Open Access Article
Open Access ArticleDetailed mechanism study of volatile organic compound decomposition and oxidation removal based on a ReaxFF MD method
Shuo
Wang
a,
Xiaoqing
Wu
*b and
Xiaozhen
Chen
 *a
*a
aChengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China
bSchool of Life Science and Engineering, Southwest Jiaotong University, Chengdu 610041, China
First published on 14th February 2024
Abstract
Volatile organic compounds (VOCs) are typical air pollutants as well as gaseous wastes that contain energy. Utilization and disposition of VOCs is currently an important research hotspot in the field of atmospheric environment. In this paper, the thermal cracking and oxidation reaction processes of typical VOCs components were modelled and analyzed by combining molecular dynamics and detailed reaction mechanisms, focusing on the effects of temperature, oxygen and other conditions on the conversion of VOCs. The results of molecular dynamics studies show that improving temperature and reaction time benefit the decomposition of VOCs. High temperatures under an inert atmosphere can sufficiently crack the VOCs themselves, but other by-products are generated, which in turn cause secondary pollution. The activation energies derived by ReaxFF-MD calculation are 328 kJ mol−1, 147 kJ mol−1 and 121 kJ mol−1 for toluene, styrene and benzaldehyde respectively, which is consistent with experimental results. Under the oxygen atmosphere, the conversion rate of VOCs is greatly increased and the reaction temperature is significantly reduced. Meanwhile, the oxidation reaction fully converts VOCs into non-polluting products such as CO2 and H2O. Detailed kinetic studies show that initial oxidation of toluene molecules raised by hydrogen abstraction reaction is the dominant step during toluene oxidation, which significantly improved the decomposition efficiency of toluene.
1. Introduction
Volatile organic compounds (VOCs) are primarily present in the gas phase in the environment due to their relatively high volatility.1 Common VOCs are benzene, toluene, xylene, styrene, trichloroethylene, trichloromethane, and others. Many VOCs have known toxicities, for example, trichloroethylene and perchloroethylene have been associated with birth defects.2 It is known that indoor VOCs can raise the risk of respiratory problems.3–5 Besides, VOCs are important precursors to urban haze and photochemical smog, and originate mainly from processes such as coal chemical, petrochemical, fuel paint manufacturing, and solvent manufacturing and use.6The development of efficient, energy-saving and green VOCs degradation technology is of great significance. Industrial VOCs treatment processes include absorption, adsorption, oxidation, low-temperature plasma decomposition, biological method and so on.7,8 Among them, combustion/oxidation method and adsorption method are most economical treatment methods.9
In practice, the combustion oxidation method is subdivided into direct combustion and thermal combustion. Direct combustion refers to the combustion of VOCs exhaust gas, air and auxiliary fuel directly in the furnace chamber, and this method is suitable for exhaust gas with high VOCs concentration. Thermal combustion is to use the heat released during fuel combustion to preheat the VOCs exhaust gas, reducing the use of auxiliary fuels, in order to achieve better VOCs exhaust gas purification performance. This method has a higher utilization efficiency of fuel combustion heat, better economic benefit. Therefore it's widely used in the industry.10,11
Regenerative Thermal Oxidation (RTO) technology, also known as thermal storage incinerator, is a typical thermal decomposition technology for VOCs treatment.12,13 The technology mainly uses the high temperature generated by auxiliary combustion (≥760 °C), oxidizing the toxic and harmful organic substances in the system to generate carbon dioxide and water, while using heat storage materials to recover the excess heat generated by the combustion process.11 In this way, the purpose of energy saving and environmental protection could be achieved. In industrial waste gas treatment, this technology is often used to deal with high concentration of organic waste gas.
The composition of VOCs is very complex, containing aliphatic hydrocarbons, aromatic hydrocarbons, halogenated hydrocarbons, and oxygenated components.6 Monocyclic aromatic hydrocarbons are the main components of VOCs in the flue gas of coal-fired power stations, accounting for 50–90% of the total VOCs content.14–17 Xie et al.18 studied the VOCs unorganized emissions from typical devices in petrochemical industry and found aromatic hydrocarbons were main compounds. Meanwhile, monoaromatics hydrocarbons are also largely produced during frying process.19 The thermal decomposition of aromatic compounds is the key problem for the VOCs treatment technology. The effect of reaction temperature, excess air coefficient, residence time is crucial for the control of the RTO reactor. During the thermal decomposition or oxidation of VOCs, the understanding of detailed reaction mechanism is crucial for the efficient conversion of VOCs compounds. However, the use of detailed kinetic mechanisms to model thermal cracking and partial oxidation is still insufficient. Most kinetic models focus on the combustion of conventional fuels.
In recent years, the molecular dynamics (MD) method based on the reaction force field has been widely applied, and ReaxFF MD has been more widely used in the thermochemical conversion process of hydrocarbon fuels. Cheng et al.20 used ReaxFF MD to study the oxidation of toluene at high temperatures and found that the initiation consumption of toluene is mainly through three ways, which is in good agreement with available chemical kinetic models. Similarly, Xin et al.21 studied the thermal decomposition of 11 typical hydrocarbons by ReaxFF MD and found the initial pyrolysis reactions of these hydrocarbons can be divided into two types: homolytic cleavage of C–H bond and C–C bond. ReaxFF MD has been proved to be an excellent method for the study of molecular reaction mechanisms. However, ReaxFF MD approach has not been sufficiently investigated in terms of the conversion mechanism of VOCs.
