine the solubility of extractants in the brine, the extraction efficiency of boron and the selectivity for boron towards sodium, lithium, magnesium, calcium and potassium for several extraction solvents composed of mono-hydroxy alcohols (1-octanol, 2-butyl-1-octanol and 2-ethyl-1-hexanol), or di-hydroxy alcohols (2-methyl-2,4-pentanediol, 2-ethyl-1,3-hexanediol and 2-butyl-2-ethyl-1,3-propanediol) diluted in kerosene or mixture of kerosene and toluene. The highest extraction efficiencies of boron were obtained with di-hydroxy alcohols since 99% boron was extracted with 1 mol L−1 2-ethyl-1,3-hexanediol diluted in 40 wt% kerosene + 60 wt% toluene at phase volume ratio O/A = 2 and pH = 5.5 while less than 55% boron was extracted with mono-alcohols. The best extraction efficiency with mono-alcohol was obtained by diluting 1 mol L−1 2-butyl-1-octanol in kerosene and by performing the boron extraction at pH = 5.5 and O/A = 4.
Despite the high efficiency of diols for boron extraction, these alcohols cannot be used in industry for two main reasons. Firstly, their low solubility in kerosene requires the addition of highly toxic toluene to enhance their solubility. Secondly, they exhibit high solubility in water. For example, the solubility of 2-ethyl-1,3-hexanediol in brine is 70 times higher than that of 2-butyl-1-octanol. Furthermore, it has been observed that the selectivity of diols for boron over sodium, lithium, magnesium, calcium, and potassium significantly decreases under alkaline conditions. Therefore, it was decided to conduct a detailed investigation into the extraction properties of 2-butyl-1-octanol diluted in kerosene instead of diols diluted in toluene.
This work distinguishes itself within the field due to its focus on a novel approach to boron extraction from brines. Unlike conventional methods reliant on solar evaporation for brine concentration before extraction, the study targets direct boron extraction from native brines. Most future lithium production plants are anticipated to adopt technologies that bypass solar evaporation (direct lithium extraction), emphasizing the need to extract boron directly from these brines—a facet rarely explored in prior studies. The aim is to identify a highly efficient and selective extraction solvent capable of recovering and maximizing the value of boron as pure boric acid or borax, without relying on solar evaporation. This introduces a new pathway for obtaining top-quality boric acid or borax directly from brines, offering a supplementary approach to the prevalent method of boron extraction. Additionally, while solvent extraction for boron retrieval from brine has encountered challenges, notably concerning alcohol solubility within the brine leading to increased effluent treatment and extractant consumption, a crucial breakthrough has been uncovered. The selected alcohol demonstrates notably lower solubility in brine compared to conventional boron extractants used in such processes, addressing a key limitation in this field. Moreover, the study breaks new ground by delving into the intricate physicochemistry of boron extraction by alcohols. Unlike previous research that often focused solely on the presence of boric acid in brines, the approach considers the complex boron speciation within the brine. This comprehensive analysis extends beyond boron to encompass the myriad of other elements present in the brine. The findings highlight the nuanced interplay between boron extraction, pH variations, and the co-extraction of elements like magnesium and calcium at different pH values. Consequently, our study contributes insights into the intricate physicochemical dynamics involved in boron extraction, elucidating its interactions with other impurities present in the brine.
In particular, this paper focuses on the use of 2-butyl-1-octanol diluted in kerosene to extract boron from a synthetic brine and two native brines from salar del Hombre Muerto, in northwest Argentina. It was shown that this extractant exhibits very low solubility in brine. The influence of 2-butyl-1-octanol concentration, phase volume ratio (O/A), pH and temperature on boron extraction and co-extraction of sodium, lithium, magnesium, calcium and potassium was systematically investigated. Boron stripping from the loaded organic phases was studied by considering three stripping solutions including hydrochloric acid, sodium hydroxide and deionized water. Finally, a simulation of continuous counter-current extraction of boron from these brines was performed and a flowsheet to extract selectively boron was proposed.
2. Experimental
2.1. Materials
Mono-hydroxy alcohols including 1-octanol (Oc, purity = 99%), 2-butyl-1-octanol (BOc, purity = 95%), 2-ethyl-1-hexanol (EH, purity = 99.6%), and di-hydroxy alcohols including 2-methyl-2,4-pentanediol (MPD, purity = 99%), 2-ethyl-1,3-hexanediol (EHD, purity = 97%) and 2-butyl-2-ethyl-1,3-propanediol (BEPD, purity = 99%), were purchased from Sigma-Aldrich (France). Kerosene (Low odour) was also provided by Sigma-Aldrich (France) while toluene (purity = 100%) was supplied by VWR (France).Magnesium chloride (MgCl2, purity = 98%, Sigma-Aldrich), calcium chloride (CaCl2, purity = 90%, Sigma-Aldrich), boric acid (H3BO3, Sigma-Aldrich, purity = 99.5%), lithium sulfate (Li2SO4, Sigma-Aldrich, purity = 98.5%), sodium chloride (NaCl, VWR, purity = 99.9%) and potassium chloride (KCl, VWR, purity = 99.5%) were dissolved in deionized water produced with the Puranity TU water system (VWR, resistivity = 18 MΩ cm) to prepare the synthetic brine mimicking the native brine which the composition is reported in Table 4.
