Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Evaluation of naked-eye sensing and anion binding studies in meso-fluorescein substituted one-walled calix[4]pyrrole (C4P)†
Shafieq Ahmad Wagaya,
Ufana Riaz b,
Manawwer Alam
b,
Manawwer Alam c and
Rashid Ali
c and
Rashid Ali *a
*a
aDepartment of Chemistry, Organic and Supramolecular Functional Materials Research Laboratory, Jamia Millia Islamia, Okhla, New Delhi 110025, India. E-mail: rali1@jmi.ac.in; Tel: +91-7011867613
bDepartment of Chemistry and Biochemistry, North Carolina Central University, 27707, USA
cDepartment of Chemistry, College of Science, King Saud University, P. O. Box 2455, Riyadh 11451, Saudi Arabia
First published on 5th March 2024
Abstract
In this paper, we have design, synthesized and fully characterized a new meso-fluorescein substituted one-walled calix[4]pyrrole (C4P7), obtained from simple and easily available starting materials such as fluorescein, 4-hydroxyacetophenone and pyrrole. The anion sensing studies reveal that the C4P7 system displays selective and sensitive naked-eye sensing towards fluoride, phosphate, and acetate anions with the limit of detection of 4.27 mg L−1, 6.4 mg L−1, and 5.94 mg L−1, respectively. Moreover, the C4P7 receptor displays good results of binding (host–guest, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) towards a variety of anions. The 1
1) towards a variety of anions. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry was further confirmed by means of Job's plots. TD-DFT calculations showed that the HOMO–LUMO gap decreases in all the complexes (C4P7@anions) in comparison to the free C4P7 system. The authors are of the opinion that this work may provide a good platform to explore calix[4]pyrrole chemistry in the arena of recognition/sensing of biologically significant analytes in future studies.
1 binding stoichiometry was further confirmed by means of Job's plots. TD-DFT calculations showed that the HOMO–LUMO gap decreases in all the complexes (C4P7@anions) in comparison to the free C4P7 system. The authors are of the opinion that this work may provide a good platform to explore calix[4]pyrrole chemistry in the arena of recognition/sensing of biologically significant analytes in future studies.
Introduction
Supramolecular chemistry, introduces various artificial receptors which have revolutionized host–guest complexes to an advanced level in the past few decades. In the realm of supramolecular chemistry, sensors in general are devices that can help to detect and enumerate the physical and/or chemical aspects of the world around us in real time.1–7 Anions are considered as vital components of various biological systems, since they regulate and/or are responsible for a myriad of environmental and biological processes in our everyday life.8–22 Importantly, anionic species exist in almost 70% of all the active sites of enzymes – playing a key role in genetic information storage, controlling osmotic pressure, generation of electrical signals, maintaining cell volume and activating signal transduction pathways.23–38As a result, anionic species in the environment might either be detrimental pollutants or indispensable for further growth. Therefore, from the last few decades, extensive research in the development of new luminous and/or colorimetric anionic sensors are being continuously accelerating – monitoring the function, concentration, and location of the anionic entities.39–44 To this context, Xu et al., have reported a naphthalimide-calix[4]arene based effective fluoride sensor – displaying selective fluorescence ‘quenching effect’ only with the fluoride anion in acetonitrile, among the various examined tetrabutylammonium anion salts.45 From the Job's plot, they have observed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry binding of the receptor with fluoride ion. Moreover, the authors have also noticed a deprotonation of the naphthalimide NH, as the fluoride ion is sufficiently basic to deprotonate the NH-proton, causing a long wavelength color change. Markedly, 1H-NMR spectra also confirmed the deprotonation of NH-proton of the naphthalimide moiety.
1 stoichiometry binding of the receptor with fluoride ion. Moreover, the authors have also noticed a deprotonation of the naphthalimide NH, as the fluoride ion is sufficiently basic to deprotonate the NH-proton, causing a long wavelength color change. Markedly, 1H-NMR spectra also confirmed the deprotonation of NH-proton of the naphthalimide moiety.
On the other hand, Fabbrizzi and teammates have reported a versatile urea-based symmetrical bis-naphthalimide-appended selective fluoride sensor, which undergo stepwise deprotonation in DMSO – resulting a new intense band development ca. at 540 nm with decreases in the intensity of the original band at 400 nm, the color turning from yellow-to-red (red-shift).46 Noticeably, on further addition of [Bu4N]F, the color turned into the blue from red, and the band at 540 nm disappeared whileas another new broad band was formed at around 600 nm. In any case, the reversible successive deprotonation of NH protons was confirmed, as directed by the fact that, on gradual addition of water, the blue solution turned first into the red and then to yellow color. Moreover, such successive deprotonation was also confirmed by virtue of 1H-NMR by these workers.
In a separate study, Fu's research group has revealed a vital guanidiniocarbonyl pyrrole functionalized 4-amino-1,8-naphthalimide fluorescent chemosensory material, which showed selective fluorescent enhancement with pyrophosphate in aqueous solution over the other anionic entities like ATP, AMP, and ADP.47 Liu et al., have reported two macrocyclic bis-benzimidazolium salts based receptors for the sensitive and selective detection of acetate and nitrate anions.48 Quite recently, a small molecule, N-tertbutyldimethylsilyl-3,6-diiodocarbazoles a conjugated organic dye, was design, constructed and employed as a selective fluoride colorimetric sensor by Zheng's research group.49 The limit of detection (LOD) for this system was found to be 3 × 10−5 M. Notably, in the year of 2023, our research group has also reported a valuable fluorescein functionalized dipyrromethane (DPM) based chemosensor for the selective detection of fluoride, phosphate and acetate ions over various other tested anions.50
From the anion binding perspective, calix[4]pyrrole (C4P) and its congeners remained among the most studied macrocyclic systems for the selective recognization of neutral molecules, anions, and/or ion-pairs. This is because of their simple-synthetic procedures, besides they also have an appropriate cavity as well as conformational flexibility.51–57 Remarkably, since the Sessler's research group, investigated the anion binding study for the first time of the parent C4P system in 1996,58 this versatile receptor rekindled pervasive interest of the researchers worldwide, as it has several possible positions (e.g. meso-, β-pyrrolic positions, & NH sites) to functionalize with ease.59–66 Therefore, taking the advantage of these tunable positions, and also to further advance the C4P-chemistry to advance level. Recently, we have revealed anion binding studies of phthalimide-based one-walled C4P architecture through the UV-vis spectroscopy and time-dependent density functional theory (TD-DFT) calculations.67
On the other hand, in another work, our group in collaboration with P.-E. Danjou (France), has reported the chemodosimetric detection of the hydrazine molecule by utilizing the β-dicyanovinyl substituted C4P system.68
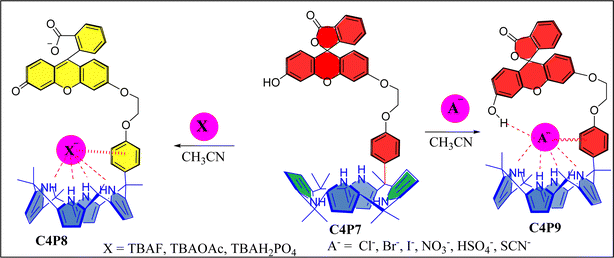
Although, in recent years, huge advancements and innovations have successfully been made in the field of chemosensors for selective and sensitive detection/identification of the physiologically significant anions.69 But, we still believe that there is always a pressing demand to design and construct novel simple-to-make yet effective sensory materials of specific interest. In the present paper, we have designed and synthesized a novel meso-substituted one-walled C4P7 receptor, and investigated its anion binding/sensing properties with the involvement of F−, Br−, Cl−, I−, AcO−, NO3−, HSO4−, SCN−, and H2PO4− anions, used as their tetrabutylammonium (TBA) salts in acetonitrile solvent. Remarkably, results have revealed that C4P7 shows colorimetric sensing with F−, AcO−, and H2PO4−, attaining a light orange color from yellowish in MeCN. In present work, the C4P7 moiety acts as the binding part whileas the fluorescein portion – acting as a signaling unit. Remarkably, the designed supramolecular structure acts as an anion sensor (e.g., for F−, AcO−, and H2PO4−) as well as anionic receptor for various other tested anions. Here, the C4P moiety is responsible for interacting and binding the anions, whereas the fluorescein moiety undergoes a detectable change in response to the binding/sensing of anions. Moreover, the receptor C4P7 displayed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding behavior (calculated by the means of an online supramolecular Bindfit v0.5 program) with all the tested anions, which were further confirmed from the Job's method, also known as a method of continuous variation.
1 binding behavior (calculated by the means of an online supramolecular Bindfit v0.5 program) with all the tested anions, which were further confirmed from the Job's method, also known as a method of continuous variation.
Results and discussion
Synthesis and characterization
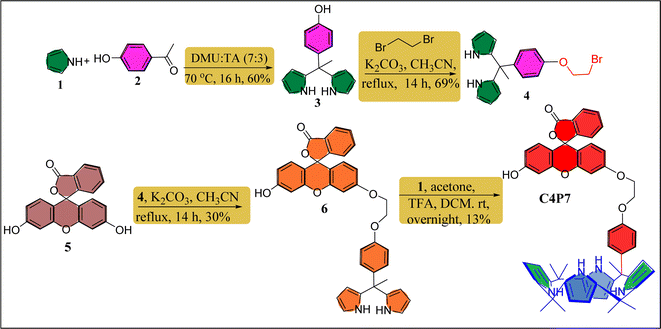
Fluorescein functionalized one-walled calix[4]pyrrole (C4P7) has been prepared through multistep reaction from easily available starting materials like fluorescein, 4-hydroxyacetophenone, acetone and freshly distilled pyrrole. To summarize, we began with the synthesis of dipyrromethane (DPM) 3 by condensing 4-hydroxyacetophenone with a freshly distilled pyrrole under eco-friendly condition using a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio of N,N′-dimethylurea (DMU) and L-(+)-tartaric acid (TA) at 70 °C using our earlier reported procedure.52 Next, the DPM 4 was achieved in good yield (69%) from the reaction of DPM 3 with 1,2-dibromoethane in the presence of K2CO3/MeCN under refluxing condition for 14 h. Later, thus prepared DPM 4 was coupled with parent fluorescein 5 using K2CO3 as a base to deliver the fluorescein functionalized DPM 6 in 30% yield. Lastly, an acid-catalyzed macrocyclization of the functionalized DPM 6 with freshly distilled pyrrole and acetone was accomplished to furnish the desired meso-fluorescein functionalized C4P7 in 13% yield (Scheme 1). The confirmation of the structure of C4P7 as well as the intermediate compounds were done by means of the standard spectroscopic techniques (see ESI†).
3 ratio of N,N′-dimethylurea (DMU) and L-(+)-tartaric acid (TA) at 70 °C using our earlier reported procedure.52 Next, the DPM 4 was achieved in good yield (69%) from the reaction of DPM 3 with 1,2-dibromoethane in the presence of K2CO3/MeCN under refluxing condition for 14 h. Later, thus prepared DPM 4 was coupled with parent fluorescein 5 using K2CO3 as a base to deliver the fluorescein functionalized DPM 6 in 30% yield. Lastly, an acid-catalyzed macrocyclization of the functionalized DPM 6 with freshly distilled pyrrole and acetone was accomplished to furnish the desired meso-fluorescein functionalized C4P7 in 13% yield (Scheme 1). The confirmation of the structure of C4P7 as well as the intermediate compounds were done by means of the standard spectroscopic techniques (see ESI†).
Naked-eye visualization of the color change (sensing studies)
The colorimetric anion sensing of fluorescein functionalized C4P7 was determined through the naked-eye, UV-vis and fluorescence experiments in acetonitrile solution. Initially, the colorimetric experiments were performed in which 60 μL of TBA salts (4.0 × 10−3 M) of F−, Cl−, Br−, I−, SCN−, NO3−, AcO−, HSO4−, and H2PO4− anions, were separately added into the 2.5 mL of C4P7 (0.5 × 10−5 M) in acetonitrile solution. Interestingly, a clear change in the color from light yellow to orange was observed by the naked-eye with AcO−, F−, and H2PO4−, whereas no color change was seen upon the addition of other examined anions under identical conditions, even by increasing the addition of anionic solution up to 100 μL (Fig. 1). | ||
| Fig. 1 Naked-eye color change visualization of the C4P7 receptor upon the addition of TBA salts of a variety of anions in CH3CN. | ||
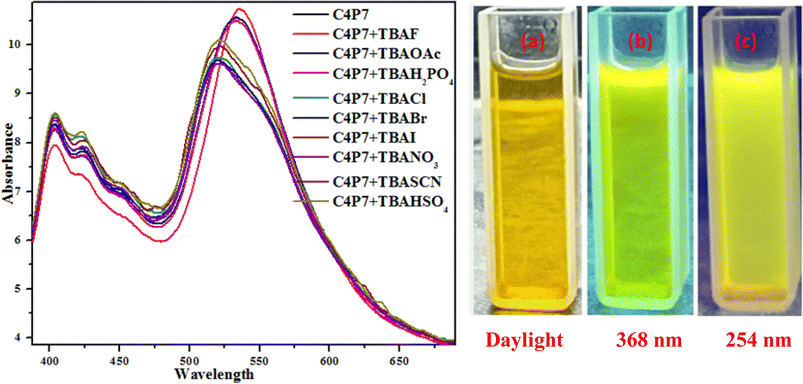
To further support these experiments, the UV-vis studies of C4P7 system (0.5 × 10−5 M) with 100 μL of tetrabutylammonium salts (4.0 × 10−3 M) of the anticipated anions were accomplished distinctly. Inspection of the Fig. 2, clearly illustrate that the free C4P7 receptor with Cl−, Br−, I−, SCN−, NO3−, and HSO4− anions exhibit a triplet like strong absorption band with high band intensity ca. at 458 nm, and two weak intensity shoulders at 489 nm and 431 nm, besides a low intensity band at 356 nm. Pleasingly, a large red-shift was perceived in the UV-vis spectrum upon addition of F−, AcO−, and H2PO4− anions – developing a new intense band at 524 nm at the expense of the original triplet like band having the maxima at 458 nm (Fig. 2).
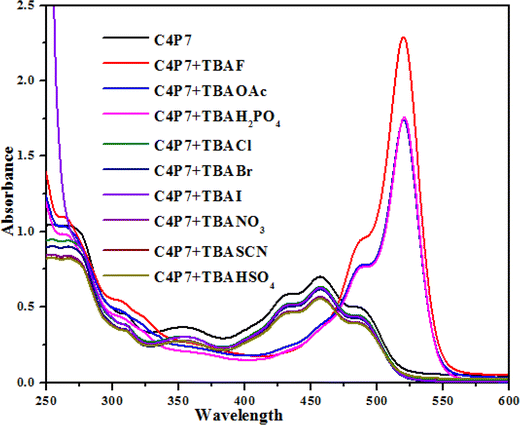 | ||
| Fig. 2 UV-vis spectra of C4P7 (0.5 × 10−5 M) after addition of 100 μL TBA salts (4.0 × 10−3 M) of various anions in CH3CN solution. | ||
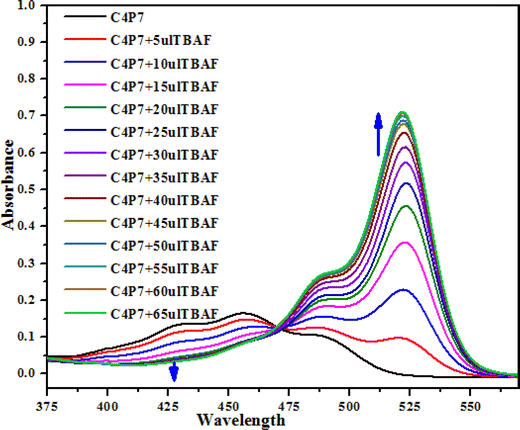
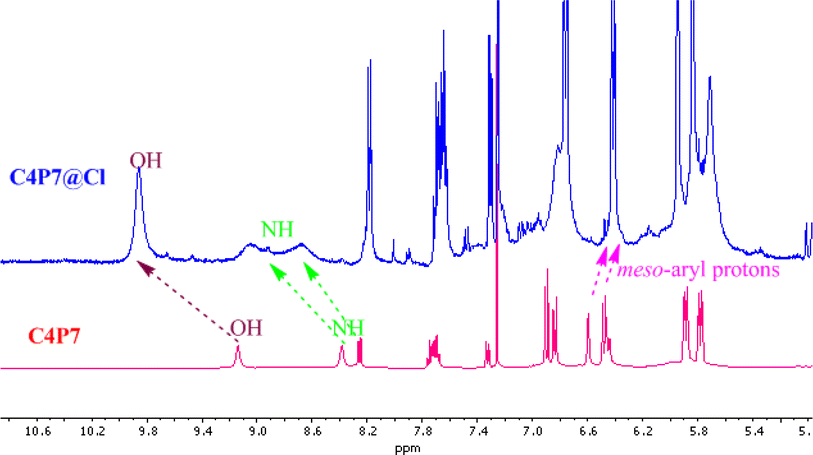
Next, UV-vis titration studies in CH3CN solvent of the fluorescein functionalized C4P7 with several anions were investigated in order to elucidate the binding/sensing behavior. Titrations were made successful with continuous addition of the solution of anions such as F−, Cl−, Br−, I−, SCN−, NO3−, AcO−, HSO4− and H2PO4− (used as their tetrabutylammonium salts). The appearance of a new intense band ca. at 524 nm, represent a bathochromic shift using the anions F− anion in comparison to the free receptor C4P7 (Fig. 3). It can clearly be seen from the Fig. 3 that gradual addition of TBAF solution, showed a continuous increase in the absorption intensity (hyperchromic shift) at 524 nm, whereas simultaneous decrease in the intensity at 430 nm and 453 nm with the development of an isosbestic point ca. at ∼471 nm. The isosbestic point, clearly indicate that the C4P7 receptor with F−/AcO−/H2PO4− is present in the equilibrium state. Interestingly, similar results were also obtained with C4P7@CH3COO− as well as C4P7@H2PO4− under identical conditions (see ESI†). On contrary, no such observations were detected with other test anions like Cl−, Br−, I−, SCN−, NO3−, and HSO4− (see ESI†). This unique behavior of F−, AcO−, and H2PO4− may be due to their more basic nature as compared to the other examined anions, resulting in the abstraction of a phenolic proton, thereby leading towards the ring-opening of the spirolactone present in the fluorescein moiety to produce a coloured quinonoid structure C4P8 (Scheme 2).70,71 To further confirm the binding and/or sensing behavior of this C4P7 system, we have also performed the comparative 1H-NMR experiments (Fig. S7, see ESI†), which reveal the abstraction of the phenolic proton – confirmed through the disappearance of OH peak, and binding of the F− ion with the calix framework through hydrogens bonds (Fig. S7,† downfield shift in the NH-protons) as depicted in the Scheme 2.
Moreover, fluorescence studies of the receptor C4P7 (0.25 × 10−5 M) with the aforesaid anions (2 × 10−4 M), used as their tetrabutylammonium (TBA) salts in acetonitrile were also accomplished to further support the naked-eye as well as UV-vis experiments (Fig. 4). To further elucidate the sensing results, fluorescence titrations of the C4P7 with 0–100 μL solution of F−, CH3COO−, and H2PO4− were performed in acetonitrile solvent (Fig. 5 and see ESI†). It can be seen from the Fig. 5 that the free receptor C4P7 shows emission bands at 402, nm 423 nm, and 520 nm. From the comparison of fluorescence analysis, it could clearly be seen that the spectra displayed a shift in wavelength from (520 nm to 536 nm), (520 nm to 532 nm), and (520 nm to 531) nm in the presence of 100 μL of F−, CH3COO−, and H2PO4− anions, respectively, whereas other anions were unable to show any spectral change in the fluorescence spectra (Fig. 4 and see ESI†). Importantly, our results were in accordance with the earlier similar types of outcomes.55,72–74 Moreover, solution phase images reveal that the C4P7 display a light green and yellowish fluorescence upon illuminating with the UV-lamp at 368 nm and 254 nm, respectively (Fig. 4a–c).
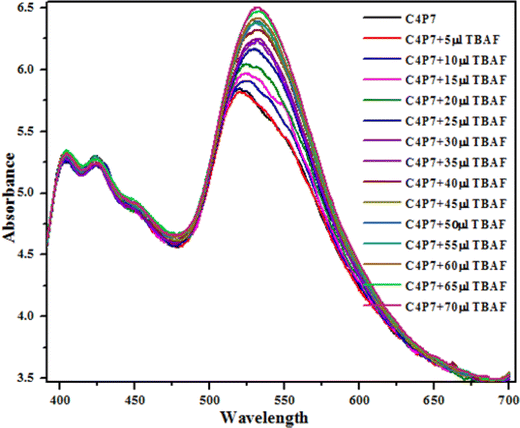 | ||
| Fig. 5 Fluorescence titration emission spectra of the C4P7 (0.25 × 10−5 M) with TBAF (2.0 × 10−3 M) in CH3CN. | ||
Limit of detection (LOD) and limit of quantification (LOQ)
The LOD/LOQ of the anticipated sensor C4P7 were calculated using the UV-vis titrations spectral data utilizing the eqn (1) and (2), respectively. For this, the first calibration curve was achieved from the plot of absorption intensity increment (A − A0) as a function of fluoride/phosphate/acetate anions concentrations (Table 1). The regression curve equation was afterward extended for the low concentration measure.75| LOD = 3.3 × S.D./k | (1) |
| LOQ = 10 × S.D./k | (2) |
| Property | TBAF | TBAH2PO4 | TBAOAc |
|---|---|---|---|
| LOD | 4.27 mg L−1 | 6.4 mg L−1 | 5.94 mg L−1 |
| LOQ | 12.95 mg L−1 | 19.41 mg L−1 | 18.01 mg L−1 |
Evaluation of anion binding studies
To investigate the anion binding studies of C4P7 receptor (0.5 × 10−5 M), the UV-vis titrations were achieved with various anions used as their tetrabutylammonium salts (4.0 × 10−3 M) in acetonitrile solution. Initially, the spectrum of free receptor C4P7 (0.5 × 10−5 M) was recorded after that solution of the guest TBACl (4.0 × 10−3 M) was added in fractions (5 μL) into it, and the spectra of each titrant was documented until the saturation point was reached, showing a notable change in the complexed (C4P7@Cl) form with a λmax at 457 nm (Fig. 6a). The data was fitted ca. at λmax 457 nm through the online supramolecular Bindfit v0.5 program, revealing the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex with a binding constant K1:1 = 32
1 host–guest complex with a binding constant K1:1 = 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 710.68 M−1 (Fig. 6b). For other anions as well, the same procedure was applied to evaluate the stoichiometry and binding constants (see ESI†), and it can clearly be seen the formation of 1
710.68 M−1 (Fig. 6b). For other anions as well, the same procedure was applied to evaluate the stoichiometry and binding constants (see ESI†), and it can clearly be seen the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes with all the tested anions, showing moderate to good binding affinities (Table 2). Noticeably, comparison of the binding strength of the present studies with the earlier reported ones, showed better results, may be due to some secondary non-covalent supramolecular interactions.41,52,67
1 complexes with all the tested anions, showing moderate to good binding affinities (Table 2). Noticeably, comparison of the binding strength of the present studies with the earlier reported ones, showed better results, may be due to some secondary non-covalent supramolecular interactions.41,52,67
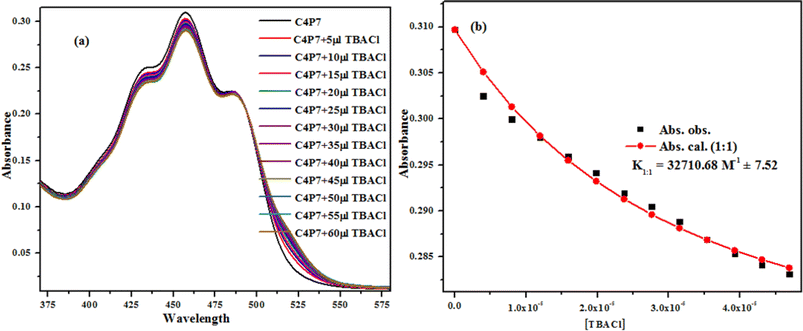 | ||
| Fig. 6 UV-vis titration (a) of the receptor C4P7 with TBACl in CH3CN, and binding isotherm (b) fitting of UV-vis titration data using Bindfit v0.5 program. | ||
| S. no. | Anion | C4P7 (Ka M−1) |
|---|---|---|
| Ka values were calculated utilizing the global fit option in Bindfit software through 1 : 1 binding model. The errors are in parenthesis with <7.52%, the anions are added as their TBA salts, [C4P7] = 0.5 × 10−5 M, [TBA salts] = 4 × 10−3 M, at T = 30 °C ± 2 °C. 1H-NMR analysis and the Job's plots. | ||
| 1 | Cl− | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 710.68 ± (7.52) 710.68 ± (7.52) |
| 2 | Br− | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 068.96 ± (6.42) 068.96 ± (6.42) |
| 3 | I− | 68.63 ± (4.52) |
| 4 | NO3− | 7055.87 ± (4.03) |
| 5 | SCN− | 5114.57 ± (2.02) |
| 6 | HSO4− | 1106.59 ± (1.99) |
As shown in Fig. 7, the OH proton signal appeared as a broad singlet at δ 9.14 ppm shifted downfield to δ 9.94 ppm, displaying the O–H–anion interaction. On the other, the NH protons also downfield shifted from δ 8.49 ppm to δ 9.12 ppm and δ 8.25 ppm to δ 8.78 ppm, which clearly indicate that the anion is bound through hydrogen bonding. However, the meso-substituted aromatic protons display slight upfield shift from δ 6.59 ppm to δ 6.44 ppm and δ 6.48 ppm to δ 6.41 ppm, which confirms anion–π interaction.50,53 Besides, these observation, β-pyrrolic protons of the C4P7 also showed upfield shift – revealing the change in the conformation of C4P7 from 1,3-alternate to the cone conformation.
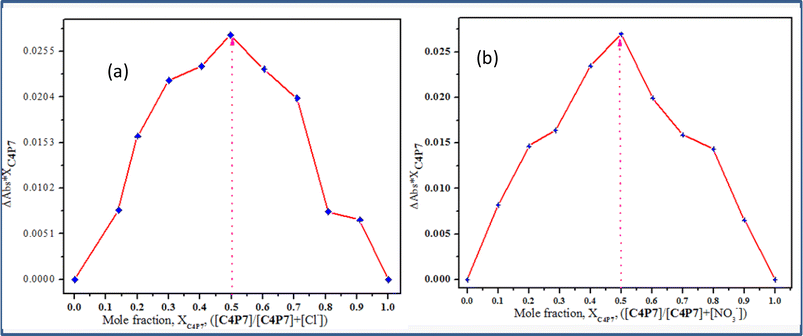
To further validate the stoichiometric results obtained from the online supramolecular Bindfit v0.5 program for C4P7 with various anions, the method of continuous variation (Job's method) was also used, plotting the graphs against the change in absorption vs. the mole-fraction of the guest molecules (Fig. 8). The concentration of both the guests (anionic entities) and the host molecule (C4P7) were held constant in acetonitrile solution at temperature 30 °C ± 2 °C, and their mole fraction were varied. It can be inspected from the Fig. 8 that the concentrations of C4P7@Cl− as well as C4P7@NO3− approaches to a maximum value at 0.5, confirming the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes between C4P7 and the tested anions. Therefore, results from the online supramolecular Bindfit v0.5 program and Job's plots are in complete agreement, depicting the formation of 1
1 complexes between C4P7 and the tested anions. Therefore, results from the online supramolecular Bindfit v0.5 program and Job's plots are in complete agreement, depicting the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes of the newly constructed C4P7 system with the assessed anions.
1 complexes of the newly constructed C4P7 system with the assessed anions.
Computational studies
On the other hand, to further support the experimental results, computational findings of C4P7 with different anions were executed utilizing the Gaussian 09 software, and the geometry optimizations for ground state (So) were accomplished by means of Becke's exchange functional coalesced with the “Lee–Yang–Parr correlation functional” designated as B3LYP. Whileas, the UV-vis absorption studies were computed through TD-B3LYP technique taking into the consideration of solvent effects through “conductor polarizable continuum model” (CPCM). Vibrational frequencies were measured to elucidate the attained geometries correspond to the minima onto the potential energy surface deprived of any imaginary frequencies. For all the optimized geometries, the time dependent density functional theory (TD-DFT) calculations were presented at the same level to develop the fundamental ‘excited states’ (ESs) of the C4P7. Moreover, vertical ionization energies have also been estimated at the identical level of theory. The building complex were adjusted at B3LYP/6-31G(d) in both gas phase as well as in solution phase and with “basis set superimposition error” (BSSE). Counterpoise correction equation of the Boys–Bernardi was employed to revenue into the description of the BSSE.67,76 This mode was involved to measure the binding energies of the anionic complexes contemplating complex compound as a fragment A and anionic species as the fragment B through following equation:| DeBSSE = (AB) = E(AB)AB − E(AAB) −E(BAB) |
Remarkably, as can be seen from the Fig. 9 and S43–48,† the HOMO–LUMO gap were found be decreased after the complexations of C4P7 with different anions (C4P7@anions), and the energy gap were found to be in the order of: C4P7 (3.19 eV) > C4P7@SCN− (2.90 eV) > C4P7@NO3− (2.64 eV) > C4P7@HSO4− (1.63 eV) > C4P7@Cl− (1.51 eV) > C4P7@Br− (1.05 eV) > C4P7@I− (1.02 eV). Moreover, it is to be pointed out that in the case of parent C4P7 (Fig. S44†) as well as C4P7@I (Fig. S46†), the HOMO lies on the calix framework, whileas the LUMO exist onto the fluorescein subunit. On the other hand, in most of the complexes (C4P7@anions), both HOMO and LUMO exits onto the calix frame except C4P7@SCN (Fig. S48†) in which both HOMO and LUMO exists onto the fluorescein unit (Fig. S48†). The optimized structures using B3LYP/6-31G(d) for the parent C4P7 and its complexes with different anions are depicted in the Fig. 10 and S36–42.†
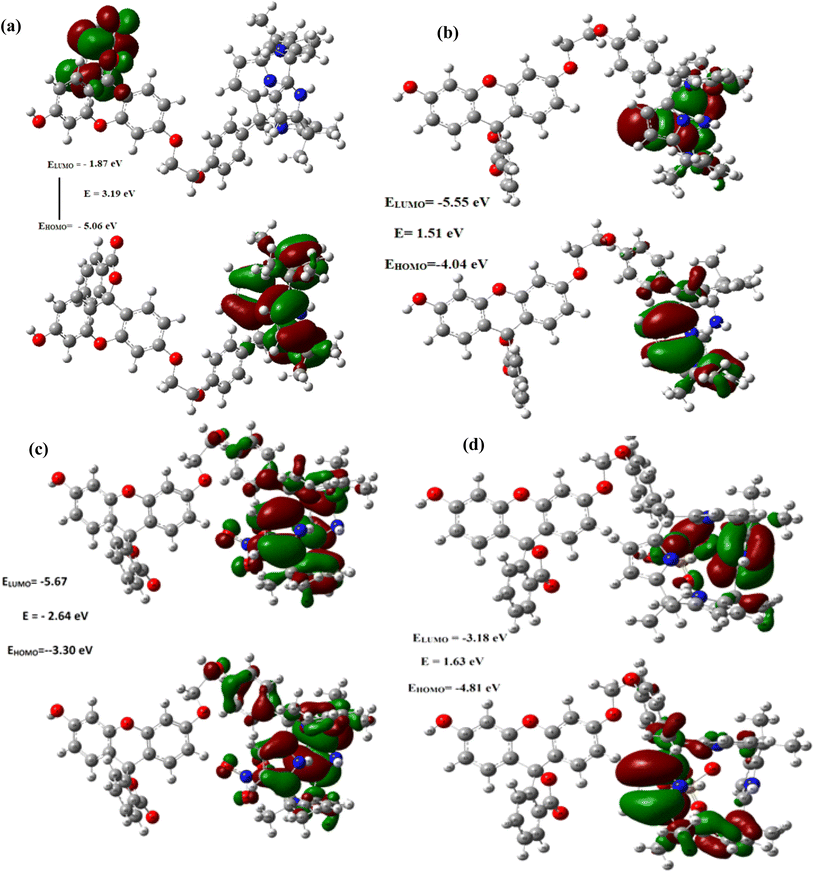 | ||
| Fig. 9 Frontier molecular orbitals distribution of (a) C4P7 (b) C4P7@TBACl (c) C4P7@TBANO3 and (d) C4P7@TBAHSO4 calculated using the TD-DFT with 6-31G (d) basis set. | ||
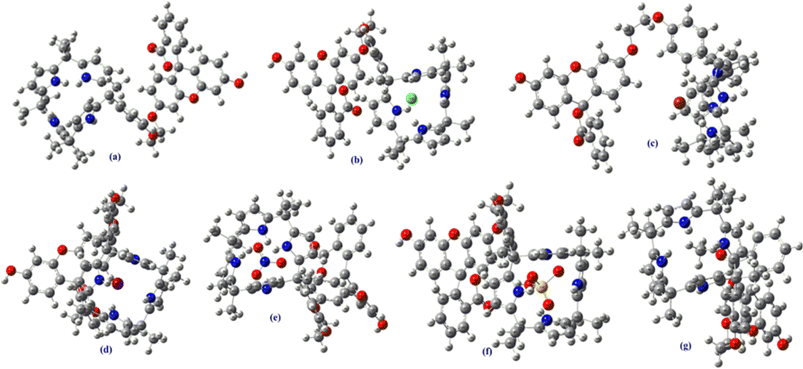 | ||
| Fig. 10 DFT optimized structures of C4P7 (a), and C4P7@anions (b–g) using 6-31G(d) basis set. The counter cation TBA has been omitted for clarity. | ||
Conclusions
In this particular contribution, we have successfully synthesized a novel meso-fluorescein functionalized one-walled calix[4]pyrrole (C4P7) system, which was confirmed by means of the standard spectroscopic techniques (e.g., 1H-NMR, 13C-NMR & HRMS). Receptor C4P7 demonstrated the selective colorimetric sensing towards fluoride, phosphate and acetate anions with the limit of detections (LODs) 4.27 mg L−1, 6.4 mg L−1, and 5.94 mg L−1, and limit of quantifications (LOQs) 12.95 mg L−1, 19.41 mg L−1, and 18.01 mg L−1, respectively. The naked-eye color change was observed from dark yellow to orange in acetonitrile solution for the above three test anions, whileas no such observation was noticed with several other examined anions.The selective sensing with F−, AcO−, and H2PO4−, may be due to strong basic nature of these anions as compared to the other assessed anions, leading to the deprotonation of the phenolic OH-group. And subsequent ring-opening of the spirolactone to produce the coloured quinonoid structure C4P8. Moreover, the receptor C4P7 have also showed worthy binding results (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex formation) with all the inspected anions in acetonitrile solution. Finally, the 1
1 complex formation) with all the inspected anions in acetonitrile solution. Finally, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 bonding stoichiometry was further validated through the Job's plots, and the involvement of OH/NH were confirmed by means of partial 1H-NMR spectra.
1 bonding stoichiometry was further validated through the Job's plots, and the involvement of OH/NH were confirmed by means of partial 1H-NMR spectra.
The HOMO–LUMO gap were found be reduced after the complexations of C4P7 with tested anions (C4P7@anions), and the energy gap were found in the order: C4P7 (3.19 eV) > C4P7@SCN− (2.90 eV) > C4P7@NO3− (2.64 eV) > C4P7@HSO4− (1.63 eV) > C4P7@Cl− (1.51 eV) > C4P7@Br− (1.05 eV) > C4P7@I− (1.02 eV). Moreover, in the case of parent C4P7 and C4P7@I, the HOMO lies on the calix framework whilesas the LUMO exist onto the fluorescein moiety. On the other hand, in all other cases, both HOMO and LUMO exists onto the calix network except C4P7@SCN, in which both HOMO and LUMO exists onto the fluorescein unit. We believe that the current results may be valid for the recognition and/or sensing of several biologically important molecules in future studies.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are indebted for the financial support from the DST-SERB, New-Delhi (Project File No. ECR/2017/000821), and are also obliged to Jamia Millia Islamia for providing the infrastructure. Dr M. Alam is thankful to King Saud University, Riyadh, Saudi Arabia, for Researchers Supporting Project (Project Number RSP2024R113). Shafieq Ahmad Wagay thanks to the University Grants Commission New Delhi, Government of India for providing doctoral fellowship.References
- T. Gunnlaugsson, P. E. Kruger, P. Jensen, J. Tierney, H. D. P. Ali and G. M. Hussey, J. Org. Chem., 2005, 70, 10875–10878 CrossRef CAS PubMed.
- H. M. Junaid, M. Batool, F. W. Harun, M. S. Akhter and N. Shabbir, Crit. Rev. Anal. Chem., 2022, 52, 463–480 CrossRef CAS PubMed.
- W. Lu, M. Zhang, K. Liu, B. Fan, Z. Xia and L. Jiang, Sens. Actuators, B, 2011, 160, 1005–1010 CrossRef CAS.
- B. Taner, A. N. Kursunlu and E. Güler, Spectrochim. Acta, Part A, 2014, 118, 903–907 CrossRef CAS PubMed.
- T. D. Thangadurai, C. J. Lee, S. H. Jeong, S. Yoon, Y. G. Seo and Y.-I. Lee, Microchem. J., 2013, 106, 27–33 CrossRef CAS.
- G. Turkoglu, New J. Chem., 2020, 44, 9485–9492 RSC.
- A. Okudan, S. Erdemir and O. Kocyigit, J. Mol. Struct., 2013, 1048, 392–398 CrossRef CAS.
- P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486–516 CrossRef CAS.
- S. Kubik, Chem. Soc. Rev., 2010, 39, 3648 RSC.
- J. R. Knowles, Annu. Rev. Biochem., 1980, 49, 877–919 CrossRef CAS PubMed.
- D. Markovich, Physiol. Rev., 2001, 81, 1499–1533 CrossRef CAS PubMed.
- J. Han, L. Kiss, H. Mei, A. M. Remete, M. Ponikvar-Svet, D. M. Sedgwick, R. Roman, S. Fustero, H. Moriwaki and V. A. Soloshonok, Chem. Rev., 2021, 121, 4678–4742 CrossRef CAS PubMed.
- W. Zhao, A. H. Flood and N. G. White, Chem. Soc. Rev., 2020, 49, 7893–7906 RSC.
- N. Busschaert, C. Caltagirone, W. Van Rossom and P. A. Gale, Chem. Rev., 2015, 115, 8038–8155 CrossRef CAS PubMed.
- W. S. Wadsworth, in Organic Reactions, John Wiley & Sons, Inc., Hoboken, NJ, USA, 1977, pp. 73–253 Search PubMed.
- J. P. Attfield, Cryst. Growth Des., 2013, 13, 4623–4629 CrossRef CAS.
- E. Pungor, Anal. Chem., 1967, 39, 28A–45A CrossRef.
- P. A. Gale, Chem. Commun., 2008, 4525 RSC.
- N. H. Evans and P. D. Beer, Angew. Chem., Int. Ed., 2014, 53, 11716–11754 CrossRef CAS PubMed.
- R. B. King, Acc. Chem. Res., 1970, 3, 417–427 CrossRef CAS.
- M. M. G. Antonisse and D. N. Reinhoudt, Chem. Commun., 1998, 443–448 RSC.
- P. A. Gale, Chem. Commun., 2011, 47, 82–86 RSC.
- N. Akhtar, O. Biswas and D. Manna, Chem. Commun., 2020, 56, 14137–14153 RSC.
- N. Busschaert and P. A. Gale, Angew. Chem., Int. Ed., 2013, 52, 1374–1382 CrossRef CAS PubMed.
- C. N. Carroll, J. J. Naleway, M. M. Haley and D. W. Johnson, Chem. Soc. Rev., 2010, 39, 3875–3888 RSC.
- D. Sharma, A. Moirangthem, S. M. Roy, A. S. K. Kumar, J. P. Nandre, U. D. Patil, A. Basu and S. K. Sahoo, J. Photochem. Photobiol., B, 2015, 148, 37–42 CrossRef CAS PubMed.
- D. Sharma, S. K. Sahoo, S. Chaudhary, R. K. Bera and J. F. Callan, Analyst, 2013, 138, 3646–3650 RSC.
- N. Kaur, G. Kaur, U. A. Fegade, A. Singh, S. K. Sahoo, A. S. Kuwar and N. Singh, TrAC, Trends Anal. Chem., 2017, 95, 86–109 CrossRef CAS.
- U. Fegade, S. K. Sahoo, A. Singh, P. Mahulikar, S. Attarde, N. Singh and A. Kuwar, RSC Adv., 2014, 4, 15288 RSC.
- D. Sharma, S. K. Ashok Kumar and S. K. Sahoo, Tetrahedron Lett., 2014, 55, 927–930 CrossRef CAS.
- D. Sharma, R. K. Bera and S. K. Sahoo, Spectrochim. Acta, Part A, 2013, 105, 477–482 CrossRef CAS PubMed.
- D. Sharma, A. R. Mistry, R. K. Bera and S. K. Sahoo, Supramol. Chem., 2013, 25, 212–220 CrossRef CAS.
- B. R. Jali, A. K. Barick, P. Mohapatra and S. K. Sahoo, J. Fluorine Chem., 2021, 244, 109744 CrossRef CAS.
- A. J. Greer, J. Jacquemin and C. Hardacre, Molecules, 2020, 25, 5207 CrossRef CAS PubMed.
- S.-Y. Jiang, A. Ma and S. Ramachandran, Int. J. Mol. Sci., 2018, 19, 2966 CrossRef PubMed.
- C.-W. Cho, T. Phuong Thuy Pham, Y.-C. Jeon and Y.-S. Yun, Green Chem., 2008, 10, 67–72 RSC.
- D. Kaniansky, M. Masár, J. Marák and R. Bodor, J. Chromatogr. A, 1999, 834, 133–178 CrossRef CAS PubMed.
- F. Geng, R. Ma and T. Sasaki, Acc. Chem. Res., 2010, 43, 1177–1185 CrossRef CAS PubMed.
- E. Mulugeta, Q. He, D. Sareen, S.-J. Hong, J. H. Oh, V. M. Lynch, J. L. Sessler, S. K. Kim and C.-H. Lee, Chem, 2017, 3, 1008–1020 CAS.
- J. Yoo, M.-S. Kim, S.-J. Hong, J. L. Sessler and C.-H. Lee, J. Org. Chem., 2009, 74, 1065–1069 CrossRef CAS PubMed.
- I. A. Rather, F. A. Sofi, M. A. Bhat and R. Ali, ACS Omega, 2022, 7, 15082–15089 CrossRef CAS PubMed.
- I. A. Rather, R. Ali and A. Ali, Org. Chem. Front., 2022, 9, 6416–6440 RSC.
- I. A. Rather, S. A. Khan, R. Ali and T. A. Khan, J. Mol. Liq., 2022, 367, 120406 CrossRef CAS.
- A. Kim, R. Ali, S. H. Park, Y.-H. Kim and J. S. Park, Chem. Commun., 2016, 52, 11139–11142 RSC.
- Z. Xu, S. Kim, H. N. Kim, S. J. Han, C. Lee, J. S. Kim, X. Qian and J. Yoon, Tetrahedron Lett., 2007, 48, 9151–9154 CrossRef CAS.
- D. E. Gómez, L. Fabbrizzi and M. Licchelli, J. Org. Chem., 2005, 14, 5717–5720 CrossRef PubMed.
- Y. Sun, C. Zhong, R. Gonga and E. Fu, Org. Biomol. Chem., 2008, 6, 3044–3047 RSC.
- Y. Liu, Z. Zhao, R. Huo and Q. Liu, Sci. Rep., 2019, 9, 502 CrossRef PubMed.
- Z. Deng, C. Wang, J. Li and M. Zheng, Front. Chem., 2021, 9, 732935 CrossRef CAS PubMed.
- S. A. Wagay and R. Ali, RSC Adv., 2023, 13, 30420–30428 RSC.
- B. Garg, T. Bisht and S. M. S. Chauhan, J. Inclusion Phenom. Macrocyclic Chem., 2011, 70, 249–255 CrossRef CAS.
- I. A. Rather and R. Ali, Green Chem., 2021, 23, 5849–5855 RSC.
- I. A. Rather, S. A. Wagay, M. S. Hasnain and R. Ali, RSC Adv., 2019, 9, 38309–38344 RSC.
- S. A. Wagay, M. Alam and R. Ali, J. Mol. Struct., 2023, 1291, 135982 CrossRef CAS.
- X. Zhang, Y. Shiraishi and T. Hirai, Tetrahedron Lett., 2007, 48, 8803–8806 CrossRef CAS.
- S. A. Wagay, I. A. Rather and R. Ali, Mater. Today: Proc., 2021, 36, 657–678 CAS.
- J. Y. Lee, H. D. Root, R. Ali, W. An, V. M. Lynch, S. Bähring, I. S. Kim, J. L. Sessler and J. S. Park, J. Am. Chem. Soc., 2020, 142, 19579–19587 CrossRef CAS PubMed.
- P. A. Gale, J. L. Sessler, V. Král and V. Lynch, J. Am. Chem. Soc., 1996, 118, 5140–5141 CrossRef CAS.
- W. Zhu, J. S. Park, J. L. Sessler and A. Gaitas, Appl. Phys. Lett., 2011, 98, 123501 CrossRef.
- C. Guo, A. C. Sedgwick, T. Hirao and J. L. Sessler, Coord. Chem. Rev., 2021, 427, 213560 CrossRef CAS PubMed.
- X. Ji, W. Chen, L. Long, F. Huang and J. L. Sessler, Chem. Sci., 2018, 9, 7746–7752 RSC.
- C.-S. Lee, W. Park, Y. U. Jo and K. Na, Chem. Commun., 2014, 50, 4354–4357 RSC.
- F. Pichierri, J. Mol. Struct.: THEOCHEM, 2002, 581, 117–127 CrossRef CAS.
- X. Ji, M. Ahmed, L. Long, N. M. Khashab, F. Huang and J. L. Sessler, Chem. Soc. Rev., 2019, 48, 2682–2697 RSC.
- C. Guo, H. Wang, V. M. Lynch, X. Ji, Z. A. Page and J. L. Sessler, Chem. Sci., 2020, 11, 5650–5657 RSC.
- S. Erdemir, S. Malkondu and O. Kocyigit, Luminescence, 2019, 34, 106–112 CrossRef CAS PubMed.
- I. A. Rather, U. Riaz and R. Ali, J. Mol. Struct., 2023, 1280, 135065 CrossRef CAS.
- A. Kasprowiak, I. A. Rather, R. Ali and P.-E. Danjou, J. Mol. Struct., 2023, 1287, 135694 CrossRef CAS.
- R. Hein, P. D. Beer and J. J. Davis, Chem. Rev., 2020, 3, 1888–1935 CrossRef PubMed.
- A. K. Mahapatra, S. K. Manna and P. Sahoo, Talanta, 2011, 85, 2673–2680 CrossRef CAS PubMed.
- M. S. Han and D. H. Kim, Angew. Chem., Int. Ed., 2002, 41, 3809–3811 CrossRef CAS PubMed.
- Z.-H. Fu, X. Han, Y. Shao, J. Fang, Z.-H. Zhang, Y.-W. Wang and Y. Peng, Anal. Chem., 2017, 89, 1937–1944 CrossRef CAS PubMed.
- S. I. Reja, N. Sharma, M. Gupta, P. Bajaj, V. Bhalla, R. D. Parihar, P. Ohri, G. Kaur and M. Kumar, Chem.–Eur. J., 2017, 23, 9872–9878 CrossRef CAS PubMed.
- J.-S. Wu, H. J. Kim, M. H. Lee, J. H. Yoon, J. H. Lee and J. S. Kim, Tetrahedron Lett., 2007, 48, 3159–3162 CrossRef CAS.
- G. Li, Y. Zhao, J. Li, J. Cao, J. Zhu, X. W. Sun and Q. Zhang, J. Org. Chem., 2015, 80, 196–203 CrossRef CAS PubMed.
- M. Gray, P. E. Bowling and J. M. Herbert, J. Chem. Theory Comput., 2022, 18, 6742–6756 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The supporting information contains material methods. Characterization part (1H-NMR, 13C-NMR, HRMS of all the synthesized compounds), anion sensing and anion binding studies spectra. Frontier molecular orbital distribution of C4P7 with various anions and their theoretically predicted FTIR spectra. See DOI: https://doi.org/10.1039/d3ra08362d |
| This journal is © The Royal Society of Chemistry 2024 |