Open Access Article
Open Access ArticleHydrogen production, storage, and transportation: recent advances
M. M. Rampai
*ab,
C. B. Mtshali
a,
N. S. Seroka
 *bc and
L. Khotseng
*bc and
L. Khotseng
 *b
*b
aTandetron Laboratory, iThemba LABS, National Research Foundation, P.O. Box 722, Somerset West 7129, South Africa. E-mail: mrampai@tlabs.ac.za; mojesirampai@gmail.com
bDepartment of Chemistry, University of the Western Cape, Private Bag X17, Bellville 7535, South Africa
cCouncil for Science and Industrial Research, Pretoria, 0001, South Africa
First published on 23rd February 2024
Abstract
One such technology is hydrogen-based which utilizes hydrogen to generate energy without emission of greenhouse gases. The advantage of such technology is the fact that the only by-product is water. Efficient storage is crucial for the practical application of hydrogen. There are several techniques to store hydrogen, each with certain advantages and disadvantages. In gaseous hydrogen storage, hydrogen gas is compressed and stored at high pressures, requiring robust and expensive pressure vessels. In liquid hydrogen storage, hydrogen is cooled to extremely low temperatures and stored as a liquid, which is energy-intensive. Researchers are exploring advanced materials for hydrogen storage, including metal hydrides, carbon-based materials, metal–organic frameworks (MOFs), and nanomaterials. These materials aim to enhance storage capacity, kinetics, and safety. The hydrogen economy envisions hydrogen as a clean energy carrier, utilized in various sectors like transportation, industry, and power generation. It can contribute to decarbonizing sectors that are challenging to electrify directly. Hydrogen can play a role in a circular economy by facilitating energy storage, supporting intermittent renewable sources, and enabling the production of synthetic fuels and chemicals. The circular economy concept promotes the recycling and reuse of materials, aligning with sustainable development goals. Hydrogen availability depends on the method of production. While it is abundant in nature, obtaining it in a clean and sustainable manner is crucial. The efficiency of hydrogen production and utilization varies among methods, with electrolysis being a cleaner but less efficient process compared to other conventional methods. Chemisorption and physisorption methods aim to enhance storage capacity and control the release of hydrogen. There are various viable options that are being explored to solve these challenges, with one option being the use of a multilayer film of advanced metals. This work provides an overview of hydrogen economy as a green and sustainable energy system for the foreseeable future, hydrogen production methods, hydrogen storage systems and mechanisms including their advantages and disadvantages, and the promising storage system for the future. In summary, hydrogen holds great promise as a clean energy carrier, and ongoing research and technological advancements are addressing challenges related to production, storage, and utilization, bringing us closer to a sustainable hydrogen economy.
1. Introduction
Hydrogen storage has been extensively researched for many decades. This technology is mostly owing to metal nanoparticles' storing capacity. Superior features of metal nanoparticles include catalytic, optical, and electrical properties. Nanotechnology has the potential to enable finer tuning of material properties at the molecular and atomic levels than their bulk counterparts. This field of hydrogen storage in nanostructured metal hydrides has the potential to be a game changer in terms of technology. The move to a green economy has inspired a lot of interest, but long-term economic growth is inhibited, and alternative, creative, and inventive structures for efficient energy storage methods and significant conversion rates are sought. Carbonaceous, 2D chalcogenides and metal oxides have unique features as novel synthetic materials, which will improve the efficacy of these systems.1,2The development, production, and study of these materials as well as material-based coatings are essential steps in the creation of novel multi-layered thin film types with exceptional performance and attributes for hydrogen storage. Hydrogen storage is widely acknowledged as one of the safer methods for storing hydrogen in gaseous form when it comes to metal hydrides. A major driver behind the increased use of fuel cells is the development of alloys for storing hydrogen that have extraordinarily large capacities. Due to their accessibility, low densities, and substantial hydrogen storage capacities, magnesium-based alloys have been the focus of increasing attention among the various possible alloys for hydrogen storage.3–5
To meet the demands of the modern world, which are growing at an alarming rate for fossil fuels, renewable energy sources must be used instead. Because it emits no pollutants and can store three times as much energy as conventional gasoline, hydrogen is hailed as the fuel of the future. But effective and safe storage presents a big obstacle. Out of the three storage techniques, solid hydrogen storage has been shown to be the safest. The common materials for electrochemical hydrogen storage are covered in this work. Examples include metal organic frameworks, metal hydrides, complex hydrides, activated carbon (aC), capillary arrays, clathrate hydrates, metal nitrides, doped polymers, and zeolites.6
One of the least expensive and greenest fuels for the future economy is hydrogen. It is simple because: (i) it is a readily available element that makes up over 90% of all atoms in the universe; (ii) it is the lightest element (molecular weight = 2.016) with the highest known energy content (calorific/heating value) of any fuel; (iii) it is sustainable; (iv) it is toxic-free; and (v) better than coal, natural gas, or petroleum, it serves as an energy carrier that is friendly to the environment and leaves water as the only exhaust product when converted into energy.6–8
Both non-renewable energy sources like coal, natural gas, and nuclear power as well as renewable energy sources like hydro, wind, wave, solar, biomass, and geothermal energy can be used to produce hydrogen. The incredible energy storage capacity of hydrogen has been demonstrated by calculations, which reveal that 1 kilogram of hydrogen contains around 120 MJ (=33.33 kW h) of energy, more than twice as much as most conventional fuels. The energy contents of hydrogen and other alternative fuels are contrasted in Table 1.6–8
| Fuel | Energy contents [MJ kg−1] | |
|---|---|---|
| Lower heating value | Higher heating value | |
| Gaseous hydrogen | 119.96 | 141.88 |
| Ethanol | 26.95 | 29.84 |
| Natural gas | 47.13 | 52.21 |
| Methanol | 20.09 | 22.88 |
| Liquified natural gas (LNG) | 48.62 | 55.19 |
| Coal (wet basis) | 22.73 | 23.96 |
| Crude oil | 42.68 | 45.53 |
| Low-sulfur diesel | 42.60 | 45.56 |
| Reformulated or low-sulfur gasoline (RFG) | 42.35 | 45.42 |
| Conventional diesel | 42.78 | 45.76 |
| Liquefied petroleum gas (LPG) | 46.60 | 50.14 |
| Conventional gasoline | 43.44 | 46.52 |
| Liquid hydrogen | 120.04 | 141.77 |
| Still gas (in refineries) | 46.89 | 50.94 |
2. Hydrogen economy overview
The Hydrogen Economy or Hydrogen Energy System is a theoretical notion of a system where hydrogen serves as the primary energy carrier. The phrase “hydrogen economy” was coined by John Bockris in 1970 during a speech at the General Motors (GM) Technical Centre. The hydrogen economy is reaching a turning point. The market requires clean and sustainable energy, and for a wide range of applications, fuel cell technologies seem practical and highly appealing. Additionally, fuel cells are effective, versatile, and clean. Solid oxide fuel cells are showing a lot of promise. The primary issue is figuring out what stage of development of different fuel cell technologies have been attained and how much they are improving each year.7The alternative to the current fossil fuels-based systems in transportation, industrial, residential, and commercial sectors, hydrogen must be produced primarily from readily accessible energy sources. Hydrogen economy as a renewable energy is the solution-problem solving facing the world today, such as (i) environmental concerns on a global scale, (ii) resource depletion, (iii) food shortage and malnutrition in third-world countries, and (iv) humongous growth of the global population. Even if the issues with the fossil fuel economy are severe and overwhelming, and contributes to the clean environment, energy security, and economy, the successful growth of the hydrogen economy will all greatly benefit the end users.8
Despite the apparent benefits, renewable energy technology has encountered severe social, scientific, and technical obstacles. Storage of hydrogen is crucial to the transition because of its extremely low density of 0.0899 g L−1.9–11 Although several industries such as chemical and refineries often employ hydrogen, storage, transport, and production costs are excessive and not suitable for the majority of nano electronics.12 But because of how exciting the hydrogen economy is, governments all around the world are making it as inexpensive as feasible. However, the hydrogen economy won't take off until it is economically and energetically viable. If not, better options will take over the market. Additionally, infrastructures are already in place for practically all synthetic liquid hydrocarbons, but a brand-new distribution system is needed for hydrogen. The whole energy supply and distribution network will be affected by the shift to a pure hydrogen economy. Therefore, before making investments, all facets of a hydrogen economy should be looked at ref. 7.
The European Commission proposed in 2003 that the European Union should transition to a hydrogen-based economy by the year 2050, and it predicts that by the year 2040, 35% of newly built vehicles will run on hydrogen with no carbon emissions, High-Level Group on Hydrogen and Fuel Cell Technologies. The Energy Efficiency and Renewable Energy, Fossil Energy, Nuclear Energy, and Science Offices of the U.S. Department of Energy, on the other hand, recommended that the transition to hydrogen-powered fuel cell cars ought to have occurred around the year 2020.8,13 There are three stages of hydrogen economy, shown in Fig. 1, that are being investigated by various research groups; these stages are, hydrogen production, transportation, and storage.14
 | ||
| Fig. 1 Illustration of hydrogen economy. Reproduced with permission from [Manmeet Kaura,14 2021 Elsevier Ltd]. | ||
To generate energy that can be delivered to all practical users with great energy efficiency, overwhelmingly positive environmental and social advantages, and competitive economics, hydrogen and/or hydrogen-containing molecules must be used.15 Given that it is the most abundant element in the universe with more than 90% of all atoms are made of it, the lightest element with molecular weight of 1.008 g m−1, has the highest known energy content (calorific or heating value) of any fuel. It can be utilized as fuel that can be stored and used for transportation, fuel cell power systems, turbines, or internal combustion engines.16–19 Generally, this technology is sought to be clean and cheap energy which can unravel the future economy. Hydrogen as a renewable, toxic-free, sustainable energy carrier and environmentally friendly, beats fuels such as petroleum, natural gas and coal. It is an energy carrier that is currently in the early stages of research and development. It is produced via renewable and non-renewable energy sources, renewable energy sources such as hydro, wind, wave, solar, biomass, and geothermal energy as well as non-renewable energy sources like coal, natural gas, and nuclear energy.
Moreover, it has been calculated that a kilogram of hydrogen has an energy content of around 120 MJ (1433.33 kW h), which is more than twice as much as most conventional fuels. Hydrogen has an incredible ability for energy storage. Additional advantages of hydrogen such as energy security through a reduction in oil imports, sustainability through the use of renewable energy sources, pollution reduction and improvement of urban air quality due to production of almost zero carbon, greenhouse gases, and oxide emissions at use, and economic viability for future prosperity of the global economy are discussed by ref. 20–22.