| Brine | pH | I (mol kgw−1) | Density (g cm−3) | Concentration (g L−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li Li |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca Ca |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg Mg |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B B |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na Na |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sr Sr |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K K |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe Fe |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl− Cl− |
SO42− | ||||
| NB1 | 6.9 | 3.6 | 1.2 | 1.27 | 0.68 | 3.09 | 1.62 | 103.24 | 0.079 | 14.21 | 0.001 | 182.85 | 11.15 |
| NB2 | 7.5 | 3.46 | 1.2 | 0.75 | 0.1 | 2.51 | 0.54 | 103.29 | 0 | 7.8 | 0 | 176.15 | 14.13 |
| SB | 5.5 | 3.6 | 1.2 | 1.27 | 0.68 | 3.09 | 1.62 | 103.24 | 0 | 14.21 | 0 | 182.25 | 8.78 |
The influence of the nature of the brine on the extraction properties of 2-butyl-1-octanol was investigated for two native brines from the Salar del Hombre Muerto (Argentina) referred to as native brine 1 (NB1) and native brine 2 (NB2) as well as a synthetic brine (SB) which the composition was the same as the composition of NB1 (Table 4). These brines were highly concentrated in monovalent elements (sodium, potassium and lithium) and divalent elements (calcium and magnesium). Iron and strontium were not accounted for preparing the synthetic brine as their concentrations were lower than 0.1 g L−1. Hydrochloric acid (HCl, purity = 37%, Sigma-Aldrich) and sodium hydroxide (NaOH, purity = 99%, VWR) were used as received to adjust the pH of the synthetic brine.
2.2. Methods
| Kerosene | Kerosene + 60% (wt) toluene | ||||
|---|---|---|---|---|---|
| Alcohol | EB (%) | Salc (mg L−1) | Alcohol | EB (%) | Salc (mg L−1) |
| BOc | 55 | 19 | EHD | 99 | 1368 |
| EH | 55 | 40 | BEPD | 97 | 259 |
| Oc | 32 | 43 | MPD | 96 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 603 603 |
Organic and aqueous phases were contacted in centrifuge tubes during the extraction and the stripping experiments in a thermostated shaker (Gherardt Laboshake THL500/1) at 200 rpm for 4 hours and 2 hours, respectively. Preliminary experiments showed that the steady state was reached under these experimental conditions. It is expected that more intensive mixing/shaking will make the equilibrium to be reached faster. However, we chose to work under reproducible experimental conditions, and in this sense, a lab shaker did not provide intensive mixing, but it allowed for high reproducibility. Afterwards, the biphasic mixtures were centrifugated at 3000 rpm for 2 min by using a SIGMA 3–16 L centrifuge to separate the organic and the aqueous phases.
Finally, the aqueous droplets were removed from the organic phases by filtering with 0.20 μm minisart made of polypropylene (Sartorius, France) and the organic droplets were removed from the aqueous phases by filtering with 0.20 μm minisart made of regenerated cellulose (Sartorius, France).
The pH values of the aqueous phases were adjusted by adding 0.5 mol L−1 hydrochloric acid (HCl) or 2 mol L−1 sodium hydroxide (NaOH). After extraction, the boron-loaded organic phase was stripped with hydrochloric acid (HCl), sodium hydroxide (NaOH) or deionized water at O/A = 4 and 25 °C.
Extraction of boron from the synthetic brine (SB) and stripping tests of boron were performed in 500 mL glass vessels under stirring during 2 hours for the extraction tests and 1 hour for the stripping tests at 700 rpm by using a three-blade propeller (higher stirring was necessary to ensure good mixing in 500 mL-glass vessels). Then, the organic–aqueous phases mixtures were settled in 500 mL separating funnels for 30 min. For these tests, the extraction solvent was composed of 2 mol L−1 2-butyl-1-octanol diluted in kerosene and 0.1 mol L−1 NaOH was used as stripping solution. All experiments were carried out at 25 °C and O/A = 4. The reproducibility of these experiments was checked by performing 10 times extraction and stripping tests in centrifuge tubes (see experimental conditions above) under the same experimental conditions. The same values for the extraction and the stripping efficiencies were obtained. The extraction and stripping equilibria were reached faster when the tests were performed in the glass vessels under stirring with a three-blade propeller at 700 rpm.