In this paper, the thermal decomposition and oxidation mechanism of aromatic hydrocarbon components in the thermal combustion process of VOCs is investigated, and the cracking and oxidation reaction paths of different aromatic hydrocarbon components are simulated and analyzed by ReaxFF MD approach, so as to obtain the key reaction paths that affect the conversion, and further to obtain the key reaction parameters controlling the decomposition and oxidation of VOCs. This work provides theoretical support for the efficient conversion of VOCs.
2. Research method
2.1 VOCs model compounds
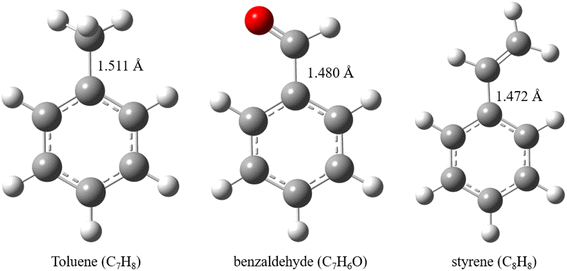
Monocyclic aromatic hydrocarbons are the main components of VOCs in the flue gas of coal-fired power stations, accounting for 50–90% of the total VOCs content.17 Shi et al.22 conducted a typical VOCs monitoring of a 300 MW coal-fired unit, and showed that VOCs in the flue gas were mainly dominated by benzene, toluene and benzaldehyde, with concentrations below 20 μg m−3. In the biomass pyrolysis gasification process, the tar is also dominated by typical aromatic hydrocarbons, including phenol, benzene, toluene, naphthalene, etc., with concentrations ranging from 10 mg Nm−5 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg Nm−3. Therefore, typical aromatic hydrocarbons, i.e., toluene, benzaldehyde and styrene, are selected as model compounds of VOCs in this paper to study their cracking and oxidative cracking transformation mechanisms at high temperatures. It provides a theoretical basis for the efficient removal of VOCs by high temperature combustion. The detailed molecular configurations of three VOCs compounds are summarized as follows (Fig. 1).
000 mg Nm−3. Therefore, typical aromatic hydrocarbons, i.e., toluene, benzaldehyde and styrene, are selected as model compounds of VOCs in this paper to study their cracking and oxidative cracking transformation mechanisms at high temperatures. It provides a theoretical basis for the efficient removal of VOCs by high temperature combustion. The detailed molecular configurations of three VOCs compounds are summarized as follows (Fig. 1).
2.2 Modeling details
Molecular dynamic simulations based on the reactive force field (ReaxFF) have been successfully used to simulate the complex reactions of hydrocarbons, such as coal, biomass and some gaseous fuels.23 ReaxFF generates results consistent with quantum-mechanical calculations and experiments for numerous hydrocarbon reactions with far less computational load, indicating the potential for simulation of large reactive systems.24 Using ReaxFF, we could obtain bond cleavage, bond formation, changes of species, and further elementary reactions in the complex thermal reactions.25In this section, the molecular dynamic modeling based on ReaxFF method was used to study the thermal decomposition of VOCs model compounds. A three-dimensional periodic box filled with 100 VOCs molecules (toluene, benzaldehyde and styrene) were built on the Material Studio software, with the box size of 40 Å × 40 Å × 40 Å. The molecular systems for three VOCs compounds are shown in Fig. 2 as follows.
Further, oxidation treatment of VOCs, which uses oxygen to combust VOCs compound into harmless products, such as CO2 and water, was studied. The mixture of VOCs compounds with oxygen molecules were constructed in Material Studio software. The equivalence ratio (ER) is controlled as 1 for three cases. The molecular systems for mixture of three VOCs compounds and oxygen are summarized in Fig. 3 as follows.
A set of non-isothermal simulations were performed using NVT ensemble in the temperature range of 500–3000 K, 500–3500 K and 500–4000 K. In the heating process, the heating time is controlled to 25 picosecond (ps) and then the systems were kept the temperature at 3000 K, 3500 K and 4000 K for 100 ps. The temperature of thermal decomposition reactions in molecular simulations is significantly higher than in experimental results. The main reason for the higher temperature in ReaxFF MD simulations is to allow the chemical reactions to occur in an extremely short time period.26 ReaxFF MD simulations typically operate on a time scale of picoseconds, which is significantly shorter than the time scale of experiments, which operate on a scale of seconds. To compensate for this difference, ReaxFF MD simulations require elevated temperatures to accelerate the reaction rate, resulting in chemical reactions occurring in an extremely short time. This is currently a common approach to reactive force field calculations.27
The first combustion reactive force field was developed by Chenoweth et al.,28 which was called CHO-2008. This force field was then used extensively to study the decomposition or combustion of different hydrocarbon fuels, including toluene,20 1-heptane,29 coal30 and lignin.31 However, CHO-2008 was not good for describing the reaction of C1 compounds.32 Ashraf et al.33 recently developed a new force field (CHO-2016), which overcomes the shortcomings of CHO-2008. In the current work, the CHO-2016 force field was used for all of the simulations. The force field has also shown good capability to describe the decomposition and the combustion of single component fuels.34
In the ReaxFF simulations, some other details are summarized as follows: the bond order cutoff and non-bonded cutoff were 0.3 and 10.0 Å, respectively; the time step was set to 0.25 fs to capture the reaction details.
