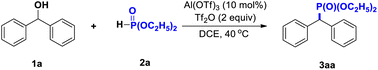Open Access Article
Open Access ArticleLewis-acid-catalyzed phosphorylation of alcohols†
Xiao-Hong Wang,
Xuan Liu,
Ya-Wen Xue,
Yan-Bin Wang,
Xiao-Hong Wei * and
Qiong Su*
* and
Qiong Su*
Key Laboratory of Environment-Friendly Composite Materials of the State Ethnic Affairs Commission, Key Laboratory for Utility of Environment-Friendly Composite Materials and Biomass in University of Gansu Province, Gansu Provincial Biomass Function Composites Engineering Research Center, College of Chemical Engineering, Northwest Minzu University, No. 1, Northwest Xincun, Lanzhou, 730030, P.R. China. E-mail: weixh12@lzu.edu.cn; hgsq@xbmu.edu.cn
First published on 24th January 2024
Abstract
An efficient method has been developed for reacting dialkyl H-phosphonates or diarylphosphine oxides with alcohols for constructing C–P bonds. This reaction was catalyzed by Lewis acid and involved nucleophilic substitution. A series of diphenylphosphonates and diphenylphosphine oxides were obtained, from the phosphorylation of alcohols, with good-to-excellent yields.
Introduction
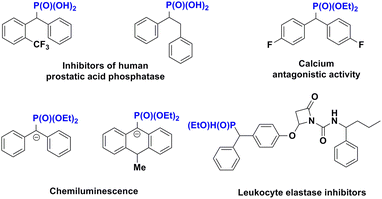
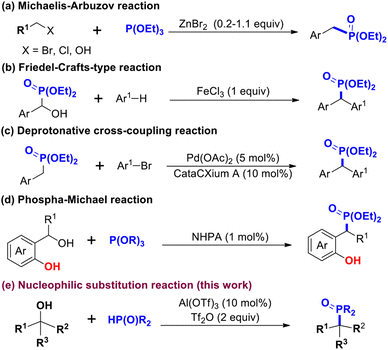
Organophosphonate compounds and their derivatives play an important role in agrochemistry,1 medicinal chemistry2 and organic chemistry.3 For example, calcium antagonist containing a diethyl phosphonate skeleton is a new calcium antagonist of the Fostedil series introduced in 1982.4 Substituted phenyl- and benzylphosphonic acids have been shown to be inhibitors of human prostatic acid phosphatase.5 In addition, they are used as general components for the preparation of functional materials, such as chemiluminescent materials,6 fluorescent materials,7 flame retardants8 and OLED transmitters9 (Fig. 1). Diaryl phosphonates are very important, but only a few methods for their synthesis have been reported. Using the classical Michaelis–Arbuzov or Michaelis–Becker reaction, dialkyl phosphonate has been synthesized from a nucleophilic phosphite and an electrophilic alkyl halide under basic conditions, but the reaction showed the disadvantages of limited availability of the starting materials and high reaction temperature.10 To overcome these limitations, the Mohanakrishnan group developed a Lewis-acid-mediated Michaelis–Arbuzov reaction to synthesize arylmethylphosphonate and heterarylmethylphosphonate from heteroarylmethylhalides/alcohols and triethyl phosphite at room temperature11 (Scheme 1a). The Chakravarty group developed a method for carrying out a Friedel–Crafts-type arylation of α-hydroxy phosphonates with arenes in the presence of stoichiometric amounts of FeCl3 to synthesize dialkyl(di-arylmethyl)phosphonates12 (Scheme 1b). The Walsh group reported a palladium-catalyzed α-arylation of benzylic phosphonates to diisopropyl phosphonate using aryl bromide and diaryl methyl phosphonate as raw materials, but this protocol was restricted to diisopropyl phosphonate derivatives13 (Scheme 1c). Recently, a novel Brønsted-acid-catalyzed phosphorylation of o-hydroxybenzyl alcohol has been developed—leading to the synthesis of diphenylphosphonates from trialkylphosphites serving as starting materials and achieved using the phosphate-Michael addition reaction14 (Scheme 1d). In addition, synthetic chemists have made remarkable progress in investigating methods for forming P–O bonds between P sources and alcohols in recent years, with examples of these methods including, the nucleophilic substitution reaction,15 Atherton–Todd reaction,16 cross-dehydrogenative coupling reaction17 and electrophile reaction.18 Our group focuses on the phosphorylation of ketones or aldehydes via the phospha-aldol-elimination (PAE) reaction catalyzed by Lewis acid or Brønsted acid.19 In this study, an effective and green nucleophilic substitution reaction for synthesizing diphenylphosphonates was studied by constructing C–P bonds with alcohol and dialkyl phosphate as raw materials under the catalysis of Tf2O and Lewis acid Al(OTf)3 (Scheme 1e).Results and discussion
After screening a series of conditions, diethyl benzhydrylphosphonate 3aa with 94% yield was obtained using diphenylmethanol 1a and diethyl phosphonate 2a as the substrates, Al(OTf)3 as a catalyst and trifluoromethanesulfonic anhydride (Tf2O) as an additive in dichloroethane (DCE) at 40 °C under air for 14 h (Table 1). Based on our previous work showing that acid as an additive plays a key role in this reaction, we were pleased to find that the yield of 3aa was significantly better when using Tf2O as an additive than when using other additives, such as TfOH, TsOH, H2SO4, and AcOH (Table 1, entry 2). Further, some Lewis acids were examined. When using Al(OTf)3 as the Lewis acid, the yield of expected product was 94% (Table 1, entry 1), but when using Cu(OTf)2, Fe(OTf)2, AgOTf, AlCl3, ZnBr2, CuBr2, or FeCl3 instead, the expected product was afforded in lower yields (Table 1, entries 3−9). Other solvents, namely CH3NO2, CH3CN, and THF, were each tested, but found to be ineffective (Table 1, entries 10–12). Finally, lower yields were observed when testing reaction temperatures and amounts of catalyst differing from those of the above optimal conditions and when carrying out certain control experiments (Table 1, entries 13–20).| Entry | Deviation | Yield (%) |
|---|---|---|
| a Reaction conditions: diphenylmethanol (0.2 mmol), Al(OTf)3 (10 mol%), diethyl phosphite (2.5 equiv.), and trifluoromethanesulfonic anhydride (2 equiv.) in dichloroethane (2 mL) at 40 °C for 14 h under air.b Isolated yield. | ||
| 1 | None | 94% |
| 2 | TfOH, TsOH, H2SO4, AcOH instead of Tf2O | Trace |
| 3 | Cu(OTf)2 instead of Al(OTf)3 | 15% |
| 4 | Fe(OTf)2 instead of Al(OTf)3 | 17% |
| 5 | AgOTf instead of Al(OTf)3 | 76% |
| 6 | AlCl3 instead of Al(OTf)3 | 50% |
| 7 | ZnBr2 instead of Al(OTf)3 | 28% |
| 8 | CuBr2 instead of Al(OTf)3 | 30% |
| 9 | FeCl3 instead of Al(OTf)3 | 36% |
| 10 | CH3NO2, CH3CN, THF instead of DCE | Trace |
| 11 | PhCH3 instead of DCE | 32% |
| 12 | Dioxane instead of DCE | 16% |
| 13 | 25 °C | 64% |
| 14 | 60 °C | 25% |
| 15 | 80 °C | 21% |
| 16 | 100 °C | 19% |
| 17 | 5 mol% Al(OTf)3 | 78% |
| 18 | 1.5 equiv. Tf2O | 84% |
| 19 | Without Tf2O | 8% |
| 20 | Without Al(OTf)3 | 37% |
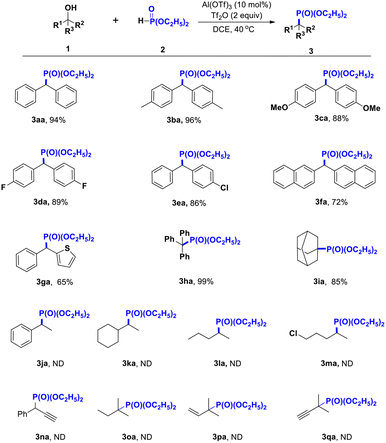
Under optimized reaction conditions, various dibenzyl alcohol and diethyl phosphite substrates were investigated, as shown in Scheme 2. Electron-donating and electron-withdrawing groups on dibenzyl alcohol afforded phosphorylation products with excellent yields (3aa–3ea). Moreover, a moderate yield of phosphorylated product 3fa was obtained with di-naphthalen-2-yl methanol in this catalytic system. In addition, other heteroaromatic alcohols, such as phenyl(thiophen-2-yl)methanol 1g, produced the desired product in moderate yields. To further verify our catalytic system, tertiary alcohols bearing aryl (1h) and alkyl (1i) groups were introduced into the reaction, and excellent yields were obtained. However, when other alcohols, such as 1-phenylethanol 1j, 1-cyclohexylethanol 1k, pentan-2-ol 1l, 5-chloropentan-2-ol 1m, 1-phenyl-2-propyn-1-ol 1n, 3-methylbutan-3-ol 1o, 2-methyl-3-buten-2-ol 1p, and 2-methyl-3-butyn-ol 1q were introduced into the reaction system in respective experiments, no target product in any of these cases was obtained, which may have been the result of the instability of the alkyl carbocation formed.
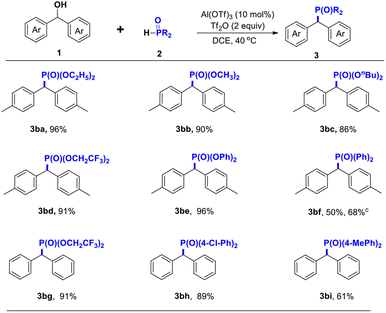
Under optimized reaction conditions, the P-source substrate scope was evaluated, as shown in Scheme 3. For example, H-phosphonate and H-phosphonate oxides were introduced into respective samples to evaluate the catalytic reaction system. To our delight, the reaction with H-phosphinate proceeded to give 3ba–3bd and 3bg in 86–96% isolated yields. Moreover, the reaction with diphenyl phosphate proceeded, and 3be was obtained with 96% yield. It is noteworthy that diphenylphosphine oxide 3bf was afforded in 50% yield under optimized reaction conditions, and when extending the reaction time, the yield was increased to 68%, but when increasing the reaction temperature using a shorter reaction time did not show the best results. However, when electron-withdrawing and electron-donating groups were introduced onto diphenylphosphine oxide, the results showed that the electron-withdrawing groups were better than the electron-donating groups, attributed to the electronic properties, for example, for bis(4-chlorophenyl)phosphine oxide 3bh and di-p-tolylphosphine oxide 3bi, the products were obtained in 89% and 61% yields, respectively.
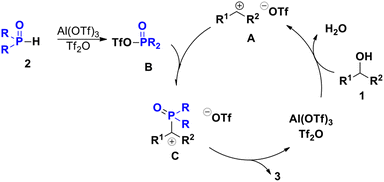
Based on previous reports,8,19a,20 we proposed a plausible reaction mechanism. According to this proposal, in the presence of Lewis acid and Brønsted acid, diaryl methanol forms diarylmethyl cations, while the more nucleophilic intermediate B is obtained under the Tf2O and Al(OTf)3 system. Intermediate B then reacts immediately with diarylmethyl cations to obtain the target product 3 while releasing the catalyst HOTf (Scheme 4).
Conclusions
In summary, we developed an efficient green method for the synthesis of diphenylphosphonates and diphenylphosphine oxide compounds with good-to-moderate yields—to construct a C–P bond by Lewis-acid-catalyzed nucleophilic substitution reaction between dialkyl H-phosphonates or diarylphosphine oxides and diarylmethanols or trisubstituted methanol.Data availability
All relevant experimental data are provided in the ESI.†Author contributions
XHW, YBW and QS directed the project. The experiments were conducted and characterized by XHW, XL and YWX. XHW prepared and revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Natural Science Foundation of China (21968032 and 22165025), Natural Science Foundation of Gansu Province (21YF1GA225) and Fundamental Research Funds for the Central Universities (31920230057 and 31920230003).Notes and references
- (a) E. R. Jackson and C. S. Dowd, Curr. Top. Med. Chem., 2012, 12, 706–728 CrossRef CAS PubMed; (b) B. Nowack, Water Res., 2003, 37, 2533–2546 CrossRef CAS PubMed; (c) S. Montel, C. Midrier, J.-N. Volle, R. Braun, K. Haaf, L. Willms, J.-L. Pirat and D. Virieux, Eur. J. Org Chem., 2012, 2012, 3237–3248 CrossRef CAS; (d) S. O. Duke and S. B. Powles, Pest Manage. Sci., 2008, 64, 319–325 CrossRef CAS PubMed.
- (a) G. P. Horsman and D. L. Zechel, Chem. Rev., 2017, 117, 5704–5783 CrossRef CAS PubMed; (b) J. W. McGrath, J. P. Chin and J. P. Quinn, Nat. Rev. Microbiol., 2013, 11, 412–419 CrossRef CAS PubMed; (c) A. Mucha, P. Kafarski and Ł. Berlicki, J. Med. Chem., 2011, 54, 5955–5980 CrossRef CAS PubMed; (d) N. Zhang and J. E. Casida, Bioorganic Med. Chem., 2002, 10, 1281–1290 CrossRef CAS PubMed; (e) A.-X. Zhou, L.-L. Mao, G.-W. Wang and S.-D. Yang, Chem. Commun., 2014, 50, 8529–8532 RSC; (f) Y. Niu, C.-X. Yan, X.-X. Yang, P.-B. Bai, P.-P. Zhou and S.-D. Yang, Org. Chem. Front., 2022, 9, 1023–1032 RSC; (g) Q. Yang and S.-D. Yang, ACS Catal., 2017, 7, 5220–5224 CrossRef CAS; (h) Y.-Y. Zhu, T. Zhang, L. Zhou and S.-D. Yang, Green Chem., 2021, 23, 9417–9421 RSC.
- (a) J.-J. Feng, X.-F. Chen, M. Shi and W.-L. Duan, J. Am. Chem. Soc., 2010, 132, 5562–5563 CrossRef CAS PubMed; (b) L. Horner, H. Hoffmann and H. G. Wippel, Chem. Ber., 1958, 91, 61–63 CrossRef CAS; (c) A. B. Juan and R. O. Liliana, Curr. Org. Chem., 2015, 19, 744–775 CrossRef; (d) Y.-N. Ma, M.-X. Cheng and S.-D. Yang, Org. Lett., 2017, 19, 600–603 CrossRef CAS PubMed; (e) Y.-N. Ma, S.-X. Li and S.-D. Yang, Acc. Chem. Res., 2017, 50, 1480–1492 CrossRef CAS PubMed; (f) W. Tang and X. Zhang, Chem. Rev., 2003, 103, 3029–3070 CrossRef CAS PubMed; (g) H.-L. Wang, R.-B. Hu, H. Zhang, A.-X. Zhou and S.-D. Yang, Org. Lett., 2013, 15, 5302–5305 CrossRef CAS PubMed; (h) Y.-N. Ma, Q.-P. Tian, H.-Y. Zhang, A.-X. Zhou and S.-D. Yang, Org. Chem. Front., 2014, 1, 284–288 RSC; (i) Z.-N. Cai, Y.-P. Han, Y. Zhang, H.-Y. Zhang, J. Zhao and S.-D. Yang, Green Chem., 2023, 25, 5721–5726 RSC.
- S. Younes, G. Baziard-Mouysset, G. de Saqui-Sannes, J. L. Stigliani, M. Payard, R. Bonnafous and J. Tisne-Versailles, Eur. J. Med. Chem., 1993, 28, 943–948 CrossRef CAS.
- C. F. Schwender, S. A. Beers, E. A. Malloy, J. J. Cinicola, D. J. Wustrow, K. D. Demarest and J. Jordan, Bioorg. Med. Chem. Lett., 1996, 6, 311–314 CrossRef CAS.
- J. Motoyoshiya, T. Ikeda, S. Tsuboi, T. Kusaura, Y. Takeuchi, S. Hayashi, S. Yoshioka, Y. Takaguchi and H. Aoyama, J. Org. Chem., 2003, 68, 5950–5955 CrossRef CAS PubMed.
- C.-L. Chiang, C.-F. Shu and C.-T. Chen, Org. Lett., 2005, 7, 3717–3720 CrossRef CAS PubMed.
- B. A. Howell and Y. J. Cho, in Fire and Polymers V, American Chemical Society, 2009, ch. 15, vol. 1013, pp. 249–265 Search PubMed.
- G. Mao, A. Orita, L. Fenenko, M. Yahiro, C. Adachi and J. Otera, Mater. Chem. Phys., 2009, 115, 378–384 CrossRef CAS.
- C. S. Demmer, N. Krogsgaard-Larsen and L. Bunch, Chem. Rev., 2011, 111, 7981–8006 CrossRef CAS PubMed.
- G. G. Rajeshwaran, M. Nandakumar, R. Sureshbabu and A. K. Mohanakrishnan, Org. Lett., 2011, 13, 1270–1273 CrossRef CAS PubMed.
- G. Pallikonda and M. Chakravarty, Eur. J. Org Chem., 2013, 2013, 944–951 CrossRef CAS.
- S. Montel, L. Raffier, Y. He and P. J. Walsh, Org. Lett., 2014, 16, 1446–1449 CrossRef CAS PubMed.
- H. Huang and J. Y. Kang, Org. Lett., 2017, 19, 5988–5991 CrossRef CAS PubMed.
- (a) J. I. Murray, R. Woscholski and A. C. Spivey, Chem. Commun., 2014, 50, 13608–13611 RSC; (b) N. P. Kenny, K. V. Rajendran and D. G. Gilheany, Chem. Commun., 2015, 51, 16561–16564 RSC.
- (a) G. Wang, R. Shen, Q. Xu, M. Goto, Y. Zhao and L.-B. Han, J. Org. Chem., 2010, 75, 3890–3892 CrossRef CAS PubMed; (b) S. Li, T. Chen, Y. Saga and L.-B. Han, RSC Adv., 2015, 5, 71544–71546 RSC; (c) B. Xiong, Y. Zhou, C. Zhao, M. Goto, S.-F. Yin and L.-B. Han, Tetrahedron, 2013, 69, 9373–9380 CrossRef CAS; (d) S. Wagner, M. Rakotomalala, Y. Bykov, O. Walter and M. Döring, Heteroat. Chem., 2012, 23, 216–222 CrossRef CAS; (e) B. A. Trofimov, N. K. Gusarova, P. A. Volkov, N. I. Ivanova and K. O. Khrapova, Heteroat. Chem., 2016, 27, 44–47 CrossRef CAS.
- (a) Q. Chen, J. Zeng, X. Yan, Y. Huang, C. Wen, X. Liu and K. Zhang, J. Org. Chem., 2016, 81, 10043–10048 CrossRef CAS PubMed; (b) B. Xiong, X. Feng, L. Zhu, T. Chen, Y. Zhou, C.-T. Au and S.-F. Yin, ACS Catal., 2015, 5, 537–543 CrossRef CAS; (c) X. Yu, S. Zhang, Z. Jiang, H.-S. Zhang and T. Wang, Eur. J. Org Chem., 2020, 2020, 3110–3113 CrossRef CAS; (d) Y. Saito, S. M. Cho, L. A. Danieli and S. Kobayashi, Org. Lett., 2020, 22, 3171–3175 CrossRef CAS PubMed; (e) J. Dhineshkumar and K. R. Prabhu, Org. Lett., 2013, 15, 6062–6065 CrossRef CAS PubMed; (f) Y. Huang, J. Tang, X. Zhao, Y. Huo, Y. Gao, X. Li and Q. Chen, Green Chem., 2023, 25, 4528–4535 RSC; (g) L. Si, B. Xiong, S. Xu, L. Zhu, Y. Liu, W. Xu and K.-W. Tang, J. Organomet. Chem., 2023, 991, 122670 CrossRef CAS; (h) Y. Tan, Y.-P. Han, Y. Zhang, H.-Y. Zhang, J. Zhao and S.-D. Yang, J. Org. Chem., 2022, 87, 3254–3264 CrossRef CAS PubMed.
- J. Shen, Q.-W. Li, X.-Y. Zhang, X. Wang, G.-Z. Li, W.-Z. Li, S.-D. Yang and B. Yang, Org. Lett., 2021, 23, 1541–1547 CrossRef CAS PubMed.
- (a) X.-H. Wei, C.-Y. Bai, L.-B. Zhao, P. Zhang, Z.-H. Li, Y.-B. Wang and Q. Su, Chin. J. Chem., 2021, 39, 1855–1860 CrossRef CAS; (b) X.-H. Wei, C.-Y. Bai, A.-J. Wang, Q.-L. Feng, L.-B. Zhao, P. Zhang, Z.-H. Li, Q. Su and Y.-B. Wang, Org. Lett., 2021, 23, 7100–7105 CrossRef CAS PubMed; (c) X.-H. Wei, X.-H. Wang, C.-Y. Bai, Y.-W. Xue, P. Zhang, Y.-B. Wang and Q. Su, Org. Chem. Front., 2023, 10, 410–415 RSC; (d) X.-H. Wang, Y.-W. Xue, C.-Y. Bai, Y.-B. Wang, X.-H. Wei and Q. Su, J. Org. Chem., 2023, 88, 16216–16228 CrossRef CAS PubMed.
- (a) L. Chen, X.-Y. Fang and Y.-X. Zou, Org. Biomol. Chem., 2018, 16, 951–956 RSC; (b) B. G. Janesko, H. C. Fisher, M. J. Bridle and J.-L. Montchamp, J. Org. Chem., 2015, 80, 10025–10032 CrossRef CAS PubMed; (c) J. P. Abell and H. Yamamoto, J. Am. Chem. Soc., 2008, 130, 10521–10523 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General information, experimental procedures and analytical data, including 1H, 31P, 13C NMR for all compounds (PDF). See DOI: https://doi.org/10.1039/d3ra08214h |
| This journal is © The Royal Society of Chemistry 2024 |