Mass-balances were checked by performing quantitative elemental analyses in the stripping solution after full back-extraction from the organic phase.
Distribution ratio of the element e (De), extraction efficiency of the element e (Ee (%)), selectivity coefficient of the element e1 towards the element e2 (Se1/e2) and stripping efficiency of boron (SEB) were calculated as follows:
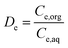 | (1) |
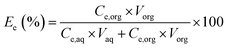 | (2) |
By combining eqn (1) and (2), the extraction efficiency can be rewritten as follows:
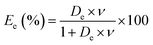 | (3) |
The selectivity coefficient for e1 towards e2 was calculated as follows:
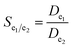 | (4) |
The boron stripping efficiency (SEB) was calculated as:
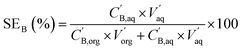 | (5) |
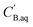 and
and 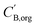 are the boron concentrations in the stripping solution and in the organic phase at equilibrium during the stripping step.
are the boron concentrations in the stripping solution and in the organic phase at equilibrium during the stripping step. 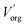 and
and 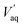 represent the volume of the organic and the aqueous phases.
represent the volume of the organic and the aqueous phases.
The error on the extraction and stripping efficiencies, and the error on the analytical method were estimated to be within 4% by repeating the experiments three times and by analysing the synthetic brine 10 times, respectively.
Dynamic viscosities of the organic phases before and after extraction were determined at 25 °C by using a Stabinger viscometer™ (Anton Paar SVM™ 2001). The viscometer was calibrated with the viscosity standards APN26, APS3, APN415 and APN7.5 provided by Anton Paar. Each sample was analysed twice. The viscometer cell was cleaned by rinsing with pure ethanol and dried with air after each viscosity measurement.
Water contents in organic phases after extraction were analysed by using a volumetric Karl Fisher Titrator (Mettler Toledo, France) after calibration with a standard containing 1% water (Sigma-Aldrich). These analyses were performed with HYDRANAL Composite 5 and Methanol Rapid (Sigma-Aldrich).
The solubilities of the alcohols (Salc) in the aqueous phase after extraction were determined by Total Organic Carbon analyses (Shimadzu TOC-VCSH), as follows:
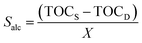 | (6) |
2.3. Speciation calculations
Speciation diagrams were calculated with PhreePlot on a PC under Windows 11. PhreePlot is a charting and plotting extension of Phreeqc (version 3.7) for creating commonly needed types of diagrams.37 The databases Pitzer and Minteq.V4 were used for speciation calculations. The relevant thermodynamic constants and the corresponding chemical reactions included in these databases are reported in Table S1 (ESI†).3. Results and discussion
3.1. Speciation calculations
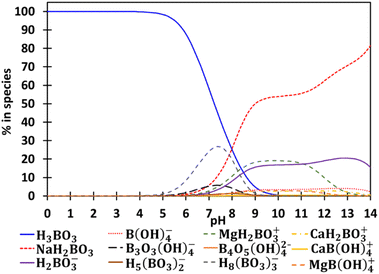
The speciation diagram of boron(III) in the synthetic brine is reported in Fig. 2 and the speciation diagrams of calcium(II), magnesium(II), potassium(I), sodium(I) and lithium(I) in the synthetic brine are gathered in Fig. S2–S6 (ESI†). These calculations show that boron exists as boric acid until pH 9.5. At higher pH, borate and several polyborate species are formed in solution as well as calcium–boron and magnesium–boron species.3.2. Boron extraction and impurities co-extraction
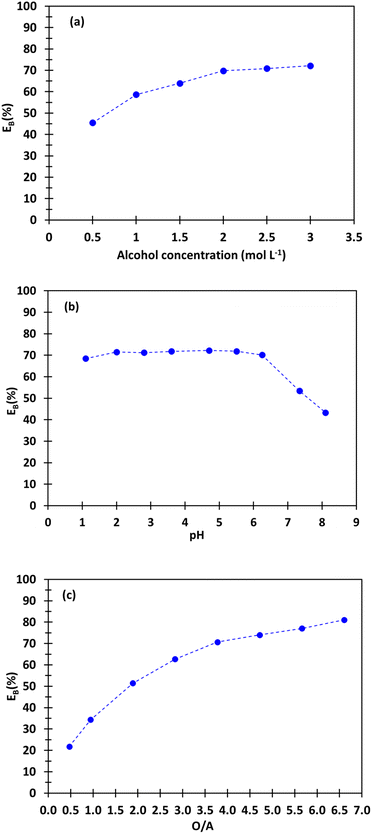
Fig. 3 shows the influence of the concentration of 2-butyl-1-octanol in kerosene, the phase volume ratio (O/A) and the pH at equilibrium on boron extraction efficiency from the synthetic brine at 25 °C. An increase of 2-butyl-1-octanol concentration is responsible for an increase of boron extraction efficiency. The curve in Fig. 3a shows that the extraction efficiency reaches a plateau (%EB = 70) when 2-butyl-1-octanol concentration is greater than 2 mol L−1.No co-extraction of lithium, calcium magnesium, sodium and potassium was observed. Therefore, the other extraction tests were performed with 2 mol L−1 2-butyl-1-octanol in kerosene.
Boron extraction efficiency does not depend on pH when pH values are lower than 5.5 (%EB = 70, Fig. 3b). Above this value, an increase of pH is responsible for a sharp decrease of boron extraction efficiency. This significant decrease in the extraction efficiency of boron can be explained by the change in boron speciation as a function of pH (Fig. 2). At pH = 1–5.5, boron exists only as boric acid in solution, i.e. a non-charged species. Above pH 5.5, boric acid concentration in the brine decreases and is almost equal to zero at pH = 9.5. Indeed, boric acid is progressively converted into borate when pH increases. Borate anions react with magnesium, sodium and calcium to form magnesium borate, sodium borate and calcium borate (see Fig. 2), which are not extractable by 2-butyl-1-octanol as no co-extraction of magnesium, calcium and sodium is observed (2-butyl-1-octanol usually extracts neutral species including acids like boric acid).
Fig. 3c shows an increase of boron extraction efficiency when the phase volume ratio (O/A) increases as it is expected according to eqn (3). Around 22% of boron is extracted at O/A = 0.5, extraction reaches 70% at O/A = 4, and even 81% at O/A = 6.6 when the pH at equilibrium is the same and equal to 1. However, O/A = 4 seems to be a good compromise to achieve high extraction efficiency even at moderate flowrate of the extraction solvent.
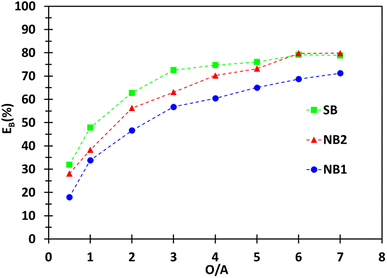
Fig. 4 compares the influence of the phase volume ratio (O/A) on boron extraction efficiency for the synthetic brine (SB) and the native brines (NB1 and NB2) under the previous optimal experimental conditions (extraction solvent: 2 mol L−1 2-butyl-1-octanol in kerosene, pH = 5.5 for SB, pH = 6.9 for NB1, pH = 7.5 for NB2, temperature = 25 °C).
Despite the difference in chemical composition of the brines, Fig. 4 shows that the extraction curves for the synthetic brine (SB) and the native brine 2 (NB2) are very similar. Surprisingly, the extraction curves obtained for SB and NB1 are different even though both brines have similar compositions. This difference may be due to the water activity of the brines that are not necessarilythe same, and/or the different values of pH between the two brines since pH influences the extraction efficiency at pH greater than 5.5 (Fig. 3b).
Under the optimal extraction conditions, i.e. 2 mol L−1 2-butyl-1-octanol in kerosene, pH = 5.5, O/A = 4 and 25 °C, 10 extraction tests from SB were performed with glass vessels. The average values of boron extraction efficiency was 66 ± 3%.
This investigation shows a high selectivity of boron extraction from brine under the optimal conditions mentioned above which leads to provide high purity boron salt when boron is co-valorised in the plants.
3.3. Boron stripping
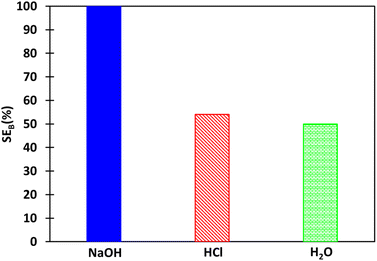
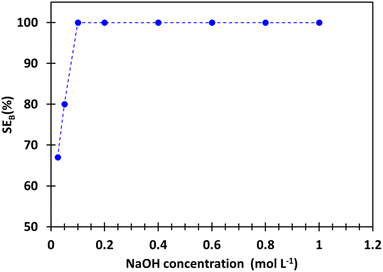
Around 20 mL of boron-loaded organic phase was prepared for stripping tests by contacting 5 mL of the synthetic brine SB with 20 mL of extraction solvent under the optimal extraction conditions, i.e. 2 mol L−1 2-butyl-1-octanol diluted in kerosenes at O/A = 4, pH 5.5 and 25 °C. Boron extraction experiments from SB were repeated 6 times and the average extraction efficiency was 72 ± 1%. Boron concentration and water content in the organic phase after extraction were 0.024 mol L−1 and 0.3 wt%, respectively.Fig. 5 shows the effect of the nature of the stripping solution (0.2 mol L−1 sodium hydroxyde (NaOH), 0.2 mol L−1 hydrochloric acid (HCl) and deionized water) on the stripping efficiency at O/A = 4 and 25 °C. The stripping efficiency of boron follows this order: NaOH (100%) ≫ HCl (54%) ≳ H2O (50%). The use of sodium hydroxide may destabilize the complex in the organic phase by favoring the formation of borate, which has no affinity towards 2-butyl-1-octanol (Fig. 2 and 3b). Low stripping efficiency with hydrochloric acid was expected as low pH favours the formation of boric acid, which is highly extractable by 2-butyl-1-octanol. However, the co-extraction of chloride as hydrochloric acid by 2-butyl-1-octanol via hydrogen bond formation could explain that the stripping efficiency is still significant since the competition between boric acid extraction and hydrochloric acid extraction favours boron stripping. The stripping efficiencies of boron with hydrochloric acid and water are similar. Such a result cannot be explained by water extraction as only 0.3% (wt) of water is extracted by 2 mol L−1 2-butyl-1-octanol diluted in kerosene. Indeed, 0.3% (wt) of water in the organic phase is not enough to destabilize the interactions between boron and 2-butyl-1-octanol in the organic phase.
Fig. 6 shows boron stripping efficiency increases when sodium hydroxide concentration increases. Full stripping is achieved with 0.1 mol L−1 sodium hydroxide and no increase of stripping efficiency is observed above this concentration. After stripping with 0.1 mol L−1 sodium hydroxide, the water content in the organic phase and the solubility of 2-butyl-1-octanol in the stripping solution were 0.4% (wt) and 181 mg L−1, respectively. The solubility of 2-butyl-1-octanol in the stripping solution (181 mg L−1) is much lower than that reported by Garrett et al.31 (2000 mg L−1). Such a low value of 2-butyl-1-octanol solubility in the stripping solution avoids the use of activated carbon to reduce its concentration and avoid additional operating and capital costs for the effluent treatement.
In order to study the reproducibility of boron stripping from the loaded extraction solvent, 10 stripping tests were performed with 0.1 mol L−1 sodium hydroxide at O/A = 4 and 25 °C. The average value of boron stripping efficiency was 95 ± 5%.
Finally, it should be highlighted that boron is fully strip in one stage from the loaded organic phase by 0.1 mol L−1 NaOH at O/A = 4 and 25 °C. Furthermore, alcohol solubility is very low in the effluent, which avoid the implementation of expensive effluent treatment to remove organic matter from the stripping solution and allows the production of pure borax.
3.4. Flowsheet for boron extraction
The boron concentration in the boron-depleted brine was set at 25 mg L−1 for McCabe–Thiele simulation as Brown et al.38 reported that boron concentration in brines should be less than 25 mg L−1 to guarantee the production of high-grade lithium carbonate for lithium-ion batteries. The McCabe–Thiele method was used to determine the number of mixers-settlers needed for boron extraction from the different brines (SB, NB1 and NB2) under optimal experimental conditions.A phase volume ratio of O/A = 6 was required to remove boron from SB and NB2 whereas O/A = 9 should be necessary to remove boron from NB1 (see Fig. S7 in ESI†).
Fig. 7 shows that four mixers-settlers are needed at O/A = 4 to extract boron from SB and NB2 so that less than 25 mg L−1 of boron remain in the effluent.
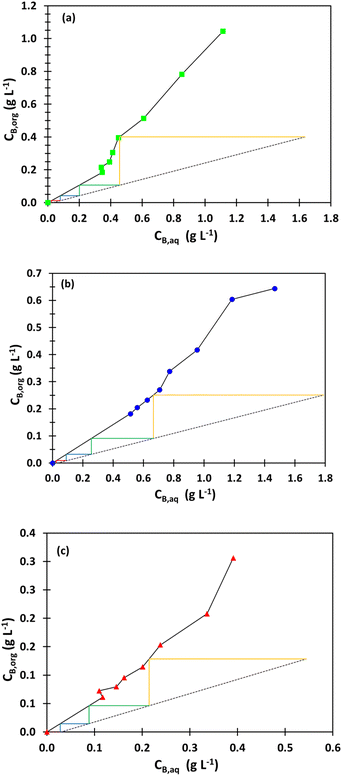 | ||
| Fig. 7 McCabe–Thiele diagrams for boron extraction from (a) the synthetic brine (SB) with O/A = 4, (b) the native brine 1 (NB1) for O/A = 4 and (c) the native brine 2 (NB2) with O/A = 4. | ||
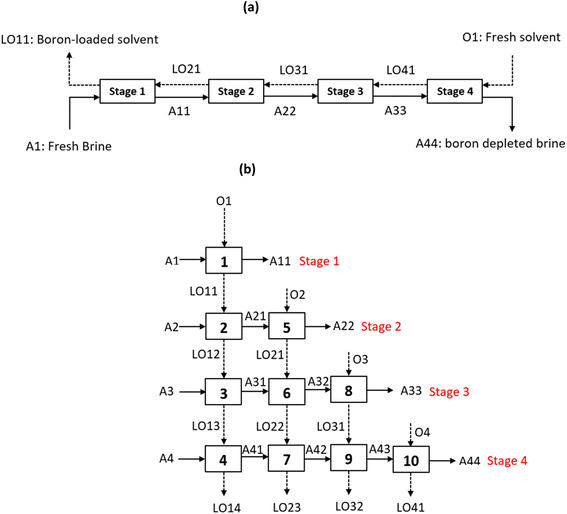
The same number of mixers-settlers can be used to process the native brine 1 (NB1) provided that O/A = 7. It is indeed necessary to increase O/A to extract boron efficiently from NB1 because the boron extraction efficiency from this brine is lower since the natural pH of NB1 (pH = 6.9) is higher than the pH of SB (pH = 5.5). Under the same extraction conditions, no significant difference in the extraction efficiency of boron for both synthetic brine (SB) and native brine 1 (NB1) was observed. For instance, when boron is extracted from SB or NB1 with 2 mol L−1 2-butyl-1-octanol diluted in kerosene at 25 °C, O/A = 4 and pH = 6.9, boron extraction efficiency reaches 60.5% (see extraction efficiency of boron under the extraction conditions mentioned above in Fig. 3b for SB and in Fig. 4 for NB1). Therefore, simulation of counter current extraction of boron from the brine following the model reported in Fig. 8 was performed to determine boron extraction efficiency in each stage when 2 mol L−1 2-butyl-1-octanol diluted in kerosene was contacted with only the synthetic brine (SB) at pH 5.5 or the native brine 2 (NB2) at pH 7.5 and O/A = 4 (Table 6).
| Stage | EB (%) | |
|---|---|---|
| Synthetic brine (SB) | Native brine 2 (NB2) | |
| 1 | 70 | 46.4 |
| 2 | 82.2 | 63.1 |
| 3 | 92.4 | 78.6 |
| 4 | 98.2 | 94.2 |
The simulation shows that the use of three stages leads to 92.4% and 78.6% of boron extraction efficiency and the addition of one more stage increases the extraction efficiency to 98.2% and 94.2% when the feed solution is the synthetic brine and the native brine 2, respectively. Therefore, the implementation of three mixers-settlers is enough to extract boron from the synthetic brine whereas 4 mixers-settlers are required to extract boron from the native brine 2.
Under these conditions (2 mol L−1 2-butyl-1-octanol diluted in kerosene contacted with the synthetic brine (SB) at pH 5.5 or the native brine 2 (NB2) at pH 7.5 and O/A = 4), the solubility of 2-butyl-1-octanol in SB was 18 mg L−1 and the dynamic viscosity of the organic phase after extraction was very close to the viscosity before extraction, i.e. 4.4 mPa s. Furthermore, it is important to highlight that, under these experimental conditions, the solubility of 2-butyl-1-octanol in the brine (18 mg L−1) is much lower than those reported in the literature31,32 (solubility between 100 mg L−1 and 200 mg L−1). Even if the initial concentration of boron was 1.60 g L−1 and 0.54 g L−1 in SB and NB2, respectively, it is interesting to point out that the residual concentration of boron in the brine was 25 mg L−1 in the last stage, as expected. Boron extraction was highly selective since no co-extraction of lithium, magnesium, calcium, sodium and potassium was observed. Long-chain mono-alcohols like heptanol, octanol, nonanol, and decanol have the capacity to extract hydrochloric acid or sulfuric acid by forming hydrogen bonds with the alcohol functional group. In this study, Table 7 does not demonstrate significant sulfate extraction, likely due to the brine's high pH value, as indicated by the negligible decrease in sulfate concentration after boron extraction.Conversely, Table 7 exhibits a slight extraction of chloride anions through the formation of hydrogen bonds between HCl and the alcohol functional group. This is evident from the decrease in chloride concentration following boron extraction, accompanied by a pH increase from 5.5 to 6.8. It's worth noting that the chloride extraction observed here is less significant compared to the findings by Garrett et al.31 (Table 2).
| Synthetic brine | pH | Density (g cm−3) | Concentration (g L−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Li Li |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca Ca |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg Mg |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B B |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na Na |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K K |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl− Cl− |
SO42− | |||
| Before extraction | 5.5 | 1.2 | 1.27 | 0.68 | 3.09 | 1.62 | 103.24 | 14.21 | 182.25 | 8.78 |
| After extraction | 6.8 | 1.2 | 1.27 | 0.68 | 3.09 | 0.025 | 103.24 | 14.21 | 181.77 | 8.76 |
4. Conclusion
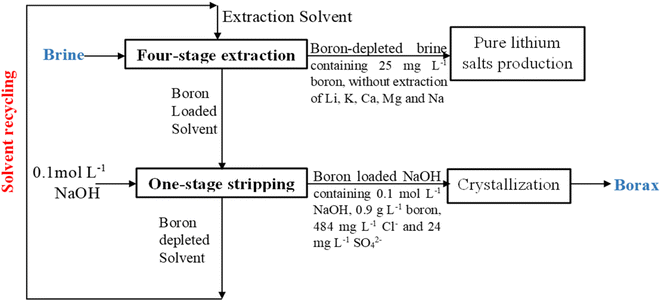
A potential method for extracting boron from brines involves using a 2 mol L−1 solution of 2-butyl-1-octanol in kerosene as the extraction solvent. Unlike previous industrial processes, this extractant has low solubility in brine (18 mg L−1), allowing easy removal using activated carbon adsorption. It shows good boron extraction efficiency, especially in counter-current continuous mode, and demonstrates high selectivity for lithium, calcium, magnesium, potassium, and sodium.Importantly, this method enables direct boron extraction from native brines without needing to concentrate the brine via evaporation. In one instance, using four stages, it removed 94.2% of boron from salar del Hombre Muerto (Argentina) brine without adjusting its pH, at an O/A ratio of 4. The boron is fully recovered from the loaded organic phase using a stripping solution containing 0.1 mol L−1 NaOH, allowing subsequent crystallization as borax.
This boron extraction process can precede direct lithium extraction (DLE) or the classic solar evaporation method, as depicted in Fig. 9, allowing co-valorization of boron with lithium. This co-production of lithium salts alongside boron products like borax or boric acid reduces waste generation, offering environmental benefits.
Traditionally, boron was extracted from brines using commercial alcohols like isooctanol, 2-ethyl-1-hexanol, or 2-chloro-4 (1,1,3,3)-tetramethylbutyl-6-methylol-phenol. However, these alcohols had high solubility in both brine and stripping solutions. Therefore, finding efficient and appropriate molecules for boron recovery from native brines is crucial.
This study highlights that an extraction solvent comprising 2 mol L−1 2-butyl-1-octanol diluted in kerosene exhibits efficient extraction, selectivity, and stripping properties for boron. Its low solubility in both brine and the stripping solution is a significant advantage. However, researchers have also suggested that using highly hydrophobic alcohols might further minimize solubility in these solutions. They showed that the length of aliphatic alcohols, the number of alcohol functional groups, and the branching of alkyl groups significantly impact solubility.19,39
Author contributions
Conceptualization: AC, AFK; formal analysis: AFK, AC, JM; funding acquisition: AC; investigation: AFK; software: AFK; project administration: AC; supervision: AC, VF; visualization: AFK, AC; writing-original draft: AFK, AC; writing-review and editing: AFK, AC, VF, JM.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the french PIA project “Lorraine Université d’Excellence”, reference ANR-15-IDEX-04-LUE and the LabEx RESSOURCES 21 through the national program “Investissements d'avenir” with the reference ANR-10-LABX-21-RESSOURCES21. The authors would like to thank Prof. Marie-Noëlle Pons (Université de Lorraine, CNRS, LRGP) for TOC analyses of the aqueous phases.References
- T. Turkbay, J. Bongono, T. Alix, B. Laratte and B. Elevli, Clean. Eng. Technol., 2022, 10, 100539 CrossRef.
- M. M. Nasseri, Ann. Nucl. Energy, 2018, 114, 603–606 CrossRef CAS.
- J. Eichler and C. Lesniak, J. Eur. Ceram. Soc., 2008, 28, 1105–1109 CrossRef CAS.
- F. Ali, N. S Hosmane and Y. Zhu, Molecules, 2020, 25, 828 CrossRef CAS PubMed.
- H. Chand, A. Kumar and V. Krishnan, Adv. Mater. Interfaces, 2021, 8, 2100045 CrossRef CAS.
- G. Moussa, R. Moury, U. B. Demirci, T. Şener and P. Miele, Int. J. Energy Res., 2013, 37, 825–842 CrossRef CAS.
- M. H. Kharita, S. Yousef and M. AlNassar, Prog. Nucl. Energy, 2011, 53, 207–211 CrossRef CAS.
- A. Mergen, M. H. Demirhan and M. Bilen, Adv. Powder Technol., 2003, 14, 279–293 CrossRef CAS.
- M. M. Harussani, S. M. Sapuan, G. Nadeem, T. Rafin and W. Kirubaanand, Def. Technol., 2022, 18, 1281–1300 CrossRef.
- D. E. Garrett, Borates: Handbook of Deposits, Processing, Properties, and Use, Elsevier, 1998 Search PubMed.
- D. E. Garrett, in Borates, ed. D. E. Garrett, Academic Press, San Diego, 1998, pp. 227–253 Search PubMed.
- A. Balinski, V. Recksiek and N. Kelly, Processes, 2021, 9, 398 CrossRef CAS.
- Y.-J. Shih, C.-H. Liu, W.-C. Lan and Y.-H. Huang, Chemosphere, 2014, 111, 232–237 CrossRef CAS PubMed.
- L. Melnik, O. Vysotskaja and B. Kornilovich, Desalination, 1999, 124, 125–130 CrossRef CAS.
- A. Farhat, F. Ahmad, N. Hilal and H. A. Arafat, Desalination, 2013, 310, 50–59 CrossRef CAS.
- Z. Guan, J. Lv, P. Bai and X. Guo, Desalination, 2016, 383, 29–37 CrossRef CAS.
- N. Bicak, M. Gazi and B. F. Senkal, React. Funct. Polym., 2005, 65, 143–148 CrossRef CAS.
- M. D. Joshi, G. Chalumot, Y. Kim and J. L. Anderson, Chem. Commun., 2012, 48, 1410–1412 RSC.
- M. Karakaplan, S. Tural, B. Tural, Y. Turgut and H. Hoşgören, Solvent Extr. Ion Exch., 2004, 22, 897–911 CrossRef CAS.
- W. L. A. Brooks, C. C. Deng and B. S. Sumerlin, ACS Omega, 2018, 3, 17863–17870 CrossRef CAS PubMed.
- A. Chagnes, Metals, 2019, 9, 211 CrossRef CAS.
- G. M. Ritcey, Tsinghua Sci. Technol., 2006, 11, 137–152 CAS.
- X. Peng, L. Li, D. Shi, L. Zhang, H. Li, F. Nie and F. Song, Hydrometallurgy, 2018, 177, 161–167 CrossRef CAS.
- Z. Xu, H. Su, J. Zhang, W. Liu, Z. Zhu, J. Wang, J. Chen and T. Qi, RSC Adv., 2021, 11, 16096–16105 RSC.
- X. Peng, D. Shi, Y. Zhang, L. Zhang, L. Ji and L. Li, J. Mol. Liq., 2021, 326, 115301 CrossRef CAS.
- J. Lü, J. Liu, Y. Sun and C. Li, Chin. J. Chem. Eng., 2014, 22, 496–502 CrossRef.
- H. Hoşgoren, S. Tural, F. Kahraman, M. Toğrul and M. Karakaplan, Solvent Extr. Ion Exch., 1997, 15, 249–257 CrossRef.
- J. Guo, Y. Yang, X. Gao and J. Yu, Hydrometallurgy, 2020, 197, 105477 CrossRef CAS.
- R. Zhang, Y. Xie, J. Song, L. Xing, D. Kong, X.-M. Li and T. He, Hydrometallurgy, 2016, 160, 129–136 CrossRef CAS.
- M. L. Vera, W. R. Torres, C. I. Galli, A. Chagnes and V. Flexer, Nat. Rev. Earth Environ., 2023, 4, 149–165 CrossRef CAS.
- D. E. Garrett, in Handbook of Lithium and Natural Calcium Chloride, ed. D. E. Garrett, Academic Press, Oxford, 2004, pp. 1–235 Search PubMed.
- D. E. Garrett, in Borates, ed. D. E. Garrett, Academic Press, San Diego, 1998, pp. 333–400 Search PubMed.
- S. Barth, Fresenius. J. Anal. Chem., 1997, 358, 854–855 CrossRef CAS.
- M. Kühn, C. Niewöhner, M. Isenbeck-Schröter and H. D. Schulz, Water Res., 1998, 32, 265–274 CrossRef.
- H. Tao, Y. Xiao-ping, G. Ya-fei, L. Ming-li, D. Ji and D. Tian-long, Spectrosc. Spectral Anal., 2020, 40, 1214–1220 Search PubMed.
- Z. Chen-ling, J. Na, L. Jia, L. Bing-bing and H. Mei, Spectrosc. Spectral Anal., 2021, 41, 3876–3880 Search PubMed.
- D. L. Parkhurst and C. A. J. Appelo, Description of Input and Examples for PHREEQC Version 3: a Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations, U.S. Geological Survey, Reston, VA, 2013, vol. 6-A43 Search PubMed.
- P. M. Brown and S. J. Beckerman, US Pat., US4980136A, 1990 Search PubMed.
- M. Karakaplan, S. Tural, M. Sunkür and H. Hoşgören, Sep. Sci. Technol., 2003, 38, 1721–1732 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra08045e |
| This journal is © The Royal Society of Chemistry 2024 |