3. Results and discussion
3.1 The thermal decomposition of VOCs compounds
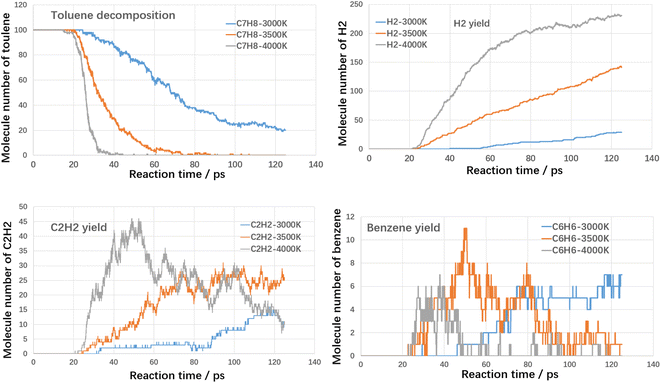
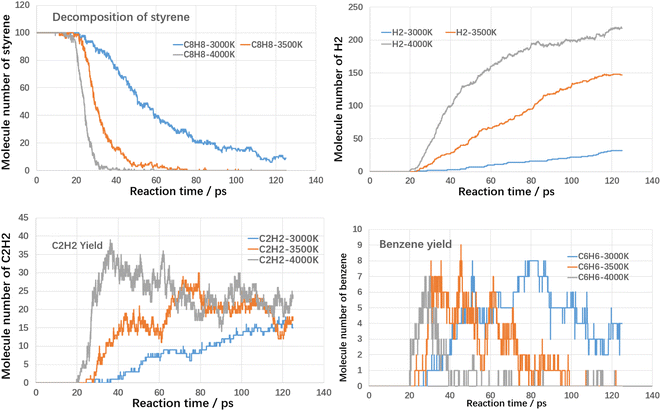
Firstly, the thermal cracking process of model compounds of VOCs was investigated. The starting temperature of thermal cracking was 500 K, and the target temperatures were 3000 K, 3500 K and 4000 K. The cracking efficiencies of toluene, styrene and benzaldehyde at different temperatures and the generation of products are shown in the following Fig. 4–6.As shown in Fig. 4, both temperature and reaction time show significant influence on toluene decomposition. The conversion ratio of toluene reached 80% at 3000 K with residence time of 125 ps. When improving temperature, the toluene conversion ratio reached 100% at 75 ps (3500 K) and 39 ps (4000 K). With respect of gaseous products, H2 and C2H2 are two main products. At high temperatures, the hydrogen atoms on the methyl and aromatic rings dissociate to form hydrogen radicals, which can combine with each other to form hydrogen, while the hydrogen radicals can further react with hydrocarbons in a bimolecular reaction to form hydrogen. As shown in Fig. 4, when temperature improving from 3000 K to 4000 K, the H2 molecules increased from 29 to 231, which indicated that higher temperature benefited the yield of H2. The effect of temperature on the formation of acetylene differs from that of hydrogen, mainly in the effect of the rate of formation. Acetylene (C2H2) is the product during aromatic ring breakage. At high temperatures (4000 K), acetylene is generated rapidly with the cleavage of toluene, reaching a maximum yield at 45 ps (45), which slowly decreases with further increases in temperature. For the final yields, the final yields at different temperatures did not differ much, being 12 (3000 K), 7 (3500 K) and 25 (4000 K). Benzene is the main aromatic hydrocarbon cracking product and it can be seen that at lower temperatures (3000 K), the benzene yield is increasing with increasing reaction time. While at higher temperatures (3500 K and 4000 K), the benzene production is increasing and then decreasing with the increase of reaction time. The molecular yields of benzene at each temperature were 7, 11 and 7. The cracking of toluene has other products of lower concentration in addition to the higher concentration products mentioned above, including small molecule hydrocarbons such as ethylene, methane and other large molecule hydrocarbons such as naphthalene, which have lower yields and will not be further analyzed here.
As shown in Fig. 5, the cracking trend of styrene with different temperatures is similar to that of toluene, the higher the temperature, the faster the cracking rate and the higher the conversion rate. The conversion ratio of toluene reached 90% at 3000 K with residence time of 125 ps. When improving temperature, the toluene conversion ratio reached 100% at 70 ps (3500 K) and 30 ps (4000 K). It is clear that the thermal cracking process of styrene is more reactive than that of toluene. When temperature improving from 3000 K to 4000 K, the H2 molecules increased from 32 to 218. At higher temperatures, the acetylene yield increases and then decreases with reaction time. The peak acetylene product occurs at 79 ps (3500 K) and 36 ps (4000 K). Benzene production has a peak in the range of 3000–4000 K. The peak point decreases from 80 ps (3000 K) to 28 ps (4000 K) with increasing temperature.
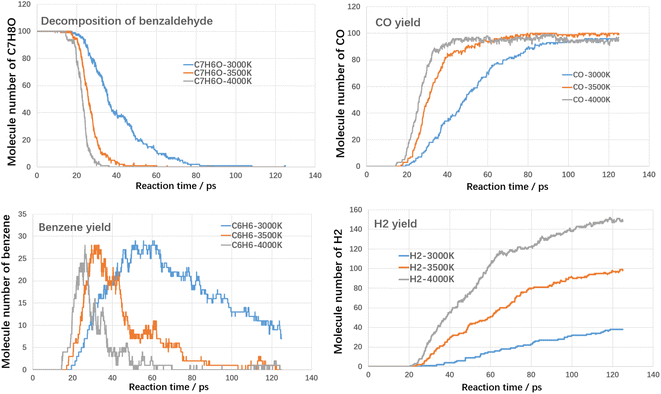
Fig. 6 shows the results of the cleavage of benzaldehyde, which is a typical oxygenated component. In terms of the cleavage process of benzaldehyde itself, its cleavage reactivity is significantly stronger than that of toluene and styrene. The complete cleavage times of benzaldehyde at different temperatures are 83 ps, 43 ps and 33 ps, respectively. Even at 3000 K, it takes only 83 ps for benzaldehyde to reach complete cleavage. CO is an important gaseous product in the benzaldehyde cleavage, which originates from the shedding of carbonyl groups. At different temperatures, the final CO yield was close to 100%, which indicated that most of the carbonyl groups were converted to CO. The hydrogen production pattern is similar to that of toluene and styrene, but the yield is significantly lower. The maximum hydrogen yield is 149 at 4000 K. In contrast, benzene yields are significantly higher than toluene and styrene. The peak yields of benzene at different temperatures are 29 (3000 K), 28 (3500 K) and 28 (4000 K), respectively. This suggests that the shedding of carbonyl group promotes the production of benzene.
Next, based on the thermal decomposition of three VOCs model compounds, the global kinetic parameters were evaluated.
To calculated the global Arrhenius parameters of the single component, the first-order kinetics were considered in this work. As pointed out in the above section, the consumption rate of the VOCs compounds largely depends on the reaction temperature and residence time. The speed of the VOCs molecules decomposition provides an indicator for the obtaining the Arrhenius parameters. The reaction rate constant at different temperatures could be determined by the following first order rate low equation:
| ln(N0) − ln(Nt) = kt | (1) |
k = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ea/RT) exp(−Ea/RT) | (2) |
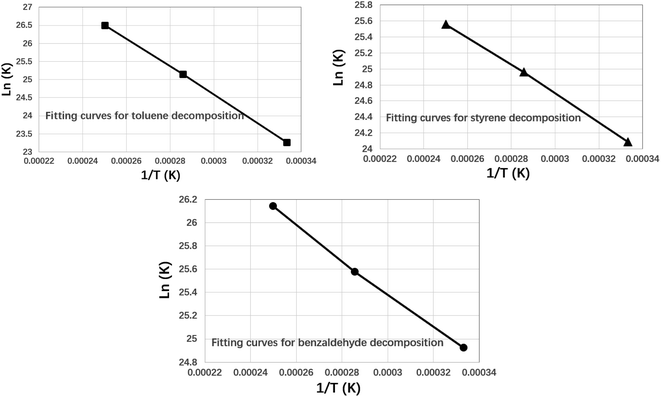
The fitting curves for three VOCs components are shown in Fig. 7 as follows.
As shown in Fig. 7, the he fitted curves for the kinetics of the cracking of the three VOCs components were all approximately straight lines. Further the reaction kinetic parameters can be calculated by eqn (2) as shown in Table 1 below.
As shown in Table 1, the activation energies derived by ReaxFF-MD calculation are 328 kJ mol−1, 147 kJ mol−1 and 121 kJ mol−1 for toluene, styrene and benzaldehyde respectively. At the same time, the experimental results derived from publications were also used to validate the reliability of theoretical calculations. There is a good agreement between the experimental results and the activation energy results from the reaction force field calculations. This indicates that the ReaxFF-MD method can be well used for the simulation and analysis of the molecular decomposition kinetics of VOCs. By comparing the activation energies of three VOCs compounds, it can be indicated that the activation energy of toluene is highest, which means toluene will decomposition at higher temperature than other two compounds. Benzaldehyde has the lowest activation energy (121 kJ mol−1), which means it will decompose at lower temperature. This conclusion is consistent with the thermal decomposition results described above.
3.2 The oxidative decomposition of VOCs compounds
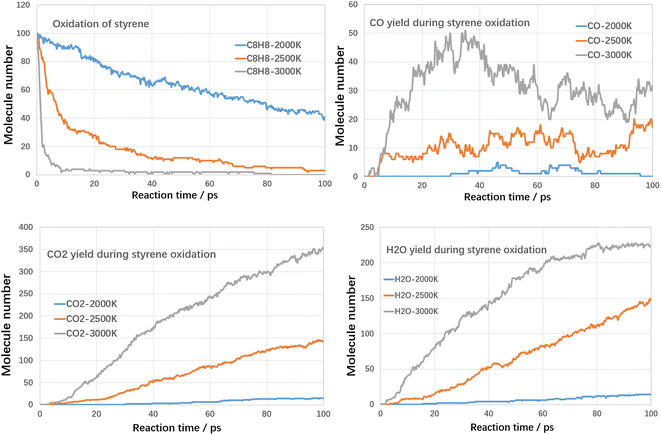
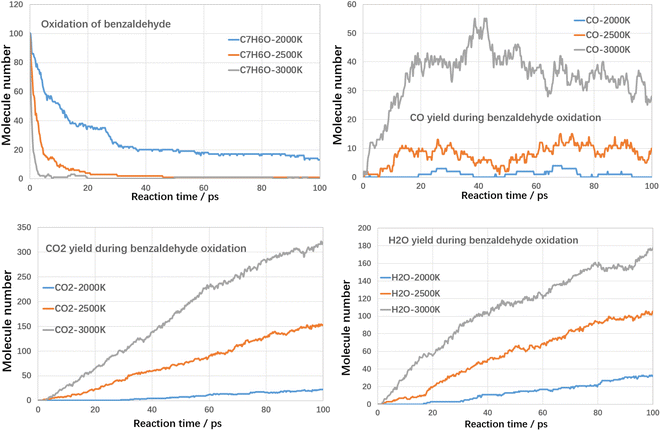
Although high temperatures can promote the cracking of VOCs, even conversion rates of up to 100% can be achieved. However, the cracking process also produces large amounts of other hydrocarbons and even large molecules of PAHs-like substances, which in turn produce other additional pollution. Therefore, in addition to using high temperatures to break down the VOCs components themselves, it is also necessary to avoid generating other secondary pollutants. Oxidation treatment uses oxygen to combust VOCs compounds, in which VOCs compounds could be fully converted into harmless products, such as CO2 and water with proper design. Next, the oxidation decomposition of VOCs compounds was studied.For each case, three kinds of oxidation temperature were used to investigate the oxidative degradation of VOCs compounds. The results are shown in Fig. 8 and 9 as follows.
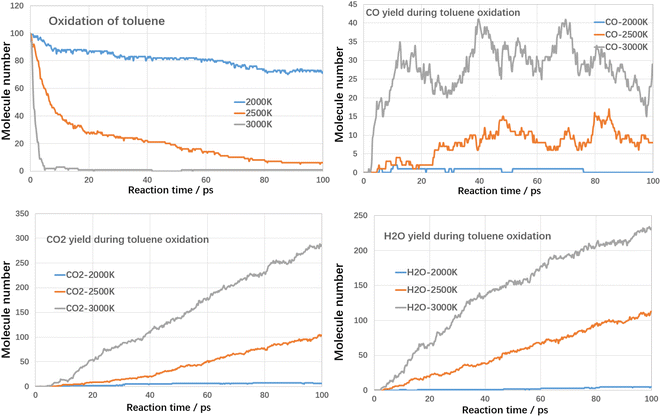
As shown in Fig. 8, the rate of toluene decomposition is greatly improved under oxygen atmosphere. At 3000 K, toluene is almost consumed at initial 5 ps, which is much quicker than thermal decomposition (conversion efficiency of 80% at 3000 K with residence time of 120 ps). Therefore, much lower reaction temperature could be adopted. At 2500 K, the conversion efficiency of toluene was about 95% with reaction time of 100 ps. When temperature is controlled at 2000 K, toluene conversion is largely weakened and the conversion ratio was about 28% with reaction time of 100 ps. The products during oxidation are also different with thermal decomposition. As shown in Fig. 8, three main kinds of oxidation products are summarized, namely CO, CO2 and H2O. The yield of CO firstly increases with reaction time and then declines. The yields of CO2 and H2O increases with reaction time at all temperature ranges. The carbon atoms are mainly oxidized to produce CO and CO2. The yield of CO2 is much higher than CO, which indicated that toluene could be fully converted into harmless products during oxidative treatment. Improving temperature and reaction time benefits the decomposition of toluene to produce CO2 and H2O, which are clean products.
The conversion tendency of styrene under oxidative atmosphere is similar with toluene, as shown in Fig. 9. At 3000 K, styrene is almost consumed at initial 8 ps. When temperature reduces to 2500 K and 2000 K, the conversion efficiency reduces to 97% and 61% respectively. Therefore, oxidative atmosphere largely enhanced the decomposition efficiency of styrene. The yields of CO2 and H2O increase with reaction time at all temperature ranges. The yield of CO firstly increases with reaction time and then decreases. Similarly, the yield of CO2 is much higher than CO. By comparing toluene and styrene, it seems that the reactivity of styrene is higher than toluene.
As shown in Fig. 10, at 3000 K, benzaldehyde is almost consumed at initial 5 ps. With temperature reduced to 2500 K, benzaldehyde could also be almost consumed at initial 45 ps. When temperature was controlled at 2000 K, the conversion ratio reduced to about 87% at 100 ps, which was much higher than toluene and styrene. It indicated that the reactivity of benzaldehyde is much higher than toluene and styrene, which was mainly attributed to the aldehyde functional group. The yields of CO, CO2 and H2O are similar to the oxidation of toluene and styrene.
Based on the thermal decomposition and oxidation of VOCs compounds, it can be indicated that oxygen promote the decomposition and conversion of VOCs. The reaction temperature is reduced and harmless products, mainly CO2 and H2O are produced. Therefore, using oxygen or air to improving the decomposition of VOCs is a promising approach for VOCs treatment.