With oil exports exceeding imports in 2020, the United States became a net exporter of the commodity, however imports of 8.47 million barrels per day in 2021 continued to play a significant role in maintaining a balance between supply and demand on both local and foreign markets. Approximately 30% of the nation's total energy requirements and 70% of its petroleum use are met by the transportation sector. Natural gas, coal, solar power, wind power, biomass, and other resources can all be used to manufacture hydrogen locally. Hydrogen provides the potential to improve national energy security, preserve petroleum, and diversify our transportation energy alternatives for a more resilient system when used to power highly efficient fuel cell electric vehicles.20
About 50% of Americans reside in locations with air pollution levels that are harmful to both the environment and public health. One of the main sources of environmental pollution is the nitrogen oxides, hydrocarbons, and particulate matter emissions from gasoline and diesel cars. Fuel cell electric vehicles powered by hydrogen emit just warm air and water (H2O), not any of these toxic chemicals. If hydrogen is produced using low- or zero-emission energy sources, such solar, wind, and nuclear power, as well as fossil fuels that have sophisticated emission controls and carbon sequestration, both the environment and human health will benefit. Utilizing these sources to generate hydrogen for transportation can reduce greenhouse gas emissions because the transportation sector is responsible for around one-third of the nation's carbon dioxide emissions.21
However, hydrogen has a poor energy density per volume. This makes hydrogen storage to be difficult since it needs extreme conditions to be stored compactly, such as high pressures, low temperatures, or chemical reactions. Light-duty vehicles frequently have restricted size and weight capacity for fuel storage, thus overcoming this obstacle is crucial. In order to satisfy consumer expectations, light-duty vehicles should typically have a driving range of more than 300 miles (482.803 km). Hydrogen can currently only be stored on a vehicle with a larger tank and higher pressure than other gaseous fuels since hydrogen has a lower volumetric energy density (0.09 kg m−3) than gasoline (45 MJ kg−1). Larger tanks can fit in medium- and heavy-duty trucks with more room, although these vehicles may be subject to weight restrictions that limit their ability to carry a total load.22,23
A circular economy concept aims to use waste valorisation to close the gap between ecosystem cycles and output. It is presented as a way to achieve sustainable development goals and “green growth” with increased resource efficiency through recycling. In order to advance a sustainable circular economy, hydrogen is essential for its production, storage, transportation, and utilization. The creation of green hydrogen via electrolysis, which is fuelled by renewable energy sources like solar or wind power, is essential for guaranteeing a clean and carbon-neutral feedstock. An efficient energy supply is ensured by techniques like subterranean salt caverns or sophisticated hydrogen carriers. Hydrogen has the potential to be a clean fuel that benefits the transportation industry by lowering reliance on fossil fuels, especially when used in fuel cells for electric vehicles. Lastly, a variety of applications in sectors such as manufacturing and electricity production support a comprehensive strategy, encouraging a circular economy that places an emphasis on resource efficiency, reduces waste, and embraces renewable energy sources. Resource recovery from waste streams has become a focal point of the resource recovery model, which is based on the reduce, reuse, and recycle principles of the circular economy. Industrial waste streams containing more than 50% hydrogen also demonstrated a potential source for hydrogen recovery through separation procedures.24
2.1 Hydrogen availability and production
Hydrogen is the most common element in the universe. However, it only occurs in very small amounts in the Earth's atmosphere, at around 500 parts per billion (or 0.5 ppm). Apart from traces of gaseous dihydrogen (H2) at the surface and above, we find hydrogen essentially bound to oxygen in water (H2O) and carbon in all hydrocarbons (CH4, C2H6). However, over time it became increasingly clear that several phenomena lead to the continuous production of H2 in the earth's crust. A water–rock interaction process known as diagenesis releases hydrogen from water during oxidation phenomena that can be observed in various geological contexts. For example, once ferrous iron (Fe2+) is present, upon contact with water (sea or rain) it oxidizes to ferric iron Fe3+ and releases H2, following the process known as the water splitting process.The same reaction can also take place with other metals such as magnesium (Mg2+ ≥ Mg3+). This process is fast and efficient at high temperatures, around 300 °C, but it can also be used at lower temperatures. Other known sources of natural H2 are reaction of water with ultrabasic rocks (serpentinization), degassing of hydrogen deep from the Earth's crust and mantle, and water interaction with exposed rock surfaces (weathering). Another production method is radiolysis, in which the H2 contained in the water is separated from the oxygen by the natural radioactivity of the earth's crust.25
Estimates of the H2 flux from the latter two sources, diagenesis and radiolysis, are important but still not very accurate. According to the literature, the H2 flux of these processes vary between a few percent and 100% of the annual H2 consumption in 2019, i.e., about 70 million tons. Other sources such as friction at fault surfaces and biogas production process also release H2, but in smaller amounts a priori.26 It is important to note that in all these cases we are dealing with H2 flux and not an accumulated fossil resource. At the same time, the preservation of large amounts of primordial H2, the H2 that was present during the formation of the solar system, in the Earth's mantle, or even in the Earth's core during Earth's formation, is also a working hypothesis being explored by some researchers. In this hypothesis, H2 is a fossil resource, but almost inexhaustible.27
Currently, most hydrogen is produced from fossil fuels, especially natural gas. Furthermore, electricity from the grid or from renewable sources such as wind, sun, geothermal energy or biomass is also used to produce hydrogen. In the longer term, solar energy and biomass can be used more directly to produce hydrogen. Combining these hydrogen producing processes with carbon capture process carbon emission can be tremendously reduced. Natural gas reforming is an advanced and mature hydrogen production process that builds on existing natural gas infrastructure. Today, ∼95% of the hydrogen produced in the world, especially in the United States, comes natural gas reforming in large central plants which is an important pathway for short-term hydrogen production.
The green pathway that involves the use of sunlight to directly or indirectly provide the energy to produce hydrogen without the emission of greenhouse gases is a viable option for the future. Even though this resource is abundant it diffuses and only available for part of the day. On the other hand, biomass is an abundant renewable resource that can be produced domestically and converted into hydrogen and other by-products using a variety of methods. The advantage of biomass over other green pathways is the removal of the carbon dioxide in the atmosphere during the process. Wind is also an abundant and variable resource for power generation. Electricity generated by wind power can drive water electrolysis to produce hydrogen, which could be used to fuel vehicles or stored and then used in fuel cells to generate electricity during daytime times when wind resources are scarce. Therefore, combining the advantages of all green pathway process of producing electricity and hydrogen i.e., biomass, geothermal, wind, and solar can curb the problem of greenhouse gases emission.28
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 113 million tonnes of oil equivalent (MTOE) made up the total global primary energy supply in 2011, this sum was met by coal (28.5%), oil (31.5%), nuclear (5.1%), natural gas (21.3%), hydro (2.3%), biofuels and waste (10%), and various other sources, for example, wind, waste heat, geothermal, solar, etc. (1%). Global power generation in the same year was 22
113 million tonnes of oil equivalent (MTOE) made up the total global primary energy supply in 2011, this sum was met by coal (28.5%), oil (31.5%), nuclear (5.1%), natural gas (21.3%), hydro (2.3%), biofuels and waste (10%), and various other sources, for example, wind, waste heat, geothermal, solar, etc. (1%). Global power generation in the same year was 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126 TW h, with the following sources providing the energy: coal (41.3%), natural gas (21.9%), hydro (15.8%), nuclear (11.7%), oil (4.8%), and additional sources (4.5%) such as geothermal, solar, wind, waste heat, biofuels, and waste materials. 2011 saw 31
126 TW h, with the following sources providing the energy: coal (41.3%), natural gas (21.9%), hydro (15.8%), nuclear (11.7%), oil (4.8%), and additional sources (4.5%) such as geothermal, solar, wind, waste heat, biofuels, and waste materials. 2011 saw 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 Mt of CO2 emissions, with coal accounting for 44%, oil for 35.3%, natural gas for 20.2%, and trash and municipal waste for 0.5% of the total. In all, fossil fuels accounted for around 85% of the world's energy supply and 70% of the power generated. A significant portion of CO2 emissions in 2011 (99.5%) came from the use of fossil fuels. Furthermore, because of their limited supply and nonhomogeneous distribution, fossil fuels are predicted to be unable to meet the world's growing energy needs. Based on the current rates of fuel depletion, oil reserves are predicted to run out in fewer than 80 years, even in the situation of fixed demand. Furthermore, it is predicted that the price of fossil fuels would rise when readily available fossil fuel supplies are depleted.29
342 Mt of CO2 emissions, with coal accounting for 44%, oil for 35.3%, natural gas for 20.2%, and trash and municipal waste for 0.5% of the total. In all, fossil fuels accounted for around 85% of the world's energy supply and 70% of the power generated. A significant portion of CO2 emissions in 2011 (99.5%) came from the use of fossil fuels. Furthermore, because of their limited supply and nonhomogeneous distribution, fossil fuels are predicted to be unable to meet the world's growing energy needs. Based on the current rates of fuel depletion, oil reserves are predicted to run out in fewer than 80 years, even in the situation of fixed demand. Furthermore, it is predicted that the price of fossil fuels would rise when readily available fossil fuel supplies are depleted.29
Even with the continuous and vigorous deployment of renewable energy sources in all major economies, fossil fuels accounted for more than 60% of the world's electricity production thus far in 2023. Supporters of the energy transition away from fossil fuels have gained momentum as the amount of power generated globally from renewable sources has increased at a rate that is over three times faster than that of fossil fuels since 2019.30
Electricity is being produced using nuclear energy as a source of energy. About 12% of the electricity produced in 2011 came from it. Hydrogen can also be produced using nuclear energy as the main energy source. The initial steps in the manufacture of nuclear-based hydrogen were steam reforming with nuclear heat and water electrolysis. Subsequently, methods such as thermochemical cycles and high temperature electrolysis were developed to produce hydrogen. Nuclear reactors can be used to run thermochemical water splitting cycles at 500 °C or higher to create hydrogen. Higher temperatures can lead to faster reaction rates and higher efficiency. Biomass has a potential to accelerate the realization of hydrogen as a major fuel of the future. Biomass sources are renewable, and they are considered as CO2 neutral in life cycle due to consumption of atmospheric CO2 during growth. When used as a hydrogen production source, it can have a small CO2 impact, but this amount is much less compared to fossil fuel-based hydrogen generation. Hydrogen can be produced from different types of biomass such as animal, forestry, industrial and municipal waste, and agricultural and industrial crops. Gasification, thermochemical, and biochemical processes are commonly used to produce hydrogen from biomass.29
Solar hydrogen can either be created via PV-based water electrolysis or direct solar water splitting. Two methods can be used to harness solar energy to manufacture hydrogen: direct solar water splitting and water electrolysis with solar power. Techniques including electrolysis, artificial photosynthesis, photo-electrolysis, and thermochemical, photocatalytic, and photo-electrochemical water splitting can all be used to produce hydrogen from solar energy. In the shift to large-scale, sustainable, renewable, and clean energy systems, wind power is crucial. With a great deal of advanced technology and a large range of sizes and capabilities, wind power can produce hydrogen from water emissions-free. This option presents a high potential among renewable sources for manufacturing pollution-free hydrogen, particularly for distributed systems, by using the power provided by wind turbines for electrolysis.
The energy and energy efficiency data of a few chosen techniques of producing hydrogen are shown in Table 2, where it is evident that gasification of coal and biomass and the reforming of fossil fuels have an advantage over other methods. However, out of all the production technologies chosen, solar-based electrolysis has the poorest performance compared to other hydrogen production methods.30
| Energy efficiency | |
|---|---|
| Fossil fuel reforming | 8.30 |
| Coal gasification | 6.30 |
| Hybrid thermochemical cycles | 5.30 |
| Electrolysis | 5.30 |
| Biomass gasification | 6.50 |
| PV electrolysis | 1.24 |
| Photocatalysis | 0.20 |
| Photoelectrochemical method | 0.70 |
| Photoelectrolysis | 0.78 |
| Ideal | 10.00 |
According to,31 world's energy demand is expected to increase, reaching approximately 778 Etta Joule annually by 2035. The oil and gas industry sector, which is a major energy producer and user, will face significant hurdles as a result. The carbon intensity of the oil and gas supply chains—which include onshore and offshore production, enhanced recovery, refining, and transportation (by road, rail, or sea)—is rising quickly as a result of the decline in so-called simple oils and the shift toward unconventional oil and gas. The supply of energy needed by some oil and gas production facilities is extremely difficult to meet because of their remote locations. Therefore, on-site renewable energy generation can lower the high cost of delivering fossil fuels to these locations while also increasing the sustainability of such processes. These kinds of solutions also apply to remote mining sectors, which mostly depend on diesel generators.
Around 33% of the world's oil and gas fields (more than 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in total) are situated offshore. Global crude oil production and refinement totals in 2015 were 4416 and 4189 million tons (Mt), respectively. In 2015, 438 Mt of oil equivalent (Mtoe) of energy were consumed by the chemical and petrochemical industries. To producing energy, the oil and gas sectors use refined petroleum products. Additionally, a certain quantity of steam or electricity is purchased and imported into the plants. For instance, 844 Mtoe was processed by US refineries in 2015, while 128
000 in total) are situated offshore. Global crude oil production and refinement totals in 2015 were 4416 and 4189 million tons (Mt), respectively. In 2015, 438 Mt of oil equivalent (Mtoe) of energy were consumed by the chemical and petrochemical industries. To producing energy, the oil and gas sectors use refined petroleum products. Additionally, a certain quantity of steam or electricity is purchased and imported into the plants. For instance, 844 Mtoe was processed by US refineries in 2015, while 128![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 339 million pounds, 46
339 million pounds, 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 860 million kW h, and 852
860 million kW h, and 852![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 067 million ft3 of natural gas were purchased, respectively. Future energy demand requires a careful analysis of diverse fuel usage in different industries. The need for energy is predicted to increase dramatically as societies continue to expand and become more urbanized. The transportation industry is about to undergo a radical change as more people choose electric vehicles, which will raise the need for electricity. The demand for fossil fuels and electricity will undoubtedly change as a result of industry using greener and more energy-efficient technology at the same time. In addition, it is anticipated that rising economic growth and population growth will raise overall energy demand, necessitating the need for diverse and sustainable energy sources.31
067 million ft3 of natural gas were purchased, respectively. Future energy demand requires a careful analysis of diverse fuel usage in different industries. The need for energy is predicted to increase dramatically as societies continue to expand and become more urbanized. The transportation industry is about to undergo a radical change as more people choose electric vehicles, which will raise the need for electricity. The demand for fossil fuels and electricity will undoubtedly change as a result of industry using greener and more energy-efficient technology at the same time. In addition, it is anticipated that rising economic growth and population growth will raise overall energy demand, necessitating the need for diverse and sustainable energy sources.31
Clean hydrogen has attracted a great deal of consideration because of its potential use as an energy carrier for future technologies by following the direction of the use of water as a source of it, with electrolytic water process being one of the most promising hydrogen production methods. There are currently four different technologies in use: (i) Proton Exchange Membrane (PEM) Technology, which has a high current density, high operating pressure, greater environmental protection, small cell size, and wide power regulation range; (ii) Alkaline Water Electrolysis Cell (AWE) technology, which is distinguished by high technological maturity and long service life. Nevertheless, PEM electrolysis is 35 times more expensive than AWE due to the use of platinum and iridium, two precious metals; (iii) the Solid Oxide Electrolytic Cell (SOEC), which runs at a high temperature (500–900 °C) and uses steam rather than liquid water.32
The current developments are concentrated on cost reduction, modernization, and commercialization. It is mostly utilized in regions with abundant thermal energy at the moment. (iv) Anion exchange membranes (AEM), a relatively sophisticated technology with a higher current density and faster response, are primary still in the laboratory research stage as are the membrane materials. It is imperative that future energy structures devise more affordable and feasible techniques for producing hydrogen with low or no carbon emissions. Natural subterranean hydrogen is abundant and has the potential to be the cornerstone of any future energy revolution. The production, storage, and transportation of hydrogen are currently the primary areas of hydrogen verification, with natural hydrogen content being relatively low. The cost of hydrogen will, however, drop dramatically as natural hydrogen reserves are discovered and developed. As a result, we must understand all aspects of natural hydrogen, such as its distribution, source and mode of occurrence.32
3. Hydrogen storage
Hydrogen can exist and be stored in two forms: gas and liquid phase. These are the only types of hydrogen storage phases that are currently being deployed on a significant scale. Notable examples are the storage of liquid hydrogen in the space industry and the large salt storage facilities in Texas (USA) and Teeside (UK).33 Hydrogen storage has always been a key issue in the development of hydrogen energy, so there are numerous research reports on hydrogen storage. For many years, the most technologically advanced countries in the world have placed hydrogen storage at the forefront of their research. The decision was made because it is believed that hydrogen can assist address the rising energy demand and slow down global climate change.Furthermore, the development of sustainable hydrogen energy is crucial for the success of the future economy, and hydrogen storage is a vital enabling technology.8 Mobile and fixed hydrogen storage systems are both necessary for the hydrogen economy to succeed. In the future hydrogen economy, the mobile sector is anticipated to use the most hydrogen. Although hydrogen is a good fuel for internal combustion engines in cars, it is anticipated that polymer electrolyte membrane fuel cells would eventually replace traditional engines in an economy based on hydrogen, in contrast to an engine which transforms chemical energy into heat before transforming heat into mechanical energy.
Polymer electrolyte membrane fuel cells can reduce greenhouse gas emissions, current energy usage, and dependency on fossil fuels since it directly and effectively converts the chemical energy of hydrogen fuel into electrical energy with water as the only exhaust product.34–36 However, permanent storage systems and transportable storage systems each have unique requirements and difficulties. In comparison to mobile applications, stationary applications' weight and volume storage problems with hydrogen are less severe. Stationary hydrogen storage systems have the capacity to accommodate slow kinetics, can operate at high temperatures and pressures, and can take up more space. However, a significant technological barrier to the development of stationary hydrogen storage systems is the lack of suitable materials for storage tanks. Nevertheless, compared to stationary applications, the criteria for hydrogen storage in mobile applications are even more demanding.22
Hydrogen may be stored for a long time due to its stable chemistry. There are several techniques to store hydrogen, each with certain advantages and disadvantages. Hydrogen storage is divided into gaseous hydrogen storage, liquid hydrogen storage and solid hydrogen storage according to the phase state of hydrogen. Fig. 2 shows a flow chart summarizing methods of hydrogen storage.8
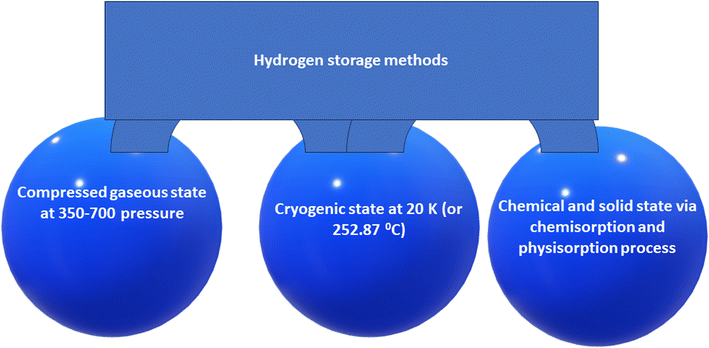 | ||
| Fig. 2 A flow chart summarizing methods of hydrogen storage [Abe8 et al. 2015]. | ||
3.1 Gaseous hydrogen storage
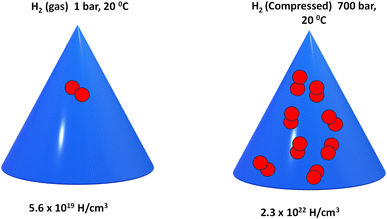
Gaseous hydrogen storage is a hydrogen storage method that uses a high-pressure vessel to store hydrogen gas at high pressure. It is suitable for large and long-distance situations. Gaseous hydrogen storage systems require high pressure gas cylinders to store hydrogen at high gravimetric/volumetric density. The latest target set by the global industry for the cylinder is 70 MPa with a mass of 110 kg, resulting in a gravimetric density of 5.06% by mass and a volumetric density of 30 kg m−3.37 Hydrogen gas can be stored in the four types of pressure vessels. The choice of the vessel is based on the final application, which requires a trade-off between technical performance and cost competitiveness.For industrial application where large amount of hydrogen is used or regulated, Type I tank is used. This type can handle compression pressure between 150–300 bar and are the cheapest in the market. Type II are mainly used for stationary applications due to their remarkable strength that allows the gas to be stored at a very high pressure. Type III and Type IV containers are intended for portable applications where weight savings are essential. However, these vessels are much more expensive.38 Their advantages include fast hydrogen speed, relatively mature technology, as well as normal temperature operation and low cost, but hydrogen can easily escape. The safety of pressure cylinders must be considered, especially in densely populated regions. In addition to pressure cylinders, there are also many studies on underground hydrogen storage, which is suitable for large-scale storage of gaseous hydrogen, such as exploiting abandoned oil fields. Fig. 3 shows a representation of H2 gas inside a cylinder.39
 | ||
| Fig. 3 Representation of a hydrogen gas inside a cylinder; a non-compressed versus compressed gas [Fraunhofer IFAM, 2023]. | ||
3.2 Liquid hydrogen storage
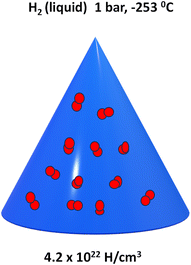
Liquid hydrogen, Fig. 4, storage is a process in which hydrogen is compressed, cooled to 21 K (−252.15 °C) and then stored in a special adiabatic vacuum vessel, such as cryotanks at 21.2 K (−251.95 °C) and ambient pressure. Due to the low critical temperature of hydrogen 33 K (−240.15 °C), liquid hydrogen can only be stored in open systems because there is no liquid phase above the critical temperature. Furthermore, the pressure in a closed storage system could increase to around 104 bar at room temperature. One advantage of this storage method is its volumetric density, which is 70.8 kg m3, 0.2 kg m3 higher than that of solid hydrogen.39The challenges in storing liquid hydrogen are the energy-efficient liquefaction process and thermal insulation of the cryogenic storage vessel to reduce hydrogen boil-off. The size, form, and thermal insulation of the tank affect the pace at which hydrogen boils off from a liquid hydrogen storage vessel because of heat leakage. A spherical shape is the ideal shape in theory since it has the lowest surface to volume ratio and the stress and strain can be evenly distributed in the walls of the tank. However, due to the difficulties in manufacturing such tanks, they are expensive. With such design that has high storage capacity, the evaporation rate decreases significantly. Boil-off losses from heat leaks are related to the surface to volume ratio.39
It has been reported that boil-off losses for double-walled vacuum-insulated spherical Dewar vessels are generally 0.4% per day for tanks with a storage volume of 50 m3, 0.2% for tanks with a volume of 100 m3, and 0.06% for tanks with a volume of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3.40 Additionally, liquid hydrogen storage systems can only be used in situations where the cost of hydrogen is not a significant factor and the hydrogen is consumed relatively quickly, due to the relatively high energy requirements for liquefaction and continuous boil-off of hydrogen. Its liquefaction process involves high energy consumption, high cost and the risk of leakage. Its advantage is that the bulk energy density of liquid hydrogen is several times higher than that of compressed storage.40
000 m3.40 Additionally, liquid hydrogen storage systems can only be used in situations where the cost of hydrogen is not a significant factor and the hydrogen is consumed relatively quickly, due to the relatively high energy requirements for liquefaction and continuous boil-off of hydrogen. Its liquefaction process involves high energy consumption, high cost and the risk of leakage. Its advantage is that the bulk energy density of liquid hydrogen is several times higher than that of compressed storage.40
3.3 Solid hydrogen storage
In contrast to pressurized hydrogen gas and cryogenic liquid hydrogen, hydrogen storage requires more significant technological advances. Solid hydrogen storage is the storage of hydrogen by means of physisorption or chemisorption processes as shown in Fig. 3. Solid hydrogen storage method has high storage capacity, safe transportation, and good economy. This method has presented some good qualities such as being very safe, effective, inexpensive, light weight, and compact, but it requires more investigation. Previous research shows that a typical pressurized hydrogen gas and cryogenic liquid hydrogen need a lot of room, a large storage system, and have safety concerns in addition to being expensive and inefficient.8,41–43 As a result, they fall short of meeting future goals for a hydrogen economy.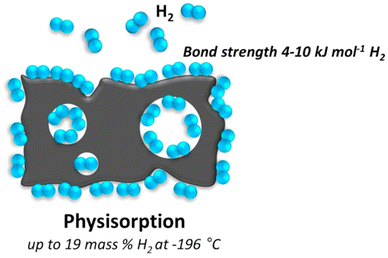 | ||
| Fig. 5 Schematic representation of current approaches to store hydrogen with materials. Reproduced with permission from [Sun Y.41 et al., 2018]. | ||
The driving forces behind physisorption are the attractive interactions between the permanent or temporary dipoles in the adsorbate and the surface. These forces are proportional to the polarizability of the molecules involved and increase with the surface area of the adsorbent. As a result, physisorption tends to occur at lower temperatures and can often be reversible with changes in temperature and pressure. This characteristic makes it useful in various applications, including gas storage, chromatography, and certain catalytic processes. Physisorption is usually characterized by its reversible nature, meaning that adsorbed molecules can be easily released from the surface when conditions change. Common techniques for studying physisorption include gas adsorption isotherms, which plot the amount of adsorbate adsorbed on a surface at different pressures and temperatures. The data obtained from these experiments can be used to determine the adsorption capacity, surface area, and pore size distribution of materials.44
Resonant fluctuations of the charge distributions, also known as dispersive interactions or van der Waals interactions, are the cause of the physical adsorption of gas molecules onto the surface of a solid. A gas molecule interacts with a number of atoms at the solid's surface during the physisorption process.45 The interaction consists of an attracting component that decreases to a power of −6 with increasing distance between the molecule and the surface, and a repulsive term that decreases to a power of −12 with increasing distance. As a result, the molecule's potential energy is at its lowest at about one molecular radius from the adsorbate, and the energy minimum is of the order of 0.01–0.1 eV (1–10 kJ mol−1).46 Significant physisorption is only detected at low temperatures (273 K (−0.15 °C)) because of the weak contact.
The interaction between a gaseous molecule and the surface of a liquid or solid adsorbate occurs once a monolayer of adsorbate molecules has formed. As a result, the latent heat of sublimation or vaporization of the adsorbate is comparable to the binding energy of the second layer of adsorbate molecules.47 As a result, one single monolayer is adsorbed when the adsorption occurs at a temperature equal to or higher than the adsorbate's boiling point at a specific pressure. The density of the liquid adsorbate and the volume of the molecule can be used to calculate the amount of adsorbate in the monolayer. The density of the liquid ρliq and the molecular mass of the adsorbate Mads can be used to determine the minimal surface area Sml for 1 mol of adsorbate in a monolayer on a substrate when a liquid is considered to have a closed packed fcc structure.
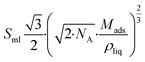 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 917 m2 mol−1. The quantity of adsorbate mads on a substrate with a given surface area Sspec, is given by mads = Mads × Sspec/Sml. Maximum specific surface area of carbon in the scenario including hydrogen as the adsorbate is Sspec = 1315 m2 g−1 (single side graphene sheet), and the maximum amount of adsorbed hydrogen is mads = 3.0 mass%. This theoretical approximation leads to the conclusion that the amount of adsorbed hydrogen is only detectable at very low temperatures and is related to the specific surface area of the adsorbent, with mads/Sspec = 2.27 × 10−3 mass% m−2 g.48
917 m2 mol−1. The quantity of adsorbate mads on a substrate with a given surface area Sspec, is given by mads = Mads × Sspec/Sml. Maximum specific surface area of carbon in the scenario including hydrogen as the adsorbate is Sspec = 1315 m2 g−1 (single side graphene sheet), and the maximum amount of adsorbed hydrogen is mads = 3.0 mass%. This theoretical approximation leads to the conclusion that the amount of adsorbed hydrogen is only detectable at very low temperatures and is related to the specific surface area of the adsorbent, with mads/Sspec = 2.27 × 10−3 mass% m−2 g.48
For instance, in catalytic converters used in automobiles, there are more reactive sites available to harmful gases like carbon monoxide and nitrogen oxides for the reaction and form less harmful compounds. Another example is the adsorption of hydrogen on metal surfaces in hydrogen storage applications.52 The process involves the dissociation of hydrogen molecules into atomic hydrogen and incorporation of hydrogen atoms into the material's lattice as demonstrated in Fig. 6(a–c), forming a new hydride phase.
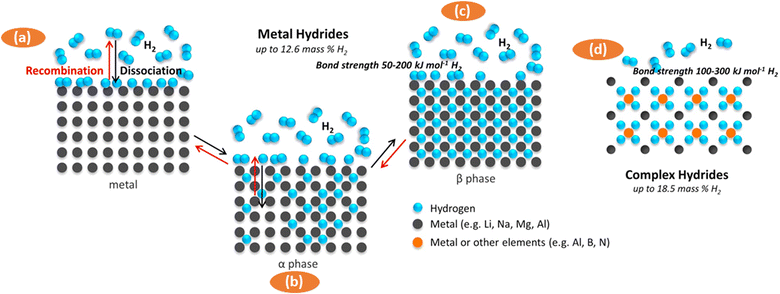 | ||
| Fig. 6 Schematic representation of current approaches to store hydrogen with materials (a)–(d). Reproduced with permission from [Sun Y.41 et al., 2018]. | ||
For example, MgH2, one of the hydrides, with a hydrogen storage density of 6.5 × 1022 H atoms per cm3 (7.6 wt% theoretical capacity) compared to liquid gas densities of 4.2 × 1022 H atoms per cm3 require larger bonding energy than hydrogen physisorption, which is greater than 40 kJ mol−1.53,54 High working temperatures (300–400 °C) are necessary for such hydrides, and these types of hydrides may be either “too stable” or “too unstable”. The “too stable” hydrides can absorb hydrogen reasonably easily but have higher breakdown temperatures. In contrast, the very unstable hydrides need high hydrogen pressures and temperatures for the absorption process to take place, but they can desorb or rapidly desorb hydrogen at ambient temperature or lower.55
Another example that requires high activation energy which is achieved at high operating temperatures (300–400 °C) is LiBH4 (such as LiBH4, 18.3 wt% theoretical capacity).56 Some reports suggested the use of composites to enhance the absorption of hydrogen such as the one demonstrated in Fig. 6(d). For instance, composite materials like Ni-based catalysts and TiO2 heterojunction have been used to improve hydride performance. Rare earth alloys like LaNi5 can absorb/desorb hydrogen at room temperature but their storage capacities are generally less than 1.8 wt%.57,58
Several new and novel solid materials for hydrogen storage, following the chemisorption process, have drawn attention in recent years due to their safest and most efficient hydrogen chemisorption properties and high hydrogen density hydrogen absorption at a very low pressure. They have been identified and broadly classified into the following categories:
(i) Metal hydrides
(ii) Light metal-based hydrides
(iii) Complex hydrides.
3.3.2.1 Metal hydrides. Metal hydrides are compounds formed by the reaction of hydrogen with metals, intermetallic compounds, and alloys.59 They can store atomic hydrogen in the interstitial sites of the metal lattice using an intermetallic alloy phase. Metal hydrides are intrinsically safer than a compressed gas or a liquid hydrogen storage. Storing hydrogen in this way depends on its properties to react reversibly with a metal and keep it safer at room temperature and standard pressure. The reaction of hydrogen with metal was first described by Graham in 1866, observing the absorption of hydrogen by palladium (Pd).
This observation led to an extensive study of metals with the ability to store hydrogen. However, under ambient conditions, physical hydrogen storage material exhibits low hydrogen storage capacity. In addition, due to their slow kinetics, metal hydrides cannot evolve hydrogen at low temperatures. Therefore, the development of hydrogen storage materials with high hydrogen storage capacity and low constraint has been a challenge for the growth of hydrogen energy industry.59 The following simple equation can be used to describe the usual interaction of metal hydrides with hydrogen:
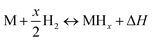 | (2) |
The strong dependence of the hydride formation on the activation energy is graphically represented by S. Liu, 2022,57 which shows the energy of interaction at interface (H2/metal surface) during the physisorption and chemisorption processes. The distance between the metal and the hydrogen atoms at the interface determine the process by which the sorption process will occur; the closer distance the surface of the metal favours the endothermic or exothermic chemisorption process depending.61
It is essential for an ideal hydrogen storage material to possess these following properties: (i) a moderate dissociation pressure and low dissociation temperature, (ii) a high hydrogen capacity per volume and unit mass, these determines the amount of energy that is available/accessible; (iii) reversibility, (iv) low heat of formation to minimize the energy required for hydrogen release, (v) safe material to use, (vi) cyclability, low reusing and charging infrastructure costs, (vii) fast kinetics, (viii) high stability against humidity for long service life, (ix) low heat release during exothermic hydride formation and (x) limited energy loss during the charging and discharging of hydrogen.62
Because of their low weight and high hydrogen atom density per metal atom, light metals like Li, Be, Na, Mg, B, and Al particularly fascinating because they produce a wide range of metal hydrogen compounds. Heavier compounds may only enter the multicomponent system as an additive in small amounts, most likely to change properties or as a catalyst. A summary of the different metal hydride materials' hydrogen storage and compression system performance is shown in Table 3. The data that was previously published by few authors was used to determine the typical values.
| Parameters (units) | Typical range/value | ||||
|---|---|---|---|---|---|
| AB5 | AB2 | AB2 | BCC-Ti–Cr–V | MgH2 | |
| Operating temperatures [°C] | 0 to 200 | −50 to 150 | 0 to 100 | −20 to 30 | 250 to 400 |
| Operating H2 pressures [atm] | 0.1 to 500 | 1 to >1000 | 1 to 30 | 10 to 300 | 1 to 20 |
| Gravimetric hydrogen storage density [wt%] | 1.50 | 1.90 | 1.75 | 2.5 | 5.5 to 7.5 |
| Volumetric hydrogen storage density [kg L−1] | 0.10 | 0.10 | 0.09 | 0.11 | 0.11 |
| Material system | 0.063 | 0.061 | 0.055 | 0.069 | 0.06 |
The most widely utilized low-temperature intermetallic hydrides have a weight hydrogen storage density that ranges from 1.5 to 1.9 wt%, the usage of Body Centered Cubic solid solution alloys based on the Ti–Cr–V system enables the capacity of hydrogen storage density of approximately 2.5 wt%. These latter materials can be utilized in hybrid hydrogen storage systems charged with H2 gas at high pressures and at subzero temperatures, along with certain AB2-type intermetallic compounds. Although MgH2 based hydrogen storage materials have far higher hydrogen storage densities, their applicability is limited to a few situations when a high-temperature heat source is present due to their high working temperature requirements.63
The volumetric hydrogen storage density on the system level will be lower due to variations in the parent material densities and the restricted safe densities for filling the materials in the containment, even though the considered hydride materials have very high volumetric hydrogen storage densities that greatly surpass the liquid hydrogen's density of ∼0.07 kg L−1. For stationary energy storage systems, the material selection criteria will primarily relate to conditions and performances of their operation, such as temperature/pressure ranges, hydrogen absorption/desorption kinetics, ease of activation and cycle stability, as opposed to hydrogen storage densities. The system volumetric hydrogen storage densities presented in the last row of Table 3, will therefore take comparable values for the various materials.
The most common used hydride materials in hydrogen compression and storage application, as well as in hydrogen supply to fuel cell systems, are AB5- and AB2-type intermetallic compounds. This is because these kinds of materials can adjust their hydrogen sorption characteristics through slight compositional changes. This presents a chance to match these materials' operating characteristics at different pressures and temperatures to the requirements of the application.64
Table 4 lists the properties of the MH materials under study, such as their capacities for hydrogen absorption at room temperature (20 °C) and at the designated H2 pressures. It also shows the temperatures that correspond to the H2 desorption plateau pressures at H concentration that match the plateau midpoint. The authors' experimental PCT data, which was then processed by the model, was used to calculate the values that were presented from hydrogen absorption and desorption isotherms for the specified materials built at various temperatures. The fitted model parameters allowed to build the isotherm at any selected temperature. The AB5-type materials, which have varying Ce/La, and the AB2-type, which have varying Zr/Ti ratios, as shown in Table 2, enable the development of diverse hydrogen storage and compression systems that can function in a range of temperature and H2 pressures. The cycle productivities of the hydrogen compressors and the useable hydrogen capacities for hydrogen storage, however, cannot be estimated using the presented data.64
| Characteristic (unit) | MH material | |||||
|---|---|---|---|---|---|---|
| LaNi5 | La0.8Ce0.2Ni5 | La0.5Ce0.5Ni5 | Ti0.55Zr0.45(Cr, Mn,Fe,Ni)2 | Ti0.65Zr0.35(Cr,Mn,Fe,Ni)2 | ||
| Hydrogen adsorption at T = 20 °C | Pressure atmosphere (atm) | 10 | 10 | 40 | 15 | 50 |
| Full H absorption capacity [NL kg−1] | 158 | 165 | 129 | 158 | 153 | |
| Hydrogen desorption temperature (°C) at plateau pressure atmosphere of (atm) | 1 | 18 | −8 | −30 | −34 | −60 |
| 10 | 77 | 52 | 24 | 42 | −10 | |
| 100 | 168 | 145 | 111 | 196 | 70 | |
| 200 | 206 | 186 | 150 | 281 | 105 | |
Some of the limitations of metal hydrides is that the energy density of metal hydride storage is very high in terms of volume, but it is relatively low in terms of weight (kW h kg−1). Values typically range from 1% to 9% of weight. As a result, metal hydride storage tanks weigh between 250 and 300 kg, or almost four times as much as gasoline tanks. Storage tanks are currently too heavy for use in passenger cars and have limited uses, even with advancements in metal hydride technology. The use of storage systems is restricted to small-scale applications due to the high cost of metals like lithium and lanthanum. Appropriate applications were further limited by the slow uptake rates exhibited by early systems. In terms of safety, handling metal hydrides can be difficult because they react violently with moist air. The purity of the hydrogen is also a concern because impurities affect how well metal hydrides work. Challenges include the management of these systems' heat as well as the efficiency and reversibility of metal hydride storage systems across a large number of cycles.65 Metal hydrides are still somewhat expensive to produce, which deters potential users from using them in commercial bids. The cost and availability of materials, as well as the intricacy and effectiveness of the hydrogen release and storage systems, can all have an impact on their practical applicability. Different performance-enhancing approaches are being developed to address these issues; for example, new synthesis techniques for metal hydrides and the utilization of innovative materials and nanostructures to increase their hydrogen storage capacity.66
3.3.2.2 Light metal-based hydrides. The lightweight hydrides, which include metal hydrides and complex hydrides, have exceptionally high gravimetric and volumetric densities and are anticipated to be the most promising candidates for on-board applications among the chemisorption materials.67 Metal elements have small atomic masses, which results in the heavy hydrides having a high gravimetric hydrogen concentration.68 The high thermodynamic stability and poor kinetics of lightweight hydrides, however, mean that they frequently need a high hydrogen desorption temperature and exhibit poor reversibility under benign conditions. In the field of hydrogen storage, the lightweight hydrides materials have attracted a lot of attention due to a strong ionic bond between the metal element and hydrogen which causes a high breakdown temperature and a high formation enthalpy.69
Furthermore, they have slow kinetic characteristics.70 Among the light metal complex hydrides are amides, alanates, and borohydrides (shown in Fig. 7). These molecules contain a remarkable amount of hydrogen and go through a difficult, multi-step dehydrogenation procedure.71 As a result of the decomposition's many component generations, dehydrogenation is challenging, and the cycle's characteristics are poor. Unsuitable thermodynamic and kinetic characteristics are another issue plaguing complex hydrides.72 Fig. 7 shows the representation of light metal complex hydrides.73
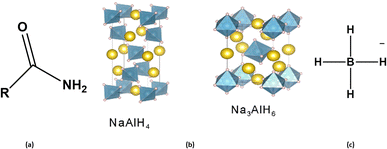 | ||
| Fig. 7 Light metal complex hydrides; (a) amides, (b) alanates, and (c) borohydrides. Reproduced with permission from [Ligterink N. F. W.,73 et al., 2018]. | ||
There are techniques such as doping and nanoscaling that have been implemented in the past to enhance the regeneration processes and raise kinetic properties of such hydrides74 but the results could not satisfy the requirements for on-board application, which include kinetic, thermodynamic, and capacity performances. Reaching large capacity, moderate thermodynamic conditions, and quick kinetic performance at the same time is quite challenging. There have been numerous attempts to control thermodynamic and kinetic performance with the least amount of capacity loss, but more research is still required.75
The use of light metal hydrides for hydrogen storage has a bright future with the continuous research and development efforts. Faster hydrogen uptake and release could be made possible by continued advances in kinetic characteristics brought on by catalyst development, nanoscale engineering, and alloy design. New light metal hydride compositions and architectures may also produce materials with improved stability and performance. Beyond storing hydrogen for transportation, light metal hydrides have numerous practical applications.76 They can balance renewable energy grids when used in stationary energy storage systems, where excess renewable energy can be stored as hydrogen and converted back to electricity. A vital step in the transition to a sustainable energy future, light metal hydrides offer a convincing solution to the problems associated with hydrogen storage. Despite improvements in knowledge of their characteristics and performance optimization, problems with kinetics, thermodynamics, and material stability still exist. Light metal hydrides have the potential to be a fundamental facilitator of hydrogen-based technologies and contribute to cleaner and more effective global energy systems with continued study and innovation.77
3.3.2.3 Chemical hydrides (complex hydrides). The light metals of groups 1, 2, and 3, such as Li, Mg, B, and Al, can form a wide range of metal–hydrogen complexes as shown in Fig. 8. They are particularly intriguing due to their light weight and the frequent presence of two hydrogen atoms per metal atom. The transformation of the metals to an ionic or covalent compound upon hydrogen absorption is the primary distinction between the complex hydrides and the metallic hydrides previously mentioned. In complicated hydrides, the hydrogen is frequently found in the corners of a tetraeder, with aluminum or boron in the center. A cation, such as Li or Na, balances out the anion's negative charge, which is caused by [BH4] and [AlH4]. The hydride complexes of borane, the tetrahydroborates M(BH4), and of alane, the tetrahydroaluminate M(AlH4), are fascinating storage compounds, but they were discovered to be stable and only disintegrate at high temperatures, frequently exceeding the melting point of the complex.78
 | ||
| Fig. 8 Overview of light metal complex hydrides; amides, alanates, and borohydrides. Reproduced with permission from [He T.82 et al., 2019]. | ||
In addition to having excessive hydrogen densities, complex metal hydrides (CMHs) made of light elements such as boron, nitrogen, or aluminum frequently have poor thermodynamic and kinetic properties as well as a restricted capacity for reversibility.79 Initially, a paradigm change in hydrogen storage research toward complex anions was brought about by the discovery of reversibility in titanium catalyzed NaAlH4.80 As a result, research was broadened to include complicated hydrides based on nitrogen, such as LiNH2, and metal borohydrides, such as LiBH4. Most of the covalent connections in the complex hydride anions have a clear orientation, whereas ionic bonding predominates when the complex is solid.81,82
An in-depth understanding of thermodynamics is required to comprehend the stability of complex hydrides. Therefore, temperature (T) and pressure (P) must be included in order to characterize the Gibbs free energy (G) of the compounds which is given by the eqn (3).
| ΔG = ΔH − TΔS | (3) |
| H = E + PV | (4) |
S = kb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ω Ω
| (5) |
It is important to understand how the complex hydride's stoichiometry varies in relation to its composition (X). If the specific heat at constant pressure (Cp) is identified, the temperature dependency can be attained. From there, it is simple to determine the enthalpy (H) and entropy (S) via integration, and as a result, G may be calculated. Cp readings from low temperatures (around 0 K) to room temperature should be supplied to characterize the compound's conventional thermodynamic properties. For condensed phases (such as solids and liquids), the pressure dependency of G is often very minimal, and for gaseous phases, it is easily attainable from partial pressure measurements. Surface energy and interfacial energy should also be understood when dealing with phase changes and scaffold interaction in complex hydrides, possibly as a function of T and P. In this instance, environmental factors, such as gas or solid phases in equilibrium with the complicated hydride, have an impact on energy levels.83,84
4. Recent developments in multi-layered thin films
A thin film is a layer of material that is in the range of nanometres to a few micrometres thick. Different deposition techniques are used by numerous industries to produce thin films. A common application of thin films in daily life is the metal-coated glass that serves as the mirror in every home. To construct various types of vacuum coating systems, Vac Coat utilizes Physical Vapour Deposition (PVD) techniques as sputtering, thermal evaporation, and pulsed laser deposition (PLD). Thin films are important because depositing them on solids assists in improving their surface properties. In this manner, forming a thin film on a bulk material is used to obtain the desired mechanical, electrical, or optical behaviours on the bulk material surface, thus achieving exceptional features such as higher conductivity, corrosion resistance, reflection, or hardness on the surface.85Many of the modern technologies of today rely heavily on thin films. Thin films are crucial to many operations, including those in the packaging, aerospace, and organic electronics sectors. There are numerous instances where a certain thin-film characteristic gave rise to a completely new technological area. Thin film engineering has evolved over the past few decades from a scientific novelty to a multi-billion-euro industry globally. Every year, new production technologies and cutting-edge methods are introduced to expand the thin film industry. To create novel thin films and unique nanostructured thin films is one of the most intriguing motives. For this reason, a variety of cutting-edge methods based on the magnetron sputtering technique have proliferated recently, including, combined vacuum processes, the use of complex precursor gases, and the integration of particle guns in the reactor, just to name a few.85
For better performance and design flexibility, thin-film transistors, or TFTs, are utilized in integrated circuits, organic electronics, and flexible displays. Thin-film solar cells, such as those made of copper indium gallium selenide (CIGS) or cadmium telluride (CdTe), provide an affordable substitute for conventional silicon-based solar cells. Thin films are used to store data in magnetic storage systems like hard disk drives because they can hold data in a stable and compact form. Thin films are used in interference filters, anti-reflection coatings, and optical coatings on lenses to obtain particular optical qualities. Solar cells, flexible electronics, touchscreens, and indium tin oxide (ITO) coatings are examples of transparent conductive sheets in use. These examples show how the special properties of thin films contributed to the creation of a wide range of technological applications, ranging from electronics and energy harvesting to optics and materials science.86
It is commonly noted that most scientific and technical advancements are strongly related to and frequently constrained by the functionality of materials and surfaces. As a result, over the past ten years, new scientific disciplines relevant to the development of intelligent materials, functional materials, biomaterials, etc. have emerged. Thus, laboratory curiosities like structured thin films are transformed into products with significant added value. Complete technologies may now be dependent on their characteristics and integration as they develop into a science in and of themselves.87
The dc and rf sputtering techniques have been utilized extensively over the past 10 years in both of their balanced and unbalanced magnetron forms. The areas of industry and science have seen the most uses. Decorative thin films, hard-wear resistant thin films, low-friction thin films, and corrosion-resistant thin films, thin films used as protective optical systems, and thin films used in the electronic industry are a few examples of thin films used in industrial settings. Additionally, researchers have worked on improving the efficiency of the system. These initiatives were started by the balanced or conventional magnetron sputtering, which was then followed by the creation of unbalanced systems and their integration into multi-source “closed field” systems.
At last, by applying a unipolar high-power pulse with a low frequency and low duty cycle to the cathode target, also known as high-power impulse magnetron sputtering (HPIMS) or high-power pulsed magnetron sputtering (HPPMS), the sputtering technique can increase the rate of deposition and ion energy. Plasma with an extremely high density is present in all highly ionized procedures. These discharges are used in sputter deposition technology to modify component surfaces, improving the material's mechanical, chemical, optical, electrical, and many other properties.88
In contrast with conventional physisorption, hydrogen can be held in the interlayer region of multilayered materials. Theoretically, previous research has shown that multilayer graphene with tight interlayer spacing has a hydrogen adsorption enthalpy of about 13.1 kJ mol−1, which is much greater than samples with large interlayer spacing.89 The interlayer space of two-dimensional (2D) materials has a quantum sieving effect that contributes to the phenomena known as the nano-pump effect. At a temperature of 300 K and a mild pressure of 4 MPa, the multilayered and mesoporous graphene could reversibly store 4.65 weight percent of hydrogen.90 The absorption of hydrogen molecules between graphite layers using first-principles calculations and traditional grand-canonical Monte Carlo simulations were investigated and according to the research, graphene has an optimal hydrogen storage density of 2.3 wt% at 298 K and 50 bar H2.91
To investigate the behaviour of hydrogen absorption within the bilayer-graphene bubble structure and the effect of layer spacing, molecular dynamics simulations were employed.92 The hydrogen storage capacity of graphene bubbles can reach 13.7 weight percent at 77 K and 100 bar H2. Due to the nano pump effect, it has also been reported that the multilayered MXene Ti2CTx (Tx, functional groups, F, O, and OH) has a large capacity (Fig. 9). At 298 K and 60 bar, Ti2CTx with an interlayer distance of 0.68 nm had a storage capacity of 8.8 weight percent. The temperature-programmed desorption (TPD) result for Ti2CTx in Fig. 5 demonstrates mild chemisorption and physisorption.93
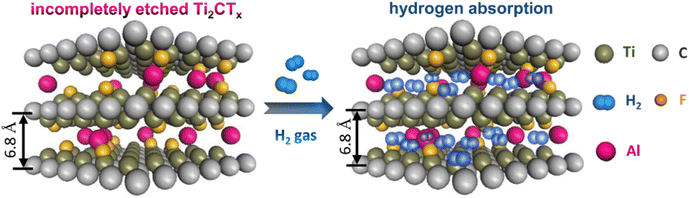 | ||
| Fig. 9 Schematic illustration of hydrogen storage in the interlayer space of Ti2CTx. Reproduced with permission from [Liu S. and Shui J.,94 2022]. | ||
The crucial structural factor that affects the effectiveness of hydrogen storage in multilayered materials is the interlayer spacing. The strength of hydrogen adsorption is influenced by the elemental compositions of the layer as well as by the functional groups on the layer. We believe that in addition to graphene and MXene, many other multilayer materials, including montmorillonite, MoS2, BN, and others, should be capable of storing hydrogen with a nanopump, but further research is required to determine the proper interlayer distances and surface functional groups.94
The growing interest in synthesizing multilayer system suitable for hydrogen storage has opened platform for various groups to engage in such research. For instance, Ouyang et al.94 have worked on MgNi/Pd multilayer thin films for hydrogen storage synthesis via DC magnetron sputtering. Each MgNi layer had a thickness of 40 nm, while each Pd layer had a thickness of 16 nm. The hydrogen absorption content of the films, measured using a Pressure Composition Isotherm technique, reached approximately 4.6 mass percentage at room temperature. The hydrogen desorption content reached 3.4 mass%, and the dehydrogenation was incomplete and left 1.2 mass% of hydrogen in the thin film. With a maximum value of 505 mA h g−1, the deposited MgNi/Pd multilayer thin films exhibited an intriguing discharge capacity behavior. After the sample had gone through the first two cycles, the PCI curves for the three cycles were compared, and the multilayer film demonstrated good hydrogen storage performance.
The multilayer structure of Pd and MgNi is only a few tens of nanometers thick and the Pd layer may serve as catalyst for hydrogen storage, improving the hydrogenation properties of MgNi/Pd multilayer thin films. Other studies revealed that the direct current magnetron sputtering-produced nanocrystalline MgNi thin film demonstrated better kinetic performances compared to the typical polycrystalline phases, for which the previous reports shown a decrease in hydrogen absorption and desorption temperatures. It was also noted in another study that in MgNi/MmM5 multilayer films, the thin MmM5 layer functions as a catalyst to speed up the hydrogenation/dehydrogenation process of the MgNi layer in MgNi/MmM5 multilayer films.95
Tarnawski et al.96 also investigated hydrogen storage in Ti–TiO2 multilayers, giving attention to all of the important parameters in both hydrogen storage and photocatalysis. In their study, several Ti–TiO2 single-, bi- and tri-layered thin films were deposited onto various substrates using the dc pulsed magnetron sputtering from a metallic Ti target in an inert Ar or reactive + O2 environment. Using Rutherford backscattering X-ray diffraction, X-ray reflectometry, and optical reflective spectra, they demonstrated that the Ti thin films which were deposited on Si(111) have a strong preferred orientation with the (001) plane parallel to the substrate, whereas TiO2 films have a columnar structure.
H charging at 1 bar and at 300 °C revealed that, in the case of the tri-layered structure of Ti/TiO2/Ti/Si (111), H diffused through the TiO2 layer without any accumulation in it. The inclusion of a Pd layer in their system enhanced hydrogen absorption up to 50% of H in the topmost and bottom Ti layers. But the preferential orientation in the Ti films was found to be destroyed upon hydrogenation at 100 bars, while the hydride TiHx phase (x < 0.66) was formed.96
In another study, Jung et al. investigated the effects of Ti interlayers on microstructures and hydrogen storage capacity in Mg/Pd multilayer thin films, in which 60 multilayer Mg/Pd and Ti/Mg/Ti/Pd films were prepared using an ultra-high-vacuum (UHV) DC magnetron sputtering system. The hydrogen absorption capacity was found to be 1.7, 3.5, and 4.7 wt% at 50, 100, and 150 °C for the Ti/Mg/Ti/Pd film. They concluded that the hydrogen absorption capacity for the Mg/Pd and Ti/Mg/Ti/Pd films depends greatly on the formation of Mg–Pd intermetallic phases.97
The Mg/Fe multi-layered film was studied by Mooij et al. In their study, they investigated the effect of microstructure on the hydrogenation of Mg/Fe thin film multilayers. Their results displayed the improved stability of the confined magnesium hydride because of elements such as Fe and Ti. For example, the hydrogenation of nanoscale magnesium layers in Fe/Mg/Fe multilayers reveals the existence of numerous plateau pressures, each of whose nature is thickness dependent, while the hydrogen desorption takes place via a single plateau. For the Ti/Mg/Fe and Ti/Mg/Ti multilayers, the MgH2 was unstable at the Fe interface and both the absorption and desorption plateau pressures increased by a factor of 2.98
Jung et al. used the catalytic actions of Pd to improve the hydrogen storage properties of a 40-layer film of Pd (x nm)/Ti (40 nm)/Mg (360 nm)/Ti (40 nm) (×1, 40, 5, 10, and 20). The Pd/Ti/Mg/Ti films had a superior hydrogen uptake of 6.42 wt% for ×1410 at 150C. The hydrogen absorption duration is substantially influenced by the thickness of the Pd layer (0–40 nm). As a result, the Pd/Ti/Mg/Ti multilayer film with the Pd interlayer can be ascribed to providing more diffusion channels and a controlled rate of hydride formation at the Pd/Ti/Mg interfaces, hence improving overall hydrogen storage qualities.99
Furthermore, an important advancement in hydrogen storage technologies in recent times has been the development of solid-state hydrogen storage materials. In comparison to traditional methods of gaseous or liquid storage, they offer a greater volumetric energy density and enhanced safety. Chemical hydrogen carriers and metal hydrides are two instances of this. Additionally, interdisciplinary engineering organizations contribute to the innovation of these materials by combining their expertise in mechanical, chemical, and materials science.100
5. Conclusion
A viable option for a future energy and economic security energy carrier that is affordable, clean, and sustainable is hydrogen. However, the storage of hydrogen continues to be the fundamental barrier to its quick integration into the global economy. Solid-state storage systems made up of metal hydrides have been acknowledged as one of the most workable techniques to store hydrogen in hydrogen-powered systems among the possibilities put up thus far. Research from the past reveals that investigating the characteristics of metal hydrides to create new types is still difficult for scientists and engineers in the sector. A decade of intensive and extensive exploration has led to the identification of several potential hydrogen storage systems. While this progress is encouraging, there are challenging issues such as thermodynamic changes and kinetic improvements that need to be carefully addressed. For a smooth further development of this field of science and technology, the urgent requirements for application-oriented research and a deep understanding of basic principles must be in a balanced relationship to each other.Conflicts of interest
There are no conflicts to declare.References
- M. K. Jangid, S. S. Sharma, J. Ray and S. Jangid, Effect of annealing and hydrogenation on optical and electrical properties of DC sputtered Mg-Ni bilayer thin films, Mater. Today: Proc., 2022, 67, 847–851 CAS.
- P. Ondrejka and M. Mikolášek, Thin Films and Coatings for Energy Storage and Conversion: From Supercapacitors and Batteries to Hydrogen Generators, Coatings, 2023, 13(4), 742 CrossRef CAS.
- L. J. Bannenberg, C. Boelsma, K. Asano, H. Schreuders and B. Dam, Metal hydride based optical hydrogen sensors, J. Phys. Soc. Jpn., 2020, 89(5), 051003 CrossRef.
- L. Ouyang, F. Liu, H. Wang, J. Liu, X. S. Yang, L. Sun and M. Zhu, Magnesium-based hydrogen storage compounds: A review, J. Alloys Compd., 2020, 832, 154865 CrossRef CAS.
- W. Cao, X. Ding, Y. Zhang, J. Zhang, R. Chen, Y. Su, J. Guo and H. Fu, Enhanced de-/hydrogenation kinetics of a hyper-eutectic Mg85Ni15-xAgx alloy facilitated by Ag dissolving in Mg2Ni, J. Alloys Compd., 2022, 917, 165457 CrossRef CAS.
- H. Jindal, A. S. Oberoi, I. S. Sandhu and M. Chitkara, Potential porous mediums for electrochemical hydrogen storage: state of art and comparative study, Mater. Today: Proc., 2020, 21, 1888–1898 CAS.
- M. Hu, G. Triulzi, and M. Sharifzadeh, Technological change in fuel cell technologies, in Design and Operation of Solid Oxide Fuel Cells, Academic Press, 2020, pp. 3–41 Search PubMed.
- J. O. Abe, A. P. Popoola, E. Ajenifuja and O. M. Popoola, Hydrogen energy, economy and storage: Review and recommendation, Int. J. Hydrogen Energy, 2019, 44(29), 15072–15086 CrossRef CAS.
- S. P. Ertem, C. E. Onuoha, H. Wang, M. A. Hillmyer, T. M. Reineke, T. P. Lodge and F. S. Bates, Hydrogenolysis of linear low-density polyethylene during heterogeneous catalytic hydrogen–deuterium exchange, Macromolecules, 2020, 53(14), 6043–6055 CrossRef CAS.
- C. E. Shields, X. Wang, T. Fellowes, R. Clowes, L. Chen, G. M. Day, A. G. Slater, J. W. Ward, M. A. Little and A. I. Cooper, Experimental Confirmation of a Predicted Porous Hydrogen-Bonded Organic Framework, Angew. Chem., Int. Ed., 2023, 62(34), e202303167 CrossRef CAS PubMed.
- L. Ouyang, F. Liu, H. Wang, J. Liu, X. S. Yang, L. Sun and M. Zhu, Magnesium-based hydrogen storage compounds: A review, J. Alloys Compd., 2020, 832, 154865 CrossRef CAS.
- M. Niermann, S. Timmerberg, S. Drünert and M. Kaltschmitt, Liquid Organic Hydrogen Carriers and alternatives for international transport of renewable hydrogen, Renewable Sustainable Energy Rev., 2021, 135, 110171 CrossRef CAS.
- K. Turoń, Hydrogen-powered vehicles in urban transport systems–current state and development, Transp. Res. Procedia, 2020, 45, 835–841 CrossRef.
- M. Kaur and K. Pal, Synthesis, characterization and electrochemical evaluation of hydrogen storage capacity of graphitic carbon nitride and its nanocomposites in an alkaline environment, J. Mater. Sci.: Mater. Electron., 2021, 32, 12475–12489 CrossRef CAS.
- P. E. de Miranda, Hydrogen energy: sustainable and perennial, in Science and Engineering of Hydrogen-Based Energy Technologies, Academic Press, 2019, pp. 1–38 Search PubMed.
- F. A. Khan, N. Pal, and S. H. Saeed, Stand-alone hybrid system of solar photovoltaics/wind energy resources: an eco-friendly sustainable approach, in Renewable Energy Systems, Academic Press, 2021, pp. 687–705 Search PubMed.
- R. Prabhukhot Prachi, M. Wagh Mahesh and C. Gangal Aneesh, A review on solid state hydrogen storage material, Adv. Energy Power, 2016, 4(11), 11–22 Search PubMed.
- B. Zhang and Y. Wu, Recent advances in improving performances of the lightweight complex hydrides Li-Mg-NH system, Prog. Nat. Sci.: Mater. Int., 2017, 27(1), 21–33 CrossRef CAS.
- T. Sadhasivam, H. T. Kim, S. Jung, S. H. Roh, J. H. Park and H. Y. Jung, Dimensional effects of nanostructured Mg/MgH2 for hydrogen storage applications: a review, Renewable Sustainable Energy Rev., 2017, 72, 523–534 CrossRef CAS.
- S. Ohta, Molecular hydrogen as a novel antioxidant: overview of the advantages of hydrogen for medical applications, Methods Enzymol., 2015, 555, 289–317 CAS.
- M. Gurz, E. Baltacioglu, Y. Hames and K. Kaya, The meeting of hydrogen and automotive: a review, Int. J. Hydrogen Energy, 2017, 42(36), 23334–23346 CrossRef CAS.
- G. D. Brewer, Hydrogen Aircraft Technology, Routledge, 2017 Search PubMed.
- H. Wang, Multicriteria sustainability ranking of biohydrogen systems, in Waste to Renewable Biohydrogen, Academic Press, 2023, pp. 195–210 Search PubMed.
- C. L. Eh, A. N. Tiong, J. Kansedo, C. H. Lim, B. S. How and W. P. Ng, Circular Hydrogen Economy and Its Challenges, Chem. Eng. Trans., 2022, 94, 1273–1278 Search PubMed.
- F. V. Donzé, L. Truche, P. Shekari Namin, N. Lefeuvre and E. F. Bazarkina, Migration of natural hydrogen from deep-seated sources in the São Francisco Basin, Brazil, Geosciences, 2020, 10(9), 346 CrossRef.
- S. L. Worman, L. F. Pratson, J. A. Karson and W. H. Schlesinger, Abiotic hydrogen (H2) sources and sinks near the Mid-Ocean Ridge (MOR) with implications for the subseafloor biosphere, Proc. Natl. Acad. Sci. U. S. A., 2020, 117(24), 13283–13293 CrossRef CAS PubMed.
- I. Moretti and M. E. Webber, Natural hydrogen: a geological curiosity or the primary energy source for a low-carbon future, Renewable Matter, 2021, 34(6), 1–6 Search PubMed.
- M. Kayfeci, A. Keçebaş, and M. Bayat, Hydrogen production, in Solar Hydrogen Production, Academic Press, 2019, pp. 45–83 Search PubMed.
- C. Acar and I. Dincer, Impact assessment and efficiency evaluation of hydrogen production methods, Int. J. Energy Res., 2015, 39(13), 1757–1768 CrossRef CAS.
- L. Cozzi, T. Gould, S. Bouckart, D. Crow, T. Y. Kim, C. McGlade, P. Olejarnik, B. Wanner, and D. Wetzel, World Energy Outlook 2020, International Energy Agency, Paris, France, 2020, pp. 1–461 Search PubMed.
- Polygeneration with Polystorage: for Chemical and Energy Hubs, ed. K. R. Khalilpour, Academic Press, 2018 Search PubMed.
- W. A. Lu, Z. Ji and S. U. Yutong, Research Progress of Several New Types of Solid Hydrogen Storage Materials, Acta Pet. Sin., 2023, 39(1), 229 Search PubMed.
- J. Andersson and S. Grönkvist, Large-scale storage of hydrogen, Int. J. Hydrogen Energy, 2019, 44(23), 11901–11919 CrossRef CAS.
- C. Y. Ahn, J. E. Park, S. Kim, O. H. Kim, W. Hwang, M. Her, S. Y. Kang, S. Park, O. J. Kwon, H. S. Park and Y. H. Cho, Differences in the electrochemical performance of Pt-based catalysts used for polymer electrolyte membrane fuel cells in liquid half-and full-cells, Chem. Rev., 2021, 121(24), 15075–15140 CrossRef CAS PubMed.
- Y. Wang, B. Seo, B. Wang, N. Zamel, K. Jiao and X. C. Adroher, Fundamentals, materials, and machine learning of polymer electrolyte membrane fuel cell technology, Energy AI, 2020, 1, 100014 CrossRef.
- Y. Wang, X. Wang, Y. Fan, W. He, J. Guan and X. Wang, Numerical investigation of tapered flow field configurations for enhanced polymer electrolyte membrane fuel cell performance, Appl. Energy, 2022, 306, 118021 CrossRef CAS.
- T. Kimura, H. Miyaoka, T. Ichikawa and Y. Kojima, Hydrogen absorption of catalyzed magnesium below room temperature, Int. J. Hydrogen Energy, 2013, 38(31), 13728–13733 CrossRef CAS.
- H. Barthélémy, AIR LIQUIDE, 75 Quai d'Orsay, Paris, 75007, France, E-mail: herve.barthelemy@airliquide.com.
- https://www.ifam.fraunhofer.de/en/Aboutus/Locations/Dresden/HydrogenTechnology/hydrides/applications-of-metal-hydrides.html.
- A. Züttel, Introduction to the Hydrogen Books, Utilization of Hydrogen for Sustainable Energy and Fuels, 2021, p. 117 Search PubMed.
- Y. Sun, C. Shen, Q. Lai, W. Liu, D. W. Wang and K. F. Aguey-Zinsou, Tailoring magnesium based materials for hydrogen storage through synthesis: Current state of the art, Energy Storage Mater., 2018, 10, 168–198 CrossRef.
- Y. H. Zhang, Z. C. Jia, Z. M. Yuan, T. Yang, Y. Qi and D. L. Zhao, Development and application of hydrogen storage, J. Iron Steel Res. Int., 2015, 22(9), 757–770 CrossRef.
- A. Eftekhari and B. Fang, Electrochemical hydrogen storage: opportunities for fuel storage, batteries, fuel cells, and supercapacitors, Int. J. Hydrogen Energy, 2017, 42(40), 25143–25165 CrossRef CAS.
- S. P. Shet, S. S. Priya, K. Sudhakar and M. Tahir, A review on current trends in potential use of metal-organic framework for hydrogen storage, Int. J. Hydrogen Energy, 2021, 46(21), 11782–11803 CrossRef CAS.
- Q. L. Zhu and Q. Xu, Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage, Energy Environ. Sci., 2015, 8(2), 478–512 RSC.
- X. Chen and G. Wang, Tuning the hydrogen evolution activity of MS 2 (M = Mo or Nb) monolayers by strain engineering, Phys. Chem. Chem. Phys., 2016, 18(14), 9388–9395 RSC.
- Y. Bao, M. Yang, S. J. Tan, Y. P. Liu, H. Xu, W. Liu, C. T. Nai, Y. P. Feng, J. Lu and K. P. Loh, Substoichiometric molybdenum sulfide phases with catalytically active basal planes, J. Am. Chem. Soc., 2016, 138(42), 14121–14128 CrossRef CAS PubMed.
- A. Züttel, Hydrogen storage and distribution systems, Mitigation Adapt. Strategies Global Change, 2007, 12, 343–365 CrossRef.
- C. Mondelli, F. Bardelli, J. G. Vitillo, M. Didier, J. Brendle, D. R. Cavicchia, J. C. Robinet and L. Charlet, Hydrogen adsorption and diffusion in synthetic Na-montmorillonites at high pressures and temperature, Int. J. Hydrogen Energy, 2015, 40(6), 2698–2709 CrossRef CAS.
- X. Zhang, R. B. Lin, J. Wang, B. Wang, B. Liang, T. Yildirim, J. Zhang, W. Zhou and B. Chen, Optimization of the pore structures of MOFs for record high hydrogen volumetric working capacity, Adv. Mater., 2020, 32(17), 1907995 CrossRef CAS PubMed.
- W. Ali, Z. Hao, Z. Li, G. Chen, Z. Wu, X. Lu and C. Li, Effects of Cu and Y substitution on hydrogen storage performance of TiFe0. 86Mn0. 1Y0. 1− xCux, Int. J. Hydrogen Energy, 2017, 42(26), 16620–16631 CrossRef CAS.
- P. Preuster, C. Papp and P. Wasserscheid, Liquid organic hydrogen carriers (LOHCs): toward a hydrogen-free hydrogen economy, Acc. Chem. Res., 2017, 50(1), 74–85 CrossRef CAS PubMed.
- T. H. Kim, J. Bae, T. H. Lee, J. Hwang, J. H. Jung, D. K. Kim, J. S. Lee, D. O. Kim, Y. H. Lee and J. Ihm, Room-temperature hydrogen storage via two-dimensional potential well in mesoporous graphene oxide, Nano Energy, 2016, 27, 402–411 CrossRef CAS.
- B. R. Barnett, H. A. Evans, G. M. Su, H. Z. Jiang, R. Chakraborty, D. Banyeretse, T. J. Hartman, M. B. Martinez, B. A. Trump, J. D. Tarver and M. N. Dods, Observation of an Intermediate to H2 Binding in a Metal–organic Framework, J. Am. Chem. Soc., 2021, 143(36), 14884–14894 CrossRef CAS PubMed.
- M. Ismail, M. S. Yahya, N. H. Idris, N. S. Mustafa, and M. F. Yap, Novel materials and technologies for hydrogen storage, in New Dimensions in Production and Utilization of Hydrogen, Elsevier, 2020, pp. 337–365 Search PubMed.
- S. Liu, J. Liu, X. Liu, J. X. Shang, R. Yu and J. Shui, Non-classical hydrogen storage mechanisms other than chemisorption and physisorption, Appl. Phys. Rev., 2022, 9(2), 213–215 Search PubMed.
- S. Liu and J. Shui, Mechanism and properties of emerging nanostructured hydrogen storage materials, Battery Energy, 2022, 1(4), 20220033 CrossRef CAS.
- H. Nishihara, F. Ohtake, A. Castro-Muñiz, H. Itoi, M. Ito, Y. Hayasaka, J. Maruyama, J. N. Kondo, R. Osuga and T. Kyotani, Enhanced hydrogen chemisorption and spillover on non-metallic nickel subnanoclusters, J. Mater. Chem. A, 2018, 6(26), 12523–12531 RSC.
- A. Züttel, Hydrogen storage methods, Naturwissenschaften, 2004, 91, 157–172 CrossRef PubMed.
- A. Kazakov, D. Blinov, I. Romanov, D. Dunikov, and V. Borzenko, Metal hydride technologies for renewable energy, in E3S Web of Conferences, EDP Sciences, 2019, vol. 114, p. 05005 Search PubMed.
- M. Dornheim, Thermodynamics of metal hydrides: tailoring reaction enthalpies of hydrogen storage materials, in Thermodynamics-Interaction Studies-Solids, Liquids and Gases, IntechOpen, 2011 Search PubMed.
- K. Shashikala, Hydrogen storage materials, Functional Materials, Elsevier, 2012, pp. 607–37 Search PubMed.
- B. Sakintuna, F. Lamari-Darkrim and M. Hirscher, Metal hydride materials for solid hydrogen storage: a review, Int. J. Hydrogen Energy, 2007, 32(9), 1121–1140 CrossRef CAS.
- C. Drawer, J. Lange and M. Kaltschmitt, Metal hydrides for hydrogen storage–Identification and evaluation of stationary and transportation applications, J. Energy Storage, 2024, 77, 109988 CrossRef.
- J. W. Sheffield, K. B. Martin, and R. Folkson, Electricity and hydrogen as energy vectors for transportation vehicles, in Alternative Fuels and Advanced Vehicle Technologies for Improved Environmental Performance, Woodhead Publishing, 2014, pp. 117–137 Search PubMed.
- V. K. Kukkapalli, S. Kim and S. A. Thomas, Thermal Management Techniques in Metal Hydrides for Hydrogen Storage Applications: A Review, Energies, 2023, 16(8), 3444 CrossRef CAS.
- Q. Lai, M. Paskevicius, D. A. Sheppard, C. E. Buckley, A. W. Thornton, M. R. Hill, Q. Gu, J. Mao, Z. Huang, H. K. Liu and Z. Guo, Hydrogen storage materials for mobile and stationary applications: current state of the art, ChemSusChem, 2015, 8(17), 2789–2825 CrossRef CAS PubMed.
- K. T. Møller, D. Sheppard, D. B. Ravnsbæk, C. E. Buckley, E. Akiba, H. W. Li and T. R. Jensen, Complex metal hydrides for hydrogen, thermal and electrochemical energy storage, Energies, 2017, 10(10), 1645 CrossRef.
- R. Mohtadi and S. I. Orimo, The renaissance of hydrides as energy materials, Nat. Rev. Mater., 2016, 2(3), 1–5 Search PubMed.
- B. Sakintuna, F. Lamari-Darkrim and M. Hirscher, Metal hydride materials for solid hydrogen storage: a review, Int. J. Hydrogen Energy, 2007, 32(9), 1121–1140 CrossRef CAS.
- S. I. Orimo, Y. Nakamori, J. R. Eliseo, A. Züttel and C. M. Jensen, Complex hydrides for hydrogen storage, Chem. Rev., 2007, 107(10), 4111–4132 CrossRef CAS PubMed.
- A. Schneemann, J. L. White, S. Kang, S. Jeong, L. F. Wan, E. S. Cho, T. W. Heo, D. Prendergast, J. J. Urban, B. C. Wood and M. D. Allendorf, Nanostructured metal hydrides for hydrogen storage, Chem. Rev., 2018, 118(22), 10775–10839 CrossRef CAS PubMed.
- N. F. W. Ligterink, J. Terwisscha van Scheltinga, V. Taquet, J. K. Jørgensen, S. Cazaux, E. F. van Dishoeck and H. Linnartz, The formation of peptide-like molecules on interstellar dust grains, Mon. Not. R. Astron. Soc., 2018, 480(3), 3628–3643 CrossRef CAS.
- M. Klose, I. Lindemann, C. B. Minella, K. Pinkert, M. Zier, L. Giebeler, P. Nolis, M. D. Baró, S. Oswald, O. Gutfleisch and H. Ehrenberg, Unusual oxidation behavior of light metal hydride by tetrahydrofuran solvent molecules confined in ordered mesoporous carbon, J. Mater. Res., 2014, 29(1), 55–63 CrossRef CAS.
- K. C. Kim, A review on design strategies for metal hydrides with enhanced reaction thermodynamics for hydrogen storage applications, Int. J. Energy Res., 2018, 42(4), 1455–1468 CrossRef CAS.
- J. Zhang, Y. Zhu, H. Lin, Y. Liu, Y. Zhang, S. Li, Z. Ma and L. Li, Metal hydride nanoparticles with ultrahigh structural stability and hydrogen storage activity derived from microencapsulated nanoconfinement, Adv. Mater., 2017, 29(24), 1700760 CrossRef PubMed.
- L. Li, Y. Huang, C. An and Y. Wang, Lightweight hydrides nanocomposites for hydrogen storage: challenges, progress and prospects, Sci. China Mater., 2019, 62(11), 1597–1625 CrossRef.
- A. Züttel, Hydrogen storage and distribution systems, Mitigation Adapt. Strategies Global Change, 2007, 12, 343–365 CrossRef.
- L. H. Jepsen, M. B. Ley, Y. S. Lee, Y. W. Cho, M. Dornheim, J. O. Jensen, Y. Filinchuk, J. E. Jørgensen, F. Besenbacher and T. R. Jensen, Boron–nitrogen based hydrides and reactive composites for hydrogen storage, Mater. Today, 2014, 17(3), 129–135 CrossRef CAS.
- Q. Lai, M. Paskevicius, D. A. Sheppard, C. E. Buckley, A. W. Thornton, M. R. Hill, Q. Gu, J. Mao, Z. Huang, H. K. Liu and Z. Guo, Hydrogen storage materials for mobile and stationary applications: current state of the art, ChemSusChem, 2015, 8(17), 2789–2825 CrossRef CAS PubMed.
- E. Grube, C. H. Olesen, D. B. Ravnsbæk and T. R. Jensen, Barium borohydride chlorides: synthesis, crystal structures and thermal properties, Dalton Trans., 2016, 45(19), 8291–8299 RSC.
- T. He, H. Cao and P. Chen, Complex hydrides for energy storage, conversion, and utilization, Adv. Mater., 2019, 31(50), 1902757 CrossRef CAS PubMed.
- E. Callini, Z. Ö. Atakli, B. C. Hauback, S. I. Orimo, C. Jensen, M. Dornheim, D. Grant, Y. W. Cho, P. Chen, B. Hjörvarsson and P. De Jongh, Complex and liquid hydrides for energy storage, Appl. Phys. A, 2016, 122, 1–22 CrossRef CAS.
- H. Grove, L. H. Rude, T. R. Jensen, M. Corno, P. Ugliengo, M. Baricco, M. H. Sørby and B. C. Hauback, Halide substitution in Ca (BH 4) 2, RSC Adv., 2014, 4(9), 4736–4742 RSC.
- O. O. Abegunde, E. T. Akinlabi, O. P. Oladijo, S. Akinlabi and A. U. Ude, Overview of thin film deposition techniques, AIMS Mater. Sci., 2019, 6(2), 174–199 CAS.
- N. Y. Abu-Thabit, Electrically conducting polyaniline smart coatings and thin films for industrial applications, in Advances in Smart Coatings and Thin Films for Future Industrial and Biomedical Engineering Applications, Elsevier, 2020, pp. 585–617 Search PubMed.
- A. Palmero and N. Martin, Advanced Strategies in Thin Films Engineering by Magnetron Sputtering, Coatings, 2020, 10(4), 419 CrossRef CAS.
- H. S. Vanegas Parra, S. Calderón Velasco, J. E. Alfonso Orjuela, J. J. Olaya Florez and S. Carvalho, Influence of Ag Doping on the Microstructural, Optical, and Electrical Properties of ZrSiN Coatings Deposited through Pulsed-DC Reactive Magnetron Sputtering, Coatings, 2023, 13(7), 1154 CrossRef CAS.
- S. Patchkovskii, J. S. Tse, S. N. Yurchenko, L. Zhechkov, T. Heine and G. Seifert, Graphene nanostructures as tunable storage media for molecular hydrogen, Proc. Natl. Acad. Sci. U. S. A., 2005, 102(30), 10439–10444 CrossRef CAS PubMed.
- S. Liu, J. Liu, X. Liu, J. Shang, L. Xu, R. Yu and J. Shui, Hydrogen storage in incompletely etched multilayer Ti2CT x at room temperature, Nat. Nanotechnol., 2021, 16(3), 331–336 CrossRef CAS PubMed.
- T. H. Kim, J. Bae, T. H. Lee, J. Hwang, J. H. Jung, D. K. Kim, J. S. Lee, D. O. Kim, Y. H. Lee and J. Ihm, Room-temperature hydrogen storage via two-dimensional potential well in mesoporous graphene oxide, Nano Energy, 2016, 27, 402–411 CrossRef CAS.
- H. Jiang, X. L. Cheng, H. Zhang, Y. J. Tang and C. X. Zhao, Molecular dynamic simulation of high-quality hydrogen storage in pillared bilayer graphene bubble structure, Comput. Theor. Chem., 2015, 1068, 97–103 CrossRef CAS.
- Q. Wang, S. R. Challa, D. S. Sholl and J. K. Johnson, Quantum sieving in carbon nanotubes and zeolites, Phys. Rev. Lett., 1999, 82(5), 956 CrossRef CAS.
- Y. Suyun, L. Ouyang and Z. Min, Hydrogen storage properties of preferentially orientated Mg-Ni multilayer film prepared by magnetron sputtering., Rare Met., 2006, 25(6), 295–299 CrossRef.
- L. Z. Ouyang, H. Wang, C. Y. Chung, J. H. Ahn and M. Zhu, MgNi/Pd multilayer hydrogen storage thin films prepared by dc magnetron sputtering, J. Alloys Compd., 2006, 422(1–2), 58–61 CrossRef CAS.
- Z. Tarnawski, N. T. Kim-Ngan, K. Zakrzewska, K. Drogowska, A. Brudnik, A. G. Balogh, R. Kužel, L. Havela and V. Sechovsky, Hydrogen storage in Ti–TiO2 multilayers, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2013, 4(2), 025004 CAS.
- H. Jung, J. Yuh, S. Cho and W. Lee, Effects of Ti interlayers on microstructures and hydrogen storage capacity in Mg/Pd multilayer thin films, J. Alloys Compd., 2014, 601, 63–66 CrossRef CAS.
- L. Mooij, T. Perkisas, G. Pálsson, H. Schreuders, M. Wolff, B. Hjörvarsson, S. Bals and B. Dam, The effect of microstructure on the hydrogenation of Mg/Fe thin film multilayers, Int. J. Hydrogen Energy, 2014, 39(30), 17092–17103 CrossRef CAS.
- H. Jung, S. Cho and W. Lee, Enhanced hydrogen storage properties of Pd/Ti/Mg/Ti multilayer films using the catalytic effects of Pd, Appl. Phys. Lett., 2015, 106(19), 193–902 CrossRef.
- L. Fan, Z. Tu and S. H. Chan, Recent development of hydrogen and fuel cell technologies: A review, Energy Rep., 2021, 7, 8421–8446 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |