Open Access Article
Open Access ArticleFrom algae to advancements: laminarin in biomedicine
Sheersha Pramanik
 a,
Anshul Singh
a,
Anshul Singh
 b,
Bassam M. Abualsoud
b,
Bassam M. Abualsoud
 c,
A. Deepakd,
Pankaj Nainwal
e,
Armen S. Sargsyan
c,
A. Deepakd,
Pankaj Nainwal
e,
Armen S. Sargsyan f and
Stefano Bellucci
*g
f and
Stefano Bellucci
*g
aDepartment of Biotechnology, Bhupat and Jyoti Mehta School of Biosciences, Indian Institute of Technology Madras, Chennai 600036, Tamil Nadu, India
bDepartment of Chemistry, Baba Mastnath University, Rohtak 124021, India
cDepartment of Pharmaceutics and Pharmaceutical Technology, College of Pharmacy, Al-Ahliyya Amman University, Amman 19328, Jordan
dSaveetha Institute of Medical and Technical Sciences, Saveetha School of Engineering, Chennai, Tamil Nadu 600128, India
eSchool of Pharmacy, Graphic Era Hill University, Dehradun, 248001, India
fScientific and Production Center “Armbiotechnology” NAS RA, 14 Gyurjyan Str., Yerevan 0056, Armenia
gINFN-Laboratori Nazionali di Frascati, Via E. Fermi 54, 00044 Frascati, Italy. E-mail: stefano.bellucci@lnf.infn.it
First published on 19th January 2024
Abstract
Laminarin, a complicated polysaccharide originating from brown algae, has emerged as a compelling candidate in the domain of biomedical research. This enigmatic molecule, composed of glucose units associated with both β-1,3 and β-1,6 glycosidic bonds, possesses an array of remarkable characteristics that render it auspicious for multifaceted biomedical applications. This review investigates the comprehensive potential of laminarin in the biomedical domain, emphasizing its remarkable biocompatibility, low cytotoxicity, and cell proliferation support. Laminarin's immunomodulatory attributes position it as an encouraging contender in immunotherapy and the development of vaccines. Moreover, its anti-inflammatory and antioxidant characteristics provide a promising avenue for combatting conditions associated with oxidative stress. In particular, laminarin excels as a drug delivery vehicle owing to its exceptional encapsulation capabilities emerging from its porous framework. Integrating pH and redox responsiveness in laminarin-based drug delivery systems is poised to redefine targeted therapies. Laminarin substantially contributes to tissue engineering by improving adhesion, migration of cells, and deposition of extracellular matrix. This augmentation magnifies the regenerative capability of tissue-engineered constructs, substantiated by the advancement of laminarin-based wound dressings and tissue scaffolds, marking considerable progress in the domain of wound healing and tissue regeneration. While laminarin exhibits substantial potential in biomedical applications, it remains in the initial phases of exploration. Comprehensive preclinical and clinical research is warranted to verify its effectiveness and safety across various applications. In essence, laminarin, a marine marvel, has the capability to remodel biomedical research, offering inventive solutions to complex difficulties.
1. Introduction
The world's oceans, with their enormous and enigmatic depths, have long enthralled the vision of explorers and investigators alike. Underneath the surface of these vast aquatic realms lies a secret treasure trove of life involving a mind-boggling diversity of marine organisms. Within this underwater domain, an outstanding category of compounds has surfaced as a subject of increasing attraction and scientific research – marine-derived polysaccharides.Polysaccharides consist of chains including approximately 30 to 50 units of monosaccharide associated with glycosidic bonds, and they amalgamate with diverse, intricate sugars to develop interconnected, large biological macromolecules with higher molecular weights.1 They are prevalent in nature, assisting crucial roles in biological procedures. The marine habitat, with its myriad of exceptional ecosystems and species, has demonstrated to be a productive ground for the breakthrough of novel polysaccharides. These biopolymers display various structures, characteristics, and functions, making them captivating subjects for investigation and capable sources of beneficial compounds.2
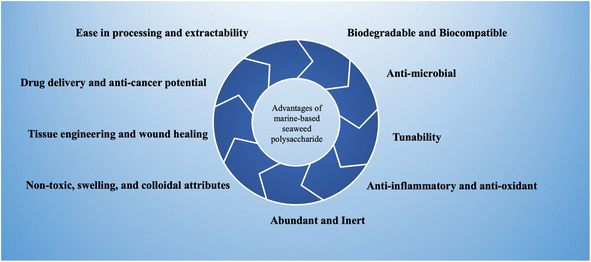
The marine habitat furnishes a diverse range of origins for these polysaccharides, with macroalgae (seaweeds), microalgae, seagrasses, and mangroves projecting as prolific producers. These creatures have adjusted to the dynamic and frequently harsh environment of the marine ecosystem, guiding the progress of a wide array of polysaccharides with definite characteristics and functions.3 Within the extensive realm of marine biological diversity, algae stand as principal reservoirs of marine polysaccharides, a class of compounds of increasing scientific and industrial fascination. Mainly, definite polysaccharides are achievable not only from macroscopic marine algae but also from marine prokaryotes, involving microalgae that can be cultivated in regulated environments like bioreactors. While red macroalgae are the most widespread sources of marine polysaccharides, it is pertinent to highlight that polysaccharides can also be sourced from green and brown macroalgae.4 Moreover, categorizing seaweeds, a distinct class of multicellular marine algae, introduces a subdivision into red, green, and brown varieties, each harboring unusual polysaccharide profiles.5 Polysaccharides originating from seaweeds have received growing attention due to their availability, decreased cost for extraction, and exceptional biological and physicochemical characteristics, for example, healing of wounds, anti-cancer, antioxidant, antibacterial, anti-inflammatory, or immunostimulatory responses.6 The significant advantages of utilization of marine-based seaweed polysaccharides have been depicted in Fig. 1. Regrettably, the potential of marine polysaccharides in the medical sector remains significantly underexplored, notwithstanding their abundant availability and promising properties.
Laminarin, an intriguing polysaccharide principally sourced from brown algae, has recently surfaced as a subject of increased scientific curiosity owing to its multifarious therapeutic characteristics. This biopolymer, even though not completely harnessed to its potential, has revealed an exceptional repertoire of bioactive functionalities. Specifically, laminarin displays pronounced anti-inflammatory properties, anti-apoptotic characteristics, anti-tumor properties, antioxidants, and anticoagulant activities.7,8 The multifarious virtues of laminarin emphasize its promising role in the biomedical field, justifying further exploration to unlatch its complete therapeutic potential.
In this review article, we commence on a voyage into the world of marine-derived polysaccharides, with a definite emphasis on laminarin. In the following sections of this review, we will embark on a comprehensive exploration of laminarin, delving into its structural characteristics, extraction methods, unique properties, and diverse biomedical applications. Through this journey, we aspire to shed light on the adaptability and promise of laminarin, highlighting its importance in the world of marine-derived polysaccharides and its potential contributions to the biomedical field.
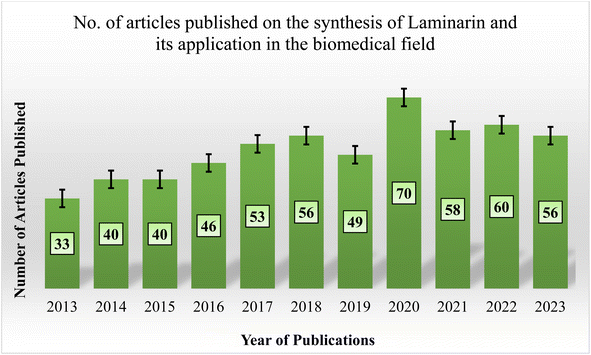
In recent decades, laminarin-based biomaterials have been widely researched in biomedical applications. Despite this, there is a noticeable gap in publications that extensively document or summarize the advancement in preparing laminarin-based composites and their unique attributes for diverse biomedical applications. A search on “PubMed” utilizing the keywords “Laminarin,” “Laminaran,” and “Biomedical Application,” with “AND” as a Boolean operator, displays a limited number of articles in recent years, as exhibited in Fig. 2. As demonstrated by the literature review, the predominant focus in existing publications lies on the synthesis of laminarin. Remarkably, only a limited number of papers have investigated the exploration of these laminarins, evaluating their potential across the biomedical field. This indicates the potential existence of uncharted research avenues under laminarin as a biomaterial, especially for biomedical applications.
2. Laminarin unearthed: source diversity and structural insight
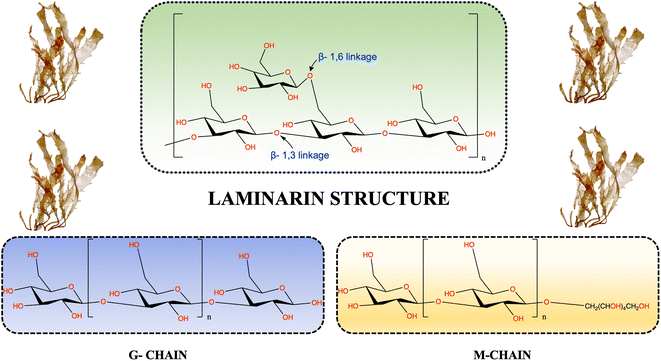
Laminarin, also referred to as laminaran or leucosin, is a naturally occurring polysaccharide that has acquired increased focus from investigators across the world owing to its exceptional properties and different applications. This biodegradable polymer principally originated from brown algae's intracellular storage (cell wall).9 It is specifically abundant in species belonging to the Laminariaceae family. Historically, laminarin was initially isolated and recognized during the 19th century, sparking scientific concern about its characteristics and promising applications. Presently, laminarin-rich brown algae can be found in diverse species, including Laminaria, Saccharina, and Eisenia. These species are usually distributed across areas in Asia and many European countries.10Generally, Laminarin establishes approximately 35% of the dry weight in diverse macroalgae species, although this proportion can demonstrate variability based on factors like variations in season, the specific species, and the ecological conditions in which they thrive.11 This alteration mainly involves the arrangement of β-glucans associated with (1,3) and (1,6) glycosidic linkages in various proportions.12 The chemical framework of macroalgae laminarin principally includes a linear backbone made up of 20 to 30 residues of β-1,3-linked-D-glucopyranose, complemented by β-1,6-linked-D-glucopyranose shaping branched chains (as depicted in Fig. 3).13,14 The accurate ratios of β-1,3 and β-1,6 linkages in laminarin can differ depending on the source brown algae and have been noticed to vary from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.15 Numerous common laminarin diversities have been determined, each with different structural characteristics. For example, laminarin extracted from Dictyota dichotoma and Sargassum fusiforme generally displays a ratio of 3
1.15 Numerous common laminarin diversities have been determined, each with different structural characteristics. For example, laminarin extracted from Dictyota dichotoma and Sargassum fusiforme generally displays a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between linkages of β-1,3 and β-1,6. In contrast, laminarin sourced from Sargassum duplicatum inclines to have a higher ratio of 6
1 between linkages of β-1,3 and β-1,6. In contrast, laminarin sourced from Sargassum duplicatum inclines to have a higher ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between linkages of β-1,3 and β-1,6.16,17 This structural multifariousness in laminarin, stemming from various algal species and their environments, provides a broad array of promising applications and bioactive characteristics related to this polysaccharide. Laminarin molecules are classified into two different types based on the framework of their reducing ends: M and G chains. M chains finish with 1-O-substituted D-mannitol as the reducing end, while G chains conclude with glucose at the reducing end. In the instance of species of Laminaria and Fucus, a substantial proportion, varying from 40% to 75%, of the reducing end groups are joined to one of the primary hydroxyl groups of D-mannitol.18 The structural properties of laminarin are subject to variations depending on the definite algal species. These variations encircle factors such as the M
1 between linkages of β-1,3 and β-1,6.16,17 This structural multifariousness in laminarin, stemming from various algal species and their environments, provides a broad array of promising applications and bioactive characteristics related to this polysaccharide. Laminarin molecules are classified into two different types based on the framework of their reducing ends: M and G chains. M chains finish with 1-O-substituted D-mannitol as the reducing end, while G chains conclude with glucose at the reducing end. In the instance of species of Laminaria and Fucus, a substantial proportion, varying from 40% to 75%, of the reducing end groups are joined to one of the primary hydroxyl groups of D-mannitol.18 The structural properties of laminarin are subject to variations depending on the definite algal species. These variations encircle factors such as the M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G ratio (sometimes, M chains may be totally absent), the extent of branching, and the degree of polymerization, generally reaching a maximum of around fifty carbohydrate residues, with an average of roughly twenty-five. Rajauria and colleagues determined that the purified laminarin usually falls within the molecular weight ranging between 5.7 and 6.2 kDa.19 Notably, laminarin displays a lower molecular weight in contrast to other seaweed-originated polysaccharides. This lower molecular weight helps with its antioxidant characteristics, principally owing to the existence of carbonyl groups, which perform a role in diminishing lipid peroxidation.
G ratio (sometimes, M chains may be totally absent), the extent of branching, and the degree of polymerization, generally reaching a maximum of around fifty carbohydrate residues, with an average of roughly twenty-five. Rajauria and colleagues determined that the purified laminarin usually falls within the molecular weight ranging between 5.7 and 6.2 kDa.19 Notably, laminarin displays a lower molecular weight in contrast to other seaweed-originated polysaccharides. This lower molecular weight helps with its antioxidant characteristics, principally owing to the existence of carbonyl groups, which perform a role in diminishing lipid peroxidation.
3. Extraction and purification of laminarin
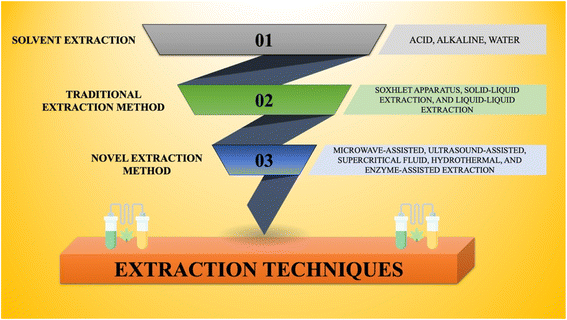
Following the cultivation of microalgae in conditions conducive to the production of high polysaccharides, it becomes vital to optimize the extraction procedure for effectiveness, sustainability, and cost-efficacy. Therefore, the worldwide scientific community should devote more attention to systematic and reliable approaches aimed at improving extraction efficiency. Additionally, in the quest to extract compounds with optimum biomedical potential, it is imperative to set up well-defined purification and structural determination methods.20Considering these factors, the extraction of brown algae polysaccharides entails a systematic procedure consisting of various stages. This involves the initial preparation and pre-treatment of the biomass prior to extraction. Afterward, a combination of conventional and modern extraction approaches is employed. Different extraction techniques have been pictured in Fig. 4. Following extraction, several purification methods are utilized to yield the desired compound.
3.1. Preparation of algal biomass prior to extraction
Preparing the biomass before the extraction of laminarin from brown algae is a pivotal step to ensure an effective extraction procedure. To start, fresh brown algae or acquired dried algae has to be collected, guaranteeing that fresh samples are rinsed completely to eliminate impurities. Afterward, the algae have to be dried employing methods like air drying or controlled-temperature oven drying, which is essential to remove moisture and improve storage stability and extraction effectiveness.21,22 Once dried, the algae should be milled or ground into smaller particles, with the degree of grinding tailored to the chosen extraction procedure. In certain circumstances, homogenization may be utilized to further break down cell walls and develop a uniform sample, which is especially useful for modern extraction approaches like enzymatic extraction or ultrasound-assisted extraction. To sustain the quality of the biomass, it should be stored in a sealed, moisture-free container to avoid absorption until the extraction procedure commences. The definite biomass preparation method may differ based on the selected extraction technique, with the characteristics of the brown algae species and the extraction procedure determining the level of preparation necessary. Assuring cleanliness and adherence to laboratory best practices is also crucial to prevent contamination during the extraction procedure.3.2. Pre-treatment of the biomass before extraction
Diverse pretreatment techniques are employed on the dehydrated algal biomass to remove pigments, proteins, lipids, mannitol, phenols, and other low molecular weight compounds that may be related to the polysaccharide. This includes utilizing a range of solvents and solvent mixtures with various polarities, assuring that they do not cause any structural modifications in the polysaccharide. For instance, in a study by Sellimi et al., the algal biomass was initially subjected to a mixture of solvents, which constituted acetone and methanol in a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v) ratio. Following that, they treated it with chloroform for 24 h at a temperature of 30 °C.23 In another investigation, Menshova et al. reported that the dried and powdered algae underwent a treatment procedure involving 70% aqueous ethanol and acetone at 23 °C for a duration of 10 days. Following this, the defatted algae were dried in air.24
3 (v/v) ratio. Following that, they treated it with chloroform for 24 h at a temperature of 30 °C.23 In another investigation, Menshova et al. reported that the dried and powdered algae underwent a treatment procedure involving 70% aqueous ethanol and acetone at 23 °C for a duration of 10 days. Following this, the defatted algae were dried in air.24
3.3. Conventional extraction techniques for algal polysaccharides
The extraction procedure stands as the primary and crucial step in the isolation of polysaccharides from algae. Therefore, to efficiently extract polysaccharides from the cell walls of various algal species, definite techniques have been utilized to develop conditions conducive to the effective extraction of the required compound.Traditional extraction approaches encompass methods such as Soxhlet extraction, solid–liquid extraction, and liquid–liquid extraction. These conventional techniques rely on the employment of organic solvents, which involve but are not limited to petroleum ether, hexane, cyclohexane, isooctane, toluene, benzene, diethyl ether, dichloromethane, isopropanol, chloroform, acetone, methanol, and ethanol. A prevailing criterion in modern extraction practices is the preference for economical and non-toxic solvents. Among these approaches, Soxhlet extraction has gained prominence owing to its operational simplicity, safety attributes, and scalability, making it an extensively adopted technique.25,26 Solvent extraction has also been utilized for the extraction of laminarin, which is described in further sections.
Though the conventional treatments are simple and straightforward, it is time-consuming and has low yields. Also, severe conditions may cause the degradation of laminarin.
3.4. Modern extraction techniques for algal polysaccharides
Recent progress in technology has paved the route for innovative and novel extraction methods that redefine effectiveness in terms of yield, time, and cost-effectiveness (as depicted in Fig. 5). These cutting-edge approaches are not only focused on optimizing resource usage but also emphasize environmental sustainability by substantially diminishing energy consumption.33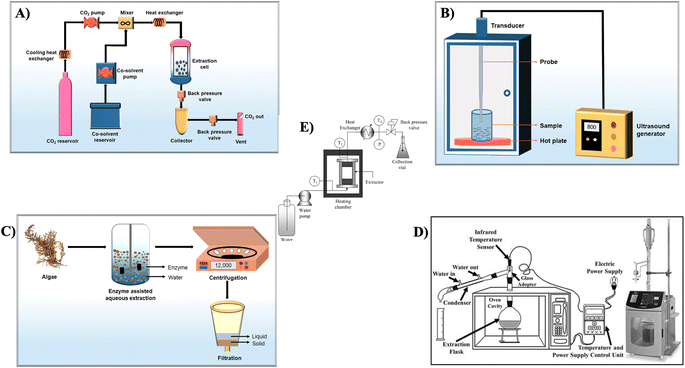 | ||
| Fig. 5 Schematic setup of (A) supercritical fluid extraction, (B) ultrasound-assisted extraction, (C) enzyme-assisted extraction, reproduced with permission from,34 copyright 2022, Springer, (D) microwave-assisted extraction,35 (E) batch-type hydrothermal extraction, reproduced with permission from,36 copyright 2009, Taylor & Francis. | ||
These steps ensure the efficient extraction and isolation of laminarin from brown algae, allowing for its utilization in various applications, including the pharmaceutical, food, and cosmetic industries. The choice of solvent and extraction conditions can be adjusted based on specific requirements and the characteristics of the brown algae source.
3.5. Purification of algal polysaccharides
After the extraction procedure, the polysaccharides are dissolved in a complex mixture with varying monosaccharide constitutions. This mixture also involves trace amounts of proteins and phenolic compounds. Intriguingly, these accompanying constituents may display diverse beneficial biological activities, both in in vitro and in vivo contexts.494. Potential biomedical applications and biological activities of laminarin
4.1. Tissue engineering
Biopolymer-based scaffolds have garnered substantial attention within the domain of tissue engineering, principally attributed to their capability to develop a conducive environment that assists vital cellular functionalities and, consequently, the regeneration of tissues.60–62 Laminarin, an underrated marine-derived polysaccharide originating from brown algae, has acquired increasing attention in the domain of tissue engineering owing to its remarkable characteristics.A fascinating piece of work was reported by Ma et al., who aimed to develop a novel polysaccharide-metal complex (as depicted in Fig. 6a), strontium Laminarin polysaccharide (LP-Sr), with the capability to employ therapeutic impact on the regulation of osteogenesis and angiogenesis, both crucial for the regeneration of bone. Extensive structural and compositional studies of the synthesized LP-Sr were carried out employing various techniques, verifying the successful fabrication of this groundbreaking polysaccharide complex. The outcomes from the biological assays revealed that LP-Sr efficiently improved the proliferation of cells and stimulated the expression of vascular endothelial growth factor (VEGF) and epidermal growth factor-like domain-containing protein 6 (EGFL6) in human umbilical vein endothelial cells (HUVECs) while substantially up-regulating the expression of Col1α1 and osteocalcin in MC3T3-E1 cells. Furthermore, LP-Sr revealed anti-inflammatory characteristics by diminishing levels of pro-inflammatory factor IL6 in both HUVECs and MC3T3-E1 cells. Specifically, the LP-Sr group demonstrated elevated expression of osteogenic and angiogenic markers, particularly alkaline phosphatase (ALP) (as shown in Fig. 6b) and CD31.63 But, the research principally relies on in vitro assays, which may not completely replicate the complex in vivo. Thus, animal studies are vital to comprehend the real-world impact of the polysaccharide complex on the regeneration of bone.
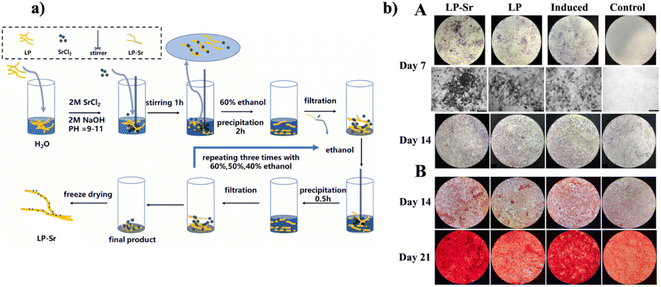 | ||
| Fig. 6 (a) Schematic diagram for the synthesis of LP-Sr (b) osteogenic differentiation of MC3T3-E1 in various sets: (A) depictions of ALP staining on the 7th and 14th days for the control, induced, LP, and LP-Sr groups. (B) Illustrative pictures of Alizarin Red-stained calcium nodules exhibiting the accumulation of extracellular calcium deposits on the 14th and 21st days, reproduced with permission from,63 copyright 2021, Elsevier. | ||
In a separate study by Amaral et al., Laminarin, a β-glucan, underwent functionalization with groups of phenylboronic acid (PBA), inserting chemical reactivity towards diol-containing polymers by esterification of boronate. The developed biopolymer displayed the ability to swiftly form boronate ester-crosslinked hydrogels with poly(vinyl alcohol) (PVA) under a physiological environment within mere seconds. These hydrogels showcased enhanced and easily tailorable rheological characteristics, and notably, they demonstrated a rapid self-healing capability upon mechanical disruption. In addition, the boronate ester bonds assisted the development of hydrogels responsive to reactive oxygen species (ROS) and capable of undergoing shear-thinning, allowing in situ administration and reactivity to the local microenvironment's state of oxidation. Most significantly, owing to their catalyst-free and mild-crosslinking characteristics, the laminarin-PBA/PVA hydrogels displayed no toxicity when it came to direct linkage with preosteoblasts for up to 48 h, interpreting them as a highly favorable platform for applications in tissue engineering and delivery of drugs.64
Laminarin (LA), a polysaccharide with a comparatively low molecular weight, has acquired attention for its potential benefits in tackling skin impairment induced by ultraviolet B (UVB) exposure, although this aspect remains relatively uncharted. In this regard, Ahn et al. focused on delving into the effect of pre-treated LA on histopathological modifications and oxidative damage in the dorsal skin of mice after frequent UVB exposure over a duration of five days. The outcomes revealed that the epidermal thickness was remarkably enhanced in the UVB-exposed group, while the LA-treated UVB group displayed considerably less thickening. Moreover, collagen fiber's density in the dermis was substantially decreased and disrupted in the UVB-exposed group, in contrast with the LA-treated UVB group, where collagen fiber density noticeably increased. Oxidative stress was drastically elevated in the UVB group, whereas the LA-treated UVB group displayed a significant decrease in oxidative stress. In terms of antioxidant enzymes, the UVB group showed a notable decrease in the expressions of superoxide dismutase 1 (SOD1), glutathione peroxidase, and catalase. On the contrary, the LA-treated UVB group showcased considerably elevated levels of these antioxidant enzymes, along with SOD2, in contrast to the control group.65
Carbon-based scaffolds in three-dimensional (3D) structures have achieved significant importance in tissue engineering owing to their extraordinary conductivity and exceptional topological frameworks. For particular in vivo implementations, it is vital to have the scaffold rigid and enhance toughness to endure compression forces from encompassing tissues. Utilizing the benefits of graphene and hydrogels, Hao et al. prepared a composite 3D scaffold comprised of graphene foam (GF) and a laminarin hydrogel (LAgel) (as shown in Fig. 7a). This scaffold was developed by immersing the GF in a precursor solution of LA hydrogel, pursued by exposure to ultraviolet (UV) radiation to persuade the establishment of a photocross-linked LAgel encapsulating the GF. Remarkably, this composite scaffold demonstrated increased toughness in contrast to individual GF or LAgel structures. The 3D GF provided an appropriate environment for the attachment and spreading of cells, assisting the adhesion of human mesenchymal stem cells (hMSCs). Concurrently, the in situ-formed LAgel, tailored with the cell adhesive peptide arginine–glycine–aspartic acid (RGD), promoted the migration of cells. These results suggested that the combination of LAgel with the 3D GF not only improved scaffold toughness but also enabled the delivery of bioactive signals to govern cell behavior, emphasizing the capability of this composite scaffold for tissue regeneration.66 Though the in vitro outcomes showed potential, validation in in vivo models are crucial to confirm the scaffold's efficiency and safety in a more complicated biological environment.
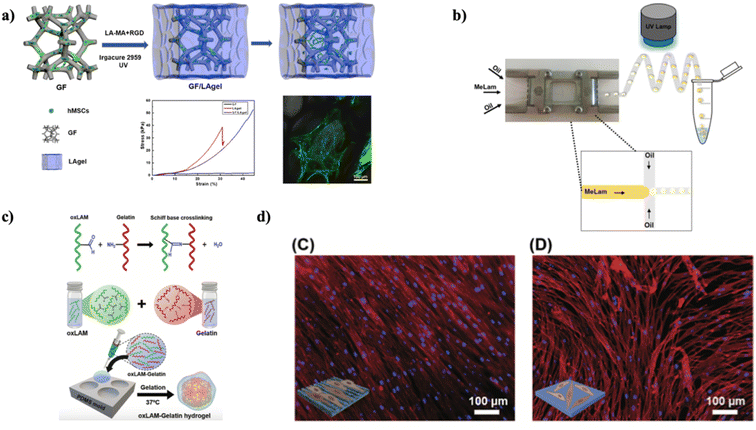 | ||
| Fig. 7 (a) Enhancing scaffold robustness and governing stem cell response via integrating laminarin-based hydrogel with graphene foam, reproduced with permission from,66 copyright 2019, ACS. (b) Diagram illustrating the microfluidic setup utilized for producing MeLam microparticles. Droplets are produced within the microfluidic chip and subsequently crosslinked employing UV light, reproduced with permission from,67 copyright 2018, Elsevier. (c) Illustrations depicting the catalyst-free crosslinking procedure between oxLAM and native gelatin, which occurs spontaneously when these two constituents are combined in an aqueous solution at a temperature of 37 °C, (d) widefield fluorescence microscopy examination of hASCs adhered to (C) nanopatterned and (D) non-patterned surfaces, following a 3 day incubation period, with F-actin labeled with rhodamine (depicted in the red channel) and nuclei staining employing DAPI (shown in the blue channel), reproduced with permission from,68 copyright 2020, Wiley. | ||
In another investigation, Martins et al. successfully prepared a highly effective one-step method for developing nearly uniform MeLam (Methacrylated Laminarin) microparticles integrated with Pluronic F-127 (PL) by utilizing a microfluidics system coupled with a source of UV light (as illustrated in Fig. 7b). The acrylate groups in MeLam also permitted the attachment of thiolated biotin via thiol-Michael addition, permitting further conjugation with RGD peptides. These versatile MeLam microparticles, incorporated with PL, were cultured with L929 cells, revealing their capability to foster cell adhesion and proliferation. MeLam microgels offered significant flexibility in regulating both their structural and chemical characteristics. They could be utilized to imitate diverse tissue environments efficiently. When cultured, these microgels could self-assemble into frameworks with various packing densities, signifying capable applications in tissue engineering and regenerative medicine.67
To recreate the extraordinary characteristics of the viscoelastic extracellular matrix (ECM) in innate human tissues, the design of hydrogel is evolving from conventional covalent crosslinking to more versatile networks with covalent bonds that can modify over time. In this context, Lavrador et al. developed an amine-reactive oxidized-laminarin biopolymer proficient in crosslinking with gelatin (referred to as oxLAM-Gelatin) network (as depicted in Fig. 7c). What's particularly intriguing is that by cautiously adjusting the aldehyde-to-amine ratios in the oxLAM-Gelatin hydrogels, it became feasible to govern the speed of crosslinking precisely, the viscoelastic characteristics, and the degradation behavior. Moreover, these hydrogels offered an exceptional opportunity to imprint definite nano- or microtopographical features onto ECM-like matrices with built-in cell-adhesive elements. These patterns can be readily generated in oxLAM-Gelatin under a physiological environment, and the complex topography stays stable over time. When human adipose-derived MSCs (hASCs) came into contact with mechanically shaped oxLAM-Gelatin hydrogels, they could sense the underlying surface's nanotopography. Consequently, the cells aligned themselves parallelly to the anisotropic nanoridge and nanogroove patterns (as shown in Fig. 6d).68 Based on the outcomes, these systems can be utilized to investigate in greater detail the substantial impact of physical stimuli on guiding cellular responses.
The utilization of bulk hydrogels has revealed substantial constraints in fostering the diffusion of oxygen, vital nutrients, and metabolites. In this regard, Zargarzadeh et al. developed laminarin-based hydrogel and presented a novel strategy for supporting cell culture, where glucose is produced within the hydrogel itself via its degradation, guaranteeing the survival and operation of cells while assisting the growth of tissue. The research demonstrated that both A549 tumor cells and hMSCs could utilize the glucose produced by the hydrogel's degradation to thrive and proliferate, even in media of cell culture without extra supplementation of glucose. Furthermore, the self-sustaining hydrogels exhibited significant potential for sustaining the survival of cells, surpassing the innate cell-laden laminarin hydrogels over a two-week period of implantation. Such scaffolds, equipped with capabilities of enzymatic degradation, have broad applications, including tissue regeneration and cell delivery systems.69
For 3D bioprinting, the preparation of hydrogel bioinks, which is dynamically crosslinked, is obtaining importance as a groundbreaking approach to improve the production of mechanically adjustable cell-laden frameworks for diverse applications in 3D in vitro disease modeling and tissue engineering. In this context, Amaral et al. explored a dynamic bioink containing boronic acid-functionalized laminarin and alginate for the bioprinting of 3D structures under a physiologically appropriate environment. This bioink leveraged a dual crosslinked network, integrating covalent yet reversible bonds of boronate ester and ionic gelation by divalent cations. Remarkably, it possessed favorable rheological characteristics and increased mechanical attributes due to its versatile chemistry of crosslinking, leading to the fabrication of stable frameworks with adaptive architecture. The cell-laden hydrogels generated via bioprinting also exhibited uniform distribution of cells post-printing and extraordinary viability of cells (>90%), which is persistent over prolonged culture periods (up to 14 days) for various cell lines.70 Although the results display potential, it is desirable to make additional refinements to the mechanical characteristics and consider the inclusion of growth factors or bioactive peptides. These adjustments could improve the adhesion and proliferation of cells even further.
4.2. Drug delivery and anti-cancer activities
The combination of drug delivery potential and anti-cancer characteristics makes laminarin a captivating subject for research in the biomedical domain, holding promise for advanced therapeutic approaches.Can et al. introduced a unique and straightforward single-step procedure for the fabrication of micro/nanogels employing poly(LAM) within a reverse micelle microemulsion method (as shown in Fig. 8A). The poly(LAM) particles were produced via the well-established Oxa-Michael addition reaction mechanism, utilizing divinyl sulfone as the crosslinking agent. The resulting poly(LAM) particles displayed spherical shapes and sizes varying from 0.3 to 10 μm, with a magnificent yield of 93 ± 7%. Moreover, these particles were chemically tailorable through functionalization with chlorosulfonic acid, permitting versatility in accommodating diverse agents, like targeting ligands. Both unmodified and modified poly(LAM) particles revealed outstanding blood compatibility, with hemolytic indices below 1% and blood clotting indices surpassing 90%. These findings highlighted the substantial potential of poly(LAM) particles as natural options for biomedical applications, especially in drug delivery systems.71 Devoted significant investigation efforts to the development of p(LAM) particles is essential. Ongoing research includes fine-tuning their size, conducting in vitro and in vivo assays, and examining their targetability, drug loading capacity, and release potentials.
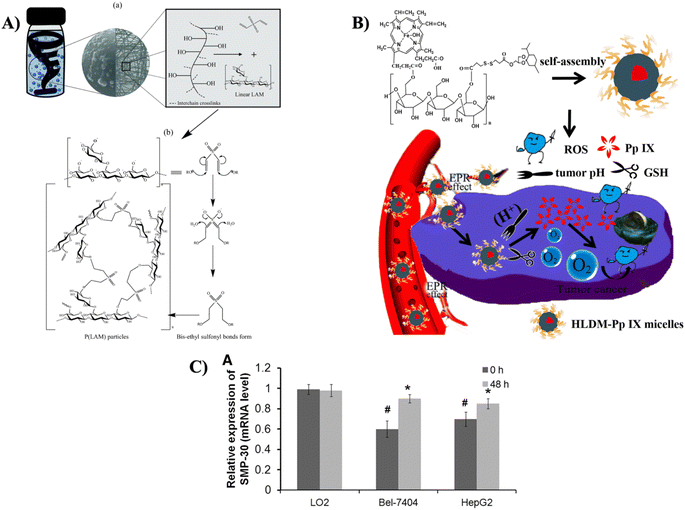 | ||
| Fig. 8 (A) (a) Diagrammatic representation of formation of p(LAM) particle in w/o microemulsion system, and (b) mechanism of particle formation via Oxa-Michael addition reaction, reproduced with permission from,71 copyright 2021, Elsevier; (B) visual representation of laminarin-based nanomedicine (HLDM) designed for delivering a photosensitizer to be employed in tumor therapy;76 (C) the mRNA expression levels of SMP-30 in different cell lines, reproduced with permission from,78 copyright 2020, Mary Ann Liebert. | ||
In a separate investigation, Liu et al. focused on investigating the promising synergistic inhibition of the formation of calcium oxalate (CaOx) crystal via the utilization of Laminarin polysaccharides (LP), specifically degraded LP (DLP) and sulfated DLP (SDLP) (both before and after sulfation), in conjunction with potassium citrate (K3cit). The primary goal was to comprehend how these compounds work together to safeguard renal epithelial cells (HK-2 cells) from the harm caused by CaOx crystals. When DLP or SDLP was mixed synergically with K3cit, the synergistic effect led to either the same amount of calcium oxalate dihydrate (COD) at a reduced concentration or more COD formation at the same concentration, highlighting the improvement observed when these constituents worked together. The synergistic groups enhanced the concentration of soluble Ca2+ ions in the supernatant, increased the surface charge on the surface of CaOx crystals, and efficiently hindered crystal aggregation. Furthermore, cell experiments revealed that the synergistic group substantially decreased the damage caused by nano-COM crystals to HK-2 cells, causing reduced levels of ROS, lower mortality rates, enhanced cell viability, and improved mitochondrial membrane potential. The synergistic groups, with SDLP–K3cit being especially noteworthy, hold potential as promising drugs for hindering the creation of CaOx kidney stones.72 The subsequent investigation could delve into comprehending the mechanism by which the two groups, individually and synergistically, hinder the formation of kidney stones. Investigating whether the synergistic group governs cellular processes via essential signaling pathways would be helpful in uncovering the primary molecular mechanisms. Moreover, it is crucial to validate the noted synergistic effects in vivo through animal tests. This extensive strategy would offer perceptions that could be instrumental in notifying strategies for the prevention and treatment of kidney stone formation.
Brown seaweeds, particularly those of the Phaeophyta (PP) group, are ample sources of beneficial polysaccharides like Laminarin and Fucoidan. Sanniyasi et al. isolated both Laminarin and Fucoidan, with the maximum yields obtained from Padina pavonica (PP) at 4.36% and Stoechospermum marginatum (STM) at 2.32%, respectively. Excluding the report for fucoidan from our side, we decided to focus on Laminarin, which was found to consist of 86.91% carbohydrates. The molecular weight of Laminarin ranged from 3 to 5 kDa. Significantly, Laminarin did not reveal cytotoxicity against Vero cells but demonstrated cytotoxicity against human colon cancer cells (HT-29) with an IC50 of 57 ± 1.2 μg mL−1. The Acriding Orange/Ethidium Bromide assay uncovered apoptosis as the mechanism of cell death induced by Laminarin. These outcomes highlighted the potential of Laminarin sourced from PP as a bioactive compounds for anticancer therapy.73 Future research will be advantageous from interventions involving molecular markers. These markers can assist as crucial indicators to comprehend and manipulate cellular processes, providing beneficial insights into the molecular mechanisms underlying diverse phenomena.
In another study, Remya et al. introduced an eco-friendly and cost-efficient strategy for producing silver nanoparticles (AgNPs) employing laminarin, a polysaccharide derived from Turbinaria ornata. The procedure involved the extraction and purification of laminarin, which was then subjected to comprehensive analysis using various techniques. Subsequently, the AgNPs were generated employing the extracted laminarin, and their characteristics were thoroughly assessed via different methods. The study also evaluated the AgNPs for their capability to scavenge free radicals and assessed their cytotoxicity against retinoblastoma Y79 cell lines in vitro. The outcomes indicated the induction of apoptosis, as revealed by the arrested cell percentage in the G2/M phase determined via flow cytometry. This finding was further substantiated by a DNA fragmentation experiment, which showed the existence of double-strand breaks in the cells. The potential applications of laminarin-based AgNPs could be extended to investigate their molecular mechanisms, opening up prospects for in vivo drug delivery and diverse medical applications in the future.74
To improve the effectiveness of hydrophobic drugs like curcumin (Cur) in anti-cancer treatment, Yu et al. developed a unique dual pH/redox-sensitive carrier biomaterial based on marine laminarin, integrating photodynamic therapy (PDT). This novel material, termed Hematin-Laminarin-Dithiodipropionic Acid-MGK (HLDM), was synthesized and characterized employing 1H-NMR and IR spectroscopy. Cur-incorporated micelles were then generated via a dialysis procedure. HLDM could self-assemble into micelles in water, with a hydrodynamic diameter of 135 ± 15 nm. Remarkably, in vitro release investigation revealed that Cur-loaded HLDM micelles could release up to 80% of the drug in pH- and redox-sensitive surroundings. Moreover, cell studies uncovered that Cur-loaded HLDM micelles demonstrated enhanced cellular uptake and cytotoxicity against MCF-7 cells in contrast to HLDM alone. This multifunctional biomaterial based on marine laminarin has the capability to serve as a drug delivery system with dual pH/redox sensitivity for the treatment of cancer.75
In a separate study, Yu et al. introduced nano-scaled particles based on laminarin conjugates as a platform for the photosensitizer protoporphyrin IX (Pp IX) in PDT for breast cancer cells (MCF-7) of humans (as illustrated in Fig. 8B). The carrier constituent, named Hematin-Laminarin-Dithiodipropionic Acid-MGK (HLDM), is amphiphilic and displays dual sensitivity to pH and redox changes. It also serves as a carrier for incorporating hydrophobic drugs, improving their solubility, and enhancing biocompatibility. In this study, the researchers developed Pp IX-loaded HLDM nanomicelles with an average diameter of 149.3 ± 35 nm in neutral water. Experimental results revealed that these micelles were capable of inducing PDT, which led to the destruction of cancer cells upon exposure to definite wavelength light, as evidenced by their phototoxicity and production of ROS. Apoptosis experiments displayed damage to the nucleus caused by the micelles. In vivo, PDT impact was evaluated employing a tumor-bearing nude mouse model with MCF-7 cells, showing substantial anti-tumor effectiveness.76
A specific oxidation procedure utilizing 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), in combination with NaBr and NaClO, led to the formation of glucoglucuronan (LAO) from Laminarin obtained from Sargassum thunbergii. Compositional analysis of LAO revealed a molar ratio of glucuronic acid (GlcA) to glucose (Glc) at 12.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. LAO's backbone consisted of (1 → 3)-linked β-D-GlcpA interspersed with (1 → 3, 1 → 6)-linked β-D-Glcp, and terminated with β-D-GlcpA. LAO revealed an inhibitory effect on the proliferation of human lung cancer A549 cells in vitro. Western blotting demonstrated up-regulation of TSC2 at an LAO concentration of 3.80 mg mL−1, while FAK, PI3K, P-AKT, and mTOR were down-regulated, signifying LAO's inhibition of proliferation of cancer cells via the FAK/PI3K/AKT/mTOR pathway. LAO also inhibited the binding of heparin to fibroblast growth factor 1 (FGF1). LAO's inhibition of heparin binding to FGF2 fluctuated between 15% and 28%, suggesting that LAO selectively interacted with FGF1, contributing to its inhibitory impact on A549 cell proliferation.77 Describing the strong interaction between LAO and FGF1, but not FGF2 poses a question due to the specific demands of these FGFs. FGF1 requires a heparan sulfate/heparin (HS/HP) with 6-sulfo groups, while FGF2 needs sHP/HS with IdoA2S residues. As a suggested explanation, it is proposed that the glucuronic acid residues of LAO may substitute for the sulfate groups, fostering interactions with both FGF1 and FGF2. To obtain a more profound knowledge of this phenomenon, further experimentation has to be planned, aiming to elucidate the particular molecular mechanisms involved in the interaction between LAO and FGFs.
1. LAO's backbone consisted of (1 → 3)-linked β-D-GlcpA interspersed with (1 → 3, 1 → 6)-linked β-D-Glcp, and terminated with β-D-GlcpA. LAO revealed an inhibitory effect on the proliferation of human lung cancer A549 cells in vitro. Western blotting demonstrated up-regulation of TSC2 at an LAO concentration of 3.80 mg mL−1, while FAK, PI3K, P-AKT, and mTOR were down-regulated, signifying LAO's inhibition of proliferation of cancer cells via the FAK/PI3K/AKT/mTOR pathway. LAO also inhibited the binding of heparin to fibroblast growth factor 1 (FGF1). LAO's inhibition of heparin binding to FGF2 fluctuated between 15% and 28%, suggesting that LAO selectively interacted with FGF1, contributing to its inhibitory impact on A549 cell proliferation.77 Describing the strong interaction between LAO and FGF1, but not FGF2 poses a question due to the specific demands of these FGFs. FGF1 requires a heparan sulfate/heparin (HS/HP) with 6-sulfo groups, while FGF2 needs sHP/HS with IdoA2S residues. As a suggested explanation, it is proposed that the glucuronic acid residues of LAO may substitute for the sulfate groups, fostering interactions with both FGF1 and FGF2. To obtain a more profound knowledge of this phenomenon, further experimentation has to be planned, aiming to elucidate the particular molecular mechanisms involved in the interaction between LAO and FGFs.
In a different investigation, Tian et al. explored the distinct functions of laminarin originating from Laminaria japonica in the context of hepatocellular carcinoma and its possible associations with senescence marker protein-30 (SMP-30). The administration of laminarin for 48 h led to a noteworthy reduction in viability and a dose-dependent enhancement in apoptosis rates of both cells of Bel-7404 and HepG2. In addition, the injection of laminarin resulted in a substantial decrease in tumor volumes (starting on the 10th day) and tumor weights (30 days post-injection) in mice, also succeeding in a dose-dependent pattern. Furthermore, treatment with laminarin at a concentration of 35 mg mL−1 for 48 h considerably upregulated expression of SMP-30 in Bel-7404 and HepG2 cells, although it had no such impact on normal liver cells (LO2) (as depicted in Fig. 8C).78 While the common effects of laminarin on hepatocellular carcinoma (HCC) cells have been examined, the precise impact on cell invasion and migration, along with the metastatic behavior of tumors in vivo, remains vague. Further research is warranted to thoroughly investigate these particular aspects and offer a more extensive understanding of the potential role of laminarin in controlling HCC cell behavior and tumor metastasis.
Over the recent decades, there has been a substantial rise in focus on the prevention of cancer and the investigation of anti-cancer mechanisms, driven by the escalating worldwide cancer mortality rates. Zhu et al. reported that optimized laminarin extracts displayed enhanced cytotoxicity against A549, A431, and Caco-2 carcinoma cells, leading to the death of cancer cells in a manner dependent on both time and dosage. Conversely, these extracts demonstrated decreased cytotoxicity in BEAS-2B normal human bronchial epithelial cells compared to commercial laminarin.42 The optimized extraction and purification techniques displayed in this research were highly scalable. Consequently, future research will concentrate on further refining the method and probing the development of a bio-refinery concept. This will include further optimization steps to improve efficacy and sustainability, laying the cornerstone for extensive applications and potential commercialization of the extracted product.
Ovarian cancer (OC) is a challenging condition to detect in its earlier stages, contributing to the higher mortality rates noticed in the United States. The conventional treatment protocol for OC includes extensive cytoreductive surgery followed by chemotherapy based on platinum compounds. Nonetheless, the progress of chemoresistance often guides relapse in advanced OC patients. Thus, Bae et al., in their study, validated that laminarin inhibited cell proliferation and obstructed cell cycle advancement in OC cells by controlling intracellular signaling pathways. Moreover, laminarin caused cell apoptosis via mechanisms involving DNA fragmentation, the production of reactive oxygen species, the commencement of apoptotic signals, endoplasmic reticulum (ER) stress, the controlling of calcium levels, and the modification of the ER-mitochondria interplay. Significantly, laminarin revealed no cytotoxicity in a zebrafish model, and in a zebrafish xenograft model, it efficiently impeded the growth of OC cells.79 Fig. 9 outlines various mechanisms of cancer prevention attributed to brown algae polysaccharides, as reported in different investigations.
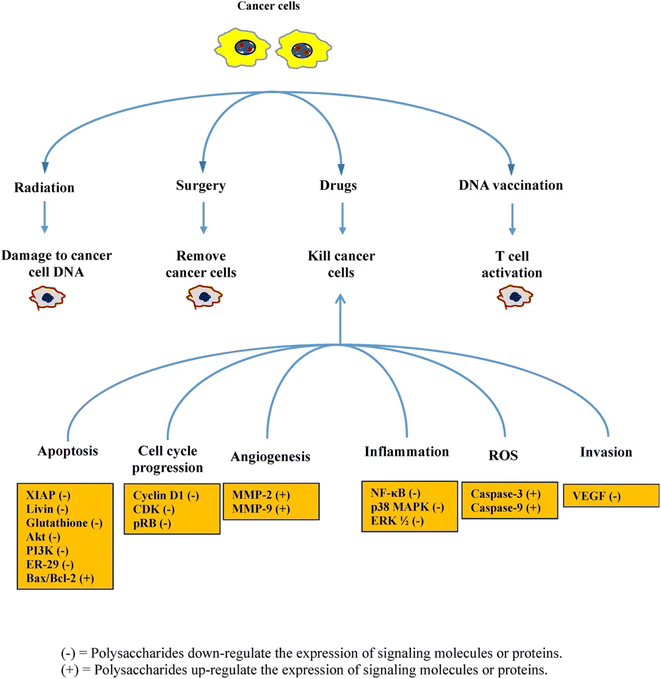 | ||
| Fig. 9 A schematic diagram showing brown-seaweed polysaccharides exhibiting various cancer prevention approaches and crucial cellular cancer-fighting mechanisms, reproduced with permission from,80 copyright 2017, Elsevier. | ||
4.3. Wound healing
Laminarin has appeared as a favorable candidate for applications in wound healing. Its potential lies in its capability to enhance the body's natural healing mechanisms and furnish a conducive environment for the recovery of wounds. Laminarin's engagement in wound healing begins with its capability to foster adhesion, migration, and proliferation of cells. These attributes are vital for the formation of granulation tissue and the re-epithelization of wounds. Laminarin's interaction with fibroblasts and endothelial cells facilitates the deposition of collagen and angiogenesis, which are fundamental procedures in the wound healing cascade. Moreover, laminarin's anti-inflammatory and antioxidant characteristics substantially contribute to its efficiency in wound healing. By decreasing inflammation and combating oxidative stress, laminarin fosters the creation of a suitable microenvironment for the repair of tissue. It aids in reducing complications linked with excessive inflammation and facilitates faster healing. Here, we explore the multifaceted role of laminarin in wound healing and its advantages in this context.Laminarin has displayed potential in the synthesis of AgNPs. Nevertheless, laminarin's innate constraints include its weak reduction potential for metal ions, resulting in the creation of AgNPs with reduced content and larger sizes. To overcome this difficulty, Sharma et al. inserted aldehyde groups to alter laminarin, thereby improving its reduction potential, decreasing the time for synthesis, and enhancing the density of AgNPs. This modification was affirmed via 1H NMR and FT-IR analyses, which established the presence of aldehyde groups on the dialdehyde-modified laminarin (DLAM). As a result, DLAM revealed the capability to promote the rapid, in situ synthesis of ultrasmall-sized spherical AgNPs (less than 10 nm), as proved by TEM images. The aldehyde and carboxyl groups in DLAM performed as reducing and anchoring agents, efficiently converting Ag ions into AgNPs-DLAM. AgNPs-DLAM demonstrated significantly improved antibacterial activity against E. coli and S. aureus in contrast to silver ions, inducing morphological alterations and pore formation in bacterial cells (as shown in Fig. 10A). Furthermore, AgNPs-DLAM revealed the capability to inhibit the formation of bacterial biofilm, while maintaining negligible toxicity towards human keratinocytes. Remarkably, AgNPs-DLAM also encouraged the migration of human keratinocytes, signifying its potential for competent wound healing.81
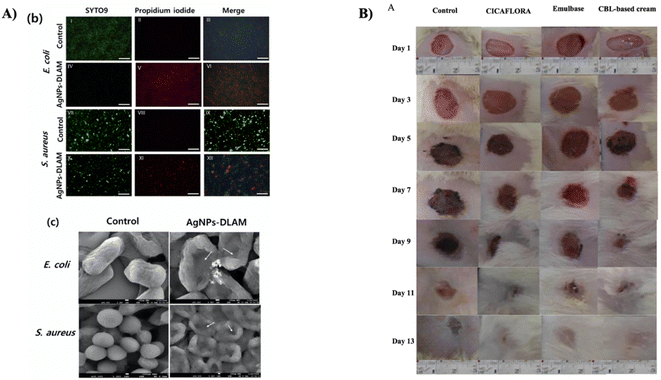 | ||
| Fig. 10 (A) (b) Fluorescence microscopy pictures displaying E. coli and S. aureus cells subjected to treatment with AgNPs-DLAM, stained with SYTO9 (in green, representing live cells) and PI (in red, indicating dead cells), (c) SEM images of E. coli and S. aureus following their exposure to AgNPs-DLAM. The arrows in the images highlight the presence of pores formed in the bacterial cells, reproduced with permission from,81 copyright 2022, Elsevier. (B) Visual illustrations of wound sites in rats over a period of 13 days, where various treatments were applied, which involved sterile physiological serum (control), emulbase, “CICAFLORA” cream, and a cream containing Cystoseira barbata laminarin (CBL), reproduced with permission from,23 copyright 2018, Elsevier. | ||
In another investigation, Amaral et al. developed a methacrylated laminarin-based (LAM-MET) micropatterned hydrogel patch incorporated with drugs and designed for applications such as wound healing. The improved adhesion characteristics are achieved by introducing hydroxypyridinone groups to the LAM-MET material, succeeded by microfabrication of the patch employing soft lithography and UV/vis-irradiation. This results in a membrane featuring micropillars with a high aspect ratio. In line with the biomimetic strategy, a drug patch is tailored by combining the microfabricated dressing with drug particles finely milled to fit the spaces between the pillars. This design allowed for the controlled release of the drug offered inherent antibacterial characteristics against E. coli and P. aeruginosa, and enhanced biocompatibility in contrast to the bare micropatterned patches.82
In a separate study, Sellimi et al. aimed to evaluate the wound-healing potential of a cream formulated with laminarin from the brown seaweed Cystoseira barbata (CBL). The antibacterial and antioxidant characteristics of CBL were assessed, demonstrating notable effects against both Gram-positive and Gram-negative bacteria. The wound coloration study (as depicted in Fig. 10B) showed an initial consistent color indicating the formation of a blood clot during the first three days. In their research, from the 3rd day, the blood clot transformed into a scab, which contracted in treated rats. Nevertheless, control group rats displayed a more substantial inflammatory reaction, with edema and wound oozing. By the 7th day, the scabs gave rise to a red coloration, corresponding to tissue granulation with the wound's expanding edges in control group rats. The CBL-based cream demonstrated substantial wound-healing effectiveness, with contraction of the wound reaching 98.57% after treatment for thirteen days. Histological examination revealed enhanced deposition of collagen, improved fibroblast and vascular densities, and well-ordered dermal tissue in the CBL-treated group in contrast to the control groups.23
In treating melanomas, surgical resection is the traditional method; nevertheless, this strategy often results in disease recurrence. Hence, there is a pressing demand for the progress of scaffold membranes integrating biological agents. In this context, Kim et al. employed an electrospinning technique to develop biocomposite nanofibrous membranes, which are constituted of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), laminarin, and depolymerized laminarin. These membranes displayed an interconnected pore framework, with alterations in pore size concerning the molecular weight of laminarin. The inclusion of hydrophilic depolymerized laminarin substantially decreased the contact angles, improving the hydrophilicity of the membranes. In vitro tests displayed that fibroblasts showed increased proliferation rates on the nanofibrous membranes as the content of depolymerized laminarin rose, principally owing to the antioxidant attributes related to low molecular weight laminarin. On the contrary, the proliferation of melanoma on the nanofibrous membranes was efficiently suppressed owing to enhanced secretion of tumor necrosis factor-α, a consequence of the existence of depolymerized laminarin. These outcomes highlighted the capability of PHBV/depolymerized laminarin biocomposite membranes as favorable biomaterials for applications in wound dressing and as well as cancer therapy.83
4.4. Anti-oxidant, anti-inflammatory, anti-diabetic, and other activities
Laminarin, a β-(1,3)-glucan, holds noteworthy characteristics, including anti-inflammatory and anti-oxidative properties. Nevertheless, its definite impact on human dermal fibroblasts adult (HDFa) and normal human epidermal keratinocytes (NHEK) remains to be interpreted. Thus, Ozanne et al. delved into the effect of laminarin on mitochondrial and antioxidant activities in skin cells. The outcomes unveiled a decrease in mitochondrial activities after 72 hours of treatment with laminarin, beginning at concentrations of 500 μg mL−1 for NHEK cells and 100 μg mL−1 for HDFa cells, all while maintaining cell viability. Hyaluronic acid and type I procollagen levels stayed unaffected across diverse laminarin concentrations. Nevertheless, a pronounced antioxidant effect was noticed at concentrations as low as 1 μg mL−1 for HDFa cells under conditions concerning both H2O2 and UVA radiation, while NHEK cells demonstrated a similar effect at concentrations of 10 μg mL−1 and 1 μg mL−1 under the respective conditions. Moreover, laminarin treatment caused modifications in cell surface glycosylation and cytokine secretions in skin cells.84 Despite the advancements made, additional experiments are essential to determine the optimal concentration of the active compound for efficient availability in the dermis or epidermis. Further research will help tune the application and dosage, ensuring the required therapeutic impacts while reducing possible side effects or limitations.In a different study, Lee et al. delved into the potential impact of topically administering laminarin employing a mouse model of Balb/c of oxazolone-induced atopic dermatitis-like skin lesions. The findings uncovered that the laminarin's topical application to the mice's ears guided substantial developments in the severity of dermatitis, involving a decrease in swelling. Further, a study through histological examination unveiled that topical laminarin efficiently mitigated the thickening of both the epidermis and dermis, along with a reduction in infiltration of mast cells within the skin lesion. Furthermore, serum immunoglobulin E (IgE) levels demonstrated a noteworthy decrease upon topical laminarin treatment. Furthermore, the application of laminarin topically led to the suppression of proinflammatory cytokines, such as interleukin-1β, tumor necrosis factor-α, monocyte chemoattractant protein-1, and macrophage inflammatory protein-1α in the skin lesion-induced by oxazolone.85 Nonetheless, to gain an extensive knowledge of the therapeutic potential of laminarin, more in-depth research is required. These investigations should dive into the particular mechanisms underlying the therapeutic impacts, providing a more detailed picture of the compound's mechanism of action.
A biodegradable and biocompatible microcarrier, sourced from laminarin, a low molecular weight marine polysaccharide known for its biological activity, such as immune modulation and antimicrobial characteristics, is proposed in a study by Castanheira and his colleagues. Through novel modifications of laminarin via click chemistry, controlled-size microparticles were generated (as depicted in Fig. 11a). These microparticles displayed a 40% release of fluorescein isothiocyanate-dextran (70 kDa) after 24 h and complete degradation within 11 days under physiological environment. When tested with human adipose stem and L929 cell lines at microparticle concentrations up to 100 μg mL−1, no cytotoxic effects or disruption to the cell membranes or nuclei were noticed.86
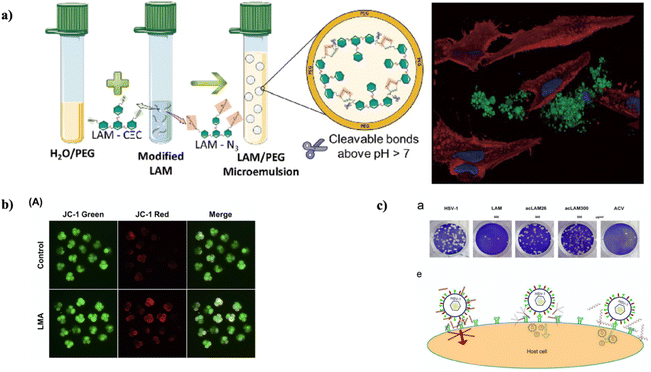 | ||
| Fig. 11 (a) Novel laminarin microparticles were synthesized via a microemulsion procedure, which showed biodegradability and non-cytotoxicity;86 (b) in 4-cell embryos, mitochondrial dysfunction was prevented by LMA. (A) Visualization of 4-cell stage embryos using JC-1 staining (100× magnification), reproduced with permission from,89 copyright 2018, Elsevier; (c) the antiviral activity of LAM and acLAMs was evaluated in a viral plaque assay. {a} Vero cells were infected with HSV-1 in the presence of LAM, acLAMs, or ACV. After infection, the cells were fixed and stained with crystal violet dye, and the number of viral plaques was quantified. {e} Potential mechanisms of action for the antiviral activity of LAM, modified with permission from,94 copyright 2022, Elsevier. | ||
In a separate investigation, Jayapala et al. enzymatically hydrolyzed laminarin utilizing a 0.2 M Hydrochloric acid (HCl) solution, resulting in the production of laminarioligosaccharides. The outcomes from 1H and 13C NMR analysis revealed that laminarioligosaccharides consist of both β(1–6)-associated and β(1–3)-associated glucose. Moreover, the 2 hour hydrolysate displayed a remarkably high reductive capability and total antioxidant activity, similar to 7.1 μg quercetin and 6.7 μg vitamin C, respectively. This hydrolysate also demonstrated significant inhibitory effects, with 42.9% inhibition in the 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) assay and 27.4% inhibition in the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS)) assay. In addition, the 2 hour laminarioligosaccharides displayed exceptional inhibitory effects on the activities of α-amylase (32.2% inhibition) and α-glucosidase (58.8% inhibition). These findings suggested that laminarin's HCl hydrolysis to produce laminarioligosaccharides could be a favorable strategy for obtaining low molecular weight saccharides with improved biological activities, especially in terms of their antioxidant and antidiabetic characteristics.87 Nevertheless, a detailed comprehension of individual hydrolyzed saccharides, involving their characterization, bioavailability, and activity, requires comprehensive investigations.
Laminarinases are enzymes belonging to the family of glycoside hydrolase 16 (GH16) that disrupt β-1,3-glycosidic bonds in laminarin. In a study by Li et al., a laminarinase obtained from the marine Flavobacteriaceae species Tamlana sp. PT2-4 was examined at both structural and functional levels. Utilizing a homology model, the researchers determined a large active groove within Lam1092, which served as a plausible pathway for hydrolyzing bent substrates. To improve its antioxidant characteristics, eight specific residues were picked for mutagenesis, guided by the interactions of Lam1092 with Lam4/Lam6. The investigation resulted in establishing eight Lam1092 mutants, and the hydrolysates produced by two of these mutants (G361A and H466A) displayed significantly improved antioxidant characteristics.88 The elucidation of the properties and catalytic mechanisms of Lam1092, as described in this research, holds the potential to serve as a beneficial reference for identifying novel laminarinases.
In another experiment, Jiang et al. focused on investigating the influence of laminarin (LMA) on the preparation of early-stage embryos of porcine and the underlying mechanisms. The outcomes revealed substantial enhancements in the developmental capability of early-stage embryos of porcine when exposed to LMA in the course of in vitro culture. Notably, the existence of 20 μg mL−1 LMA guided an enhanced rate of cleavage, rates of blastocyst formation, rate of hatching, and total number of cells in the blastocyst in contrast to the control group. LMA also diminished the production of intracellular ROS induced by H2O2. Furthermore, LMA enhanced intracellular levels of glutathione and enhanced membrane potential of mitochondria (as shown in Fig. 11b). In addition, LMA up-regulated the expression of genes related to activation of zygotic genome (YAP1), pluripotency (OCT4, NANOG, and SOX2), and hatching (COX2, GATA4, and ITGA5) during early-stage embryo development of porcine.89
Functional dyspepsia, often attributed to gastrointestinal dysmotility, is a common problem. In So, Liu et al. focused on investigating the regulatory impact of fucoidan and laminarin on mice undergoing functional dyspepsia induced via loperamide. Focusing on laminarin, the findings revealed that it efficiently alleviated the dysfunction by principally influencing gastrointestinal hormones (motilin and ghrelin), the cholinergic pathway, total bile acid levels, expression of c-kit protein, and the expression of a gene associated with contraction of gastric smooth muscle (ANO1 and RYR3). Moreover, the intervention with laminarin had a prominent impact on the composition of the gut microbiota, which involves alterations in the abundance of various species, such as Muribaculaceae, Lachnospiraceae, and Streptococcus.90 The connection between the abundance of these species and their role in the modulating of gastrointestinal motility is still unclear. Speculations concerning the functions of these bacteria remain hypothetical and should be confirmed through further experiments in the future.
In a separate investigation, Li et al. aimed to evaluate the efficiency of two functional zwitterionic laminarins, namely zwitterionic sulfonate (LZS) and zwitterionic carboxylate (LZC), in a mouse model with dextran sulfate sodium (DSS)-induced ulcerative colitis (UC). The results showed that, in contrast to UC mice, the treated mice displayed enhanced composition and diversity of gut microbiota. Remarkably, there was an improvement in Bacteroidetes and a reduction in Firmicutes. Moreover, the study revealed the alleviation of colitis by LZS and LZC, as evidenced by enhanced intestinal mucosa integrity, such as a higher number of goblet cells, increased production of mucin protein, preservation of collagen, and reduced intestinal fibrosis.91
To examine the neuroprotective effects of pre-treated laminarin against ischemia-reperfusion (IR) injury in aged animals and the underlying mechanisms, Park et al. administered laminarin intraperitoneally (at a dose of 50 mg kg−1) to aged gerbils for seven days before subjecting them to IR injury (5 minute transient ischemia). IR injury in gerbils treated with a vehicle caused the death of pyramidal neurons in the region of hippocampal CA1 five days post-IR. Nonetheless, pretreatment with laminarin efficiently shielded the CA1 pyramidal neurons from IR-induced damage. In the laminarin-treated gerbils, the generation of superoxide anions, expression of 4-hydroxy-2-nonenal, and levels of pro-inflammatory cytokines were substantially reduced in the CA1 pyramidal neurons following IR. Furthermore, laminarin treatment notably enhanced the expression of superoxide dismutase and anti-inflammatory cytokines (IL-4 and IL-13) in the CA1 pyramidal neurons both before and after IR.92 While the current results indicate a promising impact, further research is necessary to delve into more specific molecular mechanisms underlying the noticed effects.
In another investigation, Sun et al. uncovered that laminarin demonstrated the ability to hinder inflammation, oxidative stress, and apoptosis in PC12 cells subjected to oxygen-glucose deprivation and reoxygenation (OGD/R). It achieved this by regulating the PTEN/PI3K/AKT pathway. These findings offered fresh perspectives on laminarin's protective mechanisms against cerebral hypoxia and ischemia, suggesting that laminarin holds promise as a treatment alternative for ischemic stroke.93 However, the current research has not confirmed the impact of Laminaria polysaccharides on brain injury in hypoxic mice.
Long et al., in another study, synthesized a unique single helical β-glucan by introducing acetyl groups instead of some of the hydroxyl groups in Laminarin. The resulting single helical conformation displayed remarkable water stability, which was evidenced through Congo red assay depending on induced circular dichroism. The in vitro anti-viral tests revealed the efficient inhibition of replication of herpes simplex virus type 1 by the triple helical acetylated Laminarin (acLMA) (as shown in Fig. 11c). The possible mechanism for anti-viral activity of laminarin has been depicted in Fig. 11c. Remarkably, the unraveling of the triple helix framework, whether partially or entirely, governed to the loss of its anti-viral attributes.94
5. Conclusion and future outlooks
Marine polysaccharides are a diverse class of complex carbohydrates originating from different marine sources, which involve seaweed, microalgae, and other aquatic organisms. Among the various marine sources of these polysaccharides, brown algae portray a prominent category. Laminarin, an adaptable polysaccharide from brown seaweed, has garnered substantial attention in the biomedical domain owing to its extensive range of applications and beneficial properties. It assists as an invaluable resource for diverse biomedical purposes. Laminarin-based scaffolds have been utilized in tissue engineering to provide a biocompatible and biodegradable framework for cell growth and tissue regeneration. These scaffolds foster attachment and proliferation of cells, making them a suitable choice for reconstructive medicine. Laminarin's versatility as a drug delivery carrier is noteworthy. Its novel properties permit for controlled drug release, making it an outstanding candidate for targeted and sustained drug delivery systems, especially for cancer treatment. It can improve the solubility of hydrophobic drugs and enhance their bioavailability. Laminarin-based dressings have also exhibited wound healing potential. These formulations showed antibacterial properties, stimulated collagen production, and enhanced tissue regeneration. They are beneficial for treating chronic wounds and encouraging faster recovery. Laminarin's antioxidant activity is assigned to its capability to scavenge free radicals and decrease oxidative stress. This feature can have applications in avoiding or alleviating oxidative damage in diverse diseases, including neurodegenerative conditions. Laminarin also exhibits anti-inflammatory properties by modulating the immune reaction. It can decrease pro-inflammatory cytokines and alleviate inflammatory conditions, making it a potential therapeutic alternative for diseases marked by chronic inflammation. Though laminarin has shown diverse advantages, the purity of commercially available laminarin may vary, influencing its performance in various applications. The scarcity of preclinical research on laminarin employing in vitro and in vivo studies indeed emphasizes a critical gap in our comprehension of this natural polysaccharide's full potential. In particular, more comprehensive in vitro and in vivo assays can shed light on laminarin's therapeutic characteristics, mechanisms of action, and possible benefits. Further clinical studies can validate these outcomes and pave the way for the progress of new therapeutics based on laminarin. In the ever-evolving landscape of biomedicine, it is imperative that investigators explore the signaling pathways and molecular interactions via which laminarin employs its effects. This will furnish a foundation for growing targeted therapies that leverage the unique characteristics of laminarin, potentially enhancing patient outcomes across different medical conditions. Exploring laminarin's synergistic effects with other biopolymers or drugs to improve its therapeutic potential is also necessary for wound healing, anti-inflammatory treatments, and antioxidant therapies.As we look to the future, fostering collaboration between investigators, clinicians, and industry professionals will be vital in progressing our knowledge of laminarin and harnessing its biomedical potential to its fullest extent.
Abbreviations
| NaOH | Sodium hydroxide |
| EAE | Enzyme-assisted extraction |
| UAE | Ultrasonic-assisted extraction |
| MAE | Microwave-assisted extraction |
| CO2 | Carbon dioxide |
| IEC | Ion-exchange chromatography |
| SEC | Size-exclusion chromatography |
| AC | Affinity chromatography |
| MF | Membrane filtration |
| LP-Sr | Strontium laminarin polysaccharide |
| VEGF | Vascular endothelial growth factor |
| HUVECs | Human umbilical vein endothelial cells |
| EGFL6 | Epidermal growth factor-like domain-containing protein 6 |
| ALP | Alkaline phosphatase |
| PBA | Phenylboronic acid |
| PVA | Poly(vinyl alcohol) |
| ROS | Reactive oxygen species |
| LA | Laminarin |
| UVB | Ultraviolet B |
| SOD1 | Superoxide dismutase 1 |
| 3D | Three-dimensional |
| GF | Graphene foam |
| LAgel | Laminarin hydrogel |
| hMSCs | Human mesenchymal stem cells |
| RGD | Arginine–glycine–aspartic acid |
| MeLam | Methacrylated laminarin |
| PL | Pluronic F-127 |
| ECM | Extracellular matrix |
| oxLAM | Oxidized laminarin |
| hASCs | Human adipose-derived MSCs |
| CaOx | Calcium oxalate |
| LP | Laminarin polysaccharides |
| DLP | Degraded laminarin polysaccharides |
| SDLP | Sulphated degraded laminarin polysaccharides |
| K3cit | Potassium citrate |
| COD | Calcium oxalate dihydrate |
| PP | Padina pavonica |
| AgNPs | Silver nanoparticles |
| Cur | Curcumin |
| PDT | Photodynamic therapy |
| HLDM | Hematin-laminarin-dithiodipropionic acid-MGK |
| Pp IX | Protoporphyrin IX |
| LAO | Glucoglucuronan |
| GlcA | Glucuronic acid |
| Glc | Glucose |
| OC | Ovarian cancer |
| ER | Endoplasmic reticulum |
| DLAM | Dialdehyde-modified laminarin |
| LAM-MET | Methacrylated laminarin |
| CBL | Cystoseira barbata |
| PHBV | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| HDFa | Human dermal fibroblasts adult |
| NHEK | Normal human epidermal keratinocytes |
| HCl | Hydrochloric acid |
| LMA | Laminarin |
| LZS | Zwitterionic sulfonate |
| LZC | Zwitterionic carboxylate |
| DSS | Dextran sulfate sodium |
| UC | Ulcerative colitis |
| IR | Ischemia-reperfusion |
| acLMA | Acetylated laminarin |
| FGF-1 | Fibroblast growth factor-1 |
| HS/HP | Sulfate/heparin |
Author contributions
Conceptualization and supervision: Sheersha Pramanik, Stefano Bellucci; resources: Sheersha Pramanik, B. M. Abualsoud, Armen S. Sargsyan, Pankaj Nainwal, A. Deepak; literature review and writing—original draft preparation: Sheersha Pramanik, Anshul Singh, B. M. Abualsoud, A. Deepak, writing—review and editing: Sheersha Pramanik, A. Deepak, B. M. Abualsoud, Pankaj Nainwal, Armen S. Sargsyan, Stefano Bellucci. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors extend their appreciation to the Higher Education and Science Committee RA for funding this work, in the framework of the research project No. 21T-2I235. Author SP would like to thank Indian Institute of technology, Madras for providing financial assistantship and resources.References
- A. Usman, S. Khalid, A. Usman, Z. Hussain and Y. Wang, Chapter 5 – Algal polysaccharides, novel application, and outlook, in Algae Based Polymers, Blends, and Composites, ed. Zia K. M., Zuber M. and Ali M., Elsevier, 2017, pp. 115–153, ISBN 978-0-12-812360-7 Search PubMed.
- S. Pramanik, S. Kharche, N. More, D. Ranglani, G. Singh and G. Kapusetti, Natural Biopolymers for Bone Tissue Engineering: A Brief Review, Eng. Regen., 2023, 4, 193–204, DOI:10.1016/j.engreg.2022.12.002.
- M. J. Cardoso, R. R. Costa and J. F. Mano, Marine Origin Polysaccharides in Drug Delivery Systems, Mar. Drugs, 2016, 14(2), 34, DOI:10.3390/md14020034.
- S. Nigam, R. Singh, S. K. Bhardwaj, R. Sami, M. P. Nikolova, M. Chavali and S. Sinha, Perspective on the Therapeutic Applications of Algal Polysaccharides, J. Polym. Environ., 2022, 30, 785–809, DOI:10.1007/s10924-021-02231-1.
- Y. Peng, J. Hu, B. Yang, X.-P. Lin, X.-F. Zhou, X.-W. Yang and Y. Liu, Chapter 5 – Chemical composition of seaweeds, in Seaweed Sustainability, ed. Tiwari B. K. and Troy D. J., Academic Press, San Diego, 2015, pp. 79–124, ISBN 978-0-12-418697-2 Search PubMed.
- L.-E. Rioux and S. L. Turgeon, Chapter 7 – Seaweed carbohydrates, in Seaweed Sustainability, ed. Tiwari B. K. and Troy D. J., Academic Press, San Diego, 2015, pp. 141–192, ISBN 978-0-12-418697-2 Search PubMed.
- A. J. Smith, B. Graves, R. Child, P. J. Rice, Z. Ma, D. W. Lowman, H. E. Ensley, K. T. Ryter, J. T. Evans and D. L. Williams, Immunoregulatory Activity of the Natural Product Laminarin Varies Widely as a Result of Its Physical Properties, J. Immunol., 2018, 200, 788–799, DOI:10.4049/jimmunol.1701258.
- D. Cui, J. Ma, T. Liang, L. Sun, L. Meng, T. Liang and Q. Li, Selenium Nanoparticles Fabricated in Laminarin Polysaccharides Solutions Exert Their Cytotoxicities in HepG2 Cells by Inhibiting Autophagy and Promoting Apoptosis, Int. J. Biol. Macromol., 2019, 137, 829–835, DOI:10.1016/j.ijbiomac.2019.07.031.
- Z. Liu, Y. Xiong, L. Yi, R. Dai, Y. Wang, M. Sun, X. Shao, Z. Zhang and S. Yuan, Endo-β-1,3-Glucanase Digestion Combined with the HPAEC-PAD-MS/MS Analysis Reveals the Structural Differences between Two Laminarins with Different Bioactivities, Carbohydr. Polym., 2018, 194, 339–349, DOI:10.1016/j.carbpol.2018.04.044.
- S. Becker, J. Tebben, S. Coffinet, K. Wiltshire, M. H. Iversen, T. Harder, K.-U. Hinrichs and J.-H. Hehemann, Laminarin Is a Major Molecule in the Marine Carbon Cycle, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 6599–6607, DOI:10.1073/pnas.1917001117.
- L. O'Sullivan, B. Murphy, P. McLoughlin, P. Duggan, P. G. Lawlor, H. Hughes and G. E. Gardiner, Prebiotics from Marine Macroalgae for Human and Animal Health Applications, Mar. Drugs, 2010, 8, 2038–2064 CrossRef PubMed.
- J. Chen, J. Yang, H. Du, M. Aslam, W. Wang, W. Chen, T. Li, Z. Liu and X. Liu, Laminarin, a Major Polysaccharide in Stramenopiles, Mar. Drugs, 2021, 19(10), 576 CrossRef CAS PubMed.
- O. K. Lee and E. Y. Lee, Sustainable Production of Bioethanol from Renewable Brown Algae Biomass, Biomass Bioenergy, 2016, 92, 70–75, DOI:10.1016/j.biombioe.2016.03.038.
- M. Zargarzadeh, A. J. R. Amaral, C. A. Custódio and J. F. Mano, Biomedical Applications of Laminarin, Carbohydr. Polym., 2020, 232, 115774, DOI:10.1016/j.carbpol.2019.115774.
- S. Becker, A. Scheffel, M. F. Polz and J. H. Hehemann, Accurate Quantification of Laminarin in Marine Organic Matter with Enzymes from Marine Microbes, Appl. Environ. Microbiol., 2017, 83(9), e03389, DOI:10.1128/AEM.03389-16.
- O. S. Malyarenko, R. V. Usoltseva, T. N. Zvyagintseva and S. P. Ermakova, Laminaran from Brown Alga Dictyota Dichotoma and Its Sulfated Derivative as Radioprotectors and Radiosensitizers in Melanoma Therapy, Carbohydr. Polym., 2019, 206, 539–547, DOI:10.1016/j.carbpol.2018.11.008.
- R. Zhang, X. Zhang, Y. Tang and J. Mao, Composition, Isolation, Purification and Biological Activities of Sargassum Fusiforme Polysaccharides: A Review, Carbohydr. Polym., 2020, 228, 115381, DOI:10.1016/j.carbpol.2019.115381.
- M. Rinaudo, 2.21 – Seaweed polysaccharides, in Comprehensive Glycoscience, ed. Kamerling H., Elsevier, Oxford, 2007, pp. 691–735, ISBN 978-0-444-51967-2 Search PubMed.
- G. Rajauria, R. Ravindran, M. Garcia-Vaquero, D. K. Rai, T. Sweeney and J. O'Doherty, Molecular Characteristics and Antioxidant Activity of Laminarin Extracted from the Seaweed Species Laminaria Hyperborea, Using Hydrothermal-Assisted Extraction and a Multi-Step Purification Procedure, Food Hydrocolloids, 2021, 112, 106332, DOI:10.1016/j.foodhyd.2020.106332.
- L. Yang and L.-M. Zhang, Chemical Structural and Chain Conformational Characterization of Some Bioactive Polysaccharides Isolated from Natural Sources, Carbohydr. Polym., 2009, 76, 349–361, DOI:10.1016/j.carbpol.2008.12.015.
- M. Garcia-Vaquero, G. Rajauria, J. V. O'Doherty and T. Sweeney, Polysaccharides from Macroalgae: Recent Advances, Innovative Technologies and Challenges in Extraction and Purification, Food Res. Int., 2017, 99, 1011–1020, DOI:10.1016/j.foodres.2016.11.016.
- M. S. G. Mohan, A. Achary, V. Mani, E. Cicinskas, A. A. Kalitnik and M. Khotimchenko, Purification and Characterization of Fucose-Containing Sulphated Polysaccharides from Sargassum Tenerrimum and Their Biological Activity, J. Appl. Phycol., 2019, 31, 3101–3113, DOI:10.1007/s10811-019-01797-7.
- S. Sellimi, H. Maalej, D. M. Rekik, A. Benslima, G. Ksouda, M. Hamdi, Z. Sahnoun, S. Li, M. Nasri and M. Hajji, Antioxidant, Antibacterial and in Vivo Wound Healing Properties of Laminaran Purified from Cystoseira Barbata Seaweed, Int. J. Biol. Macromol., 2018, 119, 633–644, DOI:10.1016/j.ijbiomac.2018.07.171.
- R. V. Menshova, S. P. Ermakova, B. H. Um and T. N. Zvyagintseva, The Composition and Structural Characteristics of Polysaccharides of the Brown Alga Eisenia Bicyclis, Russ. J. Mar. Biol., 2013, 39, 208–213, DOI:10.1134/S1063074013030103.
- K. Ramluckan, K. G. Moodley and F. Bux, An Evaluation of the Efficacy of Using Selected Solvents for the Extraction of Lipids from Algal Biomass by the Soxhlet Extraction Method, Fuel, 2014, 116, 103–108, DOI:10.1016/j.fuel.2013.07.118.
- I. Michalak and K. Chojnacka, Algal Extracts: Technology and Advances, Eng. Life Sci., 2014, 14, 581–591 CrossRef CAS.
- K. H. Kim, Y. W. Kim, H. B. Kim, B. J. Lee and D. S. Lee, Anti-Apoptotic Activity of Laminarin Polysaccharides and Their Enzymatically Hydrolyzed Oligosaccharides from Laminaria Japonica, Biotechnol. Lett., 2006, 28, 439–446, DOI:10.1007/s10529-005-6177-9.
- P. S. Birgersson, M. Oftebro, W. I. Strand, O. A. Aarstad, G. I. Sætrom, H. Sletta, Ø. Arlov and F. L. Aachmann, Sequential Extraction and Fractionation of Four Polysaccharides from Cultivated Brown Algae Saccharina Latissima and Alaria Esculenta, Algal Res., 2023, 69, 102928, DOI:10.1016/j.algal.2022.102928.
- L. Allahgholi, R. R. R. Sardari, S. Hakvåg, K. Z. G. Ara, T. Kristjansdottir, I. M. Aasen, O. H. Fridjonsson, T. Brautaset, G. O. Hreggvidsson and E. N. Karlsson, Composition Analysis and Minimal Treatments to Solubilize Polysaccharides from the Brown Seaweed Laminaria Digitata for Microbial Growth of Thermophiles, J. Appl. Phycol., 2020, 32, 1933–1947, DOI:10.1007/s10811-020-02103-6.
- K. H. Kim, Y. W. Kim, H. B. Kim, B. J. Lee and D. S. Lee, Anti-Apoptotic Activity of Laminarin Polysaccharides and Their Enzymatically Hydrolyzed Oligosaccharides from Laminaria Japonica, Biotechnol. Lett., 2006, 28, 439–446, DOI:10.1007/s10529-005-6177-9.
- D. F. Rocher, R. A. Cripwell and M. Viljoen-Bloom, Engineered Yeast for Enzymatic Hydrolysis of Laminarin from Brown Macroalgae, Algal Res., 2021, 54, 102233, DOI:10.1016/j.algal.2021.102233.
- S. H. S. Sharma, G. Lyons, C. McRoberts, D. McCall, E. Carmichael, F. Andrews, R. Swan, R. McCormack and R. Mellon, Biostimulant Activity of Brown Seaweed Species from Strangford Lough: Compositional Analyses of Polysaccharides and Bioassay of Extracts Using Mung Bean (Vigno Mungo L.) and Pak Choi (Brassica Rapa Chinensis L.), J. Appl. Phycol., 2012, 24, 1081–1091, DOI:10.1007/s10811-011-9737-5.
- S. U. Kadam, B. K. Tiwari and C. P. O'Donnell, Application of Novel Extraction Technologies for Bioactives from Marine Algae, J. Agric. Food Chem., 2013, 61, 4667–4675, DOI:10.1021/jf400819p.
- S. Nigam, R. Singh, S. K. Bhardwaj, R. Sami, M. P. Nikolova, M. Chavali and S. Sinha, Perspective on the Therapeutic Applications of Algal Polysaccharides, J. Polym. Environ., 2022, 30, 785–809, DOI:10.1007/s10924-021-02231-1.
- C. Castro-López, R. Rojas, E. J. Sánchez-Alejo, G. Niño-Medina and G. C. G. Martínez-Ávila, Phenolic compounds recovery from grape fruit and by-products: an overview of extraction methods, in Grape and Wine Biotechnology, ed. Morata A. and Loira I., IntechOpen, Rijeka, 2016, ch. 5, ISBN 978-953-51-2693-5 Search PubMed.
- K. Kitada, S. Machmudah, M. Sasaki, M. Goto, Y. Nakashima, S. Kumamoto and T. Hasegawa, Antioxidant and Antibacterial Activity of Nutraceutical Compounds from Chlorella Vulgaris Extracted in Hydrothermal Condition, Sep. Sci. Technol., 2009, 44, 1228–1239, DOI:10.1080/01496390902729056.
- G. Kulshreshtha, A. S. Burlot, C. Marty, A. Critchley, J. Hafting, G. Bedoux, N. Bourgougnon and B. Prithiviraj, Enzyme-Assisted Extraction of Bioactive Material from Chondrus Crispus and Codium Fragile and Its Effect on Herpes Simplex Virus (HSV-1), Mar. Drugs, 2015, 13, 558–580, DOI:10.3390/md13010558.
- I. Michalak and K. Chojnacka, Algal Extracts: Technology and Advances, Eng. Life Sci., 2014, 14, 581–591, DOI:10.1002/elsc.201400139.
- E. J. Kim, A. Fathoni, G.-T. Jeong, H. Do Jeong, T.-J. Nam, I.-S. Kong and J. K. Kim, Microbacterium Oxydans, a Novel Alginate- and Laminarin-Degrading Bacterium for the Reutilization of Brown-Seaweed Waste, J. Environ. Manage., 2013, 130, 153–159, DOI:10.1016/j.jenvman.2013.08.064.
- D. Van Breda, R. Lufu and N. J. Goosen, Optimisation of Cellulase-Assisted Extraction of Laminarin from the Brown Seaweed Ecklonia Maxima, Using Response Surface Methodology, Biomass Convers. Biorefin., 2023, 13, 10399–10412, DOI:10.1007/s13399-021-01985-x.
- E. Ibañez, M. Herrero, J. A. Mendiola and M. Castro-Puyana, Extraction and characterization of bioactive compounds with health benefits from marine resources: macro and micro algae, cyanobacteria, and invertebrates, in Marine Bioactive Compounds: Sources, Characterization and Applications, ed. Hayes M., Springer US, Boston, MA, 2012, pp. 55–98, ISBN 978-1-4614-1247-2 Search PubMed.
- X. Zhu, L. Healy, J. Wanigasekara, M. Zhao, R. B. Padamati, S. Karuppusamy, J. F. Curtin, S. P. Sivagnanam, D. K. Rai and D.-W. Sun, et al., Characterisation of Laminarin Extracted from Brown Seaweed Laminaria Digitata, Using Optimized Ultrasound- and Ultrafiltration-Assisted Extraction Method, Algal Res., 2023, 75, 103277, DOI:10.1016/j.algal.2023.103277.
- S. U. Kadam, C. P. O'Donnell, D. K. Rai, M. B. Hossain, C. M. Burgess, D. Walsh and B. K. Tiwari, Laminarin from Irish Brown Seaweeds Ascophyllum Nodosum and Laminaria Hyperborea: Ultrasound Assisted Extraction, Characterization and Bioactivity, Mar. Drugs, 2015, 13, 4270–4280, DOI:10.3390/md13074270.
- J.-L. Pan, H.-M. Wang, C.-Y. Chen and J.-S. Chang, Extraction of Astaxanthin from Haematococcus Pluvialis by Supercritical Carbon Dioxide Fluid with Ethanol Modifier, Eng. Life Sci., 2012, 12, 638–647, DOI:10.1002/elsc.201100157.
- C. Crampon, O. Boutin and E. Badens, Supercritical Carbon Dioxide Extraction of Molecules of Interest from Microalgae and Seaweeds, Ind. Eng. Chem. Res., 2011, 50, 8941–8953, DOI:10.1021/ie102297d.
- E. Ibañez, M. Herrero, J. A. Mendiola and M. Castro-Puyana, Extraction and characterization of bioactive compounds with health benefits from marine resources: macro and micro algae, cyanobacteria, and invertebrates, in Marine Bioactive Compounds: Sources, Characterization and Applications, ed. Hayes M., Springer US, Boston, MA, 2012, pp. 55–98, ISBN 978-1-4614-1247-2 Search PubMed.
- S. U. Kadam, B. K. Tiwari and C. P. O'Donnell, Application of Novel Extraction Technologies for Bioactives from Marine Algae, J. Agric. Food Chem., 2013, 61, 4667–4675, DOI:10.1021/jf400819p.
- M. Garcia-Vaquero, J. V. O'Doherty, B. K. Tiwari, T. Sweeney and G. Rajauria, Enhancing the Extraction of Polysaccharides and Antioxidants from Macroalgae Using Sequential Hydrothermal-Assisted Extraction Followed by Ultrasound and Thermal Technologies, Mar. Drugs, 2019, 17(8), 457, DOI:10.3390/md17080457.
- M. T. Ale, J. D. Mikkelsen and A. S. Meyer, Designed Optimization of a Single-Step Extraction of Fucose-Containing Sulfated Polysaccharides from Sargassum Sp, J. Appl. Phycol., 2012, 24, 715–723, DOI:10.1007/s10811-011-9690-3.
- P. M. Cummins, O. Dowling and B. F. O'Connor, Ion-exchange chromatography: basic principles and application to the partial purification of soluble mammalian prolyl oligopeptidase, in Protein Chromatography: Methods and Protocols, ed. Walls D. and Loughran S. T., Humana Press, Totowa, NJ, 2011, pp. 215–228, ISBN 978-1-60761-913-0 Search PubMed.
- P. N. Nesterenko, Chapter 18 – Ion chromatography, in Liquid Chromatography, ed. Fanali S., Chankvetadze B., Haddad P. R., Poole C. F. and Riekkola M.-L., Elsevier, 3rd edn, 2023, vol. 1, pp. 465–507, ISBN 978-0-323-99968-7 Search PubMed.
- A. M. Striegel, Chapter 19 – Size-exclusion chromatography, in Liquid Chromatography, ed. Fanali S., Chankvetadze B., Haddad P. R., Poole C. F. and Riekkola M.-L., Elsevier, 3rd edn, 2023, vol. 1, pp. 509–537, ISBN 978-0-323-99968-7 Search PubMed.
- E. Lubomirsky, A. Khodabandeh, J. Preis, M. Susewind, T. Hofe, E. F. Hilder and R. D. Arrua, Polymeric Stationary Phases for Size Exclusion Chromatography: A Review, Anal. Chim. Acta, 2021, 1151, 338244, DOI:10.1016/j.aca.2021.338244.
- M. Sterner and F. Gröndahl, Extraction of Laminarin from Saccharina Latissima Seaweed Using Cross-Flow Filtration, J. Appl. Phycol., 2021, 33, 1825–1844, DOI:10.1007/s10811-021-02398-z.
- D. S. Hage and R. Matsuda, Affinity chromatography: a historical perspective, in Affinity Chromatography: Methods and Protocols, ed. Reichelt S., Springer New York, New York, NY, 2015, pp. 1–19, ISBN 978-1-4939-2447-9 Search PubMed.
- E. L. Rodriguez, S. Poddar, S. Iftekhar, K. Suh, A. G. Woolfork, S. Ovbude, A. Pekarek, M. Walters, S. Lott and D. S. Hage, Affinity Chromatography: A Review of Trends and Developments over the Past 50 Years, J. Chromatogr. B, 2020, 1157, 122332, DOI:10.1016/j.jchromb.2020.122332.
- D. J. Brose, M. Dosmar and M. W. Jornitz, Membrane filtration, in Development and Manufacture of Protein Pharmaceuticals, ed. Nail S. L. and Akers M. J., Springer US, Boston, MA, 2002, pp. 213–279, ISBN 978-1-4615-0549-5 Search PubMed.
- C. E. Askew, S. te Poele and F. Skou, Membrane filtration, in Cleaning-in-Place, 2008, pp. 195–222, ISBN 9781444302240 Search PubMed.
- X. Zhu, L. Healy, R. S. Das, M. L. Bhavya, S. Karuppusamy, D.-W. Sun, C. O'Donnell and B. K. Tiwari, Novel Biorefinery Process for Extraction of Laminarin, Alginate and Protein from Brown Seaweed Using Hydrodynamic Cavitation, Algal Res., 2023, 74, 103243, DOI:10.1016/j.algal.2023.103243.
- M. A. Abourehab, R. R. Rajendran, A. Singh, S. Pramanik, P. Shrivastav, M. J. Ansari, R. Manne, L. S. Amaral and A. Deepak, Alginate as a promising biopolymer in drug delivery and wound healing: a review of the state-of-the-art, Int. J. Mol. Sci., 2022, 23(16), 9035 CrossRef CAS PubMed.
- M. A. S. Abourehab, S. Pramanik, M. A. Abdelgawad, B. M. Abualsoud, A. Kadi, M. J. Ansari and A. Deepak, Recent Advances of Chitosan Formulations in Biomedical Applications, Int. J. Mol. Sci., 2022, 23(18), 10975 CrossRef CAS PubMed.
- M. A. S. Abourehab, S. Baisakhiya, A. Aggarwal, A. Singh, M. A. Abdelgawad, A. Deepak, M. J. Ansari and S. Pramanik, Chondroitin Sulfate-Based Composites: A Tour d’horizon of Their Biomedical Applications, J. Mater. Chem. B, 2022, 10(44), 9125–9178, 10.1039/D2TB01514E.
- F. Ma, Y. Zhang, L. Hu, Y. Peng, Y. Deng, W. He, Y. Ge and B. Tang, Strontium Laminarin Polysaccharide Modulates Osteogenesis-Angiogenesis for Bone Regeneration, Int. J. Biol. Macromol., 2021, 181, 452–461, DOI:10.1016/j.ijbiomac.2021.03.136.
- A. J. R. Amaral, V. M. Gaspar and J. F. Mano, Responsive Laminarin-Boronic Acid Self-Healing Hydrogels for Biomedical Applications, Polym. J., 2020, 52, 997–1006, DOI:10.1038/s41428-020-0348-3.
- J. H. Ahn, D. Kim, C. W. Park, B. Kim, H. Sim, H. S. Kim, T. K. Lee, J. C. Lee, G. E. Yang, Y. Her and J. H. Park, Laminarin Attenuates Ultraviolet-Induced Skin Damage by Reducing Superoxide Anion Levels and Increasing Endogenous Antioxidants in the Dorsal Skin of Mice, Mar. Drugs, 2020, 18(7), 345, DOI:10.3390/md18070345.
- L. Feng, Y. Hao, M. Zhu, Y. Zhai, L. Yang, Y. Liu and G. Cheng, Incorporation of Laminarin-Based Hydrogel with Graphene Foam To Enhance the Toughness of Scaffold and Regulate the Stem Cell Behavior, ACS Biomater. Sci. Eng., 2019, 5, 5295–5304, DOI:10.1021/acsbiomaterials.9b00752.
- C. R. Martins, C. A. Custódio and J. F. Mano, Multifunctional Laminarin Microparticles for Cell Adhesion and Expansion, Carbohydr. Polym., 2018, 202, 91–98, DOI:10.1016/j.carbpol.2018.08.029.
- P. Lavrador, V. M. Gaspar and J. F. Mano, Mechanochemical Patternable ECM-Mimetic Hydrogels for Programmed Cell Orientation, Adv. Healthcare Mater., 2020, 9, 1901860, DOI:10.1002/adhm.201901860.
- M. Zargarzadeh, A. S. Silva, C. Nunes, M. A. Coimbra, C. A. Custódio and J. F. Mano, Self-Glucose Feeding Hydrogels by Enzyme Empowered Degradation for 3D Cell Culture, Mater. Horiz., 2022, 9, 694–707, 10.1039/D0MH01982H.
- A. J. R. Amaral, V. M. Gaspar, P. Lavrador and J. F. Mano, Double Network Laminarin-Boronic/Alginate Dynamic Bioink for 3D Bioprinting Cell-Laden Constructs, Biofabrication, 2021, 13, 035045, DOI:10.1088/1758-5090/abfd79.
- M. Can and N. Sahiner, A Facile One-Pot Synthesis of Microgels and Nanogels of Laminarin for Biomedical Applications, J. Colloid Interface Sci., 2021, 588, 40–49, DOI:10.1016/j.jcis.2020.12.053.
- J.-H. Liu and J.-M. Ouyang, Synergistic Inhibition of Calcium Oxalate Crystal Formation and Synergistic Protection of HK-2 Cells from Crystal Damage by Sulfated Laminarin Polysaccharide and Potassium Citrate, Biomater. Sci., 2023, 11, 3524–3546, 10.1039/D3BM00087G.
- E. Sanniyasi, R. K. Gopal, R. Damodharan, A. Arumugam, M. Sampath Kumar, N. Senthilkumar and M. Anbalagan, In Vitro Anticancer Potential of Laminarin and Fucoidan from Brown Seaweeds, Sci. Rep., 2023, 13, 14452, DOI:10.1038/s41598-023-41327-7.
- R. R. Remya, S. R. R. Rajasree, T. Y. Suman, L. Aranganathan, S. Gayathri, M. Gobalakrishnan and M. G. Karthih, Laminarin Based AgNPs Using Brown Seaweed Turbinaria Ornata and Its Induction of Apoptosis in Human Retinoblastoma Y79 Cancer Cell Lines, Mater. Res. Express, 2018, 5, 035403, DOI:10.1088/2053-1591/aab2d8.
- Y. Yu, S. Zou, K. Wang, R. Liang, X. Fan, B. Wang, M. Liu, L. Fang, W. Liu and Z. Wu, et al., Synthesis, Characterization and in Vitro Evaluation of Dual PH/Redox Sensitive Marine Laminarin-Based Nanomedicine Carrier Biomaterial for Cancer Therapy, J. Biomed. Nanotechnol., 2018, 14, 1568–1577, DOI:10.1166/jbn.2018.2609.
- Y. Yu, B. Wang, C. Guo, F. Zhao and D. Chen, Protoporphyrin IX-Loaded Laminarin Nanoparticles for Anticancer Treatment, Their Cellular Behavior, ROS Detection, and Animal Studies, Nanoscale Res. Lett., 2019, 14, 316, DOI:10.1186/s11671-019-3138-0.
- W. Jin, X. He, W. Wu, Y. Bao, S. Wang, M. Cai, W. Zhang, C. Wang, F. Zhang and R. J. Linhardt, et al., Structural Analysis of a Glucoglucuronan Derived from Laminarin and the Mechanisms of Its Anti-Lung Cancer Activity, Int. J. Biol. Macromol., 2020, 163, 776–787, DOI:10.1016/j.ijbiomac.2020.07.069.
- L. Tian, C.-M. Li, Y.-F. Li, T.-M. Huang, N.-X. Chao, G.-R. Luo and F.-R. Mo, Laminarin from Seaweed (Laminaria Japonica) Inhibits Hepatocellular Carcinoma Through Upregulating Senescence Marker Protein-30, Cancer Biother.Radiopharm., 2020, 35, 277–283, DOI:10.1089/cbr.2019.3179.
- H. Bae, G. Song, J. Y. Lee, T. Hong, M. J. Chang and W. Lim, Laminarin-Derived from Brown Algae Suppresses the Growth of Ovarian Cancer Cells via Mitochondrial Dysfunction and Er Stress, Mar. Drugs, 2020, 18(3), 152, DOI:10.3390/md18030152.
- K. K. A. Sanjeewa, J.-S. Lee, W.-S. Kim and Y.-J. Jeon, The potential of brown-algae polysaccharides for the development of anticancer agents: An update on anticancer effects reported for fucoidan and laminaran, Carbohydr. Polym., 2017, 177, 451–459, DOI:10.1016/j.carbpol.2017.09.005.
- G. Sharma, M. Alle, H. K. Son and J.-C. Kim, Dialdehyde Modification of Laminarin for Facile Synthesis of Ultrafine Silver Nanoparticles with Excellent Antibacterial and Wound Healing Properties, Int. J. Biol. Macromol., 2022, 222, 1364–1375, DOI:10.1016/j.ijbiomssac.2022.09.228.
- K. R. Amaral, A. S. Silva, L. F. Santos, E. J. Castanheira, M. C. Mendes, D. C. S. Costa, J. M. M. Rodrigues, J. Marto and J. F. Mano, Biomimetic Adhesive Micropatterned Hydrogel Patches for Drug Release, Adv. Healthcare Mater., 2023, 2301513, DOI:10.1002/adhm.202301513.
- Y. E. Kim and Y. J. Kim, Effects of Nanofibrous Membranes Containing Low Molecular Weight β-Glucan on Normal and Cancer Cells, J. Nanosci. Nanotechnol., 2017, 17, 3597–3605, DOI:10.1166/jnn.2017.12924.
- H. Ozanne, H. Toumi, B. Roubinet, L. Landemarre, E. Lespessailles, R. Daniellou and A. Cesaro, Laminarin Effects, a β-(1,3)-Glucan, on Skin Cell Inflammation and Oxidation, Cosmetics, 2020, 7(3), 66, DOI:10.3390/COSMETICS7030066.
- T. K. Lee, D. W. Kim, J. H. Ahn, C. H. Lee, J. C. Lee, S. S. Lim, I. J. Kang, S. Hong, S. Y. Choi, M. H. Won and J. H. Park, Protective Effects of Topical Administration of Laminarin in Oxazolone-Induced Atopic Dermatitis-like Skin Lesions, Mar. Drugs, 2022, 20(11), 669, DOI:10.3390/md20110669.
- E. J. Castanheira, T. R. Correia, J. M. M. Rodrigues and J. F. Mano, Novel Biodegradable Laminarin Microparticles for Biomedical Applications, Bull. Chem. Soc. Jpn., 2020, 93, 713–719, DOI:10.1246/bcsj.20200034.
- N. Jayapala, V. Toragall, B. S. G. K. Kumar, S. R. Chaudhari and V. Baskaran, Preparation, Characterization, Radical Scavenging Property and Antidiabetic Potential of Laminarioligosaccharides Derived from Laminarin, Algal Res., 2022, 63, 102642, DOI:10.1016/j.algal.2022.102642.
- J. Li, Y. Liang, Z. He, M. Zhong and Z. Hu, Mutation of Conserved Residues in the Laminarinase Lam1092 Increases the Antioxidant Activity of the Laminarin Product Hydrolysates, Enzyme Microb. Technol., 2023, 162, 110135, DOI:10.1016/j.enzmictec.2022.110135.
- H. Jiang, S. Liang, X.-R. Yao, Y.-X. Jin, X.-H. Shen, B. Yuan, J.-B. Zhang and N.-H. Kim, Laminarin Improves Developmental Competence of Porcine Early Stage Embryos by Inhibiting Oxidative Stress, Theriogenology, 2018, 115, 38–44, DOI:10.1016/j.theriogenology.2018.04.019.
- T. Liu, M. Zhang, I. M. Asif, Y. Wu, B. Li and L. Wang, The Regulatory Effects of Fucoidan and Laminarin on Functional Dyspepsia Mice Induced by Loperamide, Food Funct., 2023, 14, 6513–6525, 10.1039/D3FO00936J.
- Y.-F. Li, V. Udayakumar, M. Sathuvan, Y. Liu, X. Liu, Y.-Q. Zhang, W.-Y. Ma, W. Zhang, S. Tang and K.-L. Cheong, Effects of Laminarin Zwitterionic Carboxylate and Sulfonate on the Intestinal Barrier Function and Gut Microbiota, Carbohydr. Polym., 2022, 278, 118898, DOI:10.1016/j.carbpol.2021.118898.
- J. H. Park, J. H. Ahn, T. K. Lee, C. W. Park, B. Kim, J. C. Lee, D. W. Kim, M. C. Shin, J. H. Cho, C. H. Lee and S. Y. Choi, Laminarin Pretreatment Provides Neuroprotection against Forebrain Ischemia/Reperfusion Injury by Reducing Oxidative Stress and Neuroinflammation in Aged Gerbils, Mar. Drugs, 2020, 18(4), 213, DOI:10.3390/md18040213.
- Z. Sun, H. Wang, W. Zhao and D. Wang, Laminarin Alleviates the Ischemia/Reperfusion Injury in PC12 Cells via Regulation of PTEN/PI3K/AKT Pathway, Adv. Polym. Technol., 2022, 2022, 9999339, DOI:10.1155/2022/9999339.
- H. Long, J. Xiao, X. Wang, M. Liang, Y. Fan, Y. Xu, M. Lin, Z. Ren, C. Wu and Y. Wang, Laminarin Acetyl Esters: Synthesis, Conformational Analysis and Anti-Viral Effects, Int. J. Biol. Macromol., 2022, 216, 528–536, DOI:10.1016/j.ijbiomac.2022.06.208.
| This journal is © The Royal Society of Chemistry 2024 |