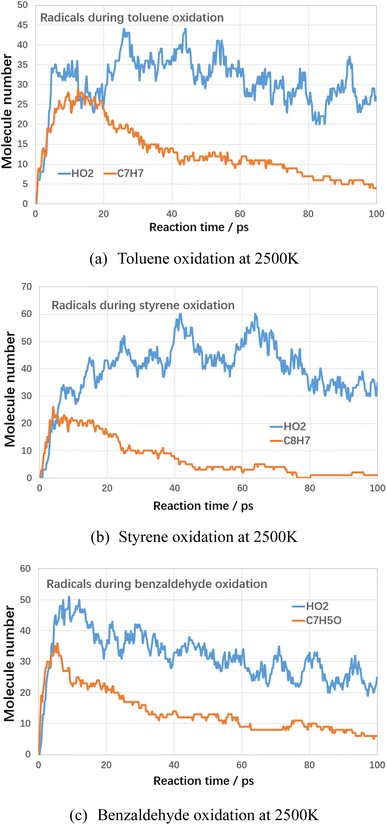
Next, the detailed mechanism for the role of oxygen on the decomposition of VOCs compounds are analyzed. In terms of electronic structure, the oxygen molecule is a triplet state structure with unpaired active electrons. Therefore, during the reaction with VOCs molecules, oxygen molecules present the characteristics of free radicals with strong oxidizing properties, which can promote the cleavage and conversion of VOCs molecules. By analyzing the intermediates and radicals during oxidation of VOCs compounds, the main radicals and intermediates were analyzed and the results are shown below.
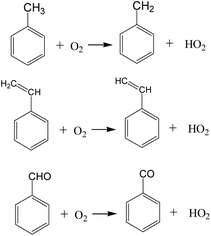
As shown in Fig. 11, during the oxidation of VOCs compounds, the hydroperoxyl radicals (HO2) are the main intermediates in the oxidation process. The possible initial oxidation of VOCs molecules raised by hydrogen abstraction reaction could be summarized in Fig. 12 as follows.
The generation of hydroperoxyl radicals starts at the initial stage of the reaction (several picosecond) and is synchronised with the decomposition reaction of the VOCs. This suggests that oxygen molecules are able to react directly with VOCs molecules in a bimolecular reaction, seizing their hydrogen atoms, forming hydroperoxyl radicals, and contributing to the rapid cracking and conversion of VOCs molecules at lower temperatures. Cheng et al.20 found the initiation consumption of toluene is mainly through three ways, namely (1) the hydrogen abstraction reactions by oxygen molecules or other small radicals to form the benzyl radical, (2) the cleavage of the C–H bond to form benzyl and hydrogen radicals, and (3) the cleavage of the C–C bond to form phenyl and methyl radicals. These basic reaction mechanisms are in good agreement with available chemical kinetic models.
3.3 The detailed kinetic decomposition of VOCs compounds
Next, the decomposition of VOCs compounds was studied by detailed kinetic mechanism. There are quite many detailed kinetic mechanisms that have been developed for the decomposition and oxidation of hydrocarbons.38,39 The detailed mechanism established by Richter et al.40,41 contains 296 components and 6663 elementary reactions, which can be described from the smallest radical H atoms to the largest carbon black particles with particle diameters up to 0.7 nm. The mechanism was used for study the decomposition and oxidation of VOCs compounds.CHEMKIN is a powerful software package for solving complex chemical reaction problems, commonly used in the simulation of combustion processes, catalytic processes, chemical vapor deposition, plasma and other chemical reactions.42 CHEMKIN is based on three core software packages, namely, gas-phase dynamics, surface dynamics, and transfer processes, and provides models and post-processing procedures for 21 common chemical reactions. In this section, toluene was used to study the detailed decomposition and oxidation mechanism for VOCs treatment. The decomposition and oxidation results at different temperatures are summarized as follows (Fig. 13).
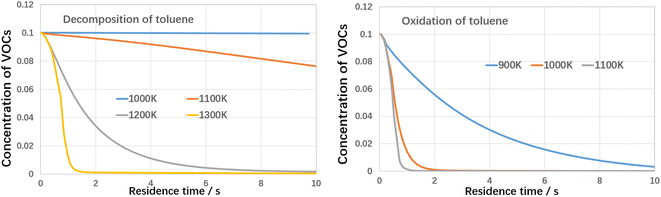
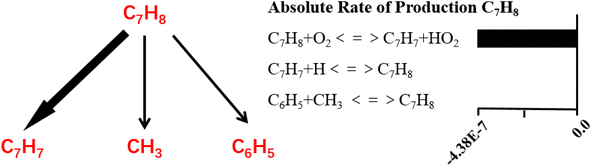
It can be indicated that toluene decomposed very slow when temperature was lower than 1100 K under inert atmosphere and when temperature increased to 1200 K, toluene was quickly consumed. When treated by oxidation, the decomposition temperature could be as low as 900 K, with nearly 100% conversion efficiency. Active oxygen largely improves the toluene conversion efficiency. Based on the kinetic calculation, the absolute rate of production for toluene and the reaction paths are built, as shown in Fig. 14 as follows.
It can be indicated the initial oxidation of toluene molecules raised by hydrogen abstraction reaction is the dominant step during toluene oxidation, which significantly improves the decomposition efficiency of toluene. Detailed reaction kinetics studies are consistent with the conclusion of molecular dynamics mechanism studies.
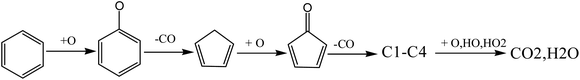
As mentioned above, the addition of oxygen reduces the reaction temperature and promotes the pyrolysis reaction of VOCs. In addition, the addition of oxygen introduced a large number of active free radicals, O, OH, HO2 and other free radicals further promoted the further oxidation of pyrolysis intermediates, and finally generated CO2 and H2O. The detailed reactions could be summarized as follows (Fig. 15).
4. Conclusion
VOCs are typical air pollutants as well as gaseous wastes that contain energy. Fully Utilizing and treating VOCs is currently an important research hotspot in the field of atmospheric environment. For efficient and low-cost treatment of VOCs, in this paper, the thermal cracking and oxidation reaction processes of typical VOCs components were modelled and analyzed by combining molecular dynamics and detailed reaction mechanisms, focusing on the effects of temperature, oxygen and other conditions on the conversion of VOCs. The main conclusions could be summarized as follows:(1) The results of molecular dynamics studies show that improving temperature and reaction time benefit the decomposition of VOCs. High temperatures under inert atmosphere can sufficiently crack the VOCs themselves, but other by-products are generated, which in turn cause secondary pollution.
(2) The activation energies derived by ReaxFF-MD calculation are 328 kJ mol−1, 147 kJ mol−1 and 121 kJ mol−1 for toluene, styrene and benzaldehyde respectively, which is consistent with experimental results.
(3) Oxidative treatment is significantly more efficient than high temperature thermal cracking. Under the oxygen atmosphere, the conversion rate of VOCs is greatly increased and the reaction temperature is significantly reduced. Meanwhile, the oxidation reaction fully converts VOCs into non-polluting products such as CO2 and H2O.
(4) Detailed kinetic studies show that initial oxidation of toluene molecules raised by hydrogen abstraction reaction is the dominant step during toluene oxidation, which significantly improved the decomposition efficiency of toluene.
Conflicts of interest
There are no conflicts to declare.References
- P. Ciccioli, VOCs and air pollution, in Chemistry and Analysis of Volatile Organic Compounds in the Environment, Springer, 1993, pp. 92–174 Search PubMed.
- P. Pandey and R. Yadav, A review on volatile organic compounds (VOCs) as environmental pollutants: fate and distribution, Int. J. Plant Environ., 2018, 4(2), 14–26 Search PubMed.
- G. P. Pappas, et al., The respiratory effects of volatile organic compounds, Int. J. Occup. Environ. Health, 2000, 6(1), 1–8 CrossRef CAS.
- S. C. Sofuoglu, et al., An assessment of indoor air concentrations and health risks of volatile organic compounds in three primary schools, Int. J. Hyg. Environ. Health, 2011, 214(1), 36–46 CrossRef CAS.
- T. Z. Maung, et al., Indoor Air Pollution and the Health of Vulnerable Groups: A Systematic Review Focused on Particulate Matter (PM), Volatile Organic Compounds (VOCs) and Their Effects on Children and People with Pre-Existing Lung Disease, Int. J. Environ. Res. Public Health, 2022, 19, 8752–8775 CrossRef CAS.
- H. Wang, et al., Characterization and assessment of volatile organic compounds (VOCs) emissions from typical industries, Chin. Sci. Bull., 2013, 58, 724–730 CrossRef CAS.
- Z. Zhang, Z. Jiang and W. Shangguan, Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review, Catal. Today, 2016, 264, 270–278 CrossRef CAS.
- X. Zhang, et al., Adsorption of VOCs onto engineered carbon materials: A review, J. Hazard. Mater., 2017, 338, 102–123 CrossRef CAS PubMed.
- Y. Shen, Biomass-derived porous carbons for sorption of Volatile organic compounds (VOCs), Fuel, 2023, 336, 126801 CrossRef CAS.
- B.-S. Choi and J. Yi, Simulation and optimization on the regenerative thermal oxidation of volatile organic compounds, Chem. Eng. J., 2000, 76(2), 103–114 CrossRef CAS.
- F. Wang, X. Lei and X. Hao, Key factors in the volatile organic compounds treatment by regenerative thermal oxidizer, J. Air Waste Manage. Assoc., 2020, 70(5), 557–567 CrossRef CAS.
- M. Tomatis, et al., Removal of VOCs from waste gases using various thermal oxidizers: a comparative study based on life cycle assessment and cost analysis in China, J. Cleaner Prod., 2019, 233, 808–818 CrossRef CAS.
- J. Liu and Z. Peng, Experimental and Numerical Investigations into Temperature Distributions and VOC Conversion Rate of RTO, in IOP Conference Series: Earth and Environmental Science, IOP Publishing, 2021 Search PubMed.
- J. Xu, et al., Formation and emission characteristics of VOCs from a coal-fired power plant, Chin. J. Chem. Eng., 2021, 35, 256–264 CrossRef CAS.
- A. G. Chmielewski, et al., VOCs emission from coal—fired power station boiler, in Environmental Engineering Studies: Polish Research on the Way to the EU, Springer, 2003 Search PubMed.
- Y. Peng, et al., VOC emissions of coal-fired power plants in China based on life cycle assessment method, Fuel, 2021, 292, 120325 CrossRef CAS.
- G. Fernández-Martínez, et al., Distribution of volatile organic compounds during the combustion process in coal-fired power stations, Atmos. Environ., 2001, 35(33), 5823–5831 CrossRef.
- H. Xie, et al., Source profile study of VOCs unorganized emissions from typical aromatic devices in petrochemical industry, Sci. Total Environ., 2023, 889, 164098 CrossRef CAS PubMed.
- A. Atamaleki, et al., The effect of frying process on the emission of the volatile organic compounds and monocyclic aromatic group (BTEX), Int. J. Environ. Anal. Chem., 2023, 103(18), 6169–6182 CrossRef CAS.
- X.-M. Cheng, et al., ReaxFF molecular dynamics simulations of oxidation of toluene at high temperatures, J. Phys. Chem. A, 2012, 116(40), 9811–9818 CrossRef CAS.
- L. Xin, et al., Thermal decomposition mechanism of some hydrocarbons by ReaxFF-based molecular dynamics and density functional theory study, Fuel, 2020, 275, 117885 CrossRef CAS.
- L. J. Shi Xiaohong and H. Liao, Research on the distribution and emission characteristics of volatile organic compounds in the flue as of coal-fired power plants, Environ. Pollut. Prev., 2021, 43(4), 405–410 Search PubMed.
- C. Ashraf and A. C. Van Duin, Extension of the ReaxFF combustion force field toward syngas combustion and initial oxidation kinetics, J. Phys. Chem. A, 2017, 121(5), 1051–1068 CrossRef CAS PubMed.
- A. C. Van Duin, et al., ReaxFF: a reactive force field for hydrocarbons, J. Phys. Chem. A, 2001, 105(41), 9396–9409 CrossRef CAS.
- Y.-Y. Li, et al., ReaxFF study on nitrogen-transfer mechanism in the oxidation process of lignite, Fuel, 2017, 193, 331–342 CrossRef CAS.
- D. Hong, et al., ReaxFF simulations of the synergistic effect mechanisms during co-pyrolysis of coal and polyethylene/polystyrene, Energy, 2021, 218, 119553 CrossRef CAS.
- J. Xu and L. Zhu, Molecular Mechanism Study of the Kinetics and Product Yields during Copyrolysis of Biomass and Solid Wastes: ReaxFF-MD Method Approach, ACS Omega, 2023, 8(39), 36126–36135 CrossRef CAS.
- K. Chenoweth, A. C. Van Duin and W. A. Goddard, ReaxFF reactive force field for molecular dynamics simulations of hydrocarbon oxidation, J. Phys. Chem. A, 2008, 112(5), 1040–1053 CrossRef CAS PubMed.
- F. Castro-Marcano and A. C. van Duin, Comparison of thermal and catalytic cracking of 1-heptene from ReaxFF reactive molecular dynamics simulations, Combust. Flame, 2013, 160(4), 766–775 CrossRef CAS.
- F. Castro-Marcano, et al., Combustion of an Illinois No. 6 coal char simulated using an atomistic char representation and the ReaxFF reactive force field, Combust. Flame, 2012, 159(3), 1272–1285 CrossRef CAS.
- A. Beste, ReaxFF study of the oxidation of lignin model compounds for the most common linkages in softwood in view of carbon fiber production, J. Phys. Chem. A, 2014, 118(5), 803–814 CrossRef CAS.
- C. Ashraf, et al., Pyrolysis of binary fuel mixtures at supercritical conditions: A ReaxFF molecular dynamics study, Fuel, 2019, 235, 194–207 CrossRef CAS.
- C. Ashraf and A. C. T. van Duin, Extension of the ReaxFF Combustion Force Field toward Syngas Combustion and Initial Oxidation Kinetics, J. Phys. Chem. A, 2017, 121(5), 1051–1068 CrossRef CAS PubMed.
- H. Kwon, et al., ReaxFF-based molecular dynamics study of bio-derived polycyclic alkanes as potential alternative jet fuels, Fuel, 2020, 279, 118548 CrossRef CAS.
- D. Baulch, et al., Evaluated kinetic data for combustion modeling: supplement II, J. Phys. Chem. Ref. Data, 2005, 34(3), 757–1397 CrossRef CAS.
- K. Pamidimukkala, et al., High-temperature pyrolysis of toluene, J. Phys. Chem., 1987, 91(8), 2148–2154 CrossRef CAS.
- O. S. L. Bruinsma, et al., Gas phase pyrolysis of coal-related aromatic compounds in a coiled tube flow reactor: 1. Benzene and derivatives, Fuel, 1988, 67(3), 327–333 CrossRef CAS.
- H. Wang and M. Frenklach, A detailed kinetic modeling study of aromatics formation in laminar premixed acetylene and ethylene flames, Combust. Flame, 1997, 110(1–2), 173–221 CrossRef CAS.
- Z. Wei, et al., Development of natural gas chemical kinetic mechanisms and application in engines: a review, ACS Omega, 2021, 6(37), 23643–23653 CrossRef CAS.
- H. Richter, et al., Detailed modeling of PAH and soot formation in a laminar premixed benzene/oxygen/argon low-pressure flame, Proc. Combust. Inst., 2005, 30(1), 1397–1405 CrossRef.
- H. Richter and J. B. Howard, Formation and consumption of single-ring aromatic hydrocarbons and their precursors in premixed acetylene, ethylene and benzene flames, Phys. Chem. Chem. Phys., 2002, 4(11), 2038–2055 RSC.
- S. Soltanimehr, M. R. Rahimpour and A. Shariati, Development of a green process based on a novel tri-reforming reactor to produce syngas for methanol synthesis: process design, modeling and multi objective optimization, J. Cleaner Prod., 2023, 418, 138167 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |